Abstract
Selected antifungal lactic acid bacteria (LAB) isolated from mature spontaneous quinoa sourdough was used as potential starter culture to produce loaf wheat bread containing controlled fermented quinoa (CFQ) supplemented with red lentil (RL) flour. Phylogenetic evolutionary tree led to the identification of Enterococcus hirae as the selected LAB isolate. Furthermore, there was no significant difference (P > 0.05) between bread containing CFQ and control in terms of hardness. The highest loaf specific volume and overall acceptability were also observed in control sample and wheat bread containing CFQ + RL, respectively. Meanwhile, the rate of surface fungal growth on wheat bread enriched with CFQ was significantly lower than the other samples. In accordance with a non-linear multivariable model, positive and negative correlations were observed between porosity and specific volume (+ 0.79), and also specific volume and crumb hardness (− 0.70), respectively. Accordingly, CFQ can be used as bio-preservative to produce clean-label supplemented wheat bread.
Keywords: Fermented quinoa, Supplemented bread, Protective culture, Antifungal activity, Textural features
Introduction
Wheat bread is staple food in all over the world, and fungal contamination is the most important microbial deterioration of this product. There are some methods to reduce fungal spoilage of bread such as addition of synthetic (calcium propionate and potassium sorbate) or biological preservatives (protective starter cultures in controlled fermentation). Fermentation as a main stage in bread-making is not a novel technique, however; fermentation with proper starter cultures can reduce the use of chemically synthesized improvers or preservatives. One of the best resources to isolate these starter cultures is sourdough ecosystem. Sourdough is a fermented mixture of water and flour, and its fermentation positively influences all aspects of the baked goods’ quality (textural and sensorial properties), nutritional value and shelf life (Arora et al. 2021). Lactic acid bacteria (LAB) in sourdough represent a large group of microorganisms that have been used not only because they significantly contribute to acidification but also due to their antagonistic activity against food spoilage agents. These protective abilities are associated with a wide variety of antimicrobial metabolites produced during LAB fermentation (Crowley et al. 2013). Quinoa as a pseudo-cereal belongs to the Chenopodiaceae family. It contains minerals, vitamins, poly-unsaturated fatty acids, antioxidants and γ-aminobutyric acid, as well as phytohormones, and there is exceptional balance between its oil and proteins, suitable to manufacture functional and enriched breads (Vega-Gálvez et al. 2010; Navruz-Varli and Sanlier 2016).
It is estimated that huge amounts of the world's total bread production is lost due to the fungal spoilage, and it costs billion dollars a year. In addition to the economic losses caused by the growth of moulds in bakery products, production of fungal toxins can lead to acute health problems (Smith et al. 2004). Today, it is common to use biological preservatives such as microorganisms or their metabolites as “clean-label” strategy to eliminate this spoilage. Proper preservative effect on bread can be achieved through the use of antifungal LAB-fermented sourdough (Axel et al. 2017). Several studies have reported prolonged bread shelf life using controlled sourdough containing selected LAB isolates as potent bio-control agents to produce synthetic preservative-free breads, coupled with other pro-functional capabilities like improved technological and nutritional properties (Sadeghi et al. 2019; Ebrahimi et al. 2020). Axel et al. (2015) reported that quinoa sourdough containing antifungal Lactobacillus amylovorus improved nutritional value, quality and microbial stability of quinoa sourdough bread. Furthermore, there are evidences showing that the use of selected LAB and quinoa sourdough enhances the quality and safety of wheat bread (Rizzello et al. 2016).
Enrichment of wheat bread with controlled fermented pseudo-cereals along with fiber-rich legumes is an important approach to produce clean-label fortified breads. Consumption of legumes decreases the risk of some types of cancer, cardiovascular disease, type-II diabetes and obesity. Meanwhile, the world consumption of legumes is below the recommended dose (McCrory et al. 2010). Furthermore, selection of proper LAB starter cultures in combination with legumes may improve the functional, nutritional, sensorial and textural features of cereal-fermented products. Cereals are poor in lysine, but this amino acid is found in abundance in legumes. Therefore, the combination of cereals and legumes is very useful in terms of nutritional quality (Temba et al. 2016).
As far as we know, this is the first report providing information on the combined effect of quinoa sourdough (containing selected antifungal LAB isolate) and red lentil (RL) flour on wheat bread properties in order to produce functional bread. The main objective of this study was also application of the selected antifungal LAB isolate as potential protective starter culture to produce clean-label wheat bread supplemented with quinoa sourdough and RL.
Materials and methods
Raw materials
Wheat flour (68% extraction rate) purchased from a local flour mill (Zahedi flour, Gorgan, Iran). Quinoa de-hulled seeds and RL (commercial brands) used in the present study were purchased from a local market of Gorgan (Iran). Then, the seeds were milled (Laboratory mill make, Asan-toose-shargh, Iran) and sieved (0.5 mm) to produce their flours. Subsequently, protein, fat, ash and moisture contents of the flours were determined according to the AACC ( 2010) approved (46–10, 30–10, 08–01 and 44–19, respectively) methods. Carbohydrate percentage was also calculated using the following equation: 100–(protein + fat + ash + moisture) percentages. Chemical reagents and microbial media were purchased with analytical grade.
Spontaneous fermentation
A mixture of quinoa flour and tap water (with quality of drinking water) was used to prepare sourdough with dough yield of 200 (DY = dough mass × 100/flour mass). Then, the mixture was incubated at 37 °C for 24 h. For daily propagation (back-slopping), 20% of the formulation (w/w) was replaced with previously fermented quinoa, and the fermentation process (37 °C for 24 h) was repeated in several continuous days until the total titratable acidity (TTA) became constant (mature spontaneous sourdough). To calculate the TTA of sourdough samples, a homogenized sourdough-distilled water mixture (10 g to 90 mL) was prepared, and then it was titrated against 0.1 N NaOH to a final pH = 8.5. The TTA was also expressed as mL of the consumed NaOH (Axel et al. 2015). In order to estimate the number of LAB, serially ten-fold dilutions of mature spontaneous sourdough was spread plated on de Man, Rogosa and Sharpe (MRS) agar and incubated at 37 °C for 24 h.
Antifungal activity of the LAB isolates
The inhibitory activities of the predominant sourdough LAB isolates were determined against Aspergillus flavus (ATCC 15.546) through overlay method (Magnusson et al. 2003). Briefly, the fungal spores were harvested from 7-day-old potato dextrose agar (PDA) plated mould, and their number was adjusted to 1 × 106 spores/mL using a haemocytometer counting-chamber device. Then, each LAB isolate was cultured on MRS agar as two 3-cm lines and incubated at 37 °C for 72 h. Next, the LAB plates were overlaid with PDA containing A. flavus spores, and incubated at 25 °C until the surface of the control plate (MRS plate without LAB) was completely covered with the fungus. Finally, the antifungal activity of LAB was determined as clear zones of inhibition every day in comparison with the control, as screening strategy.
Molecular identification of the selected LAB
To identify the selected predominant LAB isolate, the DNA was extracted (AccuPrep, Genomic DNA Extraction Kit, Bioneer, South Korea) from the pure culture, and subjected to PCR amplification (1500 bp target sequence of 16S rDNA gene was amplified using Corbett thermocycler, Australia) in accordance with Abnous et al. (Abnous et al. 2009) procedure with F44 and R1543 primers. Subsequently, agarose gel electrophoresis and sequencing (Bioneer, South Korea) of the PCR products were carried out. Next, basic local alignment search tool (BLASTn) was used to confirm the identity of the sequence and finally, evolutionary position of the isolate was determined after drawing phylogenetic tree by neighbor-joining method using MEGA6 software with 1000 bootstrap replicates (Tamura et al. 2013).
Bread making
To produce control wheat bread (without CFQ or RL), Saccharomyces cerevisiae was added (2% w/w), and then the produced dough (water absorption of 60%) was fermented at 37 °C for 2 h. Control wheat bread dough (200 g) contained wheat flour (120 g), baker’s yeast (2.5 g), sugar (1 g) edible oil (1.5 g) and water (75 mL). Then, the dough was baked at 200 °C for 25 min in an electrical oven (Feller, Germany). Wheat bread containing CFQ fermented with the LAB isolate as starter culture (DY of 160) was also produced in accordance with the two-stage procedure including production of quinoa sourdough (fermented at 37 °C for 24 h) first, and mixing it (20%, w/w) with the control wheat dough (fermented at 37 °C for 2 h), later (Rizzello et al. 2016). Furthermore, wheat breads containing spontaneous fermented quinoa (SFQ), RL flour, non-fermented quinoa (NFQ), CFQ + RL flour and NFQ + RL flour were also produced under the identical processing conditions and with the same water contents. Effect of the raw substrates (comparison between control sample and NFQ or RL added breads), effect of the fermentation (comparison between bread containing NFQ and CFQ) and combined effect of NFQ/CFQ and RL compared to their controls were evaluated. It should be noted that wheat bread containing 0.2% (w/w) calcium propionate (CP) was also produced. This synthetic preservative was added to the control dough after proofing step.
Analysis of bread properties
Crumb hardness
Texture profile analysis (TPA; TAXT, Pluse Stable Micro Systems, UK) was used to investigate crumb hardness using a cylindrical probe compressing sample to 50% of its original height, 2 h after baking in accordance with AACC (2010) approved 74-09.01 method.
Specific volume and porosity
The loaf specific volume was determined by the rapeseed displacement method, 2 h after baking according to AACC (2010) 10–05.01 procedure. Porosity was also investigated using Image J (version 1.42e, USA) analysis in accordance with the method of Farrera-Rebollo et al. (2012).
In situ antifungal activity
Artificially contaminated breads were prepared by adding 5 µL suspension containing 106 spores/mL of the target fungus on a paper disc which was placed on the center of the samples (challenge test against A. flavus). Then, the produced breads were packed in zip-lock plastic bags and stored for 7 days at 25 °C. At this time, surface expansion of the mould was used as indicator of in situ antagonistic activity in the produced breads as previously described by Sadeghi et al. (2019). To investigate mould-free shelf life (mould growth inhibition over storage period) of the samples in this challenge test, the first day of mould growth observation was also determined.
Overall acceptability (OA)
A five point hedonic scale (1 = the lowest and 5 = the highest) was used to analyze sensory properties of the produced breads by the trained panelists in terms of aroma, flavour, colour, shape, chew-ability and moth feel over a 7-day incubation period according to Rizzello et al. (2016).
Statistical analysis and correlation among bread properties
The results of this study were statistically analyzed by one-way analysis of variance (ANOVA) in three replicates using SPSS (version 20) software. The means were also compared using the least significant difference (LSD) post-hoc test at P < 0.05. Microsoft Office Excel 2013 was used to draw the charts. Then, the results were applied to “Corrplot” package in “R” software (version 4.1.2) in order to investigate the correlation among the bread characteristics (Thomas et al. 2013).
Results and discussion
pH and TTA of sourdough
Quinoa flour contained 4.6% fat, 12.7% protein, 1.8% ash, 11.5% moisture, and 69.4% total carbohydrates. RL flour had 0.51% fat, 9.2% protein and 20.7% total carbohydrates. Wheat flour had 12.2% protein, 0.45% ash and 14.2% moisture, which were consistent with the observations of previous studies (Rizzello et al. 2016; Varmola et al. 2019). As we can see in Fig. 1, the value of pH in quinoa sourdough decreased continuously from the first to 5th day, and finally reached to 3.70. Furthermore, there was a significant difference (P < 0.05) among the pH of the first to 4th day; whereas, there was no significant difference between the 4th and 5th day in terms of pH; and therefore, the 4th day was selected for isolation of predominant LAB (mature spontaneous sourdough). The TTA increased during this period; meanwhile, there was no significant difference between TTA on day 4 and day 5. Total LAB count in mature spontaneous quinoa sourdough was also equal to 2 × 108 colony forming units (CFU)/g.
Fig. 1.
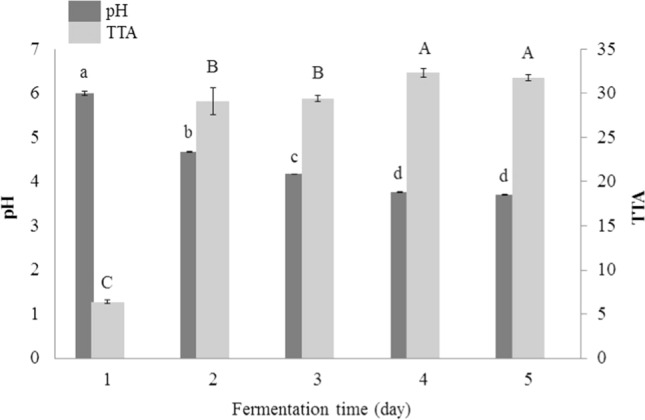
pH and total titratable acidity (TTA; as mL of the consumed 0.1 N NaOH) changes during daily back-slopping of quinoa sourdough. Different lowercase and uppercase letters indicate significant differences at P < 0.05 among the pH and TTA values, respectively
In the study of Wolter et al. (2014), TTA of quinoa fermented with Lactobacillus plantarum (DY of 200) reached to 24.6 after 24 h fermentation at 30 °C. In the work of Axel et al. (2016), the values of pH and TTA in quinoa sourdough fermented with Lactobacillus brevis were 4.14 and 32.9, respectively. Rizzello et al. (2016) also examined the pH and TTA of quinoa sourdough. In aforementioned study, pH and TTA were 5.82 and 7.4 in quinoa dough as control; whereas, their values in quinoa sourdough fermented with L. plantarum were equal to 3.83 and 30, respectively. It is announced that substrate composition, microbial metabolism, fermentation conditions (temperature, time) and process parameters (DY, back-slopping amount, refreshment period) are responsible for acidification properties during sourdough fermentation and the organic acid profiles of the resulting breads (Arora et al. 2021). Furthermore, acidification of sourdough affects several features of the produced sourdough bread including its texture, sensory and shelf life. At pH < 4, there is a significant positive charge on the sourdough proteins, and the electrostatic repulsion increases the solubility of these components. Controlled decrease in pH and increase in acid production affect the activity of indigenous enzymes during fermentation, followed by further breakdown of protein and starch. In addition, proteolysis leads to the production of volatile precursors during baking (Arendt et al. 2007).
LAB isolation and screening results
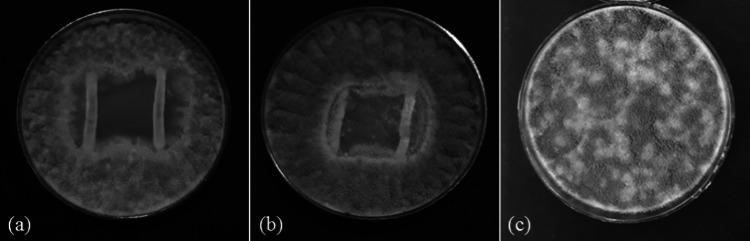
In preliminary study, two Gram-positive, catalase-negative cocci were isolated from mature spontaneous quinoa sourdough (after final back-slopping) as predominant LAB isolates. Inhibitory zone of the isolates in overlay bioassay is shown in Fig. 2. In accordance with the inhibitory activities of the isolates against A. flavus, the LAB isolate which had the highest antifungal effect was selected as potential protective starter culture for further study.
Fig. 2.
Antifungal activity of the predominant selected (a) and non-selected (b) LAB isolated from quinoa sourdough, compared to the control (c) in overlay bioassay
According to the results published by Guimarães et al. (2018), several LAB isolates were screened for antifungal activity in overlay bioassay. Among these isolates, L. plantarum had the highest antifungal activity. Pediococcus acidilactici, which had inhibitory activity against A. flavus, was also selected as protective starter culture in order to produce wheat bread supplemented with controlled fermented acorn by Purabdolah et al. (2020). Sourdough as a stressful niche is suitable ecosystem to isolate potential antifungal LAB cultures. Competition for nutrients, as well as production of organic acids and other antagonistic metabolites are the main mechanisms to explain the antimicrobial efficiency of LAB. These bacteria produce a variety of antimicrobial compounds including pH-reducing fermentation products and low molecular-weight inhibitory metabolites. Complex and synergistic activities between organic acids and peptides were already found to be responsible for the antifungal activity of sourdough LAB. Furthermore, acetic acid is believed to have a positive interaction with lactic acid in preventing fungal growth, however; acetic acid is described as more potent due to its higher pKa value. Neutralizing of the membrane electrochemical potential, increasing its permeability and fluidity, reduction of ribonuclease activity, inhibition of fungal metabolic pathways and quorum sensing are the main phenomena that have been proposed to play the key role in this antifungal effect (Crowley et al. 2013; Vermeulen et al. 2009).
Molecular identification of the selected LAB
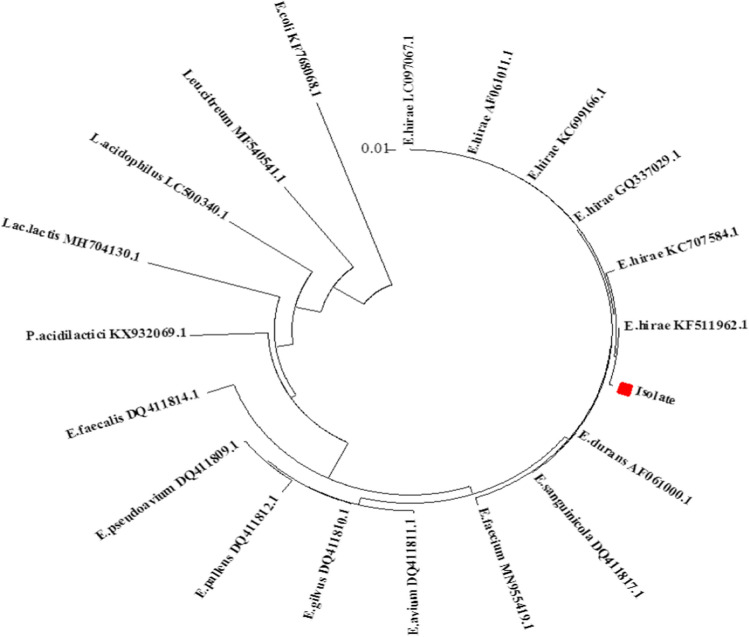
Amplification of the 1500 bp target sequence was verified according to agarose gel electrophoresis of the PCR products compared to the positive and negative controls. Sequencing results led to the identification of Enterococcus hirae RQS01as the selected isolate. Furthermore, phylogenetic evolutionary tree indicated that E. hirae KF511962.1 was the nearest LAB to the selected isolate (Fig. 3). The safety of the E. hirae isolate was also verified, and it had no haemolytic activity on blood agar.
Fig. 3.
Evolutionary position of the selected LAB isolated from quinoa sourdough in accordance with the phylogenetic tree drawn with neighbor-joining method using MEGA6 software. The bar indicates 0.01 differentiations, and E. coli was used as out group. E: Enterococcus, P: Pediococcus, Lact: Lactococcus, L: Lactobacillus and Leu: Leuconostoc
Ruiz Rodríguez et al. (2016) introduced L. brevis and Lactobacillus citreum as autochthonous predominant in spontaneously fermented quinoa sourdough. E. hirae was also isolated from quinoa grains and spontaneous sourdough in the work of Carrizo et al. (2016). Maidana et al. (2020) reported that Enterococcus was the most abundant genus in chia sourdough. The type of organisms that is found in association with each type of sourdough fermentation depends on the indigenous and process parameters. The level and the type of fermentable carbohydrates, nitrogen sources and growth factors present in the quinoa flour might have contributed to enzymatic hydrolysis during sourdough fermentation providing proper substrate for LAB metabolism and variation of this microbiota (Axel et al. 2015).
Bread properties
Crumb hardness, specific volume and porosity
According to the results of pretreatments, increasing of the CFQ led to the lower growth of A. flavus on the produced wheat bread. Whereas, the sensory properties of wheat bread supplemented with CFQ were acceptable up to 20% in formulation. Proper amount of RL flour was also determined 5% (w/w of wheat flour) in accordance with the results of pretreatment study. In the optimized formulation, the highest specific volume and the lowest crumb hardness were observed in the control sample. Application of NFQ and RL (as alone and combined) led to the significant (P < 0.05) increase of crumb hardness in supplemented wheat breads compared to the control sample. Meanwhile, the effect of RL was significantly higher than the NFQ. There was no significant difference between SFQ and NFQ added wheat breads in terms of crumb hardness. Furthermore, CFQ and CFQ + RL added breads had lower hardness and higher specific volume compared to the NFQ and NFQ + RL supplemented breads, respectively. Based on these results, controlled fermentation had noticeable effects on textural features of the enriched bread with quinoa as alone or mixed with RL (Table 1).
Table 1.
Textural features, in situ antifungal activity and overall acceptability of the supplemented wheat breads
| Bread samples | Crumb hardness (N) | Crumb porosity (%) | Specific volume (cm3/g) | Fungal growth (mm) | Overall acceptability |
|---|---|---|---|---|---|
| SFQ | 5.35 ± 0.86b | 13.45 ± 0.07b | 1.99 ± 0.08c | 44.73 ± 0.83a | 3.50 ± 0.43bc |
| CFQ | 2.82 ± 0.22c | 14.90 ± 0.71a | 2.80 ± 0.04ab | 6.15 ± 0.07f | 3.63 ± 0.40b |
| NFQ | 5.74 ± 0.79b | 12.40 ± 0.14bc | 2.55 ± 0.04b | 21.73 ± 0.18c | 3.08 ± 0.31c |
| RL | 7.58 ± 0.63a | 11.70 ± 0.71cd | 2.55 ± 0.02b | 17.14 ± 0.02d | 3.71 ± 0.20b |
| NFQ + RL | 8.88 ± 0.45a | 10.75 ± 0.49d | 2.65 ± 0.11ab | 19.44 ± 0.57d | 3.17 ± 0.48c |
| CFQ + RL | 5.20 ± 0.12b | 13.15 ± 0.35b | 2.88 ± 0.06a | 12.44 ± 0.16e | 4.13 ± 0.43a |
| Control | 2.64 ± 0.40c | 11.10 ± 0.42cd | 2.88 ± 0.02a | 25.32 ± 0.00b | 4.06 ± 0.20a |
| CP | 3.33 ± 0.38c | 14.20 ± 0.99a | 2.92 ± 0.04a | 23.55 ± 1.88b | 4.25 ± 0.24a |
The different letters in each column are significantly different at P < 0.05. SFQ: spontaneous fermented quinoa, CFQ: controlled fermented quinoa, NFQ: non-fermented quinoa, RL: red lentil and CP: calcium propionate added bread samples compared to wheat bread as control
Bourré et al. (2019) reported that the addition of RL as a pulse “the dry seed of legume” flour to wheat bread led to the reduction of loaf specific volume and increase of crumb hardness. Reduction of the dough stability due to weakening of the gluten network after addition of lentil to wheat bread was also reported by Turfani et al. (2017). Accordingly, disruption of wheat protein-starch interface after addition of legume flours caused changes in formation and hydration of gluten network. Furthermore, higher crumb hardness and lower specific volume with more compact crumb were observed in corresponding bread. High concentration of fibers, proteins and resistant starch, as well as low concentration of insoluble carbohydrates in legume flours are responsible for these phenomena. Fibers can limit the hydration of starch and proteins resulting in a compact crumb structure (Boukid et al. 2019). Addition of RL led to the higher cohesiveness of the supplemented wheat breads; whereas, it reduced the springiness (data not shown). In the same vein, TPA data showed that the chickpea and cowpea-fortified sorghum sourdough bread had higher cohesiveness and lower springiness than the control sample in the study of Olojede et al. (Olojede et al. 2020). Crumb springiness values in the study of Turkut et al. (Turkut et al. 2016) did not considerably change between quinoa added and the control sample. Lower value of springiness had been reported with denser crumb having lesser number of air bubbles. In contrast to our findings, Olojede et al. (2020) reported that chickpea-fortified sorghum sourdough bread had the lowest hardness compared to cowpea added and control breads. Furthermore, specific volume of this sample was significantly higher than the control. It is concluded that, substitution of wheat flour by legume flours leads to the dilution of gluten, and positively influences on loaf specific volume through increasing of water absorption, as well as maintenance of the gaseous bubbles due to formation of lipid monolayers at the gas/liquid interphase (Goesaert et al. 2005). Our results were also in agreement with Rizzello et al. (2016), who reported that substitution of NFQ led to the increase of crumb hardness in wheat bread. In the same respect, when quinoa sourdough was added, the hardness was significantly lower than the control, probably due to the effect of acidification and proteolysis of the selected LAB. Accordingly, the use of quinoa sourdough improved the textural features of the wheat bread in comparison with the NFQ. Sourdough acidification affects the solubility of gluten, starch and arabinoxylans, and also activity of endogenous enzymes. In addition, some metabolites produced by LAB improve water binding capacity, dough stability and gas retention through building a new structure and interaction with the gluten network.
In situ antifungal effect
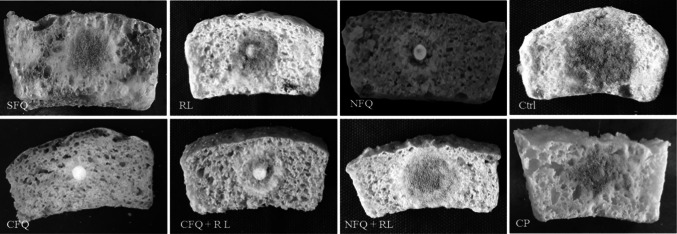
The lowest mould growth on the produced breads was observed in supplemented wheat bread with CFQ, which was significantly (P < 0.05) lower than the other samples. Furthermore, bread containing CFQ + RL had significantly higher in situ antifungal activity compared to NFQ + RL added bread. Interestingly, RL as alone had higher antifungal effect on the produced breads than the NFQ and NFQ + RL (Fig. 4 and Table 1). In addition, mould-free shelf life of almost all of the samples in challenge test (after inoculation of the target fungus) was equal to 3 days. Meanwhile, the highest mould-free shelf life was observed in CFQ added bread, which was 3 and 4 days more than CP added bread and control sample, respectively.
Fig. 4.
Surface mould growth on the produced breads containing spontaneous fermented quinoa (SFQ), controlled fermented quinoa (CFQ), non-fermented quinoa (NFQ) and red lentil (RL) powder (as alone or in combination) compared to the control sample (Ctrl) and calcium propionate added bread (CP) after 7 days of incubation at 25 °C in zip-lock plastic bags
Previous researchers similarly reported that the sourdough bread started with the association of selected LAB delayed fungal spoilage until several storage days compared to the control sample produced with baker’s yeast alone (Sadeghi et al. 2019; Ebrahimi et al. 2020). Quattrini et al. (2019) found that the shelf life of flaxseed sourdough bread fermented with Lactobacillus hammesii was 2 days longer than the control sample. Furthermore, mould-free shelf life of the produced bread challenged with Aspergillus niger increased by 6 days after addition of 4% sucrose to sourdough fermented with L. brevis. Proper protective effect (80% inhibition of A. flavus expansion) was observed when bread samples were co-fermented with L. plantarum in Russo et al. (2017) survey. It was hypothesized that fermentation of different substrates by sourdough LAB generated a complex and unique profile of bioactive antifungal metabolites (Axel et al. 2017).
Complex effect of inhibitory compounds derived from the raw substrates, released components during mixing and/or proofing steps, microbial metabolites produced during controlled fermentation, as well as synergistic interactions between these compounds could affect the in situ mould growth. The presence of antifungal compounds in legume extracts was also verified by Rizzello et al. (2017). Antifungal effect in legumes is dependent on their potential chitinolytic enzymes, which hydrolyze the chitin in fungal cell wall, and causes mould death through inhibiting the growth of fungal hyphae (Wang et al. 2009). The rate of mould growth on quinoa sourdough breads was delayed possibly due to the fact that sourdough fermentation accumulates antifungal organic acids and reduces the pH. Furthermore, the synergy between organic acids and other antifungal metabolites produced by sourdough LAB like hydroxy fatty acids (derived from unsaturated fatty acids of quinoa) play a more pronounced role in the complexity of the hurdle, and they intensify the preservative activity. In addition, production of some antifungal peptides as an effective factor in control of mould growth is related to hydrolysis of proteins during fermentation. Quinoa has a high protease activity; and therefore, it can be effective in preventing the fungal growth (Rizzello et al. 2016; Axel et al. 2016).
Sensory properties
OA of the CFQ + RL added, CP added and control samples were significantly (P < 0.05) higher than the other breads. Subsequently, RL and CFQ enriched breads had better sensory properties. Bread with fermented quinoa has yellowish crumb, and there was no significant difference between SFQ and CFQ in terms of OA. Furthermore, NFQ and NFQ + RL supplemented wheat breads had the lowest OA (Table 1). After 3 and 5 days of incubation at 25 °C, the OA had the same trend as reported for the first day. Meanwhile, after 7 days, the OA of the supplemented breads with quinoa and/or RL as alone or in combination were significantly lower than the control, and they were not acceptable for eating due to their poor chew-ability and mouth feel.
Kohajdová et al. (2013) reported that more than 10% legume flour adversely affected the OA of the supplemented wheat breads. Also, darkening of the legume added breads was attributed to higher lysine content, which led to increased Maillard reaction during processing. The use of quinoa sourdough by Wolter et al. (2014) resulted in agreeable breads with good crumb structure. The sensory analysis showed that NFQ bread had the lowest OA in the study of Ceballos‐González et al. (2018) due to high saponin content of quinoa flour and crumb hardness. In the same vein, presence of betalain pigment along with reducing sugars and lysine in whole-meal quinoa flour increases the Maillard reaction during baking. As a result, the crust of the quinoa added bread was darker than the control bread. In the study of Coda et al. (2010), sourdough bread consisting of several pseudo-cereals had the highest acceptance in terms of taste and appearance. In contrast, control wheat bread received the lowest OA. According to the researchers, the colour of quinoa sourdough bread was darker than the control, which was due to the low rate of starch hydrolysis, as well as high concentration of fiber and resistant starch. Sourdough bread OA is clearly influenced by the bread recipes, fermentation conditions and metabolic activities of the indigenous LAB, non-LAB and yeasts, as well as changes in odorant composition during thermal processing. Furthermore, degradation of amino acids, oxidation of fatty acids and production of organic acids, as well as generation of volatiles including alcohols, esters and carbonyl compounds during fermentation are the key factors in bread OA (Hansen and Schieberle 2005).
Correlation among bread characteristics
The results presented in Fig. 5 indicate the positive correlation between porosity and specific volume (+ 0.79), porosity and OA (+ 0.65), specific volume and OA (+ 0.61), as well as the mould growth and porosity (+ 0.31) of the produced breads. In contrast, negative correlations were observed between specific volume and crumb hardness (− 0.70), crumb hardness and OA (− 0.63), as well as crumb hardness and porosity (− 0.36) of the produced samples. In order to estimate the correlation among the available data and predict the mould growth using the quality characteristics of the produced breads, two linear and non-linear regression equations were applied by coding in “R” software. These models were as follow:
| 1 |
| 2 |
Fig. 5.
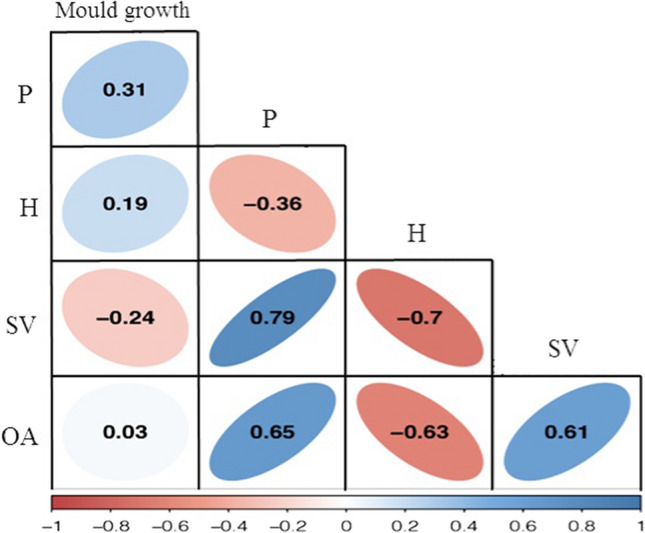
Correlation among the quality characteristics of the produced breads using “Corrplot” package in “R” software. P: porosity, H: crumb hardness, SV: loaf specific volume and OA: overall acceptability
In these equations, P, H, SV, OA, Eq. 1 and Eq. 2 are porosity, crumb hardness, loaf specific volume, overall acceptability of the produced breads, linear and non-linear multivariable models, respectively.
Monteau et al. (2017) used two models in order to explain the mechanisms of water transfer during bread staling in vapour and liquid phases. In accordance with findings of these researchers, water transfer as evaporation and condensation is important phenomenon in this process. Furthermore, it is not sufficient to consider the crust as a membrane permeable to water vapour. Ebrahimi et al. (2022) reported the synergistic effects of pumpkin puree and cereal bran sourdoughs (wheat, barley and rice bran sourdoughs) fermented with the selected P. acidilactici isolate on nutritional, textural and sensorial properties of the fortified wheat bread using response surface methodology. Moreover, linear-square model was more adequate for all responses compared to linear, linear-interactions and full quadratic models. According to our results, non-linear multivariable model had higher R2 than the linear model, and it was selected as proper model in order to analyze the correlation among different characteristics of the produced breads. Prediction of relationship between fungal spoilage and textural characteristics and/or OA of the produced bread is of great importance. Although determination of such a relationship may seem far-fetched, modeling can reveal existing relationships for better understanding of these correlations.
Conclusion
The use of sourdough LAB with suitable antifungal capabilities as protective starter cultures with the aim of controlling mould contamination and improving the quality characteristics of the produced bread is of great importance. In vitro and in situ antifungal activity of E. hirae isolate was confirmed in the present study. Accordingly, the isolate was used as protective starter culture in CFQ. Synergistic effect of CFQ and RL on properties of the produced bread was also revealed. Among the samples containing CFQ and RL (alone or combined), the sample containing CFQ had the lowest rate of surface fungal growth. Furthermore, specific volume and OA of the sample containing CFQ + RL were not significantly different from the control. To sum up, quinoa sourdough serves as a great bio-additive to produce functional wheat bread. In addition, the simultaneous application of legume flour and quinoa sourdough, which are rich in fiber and micronutrients, is important to improve quality characteristics of wheat bread as the main staple food in all over the world.
Acknowledgements
Not applicable.
Abbreviations
- ANOVA
One-way analysis of variance
- BLAST
Basic local alignment search tool
- CFQ
Controlled fermented quinoa
- CP
Calcium propionate
- DY
Dough yield
- LAB
Lactic acid bacteria
- LSD
Least significant difference
- MRS
De Man, Rogosa and Sharpe
- NFQ
Non-fermented quinoa
- OA
Overall acceptability
- PDA
Potato dextrose agar
- RL
Red lentil
- SFQ
Spontaneous fermented quinoa
- TTA
Total titratable acidity
Authors' contributions
Elham Rouhi: Formal analysis. Alireza Sadeghi: Supervision, Project administration, Methodology, Validation, Writing—review & editing. Seid Mahdi Jafari: Methodology, Validation, Writing—review & editing. Mohammad Abdolhoseini: Methodology, Validation. Elham Assadpour: Methodology, Validation.
Funding
Not applicable.
Availability of data and materials
All the data are available in the manuscript.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- AACC, Moisture 44-19, protein 46-10, fat 30-10, ash 08-01, crumb hardness 74-09.01 and loaf specific volume 10–05.01 methods. In. St. Paul, MN, USA: American association of cereal chemists (AACC) international (2010).
- Abnous K, Brooks SP, Kwan J, Matias F, Green-Johnson J, Selinger LB, Thomas M, Kalmokoff M. Diets enriched in oat bran or wheat bran temporally and differentially alter the composition of the fecal community of rats. J Nutr. 2009;139(11):2024–2031. doi: 10.3945/jn.109.109470. [DOI] [PubMed] [Google Scholar]
- Arendt EK, Ryan LA, Dal Bello F. Impact of sourdough on the texture of bread. Food Microbiol. 2007;24(2):165–174. doi: 10.1016/j.fm.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Arora K, Ameur H, Polo A, Di Cagno R, Rizzello CG, Gobbetti M. Thirty years of knowledge on sourdough fermentation: a systematic review. Trends Food Sci Technol. 2021;108:71–83. doi: 10.1016/j.tifs.2020.12.008. [DOI] [Google Scholar]
- Axel C, Röcker B, Brosnan B, Zannini E, Furey A, Coffey A, Arendt EK. Application of Lactobacillus amylovorus DSM19280 in gluten-free sourdough bread to improve the microbial shelf life. Food Microbiol. 2015;47:36–44. doi: 10.1016/j.fm.2014.10.005. [DOI] [PubMed] [Google Scholar]
- Axel C, Brosnan B, Zannini E, Furey A, Coffey A, Arendt EK. Antifungal sourdough lactic acid bacteria as biopreservation tool in quinoa and rice bread. Int J Food Microbiol. 2016;239:86–94. doi: 10.1016/j.ijfoodmicro.2016.05.006. [DOI] [PubMed] [Google Scholar]
- Axel C, Zannini E, Arendt EK. Mold spoilage of bread and its biopreservation: a review of current strategies for bread shelf life extension. Crit Rev Food Sci Nutr. 2017;57(16):3528–3542. doi: 10.1080/10408398.2016.1147417. [DOI] [PubMed] [Google Scholar]
- Boukid F, Vittadini E, Lusuardi F, Ganino T, Carini E, Morreale F, Pellegrini N. Does cell wall integrity in legumes flours modulate physiochemical quality and in vitro starch hydrolysis of gluten-free bread? J Funct Foods. 2019;59:110–118. doi: 10.1016/j.jff.2019.05.034. [DOI] [Google Scholar]
- Bourré L, Frohlich P, Young G, Borsuk Y, Sopiwnyk E, Sarkar A, Nickerson MT, Ai Y, Dyck A, Malcolmson L. Influence of particle size on flour and baking properties of yellow pea, navy bean, and red lentil flours. Cereal Chem. 2019;96(4):655–667. doi: 10.1002/cche.10161. [DOI] [Google Scholar]
- Carrizo SL, de Oca CEM, Laiño JE, Suarez NE, Vignolo G, LeBlanc JG, Rollán G. Ancestral Andean grain quinoa as source of lactic acid bacteria capable to degrade phytate and produce B-group vitamins. Food Res Int. 2016;89:488–494. doi: 10.1016/j.foodres.2016.08.013. [DOI] [PubMed] [Google Scholar]
- Ceballos-González C, Bolívar-Monsalve J, Ramírez-Toro C, Bolívar GA. Effect of lactic acid fermentation on quinoa dough to prepare gluten-free breads with high nutritional and sensory quality. J Food Process Preserv. 2018;42(3):e13551. doi: 10.1111/jfpp.13551. [DOI] [Google Scholar]
- Coda R, Rizzello CG, Gobbetti M. Use of sourdough fermentation and pseudo-cereals and leguminous flours for the making of a functional bread enriched of γ-aminobutyric acid (GABA) Int J Food Microbiol. 2010;137(2–3):236–245. doi: 10.1016/j.ijfoodmicro.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Crowley S, Mahony J, van Sinderen D. Current perspectives on antifungal lactic acid bacteria as natural bio-preservatives. Trends Food Sci Technol. 2013;33(2):93–109. doi: 10.1016/j.tifs.2013.07.004. [DOI] [Google Scholar]
- Ebrahimi M, Sadeghi A, Mortazavi SA. The use of cyclic dipeptide producing LAB with potent anti-aflatoxigenic capability to improve techno-functional properties of clean-label bread. Ann Microbiol. 2020;70(1):1–12. doi: 10.1186/s13213-020-01571-y. [DOI] [Google Scholar]
- Ebrahimi M, Noori SMA, Sadeghi A, Emir Coban O, Zanganeh J, Ghodsmofidi SM, Malvandi Z, Raeisi M. Application of cereal-bran sourdoughs to enhance technological functionality of white wheat bread supplemented with pumpkin (Cucurbita pepo) puree. LWT Food Sci Technol. 2022 doi: 10.1016/j.lwt.2022.113079. [DOI] [Google Scholar]
- Farrera-Rebollo RR, Salgado-Cruz M, Chanona-Pérez J, Gutiérrez-López GF, Alamilla-Beltrán L, Calderón-Domínguez G. Evaluation of image analysis tools for characterization of sweet bread crumb structure. Food Bioprocess Tech. 2012;5(2):474–484. doi: 10.1007/s11947-011-0513-y. [DOI] [Google Scholar]
- Goesaert H, Brijs K, Veraverbeke W, Courtin C, Gebruers K, Delcour J. Wheat flour constituents: how they impact bread quality, and how to impact their functionality. Trends Food Sci Technol. 2005;16(1–3):12–30. doi: 10.1016/j.tifs.2004.02.011. [DOI] [Google Scholar]
- Guimarães A, Santiago A, Teixeira JA, Venâncio A, Abrunhosa L. Anti-aflatoxigenic effect of organic acids produced by Lactobacillus plantarum. Int J Food Microbiol. 2018;264:31–38. doi: 10.1016/j.ijfoodmicro.2017.10.025. [DOI] [PubMed] [Google Scholar]
- Hansen A, Schieberle P. Generation of aroma compounds during sourdough fermentation: applied and fundamental aspects. Trends Food Sci Technol. 2005;16(1–3):85–94. doi: 10.1016/j.tifs.2004.03.007. [DOI] [Google Scholar]
- Kohajdová Z, Karovičová J, Magala M. Effect of lentil and bean flours on rheological and baking properties of wheat dough. Chem Pap. 2013;67(4):398–407. doi: 10.2478/s11696-012-0295-3. [DOI] [Google Scholar]
- Magnusson J, Ström K, Roos S, Sjögren J, Schnürer J. Broad and complex antifungal activity among environmental isolates of lactic acid bacteria. FEMS Microbiol Lett. 2003;219(1):129–135. doi: 10.1016/S0378-1097(02)01207-7. [DOI] [PubMed] [Google Scholar]
- Maidana SD, Ficoseco CA, Bassi D, Cocconcelli PS, Puglisi E, Savoy G, Vignolo G, Fontana C. Biodiversity and technological-functional potential of lactic acid bacteria isolated from spontaneously fermented chia sourdough. Int J Food Microbiol. 2020;316:108425. doi: 10.1016/j.ijfoodmicro.2019.108425. [DOI] [PubMed] [Google Scholar]
- McCrory MA, Hamaker BR, Lovejoy JC, Eichelsdoerfer PE. Pulse consumption, satiety, and weight management. Adv Nutr. 2010;1(1):17–30. doi: 10.3945/an.110.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteau JY, Purlis E, Besbes E, Jury V, Le-Bail A. Water transfer in bread during staling: physical phenomena and modelling. J Food Eng. 2017;211:95–103. doi: 10.1016/j.jfoodeng.2017.04.016. [DOI] [Google Scholar]
- Navruz-Varli S, Sanlier N. Nutritional and health benefits of quinoa (Chenopodium quinoa Willd.) J Cereal Sci. 2016;69:371–376. doi: 10.1016/j.jcs.2016.05.004. [DOI] [Google Scholar]
- Olojede A, Sanni A, Banwo K. Effect of legume addition on the physiochemical and sensorial attributes of sorghum-based sourdough bread. LWT Food Sci Technol. 2020;118:108769. doi: 10.1016/j.lwt.2019.108769. [DOI] [Google Scholar]
- Purabdolah H, Sadeghi A, Ebrahimi M, Kashaninejad M, Shahiri Tabarestani H, Mohamadzadeh J. Techno-functional properties of the selected antifungal predominant LAB isolated from fermented acorn (Quercus persica) J Food Meas Charact. 2020;14(3):1754–1764. doi: 10.1007/s11694-020-00423-2. [DOI] [Google Scholar]
- Quattrini M, Liang N, Fortina MG, Xiang S, Curtis JM, Gänzle M. Exploiting synergies of sourdough and antifungal organic acids to delay fungal spoilage of bread. Int J Food Microbiol. 2019;302:8–14. doi: 10.1016/j.ijfoodmicro.2018.09.007. [DOI] [PubMed] [Google Scholar]
- Rizzello CG, Lorusso A, Montemurro M, Gobbetti M. Use of sourdough made with quinoa (Chenopodium quinoa) flour and autochthonous selected lactic acid bacteria for enhancing the nutritional, textural and sensory features of white bread. Food Microbiol. 2016;56:1–13. doi: 10.1016/j.fm.2015.11.018. [DOI] [PubMed] [Google Scholar]
- Rizzello CG, Verni M, Bordignon S, Gramaglia V, Gobbetti M. Hydrolysate from a mixture of legume flours with antifungal activity as an ingredient for prolonging the shelf-life of wheat bread. Food Microbiol. 2017;64:72–82. doi: 10.1016/j.fm.2016.12.003. [DOI] [PubMed] [Google Scholar]
- Ruiz Rodríguez L, Vera Pingitore E, Rollan G, Cocconcelli PS, Fontana C, Saavedra L, Vignolo G, Hebert EM. Biodiversity and technological-functional potential of lactic acid bacteria isolated from spontaneously fermented quinoa sourdoughs. J App Microbiol. 2016;120(5):1289–1301. doi: 10.1111/jam.13104. [DOI] [PubMed] [Google Scholar]
- Russo P, Arena MP, Fiocco D, Capozzi V, Drider D, Spano G. Lactobacillus plantarum with broad antifungal activity: a promising approach to increase safety and shelf-life of cereal-based products. Int J Food Microbiol. 2017;247:48–54. doi: 10.1016/j.ijfoodmicro.2016.04.027. [DOI] [PubMed] [Google Scholar]
- Sadeghi A, Ebrahimi M, Mortazavi SA, Abedfar A. Application of the selected antifungal LAB isolate as a protective starter culture in pan whole-wheat sourdough bread. Food Control. 2019;95:298–307. doi: 10.1016/j.foodcont.2018.08.013. [DOI] [Google Scholar]
- Smith JP, Daifas DP, El-Khoury W, Koukoutsis J, El-Khoury A. Shelf life and safety concerns of bakery products-a review. Crit Rev Food Sci Nutr. 2004;44(1):19–55. doi: 10.1080/10408690490263774. [DOI] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 60. Mol Biol Evol. 2013;30(12):2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temba MC, Njobeh PB, Adebo OA, Olugbile AO, Kayitesi E. The role of compositing cereals with legumes to alleviate protein energy malnutrition in Africa. Int J Food Sci. 2016;51(3):543–554. doi: 10.1111/ijfs.13035. [DOI] [Google Scholar]
- Thomas R, Vaughan J, Lello I (2013) Data analysis with R statistical software. A guidebook for scientists. Ecoexplore
- Turfani V, Narducci V, Durazzo A, Galli V, Carcea M. Technological, nutritional and functional properties of wheat bread enriched with lentil or carob flours. LWT Food Sci Technol. 2017;78:361–366. doi: 10.1016/j.lwt.2016.12.030. [DOI] [Google Scholar]
- Turkut GM, Cakmak H, Kumcuoglu S, Tavman S. Effect of quinoa flour on gluten-free bread batter rheology and bread quality. J Cereal Sci. 2016;69:174–181. doi: 10.1016/j.jcs.2016.03.005. [DOI] [Google Scholar]
- Varmola E, Bedade D, Deshaware S, Ojamo H, El Haj AM, Shamekh S. Evaluation of baking conditions for frozen doughs. J Food Meas Charact. 2019;13(4):3307–3317. doi: 10.1007/s11694-019-00253-x. [DOI] [Google Scholar]
- Vega-Gálvez A, Miranda M, Vergara J, Uribe E, Puente L, Martínez EA. Nutrition facts and functional potential of quinoa (Chenopodium quinoa willd.) an ancient Andean grain: a review. J Sci Food Agric. 2010;90(15):2541–2547. doi: 10.1002/jsfa.4158. [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Gysemans K, Bernaerts K, Geeraerd A, Debevere J, Devlieghere F, Van Impe J. Modelling the influence of the inoculation level on the growth/no growth interface of Listeria monocytogenes as a function of pH, aw and acetic acid. Int J Food Microbiol. 2009;135(2):83–89. doi: 10.1016/j.ijfoodmicro.2009.07.038. [DOI] [PubMed] [Google Scholar]
- Wang S, Shao B, Fu H, Rao P. Isolation of a thermostable legume chitinase and study on the antifungal activity. Appl Microbiol Biotechnol. 2009;85(2):313–321. doi: 10.1007/s00253-009-2074-9. [DOI] [PubMed] [Google Scholar]
- Wolter A, Hager AS, Zannini E, Czerny M, Arendt EK. Impact of sourdough fermented with Lactobacillus plantarum FST 17 on baking and sensory properties of gluten-free breads. Eur Food Res Technol. 2014;239(1):1–12. doi: 10.1007/s00217-014-2184-1. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data are available in the manuscript.
Not applicable.