Abstract
Chitosan oligosaccharides (COSs) have been reported to possess a broad range of activities such as antitumor, antioxidant and neuroprotective activities. In this study, the protective effects and mechanisms of peracetylated chitosan oligosaccharides (PACOs) against Aβ-induced cognitive deficits were investigated in Sprague–Dawley (SD) rats. PACOs treatment significantly improved the learning and memory function of Alzheimer’s disease (AD) rats and attenuated the neuron cell damage caused by Aβ. PACOs also markedly reduced the levels of lactate dehydrogenase (LDH) and Malondialdehyde (MDA) and decreased the phosphorylation of Tau protein to inhibit oxidative injury and inflammatory responses in AD rats. Further studies indicated that PACOs may promote the repair of Aβ induced nerve damage and inhibit neuronal apoptosis mainly through regulating PI3K/Akt/GSK3β signaling pathway. Consistently, the transcriptome analysis verified that the differentially expressed genes (DEGs) were mainly involved in neuron development and the PI3K-Akt signaling pathway. Taken together, peracetylated chitosan oligosaccharides (PACOs) have the potential to be developed into novel anti-AD agents targeting the cellular PI3K/Akt/GSK3β signaling pathway.
Supplementary Information
The online version contains supplementary material available at 10.1007/s42995-023-00172-3.
Keywords: Peracetylated chitosan oligosaccharides, Neuroprotective, Amyloid, Cognitive deficit, PI3K/Akt/GSK3β pathway
Introduction
Dementia affects more than 47 million of the world's population and brings great burden to individuals and society. Alzheimer’s disease (AD) accounts for about 2/3 of dementia cases. According to a recent WHO statistic, there are more than 55 million people suffering from Alzheimer's disease (AD) in the world and over 10 million AD patients in China, which has been the country with the most AD patients (Corriveau et al. 2017; Ren et al. 2022). The incidence of AD continues to increase, and its social and economic burden is increasing, which constitutes a major medical and social problem threatening urban and rural residents in China (Jia et al. 2020a, b; Ren et al. 2022). Aβ aggregates in the brain are the pathological hallmark of AD and associated with cognitive impairment. However, the underlying pathogenesis mechanisms of AD are unclear (Haass and Selkoe 2022; Thal and Fändrich 2015). The mostly accepted theories of AD pathogenesis include Aβ cascade hypothesis, Tau over-phosphorylation theory, cholinergic theory, neuronal cell apoptosis theory, oxidative stress theory, and inflammatory reaction theory (Alzheimer's Association 2016). Accordingly, tackling not only Aβ aggregates but also Tau over-phosphorylation, neuronal cell apoptosis, and inflammatory reaction may offer comprehensive targets for AD prevention and therapy. However, until now there is still a lack of effective long-term drugs to improve cognitive function and reduce disease progression (Athar et al. 2021). Thus, it is urgent to develop novel multi-target pharmacological agents for the prevention and therapy of AD.
Chitosan oligosaccharides (COSs) are degradation products of chitosan, which have various biological activities, including antioxidant, antimicrobial, and antitumor activities (Hao et al. 2017; Yi et al. 2020; Zhang et al. 2010). Recently, it has been reported that the COSs possess good neuroprotective properties such as β-amyloid and acetylcholinesterase inhibitory activities, anti-neuroinflammation, and anti-apoptosis effects, which suggest the COSs had all the needed properties for AD prevention and therapy (Lee et al. 2009; Ouyang et al. 2017; Saxena and Dubey 2019; Zhou et al. 2008). Our previous studies showed that the derivatives of COSs-acetylated chitosan oligosaccharides have better neuroprotective effects than COSs in vitro, and they can inhibit high glutamate induced PC12 cell death mainly through preventing neuronal cell apoptosis, suggesting that the acetylated chitosan oligosaccharides might be promising antagonists against neural cell death (Hao et al. 2015).
To further correlate the neuroprotective activities of acetylated chitosan oligosaccharides with their underlying molecular mechanisms, the peracetylated chitosan oligosaccharides (PACOs) were produced and their anti-AD effects and mechanisms were investigated in vivo in this study. The results showed that PACOs treatment significantly improved the learning and memory function of AD rats and may promote repair of Aβ induced nerve damage and inhibit neuronal apoptosis mainly though regulating the PI3K/Akt/GSK3β signaling pathway, suggesting that PACOs merit further investigation to be developed into a novel anti-AD agent in the future.
Results
Characterization of peracetylated chitosan oligosaccharides (PACOs)
Peracetylated chitosan oligosaccharides (PACOs) were a mixture of oligosaccharides (Supplementary Fig. S1A) with different degree of polymerization (DP), which were prepared using the methods described previously (Gao et al. 2013; Hao et al. 2015; Wang et al. 2005). The compositional and structural characterization of PACOs by MS, HPTLC, IR, 1H-NMR and 13C-NMR are shown in Supplementary Fig. S1B–F, respectively. There were mainly five components in PACOs as shown in Supplementary Fig. S1C based on HPTLC analysis. The main ions observed in ESI–MS spectrum (Supplementary Fig. S1B) were m/z 964.62 [M]+, 965.65 [M + H]+, and 986.69 [M + Na]+ corresponding to the trimer (DP = 3) of PACOs, 1251.84 [M + H]+ and 1273.89 [M + Na]+ corresponding to the tetramer (DP = 4) of PACOs, and 1539.10 [M + H]+ corresponding to the pentamer (DP = 5) of PACOs. Thus, PACOs consist mainly of DP3, DP4, and DP5 in addition to a small amount of DP2 and DP6 based on MS and HPTLC analyses (Supplementary Fig. S1B, C).
The structure of PACOs was characterized by FT-IR and NMR. As shown in Supplementary Fig. S1D, the bands at 3338 cm−1, 1662 cm−1 and 1536 cm−1 corresponded to the νN–H, νC=O and δνN–H of the –NHAc, respectively. The bands at 1746 cm−1, 1231 cm−1 and 1049 cm−1 were attributed to the νC=O, νas C(=O)–O–C and νs C(=O)–O–C of the –OAc, respectively. In the 1H-NMR spectrum of PACOs (Supplementary Fig. S1E), the peaks at δ 7.97–7.86 × 10–6 were attributed to NH, and the peaks at 5.81 × 10–6 and 5.60 × 10–6 were assigned to the H-1(α-isomer) and H-1(β-isomer). The signals of H-2, H-3, H-4, H-5 and H-6 were in the region of 5.13–3.55 × 10–6. The peaks at 2.14–1.72 × 10–6 were attributed to CH3. There were no signals of OH in 1H-NMR, indicating that all the OHs were acetylated. In the 13C NMR spectrum (Supplementary Fig. S1F), the peaks at δ 170.2–169.2 × 10–6 were attributed to C = O, the peaks at δ 100.1–99.7 × 10–6 were attributed to C-1 of non-reducing end residues, and the peaks at 91.6 × 10–6 and 89.7 × 10–6 were assigned to the resonance of C-1 (β-isomer) and C-1(α-isomer) of reducing end residues, respectively. The signals of C-2, C-3, C-4, C-5 and C-6 were packed in a narrow region between 75.7 and 50.0 × 10–6. The peaks at 22.6–20.3 × 10–6 were attributed to the resonances of CH3. Thus, PACOs had the structures shown in Supplementary Fig. S1A.
The improvement effects of PACOs on Aβ25–35 induced impairments of spatial learning and memory ability in AD rats
Our previous studies indicated that the peracetylated chitosan oligosaccharides (PACOs) possessed protective effects against glutamate-induced PC12 cell death (Hao et al. 2015); thus, we further explored whether PACOs possess anti-AD effects in vivo in this study. It has been reported that Aβ25–35 can induce neuronal malnutrition, synaptic loss and neuronal apoptosis (Li et al. 2017). As such, we further investigated the neuroprotective effects and mechanisms of PACOs in vivo by using the traditional animal model of injecting Aβ25–35 into the lateral ventricle of SD rats (Supplementary Fig. S2A). The Morris water maze (MWM) test was performed to analyze the protective effects of PACOs in AD rats induced by Aβ25–35 (Supplementary Fig. S2). After training, rats in the sham operation group (control) used the least time to escape the latent period, and the rats in the model group (Aβ) showed significantly longer escape latencies compared to those in the sham control group, indicating that the Aβ25–35 truly induced learning impairment of rats (Supplementary Fig. S2B). However, after PACOs treatment (25, 50, 100 mg/kg), the escape latencies were significantly shortened on Day 1 to Day 4, compared to the model group (Aβ) (Supplementary Fig. S2B). Rats in the PACO-50 group (50 mg/kg) had the least escape latency among three PACOs treated groups, even better than that in the sham group (control) on Day 1 and Day 2 (Supplementary Fig. S2B).
Moreover, the spatial probe test was also performed to explore the effect of PACOs on the spatial memory ability of AD rats. As shown in Supplementary Fig. S2C and S2D, after training, the rats in the sham operation group (control) possessed the most times of platform crossing in 120 s and the longest time of duration in the target quadrant. Consistent with the result of the orientation navigation test, in the probe trials, the duration in the target quadrant or the number of crossings over the platform was significantly reduced in the model group (Aβ), compared to the Sham control group (P < 0.001). However, after treatment with 50 or 100 mg/kg of PACOs, the duration time in the target quadrant and the number of crossings were significantly increased, as compared to the model group (P < 0.05) (Supplementary Fig. S2C, D). PACOs (25 mg/kg) treatment also improved the performance but without significance (P > 0.05) (Supplementary Fig. S2C, D). In summary, these results indicated that PACOs could decrease the escape latencies, increase the target quadrant duration and number of crossings, suggesting that PACOs may be able to improve the learning and memory ability of AD rats.
PACOs improved pathological changes and cell apoptosis in rats’ hippocampus
To further evaluate the effects of PACOs on Aβ induced neuronal loss in the hippocampus of AD rats, HE staining and immunohistochemistry assay were applied as described previously (Feldman and Wolfe 2014). As shown in Supplementary Fig. S3, the results of HE staining indicated that the hippocampal neurons of the sham group (control) were intact, neatly and tightly arranged, with large and clear nucleolus. However, after Aβ25–35 injections, the cell number in the CA1 region was decreased and some neurons arranged disorderly with dark staining, membrane shrinkage, and even disappearance (Supplementary Fig. S3). However, these neuronal damages were significantly attenuated after treatment with PACOs compared to the model group (Aβ) (Supplementary Fig. S3). In the 50 mg/kg of PACOs treated group, the nerve cells arranged in a more orderly manner, and the cell morphology and nucleolus were clearer than other PACOs treated group (25 or 100 mg/kg), very similar to that in the sham group (Supplementary Fig. S3).
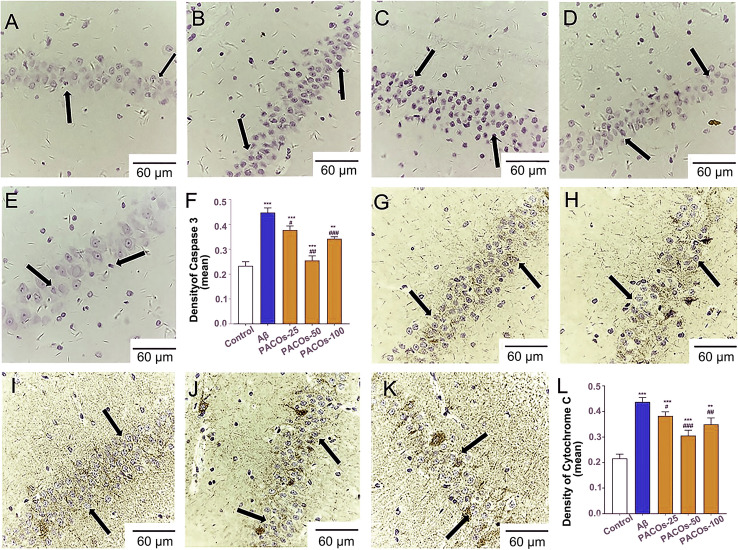
To further determine the effects of PACOs on cell apoptosis in rats’ hippocampus, we detected the expression levels of activated (cleaved) caspase-3 protein, which is a critical early marker of cell apoptosis. Consistent to the results of HE staining, the immunohistochemistry assay showed that the level of cleaved caspase-3 was significantly increased after Aβ injection compared to the sham group (control) (P < 0.001) (Fig. 1A–E), and treatment with PACOs (25, 50 or 100 mg/kg) significantly reduced the Aβ-induced caspase-3 activation (P < 0.05) (Fig. 1F). Moreover, treatment with PACOs (25, 50 or 100 mg/kg) also significantly decreased the Aβ induced cytochrome C release, as compared to the model group (Aβ) (P < 0.05) (Fig. 1G–L). Thus, PACOs had neuroprotective effects against the neuron cell damage caused by Aβ.
Fig. 1.
IHC assay of cleaved Caspase 3 and Cytochrome C in hippocampal of AD model rats. The contents of cleaved Caspase 3 and Cytochrome C in hippocampal of AD model rats with or without PACOs (25, 50, 100 mg/kg) treatment were detected by immunohistochemistry (IHC) assay. A, G Control group; B, H Aβ group; C, I PACOs-25 group; D, J PACOs-50 group; E, K PACOs-100 group (magnification ×200, scale bar represents 60 µm). Quantitative analysis of IHC assay of cleaved Caspase 3 (F) and Cytochrome C (L) was also shown. *P < 0.05, **P < 0.01, ***P < 0.001 versus control group; #P < 0.05, ##P < 0.01, ###P < 0.001 versus AD group (Aβ), scale bar represents 60 µm
Effects of PACOs on the production of Aβ aggregation and inflammatory factors
It has been known that the presence of amyloid plaques and neurofibrillary tangles are often required for a pathological diagnosis of AD (DeTure and Dickson 2019), and the Aβ aggregation and tau-positive neurites are believed to be closely related to the neuronal loss and cognitive decline in AD (Knowles et al. 1999; Malek-Ahmadi et al. 2016). Since PACOs could reduce neuronal loss and apoptosis in Aβ treated rat hippocampus, we further investigated the inhibition of PACOs against Aβ aggregation and its induced cytokine secretion in rat hippocampus. First, the content of Aβ1–42 in rat’s hippocampus was determined by using enzyme linked immunosorbent assay (ELISA) to further explore the effect of PACOs on Aβ aggregation. As shown in Fig. 2A, compared with the sham group (control), the level of Aβ1–42 protein in rat hippocampus was significantly increased in the model group (Aβ) (P < 0.001). However, after PACOs treatment (25, 50, 100 mg/kg), the levels of Aβ protein were significantly reduced in a dose-dependent manner, as compared to the model group (P < 0.05) (Fig. 2A), suggesting that PACOs could reduce the production of Aβ aggregation in rat hippocampus.
Fig. 2.

Effects of PACOs on the production of Aβ aggregation and inflammatory factors. A The effects of PACOs on Aβ aggregation in rat hippocampus were evaluated by ELISA assay. B The effects of PACOs on the production of p-Tau protein in rat’s hippocampus were evaluated by using ELISA assay. C, D The inhibition of PACOs against Aβ-induced cytokine TNF-α (C) and IL-6 D secretion in rat hippocampus was determined by ELISA assay. All the data presented as mean ± SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001 versus control group; #P < 0.05, ##P < 0.01, ###P < 0.001 versus AD group (Aβ)
The anatomic localization of NFT in AD is closely related to the most severe brain regions with neuron loss, so the phosphorylated Tau protein is thought to play a key role in the pathogenesis of AD (DeTure and Dickson 2019). The effects of PACOs on the phosphorylation of Tau protein in rat’s hippocampus were also evaluated by using ELISA assay. As shown in Fig. 2B, compared with the sham group (control), the expression of phosphorylated Tau protein in rat hippocampus significantly increased to about 1850 pg/mL in the model group (Aβ) (P < 0.001). After PACOs treatment (25, 50, 100 mg/kg), the levels of phosphorylated Tau protein significantly reduced to about 1660, 1500, and 1350 pg/mL, respectively, as compared to the model group (P < 0.05) (Fig. 2B). Thus, PACOs may reduce Aβ aggregation via inhibiting the phosphorylation of Tau.
Furthermore, the inhibition of PACOs on the production of inflammation factors in rat hippocampus was also explored by ELISA assay. As shown in Fig. 2C, the production of cytokine TNF-α significantly increased after Aβ treatment as compared to that in the sham control group (P < 0.001). After treatment with PACOs (50 or 100 mg/kg), the production of TNF-α significantly decreased from over 290 pg/mL to about 240 and 225 pg/mL, respectively, as compared to the model group (P < 0.05) (Fig. 2C). In addition, PACOs (25, 50, 100 mg/kg) treatment also significantly reduced the production of IL-6 from about 110 pg/mL to about 95, 90, and 85 pg/mL, respectively, as compared to that in the model group (P < 0.05) (Fig. 2D). Thus, PACOs may be able to attenuate the inflammatory responses accompanying neuron damage.
Effect of PACOs on the oxidative stress induced by Aβ25–35 in AD rats
MDA is usually considered a reliable marker for the detection of oxidative stress since the level of MDA is positively correlated with the level of oxidative stress (Ferreiro et al. 2012). Thus, the effect of PACOs on MDA production was evaluated by ELISA assay to further explore whether PACOs can influence oxidative stress. The results showed that the MDA content in hippocampus of the model group (Aβ) increased significantly (P < 0.001) (Fig. 3A) compared to the sham group (control). However, after PACOs (25, 50, 100 mg/kg) treatment, the levels of MDA significantly decreased as compared to that in the model group (P < 0.001) (Fig. 3A).
Fig. 3.
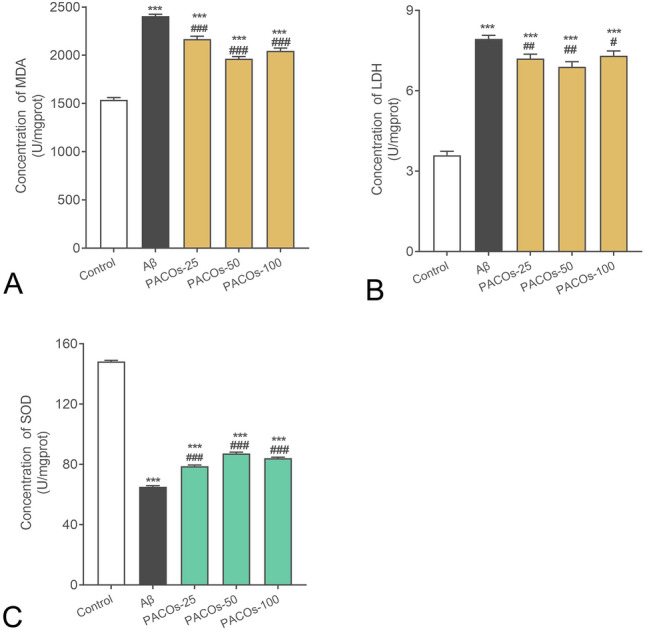
Effects of PACOs on the oxidative stress induced by Aβ. A The MDA production in the hippocampus of AD model rats was evaluated by using ELISA assay. B The LDH release, an indicator of oxidative stress, was evaluated by using LDH assay kit. C The activities of SOD in Aβ treated rat hippocampus were determined by ELISA assay. Values are expressed as mean ± SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001 versus control group; #P < 0.05, ##P < 0.01, ###P < 0.001 versus AD group (Aβ)
The LDH release, another indicator of oxidative stress, was also determined by using an LDH assay kit according to the manufacturer’s protocol. As shown in Fig. 3B, treatment with Aβ (model group) resulted in a significant increase of LDH release into the rats’ hippocampus, as compared to the sham control group (P < 0.001). However, oral administration of PACOs at different doses (25, 50, and 100 mg/kg) significantly reduced LDH release into the hippocampus (P < 0.05), which decreased from 1.6-fold to about 1.4, 1.2, and 1.3-fold, respectively, as compared to that in the model group (Fig. 3B).
Superoxide dismutase (SOD) is an important enzyme that is crucial for the prevention of diseases linked to oxidative stress and is also important in the prevention of some neurodegenerative disorders (Maier and Chan 2002). The activities of SOD in Aβ treated rat hippocampus were determined by ELISA assay. The results showed that Aβ treatment resulted in a significant decrease of SOD activities in hippocampus (P < 0.001), as compared to the sham group (control). In contrast, after treatment with PACOs (25, 50, 100 mg/kg), the SOD activities significantly increased from 40% to about 50%, 60%, and 55% of the sham control group (100%), respectively, as compared to the model group (Aβ) (P < 0.001) (Fig. 3C). Therefore, PACOs may also be able to reduce oxidative injury in Aβ treated rat hippocampus.
Influence of PACOs on the inflammation related signaling pathways in AD rats
The MAPK/ERK signaling pathway was reported to be associated with the phosphorylation of Tau protein and inflammatory response; thus, the influence of PACOs on the activation of ERK1/2 was determined by western blot. As shown in Fig. 4A, B, Aβ injection significantly increased the phosphorylation of ERK1/2 as compared to the sham control group (P < 0.001). However, treatment with PACOs (50, 100 mg/kg) significantly reduced the phosphorylation of ERK1/2 in rat hippocampus (P < 0.001) (Fig. 4B). Moreover, PACOs (50, 100 mg/kg) treatment also significantly reduced the expression levels of cleaved caspase 3 in rat hippocampus as compared to the non-treated control group (P < 0.001) (Fig. 4C, D). Thus, PACOs may be able to down-regulate the activation of ERK and caspase 3 to inhibit Aβ aggregation and cell apoptosis.
Fig. 4.
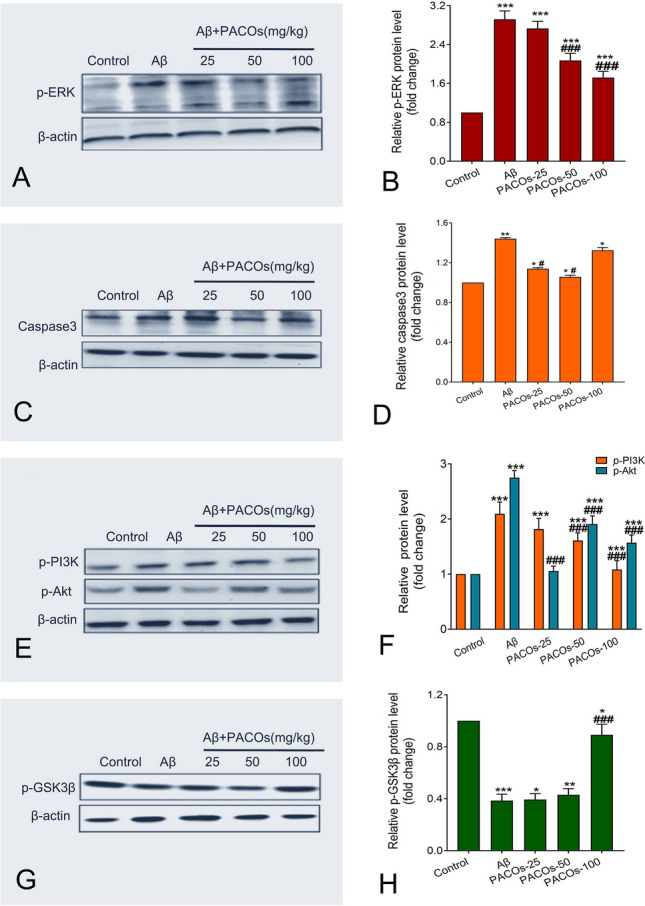
Effects of PACOs on the activation of the PI3K/Akt/GSK3 signaling pathway in Aβ treated rat hippocampus. A, C, E, G The expression of p-ERK (A), cleaved caspase 3 (C), p-PI3K (E), p-Akt (E), and p-GSK3β (G) in rat hippocampus was determined by western blot. β-actin was also detected as a control. B, D, F, H Plots quantifying the immunoblots (as ratios to actin) for p-ERK (B), cleaved caspase 3 (D), p-PI3K (F), p-Akt (F), and p-GSK3β (H) proteins were shown. The ratios for sham control cells (control) were assigned values of 1.0 and the data presented as mean ± SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001 versus control group; #P < 0.05, ##P < 0.01, ###P < 0.001 versus AD group (Aβ)
Moreover, the activation of the PI3K/Akt signaling pathway has been reported to be associated with the inflammatory response after amyloid β stimulation in AD models, and the compound Roflupram could decrease the expression of apoptosis-related molecules via inhibition of the PI3K/Akt/mTOR signaling pathway (Wang et al. 2020a, b). Thus, the effect of PACOs on the PI3K/Akt pathway was also explored in this study by using western blot assay. As shown in Fig. 4E, F, after Aβ injection, the levels of phosphorylated PI3K were significantly increased in rat hippocampus as compared to the sham control group (P < 0.001). However, after oral treatment of PACOs (50, 100 mg/kg), the level of phosphorylated PI3K significantly decreased from about 2.1-fold to about 1.6- and 1.1-fold of the sham control group, respectively, as compared to the model group (Aβ) (P < 0.001) (Fig. 4E, F). PACOs treatment also significantly reduced the levels of phosphorylated Akt from about 2.8-fold to about 1.0, 1.9, and 1.7-fold of the control group, respectively, as compared to the model group (P < 0.001) (Fig. 4E, F). These data indicate a crucial role of PI3K/Akt signaling in the neuroprotective mechanism of PACOs.
It is known that the phosphorylation of GSK3β can inactivate itself to inhibit the activation of caspase-3 and the release of cytochrome C (Llorens-Martín et al. 2014; Ma 2014). Thus, the effect of PACOs on the activation of GSK3β was also evaluated in this study. The results of western blot showed that PACOs treatment (25, 50, 100 mg/kg) increased the levels of phosphorylated GSK3β from about 0.4-fold to about 0.5, 0.5 and 0.9-fold of the sham control, respectively (Fig. 4G, H). PACOs treatment at the dose of 100 mg/kg possessed the most significant enhancement effect on the phosphorylation of GSK3β to inhibit its activity (P < 0.001) (Fig. 4H). Considering that PACOs treatment also reduced the activation of caspase-3 in rat hippocampus (Figs. 1, 4D), we propose that PACOs may be able to inhibit the Aβ-induced inflammation response and neuronal apoptosis mainly though regulating the PI3K/Akt/GSK3β signaling pathway.
Transcriptome analysis identifies the differential expressed genes in PACOs treated AD rats
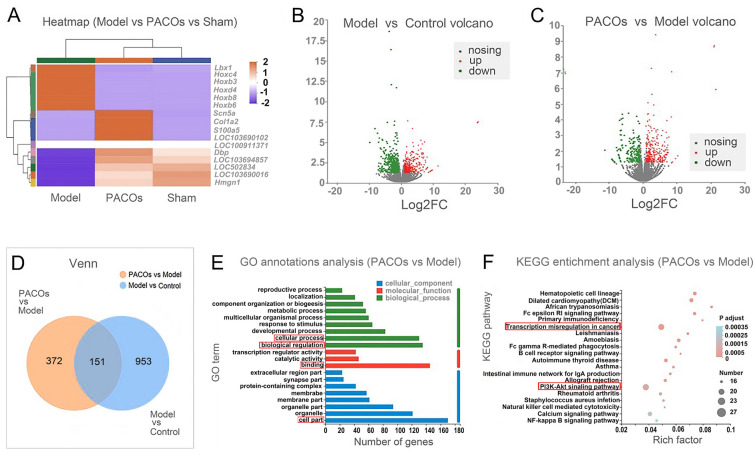
To further explore the protective mechanism of PACOs against neuron damage in vivo, the transcriptome analysis of rat brain was conducted after PACOs (50 mg·kg−1·d−1) treatment for 7 days (Assisted by Majorbio). As shown in Fig. 5A, B, there were about 436 upregulated genes and 668 downregulated genes identified (fold change ≥ 2 and P < 0.001) in the model group (Aβ) relative to the control group (sham). However, after PACOs (50 mg·kg−1·d−1) treatment, the change trend of gene differential expression in brain cells (model) caused by Aβ was significantly reversed, and the gene expression level basically recovered to be similar to that of the sham control group (Fig. 5A, B). There were about 297 upregulated genes and 226 downregulated genes identified (fold change ≥ 2 and P < 0.001) in PACOs treated group (PACOs) relative to the model group (Fig. 5C). The Venn diagram shown in Fig. 5D indicated that there were about 151 differentially expressed genes (DEGs) in both the PACOs versus model group and model versus control group. Among them, the ladybird homebox 1 (lbx1), homeobox D4 (Hoxd4), homeobox C4 (Hoxc4), homeobox B6 (Hoxb6), homeobox B3 (Hoxb3) and homeobox B8 (Hoxb8) were all significantly up-regulated in the model group and down-regulated after PACOs treatment (PACOs) (Fig. 5A). Considering that all of these DEGs are mainly involved in neuron development and cell proliferation, we speculate that the host signaling pathways related to development and differentiation processes may be the targets of PACOs in brain cells.
Fig. 5.
Transcriptome analysis of PACOs treated brain cells in AD rats. A Significantly up-regulated and down-regulated genes of brain cells after PACOs treatment in AD rats (fold change ≥ 2 and P < 0.01) shown by heat map. B, C Volcano plots indicate up-regulated (red) and down-regulated (green) mRNA transcripts in the Model versus Control groups (B) or PACOs versus Model groups (C). D Venn diagram indicates the differentially expressed genes (DEGs) in both Model versus Control groups and PACOs versus Model groups. E The GO annotation analysis was performed to display the DEGs related biological processes. The red rectangle indicates the major DEGs related biological process, cellular component, and molecular functions. F KEGG pathway enrichment analysis of DEGs in AD rat brain after PACOs treatment was shown (P < 0.01). The red rectangle indicates the two major activation pathways based on the rich factor and DEGs numbers
Moreover, the Gene Ontology (GO) annotation analysis was performed to further display the differentially expressed genes (DEGs) related to biological processes. The results showed that the genes related to “cell part”, “binding”, “biological regulation” and “cellular process” are the most highly represented in terms of both − log10(FDR) and number of genes (> 140) (Fig. 5E). Thus, PACOs may affect cell cellular processes responsible for regulation of neuron injuries.
Furthermore, the KEGG enrichment assay was then performed to determine the signaling pathways involved in the neuroprotective actions of PACOs. The bubble map of the differential genes provided a graphical representation of the top 20 most enriched pathways of DEGs (Fig. 5F). Among the enriched signaling pathways, the “transcriptional mis-regulation in cancer” and “PI3K-Akt signaling pathway” were the most significant (Fig. 5F). Importantly, there were more than 27 DEGs significantly enriched in the signaling pathways related to “PI3K-Akt signaling pathway” (P < 0.00005) (Fig. 5F). In summary, PACOs may largely affect PI3K-Akt signaling pathways and cell proliferation related pathways to improve the repair of β-amyloid-induced cognitive deficits in rats.
Discussion
Dementia especially affects those suffering from Alzheimer’s disease and brings a great burden to both individuals and society. Until now, there is still a lack of effective long-term drug options to improve cognitive function and reduce or halt disease progression (Salehipour et al. 2022). In 2019, the novel anti-AD drug GV-971 derived from seaweed polysaccharide has been approved for clinical use in China, suggesting that the marine glycans have the potential to be developed into novel multitarget-agents for prevention and treatment of AD (Wang et al. 2020a, b). Our previous studies demonstrated that the peracetylated chitosan oligosaccharides (PACOs) may be used as antagonists against glutamate-induced PC12 cell death (Hao et al. 2015). In this study, the Aβ-induced neuronal injury model was utilized to further explore the neuroprotective effects and mechanisms of PACOs in vivo. Consistent with previous reports (Aghsami et al. 2018), Aβ injection successfully induced cognitive impairments and neuronal injury in rats. Oral administration of PACOs at the dose of 25–100 mg/kg significantly improved the learning and memory of AD rats induced by Aβ and attenuated Aβ-induced neuron injury and the inflammation response mainly through regulating the PI3K/Akt/GSK3 signaling pathway.
The key point of oxidative stress theory is that the elevated levels of highly active oxygen radicals can lead to the impairment of neurons which subsequently causes the cognitive and learning dysfunction in AD patients (Butterfield 2018; Tamagno et al. 2021; Zhao and Zhao 2013). The neurotoxicity of Aβ is related to the action of free radicals, and oxidative stress is involved in the regulation of Aβ neurotoxicity, which causes the damage of cell membrane (Eftekharzadeh et al. 2012). It has been reported that the preconditioning signal leading to cellular protection through hormesis can lead to an important redox dependent aging, which is associated with free radical accumulation and inflammatory responses involved in the pathophysiology of AD (Calabrese et al. 2009, 2010, 2012; Zhang et al. 2011). In addition, the vitagene network was reported to be emerging as a neurohormetic potential target for cytoprotective interventions and that dietary antioxidants may be used in the prevention and treatment of neurodegenerative disorders (Calabrese et al. 2007; Drake et al. 2003; Mancuso et al. 2006). A variety of compounds with antioxidant properties have been used for prophylactic or treatment of AD in the laboratory or clinic, such as curcumin and green tea polyphenol, but the exact therapeutic effects and mechanisms of these compounds are still controversial (Cong et al. 2016; Mazza et al. 2006; Nisbet and Götz 2018; Tamagno et al. 2021; Tang and Taghibiglou 2017). In this study, we found that PACOs treatment significantly improved the learning and memory function of AD rats and significantly reduced the level of MDA, inhibited the LDH leakage, and increased the SOD activities in rat hippocampus, suggesting that PACOs may be able to down-regulate Aβ-induced oxidative stress to inhibit neuron injury.
The plaque and neurofibrillary tangles on the brain slices of AD patients are the main pathological features of AD. The main composition of plaque is β amyloid protein, and the main component of the fiber entanglement is phosphorylated Tau protein (Cong et al. 2016). Thus, the compounds which can accelerate the clearance of Aβ and phosphorylated Tau can be used for AD therapy. Herein, our studies indicated that PACOs may be able to reduce the aggregation of Aβ and inhibit the phosphorylation of Tau protein to attenuate the production of plaque and neurofibrillary tangles on the brain. The MAPK/ERK signaling pathway has been reported to be associated with the phosphorylation of Tau protein in the pathogenesis of AD (Morroni et al. 2016; Xia et al. 2015). Consistently, our data indicated that PACOs significantly inhibited the phosphorylation of ERK1/2 in a dose-dependent manner, suggesting that PACOs may be able to down-regulate the MAPK/ERK pathway to inhibit the phosphorylation of the Tau protein. Thus, PACOs may promote the clearance of Aβ and down-regulate the MAPK/ERK pathway to inhibit the phosphorylation of the Tau protein.
The activation of the PI3K/Akt signaling pathway was reported to be associated with the inflammatory response after amyloid β stimulation in AD models (Morroni et al. 2016; Wang et al. 2020a, b), and the phosphorylation of its downstream signal GSK3β can inactivate itself to inhibit the activation of caspase-3 and the release of cytochrome C (Ferreiro et al. 2012; Llorens-Martín et al. 2014; Maier and Chan 2002). Interestingly, we found that PACOs treatment significantly inhibited the activation of PI3K-Akt signaling and down-regulated the activation of GSK3β in hippocampus (Fig. 4), suggesting that the PI3K/Akt/GSK3 pathway may be involved in the neuroprotective actions of PACOs in AD rats. Consistently, the transcriptome analysis further verified that the differentially expressed genes (DEGs) are mainly involved in neuron development and the PI3K-Akt signaling pathway in PACOs treated AD rats (Fig. 5). It has been reported that highly N-acetylated chitooligosaccharide (NACOS) rather than COS remarkably inhibited the phosphorylation of PI3K and Akt to exert its anti-inflammatory activity (Xu et al. 2017). Similarly, peracetylation was reported to be able to facilitate passive diffusion of disaccharides across cell membranes, allowing them to enter the Golgi (Brown et al. 2006), suggesting that the peracetylation may facilitate the chitosan oligosaccharides to enter the cells and reduce ROS induced apoptosis. Taken together, PACOs may be able to improve the repair of Aβ-induced neuron injury and inhibit the apoptosis of neuron cells mainly through down-regulating the PI3K/Akt/GSK3 signaling pathway.
In conclusion, PACOs possessed a protective effect against Aβ-induced cognitive deficits in rats mainly through regulating the PI3K/Akt/GSK3 signal pathway to promote repair of nerve damage and inhibit neuronal apoptosis. Further studies need to be done in gene knock-out rats or other animal models before clinical trials to fully elucidate the molecular targets of PACOs and advance their prospects for drug development. Nevertheless, PACOs have the potential to be developed into novel agents for prevention and treatment of AD in the future.
Materials and methods
Compounds and reagents
Peracetylated chitosan oligosaccharides (PACOs, ≥ 98% purity) were prepared in the School of Medicine and Pharmacy, Ocean University of China. Aβ25–35 (APN16021-1-1) was purchased from Abcam (Cambridge, England). Lactate dehydrogenase (LDH) and superoxide dismutase (SOD) activity assay kits, malondialdehyde (MDA), interleukin 6 (IL-6) and tumor necrosis factor alpha (TNFα) detection kits were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The phosphorylated Tau (p-Tau) and Aβ assay kits were purchased from Wuhan Colorful Gene Biotech (Wuhan, China). Antibodies for detecting cleaved caspase-3, β-actin, phosphorylated PI3K (phospho Y607), Akt (phospho S474), ERK1/2 (phospho T202 + phospho T185), and GSK3β (phospho S9) proteins were obtained from Abcam (Cambridge, MA). Other reagents were of analytical grade.
PACOs preparation and physicochemical analysis
The peracetylated chitosan oligosaccharides (PACOs) were prepared using our published method with modifications (Hao et al. 2015; Wang et al. 2005). In brief, the dried COSs mixture (1 g) was suspended in acetic anhydride (10 mL) at room temperature with stirring, and 0.5 mL concentrated H2SO4 was added (Gao et al. 2013). The reaction mixture was placed in a microwave synthesizer (Discover SP) and reacted for 0.5 h at 50 °C using thin layer chromatography (TLC, CH2Cl2:CH3OH, 12:1, v/v) to monitor the completion of the acetylation reaction. The reaction mixture was added into 20 mL 15% sodium acetate solution at 0 °C and extracted with CH2Cl2 (40 mL × 3). The organic layer was dried over anhydrous sodium sulphate and further removed under reduced pressure. The residue was purified by silica gel column chromatography (CH2Cl2:CH3OH, 50:1–30:1) to give PACOs.
The consistency and quality of PACOs were checked by high performance thin layer chromatography (HPTLC), Fourier transform infrared spectroscopy (FT-IR), mass spectrometry (MS), and nuclear magnetic resonance (NMR), respectively. For HPTLC analysis, the solvent system used was CH2Cl2/CH3OH (10:1, v/v/v) and a trimer of PACOs was used as a standard, followed by staining with aniline-diphenylamine. Fourier transform IR was performed with a Nicolet Nexus 470 IR spectrometer using the KBr pellet technique. The spectra were recorded in the range between 400 and 4000 cm−1, and the resolution was 0.5 cm−1. MS was conducted using Micromass Global Q-TOF Mass Spectrometer (Indian Trail, NC, USA). ESI technique was used to determinate the molecular mass of each peracetylated oligosaccharide. 1H and 13C NMR spectra were recorded at 25 °C on an Agilent 500 MHz DD2 spectrometer with the sample (30 mg) dissolved in 0.5 mL of DMSO-d6. Tetramethylsilane (Me4Si) was used as the internal standard.
Animals
Specific pathogen free (SPF)-grade adult male Sprague–Dawley (SD) rats (300–330 g) were purchased from Jinan Pengyue Experimental Animal Center (license No. SCXK 2014-0007). The protocol was approved by the Research Review Committee of the affiliated hospital of Qingdao University (No. 201612A001). All rats were housed in 12-h light and 12-h dark conditions in which the humidity was 60% ± 5% and the temperature was 25 ± 2 °C. All rats had free access to food and water.
Experimental design
The 53 rats housed for seven days were subjected to the dark shuttle test to eliminate the congenital dull rats (Yamaguchi and Kawashima 2001). Rats received bilateral hippocampal injection of 5 μL of Aβ25–35 (4 μg/μL in PBS) or 5 μL PBS solution (sham group) according to the coordinates: posterior 1.0 mm relative to the bregma, lateral 2.6 mm to the midline, and ventral 3.8 mm beneath the dura. The injection was infused by Hamilton syringe over 5 min and remained for 5 min before removal. Animals were then randomly divided into five groups: Sham (n = 11), Model (Aβ) (n = 9), Aβ + PACOs-L (25 mg/kg) (n = 11), Aβ + PACOs-M (50 mg/kg) (n = 11), and Aβ + PACOs-H (100 mg/kg) (n = 11). Rats then received intragastrically the administration of 25, 50 and 100 mg/kg PACOs or PBS from the first day after surgery by gavage, once per day for 21 days continuously before commencing the training blocks.
Morris water maze test
Spatial memory function was assessed using the Morris water maze (MWM) task. Water maze test equipment mainly consisted of the platform, camera and a black circular pool (80 cm in radius and 50 cm deep). The pool was artificially divided into I, II, III, VI four quadrants with four start positions. All the animals were subjected to four successive days of training in the MWM. In the location navigation training experiment, the time of escaping latency was observed dynamically, and a short escaping time correlates to strong memory function. In the space exploration experiment, the greater ratio of the target's quadrant time to the total time in the water maze and the greater number of times of platform crossing were used to indicate the better ability of spatial learning and memory function of rats. Each rat received three trials daily, the escape latency and swimming speed were automatically recorded during the period.
Tissue preparation
After the MWM tests, animals were euthanized by deep anesthesia using overdose sodium pentobarbital, and five of them from each group were perfused with 0.1 M PBS and then fixed by the precooled 4% paraformaldehyde for a week followed by paraffin embedding. For the remaining animals, hippocampuses were quickly separated, homogenized and kept at − 80 °C for further western blot and ELISA assays. The embedded brain tissues were serially sliced in sections of 4 μm for hematoxylin–eosin (HE) staining (Feldman and Wolfe 2014). The morphological changes in hippocampus were observed by microscope (Revolve FL, Echo-Labs, USA).
ELISA assay
The hippocampal tissue was weighed, homogenized and centrifuged at 4 °C, and the total protein content in the supernatant was determined by BCA assay. After that, the relative activities of LDH and SOD were evaluated by determination of the absorbance at 440 nm and 450 nm, respectively, using a microplate reader (Zhang et al. 2015). The relative levels of MDA, TNFα, IL-6, p-Tau, and Aβ1–42 proteins were detected by using ELISA assay kits according to the manual’s instructions.
Immunohistochemistry
Seven days after injection of Aβ25–35, 4% paraformaldehyde was used to fix the rat brains for 24 h, and then the fixed brains were paraffin embedded. To conduct the immunohistochemistry, 5 μm sections were made, and then the specimens were incubated with antibodies against cleaved caspase-3 or cytochrome C protein (dilution, 1:500) at 4 °C overnight. After washing, the rabbit/mouse HRP kits (DAB) (Beyotime Biotechnology, Shanghai, China) containing the secondary antibodies were used to detect the expression and localization of proteins. To count the staining positive cells throughout four fields in the CA1 region of hippocampal, an examiner blinded to the experimental groups performed the cell count under a × 200 light microscope (Revolve FL, Echo-Labs, USA). Each group consisted of five rats and from each rat three coronal hippocampal sections were taken (Ryu et al. 2009).
mRNA-seq assay and bioinformatics analysis
Library preparation and high throughput sequencing were conducted as described previously (Zeng et al. 2019). Briefly, purified RNA was subjected to cDNA library construction using the KAPA Stranded RNA-Seq Library Preparation Kit for Illumina Platforms (KAPA biosystems) following the manufacturer’s protocol. After purification and quantification, the prepared libraries were subjected to high throughput sequencing on an Illumina HiSeq Xten platform by Majorbio technology Inc. Differential gene expression was calculated by SAM analysis. Identified genes with significant upregulation and downregulation were mapped (fold change ≥ 2 and P < 0.05). The data were analyzed on the free online platform Majorbio I-Sanger Cloud Platform (http://www.i-sanger.com). Heatmap was plotted using the OmicShare tools (http://www.omicshare.com/tools). Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway annotations of DEGs were applied using the DAVID Bioinformatics Resources (https://david.ncifcrf.gov/).
Western blot
Western blot analysis was performed as described previously (Sugawara et al. 2002). Proteins were separated using SDS-PAGE and electrically transferred to a NC membrane (Pall, New York, NY, USA). After that, the membranes were blocked with TBST (50 mmol/L Tris–HCl, pH 7.4, 0.15 mol/L NaCl, 0.1% Tween-20) containing 5% BSA (Sigma, St. Louis, MO, USA) for 2 h. Then the membranes were incubated with primary antibodies against phosphorylated PI3K, Akt, ERK1/2, and GSK3β or β-actin diluted at 1:1000 at 4 °C overnight. After washing with TBST three times, the membranes were incubated with AP-labeled secondary antibodies (1:3000 dilutions) at room temperature for 1.5 h. The protein bands were then visualized by incubating with the developing solution [p-nitro blue tetrazolium chloride (NBT) and 5-bromo-4-chloro-3-indolyl phosphate toluidine (BCIP)] at room temperature for 30 min. The relative densities of proteins were all determined by using Image J (NIH) V.1.33u (USA).
Statistical analysis
All data are represented as the mean ± SD, and significance was calculated by using GraphPad Prism 7.0 (San Diego, CA, USA). The escape latency of MWM data were analyzed using a repeated measures analysis of variance (two-way ANOVA) process. Comparison between groups was made by one-way analysis of variance (ANOVA) followed by post hoc Tukey's tests if F achieved statistical significance (P < 0.05) and there was no significant variance in homogeneity. Differences were considered statistically significant at P < 0.05.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by National Natural Science Foundation of China (31500646, 81874320, and 81672585), Shandong Major Science and Technology Project (2021ZDSYS22), the Promotive Research Fund for Excellent Young and Middle-aged Scientists of Shandong Province (BS2015YY040), Qingdao Science and Technology Development project (15-9-1-67-JCH) and Youth Research Fund of Affiliated Hospital of Qingdao University (QDFYQN202101003).
Abbreviations
- Aβ
β-Amyloid
- AD
Alzheimer’s disease
- COSs
Chitosan oligosaccharides
- DEGs
Differentially expressed genes
- GSK3β
Glycogen synthase kinase 3β
- HE
Hematoxylin–eosin
- MDA
Malondialdehyde
- MWM
Morris water maze
- PACOs
Peracetylated chitosan oligosaccharides
- PI3K
Phosphoinositide 3-kinase
- SOD
Superoxide dismutase
Author contributions
Conceptualization: CH, CXL and TX; Formal Analysis and Investigation: MMH, CY, QHZ; Validation and Visualization: MMH, CH, WW; Project Administration and Funding Acquisition: CH, WW, CXL; Writing-original draft: CH, WW; Writing-review and editing: WW, CXL, YLG, LJZ. All authors have read and agreed to the published version of the manuscript.
Data availability
The data that supports the findings of this study are included in this published article (and its supplementary information file).
Declarations
Conflict of interest
The authors declare no conflict of interest.
Animal and human rights statement
This article does not contain any studies with human participants performed by any of the authors. All animal experimental protocols in this study were approved by the Research Review Committee of the affiliated hospital of Qingdao University (No. 201612A001). All animal experiments were carried out in accordance with internationally valid guidelines of the Standards for Laboratory Animals of China (GB 14922.2-2001, GB 14923-2001, and GB 14925-2001).
Footnotes
Cui Hao and Minmin Han have contributed equally to this work.
Contributor Information
Cui Hao, Email: haocui@qduhospital.cn.
Chunxia Li, Email: lchunxia@ouc.edu.cn.
References
- Aghsami M, Sharifzadeh M, Sepand MR, Yazdankhah M, Seyednejad SA, Pourahmad J. A cAMP analog attenuates beta-amyloid (1–42)-induced mitochondrial dysfunction and spatial learning and memory deficits. Brain Res Bull. 2018;140:34–42. doi: 10.1016/j.brainresbull.2018.03.016. [DOI] [PubMed] [Google Scholar]
- Alzheimer's Association Alzheimer's disease facts and figures. Alzheimers Dement. 2016;12:459–508. doi: 10.1016/j.jalz.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Athar T, Al Balushi K, Khan SA. Recent advances on drug development and emerging therapeutic agents for Alzheimer's disease. Mol Biol Rep. 2021;48:5629–5645. doi: 10.1007/s11033-021-06512-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JR, Fuster MM, Li RX, Varki N, Glass CA, Esko JD. A disaccharide-based inhibitor of glycosylation attenuates metastatic tumor cell dissemination. Clin Cancer Res. 2006;12:2894–2901. doi: 10.1158/1078-0432.CCR-05-2745. [DOI] [PubMed] [Google Scholar]
- Butterfield DA. Perspectives on oxidative stress in Alzheimer’s disease and predictions of future research emphases. J Alzheimers Dis. 2018;64:S469–S479. doi: 10.3233/JAD-179912. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Mancuso C, Calvani M, Rizzarelli E, Butterfield DA, Stella AM. Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nat Rev Neurosci. 2007;8:766–775. doi: 10.1038/nrn2214. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Cornelius C, Dinkova-Kostova AT, Calabrese EJ. Vitagenes, cellular stress response, and acetylcarnitine: relevance to hormesis. BioFactors. 2009;35:146–160. doi: 10.1002/biof.22. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Cornelius C, Dinkova-Kostova AT, Calabrese EJ, Mattson MP. Cellular stress responses, the hormesis paradigm, and vitagenes: novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid Redox Signal. 2010;13:1763–1811. doi: 10.1089/ars.2009.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese EJ, Iavicoli I, Calabrese V. Hormesis: why it is important to biogerontologists. Biogerontology. 2012;13:215–235. doi: 10.1007/s10522-012-9374-7. [DOI] [PubMed] [Google Scholar]
- Cong L, Cao C, Cheng Y, Qin XY. Green tea polyphenols attenuated glutamate excitotoxicity via antioxidative and antiapoptotic pathway in the primary cultured cortical neurons. Oxid Med Cell Longev. 2016;2016:1–8. doi: 10.1155/2016/2050435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corriveau RA, Koroshetz WJ, Gladman JT, Jeon S, Babcock D, Bennett DA, Carmichael ST, Dickinson SL, Dickson DW, Emr M, Fillit H, Greenberg SM, Hutton ML, Knopman DS, Manly JJ, Marder KS, Moy CS, Phelps CH, Scott PA, Seeley WW, et al. Alzheimer's disease-related dementias summit 2016 national research priorities. Neurology. 2017;89:2381–2391. doi: 10.1212/WNL.0000000000004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeTure MA, Dickson DW. The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener. 2019;14:32. doi: 10.1186/s13024-019-0333-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake J, Sultana R, Aksenova M, Calabrese V, Butterfield DA. Elevation of mitochondrial glutathione by gamma-glutamylcysteine ethyl ester protects mitochondria against peroxynitrite-induced oxidative stress. J Neurosci Res. 2003;74:917–927. doi: 10.1002/jnr.10810. [DOI] [PubMed] [Google Scholar]
- Eftekharzadeh B, Ramin M, Khodagholi F, Moradi S, Tabrizian K, Sharif R, Azami K, Beyer C, Sharifzadeh M. Inhibition of PKA attenuates memory deficits induced by β-amyloid (1–42), and decreases oxidative stress and NF-κB transcription factors. Behav Brain Res. 2012;226:301–308. doi: 10.1016/j.bbr.2011.08.015. [DOI] [PubMed] [Google Scholar]
- Feldman AT, Wolfe D. Tissue processing and hematoxylin and eosin staining. Methods Mol Biol. 2014;1180:31–34. doi: 10.1007/978-1-4939-1050-2_3. [DOI] [PubMed] [Google Scholar]
- Ferreiro E, Baldeiras I, Ferreira IL, Costa RO, Rego AC, Pereira CF, Oliveira CR. Mitochondrial- and endoplasmic reticulum-associated oxidative stress in Alzheimer's disease: from pathogenesis to biomarkers. Int J Cell Biol. 2012;2012:1–23. doi: 10.1155/2012/735206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao LX, Li CX, Wang SX, Zhao X, Guan HS (2013) Preparation and analysis of chitooligosaccharide isomers with different degree. Chin J Mar Drugs 32:21–27
- Haass C, Selkoe D. If amyloid drives Alzheimer disease, why have anti-amyloid therapies not yet slowed cognitive decline? PLoS Biol. 2022;20:e3001694. doi: 10.1371/journal.pbio.3001694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao C, Gao LX, Zhang YR, Wang W, Yu GL, Guan HS, Zhang LJ, Li CX. Acetylated chitosan oligosaccharides act as antagonists against glutamate-induced PC12 cell death via Bcl-2/Bax signal pathway. Mar Drugs. 2015;13:1267–1289. doi: 10.3390/md13031267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao C, Wang W, Wang SX, Zhang LJ, Guo YL. An overview of the protective effects of chitosan and acetylated chitosan oligosaccharides against neuronal disorders. Mar Drugs. 2017;15:89–104. doi: 10.3390/md15040089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia LF, Du YF, Chu L, Zhang ZJ, Li FY, Lyu DY, Li Y, Li Y, Zhu M, Jiao HS, Song Y, Shi YP, Zhang H, Gong M, Wei CB, Tang Y, Fang BY, Guo DM, Wang F, Zhou AH, et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: a cross-sectional study. Lancet Public Health. 2020;5:e661–e671. doi: 10.1016/S2468-2667(20)30185-7. [DOI] [PubMed] [Google Scholar]
- Jia L, Quan MN, Fu Y, Zhao T, Li Y, Wei CB, Tang Y, Qin Q, Wang F, Qiao YCH, Shi SL, Wang YJ, Du YF, Zhang JW, Zhang JJ, Luo BY, Qu QM, Zhou CK, Gauthier S, Jia JP, Group for the Project of Dementia Situation in China (2020b) Dementia in China: epidemiology, clinical management, and research advances. Lancet Neurol 19:81–92 [DOI] [PubMed]
- Knowles RB, Wyart C, Buldyrev SV, Cruz L, Urbanc B, Hasselmo ME, Stanley HE, Hyman BT. Plaque-induced neurite abnormalities: implications for disruption of neural networks in Alzheimer's disease. Proc Natl Acad Sci USA. 1999;96:5274–5279. doi: 10.1073/pnas.96.9.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Park JS, Kim SK, Ahn CB, Je JY. Chitooligosaccharides suppress the level of protein expression and acetylcholinesterase activity induced by Aβ25-35 in PC12 cells. Med Chem Let. 2009;19:860–862. doi: 10.1016/j.bmcl.2008.12.019. [DOI] [PubMed] [Google Scholar]
- Li L, Xu SF, Liu LF, Feng RT, Gong YX, Zhao XY, Li J, Cai J, Feng N, Wang L, Wang XL, Peng Y. Multifunctional compound ad-35 improves cognitive impairment and attenuates the production of TNF-α and IL-1β in an Aβ25-35-induced rat model of Alzheimer's disease. J Alzheimers Dis. 2017;56:1403–1417. doi: 10.3233/JAD-160587. [DOI] [PubMed] [Google Scholar]
- Llorens-Martín M, Jurado J, Hernández F, Avila J. GSK-3beta, a pivotal kinase in Alzheimer disease. Front Mol Neurosci. 2014;7:46. doi: 10.3389/fnmol.2014.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T. GSK3 in Alzheimer's disease: mind the isoforms. J Alzheimers Dis. 2014;39:707–710. doi: 10.3233/JAD-131661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier CM, Chan PH (2002) Book review: role of superoxide dismutases in oxidative damage and neurodegenerative disorders. Neuroscientist 8:323–334 [DOI] [PubMed]
- Malek-Ahmadi M, Perez SE, Chen K, Mufson EJ. Neuritic and diffuse plaque associations with memory in non-cognitively impaired elderly. J Alzheimers Dis. 2016;53:1641–1652. doi: 10.3233/JAD-160365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso C, Pani G, Calabrese V. Bilirubin: an endogenous scavenger of nitric oxide and reactive nitrogen species. Redox Rep. 2006;11:207–213. doi: 10.1179/135100006X154978. [DOI] [PubMed] [Google Scholar]
- Mazza M, Capuano A, Bria P, Mazza S. Ginkgo biloba and donepezil: a comparison in the treatment of Alzheimer's dementia in a randomized placebo-controlled double-blind study. Eur J Neurol. 2006;13:981–985. doi: 10.1111/j.1468-1331.2006.01409.x. [DOI] [PubMed] [Google Scholar]
- Morroni F, Sita G, Tarozzi A, Rimondini R, Hrelia P. Early effects of Aβ1-42 oligomers injection in mice: involvement of PI3K/Akt/GSK3 and MAPK/ERK1/2 pathways. Behav Brain Res. 2016;314:106–115. doi: 10.1016/j.bbr.2016.08.002. [DOI] [PubMed] [Google Scholar]
- Nisbet RM, Götz J. Amyloid-β and Tau in Alzheimer’s disease: novel pathomechanisms and non-pharmacological treatment strategies. J Alzheimers Dis. 2018;64:S517–S524. doi: 10.3233/JAD-179907. [DOI] [PubMed] [Google Scholar]
- Ouyang QQ, Zhao S, Li SD, Song C. Application of chitosan, chitooligosaccharide, and their derivatives in the treatment of Alzheimer's disease. Mar Drugs. 2017;15:322. doi: 10.3390/md15110322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren RJ, Qi JL, Lin SH, Liu XY, Yin P, Wang ZH, Tang R, Wang JT, Huang Q, Li JP, Xie YX, Hu YB, Cui SH, Zhu Y, Yu XP, Wang PF, Zhu YK, Wang YR, Huang YY, Hu YS, et al. The china alzheimer report 2022. Gen Psychiatr. 2022;35:e100751. doi: 10.1136/gpsych-2022-100751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu JK, Cho T, Choi HB, Wang YT, McLarnon JG. Microglial VEGF receptor response is an integral chemotactic component in Alzheimer's disease pathology. J Neurosci. 2009;29:3–13. doi: 10.1523/JNEUROSCI.2888-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehipour A, Bagheri M, Sabahi M, Dolatshahi M, Boche D. Combination therapy in Alzheimer's disease: Is it time? J Alzheimers Dis. 2022;87:1433–1449. doi: 10.3233/JAD-215680. [DOI] [PubMed] [Google Scholar]
- Saxena M, Dubey R. Target enzyme in Alzheimer's disease: acetylcholinesterase inhibitors. Curr Top Med Chem. 2019;19:264–275. doi: 10.2174/1568026619666190128125912. [DOI] [PubMed] [Google Scholar]
- Sugawara T, Noshita N, Lewén A, Gasche Y, Ferrand-Drake M, Fujimura M, Morita-Fujimura Y, Chan PH. Overexpression of copper/zinc superoxide dismutase in transgenic rats protects vulnerable neurons against ischemic damage by blocking the mitochondrial pathway of caspase activation. J Neurosci. 2002;22:209–217. doi: 10.1523/JNEUROSCI.22-01-00209.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamagno E, Guglielmotto M, Vasciaveo V, Tabaton M (2021) Oxidative stress and beta amyloid in Alzheimer’s disease. Which comes first: The chicken or the egg? Antioxidants (Basel) 10:1479 [DOI] [PMC free article] [PubMed]
- Tang M, Taghibiglou C (2017) The mechanisms of action of curcumin in Alzheimer’s disease. J Alzheimers Dis 58:1003–1016 [DOI] [PubMed]
- Thal DR, Fändrich M. Protein aggregation in Alzheimer's disease: Abeta and tau and their potential roles in the pathogenesis of AD. Acta Neuropathol. 2015;129:163–165. doi: 10.1007/s00401-015-1387-2. [DOI] [PubMed] [Google Scholar]
- Wang J, Li YX, Song N, Guan HS. Preparation and characterization of chito-oligosaccharide and peracetylated-chito-oligosaccharides. Periodical Ocean U China. 2005;35:994–1000. [Google Scholar]
- Wang T, Kuang WH, Chen W, Xu WW, Zhang LM, Li YJ, Li HL, Peng Y, Chen YM, Wang BJ, Xiao JS, Li HH, Yan CZ, Du YF, Tang MN, He ZY, Chen HB, Li W, Lin H, Shi SG, et al. A phase II randomized trial of sodium oligomannate in Alzheimer's dementia. Alzheimers Res Ther. 2020;12:110–120. doi: 10.1186/s13195-020-00678-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XQ, Zhang R, Lin YQ, Shi P. Inhibition of NF-κB might enhance the protective role of roflupram on SH-SY5Y cells under amyloid β stimulation via PI3K/AKT/mTOR signaling pathway. Int J Neurosci. 2020;131:864–874. doi: 10.1080/00207454.2020.1759588. [DOI] [PubMed] [Google Scholar]
- Xia Q, Hu Q, Wang H, Yang H, Gao F, Ren H, Chen D, Fu C, Zheng L, Zhen X, Ying Z, Wang G. Induction of COX-2-PGE2 synthesis by activation of the MAPK/ERK pathway contributes to neuronal death triggered by TDP-43-depleted microglia. Cell Death Dis. 2015;6:e1702. doi: 10.1038/cddis.2015.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu QS, Liu MS, Liu QS, Wang WX, Du YG, Yin H. The inhibition of LPS-induced inflammation in RAW264.7 macrophages via the PI3K/Akt pathway by highly N-acetylated chitooligosaccharide. Carbohydr Polym. 2017;174:1138–1143. doi: 10.1016/j.carbpol.2017.07.051. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Kawashima S. Effects of amyloid-beta-(25–35) on passive avoidance, radial-arm maze learning and choline acetyltransferase activity in the rat. Eur J PharmacoL. 2001;412:265–272. doi: 10.1016/S0014-2999(01)00730-0. [DOI] [PubMed] [Google Scholar]
- Yi Z, Luo X, Zhao L. Research advances in chitosan oligosaccharides: from multiple biological activities to clinical applications. Curr Med Chem. 2020;27:5037–5055. doi: 10.2174/0929867326666190712180147. [DOI] [PubMed] [Google Scholar]
- Zeng H, Yu BF, Liu N, Yang YY, Xing HY, Liu XX, Zhou MW. Transcriptomic analysis of α-synuclein knockdown after T3 spinal cord injury in rats. BMC Genomics. 2019;20:851–869. doi: 10.1186/s12864-019-6244-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JL, Xia WS, Liu P, Cheng QY, Tahirou T, Gu WX, Li B. Chitosan modification and pharmaceutical/biomedical applications. Mar Drugs. 2010;8:1962–1986. doi: 10.3390/md8071962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Ahn YH, Benjamin IJ, Honda T, Hicks RJ, Calabrese V, Cole PA, Dinkova-Kostova AT. HSF1-dependent upregulation of Hsp70 by sulfhydryl-reactive inducers of the KEAP1/NRF2/ARE pathway. Chem Biol. 2011;18:1355–1361. doi: 10.1016/j.chembiol.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YR, Wang W, Hao C, Mao XZ, Zhang LJ. Astaxanthin protects PC12 cells from glutamate-induced neurotoxicity through multiple signaling pathways. J Funct Foods. 2015;16:137–152. doi: 10.1016/j.jff.2015.04.008. [DOI] [Google Scholar]
- Zhao Y, Zhao BL. Oxidative stress and the pathogenesis of Alzheimer's disease. Oxid Med Cell Longev. 2013;2013:316523. doi: 10.1155/2013/316523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou SL, Yang YM, Gu XS, Ding F. Chitooligosaccharides protect cultured hippocampal neurons against glutamate-induced neurotoxicity. Neurosci Lett. 2008;444:270–274. doi: 10.1016/j.neulet.2008.08.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that supports the findings of this study are included in this published article (and its supplementary information file).