Introduction
Xylazine, commonly referred to by its street name “tranq”, is a nonopioid veterinary tranquilizer increasingly found in the illicit drug supply. First detected in 2006, xylazine is now found as an adulterant in over 90% of illegal drug samples tested in Philadelphia and has been detected in the drug supply of 48 states nationwide.1,2 Between 2010 and 2020, drug overdose deaths involving illicit opioids containing xylazine have increased from 2% to 31% in Philadelphia.3 In 2019, xylazine was implicated in overdose deaths in 25 of 38 states that performed xylazine testing.4 Severe skin ulceration is a common complication of xylazine use.
Herein, we report 6 cases of xylazine-associated skin ulcerations (Table I).
Table I.
Clinical characteristics of reported cases
| Case #/Gender/Age | Drugs endorsed | UDS results | Xylazine confirmation∗ | Wound directed treatment | Complications | Number of presentations February 2022-February 2023 | Cumulative days in ED or hospital February 2022-February 2023 |
|---|---|---|---|---|---|---|---|
| 1/F/28 | Benzodiazepines, cocaine, fentanyl, xylazine | Benzodiazepines, cocaine, fentanyl, norfentanyl |
Not collected | Antibiotics, wound care |
None | 1 | 10 |
| 2/M/32 | Cocaine, fentanyl | Cocaine metabolites, fentanyl, norfentanyl, oxycodone, opiates |
Positive | Antibiotics, wound care |
Abscess, bacteremia, endocarditis, empyema, septic emboli, osteomyelitis | 5 | 89 |
| 3/M/51 | Fentanyl, heroin | Fentanyl, norfentanyl, hydromorphone, oxycodone, oxymorphone, opiates | Not collected | Surgical debridement, antibiotics, wound care |
MRSA bacteremia, myositis, osteomyelitis | 1 | 22 |
| 4/F/36 | Benzodiazepines, cocaine, fentanyl | Amphetamines, methamphetamines, benzodiazepines, benzoylecgonine, cocaine metabolites, fentanyl, norfentanyl, morphine, opiates |
Positive | Surgical debridement, antibiotics, wound care |
Abscess, bacteremia | 25 | 56 |
| 5/M/36 | Cocaine, fentanyl, xylazine | Benzoylecgonine, cocaine metabolites fentanyl, norfentanyl, hydromorphone, oxycodone, oxymorphone, opiates |
Positive | Antibiotics, wound care |
Bacteremia, osteomyelitis | 34 | 100 |
| 6/TGF†/39 | Cocaine, fentanyl, xylazine | Benzoylecgonine, cocaine metabolites, fentanyl, norfentanyl, hydromorphone, oxycodone, oxymorphone, methadone, opiates |
Positive | Antibiotics, wound care |
None | 2 | 14 |
ED, Emergency department; UDS, urine drug screen; MRSA, methicillin-resistant Staphylococcus aureus.
Xylazine confirmation screen is a urine gas-chromotography mass-spectrometry test developed by and used internally at the University of Pennsylvania Health System.
Transgender female individual born biological male.
Case Summary
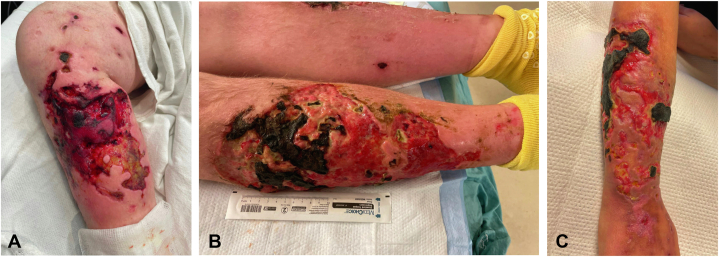
All 6 cases involved intravenous drug use (IVDU) of opioids with subsequent ulcer development on the distal extremities. Two patients also endorsed skin popping. Most patients had a long-term history of IVDU and reported limited cutaneous complications until recent addition of a new substance, “tranq dope”, in their drug supply. These ulcerations were described by dermatology consultants as jagged, angulated ulcers often with areas of eschar formation (Fig 1). Urine drug screens (UDS) detected fentanyl usage in all 6 patients. Xylazine confirmation screen was performed in 4 of the patients and verified exposure. Wound-directed treatments included surgical debridement, systemic antibiotics, and topical wound care. Four patients experienced complications including but not limited to bacteremia, osteomyelitis, and endocarditis. Many patients requested discharges against medical advice with frequent presentations to emergency departments (ED), resulting in numerous fragmented hospital admissions.
Fig 1.
Representative clinical photos of xylazine-associated cutaneous ulcerations (A, Case 4, B, Case 5, C, Case 1): angulated ulcerations with eschar and islands of spared normal skin.
Discussion
We report 6 cases of cutaneous ulcerations likely secondary to xylazine exposure. As an alpha-2-adrenergic agonist, xylazine causes heavy sedation and is thought to extend the effects of fentanyl and delay the symptoms of withdrawal.5 The mechanism by which xylazine causes skin wounds is not well understood, and wounds have been reported beyond the sites of local injection. It has been postulated that the pathogenesis involves peripheral vasoconstriction from α2-receptor activation.
In our reported cases, patients presented with angulated ulcerations on the distal extremities, often with eschar and islands of normal-appearing skin (Fig 1). Although the xylazine confirmation screen was not performed in 2 cases, all patients tested positive for fentanyl, which is likely contaminated with xylazine. Additionally, the morphology of the wounds supports the diagnosis of xylazine-related ulceration. This highlights the important role of the dermatologist to guide diagnosis and management of these patients in situations where xylazine testing may not be readily available.
The treatment of these ulcerations requires immediate cessation of xylazine and other illicit drug use. However, withdrawal from xylazine is oftentimes not adequately controlled by standard pharmacological opioid use disorder treatments, which adds an additional layer of complexity in managing these patients. Suspected superinfections should be treated with the appropriate antimicrobial agent. Patients should also be instructed on local wound care and provided with adequate supplies and follow-up to continue outpatient management.
Our cases highlight the cutaneous findings associated with xylazine exposure. As xylazine continues to infiltrate the illicit drug supply across the United States, it is important for clinicians to recognize its presentation for the adequate treatment and coordination of multidisciplinary patient care.
Conflicts of interest
None disclosed.
Footnotes
Dr Wei and Mr Wachuku contributed equally to this work. Drs Rosenbach and Messenger contributed equally to this work.
Funding sources: None.
IRB approval status: IRB exempt.
References
- 1.Soslow Z., Monterroso K. City of Philadelphia overdose fatality review. 2021. https://static1.squarespace.com/static/59ffe528d74cff8c750763fb/t/62bcbc36d7b44c0087ab6778/1656536119103/OD+Stat+Annual+Report+2021+FINAL.pdf
- 2.United States Drug Enforcement Administration DEA reports widespread threat of fentanyl mixed with xylazine | DEA.gov, Drug Enforcement Administration. 2023. https://www.dea.gov/alert/dea-reports-widespread-threat-fentanyl-mixed-xylazine
- 3.Johnson J., Pizzicato L., Johnson C., Viner K. Increasing presence of xylazine in heroin and/or fentanyl deaths, Philadelphia, Pennsylvania, 2010–2019. Inj Prev. 2021;27(4):395–398. doi: 10.1136/injuryprev-2020-043968. [DOI] [PubMed] [Google Scholar]
- 4.Kariisa M., Patel P., Smith H., Bitting J. Notes from the field: xylazine detection and involvement in drug overdose deaths — United States, 2019. MMWR Morb Mortal Wkly Rep. 2021;70:1300–1302. doi: 10.15585/mmwr.mm7037a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman J., Montero F., Bourgois P., et al. Xylazine spreads across the US: a growing component of the increasingly synthetic and polysubstance overdose crisis. Drug and Alcohol Dependence. 2022;233 doi: 10.1016/j.drugalcdep.2022.109380. [DOI] [PMC free article] [PubMed] [Google Scholar]