Abstract
Individuals with lower-limb amputations, many of whom have type 2 diabetes, experience impaired musculoskeletal health. This study: (1) compared residual and intact limbs of diabetic and non-diabetic post-mortem individuals with amputation to identify structures vulnerable to injury, and (2) compared findings to diabetic and healthy control groups to differentiate influences of amputation and diabetes on musculoskeletal health. Postmortem CT scans of three groups, ten individuals each, were included: (1) individuals with transtibial or transfemoral amputations, half with diabetes (2) diabetic controls, and (3) healthy controls. Hip and knee joint spaces, cross-sectional thigh muscle and fat areas, and cross-sectional bone properties (e.g. area, thickness, geometry) were measured. Wilcoxon Signed-Rank and Kruskal–Wallis tests assessed statistical significance. Asymmetry percentages between limbs assessed clinical significance. Residual limbs of individuals with amputation, particularly those with diabetes, had significantly less thigh muscle area and thinner distal femoral cortical bone compared to intact limbs. Compared to control groups, individuals with amputation had significantly narrower joint spaces, less thigh muscle area bilaterally, and thinner proximal femoral cortical bone in the residual limb. Diabetic individuals with amputation had the most clinically significant asymmetry. Findings tended to align with those of living individuals. However, lack of available medical information and small sample sizes reduced the anticipated clinical utility. Larger sample sizes of living individuals are needed to assess generalizability of findings. Quantifying musculoskeletal properties and differentiating influences of amputation and diabetes could eventually help direct rehabilitation techniques.
Subject terms: Anatomy, Musculoskeletal system, Bone, Cartilage, Muscle, Skeleton
Introduction
Forces acting on the human body during gait help maintain musculoskeletal health. Individuals with lower-limb amputation (IWAs) experience increased forces on the intact limb and reduced forces on the amputated, or residual, limb while walking with a prosthesis1. Chronic over or underuse of musculoskeletal structures alters patterns of musculoskeletal remodeling and can contribute to asymmetries between residual and intact limbs2. These asymmetries have been associated with adverse health outcomes such as increased risks of hip and knee osteoarthritis2,3, thigh muscle atrophy4, and femoral fracture5.
While gait asymmetries between residual and intact limbs have been studied extensively in this population, little attention has been paid to examining underlying structural musculoskeletal adaptations that have been associated with gait deviations2. Understanding structural musculoskeletal properties in this population could help reduce these risks by improving rehabilitation foci and improving clinical screening metrics. Specifically, these risks may be assessed by quantifying structural musculoskeletal properties, such as narrower hip and knee joint spaces to indicate osteoarthritis6, reduced muscle mass and fat tissue area to indicate muscle atrophy7, and reduced cross-sectional bone properties and moment of inertia to indicate fracture risk8.
Type 2 diabetes is a common comorbidity of IWAs9 and is also associated with increased risks of hip and knee osteoarthritis, thigh muscle atrophy, and femoral fracture risk10–12. However, diabetic IWAs are underrepresented in musculoskeletal properties literature that used anatomical imaging2,13. Determining how lower-limb amputation and diabetes differentially influence these risks can help inform rehabilitation techniques according to diabetic status in this population. As healthcare technology improves, quantifying musculoskeletal properties by pathology could help clinicians assess typical levels of musculoskeletal health, and implement rehabilitation exercises to reduce risks of hip and knee osteoarthritis, thigh muscle atrophy, and femoral fracture.
Therefore, this study quantified influences of amputation and diabetes on musculoskeletal parameters indicative of hip and knee osteoarthritis, thigh muscle atrophy, and femoral fracture risk. Our first aim was to determine if significant differences exist between residual and intact limbs of IWAs. We hypothesized that residual limbs, compared to intact limbs, would have significantly wider hip and knee joint spaces, reduced thigh muscle mass, and reduced cross-sectional bone properties (e.g. area, thickness, geometry). Our second aim was to compare IWA findings to diabetic and healthy control groups to differentiate interactions between amputation and diabetes on musculoskeletal health. We hypothesized IWAs, compared to diabetic and healthy control groups, would have significantly narrower hip and knee joint spaces, reduced muscle mass, and reduced cross-sectional bone properties. Overall, we expected diabetic IWAs would show the most impaired musculoskeletal health, followed by non-diabetic IWAs, diabetic controls, then healthy controls.
Methods
Study population
Deidentified data from deceased individuals did not require ethics approval from the North Texas Regional Institutional Review Board, per the institution’s policy. Computed tomography (CT) scans from deceased individuals were obtained with permission from the New Mexico Decedent Image Database (NMDID)14 from June to October 2021. The NMDID’s IRB approval is publicly posted on their website14. The NMDID used a Phillips Brilliance Big Bore 16 slice CT scanner with Z-position accuracy of ± 0.25 mm and resolution of up to 24 Lp/cm. Additional specifications for lower-limb scans are detailed on their website. Three groups were included: transtibial or transfemoral IWAs (half with diabetes), diabetic controls without amputation, and healthy controls without amputation.
To find IWAs, our preliminary search criteria was narrowed to males 40–90 years of age with potentially relevant causes of death (e.g., “diabetes”, which is listed as a selection for cause of death in the database as determined by the medical examiner) or record of lower limb surgery, as indicated by the NMDID records. IWAs were included if they had limb loss upon visual inspection of scout images, and excluded if they had advanced decomposition (determined by the CT technologist per NMDID, from a scale of no decomposition to mild, moderate, and advanced). A total of 10 IWAs were found in the NMDID database as of October 10th, 2021. Presence or absence of diabetes for was determined by a diagnosis of type II diabetes listed in the medical history. Once sex, age, and BMI of all 10 IWAs were recorded, diabetic and healthy controls were identified that were sex, age, and BMI matched to each of the 10 IWAs. Healthy controls were excluded if they had: amputation, diabetes, advanced body decomposition, cancer, or an age or BMI outside the range of identified IWAs. Diabetic controls were excluded for any of the above criteria for healthy controls, except diabetes.
Sample demographics
In total, thirty males 42–79 years of age with BMIs of 19.7–48.9 kg/m2 were included. Summary demographics are provided in Table 1. BMIs for IWAs were adjusted according to equations in previous literature15. IWAs were added to the study until no more could be found, which resulted in ten IWAs (6 transtibial, 4 transfemoral). Five IWAs had a diagnosis of diabetes, and five did not have a diagnosis of diabetes. Therefore, ten diabetic controls and ten healthy controls were included in this study. Two IWAs were affected bilaterally. For these individuals, the residual limb refers to the most proximally amputated limb and the intact limb refers to the most distally amputated limb. Limitations regarding sample size, individuals affected bilaterally, and available medical history information are discussed in “Limitations and future work”. Supplemental Tables 1 and 2 contain data from each IWA, classified by level of amputation and whether they were affected unilaterally or bilaterally. Supplemental Tables 3–7 present all summarized data and p values.
Table 1.
Summarized demographics.
| Sex | Age (years) | BMI (kg/m2) | Time from death to scan (days) | Race | Ethnicity | Primary cause of death | Side/Level of Amputation | |
|---|---|---|---|---|---|---|---|---|
| Healthy controls (n = 10) | All M | 57.50 ± 11.17 | 30.93 ± 8.05 | 61.20 ± 130.42 | All White | 4 of 10 Hispanic, Latino, or Middle Eastern | All natural | N/A |
| Diabetic controls (n = 10) | All M | 54.00 ± 9.18 | 29.22 ± 6.89 | 26.20 ± 38.71 | 9 White; 1 Native American | 2 of 10 Hispanic, Latino, or Middle Eastern | All diabetes | N/A |
| All IWAs (n = 10) | All M | 61.90 ± 11.05 | 33.80 ± 8.31 | 23.11 ± 15.83 | 7 White; 1 Black or African American; 1 Hispanic; 1 Native American | 5 of 10 Hispanic, Latino, or Middle Eastern | 5 diabetes; 2 natural; 3 sepsis |
5 unilateral TT; 3 unilateral TF; 1 bilateral TT; 1 bilateral TF |
| Diabetic IWAs (n = 5) | All M | 67.00 ± 11.00 | 32.58 ± 8.81 | 14.00 ± 4.24 | 3 White; 1 Hispanic; 1 Native American | 1 of 5 Hispanic, Latino, or Middle Eastern | 2 natural; 3 sepsis |
2 unilateral TT; 1 bilateral TT; 1 bilateral TF; 1 unilateral TF |
| Non-diabetic IWAs (n = 5) | All M | 56.80 ± 9.42 | 35.01 ± 8.61 | 30.40 ± 18.20 | 1 Black or African American; 4 White | 0 of 5 Hispanic, Latino, or Middle Eastern | All diabetes | 3 unilateral TT; 2 unilateral TF; |
| p values between Healthy controls, diabetic controls, and All IWAs | 0.18 | 0.98 | 0.08 |
Summarized demographic characteristics of all individuals included in this study as reported in the New Mexico Decedent Image Database. Data depicted as means ± standard deviations. Differences among groups between age, BMI, and time from death to scan were calculated using a Kruskal–Wallis test. All p values are two-tailed, with significance set at 0.05. IWAs individuals with lower-limb amputation, TT transtibial, TF transfemoral, N/A not applicable.
Joint space, tissue area, and femur morphology
CT scans were imported into 3D Slicer software (version 4.10) for the following bilateral measurements16. Author CF measured all musculoskeletal properties in the IWAs. Authors CF and WN were each randomly assigned to measure all musculoskeletal properties in half of the diabetic controls and half of the healthy controls.
Hip and knee joint spaces were measured from femoral head to acetabulum, and from femoral to tibial condyles, respectively. Fourteen measurements were averaged per hip and knee joint space, divided equally between anterior and posterior aspects or medial and lateral aspects, respectively, to provide a more accurate representation of the entire knee joint space.
Cross-sectional thigh muscle and fat tissue areas were measured at the femoral midshaft, defined as 50% of overall femur length. Within the midshaft slice, muscle and fat were identified using the threshold function within the Segmentation Editor module. Tissue areas were calculated using the Segmentation Statistics module.
Femur lengths were measured from greater trochanter to lateral condyle in intact limbs, and from greater trochanter to the lateral aspect of the distal end in residual limbs. Femoral head diameters were measured from anterior to posterior aspects and medial to lateral aspects. These two dimensions were averaged and expressed as a ratio of anterior–posterior to medial–lateral diameter. Femoral neck widths were measured superoinferiorly at the narrowest aspect. Femoral diaphysis width was measured anteroposteriorly at the midshaft.
Femur geometry
Cross-sectional bone properties were also measured for the femur. CT scan slices were loaded into the BoneJ plugin (version 2) for Fiji to quantify cortical bone thickness and moment of inertia17. Three slices per femur were used: the proximal (25% of total femoral length), middle (50%), and distal (75%) femoral shaft. The slice was cropped to enclose the cortical bone and converted into an 8-bit image. The grayscale threshold range was adjusted to encompass the bone while excluding air and soft tissue. Threshold values were imputed into the Slice Geometry module of BoneJ to calculate biomechanical properties of the bone at the specified cross-section.
Repeatability study
A repeatability study was conducted to ensure intra and inter-observer reliability between CF and WN’s control group measurements. CF and WN were assigned the same three scans, and collected all measurements three times with at least 24 h between each collection. Intra- and inter-observer reliability of CF and WN were determined using paired and unpaired t-tests, respectively. Percent differences in measurements were also calculated. No significant differences were found, and all reliability measurements differed by less than 10%.
Statistics
NCSS Statistical Software (2021, LCC, Kaysville, UT, USA) was used for statistical analysis18. Missing data points (e.g. knee joint space absent due to transfemoral amputation) were not included. For Aim 1, Wilcoxon Signed-Rank tests assessed differences between residual and intact limbs of IWAs, right and left limbs of diabetic controls, and right and left limbs of healthy controls. For Aim 2, Kruskal–Wallis tests with Bonferroni corrections assessed differences between groups. Right and left limbs were averaged for diabetic and healthy controls to provide a single limb value. Two Kruskal–Wallis tests were performed: (1) compared the intact limbs of IWAs to diabetic and healthy controls, and (2) compared the residual limbs of IWAs to diabetic and healthy controls. Mann–Whitney p values were performed for significant Kruskal Wallis group comparisons. All p values are reported as two-tailed, with significance levels set at α ≤ 0.05.
Calculation of asymmetry
Asymmetry between limbs was calculated to assess clinical significance. A threshold of 10% asymmetry is considered a clinically relevant threshold, where 0% is perfectly symmetrical and 100% is perfectly asymmetrical13. Asymmetry percentages were calculated using the following equation:
| 1 |
For IWAs, I represents the intact limb value and R represents the residual limb value. For diabetic and healthy controls, the left limb value replaced I and the right limb value replaced R.
Results
Joint space, tissue area, and femur morphology
Between-limb comparisons
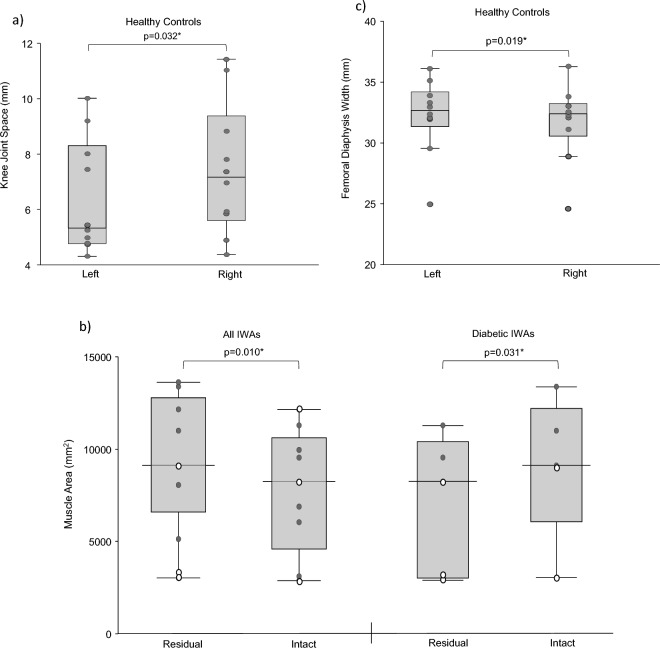
Data are presented in Fig. 1 and Supplemental Table 3. Healthy controls had significantly different knee joint spaces (p = 0.032) between right and left limbs. IWAs had significantly less muscle tissue area (p = 0.010) in residual limbs compared to intact limbs. Diabetic IWAs also showed this significance in muscle tissue area (p = 0.031). Diabetic controls had significantly different anterior–posterior femoral head width between right and left limbs (p = 0.014), and significant differences in femoral head ratio (p = 0.032). Healthy controls had significantly different femoral diaphysis widths (p = 0.019) between right and left limbs.
Figure 1.
Between-limb comparisons with statistical significance for: (a) joint space, (b) tissue area, and (c) femur morphology. Dark grey points indicate data from transtibial participants, and white data points indicate data from transfemoral participants.
Healthy controls had clinically significant knee joint space asymmetry (14.85%). Non-diabetic IWAs had clinically significant hip (21.01%) and knee (17.90%) joint space asymmetry. IWAs had clinically significant muscle area (18.69%) asymmetry, higher in diabetic (26.09%) compared to non-diabetic (10.63%) IWAs.
Between-group comparisons
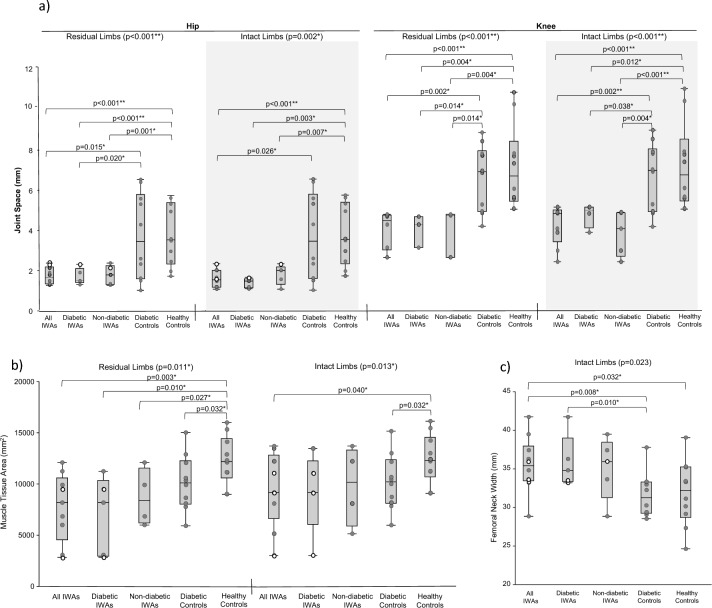
Data are presented in Fig. 2 and Supplemental Table 4. Mann–Whitney post hoc comparisons for significant differences are presented in Supplemental Table 5. Compared to diabetic and healthy controls, IWAs had significantly narrower hip joint space on intact (p = 0.002) and residual (p < 0.001) limbs, narrower knee joint space on intact (p < 0.001) limbs, and wider femoral diaphysis on the intact limb (p = 0.023). Compared to healthy controls, IWAs had significantly narrower knee joint space on residual (p < 0.001) limbs, and reduced muscle area on intact (p = 0.013) and residual (p = 0.011) limbs.
Figure 2.
Between-group comparisons with statistical significance for: (a) joint space, (b) tissue area, and (c) femur morphology. Dark grey points indicate data from transtibial participants, and white data points indicate data from transfemoral participants.
Femur geometry
Between-limb comparisons
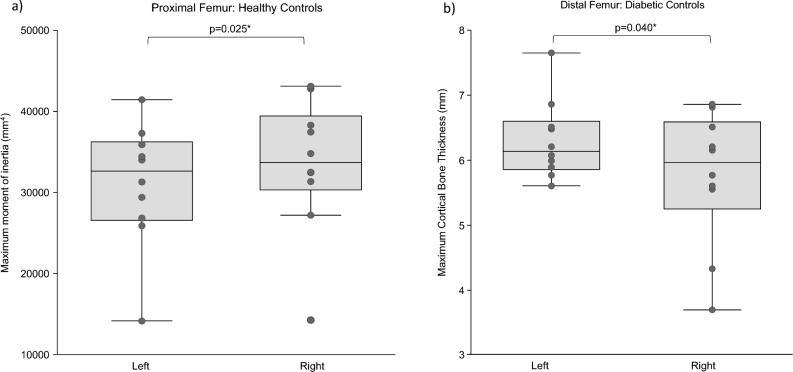
Data are presented in Fig. 3 and Supplemental Table 5. IWAs had significantly higher standard deviation cortical bone thickness (p = 0.037), which indicates higher variability in cortical bone thickness, at the femoral midshaft in residual limbs compared to intact limbs. Healthy controls had significantly different maximum moments of inertia (p = 0.025) at the proximal femur between right and left limbs. Diabetic controls had significantly different maximum (p = 0.040) and standard deviation (p = 0.025) thicknesses at the distal femur between right and left limbs.
Figure 3.
Between-limb comparisons with statistical significance for: (a) proximal femur geometry, and (b) distal femur geometry.
At the proximal femur, non-diabetic IWAs had clinically significant cross-sectional area (10.37%), maximum (10.39%), and mean (12.41%) cortical bone thickness asymmetry. At the femoral midshaft, IWAs and diabetic IWAs had clinically significant standard deviation cortical bone thickness asymmetry (16.80% and 28.49%, respectively). At the distal femur, IWAs and non-diabetic IWAs had clinically significant standard deviation cortical bone thickness asymmetry (10.87% and 21.53%, respectively). Further, at the distal femur, diabetic IWAs had clinically significant cross-sectional area (15.15%), mean (16.13%), and standard deviation (11.73%) cortical thickness asymmetry. Additionally, non-diabetic IWAs had clinically significant asymmetry in minimum (10.53%) and maximum (17.15%) moments of inertia.
Between-group comparisons
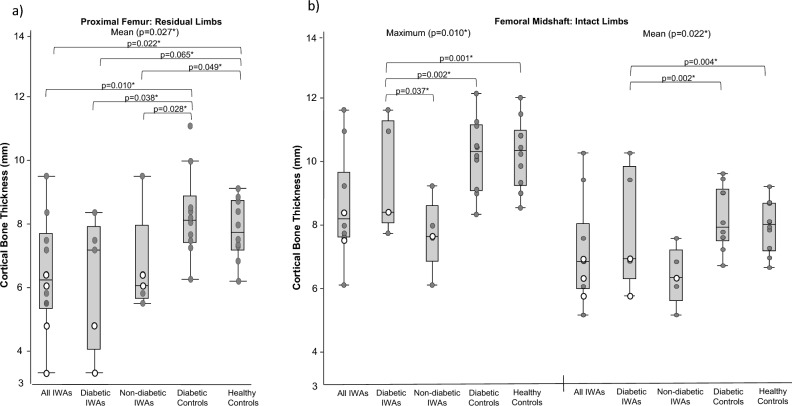
Data are presented in Fig. 4 and Supplemental Table 6. At the proximal femur, residual limbs of IWAs had significantly narrower mean cortical thickness (p = 0.027) than diabetic and healthy controls. At the femoral midshaft, residual limbs of diabetic IWAs had significantly narrower maximum (p = 0.004) and mean (p = 0.017) cortical thickness than diabetic and healthy controls. At the distal femur, residual limbs of non-diabetic IWAs had significantly higher standard deviation cortical thickness (p = 0.020) than all groups.
Figure 4.
Between-group comparisons with statistical significance for: (a) proximal femur geometry, and (b) femoral midshaft geometry. Dark grey points indicate data from transtibial participants, and white data points indicate data from transfemoral participants.
Discussion
Obtaining large cohorts of CT scans in living individuals with amputations can be challenging. Using the NMDID to collect musculoskeletal data from deceased individuals with amputations could help inform which properties might be most beneficial to examine in living individuals. To our knowledge, this was the first study to use CT scans to quantify musculoskeletal health in individuals with amputation, compare findings to diabetic and healthy controls, and is of the largest sample sizes to examine musculoskeletal health from an anatomical perspective in individuals with amputation. Our findings aligned with those from other musculoskeletal methodologies, (e.g. MRI, ultrasound) as well as known risk factors in living individuals2. Residual limbs of IWAs had higher indications of thigh muscle atrophy and distal femoral fracture risk compared to intact limbs. Compared to control groups, IWAs had higher indications of muscle atrophy and osteoarthritis bilaterally, along with proximal femoral fracture risk on the residual limb. Intact limbs of IWAs had wider femoral necks, indicative of increased femoral loading compared to diabetic and healthy controls. Diabetic IWAs tended to show the most impaired musculoskeletal health. Findings align with previous literature surrounding risk factors of hip and knee osteoarthritis, thigh muscle atrophy, and femoral fracture risk in living individuals with amputation1–3. However, major limitations include lack of healthcare information in the database related to amputation and prosthesis use, as well as our small sample size. Future research could examine larger sample sizes of living individuals to determine if findings are generalizable.
Hip and knee osteoarthritis
Individuals who are unilateral lower-limb prosthesis users are more likely to develop hip and knee osteoarthritis on the intact limb, compared to the residual limb and nonamputee1. Prosthesis users rely on their intact limb, resulting in more biomechanical load, narrow joint space, and accelerated cartilage degeneration1. IWAs showed narrower hip and knee joint spaces bilaterally compared to controls, in line with prior work on the prevalence of osteoarthritis in the IWAs population compared to the general population1. However, in contrast with previous work19,20, hip or knee joint space were only significant between residual and intact limbs of non-diabetic IWAs. This may be due to our limited sample size.
Thigh muscle atrophy
Thigh atrophy can hinder prosthetic control21 and balance22. IWAs, particularly those with diabetes, showed more thigh muscle atrophy in residual limbs compared to intact limbs. IWAs also showed indications of more thigh muscle atrophy bilaterally compared to diabetic and healthy controls. This aligns with work in living IWAs that used other methodologies (e.g. ultrasound, MRI)4,23,24. Elevated blood sugar levels, as seen in type 2 diabetes, may be associated with muscle atrophy25, which may explain why diabetic IWAs had the most thigh muscle atrophy. No significant differences in fat mass were found in any group, in contrast with previous work23,26–28, potentially due to our small sample sizes.
Femoral fractures
Falls and fall-related injuries are prevalent in IWAs, so preventing fall-related fractures are of particular concern in this population29,30. In alignment with prior work, intact limbs of IWAs, compared to controls, had wider femoral necks and thinner cortical bone at the proximal femur31,32. Clinical significance among diabetic and non-diabetic IWAs suggest femoral adaptations may differ according to diabetic status. In nonamputees, proximal and distal femoral diaphysis are most adaptive to changes in biomechanical loading in nonamputees33. This may also occur in IWAs, as our findings and previous literature have shown thinner cortical bone and increased fracture rates at the proximal and distal femoral diaphysis in the residual limb33,34. This finding also supports previous literature regarding this population experiencing increased loading in the intact limb and decreased loading in the residual limb1–3,32. Proactive skeletal screening, rehabilitation exercises to promote residual limb loading, and earlier ambulation may help maintain skeletal health.
Control groups
Leg dominance, combined with outliers or small sample size, may underlie significant differences between right and left limbs in healthy and diabetic control groups. Healthy controls had significant asymmetries between limbs in knee joint space and femoral diaphysis width, while diabetic controls had significant asymmetries between limbs in AP femoral head width and ratio. Additionally, healthy controls tended to have larger right-limb values for all cross-sectional data, and diabetic controls tended to have larger left-limb values for gross and cross-sectional data.
Influence of diabetes
Diabetes, similar to amputation, has been associated with increased risks of developing sarcopenia, osteoarthritis, and osteopenia10–12. Despite having no statistical significance between diabetic and non-diabetic IWAs, diabetic IWAs showed the most clinical significance in hip and knee joint space as well as muscle tissue area. Diabetic IWAs also had the most impaired musculoskeletal health in several parameters, including knee joint space bilaterally, intact limb femoral neck width, and thinner intact femoral midshaft cortical bone. These trends suggest additive effects of amputation and diabetes on impaired musculoskeletal health, despite a lack of statistical significance. Clinicians should still consider other diabetic complications, such as retinopathy and peripheral neuropathy, which can lead to fall-related fractures35.
Limitations and future work
A major limitation of this study was the amount of medical history information available from the NMDID. While potential confounding factors of musculoskeletal health were excluded (e.g. cancer, advanced body decomposition), information on prosthesis use was absent. For instance, all of the IWAs had a confirmed amputation surgery, but no record of time since amputation, diabetic onset, prosthesis use, or activity level. Therefore, musculoskeletal findings could not be assessed by these factors. This study included two individuals with bilateral amputations, as well as transtibial and transfemoral amputations, so findings should not be applied to one subset of amputation level.
Several properties on two individuals could not be measured, since the limb was outside the frame of the CT scan. Similarly, several femoral properties could not be measured in individuals with transfemoral amputation, particularly hip and knee joint space for one individual with bilateral hip replacements. Also, transfemoral residual limbs were measured at midshaft of the overall femur length. Further, while time between death and CT scan typically ranged between 10 and 30 days, two of the control individuals were scanned over 100 days after death. While hip joint replacements, transfemoral femur length, and time delay could theoretically have influenced findings, outliers were not observed in the data for these individuals.
The authors located, to the best of our knowledge, all available scans of IWAs in the database for this study. Future work can include a larger sample size to assess the generalizability of our findings as individuals are added to the database, compare our results to CT scans of living individuals, or examine individuals affected unilaterally or bilaterally separately. Additionally, application of findings are limited to males. Future work could examine effects of aging or include females, particularly to examine additive effects of osteoporosis risk due to hormonal changes during menopause.
Conclusion
This study is currently among the largest sample sizes of musculoskeletal health in this population, and is the first to use CT scans of deceased individuals. While musculoskeletal health has been assessed in prior literature, few studies have collected anatomical properties underlying musculoskeletal health risks prevalent in this population. Findings tended to align with previously reported health risks in living individuals. Residual limbs of IWAs showed higher indications of muscle atrophy and fracture risk at the distal femur compared to intact limbs. IWAs, compared to control groups, showed higher indications of muscle atrophy and osteoarthritis bilaterally, as well as higher indications of fracture risk at the proximal femur on the residual limb. Proactive musculoskeletal screenings, targeted rehabilitation exercises, shortening time from amputation to ambulation, and ensuring optimal prosthetic fit and alignment could help clinicians reduce these risks. Musculoskeletal adaptations from amputation and diabetes tended to show an additive effect, but were not statistically significant. However, lack of available medical information and our small sample size greatly reduced the anticipated clinical utility of this study. Larger sample sizes and living individuals should be included in future work to assess generalizability of findings.
Supplementary Information
Acknowledgements
The authors wish to sincerely thank those who donated their bodies to science so that anatomical research could be performed. Results from such research can potentially improve patient care and increase overall knowledge. Therefore, these donors and their families deserve our highest gratitude. Thanks to Bethany Holley for identifying the majority of diabetic individuals used in this study. This project is taken in part from a dissertation submitted to the University of North Texas Health Science Center in partial fulfillment of the requirements for the degree of Doctor of Philosophy.
Author contributions
Conceptualization: M.G.F., R.A.M.; methodology: M.G.F., R.A.M.; data curation: C.F., W.N.; investigation: C.F., W.N.; formal analysis: M.G.F.; validation: C.F., W.N.; visualization: M.G.F.; resources: R.A.M.; supervision: R.A.M.; writing—original draft: M.G.F.; writing—review and editing: all authors.
Funding
The Free Access Decedent Database was funded by the National Institute of Justice grant number 2016-DN-BX-0144. MGF was supported by the National Institutes of Health/National Institute on Aging (T32 AG020494) and the Institute for Healthy Aging. CF was supported by the Summer Opportunities in Anatomy Research at the University of North Texas Health Science Center through the American Association for Anatomy Innovations Program.
Data availability
All data can be made available upon request by contacting the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: M. G. Finco and Caitlyn Finnerty.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-34773-w.
References
- 1.Gailey R, Allen K, Castles J, Kucharik J, Roeder M. Review of secondary physical conditions associated with lower-limb amputation and long-term prosthesis use. J. Rehabil. Res. Dev. 2008;45:15–29. doi: 10.1682/JRRD.2006.11.0147. [DOI] [PubMed] [Google Scholar]
- 2.Finco MG, Kim S, Ngo W, Menegaz RA. A review of musculoskeletal adaptations in individuals following major lower-limb amputation. J. Musculoskelet. Neuronal. Interact. 2022;22:269–283. [PMC free article] [PubMed] [Google Scholar]
- 3.Lloyd CH, Stanhope SJ, Davis IS, Royer TD. Strength asymmetry and osteoarthritis risk factors in unilateral trans-tibial, amputee gait. Gait Posture. 2010;32:296–300. doi: 10.1016/j.gaitpost.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Sibley AR, Strike S, Moudy SC, Tillin NA. The effects of long-term muscle disuse on neuromuscular function in unilateral transtibial amputees. Exp. Physiol. 2020;105:408–418. doi: 10.1113/EP088087. [DOI] [PubMed] [Google Scholar]
- 5.Finco MG, Menegaz RA. Skeletal asymmetries in anatomical donors with lower-limb amputations. PM&R. 2022;14:406–408. doi: 10.1002/pmrj.12599. [DOI] [PubMed] [Google Scholar]
- 6.Braun HJ, Gold G. E. Diagnosis of osteoarthritis: Imaging. Bone. 2012;51:278–288. doi: 10.1016/j.bone.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engelke K, Museyko O, Wang L, Laredo JD. Quantitative analysis of skeletal muscle by computed tomography imaging-state of the art. J. Orthop. Translat. 2018;15:91–103. doi: 10.1016/j.jot.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Treece GM, Gee AH. Independent measurement of femoral cortical thickness and cortical bone density using clinical ct. Med. Image Anal. 2015;20:249–264. doi: 10.1016/j.media.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the united states: 2005 to 2050. Arch. Phys. Med. Rehabil. 2008;89:422–429. doi: 10.1016/j.apmr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Kim YK, Lee HS, Ryu JJ, In Lee H, Seo SG. Sarcopenia increases the risk for mortality in patients who undergo amputation for diabetic foot. J. Foot Ankle Res. 2018;11:32. doi: 10.1186/s13047-018-0274-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piva SR, Susko AM, Khoja SS, Josbeno DA, Fitzgerald GK, Toledo FG. Links between osteoarthritis and diabetes: Implications for management from a physical activity perspective. Clin. Geriatr. Med. 2015;31:67–87. doi: 10.1016/j.cger.2014.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duarte VMG, Ramos AMO, Rezende LA, Macedo UBO, Brandão-Neto J, Almeida MG, et al. Osteopenia: A bone disorder associated with diabetes mellitus. J. Bone Miner. Metab. 2005;23:58–68. doi: 10.1007/s00774-004-0542-y. [DOI] [PubMed] [Google Scholar]
- 13.Finco MG, Moudy SC, Patterson RM. Normalized kinematic walking symmetry data for individuals who use unilateral lower-limb prostheses: Considerations for clinical practice and future research. J. Prosthet. Orthot. 2023;35:1–17. doi: 10.1097/JPO.0000000000000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edgar, H. D. B.; Moes, E., Adolphi, N. L., Bridges, P., & Nolte, K. B. New Mexico decedent image database: Office of the Medical Investigator, University of New Mexico; 2020.
- 15.Himes JH. New equation to estimate body mass index in amputees. J. Am. Diet Assoc. 1995;95:646. doi: 10.1016/S0002-8223(95)00175-1. [DOI] [PubMed] [Google Scholar]
- 16.Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC, Pujol S, et al. 3d slicer as an image computing platform for the quantitative imaging network. Magn. Reson. Imaging. 2012;30:1323–1341. doi: 10.1016/j.mri.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doube M, Kłosowski MM, Arganda-Carreras I, Cordelières FP, Dougherty RP, Jackson JS, et al. Bonej: Free and extensible bone image analysis in imagej. Bone. 2010;47:1076–1079. doi: 10.1016/j.bone.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ncss 2021 statistical software (2021). NCSS, LLC. Kaysville, Utah, USA. http://ncss.com/software/ncss2021.
- 19.Welke B, Jakubowitz E, Seehaus F, Daniilidis K, Timpner M, Tremer N, et al. The prevalence of osteoarthritis: Higher risk after transfemoral amputation?—a database analysis with 1,569 amputees and matched controls. PLoS One. 2019;14:25. doi: 10.1371/journal.pone.0210868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farrokhi S, Mazzone B, Yoder A, Grant K, Wyatt M. A narrative review of the prevalence and risk factors associated with development of knee osteoarthritis after traumatic unilateral lower limb amputation. Mil. Med. 2016;181:38–44. doi: 10.7205/MILMED-D-15-00510. [DOI] [PubMed] [Google Scholar]
- 21.Isakov E, Burger H, Gregorič M, Marinček Č. Stump length as related to atrophy and strength of the thigh muscles in trans-tibial amputees. Prosthet. Orthot. Int. 1996;20:96–100. doi: 10.3109/03093649609164425. [DOI] [PubMed] [Google Scholar]
- 22.Hewson A, Dent S, Sawers A. Strength deficits in lower limb prosthesis users: A scoping review. Prosthet. Orthot. Int. 2020;44:323–340. doi: 10.1177/0309364620930176. [DOI] [PubMed] [Google Scholar]
- 23.Sherk VD, Bemben MG, Bemben DA. Interlimb muscle and fat comparisons in persons with lower-limb amputation. Arch. Phys. Med. Rehabil. 2010;91:1077–1081. doi: 10.1016/j.apmr.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Henson DP, Edgar C, Ding Z, Sivapuratharasu B, Le Feuvre P, Finnegan ME, et al. Understanding lower limb muscle volume adaptations to amputation. J. Biomech. 2021;125:25. doi: 10.1016/j.jbiomech.2021.110599. [DOI] [PubMed] [Google Scholar]
- 25.Bianchi L, Volpato S. Muscle dysfunction in type 2 diabetes: A major threat to patient's mobility and independence. Acta Diabetol. 2016;53:879–889. doi: 10.1007/s00592-016-0880-y. [DOI] [PubMed] [Google Scholar]
- 26.Bramley JL, Worsley PR, Bader DL, Everitt C, Darekar A, King L, et al. Changes in tissue composition and load response after transtibial amputation indicate biomechanical adaptation. Ann. Biomed. Eng. 2021;49:3176–3188. doi: 10.1007/s10439-021-02858-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cavedon V, Sandri M, Peluso I, Zancanaro C, Milanese C. Body composition and bone mineral density in athletes with a physical impairment. PeerJ. 2021;9:25. doi: 10.7717/peerj.11296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Putz C, Block J, Gantz S, Heitzmann DWW, Dreher T, Lehner B, et al. Structural changes in the thigh muscles following trans-femoral amputation. Eur. J. Orthop. Surg. Traumatol. 2017;27:829–835. doi: 10.1007/s00590-017-1929-5. [DOI] [PubMed] [Google Scholar]
- 29.Kim J, Major MJ, Hafner B, Sawers A. Frequency and circumstances of falls reported by ambulatory unilateral lower limb prosthesis users: A secondary analysis. PM&R. 2019;11:344–353. doi: 10.1016/j.pmrj.2018.08.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clemens S, Doerger C, Lee SP. Current and emerging trends in the management of fall risk in people with lower limb amputation. Curr. Geriatr. Rep. 2020;9:134–141. doi: 10.1007/s13670-020-00328-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Royer T, Koenig M. Joint loading and bone mineral density in persons with unilateral, trans-tibial amputation. Clin. Biomech. (Bristol, Avon) 2005;20:1119–1125. doi: 10.1016/j.clinbiomech.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Sherk VD, Bemben MG, Bemben DA. BMD and bone geometry in transtibial and transfemoral amputees. J. Bone Miner. Res. 2008;23:1449–1457. doi: 10.1359/jbmr.080402. [DOI] [PubMed] [Google Scholar]
- 33.Gosman JH, Hubbell ZR, Shaw CN, Ryan TM. Development of cortical bone geometry in the human femoral and tibial diaphysis. Anat. Rec. 2013;296:774–787. doi: 10.1002/ar.22688. [DOI] [PubMed] [Google Scholar]
- 34.Haleem S, Yousaf S, Hamid T, Nagappa S, Parker MJ. Characteristics and outcomes of hip fractures in lower limb amputees. Injury. 2021;52:914–917. doi: 10.1016/j.injury.2020.10.017. [DOI] [PubMed] [Google Scholar]
- 35.Moayeri A, Mohamadpour M, Mousavi SF, Shirzadpour E, Mohamadpour S, Amraei M. Fracture risk in patients with type 2 diabetes mellitus and possible risk factors: A systematic review and meta-analysis. Ther. Clin. Risk Manage. 2017;13:455–468. doi: 10.2147/TCRM.S131945. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data can be made available upon request by contacting the corresponding author.