Abstract
Glioblastoma is acknowledged as the most aggressive cerebral tumor in adults. However, the efficacy of current standard therapy is seriously undermined by drug resistance and suppressive immune microenvironment. Ferroptosis is a recently discovered form of iron-dependent cell death that may have excellent prospect as chemosensitizer. The utilization of ferropotosis inducer Erastin could significantly mediate chemotherapy sensitization of Temozolomide and exert anti-tumor effects in glioblastoma. In this study, a combination of hydrogel-liposome nanoplatform encapsulated with Temozolomide and ferroptosis inducer Erastin was constructed. The αvβ3 integrin-binding peptide cyclic RGD was utilized to modify codelivery system to achieve glioblastoma targeting strategy. As biocompatible drug reservoirs, cross-linked GelMA (gelatin methacrylamide) hydrogel and cRGD-coated liposome realized the sustained release of internal contents. In the modified intracranial tumor resection model, GelMA-liposome system achieved slow release of Temozolomide and Erastin in situ for more than 14 d. The results indicated that nanoplatform (T+E@LPs-cRGD+GelMA) improved glioblastoma sensitivity to chemotherapeutic temozolomide and exerted satisfactory anti-tumor effects. It was demonstrated that the induction of ferroptosis could be utilized as a therapeutic strategy to overcome drug resistance. Furthermore, transcriptome sequencing was conducted to reveal the underlying mechanism that the nanoplatform (T+E@LPs-cRGD+GelMA) implicated in. It is suggested that GelMA-liposome system participated in the immune response and immunomodulation of glioblastoma via interferon/PD-L1 pathway. Collectively, this study proposed a potential combinatory therapeutic strategy for glioblastoma treatment.
Keywords: Glioblastoma, Relapse, Hydrogel-liposome, Ferroptosis, Drug resistance, Immunomodulation
Graphical abstract
1. Introduction
Glioblastoma multiforme (GBM) is acknowledged to be the most common and aggressive cerebral malignant tumor in adults, and the mortality rate of GBM is second only to that of pancreatic cancer. Even with standard comprehensive therapy (surgery combined with radiation and oral temozolomide), the patients’ life expectancy is only extended by 16 to 18 months, and five-year survival rate of patients is 6.8% [1]. The primary reason accounting for these is drug resistance. Temozolomide (TMZ) is the most effective chemotherapeutic drug for GBM, however, researches have revealed that insensitive GBM cells can gradually develop drug resistance after long-term TMZ application [2,3]. Despite the fact that surgery has been recognized as the primary treatment, resecting GBM mass completely in microsurgery is an unfeasible task since tumor satellites have the propensity to infiltrate and reside in normal tissues [1]. Therefore, inexorable postoperative tumor recurrence happens in almost all GBM patients [3]. At relapse, the genetic alterations associated with a signature of TMZ-induced mutations lead to malignant transformation and non-response to therapeutic intervention [4]. Hence, it is of significance to explore an effective therapeutic strategy to target infiltrating tumor cells and overcome drug resistance [5], [6], [7], [8], [9].
Ferroptosis is a recently discovered regulatory form of iron-dependent cell death being distinct from apoptosis, necroptosis, pyroptosis and autophagy [10]. The typical characteristics of ferroptosis include reduced glutathione (GSH) level, accumulation of cellular reactive oxygen species (ROS)and augmented lipid peroxidation [11]. In the past few years, it has been increasingly reckoned that ferroptosis is implicated in the therapeutic response of GBM therapy [12,13]. Recent advances have identified that the hub genes of ferroptosis pathway, including cysteine-glutamate transporter (xCT) and glutathione peroxidase (GPX), play indispensable roles in the therapeutic resistance of tumors, especially for GBM [14], [15], [16]. Intervention of these key factors is proved to enhance the sensitivity of chemotherapy and radiotherapy, thus improving the prognosis of GBM [17], [18]. Erastin (ERA) is the first small molecule inhibitor found to selectively and effectively mediate ferroptosis through xCT, voltage-dependent anion channel (VDAC) and p53. It is also suggested that ERA has excellent prospects as novel radiosensitizer and chemosensitizer [19]. However, the extremely poor solubility of ERA in common solvents limited its efficient applications in GBM treatment. What's more, whether ERA would enhance the efficacy of TMZ in resistant GBM via activating ferroptotic pathways remains to be further investigated.
Hydrogel is cross-linked network composed by hydrophilic polymers. It has been widely used in pharmaceutical processing area due to its biocompatibility and safety [20]. Surgery is the standard therapy for GBM, therefore, the most extensive application of hydrogel in GBM area is in situ drug delivery within postresection intracavity. The internal drugs are released mainly through passive diffusion, in which the swelling or degradation of networks enlarge the interior mesh size of hydrogel and trigger rapid uncontrolled release of encapsulated micro-molecules [5]. It has been demonstrated that the incorporation of liposome into hydrogel can realize prolonged drug release [20], [21]. The presence of liposome strengthens the mechanical bridging within hydrogel, and the large size of liposome contributes to slow diffusion coefficient [22]. Three-dimensional (3D) printing is an emerging technology with the ability to fabricate prototypes from computer modeling. Previous studies in treating GBM by hydrogel system were mainly focused on those with self-assembly or thermal-sensitivity properties [23], [24]. Comparing to intraoperative cross-linking procedures, 3D printing with light curing achieves the prefabrication of hydrogel, which reduces the overall operation time and prevents liquid hydrogel from entering cerebral ventricular circulation system.
Herein, to achieve successful drug delivery and to inhibit postresection glioblastoma relapse, we fabricated a 3D-printing light-curing hydrogel as the drug reservoir for the delivery of cRGD-decorated liposomes containing TMZ and ERA (T+E@LPs-cRGD+GelMA) (Fig. 1). The cyclic RGD peptide has been demonstrated as a promising targeting ligand for drug delivery in GBM therapy [25], [26], [27]. After intracavity implantation, gelatin methacrylamide (GelMA) hydrogel could release the hybrid liposomes in a sustained manner for 14 d. Subsequently, the liberalized cRGD-liposomes could specifically target residual GBM cells and continuously deliver internalized cargos including TMZ and ERA. Indubitably, ERA activated ferroptosis and exhibited remarkable anti-tumor efficiency synergistically with TMZ to chemo-resistance GBM cells. Immunosurveillance escape is increasingly recognized as a landmark event in GBM biology [28]. Intriguingly, the GelMA-liposome drug delivery platform exerted an immunomodulating effect on GBM cells, which could in turn regulate the tumor immune microenvironment via interferon/PD-L1 signaling pathway and suppress the recurrence of postoperative glioblastoma. Collectively, our investigation provides a synergistic and GBM-tropic therapeutic strategy to achieve the sustained release of TMZ and ERA in postresection intracavity, which potently inhibited GBM relapse.
Fig. 1.
Schematic illustration of the overall study design of T+E@LPs-cRGD+GelMA for the treatment of postoperative GBM recurrence in mouse.
2. Materials and methods
2.1. Materials
DSPE-PEG-cRGD were purchased from A.V.T company (Shanghai, China). GelMA in this study were provided by EngineeringForLife (Suzhou, China). ERA and TMZ were purchased from MCE (NJ, USA). Cell-Light™ EdU Apollo567 In Vitro Imaging Kit were purchased from RiboBio company (Guangzhou, China). Calcein-AM/PI Double Dye Kit were purchased from Yuanye company (Shanghai, China). Cell Counting Kit-8 were purchased from YEASEN (Shanghai, China). Annexin V-FITC Apoptosis Detection Kit and RNA-easy™ Isolation Reagent were purchased from Vazyme company (Nanjing, China). GSH/GSSG Assay Kit and JC-1 Assay Kit were purchased from Beyotime company (Shanghai, China). 4,6-diamidino-2-phenylindole (DAPI) were purchased from Beyotime Biotechnology (Shanghai, China). Immunohistochemistry Kit were provided by Sangon Biotech (Shanghai, China).
2.2. GelMA and liposomal drug delivery system
Prepared by reverse phase evaporation. HSPC (Hydrogenated Soybean Phosphotidylcholine), cholesterol, cRGD-coated DSPE-PEG and ERA were dissolved in 4 ml chloroform in a ratio of 4:1:1:1. The emulsion was formed by the mixture of lipids with TMZ-containing ddH2O after 2 min ultrasonic treatment. Then consecutive rotary evaporation for 20 min was conducted to remove chloroform. The liposome system was formed through ultrasonic and extruder processing. Unloaded ERA and TMZ were removed through 0.05 µm dialysis membrane, and the loaded drug amount was estimated by spectrometer (TMZ at 329 nm, ERA at 276 nm). The concentration ratio of two drugs was in line with the proportion range of synergistic concentration.
Synthetic liposomes were gently mixed with 15% (w/v) solution of lyophilized GelMA (EFL-GM-60, ) at 1:1 ratio. The amount of loaded TMZ in T@LPs-cRGD+GelMA and T+E@LPs-cRGD+GelMA is ∼38 mg in 1 ml nanoplatforms. The amount of loaded ERA in E@LPs-cRGD+GelMA and T+E@LPs-cRGD+GelMA groups is ∼2.6 mg in 1 ml nanoplatform. The mixture was light-cured by 3D printing workstation (BP8601 pro, EngineeringForLife, Suzhou, China) for 20 s using cylinder with height of 12 mm and bottom of 5 mm (for in vitro experiment) and 1.2 mm radius sphere template (for in vivo experiment) in PotatoP software.
2.3. Characterization of GelMA-liposome system
Morphological and mechanical characterization of GelMA-liposome was performed using Tescan MIRA scanning electron microscopy (SEM; Brno, Chech), JEM-1400FLASH transmission electron microscope (TEM; Tokyo, Japan), and Dimension icon atomic force microscopy (AFM; Bruker, Germany). The solidified GelMA-liposome was detected by Thermo Nicolet iS20 Fourier transform infrared spectroscopy (FTIR; MA, USA).
2.4. Cell culture
The human glioblastoma cells (U251, LN229) were obtained from iCell Bioscience (Shanghai, China). Mouse glioblastoma cells (GL261) were obtained from JiHe biotechnology (Shanghai, China). Normal human astrocytes (NHA) were purchased from the Sciencell Research Laboratories (Carlsbad, CA, USA). The TMZ-resistant variant U251TR, LN229TR and GL261TR were established in our laboratory by repetitive exposure to TMZ with increasing concentrations (from 50 to 500 µM) for 6 months. To maintain the TMZ-resistant phenotype, U251TR, LN229TR and GL261TR were alternately treated with drug-free medium and TMZ medium (500 µM) for 72 h. The cells were cultured with Dulbecco's Modified Eagle's Medium (BI, NY, USA) and incubated in a humidified incubator with a 5% CO2 atmosphere at 37 °C. The cells number was counted by automated cell counter IC1000 (Shanghai, China). Culture medium was supplemented with 10% fetal bovine serum (BI, NY, USA), 100 µg/ml streptomycin and 100 Units/ml penicillin (BI, NY, USA).
2.5. In vitro release and cellular uptake of GelMA-liposome system
Fluorescence dye Cy5.5 was utilized to fabricate GelMA-liposome system. Cy5.5@LPs-cRGD+GelMA was placed in a 6-well plate to evaluate the in vitro release efficiency. The fluorescence densities at different time point were measured via IVIS® (in vivo imaging system) Spectrum (PerkinElmer, MA, USA).
A transwell model was used to assess the cellular uptake efficiency. In brief, U251TR, LN229TR and NHA cells were cultured in the bottom surface of 24-well plates, and Cy5.5@LPs+GelMA and Cy5.5@LPs-cRGD+GelMA was placed in the upper transwell chambers (Corning star, MA, USA). The GelMA-liposome system was completely immersed in medium. After co-culture for 2 h, GelMA-liposome system was removed, the cells were stained with Hoechst (Solarbio, Beijing, China) and F-actin (abcam, Shanghai, China) dye and observed under fluorescence microscope.
2.6. Cell proliferation/viability assay
The cell proliferation ability was assessed by utilizing Cell-Light™ EdU Apollo567 In Vitro Imaging Kit. The cells after treatment were seeded at 1 104 cells/well in 96-well plates. After fluorescent labeling for 24 h, fluorescence microscope (Olympus 1×71, Tokyo, Japan) was utilized to visualize EdU-positive cells. The live/dead cell assay was assessed by Calcein-AM/PI double dye kit (Yuanye, Shanghai, China) according to the instructions.
Cell viability was determined by utilizing The Cell Counting Kit-8 (CCK-8). Cells seeded at 1 × 103 cells/well in 96-well plates (Corning, NY, USA) were incubated with 10 µl CCK-8 solution and 100 µl medium at 37 °C for 1 h. The results were measured with Infinite M Nano (Tecan, Switzerland) at optical density (OD) 450 nm.
2.7. Apoptosis assay
Cell apoptosis was explored by utilizing Annexin V-FITC Apoptosis Detection Kit. The cells were seeded in 6-well plates at 2 × 105 cells/well. After treatments with blank group or different liposome-GelMA systems, the cells were collected by 0.25% EDTA-free trypsin (Servicebio, Wuhan, China) and washed 3 times with 4 °C PBS (Servicebio, Wuhan, China). Then, cells were incubated with Annexin V-FITC and PI dye for 10 min at room temperature. The ratios of FITC/PI staining were recorded by Cytoflex S (Beckman coulter, CA, USA).
2.8. Wound-healing and transwell assay
The migration ability was assessed with wound-healing assay. After treatments with blank group or different liposome-GelMA systems, the cells were seeded in 6-well plates at 100% confluence. The scratching step was conducted vertically by 200 µl pipettes on the center of each well. The pictures of scratch region were taken at different time points on the microscope at 200×, and the area of scratch was measured by Image J (v 1.8.0).
The invasive ability was assessed by performing Transwell assay. After treatments with blank group or different liposome-GelMA systems, the cells were seeded in the upper transwell chambers with 8 µm pores in the 24-well plates at 2 × 103 cells/well. After 12 h incubation, 4% paraformaldehyde (Biosharp, Hefei, China) was utilized to fix the migrated cells on the membrane of lower chamber. Then, the cells were stained by 0.25% crystal violet dye solution (Servicebio, Wuhan, China), and the numbers of migrated cells were counted at 200× magnification using microscope in five randomly selected fields.
2.9. Ferroptosis evaluation
The lipid peroxidation level was evaluated by conducting Malondialdehyde (MDA) assay (Beyotime, Shanghai, China). MDA can react with thiobarbituric acid (TBA) under acidic and high temperature conditions to produce a brownish red trimethyl (3,5,5-trimethyloxazole-2,4‑dione) with a maximum absorption wavelength of 532 nm. The lipid peroxidation levels of lysed cells were detected as MDA reacting with TBA. The results of MDA level were presented as nmol/mg prot.
Oxidative glutathione (GSSG) is reduced to reduced glutathione (GSH) by glutathione reductase, and GSH can react with chromogenic substrate DTNB to produce yellow TNB and GSSG. The redox status was indicated by GSH/GSSG assay kit (Beyotime, Shanghai, China). 5×104 cells were lysed, and the amounts of GSH and GSSG were measured according to the instructions of manufacturer.
Mitochondrial membrane potentials (ΔΨm) were assessed by enhanced JC-1 assay kit (Beyotime, Shanghai, China). JC-1 monomers and aggregates were recorded by fluorescent microscope and flow cytometry according to the instructions of manufacturer. The ratio of red/green fluorescent densities were calculated.
2.10. Quantitative reverse transcription PCR (qRT-PCR) assay
The total RNA was extracted by RNA-easy™ Isolation Reagent. The concentration and purity of RNA were determined by NanoDrop One/OneC Spectrophotometer (Thermo Scientific, DE, USA). 1 µg RNA was reverse transcribed into cDNA utilizing cDNA synthesis kit (Transgen, Beijing, China). qRT-PCR was performed by using TransStart Tip Green qPCR SuperMix Kit (Transgen, Beijing, China) and CFX Connect Real-time PCR System (Bio-rad, CA, USA). Relative gene expression levels were analyzed with the normalizing data of threshold cycles. Experimental related primers were designed by General Biol (Anhui, China) and were listed at Supplementary Table S3.
2.11. Western blot assay
Total proteins of cells were extracted by the RIPA lysis buffer (Beyotime, Shanghai, China) containing 1:1,000 protease/phosphates inhibitors (Apexbio, TX, USA). BCA Protein Quantification Kit (Vazyme, Jiangsu, China) was used to measure the concentration of proteins. 25–50 µg proteins mixing with loading buffer (Epizyme, Shanghai, China) were separated by 7.5%−12.5% SDS-PAGE (Epizyme, Shanghai, China) gel electrophoresis. The proteins were then transferred to PVDF membranes (Millipore, MA, USA), blocked with 5% nonfat milk (Cell signaling technology, MA, USA) for 2 h and incubated with primary antibodies at 4 °C overnight. The PVDF membranes were subsequently incubated with horseradish peroxidase-conjugated secondary antibodies for 2 h. The proteins were visualized using ultra-sensitive chemiluminescence (ECL) reagent (Uubio, Jiangsu, China) and detected by image analysis system Tanon 4800 (Tanon, Shanghai, China). Anti-GAPDH/β-actin antibody was used for normalization. The detailed antibody information was displayed in Supplementary Table S4.
2.12. Animal experiments
Male C57BL/6 J and NU/NU mice (4–6 weeks old) were purchased from Charles River (Beijing, China) and were assigned randomly to different groups (n = 5 each group). Experimental cell line luciferase-expressing GL261TR/U251TR was established through lentivirus (constructed by Cyagen, Guangzhou, China) infection and puromycin (Solarbio, Beijing, China) selection. The partial craniectomy of the dextral frontal and parietal bone was performed in advance. The stereotactic injection of GL261TR/U251TR-luciferase cells (2.5 × 106 cells in 5 µl) was performed at the location where 2 mm lateral and 2 mm posterior to the bregma. The orthotopic injection was conducted at depth of 2 mm from pia mater with speed of 0.5 µl per 15 s (total 5 µl). The microsyringe was left for 2 min and withdrawn with speed of 1 mm every minute. At Day 7 post-inoculation, the surgical resection of tumor was operated under 20× microscope (Olympus SZX16, Tokyo, Japan) using 1.0 mm excavators spoon (Majestic, WY, United Kingdom), and ∼7 µl hydrogel-liposome system was implanted into the resection cavity. Then, α-cyanoacrylate medical adhesive (508 Baiyun EC type, Guangzhou, China) was utilized for wound closure. Two mice in each group were euthanized for H&E stain and immunohistochemistry stain (IHC) at Day 25 post-inoculation, and three mice were further monitored for weight and survival assessment. Tumor growth was evaluated at the Day 6, 12 and 24 via IVIS® Spectrum.
All the animals were housed in the laboratory animal center of Cheeloo College of Medicine, Shandong University. The animal experimental procedures were carried out with permission of the Animal Care Committee of Cheeloo College of Medicine.
2.13. Immunofluorescence and immunohistochemistry
Immunofluorescence (IF) analysis was performed using paraffin-embedded brain sections. After fixation, tissue sections were permeabilized with 0.1% Triton X-100 (in PBS) and then blocked with 3% BSA. Thereafter, brain sections were probed with primary antibody PD-L1 (1:500) and followed by the incubation with secondary antibody and DAPI. Image acquisition was performed on fluorescence microscope.
Immunohistochemistry (IHC)staining was performed by using Immunohistochemistry Kit (D601037–0050, Sangon Biotech, Shanghai, China). After antigen retrieval, the brain sections were blocked with BSA and then probed with 50 µl primary antibody ki-67 (1:200) overnight at 4 °C. Thereafter, samples were probed with 25 µl enzyme-labeled secondary antibody for 30 min at 37 °C. The H scoring of brain section was automatically measured by Aipathwell software ranging from 0 to 300.
2.14. Statistical analysis
Data was presented as the mean ± standard deviation (SD). GraphPad Prism 9 (La Jolla, CA, USA) was utilized for the statistical analyses and figure productions. P-values less than 0.05 were considered to be statistically significant (*P < 0.05, **P < 0.01, and ***P < 0.001).
3. Results and discussion
3.1. Characterization of GelMA and cRGD-liposomes
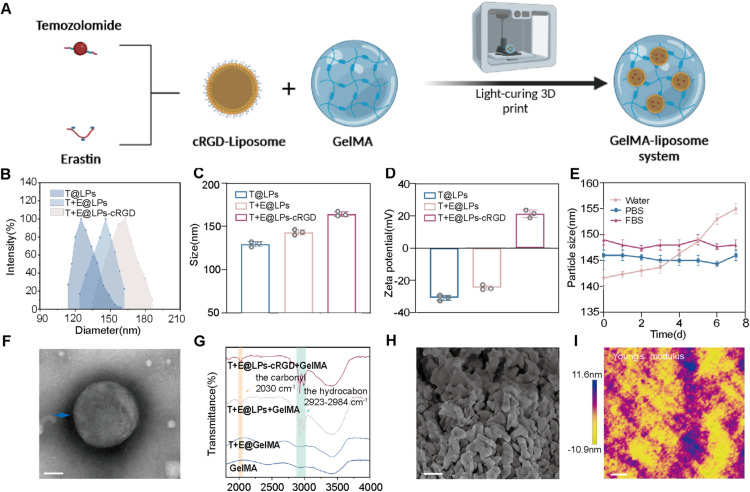
We developed TMZ and ERA co-encapsulated GelMA-liposome nanocarriers (Fig. 2A). DSPE-PEG2000-cRGD was syn-thesized and confirmed by HNMR (=H Nuclear Magnetic Resonance) spectrum assay and was utilized as a targeting ligand to modify the liposomes (Fig. S1). The combo drugs TMZ and ERA were encapsulated into the cRGD-decorated liposomes. The size and zeta-potential of different liposomes were shown in Fig. 2B-2D. Similar hydrodynamic size with uniform distribution was observed in different liposomes, demonstrating that cRGD functionalization has minor effect on the particle size. The zeta potential difference between T+E@LPs-cRGD and other liposomes is mainly attributed to the positively charged peptide cRGD (Fig. 2D). The stability of liposome nanocarrier was assessed. T+E@LPs-cRGD remained stable in PBS and in fetal bovine serum (FBS) (Fig. 2E). The TEM images showed a spherical nanostructure morphology of cRGD-liposomes (Fig. 2F). The TEM images in multiple liposomes of T@LPs, T+E@LPs and T+E@LPs-cRGD were in Fig. S2. Drug EE of TMZ and ERA in different liposomes were shown in Table S1. DLC for both drugs was more than 3%. As shown in Fig. 2G, two characteristic vibrational bands ( -CH, 2923–2984 cm−1; C = O, 2030 cm−1) were identified in the spectrum of liposomes-loaded GelMA, which proved the encapsulation of liposomes (which possess a large content of DSPE) and suggested a generation of strong chemical binding between GelMA and liposomes. The high-resolution SEM image indicated that the GelMA hydrogel had smooth structure (Fig. 2H). The SEM image of GelMA was in Fig. S2. The mechanical characterization of T+E@LPs-cRGD+GelMA was performed using AFM. T+E@LPs-cRGD+GelMA exhibits an overall average Young's modulus of approximately 500 kPa (Fig. 2I).
Fig. 2.
Characterization of T+E@LPs-cRGD+GelMA: (A) Schematic illustration of the preparation of T+E@LPs-cRGD+GelMA. (B) Size distributions of T@LPs, T+E@LPs and T+E@LPs-cRGD. Size distributions (C) and zeta potential (D) of each nanoformulation. (The data are shown as means ± SD, n = 3) (E) Dispersibility and stability of T+E@LPs-cRGD+GelMA in water, PBS and FBS. (The data are shown as means ± SD, n = 3) (F) TEM image of T+E@LPs-cRGD. Scale bar: 100 nm. (G) FTIR of GelMA, T+E@GelMA, T+E@LPs+GelMA and T+E@LPs-cRGD+GelMA. The infrared peaks at 2030 cm−1 and 2923–2984 cm−1 were identified. (H) SEM image of T+E@LPs-cRGD+GelMA. Scale bar: 1 µm (I) Young's modulus analysis for T+E@LPs-cRGD+GelMA. Scale bar: 5 µm.
3.2. Sustained release and cellular uptake of GelMA-liposome platform
The hydrogel and liposome remained stable in the PBS containing 5% FBS. Their release profiles were evaluated in vitro and the remaining mass of hydrogel system indicated that the gel degradation could last more than 3 weeks (Fig. 3A). The cumulative release of TMZ and ERA was assessed by measuring drug concentrations with spectrophotometer. As shown in Fig. 3B, both two drugs exhibited a sustained release manner that attributed to the slow degradation of GelMA and liposome. And the release profile in liposomes without the hydrogel was in Fig. S3. The detection of maximum absorption wavelength of both two drugs were presented in Fig. S4. Fluorescence dye (Cy5.5) was encapsulated in the liposomes to evaluate release and cellular uptake efficiency. The results verified a sustained and slow release of fluorescence from Cy5.5@LPs-cRGD+GelMA to PBS for a duration of more than 14 d in vitro (Fig. 3C). Moreover, the fluorescent GelMA-liposome platform was implanted in C57BL/6 J mouse, and the imaging results suggested that the delivery system could maintain proper stability in situ (Fig. 3D). Tumor cells U251TR/LN229TR and normal astrocyte cells NHA were used to investigate the targeting ability of GelMA-liposome platform. Owing to the overexpressed αvβ3 integrin in tumor cells that cRGD could specifically associate with, Cy5.5@LPs-cRGD+GelMA possessed a higher efficiency of fluorescence accumulation in U251TR and LN229TR (Fig. 3E&3F). Additionally, no significant differences of cellular uptake were observed in NHA cell line between control liposome and cRGD-decorated liposome, further suggesting that cRGD peptide could effectively promote tumor targeting (Fig. 3G). Chloroquine (CQ) is an endocytosis blocking agent. Pre-treatment of cells with CQ reduced the cellular uptake of fluorescence dye substantially, indicating that the internalization of Cy5.5@LPs-cRGD+GelMA is primarily mediated by clathrin-mediated endocytosis (Fig. 3H).
Fig. 3.
Release and uptake of T+E@LPs-cRG+GelMA: (A) Remaining mass of the hydrogel system in vitro. (The data are shown as means ± SD, n = 3 independent experiments.) (B) Release profile of each agent from the hydrogel. (The data are shown as means ± SD, n = 3) (C) Release efficiency of LPs-cRGD@GelMA through measuring the fluorescence intensity of Cy5.5 dye in PBS. (D) Release efficiency of LPs-cRGD@GelMA through measuring the fluorescence intensity of Cy5.5 dye by IVIS®. (E, F and G) Uptake of T+E@LPs-cRGD+GelMA by U251TR cells, LN229TR cells and NHA cells observed by fluorescence microscope. Blue, cell nuclei stained with Hoechst; Green, cytoskeleton stained with F-actin; Red, liposome stained with Cy5.5@LPs-cRGD+GelMA. Scale bar: 20 µm. (H) Cellular uptake of T+E@LPs-cRGD+GelMA by U251TR cells in the presence of intracellular uptake inhibitor CQ in 5 µM and 10 µM as measured through flow cytometry analysis.
3.3. Synergistic anti-tumor functions of GelMA-liposome platform
U251 and LN229 TMZ-resistance (TR) cell lines were established by repetitive TMZ exposure. The resistance index (RI) of U251TR and LN229TR were 4.07 and 3.47, respectively. The dose-response curves of TMZ and ERA in U251/LN229 cell lines were presented in Fig. S5. The combinatorial synergistic effect of free TMZ and ERA was analyzed by CompuSyn (Table S2). The calculated combination index (CI) value after treatment with escalating doses (at 40:1 ratio) of TMZ and ERA was 0.67 to 0.73 for the fraction inhibition of Fa=0.5∼0.9, demonstrating a synergistic cytotoxic effect against U251TR and LN229TR (Fig. 4A&4B). We initially investigated the combinatorial anti-tumor effects of GelMA-liposome by EdU proliferation assays. The results showed that the proliferation of GBM cells was partially impeded after treatment of T@LPs-cRGD+GelMA and E@LPs-cRGD+GelMA, and the inhibition effect was more significant in T+E@LPs-cRGD+GelMA group (Fig. 4C-4E). We further explored the cell viability via live/dead cells stain and Annexin V/PI apoptosis assay. These results suggested that T+E@LPs-cRGD+GelMA induced superior cytotoxicity against TMZ-resistant cells U251TR and LN229TR (Fig. 4F&4G and S6&S7). Additionally, the Transwell and scratch wound healing assays showed that T+E@LPs-cRGD+GelMA significantly diminished the migration and invasion abilities of U251TR and LN229TR comparing with other groups (Fig. 4H-4M). And as comparison, the Transwell and scratch wound healing assays in LN229 were in Fig. S8. Collectively, these in vitro results indicated the great potential that T+E@LPs-cRGD+GelMA system have for overcoming the challenges of drug resistance, and demonstrated the superiority of GelMA-liposome platform in targeting tumor cells and inhibiting GBM.
Fig. 4.
Effect of T+E@LPs-cRGD+GelMA on synergistic anti-tumor: (A and B) Effect of escalating doses of TMZ, ERA and TMZ+ERA on the viability of U251TR and LN229TR cells. (C) Proliferation rate of U251TR cells and LN229TR cells treated with T@LPs-cRGD+GelMA, E@LPs-cRGD+GelMA and T+E@LPs-cRGD+GelMA for 48 h, as measured using the EdU (red) assay. The nuclei were stained with DAPI (blue). Scale bar: 100 µm. (D and E) Graphical representation of the ratios of EdU-positive U251TR and LN229TR cells treated with each formulation (The data are shown as means ± SD, n = 3) (F) Microscopy images of Live/Dead staining of U251TR cells and LN229TR cells treated with each formulation after 48 h. T+E@LPs-cRGD+GelMA significantly reduced the density of live cells (green) and increased the number of dead cells (red). Scale bar: 100 µm. (G) Apoptosis assay 48 h after treatment with each formulation in U251TR and LN229TR cells. Annexin V-FITC (-)/PI (-) cells were alive. Annexin V-FITC (+)/PI (-) cells were considered in the early stage of apoptosis, while Annexin V-FITC (+)/PI (+) cells were in the late stage. Annexin V-FITC (-)/PI(+) cells were necrotic. FITC, fluorescein isothiocyanate. (H) The migration ability of U251TR and LN229TR cells treated with each formulation. Scale bar: 100 µm. (I and J) Statistical chart of the number of transmembrane cells in transwell analysis. (The data are shown as means ± SD, n = 3) (K, L and M) Representative images (K) and quantification of scratch wound healing assays in U251TR cells (L) and LN229TR cells (M). Scale bar: 200 µm. (The data are shown as means ± SD, n = 3) *P < 0.05; **P < 0.01; ***P < 0.001.
3.4. Ferroptosis induced by GelMA-liposome platform
It is acknowledged that ERA mediates the ferroptosis of cancer cells by regulating xCT and GPX4 pathway [29,30]. Therefore, we attempted to determine whether the resistance reversal and enhanced anti-tumor effects of GelMA-liposome platform were associated with ferroptosis. Western blot analysis showed that treatment of U251TR and LN229TR with T@LPs-cRGD+GelMA and E@LPs-cRGD+GelMA for 48 h could reduce the protein levels of GPX4, xCT and ferritin, and the inhibition effect was more significant in T+E@LPs-cRGD+GelMA group (Fig. 5A-5C). Additionally, the transcriptional expression levels of those ferroptotic genes assessed by qRT-PCR showed consistent results (Fig. 5D). MGMT (O-6-methylguanine-DNA methyltransferase) represents the most substantial factor that compromises the therapeutic efficacy of TMZ [31]. p53 is a central tumor suppressor protein and can be activated by ERA [32]. After utilization of T+E@LPs-cRGD+GelMA system, the MGMT protein expression was significantly inhibited and the p53 expression was enhanced (Fig. 5E&5F). These results demonstrated the promising potential of GelMA-liposome system in reversing drug resistance and suppressing GBM. As previously described, the accumulation of cellular ROS, reduced GSH level and augmented lipid peroxidation are the most typical characteristics of ferroptosis. Therefore, the GSH and MDA levels were also evaluated after treatment of GelMA-liposome system. The results showed that the E@LPs-cRGD+GelMA and T+E@LPs-cRGD+GelMA system could potently decrease the cellular GSH levels and increase the MDA levels (lipid peroxidation) in U251TR and LN229TR (Fig. 5G&5H). The changes in mitochondrial membrane potential (JC-1) and ROS level were evaluated as well (Fig. 5I-5L), suggesting the accumulated oxidative stress occurred in TMZ-resistant GBM cells. Additionally, the TEM images of U251TR and LN229TR showed that GelMA-liposome system could trigger mitochondrial atrophy, decreased mitochondrial cristae, and increased membrane density (Fig. 5M). Collectively, these results elucidated that T + E@LPs-cRGD+GelMA could significantly induce the ferroptosis of GBM cells, highlighting the involvement of ferroptosis in the synergistic anti-tumor effects and the reversal of TMZ resistance.
Fig. 5.
The ferroptosis induced by T+E@LPs-cRG+GelMA: (A, B and C) Effect of each formulation on the protein expression of GPX4 (A), xCT (B) and ferritin (C) in U251TR cells. ) (D) qRT-PCR analyses of GPX4, SLC7A11 and ferritin in U251TR cells following treatment with each formulation. ) (E and F) Effect of each formulation on the protein expression of MGMT (E) and p53 (F) in U251TR cells.) (G) MDA levels in U251TR cells and LN229TR cells treated with each formulation for 48 h. (H) GSH levels in U251TR cells and LN229TR cells treated with each formulation for 48 h. (I and K) Representative images (I) and quantification (K) of JC-1 in U251TR cells and LN229TR cells treated with each formulation for 48 h. Red JC-1 aggregates represent normal mitochondrial membrane potential; Green JC-1 monomers represent depolarized mitochondrial membrane potential. Scale bar: 100 µm. (J and L) Representative images (J) and quantification (L) of ROS production in U251TR cells and LN229TR cells treated with each formulation for 48 h. Green fluorescence (DCF) indicates a dramatic increase in cellular reactive oxygen species. Scale bar: 100 µm. (M) TEM images of U251TR cells and LN229TR cells treated with T+E@LPs-cRGD+GelMA. The scale bar of the top image is 2 µm, and of the bottom image is 1 µm. *P < 0.05; **P < 0.01; ***P < 0.001. (All datas are shown as means ± SD, n = 3).
3.5. Immunoregulatory functions of GelMA-liposome platform
To investigate the underlying mechanisms that T+E@LPs-cRGD+GelMA involved in, transcriptome sequencing analysis was performed in U251TR cells (Fig. 6A). The differentially expressed genes were illustrated and presented in volcano map (Fig. 6B). As shown in Fig. 6C, the cluster analysis of top 20 biological process (BP) was significantly enriched in immune system. The differential expressions (DEs) analyzed by Metascape (https://metascape.org/) were mainly enriched in Interferon Signaling (R-HSA-913,531) and type II interferon-mediated signaling pathway (GO:0,060,333). Therefore, we shifted in focus to the exploration of interferon-γ (IFN-γ) signaling pathway in GBM. IFN-γ is a secreted factor that can be generated by most nucleated cells (including tumor cells and immune cells) [33]. IFN-γ receptors IFNGR1 and IFNGR2 are supposed to be responsible for inducing intracellular cascades (JAK-STAT signaling pathway) and mediating anti-tumor immunity. Recent advances have proposed that altered IFNGR1/2 protein could affect the integrity of IFN-signaling and thereby influencing immunotherapy sensitivity [34,35]. The correlation between GBM clinicopathological characteristics (overall survival) and IFN signaling receptor was analyzed by CGGA database (http://www.cgga.org.cn/). As shown in Fig. 6D&6E, the transcriptional expression levels of IFNGR1/2 were negatively correlated with survival probability, suggesting that IFN signaling pathway is essential to GBM tumorigenesis. qRT-PCR and western blot assays were conducted for the further validation of DEs. The result showed that the constitutive expressions of IFNGR1/2 were significantly inhibited after co-culture with GelMA-liposome (Fig. 6F&6 G). Additionally, the Elisa assay of supernatant showed that the tumoral secretion of IFN-γ was impeded by GelMA-liposome system (Fig. 6H). PD-L1 is an immune inhibitory receptor ligand expressed on tumor cells and is well acknowledged as a negative prognostic indicator for GBM. Intriguingly, emerging advances demonstrated that the activation of IFN signaling pathway was associated with PD-L1 axis and immune escape in GBM [36], [37], [38]. Therefore, we evaluated the PD-L1 (programmed death-ligand 1) levels in U251TR and LN229TR utilizing western blot and immuno-fluorescent flow cytometry. The results showed that T@LPs+GelMA could enhance the PD-L1 expression while T+E@LPs-cRGD+GelMA reversed the elevated expression level of PD-L1, which was consistent with the tendency of IFN signaling (Fig. 6I&6J). Additionally, after treatment of GelMA-liposome platform, immunolysis assay indicated that the induced immunogenic cell deaths in U251TR and LN229TR were increased (Fig. 6K). These results demonstrated the immunoregulatory function of GelMA-liposome, which reversed the immune evasion of GBM in microenvironments.
Fig. 6.
Functions of T+E@LPs-cRG+GelMA on immunoregulatory: (A) Hierarchical clustering of differentially expressed genes in U251TR and U251TR cells treated with T+E@LPs-cRGD+GelMA after 48 h. The significance criteria for DEGs were set as P < 0.05 and |log2FC|>1. (B) Volcano plot of differentially expressed genes in U251TR cells treated with T+E@LPs-cRGD+GelMA. The DEGs associated with IFN signaling were marked. Yellow dots indicate significantly up-regulated genes, and blue dots indicate down-regulated genes. (C) The top 20 biological enrichment analyses of gene ontology in U251TR cells treated with T+E@LPs-cRGD+GelMA after 48 h. (D and E) Kaplan–Meier analysis of the correlation between overall survival with IFN signaling receptor IFNGR1 and IFNGR2. (F) Effect of each formulation on the protein expression of IFN-γ in U251TR cells. (G) qRT-PCR analyses of IFN-γ in U251TR cells following treatment with each formulation (The data are shown as means ± SD, n = 3) (H) The content of IFN-γ in U251TR cell supernatant following treatment with each formulation. (The data are shown as means ± SD, n = 3) (I) Effect of each formulation on the protein expression of PD-L1 in U251TR cells. (J) PD-L1 levels in U251TR cells treated with each formulation for 48 h as measured through immuno-fluorescent flow cytometry analysis (The data are shown as means ± SD, n = 3). (K) Immunolysis assays of each formulation. TCL cells were used in a 4 h immune cell lysis assay with U251TR cells and LN229TR cells, treated with each formulation for 48 h, as target cells. *P < 0.05; **P < 0.01; ***P < 0.001.
3.6. Therapeutic efficacy of GelMA-liposome platform in vivo
The therapeutic potential of T+E@LPs-cRGD+GelMA was assessed in intracranial U251TR C57BL/6 J and NU/NU mouse model. We applied a modified method to effectively expand the surgical field for tumor resection, and the partial craniectomy of mouse model was performed in advance. As schedule illustration presented in Fig. 7A, at Day 7 post tumor inoculation, GelMA-liposome was implanted into the intracavity after surgical debulking. Tumor responses were observed as tumor fluorescence intensity/tumor size (Fig. 7B&7C and S4), body weight loss (Fig. 7D) and survival time (Fig. 7E). These results collectively showed that the mice treated with T+E@LPs-cRGD+GelMA exhibited significant GBM retardation compared to other groups. Notably, the median survival time of tumor-bearing mice in T+E@LPs-cRGD+GelMA group was 76.5 d, which was significantly longer than that of the mice in NC group (27 d, P<0.0001), T@GelMA group (38.5 d, P<0.0001), T+E@GelMA group (53.5 d, P<0.0001), and T+E@LPs@GelMA group (66.5 d, P<0.05). Moreover, H&E staining results showed that the recurrent tumor size in GelMA-liposome group was smaller than that of in NC group at end point (Fig. 7F). Indubitably, the combination of TMZ and ERA achieved a satisfactory synergistic anti-tumor effect, and the structural combination of hydrogel and liposome played an indispensable role in extending drug release, inhibiting tumorigenesis, and alleviating relapse. Immunohistochemistry (IHC) staining of ki-67 was assessed in different groups. The H-score of T+E@LPs-cRGD+GelMA group was significantly lower than that of in NC group, indicating that the proliferation of GBM cells was profoundly inhibited (Fig. 7G). The immunofluorescence staining of PD-L1 in recurrent GBM tissue was assessed in GL261TR-bearing C57BL/6 J mice. The PD-L1 expression was slightly enhanced after TMZ exposure, and the utilization of GelMA-liposome system inhibited PD-L1 expression potently (Fig. 7H). These results were consistent with the corresponding protein expression levels verified in vitro.
Fig. 7.
Therapeutic efficacy of T+E@LPs-cRG+GelMA on postoperative recurrent GBM in mice: (A) Schematic illustration of the animal experimental design. (B) Quantification of the bioluminescence in tumor-bearing mice. Statistical significance was calculated using a two-way ANOVA. (C) The inhibitory effect of each formulation on the growth of postoperative recurrent GBM observed by in vivo bioluminescence imaging. (D) Changes in the bodyweight of mice during the treatment process. (The data are shown as means ± SD, n = 5) (E) Kaplan-Meier survival curves of mice after each treatment. Data were analyzed by using the log-rank (Mantel-Cox) test. (F) H&E staining of the brain tissue from each group. (G) Representative images of IHC staining for Ki-67 in mouse brain sections. Scale bar: 50 µm. (H) The protein expression of PD-L1 level in GBM tissue observed by immunofluorescence staining. Scale bar: 20 µm. *P < 0.05; **P < 0.01; ***P < 0.001.
3.7. Discussion
Currently, nearly all GBM will eventually progress or relapse and there is no standard therapy for recurrent GBM [39]. Several studies [30,40] have shown that ferroptosis inducer ERA has a great prospect in treatment of GBM. However, the solubility of ERA in conventional solvents is extremely poor and thereby limits its further application. With remarkable advancements in nanotechnology, the liposomes characterized with high biocompatibility and low immunogenicity have been widely used as drug delivery systems [41], [42], [43]. Gelatin is recognized to be safe (GRAS) by FDA [20]. The GelMA hydrogel is characterized with excellent biocompatibility and has been applied in various fields with no safety issues. The application of GelMA includes cell culture, tissue regeneration, tumor therapy, etc. [44], [45], [46] In this study, we utilized hydrogel-liposome system to encapsulate combo drugs TMZ/ERA, which reduced the administrated dosage of TMZ and alleviated the side effects comparing with intravenous route. Liposomes modified with cell penetrating peptide cRGD were developed for targeting αvβ3 integrins that were overexpressed in GBM cells. After surgical debulking, the intracranial implantation of GelMA-liposomes nanoplatform realized superior properties including sustained release and GBM targeting. These results evidently suggested that the hydrogel-liposome nanoplatform (T+E@LPs-cRGD+GelMA) might be a potential strategy for GBM therapy.
TMZ is an alkylating agent inducted as the first-choice chemotherapeutic drug for GBM therapy. A majority of patients develop TMZ resistance during the course of treatment since several DNA repair pathways (such as mismatch repair, base excision repair and double-strand break repair) are activated to restore genomic integrity [47]. Pharmacological or genetic regulation of ferroptosis has been demonstrated as a novel approach to reversing chemotherapy resistance [48], [49], [50]. In this study, ferroptosis inducer ERA was applied, and the canonical xCT/GPX4-regulated pathway was inhibited, which played an essential role in reversing TMZ resistance. In order to explore whether ERA has an underlying mechanism to overcome drug resistance other than the ferroptosis pathway, we utilized target identification server PharmMapper (http://www.lilab-ecust.cn/pharmmapper/) to find downstream factors. Notably, the most significant binding candidate for both 2D and 3D structure of ERA is Acyl-CoA Dehydrogenase Very Long Chain (ACADVL), which mediates the β-oxidation step of Acetyl-CoA (Fig. S10). The western blot and qRT-PCR results suggested that the protein and RNA expression levels of ACADVL were decreased after the application of ERA (Fig. S11A&S11B). Elisa analysis showed that the cellular Acetyl-CoA level was significantly reduced by ERA (Fig. S11C). Since epigenetic modifications have potent impacts on adaptive drug resistance [51], we assume that the TMZ resistance reversing induced by ERA may be associated with the altered acetylation levels of intracellular proteins. This supposition still needs further deep investigations.
PD-L1 (B7-H1, CD274) is a coinhibitory ligand expressed predominantly on tumor cells and act as a shield to protect tumor from T cell-mediated elimination [52]. The upregulation of PD-L1 on tumor cells is associated with immune escape and thus favors the growth of GBM [53]. TMZ can trigger STAT3 activation, which acts as a key transcription factor to promote the expression of PD-L1 [54]. Recent advance showed that the inhibition of PD-L1 expression in GBM by hydrogel could ameliorate tumor immune microenvironment and exert anti-tumor effects [55]. Extrinsic induction mediated by T cell-secreted IFN has been considered as the primary reason accounting for PD-L1 overexpression. More recently, autocrine activation of the IFN signaling pathway has been reported, indicating the significance of constitutive IFN signaling to modulate immune microenvironment in GBM cells. Fascinatingly, our high throughput sequencing demonstrated that GelMA-liposome nanoplatform contributes to the loss of IFN signaling pathway and PD-L1 expression in TMZ resistant cells, thus realized the combined chemo-inmmunotherapy of GBM. p53 is a central tumor suppressor protein and can be activated by ERA [56]. It is suggested that both constitutive and IFN-induced PD-L1 protein expression levels were negatively regulated by p53 [57]. Collectively, the hydrogel-liposome nanoplatform participated in the regulation of GBM immune microenvironment via IFN/PD-L1 pathway.
4. Conclusion
Given the challenges associated with ineffective drug delivery and extensive chemotherapeutic resistance in GBM, we developed a novel 3D-print hydrogel-liposome nanoplatform (T+E@LPs-cRGD+GelMA) for intracranial implantation. As a biocompatible drug reservoir, cross-linked GelMA and cRGD-coated liposome realized the sustained release of internal contents. The liposomes coated with cRGD peptide increased the solubility of ERA significantly and played an essential role in GBM targeting. Taken together, the in situ implantation method could profoundly reduce systemic side effects comparing to traditional administration route. We confirmed that the synergistic effects of TMZ and ERA combination were persevered after encapsulated in liposomes and hydrogel. The chemosensitivity to TMZ was ameliorated and the synergistic anti-tumor effects were observed in TMZ-resistance cell lines U251TR and LN229TR after the application of ferroptosis inducer ERA. On account of our findings, GelMA-liposome system could effectively induce ferroptosis and modulate tumor immune microenvironment via interferon/PD-L1 signaling pathway, which played significant roles in intervening. In summary, the GelMA-liposome nanoplatform showed significant potentials for anti-GBM therapy, which offered great promise for the prospective clinical translation.
Conflicts of interest
The authors declare that they have no competing interests.
Acknowledgements
This work was supported by Natural Science Foundation of China (Grant NO. 81972340, 82173140, 81871196), Shandong Provincial Natural Science Foundation, China (Grant No. ZR202010300086), Academic promotion program of Shandong First Medical University (Grant NO. 2019LJ005). We appreciate Translational Medicine Core Facility of Shandong University for consultation and instrument availability that supported this work.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajps.2023.100800.
Contributor Information
Tao Xin, Email: xintao@sdfmu.edu.cn.
Qian Liu, Email: cardioqian@sdu.edu.cn.
Appendix. Supplementary materials
References
- 1.Wen P.Y., Weller M., Lee E.Q., Alexander B.M., Barnholtz-Sloan J.S., Barthel F.P., et al. Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 2020;22(8):1073–1113. doi: 10.1093/neuonc/noaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li H., Liu Q., Chen Z., Wu M., Zhang C., Su J., et al. Hsa_circ_0110757 upregulates ITGA1 to facilitate temozolomide resistance in glioma by suppressing hsa-miR-1298-5p. Cell Death Dis. 2021;12(3):252. doi: 10.1038/s41419-021-03533-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen J., Li Y., Yu T.S., McKay R.M., Burns D.K., Kernie S.G., et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488(7412):522–526. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi S., Yu Y., Grimmer M.R., Wahl M., Chang S.M., Costello J.F. Temozolomide-associated hypermutation in gliomas. Neuro Oncol. 2018;20(10):1300–1309. doi: 10.1093/neuonc/noy016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Z., Ji X., He D., Zhang R., Liu Q., Xin T. Nanoscale drug delivery systems in Glioblastoma. Nanoscale Res Lett. 2022;17(1):27. doi: 10.1186/s11671-022-03668-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y., Zhang L., Hu Y., Jiang K., Li Z., Lin Y.Z., et al. Cell-permeable NF-kappaB inhibitor-conjugated liposomes for treatment of glioma. J Control Release. 2018;289:102–113. doi: 10.1016/j.jconrel.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 7.Sun X., Pang Z., Ye H., Qiu B., Guo L., Li J., et al. Co-delivery of pEGFP-hTRAIL and paclitaxel to brain glioma mediated by an angiopep-conjugated liposome. Biomaterials. 2012;33(3):916–924. doi: 10.1016/j.biomaterials.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 8.Liu C., Wang Y., Li L., He D., Chi J., Li Q., et al. Engineered extracellular vesicles and their mimetics for cancer immunotherapy. J Control Release. 2022;349:679–698. doi: 10.1016/j.jconrel.2022.05.062. [DOI] [PubMed] [Google Scholar]
- 9.Hu Y., Xu Y., Mintz R.L., Luo X., Fang Y., Lao Y.H., et al. Self-intensified synergy of a versatile biomimetic nanozyme and doxorubicin on electrospun fibers to inhibit postsurgical tumor recurrence and metastasis. Biomaterials. 2023;293 doi: 10.1016/j.biomaterials.2022.121942. [DOI] [PubMed] [Google Scholar]
- 10.Stockwell B.R. Ferroptosis turns 10: emerging mechanisms, physiological functions, and therapeutic applications. Cell. 2022;185(14):2401–2421. doi: 10.1016/j.cell.2022.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stockwell B.R., Friedmann Angeli J.P., Bayir H., Bush A.I., Conrad M., Dixon S.J., et al. Ferroptosis: a regulated cell death Nexus linking metabolism, redox biology, and disease. Cell. 2017;171(2):273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu T., Zhu C., Chen X., Guan G., Zou C., Shen S., et al. Ferroptosis, as the most enriched programmed cell death process in glioma, induces immunosuppression and immunotherapy resistance. Neuro Oncol. 2022;24(7):1113–1125. doi: 10.1093/neuonc/noac033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Efimova I., Catanzaro E., Van der Meeren L., Turubanova V.D., Hammad H., Mishchenko T.A., et al. Vaccination with early ferroptotic cancer cells induces efficient antitumor immunity. J Immunother Cancer. 2020;8(2) doi: 10.1136/jitc-2020-001369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polewski M.D., Reveron-Thornton R.F., Cherryholmes G.A., Marinov G.K., Cassady K., Aboody K.S. Increased expression of system xc- in Glioblastoma confers an altered metabolic state and temozolomide resistance. Mol Cancer Res. 2016;14(12):1229–1242. doi: 10.1158/1541-7786.MCR-16-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hangauer M.J., Viswanathan V.S., Ryan M.J., Bole D., Eaton J.K., Matov A., et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature. 2017;551(7679):247–250. doi: 10.1038/nature24297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L., Li X., Liu L., Yu B., Xue Y., Liu Y. Erastin sensitizes glioblastoma cells to temozolomide by restraining xCT and cystathionine-gamma-lyase function. Oncol Rep. 2015;33(3):1465–1474. doi: 10.3892/or.2015.3712. [DOI] [PubMed] [Google Scholar]
- 17.Su X., Xie Y., Zhang J., Li M., Zhang Q., Jin G., et al. HIF-α activation by the prolyl hydroxylase inhibitor roxadustat suppresses chemoresistant glioblastoma growth by inducing ferroptosis. Cell Death Dis. 2022;13(10):861. doi: 10.1038/s41419-022-05304-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhuo S., He G., Chen T., Li X., Liang Y., Wu W., et al. Emerging role of ferroptosis in glioblastoma: therapeutic opportunities and challenges. Front Mol Biosci. 2022;9 doi: 10.3389/fmolb.2022.974156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Y., Li Y., Zhang R., Wang F., Wang T., Jiao Y. The role of erastin in ferroptosis and its prospects in cancer therapy. Onco Targets Ther. 2020;13:5429–5441. doi: 10.2147/OTT.S254995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klotz B.J., Gawlitta D., Rosenberg A.J.E.P., Malda J., Melchels F.P.W. Gelatin-methacryloyl hydrogels: towards biofabrication-based tissue repair. Trends Biotechnol. 2016;34(5):394–407. doi: 10.1016/j.tibtech.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Billard A., Pourchet L., Malaise S., Alcouffe P., Montembault A., Ladavière C. Liposome-loaded chitosan physical hydrogel: toward a promising delayed-release biosystem. Carbohydr Polym. 2015;115:651–657. doi: 10.1016/j.carbpol.2014.08.120. [DOI] [PubMed] [Google Scholar]
- 22.Veloso S.R.S., Andrade R.G.D., Castanheira S.M.S. Review on the advancements of magnetic gels: towards multifunctional magnetic liposome-hydrogel composites for biomedical applications. Adv Colloid Interface Sci. 2021;288 doi: 10.1016/j.cis.2020.102351. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J., Chen C., Li A., Jing W., Sun P., Huang X., et al. Immunostimulant hydrogel for the inhibition of malignant glioma relapse post-resection. Nat Nanotechnol. 2021;16(5):538–548. doi: 10.1038/s41565-020-00843-7. [DOI] [PubMed] [Google Scholar]
- 24.Gu W., Fan R., Quan J., Cheng Y., Wang S., Zhang H., et al. Intracranial in situ thermosensitive hydrogel delivery of temozolomide accomplished by PLGA-PEG-PLGA triblock copolymer blending for GBM treatment. Polymers (Basel) 2022;14(16):3368. doi: 10.3390/polym14163368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Almeida B., Nag O.K., Rogers K.E., Delehanty J.B. Recent progress in bioconjugation strategies for liposome-mediated drug delivery. Molecules. 2020;25(23):5672. doi: 10.3390/molecules25235672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang R., Wang Z., Yuan Y., Qian T., Zhou Q. cRGD target liposome delivery system promoted immunogenic cell death through enhanced anticancer potency of a thymidine conjugate under UVA activation as a cancer vaccine. Eur J Med Chem. 2019;167:499–509. doi: 10.1016/j.ejmech.2019.02.031. [DOI] [PubMed] [Google Scholar]
- 27.Liu H., Zhang R., Zhang D., Zhang C., Zhang Z., Fu X., et al. Cyclic RGD-decorated liposomal gossypol AT-101 targeting for enhanced antitumor effect. Int J Nanomedicine. 2022;17:227–244. doi: 10.2147/IJN.S341824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jackson C.M., Choi J., Lim M. Mechanisms of immunotherapy resistance: lessons from glioblastoma. Nat Immunol. 2019;20(9):1100–1109. doi: 10.1038/s41590-019-0433-y. [DOI] [PubMed] [Google Scholar]
- 29.Weller M., Stupp R., Reifenberger G., Brandes A.A., Bent van den M.J., Wick W., et al. MGMT promoter methylation in malignant gliomas: ready for personalized medicine? Nat Rev Neurol. 2010;6(1):39–51. doi: 10.1038/nrneurol.2009.197. [DOI] [PubMed] [Google Scholar]
- 30.Yang W.S., SriRamaratnam R., Welsch M.E., Shimada K., Skouta R., Viswanathan V.S., et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156(1–2):317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan R., Xie E., Li Y., Li J., Zhang Y., Chi X., et al. The structure of erastin-bound xCT-4F2hc complex reveals molecular mechanisms underlying erastin-induced ferroptosis. Cell Res. 2022;32(7):687–690. doi: 10.1038/s41422-022-00642-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao J.Y., Dixon S.J. Mechanisms of ferroptosis. Cell Mol Life Sci. 2016;73(11–12):2195–2209. doi: 10.1007/s00018-016-2194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pyonteck S.M., Akkari L., Schuhmacher A.J., Bowman R.L., Sevenich L., Quail D.F., et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19(10):1264–1272. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams J.B., Li S., Higgs E.F., Cabanov A., Wang X., Huang H., et al. Tumor heterogeneity and clonal cooperation influence the immune selection of IFN-gamma-signaling mutant cancer cells. Nat Commun. 2020;11(1):602. doi: 10.1038/s41467-020-14290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y., Ji H., Gao X. A 2-Gene signature related to interferon-gamma predicts prognosis and responsiveness to immune checkpoint blockade of glioma. Front Med (Lausanne) 2022;9 doi: 10.3389/fmed.2022.846847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silginer M., Nagy S., Happold C., Schneider H., Weller M., Roth P. Autocrine activation of the IFN signaling pathway may promote immune escape in glioblastoma. Neuro Oncol. 2017;19(10):1338–1349. doi: 10.1093/neuonc/nox051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qian J., Wang C., Wang B., Yang J., Wang Y., Luo F., et al. The IFN-gamma/PD-L1 axis between T cells and tumor microenvironment: hints for glioma anti-PD-1/PD-L1 therapy. J Neuroinflammation. 2018;15(1):290. doi: 10.1186/s12974-018-1330-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jia H., Xie X., Wang L., Wang L., Che F. IFN-gamma induces PD-L1 through p38/JNK/ERK signaling pathways and counteracts the tumor promoting effect mediated by PD-L1 in Glioblastoma. Comput Intell Neurosci. 2022;2022 doi: 10.1155/2022/5492602. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Yang K., Wu Z., Zhang H., Zhang N., Wu W., Wang Z., et al. Glioma targeted therapy: insight into future of molecular approaches. Mol Cancer. 2022;21(1):39. doi: 10.1186/s12943-022-01513-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moujalled D., Southon A.G., Saleh E., Brinkmann K., Ke F., Iliopoulos M., et al. BH3 mimetic drugs cooperate with Temozolomide, JQ1 and inducers of ferroptosis in killing glioblastoma multiforme cells. Cell Death Differ. 2022;29(7):1335–1348. doi: 10.1038/s41418-022-00977-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song J., Zhang H., Wang D., Wang J., Zhou J., Zhang Z., et al. Hydrogel loading functionalized PAMAM/shRNA complex for postsurgical glioblastoma treatment. J Control Release. 2021;338:583–592. doi: 10.1016/j.jconrel.2021.08.052. [DOI] [PubMed] [Google Scholar]
- 42.Zhu Y., Jia J., Zhao G., Huang X., Wang L., Zhang Y., et al. Multi-responsive nanofibers composite gel for local drug delivery to inhibit recurrence of glioma after operation. J Nanobiotechnology. 2021;19(1):198. doi: 10.1186/s12951-021-00943-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Norouzi M., Nazari B., Miller D.W. Injectable hydrogel-based drug delivery systems for local cancer therapy. Drug Discov Today. 2016;21(11):1835–1849. doi: 10.1016/j.drudis.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 44.Huang Y., Xu Y., Zhu J., Wan J., Xiong Y., Jiang Z., et al. An artificial LAMA2-GelMA hydrogel microenvironment for the development of pancreatic endocrine progenitors. Biomaterials. 2022;291 doi: 10.1016/j.biomaterials.2022.121882. [DOI] [PubMed] [Google Scholar]
- 45.Kurian A.G., Singh P.K., Patel K.D., Lee J.H., Kim H.W. Multifunctional GelMA platforms with nanomaterials for advanced tissue therapeutics. Bioact Mater. 2021;8:267–295. doi: 10.1016/j.bioactmat.2021.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dang W., Chen W.C., Ju E., Xu Y., Li K., Wang H., et al. 3D printed hydrogel scaffolds combining glutathione depletion-induced ferroptosis and photothermia-augmented chemodynamic therapy for efficiently inhibiting postoperative tumor recurrence. J Nanobiotechnology. 2022;20(1):266. doi: 10.1186/s12951-022-01454-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tomar M.S., Kumar A., Srivastava C., Shrivastava A. Elucidating the mechanisms of Temozolomide resistance in gliomas and the strategies to overcome the resistance. Biochim Biophys Acta Rev Cancer. 2021;1876(2) doi: 10.1016/j.bbcan.2021.188616. [DOI] [PubMed] [Google Scholar]
- 48.Zhang C., Liu X., Jin S., Chen Y., Guo R. Ferroptosis in cancer therapy: a novel approach to reversing drug resistance. Mol Cancer. 2022;21(1):47. doi: 10.1186/s12943-022-01530-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen T.C., Chuang J.Y., Ko C.Y., Kao T.J., Yang P.Y., Yu C.H., et al. AR ubiquitination induced by the curcumin analog suppresses growth of temozolomide-resistant glioblastoma through disrupting GPX4-Mediated redox homeostasis. Redox Biol. 2020;30 doi: 10.1016/j.redox.2019.101413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roh J.L., Kim E.H., Jang H.J., Park J.Y., Shin D. Induction of ferroptotic cell death for overcoming cisplatin resistance of head and neck cancer. Cancer Lett. 2016;381(1):96–103. doi: 10.1016/j.canlet.2016.07.035. [DOI] [PubMed] [Google Scholar]
- 51.Wu Q., Berglund A.E., Etame A.B. The impact of epigenetic modifications on adaptive resistance evolution in glioblastoma. Int J Mol Sci. 2021;22(15):8324. doi: 10.3390/ijms22158324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xue S., Hu M., Iyer V., Yu J. Blocking the PD-1/PD-L1 pathway in glioma: a potential new treatment strategy. J Hematol Oncol. 2017;10(1):81. doi: 10.1186/s13045-017-0455-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen X., Pan X., Zhang W., Guo H., Cheng S., He Q., et al. Epigenetic strategies synergize with PD-L1/PD-1 targeted cancer immunotherapies to enhance antitumor responses. Acta Pharm Sin B. 2020;10(5):723–733. doi: 10.1016/j.apsb.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang S., Yao F., Lu X., Li Q., Su Z., Lee J.H., et al. Temozolomide promotes immune escape of GBM cells via upregulating PD-L1. Am J Cancer Res. 2019;9(6):1161–1171. [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu L., Liu J., Qiu M., Chen J., Liang Q., Peng G., et al. Bacteria-mediated metformin-loaded peptide hydrogel reprograms the tumor immune microenvironment in glioblastoma. Biomaterials. 2022;288 doi: 10.1016/j.biomaterials.2022.121711. [DOI] [PubMed] [Google Scholar]
- 56.Huang C., Yang M., Deng J., Li P., Su W., Jiang R. Upregulation and activation of p53 by erastininduced reactive oxygen species contribute to cytotoxic and cytostatic effects in A549 lung cancer cells. Oncol Rep. 2018;40(4):2363–2370. doi: 10.3892/or.2018.6585. [DOI] [PubMed] [Google Scholar]
- 57.Thiem A., Hesbacher S., Kneitz H., di Primio T., Heppt M.V., Hermanns H.M., et al. IFN-gamma-induced PD-L1 expression in melanoma depends on p53 expression. J Exp Clin Cancer Res. 2019;38(1):397. doi: 10.1186/s13046-019-1403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.