Abstract
Avascular necrosis (AVN) involves ischemic cell death of the bone. AVN leaves an abundance of necrotic lipids and debris in the bone marrow, which instigates inflammatory bone repair. Consequently, the necrotic bone microenvironment stimulates excessive bone resorption, leading to joint deformities and osteoarthritis. Here, we performed a detergent-assisted bone wash using Poloxamer 407 (P407) to clean the necrotic bone environment by removing lipids and necrotic debris. The new concept was tested using an established ex vivo AVN model of porcine cadaver humeral heads. The P407 wash was performed using P407 solution and followed with saline via two intraosseous needles. Visual inspection and image analyses of average pixel light intensity showed that the P407 wash produced a better-cleaned bone than the saline wash. Analyses of the collected bone wash solution showed a two-fold increase in triglycerides (101 vs. 53 mmol/head, p=0.006) and a ten-fold increase in the dry weight of the removed debris (1.34 vs. 0.13 g/head, p=0.02) with the P407 wash compared to saline. The histological evaluation showed significantly decreased Oil-Red-O (fats) staining in the P407-washed bone compared to the saline-washed bone. The in vitro assays of Alizarin red and qPCR showed the P407 wash neither altered the osteogenic behaviors of porcine bone marrow-derived mesenchymal cells (pBMMCs) nor raised inflammatory responses of porcine bone marrow-derived macrophages (pBMMs). In conclusion, detergent-assisted bone wash using P407 produced a better removal of non-soluble debris from the bone marrow space than the saline wash without causing changes to osteogenesis or inflammatory reactions.
Keywords: poloxamer 407, intraosseous bone wash, avascular necrosis
Graphical Abstract

Introduction
Avascular necrosis (AVN) is the death of bone, commonly affecting the hip, shoulder, and knee joints.1–3 The annual incidence of hip AVN in the United States is about 10,000 to 20,000 per year 4, and over 20 million people in the world will suffer from AVN in the next ten years.5 Without treatment, over 50% of the affected joints will develop severe disabling osteoarthritis (OA)2,6,7, which significantly reduces the quality of life. Total joint replacement (TJR) is often required due to AVN progression, but TJR is a suboptimal treatment in young patients who have a long life expectancy and high activity demands.8
AVN produces and leaves an abundance of necrotic debris and inflammatory factors in the bone marrow space, including necrotic fat and debris, and damage-associated molecular patterns. The necrotic components activate and sustain local innate immune responses, leading to chronic inflammation, increased bone resorption, and decreased bone formation.9,10 Recently, a minimally invasive technique was developed to wash out the necrotic bone marrow as a treatment for AVN.11 This technique involves the placement of intraosseous needles, which are used to infuse and collect the saline wash solution. It showed that saline wash could remove soluble debris, such as DNA fragments and proteins.11 Furthermore, a follow-up in vivo study using a piglet model of ischemic osteonecrosis in the femoral head showed a decrease in bone resorption and an increase in new bone formation.12 Given the positive results from the in vivo study, we set out to find a bone wash solution that can increase the efficacy of debris removal for optimization of the bone wash procedure.
Poloxamers are a class of water-soluble nonionic triblock copolymers consisting of a central hydrophobic polypropylene block flanked by two hydrophilic blocks of polyethylene glycol. Poloxamer 407 (P407) is one of the derivatives capable of assembling into micelles and loading non-soluble ingredients within its hydrophobic core. The outer hydrophilic blocks form a shell, which allows the micelles to disperse in an aqueous system.13 P407 is utilized in the pharmaceutical industry to increase the solubilization of hydrophobic drug molecules, promoting their rapid and complete dispersion.13,14 The concentrations of hydrophobic drugs such as piroxicam and nifedipine can be increased 11- and 27-fold, respectively, when adding 22.5% (w/w) and 4% (w/v) P407.15,16 Fifteen percent P407 also increased the concentration of lipid drug-carriers by 7.5-fold in plasma.17
P407 has been shown to be biologically inert without cytotoxicity or immunogenic responses, and it can be cleared via renal circulation.13,18 The FDA guide has presented P407 as an inactive ingredient for various preparations such as inhalation, oral solution, suspension, ophthalmic, etc.13 A Sol-Gel made by 22% P407 has been approved by FDA for use in temporary endovascular occlusion.19 In the application of bone regeneration, P407 was used as an additive to improve the handling of bone grafts.20–22 P407 was also tested as a bone hemostatic agent.23,24 Compared to bone wax, P407 can be absorbed much faster without causing an inflammatory reaction.
The current study aimed to compare the wash efficiency of P407 solution and saline in the removal of lipid and debris, and to test the biological effects of P407 wash on osteogenesis and inflammatory responses in vitro. We applied an established ex vivo AVN model of pig.11 To mimic the AVN condition, the porcine cadaveric humeral head (HH) underwent three freeze-thaw cycles to induce cell lysis.11,25,26 To assess the wash efficiency, we evaluated the average pixel light intensity of digital images of the sectioned HH, the dry weight of washout debris, and the triglyceride concentration of washout solution. We applied histology to examine debris and fat remaining in the marrow space. We also determined the effects of P407 wash on the elemental components of the bone matrix via energy-dispersive X-ray spectroscopy. To assess the biological effects of P407 wash, we tested the effect of different concentrations of the P407 and post-wash solution on the inflammatory responses of porcine macrophages via measuring the inflammatory-related gene expressions. We also tested the effect of different concentrations of P407 and post-wash solution on the osteogenesis of porcine mesenchymal cells via Alizarin red assay and qPCR.
Materials and Methods
Materials
Sodium Chloride (Cat: S9888, Sigma), Poloxamer 407(Cat: 16758, Sigma), Alizarin Red S (Cat: A5533, Sigma)
Sample collection and preparation
Eighteen cadaveric HHs from pigs weighing 37–42 lbs were obtained from another study approved by the local IACUC.12 Only the femoral heads were used in the previous study, so HHs were readily available and minimized unnecessary use and sacrifice of animals. The samples underwent three freeze-thaw cycles to mimic AVN conditions. 11,20,21 For each cycle, the samples were placed in a 37 °C water bath for 6 hours, followed by −20°C freezer for 6 hours.
Bone wash procedure
This study utilized a previously described bone wash procedure with minor changes.11 The HH was cut from the humerus, leaving a metaphyseal portion 1–2 cm below the growth plate. Two 15-gauge trocar needles with fenestrations at the tips were drilled into the HH with a 12mm inter-needle distance using a 3D printed guide (Figure 1A). The needles were placed at least 3 mm past the growth plate without penetrating the subchondral bone. The placement of needles was confirmed via X-ray (Figure 1B). After that, the two needle cannulas were used as inflow and outflow wash portals alternatively (Figure 1C). Manual pressure was applied to the inflow syringe, while negative pressure was applied to the outflow syringe to extract the wash solution. In the saline wash group, 15 washes were performed with 30ml per wash. In the P407 wash group, 10% P407 solution was used for the first 4 washes, followed by 11 washes of saline. Before the procedure, all bone wash solutions were pre-warmed at 37°C. After the bone wash, all post-wash solutions were collected and stored at −20°C for analysis.
Figure 1.

The ex vivo bone wash method. A) A HH that was cut parallel to the humeral physis using a band saw, with the placement of parallel wash needles into the HH at an inter-needle distance of 12mm using a 3D printed guide. B) X-ray image showed the needles pasted the growth plate without penetrating the subchondral bone. C) After drillings, the 3D guide and trocar of each needle were removed, and two 30ml syringes attached. The syringes alternated inflow for injection of the wash solution and outflow for collection of the post-wash solution.
Analysis of humeral head gross sections
After the bone wash procedure, the HHs were fixed in 10% formalin and cut into six 6mm thick sections along the coronal plane. The sections were labeled as A3, A2, A1, P1, P2, P3 from the anterior to posterior of the HH. All sections were imaged using a Nikon DSLR camera. The digital images were analyzed with OSTEOMAGER (Nashville, TN). The average pixel light intensity was measured within the epiphysis, with higher pixel intensities indicating a cleaner bone. The average pixel light intensity was used to set a threshold to determine the clean and unclean bone (Supplementary Information Figure S1).
Histological assessment
The sections of A1, A3, P3 were decalcified in EDTA. The specimens were processed with 30% sucrose and cut for 10μm sections. The sections were stained with Hematoxylin and Eosin (H&E) or Oil-Red-O. H&E sections were imaged for qualitative evaluation of debris remaining in the HHs, and Oil-Red-O stained sections were imaged and analyzed to quantify residual lipids remaining using OSTEOMAGER. The percentage of lipid area was calculated by dividing the Oil-Red-O-stained lipid area with the total area.
Element assessments of bone using backscattered scanning electron microscopy (SEM) with Energy Dispersive X-Ray Analysis (EDX)
The sections of A2, P1, P2 were embedded with plastic without decalcification. Each block was processed by polishing, cleaning in an ultrasonic bath, air-drying, sputter coating with carbon, and scanned with a backscattered electron detector in a JEOL JSM-6300SEM (JEOL Limited, Japan).27
Dry weight of the extracted debris
Five ml of post-wash solution was collected from each of the first four washes and mixed into a single test tube to obtain a total of 20ml. The mixed solution was freeze-dried using a lyophilizer (LABCONCO, Kansas City, MO). The dry weight of the extracted debris was calculated by subtracting the dry weight of saline salts or P407 from the dry weight of the post-wash solution.
Triglyceride assay and absorbance quantification
To measure the extracted lipids, the post-wash solution was used to perform a triglyceride assay, 1ml of a mixed solution obtained by combining 15 post-wash solutions was used to measure lipid content using a triglyceride kit (ab65336, Sigma Aldrich). To measure the turbidity of the post-wash solution, the mixed solution was measured at 420 nm using a spectrophotometer.
Osteogenic assessment of the post-wash solution
The porcine bone marrow-derived mesenchymal cells (pBMMCs) were isolated from the iliac crest of pigs.28 Passages 3–5 were used for the osteogenic assays. 5×105 cells were seeded into 12-well plates and cultured with the growth medium (GM, minimum essential medium with 10% fetal bovine serum and 1% penicillin/streptomycin) in 37 °C with 5% CO2. When cells reached 70–80% confluence, osteogenic medium (OM, GM with 10 mM Beta-glyceral phosphate, 50 mM ascorbic acid, and 100 nM dexamethasone) was applied to initiate osteogenesis. The cells cultured in OM were applied as the control group. The cells cultured in GM were applied as the negative control group. The OM was added 1% or 0.1% of P407, or 10% of the 15th P407 post-wash solution to test the effects of P407 on osteogenesis of pBMMCs. The culture medium was replaced every two days. The cells were harvested after nine days of culture for qPCR analysis and Alizarin Red staining28, respectively. The primers for qPCR were listed in Supplementary Information Table S1.
Macrophages responses to the post-wash solution
The porcine bone marrow-derived macrophages (pBMMs) were derived from the ribs of pigs. Briefly, the mononuclear cells were isolated from the bone marrow via a series of Ficoll processing steps.29 After that, 2×107 cells were seeded in 6-well plates and cultured with the macrophage culture medium (RPMI 1640 with 20% porcine serum and 1% penicillin/streptomycin) in 37 °C with 5% CO2. The macrophage induction was initiated on day 7. The macrophage culture medium was applied as the control group. The medium was added 100 ng/ml of LPS to induce the inflammatory macrophages for the positive control. The medium was added 1% or 0.1% of P407, or 10% of the 15th P407 post-wash solution to test the effects of P407 wash on the inflammatory responses of macrophages. After induction for 24 hours, the cells were harvested for qPCR analyses. The expressions of IL1β, IL6, IL8, and TNFα were tested. Primers used in the study are listed in Supplementary Information Table S1.
Statistical analysis
GraphPad Prism 7.0 (GraphPad Software Inc.) was used to present data as mean ± standard deviation (SD) for statistical analysis. For three or more group comparisons, one-way ANOVA with post-hoc Bonferroni multiple comparison test was used. (e.g., the average pixel light intensity, Oil-Red-O staining, Alizarin red staining, and qPCR.) For two group comparisons, the unpaired t-test was used. (e.g., the dry weight and triglyceride of post-wash solutions). P-value < 0.05 was considered statistically significant.
Results
Visual inspection of the bone sections
The hemoglobin located in red blood cells distributes throughout the whole bone marrow and vascular tissue. After the bone wash, the reddish bone marrow color fades to reveal trabecular bone, indicating the removal of red blood cells and debris from the bone marrow space. As shown in Figure 2A, the unwashed bone section showed a typical red/brown bone marrow. In Figure 2B, the saline-washed HH showed a decrease in color at the central region. However, the periphery remained reddish/brownish. The P407-washed sample showed white bone throughout the HH, indicating an improved clearance of marrow space debris (Figure 2C). The average pixel light intensity in the epiphyseal region of the gross images was analyzed to evaluate debris clearance. The average pixel light intensity of the P407-washed epiphysis was significantly higher than unwashed epiphysis (190 ± 22 versus 120 ± 8, p<0.0001) and saline-washed epiphysis (190 ± 22 versus 159 ± 23, p=0.03) (Figure 2D). An average pixel light intensity threshold was used to indicate that trabecular bone was void of all debris. Trabecular areas that met this threshold were considered clean (Supplementary Information Figure S1). Through this method, the P407-washed HHs displayed a significant increase of the cleaned area compared to unwashed (87%± 7.5% versus 23% ± 3.6%) and saline-washed HHs (87% ± 7.5 versus 78% ± 2.0%, p=0.03) (Figure 2E).
Figure 2.

Wash efficiency comparisons by visual inspection of HH cross-sections. Gross coronal images showed (A) the unwashed, (B) saline-washed, and (C) P407-washed HHs; D) Bar graph compared the average pixel light intensity of HHs that were unwashed, or washed with saline or P407. E) Bar graph comparing the percent area of the cleaned region for HHs that were unwashed, or washed with saline or P407. *p<0.05; ** p<0.01; ****p<0.0001
Histological evaluation
H&E staining showed a diffuse presence of hematopoietic cells and adipocytes throughout all three regions of the unwashed HH epiphysis (Figure 3A–C). With the saline wash, there was a sharp decrease of bone marrow debris in the central region (Figure 3D), but the posterior and anterior regions still had a considerable amount of marrow contents (Figure 3E–F). After the P407 wash, the trabecular space was much cleaner with little debris remaining in all three regions. (Figure 3H–J).
Figure 3.

Wash efficiency comparisons by histology. Representative images of H&E stained sections showed (A-C) the unwashed HH sections, (D-F) saline-washed HH sections, (H-J) P407-washed HH sections. A, D, H were from the anterior HH; B, E, I were from the central HH; C, F, J were from the posterior HH.
To evaluate lipid removal, the HH sections were stained with Oil-Red-O. The unwashed HH sections showed a diffuse and homogenous presence of red staining throughout the epiphysis (Figure 4A). The saline-washed HHs showed a decreased amount of lipids in the central epiphyseal area while lipids still remained in the peripheral area (Figure 4B). In contrast, P407-washed HHs displayed an even reduction in Oil-Red-O staining throughout the epiphysis (Figure 4C). The quantitation result showed that there was a 63% decrease in lipid-stained areas in the P407-washed HHs (8.4 ± 4.4%, p=<0.0001), compared to the unwashed HHs (23% ± 2.9%). And the P407-washed HHs exhibited a 44% decrease (p=0.005), compared to the saline-washed HHs (15% ± 5.0%, Figure 4D).
Figure 4.

Wash efficiency comparisons by Oil-Red-O staining. Low and high magnification images of Oil Red O staining showed (A) the unwashed, (B) saline-washed, and (C) P407-washed HH; D) Bar graph showing the percentage of lipid remaining in HH. *p < 0.05; ** p < 0.01; **** p < 0.0001
Turbidity, dry weight, and lipid content of the post-wash solution
The saline post-wash solution had a slight reddish tint (Figure 5A, upper panel), whereas the P407 post-wash solution had a darker red appearance (Figure 5B, upper panel). The turbidity of the post-wash solutions was assessed by measuring absorbance at 420 nm. The quantitation result showed that the post-wash P407 solution had a significant increase in absorbance compared to the post-wash saline solution (0.70 ± 0.30 versus 0.22 ± 0.077, p=0.0087) (Figure 5C).
Figure 5.
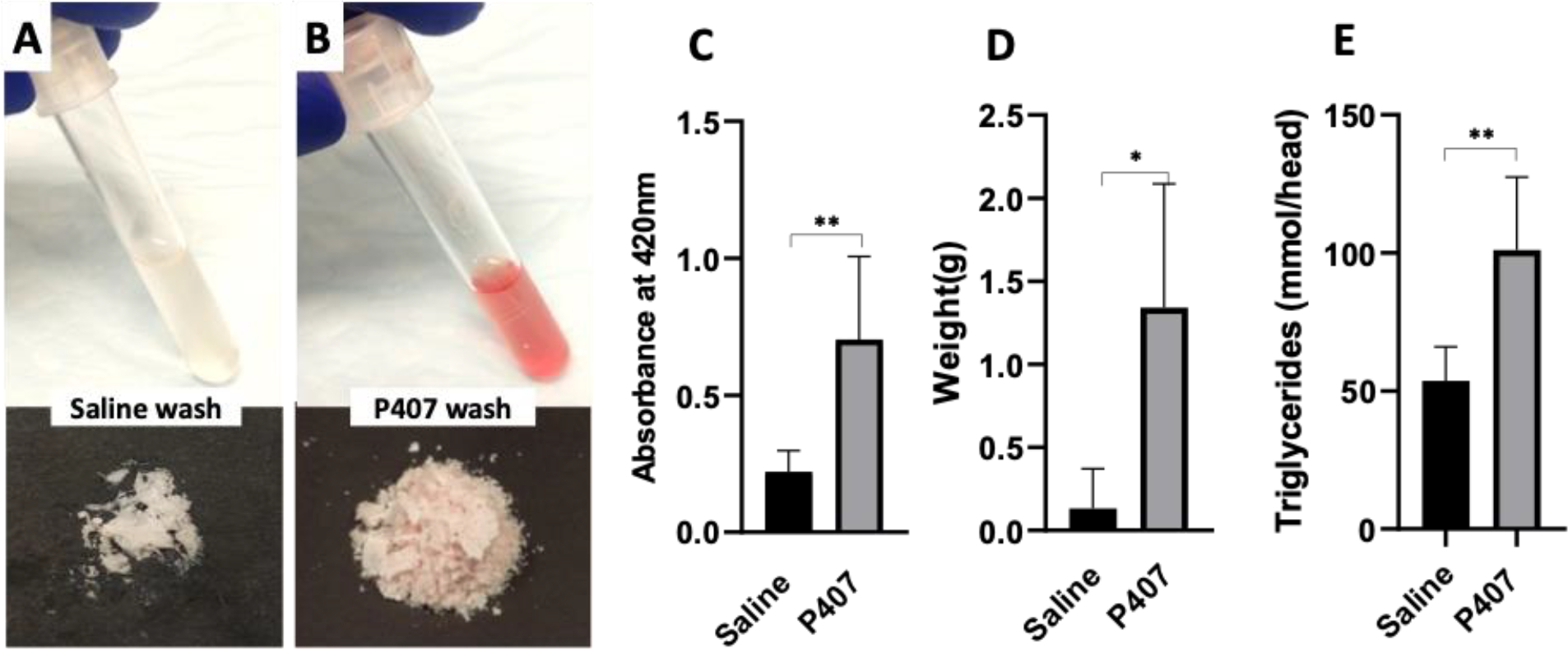
Wash efficiency comparisons by analysis of post-wash solution components of turbidity, dry weight, and lipid content. A-B) Digital images showed the collected post-wash solutions (upper panel) and the dried debris (lower panel) from (A) the saline-washed, and (B) P407-washed HH; C) Bar graph showed the turbidity of post-wash solutions from the saline-washed and P407-washed HH; D) Bar graph showed the extracted debris in grams from the saline-washed and P407-washed HH; E) Bar graph showed the amount of triglycerides from the saline-washed and P407-washed HH. *p<0.05; **p<0.01
The images of the dried debris from the post-wash solution showed P407 group showed more debris extracted compared to the saline group (Figure 5A&B, lower panel). The quantitation result confirmed that P407 post-wash solution had a significantly higher dry weight of the extracted debris than saline post-wash solution (1.34g/head ± 0.74 versus 0.13g/head ± 0.2, p=0.02, Figure 5D).
Triglyceride assays were performed to quantify lipids in the post-wash solutions (Figure 5E). P407 post-wash solution possessed a significantly higher lipid content than saline post-wash solution (101 mmol/head versus 53 mmol/head, p=0.006).
Chemical element analysis of bone matrix after P407 wash
The SEM was used to evaluate the morphology and element changes of trabecular matrix following the P407 wash. The bright area indicates the trabecula minerals on the SEM images (Figure 6A&B). The surface of the trabecular bone from the epiphysis of normal HH was smooth with even signal intensity at the edge and central area of the trabeculae (Figure 6A). There were no visible differences in the morphology and signal intensity of the trabecular bone from P407-washed HH (Figure 6B). Furthermore, SEM-EDX analysis showed no significant difference in the elemental components of Carbon, Oxygen, Phosphorus, and Calcium in the trabecular bone of the normal and P407-washed HH (Figure 6C). Additionally, no significant differences were detected in the atom ratios of Calcium and Phosphorus between normal and P407-washed bone (Figure 6D). These results indicate P407 did not alter the morphology and element components of the trabecular matrix.
Figure 6.

Evaluating the chemical effects of the P407 wash on the bone matrix via SEM-EDX. A-B) The SEM images showed the epiphyseal trabeculae from (A) the unwashed and (B) P407-washed HH; C) Bar graph showed the element mass percentage of carbon (C), oxygen (O), phosphorus (P), calcium (Ca) in the groups of control and P407 wash; D) Bar graph showed the atom ratio of calcium and phosphorus (Ca/P) in the groups of control and P407 wash.
Assessment of macrophages inflammatory responses to the post-wash solutions
To test the P407 wash effects on macrophage polarization, 1% or 0.1% of P407, or 10% of 15th P407 post-wash solution were added to the macrophage culture medium. As shown in Figure 7A, the pBMMs displayed a typical fried-egg morphology of macrophages in the control group. With the addition of LPS, the pBMMs fused into large multinucleated cells. When adding 1% or 0.1% of P407 into the culture medium, the pBMMs were less spreading compared to the control. When adding 10% of the 15th P407 post-wash solution, the morphology of pBMMs had no apparent difference compared to the control. The inflammation-related genes, including IL1β, IL6, IL8, and TNFα, were up-regulated in LPS-treated pBMMs compared to the control. In contrast, the gene expressions did not significantly change in the groups of 1% P407, 0.1% P407, and 10% of the 15th P407 post-wash solution compared to the control (Figure 7B–E). These results indicate that P407 did not activate the inflammatory responses of the macrophages.
Figure 7.

Testing inflammatory responses of the pBMMs following the P407 wash in vitro. A) The microscope images showed the typical pBMMs morphologies after 24 hours of induction under different induction mediums, including the macrophage culture medium and the medium with 100ng/ml of LPS, 1% P407, 0.1% P407, and 10% of 15th P407 post-wash solution. B-D) Bar graphs showed the comparisons of the expressions of IL1β (Β), IL6 (C), IL8 (D), and TNFα (E) in the pBMMs from different induction mediums. *p<0.05, when comparing with the group of control; # p<0.05, when comparing with the group of LPS; Double black arrows representing the multinucleated cells.
Assessment of the post-wash solutions on the osteogenesis of pBMMCs
As shown in Figure 8A, the OM group showed a dark Alizarin red staining. There was no apparent difference in the staining intensity between the 0.1% P407 and OM groups, but a decrease in the 1% P407 group compared to the OM group. The staining in the group of 10% of the 15th P407 post-wash showed a minor increase compared to the OM group. The quantitation of Alizarin red staining confirmed the above observations. The group of 10% of the 15th P407 post-wash showed the highest minerals among the groups (Figure 8B). The qPCR analyses of osteogenic markers showed that 1% P407 significantly down-regulated the expressions of Osterix, Col 1, OCN, and BSP (p<0.0001), while no significant changes were founded in the 0.1% P407 group compared to the OM group (Figure 8C–F). These results indicate that a high concentration of P407 (1%) inhibits the osteogenesis of pBMMCs, but a low concentration of P407 has a neglectable effect on osteogenesis.
Figure 8.

Testing osteogenesis of pBMMCs following the P407 wash in vitro. A) Images of the Alizarin red staining for pBMMCs cultured in different mediums, including GM, OM, 1% P407, 0.1% P407, and 10% of the 15th P407 post-wash solution; B) Bar graph showed the quantitation of the Alizarin red stained minerals from the pBMMCs cultured in different mediums; C-F) Bar graph showed the gene expressions of (C) Osterix (OSX), (D) Col 1, (E) OCN, and (F) BSP from pBMMCs cultured in different media. *p<0.05 when comparing with OM; # p<0.05 when comparing with GM.
Discussion
The harsh necrotic microenvironment is one of the major challenges for bone repair following AVN. It produces abundant necrotic debris and inflammatory factors in the bone marrow space. Thus, to develop an effective local treatment for osteonecrosis, the prerequisite step is to establish an efficient method to clean the necrotic bone marrow space. The results showed that the P407 wash has a 2-fold increase in lipid removal (Figure 4) and a 10-fold increase in total debris removal (Figure 5) compared to the saline wash. The histological assessment further confirmed that the P407 wash produced a cleaner bone marrow space compared to the saline wash. It indicated that P407, an amphipathic molecule, acts as a detergent that can efficiently elute out non-soluble debris (Figure 9). Moreover, the P407 wash neither raised the inflammatory responses of pBMMs (Figure 7) nor altered the osteogenic behaviors of pBMMCs (Figure 8). These findings demonstrate that P407 wash is a more effective method than a saline wash, which can improve the reconditioning of the necrotic bone microenvironment.
Figure 9.

Washing model for the extraction of lipids and non-soluble debris. The box shows the molecular structure of poloxamer 407 and the magnified region shows micelle formation.
Ex vivo model of AVN and chemical consideration for intraosseous washing
An ex vivo model of AVN was used to study the debris removal by the bone wash. To recapitulate the necrotic bone marrow, we performed frozen-thaw cycles on the cadaveric humeral head. The frozen-thaw cycles can induce physical dissociation of the bone marrow, which leads to the cell membrane disruption and release of intracellular molecules such as lipids, proteins, and DNA.30 It also changes the rheological properties of bone marrow. One frozen-thaw cycle would decrease the viscosity by an order of magnitude.30 The method has been used to simulate the necrotic bone marrow microenvironment in previous studies. 25,31,32
Based on the chemical and physical characteristics of the necrotic bone marrow, two variables may affect the clearance of bone marrow content: solubility of the debris in the wash solution and the local shear stress induced by liquid flow. Saline may not be the most efficient wash solution for the following reasons. First, the hydrophilic nature of saline is unsuitable for dissolving and dispersing lipids and other non-soluble debris. Second, the low viscosity of saline and the limited injection pressure of hand injection generate insufficient local disturbance to the bone marrow debris. In support of this point, we found that only the central region of the HH (between the two wash needles) showed good clearance of debris, leaving a large amount of bone marrow debris in the peripheral regions of the HH (Figure 3).
P407 is an amphipathic molecule that can form micelles at low concentrations. It has a critical micelle concentration of 2.8 × 10−6 M.26 The 10% solution provided a high concentration of P407 to maintain its detergent feature for packing lipids and non-soluble debris in micelles. Moreover, the viscosity of 10% P407 (10 mPa.s) 33 is 10-fold higher than saline (0.818 mPa.s).34 Thus, the P407 wash would produce higher shear stress, which could disturb and remove more debris than saline. In addition, unlike a high concentration of P407 (> 12.6% w/v) exhibits a typical thermo-gelling behavior, 33 10% P407 makes the wash solution liquid that it easily mixes with saline and avoids any leftover in the bone marrow. Therefore, the P407 wash removed necrotic debris from a wider region compared to the saline wash, and no gel-like residue was found in the P407-washed HHs (Figure 3).
P407 is a nonionic polymer with a neutral pH value. The SEM-EDX analyses demonstrated that the contents of Carbon, Oxygen, Calcium, and Phosphorus, and the ratio of Calcium/Phosphorus had no significant changes after P407 wash (Figure 6). These data confirmed that P407 did not alter the structure and chemical compositions of the trabecular matrix.
Biological consideration of P407 wash
It has been reported that P407 did not stimulate inflammatory responses.35 Consequently, P407 has been commonly used as a drug/cell carrier, or as an additive to decrease inflammatory responses.36–38 The bioinert features of P407 indicate a safe application as a bone wash detergent. The study showed that the inflammatory gene expressions of pBMMs did not significantly up-regulate after supplying the P407 into the culture medium (Figure 7). However, it has been reported that the application of P407 led to hyperlipidemia on rodents.39,40 Another study on pigs showed a transient elevation of triglyceride when using P407 gel for temporary vascular occlusion.19 Moreover, applying a high dose of P407 (1%) in the culture medium significantly inhibited the osteogenesis of pBMMCs, whereas there was only a minor effect when applying a low dose of P407 (0.1%) (Figure 8). Therefore, it is necessary to perform additional washes to remove the P407. The current study performed four P407 washes followed with eleven saline washes. The osteogenic assays showed no negative effects on osteogenesis when adding the 15th P407 post-wash solution to the culture medium, indicating a completed removal of P407 after the P407 wash (Figure 8).
Clinical Relevance
There is a lack of effective treatments for AVN. Treatments based on mechanical concepts, like weight-bearing restriction and osteotomies, have had limited and unreliable therapeutic effects.41,42 Systemic drug therapy, such as systemic bisphosphonate or statin therapy, cannot ensure adequate local drug bioavailability due to the lack of blood flow to the necrotic region.43–45 Operative interventions like core decompression, create large bone tunnel(s) to facilitate the restoration of vasculature. However, the procedures significantly disrupt the native trabecular network and can lead to iatrogenic collapse.46 A recent systematic review and meta-analysis indicated that core decompression treatment only reached a modest success rate (65%).47
In the previous in vivo study, the saline wash was tested as a surgical treatment of AVN. It improved bone regeneration in the central region where the intraosseous needles were placed.12 However, the necrotic bone in the peripheral regions where bone wash was not adequate showed large bone void areas (Supplementary Information Figure S2A versus Figure 3). Moreover, the unwashed necrotic bone samples showed extensive bone resorption throughout the joint head. This evidence indicates that the incomplete removal of necrotic debris resulted in bone resorption in the unwashed area, leading to an uneven bone repair.
The current study was designed to improve the removal of necrotic debris using P407 wash. The new design has many advantages for clinical application. First, the wash procedure was prepared via intraosseous drillings with small needles (15G, 1.8mm in outer diameter). It minimizes the disruption of the native trabecular structure and avoids weakening the bone. Second, P407 wash significantly increased the washing efficiency compared to saline wash, with no apparent effects on bone morphology and components, osteogenesis, and inflammation responses. (Figure 6, 7 and 8) Third, P407 wash prepared a “porous bony scaffold” (Figure 3). It is anticipated to facilitate local tissue engineering strategies. A clean washed bone marrow space allows biologicals, such as cells48 and biomaterial carriers with bioactive factors (e.g., injectable hydrogel49 and microsphere50 system, etc.) to be injected and distributed broadly within the bone for a uniformed bone repair. Both the drilling procedures and P407 are available in clinical settings. Therefore, P407 is expected to have great potential for clinical translation.
Limitations
AVN affects all age groups, and there are anatomic differences in the epiphyses of pediatric and adult patients. The present study only performed bone washes on the pediatric epiphysis of porcine, in which the epiphysis is separated from the long bone shaft while the adult epiphysis is fused to it. This difference may affect the wash flow within the trabeculae and further change the wash efficacy. Therefore, more studies will be required to target different age groups. In addition, the bone marrow dissociation mechanisms/levels are different between ex vivo and in vivo conditions. This may also lead to differences in bone wash outcome. Future studies with in vivo model will be necessary to further evaluate the wash efficiency and treatment effects of P407 bone wash.
Conclusion
In summary, our study demonstrates that 10% P407 solution resulted in a superior clearance of lipids and other non-soluble debris from the bone marrow space with minimal changes in the morphology, elemental composition, inflammation responses, and osteogenesis of trabecular bone. The current study laid groundwork for the new AVN treatment of P407 wash.
Supplementary Material
Acknowledgments
The study is supported by an internal grant from the Scottish Rite for Children, a microgrant from the Pediatric Orthopedic Society of North America, and NIAMS 1R01AR078311. The authors would like to thank Ila Oxendine, Reuel Cornelia, Richard Banlaygas, Amanda McLerran for their help in the triglyceride assays, histology processing, and collection of porcine humeri, and Alice Liang for proofreading the article.
Footnotes
All authors have read and approved the final submitted manuscript. Each author believes that the manuscript represents honest work.
Contributor Information
Graham Andre, Center for Excellence in Hip, Scottish Rite for Children, Dallas, Texas 75219. This author had a substantial contribution to the research design, data acquisition, and this paper by revising it critically..
Francesco Boschetto, Center for Excellence in Hip, Scottish Rite for Children, Dallas, Texas 75219. This author had a substantial contribution to the data acquisition..
Vishal Gokani, Center for Excellence in Hip, Scottish Rite for Children, Dallas, Texas 75219. This author had a substantial contribution to the research design..
Mo Singhal, Center for Excellence in Hip, Scottish Rite for Children, Dallas, Texas 75219. This author had a substantial contribution to the data acquisition..
Yan Jing, Department of Orthodontics, Texas A&M School of Dentistry, Dallas, Texas 75246. This author had a substantial contribution to the data acquisition..
Harry K.W. Kim, Center for Excellence in Hip, Scottish Rite for Children, Dallas, Texas 75219, Department of Orthopedic Surgery, University of Texas Southwestern Medical Center, Dallas, Texas 75390. This author had a substantial contribution to the research design, interpretation of data, and this paper by revising it critically..
Chi Ma, Center for Excellence in Hip, Scottish Rite for Children, Dallas, Texas 75219, Department of Orthopedic Surgery, University of Texas Southwestern Medical Center, Dallas, Texas 75390. This author had a substantial contribution to the research design, data acquisition, interpretation of data, and drafting the paper..
Reference
- 1.Hernigou P, Hernigou J, Scarlat M. 2020. Shoulder Osteonecrosis: Pathogenesis, Causes, Clinical Evaluation, Imaging, and Classification. Orthop Surg 12:1340–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karim AR, Cherian JJ, Jauregui JJ, et al. 2015. Osteonecrosis of the knee: review. Ann Transl Med 3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim HK, Herring JA. 2011. Pathophysiology, classifications, and natural history of Perthes disease. The Orthopedic clinics of North America 42:285–295, v. [DOI] [PubMed] [Google Scholar]
- 4.Mont MA, Hungerford DS. 1995. Non-traumatic avascular necrosis of the femoral head. J Bone Joint Surg Am 77:459–474. [DOI] [PubMed] [Google Scholar]
- 5.Mont MA, Zywiel MG, Marker DR, et al. 2010. The natural history of untreated asymptomatic osteonecrosis of the femoral head: a systematic literature review. J Bone Joint Surg Am 92:2165–2170. [DOI] [PubMed] [Google Scholar]
- 6.Joseph B, Mulpuri K, Varghese G. 2001. Perthes’ disease in the adolescent. J Bone Joint Surg Br 83:715–720. [DOI] [PubMed] [Google Scholar]
- 7.Shah KN, Racine J, Jones LC, et al. 2015. Pathophysiology and risk factors for osteonecrosis. Curr Rev Musculoskelet Med 8:201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scaglione M, Fabbri L, Celli F, et al. 2015. Hip replacement in femoral head osteonecrosis: current concepts. Clin Cases Miner Bone Metab 12:51–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andreev D, Liu M, Weidner D, et al. 2020. Osteocyte necrosis triggers osteoclast-mediated bone loss through macrophage-inducible C-type lectin. J Clin Invest 130:4811–4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao JJ. 2011. Effects of obesity on bone metabolism. Journal of Orthopaedic Surgery and Research 6:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alves do Monte F, Sung Park M, Gokani V, et al. 2020. Development of a novel minimally invasive technique to washout necrotic bone marrow content from epiphyseal bone: A preliminary cadaveric bone study. Orthop Traumatol Surg Res 106:709–715. [DOI] [PubMed] [Google Scholar]
- 12.Kim HKW, Park MS, Alves do Monte F, et al. 2021. Minimally Invasive Necrotic Bone Washing Improves Bone Healing After Femoral Head Ischemic Osteonecrosis: An Experimental Investigation in Immature Pigs. J Bone Joint Surg Am. [DOI] [PubMed] [Google Scholar]
- 13.Dumortier G, Grossiord JL, Agnely F, et al. 2006. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm Res 23:2709–2728. [DOI] [PubMed] [Google Scholar]
- 14.Chiappetta DA, Sosnik A. 2007. Poly(ethylene oxide)-poly(propylene oxide) block copolymer micelles as drug delivery agents: improved hydrosolubility, stability and bioavailability of drugs. Eur J Pharm Biopharm 66:303–317. [DOI] [PubMed] [Google Scholar]
- 15.Shin SC, Cho CW. 1997. Physicochemical characterizations of piroxicam-poloxamer solid dispersion. Pharm Dev Technol 2:403–407. [DOI] [PubMed] [Google Scholar]
- 16.Chutimaworapan S, Ritthidej GC, Yonemochi E, et al. 2000. Effect of water-soluble carriers on dissolution characteristics of nifedipine solid dispersions. Drug Dev Ind Pharm 26:1141–1150. [DOI] [PubMed] [Google Scholar]
- 17.Fu Din, Mustapha O Kim DW, et al. 2015. Novel dual-reverse thermosensitive solid lipid nanoparticle-loaded hydrogel for rectal administration of flurbiprofen with improved bioavailability and reduced initial burst effect. European Journal of Pharmaceutics and Biopharmaceutics 94:64–72. [DOI] [PubMed] [Google Scholar]
- 18.Pec EA, Wout ZG, Johnston TP. 1992. Biological Activity of Urease Formulated in Poloxamer 407 after Intraperitoneal Injection in the Rat. Journal of Pharmaceutical Sciences 81:626–630. [DOI] [PubMed] [Google Scholar]
- 19.Raymond J, Metcalfe A, Salazkin I, et al. 2004. Temporary vascular occlusion with poloxamer 407. Biomaterials 25:3983–3989. [DOI] [PubMed] [Google Scholar]
- 20.Lee JH, Baek HR, Lee KM, et al. 2014. The effect of poloxamer 407-based hydrogel on the osteoinductivity of demineralized bone matrix. Clin Orthop Surg 6:455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fowler EB, Cuenin MF, Hokett SD, et al. 2002. Evaluation of pluronic polyols as carriers for grafting materials: study in rat calvaria defects. J Periodontol 73:191–197. [DOI] [PubMed] [Google Scholar]
- 22.Zhou AJ, Clokie CM, Peel SA. 2013. Bone formation in algae-derived and synthetic calcium phosphates with or without poloxamer. J Craniofac Surg 24:354–359. [DOI] [PubMed] [Google Scholar]
- 23.Wang MY, Armstrong JK, Fisher TC, et al. 2001. A new, pluronic-based, bone hemostatic agent that does not impair osteogenesis. Neurosurgery 49:962–967; discussion 968. [DOI] [PubMed] [Google Scholar]
- 24.Choi SY, Rhim J, Heo SA, et al. 2021. Efficacy and safety of a novel hemostatic material, BoneStat, compared with Ostene and Bone Wax in a rat calvarial defect model. Int J Artif Organs 44:734–747. [DOI] [PubMed] [Google Scholar]
- 25.Poignard A, Lebouvier A, Cavet M, et al. 2014. New preclinical porcine model of femoral head osteonecrosis to test mesenchymal stromal cell efficiency in regenerative medicine. Int Orthop 38:1837–1844. [DOI] [PubMed] [Google Scholar]
- 26.Iyer SS, Pulskens WP, Sadler JJ, et al. 2009. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci U S A 106:20388–20393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamiya N, Shuxian L, Yamaguchi R, et al. 2016. Targeted disruption of BMP signaling through type IA receptor (BMPR1A) in osteocyte suppresses SOST and RANKL, leading to dramatic increase in bone mass, bone mineral density and mechanical strength. Bone 91:53–63. [DOI] [PubMed] [Google Scholar]
- 28.Deng Z, Ren Y, Park MS, et al. 2022. Damage associated molecular patterns in necrotic femoral head inhibit osteogenesis and promote fibrogenesis of mesenchymal stem cells. Bone 154:116215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao J, Scheenstra MR, van Dijk A, et al. 2018. A new and efficient culture method for porcine bone marrow-derived M1- and M2-polarized macrophages. Vet Immunol Immunopathol 200:7–15. [DOI] [PubMed] [Google Scholar]
- 30.Metzger TA, Shudick JM, Seekell R, et al. 2014. Rheological behavior of fresh bone marrow and the effects of storage. J Mech Behav Biomed Mater 40:307–313. [DOI] [PubMed] [Google Scholar]
- 31.Goetz JE, Robinson DA, Pedersen DR, et al. 2011. Cryoinsult parameter effects on the histologically apparent volume of experimentally induced osteonecrotic lesions. J Orthop Res 29:931–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adapala NS, Yamaguchi R, Phipps M, et al. 2016. Necrotic Bone Stimulates Proinflammatory Responses in Macrophages through the Activation of Toll-Like Receptor 4. Am J Pathol 186:2987–2999. [DOI] [PubMed] [Google Scholar]
- 33.Fakhari A, Corcoran M, Schwarz A. 2017. Thermogelling properties of purified poloxamer 407. Heliyon 3:e00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kestin J, Shankland IR. 1984. Viscosity of aqueous NaCl solutions in the temperature range 25–200 °C and in the pressure range 0.1–30 MPa. International Journal of Thermophysics 5:241–263. [Google Scholar]
- 35.Johnston TP, Li Y, Jamal AS, et al. 2003. Poloxamer 407-induced atherosclerosis in mice appears to be due to lipid derangements and not due to its direct effects on endothelial cells and macrophages. Mediators Inflamm 12:147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Popescu I, Turtoi M, Suflet DM, et al. 2021. Alginate/poloxamer hydrogel obtained by thiol-acrylate photopolymerization for the alleviation of the inflammatory response of human keratinocytes. Int J Biol Macromol 180:418–431. [DOI] [PubMed] [Google Scholar]
- 37.Monteiro do Nascimento MH, Ambrosio FN, Ferraraz DC, et al. 2021. Sulforaphane-loaded hyaluronic acid-poloxamer hybrid hydrogel enhances cartilage protection in osteoarthritis models. Mater Sci Eng C Mater Biol Appl 128:112345. [DOI] [PubMed] [Google Scholar]
- 38.Yang X, Yang R, Chen M, et al. 2020. KGF-2 and FGF-21 poloxamer 407 hydrogel coordinates inflammation and proliferation homeostasis to enhance wound repair of scalded skin in diabetic rats. BMJ Open Diabetes Res Care 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnston TP, Korolenko TA, Sahebkar A. 2017. P-407-induced Mouse Model of Dose-controlled Hyperlipidemia and Atherosclerosis: 25 Years Later. J Cardiovasc Pharmacol 70:339–352. [DOI] [PubMed] [Google Scholar]
- 40.Yeom M, Park J, Lee B, et al. 2018. Electroacupuncture ameliorates poloxamer 407-induced hyperlipidemia through suppressing hepatic SREBP-2 expression in rats. Life Sci 203:20–26. [DOI] [PubMed] [Google Scholar]
- 41.Sadile F, Bernasconi A, Russo S, et al. 2016. Core decompression versus other joint preserving treatments for osteonecrosis of the femoral head: a meta-analysis. British Medical Bulletin 118:33–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mont MA, Carbone JJ, Fairbank AC. 1996. Core decompression versus nonoperative management for osteonecrosis of the hip. Clinical orthopaedics and related research:169–178. [DOI] [PubMed] [Google Scholar]
- 43.Ajmal M, Matas AJ, Kuskowski M, et al. 2009. Does Statin Usage Reduce the Risk of Corticosteroid-Related Osteonecrosis in Renal Transplant Population? Orthopedic Clinics of North America 40:235–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Disch AC, Matziolis G, Perka C. 2005. The management of necrosis-associated and idiopathic bone-marrow oedema of the proximal femur by intravenous iloprost. J Bone Joint Surg Br 87:560–564. [DOI] [PubMed] [Google Scholar]
- 45.Lai KA, Shen WJ, Yang CY, et al. 2005. The use of alendronate to prevent early collapse of the femoral head in patients with nontraumatic osteonecrosis. A randomized clinical study. J Bone Joint Surg Am 87:2155–2159. [DOI] [PubMed] [Google Scholar]
- 46.Beltran J, Knight CT, Zuelzer WA, et al. 1990. Core decompression for avascular necrosis of the femoral head: correlation between long-term results and preoperative MR staging. Radiology 175:533–536. [DOI] [PubMed] [Google Scholar]
- 47.Hua KC, Yang XG, Feng JT, et al. 2019. The efficacy and safety of core decompression for the treatment of femoral head necrosis: a systematic review and meta-analysis. J Orthop Surg Res 14:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elgaz S, Bonig H, Bader P. 2020. Mesenchymal stromal cells for osteonecrosis. J Transl Med 18:399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Phipps MC, Monte F, Mehta M, et al. 2016. Intraosseous Delivery of Bone Morphogenic Protein-2 Using a Self-Assembling Peptide Hydrogel. Biomacromolecules 17:2329–2336. [DOI] [PubMed] [Google Scholar]
- 50.Ma C, Andre G, Edwards D, et al. 2021. A rat model of ischemic osteonecrosis for investigating local therapeutics using biomaterials. Acta Biomater 132:260–271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


