Abstract
Background
Olaparib, a poly(ADP-ribose) polymerase inhibitor, was approved by the European Commission in June 2019, following the results of the SOLO-1/GOG 3004 trial as maintenance monotherapy in adult patients with BRCA-mutated epithelial ovarian cancer.
Objective
This study aimed to provide a descriptive analysis of the first real-world data from patients with BRCA-mutated ovarian cancer who received olaparib as first-line maintenance monotherapy in the French cohort Temporary Authorisation for Use (Autorisation Temporaire d’Utilisation de cohorte, ATUc) programme from 11 March, 2019 to 16 January, 2020.
Methods
Eligible patients were aged 18 years and over with confirmed epithelial ovarian, primary peritoneal or Fallopian tube cancer and a deleterious or suspected deleterious germline or somatic BRCA 1/2 mutation. Patients were in complete or partial clinical response at the end of first-line platinum-based chemotherapy. Olaparib maintenance therapy was initiated within 8 weeks of the patients’ last dose of chemotherapy. Real-world data were collected through treatment access request forms completed by physicians. Clinical and safety data were collected monthly until the end of the ATUc programme.
Results
A total of 107 centres in metropolitan France and the French Overseas Departments and Territories requested the inclusion for 238 patients, of whom 194 received maintenance olaparib. In total, 87.6% of the primary tumour locations were ovary, the most common histology was high-grade serous (93.0%) and the most common International Federation of Gynaecology and Obstetrics (Fédération Internationale de Gynécologie et d’Obstétrique) stage was IIIC (56.8%). BRCA testing was performed in routine practice, prior to inclusion into the ATUc programme. All patients had a BRCA mutation: 52.5% had a somatic mutation, 38.4% had a germinal mutation and 9.1% had germinal and somatic mutations. Twenty-four (12%) patients experienced serious adverse drug reactions at the last safety follow-up (17 February, 2020). The most common were anaemia (12 [6%] patients), neutropenia (3 [2%] patients) and thrombocytopenia (3 [2%] patients).
Conclusions
The rapid enrolment into the ATUc programme highlighted the strong unmet need for patients with ovarian cancer and a BRCA mutation in first-line maintenance treatment. Olaparib was well tolerated and no new safety signals were observed in this real-world patient population.
Key Points
| The results of the Autorisation Temporaire d’Utilisation de cohorte programme correlate with the clinical safety profile of olaparib previously demonstrated and highlight the strong unmet need for patients with ovarian cancer and a BRCA mutation in first-line maintenance treatment. |
| Our “real-world” study provides valuable information on olaparib as maintenance therapy for physicians, patients and policy makers to further improve the outcomes of patients with ovarian cancer. |
| In France, the regulatory procedure for the Autorisation Temporaire d’Utilisation de cohorte allows rapid and fair access to investigational drugs on the presumption of efficacy and safety, bringing life-changing treatments to patients as early as possible. |
Introduction
Advanced ovarian cancer is a serious and life-threatening disease, and is the leading cause of death from gynaecological cancers in France, ranking as the fourth most common cause of cancer death in women [1]. Currently, the 5-year survival rate for patients with advanced ovarian cancer is 38% [2]. Standard of care for patients with newly diagnosed, advanced ovarian cancer consists of cytoreductive surgery and platinum-based chemotherapy, which is generally given for six cycles [3–5]. However, before this cohort Temporary Authorisation for Use (Autorisation Temporaire d’Utilisation de cohorte, ATUc), no maintenance therapy had been approved specifically for women with newly diagnosed, advanced ovarian cancer and a BRCA mutation and these patients had the same treatment options as patients without a BRCA mutation [3]. Bevacizumab, a promising anti-angiogenic drug, was approved in December 2011 by the European Medicines Agency and in June 2018 by the US Food and Drug Administration in combination with chemotherapy (carboplatin and paclitaxel) for front-line and maintenance therapy for patients with newly diagnosed ovarian cancer, based on the results of the GOG-0218 and ICON-7 phase III trials that demonstrated a progression-free survival (PFS) benefit [6–8]. However, the correlation between BRCA mutational status and the response to bevacizumab in women with advanced ovarian cancer suggested no evidence of benefit in terms of PFS and overall survival (OS) with regard to bevacizumab maintenance treatment in patients with the BRCA mutation [9]. The French Health Agency, ANSM (Agence Nationale de Sécurité du Médicament et des Produits de Santé), defines the ATUc as a regulatory procedure that allows rapid and fair access to investigational drugs that have demonstrated promising efficacy and safety in clinical trials to patients with an unmet medical need. Until 2021 in France, ANSM evaluated access to innovative drugs prior to their marketing authorisation and could grant an ATUc. Olaparib is a highly selective poly(adenosine diphosphate-ribose) polymerase enzyme inhibitor that generates an accumulation of DNA damage in tumours that have defects in homologous recombination repair, such as those with a mutation in BRCA1 or BRCA2, leading to tumour cell death [10]. Maintenance treatment with olaparib has been shown to provide a substantial benefit in PFS among patients with platinum-sensitive relapsed ovarian cancer in two pivotal trials [11, 12].
The SOLO-1 trial (NCT01844986) aimed to evaluate the efficacy of maintenance monotherapy with olaparib in patients with newly diagnosed, advanced ovarian, primary peritoneal or Fallopian tube cancer with a BRCA1/BRCA2 mutation, who had a complete or partial clinical response after first line platinum-based chemotherapy [13, 14]. In SOLO-1, olaparib demonstrated a significant improvement in PFS compared with placebo: after a median follow-up of 41 months, median PFS was not reached in the olaparib group, as compared with 13.8 months in the placebo group (hazard ratio for disease progression or death, 0.30; 95% confidence interval 0.23–0.41; p < 0.001) [13]. The safety profile in the olaparib group of SOLO-1 was consistent with data from previous olaparib trials [13]. Olaparib was approved by the European Commission on 12 June, 2019, following the SOLO-1 results, as maintenance therapy for adult patients with advanced (International Federation of Gynaecology and Obstetrics, Fédération Internationale de Gynécologie et d’Obstétrique [FIGO] stages III and IV) BRCA-mutated epithelial ovarian, Fallopian tube or primary peritoneal cancer, who had a complete or partial response to their first line of platinum-based chemotherapy. Olaparib is the first poly(adenosine diphosphate-ribose) polymerase inhibitor that has been granted the ATUc. Here, we describe the first real-world data from patients with BRCA-mutated epithelial ovarian cancer who had received maintenance olaparib in the French ATUc programme from 11 March, 2019 to 16 January, 2020.
Methods
ATUc Programme
ANSM granted an ATUc for olaparib on 11 March, 2019, as maintenance monotherapy for adult patients with newly diagnosed, advanced (FIGO stages III or IV), BRCA-mutated high-grade epithelial ovarian, Fallopian tube or primary peritoneal cancer, who were in response (complete or partial) following completion of first-line platinum-based chemotherapy. Each request for the ATUc was assessed and managed by the Regulatory Affairs, Patient Safety and the Medical Oncology Departments of AstraZeneca France, according to the Protocol for Therapeutic Use approved by ANSM.
Study Design
Real-world data were collected as part of the olaparib ATUc. Clinical and safety data from these patients were collected each month until the end of the ATUc (17 February, 2020).
Patients
Patients were eligible if they were 18 years of age and over and had ovarian cancer. Patients had a deleterious or suspected deleterious germline or somatic BRCA 1/2 mutation, had a complete or partial clinical response after first line platinum-based chemotherapy, showed no radiological signs of disease progression on a post-treatment imaging examination and had a normal CA-125 level at the end of chemotherapy. Maintenance therapy with olaparib was planned to start within 8 weeks of the last dose of chemotherapy. Main exclusion criteria included: patient eligible in a clinical trial with ongoing recruitment; haemoglobin <10.0 g/dL; and a blood transfusion occurring within the past 28 days.
Dose and Duration of Treatment
Patients received olaparib tablets (300 mg as two 150-mg tablets), twice daily. The 100-mg tablet was available in case of a dose reduction. Patients could continue their treatment for 2 years or until disease progression. Patients who were in complete response (no radiological signs of the disease) at 2 years should have discontinued their treatment. Patients with signs of disease at 2 years who, according to the physician’s opinion, may have benefited from continuing treatment, could be treated longer than 2 years.
Data Collection
Data were collected in treatment access request forms completed by physicians at the time they requested the ATUc of olaparib. Physicians were also invited to spontaneously report any adverse drug reactions (ADRs) with olaparib to their Regional Pharmacovigilance Centres or to AstraZeneca. Reasons for discontinuation of olaparib treatment could be an adverse event (AE), which is defined as any untoward medical occurrence in a patient participating in the ATUc and that does not necessarily have a causal relationship with olaparib, or an ADR, defined as an AE considered by the treating physician or by AstraZeneca as causally related to olaparib, or a serious ADR, defined as an adverse reaction that results in death, is life threatening, requires in-patient hospitalisation or prolongation of existing hospitalisation, results in persistent or significant disability or incapacity, is a congenital anomaly/birth defect or is considered an important medical event.
Results
Patients
From 11 March, 2019 to 16 January, 2020, 107 centres requested inclusion for 238 patients; 201 requests were accepted, 28 were denied because they did not meet the ATUc criteria and 9 requests were awaiting additional information on 16 January, 2020, and were ultimately deemed as not acceptable for inclusion in the ATUc. The main reason for the ATUc exclusion was that the time between the last chemotherapy cycle and the request was greater than 8 weeks (Table 1) [15]. A total of 194 patients received olaparib maintenance therapy.
Table 1.
Reasons for ATUc non inclusion
| Inclusion criteria not met | N = 28 |
|---|---|
| Start of olaparib maintenance treatment planned more than 8 weeks after day of the last administration of chemotherapy | 17 |
| Patient relapsing from epithelial ovarian cancer | 7 |
| Epithelial ovarian cancer without BRCA mutation | 1 |
| Blood transfusion performed in the last 28 days | 1 |
| Only 3 cycles of chemotherapy | 1 |
| Hemoglobin < 10.0 g / dL | 1 |
Requesters
In total, 164 specialists made at least one request for the ATUc inclusion, across 107 centres in metropolitan France and the French Overseas Departments and Territories. Specialists who requested access for patients in the ATUc were oncologists (89.0%), haemato-oncologists (4.9%) or gynaecologists with cancer expertise (4.3%). Of the 107 sites, 39 (36.4%) were private centres, 34 (31.8%) were general hospitals (CHGs), 20 (18.7%) were comprehensive cancer centres and 13 (12.1%) were university hospitals (CHUs). National coverage is shown in Fig. 1 [15]. Five requests (3.0%) were made by physicians from the French Overseas Departments and Territories: Guadeloupe and Ile de la Réunion.
Fig. 1.
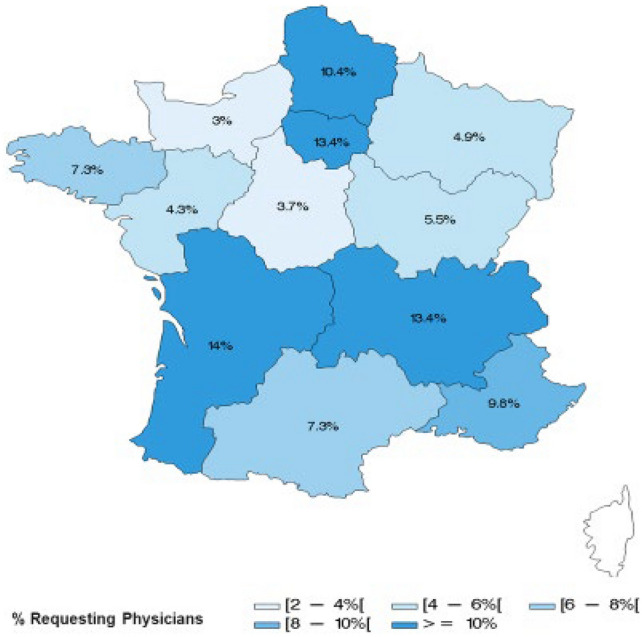
Geographical distribution of requesting physicians in metropolitan France
Patient Characteristics
The mean age of patients included in the ATUc was 62.5 ± 11.0 years (range 32–91 years). Overall, 87.6% of primary tumours were located in the ovary, the most common histology was high-grade serous (93.0%) and the most common FIGO stage was IIIC (56.8%). BRCA testing was performed in routine practice, prior to the ATUc inclusion, and 38.4% of patients had a germline BRCA mutation, 52.5% had a somatic BRCA mutation and 9.1% had germline and somatic BRCA mutations. After platinum-based chemotherapy, 83.8% of patients had a complete clinical response and 16.2% had a partial response (Table 2) [15].
Table 2.
Patient characteristics
| Characteristics | N = 201 (%) |
|---|---|
| Age, mean years (range) | 62.5 ± 11 (32–91) |
| ECOG performance status, n (%) | |
| 0 | 113 (56.2) |
| 1 | 83 (41.3) |
| 2 | 5 (2.5) |
| Primary tumor location, n (%) | |
| Ovary | 176 (87.6) |
| Primary peritoneal | 13 (6.5) |
| Fallopian tubes | 6 (3.0) |
| Histology, n (%) | |
| High-grade serous | 187 (93.0) |
| High-grade endometrioid | 3 (1.5) |
| Other | 11 (5.5) |
| FIGO stage, n (%) | |
| IIIA | 17 (8.5) |
| IIIB | 13 (6.5) |
| IIIC | 113 (56.8) |
| IV | 56 (28.1) |
| BRCAm status, n (%) | |
| Germinal mutation | 76 (38.4) |
| Somatic mutation | 104 (52.5) |
| Germinal & somatic mutations | 18 (9.1) |
| BRCA1 mutation | 126 (62.7) |
| BRCA2 mutation | 72 (35.8) |
| BRCA1 & BRCA2 mutations | 3 (1.5) |
| History of cytoreductive surgery, n (%) | 168 (83.6) |
| Upfront surgery | 53 (31.9) |
| Interval cytoreductive surgery | 108 (65.1) |
| Complete resection after surgery (of the 168 patients) | 148 (90.8) |
| Response after platinum-based chemotherapy, n (%) | |
| Complete response | 166 (83.8) |
| Partial response | 32 (16.2) |
Previous Treatments for Ovarian Cancer
All patients included in the ATUc received carboplatin as platinum-based chemotherapy prior to the initiation of olaparib. Carboplatin was administered alone in 1.5% of patients, or in combination with paclitaxel in 96.5% of patients, or with pegylated liposomal doxorubicin in 1.5% of patients, or with gemcitabine in 0.5% of patients. The median time from the end of chemotherapy to the ATUc inclusion was 6.4 weeks. Of the 201 patients included, 14 (7.0%) received platinum-based chemotherapy in combination with bevacizumab. Treatment with bevacizumab was discontinued prior to the initiation of olaparib in the ATUc.
Duration of Olaparib Treatment
The duration of treatment with olaparib was defined as the time from the olaparib initiation and either the discontinuation of treatment or the end of the ATUc period (17 February, 2020), also corresponding to the median duration of follow-up. A total of 194 patients were exposed to olaparib as part of the ATUc programme. On 17 February, 2020, 180 (92.8%) patients were still receiving treatment and the median duration of follow-up was 5.31 months (range 0.03–11.07 months). At the last safety follow-up (17 February, 2020), 14 patients (7.8%) had discontinued olaparib treatment for one or more of the following reasons: three (1.7%) adverse events (AEs) [asthenia (n = 2) and dysgeusia (n = 1)]; although dysgeusia and asthenia are known ADRs of olaparib, both were collected as AEs in this analysis, five (2.8%) ADRs (olaparib-related AEs assessed by the reporting physician or AstraZeneca if not reported) [anaemia (n = 2), hypersensitivity (n = 1), dizziness (n = 1) and fatigue (n = 1)], five (2.8%) disease progressions, one (0.56%) patient decision and two (1.1%) unknown reasons.
Safety and Tolerability
The safety population was defined as all patients treated with olaparib as part of the ATUc programme or for whom a compassionate use was granted by AstraZeneca (six patients who were integrated in the safety analysis) and who experienced one or several AEs or ADRs. There was no active collection of AEs or ADRs and participating physicians were requested to report ADRs that occurred with olaparib to their Regional Pharmacovigilance Centres.
The safety data presented (Table 3) include all AEs reported spontaneously to AstraZeneca, with a reporting period from the beginning of the ATUc to 17 February, 2020. Adverse events of any cause and grade occurred in 128 patients who received olaparib. Adverse drug reactions of any grade occurred in 70 patients. Serious ADRs occurred in 24 patients (Table 3) [15]. The most common reported serious ADRs with olaparib were anaemia (n = 12), neutropenia (n = 3) and thrombocytopenia (n = 3). No myelodysplastic syndrome (MDS)/acute myeloid leukaemia (AML) was reported, and there were no reports of AEs leading to death. One or more serious ADRs may have occurred in the same patient.
Table 3.
Serious ADRs reported at the latest safety follow-up. One or more serious ADRs may have occurred in the same patient. N = 24 patients, N total events = 27 (n = 24 patients; February 17, 2020)
| Preferred Term | Number of events |
|---|---|
| Anaemia | 12 |
| Neutropenia | 3 |
| Thrombocytopenia | 3 |
| Asthenia | 1 |
| Hypersensitivity | 1 |
| Creatinine renal clearance decreased | 1 |
| Full blood count abnormal | 1 |
| Hemoglobin decreased | 1 |
| Platelet count decreased | 1 |
| White blood cell count decreased | 1 |
| Neuropathy peripheral | 1 |
| Lung disorder | 1 |
Discussion
“Real-world data” refer to information on the utilisation and outcome of specific new treatments and technologies in clinical practice [16]. While real-world evidence including population-based studies will never replace randomised clinical trials, real-world evidence is valuable as it reflects the safety and effectiveness of treatments from clinical practice and thereby provides an assessment of benefit to the population treated outside of clinical trials [17, 18]. Valuable additional information for physicians, patients and policy makers is provided through an understanding of the outcomes in the “real-world” population treated. There is indeed evidence of improved population outcomes from ovarian cancer treatment, and more such studies are needed [16]. The non-interventional ENCOURAGE prospective cohort study assessed bevacizumab administration as first-line treatment for ovarian cancer and outcomes in the French real-world setting. Results indicated consistency between clinical outcomes and tolerability with bevacizumab in a real-world setting and randomised clinical trials [19].
We described here the ATUc programme, the first French real-world data through the ATUc with olaparib as maintenance therapy of adult patients with advanced BRCA-mutated epithelial ovarian, Fallopian tube and peritoneal cancer who were in response following completion of first line platinum-based chemotherapy. In the phase III SOLO-1 trial, 391 patients underwent randomisation (2:1); 260 were assigned to receive olaparib tablets (300 mg twice daily) and 131 to receive placebo; 391 patients were included in the safety analyses. The primary endpoint was PFS as assessed by investigators [13]. In the ATUc programme, 83.8% of patients had a complete clinical response after platinum-based chemotherapy compared with 82.0% in SOLO-1, and 16.2% had a partial response (vs 18.0% in SOLO-1). Regarding the tumour, 87.6% of primary locations were in the ovary in the ATUc programme, as compared with 85.0% in SOLO-1; the most common histology was high-grade serous (93.0% in the ATUc programme, as compared with 95.0% in SOLO-1); and the most common tumour FIGO stage was III (71.8% in the ATUc, as compared with 85.0% in SOLO-1). The BRCA1 mutation was found in 62.7% of patients in the ATUc versus 73.0% in SOLO-1, the BRCA2 mutation in 35.8% in the ATUc versus 25.0% in SOLO-1, and BRCA1 and BRCA2 mutations in 1.5% of patients in the ATUc versus 1% in SOLO-1. The germinal, somatic, and germinal and somatic mutation proportions in patients were not specified in SOLO-1 [13]. As previously reported, the use of maintenance therapy with olaparib in SOLO-1 led to a significant increase in PFS compared with placebo, both in the primary analysis (median follow-up of 41 months) and after 5 years [13, 14]. Despite similar hazard ratios for disease progression or death in SOLO-1 regardless of the Eastern Cooperative Oncology Group Performance Status (ECOG PS) 0 or 1 [13], we can notice the difference in the ECOG PS 0 population (77% in SOLO-1 vs 56.2% in the ATUc), suggesting an impact on tolerance, yet the safety profile of olaparib observed in the ATUc was consistent with the known safety profile of SOLO-1. Indeed, if the patients are in a poorer general condition or with residual toxicities linked to chemotherapy with consequently an ECOG PS of 1 or 2, then the tolerance of olaparib may be worse compared with patients with an ECOG PS of 0. Regarding the safety and tolerability, the safety profile in the olaparib group of the phase III SOLO-1 trial was consistent with previous olaparib data [13]. Acute myeloid leukaemia occurred in 1.0% of patients in the olaparib group and in none in the placebo group. No AEs that occurred during the trial intervention resulted in death [13].
After a 7-year follow-up, the safety profile of olaparib as maintenance therapy was consistent with that previously reported at the data cut-offs [13, 14]. A total of four (1.5%) cases of MDS/AML were reported in the olaparib group and one (0.8%) case of MDS/AML was reported in the placebo group after a 7-year follow-up. This highlights the low incidence [20]. In the ATUc programme, the most common serious ADRs reported in patients with olaparib were anaemia (6.0%), neutropenia (2.0%) and thrombocytopenia (2.0%). No MDS/AML was reported and no AE resulted in death. As of 17 February, 2020, 180 out of 194 patients from the ATUc were still receiving treatment and could continue to receive optimal treatment until disease progression; 14 patients had discontinued olaparib treatment at the last safety follow-up (17 February, 2020). In terms of health-related quality of life in SOLO-1, the substantial PFS benefit was reported to be achieved with no detrimental effect on patients’ health-related quality of life and was supported by clinically meaningful quality-adjusted PFS and time without significant symptoms of toxicity benefits with maintenance olaparib versus placebo [21].
In addition to a statistically and clinically significant improvement in PFS compared with placebo, a median OS advantage was seen with maintenance olaparib versus placebo in patients with the BRCA mutation [22, 23]. Furthermore, a descriptive analysis of OS after a 7-year follow-up in SOLO-1 reported the longest follow-up for any poly(adenosine diphosphate-ribose) polymerase inhibitor in newly diagnosed, advanced ovarian cancer. At the time of the data cut-off (the 7-year timepoint), the median OS was not reached in the olaparib group, compared with a median OS of 75.2 months in the placebo group [20]. Finally, results from the 7-year follow-up indicated a clinically meaningful improvement in OS with maintenance olaparib versus placebo in women with newly diagnosed, advanced ovarian cancer and a BRCA mutation, with no new safety signals detected [20].
Conclusions
The enrolment of 201 patients in the ATUc programme in only 10 months highlighted the strong unmet need for new first-line maintenance treatments in patients with BRCA-mutated ovarian cancer. Olaparib was well tolerated and no new safety signal was observed in this real-world patient population. These results correlate with the long-term safety profile of olaparib observed with the updated SOLO-1 results [20].
Declarations
Funding
The work was funded by AstraZeneca France (part of an alliance between AstraZeneca and Merck Sharp & Dohme (MSD)).
Conflicts of Interest
CB, DB and CF declare no conflicts of interest. LG declares honoraria and consulting/advisory board work for AstraZeneca. FL declares honoraria for consulting or advisory board work for Daiichi Sankyo, Lilly, Seagen, Novartis, Pfizer, Roche and Sandoz and travel fees from Daiichi Sankyo, Lilly, Novartis, Pfizer and Pierre Fabre and serves as a speaker for Amgen, Lilly, Novartis and Pierre Fabre. GF declares honoraria and advisory board work for AstraZeneca, BMS, Clovis Oncology, GSK, Lilly, MSD, Novartis and Pfizer. AF declares honoraria and congress and presentation work for AstraZeneca, GSK and Clovis Oncology. MK declares honoraria as a speaker for AstraZeneca and consultancy work for GSK.
Ethics Approval
Not applicable. The ATUc programme is not a clinical trial and thus does not require ethics approval.
Consent to Participate
Not applicable. The ATUc programme is not a clinical trial and therefore patient consent was not required. The patients did receive a patient information leaflet.
Consent for Publication
Not applicable.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Authors’ Contributions
The ATUc programme is not a clinical trial. Therefore, there is no study conception, design or material preparation. However all authors contributed to the data collection and analysis and writing of the article and the current version is approved by all authors.
References
- 1.Institut National du Cancer. Recommandations professionnelles: cancer de l’ovaire. Traitement chirurgical. Juin 2019. Available from: www.e-cancer.fr. [Accessed 19 Dec 2022].
- 2.De Angelis R, Sant M, Coleman MP, Francisci S, Baili P, Pierannunzio D, et al. Cancer survival in Europe 1999–2007 by country and age: results of EUROCARE-5: a population-based study. Lancet Oncol. 2014;15(1):23–34. doi: 10.1016/S1470-2045(13)70546-1. [DOI] [PubMed] [Google Scholar]
- 3.Ledermann JA, Raja FA, Fotopoulou C, Gonzalez-Martin A, Colombo N, Sessa C, et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(6):24–32. doi: 10.1093/annonc/mdt333. [DOI] [PubMed] [Google Scholar]
- 4.Lambert HE, Rustin GJ, Gregory WM, Nelstrop AE. A randomized trial of five versus eight courses of cisplatin or carboplatin in advanced epithelial ovarian carcinoma: a North Thames Ovary Group study. Ann Oncol. 1997;8(4):327–333. doi: 10.1023/a:1008256431090. [DOI] [PubMed] [Google Scholar]
- 5.Bookman MA, Brady MF, McGuire WP, Harper PG, Alberts DS, Friedlander M, et al. Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: a phase III trial of the Gynecologic Cancer Intergroup. J Clin Oncol. 2009;27(9):1419–1425. doi: 10.1200/JCO.2008.19.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haunschild CE, Tewari KS. Bevacizumab use in the frontline, maintenance and recurrent settings for ovarian cancer. Future Oncol. 2020;16(7):225–246. doi: 10.2217/fon-2019-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marchetti C, Muzii L, Romito A, Benedetti PP. First-line treatment of women with advanced ovarian cancer: focus on bevacizumab. Onco Targets Ther. 2019;8(12):1095–1103. doi: 10.2147/OTT.S155425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colomban O, Tod M, Peron J, Perren TJ, Leary A, Cook AD, et al. Bevacizumab for newly diagnosed ovarian cancers: best candidates among high-risk disease patients (ICON-7) JNCI Cancer Spectr. 2020;4(3):26. doi: 10.1093/jncics/pkaa026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lorusso D, Marchetti C, Conte C, Giudice E, Bolomini G, Vertechy L, et al. Bevacizumab as maintenance treatment in BRCA mutated patients with advanced ovarian cancer: a large, retrospective, multicenter case-control study. Gynecol Oncol. 2020;159(1):95–100. doi: 10.1016/j.ygyno.2020.07.022. [DOI] [PubMed] [Google Scholar]
- 10.O'Connor MJ. Targeting the DNA damage response in cancer. Mol Cell. 2015;60(4):547–560. doi: 10.1016/j.molcel.2015.10.040. [DOI] [PubMed] [Google Scholar]
- 11.Pujade-Lauraine E, Ledermann JA, Selle F, Gebski V, Penson RT, Oza AM, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(9):1274–1284. doi: 10.1016/S1470-2045(17)30469-2. [DOI] [PubMed] [Google Scholar]
- 12.Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366(15):1382–1392. doi: 10.1056/NEJMoa1105535. [DOI] [PubMed] [Google Scholar]
- 13.Moore K, Colombo N, Scambia G, Kim BG, Oaknin A, Friedlander M, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379(26):2495–2505. doi: 10.1056/NEJMoa1810858. [DOI] [PubMed] [Google Scholar]
- 14.Banerjee S, Moore KN, Colombo N, Scambia G, Kim BG, Oaknin A, et al. Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021;22(12):1721–1731. doi: 10.1016/S1470-2045(21)00531-3. [DOI] [PubMed] [Google Scholar]
- 15.Bellier C, Gladieff L, Le Du F, Garnier C, Berton D, Bonnard C, et al. First real-life data on olaparib in 1st line maintenance BRCA1/2 mutated epithelial ovarian cancer in France: descriptive analysis of 201 patients enrolled in the Cohort Temporary Authorization for Use (ATUc). Presented at the Virtual European Society for Medical Oncology Congress; 2020.
- 16.Eisenhauer EA. Real-world evidence in the treatment of ovarian cancer. Ann Oncol. 2017;28(8):61–65. doi: 10.1093/annonc/mdx443. [DOI] [PubMed] [Google Scholar]
- 17.Cecere SC, Giannone G, Salutari V, Arenare L, Lorusso D, Ronzino G, et al. Olaparib as maintenance therapy in patients with BRCA 1–2 mutated recurrent platinum sensitive ovarian cancer: real world data and post progression outcome. Gynecol Oncol. 2020;156(1):38–44. doi: 10.1016/j.ygyno.2019.10.023. [DOI] [PubMed] [Google Scholar]
- 18.Romero I, López-Guerrero JA, Pignata S. Real-world experience with trabectedin for the treatment of recurrent ovarian cancer. Expert Rev Anticancer Ther. 2021;21(10):1089–1095. doi: 10.1080/14737140.2021.1941890. [DOI] [PubMed] [Google Scholar]
- 19.Berton D, Floquet A, Lescaut W, Baron G, Kaminsky MC, Toussaint P, et al. Real-world experience of bevacizumab as first-line treatment for ovarian cancer: the GINECO ENCOURAGE cohort of 468 French patients. Front Pharmacol. 2021;20(12):711813. doi: 10.3389/fphar.2021.711813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DiSilvestro P, Banerjee S, Colombo N, Scambia G, Kim BG, Oaknin A, et al. Overall survival with maintenance olaparib at a 7-year follow-up in patients with newly diagnosed advanced ovarian cancer and a BRCA mutation: the SOLO1/GOG 3004 trial. J Clin Oncol. 2022;2:2201549. doi: 10.1200/JCO.22.01549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedlander M, Moore KN, Colombo N, Scambia G, Kim BG, Oaknin A, et al. Patient-centred outcomes and effect of disease progression on health status in patients with newly diagnosed advanced ovarian cancer and a BRCA mutation receiving maintenance olaparib or placebo (SOLO1): a randomised, phase 3 trial. Lancet Oncol. 2021;22(5):632–642. doi: 10.1016/S1470-2045(21)00098-X. [DOI] [PubMed] [Google Scholar]
- 22.Poveda A, Floquet A, Ledermann JA, Asher R, Penson RT, Oza AM, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a final analysis of a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2021;22(5):620–631. doi: 10.1016/S1470-2045(21)00073-5. [DOI] [PubMed] [Google Scholar]
- 23.Ledermann JA, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Overall survival in patients with platinum-sensitive recurrent serous ovarian cancer receiving olaparib maintenance monotherapy: an updated analysis from a randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Oncol. 2016;17(11):1579–1589. doi: 10.1016/S1470-2045(16)30376-X. [DOI] [PubMed] [Google Scholar]


