Abstract
Pullulan is a commercially available exopolymer biosynthesized by Aureobasidium pullulans supplemented with nitrogen, carbon and other vital components through submerged and solid-state fermentation. These nutrients are very expensive and it raises the cost for the production of pullulan. Hence, the need of alternative cost-effective raw materials for its production is a prerequisite. Owing to its unique physicochemical features, pullulan has various applications in the food, pharmacological, and biomedical domains. Food industrial wastes generate a considerable number of by-products which accumulates and has a negative influence on the environment. These by-products are made up of proteins, carbohydrates, and other components, can be employed as substrates for the production of pullulan. The present review briefs on the pullulan production using food processing waste and by-products and the elements that impact it. It provides an insight into versatile applications of pullulan in food industries. Various challenges and future prospects in the field of research on pullulan production have been uncovered.
Keywords: Pullulan, Aureobasidium pullulans, Food processing wastes, Sustainability
Introduction
Pullulan is an inert, linear polysaccharide generated aerobically over sugar and starch conditions by Aureobasidium pullulans, a yeast-like microorganism with gene mutations. The molecule is structured of repeated units of maltotriose coupled by 1,6-glycosidic interconnections to three 1,4-linked glucose molecules (Singh et al. 2017, 2021; Mishra and Varjani 2019). A stair-step structure is formed by this repeating pattern. Chain flexibility and solubility are enhanced by the regular modification of -1,4 and -1,6 bonds (Hamidi et al. 2019; Vivek et al. 2020). Nitrogen source, carbon source, and other functional ingredients for A. pullulans are required for fermentative biosynthesis of pullulan. It is a ‘generally considered as safe’ (GRAS) excipient since it is innocuous, non-immunogenic, noncarcinogenic, and non-mutagenic (Mishra and Suneetha 2014; Mishra et al. 2018; Liu et al. 2020). It serves as a low-calorie dietary fibre alternative for starch in food preparations. Molding a wet pullulan solution on a flat surface produces good films with minimal oxygen permeability (Raychaudhuri et al. 2020; Priyadarshi et al. 2021). Pullulan granules are crystalline, non-hygroscopic, whitish, and breakdown promptly in both hot and cold water. In opposed to dextran, pullulan degrades much more quickly in blood serum (Tabasum et al. 2018). For its non-animal origin, pullulan is appropriate for all consumer groups. Chewing gum and bubble gum contain this as an exfoliant and glazing agent. It’s also utilised in milk-based sweets as a foaming ingredient (Singh et al. 2017; Mishra and Varjani 2019).
The nutrients needed in the synthesis of pullulan are costly. It adds to the expense of production (Mishra and Varjani 2019). However, many food processing industries generate waste enriched with inorganic and organic compounds essential for A. pullulans to flourish. Food processing and agribusiness dwellers engender a significant amount of waste, which, if disposed of untreated, can result in serious ecological concerns (Mishra et al. 2018; Varjani et al. 2020, 2021; Vyas et al. 2022; Yaashikaa et al. 2022). On a global scale, it is statistically found that nearly one-third of all food residues is wasted, equivalent to 1.3 billion tonnes of food every year. Furthermore, lost or wasted food generates roughly 3.49 billion tonnes of greenhouse emissions across the supply chain (FAO 2019). Landfilling, composting, thermal treatment is among the most common waste management technique now in use. A multitude of food industrial by-products has been documented to produce pullulan (Mishra et al. 2018; Vivek et al. 2020; Abdeshahian et al. 2021; Wani et al. 2021). The volarization methods for food industrial wastes have been illustrated in Fig. 1.
Fig. 1.

Food industrial waste volarization methods
Due to its higher cost (Approximately, Rs 3000–6000 per kg in India), pullulan is underutilised in comparison to other exopolysaccharides. This biopolymer is imported into India from China, Japan, and the United States. To meet market demand, it is necessary to boost pullulan production on a pilot scale using low-cost and environmentally friendly methods. The present review describes the utilization of various food processing waste and its by-products for efficient production of pullulan and its applications. These residues can be utilized as an alternate substrate to produce pullulan through solid-state fermentation or submerged fermentation.
Biosynthesis of pullulan
Despite the fact that pullulan’s chemical composition was discovered in the 1960s and it has been involved in the production and exploited in the medicaments, cosmetics, and food sectors for over 40 years, its biosynthetic mechanism had remained a mystery for decades (Mishra et al. 2011). Despite this, many efforts have been made to decipher its synthesis route, as well as the necessary enzymes and genes that encode it.
Microbial sources
Because of its high yield and excellent pullulan characteristics, Aureobasidium pullulans is one of the most extensively utilised strains in commercial pullulan production. Aureobasidium pullulans is a genetically distinct yeast-like fungus that can often be encountered in freshwater, wood, soils, rock, and animals and plants tissues, besides other places. It is harmful to plants but non-pathogenic to people, but only a few strains of A. pullulans are pathogenic and can cause health problems (Singh et al. 2019). Amylases, esterases, hemicellulases, pectinases, proteases, and other enzymes are produced by A. pullulans isolates (Singh and Saini 2012). The synthesis of pullulan via, blastospores and hyphal cells in submerged fermentation, and other aspects of A. pullulans’ development cycle were examined. A few investigations have also shown that different A. pullulans strains produce dissimilar pullulans (composition and structure). Apart from the polysaccharide pullulan, A. pullulans generate a dark pigment known as melanin, which gives antimicrobial properties to phagocytosis in the recipient and also causes polysaccharide chlorosis (Mishra et al. 2018; Singh et al. 2019; Wani et al. 2021). Various parameters, such as the ATP/ADP ratio, knocking out the PKSIII (Polyketide Synthase III) gene, incorporating desired genes into genomic DNA, and others, have been found to boost pullulan productivity and diminish melanin synthesis in metabolic engineering (Li et al. 2016).
There are several physicochemical ways for removing melanin from fermented media (adsorption with solvents, activated charcoal, and salts), however, the cost must be considered. To reduce capital investment, strains must be altered, metabolisms must be engineered. However, care must be taken to preserve the strain’s potential for producing pullulan with good viscosity, molecular weight distribution, and other physical features (Seviour et al. 2011; Castillo et al. 2015; Reddy et al. 2021). Rhodotorula bacarum, Rhodosporidium paludigenum, Cyttaria darwinii, Cyttaria harioti, Cryphonectria parasitica, Aspergillus japonicus, Teloschistes flavicans, Tremella mesenterica, Micrococcus leuteus are among the strains capable of producing pullulan (Mishra et al. 2018). In an attempt to optimise A. pullulans' pullulan productivity, the strain must be investigated through mutation and metabolic engineering. Some of the strains, like Aureobasidium mousonni (NCIM 1226), Aspergillus japonicus-VITSB1, were modified utilising Ethyl methane sulfonate (EMS) and UV rays’ mutagenesis for good yields and enhanced level of pullulan (Mishra and Suneetha 2014).
Mechanism of pullulan synthesis
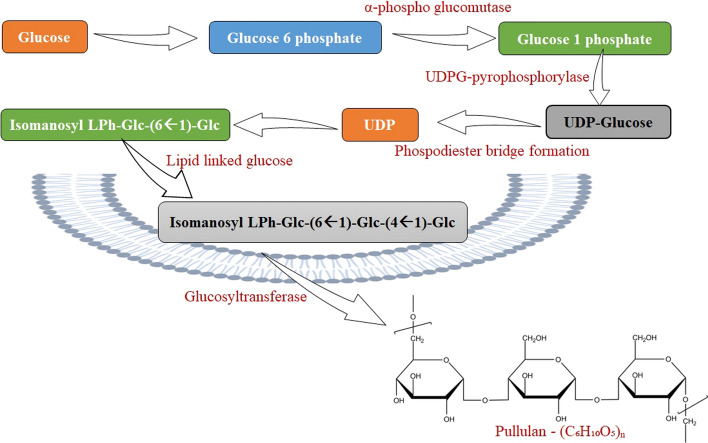
Within the cell, pullulan is produced and extravasated into the medium as a slimy, loose, and amorphous layer through the -glucan layer. The microbe’s creation of the precursor will speed up the formation of pullulan. Pullulan is made up of units of maltitriose joined together by a -1,4 glycosidic connection, whereas -1,6 glycosidic bonds connect the succeeding maltotriose units. The connection offers great structural flexibility as well as increased pullulan solubility (Dailin et al. 2019; Liu et al. 2021). Pullulan biosynthesis is a multistep biological reaction in A. pullulans. Pullulan is synthesised through the adjudication of sugar-nucleotide-lipid transport medians in the membrane of the cell section. Because of the complex properties of the microorganism that generates pullulan, the specific method of the pullulan biosynthetic pathway has not been fully elucidated. Pullulan synthesis is aided by the accumulation of glucose in the cell during the early stages of fermentation. Phosphoglucomutase, glucosyltransferase, and Uridine diphosphate glucose pyrophosphorylase (UDPG-pyrophosphorylase) are the major enzymes engaged in the synthesis of pullulan. The enzymes phosphoglucomutase and UDPG-pyrophosphorylase convert subtle carbon (glucose) to Uridine Diphosphate glucose, which is a necessary prerequisite of pullulan synthesis. Hexokinase helps to combine glucose-6-phosphate with glucose, further converted to glucose-1-phosphate by the enzyme -phosphoglucomutase. The D-glucose in Uridine Diphosphate-glucose generates an isomaltosyl residue when it mixes with additional glucose units. The isopanosyl moiety is produced by the metabolic interaction between iso-maltosyl and lipid-linked glucose, which is subsequently polymerized by the glucosyltransferase enzyme to make pullulan polysaccharide (Duan et al. 2008; Sugumaran and Ponnusami 2017; Mishra et al. 2018; Singh et al. 2019). The generation of phosphodiester links from UDP-glucose, the development of isomaltose monomers, and the manufacture of iso-panosyl molecules are the different phases of pullulan chain biosynthesis. Figure 2 depicts the biosynthetic routes for the formation of pullulan.
Fig. 2.
Mechanism of pullulan synthesis
Utilization of food processing waste for pullulan production
Sugarcane bagasse and molasses
Sugarcane is among the most widely grown in cultivation in India and other parts of the world. Sugarcane bagasse is produced when cane pulp is harvested for the production of refined sugar and its by-products. Bagasse is produced in the amount of 280 kg each tonne of sugar churns out amounting to about 10 crore tonnes annually. Cane biomass is a lignocellulosic substance made up of, hemicellulose (27.89 ± 2.68%), cellulose (38.59 ± 3.45%), organic matter (1.61 ± 0.16%), lignin (17.79 ± 0.62%), and ashes (8.80 ± 0.02%) (Cheng and Zhu 2013). Hydrolysis of cellulose from cane biomass transforms plant-derived dry sugars into basic sugars that can be used by a wide range of microorganisms. Sulfuric acid was used to hydrolyse the vaporised cane biomass at 100 °C for 30 min, and at 28 °C, activated charcoal was used to detoxify the digestate, with continuous vertexing (50 rpm) for 4 h. The hydrolysate was 12% glucose, 7% arabinose, 70% xylose, and 11% other chemicals, and it was utilised by A. pullulans to produce pullulan. The addition of DL-dithiothreitol (1.0 mM) to a sugarcane bagasse hydrolysate-based medium and pH control improved pullulan generation in shake-flask fermentation processes (Chen et al. 2014). Pullulan generation by Aureobasidium pullulans is coupled with the creation of melanin, which drives up the cost of downstream treatments. Deploying a blue LED entirely prevents melanin formation throughout the fermentation procedure, while a red LED promotes A. pullulans development. In shake-flask fermentation processes and column bubble photobioreactors, sugarcane bagasse hydrolysate was employed to produce pullulan by A. pullulans. Pullulan yield in column bubble photobioreactors (25.19 g/L) was comparable to shake-flask fermentations (Hilares et al. 2019).
Molasses is a dusky viscous fluid that forms as an offshoot of the sugarcane juice refining process. The sugar factory releases a large quantity of molasses into the local water source, causing significant contamination. Molasses is made up of fermentable sugars like total solids (70–85%), glucose and fructose (48–60%), organic content (9–12%) (Singh et al. 2019). Molasses may be readily absorbed as a substrate of carbon for the formation of pullulan by A. pullulans because of these sugars. Molasses, on the other hand, contains heavy metals (iron, manganese, copper, zinc, magnesium, calcium, and so on), which inhibit the development of microbes, suppress beneficial enzymes, and reduce the end yield of the product (Mishra et al. 2018). As a result, molasses pretreatment is an important step in achieving a high-quality and high-quantity product output. The best approach for removing heavy metals is to treat molasses with sulphuric acid. Sulphuric acid (1 N) was appended to molasses as a pretreatment, after which the mixture has been left to exist for 24 h before centrifugation was used to extract the supernatant (Singh et al. 2019). The use of activated carbon in conjunction with sulfuric acid aids in the expelling of excess colouring compounds, amino acids, and heavy metals, improving pullulan synthesis at the shake-flask level. Pullulan manufacture is cost-effective when pretreated molasses is used as the production medium (Srikanth et al. 2014).
Potatoes and sweet potatoes residues
The starch grain is found in the cells of the potato root tuber. The potato starch business has released a significant quantity of waste residue, which comprises leachates and potato residues. This has big repercussions for the ecosystem. Carbohydrates are the primary elements of potato starch waste. These effluents have a chemical oxygen demand (COD) that was found to be greater than 30 g/L, indicating that they are high in eco-friendly elements (cellulose, starch, and proteins) that microorganisms may use. The utilization of potato starch waste for the synthesis of pullulan by using the strain of A. pullulan P56 was investigated by some researchers (Mishra et al. 2018). Amyloglucosidase and Pullulanase enzymes (Ca-alginate immobilised form) were used to liquefy potato starch in a packed bed reactor. The threshold pullulan generation was discovered to be 19.2 g/L, and after optimising several course criteria, the output was enhanced by 20% over the preliminary level (Mishra et al. 2018). It was observed by combining potato starch hydrolysate with sucrose improved pullulan synthesis, and that a minuscule portion of sucrose could trigger the enzymes required for pullulan fabrication, allowing for more effective potato starch hydrolysate conversion. It was also looked at using crude potato starch hydrolysates for pullulan synthesis. After 96 h of fermentation, the highest pullulan manufacture was reported to be 36.17 g/L. Pullulan production was compared using glucose and sucrose as carbon sources, yielding 22.07 g/L and 31.42 g/L of pullulan, respectively (Wu et al. 2016). These observations highlight the possibility of using fresh potato starch hydrolysates as an affordable provenance of carbon for producing pullulan.
Sweet potato is a carbohydrate-rich, beta-carotene-rich, vitamin-rich, and fibre-rich tuberous root vegetable. Proteins account for 87% of the sweet potato hydrolysate, followed by sugar (1.56%), blubber (0.6%), coarse fibre (0.16%), and cinders (2.19%). Sweet potato is mostly made up of starch, which is well suited to industrial fermentation despite the fact that many industrially significant microbes cannot use it in its natural state. The same procedure is used to hydrolyse sweet potato starch as it is for potato starch. Small bits of sweet potato are treated with separate enzymes (amylase, pullulanase, and β-amylase) during the saccharification process. Because sweet potatoes contain a significant quantity of β-amylase, it is not necessary for accentuating another resource. The sweet potatoes are treated with β-amylase and pullulanase in the first phase of hydrolysis. β-amylase, which is found in sweet potatoes, might further saccharify the hydrolysate. In fermentation processes, saccharine potato hydrolysate can be employed as an economical base for carbon. A. pullulans used sweet potato hydrolysate in shake-flask fermentation to produce pullulans (Wu et al. 2009; Mishra et al. 2018). Pullulan derived using sweet potato hydrolysate (3.4 105 Da) had a mol. wt. larger than that obtained from glucose (1.3 105 Da) and sucrose (1.7 105 Da) media. Marine cold-adapted -amylase can successfully hydrolyze sweet potato starch (Wu et al. 2009). Various sugars like isomaltose, maltose, maltotriose, glucose, and other maltooligosaccharides make up the sweet potato hydrolysate. These hydrolysate components have a high interfacial adhesion. In a study, A. pullulans produced more pullulans (36.17 g/L) from sweet potato hydrolysate than it did from glucose (22.07 g/L) or sucrose (31.42 g/L). As a result, sweet potato hydrolysate would be used to produce pullulan at a low cost (Wu et al. 2016).
Grape residues
Grapes are a vital component of the wine and juice industries. Grapes are processed by removing the exocarp and extracting the taille from the mash. Grape extract is generally employed in the creation of bottled goods; however, grape peel and the slash are discarded as grape pomace after processing. Total sugars (85.20%), reducing sugars (3.40%), protein (7.80%), and glucose (1.280%) are all present in grape pomace (Mishra et al. 2018). Acids, colours, and specific salts are also abundant, all of which are employed in the food sector. In its solid form, a grape poultice is difficult to use; however, grape peel and slop extricate is much easier to ply. The grape poultice harvest can be made by pouring boiling water into the grape pomace, blending for 30 min, and then filtering (Singh et al. 2019). Pullulan production by Aureobasidium pullulans using shake-flask fermentation processes was achieved using grape poultice extricate, with a pullulan yield of 22.3 g/L (Israilides et al. 1998). Pullulan made from grape pomace extract is uniformly composed, has a high molecular weight, and has a higher yield.
Other food industrial residues
Sugumaran et al. (2014) conducted research in which four food waste by-products, namely rice and wheat bran, coconut and palm kernels, were identified as nadir carbon sources for A. pullulans pullulan synthesis in the solid state for fermentation (50% moisture content). The ideal carbon source amongst four food waste by-products was palm kernel, which yielded 16 g/L pullulan. Later, using Response Surface Methodology (RSM) with Asian Palm Kernel as a carbon source, they have improved the process variables for pullulan production. The output of pullulan was raised to 30.4 g/L. In conclusion, palm kernel proves to be a minimal substrate for pullulan biosynthesis.
The soy sauce industry produces a lot of soybean pomace, which is a key food waste by-product. Carbohydrates and proteins are the two main components. Despite the fact that soybean pomace is quite useful, it is dumped as dissipate due to the extreme sodium chlorite level (NaCl). This has major consequences for the ecosystem. Furthermore, discarding soybean pomace, which is an abundant wellspring of carbs and proteins, is a major waste of natural deposits. So many studies had been performed with soybean pomace as a source of nitrogen pullulan production by A. pullulan HP-2001 (Mishra et al. 2018; Singh et al. 2019).
Coconut water is indeed a transparent beverage found in the centre of the coconut. It is made up of simple sugars and electrolytes, which are easily absorbed carbohydrates. Coconut milk is made by grating the meat of a ripe coconut into a liquid. Various industries that produce desiccated coconut, copra, as well as items made from coconut meat (Coconut honey, Coco sauce, roasted young coconut, coconut chips, cream, candy, and flour, for example) coconut water and coconut milk are produced as waste. Coconut offshoot is classified as a vital contaminant in nature due to its greater Biological Oxygen Demand (BOD). This environmental issue has piqued current academics’ interest in coconut by-products and prompted their use in the manufacturing of such a pivotal industrial product. Thirumavalavan et al. (2009) investigated utilised coconut milk and water to develop pullulan. Since coconut milk has a greater C/N ratio than coconut water, it has been demonstrated to be somewhat more beneficial for pullulan synthesis.
Jaggery was employed as a carbon source for the manufacture of pullulan by various researchers with A. pullulans CFR-77 and A. pullulans MTCC 2195 (Mishra et al. 2018). A concise delineation regarding the utilization of food processing waste for pullulan production has been highlighted in Table 1.
Table 1.
Comprehensive report on the utilization of food processing waste for pullulan production
| Food processing waste | Microorganism | Fermentation process and process parameters | Pullulan (g/L) | References |
|---|---|---|---|---|
| Beet Molasses | A. pullulans P 56 |
Shake and flask (Submerged) 8 days, 200 rpm, 28 °C |
24.00 | Lazaridou et al. (2002) |
| Beet Molasses | A. pullulans P 56 |
Shake and flask (Submerged) 5 days, 400 rpm, 28 °C, aeration rate 2 vvm |
6.6 | Goksungur et al. (2004) |
| Cassava bagasse | A. pullulans MTCC 2195 |
Shake and flask (Submerged) 5 days, 35 °C, 150 rpm, |
45.00 | Srikanth et al. (2014) |
| Cassava bagasse | A. pullulans MTCC 1991 |
Solid-state fermentation in flask 7 days, 28 °C |
32.00 | Ray and Moorthy (2007) |
| Cassava bagasse | A. pullulans MTCC 2670 |
Solid-state fermentation in flask 5 days, 30 °C |
19.00 | Sugumaran and Ponnusami (2017) |
| Corn steep liquor | A. pullulans RBF 4A3 |
Shake and flask (Submerged) 5 days, 28 °C, 200 rpm |
88.59 | Sharma et al. (2013) |
| Corn steep liquor | A. pullulans ATCC 42,023 |
Shake and flask (Submerged) 5 days, 28 °C, 210 rpm |
65.30 | Hafez et al. (2007) |
| Coconut milk | A. pullulans MTCC 2195 |
Shake and flask (Submerged) 7 days, 28 °C, 200 rpm |
58.00 | Thirumavalavan et al. (2009) |
| De-oiled rice bran | A. pullulans MTCC 6994 |
Shake and flask (Submerged) 7 days, 30 °C, 150 rpm |
54.8 | Singh and Kaur (2019) |
| Grape skin pulp extract | A. pullulans NRRLY 6220 |
Shake and flask (Submerged) 5 days, 28 °C, 200 rpm |
22.3 | Israilides et al. (1998) |
| Jackfruit seeds | A. pullulans NCIM 1049 |
Solid-state in flask 7 days 28 °C |
34.2 | Sugumaran et al. (2013) |
| Jackfruit seeds | A. pullulans MTCC 2195 |
Shake and flask (Submerged) 7 days, 30 °C, 200 rpm |
18.76 | Sharmila et al. (2013) |
| Jatropha seedcake | A. pullulans RBF 4A3 |
Shake and flask (Submerged) 5 days, 28 °C, 200 rpm |
83.98 | Choudhury et al. (2012) |
| Palm kernel | A. pullulans MTCC 2670 |
Solid-state in flask 7 days 30 °C |
18.43 | Sugumaran et al. (2013) |
| Potato starch water | A. pullulans 201,253 |
Stirred tank reactor fermentation (Submerged) 5 days,28 °C, 500 rpm |
54.57 | An et al. (2017) |
| Potato starch water | A. pullulans P 56 |
Shake and flask (Submerged) 5 days, 28 °C, 200 rpm |
19.20 | Goksungur et al. (2011) |
| Rice hull | A. pullulans CCTCCM2012259 |
Stirred tank reactor fermentation (Submerged) 3 days, 28 °C, 400 rpm, aeration rate 3 L min−1 |
22.20 | Wang et al. (2014) |
| Soybean pomace | A. pullulans MTCC 1991 |
Stirred tank reactor fermentation (Submerged) 7 days, 27 °C, 210 rpm, aeration rate 1.25 vvm |
125.7 | Sheoran et al. (2012) |
| Sugarcane bagasse | A. pullulans LB83 |
Shake and flask (Submerged) 4 days, 28 °C, 200 rpm |
15.70 | Hilares et al. (2017) |
| Sugarcane bagasse | A. pullulans LB83 |
Shake and flask (Submerged) 4 days, 25.3 °C, 232 rpm |
25.19 | Hilares et al. (2019) |
| Sugarcane molasses | A. pullulans MTCC 2195 |
Shake and flask (Submerged) 5 days, 35 °C, 150 rpm |
45.0 | Srikanth et al. (2014) |
| Sweet potato hydrolysate | A. pullulans AP329 |
Shake and flask (Submerged) 4 days, 28 °C, 200 rpm |
29.43 | Wu et al. (2009) |
| Whey | A. pullulans ATCC 42,023 |
Shake and flask (Submerged) 5 days, 28 °C, 210 rpm |
12.00 | Hafez et al. (2007) |
| Sesame seed oil cake | A. pullulans KY767024 |
Solid-state in flask 25 °C, 2 h, 200 rpm, |
54.5 | Khodaiyan et al. (2020) |
Fermentative production of pullulan
Different media as well as other process variables influence the pullulan fermentation process. Fermentation media structure, fermentation pattern and duration, arrangement, bioreactor construction, microbial entities, moisture levels, physical properties, morphogenesis, deployable temperature, pH, illuminance, oxygen profile, and other factors might very well impact the efficient implementation of the fermentation process for increased pullulan productivity.
Microbial culture
The form of microbial culture is another crucial aspect that influences pullulan productivity. According to prior publications, A. pullulans seems to be the highest pullulan-producing wild strain ever discovered. The mutant strains facilitated the large-scale execution of reactions under ideal conditions. Other mutant strains aided with the manufacturing of high-molecular-weight pullulan, which increased cell proliferation while reducing melanin pigmentation (Liu et al. 2020). Pullulan was synthesized through coculturing of a strain that produces pullulans, A. Kluyveromyces fragile ATCC 52,466, an insulin degradation strain, and A. pullulans SH 8646. The efficacy of fermentation suggests that the polymer synthesising activity of the currently employed genetically mutated isolates of A. pullulans is practically indistinguishable (Mishra et al. 2018).
Type of fermentation
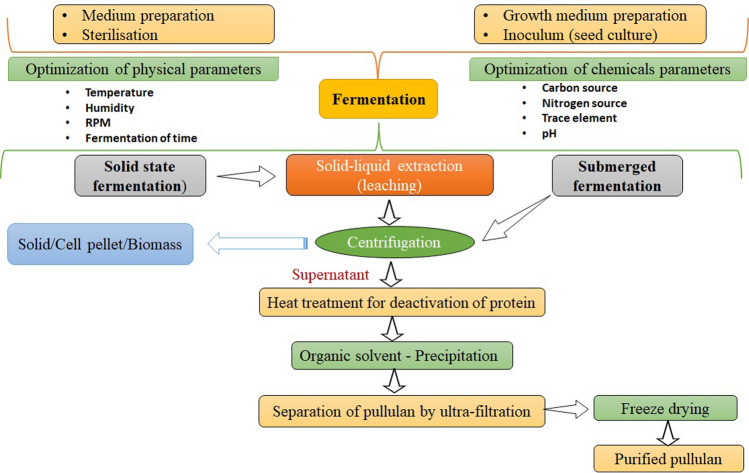
Multiple investigations examined the repercussions of fermentation formats, such as batch, fed-batch, and continuous, on competence of pullulan production. The problem of suppressing the effect of increased concentration of substrate could be avoided by supplying restricting substrates to the medium on an irregular basis. The fed-batch mode, on the other hand, boosted productivity until a certain point but did not exhibit a significant improvement in yield after adding sucrose (Singh et al. 2019; Reddy et al. 2021). Furthermore, within a week of cultivation, the fed-batch technique showed a negligible decline in pullulan concentration. Several investigations have shown that continuous mode is used to produce pullulan Exopolysaccharide production was said to have increased for a long period without causing any difficulties, according to reports. However, in the continual modus operandi, the dilution rates were exceptionally low. In a chemostat, the rates of dilution are indeed a significant parameter that determines biopolymer production. According to the literature, using a chemostat system increased pullulan output albeit at lower dilution rates. Long-term production is possible with continuous fermentation procedures combined with increased cell biomass (Reddy et al. 2021). The process of production of pullulan has been illustrated in Fig. 3.
Fig. 3.
Schematic way to show the fermentative production of pullulan
Bioreactor operation and configuration
The broth makeup and behaviour at various agitation speeds, firm airflow access, and low shear rate, among other aspects, all have a significant impact on the synthesis process in submerged fermentation, resulting in ideal conditions for microbe development. All of the parameters listed above could be manipulated in the bioreactor. As a result, bioreactor configuration plays a critical role in improving pullulan production efficiency. High productivity will be aided by the development of novel and revolutionary fermentation reactors. Different bioreactors, such as the reciprocating plate bioreactor, have been created to accommodate the fermentation process and produce high pullulan productivity. The configuration of the reactor, such as biofilm and suspended culture, has an impact on the biological system’s function and regulates the process (Reddy et al. 2021). To immobilise the strain, transporters for biofilm configuration has been widely used. Despite the multiple benefits of biofilm structure, substrate clumping and other parameters such as inadequate free volume, aeration rate, and so on had an impact on metabolite production (Seviour et al. 2011; Wani et al. 2021).
With the passage of time, the quantity of pullulan generated and its yield change. According to reports, the fermentation period required to achieve optimum pullulan output varies depending on operational circumstances and microbial cultures. As a result, depending on the microbial populations and operating conditions, the best period for producing high pullulan yields ranges from 48 h to 5.36 days (Sugumaran et al. 2014).
Pullulan supplication in food industries
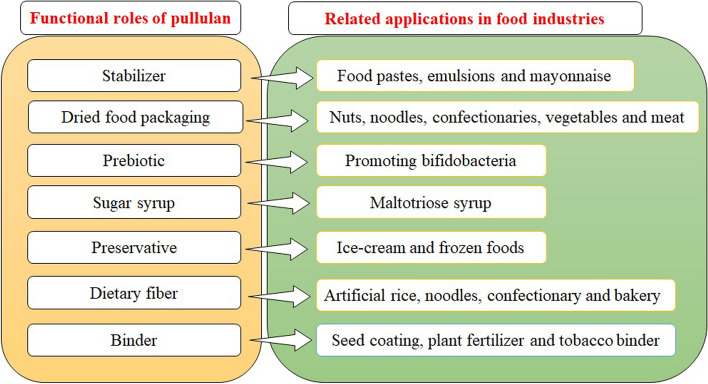
Pullulan is also useful for making edible coatings because it is simultaneously a food ingredient and has the potential to form films due to its properties. In the food industry, Pullulan can be used as a starch substitute in low-calorie food recipes, as well as a food deposition and bottling material. It can also be utilised as a spice and flavouring in microencapsulated seasoning agents (Priyadarshi et al. 2021). Because of its intensifier qualities, it is commonly used in sauces, soups, and beverages. It is often used to keep mayonnaise’s grade and appearance stable (Singh et al. 2019). Pullulan is sometimes used to stick nuts to cookies, as a dental implant adhesive, as a binding material and stabilizing agent in food pastes.
Pullulan can be used as a tobacco binder, seed coat, and plant nutrients (Priyadarshi et al. 2021). Because of its inclusion in the GRAS list and its slow digestion, pullulan can be employed effectively in the development of light (diet) meals. Distributable films dissolve easily in water, giving them the ability to soften as orally potable food toppings. Pullulan films are suitable for protecting rapidly oxidised lipids and vitamins in food because of their oxygen resistance (Abdeshahian et al. 2021). The involvement of pullulan in various foods along with functional roles and related applications have been illustrated in Fig. 4.
Fig. 4.
Various functional roles and applications of pullulan in food industries
Pullulan films can be used to coat or package dried items such as noodles, confections, nuts, meats, and vegetables. As a protective coating, pullulan can be applied directly to food. To stabilise fatty emulsions, pullulan can be replaced by cholesterol or fatty acids (Priyadarshi et al. 2021). Maltotriose syrup can be made utilising the debranching enzyme pullulanase and enzymatic hydrolysis of a polysaccharide pullulan. The following characteristics were used to make maltotriose syrup using pullulanase from pullulan: a. extremely low freezing point recession; b. gentle sweetness; c. moisture retention; d. mitigation of starch retrogradation in foodstuffs; e. less palette formation when collated to maltose or glucose syrups, or sucrose; f. good heat stability. These characteristics are advantageous in the food industry for utilizing pullulan as a substrate as compared to other polysaccharides (Priyadarshi et al. 2021).
Future prospects
Despite its many useful applications, pullulan’s cost, which is 3 times that of other polysaccharides like Xanthan and Dextran, is a major barrier to its utilisation. Previous research has looked into the melanin derivate in generating pullulan, but the cost (25–30 USD/Kg) is a bigger issue. Engineering breakthroughs or effective production lines, particularly with lower melanin production, could help to enhance production economics, hence offering new paths for pullulan use. To improve product quality and to research pullulan biosynthesis in Metabolic Engineering and Molecular Editing, a thorough understanding of the mechanism is essential. Pullulan’s biology holds the key to solving critical downstream and manufacturing issues. Pullulan production in connection to molecular characteristics, upstream genetic regulators, and downstream processes, encompassing innovative bioreactor design, cultivation settings, and uses, has yet to be thoroughly investigated. Pullulan could be a potential source of novel bioactive derivatives in a variety of sectors with further chemical changes. Modified pullulan analogues with various material qualities and pullulan with a specific size distribution can be developed using cutting-edge modification and cultivation technologies. Pullulan is becoming more popular in cancer therapy as a result of new research. The modified pullulan has strong bioactivity with several cytotoxic chemicals and is known to form complexes with those compounds. The build-up of these inclusion complexes at target areas aids the slow release of cytotoxic chemicals. Pullulan is used to replace other synthetic materials that produce CO2 in the medical cosmetics industry because it has no negative side effects. It’s important to see if they can be used in additional personal maintenance and aesthetic purposes with the same polymer, not only as a groundbreaking active component but more as a harmless component for environmentally friendly materials and packaging. Anti-ageing cosmetics appear to have a strong demand. Personal hygiene and aesthetic items should be packaged in environment friendly containers to minimise environmental impact. The biomedical engineering market is another rising sector, as pullulan has a high absorption capacity. For the biosynthesis of pullulan, safer and more novel approaches are being developed. Pullulan has been used in drug delivery in a variety of ways, including subcellular attacking, stimulus-responsive drug delivery devices, and nanoplatforms. Pullulan derived nanostructures or gels have a broad spectrum of supplications in the pharmaceutical and food sectors for medication delivery and gene transfer. Pullulan is being used in regenerative medicine, visualization, cancer cell targeting, and other applications. In light of these considerations, pullulan has a promising future in the healthcare industry for the benefit of humanity. Pullulan can have its surface modified to broaden its applicability. Future studies could focus on providing surface adhesion for cell attachment in bone tissue culture applications via osteogenesis.
Despite the fact that pullulan has numerous uses in biotechnology, its production and control have remained a mystery. Pullulan biosynthesis and its regulation have recently been described biochemically, along with their genes and encoding proteins. Presently, major research is going on regulating such a metabolic process through the important enzymes and genes manipulation. Any other transcriptional factors or signalling mechanisms that regulate pullulan production are likewise yet to be discovered.
Conclusion
Every day, a large pile of waste products is produced in the food processing industries and its improper management results in serious issues impacting the environment. These wastes should be investigated for use in the manufacturing of pullulan on a large scale. For the selection of the appropriate biotransformation, it’s critical to understand the biochemical makeup and microbial growth requirements. The key constituents in food processing wastes are unavailable, and these wastes must be pre-treated in order to provide a fermentable sugar and nitrogen source. Pullulan production costs have been reduced in half owing to the use of food-industrial waste. Pullulan’s practical application in food have mostly been discovered and accepted, but they have yet to be tested on a large scale. The eventual goal will be to define pullulan usage at the industrial level and to determine whether or not pullulan will be effective in the food industry.
Acknowledgements
Bishwambhar Mishra wants to acknowledge Chaitanya Bharathi Institute of Technology, Hyderabad, India for providing infrastructure and facility to carry out this work.
Abbreviations
- GRAS
Generally considered as safe
- EMS
Ethyl methane sulfonate
- UV
Ultra violate
- ATP
Adenosine triphosphate
- ADP
Adenosine diphosphate
- PKSIII
Polyketide synthase III
- DNA
Deoxyribonucleic acid
- UDPG
Uridine diphosphate glucose
- UDP
Uridine diphosphate
- LED
Light emitting diode
- COD
Chemical oxygen demand
- RSM
Response surface methodology
- BOD
Biological oxygen demand
- DO
Dissolved oxygen
Author contributions
BM: Conceptualization, literature review, experimentation, writing—original draft; YKM: Literature review, writing original draft, data curation, data analysis; SV: Conceptualization, Supervision, data curation, review and editing, Resources; SKM: Writing original draft, review and editing, data curation; LNSV: Formal analysis, supervision, review and editing; PC: Review and editing; MKA: Review and editing; ZZ Review and editing; RS: Review and editing; PB: review and editing; RRS: Review and editing; VK: Review and editing.
Funding
None.
Data availability
All data, models, and code generated are used during the study appear in the submitted article.
Code availability
None.
Declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
Not required.
Consent to participate
None.
Consent for publication
None.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Bishwambhar Mishra and Yugal Kishore Mohanta have been contributed equally to this work.
References
- Abdeshahian P, Ascencio JJ, Philippini RR, Antunes FA, de Carvalho AS, Abdeshahian M, dos Santos JC, da Silva SS. Valorization of lignocellulosic biomass and agri-food processing wastes for production of glucan polymer. Waste Biomass Valorization. 2021;12:2915–2931. doi: 10.1007/s12649-020-01267-z. [DOI] [Google Scholar]
- An C, Ma SJ, Chang F, Xue WJ. Efficient production of pullulan by Aureobasidium pullulans grown on mixtures of potato starch hydrolysate and sucrose. Braz J Microbiol. 2017;48:180–185. doi: 10.1016/j.bjm.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo NA, Valdez AL, Fariña JI. Microbial production of scleroglucan and downstream processing. Front Microbiol. 2015;6:1106. doi: 10.3389/fmicb.2015.01106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Guo J, Li F, Liu M, Zhang X, Guo X, Xiao D. Production of pullulan from xylose and hemicellulose hydrolysate by Aureobasidium pullulans AY82 with pH control and DL-dithiothreitol addition. Biotechnol Bioproc E. 2014;288:282–288. doi: 10.1007/s12257-013-0715-4. [DOI] [Google Scholar]
- Cheng J, Zhu M. A novel anaerobic co-culture system for bio-hydrogen production from sugarcane bagasse. Bioresour Technol. 2013;144:623–631. doi: 10.1016/j.biortech.2013.07.018. [DOI] [PubMed] [Google Scholar]
- Choudhury AR, Sharma N, Prasad G. Deoiledjatropha seed cake is a useful nutrient for pullulan production. Microb Cell Fact. 2012;11:39. doi: 10.1186/1475-2859-11-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailin DJ, Low LZ, Kumar K, Abd Malek R, Natasya KH, Keat HC. Agro-Industrial waste: a potential feedstock for pullulan production. Biosci Biotech Res Asia. 2019;16:229–250. doi: 10.13005/bbra/2740. [DOI] [Google Scholar]
- Duan X, Chi Z, Wang L, Wang X. Influence of different sugars on pullulan production and activities of α-phosphoglucose mutase, UDPG-pyrophosphorylase and glucosyltransferase involved in pullulan synthesis in Aureobasidium pullulans Y68. Carbohyd Polym. 2008;73:587–593. doi: 10.1016/j.carbpol.2007.12.028. [DOI] [PubMed] [Google Scholar]
- FAO (2019) The state of food and agriculture 2019. moving forward on food loss and waste reduction. Rome. Licence: CC BY-NC-SA 3.0 IGO. Available online: http://www.fao.org/3/ca6030en/ca6030en.pdf. Accessed 22 Mar 2022
- Göksungur Y, Uçan A, Güvenç U. Production of pullulan from beet molasses and synthetic medium by Aureobasidium pullulans. Turk J Biol. 2004;28(1):23–30. [Google Scholar]
- Göksungur Y, Uzunoğulları P, Dağbağlı S. Optimization of pullulan production from hydrolysed potato starch waste by response surface methodology. Carbohyd Polym. 2011;83:1330–1337. doi: 10.1016/j.carbpol.2010.09.047. [DOI] [Google Scholar]
- Hafez AA, Abdelhady HM, Sharaf MS, El-Tayeb TS, El-Kheima S. Bioconversion of various industrial by-products and agricultural wastes into pullulan. J Appl Sci Res. 2007;3:1416–1425. [Google Scholar]
- Hamidi M, Kennedy JF, Khodaiyan F, Mousavi Z, Hosseini SS. Production optimization, characterization and gene expression of pullulan from a new strain of Aureobasidium pullulans. Int J Biol Macromol. 2019;138:725–735. doi: 10.1016/j.ijbiomac.2019.07.123. [DOI] [PubMed] [Google Scholar]
- Hilares RT, Orsi CA, Ahmed MA, Marcelino PF, Menegatti CR, da Silva SS, Dos Santos JC. Low-melanin containing pullulan production from sugarcane bagasse hydrolysate by Aureobasidium pullulans in fermentations assisted by light-emitting diode. Bioresour Technol. 2017;230:76–81. doi: 10.1016/j.biortech.2017.01.052. [DOI] [PubMed] [Google Scholar]
- Hilares RT, Resende J, Orsi CA, Ahmed MA, Lacerda TM, da Silva SS, Santos JC. Exopolysaccharide (pullulan) production from sugarcane bagasse hydrolysate aiming to favor the development of biorefineries. Int J Biol Macromol. 2019;127:169–177. doi: 10.1016/j.ijbiomac.2019.01.038. [DOI] [PubMed] [Google Scholar]
- Israilides CJ, Smith A, Harthill JE, Barnett C, Bambalov G, Scanlon B. Pullulan content of the ethanol precipitate from fermented agro-industrial wastes. Appl Microbiol Biotechnol. 1998;49(5):613–617. doi: 10.1007/s002530051222. [DOI] [Google Scholar]
- Israilides C, Smith A, Scanlon B, Barnett C. Pullulan from agro-industrial wastes. Biotechnol Genet Eng Rev. 1999;16:309–324. doi: 10.1080/02648725.1999.10647981. [DOI] [Google Scholar]
- Lazaridou A, Biliaderis CG, Roukas T, Izydorczyk M. Production and characterization of pullulan from beet molasses using a nonpigmented strain of Aureobasidium pullulans in batch culture. Appl Biochem Biotechnol. 2002;97:1–22. doi: 10.1385/ABAB:97:1:01. [DOI] [PubMed] [Google Scholar]
- Li H, Zhang Y, Gao Y, Lan Y, Yin X, Huang L. Characterization of UGPase from Aureobasidium pullulans NRRL Y-12974 and application in enhanced pullulan production. Appl Biochem Biotech. 2016;178:1141–1153. doi: 10.1007/s12010-015-1934-2. [DOI] [PubMed] [Google Scholar]
- Liu G, Zhao X, Chen C, Chi Z, Zhang Y, Cui Q, Chi Z, Liu YJ. Robust production of pigment-free pullulan from lignocellulosic hydrolysate by a new fungus co-utilizing glucose and xylose. Carbohyd Polym. 2020;241:116400. doi: 10.1016/j.carbpol.2020.116400. [DOI] [PubMed] [Google Scholar]
- Liu F, Zhang J, Zhang L, Diao M, Ling P, Wang F. Correlation between the synthesis of pullulan and melanin in Aureobasidium pullulans. Int J Biol Macromol. 2021;177:252–260. doi: 10.1016/j.ijbiomac.2021.02.108. [DOI] [PubMed] [Google Scholar]
- Mirzaee H, Khodaiyan F, Kennedy JF, Hosseini SS. Production, optimization and characterization of pullulan from sesame seed oil cake as a new substrate by Aureobasidium pullulans. Carbohyd Polym Technol Appl. 2020;1:100004. doi: 10.1016/j.carpta.2020.100004. [DOI] [Google Scholar]
- Mishra B, Suneetha V. Biosynthesis and hyper production of pullulan by a newly isolated strain of Aspergillus japonicus-VIT-SB1. World J Microb Biot. 2014;30:2045–2052. doi: 10.1007/s11274-014-1629-9. [DOI] [PubMed] [Google Scholar]
- Mishra B, Suneetha V. Strain improvement and statistical analysis of pullulan producing strain of Aspergillus japonicus-VIT-SB1 for maximum yield. J Pure Appl Microbiol. 2014;8:1535–1545. doi: 10.1007/s11274-014-1629-9. [DOI] [Google Scholar]
- Mishra B, Vuppu S, Rath K. The role of microbial pullulan, a biopolymer in pharmaceutical approaches: a review. J Appl Pharm Sci. 2011;1:45–50. [Google Scholar]
- Mishra B, Varjani S. Evaluation of pullulan production by a newly isolated Micrococcus luteus. Indian J Exp Biol. 2019;57:813–820. [Google Scholar]
- Mishra B, Zamare D, Manikanta A. Biosynthetic technology and environmental challenges. Singapore: Springer; 2018. Selection and utilization of agro-industrial waste for biosynthesis and hyper-production of pullulan: a review; pp. 89–103. [Google Scholar]
- Priyadarshi R, Kim SM, Rhim JW. Pectin/pullulan blend films for food packaging: effect of blending ratio. Food Chem. 2021;347:129022. doi: 10.1016/j.foodchem.2021.129022. [DOI] [PubMed] [Google Scholar]
- Ray RC, Moorthy SN (2007) Exopolysaccharide (pullulan) production from cassava starch residue by Aureobasidium pullulans strain MTTC 1991. J Sci Ind Res 252–255. http://hdl.handle.net/123456789/1237
- Raychaudhuri R, Naik S, Shreya AB, Kandpal N, Pandey A, Kalthur G, Mutalik S. Pullulan based stimuli responsive and sub cellular targeted nanoplatforms for biomedical application: synthesis, nanoformulations and toxicological perspective. Int J Biol Macromol. 2020;161:1189–1205. doi: 10.1016/j.ijbiomac.2020.05.262. [DOI] [PubMed] [Google Scholar]
- Reddy CN, Mishra B, Mandal SK, Agrawal DC, Kruthiventi C. Polysaccharides of microbial origin: biomedical applications. Cham: Springer; 2021. An Insight into pullulan and its potential applications; pp. 1–32. [Google Scholar]
- Seviour RJ, McNeil B, Fazenda ML, Harvey LM. Operating bioreactors for microbial exopolysaccharide production. Crit Rev Biotechn. 2011;31:170–185. doi: 10.3109/07388551.2010.505909. [DOI] [PubMed] [Google Scholar]
- Sharma N, Prasad GS, Choudhury AR. Utilization of corn steep liquor for biosynthesis of pullulan, an important exopolysaccharide. Carbohyd Polym. 2013;93:95–101. doi: 10.1016/j.carbpol.2012.06.059. [DOI] [PubMed] [Google Scholar]
- Sharmila G, Muthukumaran C, Nayan G, Nidhi B. Extracellular biopolymer production by Aureobasidium pullulans MTCC 2195 using jackfruit seed powder. J Polym Environ. 2013;21:487–494. doi: 10.1007/s10924-012-0459-9. [DOI] [Google Scholar]
- Sheoran SK, Dubey KK, Tiwari DP, Singh BP. Directive production of pullulan by altering cheap source of carbons and nitrogen at 5 l bioreactor level. Int Sch Res Notice. 2012;2012:867198. doi: 10.5402/2012/867198. [DOI] [Google Scholar]
- Singh RS, Kaur N. Understanding response surface optimization of medium composition for pullulan production from de-oiled rice bran by Aureobasidium pullulans. Food Sci Biotechnol. 2019;28:1507–1520. doi: 10.1007/s10068-019-00585-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RS, Kaur N, Rana V, Kennedy JF. Pullulan: a novel molecule for biomedical applications. Carbohyd Polym. 2017;171:102–121. doi: 10.1016/j.carbpol.2017.04.089. [DOI] [PubMed] [Google Scholar]
- Singh RS, Saini GK, Kennedy JF. Pullulan production in stirred tank reactor by a colour-variant strain of Aureobasidium pullulans FB-1. Carbohydr Polym Tech Appl. 2021;2:100086. doi: 10.1016/j.carpta.2021.100086. [DOI] [Google Scholar]
- Singh RS, Saini GK. Microorganisms in sustainable agriculture and biotechnology. Dordrecht: Springer; 2012. Biosynthesis of pullulan and its applications in food and pharmaceutical industry; pp. 509–553. [Google Scholar]
- Srikanth S, Swathi M, Tejaswini M, Sharmila G, Muthukumaran C, Jaganathan MK, Tamilarasan K. Statistical optimization of molasses based exopolysaccharide and biomass production by Aureobasidium pullulans MTCC 2195. Biocatal Agr Biotechnol. 2014;3:7–12. doi: 10.1016/j.bcab.2013.11.011. [DOI] [Google Scholar]
- Sugumaran KR, Ponnusami V. Review on production, downstream processing and characterization of microbial pullulan. Carbohyd Polym. 2017;173:573–591. doi: 10.1016/j.carbpol.2017.06.022. [DOI] [PubMed] [Google Scholar]
- Sugumaran KR, Gowthami E, Swathi B, Elakkiya S, Srivastava SN, Ravikumar R, Gowdhaman D, Ponnusami V. Production of pullulan by Aureobasidium pullulans from Asian palm kernel: a novel substrate. Carbohyd Polym. 2013;92:697–703. doi: 10.1016/j.carbpol.2012.09.062. [DOI] [PubMed] [Google Scholar]
- Sugumaran KR, Jothi P, Ponnusami V. Bioconversion of industrial solid waste-cassava bagasse for pullulan production in solid state fermentation. Carbohyd Polym. 2014;99:22–30. doi: 10.1016/j.carbpol.2013.08.039. [DOI] [PubMed] [Google Scholar]
- Tabasum S, Noreen A, Maqsood MF, Umar H, Akram N, Chatha SA, Zia KM. A review on versatile applications of blends and composites of pullulan with natural and synthetic polymers. Int J Biol Macromol. 2018;120:603–632. doi: 10.1016/j.ijbiomac.2018.07.154. [DOI] [PubMed] [Google Scholar]
- Thirumavalavan K, Manikkadan TR, Dhanasekar R. Pullulan production from coconut by-products by Aureobasidium pullulans. Afr J Biotechnol. 2009;8(2):254–258. [Google Scholar]
- Varjani S, Lee DJ, Zhang ZQ. Valorizing agricultural biomass for sustainable development: biological engineering aspects. Bioengineered. 2020;11:522–523. doi: 10.1080/21655979.2020.1759185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varjani S, Shah AV, Vyas S, Srivastava VK. Processes and prospects on valorizing solid waste for the production of valuable products employing bio-routes: a systematic review. Chemosphere. 2021;282:130954. doi: 10.1016/j.chemosphere.2021.130954. [DOI] [PubMed] [Google Scholar]
- Viveka R, Varjani S, Ekambaram N. Valorization of cassava waste for pullulan production by Aureobasidium pullulans MTCC 1991. Energy Environ. 2020;32:1086–1102. doi: 10.1177/0958305X20908065. [DOI] [Google Scholar]
- Vyas S, Prajapati P, Shah AV, Srivastava VK, Varjani S. Opportunities and knowledge gaps in biochemical interventions for mining of resources from solid waste: a special focus on anaerobic digestion. Fuel. 2022;311:122625. doi: 10.1016/j.fuel.2021.122625. [DOI] [Google Scholar]
- Wang D, Ju X, Zhou D, Wei G. Efficient production of pullulan using rice hull hydrolysate by adaptive laboratory evolution of Aureobasidium pullulans. Bioresource Technol. 2014;164:12–19. doi: 10.1016/j.biortech.2014.04.036. [DOI] [PubMed] [Google Scholar]
- Wani SM, Mir SA, Khanday FA, Masoodi FA. Advances in pullulan production from agro-based wastes by Aureobasidium pullulans and its applications. Innov Food Sci Emerg. 2021;74:102846. doi: 10.1016/j.ifset.2021.102846. [DOI] [Google Scholar]
- Wu S, Jin Z, Tong Q, Chen H. Sweet potato: a novel substrate for pullulan production by Aureobasidium pullulans. Carbohyd Polym. 2009;76:645–649. doi: 10.1016/j.carbpol.2008.11.034. [DOI] [Google Scholar]
- Wu S, Lu M, Chen J, Fang Y, Wu L, Xu Y, Wang S. Production of pullulan from raw potato starch hydrolysates by a new strain of Auerobasidium pullulans. Int J Biol Macromol. 2016;82:740–743. doi: 10.1016/j.ijbiomac.2015.09.075. [DOI] [PubMed] [Google Scholar]
- Yaashikaa PR, Kumar PS, Varjani S. Valorization of agro-industrial wastes for biorefinery process and circular bioeconomy: a critical review. Bioresour Technol. 2022;343:126126. doi: 10.1016/j.biortech.2021.126126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data, models, and code generated are used during the study appear in the submitted article.
None.