Abstract
This study investigated the synergy of ultrasonic and transglutaminase (TGase) treatment on the structural, physicochemical, rheological, gelation properties and controlled release properties of dehulled walnut proteins (DWP). The results showed that after ultrasonic-TGase treatment, the surface hydrophobicity was decreased, indicating the involvement of disulfide bonds in gel formation. Scanning electron microscopy (SEM) showed that ultrasonic-TGase treatment resulted in a more uniform and denser microstructure of DWP gels. Ultrasonic-TGase treatment changed the secondary structure of the DWP gels as determined by Fourier transform infrared spectroscopy, with an increase in α-helix, β-turn and random coils and a decrease in β-sheets. In addition, in vitro drug release profiles showed that ultrasonic-TGase treatment promoted the cross-linking of protein molecules and formed a dense network to embed tea polyphenols (TP), thereby slowing down the digestion of TP in simulated gastric fluid and achieving the purpose of slow-release in simulated intestinal fluid. Thus, the synergy of ultrasonic and TGase treatment might be an effective method to improve gel properties and expand the application of protein gels in the food industries.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13197-023-05756-6.
Keywords: Ultrasonic, Transglutaminase, Dehulled walnut protein gels, Structural properties, Tea polyphenols
Introduction
Walnuts (Juglans regia L.) are widely distributed in Southern Europe, North Africa, East Asia, the United States, Western South America and other regions (Tapia et al. 2013). China is the main producer of walnuts. According to recent statistical data, walnut production in the world was 2.83 million tons in 2020 (UN Food & Agriculture Organization, 2020). As a nut with nutritional value, walnuts contain > 24% protein and ~ 70% oil. In addition, walnuts also contain a variety of biologically active substances required by the human body, such as phenols, B vitamins, phytosterols, dietary fiber and non-sodium minerals (Chen et al. 2022a, b). Walnut protein is the main by-product of oil extraction (Rabadán et al. 2018) and is often used as a low-value animal feed and fertilizer (Yan et al. 2021). Walnut protein is weak in the emulsifying properties, and TGase cross-linking could improve the emulsification of walnut protein (Jin et al. 2020; Wen et al. 2018).
Protein gel is a state in which protein molecules aggregate to form a gel or solid under certain conditions. Proteins can form gels through various physical modifications (such as microwave, electric field, ultrasound, etc.) or chemical modifications (such as adding chemical modifiers, changing pH values, etc.). In recent years, synergistic modification (physical and chemical modification) has been used to prepare protein gels. Xue et al. (2022) reported that ultrasound and synergetic phosphorylation/ultrasound treatments improved duck egg’s stability, water binding, and gel properties of duck egg. Wang et al. (2022) studied the effect of preheating cooperation with ultrasound treatment on wooden breast myofibrillar protein's physicochemical and gelling properties. Transglutaminase is a protease that can catalyze acyl transfer reactions. TGase-induced cross-linking is a specific, efficient and safe gel preparation method that meets the requirements of green science. TGase can promote the formation of covalent bonds between glutamine and lysine residues. The cross-linking induced By TGase could improve the gel properties of plant proteins, such as soy protein isolate (Qin et al. 2016). TGase, in combination with other technologies, could improve the texture, stability and hydration capacity of food-grade protein gels without changing the food’s flavor and nutritional quality (Gharibzahedi et al. 2018). Silva et al. (2018) found that TGase pretreatment significantly affected the rheological properties, WHC and microstructure of the gels in the process of acidifying skimmed microfiltered milk. TGase induced intermolecular chemical cross-linking, which increased the gels' hardness and storage modulus. However, there are few studies on the properties of plant protein gels with different concentrations of TGase. In addition, applying of Tgase-induced plant protein gels in the entrapment and controlled release of bioactive substances may have commercial benefits. Riboflavin, for example, is a low molecular weight and partially water-soluble vitamin. Low water solubility and sensitivity to external environment lead to decreased riboflavin bioactivity. Tgase-induced walnut protein gels as materials to encapsulate riboflavin may achieve its targeted release in the intestinal (Wen et al. 2018).
Ultrasound is a safe and efficient non-thermal processing method, which has been widely used to modify of protein in the food industry (Lin et al. 2021; Chen et al. 2022a, b; Yang et al. 2023). Ultrasonic affects the structure of the protein through cavitation, which refers to the formation and collapse of cavitation bubbles under the ultrasonic treatment, leading to changes in the structure and properties of the protein (Zhang et al. 2021). Ahmadi et al. (2017) indicated that ultrasonic treatment could modify the structure of whey protein and make it easier to cross-link TGase, thus improving the functional properties of whey protein.
Bioactive compounds such as carotenoids and tea polyphenols have some nutraceutical and pharmacological properties, making them potent to prevent numerous diseases in humans. TP has health-elevating functions like antioxidant (Yan et al. 2020), anti-inflammatory (Truong & Jeong 2022), antibacterial (Chacko et al. 2010) and antiparasitic (Saeed et al. 2017) properties. However, TP is a hydrophobic constituent with poor water solubility, chemical stability, and rapid degradation in the physiological pH of the body which limits its applications in food systems. To overcome these finitudes, various delivery systems are recognized like emulsions, nanoparticles, and nano-emulsions named entrapment systems. Recently, encapsulating method attracted more attention and tendency than other techniques in which water-soluble proteins like bovine serum albumin (BSA), human serum albumin (HSA), and β-lactoglobulin (β-LG) or water-insoluble proteins such as gliadin, zein, and barley proteins were employed as the outer cover of such lipophilic compounds in delivery systems (Liu et al. 2018).
In this study, the ultrasound-TGase synergy technology was used to prepare the walnut protein gels. The effect of ultrasound-TGase treatment on walnut protein gels' structure and functional properties was explored. Further, the DWP gels were used to load TP, and its slow release in the simulated gastric fluid was analyzed. This study aims to provide insights for the preparation of walnut protein gel and improve the bioavailability of bioactive substances in the human body. This study aims to provide insights for the preparation of walnut protein gel and improve the bioavailability of bioactive substances in the human body.
Materials and methods
Materials
Walnuts were purchased from Huize (Yunnan, China) in 2019. Transglutaminase (CAS: 80146-85-6, 100,000 U/g) was bought from Hebei Colodo Biotechnology Co. Ltd. (Handan, Hebei, China). TP (98%, derived from green tea) were purchased from Beijing Kebaiao Biotechnology Co. Ltd. (Beijing, China). All other chemicals and reagents used were of analytical grade and obtained from Beijing Kebaiao Biotechnology.
Extraction of dehulled walnut protein (DWP)
The walnuts were dehulled manually, and their kernels were fractured for protein extraction. TZ-230 hydraulic oil expeller (Henan Taizheng Grain and Oil Machinery Equipment Co. Ltd., Anyang, Henan, China) was used to remove the oil at room temperature. The defatted walnut meal was dissolved in deionized water (DW) with a ratio of 1:20 (w/v) by adjusting the pH to 11.0 with 1.0 M NaOH. After that, the mixture was magnetically stirred (HJ-3, Jiangsu Guohua Electric Appliance Co. Ltd., Changzhou, Jiangsu, China) for 90 min at 53 °C and centrifuged (3–5 N, Hunan Hengnuo Instrument Equipment Co. Ltd., Changsha, Hunan, China) at 4000 × g for 15 min at 25 °C. After that, by adjusting the pH of supernatant to 4.5 with 1.0 M HCl, the slurry was centrifuged at 4000 × g for 15 min, the precipitate was collected, lyophilized (FDU-2110, Tokyo Physiochemical Machinery, Tokyo, Japan) and kept in plastic tubes at − 20 °C before use. The crude protein content (N × 5.3) of DWP was 81.2%, as determined using the Kjeldahl method.
Ultrasonic treatment of DWP solutions
The solutions (20% w/v, pH 8.0) were prepared by adding DWP flour into distilled water, followed by gentle agitation for 1 h at room temperature (25 ± 1 °C) until the DWP was completely dissolved, and then ultrasonic treatment was performed. Refereed to Liang et al. (2021) for ultrasonic treatment method with slight modifications. An ultrasonic processor (VCX800, Shanghai Shujun Instrument Equipment Co., Ltd., Shanghai, China) was used to sonicate 100 mL of DWP solution. The DWP solutions were treated with a probe-type ultrasonic processor at 500 W for 23 min. To avoid the heat generated by ultrasonic treatment, the DWP solutions were carried out with an ice bath after ultrasonic process.
TGase cross-linking of DWP gels
The samples pretreated with ultrasonic were adjusted to pH 7.0, then TGase was slowly added with different concentration (0, 1, 3, 5, 7 and 9 U/g protein) and stirred magnetically for 1 h. The DWP gels were obtained by incubating the mixture at 40 °C for 2 h in a water bath. The DWP gels were subsequently heated at 90 °C for 10 min to inactivated the TGase and cooled in an ice bath (SHZ-82 A, Ruihua Instrument, Changzhou, Jiangsu, China), then stored in a refrigerator (~ 4 °C) for 24 h. To measure the properties of the gel, it was lyophilized at −40 °C for 12 h and stored at 4 °C until use for a maximum of 4 weeks.
Fourier-transform infrared spectroscopy (FTIR) of DWP gels
The conformations of DWP gels were measured by FTIR (IRTracer-100, Shimadzu Corporation, Kyoto, Japan). The samples were mixed with KBr with the ratio of 1:5 (w/w) and pressed into the tablet, and then the mixtures were place in the sample holder. The spectra were scanned from 4000 to 400 cm−1 wavenumbers in 32 scans with a resolution of 4 cm−1. The data was analyzed using the Peak Fit 4.12 software (SeaSolve software Inc, Framingham, USA). The parameters were set as follows: Xi: 1600, Xf: 1700, Resp Fn Width: 3.36024, Iter action: 7 (Hu et al., 2022).
Fluorescence spectroscopy of DWP gels
Fluorescence measurements of DWP gels were carried out on a F-7000 fluorescence spectrophotometer (Hitachi Ltd., Tokyo, Japan). The DWP gels (0.1 mg/mL) was dispersed in 0.01 M phosphate buffer (pH 7.0). To minimize the effect of tyrosine residues to the spectra, the DWP gels were excited at 295 nm and the emission spectra were recorded from 300 to 400 nm at a constant slit of 5 nm. All the determinations were conducted in triplicate.
Surface hydrophobicity (H0)
The surface hydrophobicity of DWP gels was determined by 1-anilino-8-naphthalenesulfonate (ANS) fluorescence probe, according to the method of Kato and Nakai (1980). The DWP gels were dispersed in 0.01 M sodium phosphate buffer (pH 7.0), and the concentrations of gel were 0.05, 0.1, 0.2, 0.4 and 0.8 mg/mL. The gel samples (4 mL) were mixture with 20 μL of ANS solution (8.0 0.008 M in 0.01 M sodium phosphate buffer, pH 7.2). The fluorescence intensity (excitation wavelength: 390 nm and emission wavelength: 470 nm) was measured using a F-7000 spectrofluorometer (Hitachi Ltd., Tokyo, Japan). The surface hydrophobicity index (H0) was calculated from the initial slope of the fluorescence intensity versus protein concentration.
Scanning electron microscopy (SEM)
A JSM-6700F scanning electron microscope (JEOL Ltd., Tokyo, Japan) was used for visualizing the microstructures of gels. Dried gels were fixed on aluminum stubs and sprayed with 10 nm gold (208HR, Cressington Scientific Instruments Ltd., Watford, UK). The samples were observed under the vacuum conditions, and then photographed at an acceleration voltage of 10.0 kV and a magnification of 5000.
Rheological properties of DWP gels
Rheological properties of gels were measured with a rheometer (MCR301, Anton Paar Ltd., Graz, Austria). The fresh samples (1 mL) were placed on the plate with a diameter of 40 mm for scanning immediately. The slit distance was set at 1 mm and the test was carried out at room temperature. The experiments were carried out three times for each sample. Gels were continuously sheared at a fixed frequency of 0.1 Hz and the strain was controlled at 1%. Apparent (shear) viscosity, storage modulus (G′) and loss modulus (G″) were recorded during the measurement.
Preparation of tea polyphenols (TP)-loaded gels
Gels with TP were prepared by dissolving the TP powder (5%, w/w, based on the dry weight of DWP gels) in gel solutions (20%, w/v) and stirred for 1 h at 25 °C. Different concentrations (0, 1, 3, 5, 7 and 9 U/g) of TGase were added into the mixtures and incubated at 40 °C for 2 h to form gels. After inactivated the TGase at 90 °C for 10 min, the TP loaded gels with different concentration of TGase were obtained. The gels were lyophilized and stored at 4 °C for further use.
The entrapment efficiency (EE) and loading capacity (LC) were calculated according to the method of Chen and Subirade (2009) with a slight modification. About 160 mg of dry gels were weighted precisely and mixture with 20 mL of distilled water, then the mixture was put in a 37 °C water bath for 6 h. After centrifuged at 8000 × g for 15 min at 4 °C, the TP concentration in the supernatant was determined as the absorbance at 540 nm with a UV–visible spectrophotometer (HC-2518R, Anhui ZhongkeZhongjia Scientific Instrument Co. Ltd., Heifei, China). The EE and LC were calculated using the following formulae:
In vitro gastrointestinal digestion of TP-loaded gels
In vitro gastric digestion was carried out using a standardized method (Minekus et al. 2014). Simulated gastric fluid (SGF) was prepared by adding 2 g NaCl into distilled water and mixing with 7 mL 37% HCl. The pH was adjusted to 1.2, the volume was brought to 1000 mL with distilled water and left for 1 h at 25 °C. Pepsin (4%, w/v, 3000 U/g, Hebei Hongtao Biological Engineering) was added and an aliquot (5 mL) removed after 1 h at 37 °C. Simulated intestinal fluid (SIF) was prepared by dissolving 6.8 g potassium dihydrogen phosphate in 250 mL distilled water, adding 200 mL 0.2 mol/L NaOH and 300 mL distilled water. The pH was adjusted to 7.4 with 0.2 mol/L NaOH. Dry trypsin (4%, w/v) was added and the reaction stopped by adding an equal volume of trichloroacetic acid (20%) and placed in a 90 °C water bath for 5 min to denature the TGase after 1 h heating.
The lyophilized gels (3 g) were dispersed in 50 mL SGF and placed in a shaker, preheated at 37 °C for 5 min, the reaction lasted for 1 h to conduct the simulated gastric digestion. The digestive fluid (5 mL) was collected every 30 min, and then centrifuged at 8000 × g at 25 °C for 10 min. The supernatant was passed through a 0.45 μm filter membrane (Tianjin Jinteng Experimental Equipment Co., Ltd., Tianjin, China) and the absorbance was measured at 540 nm.
Simulated intestinal digestion was followed by adding 50 mL SIF in simulated gastric digestive fluids, and the pH was adjusted to 7.5 with 0.5 M NaOH, the reaction continued at 37 °C for 2 h. After enzyme deactivation at 90 °C for 5 min, the intestinal digestion products were centrifuged at 8000 × g at 25 °C for 10 min, the precipitate was discarded and the supernatant was passed through a 0.45 μm filter membrane (Tianjin Jinteng Experimental Equipment Co., Ltd., Tianjin, China). The absorbance value was measured at 540 nm with simulated intestinal fluid as a blank and the release rate of TP was calculated.
Statistical analysis
Each experiment was carried out at least in triplicate. The results are reported as the mean ± standard deviation. The difference of experimental data was analyzed by Ducan method for significance. The level of significance was set at p < 0.05. The experimental data were analyzed using SPSS software version 22.0 (SPSS Inc., Chicago, Illinois, USA).
Results and discussion
Fourier transform infrared spectroscopy (FTIR) analysis of DWP gels
FTIR spectroscopy was used to investigate the effect of the ultrasonic-TGase treatment on the secondary structure of the protein. FTIR spectroscopy of DWP gels treated with different concentrations of TGase (0–9 U/g) is shown in Table 1. It could be inferred from Table 1. that the dominant secondary structure of DWP gels was β-sheet, which was consistent with the previous study (Maleki et al. 2011). After ultrasonic-TGase treatment, the content of β-sheet was decreased from 53.71 to 51.97% (p < 0.05), while β-turn was increased from 15.45 to 16.41% (p < 0.05), α-helix was increased from 15.05 to 15.61% (p < 0.05) and the contents of the random coil was increased from 15.79% to 15% (p < 0.05). The decrease of β-sheet indicated the exposure of hydrophobic sites on the protein, which leads to an increase in surface hydrophobicity (Wang et al. 2014). The increase of β-turn could improve the structural stability of protein (Moreno et al. 2015). The increase of α-helix content might be related to the aggregation of protein molecules caused by ultrasonic cavitation (Cui et al. 2013). In addition, the increase of random coil content promoted the conversion of β-turn into random coil. To some extent, ultrasonic pretreatment changed the molecular structure of DWP and enhanced the accessibility of proteins to TGase cross-linking.
Table 1.
FTIR results for the secondary structure contents of DWP gels with different concentration of TGase
| Sample (U/g protein) | α-helix (%) | β-sheet (%) | β-turn (%) | Random coil (%) |
|---|---|---|---|---|
| 0 | 15.05 ± 0.18e | 53.71 ± 0.20a | 15.45 ± 0.19d | 15.79 ± 0.27c |
| 1 | 15.20 ± 0.25d | 53.28 ± 0.13b | 15.49 ± 0.11d | 15.99 ± 0.31a |
| 3 | 15.24 ± 0.11d | 53.23 ± 0.22b | 15.75 ± 0.21c | 15.82 ± 0.15b |
| 5 | 15.30 ± 0.21c | 52.41 ± 0.35c | 16.34 ± 0.26b | 15.95 ± 0.18a |
| 7 | 15.54 ± 0.10b | 52.05 ± 0.19d | 16.37 ± 0.16b | 15.97 ± 0.21a |
| 9 | 15.61 ± 0.22a | 51.97 ± 0.32e | 16.51 ± 0.20a | 15.98 ± 0.11a |
Note: The different letters (a–d) indicate significant differences between treatments with different concentrations (p < 0.05). Data are the means ± standard deviation (n = 3)
Fluorescence spectra of DWP gels
The fluorescence spectroscopy was used to characterize the tertiary structural changes of ultrasonic-TGase induced DWP gels. In native proteins, only tryptophan (Trp), tyrosine (Tyr) and phenylalanine (Phe) residues can produce fluorescence, these three amino acid residues have different fluorescence spectra due to the different aromatic groups in their side chains (Tang et al. 2005), which reflect the change in the tertiary structure of protein. The aromatic amino acid residues absorb the incident light in the ultraviolet region and emit fluorescence characteristics. In proteins, the energy transfer from tyrosine to tryptophan residues frequently occurred, resulting in fluorescence quenching of tyrosine residues and fluorescence enhancement of tryptophan residues. Thus, tryptophan is mostly used as an endogenous fluorescent probe to study the texture structure of proteins (Yan et al. 2022). Fluorescence intensities of ultrasonic-TGase induced DWP gels were different from those in the control group (Fig. 1). The fluorescence emission maximum wavelength (λmax) of control group was 330 nm. With the increase of TGase (0–9 U/g), λmax for the ultrasonic-TGase treated DWP gels were 336.4 nm (1 U/g), 335.6 nm (3 U/g), 335.6 nm (5 U/g), 338.2 nm (7 U/g) and 336.4 nm (9 U/g), respectively. The increase of fluorescence intensity was due to the protein structure was fully stretched after ultrasonic treatment, more active sites for TGase were exposed and increased the degree of cross-linking, which led to the exposure of chromophore group embedded in the protein. These results were consistent with Zang et al. (2019) and they reported that the intrinsic fluorescence of rice bran protein was shifted to higher wavelength after trypsin treatment, which could be attributed to exposure of hydrophobic groups through the action of trypsin.
Fig. 1.
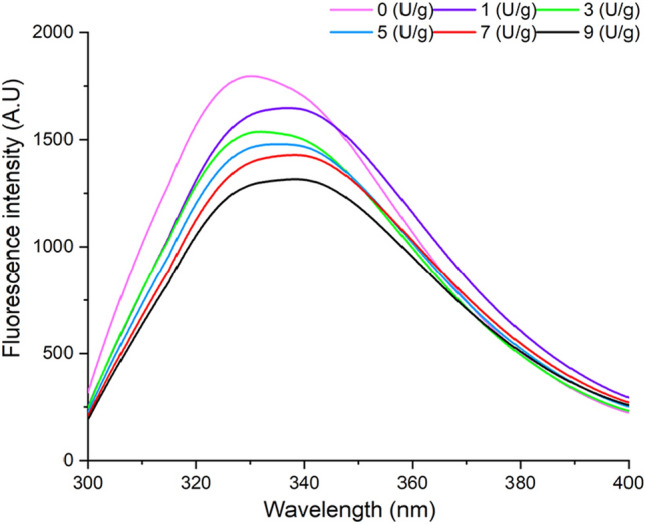
Fluorescence spectra of DWP gels with different concentration of TGase
Surface hydrophobicity (H0)
The effect of ultrasonic-TGase treatment on H0 of DWP gels are shown in Table 2. After ultrasonic-TGase treatments, the H0 of DWP gels was greatly increased (p < 0.05) from 89.7 to 548.2 with the increase of TGase from 0 to 9 (U/g). This result indicated that, on the one hand, TGase cross-linking could promote the exposure of hydrophobic amino acid residues in DWP, which increased the surface hydrophobicity, on the other hand, the high-intensity shock wave generated by ultrasonic energy exposed more active sites in DWP, which is helpful to improve the cross-linking reaction of TGase, thereby increased the degree of cross-linking of DWP and exposed more hydrophobic amino acid residues. However, others reported that the decrease of H0 after TGase and ultrasonic treatment. As examples, Cui et al. (2020) reported that the H0 of soybean-whey mixed protein decrease after the ultrasonic treatment, which could be attributed to the hydrophobic groups aggregation of proteins caused by ultrasonic treatment. Chandrapala et al. (2011) also studied the effect of ultrasonic and TGase treatment on the H0 of whey protein concentrate, and the results indicated that the after ultrasonic treatment, the TGase crosslinked whey protein concentrate after ultrasonic treatment showed the lower value of H0. The decrease of H0 might be related to the aggregation of proteins to protect the hydrophobic groups. Besides, the deamidation of TGase increased the content of negatively charged amino acids such as Glu and Asp, resulting in the decrease of surface hydrophobicity.
Table 2.
Free SH contents and surface hydrophobicity of DWP gels treated with different concentration of TGase
| TGase concentration U/g | Surface free -SH (SHF) content μmol/g protein | Surface hydrophobicity (H0) |
|---|---|---|
| 0 | 8.9 ± 0.4a | 548.2 ± 20.3a |
| 1 | 7.7 ± 1.0b | 222.8 ± 15.1b |
| 3 | 7.7 ± 0.4b | 168.3 ± 8.7c |
| 5 | 6.4 ± 0.3c | 104.7 ± 7.2d |
| 7 | 6.4 ± 1.3c | 92.6 ± 10.5e |
| 9 | 6.1 ± 0.3d | 89.7 ± 6.4f |
The different letters (a–d) indicate significant differences between treatments with different concentrations (p < 0.05). Data are the means ± standard deviation (n = 3)
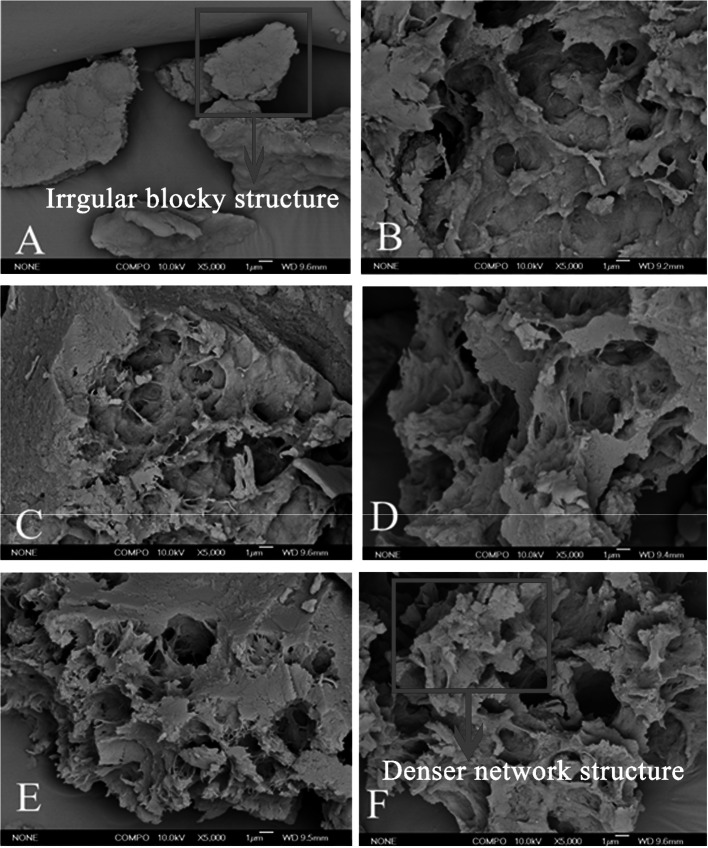
Microstructural properties of the DWP gels
SEM was performed to explore the effect of ultrasonic and TGase treatment on the microstructure of DWP gels (Fig. 2A), and the DWP gels before and after lyophilization were shown in Fig. S1. Shows that the untreated DWP gels had an irregular block structure, while ultrasonic and TGase tresatment resulted in a denser and more homogeneous network structure of DWP gels. These results were similar to the results reported by Zhu et al. (2018). This change could be attributed to the reduction of protein particle size caused by ultrasonic cavitation, which promoted the formation of disulfide bonds and a denser three-dimensional network structure during TGase cross-linking. TGase promoted intermolecular or intramolecular cross-linking of protein by inserting permanent ɛ-(γ-Glu)-Lys bonds between proteins and stabilized the three-dimensional networks of gels (Abou-Soliman et al. 2017). Furthermore, with the increase of TGase, the gel pores became larger, possibly due to the extensive cross-linking that resulted in the formation of polymer and coarse networks (Luo et al. 2019). Another possible reason for this result might be that ultrasound facilitated the formation of protein aggregates, which affected the microstructure of the gel induced by TGase (Arzeni et al. 2012).
Fig. 2.
The scanning electron microscope pictures of the gels. Magnification 1000x. (A) Without TGase. (B) With 1 U/g TGase. (C) With 3 U/g TGase. (D) With 5 U/g TGase. (E) With 7 U/g TGase. (F) With 9 U/g TGase
Rheological characteristics of DWP gels
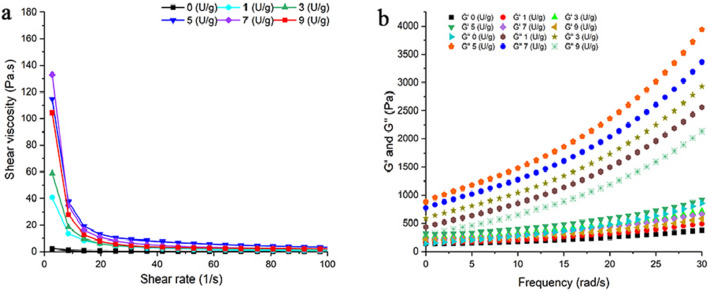
Figure 3a shows the shear viscosity of DWP gels after ultrasonic and TGase treatment. The viscosity of all samples (0–9 U/g) decreased rapidly in the low shear rate range (0–20 s−1) and decreased smoothly in the high shear rate range (20–100 s−1). All samples exhibited non-Newtonian fluid behavior with the shear rate ranging from 1 to 100 s−1. Ultrasonic treatment prominently increased the apparent viscosity of TGase cross-linked DWP gels cross-linked by TGase, which might be due to the fact that ultrasonic treatment catalyzed the cross-linking of proteins and made them generated high-molecular-weight polymers, leading to higher apparent viscosity (Jiang et al. 2019). Ahmadi et al. (2017) indicated that the combination of ultrasonic and TGase treatment improved the rheological of whey protein concentrate, which was consistent with the results of this study.
Fig. 3.
Rheological properties of DWP gels. Shear viscosity of unloaded DWP gels (a), storage modulus (G′) and loss modulus (G″) of unloaded DWP gels (b), Shear viscosity of TP loaded DWP gels (c), storage modulus (G′) and loss modulus (G″) of TP loaded DWP gels (d)
The profiles of G′ and G″ at different concentrations of TGase are shown in Fig. 3b. Both the G′ and G″ modulus showed a linear increase with the increase of angular frequency, but the slope of G″ was lower than that of G′. When G' is higher than G'', the elastic deformation dominated and the gel behaved as solid (Zhao et al. 2022). Ultrasonic treatment destroyed the non-covalent interactions between walnut proteins and exposed more TGase active areas, thus promoting proteins to form high molecular weight polymers, which was beneficial to improve the rheology of DWP gels.
Entrapment efficiency and loading rates of TP
The amount of TGase (from 0 to 9 U/g) affected the entrapment efficiencies and the loading rates of TP-loaded DWP gels, respectively. Table 3 shows that the entrapment efficiencies of TP-loaded gels were from 75.28% to 93.41%, and loading rate of TP-loaded gels were from 3.76% to 4.67%. TGase crosslinked DWP gels treated by ultrasonic had significant effects on the entrapment and loading efficiencies of TP. After sonication, the viscosity of protein molecules was increased and the TP could be embedded in the three-dimensional network structure of the gels, which resulted in higher entrapment efficiency and loading rates. Ren et al. (2019) also discovered that ultrasound treatment increased the entrapment rate and loading rate of resveratrol by the zein-chitosan complex, which might be due to cavitation and thermal effects caused by ultrasound. It was consistent with the results of this study.
Table 3.
Entrapment efficiency and loading rate of TP induced by different concentration of TGase
| Concentration of TGase (U/g) | Entrapment efficiency of TP (%) | Loading rate of TP (%) |
|---|---|---|
| 0 | 75.28 ± 3.26a | 3.67 ± 0.16a |
| 1 | 84.35 ± 4.25b | 4.22 ± 0.22b |
| 3 | 85.71 ± 1.34b | 4.29 ± 0.19c |
| 5 | 87.89 ± 2.36c | 4.40 ± 0.24c |
| 7 | 90.24 ± 2.15d | 4.51 ± 0.21d |
| 9 | 93.41 ± 2.75e | 4.67 ± 0.23e |
The different letters (a–d) indicate significant differences between treatments with different concentrations (p < 0.05). Data are the means ± standard deviation (n = 3)
In vitro release properties
To investigate the digestion of bioactive substances in human body, the digestion of TP was investigated in simulated gastric fluid and simulated intestinal fluid. Figure 4 shows the effect of TGase addition (0 to 9 U/g) on the controlled release behavior of TP-loadesd DWP gels. The release rate of TP-loaded DWP gels without TGase treatment was already as high as 52.12% after 1 h of release in the simulated gastric juice, and that of TP-loaded DWP gels treated by TGase was lower. The slow-release ability of TP loaded by DWP gels increased with the increase of the amount of TGase. When the amount of TGase was 9 U/g, the release rate of TP-loaded DWP gels was 35.73%, it showed that DWP gels have good slow-release property. When the TP-loaded DWP gels was in the simulated intestinal fluid, the release rate of TP increased significantly due to the change of pH of juices and the effect of trypsin. With the increase of the amounts of TGase and the slow-release ability of TP-loaded DWP gels increased. When the digestion time was 180 min, the release rate of TP-loaded DWP gels without TGase treatment was 99%, and the release rate of TP-loaded DWP gels treated by TGase (9 U/g) was 87.18%, which prolonged the release time of TP. These results indicate that TGase induced DWP gels could effectively encapsulate the bioactive substances and prevent them from the early release in stomach, while allowing them to be released upon arrival in the small intestine. Wen et al. (2018) found that APC gels without MTGase increased the release of riboflavin, reaching 27% compared to < 16% with MTGase induced gels. When transferred to SIF, the riboflavin release was accelerated in gels. The obtained results in our study were consist with Wen et al. (2018).
Fig. 4.
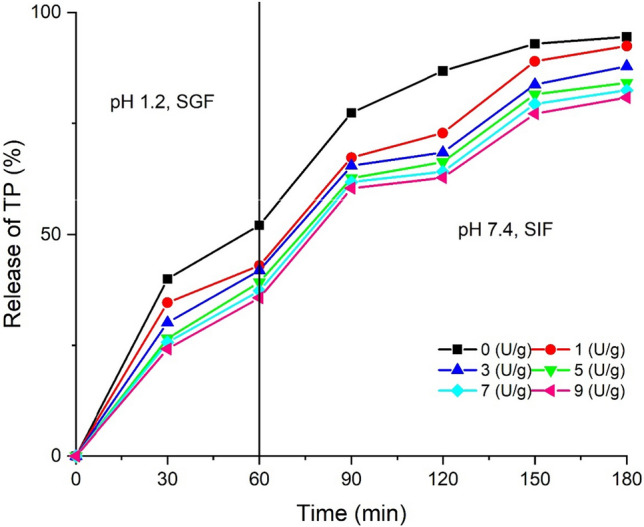
Effect of Tgase addition on the controlled release properties of TP loaded gelsSS
Conclusion
In the present study, the ultrasound-TGase treatment was utilized to construct the DWP gels, and the gel’s structure and textural properties were investigated. FTIR revealed that DWP gels were mainly composed of β-sheet, and the secondary structure was changed after ultrasound-TGase treatment. The fluorescence spectroscopy showed that adding TGase improved the texture properties of walnut protein gels. With the increase of TGase, the surface hydrophobicity and viscosity increased, while the storage modulus of gels was always higher than the loss modulus. The SEM found that a better three-dimensional network structure was formed inside the walnut protein gels after ultrasonic-TGase treatment, presenting a uniform density and pore size. Then, the DWP gels were utilized to encapsulate TP. The results showed that the ultrasound-TGase treatment could improve the entrapment efficiency and loading capacity of TP, accompanied by increased resistance to the simulated gastric and intestinal digestion. Our study could provide insights into applying ultrasound-TGase DWP gels in delivering sensitive compounds into functional foods.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are thankful to the National Key Research and Development Program (2022YFD1000104)
Abbreviations
- DWP
Dehulled walnut protein
- TP
Tea polyphenol
- SEM
Scanning electron microscopy
- TGase
Transglutaminase
- BSA
Bovine serum albumin
- HAS
Human serum albumin
- β-LG
β-Lactoglobulin
- DW
Deionized water
- ANS
1-Anilino-8-naphthalenesulfonate
- SGF
Simulated gastric fluid
- SIF
Simulated intestinal fluid
- FTIR
Fourier transform infrared spectroscopy
Author’s contributions
PSP Experiments, Methodology, Software, Writing-Original Draft. WY Experiments, Validation, Visualization, Investigation. ZY Writing-Review & Editing, Data Curation. WFJ Resources, Writing—Review & Editing, Supervision, Data Curation.
Funding
This research was supported by the National Key Research and Development Program (2022YFD1000104).
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Declarations
Conflicts of interest
The authors declare no conflict of interest.
Consent to participate
We are giving our consent for participation. Fengjun Wang, having a specialization in Walnut Protein Processing, will be grateful to review at least three manuscripts of JFST in future.
Consent for publication
The work was original research that has not been published previously, and is not under consideration for publication elsewhere, in whole or in part.
Ethics approval
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Suping Pei and Ying Wang have contributed equally to the work and should be regarded as co-first authors.
References
- Abou-Soliman NHI, Sakr SS, Awad S. Physico-chemical, microstructural and rheological properties of camel-milk yogurt as enhanced by microbial transglutaminase. J Food Sci Technol. 2017;54(6):1616–1627. doi: 10.1007/s13197-017-2593-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi Z, Razavi SMA, Varidi M. Sequential ultrasound and transglutaminase treatments improve functional, rheological, and textural properties of whey protein concentrate. Innov Food Sci Emerg. 2017;43:207–215. doi: 10.1016/j.ifset.2017.08.013. [DOI] [Google Scholar]
- Arzeni C, Martínez K, Zema P, Arias A, Pérez OE, Pilosof AMR. Comparative study of high intensity ultrasound effects on food proteins functionality. J Food Eng. 2012;108(3):463–472. doi: 10.1016/j.jfoodeng.2011.08.018. [DOI] [Google Scholar]
- Chacko SM, Thambi PT, Kuttan R, Nishigaki I. Beneficial effects of green tea: a literature review. Chin Med. 2010;5:13. doi: 10.1186/1749-8546-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrapala J, Zisu B, Palmer M, Kentish S, Ashokkumar M. Effects of ultrasound on the thermal and structural characteristics of proteins in reconstituted whey protein concentrate. Ultrason Sonochem. 2011;18(5):951–957. doi: 10.1016/j.ultsonch.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Chen L, Subirade M. Elaboration and characterization of soy/zein protein microspheres for controlled nutraceutical delivery. Biomacromol. 2009;10(12):3327–3334. doi: 10.1021/bm900989y. [DOI] [PubMed] [Google Scholar]
- Chen W, Ma H, Wang YY. Recent advances in modified food proteins by high intensity ultrasound for enhancing functionality: Potential mechanisms, combination with other methods, equipment innovations and future directions. Ultrason Sonochem. 2022;85:105993. doi: 10.1016/j.foodchem.2021.130961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Pei H, Dai Q, Zhang C, Kong X, Hua Y. Raw walnut kernel: A natural source for dietary proteases and bioactive proteins. Food Chem. 2022;369:130961. doi: 10.1016/j.foodchem.2021.130961. [DOI] [PubMed] [Google Scholar]
- Cui C, Hu Q, Ren J, Zhao H, You L, Zhao M. Effect of the structural features of hydrochloric acid-deamidated wheat gluten on its susceptibility to enzymatic hydrolysis. J Agric Food Chem. 2013;61(24):5706–5714. doi: 10.1021/jf400281v. [DOI] [PubMed] [Google Scholar]
- Cui Q, Wang G, Gao D, Wang L, Zhang A, Wang X, Xu N, Jiang L. Improving the gel properties of transgenic microbial transglutaminase cross-linked soybean-whey mixed protein by ultrasonic pretreatment. Process Biochem. 2020;91:104–112. doi: 10.1016/j.procbio.2019.12.001. [DOI] [Google Scholar]
- Gharibzahedi SMT, Roohinejad S, George S, Barba FJ, Greiner R, Barbosa-Cánovas GV, Mallikarjunan K. Innovative food processing technologies on the transglutaminase functionality in protein-based food products: trends, opportunities and drawbacks. Trends in Food Sci Technol. 2018;75:194–205. doi: 10.1016/j.tifs.2018.03.014. [DOI] [Google Scholar]
- Hu G, Ma M, Batool Z, Sheng L, Cai Z, Liu Y, Jin Y. Gel properties of heat-induced transparent hydrogels from ovalbumin by acylation modifications. Food Chem. 2022;369:130912. doi: 10.1016/j.foodchem.2021.130912. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Wang C, Li T, Sun D, Gao H, Gao Z, Mu Z. Effect of ultrasound on the structure and functional properties of transglutaminase-crosslinked whey protein isolate exposed to prior heat treatment. Int Dairy J. 2019;88:79–88. doi: 10.1016/j.idairyj.2018.08.007. [DOI] [Google Scholar]
- Jin F, Wang Y, Tang H, Regenstein JM, Wang F. Limited hydrolysis of dehulled walnut (Juglans regia L.) proteins using trypsin: Functional properties and structural characteristics. LWT-Food Sci Technol. 2020;133:110035. doi: 10.1016/j.lwt.2020.110035. [DOI] [Google Scholar]
- Liang Y, Teng F, He M, Jiang L, Yu J, Wang X, Li Y, Wang Z. Effects of ultrasonic treatment on the structure and rehydration peculiarity of freeze-dried soy protein isolate gel. Food Struct. 2021 doi: 10.1016/j.foostr.2020.100169. [DOI] [Google Scholar]
- Lin D, Zhang Q, Xiao L, Huang Y, Yang Z, Wu Z, Tu Z, Qin W, Chen H, Wu D, Zhang Q, Li S. Effects of ultrasound on functional properties, structure and glycation properties of proteins: a review. Crit Rev Food Sci Nutr. 2021;61(15):2471–2481. doi: 10.1080/10408398.2020.1778632. [DOI] [PubMed] [Google Scholar]
- Liu C, Yang X, Wu W, Long Z, Xiao H, Luo F, Shen Y, Lin Q. Elaboration of curcumin-loaded rice bran albumin nanoparticles formulation with increased in vitro bioactivity and in vivo bioavailability. Food Hydrocoll. 2018;77:834–842. doi: 10.1016/j.foodhyd.2017.11.027. [DOI] [Google Scholar]
- Luo K, Liu S, Miao S, Adhikari B, Wang X, Chen J. Effects of transglutaminase pre-crosslinking on salt-induced gelation of soy protein isolate emulsion. J Food Eng. 2019;263:280–287. doi: 10.1016/j.jfoodeng.2019.07.008. [DOI] [Google Scholar]
- Kato A, Nakai S. (1980). Hydrophobicity determined by a fluorescence probe method and its correlation with surface properties of proteins. Biochim Biophys Acta (BBA) Protein Struct 624(1), 13–20. doi: 10.1016/0005-2795(80)90220-2 [DOI] [PubMed]
- Maleki SJ, Teuber SS, Cheng H, Chen D, Comstock SS, Ruan S, Schein CH. Computationally predicted IgE epitopes of walnut allergens contribute to cross-reactivity with peanuts. Allergy. 2011;66(12):1522–1529. doi: 10.1111/j.1398-9995.2011.02692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minekus M, Alminger M, Alvito P, Ballance S, Bohn T, Bourlieu C, Carrière F, Boutrou R, Corredig M, Dupont D, Dufour C, Egger L, Golding M, Karakaya S, Kirkhus B, Feunteun SL, Lesmes U, Macierzanka A, Mackie A, Marze S, McClements DJ, Ménard O, Recio I, Santos CN, Singh RP, Vegarud GE, Wickham MSJ, Weitschies W, Brodkorb A. A standardised static in vitro digestion method suitable for food - an international consensus. Food Funct. 2014;5(6):1113–1124. doi: 10.1039/c3fo60702j. [DOI] [PubMed] [Google Scholar]
- Moreno HM, Bargiela V, Tovar CA, Cando D, Borderias AJ, Herranz B. High pressure applied to frozen flying fish (Parexocoetus brachyterus) surimi: Effect on physicochemical and rheological properties of gels. Food Hydrocoll. 2015;48:127–134. doi: 10.1016/j.foodhyd.2015.01.029. [DOI] [Google Scholar]
- Qin XS, Luo SZ, Cai J, Zhong XY, Jiang ST, Zhao YY, Zheng Z. Transglutaminase-induced gelation properties of soy protein isolate and wheat gluten mixtures with high intensity ultrasonic pretreatment. Ultrason Sonochem. 2016;31:590–597. doi: 10.1016/j.ultsonch.2016.02.010. [DOI] [PubMed] [Google Scholar]
- Rabadán A, Pardo JE, Gómez R, Álvarez-Ortí M. Evaluation of physical parameters of walnut and walnut products obtained by cold pressing. Lwt. 2018;91:308–314. doi: 10.1016/j.lwt.2018.01.061. [DOI] [Google Scholar]
- Ren X, Hou T, Liang Q, Zhang X, Hu D, Xu B, Chen X, Chalamaiah M, Ma H. Effects of frequency ultrasound on the properties of zein-chitosan complex coacervation for resveratrol encapsulation. Food Chem. 2019;279:223–230. doi: 10.1016/j.foodchem.2018.11.025. [DOI] [PubMed] [Google Scholar]
- Saeed M, Naveed M, Arif M, Kakar MU, Manzoor R, Abd El-Hack ME, Alagawany M, Tiwari R, Khandia R, Munjal A, Karthik K, Dhama K, Iqbal HMN, Dadar M, Sun C. Green tea (Camellia sinensis) and l-theanine: medicinal values and beneficial applications in humans-A comprehensive review. Biomed Pharmacother. 2017;95:1260–1275. doi: 10.1016/j.biopha.2017.09.024. [DOI] [PubMed] [Google Scholar]
- Silva NFN, Casanova F, Gaucheron F, Teixeira AVNdC, da Silva GM, Minim LA, Carvalho AFd. Combined effect of transglutaminase and sodium citrate on the microstructure and rheological properties of acid milk gel. Food Hydrocoll. 2018;82:304–311. doi: 10.1016/j.foodhyd.2018.03.038. [DOI] [Google Scholar]
- Tang CH, Wu H, Chen Z, Yang XQ, Peng ZY. Coagulation and gelation of soy protein isolates induced by microbial transglutaminase. J Food Biochem. 2005;29:402–421. doi: 10.1111/j.1745-4514.2005.00049.x. [DOI] [Google Scholar]
- Tapia MI, Sánchez-Morgado JR, García-Parra J, Ramírez R, Hernández T, González-Gómez D. Comparative study of the nutritional and bioactive compounds content of four walnut (Juglans regia L.) cultivars. J Food Compos Anal. 2013;31(2):232–237. doi: 10.1016/j.jfca.2013.06.004. [DOI] [Google Scholar]
- Truong VL, Jeong WS. Antioxidant and anti-inflammatory roles of tea polyphenols in inflammatory bowel diseases. Food Sci Human Wellness. 2022;11(3):502–511. doi: 10.1016/j.fshw.2021.12.008. [DOI] [Google Scholar]
- UN Food & Agriculture Organization (2020) Statistics Division (FAOSTAT). Production of walnut with shell by countries. FAO: Rome. http://www.fao.org/faostat/en/#data/QC.
- Wang Z, Li Y, Jiang L, Qi B, Zhou L. Relationship between secondary structure and surface hydrophobicity of soybean protein isolate subjected to heat treatment. J Chem. 2014;2014:1–10. doi: 10.1155/2014/475389. [DOI] [Google Scholar]
- Wang K, Li Y, Zhang Y, Sun J, Qiao C. Preheating and high-intensity ultrasound synergistically affect the physicochemical, structural, and gelling properties of chicken wooden breast myofibrillar protein. Food Res Int. 2022;162:111975. doi: 10.1016/j.foodres.2022.111975. [DOI] [PubMed] [Google Scholar]
- Wen X, Jin F, Regenstein JM, Wang F. Transglutaminase induced gels using bitter apricot kernel protein: chemical, textural and release properties. Food Biosci. 2018;26:15–22. doi: 10.1016/j.fbio.2018.09.002. [DOI] [Google Scholar]
- Xue H, Liu H, Wu N, Zhang G, Tu Y, Zhao Y. Improving the gel properties of duck egg white by synergetic phosphorylation/ultrasound: gel properties, crystalline structures, and protein structure. Ultrason Sonochem. 2022;89:106149. doi: 10.1016/j.ultsonch.2022.106149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C, Zhou Z. Walnut pellicle phenolics greatly influence the extraction and structural properties of walnut protein isolates. Food Res Int. 2021;141:110163. doi: 10.1016/j.foodres.2021.110163. [DOI] [PubMed] [Google Scholar]
- Yan ZM, Zhong YZ, Duan YH, Chen QH, Li FN. Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim Nutr. 2020;6(2):115–123. doi: 10.1016/j.aninu.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Yin L, Qu Y, Yan W, Zhang M, Su J, Jia X. Effect of calcium ions concentration on the properties and microstructures of doubly induced sorghum arabinoxylan/soy protein isolate mixed gels. Food Hydrocoll. 2022;133:107997. doi: 10.1016/j.foodhyd.2022.107997. [DOI] [Google Scholar]
- Yang J, Huang F, Huang Q, Ma D, Chen Y, Peng D, Yu X, Deng Q, Geng F. Physical and emulsifying properties of pea protein influence of combined physical modification by flaxseed gum and ultrasonic treatment. Food Sci Human Wellness. 2023;12:431–441. doi: 10.1016/j.fshw.2022.07.045. [DOI] [Google Scholar]
- Zang X, Yue C, Wang Y, Shao M, Yu G. Effect of limited enzymatic hydrolysis on the structure and emulsifying properties of rice bran protein. J Cereal Sci. 2019;85:168–174. doi: 10.1016/j.jcs.2018.09.001. [DOI] [Google Scholar]
- Zhang T, Zhao Y, Tian X, Liu J, Ye H, Shen X. Effect of ultrasound pretreatment on structural, physicochemical, rheological and gelation properties of transglutaminase cross-linked whey protein soluble aggregates. Ultrason Sonochem. 2021;74:105553. doi: 10.1016/j.ultsonch.2021.105553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Liu X, Liu W, Liu Q, Zhang L, Hu H. Effect of high-intensity ultrasound on the structural, rheological, emulsifying and gelling properties of insoluble potato protein isolates. Ultrason Sonochem. 2022;85:105969. doi: 10.1016/j.ultsonch.2022.105969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Zhu W, Yi J, Liu N, Cao Y, Lu J, Decker EA, McClements DJ. Effects of sonication on the physicochemical and functional properties of walnut protein isolate. Food Res Int. 2018;106:853–861. doi: 10.1016/j.foodres.2018.01.060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Not applicable.