Abstract
Lateral flow immunoassay (LFIA) is widely used as a rapid point-of-care testing (POCT) technique in food safety, veterinary and clinical detection on account of the accessible, fast and low-cost characteristics. After the outbreak of the coronavirus disease 2019 (COVID-19), different types of LFIAs have attracted considerable interest because of their ability of providing immediate diagnosis directly to users, thereby effectively controlling the outbreak. Based on the introduction of the principles and key components of LFIAs, this review focuses on the major detection formats of LFIAs for antigens, antibodies and haptens. With the rapid innovation of detection technologies, new trends of novel labels, multiplex and digital assays are increasingly integrated with LFIAs. Therefore, this review will also introduce the development of new trends of LFIAs as well as its future perspectives.
Keywords: Lateral flow immunoassays, Antigens, Antibodies, Haptens, Detection format, New trends
1. Introduction
Lateral flow immunoassays (LFIAs) develop on the basis of monoclonal antibody (mAb) technologies, immunochromatography technologies, new materials and labeling technologies [1,2]. LFIAs can realize qualitative and semi-quantitative detection of various analytes such as antigens, antibodies and haptens without professional skills and expensive instruments [[3], [4], [5], [6]]. The LFIA is one of the ideal immune rapid detection technologies, which is widely used in rapid detection of hormones [7], pathogenic microorganisms [8,9], veterinary drugs [8], pesticides [10], biotoxins and other targets [11,12]. LFIAs show broad application prospects in on-site real-time detection, which are especially suitable for hospitals, veterinary clinics, farms, dairies and other fields [[13], [14], [15]].
LFIAs were derived from the latex agglutination test established by Plotz and Singer [16], it is in the same period of time as radio-immunoassay and enzyme immunoassay. The early development and application of LFIA was the determination of human chorionic gonadotropin in pregnant women urine and serum/plasma in the late 1980s. LFIAs achieved the long-sought standard of “assurance” in diagnostic technology (affordable, sensitive, specific, user-friendly, fast and robust, no equipment required), which significantly promoted the development of immune diagnostic technology [17].
According to the different detection formats, LFIAs can be divided into direct detection and competitive detection. The direct detection can be used for the detection of antigens (such as human or animal proteins and pathogenic microorganism proteins, etc.) and antibodies (such as immunoglobulins). It is mostly applied in the early diagnosis of human and animal diseases, biochemical analysis and antibody titer monitoring. The competitive detection is a competitive inhibitory immunological binding reaction and mainly used for the detection of small molecule compounds with few antigenic sites or only a single antigenic site. These small molecule compounds are usually reactogenic but not immunogenic, they are also called haptens. Therefore, competitive detection is of great convenience in testing residues of pesticide, veterinary drug or mycotoxin.
With the rapid innovation of diagnostics, extensive efforts have been invested into carrying out novel labels to improve the sensitivity, multiplex detection to realize simultaneous detection of multiple targets and digital detection to realize visual reading and quantitative detection [18,19]. Hence, new trends of new labels, multiplex assays and digital assays are introduced. Finally, the future perspectives of LFIAs are also discussed.
2. Principles and components of the LFIAs
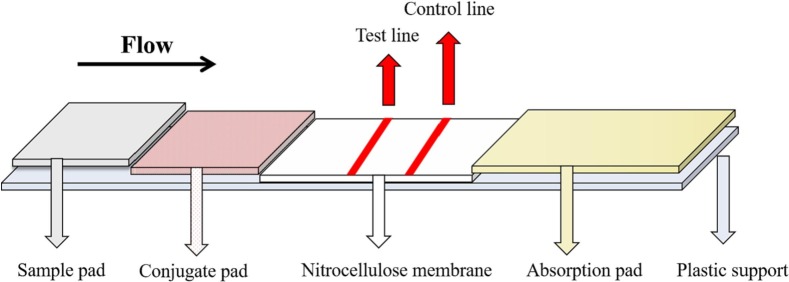
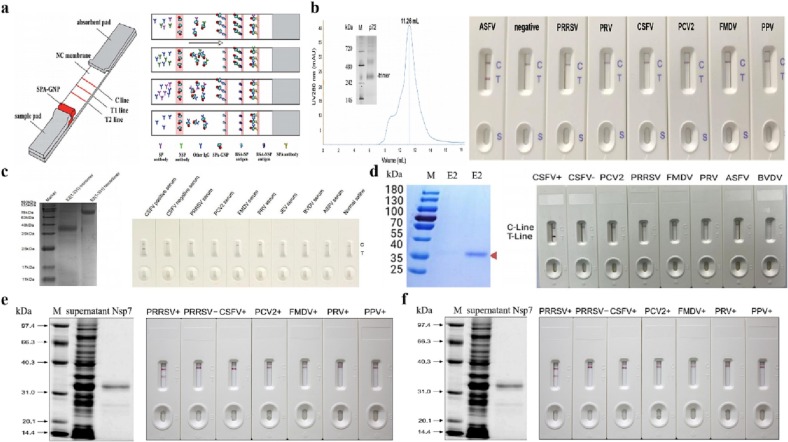
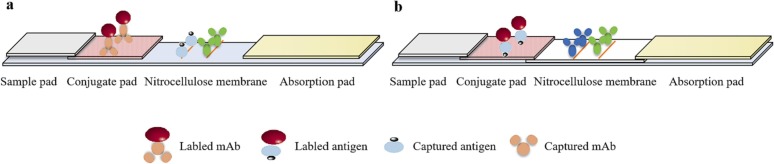
The test strip is the commonest form of LFIA, it consists of four basic structures: sample pad, conjugate pad, nitrocellulose (NC) membrane and absorption pad [1,20], which are stacked on a support plate in sequence from the test end to the handle end (Fig. 1 ). The sample pad absorbs the sample solution and makes it flow laterally to the conjugate pad by capillary force. The conjugate pad is labeled with bioactive materials (such as colloidal gold-labeled antibodies), which can bind to the target from sample solution to generate immunological complexes. The NC membrane intercepts labeled immune complexes, and visually displays the result. Two or more different biologically active materials (such as antigens or antibodies) are immobilized to form a “test line” (T line) and a “control line” (C line) on the NC membrane. The absorption pad absorbs the sample solution flowing through the strip, which maintains the pressure difference between the two ends and promotes more sample solution to laterally flow on the NC membrane.
Fig. 1.
Schematic diagram of a conventional LFIA structure.
3. LFIAs for antigens detection
Sandwich LFIA based on the principle of two different antibodies bind to antigen simultaneously is mainly detecting antigen with multiple antigenic sites, such as pathogenic bacteria, viruses and proteins. Most natural antigen molecules have complex structures with multiple antigenic epitopes on the surface. They can bind to different antibodies simultaneously and be used as detection targets. There are three LFIA formats for antigen detection.
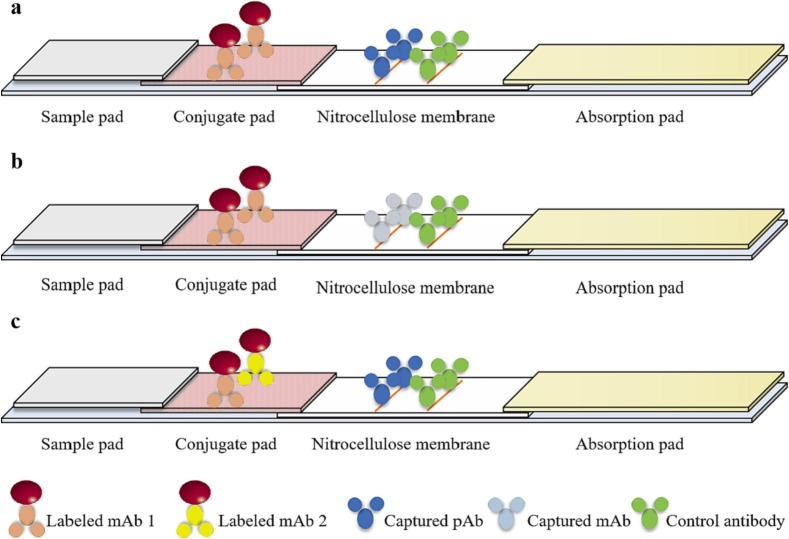
3.1. MAb labeling-polyclonal antibody capture format
The mAb labeling-polyclonal antibody (pAb) capture format is to prepare the conjugate pad by labeling the mAb that specifically recognizes the analyte with nanomaterial. PAb works as a capture antibody for immunological complexes, anti-mouse IgG antibody or Staphylococcal protein A (SPA) has the ability to bind immunoglobulin and work as the control antibody. They are coated on the NC membrane as the T line and the C line, respectively (Fig. 2a). During detection, the analyte in the sample is combined with the labeled antibody to form the immunological complexes, which are specifically recognized by the capture antibody, presenting a colored band at the T line. The excess labeled antibody and part of the immunological complexes are recognized by the control antibody, forming the C line, which gives a positive result. In addition, the color intensity is correlated with antigen concentration.
Fig. 2.
Antigen detection format diagram of LFIAs. a: mAb labeling-pAb capture format; b: mAb labeling-mAb capture format; c: combined mAb labeling-mAb capure format.
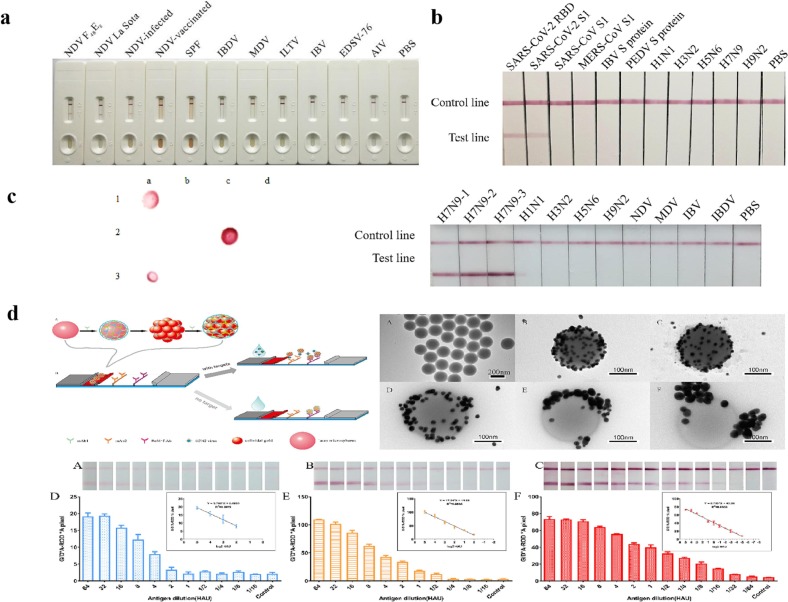
An immunochromatographic strip for the detection of avian avulavirus 1 (Newcastle disease virus, NDV) based on one anti-NDV mAb and one pAb was established by Li et al. in Key Laboratory of Animal Immunology (KLAI) of Henan Academy of Agricultural Sciences (HAAS) [21]. The strip can detect NDV with a detection limit of 104.9 EID50 viruses/0.1 mL in the NDV infected sample, whose performance is as good as hemagglutinin (HA) test (Fig. 3a). This strip can detect NDV in infected tissue as early as 36 h, before clinical symptoms and gross anatomical lesions.
Fig. 3.
LFIA for antigens detection developed in KLAI of HAAS. a: The LFIA for NDV antigen detection [21]; b: The LFIA for SARS-CoV-2 antigen detection [22]; c: The LFIA for H7 subtype avain influenza virus detection [23]; d: The LFIA for H3 subtype influenza virus detection with high sensitivity [24].
3.2. MAb labeling-mAb capture format
The mAb recognizes specific epitope of antigen coated on the NC membrane can effectively improve the specificity of the detection. Meanwhile, the use of mAbs can overcome the bias between batches of pAbs, thus it is suitable for standardized production of antigen test strips (Fig. 2b). Li et al. had established an immunochromatographic strip for the detection of the spike protein of SARS-CoV-2 [22]. This strip can detect SARS-CoV-2 spike protein in subunit vaccine with a detection limit of 62.5 ng/mL within 15–30 min (Fig. 3b). This strip monitors vaccine quality by detecting the antigen content of spike protein, providing technical support for the early diagnosis of the outbreak of COVID-19.
Li et al. [23] had developed an immunochromatographic strip based on two mAbs against HA protein for rapid detection of H7 subtype avain influenza viruses. The detection limit of the strip was 2.4 log10EID50/0.1 mL for chicken swab samples, which provided an effective approach for the rapid and early detection of H7 subtype avain influenza viruses (Fig. 3c). Liu et al. [24] has developed a strip for the optical determination of influenza virus H3 subtype. The strip utilized gold nanoparticles (AuNP)-coated polystyrene latex microspheres (PS) based on two mAbs against HA protein of H3. It showed a detection limit of 0.016 hemagglutination unit, assisting early determination of influenza virus infection (Fig. 3d).
3.3. Combined mAb labeling-mAb capture format
For pathogenic microorganisms with large antigenic epitopes, the variation of epitopes may lead to evasion of pathogenic microorganisms by the above two detection formats. The combination mAbs that recognize different antigenic sites are labeled with colloidal gold. Its efficiency of epitope recognition greatly improves the sensitivity of the test (Fig. 2c).
4. LFIAs for antibodies detection
Indirect LFIA is used to capture antibodies such as IgG, IgM and IgA. IgM antibodies are commonly used as early detection of infection; IgG antibodies are for the monitoring of immune antibody titers or the detection of differential diagnosis marker antibodies; IgA antibodies are applied to evaluation of mucosal immunity. There are four formats of LFIAs for antibodies detection.
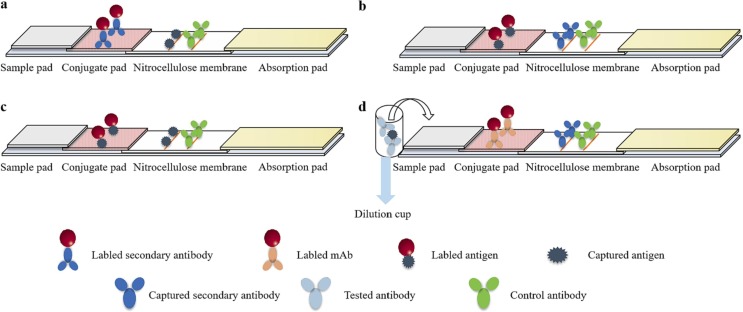
4.1. Secondary antibody labeling-antigen capture format
The conjugate pad is prepared by labeling anti-species IgG antibody or bacterial immunoglobulin binding protein A/G with nanomaterial. The specific antigen works as a capture antigen and protein A/G or IgG antibody works as a control antibody, which are coated on the NC membrane as T line and C line, respectively (Fig. 4a). The labeled antibody can bind to all immunoglobulins in blood or serum samples, non-specific antibodies with immunoglobulins significantly affect the generation of specific immunological complexes, thus affecting the results. In particular, low sensitivity to trace specific antibody often cause false negative results. A gold nanoparticle strip discriminating Foot-and-Mouth disease virus (FMDV) vaccinated animals from infected animals was established [25]. SPA conjugated with colloidal gold nanoparticles were used as a probe. Two proteins of FMDV were coated as test line 1 and test line 2 (T1 and T2) and the goat anti-pig antibody IgG was coated as a control line, as shown in Fig. 5a. The strip showed the specificity of T1 and T2 were 95.17 % and 100 %, which was in accordance with commercial ELISA kits. This strip enables monitoring titers of O-type FMDV antibody on site.
Fig. 4.
Antibodies detection format diagram of LFIAs. a: Secondary antibody labeling-antigen capture format; b: Antigen labeling-secondary antibody capture format; c: Antigen sandwich format; d: Antibody blocking format.
Fig. 5.
LFIAs for antibodies detection. a: The LFIA for FMDV antibody detection; b: The LFIA for African swine fever virus (ASFV) antibody detection; c and d: LFIAs for Classical swine fever virus (CSFV) antibody detection; e: The LFIA for Porcine reproductive and respiratory syndrome virus (PRRSV) antibody detection; f: The LFIA for Pseudorabies virus (PRV) antibody detection.
4.2. Antigen labeling-secondary antibody capture format
Nanomaterial labeled antigen as a probe can interact with analyte in the sample to form immunological complexes, which captured by anti-species IgG antibody or protein A/G on T line, and then react with analyte-specific antibody on C line (Fig. 4b). Antigens as probe reduce the interference of non-specific immunoglobulins and improve the sensitivity. Non-specific antibodies have chances to bind to the secondary antibody or protein A/G, which affects the generation efficiency of specific complexes. The affinity of the capture antibody to immunoglobulin is much higher than that of labeled antibody (about 10 times), thus reducing the non-specific bindings. Meanwhile, the diluted serum with less interference of non-specific antibody boost sensitivity. Varieties of strips aiming at detection of diseases, particularly swine diseases have been established. (Table 1 and Fig. 5).
Table 1.
Antibody detection strips for different diseases.
| Diseases | Antigens | Expression systems of antigens | Test line | Control line | Detection limit | References |
|---|---|---|---|---|---|---|
| African swine fever virus | P72 protein | HEK 293 | SPA | Anti-p72 pAbs | 1: 409,600 | [26] |
| Classical swine fever virus | E2 protein | Bac-to-Bac baculovirus | SPA | Rabbit anti-E2 pAbs | 1: 102,400 | [27] |
| Classical swine fever virus | E2 protein | Transgenic rice endosperm | SPA | Anti IgG | 1:128,000 | [28] |
| Porcine reproductive and respiratory syndrome virus | Nsp7 protein | Escherichia coli | SPA | Anti-Nsp7 antibodies | 1: 3200 | [29] |
| Pseudorabies virus | GB protein | Escherichia coli | SPA | Swine anti-PRV antibody IgG | 96 % compared with commercial ELISA | [30] |
4.3. Antigen sandwich format
Most of the antibodies are multivalent antibodies (IgG, IgE and serum IgA are bivalent, and IgM is tenvalent), which can form antigen-specific antibody-labeled antigen complexes at T line. Nanomaterial labeled one antigen as a probe can interact with analyte in the sample to form immunological complexes, which captured by the other antigen on T line, and then reacted with antigen-specific antibody on C line (Fig. 4c). The color intensity is positively correlated with the antibody level. The double-antigen sandwich format eliminates the interference of non-specific immunoglobulins on the formation of labeled antigen-antibody complexes. When the tested serum contains a small amount of target antibody, the excess labeled antigen may block the binding site of the specific antibody resulting in false negative results.
4.4. Antibody blocking format
Antibody blocking format enables detection of specific antigenic epitopes or differential diagnosis of labeled antibodies through high-affinity mAbs. The blocking test strip established on the base of neutralizing mAbs. It detects the neutralizing antibody and evaluates the titer of the neutralizing antibody. Also, the antibody blocking format can effectively exclude the interference of non-specific antibodies. Nanomaterial-labeled antigens or neutralizing antibodies are key components of this test. PAb or mAb plays a role as blocking antibody immobilized on T line, and anti-mouse IgG antibody is coated on C line (Fig. 4d). An optimal concentration of antigen is critical to antibody diluent. When the sample does not contain analyte-specific antibodies, the antigens in the diluent bind to labeled antibodies from the conjugate pad and then react with capture antibody on T line, and those of rest intercept on control line, representing a negative result. When the sample contains the analyte-specific antibodies, antigen-antibody binding occurs in the first step, and terminates the following reaction, only C line displays, indicating a positive result. The presence of specific antibodies can significantly inhibit or completely block the formation of antigen-labeled antibody complexes, which cannot be effectively captured by the detection antibodies at the test blot, leading to the T line significantly weakened or completely disappeared. The labeled antibody, however, can be capture by the control antibody at C line, which represents a positive result. The color intensity of the detection line is negatively correlated with the antibody level.
Ma [31] has developed an immunochromatographic strip for the detection of NDV antibody, which can distinguish vaccine-immunized animals from naturally infected animals within 10 min. The recombinant HN or F protein was adsorpted in an antigen pad or pre-incubation with serum. Then the anti-HN or anti-F mAb was labeled with colloidal gold as the detector. A chicken anti-NDV pAb and SPA were used as the T and C line, respectively. The colored C line without T line indicates a positive result, while both colored T and C lines indicates a negative result.
5. LFIAs for haptens detection
The haptens detection test strip mainly detects small molecule compounds of single antigenic epitope. The small molecule compounds are non-immunogenic or poorly immunogenic with small molecular weights. The hapten test strips are widely applied to the determination of antibiotics, pesticides, veterinary drugs, toxins, banned additives, drugs, hormones and heavy metals, etc. There are two formats of LFIAs for haptens detection.
5.1. Antibody labeling format
In the antibody labeling format, the conjugate pad is prepared by labeling the mAb that specifically recognizes the target antigen with nanomaterial. The target antigen coupled to a carrier protein (artificial antigen) is used as the capture antigen on T line, and the anti-species IgG antibody coated as C line (Fig. 6a). Sample without target leads to conjugated antibody captured by antigen on T line and then C line, indicating a negative result. Sample with target can be specifically recognized by conjugated antibody, thereby blocking the binding of the conjugated antibody to the capture antigen on T line. The presence of the target antigens interfere with blocking of antibody captured or completely blocked, significantly reducing the color appearing on T line. The unblocked labeled-antibody is recognized by C line, indicating a positive result. There were a series of test strips for the detection of haptens developed in KLAI of HAAS (Table 2 ).
Fig. 6.
Haptens detection formats diagram of LFIAs. a: Antibody labeling format; b: Antigen labeling format.
Table 2.
Examples of haptens detection strips developed in the past five years.
| Detection targets | Samples | IC50 values | Detection limits | Developed time | References |
|---|---|---|---|---|---|
| Xylazine | Milk | 0.590 ng/mL | 0.100 ng/mL | 2022 | [32] |
| Imidocarb | Milk | 0.400 ng/mL | 0.078 ng/mL | 2021 | [33] |
| Diminazene | Milk | 5.200 ng/mL | 1.200 ng/mL | 2020 | [34] |
| Bacitracin zinc | Milk | 3.160 ng/mL | 0.820 ng/mL | 2020 | [35] |
| Nitroxynil | Milk | 5.716 ng/mL | 1.146 ng/mL | 2020 | [36] |
| Danofloxacin | Milk | 0.513 ng/mL | 0.092 ng/mL | 2019 | [37] |
| Antibiotics | Milk | 0.040 ng/mL | 0.216 pg/mL | 2018 | [38] |
| Oseltamivir phosphate | Egg and chicken meat | 2.56 and 2.63 μg/kg | 0.43 and 0.42 μg/kg | 2018 | [39] |
5.2. Antigen labeling format
In the antigen labeling format, the conjugate pad is prepared by labeling the target antigen (artificial antigen) with nanomaterial. The mAb specific for the target antigen is used as the capture antibody on T line, and the anti-carrier protein antibody immobilized as C line (Fig. 6b). Sample without target leads to conjugated antigen captured by antibody on T line and then C line, indicating a negative result. Sample with target can compete with the conjugated antigen to bind the detection antibody on T line making the antibody unable to be captured or completely blocked, reducing the color appearing on T line. The unblocked conjugated-antigen is captured by C line, which represents a positive result.
6. New trends of LFIAs
6.1. LFIAs for hyper sensitivity
With the continuous development of material technologies, more and more nanoparticles have been developed and applied to LFIAs to improve the sensitivity of conventional test strip. Nanomaterials have shown the great potential in the labeling of antigens and antibodies owing to the special structure level, strong adsorption capacity, good orientation performance, biocompatibility and structural compatibility [40]. Nanomaterials have been used in developing LFIA for various kind, including gold nanoparticles (GNPs), carbon nanoparticles (CNPs), colloidal selenium nanoparticles (SNPs), quantum dots (QDs), fluorescent microspheres and magnetic nanoparticles (MNPs). The advantages and disadvantages of different nanoparticles used in LFIA are shown in Table 3 .
Table 3.
Advantages and disadvantages of different nanoparticles used in LFIAs.
| Nanoparticles | Advantages | Disadvantages | References |
|---|---|---|---|
| Gold nanoparticles | Easy to prepare and functionalize, good biocompatibility, low cost, easy-to-read results, detectable with the naked eye | Low sensitivity, requires reader for quantification | [[41], [42], [43]] |
| Carbon nanoparticles | High signal-to-noise ratio, stable, functional, non-toxic | Qualitative or semi-quantitative, background interference | [44,45] |
| Colloidal selenium nanoparticles | Rust-colored, easy to prepare and handle | Difficult to discern the results with the naked eye | [46] |
| Quantum dots | High optical stability, wide absorption spectrum, narrow emission band, strong stability and high sensitivity | Easily quenched of fluorescence, toxic, high fluorescence background, require a fluorescence reader for quantification | [[47], [48], [49], [50], [51]] |
| Up conversion nanoparticles | Long fluorescence lifetime, easy functionalization, stable, tunable emission color, no background fluorescence interference, high sensitivity | require a fluorescence reader for quantification | [[52], [53], [54]] |
| Magnetic nanoparticles | Easy surface functionalization, magnetic properties, long signal duration and low background noise | Require a magnetic readeror magnetic field sensor | [55,56] |
GNPs are in a stable colloidal state formed by electrostatic action and the polymerization of chloroauric acid under the reduction of trisodium citrate into gold particles of a specific size. GNP has a diameter of 1–150 nm and is purple color. Colloidal gold is widely used as LFIA-labeled probe on account of the characteristics of bright color, easy preparation, good biocompatibility, high stability, chemical traceability, and fine optical properties [57]. GNPs are a class of colored particle markers that can realize qualitative or semi-quantitative detection, with particle sizes ranging from 10 to 100 nm. The high stability, easy preparation, non-toxicity, easy conjugation and no activation characteristics of GNPs make them widely used in LFIAs [58]. Colloidal selenium is a nanoscale selenium particle with surface effect and small size effect obtained by adjusting the reaction conditions. It is widely used in LFIAs with characteristics of low cost, simple labeling with proteins, uneasy to coagulate after labeling, and easy adjustment of particle size range. Qds are spherical or quasi-spherical fluorescent semiconductor nanocrystals with a particle size ranging from 2 to 20 nm. QDs are considered to be the most potential biomarkers in LFIA, because of excellent fluorescence properties such as strong photochemical stability, long fluorescence lifetime, narrow and symmetrical emission spectrum [59]. Up conversion nanoparticles (UCPs) are fluorescent substances consisting of a host matrix, absorbers and emitters. UCPs are newly developed fluorescent probes that excited by low-energy near-infrared light and emits high-energy visible light. UCPs are ideal fluorescent nanomaterials with little damage to biological tissues and strong penetrating ability [60]. MNPs refer to nano-sized magnetic materials (represented by Fe3O4), which show satisfactory biocompatibility and magnetic orientation. MNPs are easy to prepare to couple with antibodies coated on immunomagnetic beads [61].
6.2. LFIAs for high-throughput detection
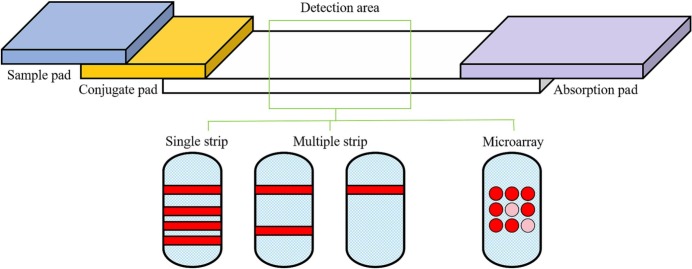
Typical LFIAs are suitable for point-of-care tests due to their portability, simplicity, cost-effectiveness, and rapid detection of target biomarkers [62]. However, detecting a single biomarker in typical LFIAs is not conducive to high-throughput diagnosis. Consequently, multi-biomarkers detection of LFIAs have been extensively studied in recent years [63]. Three typical structures are detection of multi-targets in a single-strip, a dual-strip with multi-targets, and microarray, integration of lateral flow with microanalysis. (Fig. 7 ) [64].
Fig. 7.
Schematic of three multiplex LFIAs.
The popular strategy for the LFIA multiplexing is to design several T lines or dots on the immunochromatographic strip by using gold nanoparticles (GNPs), quantum dots, or colored/fluorescent microspheres as carriers. Fluorescent nanoparticles require additional equipment for excitation, thus colored nanoparticles visible to naked eyes have been more extensively researched [[65], [66], [67], [68]]. Several individual strips can be combined into a special strip for samples to be collected only once and distributed to each strip in parallel flow. The advantages of this method rely on the large multiplexing capability and the absence of mutual interference between analytes that occur independently on a single strip [69]. Since a single test strip or a multi-test strip limited by quantitative capability and diagnostic validity, the combination of lateral flow and protein chip technology has been developed to achieve rapid and accurate detection of diagnosis of infectious diseases [70].
6.3. LFIAs for digital detection
Conventional LFIAs are convenient yet unable to achieve quantitative detection, and the bias in visual readings limit the application of LFIAs [71]. Thus, LFIAs with integrated optical readers to convert visual signals into more precise quantitative results is an acceptable approach. Instrument-based optical detection and electrochemical detection in conjunction with LFIAs are discussed.
Combining optical readers with LFIA make results to be easily obtained, especially in fields of genotyping and healthcare [72]. The imaging system accommodates a wider range of labeling systems with various color or optical properties, providing a more versatile instrument platform for LFIA. It is performed in conjunction with LFIAs by placing the test strip in a controlled lighting environment, where a complementary metal-oxide-semiconductor camera or high-density charge-coupled device captures images of the test and control lines [73]. Hundreds of millions of mobile phone users worldwide make mobile an attractive distributed platform for the future diagnostic market. The mobile phone camera based on CMOS imaging sensor can provide high image quality. Optical reader added up to LFIA will greatly improve its efficiency and convenience.
7. Outlooks
LFIAs based on three conventional systems of antigen, antibody and hapten have flourished since the establishment. They are extensively available in diagnosis, prognosis, screening, and monitoring of diseases in veterinary industry and human healthcare in hospital wards, clinics, health centers and self-diagnosis. IgM or IgG antibody test strips known to world since the outbreak of the COVID-19 in 2019. In order to monitor the SARS-CoV-2 and achieve effective control of the epidemic, antigen test strips based on antigen-antibody responses have been entrusted with detecting throat swabs or nasal swabs. However, a series of mutant viruses with stronger transmission of COVID have been derived, including the Alpha, Beta, Delta and Omicron variants.
The demands for quantitative LFIAs increase as the LFIA market expands [74]. New trends of novel labels, multiplex and digital assays are increasingly integrated with LFIAs with the rapid innovation of diagnostic technologies. Through the reader, nanoparticle tags captured in the test area are excited by external physical stimuli, such as lasers, electrical potentials or magnetic fields, resulting in amplified signals [75]. LFIAs are the backbone of rapid point-of-care diagnostics, with the potential to enable early case management and change in the epidemiology of infectious diseases. Emergent requirements from users for multiplexed systems capable of detecting multiple biomarkers simultaneously due to the complexity, multiple symptoms, and multiple infectious states of human disease. Imaging systems are also used for quantitative analysis of LFIAs. Cho I [76] used confocal Raman imaging combined with silver-enhanced technology to detect influenza B virus with sensitivity 1000 times higher than that of ordinary method. Mobile telephony (cell phone) healthcare is an emerging field as a next-generation point-of-care diagnostic platform.
8. Conclusions
The unique and remarkable properties of LFIAs have contributed to the detection of disease biomarkers and infectious agents in the fields of drugs, food, agriculture and environmental safety through three major detection systems. The principles of LFIAs have remained for decades, while the updates continue to occur. The sensitivity and reproducibility of LFIAs are promoted through novel labels and digitized devices, multiplex. Moreover, LFIAs effectively performed outside the laboratory with continued refinement. They offer the point-of-care diagnostics for developing countries both on site and in clinical settings.
Funding
This work was supported by the Science and Technology Development Project of Henan Province (212102110091, 222102110453), and Science and Technology Innovation Team of Henan Academy of Agricultural Sciences (2023TD03).
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
CRediT authorship contribution statement
Ge Li: Writing – original draft. Qingmei Li: Supervision. Xun Wang: Data curation. Xiao Liu: Visualization, Investigation. Yuhang Zhang: Writing – review & editing. Rui Li: Supervision. Junqing Guo: Conceptualization, Methodology, Software. Gaiping Zhang: Writing – review & editing.
Declaration of competing interest
The authors declare no competing interests.
Data availability
Datasets that support the current study are available from the corresponding author, [RS], upon reasonable request.
References
- 1.Zhang G., Guo J., Wang X. Immunochromatographic lateral flow strip tests. Methods Mol. Biol. 2009;504:169–183. doi: 10.1007/978-1-60327-569-9_12. [DOI] [PubMed] [Google Scholar]
- 2.Di Nardo F., Chiarello M., Cavalera S., et al. Ten years of lateral flow immunoassay technique applications: trends, challenges and future perspectives. Sensors (Basel) 2021;21(15):5185. doi: 10.3390/s21155185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park J.H., Park E.K., Cho Y.K., et al. Normalizing the optical signal enables robust assays with lateral flow biosensors. Acs Omega. 2022;7(21):17723–17731. doi: 10.1021/acsomega.2c00793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ali Z., Sanchez E., Tehseen M., et al. Bio-SCAN: a CRISPR/dCas9-based lateral flow assay for rapid, specific, and sensitive detection of SARS-CoV-2. ACS Synth. Biol. 2022;11(1):406–419. doi: 10.1021/acssynbio.1c00499. [DOI] [PubMed] [Google Scholar]
- 5.Karachaliou C.E., Koukouvinos G., Goustouridis D., et al. Recent developments in the field of optical immunosensors focusing on a label-free, white light reflectance spectroscopy-based immunosensing platform. Sensors (Basel) 2022;22(14):5114. doi: 10.3390/s22145114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh H., Singh S., Bhardwaj S.K., et al. Development of carbon quantum dot-based lateral flow immunoassay for sensitive detection of aflatoxin M1 in milk. Food Chem. 2022;393 doi: 10.1016/j.foodchem.2022.133374. [DOI] [PubMed] [Google Scholar]
- 7.Shyu R.H., Shyu H.F., Liu H.W., et al. Colloidal gold-based immunochromatographic assay for detection of ricin. Toxicon. 2002;40(3):255–258. doi: 10.1016/s0041-0101(01)00193-3. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen K., Yu W.L., Kelly L., et al. Validation and field assessment of a rapid lateral flow assay for detection of bovine antibody to Anaplasma marginale. J. Immunoass. Immunochem. 2009;30(3):313–321. doi: 10.1080/15321810903084749. [DOI] [PubMed] [Google Scholar]
- 9.Morales-Narvaez E., Naghdi T., Zor E., et al. Photoluminescent lateral-flow immunoassay revealed by graphene oxide: highly sensitive paper-based pathogen detection. Anal. Chem. 2015;87(16):8573–8577. doi: 10.1021/acs.analchem.5b02383. [DOI] [PubMed] [Google Scholar]
- 10.Mei Z., Qu W., Deng Y., et al. One-step signal amplified lateral flow strip biosensor for ultrasensitive and on-site detection of bisphenol A (BPA) in aqueous samples. Biosens. Bioelectron. 2013;49:457–461. doi: 10.1016/j.bios.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Ngom B., Guo Y., Wang X., et al. Development and application of lateral flow test strip technology for detection of infectious agents and chemical contaminants: a review. Anal. Bioanal. Chem. 2010;397(3):1113–1135. doi: 10.1007/s00216-010-3661-4. [DOI] [PubMed] [Google Scholar]
- 12.Kuang H., Xing C., Hao C., et al. Rapid and highly sensitive detection of lead ions in drinking water based on a strip immunosensor. Sensors (Basel) 2013;13(4):4214–4224. doi: 10.3390/s130404214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alhabbab R.Y. Lateral flow immunoassays for detecting viral infectious antigens and antibodies. Micromachines (Basel). 2022;13(11):1901. doi: 10.3390/mi13111901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moshe M., Daunt A., Flower B., et al. SARS-CoV-2 lateral flow assays for possible use in national covid-19 seroprevalence surveys (react 2): diagnostic accuracy study. Bmj. 2021;372 doi: 10.1136/bmj.n423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vashist S.K. Trends in multiplex immunoassays for in vitro diagnostics and point-of-care testing. Diagnostics (Basel) 2021;11(9):1630. doi: 10.3390/diagnostics11091630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plotz C.M., Singer J.M. The latex fixation test. I. Application to the serologic diagnosis of rheumatoid arthritis. Am. J. Med. 1956;21(6):888–892. doi: 10.1016/0002-9343(56)90103-6. [DOI] [PubMed] [Google Scholar]
- 17.Boehringer H.R., O’Farrell B.J. Lateral flow assays in infectious disease diagnosis. Clin. Chem. 2021;68(1):52–58. doi: 10.1093/clinchem/hvab194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y., He Z., Ablimit P., et al. Development of multiplex cross displacement amplification combined with lateral flow biosensor assay for detection of virulent shigella sonnei. Front. Cell. Infect. Microbiol. 2022;12:1012105. doi: 10.3389/fcimb.2022.1012105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bishop J.D., Hsieh H.V., Gasperino D.J., et al. Sensitivity enhancement in lateral flow assays: a systems perspective. Lab Chip. 2019;19(15):2486–2499. doi: 10.1039/c9lc00104b. [DOI] [PubMed] [Google Scholar]
- 20.Ching K.H. Lateral flow immunoassay. Methods Mol. Biol. 2015;1318:127–137. doi: 10.1007/978-1-4939-2742-5_13. [DOI] [PubMed] [Google Scholar]
- 21.Li Q., Wang L., Sun Y., et al. Evaluation of an immunochromatographic strip for detection of avian avulavirus 1 (Newcastle disease virus) J. Vet. Diagn. Investig. 2019;31(3):475–480. doi: 10.1177/1040638719837320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li G., Wang A., Chen Y., et al. Development of a colloidal gold-based Immunochromatographic strip for rapid detection of severe acute respiratory syndrome coronavirus 2 spike protein. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.635677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li G., Wang X., Li Q., et al. Development of an immunochromatographic strip for rapid detection of H7 subtype avian influenza viruses. Virol. J. 2021;18(1):68. doi: 10.1186/s12985-021-01537-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu X., Yang J., Li Q., et al. A strip test for the optical determination of influenza virus H3 subtype using gold nanoparticle coated polystyrene latex microspheres. Mikrochim Acta. 2020;187(5):306. doi: 10.1007/s00604-020-04255-1. [DOI] [PubMed] [Google Scholar]
- 25.Yang S., Sun Y., Yang J., et al. A gold nanoparticle strip for simultaneously evaluating FMDV immunized antibody level and discriminating FMDV vaccinated animals from infected animals. RSC Adv. 2019;9(52):30164–30170. doi: 10.1039/c9ra04810c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geng R., Sun Y., Li R., et al. Development of a p72 trimer-based colloidal gold strip for detection of antibodies against African swine fever virus. Appl. Microbiol. Biotechnol. 2022;106(7):2703–2714. doi: 10.1007/s00253-022-11851-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bai Y., Jia R., Wei Q., et al. Development and application of a high-sensitivity immunochromatographic test strip for detecting classical swine fever virus antibodies. Transbound. Emerg. Dis. 2022;69(4):e788–e798. doi: 10.1111/tbed.14367. [DOI] [PubMed] [Google Scholar]
- 28.Xu Q., Sun Y., Yang J., et al. An improved immunochromatographic strip based on plant-derived E2 for detection of antibodies against classical swine fever virus. Microbiol. Spectr. 2022 doi: 10.1128/spectrum.01050-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H., Yang J., Bao D., et al. Development of an immunochromatographic strip for detection of antibodies against porcine reproductive and respiratory syndrome virus. J. Vet. Sci. 2017;18(3):307–316. doi: 10.4142/jvs.2017.18.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X., Sun Y., Yang S., et al. Development of an immunochromatographic strip for antibody detection of pseudorabies virus in swine. J. Vet. Diagn. Investig. 2015;27(6):739–742. doi: 10.1007/s00604-020-04255-1. [DOI] [PubMed] [Google Scholar]
- 31.Ma F., Zhang E., Li Q., et al. A plant-produced recombinant fusion protein-based Newcastle disease subunit vaccine and rapid differential diagnosis platform. Vaccines (Basel) 2020;8(1):122. doi: 10.3390/vaccines8010122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen L., Hu X., Sun Y., et al. An ultrasensitive monoclonal antibody-based lateral flow immunoassay for the rapid detection of xylazine in milk. Food Chem. 2022;383 doi: 10.1016/j.foodchem.2022.132293. [DOI] [PubMed] [Google Scholar]
- 33.Chen L., Hu X., Sun Y., et al. Immunochromatographic assay based on high-affine monoclonal antibody for the detection of imidocarb in milk. J. Food Sci. 2021;86(8):3413–3421. doi: 10.1111/1750-3841.15831. [DOI] [PubMed] [Google Scholar]
- 34.Chen L., Sun Y., Hu X., et al. Colloidal gold-based immunochromatographic strip assay for the rapid detection of diminazene in milk. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2020;37(10):1667–1677. doi: 10.1080/19440049.2020.1778185. [DOI] [PubMed] [Google Scholar]
- 35.Na G., Hu X., Yang J., et al. Colloidal gold-based immunochromatographic strip assay for the rapid detection of bacitracin zinc in milk. Food Chem. 2020;327 doi: 10.1016/j.foodchem.2020.126879. [DOI] [PubMed] [Google Scholar]
- 36.Na G., Hu X., Yang J., et al. A rapid colloidal gold-based immunochromatographic strip assay for monitoring nitroxynil in milk. J. Sci. Food Agric. 2020;100(5):1860–1866. doi: 10.1002/jsfa.10074. [DOI] [PubMed] [Google Scholar]
- 37.Yang X., Wang Y., Yang J., et al. An immunochromatographic lateral flow strip test for the rapid detection of danofloxacin in milk. Food Anal. Methods. 2019;12(11):2430–2437. doi: 10.1007/s12161-019-01601-9. [DOI] [Google Scholar]
- 38.Shi Q., Huang J., Sun Y., et al. Utilization of a lateral flow colloidal gold immunoassay strip based on surface-enhanced Raman spectroscopy for ultrasensitive detection of antibiotics in milk. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018;197:107–113. doi: 10.1016/j.saa.2017.11.045. [DOI] [PubMed] [Google Scholar]
- 39.Yang X., Yang J., Wang Y., et al. A lateral flow Immunochromato-graphic strip test for rapid detection of oseltamivir phosphate in egg and chicken meat. Sci. Rep. 2018;8(1):16680. doi: 10.1038/s41598-018-35080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xue Z., Zhang Y., Yu W., et al. Recent advances in aflatoxin B1 detection based on nanotechnology and nanomaterials-a review. Anal. Chim. Acta. 2019;1069:1–27. doi: 10.1016/j.aca.2019.04.032. [DOI] [PubMed] [Google Scholar]
- 41.Zhou S., Hu J., Chen X., et al. Hydrazide-assisted directional antibody conjugation of gold nanoparticles to enhance immunochromatographic assay. Anal. Chim. Acta. 2021;1168 doi: 10.1016/j.aca.2021.338623. [DOI] [PubMed] [Google Scholar]
- 42.Zhang L., Mazouzi Y., Salmain M., et al. Antibody-gold nanoparticle bioconjugates for biosensors: synthesis, characterization and selected applications. Biosens. Bioelectron. 2020;165 doi: 10.1016/j.bios.2020.112370. [DOI] [PubMed] [Google Scholar]
- 43.Okyem S., Awotunde O., Ogunlusi T., et al. High-affinity points of interaction on antibody allow synthesis of stable and highly functional antibody-gold nanoparticle conjugates. Bioconjug. Chem. 2021;32(8):1753–1762. doi: 10.1021/acs.bioconjchem.1c00261. [DOI] [PubMed] [Google Scholar]
- 44.Xu L.D., Du F.L., Zhu J., et al. Luminous silica colloids with carbon dot incorporation for sensitive immunochromatographic assay of Zika virus. Analyst. 2021;146(2):706–713. doi: 10.1039/d0an02017f. [DOI] [PubMed] [Google Scholar]
- 45.Xu L.D., Zhu J., Ding S.N. Immunoassay of SARS-CoV-2 nucleocapsid proteins using novel red emission-enhanced carbon dot-based silica spheres. Analyst. 2021;146(16):5055–5060. doi: 10.1039/d1an01010g. [DOI] [PubMed] [Google Scholar]
- 46.Wang Z., Zhou Q., Guo Y., et al. Rapid detection of ractopamine and salbutamol in swine urine by immunochromatography based on selenium nanoparticles. Int. J. Nanomedicine. 2021;16:2059–2070. doi: 10.2147/IJN.S292648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou J., Yang Q., Liang C., et al. Detection of ochratoxin a by quantum dots-based fluorescent immunochromatographic assay. Anal. Bioanal. Chem. 2021;413(1):183–192. doi: 10.1007/s00216-020-02990-1. [DOI] [PubMed] [Google Scholar]
- 48.Zhou J., Qian W., Yang Q., et al. Analysis of virginiamycin M1 in swine feed, muscle and liver samples by quantum dots-based fluorescent immunochromatographic assay. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2022;39(8):1390–1400. doi: 10.1080/19440049.2022.2081366. [DOI] [PubMed] [Google Scholar]
- 49.Niu Y., Zhang G., Zhou J., et al. Differential diagnosis of the infection caused by wild-type or CD2v-deleted ASFV strains by quantum dots-based immunochromatographic assay. Lett. Appl. Microbiol. 2022;74(6):1001–1007. doi: 10.1111/lam.13691. [DOI] [PubMed] [Google Scholar]
- 50.Chen P., Zhou M., Chen X., et al. Quantum dot bead-based competitive immunochromatographic assay for enterotoxin aureus a detection in pasteurized milk. J. Dairy Sci. 2022;105(6):4938–4945. doi: 10.3168/jds.2021-21568. [DOI] [PubMed] [Google Scholar]
- 51.Jahangir M.A., Gilani S.J., Muheem A., et al. Quantum dots: next generation of smart nano-systems. Pharm. Nanotechnol. 2019;7(3):234–245. doi: 10.2174/2211738507666190429113906. [DOI] [PubMed] [Google Scholar]
- 52.Lu X., Chen Y., Zou R., et al. Novel immunochromatographic strip assay based on up-conversion nanoparticles for sensitive detection of imidacloprid in agricultural and environmental samples. Environ. Sci. Pollut. Res. Int. 2021;28(35):49268–49277. doi: 10.1007/s11356-021-14143-7. [DOI] [PubMed] [Google Scholar]
- 53.Chen Y., Lu H., Shi H., et al. An improved up-conversion nanoparticles-based immunochromatographic assay for rapid detection of zearalenone in cereals. Food Chem. 2023;412 doi: 10.1016/j.foodchem.2023.135555. [DOI] [PubMed] [Google Scholar]
- 54.Zhang S., Sun Y., Sun Y., et al. Semiquantitative immunochromatographic colorimetric biosensor for the detection of dexamethasone based on up-conversion fluorescent nanoparticles. Mikrochim Acta. 2020;187(8):447. doi: 10.1007/s00604-020-04418-0. [DOI] [PubMed] [Google Scholar]
- 55.Yin H.Y., Li Y.T., Tsai W.C., et al. An immunochromatographic assay utilizing magnetic nanoparticles to detect major peanut allergen Ara h 1 in processed foods. Food Chem. 2022;375 doi: 10.1016/j.foodchem.2021.131844. [DOI] [PubMed] [Google Scholar]
- 56.Lai X., Zhang G., Zeng L., et al. Synthesis of PDA-mediated magnetic bimetallic Nanozyme and its application in immunochromatographic assay. ACS Appl. Mater. Interfaces. 2021;13(1):1413–1423. doi: 10.1021/acsami.0c17957. [DOI] [PubMed] [Google Scholar]
- 57.Goudarzi S., Ahmadi A., Farhadi M., et al. Development of a new immunochromatographic assay using gold nanoparticles for screening of IgA deficiency. Iran J. Allergy Asthma Immunol. 2015;14(1):105–112. doi:ijaai.tums.ac.ir/index.php/ijaai/article/view/405. [PubMed] [Google Scholar]
- 58.Zhang X., Yu X., Wen K., et al. Multiplex lateral flow immunoassays based on amorphous carbon nanoparticles for detecting three fusarium mycotoxins in maize. J. Agric. Food Chem. 2017;65(36):8063–8071. doi: 10.1021/acs.jafc.7b02827. [DOI] [PubMed] [Google Scholar]
- 59.Chen H., Zhang X., Jin Z., et al. Differential diagnosis of PRV-infected versus vaccinated pigs using a novel EuNPs-virus antigen probe-based blocking fluorescent lateral flow immunoassay. Biosens. Bioelectron. 2020;155 doi: 10.1016/j.bios.2020.112101. [DOI] [PubMed] [Google Scholar]
- 60.Lian Y., Ding L.J., Zhang W., et al. Synthesis of highly stable cyanine-dye-doped silica nanoparticle for biological applications. Methods Appl. Fluoresc. 2018;6(3):34002. doi: 10.1088/2050-6120/aab930. [DOI] [PubMed] [Google Scholar]
- 61.Huang X., Aguilar Z.P., Xu H., et al. Membrane-based lateral flow immunochromatographic strip with nanoparticles as reporters for detection: a review. Biosens. Bioelectron. 2016;75:166–180. doi: 10.1016/j.bios.2015.08.032. [DOI] [PubMed] [Google Scholar]
- 62.Huang L., Tian S., Zhao W., et al. Multiplexed detection of biomarkers in lateral-flow immunoassays. Analyst. 2020;145(8):2828–2840. doi: 10.1039/c9an02485a. [DOI] [PubMed] [Google Scholar]
- 63.Byzova N.A., Urusov A.E., Zherdev A.V., et al. Multiplex highly sensitive immunochromatographic assay based on the use of nonprocessed antisera. Anal. Bioanal. Chem. 2018;410(7):1903–1910. doi: 10.1007/s00216-018-0853-9. [DOI] [PubMed] [Google Scholar]
- 64.Kim H., Chung D.R., Kang M. A new point-of-care test for the diagnosis of infectious diseases based on multiplex lateral flow immunoassays. Analyst. 2019;144(8):2460–2466. doi: 10.1039/c8an02295j. [DOI] [PubMed] [Google Scholar]
- 65.Peng J., Wang Y., Liu L., et al. Multiplex lateral flow immunoassay for five antibiotics detection based on gold nanoparticle aggregations. RSC Adv. 2016;6(10):7798–7805. doi: 10.1039/C5RA22583C. [DOI] [Google Scholar]
- 66.Wang Q., Liu Y., Wang M., et al. A multiplex immunochromatographic test using gold nanoparticles for the rapid and simultaneous detection of four nitrofuran metabolites in fish samples. Anal. Bioanal. Chem. 2018;410(1):223–233. doi: 10.1007/s00216-017-0714-y. [DOI] [PubMed] [Google Scholar]
- 67.Sun Y., Yang J., Yang S., et al. Development of an immunochromatographic lateral flow strip for the simultaneous detection of aminoglycoside residues in milk. RSC Adv. 2018;8(17):9580–9586. doi: 10.1039/c8ra01116h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu B., Gong H., Wang Y., et al. A gold immunochromatographic assay for simultaneous detection of parathion and triazophos in agricultural products. Anal. Methods. 2018;10(4):422–428. doi: 10.1039/C7AY02481A. [DOI] [Google Scholar]
- 69.Carrio A., Sampedro C., Sanchez-Lopez J.L., et al. Automated low-cost smartphone-based lateral flow saliva test reader for drugs-of-abuse detection. Sensors (Basel) 2015;15(11):29569–29593. doi: 10.3390/s151129569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chinnasamy T., Segerink L.I., Nystrand M., et al. A lateral flow paper microarray for rapid allergy point of care diagnostics. Analyst. 2014;139(10):2348–2354. doi: 10.1039/c3an01806g. [DOI] [PubMed] [Google Scholar]
- 71.Mak W.C., Beni V., Turner A. Lateral-flow technology: from visual to instrumental. Trends Anal. Chem. 2016;79:297–305. doi: 10.1016/j.trac.2015.10.017. [DOI] [Google Scholar]
- 72.Chan C.P., Mak W.C., Cheung K.Y., et al. Evidence-based point-of-care diagnostics: current status and emerging technologies. Annu Rev Anal Chem (Palo Alto, Calif) 2013;6:191–211. doi: 10.1146/annurev-anchem-062012-092641. [DOI] [PubMed] [Google Scholar]
- 73.Tahir M.A., Dina N.E., Cheng H., et al. Surface-enhanced Raman spectroscopy for bioanalysis and diagnosis. Nanoscale. 2021;13(27):11593–11634. doi: 10.1039/d1nr00708d. [DOI] [PubMed] [Google Scholar]
- 74.Kim J., Mohamed M., Zagorovsky K., et al. State of diagnosing infectious pathogens using colloidal nanomaterials. Biomaterials. 2017;146:97–114. doi: 10.1016/j.biomaterials.2017.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu Y., Liu Y., Wu Y., et al. Fluorescent probe-based lateral flow assay for multiplex nucleic acid detection. Anal. Chem. 2014;86(12):5611–5614. doi: 10.1021/ac5010458. [DOI] [PubMed] [Google Scholar]
- 76.Gozdzialski L., Rowley A., Borden S.A., et al. Rapid and accurate etizolam detection using surface-enhanced Raman spectroscopy for community drug checking. Int. J. Drug Policy. 2022;102 doi: 10.1016/j.drugpo.2022.103611. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Datasets that support the current study are available from the corresponding author, [RS], upon reasonable request.