Abstract
While cord blood (CB) is primarily utilized in allogeneic hematopoietic cell transplantation (HCT), the development of novel cell therapy products from CB is a growing and developing field. Compared to adult blood, CB is characterized by a higher percentage of hematopoietic stem cells (HSCs) and progenitor cells, less mature immune cells that retain a high capacity of proliferation, and stronger immune tolerance that requires less stringent HLA-matching when used in the allogenic setting. Given that CB is an FDA regulated product and along with its unique cellular composition, CB lends itself as a readily available and safe starting material for the development of off-the-shelf cell therapies. Moreover, non-hematologic cells such as mesenchymal stem cell (MSCs) residing in CB or CB tissue also have potential in regenerative medicine and inflammatory and autoimmune conditions. In this review, we will focus on recent clinical development on CB-derived cellular therapies in the field of oncology, including T-cell therapies such as chimeric antigen receptor (CAR) T-cells, regulatory T-cells, and virus-specific T-cells; NK-cell therapies, such as NK cell engagers and CAR NK-cells; CB-HCT and various modifications; as well as applications of MSCs in HCT.
Keywords: cord blood, chimeric antigen receptor, hematopoietic stem cell transplantation, NK-cells, mesenchymal stem cells
1. Introduction
The umbilical cord (UC) connects the fetus to the placenta for nutrient uptake, waste elimination, and gas exchange. The UC contains one vein, two arteries, and is protected by a surrounding gelatinous substance called Wharton’s jelly. At term, the UC has a mean length of 50cm, a mean diameter of 14mm, an approximate weight of 40g, and together with the placenta contains 50 to 200mL of cord blood (CB) (1). Compared to adult blood, CB contains a higher percentage of hematopoietic stem cells (HSCs) (2); moreover, the immune cells, such as T-cells and NK-cells, are phenotypically less mature (3, 4) These CB-specific features allow for more robust ex vivo and in vivo hematopoietic expansions and greater immune tolerance when used as a source for allogeneic cellular therapies. Apart from blood cells, nonhematopoietic progenitor cells such as mesenchymal stem cells (MSCs) can also be readily obtained from CB or CB tissue compartments such as Wharton’s jelly, which have great potential in regenerative medicine and immune modulation.
Following the first successful CB hematopoietic cell transplantation (CB-HCT) in 1988 and the establishment of the first public CB bank in New York in 1991 (5), more than 100 public CB banks were established worldwide with millions of CB units donated altruistically, greatly expanding the availability of CB (6). Moreover, it was estimated that more than 4 million units are being cryopreserved in private CB banks (7). Additionally, CB is an FDA regulated product that adheres to regulatory requirements. Therefore, the CB banks can provide readily available, high quality, and immunologically compatible hematopoietic cells to the majority of the population (8). CB is a particularly important cell source for patients who are racial or ethnic minorities, where matched adult donors are lacking in the registry (9). Although CB-HCT remains the main application of public CB banks, recent progress in cellular therapy has shown great potential in other CB-derived cellular products. Therefore, CB banks are an important resource to quickly upscale CB-derived cellular therapy. In addition to public cord blood banks, private cord blood banks offer the option of storing and using autologous cells for future use. However, preserving cord blood cells at birth for personal use poses several challenges. Firstly, the likelihood of needing CB-HCT, which is currently the only FDA-approved use for CB cells, is very low for people without a family history of blood disorders, estimated to be around 1 in 20,000 in their lifetime (10). Moreover, autologous CB-HCT is less effective than allogeneic CB-HCT in treating malignancies due to the absence of GvL effect. Thirdly, the quantities of stored single unit autologous cells may not be sufficient for cellular therapy. Finally, the impact of prolonged cryopreservation on the functionality of CB cells remains unclear. While the transplantation outcomes appear unaffected when CB cells were cryopreserved for up to 10 years, longer preservation may affect the viability of CB cells (11).
CB-derived cellular therapy harbors certain limitations. First, biologically, CB might not be the ideal source for all cellular therapies. For example, virus-specific T-cells (VSTs) have been used in severely immunosuppressed patients with resistant viral infections. However, given the naïve nature of CB, it lacks virus-experienced T-cells to manufacture VSTs (12). Second, practically, CB is limited by the small volume and total amount of hematopoietic cells. Therefore, ex vivo expansion may be required prior to adoptive cellular transfer. Moreover, good manufacturing practice (GMP)-compliant expansion protocols need to be established for each type of cellular therapy (13). Third, acquisition, processing, and storage of CB are expensive, which may result in low utilization of CB units from CB banks (7). Therefore, the high cost of each CB unit may translate into expensive CB-derived cellular products. Indeed, the current cost of a CB unit for HCT in developed countries ranges from thirty- to sixty-thousand US dollars (14).
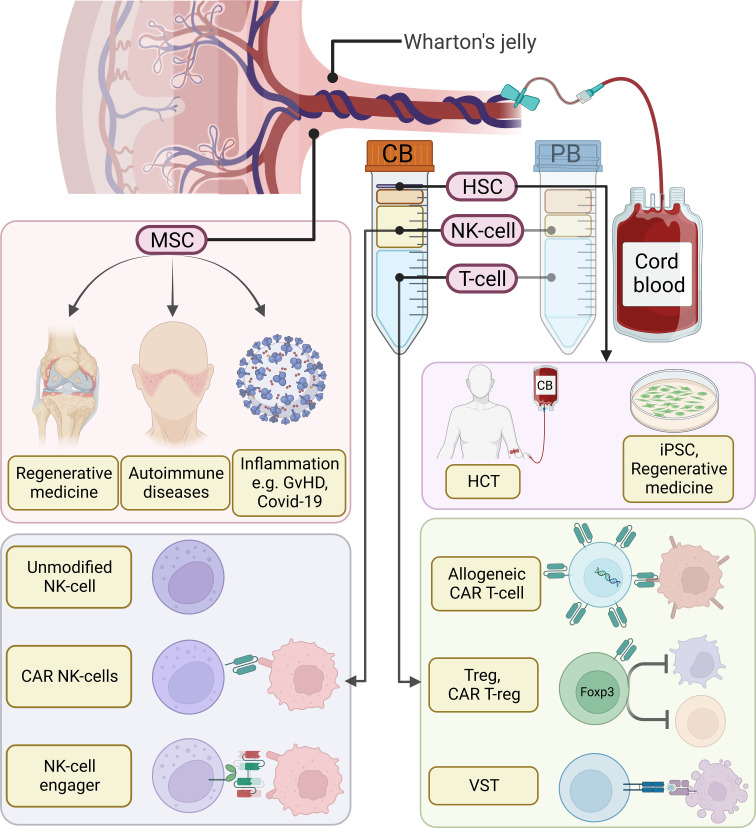
In this review, we will discuss cellular therapies utilizing different hematologic components of CB, including T-cells, NK-cells, HSCs, and hematopoietic progenitor cells, as well as non-hematologic components such as MSCs ( Figure 1 ). This review will focus on their applications in oncology based on clinical evidence from published clinical trials, and discuss advantages, challenges, and limitations of each CB-derived cellular product ( Table 1 )
Figure 1.
Overview of cord blood (CB) derived cellular therapies. Compared to adult peripheral blood (PB), CB is characterized by a higher percentage of NK-cells and the presence of hematopoietic stem cells (HSCs). Moreover, blood cells in CB are more naïve and harbor greater proliferation potential. Among them, HSCs can be used in hematopoietic stem cell transplantation (HCT) or as a source for induced pluripotent stem cells (iPSCs) and regenerative medicine. T-cells can be utilized to generate allogeneic chimeric antigen receptor (CAR) T-cells, regulatory T-cells (Tregs), CAR T-regs, or virus-specific T-cells (VSTs). CB-derived NK-cells have been shown to be a great source for allogeneic NK-cell infusion, CAR NK-cells, as well as pre-activated NK-cells pre-complexed with NK-cell engagers. In addition to CB-derived blood cells, nonhematologic cells in the Wharton’s jelly, such as mesenchymal stem cells (MSCs), process regenerative and immunomodulatory effects. MSCs can be used in tissue engineering, autoimmune diseases, as well as inflammatory diseases.
Table 1.
Overview of advantages and challenges of cord blood-derived cellular therapies .
| Cellular component | Product or indication | Clinical development phase | Advantages | Challenges | Future development |
|---|---|---|---|---|---|
| T-cell | CAR T | Ongoing Phase I | • More naïve T-cell phenotype, may translate into better in vivo expansion and persistence (15) • Ability to generate an off-the-shelf bank matching HLA of most population, avoiding GvH and HvG effects without genome editing (16) • Able to incorporate CB-HCT before or after CAR T-cell therapy (17) • CB stored at birth can be used autologously avoiding contamination from T-cell leukemia |
• Limited starting material • Lack proof-of-concept early phase clinical trial |
• Proof-of-concept clinical trials with HLA-matched CB-derived CAR T-cell product demonstrating in vivo expansion and persistence • Use techniques such as iPSC to generate bulks of T-cells |
| Treg | Phase I/II, preclinical for CAR-Tregs | • Higher percentage of Tregs in CB (18) • Allogeneic and off-the-shelf ability given HLA-mismatch tolerance |
• Limited starting material • Limited persistence in blood, may need repeated infusion (19) |
• CAR-Tregs to target specific antigen • Use techniques such as iPSC to generate bulks of Tregs |
|
| VST | Phase I/II | • Able to utilize 20% of CB units for CB-HCT to generate VST (12) | • Naïve CB T-cells lacking virus-experienced T-cells • Prolonged production time due to limited starting material (12) |
• Allogeneic PB-derived VST likely a better source than CB-derived VST | |
| NK-cell | NK infusion | Phase I/II | • Higher percentage of NK cells in CB (2) • Improved persistence of HLA-matched CB-derived NK cells • Bulk production of HLA-matched CB-derived NK cells through induced differentiation of HSCs (20) |
• Limited activity in early phase trials (21) • Reduced cytotoxicity compared to PB NK cells if without cytokine stimulation (22) • Lack of in vivo expansion and persistence (23) • Poor intra-tumoral infiltration (24) • Cryopreservation of CB can damage NK cell cytotoxicity (25) |
• Combine other agents such as interleukin, TGFβ receptor inhibitor to improve efficacy • Genetically engineered NK cells, such as self-IL-15 secretion, to improve expansion and persistence |
| CAR NK | Phase I/II | • Favorable safety profile without risk of GvHD, CRS, or ICANS (26) • Enhanced killing through both CAR and innate receptor, theoretically reducing CAR antigen escape (27) • Higher percentage of phenotypically naïve NK cells in CB promoting in vivo expansion (28) • Lack of innate inhibitory NK signal given the allogeneic nature • As a CB-derived product, readily available cord banks and high proliferative potential |
• Requires ex vivo expansion • Limited in vivo persistence, leading to early relapse (26) • Tumor suppressive environment hindering efficacy (29) • Feasibility of large-scale manufacturing |
• Genetically engineered NK cells to improve expansion and persistence • Approaches to tackle trogocytosis |
|
| NKCE+NK | Phase I/II | • Proven off-the-shelf ability (30) • Favorable safety profile without risk of GvHD, CRS, or ICANS (30) • Relatively lower cost than genetically engineered cells • Multiple indications with the same platform |
• Limited efficacy if without co-infusion of preactivated NK cells; cumbersome to administer (31) | • Confirmation trials with a larger population • Expansion of indications in other types of malignancies |
|
| Stem cell | CB-HCT | FDA-approved | • Readily available source • Less stringent HLA-matching requirement (32) • Less chronic GvHD (33) • FDA-approved ex vivo expanded CB products enhancing engraftment (34) |
• Low stem cell dose • Slow engraftment, leading to increased transplant-related morbidity and mortality (35) • Increased resource utilization • Extra processing time and cost for ex vivo expanded CB products |
• Increased utilization of ex vivo expanded CB products, such as omidubicel, in the clinical setting • Further development of ex vivo expanded CB products • Reducing cost for ex vivo expanded CB products |
| MSC | GvHD | Phase II | • Easily obtained source • Good safety profile (36) • Efficacy in early phase trials (37–39) • Off-the-shelf ability |
• A phase III trial of BM-derived MSCs failed to show improved efficacy over existing care (40) | • Carefully designed phase III trials in later lines of therapy for GvHD • Efficacy in chronic GvHD • Identify markers to predict responders |
CAR, chimeric antigen receptor; HLA, human leukocyte antigen; GvH, graft-versus-host; HvG, host-versus-graft; CB, cord blood; HCT, hematopoietic cell transplantation; iPSC, induced pluripotent stem cells; VST, virus-specific T-cells; PB, peripheral blood; HSC, hematopoietic stem cells; CRS, cytokine release syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome; NKCE, natural killer cell engager; ARDS, acute respiratory distress syndrome; BM, bone marrow.
2. Cord blood-derived T-cell therapies
CB T-cells differ in composition and functional properties when compared to peripheral blood (PB) T-cells. Close to 90% of T-cells in the CB are naïve T-cells since they have not encountered any foreign antigens (3). Moreover, CB harbors a higher percentage of CD8+CCR7+ T-central memory (TCM) cells, which mediates enhanced antitumor responses through increased tumor-homing and rapid gain of cytotoxic function, making it an excellent source for adoptive T-cell therapies (41, 42). However, CB T-cells are not simply immature versions of adult T-cells, but a distinct population with different gene expression profile and functional properties (43). They are more tolerant-prone and poised for rapid effector differentiation (44), which are ideally suited for allogeneic T-cell therapies.
T-cell therapies in CB can be broadly divided into two categories, one involves isolation and expansion of existing T-cells (Tregs, or virus-specific or tumor antigen-specific cytotoxic T-cells), and the other involves genetic engineering of T-cells. The latter, which in the early years utilized T-cell receptor (TCR) engineering, has been associated with suboptimal clinical efficacy and considerable toxicities (45). The recent years have witnessed a rapid development of chimeric antigen receptor (CAR) T-cell therapy, which has demonstrated superior clinical efficacy especially in B-cell malignancies (46), making it an ideal platform for CB-based T-cell therapies.
2.1. Allogeneic CAR T-cell therapy
CAR T-cell therapy has revolutionized the treatment of refractory/relapsed B-cell malignancies. However, current FDA-approved CAR T-cells are all autologous products derived from patients, limited by long production time, chance of production failure, product variability, possible contamination by tumor cells, and high production cost. Therefore, “off-the-shelf” allogeneic CAR T-cells have attracted great attention in recent years, albeit encountering many challenges, such as graft (CAR T)-versus-host (GvH) effects due to Human Leukocyte Antigens (HLA) mismatch, and lack of in vivo expansion and persistence due to host-versus-graft (HvG) effects (47). Using CB as the source for allogeneic CAR T-cells may overcome some of the challenges and offer unique advantages over PB from healthy donors. First, CB CAR T-cells retained a less differentiated phenotype (such as TSCM and TCM) than PB CAR T-cells (15), which could translate into a longer persistence of CAR T-cells. Second, CB T-cells have been known to have greater immune tolerance to HLA mismatch (16). In CB HCT, matching for alleles HLA-A, HLA-B and HLA-DR (i.e. ≥ 4 of 6 HLA match) is sufficient to reduce the incidence of graft-versus-host disease (GvHD) to a low level (48). Therefore, it has been demonstrated that an CB bank of only 150 selected samples could provide full matches (6 of 6 HLA loci) for 93% of the UK population (49); if partial mismatch is allowed (4 or 5 of 6 HLA loci), the required number can be further reduced. Therefore, it is possible to generate an “off-the-shelf” allogeneic CB-derived CAR T-cell bank that can be readily used for most of the population with minimal GvH effects. On the other hand, to reduce GvH effects in PB-derived CAR T-cells, T-cell receptor (TCR) knockout using genome editing is required, for which the long-term safety is still unknown (50). Another advantage of CB-derived CAR T-cell therapy is the ease to incorporate consolidative CB HCT after CB CAR T-cell therapy, which might provide improved progression-free survival (17).
Several preclinical studies have demonstrated the feasibility of manufacturing CAR T-cells from CB (15, 41, 51). In an earlier study by Pegram et al., banked CB T-cells were expanded with IL-12/IL-15 stimulation, and transduced with anti-CD19 and IL-12 secreting modules (51). The CAR T-cells retained a central memory-effector phenotype, and demonstrated activity in a B-cell acute lymphoblastic leukemia mouse model. In a study by Cael et al., fresh or thawed CB T-cells were transduced by lentivirus encoding CD123-CAR to target blastic plasmacytoid dendritic cell neoplasm (BPDCN) mouse models (15). They found that CB CAR T-cells exhibited potent anti-tumor activities while retaining a less differentiated profile than PB CAR T-cells; moreover, fresh or cryopreserved CB CAR T-cells demonstrated similar functionalities. In another study by Liu et al., cryopreserved, CB-derived, CD19-targeting CAR T-cells had more TN and TCM than PB CAR T-cells derived from the patients, and harbored fewer T-cell exhaustion markers, leading to a better antitumor efficacy and longer tumor-suppressive effects (41).
However, allogeneic use of CB as an “off-the-shelf” product in the clinical setting is still lacking. A search of ClinicalTrials.gov in December 2022 returned only one ongoing clinical trial (NCT03881774). Indeed, several limitations of CB-derived CAR T-cells need be addressed before broader clinical applications. First, like other allogeneic CAR T-cell therapies, solutions need be developed to tackle GvH and HvG effects. Although TCR knockout has been effective to reduce GvH in PB-derived (52) and CB-derived (53) CAR T-cells, it is possible to avoid genome editing, as mentioned above, by obtaining an HLA-matched CB unit from a limited and highly selected CB bank as the cell source for CAR T-cells (49). However, proof-of-concept clinical studies are lacking at this point. Second, due to limited availability of selected CB units, techniques such as induced pluripotent stem cells (iPSCs) (54) or directed differentiation of hematopoietic stem cells (55) are required to produce the bulk population of T-cells. However, further developments of these methods are still needed to generate a self-renewing and clinically applicable source of T-cells.
2.2. Regulatory T-cells (Tregs)
Tregs are a subset of natural occurring CD4+ T-cells that suppresses the activation and expansion of overactive lymphocytes, thus preventing excessive immune activation and autoimmunity. While Tregs comprise less than 3% of mononuclear cells in the PB, CB contains a high proportion of circulating natural Tregs, obviating multiple selection processes to reach the required purity of Tregs (18). Moreover, compared to PB-derived Tregs, CB-derived Tregs have greater post-expansion purity, FOXP3 stability, and proliferative capacity (56). Another advantage is the potential of CB units as an allogeneic source of Tregs due to great tolerability of HLA-mismatch. Adoptive cellular therapy using Tregs can be divided into two categories: selection and expansion of natural occurring polyclonal Tregs, and manufacturing antigen specific Tregs using TCR or CAR gene transfer.
Polyclonal Tregs have been reported in human trials in treating autoimmune diseases such as multiple sclerosis (57) and type 1 diabetes mellitus (58, 59), preventing rejection and reducing needs for immunosuppressant agents after solid-organ transplantation (20, 60, 61), and preventing and treating GvHD after HCT (19, 62–64). While most of the aforementioned studies used autologous PB mononuclear cells (PBMC) as the source of Treg, in a study by Brunstein et al., 11 patients undergoing CB-HCT received CB-derived, HLA-matched Tregs from a cord bank; they observed very low incidence of Grade II-IV acute GvHD of only 9%, and none developed chronic GvHD (19). However, although Tregs have shown a favorable safety profile and early signs of clinical efficacy, several challenges must be overcome before broader applications. The first challenge is to determine the timing and frequency of administration. Although in some studies Tregs can still be detected in the PB one year after administration (59), other studies have shown the persistence of only 14 days (19). Therefore, for conditions like autoimmune disease or solid-organ transplantation where persistent immunosuppression is required, it is unknown whether and when Treg products should be re-dosed after the initial infusion. Indeed, in the landmark ONE trial among patients with kidney transplantation, after a single dose of autologous Treg infusion, immunosuppressants were able to be tapered but not completely weaned off (60). Second, due to limited volume of CB, it is not uncommon to fail to achieve the targeted cell dose, which occurres in a frequency of more than 10% in previous studies (18, 19). Therefore, a better understanding of Treg biology and improvements of manufacturing process are needed for future larger trials. While identifying new Treg markers can simplify Treg selection and purification, techniques such as 3D culture, automated bioreactors, or robotic platforms may aid in the large-scale production of lineage-stable CB-derived Treg cells.
While polyclonal Tregs may be suitable for the treatment of autoimmune conditions where a variety of tissues are targeted, antigen-specific Tregs may be particularly useful in situations where the disease-relevant antigens are known or limited tissues are involved (65). Through engineered antigen-specific receptors (CARs or TCRs), Tregs have enhanced trafficking and targeting to specific microenvironment. With the success of CD19-targeting CAR T-cell therapy, CAR-Tregs have attracted great attention in recent years, albeit current studies have been limited to the preclinical phase. Major conditions being studied include HCT/solid organ transplantation (66), type 1 diabetes (67), inflammatory bowel disease (68), and hemophilia (69), targeting HLA-A2, insulin/HiP2, carcinoembryonic antigen (CEA), and factor VIII, respectively. A search of ClinicalTrials.gov in December 2022 showed two ongoing clinical trials (NCT04817774 and NCT05234190), both using autologous, HLA-A2 targeting CAR Tregs to prevent rejection in solid-organ transplantation recipients who are HLA-A2 negative receiving an A2 positive graft. Hurdles for manufacturing autologous CAR Tregs for clinical use include prolonged production time, contamination of conventional T-cells, and higher chance of production failure due to limited amount in the PB (70). Moreover, in a population often receiving high dose immunosuppressants, the function of Tregs might be impaired. Therefore, “Off-the-shelf” allogeneic CAR Tregs products, such as those manufactured from CB, are promising alternatives. However, further studies are needed to demonstrate their safety and efficacy.
2.3. Virus-specific T-cells (VSTs)
Over the past three decades, adoptive transfer of VSTs has been proven to be safe and effective for the treatment of refractory viral infections in patients with severely compromised immune system, such as those underwent HCT/solid-organ transplantation (71) or with primary immunodeficiency disorders (72). Moreover, the indications of VSTs have been extended to viral-associated malignancies such as EBV-positive post-transplantation lymphoma (73–75). These VSTs are usually organ or stem-cell donor derived, either through ex vivo stimulation and expansion, or direct sorting and selection from apheresis products of seropositive donors (76). True allogeneic VSTs obtained from healthy third-party donors have been increasingly reported in recent years. Even with low level HLA match (1 or 2 out of 6 or 8), studies have shown excellent clinical efficacy without causing significant GvHD (77–79). The extremely low rates of acute GvHD (<10%) are probably due to the fact that VSTs are largely central or effector memory T cells that are less likely to be alloreactive (80). However, studies have shown that persistence of third-party allogenic VSTs was only 12 weeks (78), which might not be enough to cover the whole immune reconstitution phase. Therefore, future studies are needed to examine the necessity and timing of redosing or investigate methods to promote allogeneic VST’s persistence.
However, CB has several major limitations as a source for VSTs. First, CB contains predominantly naïve T-cells lacking virus-experienced T-cells, which makes direct sorting and selection difficult. Second, a single CB unit has a small volume; with the inability to procure further donations, this leads to prolonged expansion and production time with high chances of production failure. However, for patients undergoing CB HCT, CB becomes the only available source for autologous VSTs. Abraham et al. reported the use of 20% fraction of CB units that were reserved prior to the stem cell infusion, and they used them to manufacture CB-derived multivirus-specific T-cells in pediatric patients (12). Using ex vivo antigen stimulation, production was successful in 18 of the 21 pediatric patients. However, the median production time was 52.5 days, which remains the major obstacle for widespread application given the urgency of antiviral treatment. Further development has resulted in reduced production time, but it could still take around 30 days (81). Moreover, the expanded cell dose is likely insufficient for adult patients. Therefore, although it is feasible to produce VSTs from CB, challenges remain; given the efficacy and safety of allogeneic PBMC-derived VSTs, they will likely come out as a better option for patients undergoing CB HCT complicated by refractory viral infections.
2.4. Other T-cell therapies
Autologous HCT using stored self CB have been reported (82). Therefore, it is also possible to manufacture autologous CAR T-cells from preserved CB at birth. This may be particularly useful for patients with T-cell leukemia where it is vital to avoid CAR T-cell product contamination from circulating leukemic T-cells.
γδ T-cells is a unique subpopulation of T-cells that function in between innate and adaptive immunity – they mediate cytotoxicity through antigen-pattern recognition independent of MHC presentation (83). Over the past two decades, adoptive transfer of PB-derived autologous γδ T-cells have been tested in a wide range of malignancies without good clinical response (84). Interestingly, given the HLA-independent killing, allogenic γδ T-cells have been proven to be safe with a low risk of GvHD. Therefore, repeated infusion of healthy donor derived allogeneic γδ T-cells has become possible. Indeed, two recent studies showed improved clinical efficacy with repeated γδ T-cells infusion in patients with late-stage malignancies (85, 86). Furthermore, targeted therapy using CAR-engineered γδ T-cells have attracted great attention, with a recent phase I study showed promising safety and efficacy (87). On the other hand, CB has several disadvantages as the source for γδ T-cell therapies, including low frequency of γδ T-cells (<1%) and lack of the functional subset of γδ T-cells (88). Although successful expansion of CB-derived γδ T-cells has been reported (89, 90), its clinical usage remains to be defined, given that PB-derived γδ T-cells can be more easily manufactured.
A broader application of CAR T-cell therapy in solid tumors is limited due to the lack of tumor-specific surface antigens. However, frequently mutated intracellular protein can be presented to the cell surface through coupling with MHC I molecules, forming neoantigens that can be recognized by TCRs; this provides an opportunity for genetically engineered TCR-T cell therapy. A recent study using autologous T cells transduced with TCRs recognizing the mutant KRAS G12D-MHC I complex showed a partial response in a patient with metastatic pancreatic cancer (91). CB-derived TCR T-cell therapy has remained in the pre-clinical phase (92).
Lastly, tumor antigen-specific cytotoxic lymphocytes (CTLs) can be selectively expanded (93) or primed (94) using techniques similar to manufacturing VSTs. Compared to CTLs obtained from PB, CB-derived CTLs showed higher proliferative and cytotoxic activities in samples obtained from patients (95). Antigen-specific CTLs might be useful in patients who have received CB HCT to prevent or treat cancer relapse, in a role similar to donor lymphocyte infusion (DLI). However, research in this field is extremely limited.
3. CB-Derived NK-cell therapies
NK cells are part of the innate immune system that target malignant or virus-infected cells. Unlike T cells, NK cells lack specificity to a certain antigen. Rather, their activation is based on a “signal threshold” from the integration of inhibitory and activating receptors – bindings between NK inhibitory receptors and target cell HLA molecules provide the inhibitory signals, while the engagements of NK activating receptors with stress-induced self-ligands provide the activating signals (96). When HLA expression is decreased due to infection or malignant transformation, or when NK cells are used in the allogeneic setting, the inhibitory signals are weakened; along with increased activating signals, NK cells are activated.
While NK cells are the main effector for the early graft-versus-leukemia reactions, they do not directly cause GvHD even with HLA-mismatch (97), making them an ideal source for allogeneic adoptive cell therapy (98). NK cells are enriched in CB comprising up to 30%, whereas in the PB they only constitute 10% of lymphocytes (2). Furthermore, it has been observed that NK cells reconstitute more rapidly after CB HCT than PB HCT (99), which is likely due to better homing capability of HCB NK cells to the bone marrow (4) and the presence of a unique progenitor-like subpopulation (28). In addition, much like CB T cells, CB NK cells are less mature than PB NK cells, as characterized by the reduced expression of activation receptors (4). While this leads to reduced cytotoxicity compared to PB NK cells, their activities can be enhanced with cytokine stimulation (22). Therefore, allogeneic NK cell therapies especially using CB as the source have attracted great attention in recent years. However, limited persistence and modest antitumor activities remain the two biggest barriers in the field of NK cell therapy.
3.1. Allogeneic NK cell infusion from a CB source
Earlier clinical trials using unmodified autologous NK cell infusion demonstrated only modest success (21). Advancement in allogeneic HCT has helped us better understand the physiology of NK cells – the mismatch between NK receptors and ligands are the key for the graft-versus-leukemia effect. In the past two decades, haploidentical NK cell therapies tested in patients with hematologic malignancies with or without prior HCT, have demonstrated feasibility, safety, and signals of efficacy (100–108). However, most of the studies used PB from healthy donors as the source for NK cells; clinical studies on infusion of unmodified NK cells from a CB source are scarce. Shah et al. reported the infusion of ex vivo expanded, 4/6 HLA-matched, cryopreserved CB-derived NK cells to patients with multiple myeloma, in between high-dose melphalan conditioning and autologous HCT (109). They observed no dose-limiting toxicities. Recently, our group reported the use of HLA-mismatched NK cells in patients with refractory malignancies; while we observed a good safety profile without GVHD, NK cells only persisted less than 4 weeks (97). Therefore, HLA-match or partial match is likely not required from the safety standpoint, but it may play a role in preventing allorejection and ensuring long-term persistence.
In addition to the “isolation-expansion” approach described above, NK cells can also be obtained through differentiation of hematopoietic stem and progenitor cells (HSPCs). This approach has been shown to result in a higher purity of NK cells with an improved bone marrow homing capacity (110). Dolstra et al. reported the first-in-human use of isolating CD34+ HSPCs from partially HLA-matched CB units, and inducing ex vivo NK cell differentiation by applying IL-2 and IL-15 (111). The final product was infused in 10 elderly patients with acute myeloid leukemia in remission, and they observed a good safety profile with preliminary efficacy as 2 of the 4 patients with positive measurable residual disease (MRD) turned negative. Nevertheless, larger and comparative studies are needed to determine whether induced NK cells have better clinical outcomes than isolated NK cells.
3.2. CB-derived NK cells complexed with NK cell engagers
Due to the modest antitumor activities of unmodified NK cell infusion, various strategies have been developed in the past few years to improve NK cell recognition and activation, including overexpressing chemokines or chemokine receptors to enhance NK cell tumor infiltration (112, 113), concurrent use of immune checkpoint inhibitors to reverse NK cell exhaustion (114), blocking the immunosuppressive TGF-β signaling from the TME (115), and promoting NK cell tumor-specific engagement through NK cell engagers (NKCEs) (31, 116–119). Of note, almost all have remained in the preclinical/early clinical phase.
Among them, NKCEs have received most attention in recent years due to excellent preclinical results, with multiple ongoing early phase clinical trials in various types of malignancies ( Table 2 ). NK cells exert their cytotoxic activities through three main mechanisms: direct killing by perforin/granzyme release, cytokine secretion, and antibody-dependent cell-mediated cytotoxicity (ADCC) though Fc-region binding receptor CD16 (FcγRIIA). The third mechanism provides a unique opportunity to pair monoclonal antibodies and NK cells to enhance their anti-tumor activities, prompting the development of NKCEs. Depending on the number of modules, NKCEs can be bispecific killer cell engagers (BiKEs) targeting CD16 expressed on NK cells and antigens of interest on tumor cells, or trispecific killer cell engagers (TriKEs), where an additional IL-15 module was added to promote NK cell expansion and persistence (31). Although NKCEs can be given as a monotherapy, the response rate was suboptimal. For example, when an anti-CD16/anti-CD30 bispecific NKCE, AFM13, was administered in patients with relapsed/refractory Hodgkin lymphoma, only 16.7% response rate was observed (120). However, a preclinical study by Kerbauy et al. combined AFM 13 with cytokine-induced memory-like NK cells and infused them in a pre-complexed manner (117). The authors found the combination of NKCE and pre-activated NK cells significantly improved the efficacy against CD30+ cancers compared to using either NKCE or NK cells alone. Moreover, when CB-derived NK cells were pre-activated by a set of cytokines and complexed with NKCE, they mounted an augmented response against CD30+ cancers (117). Currently, a phase I/II clinical trial (NCT04074746) is actively investigating this approach using CB-derived NK cells in patients with CD30+ T-cell lymphoma and Hodgkin lymphoma. Preliminary results of 30 patients with relapsed/refractory CD30+ lymphoma showed that this approach was safe – without infusion reaction, CRS, ICANS, or GvHD of any grade – and demonstrated an impressive efficacy with an 97% overall response rate and 63% complete response rate (30).
Table 2.
Ongoing trials of cord blood-derived CAR NK-cells or NK-cells pre-complexed with NK-cell engagers (NKCEs).
| Reference | Sponsor | Phase of trial | Product type | Product design | Indication | Dose of NK cells | Pre-conditioning prior to infusion | Safety outcome | Efficacy outcome |
|---|---|---|---|---|---|---|---|---|---|
| NCT05472558 | Second Affiliated Hospital, Zhejiang University, China | Phase I recruiting (n=48) |
CAR NK | Anti-CD19 | R/R B-NHL | 2×106/kg, 4×106/kg, 8×106/kg | N/A | N/A | N/A |
| NCT04796675 | Wuhan Union Hospital, China | Phase I recruiting (n=27) | CAR NK | Anti-CD19, IL-15 co-expression | R/R B-NHL | 0.1×106/kg, 1×106/kg, 10×106/kg |
Fludarabine (30mg/kg), Cyclophosphamide (300mg/kg) | N/A | N/A |
| NCT05247957 | Lu Daopei Hospital, China | Phase I recruiting (n=9) | CAR NK | Anti-NKG2D ligand | R/R AML | 2×106/kg, 6×106/kg, 18×106/kg | N/A | N/A | N/A |
| NCT05008536 | Xinqiao Hospital, China | Phase I recruiting (n=27) | CAR NK | Anti-BCMA | R/R MM | 1×106/kg, 6×106/kg, 18×106/kg | Fludarabine (30mg/kg), Cyclophosphamide (300mg/kg) | N/A | N/A |
| NCT05092451 | MD Anderson | Phase I/II recruiting (n=94) |
CAR NK | Anti-CD70, IL-15 co-expression | CD70-expressing R/R hematologic malignancies | N/A | Fludarabine, Cyclophosphamide | N/A | N/A |
| NCT05110742 | MD Anderson | Phase I/II recruiting (n=48) |
CAR NK | Anti-CD5, IL-15 co-expression | CD5-expressing R/R hematologic malignancies | 1×107, 1×108, 1×109, 1×1010 |
Fludarabine, Cyclophosphamide | N/A | N/A |
| NCT03056339 (26) | MD Anderson | Phase I published (n=11) | CAR NK | Anti-CD19, IL-15 co-expression | R/R B-NHL | 0.1×106/kg, 1×106/kg, 10×106/kg |
Fludarabine (30mg/kg), Cyclophosphamide (300mg/kg) | No GvHD, CRS, or ICANS | ORR 73%, CR 64% |
| NCT04074746 (30) | MD Anderson | Phase I/II recruiting (n=30) | NKCE | AFM13 precomplexed with CB-derived NK cells | CD30-expressing R/R lymphoma | 1×106/kg, 1×107/kg, 1×108/kg |
Fludarabine (30mg/kg), Cyclophosphamide (300mg/kg) | No GvHD, CRS, or ICANS. No DLT. | ORR 97%, CR 63% |
Method: the website clinicaltrials.gov was accessed on 1/16/2023, and search terms “CAR+NK”, “Chimeric+antigen+NK”, “CAR+natural+killer”, and “Chimeric+antigen+natural+killer” were used to identify ongoing CAR NK-cell studies; each study was further reviewed and those using CB as the source were included in the table. Likewise, search terms “natural+killer+engager”, “NK+engager” were used to identify ongoing NKCE trials; studies that used precomplexed CB-derived NK-cells were included in the table. R/R, relapsed/refractory. NHL, non-Hodgkin lymphoma. N/A, not available; AML, acute myeloid leukemia; MM, multiple myeloma; GvHD, graft-versus-host disease; CRS, cytokine release syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome; ORR, overall response rate; CR, complete response rate; DLT, dose-limiting toxicity.
3.3. CB-derived CAR NK-cells
CAR NK-cells have several advantages over CAR T-cells. First, they have a favorable safety profile due to the lack of TCR, with low risks of GvHD, cytokine release syndrome and neurotoxicity (26). Second, CAR NK-cells possess dual mechanisms of killing, through both CAR and innate receptors (27), which can potentially reduce the chance of CAR-targeted antigen escape. Compared with autologous CAR NK-cells, allogeneic CAR NK-cells also have advantages. First, “off-the-shelf” allogeneic products can significantly reduce the cost and increase availability. Second, as the activation of autologous CAR NK-cells is negatively affected by their innate inhibitory receptors (27); the use of allogeneic NK-cells can avoid the innate inhibition signal and enhance CAR NK activities.
Liu et al. reported the first-in-human trial of CAR-NK cells derived from unmatched or partially matched HLA cord blood in 2020 (26). The CAR-NK cells were transduced with a vector encoding anti-CD19 CAR, CD28 co-stimulatory domain, IL-15, and a safety switch of inducible caspase 9, and infused in 11 patients with B-cell lymphoma. No GvHD, CRS, or neurotoxicity was observed even at the highest dose; a response rate of 73% was reported, although the duration of response was unable to be assessed due to subsequent treatments. Of note, although CAR copies were still detectable in the peripheral blood after one year, on flow cytometry, CAR NK-cell rapidly declined after two weeks. Li et al. from the same group found that trogocytosis, a process involving transfer of tumor surface proteins (CD19) to CAR NK-cells upon anti-CD19 CAR/CD19 ligation, resulted in fratricide among CAR NK-cells and led to limited persistence (29). Co-infusion of KIR-based inhibitory CARs were able to counteract this process (29). Other ongoing CB-derived CAR NK-cell are summarized in Table 2 .
The limited persistence of CAR NK-cells can also be an advantage from a safety standpoint. Therefore, CAR NK-cells may be an ideal tool for bridging to HCT. If a patient was ineligible for HCT, repeated infusions of CAR NK-cells might be needed to achieve long-term immune surveillance. However, previous studies using allogeneic unmodified NK cells have demonstrated that repeated NK-cell infusion could result in even shorter persistence (106). Therefore, clinical studies are needed to test whether repeated CAR NK-cell infusions are safe and effective.
3.4. Current challenges with CB-derived NK cell therapy
Autologous NK cells obtained from patients with cancer have suppressed effector function and poor clinical activities, likely due to the presence of “self” MHC molecules and NK cell exhaustion from cancer TME (121). Allogeneic NK cells provide an intriguing alternative despite still facing many challenges – some are intrinsic to the NK cell therapy; others are specific to the allogeneic nature.
First, in the absence of cytokine support, NK cells lack initial expansion after adoptive transfer (23). Although this can be improved by co-administration of IL-2, it is associated with increased toxicity (100, 122). In recent years, applying high intensity lymphodepleting chemotherapy prior to NK cell infusion has become a standard protocol, which creates a cytokine sink and leads to a surge of IL-15, facilitating NK cell expansion (104). However, the overall expansion is still poor especially in certain solid tumors (104).
Second, long-term persistence of functional allogeneic NK cells has not been established. On the other hand, it has been shown that CAR T-cells are able to form a stable clone persisting over 10 years, which is likely the key to long-term remissions in certain indolent cancers (123). Without long-term tumor immunosurveillance, a high relapse rate from NK cell therapy has been observed (124). Therefore, most clinical studies have only us`ed allogeneic NK cells as a bridging treatment to more definitive therapies such as HCT (26, 125). In recent years, a combination of cytokines were found to be able to induce NK-cell differentiation into memory-like NK cells, which have improved persistence to months in the matched-donor setting after HCT (126, 127). However, its use in the true allogeneic setting needs to be further explored (107). In another attempt, NK cells transduced with an IL-15 gene with the ability of endogenous IL-15 secretion have shown improved persistence to over a year (26, 128). Moreover, knockout of a negative regular of IL-15 signaling (cytokine checkpoint) can further improve NK cell persistence (129). However, more clinical studies are needed to confirm the functional status of persistent NK cells. Another factor leading to the short persistence is T-cell and host NK cell allorejection. To overcome this, knockout of HLA class I molecule and overexpression of inhibitory NK receptors have been proposed (130).
Third, as shown in tumor biopsy samples, NK cells have limited intra-tumoral infiltration or homing ability (24); moreover, NK cells are usually dysfunctional within the tumor due to its immunosuppressive TME (131). These factors likely lead to limited clinical activities of NK cell therapy in solid tumors. Strategies to enhance NK cell trafficking to tumor tissue and restore their function within TME are being developed in animal models, awaiting to be tested in the clinical setting (132).
Fourth, NK cells are subject to cryoinjury – cryopreserved and thawed post-expansion NK cells have reduced cytotoxicity which might become a hurdle for large-scale allogeneic NK cell production – although the significance of such impaired activity remains to be studied in the clinical setting (25).
4. CB-derived stem cell therapy
The most common application of banked CB is to use as a source for allogeneic HCT — around 500 CB-HCT are performed in the US each year (133). Although limited by the quantity as a single unit, CB hematopoietic stem cells possess a higher capability for self-renewal and proliferation than bone marrow stem cells (134). Moreover, the immune tolerant state and low frequency of alloreactive T cells in the graft have likely led to a lower incidence of GVHD and less restrictive HLA matching for successful engraftment (33, 135). Ever since CB-HCT was first performed in 1988 (5), several landmark studies have significantly improved the safety and efficacy of CB-HCT. For example, studies have shown that 1 or 2 allele mismatches did not impair engraftment, albeit a slightly increased risk of aGVHD and non-relapse mortality (32, 136); the development of double partially HLA-matched CB units (dCB) has expanded the use of CB-HCT to adults, where the cell dose in a single unit is frequently insufficient (137). Nevertheless, limited number of initial stem cells and slow engraftment remain the two biggest challenges in CB-HCT. Indeed, the current guideline from American Society for Transplantation and Cellular Therapy (ASTCT) recommends filtering out CB units with low total nucleated cell and CD34+ cell count as the initial steps for selection, irrespective of good HLA matching (138). Moreover, a study found that only 4% of CB units in the US CB bank inventory were suitable for a single unit CB-HCT (139). Therefore, techniques to improve CB ex vivo and in vivo expansion are needed to expand the use of CB-HCT.
With recent advancements in haploidentical HCT, which greatly expanded the donor source, the need for CB-HCT has been questioned due to its high cost and limitations. Indeed, the recently published prospective BMT CTN 1101 trial showed that compared to haploidentical HCT with post-transplantation cyclophosphamide, dCB-HCT had similar rates of GVHD, but a higher non-relapse mortality rate and lower overall survival, in even experienced CB transplant centers (35). However, CB-HCT still has its role in certain situations. First, about 10% patients in the US do not have a sibling, matched unrelated, or haploidentical donor, making CB the only feasible choice (140). Second, the BMT CTN 1101 trial allowed partially mismatched CB units and the median infused CD34+ cell dose was notably lower than the current ASTCT guideline (138); it is unknown if patients had fully matched CB units and received higher CB doses, their outcomes would be improved. Third, different strategies have been developed or are being developed to improve CB expansion and early hematopoietic recovery, which can further improve the safety of CB-HCT.
In addition to HCT, CB stem cells have also been studied in regenerative medicine, including organs that typically lack self-regenerative abilities, such as nerve, heart, or cirrhotic liver. However, a detailed discussion on this topic is beyond the scope of this review; we will refer reader to other updated review on this topic (141).
4.1. Strategies to improve in vivo expansion
To improve the in vivo expansion of CB after transplantation, several strategies have been tested in clinical trials. One attempt was to directly inject CB stem cells into the bone marrow (intrabone injection). It was first developed by Frassoni et al. with the hypothesis of reducing trapping in organs such as liver after intravenous injection, improving homing to the bone marrow, and lowering required cell dose for CB HCT (142). Multiple phase I/II studies have confirmed feasibility of this approach, with neutrophil engraftment time ranging from 17 to 23 days with lower than standard total nucleated cell (TNC) and CD34+ cell doses (142–148). In a retrospective study comparing intrabone injection with traditional dCB-HCT, the intrabone approach was associated with an improved neutrophil engraftment (23 vs 28 days) and a lower relapse rate (25 vs 29% at 2 years), despite receiving significantly lower cell doses (median TNC count 2.5 vs 3.9 × 107/kg) (149). Therefore, intrabone injection is particularly useful when CB cell dose is insufficient and no alternative HCT approach is available. Phase III studies are needed to further investigate the efficacy of this approach.
Another method is to combine a small dose of CB with CD34-selected PB HSCs from a haploidentical donor. This haplo-cord approach resulted in a rapid early engraftment from the haploidentical donor, which was later replaced by the more durable engraftment of CB cells (150, 151). A retrospective study compared haplo-cord approach with haploidentical HCT with BM graft and post-transplantation cyclophosphamide, and found that the two approaches were associated with similar progression-free survival and overall survival, but the haplo-cord approach was associated with faster neutrophil engraftment and lower incidences of GvHD (152). However, the haplo-cord approach requires extra resources to obtain both CB and haploidentical donor cells; further, its efficacy needs to be confirmed in a prospective trial. Another approach to augment CB-HCT that has been developed recently was to infuse third-party granulocytes, with the goal to provide allogeneic MHC class I signaling, which would stimulate CD8+ T-cell proliferation to increase graft-versus-leukemia effects (153). In a proof-of-concept trial of 4 pediatric patients undergoing CB-HCT, the infusion of granulocytes led to more than 20-folds of CD8+ T-cell expansion (153). However, the safety of this approach, such as the occurrence of pre-engraftment syndrome, needs to be further studied.
4.2. Strategies to improve ex vivo expansion
Progress has been made in the past few years to improve the ex vivo expansion of CB through manipulation of culture conditions. These techniques have resulted in a higher stem cell dose at the time of infusion, with accumulating clinical studies demonstrating improved outcomes.
One strategy to optimize CB expansion is to co-culture with mesenchymal stem cells (MSCs), which can provide necessary microenvironment and cytokine support (154, 155). de Lima et al. showed that this approach led to a 12-fold increase in nucleated cells and a 30-fold increase in CD34+ stem cells, with the final product containing a median of 1.8×106 CD34+ cells per kilogram (154). The improvement of initial cell dose translated into a significantly faster neutrophils engraftment than those undergoing single unit CB HCT (15 days vs 24 days) (154).
Another strategy to improve CB expansion is to add certain agents to the culture media (156). A variety of molecules, including Notch ligand (157, 158), copper chelation agents such as TEPA (159), vitamin B derivatives such as nicotinamide (160), aryl hydrocarbon receptor antagonists (AHRs) (161), and pyrimidoindole derivative UM171 (162), have shown improved expansion in clinical studies ( Table 3 ). For example, nicotinamide can inhibit differentiation of hematopoietic stem cells; when added to CB culture media, an outgrowth of phenotypically primitive stem cells has been observed (163). A phase III clinical trial using nicotinamide expanded CB stem cells, known as the omidubicel, has shown significantly improved engraftment when compared with single or double-unit standard CB HCT (median neutrophil engraftment time 10 vs 20 days) (34). Omidubicel has also resulted in fewer bacterial and viral infections and less time in the hospital. Based on the trial results, omidubicel is currently seeking FDA approval for patients with hematologic malignancies in need of allo-HCT. However, it is important to note that these techniques require ex vivo manipulation of CB, which can lead to increased complexity and cost.
Table 3.
Completed trials of ex vivo expansion of cord blood for hematopoietic stem cell transplantation.
| Reference | Phase of trial | Expansion method | Expanded TNC, median, ×107/kg | Expanded CD34+ cells, median, ×106/kg | Conditioning and transplant | Neutrophils engraftment time, median (range), days | Platelet engraftment time, median (range), days | Infection outcome | Survival outcome |
|---|---|---|---|---|---|---|---|---|---|
| de Lima (154) | Phase I (n=31) | MSC | 5.8 | 0.95 | MAC | 15 (9 to 42) | 42 (15 to 62) | 1-yr f/u: bacteria 9.7%, fungal 16.1% | 1-yr OS: 32.2% |
| Mehta (155) | Phase I (n=27) |
MSC | 5.7 | 1.6 | RIC | 12 (1 to 28) | 31 (9 to 25) | 5 deaths due to infection | 2-yr OS: 32% |
| Delaney (157) | Phase I (n=10) |
Notch ligand | 4.6 | 6 | MAC, co-infusion of 1u unmanipulated CB | 16 (7 to 34) | N.R. | N.R. | 1-yr OS: 70% |
| Milano (158) | Phase II (n=15) |
Notch ligand | 5.8 | 5.2 | MAC, non-HLA matched product, co-infusion with standard CB | 19 (9 to 31) | 35 (21 to 86) | N.R. | 3-yr DFS: 86% |
| Stiff (159) | Phase II/III (n=101) |
Copper chelator | 3.1 | 0.14 | MAC | 21 (95%CI 18 to 24) | 54 (95%CI 43 to 62) | 5 deaths due to infection within 100 days | 100-day OS: 84% |
| Horwitz (160) | Phase I/II (n=36) |
Nicotinamide | 4.9 | 6.3 | MAC | 11.5 (95%CI 9 to 14) | 34 (95%CI 32 to 42) | 5 deaths due to infection | 2-yr OS: 51% |
| Horwitz (34) | Phase III RCT (n=125, 1:1 assigned) |
Nicotinamide | 4.7 vs 5.0 | 9.0 vs 0.3 | MAC, omidubicel vs standard CB | 10.0 (95%CI 8 to 13), vs control 20.5 (95%CI 18 to 24) | 37.0 (95%CI 33 to 42), vs control 50 (95%CI 42 to 58) | Grade 2/3 infection 37% vs 57% in control within 100 days | 210-day NRM: 11% vs 24% |
| Wagner (161) | Phase I/II (n=17) |
SR-1 | 5 | 17.5 | MAC, co-infusion of 1u unmanipulated CB | 15 (6 to 30) | 49 (28 to 136) | N.R. | 1-yr OS: 55% |
| Cohen (162) | Phase I/II (n=22) |
UM171 | 2.92 | 0.14 | MAC | 18 (12.5 to 20) | 42 (35 to 47) | No infection related mortality | 1-yr OS: 90% |
TNC, total nucleated cells; MSC, mesenchymal stem cells; MAC, myeloablative conditioning; RIC, reduced intensity conditioning; f/u, follow up; OS, overall survival; N;R;, not reported; CB, cord blood; DFS, disease-free survival; RCT, randomized controlled trial; NRM, non-relapse mortality.
5. Cord-derived mesenchymal stem cells
Mesenchymal stem cells (MSCs) are multipotent perivascular progenitors that can differentiate into osteogenic, adipogenic, myogenic, or chondrogenic cells, making them an ideal resource for regenerative medicine. In addition, as part of the bone marrow niche microenvironment, MSCs possess immune modulatory properties by secreting cytokines, chemokines, and extracellular vesicles, rendering them great potential in treating inflammatory or autoimmune conditions (164). Although MSCs can be obtained from adult tissues such as the bone marrow or adipose tissue, CB and its compartments derived MSCs have advantages of ease in harvesting, increased stemness potential, and improved expansion (165). MSC therapies have consistently been proven safe, but clinical outcomes have been mixed and later phase studies are often disappointing despite efficacy signals in early phase studies. The discrepancy can be partially explained by MSC heterogeneity between donors, different manufacturing protocols for MSCs, and handling protocols after manufacture (166, 167). Moreover, due to short persistence of MSCs after infusion, repeated administrations are needed to achieve long-term effects. Despite these limitations, MSCs have been increasingly studied in regenerative medicine, inflammatory and autoimmune diseases including Covid-19. A detailed discussion on these topics is beyond the scope of this review, and we refer readers to other recently published review articles in this journal (168–170).MSCs have demonstrated two clinical applications in the field HCT. One is to promote ex vivo expansion and sustain in vivo hematopoiesis of HSCs, and the other is to prevent and treat GvHD given their immunomodulation ability. Several studies utilized co-infusion of CB-derived MSCs with allogeneic HSCs in patients with aplastic anemia – a condition known to be associated with poor graft function and a high risk of graft failure – in order to improve transplant outcomes (171–173). For example, Wang et al. showed that in 22 patients undergoing alloHCT for aplastic anemia who also received co-infusion of CB-derived MSCs, all achieved successful neutrophil engraftment in a median of 14 days; after 15 months’ follow-up, 21 patients were still alive and all maintained full donor chimerism (173).
MSCs have also been applied in GvHD. In the seminal study by Le Blanc et al. in 2004, bone marrow derived MSCs were first used in a patient with grade IV treatment-resistant acute GvHD and achieved great outcomes (36). Since the initial case report, numerous studies have been conducted to investigate various sources of MSCs in both acute and chronic GvHD. Although most studies were performed with BM-derived MSCs (174), a few studies have shown promising results from CB or its compartments derived MSCs. For example, in a phase I trial of 10 patients with de novo high-risk or steroid-refractory acute GvHD, Soder et al. showed that Wharton’s jelly-derived MSCs achieved an overall response and complete response rate of 70% and 40%, respectively (175). However, in a large phase III trial of 260 patients with steroid-refractory acute GvHD who were randomized to receive BM-derived MSCs versus placebo added to second-line therapy, there was no significant improvement of durable complete response (40). Therefore, more research is needed to fully understand the potential of these cells in the treatment of GvHD. CB-derived MSCs have also been tested to prevent GvHD (37, 38). In a phase II trial by Gao et al., 124 patients were randomized to receive monthly CB-derived MSCs or placebo for a maximum of 4 cycles starting at least 4 months after haploidentical alloHCT (38). They reported that the MSC group had significantly lower 2-year cumulative incidence of chronic GvHD than the placebo (27.4 vs 49.0%). Of note, the optimal timing of MSC infusion to prevent GvHD especially acute GvHD needs to be further investigated, as too early infusion might increase the risk of disease relapse (39).
6. Concluding remarks
With recent advancement in adoptive cell transfer, CB has demonstrated great and unique potential in various types of cellular therapy. As a source for allogeneic CAR T-cell therapy, it is possible to generate an HLA-homozygous CAR T-cell bank that matches most of the population, providing off-the-shelf CAR T-cell products with minimal GvH side effect and without genome editing. As a source for NK-cell therapy, recent early-phase studies have shown promising results with CAR NK-cells, as well as co-infusion of NK-cell engager and preactivated NK-cells. As a source for HCT, recent development in various techniques have greatly shorten the engraftment time resulting in improved safety. As a source of MSC, clinical studies for various autoimmune and inflammatory conditions such as GvHD are underway. However, most of the clinical studies are early phase; further developments are needed to facilitate more CB-derived cellular therapies into the market. Moreover, many of the CB-derived cellular products are regulated as advanced therapy medicinal products (ATMPs) both in the US and in the EU due to more-than-minimal or substantial biological manipulations of the CB cells. Although the regulatory frameworks were installed to ensure the safety and quality of cellular products, the complexity of regulatory requirements imposes significant challenges particularly for academic researchers who lack the resources to meet them.
Author contributions
LM conceived the project. JW and LM reviewed the literature and drafted the article. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Spurway J, Logan P, Pak S. The development, structure and blood flow within the umbilical cord with particular reference to the venous system. Australas J Ultrasound Med (2012) 15(3):97–102. doi: 10.1002/j.2205-0140.2012.tb00013.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Theilgaard-Monch K, Raaschou-Jensen K, Palm H, Schjodt K, Heilmann C, Vindelov L, et al. Flow cytometric assessment of lymphocyte subsets, lymphoid progenitors, and hematopoietic stem cells in allogeneic stem cell grafts. Bone Marrow Transplant (2001) 28(11):1073–82. doi: 10.1038/sj.bmt.1703270 [DOI] [PubMed] [Google Scholar]
- 3. Szabolcs P, Park KD, Reese M, Marti L, Broadwater G, Kurtzberg J. Coexistent naive phenotype and higher cycling rate of cord blood T cells as compared to adult peripheral blood. Exp Hematol (2003) 31(8):708–14. doi: 10.1016/S0301-472X(03)00160-7 [DOI] [PubMed] [Google Scholar]
- 4. Luevano M, Daryouzeh M, Alnabhan R, Querol S, Khakoo S, Madrigal A, et al. The unique profile of cord blood natural killer cells balances incomplete maturation and effective killing function upon activation. Hum Immunol (2012) 73(3):248–57. doi: 10.1016/j.humimm.2011.12.015 [DOI] [PubMed] [Google Scholar]
- 5. Gluckman E, Broxmeyer HA, Auerbach AD, Friedman HS, Douglas GW, Devergie A, et al. Hematopoietic reconstitution in a patient with fanconi’s anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med (1989) 321(17):1174–8. doi: 10.1056/NEJM198910263211707 [DOI] [PubMed] [Google Scholar]
- 6. Querol S, Rubinstein P, Madrigal A. The wider perspective: cord blood banks and their future prospects. Br J Haematol (2021) 195(4):507–17. doi: 10.1111/bjh.17468 [DOI] [PubMed] [Google Scholar]
- 7. Dessels C, Alessandrini M, Pepper MS. Factors influencing the umbilical cord blood stem cell industry: an evolving treatment landscape. Stem Cells Transl Med (2018) 7(9):643–50. doi: 10.1002/sctm.17-0244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gragert L, Eapen M, Williams E, Freeman J, Spellman S, Baitty R, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U. S Registry N Engl J Med (2014) 371(4):339–48. doi: 10.1056/NEJMsa1311707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barker JN, Boughan K, Dahi PB, Devlin SM, Maloy MA, Naputo K, et al. Racial disparities in access to HLA-matched unrelated donor transplants: a prospective 1312-patient analysis. Blood Adv (2019) 3(7):939–44. doi: 10.1182/bloodadvances.2018028662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Annas GJ. Waste and longing–the legal status of placental-blood banking. N Engl J Med (1999) 340(19):1521–4. doi: 10.1056/NEJM199905133401923 [DOI] [PubMed] [Google Scholar]
- 11. Mitchell R, Wagner JE, Brunstein CG, Cao Q, McKenna DH, Lund TC, et al. Impact of long-term cryopreservation on single umbilical cord blood transplantation outcomes. Biol Blood Marrow Transplant (2015) 21(1):50–4. doi: 10.1016/j.bbmt.2014.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abraham AA, John TD, Keller MD, Cruz CRN, Salem B, Roesch L, et al. Safety and feasibility of virus-specific T cells derived from umbilical cord blood in cord blood transplant recipients. Blood Adv (2019) 3(14):2057–68. doi: 10.1182/bloodadvances.2019000201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pham PV, Vu NB, Pham VM, Truong NH, Pham TL, Dang LT, et al. Good manufacturing practice-compliant isolation and culture of human umbilical cord blood-derived mesenchymal stem cells. J Transl Med (2014) 12:56. doi: 10.1186/1479-5876-12-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bart T. Cost effectiveness of cord blood versus bone marrow and peripheral blood stem cells. Clinicoecon Outcomes Res (2010) 2:141–7. doi: 10.2147/CEOR.S11210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cael B, Galaine J, Bardey I, Marton C, Fredon M, Biichle S, et al. Umbilical cord blood as a source of less differentiated T cells to produce CD123 CAR-T cells. Cancers (Basel) (2022) 14(13). doi: 10.3390/cancers14133168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barker JN, Davies SM, DeFor T, Ramsay NK, Weisdorf DJ, Wagner JE. Survival after transplantation of unrelated donor umbilical cord blood is comparable to that of human leukocyte antigen-matched unrelated donor bone marrow: results of a matched-pair analysis. Blood (2001) 97(10):2957–61. doi: 10.1182/blood.V97.10.2957 [DOI] [PubMed] [Google Scholar]
- 17. Sun G, Tang B, Wan X, Yao W, Song K, Tu M, et al. Chimeric antigen receptor T cell therapy followed by unrelated cord blood transplantation for the treatment of Relapsed/Refractory b cell acute lymphoblastic leukemia in children and young adults: superior survival but relatively high post-transplantation relapse. Transplant Cell Ther (2022) 28(2):71 e1– e8. doi: 10.1016/j.jtct.2021.11.011 [DOI] [PubMed] [Google Scholar]
- 18. Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, Curtsinger J, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood (2011) 117(3):1061–70. doi: 10.1182/blood-2010-07-293795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brunstein CG, Miller JS, McKenna DH, Hippen KL, DeFor TE, Sumstad D, et al. Umbilical cord blood-derived T regulatory cells to prevent GVHD: kinetics, toxicity profile, and clinical effect. Blood (2016) 127(8):1044–51. doi: 10.1182/blood-2015-06-653667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roemhild A, Otto NM, Moll G, Abou-El-Enein M, Kaiser D, Bold G, et al. Regulatory T cells for minimising immune suppression in kidney transplantation: phase I/IIa clinical trial. BMJ (2020) 371:m3734. doi: 10.1136/bmj.m3734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rosenberg SA, Lotze MT, Muul LM, Chang AE, Avis FP, Leitman S, et al. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med (1987) 316(15):889–97. doi: 10.1056/NEJM198704093161501 [DOI] [PubMed] [Google Scholar]
- 22. Dalle JH, Menezes J, Wagner E, Blagdon M, Champagne J, Champagne MA, et al. Characterization of cord blood natural killer cells: implications for transplantation and neonatal infections. Pediatr Res (2005) 57(5 Pt 1):649–55. doi: 10.1203/01.PDR.0000156501.55431.20 [DOI] [PubMed] [Google Scholar]
- 23. Fujisaki H, Kakuda H, Shimasaki N, Imai C, Ma J, Lockey T, et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res (2009) 69(9):4010–7. doi: 10.1158/0008-5472.CAN-08-3712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stankovic B, Bjorhovde HAK, Skarshaug R, Aamodt H, Frafjord A, Muller E, et al. Immune cell composition in human non-small cell lung cancer. Front Immunol (2018) 9:3101. doi: 10.3389/fimmu.2018.03101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mark C, Czerwinski T, Roessner S, Mainka A, Horsch F, Heublein L, et al. Cryopreservation impairs 3-d migration and cytotoxicity of natural killer cells. Nat Commun (2020) 11(1):5224. doi: 10.1038/s41467-020-19094-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu E, Marin D, Banerjee P, Macapinlac HA, Thompson P, Basar R, et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med (2020) 382(6):545–53. doi: 10.1056/NEJMoa1910607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oei VYS, Siernicka M, Graczyk-Jarzynka A, Hoel HJ, Yang W, Palacios D, et al. Intrinsic functional potential of NK-cell subsets constrains retargeting driven by chimeric antigen receptors. Cancer Immunol Res (2018) 6(4):467–80. doi: 10.1158/2326-6066.CIR-17-0207 [DOI] [PubMed] [Google Scholar]
- 28. Rutella S, Bonanno G, Marone M, De Ritis D, Mariotti A, Voso MT, et al. Identification of a novel subpopulation of human cord blood CD34-CD133-CD7-CD45+lineage- cells capable of lymphoid/NK cell differentiation after in vitro exposure to IL-15. J Immunol (2003) 171(6):2977–88. doi: 10.4049/jimmunol.171.6.2977 [DOI] [PubMed] [Google Scholar]
- 29. Li Y, Basar R, Wang G, Liu E, Moyes JS, Li L, et al. KIR-based inhibitory CARs overcome CAR-NK cell trogocytosis-mediated fratricide and tumor escape. Nat Med (2022) 28(10):2133–44. doi: 10.1038/s41591-022-02003-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nieto Y, Banerjee PP, Kaur I, Griffin L, Ganesh C, Kerbauy L, et al. Innate cell engager AFM13 combined with preactivated and expanded cord blood-derived NK cells for patients with double refractory CD30+ lymphoma. Blood (2022) 140(Supplement 1):415–6. doi: 10.1182/blood-2022-156125 [DOI] [Google Scholar]
- 31. Felices M, Lenvik TR, Davis ZB, Miller JS, Vallera DA. Generation of BiKEs and TriKEs to improve NK cell-mediated targeting of tumor cells. Methods Mol Biol (2016) 1441:333–46. doi: 10.1007/978-1-4939-3684-7_28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Eapen M, Klein JP, Sanz GF, Spellman S, Ruggeri A, Anasetti C, et al. Effect of donor-recipient HLA matching at HLA a, b, c, and DRB1 on outcomes after umbilical-cord blood transplantation for leukaemia and myelodysplastic syndrome: a retrospective analysis. Lancet Oncol (2011) 12(13):1214–21. doi: 10.1016/S1470-2045(11)70260-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rocha V, Wagner JE, Jr., Sobocinski KA, Klein JP, Zhang MJ, Horowitz MM, et al. Graft-versus-host disease in children who have received a cord-blood or bone marrow transplant from an HLA-identical sibling. eurocord and international bone marrow transplant registry working committee on alternative donor and stem cell sources. N Engl J Med (2000) 342(25):1846–54. doi: 10.1056/NEJM200006223422501 [DOI] [PubMed] [Google Scholar]
- 34. Horwitz ME, Stiff PJ, Cutler C, Brunstein C, Hanna R, Maziarz RT, et al. Omidubicel vs standard myeloablative umbilical cord blood transplantation: results of a phase 3 randomized study. Blood (2021) 138(16):1429–40. doi: 10.1182/blood.2021011719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fuchs EJ, O’Donnell PV, Eapen M, Logan B, Antin JH, Dawson P, et al. Double unrelated umbilical cord blood vs HLA-haploidentical bone marrow transplantation: the BMT CTN 1101 trial. Blood (2021) 137(3):420–8. doi: 10.1182/blood.2020007535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet (2004) 363(9419):1439–41. doi: 10.1016/S0140-6736(04)16104-7 [DOI] [PubMed] [Google Scholar]
- 37. Wu Y, Wang Z, Cao Y, Xu L, Li X, Liu P, et al. Cotransplantation of haploidentical hematopoietic and umbilical cord mesenchymal stem cells with a myeloablative regimen for refractory/relapsed hematologic malignancy. Ann Hematol (2013) 92(12):1675–84. doi: 10.1007/s00277-013-1831-0 [DOI] [PubMed] [Google Scholar]
- 38. Gao L, Zhang Y, Hu B, Liu J, Kong P, Lou S, et al. Phase II multicenter, randomized, double-blind controlled study of efficacy and safety of umbilical cord-derived mesenchymal stromal cells in the prophylaxis of chronic graft-Versus-Host disease after HLA-haploidentical stem-cell transplantation. J Clin Oncol (2016) 34(24):2843–50. doi: 10.1200/JCO.2015.65.3642 [DOI] [PubMed] [Google Scholar]
- 39. Zhu L, Wang Z, Zheng X, Ding L, Han D, Yan H, et al. Haploidentical hematopoietic stem cell transplant with umbilical cord-derived multipotent mesenchymal cell infusion for the treatment of high-risk acute leukemia in children. Leuk Lymphoma (2015) 56(5):1346–52. doi: 10.3109/10428194.2014.939970 [DOI] [PubMed] [Google Scholar]
- 40. Kebriaei P, Hayes J, Daly A, Uberti J, Marks DI, Soiffer R, et al. A phase 3 randomized study of remestemcel-l versus placebo added to second-line therapy in patients with steroid-refractory acute graft-versus-Host disease. Biol Blood Marrow Transplant (2020) 26(5):835–44. doi: 10.1016/j.bbmt.2019.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu DD, Hong WC, Qiu KY, Li XY, Liu Y, Zhu LW, et al. Umbilical cord blood: a promising source for allogeneic CAR-T cells. Front Oncol (2022) 12:944248. doi: 10.3389/fonc.2022.944248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hiwarkar P, Qasim W, Ricciardelli I, Gilmour K, Quezada S, Saudemont A, et al. Cord blood T cells mediate enhanced antitumor effects compared with adult peripheral blood T cells. Blood (2015) 126(26):2882–91. doi: 10.1182/blood-2015-06-654780 [DOI] [PubMed] [Google Scholar]
- 43. Mold JE, Venkatasubrahmanyam S, Burt TD, Michaelsson J, Rivera JM, Galkina SA, et al. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science (2010) 330(6011):1695–9. doi: 10.1126/science.1196509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rudd BD. Neonatal T cells: a reinterpretation. Annu Rev Immunol (2020) 38:229–47. doi: 10.1146/annurev-immunol-091319-083608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kershaw MH, Westwood JA, Darcy PK. Gene-engineered T cells for cancer therapy. Nat Rev Cancer (2013) 13(8):525–41. doi: 10.1038/nrc3565 [DOI] [PubMed] [Google Scholar]
- 46. Wang J, Hu Y, Huang H. Current development of chimeric antigen receptor T-cell therapy. Stem Cell Investig (2018) 5:44. doi: 10.21037/sci.2018.11.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Caldwell KJ, Gottschalk S, Talleur AC. Allogeneic CAR cell therapy-more than a pipe dream. Front Immunol (2020) 11:618427. doi: 10.3389/fimmu.2020.618427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rubinstein P, Carrier C, Scaradavou A, Kurtzberg J, Adamson J, Migliaccio AR, et al. Outcomes among 562 recipients of placental-blood transplants from unrelated donors. N Engl J Med (1998) 339(22):1565–77. doi: 10.1056/NEJM199811263392201 [DOI] [PubMed] [Google Scholar]
- 49. Taylor CJ, Peacock S, Chaudhry AN, Bradley JA, Bolton EM. Generating an iPSC bank for HLA-matched tissue transplantation based on known donor and recipient HLA types. Cell Stem Cell (2012) 11(2):147–52. doi: 10.1016/j.stem.2012.07.014 [DOI] [PubMed] [Google Scholar]
- 50. Razeghian E, Nasution MKM, Rahman HS, Gardanova ZR, Abdelbasset WK, Aravindhan S, et al. A deep insight into CRISPR/Cas9 application in CAR-T cell-based tumor immunotherapies. Stem Cell Res Ther (2021) 12(1):428. doi: 10.1186/s13287-021-02510-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pegram HJ, Purdon TJ, van Leeuwen DG, Curran KJ, Giralt SA, Barker JN, et al. IL-12-secreting CD19-targeted cord blood-derived T cells for the immunotherapy of b-cell acute lymphoblastic leukemia. Leukemia (2015) 29(2):415–22. doi: 10.1038/leu.2014.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lekakis LJ, Locke FL, Tees M, Neelapu SS, Malik SA, Hamadani M, et al. ALPHA2 study: ALLO-501A allogeneic CAR T in LBCL, updated results continue to show encouraging safety and efficacy with consolidation dosing. Blood (2021) 138(Supplement 1):649–. doi: 10.1182/blood-2021-146045 [DOI] [Google Scholar]
- 53. Karasiewicz K, He S, Ng M, Tess K, Ling W, Kaufmann GF, et al. Preclinical evaluation of human placental-derived allogeneic CD19 CAR-T cells against b cell malignancies. Blood (2019) 134(Supplement_1):3222–. doi: 10.1182/blood-2019-130782 [DOI] [Google Scholar]
- 54. Iriguchi S, Yasui Y, Kawai Y, Arima S, Kunitomo M, Sato T, et al. A clinically applicable and scalable method to regenerate T-cells from iPSCs for off-the-shelf T-cell immunotherapy. Nat Commun (2021) 12(1):430. doi: 10.1038/s41467-020-20658-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Awong G, Herer E, La Motte-Mohs RN, Zuniga-Pflucker JC. Human CD8 T cells generated in vitro from hematopoietic stem cells are functionally mature. BMC Immunol (2011) 12:22. doi: 10.1186/1471-2172-12-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Seay HR, Putnam AL, Cserny J, Posgai AL, Rosenau EH, Wingard JR, et al. Expansion of human tregs from cryopreserved umbilical cord blood for GMP-compliant autologous adoptive cell transfer therapy. Mol Ther Methods Clin Dev (2017) 4:178–91. doi: 10.1016/j.omtm.2016.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chwojnicki K, Iwaszkiewicz-Grzes D, Jankowska A, Zielinski M, Lowiec P, Gliwinski M, et al. Administration of CD4(+)CD25(high)CD127(-)FoxP3(+) regulatory T cells for relapsing-remitting multiple sclerosis: a phase 1 study. BioDrugs (2021) 35(1):47–60. doi: 10.1007/s40259-020-00462-7 [DOI] [PubMed] [Google Scholar]
- 58. Marek-Trzonkowska N, Mysliwiec M, Dobyszuk A, Grabowska M, Techmanska I, Juscinska J, et al. Administration of CD4+CD25highCD127- regulatory T cells preserves beta-cell function in type 1 diabetes in children. Diabetes Care (2012) 35(9):1817–20. doi: 10.2337/dc12-0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK, et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med (2015) 7(315):315ra189. doi: 10.1126/scitranslmed.aad4134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sawitzki B, Harden PN, Reinke P, Moreau A, Hutchinson JA, Game DS, et al. Regulatory cell therapy in kidney transplantation (The ONE study): a harmonised design and analysis of seven non-randomised, single-arm, phase 1/2A trials. Lancet (2020) 395(10237):1627–39. doi: 10.1016/S0140-6736(20)30167-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sanchez-Fueyo A, Whitehouse G, Grageda N, Cramp ME, Lim TY, Romano M, et al. Applicability, safety, and biological activity of regulatory T cell therapy in liver transplantation. Am J Transplant (2020) 20(4):1125–36. doi: 10.1111/ajt.15700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. MacMillan ML, Hippen KL, McKenna DH, Kadidlo D, Sumstad D, DeFor TE, et al. First-in-human phase 1 trial of induced regulatory T cells for graft-versus-host disease prophylaxis in HLA-matched siblings. Blood Adv (2021) 5(5):1425–36. doi: 10.1182/bloodadvances.2020003219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Theil A, Tuve S, Oelschlagel U, Maiwald A, Dohler D, Ossmann D, et al. Adoptive transfer of allogeneic regulatory T cells into patients with chronic graft-versus-host disease. Cytotherapy (2015) 17(4):473–86. doi: 10.1016/j.jcyt.2014.11.005 [DOI] [PubMed] [Google Scholar]
- 64. Trzonkowski P, Bieniaszewska M, Juscinska J, Dobyszuk A, Krzystyniak A, Marek N, et al. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127- T regulatory cells. Clin Immunol (2009) 133(1):22–6. doi: 10.1016/j.clim.2009.06.001 [DOI] [PubMed] [Google Scholar]
- 65. Bluestone JA, Tang Q. Treg cells-the next frontier of cell therapy. Science (2018) 362(6411):154–5. doi: 10.1126/science.aau2688 [DOI] [PubMed] [Google Scholar]
- 66. Bezie S, Charreau B, Vimond N, Lasselin J, Gerard N, Nerriere-Daguin V, et al. Human CD8+ tregs expressing a MHC-specific CAR display enhanced suppression of human skin rejection and GVHD in NSG mice. Blood Adv (2019) 3(22):3522–38. doi: 10.1182/bloodadvances.2019000411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Radichev IA, Yoon J, Scott DW, Griffin K, Savinov AY. Towards antigen-specific tregs for type 1 diabetes: construction and functional assessment of pancreatic endocrine marker, HPi2-based chimeric antigen receptor. Cell Immunol (2020) 358:104224. doi: 10.1016/j.cellimm.2020.104224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Blat D, Zigmond E, Alteber Z, Waks T, Eshhar Z. Suppression of murine colitis and its associated cancer by carcinoembryonic antigen-specific regulatory T cells. Mol Ther (2014) 22(5):1018–28. doi: 10.1038/mt.2014.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yoon J, Schmidt A, Zhang AH, Konigs C, Kim YC, Scott DW. FVIII-specific human chimeric antigen receptor T-regulatory cells suppress T- and b-cell responses to FVIII. Blood (2017) 129(2):238–45. doi: 10.1182/blood-2016-07-727834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fritsche E, Volk HD, Reinke P, Abou-El-Enein M. Toward an optimized process for clinical manufacturing of CAR-treg cell therapy. Trends Biotechnol (2020) 38(10):1099–112. doi: 10.1016/j.tibtech.2019.12.009 [DOI] [PubMed] [Google Scholar]
- 71. Kaeuferle T, Krauss R, Blaeschke F, Willier S, Feuchtinger T. Strategies of adoptive T -cell transfer to treat refractory viral infections post allogeneic stem cell transplantation. J Hematol Oncol (2019) 12(1):13. doi: 10.1186/s13045-019-0701-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Keller MD, Bollard CM. Virus-specific T-cell therapies for patients with primary immune deficiency. Blood (2020) 135(9):620–8. doi: 10.1182/blood.2019000924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. McLaughlin LP, Rouce R, Gottschalk S, Torrano V, Carrum G, Wu MF, et al. EBV/LMP-specific T cells maintain remissions of T- and b-cell EBV lymphomas after allogeneic bone marrow transplantation. Blood (2018) 132(22):2351–61. doi: 10.1182/blood-2018-07-863654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bollard CM, Gottschalk S, Torrano V, Diouf O, Ku S, Hazrat Y, et al. Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting Epstein-Barr virus latent membrane proteins. J Clin Oncol (2014) 32(8):798–808. doi: 10.1200/JCO.2013.51.5304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Prockop S, Doubrovina E, Suser S, Heller G, Barker J, Dahi P, et al. Off-the-shelf EBV-specific T cell immunotherapy for rituximab-refractory EBV-associated lymphoma following transplantation. J Clin Invest (2020) 130(2):733–47. doi: 10.1172/JCI121127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Peggs KS, Thomson K, Samuel E, Dyer G, Armoogum J, Chakraverty R, et al. Directly selected cytomegalovirus-reactive donor T cells confer rapid and safe systemic reconstitution of virus-specific immunity following stem cell transplantation. Clin Infect Dis (2011) 52(1):49–57. doi: 10.1093/cid/ciq042 [DOI] [PubMed] [Google Scholar]
- 77. Withers B, Blyth E, Clancy LE, Yong A, Fraser C, Burgess J, et al. Long-term control of recurrent or refractory viral infections after allogeneic HSCT with third-party virus-specific T cells. Blood Adv (2017) 1(24):2193–205. doi: 10.1182/bloodadvances.2017010223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tzannou I, Papadopoulou A, Naik S, Leung K, Martinez CA, Ramos CA, et al. Off-the-Shelf virus-specific T cells to treat BK virus, human herpesvirus 6, cytomegalovirus, Epstein-Barr virus, and adenovirus infections after allogeneic hematopoietic stem-cell transplantation. J Clin Oncol (2017) 35(31):3547–57. doi: 10.1200/JCO.2017.73.0655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Leen AM, Bollard CM, Mendizabal AM, Shpall EJ, Szabolcs P, Antin JH, et al. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood (2013) 121(26):5113–23. doi: 10.1182/blood-2013-02-486324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Melenhorst JJ, Leen AM, Bollard CM, Quigley MF, Price DA, Rooney CM, et al. Allogeneic virus-specific T cells with HLA alloreactivity do not produce GVHD in human subjects. Blood (2010) 116(22):4700–2. doi: 10.1182/blood-2010-06-289991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Dave H, Luo M, Blaney JW, Patel S, Barese C, Cruz CR, et al. Toward a rapid production of multivirus-specific T cells targeting BKV, adenovirus, CMV, and EBV from umbilical cord blood. Mol Ther Methods Clin Dev (2017) 5:13–21. doi: 10.1016/j.omtm.2017.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Fruchtman SM, Hurlet A, Dracker R, Isola L, Goldman B, Schneider BL, et al. The successful treatment of severe aplastic anemia with autologous cord blood transplantation. Biol Blood Marrow Transplant (2004) 10(11):741–2. doi: 10.1016/j.bbmt.2004.07.003 [DOI] [PubMed] [Google Scholar]
- 83. Barros MS, de Araujo ND, Magalhaes-Gama F, Pereira Ribeiro TL, Alves Hanna FS, Tarrago AM, et al. Gammadelta T cells for leukemia immunotherapy: new and expanding trends. Front Immunol (2021) 12:729085. doi: 10.3389/fimmu.2021.729085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Saura-Esteller J, de Jong M, King LA, Ensing E, Winograd B, de Gruijl TD, et al. Gamma delta T-cell based cancer immunotherapy: past-Present-Future. Front Immunol (2022) 13:915837. doi: 10.3389/fimmu.2022.915837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lin M, Zhang X, Liang S, Luo H, Alnaggar M, Liu A, et al. Irreversible electroporation plus allogenic Vgamma9Vdelta2 T cells enhances antitumor effect for locally advanced pancreatic cancer patients. Signal Transduct Target Ther (2020) 5(1):215. doi: 10.1038/s41392-020-00260-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Xu Y, Xiang Z, Alnaggar M, Kouakanou L, Li J, He J, et al. Allogeneic Vgamma9Vdelta2 T-cell immunotherapy exhibits promising clinical safety and prolongs the survival of patients with late-stage lung or liver cancer. Cell Mol Immunol (2021) 18(2):427–39. doi: 10.1038/s41423-020-0515-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Neelapu SS, Hamadani M, Miklos DB, Holmes H, Hinkle J, Kennedy-Wilde J, et al. A phase 1 study of ADI-001: anti-CD20 CAR-engineered allogeneic gamma delta (γδ) T cells in adults with b-cell malignancies. J Clin Oncol (2022) 40(16_suppl):7509–. doi: 10.1200/JCO.2022.40.16_suppl.7509 [DOI] [Google Scholar]
- 88. Willcox CR, Davey MS, Willcox BE. Development and selection of the human Vgamma9Vdelta2(+) T-cell repertoire. Front Immunol (2018) 9:1501. doi: 10.3389/fimmu.2018.01501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Berglund S, Gaballa A, Sawaisorn P, Sundberg B, Uhlin M. Expansion of gammadelta T cells from cord blood: a therapeutical possibility. Stem Cells Int (2018) 2018:8529104. doi: 10.1155/2018/8529104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Cairo C, Sagnia B, Cappelli G, Colizzi V, Leke RG, Leke RJ, et al. Human cord blood gammadelta T cells expressing public Vgamma2 chains dominate the response to bisphosphonate plus interleukin-15. Immunology (2013) 138(4):346–60. doi: 10.1111/imm.12039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Leidner R, Sanjuan Silva N, Huang H, Sprott D, Zheng C, Shih YP, et al. Neoantigen T-cell receptor gene therapy in pancreatic cancer. N Engl J Med (2022) 386(22):2112–9. doi: 10.1056/NEJMoa2119662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ma Q, Garber HR, Lu S, He H, Tallis E, Ding X, et al. A novel TCR-like CAR with specificity for PR1/HLA-A2 effectively targets myeloid leukemia in vitro when expressed in human adult peripheral blood and cord blood T cells. Cytotherapy (2016) 18(8):985–94. doi: 10.1016/j.jcyt.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. St John LS, Wan L, He H, Garber HR, Clise-Dwyer K, Alatrash G, et al. PR1-specific cytotoxic T lymphocytes are relatively frequent in umbilical cord blood and can be effectively expanded to target myeloid leukemia. Cytotherapy (2016) 18(8):995–1001. doi: 10.1016/j.jcyt.2016.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kwoczek J, Riese SB, Tischer S, Bak S, Lahrberg J, Oelke M, et al. Cord blood-derived T cells allow the generation of a more naive tumor-reactive cytotoxic T-cell phenotype. Transfusion (2018) 58(1):88–99. doi: 10.1111/trf.14365 [DOI] [PubMed] [Google Scholar]
- 95. Okas M, Gertow J, Uzunel M, Karlsson H, Westgren M, Karre K, et al. Clinical expansion of cord blood-derived T cells for use as donor lymphocyte infusion after cord blood transplantation. J Immunother (2010) 33(1):96–105. doi: 10.1097/CJI.0b013e3181b291a4 [DOI] [PubMed] [Google Scholar]
- 96. Paul S, Lal G. The molecular mechanism of natural killer cells function and its importance in cancer immunotherapy. Front Immunol (2017) 8:1124. doi: 10.3389/fimmu.2017.01124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Otegbeye F, Cooper B, Caimi P, Zamborsky K, Reese-Koc J, Hillian A, et al. A phase I study to determine the maximum tolerated dose of ex vivo expanded natural killer cells derived from unrelated, HLA-disparate adult donors. Transplant Cell Ther (2022) 28(5):250 e1– e8. doi: 10.1016/j.jtct.2022.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Simonetta F, Alvarez M, Negrin RS. Natural killer cells in graft-versus-Host-Disease after allogeneic hematopoietic cell transplantation. Front Immunol (2017) 8:465. doi: 10.3389/fimmu.2017.00465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Jacobson CA, Turki AT, McDonough SM, Stevenson KE, Kim HT, Kao G, et al. Immune reconstitution after double umbilical cord blood stem cell transplantation: comparison with unrelated peripheral blood stem cell transplantation. Biol Blood Marrow Transplant (2012) 18(4):565–74. doi: 10.1016/j.bbmt.2011.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bachanova V, Cooley S, Defor TE, Verneris MR, Zhang B, McKenna DH, et al. Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood (2014) 123(25):3855–63. doi: 10.1182/blood-2013-10-532531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Bachanova V, Sarhan D, DeFor TE, Cooley S, Panoskaltsis-Mortari A, Blazar BR, et al. Haploidentical natural killer cells induce remissions in non-Hodgkin lymphoma patients with low levels of immune-suppressor cells. Cancer Immunol Immunother (2018) 67(3):483–94. doi: 10.1007/s00262-017-2100-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Shaffer BC, Le Luduec JB, Forlenza C, Jakubowski AA, Perales MA, Young JW, et al. Phase II study of haploidentical natural killer cell infusion for treatment of relapsed or persistent myeloid malignancies following allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant (2016) 22(4):705–9. doi: 10.1016/j.bbmt.2015.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Rizzieri DA, Storms R, Chen DF, Long G, Yang Y, Nikcevich DA, et al. Natural killer cell-enriched donor lymphocyte infusions from a 3-6/6 HLA matched family member following nonmyeloablative allogeneic stem cell transplantation. Biol Blood Marrow Transplant (2010) 16(8):1107–14. doi: 10.1016/j.bbmt.2010.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood (2005) 105(8):3051–7. doi: 10.1182/blood-2004-07-2974 [DOI] [PubMed] [Google Scholar]
- 105. Rubnitz JE, Inaba H, Ribeiro RC, Pounds S, Rooney B, Bell T, et al. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol (2010) 28(6):955–9. doi: 10.1200/JCO.2009.24.4590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Curti A, Ruggeri L, D’Addio A, Bontadini A, Dan E, Motta MR, et al. Successful transfer of alloreactive haploidentical KIR ligand-mismatched natural killer cells after infusion in elderly high risk acute myeloid leukemia patients. Blood (2011) 118(12):3273–9. doi: 10.1182/blood-2011-01-329508 [DOI] [PubMed] [Google Scholar]
- 107. Romee R, Rosario M, Berrien-Elliott MM, Wagner JA, Jewell BA, Schappe T, et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci Transl Med (2016) 8(357):357ra123. doi: 10.1126/scitranslmed.aaf2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Shi J, Tricot G, Szmania S, Rosen N, Garg TK, Malaviarachchi PA, et al. Infusion of haplo-identical killer immunoglobulin-like receptor ligand mismatched NK cells for relapsed myeloma in the setting of autologous stem cell transplantation. Br J Haematol (2008) 143(5):641–53. doi: 10.1111/j.1365-2141.2008.07340.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Shah N, Li L, McCarty J, Kaur I, Yvon E, Shaim H, et al. Phase I study of cord blood-derived natural killer cells combined with autologous stem cell transplantation in multiple myeloma. Br J Haematol (2017) 177(3):457–66. doi: 10.1111/bjh.14570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Cany J, van der Waart AB, Tordoir M, Franssen GM, Hangalapura BN, de Vries J, et al. Natural killer cells generated from cord blood hematopoietic progenitor cells efficiently target bone marrow-residing human leukemia cells in NOD/SCID/IL2Rg(null) mice. PloS One (2013) 8(6):e64384. doi: 10.1371/journal.pone.0064384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Dolstra H, Roeven MWH, Spanholtz J, Hangalapura BN, Tordoir M, Maas F, et al. Successful transfer of umbilical cord blood CD34(+) hematopoietic stem and progenitor-derived NK cells in older acute myeloid leukemia patients. Clin Cancer Res (2017) 23(15):4107–18. doi: 10.1158/1078-0432.CCR-16-2981 [DOI] [PubMed] [Google Scholar]
- 112. Kremer V, Ligtenberg MA, Zendehdel R, Seitz C, Duivenvoorden A, Wennerberg E, et al. Genetic engineering of human NK cells to express CXCR2 improves migration to renal cell carcinoma. J Immunother Cancer (2017) 5(1):73. doi: 10.1186/s40425-017-0275-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Lavergne E, Combadiere C, Iga M, Boissonnas A, Bonduelle O, Maho M, et al. Intratumoral CC chemokine ligand 5 overexpression delays tumor growth and increases tumor cell infiltration. J Immunol (2004) 173(6):3755–62. doi: 10.4049/jimmunol.173.6.3755 [DOI] [PubMed] [Google Scholar]
- 114. Dong W, Wu X, Ma S, Wang Y, Nalin AP, Zhu Z, et al. The mechanism of anti-PD-L1 antibody efficacy against PD-L1-Negative tumors identifies NK cells expressing PD-L1 as a cytolytic effector. Cancer Discovery (2019) 9(10):1422–37. doi: 10.1158/2159-8290.CD-18-1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ravi R, Noonan KA, Pham V, Bedi R, Zhavoronkov A, Ozerov IV, et al. Bifunctional immune checkpoint-targeted antibody-ligand traps that simultaneously disable TGFbeta enhance the efficacy of cancer immunotherapy. Nat Commun (2018) 9(1):741. doi: 10.1038/s41467-017-02696-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Yun HD, Felices M, Vallera DA, Hinderlie P, Cooley S, Arock M, et al. Trispecific killer engager CD16xIL15xCD33 potently induces NK cell activation and cytotoxicity against neoplastic mast cells. Blood Adv (2018) 2(13):1580–4. doi: 10.1182/bloodadvances.2018018176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Kerbauy LN, Marin ND, Kaplan M, Banerjee PP, Berrien-Elliott MM, Becker-Hapak M, et al. Combining AFM13, a bispecific CD30/CD16 antibody, with cytokine-activated blood and cord blood-derived NK cells facilitates CAR-like responses against CD30(+) malignancies. Clin Cancer Res (2021) 27(13):3744–56. doi: 10.1158/1078-0432.CCR-21-0164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Felices M, Kodal B, Hinderlie P, Kaminski MF, Cooley S, Weisdorf DJ, et al. Novel CD19-targeted TriKE restores NK cell function and proliferative capacity in CLL. Blood Adv (2019) 3(6):897–907. doi: 10.1182/bloodadvances.2018029371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. McCall AM, Adams GP, Amoroso AR, Nielsen UB, Zhang L, Horak E, et al. Isolation and characterization of an anti-CD16 single-chain fv fragment and construction of an anti-HER2/neu/anti-CD16 bispecific scFv that triggers CD16-dependent tumor cytolysis. Mol Immunol (1999) 36(7):433–45. doi: 10.1016/S0161-5890(99)00057-7 [DOI] [PubMed] [Google Scholar]
- 120. Sasse S, Brockelmann PJ, Momotow J, Plutschow A, Huttmann A, Basara N, et al. AFM13 in patients with relapsed or refractory classical Hodgkin lymphoma: final results of an open-label, randomized, multicenter phase II trial. Leuk Lymphoma (2022) 63(8):1871–8. doi: 10.1080/10428194.2022.2095623 [DOI] [PubMed] [Google Scholar]
- 121. Bi J, Tian Z. NK cell dysfunction and checkpoint immunotherapy. Front Immunol (2019) 10:1999. doi: 10.3389/fimmu.2019.01999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Gluck WL, Hurst D, Yuen A, Levine AM, Dayton MA, Gockerman JP, et al. Phase I studies of interleukin (IL)-2 and rituximab in b-cell non-hodgkin’s lymphoma: IL-2 mediated natural killer cell expansion correlations with clinical response. Clin Cancer Res (2004) 10(7):2253–64. doi: 10.1158/1078-0432.CCR-1087-3 [DOI] [PubMed] [Google Scholar]
- 123. Melenhorst JJ, Chen GM, Wang M, Porter DL, Chen C, Collins MA, et al. Decade-long leukaemia remissions with persistence of CD4(+) CAR T cells. Nature (2022) 602(7897):503–9. doi: 10.1038/s41586-021-04390-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Tang X, Yang L, Li Z, Nalin AP, Dai H, Xu T, et al. First-in-man clinical trial of CAR NK-92 cells: safety test of CD33-CAR NK-92 cells in patients with relapsed and refractory acute myeloid leukemia. Am J Cancer Res (2018) 8(6):1083–9. [PMC free article] [PubMed] [Google Scholar]
- 125. Vela M, Corral D, Carrasco P, Fernandez L, Valentin J, Gonzalez B, et al. Haploidentical IL-15/41BBL activated and expanded natural killer cell infusion therapy after salvage chemotherapy in children with relapsed and refractory leukemia. Cancer Lett (2018) 422:107–17. doi: 10.1016/j.canlet.2018.02.033 [DOI] [PubMed] [Google Scholar]
- 126. Shapiro RM, Birch GC, Hu G, Vergara Cadavid J, Nikiforow S, Baginska J, et al. Expansion, persistence, and efficacy of donor memory-like NK cells infused for posttransplant relapse. J Clin Invest (2022) 132(11). doi: 10.1172/JCI154334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Bednarski JJ, Zimmerman C, Berrien-Elliott MM, Foltz JA, Becker-Hapak M, Neal CC, et al. Donor memory-like NK cells persist and induce remissions in pediatric patients with relapsed AML after transplant. Blood (2022) 139(11):1670–83. doi: 10.1182/blood.2021013972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Liu E, Tong Y, Dotti G, Shaim H, Savoldo B, Mukherjee M, et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia (2018) 32(2):520–31. doi: 10.1038/leu.2017.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Daher M, Basar R, Gokdemir E, Baran N, Uprety N, Nunez Cortes AK, et al. Targeting a cytokine checkpoint enhances the fitness of armored cord blood CAR-NK cells. Blood (2021) 137(5):624–36. doi: 10.1182/blood.2020007748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Hoerster K, Uhrberg M, Wiek C, Horn PA, Hanenberg H, Heinrichs S. HLA class I knockout converts allogeneic primary NK cells into suitable effectors for “Off-the-Shelf” immunotherapy. Front Immunol (2020) 11:586168. doi: 10.3389/fimmu.2020.586168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Melero I, Rouzaut A, Motz GT, Coukos G. T-Cell and NK-cell infiltration into solid tumors: a key limiting factor for efficacious cancer immunotherapy. Cancer Discovery (2014) 4(5):522–6. doi: 10.1158/2159-8290.CD-13-0985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Cozar B, Greppi M, Carpentier S, Narni-Mancinelli E, Chiossone L, Vivier E. Tumor-infiltrating natural killer cells. Cancer Discovery (2021) 11(1):34–44. doi: 10.1158/2159-8290.CD-20-0655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. D’Souza A, Fretham C, Lee SJ, Arora M, Brunner J, Chhabra S, et al. Current use of and trends in hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant (2020) 26:e177–e82. doi: 10.1016/j.bbmt.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Wang JC, Doedens M, Dick JE. Primitive human hematopoietic cells are enriched in cord blood compared with adult bone marrow or mobilized peripheral blood as measured by the quantitative in vivo SCID-repopulating cell assay. Blood (1997) 89(11):3919–24. doi: 10.1182/blood.V89.11.3919 [DOI] [PubMed] [Google Scholar]
- 135. Eapen M, Rubinstein P, Zhang MJ, Stevens C, Kurtzberg J, Scaradavou A, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet (2007) 369(9577):1947–54. doi: 10.1016/S0140-6736(07)60915-5 [DOI] [PubMed] [Google Scholar]
- 136. Eapen M, Klein JP, Ruggeri A, Spellman S, Lee SJ, Anasetti C, et al. Impact of allele-level HLA matching on outcomes after myeloablative single unit umbilical cord blood transplantation for hematologic malignancy. Blood (2014) 123(1):133–40. doi: 10.1182/blood-2013-05-506253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Wagner JE, Jr., Eapen M, Carter S, Wang Y, Schultz KR, Wall DA, et al. One-unit versus two-unit cord-blood transplantation for hematologic cancers. N Engl J Med (2014) 371(18):1685–94. doi: 10.1056/NEJMoa1405584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Politikos I, Davis E, Nhaissi M, Wagner JE, Brunstein CG, Cohen S, et al. Guidelines for cord blood unit selection. Biol Blood Marrow Transplant (2020) 26(12):2190–6. doi: 10.1016/j.bbmt.2020.07.030 [DOI] [PubMed] [Google Scholar]
- 139. Barker JN, Kempenich J, Kurtzberg J, Brunstein CG, Delaney C, Milano F, et al. CD34(+) cell content of 126 341 cord blood units in the US inventory: implications for transplantation and banking. Blood Adv (2019) 3(8):1267–71. doi: 10.1182/bloodadvances.2018029157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Kosuri S, Wolff T, Devlin SM, Byam C, Mazis CM, Naputo K, et al. Prospective evaluation of unrelated donor cord blood and haploidentical donor access reveals graft availability varies by patient ancestry: practical implications for donor selection. Biol Blood Marrow Transplant (2017) 23(6):965–70. doi: 10.1016/j.bbmt.2017.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Rizk M, Aziz J, Shorr R, Allan DS. Cell-based therapy using umbilical cord blood for novel indications in regenerative therapy and immune modulation: an updated systematic scoping review of the literature. Biol Blood Marrow Transplant (2017) 23(10):1607–13. doi: 10.1016/j.bbmt.2017.05.032 [DOI] [PubMed] [Google Scholar]
- 142. Frassoni F, Gualandi F, Podesta M, Raiola AM, Ibatici A, Piaggio G, et al. Direct intrabone transplant of unrelated cord-blood cells in acute leukaemia: a phase I/II study. Lancet Oncol (2008) 9(9):831–9. doi: 10.1016/S1470-2045(08)70180-3 [DOI] [PubMed] [Google Scholar]
- 143. Brunstein CG, Barker JN, Weisdorf DJ, Defor TE, McKenna D, Chong SY, et al. Intra-BM injection to enhance engraftment after myeloablative umbilical cord blood transplantation with two partially HLA-matched units. Bone Marrow Transplant (2009) 43(12):935–40. doi: 10.1038/bmt.2008.417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Nishida T, Kobayashi T, Sawa M, Masuda S, Shibasaki Y, Goto T, et al. A multicenter phase II study of intrabone single-unit cord blood transplantation without antithymocyte globulin. Ann Hematol (2021) 100(3):743–52. doi: 10.1007/s00277-020-04365-z [DOI] [PubMed] [Google Scholar]
- 145. Murata M, Maeda Y, Masuko M, Onishi Y, Endo T, Terakura S, et al. Phase II study of intrabone single unit cord blood transplantation for hematological malignancies. Cancer Sci (2017) 108(8):1634–9. doi: 10.1111/cas.13291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Kurita N, Gosho M, Yokoyama Y, Kato T, Obara N, Sakata-Yanagimoto M, et al. A phase I/II trial of intrabone marrow cord blood transplantation and comparison of the hematological recovery with the Japanese nationwide database. Bone Marrow Transplant (2017) 52(4):574–9. doi: 10.1038/bmt.2016.319 [DOI] [PubMed] [Google Scholar]
- 147. Okada M, Tasaka T, Ikegame K, Aotsuka N, Kobayashi T, Najima Y, et al. A prospective multicenter phase II study of intrabone marrow transplantation of unwashed cord blood using reduced-intensity conditioning. Eur J Haematol (2018) 100(4):335–43. doi: 10.1111/ejh.12999 [DOI] [PubMed] [Google Scholar]
- 148. Bonifazi F, Dan E, Labopin M, Sessa M, Guadagnuolo V, Ferioli M, et al. Intrabone transplant provides full stemness of cord blood stem cells with fast hematopoietic recovery and low GVHD rate: results from a prospective study. Bone Marrow Transplant (2019) 54(5):717–25. doi: 10.1038/s41409-018-0335-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Rocha V, Labopin M, Ruggeri A, Podesta M, Gallamini A, Bonifazi F, et al. Unrelated cord blood transplantation: outcomes after single-unit intrabone injection compared with double-unit intravenous injection in patients with hematological malignancies. Transplantation (2013) 95(10):1284–91. doi: 10.1097/TP.0b013e318288ca4d [DOI] [PubMed] [Google Scholar]
- 150. Liu H, Rich ES, Godley L, Odenike O, Joseph L, Marino S, et al. Reduced-intensity conditioning with combined haploidentical and cord blood transplantation results in rapid engraftment, low GVHD, and durable remissions. Blood (2011) 118(24):6438–45. doi: 10.1182/blood-2011-08-372508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Hsu J, Artz A, Mayer SA, Guarner D, Bishop MR, Reich-Slotky R, et al. Combined haploidentical and umbilical cord blood allogeneic stem cell transplantation for high-risk lymphoma and chronic lymphoblastic leukemia. Biol Blood Marrow Transplant (2018) 24(2):359–65. doi: 10.1016/j.bbmt.2017.10.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. van Besien K, Artz A, Champlin RE, Guarneri D, Bishop MR, Chen J, et al. Haploidentical vs haplo-cord transplant in adults under 60 years receiving fludarabine and melphalan conditioning. Blood Adv (2019) 3(12):1858–67. doi: 10.1182/bloodadvances.2019000200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Hiwarkar P, Adams S, Gilmour K, Nataraj R, Bonney D, Poulton K, et al. Cord blood CD8+ T-cell expansion following granulocyte transfusions eradicates refractory leukemia. Blood Adv (2020) 4(17):4165–74. doi: 10.1182/bloodadvances.2020001737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. de Lima M, McNiece I, Robinson SN, Munsell M, Eapen M, Horowitz M, et al. Cord-blood engraftment with ex vivo mesenchymal-cell coculture. N Engl J Med (2012) 367(24):2305–15. doi: 10.1056/NEJMoa1207285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Mehta RS, Saliba RM, Cao K, Kaur I, Rezvani K, Chen J, et al. Ex vivo mesenchymal precursor cell-expanded cord blood transplantation after reduced-intensity conditioning regimens improves time to neutrophil recovery. Biol Blood Marrow Transplant (2017) 23(8):1359–66. doi: 10.1016/j.bbmt.2017.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Saiyin T, Kirkham AM, Bailey AJM, Shorr R, Pineault N, Maganti HB, et al. Clinical outcomes of umbilical cord blood transplantation using ex vivo expansion: a systematic review and meta-analysis of controlled studies. Transplant Cell Ther (2023) 29(2):129 e1– e9. doi: 10.1016/j.jtct.2022.11.007 [DOI] [PubMed] [Google Scholar]
- 157. Delaney C, Heimfeld S, Brashem-Stein C, Voorhies H, Manger RL, Bernstein ID. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med (2010) 16(2):232–6. doi: 10.1038/nm.2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Milano F, Thur LA, Blake J, Delaney C. Infusion of non-HLA-Matched off-the-Shelf ex vivo expanded cord blood progenitors in patients undergoing cord blood transplantation: result of a phase II clinical trial. Front Cell Dev Biol (2022) 10:835793. doi: 10.3389/fcell.2022.835793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Stiff PJ, Montesinos P, Peled T, Landau E, Goudsmid NR, Mandel J, et al. Cohort-controlled comparison of umbilical cord blood transplantation using carlecortemcel-l, a single progenitor-enriched cord blood, to double cord blood unit transplantation. Biol Blood Marrow Transplant (2018) 24(7):1463–70. doi: 10.1016/j.bbmt.2018.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Horwitz ME, Wease S, Blackwell B, Valcarcel D, Frassoni F, Boelens JJ, et al. Phase I/II study of stem-cell transplantation using a single cord blood unit expanded ex vivo with nicotinamide. J Clin Oncol (2019) 37(5):367–74. doi: 10.1200/JCO.18.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Wagner JE, Jr., Brunstein CG, Boitano AE, DeFor TE, McKenna D, Sumstad D, et al. Phase I/II trial of StemRegenin-1 expanded umbilical cord blood hematopoietic stem cells supports testing as a stand-alone graft. Cell Stem Cell (2016) 18(1):144–55. doi: 10.1016/j.stem.2015.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Cohen S, Roy J, Lachance S, Delisle JS, Marinier A, Busque L, et al. Hematopoietic stem cell transplantation using single UM171-expanded cord blood: a single-arm, phase 1-2 safety and feasibility study. Lancet Haematol (2020) 7(2):e134–e45. doi: 10.1016/S2352-3026(19)30202-9 [DOI] [PubMed] [Google Scholar]
- 163. Peled T, Shoham H, Aschengrau D, Yackoubov D, Frei G, Rosenheimer GN, et al. Nicotinamide, a SIRT1 inhibitor, inhibits differentiation and facilitates expansion of hematopoietic progenitor cells with enhanced bone marrow homing and engraftment. Exp Hematol (2012) 40(4):342–55 e1. doi: 10.1016/j.exphem.2011.12.005 [DOI] [PubMed] [Google Scholar]
- 164. Harrell CR, Jovicic N, Djonov V, Arsenijevic N, Volarevic V. Mesenchymal stem cell-derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cells (2019) 8(12). doi: 10.3390/cells8121605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol (2000) 109(1):235–42. doi: 10.1046/j.1365-2141.2000.01986.x [DOI] [PubMed] [Google Scholar]
- 166. de Wolf C, van de Bovenkamp M, Hoefnagel M. Regulatory perspective on in vitro potency assays for human mesenchymal stromal cells used in immunotherapy. Cytotherapy (2017) 19(7):784–97. doi: 10.1016/j.jcyt.2017.03.076 [DOI] [PubMed] [Google Scholar]
- 167. Wiese DM, Wood CA, Braid LR. From vial to vein: crucial gaps in mesenchymal stromal cell clinical trial reporting. Front Cell Dev Biol (2022) 10:867426. doi: 10.3389/fcell.2022.867426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Merimi M, El-Majzoub R, Lagneaux L, Moussa Agha D, Bouhtit F, Meuleman N, et al. The therapeutic potential of mesenchymal stromal cells for regenerative medicine: current knowledge and future understandings. Front Cell Dev Biol (2021) 9:661532. doi: 10.3389/fcell.2021.661532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Garcia-Bernal D, Garcia-Arranz M, Yanez RM, Hervas-Salcedo R, Cortes A, Fernandez-Garcia M, et al. The current status of mesenchymal stromal cells: controversies, unresolved issues and some promising solutions to improve their therapeutic efficacy. Front Cell Dev Biol (2021) 9:650664. doi: 10.3389/fcell.2021.650664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Xu Z, Huang Y, Zhou J, Deng X, He W, Liu X, et al. Current status of cell-based therapies for COVID-19: evidence from mesenchymal stromal cells in sepsis and ARDS. Front Immunol (2021) 12:738697. doi: 10.3389/fimmu.2021.738697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Wu Y, Cao Y, Li X, Xu L, Wang Z, Liu P, et al. Cotransplantation of haploidentical hematopoietic and umbilical cord mesenchymal stem cells for severe aplastic anemia: successful engraftment and mild GVHD. Stem Cell Res (2014) 12(1):132–8. doi: 10.1016/j.scr.2013.10.001 [DOI] [PubMed] [Google Scholar]
- 172. Wu KH, Tsai C, Wu HP, Sieber M, Peng CT, Chao YH. Human application of ex vivo expanded umbilical cord-derived mesenchymal stem cells: enhance hematopoiesis after cord blood transplantation. Cell Transplant (2013) 22(11):2041–51. doi: 10.3727/096368912X663533 [DOI] [PubMed] [Google Scholar]
- 173. Wang H, Wang Z, Zheng X, Ding L, Zhu L, Yan H, et al. Hematopoietic stem cell transplantation with umbilical cord multipotent stromal cell infusion for the treatment of aplastic anemia–a single-center experience. Cytotherapy (2013) 15(9):1118–25. doi: 10.1016/j.jcyt.2013.04.007 [DOI] [PubMed] [Google Scholar]
- 174. Li Y, Hao J, Hu Z, Yang YG, Zhou Q, Sun L, et al. Current status of clinical trials assessing mesenchymal stem cell therapy for graft versus host disease: a systematic review. Stem Cell Res Ther (2022) 13(1):93. doi: 10.1186/s13287-021-02683-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Soder RP, Dawn B, Weiss ML, Dunavin N, Weir S, Mitchell J, et al. A phase I study to evaluate two doses of wharton’s jelly-derived mesenchymal stromal cells for the treatment of De novo high-risk or steroid-refractory acute graft versus host disease. Stem Cell Rev Rep (2020) 16(5):979–91. doi: 10.1007/s12015-020-10015-8 [DOI] [PMC free article] [PubMed] [Google Scholar]