Abstract
Gaucher disease (GD) is caused by biallelic pathogenic variants in GBA1 gene that encodes the lysosomal enzyme glucocerebrosidase. Up to now, specific treatment for GD cannot completely reverse bone complications. Bone is composed of different cell types; including osteoblasts, osteocytes and osteoclasts. Osteoblasts are present on bone surfaces and are derived from local mesenchymal stem cells (MSCs). Depending on environment conditions, MSCs could differentiate into osteoblasts and adipocytes. Mature adipocytes-secreted adipokines and free fatty acids affect both osteoblasts and osteoclasts formation/activity and therefore mediate skeletal homeostasis. The aim of this study was to evaluate possible alterations in GD adipocyte (GD Ad) that could contribute to bone complications. MSCs were grown in adipogenic media in order to evaluate expression of differentiation markers as PPAR-γ. PPAR-γ was observed into the nucleus of GD Ad, indicating that these cells are properly stimulated. However, these cells accumulate lesser lipid droplets (LDs) than Control Ad. In order to study lipid droplet metabolism, we evaluated the lipolysis of these structures by the measurement of free glycerol in culture supernatant. Our results indicated that GD Ad had an alteration in this process, evidenced by an increase in glycerol release. We have also evaluated two enzymes involved in LDs synthesis: fatty acid synthase (FASN) and stearoyl-coenzyme A desaturase 1 (SCD1). The transcription of these genes was decreased in GD Ad, suggesting a dysfunction in the synthesis of LDs. In conclusion, our results show an alteration in LDs metabolism of GD Ad, independent of adipocyte differentiation process. This alteration would be caused by an increase in lipolysis in early stages of differentiation and also by a reduction of lipid synthesis, which could contribute with the skeletal imbalance in GD.
Keywords: Gaucher, MSCs, Adipocytes, Bone, Lipid droplets
1. Introduction
Gaucher disease (GD), one of the most common lysosomal storage disorders, is an autosomal recessive disorder caused by biallelic pathogenic variants in GBA1 which encodes for lysosomal β-glucocerebrosidase (GCase) (Enzyme Commission 3.2.1.45) enzyme. Three main clinical forms have been identified. Types II and III are neuronopathic, whereas type I (the most frequent presentation in Western countries), lacks early neuropathology and is characterized by splenomegaly, hepatomegaly, anemia, thrombocytopenia, and skeletal complications that include acute or chronic pain, avascular necrosis, osteoarthritis, osteopenia and osteoporosis. The pathophysiology of skeletal density affection in GD is matter of intense research due to the diverse mechanisms that could be involved, including rate of bone remodeling, development and differentiation of mesenchymal stem cells (MSCs) and osteoimmunology [1].
Bone marrow MSCs are a population of self-renewing multipotent stem cells with the ability to differentiate into adipocytes or bone-forming osteoblasts, among other cell types. Hematopoietic stem cells (HSCs) give rise to blood cells of the lymphoid and myeloid lineage as well as bone-resorbing osteoclasts. Adipocytes have been found to influence the development and function of other cells types through paracrine actions, which impact in the suppression of osteogenic lineages from MSCs as well as the promotion of osteoclast formation from HSCs [2]. We have reported that Gaucher patients have higher numbers of circulating monocytes expressing markers of osteoclast precursors that potentially migrate into bone, differentiate into mature osteoclasts and produce an increment in bone resorption activity [3]. Additionally, commitment of MSCs to osteoblastic differentiation is reduced and several in vivo and in vitro GD models showed that osteoblasts have a deficient bone formation function as evidenced by a low level of bone matrix deposition [[4], [5], [6]]. Moreover, GD MSCs showed an imbalanced tendency to differentiate into adipocytes instead of osteoblasts [7]. All these alterations could be favored due to the existence of a proinflammatory profile associated with GD, where immune cells produce higher levels of proinflammatory cytokines such as RANKL and IL-1β [8,9].
It is well known that a proinflammatory bone environment favors adipogenic over osteogenic differentiation of MSCs, evidenced by higher expression of PPARγ and lower expression of RUNX2 [10]. Indeed, in the context of GD, we have shown that GD MSCs expressed low levels of Sirtuin 1 (SIRT1) and RUNX2, but increased levels of NLRP3 and PPARγ. Intriguingly, although GD adipocytes (GD Ad) express higher levels of PPARγ, these cells accumulate fewer lipid droplets than control Ad (Ctrl Ad), reflecting a probable disconnection between the observed increased tendency to differentiate into adipocytes and the possibility to acquire full phenotype of an adypocite with LDs accumulation [7]. The clinical relevance of these in vitro observations would be that Gaucher treatment-naïve patients often display a reduced bone marrow fat fraction as analyzed by quantitative chemical shift imaging (QCSI) [[11], [12], [13]]. However, it was originally suggested that the reduced fat fraction was the result of displacement of adipocyte cells by progressive infiltration with Gaucher cells [11].
In general terms, adipogenesis is a two-step developmental process that combines commitment and terminal differentiation of cells [14]. At first, a MSC differentiates into preadipocyte and then undergoes terminal differentiation to become a mature adipocyte with lipid droplet accumulation. During early stages, multiple inducers activate PPARγ expression which activates C/EBPα toward adipogenic differentiation [15]. On the contrary, it has been demonstrated that TGF-β has an important role in the suppression of adipocyte commitment of MSCs through phosphorylation of PPARγ [16,17]. During terminal differentiation, mature adipocytes undergo morphological and functional changes [18] as they store neutral lipids into lipid droplets (LDs). The formation of LDs in the ER is initiated with the synthesis of neutral lipids which are covered by a monolayer of phospholipids [19]. On the other hand, the catabolism of LDs into free fatty acids is a crucial cellular pathway that is required to generate energy, biological membranes and hormone synthesis. LDs are broken down mostly by lipolysis, which is a biochemical catabolic pathway that relies on the direct activation of LD-associated lipases, such as adipose triglyceride lipase, hormone-sensitive lipase and monoglyceride lipase [19].
It has been observed that basal adipocyte lipolysis is closely associated with insulin resistance due to excessive circulating fatty acids, which can ectopically accumulate in insulin-sensitive organs an impair insulin action. Furthermore, excessive fatty acid release contributes to adipose tissue inflammation that also worsen insulin resistance [20]. Langeveld et al. described a 6% prevalence of insulin resistance in ERT-treated GD1 patients, possibly associated to the altered sphingolipid metabolism [21]. There is evidence that increase in GM3 ganglioside, as has been reported in GD patients [22], has a prominent influence on osteoclast activation [23] and the development of insulin resistance in patients with GD1 due to the loss of insulin receptors from lipid rafts [24].
With this background in mind, the aim of this work is to understand the molecular basis of the reduced lipid droplet accumulation in GD Ads.
2. Materials and methods
2.1. Cell lines
The type 3 GD L444P/L444P and control Mesenchymal Stem Cells (MSCs) used in this study are iPSC derived-MSCs that has previously been described [25,26]. These cells were obtained from healthy and GD patient’s fibroblasts that were dedifferentiated into iPSCs and then differentiated into MSCs [27].
2.2. Cell culture
MSCs were grown in DMEM-GlutaMAX™ (GIBCO, Grand Island, NY) supplemented with 20% heat inactivated fetal bovine serum (Gibco-BRL, Life technologies, Grand Island, NY), 100 units/ml of penicillin and 100 μg/ml of streptomycin (complete media). Cultures were grown at 37 °C in 5% CO2 atmosphere, replacing the media every 48 h.MSCs can differentiate into adipocytes in adipogenic media. To obtain adipogenic media, complete media was supplemented with 500 μM 3-isobutyl-1-methylxanthine (IBMX, Sigma), 0,1 μM dexamethasone (Sigma), 50 μM Indomethacin (Sigma) and 10 μg/ml insulin (Densulin R, Argentina). MSCs were seeded at 20.000 cells/well in a 48 well-plate and were grown in MSC media until confluence. Then, we replaced the complete media with adipogenic media for 7 and 14 days. Differentiated adipocytes will be referred as Ad.
For reduction substrate treatment in GD Ad, we added D-threo-1-phenyl-2-decanoylamino-3-morpholino-propanol (PDMP, commercially known as eliglustat) 10 μM (Matreya, LLC), to the adipogenic media during differentiation time.
2.3. Immunofluorescence Microscopy
Adipocytes were grown in chamber slides for 7 days as previously described and fixed in 4% paraformaldehyde for 20 min at room temperature. For lipid staining, cells were fixed with paraformaldehyde, permeabilized with 0.3% Triton X-100, and then lipid droplets were stained with 1 μg/ml of Bodipy 493/503 (Invitrogen, cat. D3922). For PPARγ visualization, cells were incubated with a rabbit polyclonal antibody specific for PPARγ (1:250 dilution) (Invitrogen, pa5-25757) and then with a 1:300 dilution of Alexa Fluor488-conjugated Fab´fragment of goat anti-rabbit IgG (H + L) (Life Technology, cat A11008). The primary antibody was incubated overnight at 4°C, and three washes between incubations were performed with 0.1% Tween-20 in PBS. Secondary antibodies were incubated for 1 h at room temperature. Nuclei were stained with Methil Green 1:500 (stock 2 % p/v) added into the Fluorescent Mounting Medium (DakoCytomation, Glostrup, Denmark, cat S3023). Cells were visualized in a TCS SP5 Leica confocal microscope (Leica Microsystems, Wetzlar, Germany). Images were taken using the Leica LAS AF software (Leica Microsystems,Wetzlar, Germany) and image analysis was performed using the FIJI software (National Institutes of Health, Bethesda, MD, USA).
2.4. mRNA extraction and Real-time quantitative PCR
Total RNA was isolated from MSCs or Ad using a total RNA isolation system (GE Healthcare, Piscataway, NJ, USA) following the manufacturer's protocols. The isolated total RNA samples were subjected to reverse transcription using SuperScript™ III Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA). Real-time quantitative PCR (qPCR) was performed using SYBR GreenER PCR Master Mix (Invitrogen) in an iQ-Cycler equipment (Bio-Rad, Hercules, CA, USA). The sequence-specific primers were obtained from OriGene website and checked with PrimerBlast on NCBI. The sequences used are shown in Table 1. A human β-Actin gene was used as an internal control. The comparative threshold (Ct) method was used for data analysis, expressed as 2– (dCT).
Table 1.
Primer sequences.
| Name | Sequence (5′ to 3′) |
|---|---|
| hFASN_Fw | AAGGACCTGTCTAGGTTTGATGC |
| hFASN_Rv | TGGCTTCATAGGTGACTTCCA |
| hSCD1_Fw | TTCCTACCTGCAAGTTCTACACC |
| hSCD1_Rv | CCGAGCTTTGTAAGAGCGGT |
| hTGFβ_Fw | CCCACAACGAAATCTATGAC |
| hTGFβ_Rv | CTGTATTTCTGGTACAGCTC |
2.5. Adipocytes cultured with exogenous lipids
Control MSCs were incubated for 7 days with Gaucher specific lipids (Matreya, LLC): glucosylceramide (GlcCer 20μM); glucosylesphingosine (GlcSph 0,1μM; 1μM); or non-specific Gaucher related Lipids (Matreya, LLC): Globotriaosylceramide (Gb3 20μM); Globotriaosylsphingosine (Lyso-Gb3 1μM). Lipids were dissolved in solvents as follows: GlcCer, Gb3 and Lyso-Gb3 in chloroform/methanol (2:1); GlcSph in Ethanol (EtOH). The respective solvent controls were included in the assay. Cultures were grown at 37 °C in 5% CO2 atmosphere, replacing the media every 48 h.
2.6. Lipolysis assay
MSCs were cultured and differentiated into adipocytes as described. Cell supernatants were collected at every media change: at start of differentiation, 3 days, 5 days and 7 days. A glycerol assay kit (ab133130) was used according to manufacturer protocol in order to determine adipocyte lipolysis.
2.7. Glucose uptake
Cells were grown in a 96 well plate to confluence. Then, cells were washed with PBS and were incubated with: PBS + Fetal Bovine Serum 20% (Gibco) + (2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-il)amino)-2-desoxiglucosa) (2NBDG) 100 μM (ab146200) and Fetal Bovine Serum 20% (Gibco) + 2NBDG 100 μM + insulin at three different concentrations (10 μg/ml; 500 μg/ml or 2000 μg/ml insulin) during 15 min at 37 °C in 5% CO2 atmosphere [28]. After incubation, cells were washed with cold PBS, fixed with PFA 4% for 10 min and left in PBS to measure fluorescence intensity in Varioskan™ LUX multimode microplate reader (Thermo Scientific™).
2.8. Oil Red-O Staining
Cultured adipocytes were fixed with 4% (p/v) paraformaldehyde and stained with Oil Red-O solution (SIGMA). For quantitative analysis, the stained material was dissolved in 150ul of isopropanol 100%. The dye solution was transferred to microtiter plates, and the optical density (OD) measured with a microplate reader (BD Pharmingen, San Diego, CA, USA) at 492 nm against isopropanol 100% as a blank.
2.9. Statistical analysis
Statistical analyses were performed using GraphPad Prism 7.0 software (Graphpad Software, La Jolla, CA, USA) applying unpaired t-test and one-way ANOVA followed by Sidak test. Data are expressed as mean ± SD (n = 4) and are representative of three independent experiments.
3. Results
3.1. Gaucher adipocytes cannot properly accumulate LDs
The main function of an adipocyte is to store energy in the form of lipids that is reflected by volume increase [29]. Based on our previous result showing an increased expression of PPARγ in GD MSCs [7] and the role of PPARγ in the promotion of differentiation of MSCs into adipocytes, we decided to analyze the presence of LDs in GD Ad. MSCs were differentiated with adipogenic media and LDs were stained with Bodipy 493/503 and visualized by confocal microscopy (Fig 1A). Quantification of both number of LDs (Fig 1B) and relative fluorescence intensity (Fig 1C) has revealed a significant reduced number and amount of lipid droplets in GD Ad as compared to control ones.
Fig 1.
Gaucher adipocytes cannot properly accumulate lipid droplets. Localization (A), lipid droplets quantification (B) and the fluorescence intensity (C) of the lipid droplets are shown in adipocytes of 7 days of differentiation. Five microscopic fields per condition were quantified for each experiment. Scale bar: 100μm. Unpaired t-test ** p < 0.005, **** p < 0.0001
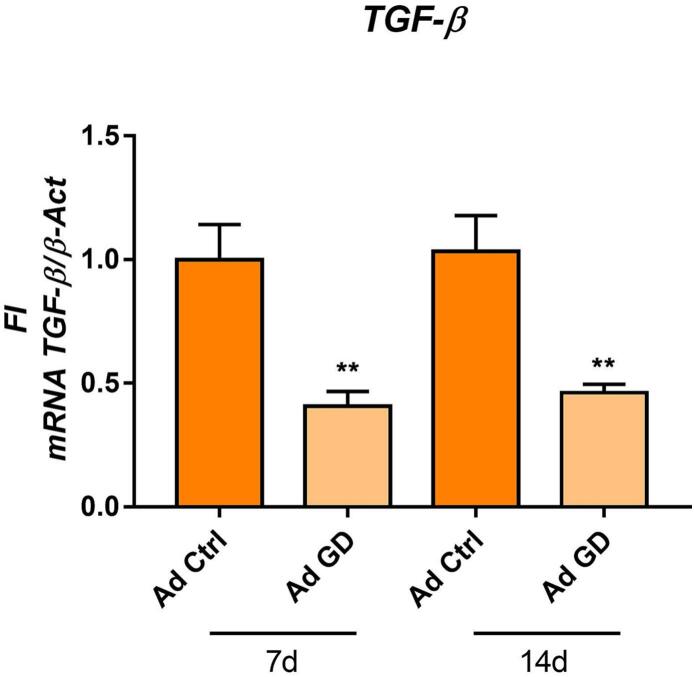
3.2. TGF-β1 is downregulated in GD Ad
It is known that TGF-β has a critical role in regulating adipocyte commitment of MSCs [30], thus we decided to evaluate TGF-β1 expression in GD Ad. We found a reduced TGF-β1 mRNA transcription in GD Ad as compared to control ones (Fig. 2). This result indicates that TGF-β1 could be an associated factor that may have an influence in the upregulation of GD Ad differentiation.
Fig 2.
TGF-β1 is downregulated in Gaucher adipocytes. TGF-β mRNA transcription in Ad at 7 and 14 days of differentiation. All bars are referred at Ad ctrl at 7d. Statistics are referred at the control of the corresponding day. Unpaired t-test **p < 0.005
3.3. PPARγ is increased and present in the nucleus in GD Ad
PPARγ is considered the master nuclear transcription factor that regulates adipogenesis [31]. One hypothesis to explain the lower levels of LDs in the context of high PPARγ expression could be that PPARγ do not translocate into the nucleus. In order to evidence its localization within adipocytes, we differentiated MSCs in adipogenic media and PPARγ was observed by confocal microscopy. We observed that PPARγ is localized in the nucleus of both control and GD Ad (Fig. 3A). Moreover, the expression of PPARγ is increased in GD Ad as compared to control cells (Fig. 3B). These results would rule out a PPARγ-associated suppression of GD Ad differentiation. Therefore we hypothesized that reduction of LDs could be a consequence of PPARγ effect on lipolysis stimulation in mature adipocytes [32].
Fig 3.
PPARγ is increased in GD Ad. Localization (A) and fluorescence quantification per cell (B) is shown in adipocytes of 7 days of differentiation. Five microscopic fields per condition were quantified for each experiment. Scale bar: 50μm. Unpaired t-test *p < 0.05
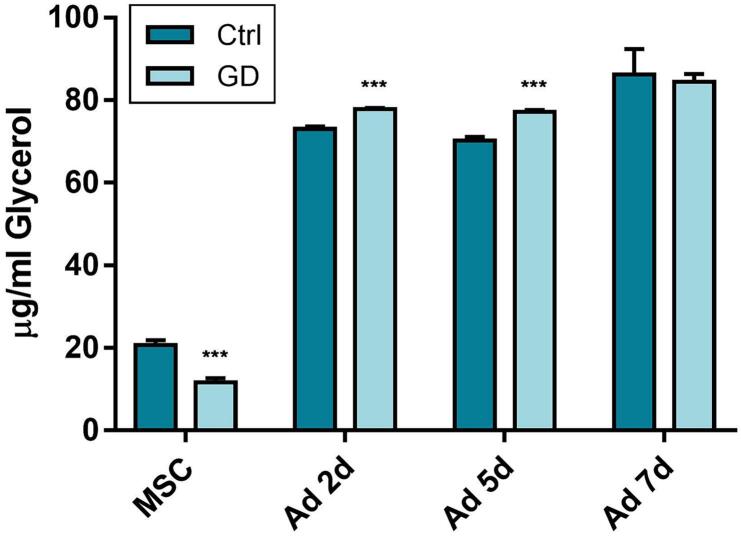
3.4. Lipolysis is upregulated at early stages of differentiation
Based on our results that GD MSCs preferentially differentiate into adipocytes rather than osteoblast, and the evidence that PPARγ promotes lipolysis, we studied if LDs are being degraded at a higher rate in GD Ad by a lipolytic mechanism. This pathway of lipid degradation converts triglycerides into fatty acids and glycerol, where glycerol is secreted proportionally to lipolysis activity. We therefore differentiated MSCs to Ad, and collected the supernatants at each media change. Our results revealed increased glycerol levels during early adipocyte differentiation in both cell types at day 2 and 5 of differentiation (Fig. 4). However, at day 7 this difference is not observed, thus we cannot certainly attribute the fewer LDs in GD Ad only at lipolytic mechanisms.
Fig 4.
Lipolysis is upregulated at early stages of differentiation. Free glycerol was measured in MSCs and different stages of adipocyte differentiation culture media supernatants. Statistics are referred at the control of the corresponding day. Unpaired t-test *** p < 0.0005
3.5. Lipid synthesis is affected in GD Ad
Another aspect that could be relevant to study the accumulation of LDs is the synthesis of lipids. In order to study if the transcriptions of the enzymes involved in this process are affected in GD adipocytes, we decided to evaluate transcription of fatty acid synthase (FASN) and stearoyl-coenzyme A desaturase 1 (SCD1) [33]. Both FASN and SCD1 mRNA transcription was downregulated in GD Ad (Fig. 5). A reduction in the transcription of genes associated to lipid synthesis may have a deleterious effect in the formation of LDs which could explain, at least in part, the lesser LDs amounts observed in these cells.
Fig 5.
Lipid synthesis is affected in Gaucher adipocytes. mRNA transcription was measured by RT-qPCR in control and GD adipocytes at 7 and 14 days of differentiation. All bars are referred at Ad ctrl at 7d. Statistics shown in Ad GD are referred at the control of the corresponding day. Unpaired t-test * p < 0.05, ** p < 0.005, *** p < 0.0005, **** p < 0.0001
3.6. Glucose uptake and metabolism is not altered in GD MSCs
Glucose is converted to fatty acids and cholesterol through de novo lipid biosynthesis pathway. Therefore glucose uptake and metabolism is a critical point for LD formation. Several studies indicate that glycosphingolipids are involved in the development of insulin resistance and directly interfere with insulin signaling. It has been shown that gangliosides block phosphorylation of the insulin receptor and its downstream signaling [34]. In order to evaluate a possible alteration in insulin signaling, MSCs were incubated with insulin at 10 μg/ml (same as needed for adipocyte differentiation), 500 μg/ml and 2000 μg/ml. At the same time, we used 2NBDG that enters into the cells through glucose transporters and is subsequently phosphorylated by hexokinase, keeping glucose trapped inside the cells. Results showed an uptake of 2NBDG in both control and GD MSCs, which indicates that these cells are sensitive to insulin. Also, higher levels of 2NBDG were detected in GD MSCs (Fig. 6).
Fig 6.
Glucose uptake and metabolism in GD MSCs is not altered. Glucose uptake and metabolism was evidenced by using 2NBGD. A dose response curve was done with different insulin concentrations (10, 500 and 2000 μg/ml). Statistics that is showed over bars are referred at Ctrl or GD cells without insulin. One-way ANOVA followed by Sidak test *** p < 0.0005, **** p < 0.0001
3.7. Glucocerebrosides do not affect lipid droplet accumulation in control adipocytes
It is known that glycosphingolipids can directly inhibit insulin signaling pathway contributing to insulin resistance [34]. As GD is characterized by a primary glucosylceramide accumulation and secondary elevation of gangliosides, we investigated whether high glycosphingolipids concentration are associated with the altered adipogenic phenotype in GD adipocytes. For that purpose, we incubated control MSCs with glucosylceramide (GlcCer) and glucosylsphingosine (GlcSph) and differentiated them in adipogenic media. Lipids that are not directly associated to GD were included to dismiss any effect caused by the lipid per se, and lipid concentrations were chosen in concordance to lipid levels observed in Gaucher patient’s plasma and serum [35,36]. We found no difference in LD formation in control adipocytes due to the exposure of these cells to any lipid, not only alone but also in combination (Fig. 7A). To clarify the role of Gaucher-related lipid accumulation, we treated GD Ad with eliglustat. This inhibitor of glucosylceramide synthase prevents substrate accumulation and, therefore, the accumulation of glycolipids related to the disease. Our results showed that treatment with eliglustat did not change the capacity of GD Ad to accumulate lipid droplets, as determined by Oil Red-O staining (data not shown). However, when cells were observed by light microscopy, some cells showed differences in their morphology and their lipid droplets distribution resembling control Ad (Fig. 7B).
Fig 7.
Glucocerebrosides do not affect lipid droplet accumulation in Control Ad. Control MSCs were differentiated into adipocytes for 7 days. A) During differentiation cells were incubated with GlcCer (20 μM), GlcSph (0,1 μM; 1 μM) or its combination. Gb3 and Lyso-Gb3 are non Gaucher specific lipids. As assay controls, EtOH, chloroform and methanol were tested at the same combination and volume than the ones used in assays with lipids. All bars and statics are referred to Ctrl Ad without treatment (-). Unpaired t-test: ns B) Light microscopy visualization of Ctrl and GD Ad with or without Eliglustat (10 μg/ml) treatment. Scale bar: 100 μm.
4. Discussion
Over a thirty year observation period, pharmacologic, recombinant GCase treatment (ERT) effectively reverses GD-associated hepatosplenomegaly, anemia and thrombocytopenia in most treated patients. However, despite ERT in recognized effective doses, high percentage of patients continue to have skeletal signs and symptoms. Bone health is maintained by a delicate homeostasis among several cell types including osteoblasts, osteoclasts and bone marrow adipocytes, which have a functional relationship with bone tissue and blood cell production [37]. Continued bone structure abnormalities in GD may be attributable to persistent infiltration due to incomplete clearance of Gaucher cells in the bone marrow. Failure to normalize a reduced fat fraction is a surrogate indication of the extent of displacement of adipocytes by Gaucher cells infiltrates [11]. In this context, the absence or paucity of bone marrow adipocytes, their bad distribution in the bone marrow environment, or the failure in their function, could contribute to bone fragility.
MSCs differentiate into osteoblasts or bone marrow adipocytes depending on molecular and environmental signals [38,39]. We have previously reported that GD MSCs showed an imbalanced tendency to differentiate into adipocytes rather than of osteoblasts [7] and that GD Ad have reduced number of LDs. Therefore, the main goal of the present study was to elucidate the molecular mechanisms causing the reduced lipid droplet accumulation in GD adipocytes as an index of GD Ad dysfunction.
PPARγ is a master regulator of adipogenesis [31] and our previous results indicated that its expression is elevated in GD adipocytes with respect to control cells. Therefore, we expected to see an increase of adipocyte size as a signal of mature adipocytes. However, we observed GD Ad had a reduced number of lipid droplets as compared to control cells. This finding presents a paradox: GD MSCs are prone to differentiate into adipocytes but these cells are structurally abnormal, presenting a reduced content of lipid droplets.
It is known that TGF-β has an important role in the suppression of adipocyte commitment of MSCs through phosphorylation of PPARγ[16.17]. It has been reported that TGF-β is increased in plasma from GD patients respect to healthy controls [40]. On the contrary we have reported that serum from GD patients present lower levels of TGB-β than healthy controls [3]. indicating that the issue is still controversial. Other factors such as phenotype, age and course of the disease, could be involved in the regulation of TGF-β levels. In our model, we detected a reduced expression of TGF-β in GD adipocytes respect to healthy controls. In this aspect, the downregulation of TGF-β could be associated with decrease phosphorylation of PPARγ, a process that should favor adipogenesis over osteoblastogenesis. Furthermore, PPARγ needs to be localized into the nucleus in order to initiate this process [31]. In this work we observed that PPARγ in GD adipocytes was localized into the nucleus, even in higher levels than control cells, thus allowing GD Ad differentiation to proceed normally. On the contrary, our results indicated that the lipid droplets content diminished in GD ad. This could be explained at least in part by previous findings indicating that persistent higher concentrations of unphosphorylated PPARγ secondarily stimulate hydrolysis of lipid droplets through lipolysis [32].
Lipolysis is one of the mechanisms of lipid droplet degradation and this process is quantifiable by measuring glycerol release [41]. Per our hypothesis, we found higher levels of glycerol in GD adipocytes supernatants. Additionally, we evaluated the expression of FASN and SCD1, two enzymes involved in lipid synthesis, and both of them were reduced in GD adipocytes, indicating a second mechanism limiting lipid accumulation in GD adipocytes. Indeed, SCD1 inhibition in differentiated adipocytes is associated with downregulation of genes involved in triacylglycerol biosynthesis [42]. De novo lipogenesis is also limited by epigenetic downregulation [43]. Thus, the reduced size and numbers of lipid droplets in GD Ad is attributable to a combination of reduced synthesis and incipient increased lipolysis.
Evidence suggests glycosphingolipids are involved in the development of insulin resistance. Complex glycosphingolipids and gangliosides block phosphorylation of the insulin receptor and down-stream signalling in a mechanism that could involve the exclusion of the insulin receptor from specific membrane domains [34]. It has been hypothesized that accumulation of such lipids may induce development of insulin resistance in patients with GD1 [24]. Langeveld et al. described a 6% prevalence of insulin resistance in ERT-treated GD1 patients, possibly associated to the altered sphingolipid metabolism [21]. Therefore, we investigated whether GD MSCs in our model were altered in regard to insulin-mediated glucose uptake. Results showed that both cell types respond to insulin stimulus due to 2NBDG levels in both cell types are similar, indicating that glucose would be entering into the cell at the same rate. And, at higher insulin dose, GD Ad show higher levels of 2NBDG compare to control Ad. This effect could be explained by a decrease in glucose metabolism of GD cells where 2NBDG is not consumed but accumulate; or by an increase of insulin receptors in GD Ad’s plasma membrane which allow entry of higher amounts of 2NBDG than its consumption rate. By this experiment we exclude the possibility of a defect in insulin signalling. More experiments regarding expression and localization of insulin receptors should be done to deepen these studies.
Glycosphingolipids affect bone mineral density by reducing osteoblast activity and stimulating bone resorption [44]. In this regard, we wanted to evaluate if exposure to specific Gaucher glycosphingolipids (GlcCer and GlcSph) could alter the accumulation of LDs in control adipocytes emulating a GD phenotype. We found no difference in the generation of lipid droplets between C Ad and C Ad cultured with exogenous GlcCer and GlcSph alone or in combination. This lack of effect could be explained by the insufficient incubation time to observe the consequences of glycolipid accumulation on lipid droplet metabolism. To clarify the role of Gaucher-related glycolipid accumulation, we treated GD Ad with eliglustat. This inhibitor of glucosylceramide synthase prevents substrate accumulation and, therefore, the accumulation of glycolipids related to the disease. Although we did not see a significant improvement of GD Ad in lipid droplets, we observed a change in cell morphology resembling that of wild type cells. This result would suggest that Gaucher related glycolipid accumulation has an impact in the observed phenotype. Further studies should be done to deepen these findings, such as using different doses of eliglustat and prolonged treatment time.
These studies using MSCs derived from iPSCs provide clues regarding potential factors that could alter adipocyte phenotype in GD Ad. Despite the use of MSCs derived from GD patients represents an excellent tool, this study has some limitations because the GD cell line was obtained from only one GD3 patient, as extensively described in other reports [25.26]. Therefore, these findings are limited to this particular genotype, and studies with iPCS derived-MSC from other patient genotypes are required to extrapolate these results and definitively associate them with Gaucher alterations.
Our results indicate that TGF-β1, PPARγ and the enzymes involved in lipid synthesis, appear to be altered in GD cells. These results also do not support an insulin resistance mechanism. Further studies using diverse iPCS derived-MSCs from other patient genotypes and in vivo models will be needed to confirm to what extent the mediators described here have a “real life” role in the pathophysiology of GD disease.
Funding source
This work was supported by the CONICET (PIP0330) and Universidad Nacional de La Plata (11/X814). La Plata, Argentina
Declaration of Competing Interest
None.
Acknowledgements
We thank Dr. Ricardo A. Feldman (University of Maryland School of Medicine, Baltimore MD, USA) for providing the MSCs used in this study.
Data availability
Data will be made available on request.
References
- 1.Rozenfeld P.A., Crivaro A.N., Ormazabal M., Mucci J.M., Bondar C., Delpino M.V. Unraveling the mystery of Gaucher bone density pathophysiology [Internet] Mol. Genet. Metab. 2021;132:76–85. doi: 10.1016/j.ymgme.2020.07.011. [DOI] [PubMed] [Google Scholar]
- 2.Shanmugam M., Govindarajan R., Sinal C.J. Bone marrow adipose tissue and skeletal health. Curr Osteoporos Rep. 2018;16(4):434–442. doi: 10.1007/s11914-018-0451-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bondar C., Mucci J., Crivaro A., Ormazabal M., Ceci R., Oliveri B., et al. In vitro osteoclastogenesis from Gaucher patients’ cells correlates with bone mineral density but not with Chitotriosidase. Bone [Internet]. 2017;103:262–269. doi: 10.1016/j.bone.2017.07.020. [cited 2017 Aug 18] [DOI] [PubMed] [Google Scholar]
- 4.Mistrya P.K., Liua J., Yanga M., Nottolic T., McGratha J., Jaine D., et al. Glucocerebrosidase gene-deficient mouse recapitulates gaucher disease displaying cellular and molecular dysregulation beyond the macrophage. Proc. Natl. Acad. Sci. U. S. A. 2010;107(45):19473–19478. doi: 10.1073/pnas.1003308107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sgambato J.A., Park T.S., Miller D., Panicker L.M., Sidransky E., Lun Y., et al. Gaucher disease-induced pluripotent stem cells display decreased erythroid potential and aberrant myelopoiesis. Stem Cells Transl. Med. 2015;4(8):878–886. doi: 10.5966/sctm.2014-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zancan I., Bellesso S., Costa R., Salvalaio M., Stroppiano M., Hammond C., et al. Glucocerebrosidase deficiency in zebrafish affects primary bone ossification through increased oxidative stress and reduced Wnt/β-catenin signaling. Hum Mol Genet [Internet] 2015 Mar 1;24(5):1280–1294. doi: 10.1093/hmg/ddu538. [cited 2015 Apr 1] [DOI] [PubMed] [Google Scholar]
- 7.Crivaro A., Bondar C., Mucci J.M., Ormazabal M., Feldman R.A., Delpino M.V., et al. Gaucher disease-associated alterations in mesenchymal stem cells reduce osteogenesis and favour adipogenesis processes with concomitant increased osteoclastogenesis. Mol Genet Metab [Internet]. 2020 Aug 1;130(4):274–282. doi: 10.1016/j.ymgme.2020.06.003. [cited 2020 Sep 21] [DOI] [PubMed] [Google Scholar]
- 8.Mucci J.M., Rozenfeld P. Pathogenesis of bone alterations in gaucher disease: the role of immune system. J Immunol Res. 2015:2015. doi: 10.1155/2015/192761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campeau P.M., Rafei M., Boivin M.N., Sun Y., Grabowski G.A., Galipeau J. Characterization of Gaucher disease bone marrow mesenchymal stromal cells reveals an altered inflammatory secretome. Blood. 2009;114(15):3181–3190. doi: 10.1182/blood-2009-02-205708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L., Chen K., Wan X., Wang F., Guo Z., Mo Z. NLRP3 inflammasome activation in mesenchymal stem cells inhibits osteogenic differentiation and enhances adipogenic differentiation. Biochem. Biophys. Res. Commun. 2017;484(4):871–877. doi: 10.1016/j.bbrc.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Hollak C., Maas M., Akkerman E., den Heeten A., Aerts H. Dixon quantitative chemical shift imaging is a sensitive tool for the evaluation of bone marrow responses to individualized doses of enzyme supplementation therapy in Type 1 Gaucher Disease. Blood Cells, Mol Dis [Internet]. 2001 Nov 1;27(6):1005–1012. doi: 10.1006/bcmd.2001.0474. [cited 2018 Jul 19] [DOI] [PubMed] [Google Scholar]
- 12.van Dussen L., Zimran A., Akkerman E.M., Aerts J.M.F.G., Petakov M., Elstein D., et al. Taliglucerase alfa leads to favorable bone marrow responses in patients with type I Gaucher disease. Blood Cells, Mol Dis [Internet] 2013 Mar 1;50(3):206–211. doi: 10.1016/j.bcmd.2012.11.001. [cited 2018 Jul 23] [DOI] [PubMed] [Google Scholar]
- 13.Roca Espiau M. Aspectos óseos de la enfermedad de Gaucher. Med Clin (Barc) [Internet]. 2011;137(Suppl. 1):23–31. doi: 10.1016/S0025-7753(11)70013-6. [DOI] [PubMed] [Google Scholar]
- 14.Ambele M.A., Dhanraj P., Giles R., Pepper M.S. Adipogenesis: a complex interplay of multiple molecular determinants and pathways. Int J Mol Sci [Internet] 2020 Jun 2;21(12):1–27. doi: 10.3390/ijms21124283. [cited 2022 Jun 8] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang N., Li Y., Shu T., Wang J. Vol. 13. Frontiers of Medicine; 2018. Cytokines and inflammation in adipogenesis: an updated review; pp. 314–329. Higher Education Press. [DOI] [PubMed] [Google Scholar]
- 16.Ahdjoudj S., Kaabeche K., Holy X., Fromigué O., Modrowski D., Zérath E., et al. Transforming growth factor-beta inhibits CCAAT/enhancer-binding protein expression and PPARgamma activity in unloaded bone marrow stromal cells. Exp Cell Res [Internet] 2005 Feb 1;303(1):138–147. doi: 10.1016/j.yexcr.2004.09.013. [cited 2022 Mar 3] [DOI] [PubMed] [Google Scholar]
- 17.Li S.N., Wu J.F. TGF-β/SMAD signaling regulation of mesenchymal stem cells in adipocyte commitment. Stem Cell Res Ther [Internet] 2020 Jan 29;11(1) doi: 10.1186/s13287-020-1552-y. [cited 2022 Feb 24] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nic-Can G.I., Rodas-Junco B.A., Carrillo-Cocom L.M., Zepeda-Pedreguera A., Peñaloza-Cuevas R., Aguilar-Ayala F.J., et al. Epigenetic regulation of adipogenic differentiation by histone lysine demethylation. Int J Mol Sci [Internet]. 2019 Aug 2;20(16) doi: 10.3390/ijms20163918. [cited 2022 Jun 8] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onal G., Kutlu O., Gozuacik D., Dokmeci Emre S. Lipid droplets in health and disease. Lipids Health Dis. 2017;16(1):1–15. doi: 10.1186/s12944-017-0521-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morigny P., Houssier M., Mouisel E., Langin D. Adipocyte lipolysis and insulin resistance. Biochimie. 2016;125:259–266. doi: 10.1016/j.biochi.2015.10.024. Elsevier B.V. [DOI] [PubMed] [Google Scholar]
- 21.Langeveld M., Ghauharali K.J.M., Sauerwein H.P., Ackermans M.T., Groener J.E.M., Hollak C.E.M., et al. Type I Gaucher disease, a glycosphingolipid storage disorder, is associated with insulin resistance. J Clin Endocrinol Metab [Internet]. 2008;93(3):845–851. doi: 10.1210/jc.2007-1702. [cited 2022 Jun 8. [DOI] [PubMed] [Google Scholar]
- 22.Ghauharali-van der Vlugt K., Langeveld M., Poppema A., Kuiper S., CEM Hollak, Aerts J.M., et al. Prominent increase in plasma ganglioside GM3 is associated with clinical manifestations of type I Gaucher disease. Clin Chim Acta [Internet] 2008 Mar;389(1–2):109–113. doi: 10.1016/j.cca.2007.12.001. [cited 2023 Apr 5] [DOI] [PubMed] [Google Scholar]
- 23.Ersek A., Xu K., Antonopoulos A., Butters T.D., Santo A.E., Vattakuzhi Y., et al. Glycosphingolipid synthesis inhibition limits osteoclast activation and myeloma bone disease. J. Clin. Invest. 2015;125(6):2279–2292. doi: 10.1172/JCI59987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kałużna M., Trzeciak I., Ziemnicka K., Machaczka M., Ruchała M. Endocrine and metabolic disorders in patients with Gaucher disease type 1: a review. Orphanet J Rare Dis [Internet]. 2019 Dec 2;14(1) doi: 10.1186/s13023-019-1211-5. [cited 2022 Jun 8] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panicker L.M., Miller D., Park T.S., Patel B., Azevedo J.L., Awad O., et al. Induced pluripotent stem cell model recapitulates pathologic hallmarks of Gaucher disease. Proc. Natl. Acad. Sci. U. S. A. 2012;109(44):18054–18059. doi: 10.1073/pnas.1207889109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panicker L.M., Miller D., Awad O., Bose V., Lun Y., Park T.S., et al. Gaucher iPSC-derived macrophages produce elevated levels of inflammatory mediators and serve as a new platform for therapeutic development. Stem Cells. 2014;32(9):2338–2349. doi: 10.1002/stem.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panicker L.M., Srikanth M.P., Castro-Gomes T., Miller D., Andrews N.W., Feldman R.A. Gaucher disease iPSC-derived osteoblasts have developmental and lysosomal defects that impair bone matrix deposition. Hum. Mol. Genet. 2018;27(5):811–822. doi: 10.1093/hmg/ddx442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanap A., Bhonde R., Joshi K. Mesenchymal stem cell conditioned medium ameliorates diabetic serum-induced insulin resistance in 3T3-L1 cells. Chronic Dis Transl Med. 2021 Mar 1;7(1):47–56. doi: 10.1016/j.cdtm.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gregoire F.M., Smas C.M., Sul H.S. Understanding adipocyte differentiation. Physiol Rev [Internet]. 1998 Jan 7;78(3):783–809. doi: 10.1152/physrev.1998.78.3.783. [cited 2019 Jul 26] [DOI] [PubMed] [Google Scholar]
- 30.Zhao L., Hantash B.M. TGF-β1 regulates differentiation of bone marrow mesenchymal stem cells [Internet] Vitam. Horm. 2011;87:127–141. doi: 10.1016/B978-0-12-386015-6.00042-1. 1st ed. Elsevier Inc. [DOI] [PubMed] [Google Scholar]
- 31.Lefterova M.I., Haakonsson A.K., Lazar M.A., Mandrup S. PPARγ and the global map of adipogenesis and beyond. Trends Endocrinol. Metab. 2014;25(6):293–302. doi: 10.1016/j.tem.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tamori Y., Masugi J., Nishino N., Kasuga M. Role of peroxisome proliferator-activated receptor-γ in maintenance of the characteristics of mature 3T3-L1 adipocytes. Diabetes [Internet]. 2002 Jul 1;51(7):2045–2055. doi: 10.2337/diabetes.51.7.2045. [cited 2022 Aug 4] [DOI] [PubMed] [Google Scholar]
- 33.Song Z., Xiaoli A.M., Yang F. Regulation and metabolic significance of de novo lipogenesis in adipose tissues. Nutrients [Internet]. 2018 Oct 1;10(10) doi: 10.3390/nu10101383. [cited 2022 Feb 25] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langeveld M., Aerts J.M.F.G. Glycosphingolipids and insulin resistance. Prog Lipid Res [Internet] 2009 May;48(3–4):196–205. doi: 10.1016/j.plipres.2009.03.002. [cited 2022 Jun 8] [DOI] [PubMed] [Google Scholar]
- 35.Dekker N., Van Dussen L., CEM Hollak, Overkleeft H., Scheij S., Ghauharali K., et al. Elevated plasma glucosylsphingosine in Gaucher disease : relation to phenotype, storage cell markers, and therapeutic response. 2015;118(16):118–128. doi: 10.1182/blood-2011-05-352971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murugesan V., Chuang W.L., Liu J., Lischuk A., Kacena K., Lin H., et al. Glucosylsphingosine is a key biomarker of Gaucher disease. Am J Hematol [Internet]. 2016 Nov 1;91(11):1082–1089. doi: 10.1002/ajh.24491. [cited 2022 Jun 8] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pino A.M., Rodríguez J.P. Is fatty acid composition of human bone marrow significant to bone health? Bone [Internet]. 2017;118:53–61. doi: 10.1016/j.bone.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 38.Chen Q., Shou P., Zheng C., Jiang M., Cao G., Yang Q., et al. Fate decision of mesenchymal stem cells: adipocytes or osteoblasts&quest. Cell Death Differ [Internet]. 2016;23:1128–1139. doi: 10.1038/cdd.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu L., Yin C., Zhao F., Ali A., Ma J., Qian A. Molecular Sciences Mesenchymal Stem Cells: Cell Fate Decision to Osteoblast or Adipocyte and Application in Osteoporosis Treatment. Int J Mol Sci [Internet] 2018;19(2):360. doi: 10.3390/ijms19020360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pérez Calvo J.I., Gil P.I., Giraldo Castellano P., Torralba Cabeza M.A., Civeira F., García S.L., et al. Transforming growth factor β (TGF-β) en la enfermedad de Gaucher. Resultados preliminares en un grupo de enfermos y familiares portadores y no portadores. Med. Clin. (Barc.) 2000;115(16):601–604. doi: 10.1016/s0025-7753(00)71637-x. [DOI] [PubMed] [Google Scholar]
- 41.Grabner G.F., Xie H., Schweiger M., Zechner R. Lipolysis: cellular mechanisms for lipid mobilization from fat stores. Nat Metab [Internet]. 2021 Nov 1;3(11):1445–1465. doi: 10.1038/s42255-021-00493-6. [cited 2022 Sep 1] [DOI] [PubMed] [Google Scholar]
- 42.Ralston J.C., Mutch D.M. SCD1 inhibition during 3T3-L1 adipocyte differentiation remodels triacylglycerol, diacylglycerol and phospholipid fatty acid composition. Prostaglandins Leukot. Essent. Fat. Acids. 2015 Jul 1;(98):29–37. doi: 10.1016/j.plefa.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 43.Sievert H., Krause C., Geiβler C., Grohs M., El-Gammal A.T., Wolter S., et al. Epigenetic downregulation of FASN in visceral adipose tissue of insulin resistant subjects. Exp. Clin. Endocrinol. Diabetes. 2021;129(9):674–682. doi: 10.1055/a-1150-7446. [DOI] [PubMed] [Google Scholar]
- 44.Reed M.C., Schiffer C., Heales S., Mehta A.B., Hughes D.A. Impact of sphingolipids on osteoblast and osteoclast activity in Gaucher disease. Mol. Genet. Metab. 2018;124(4):278–286. doi: 10.1016/j.ymgme.2018.06.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.