Abstract
While immunotherapy for pediatric cancer has made great strides in recent decades, including the FDA approval of agents such as dinutuximab and tisgenlecleucel, these successes have rarely impacted children with pediatric central nervous system (CNS) tumors. As our understanding of the biological underpinnings of these tumors evolves, new immunotherapeutics are undergoing rapid clinical translation specifically designed for children with CNS tumors. Most recently, there have been notable clinical successes with oncolytic viruses, vaccines, adoptive cellular therapy, and immune checkpoint inhibition. In this article, the immunotherapy working group of the Pacific Pediatric Neuro-Oncology Consortium (PNOC) reviews the current and future state of immunotherapeutic CNS clinical trials with a focus on clinical trial development. Based on recent therapeutic trials, we discuss unique immunotherapy clinical trial challenges, including toxicity considerations, disease assessment, and correlative studies. Combinatorial strategies and future directions will be addressed. Through internationally collaborative efforts and consortia, we aim to direct this promising field of immuno-oncology to the next frontier of successful application against pediatric CNS tumors.
Keywords: Immunotherapy, Pediatric cancer, Brain tumor, Central nervous system tumor
Introduction
The immune system has long garnered attention as a potentially powerful ally in the fight against cancer. Recent decades have seen significant advances in the clinical implementation of immunotherapy for a wide range of tumors, including pediatric cancers. However, these advances are only beginning to reach children with central nervous system (CNS) tumors, the deadliest pediatric cancers. The initial clinical implementation of immunotherapeutics against CNS tumors has been hampered by unique considerations forced by the clinical, biologic, and immunosuppressive features of solid tumors in the brain and spine. This following review from the immunotherapy working group within the Pacific Pediatric Neuro-Oncology Consortium (PNOC) and Children's Brain Tumor Network (CBTN) focuses on highlighting themes on translation of immunotherapies to clinical trials for pediatric CNS tumors. We review recent trials and advances in oncolytic viruses, vaccines, adoptive cell therapy, and checkpoint inhibition, followed by a discussion of important aspects in trial development, including toxicity, correlative studies, and combinatorial opportunities.
The immunotherapy landscape in pediatric CNS tumors
Oncolytic viruses
Oncolytic viruses (OVs) represent a unique type of anticancer agents at the interface of biological- and immunotherapies. The field achieved a milestone in 2015 with the approval by the US Food and Drug Administration (FDA) of the herpes simplex virus 1 (HSV-1) expressing granulocyte-macrophage colony-stimulating factor (GM-CSF) named T-VEC (talimogene laherparepvec) for the treatment of advanced melanoma [1]. More recently Teserpaturev, a triple-mutated HSV-1 OV, has been approved in Japan for the treatment of malignant glioma in adults [2]. OVs employ either naturally occurring viruses that take advantage of the molecular makeup of the cancer cells or viruses modified in the laboratory to achieve cancer specificity. OVs have the ability to convert tumors from immunologically ‘cold’ to ‘hot’ through induction of proinflammatory pathways within the tumor microenvironment (TME) [3]. This characteristic makes them very attractive for treating pediatric CNS tumors considering their low tumor mutational burden (TMB) and paucity of infiltrating immune cells. In preclinical studies, adenoviruses and herpes viruses are the most extensively studied, demonstrating tolerability and efficacy in different models including atypical teratoid rhabdoid tumor (ATRT), diffuse intrinsic pontine glioma (DIPG), high-grade glioma (HGG), and medulloblastoma [4], [5], [6], [7]. Most of these approaches utilize intratumoral administration rather than systemic, with viral carriers such as neural or mesenchymal cells also evaluated [8]. Recent trials using either the HSV-1 G207 for patients with recurrent or progressive HGG (NCT02457845) [9] or DNX-2401 for newly diagnosed DIPG (NCT03178032) [10] reported safety and a degree of efficacy across both studies. Additional viruses are also being used in ongoing and recent trials including modified measles virus (PNOC005, NCT02962167; Table 1), as well as modified polio and rhinovirus viruses (PVSRIPO, NCT03043391). Refinements aimed at enhanced efficacy may be achieved through combinatorial therapeutic partners, repetitive intratumoral dosing regimens, or improved potency of the virus (e.g. armed with ligands that further drive the immune system) [11].
Table 1.
Immune based clinical trials through the Pacific Pediatric Neuro-Oncology Consortium (PNOC).
| Trial # | Study Name | NCT |
|---|---|---|
| PNOC005 | A phase 1 study of modified measles virus for the treatment of children and young adults with recurrent medulloblastoma or recurrent atypical teratoid rhabdoid tumors (ATRT) | NCT02962167 |
| PNOC007 | H3.3K27M Specific Peptide Vaccine Combined with poly-ICLC and Nivolumab for the Treatment of newly diagnosed HLA-A2 (02:01)+ H3.3K27M Positive Diffuse Intrinsic Pontine Glioma (DIPG) and newly diagnosed HLA-A2 (02:01)+ H3.3K27M Positive Midline Gliomas | NCT02960230[14] |
| PNOC013 | A Safety and Pharmacokinetic Study of Single Agent REGN2810 in Pediatric Patients with Relapsed or Refractory Solid or Central Nervous System (CNS) Tumors and a Safety and Efficacy Trial of REGN2810 in Combination with Radiotherapy in Pediatric Patients | NCT03690869 |
| PNOC018 | Genetically Modified Cells (KIND T Cells) for the Treatment of HLA-A*0201-Positive Patients With H3.3K27M-Mutated Glioma | NCT05478837 |
| PNOC019 | A Randomized, Double-Blinded, Pilot Trial of Neoadjuvant Checkpoint Inhibition followed by Combination Adjuvant Checkpoint Inhibition in Children and Young Adults with Recurrent or Progressive High Grade Glioma (HGG) | NCT04323046 |
| PNOC020 | A Study of RNA-lipid Particle (RNA-LP) Vaccines for Newly Diagnosed Pediatric High-Grade Gliomas (pHGG) and Adult Glioblastoma (GBM) | NCT04573140 |
| PNOC025 | A Phase 1 Study of Magrolimab in Children and Adults with Recurrent or Progressive Malignant Brain Tumors | NCT05169944 |
| PNOC028 | A Phase 1 Study of Intra-Tumoral Injections of Ex Vivo Expanded Natural Killer Cells in Children and Young Adults with Recurrent or Progressive Supratentorial Malignant Brain Tumors | Not available |
| PNOC029 | Nivolumab and DAY101 for the treatment of newly diagnosed or recurrent craniopharyngioma in children and young adults | NCT05465174 |
Vaccine therapies
Cancer vaccines have had a long history since Coley's toxins in the late 1800′s but have yet to be fully realized against CNS tumors. Peptide vaccines have been traditionally employed as a source of antigenic material to induce response in malignant CNS tumors, but tumor specific antigen targeting remains challenging. EGFRVIII, a tumor specific antigen, has been utilized as a peptide target (conjugated to keyhole limpet hemocyanin) in adult glioblastoma (GBM) but did not meet efficacy goals in a phase III study [12]. Multiple overexpressed antigens (e.g. survivin, EphA2, interleukin (IL)-13 receptor-α2, YKL-40, and gp100) are also being leveraged as therapeutic targets with promising activity [13]. PNOC utilized an H3.3K27M specific peptide vaccine coupled with polyinosinic:polycytidylic acid (poly(I:C)) for HLA-A2 restricted patients with DMG (PNOC007, NCT02960230; Table 1). While overall survival was similar to historical controls, a subset of patients with expansion of vaccine-induced specific cytotoxic T cells had a superior median survival (>16 months) [14]. This report included characterization of peripheral PD-1+ CD8+ T cells that were exhausted following the vaccine, suggesting potential efficacy from a combination with anti-PD-1 therapy and spurring the addition of nivolumab to the clinical trial (PNOC007, NCT02960230; Table 1). Alternatively, some studies have used autologous cellular vaccines targeting total tumor material using irradiated cells [15], cell lysate [16], and whole tumor mRNA [17]. Vaccine trials using mRNA loaded dendritic cells are underway in medulloblastoma (Re-MATCH trial; NCT01326104), HGG (ACTION trial; NCT03334305), and DIPG (NCT03396575). While promising, HLA-restricted epitopes make development of peptide vaccines for the general population challenging, which can be especially limiting for relatively rare diseases such as pediatric CNS tumors. However, mRNA vaccines in lipid-nanoparticles could be available to all patient haplotypes, tailored for flexibility in commercialization, and available for rapid deployment. These systems have been piloted against adult GBM, but are now underway for children with HGG (PNOC020, NCT04573140; Table 1).
Adoptive cellular therapy
Adoptive cellular therapy (ACT) has become one of the fastest-growing fields under investigation against pediatric CNS tumors. ACT involves ex vivo manipulation of immune cells that are then administered to a patient for anti-tumor effect. The majority of cellular therapy has primarily focused on T cell manipulation for anti-cancer activity, though similar technology can be applied to other effector cells, such as NK cells [18] and macrophages [19]. Manipulation can include simple expansion of the immune cells, stimulation with a particular tumor-associated target, or modification to introduce a T cell receptor (TCR) or chimeric antigen receptor (CAR) on the cell surface. Infusion of expanded T and NK cells for a cohort of relapsed medulloblastoma patients was initially published over thirty years ago [20], with newer expansion techniques being utilized in recent [21] and upcoming clinical trials (PNOC028, registration pending; Table 1). T cells expanded and selected for the targets WT1, PRAME, and survivin are also currently being tested (REMIND trial, NCT03652545) [22]. Building on the momentum of six FDA-approved CAR T cell therapies for hematologic malignancies, CAR T cells are now being tested against pediatric CNS tumors with exciting initial reports. CAR T cells targeting HER2 were the first to be intracranially delivered and reported for pediatric CNS tumor patients (BrainChild-01, NCT03500991) [23], with additional ongoing trials targeting EGFR806 (BrainChild-02, NCT03638167), B7-H3 (Brainchild-03, NCT04185038) [24], GD2 (NCT04196413, GAIL-B; NCT04099797) [25], IL-13RA2 (NCT04510051), and the first multi-antigen targeting trial against pediatric CNS tumors (BrainChild-04, NCT05768880). Preliminary reports from the first patients treated with B7-H3-, GD2-, and HER2-specific CAR T cells have shown that it is feasible to manufacture and deliver fractionated doses that allow for repeated intracranial infusions and can induce locoregional immune activation [23], [24], [25]. B7-H3- and GD2-specific CAR T cells may also be demonstrating early evidence of efficacy against both pontine and spinal DMG, though, if there has been clinical benefit, it has not been universal [24,25]. Ultimately, these initial studies are still ongoing and as more patients are treated at higher dose levels there may be a clearer signal of clinical efficacy. Regardless, the preliminary feasibility of these trials has invigorated international efforts to build the next generation of these therapies that will be directed against novel targets, incorporate multi-antigen targeting, and feature secondary genetic modifications to boost efficacy and chemotaxis. For example, a novel targeting approach will be featured in an upcoming trial using TCR targeting H3K27M, the pathognomonic mutation in DMG (PNOC018, NCT05478837; Table 1). Future trials will also look to optimize delivery. While initial studies are using systemic and intracranial dosing, the optimal route of delivery remains an ongoing question in the field. When given intravenously, CAR T cells certainly can traffic to the brain; however, this approach likely requires larger effector cell doses and increases the risk of systemic toxicities [25,26]. Even if intracranial delivery is determined to be superior to systemic dosing, there is no consensus on where in the CNS ACT should be delivered. It is possible delivery will depend on location and extent of disease, as well as potentially tumor biology-driven microenvironmental considerations.
Checkpoint blockade and immunoregulatory antibodies
Immune checkpoint inhibitors (ICIs) are a class of humanized monoclonal antibodies that target inhibitory receptors and ligands (i.e., PD-1, PD-L1 and CTLA-4) expressed on tumor cells as well as T lymphocytes and antigen presenting cells to restore anti-tumor immune responses and allow for persistent activation of T cells. ICIs have been investigated in pediatric CNS tumors due to the successful results seen in adult patients with metastatic melanoma, non-small cell lung cancer and renal cell carcinoma, but have demonstrated limited efficacy except in patients with congenital mismatch repair deficiency (PBTC-045, NCT02359565) [27], [28], [29], [30]. Indeed, replication repair deficient pediatric HGG tumors treated with ICIs had objective responses and a promising median survival of 2 years when compared to historical controls with median post-relapse survival of only 2.6 months [31]. Alternative dosing schedules, such as neo-adjuvant approaches (PNOC019, NCT04323046; Table 1) and immunotherapy combinations (PNOC013, NCT03690869 and PNOC029, NCT05465174; Table 1), continue to be investigated. In addition, immune modulatory antibodies targeting cells beyond T cells are actively being explored, such as magrolimab, an anti-CD47 antibody that disrupts the “don't eat me” signal to macrophages (PNOC025, NCT05169944; Table 1), and CD40-agonists that stimulate dendritic cells, lymphocytes and macrophages (NCT03389802). Ultimately, the efficacy of antibody-based therapy may be hindered by the blood-brain barrier and may require additional disruption, such as focused ultrasound or intratumoral administration.
Toxicity considerations
Systemic inflammation
Immune-based therapies have the potential to induce systemic inflammation from activation of different facets of the immune system. This is well described in the trials of CAR T cell therapy and bi-specific T cell engagers for hematologic malignancies, in which patients can develop symptoms ranging from isolated fever to those mirroring macrophage activating syndrome/hemophagocytic lymphohistiocytosis [32], [33], [34]. These adverse events have been termed cytokine release syndrome (CRS) and are driven by elevated levels of circulating cytokines, such as IL-6. Similarly, immune-effector cell associated neurotoxicity syndrome (ICANS) is an acute constellation of neurologic symptoms that most often includes confusion, headaches, and language disturbances, but can involve encephalopathy, seizures, and fatal cerebral edema [35]. In a prospective study aiming to assess the occurrence of CRS and ICANS in CD19-directed CAR T cell therapy, 44% (19/43) of patients experienced neurotoxicity, and ICANS correlated with severity of CRS, prior abnormal CNS neuroimaging findings, and higher systemic (but not CSF) CAR T cell numbers [36]. The study suggests that ICANS is the result of systemic inflammation affecting the brain. CRS and ICANS do not seem to occur nearly as frequently in intracranially delivered CAR T cells for CNS tumors [[23], [24], [25],37], which may have been anticipated due to the lack of systemic inflammation from locoregional CNS delivery.
ICIs have been well tolerated in pediatric CNS tumor clinical trials thus far, but ICIs are also associated with systemic immune-related adverse events (irAEs). Inhibition of immune checkpoints, necessary for balanced immune homeostasis, can lead to activation of autoreactive T cells and resultant autoimmune related activity [38], [39], [40]. The exact mechanism that leads to irAEs is unknown but is thought to be secondary to ICIs reduction of T cell self-tolerance and an increase in autoantibody production as well as inflammatory cytokines. IrAEs can occur in any organ system, but most commonly present in the gastrointestinal tract, skin, lungs, endocrine glands, and liver [41]. Rare fatal irAEs include autoimmune cardiac and neurologic toxicities. Severe irAEs occur more commonly when anti-PD1 monoclonal antibody is given in combination with anti-CTLA4 monoclonal antibody rather than as monotherapies [40,42]. While several studies in melanoma and small cell lung cancer have postulated a positive association with the development of irAEs and anti-tumor responses using ICIs, this remains controversial and not universally observed across all tumor types [43].
Local inflammation
Pseudoprogression is a term frequently used in immunotherapy to describe transient local inflammatory changes at the site of CNS tumors following therapy. CAR T cells targeting tumors in the brain and spinal cord have the potential to cause local inflammation at the site of the tumor as T cells are activated, with subsequent neurologic sequelae. Preclinically, this raised concerns with GD2-directed CAR T cells in murine models of DMG where a small portion of mice with pontine tumors and all mice with thalamic tumors died secondary to localized swelling, hydrocephalus, and herniation [44]. Early clinical data suggests there may be a risk of worsening existing neurologic deficits, which is often self-limiting or responds to anti-inflammatory interventions [23], [24], [25]. Localized inflammation in the tumor in the setting of CAR T cells for CNS tumors has recently been termed tumor inflammation-associated neurotoxicity (TIAN) [45]. There are relevant questions about how to distinguish these symptoms from clinical progression or if truly localized neurologic toxicity composes a distinct constellation that deserves unique consideration, as with CRS and ICANS. On-target/off-tumor effects from CAR T cells may also produce local inflammation in the brain, and must be carefully assessed, although additional biopsy or post-mortem analysis may be necessary for full characterization.
The use of ICIs have also been associated with symptoms of local tumor inflammation or pseudoprogression due to infiltration of activated immune cells in a number of cancers, such as metastatic melanoma and non-small cell lung cancer, but the incidence in primary CNS tumors is unknown [46]. Patients with DIPG treated with the PD-1 inhibitor pembrolizumab on the PBT-045 trial experienced new/increased neurological symptoms and decreased progression-free and overall survival compared to historical controls [47], causing their subsequent exclusion from the trial. The incidence in other CNS tumors is less well described.
Similarly, data on local inflammation after treatment with oncolytic viruses is still scarce. Pseudoprogression was observed in several patients treated with the G207 virus (NCT02457845), suggesting local inflammation that was confirmed on paired samples pre- and post-treatment with increased tumor infiltration [9]. In DIPG patients treated with DNX-2401 (NCT03178032), local inflammation after treatment was not specifically noted, but two patients developed either hemiparesis or tetraparesis following biopsy and therapy [10]. Since gadolinium was not used in the imaging protocol, pseudoprogression could not be analyzed in this trial, although access to a paired pre-post biopsy also showed increased immune infiltration in the tumor.
Approach to toxicity management
As the initial wave of phase 1 adoptive cellular therapy trials for children with CNS tumors is still ongoing, our understanding of irAEs is evolving. The treatment for severe irAEs includes suspending treatment such as ICIs and beginning corticosteroid treatment. Additional immunomodulators may be necessary if clinical symptoms do not improve with steroids. Specific immunomodulators will be recommended based on the irAEs experienced, e.g. immune-related-colitis can be treated with infliximab and immune-related-hepatitis with agents like mycophenolate mofetil or budesonide.
Bevacizumab can also be used as an alternative to corticosteroids to treat pseudoprogression, thereby limiting side effects and direct inhibition of lymphocytes. The relationship to recent surgery, potential for upcoming urgent surgery, and risk of hemorrhage may be relevant to a discussion about the role and timing of bevacizumab. Elevated intracranial pressure may need to be relieved through pharmacologic or neurosurgical interventions and these discussions may be altered based on the presence and type of CNS catheter that a patient may have in place.
As noted above, preliminary evidence of children receiving CAR T cells for CNS tumors, in particular for those receiving intracranial dosing, suggests that severe CRS may not be a risk. Notably, different research teams may capture self-resolving metronomic fever immediately following CAR T cell infusions individually as fever or as grade 1 CRS. However, as individual cellular therapies may have a distinct risk of trafficking into the serum and inducing systemic toxicity, either due to the target or the CAR construction, vigilance in monitoring for CRS will continue to be important. Treatment of CRS may include anti-pyrectics, inhibition of IL-6 receptor, steroids, and supportive care as reviewed in the EBMT/EHA CAR-T Cell Handbook [48] Lymphodepleting chemotherapy such as cyclophosphamide and fludarabine used in preparation for adoptive cellular therapy has been linked to increased incidence of CRS and neurotoxicity in the past, however further investigation has shown this may be more related to the degree of systemic CAR T cell proliferation of specific products rather than conditioning regimens [49], [50], [51]. Nevertheless, there is inconsistency in the use of lymphodepleting chemotherapy preparative regimens in CAR T cell trials for CNS tumors, with many trials avoiding their use.
Clinical trials of CAR T cells for pediatric CNS tumors may also have different approaches to preemptive management, including required anti-epileptic medications or mandated inpatient admissions to the oncology team, transplant team, or intensive care unit (ICU). Therefore, acute management will be impacted by the level of baseline care. In general, detailed neurologic exams are critical and early neurology team involvement for toxicities is recommended. Cooperative groups are now working on consensus guidelines for management and a better understanding of the pathophysiology of these neurological findings that are most likely distinct from ICANS.
Disease assessment and correlative studies
A major obstacle to clinical trials using immune-based therapeutics is accurately identifying disease response. Imaging in this context can be challenging to interpret. As first described in the setting of radiotherapy [52], pseudoprogression describes increased contrast-enhancement and lesion size following therapy, but ultimately reflects only transient changes that remain stable or improve over time without a change in therapy. Tumor directed immunotherapy can lead to pseudoprogression and other atypical radiographic patterns which can be confused with true tumor progression or treatment failure. In addition, clinical symptoms from tumor pseudoprogression mimic true progression, further muddying the picture in real time. Additionally, anti-inflammatory agents given for symptom management following immunotherapy may affect intra- or peri-tumoral inflammation which decreases tumor bulk on neuroimaging rather than classic disease response.
Traditional response criteria based on conventional imaging such as magnetic resonance imaging (MRI) is not reliably predictive of immunotherapeutic outcomes [46,[53], [54], [55]]. This can lead to premature discontinuation of immunotherapy as well as inaccurate conclusions from new immune mediated strategies. Therefore, novel imaging techniques and serially evaluable biomarkers are needed to better assess immunotherapeutic efficacy and safety, as well as tumor evolution and immune response.
Specimens for tumor and immune monitoring
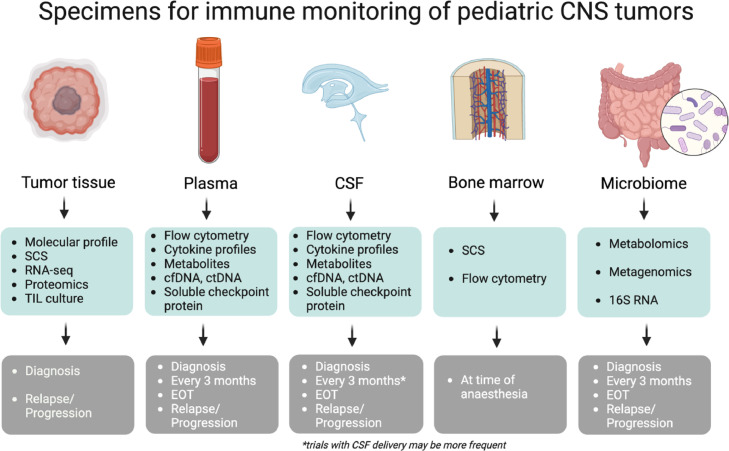
To get a better understanding of the immunological changes and select possible predictive markers for response to immunotherapy, multiple techniques can be applied on several specimens to enrich the clinical trial setting (Fig. 1). Molecular profiling of the tumor at diagnosis will offer unique insight into the immune composition at diagnosis, and we propose additional subsequent monitoring of immune changes through serial sampling of other compartments including peripheral blood, CSF, bone marrow, and stool (Fig. 1). These modalities are aimed at harm minimization whilst also providing clinically relevant information in real-time throughout a patient's disease journey.
Fig. 1.
Specimens for immune monitoring of pediatric CNS tumors. Characterization of the immune landscape from multiple compartments for application in clinical trials for pediatric CNS malignancies. Longitudinal liquid biopsies (blood, tumor tissue, CSF), microbiome and bone marrow can be utilized for a comprehensive evaluation with the suggested techniques. CSF: Cerebral Spinal Fluid; SCS: Single Cell Sequencing (different techniques available); ctDNA: circulating tumor DNA; cfDNA: circulating free DNA; RNA-seq: RNA-sequencing (bulk); TIL: tumor infiltrating lymphocytes; EOT: End Of Treatment. Created with BioRender.com.
Flow cytometry
Unlike hematological malignancies, flow cytometry is not frequently used for CNS malignancies. However, this technique has been explored in fresh tissue to characterize immune cell infiltration across multiple pediatric CNS malignancies [56,57]. To fully appreciate the effect of a therapy on the tumor and its TME, repetitive sampling of the tumor could be performed. However, repetitive sampling is not standard practice and is invasive for the patient. Studying more accessible immune compartments, such as blood, CSF, or bone marrow, could provide valuable information on changes in the immune landscape and functional dynamics in a patient with a CNS tumor. Ideally, this correlates with the TME and/or disease response or can discriminate between real progression and pseudoprogression. In a study for adult brain tumor patients, flow cytometry and CyTOF on blood samples showed an increase of myeloid derived suppressor cells (MDSCs), but not regulatory T cells, for glioblastoma patients compared to other CNS tumor entities [58]. Higher intratumoral MDSCs correlated with poorer survival, underpinning the MDSC as an important target for immunotherapy [58]. Furthermore, they demonstrated that the immune signature in the blood changed during treatment [58]. More recently CNS CAR T cell trials have utilized flow cytometry on patient blood and CSF to identify circulating CAR T cells [23], [24], [25].
The role of the bone marrow compartment and its interplay with CNS malignancies needs to be further clarified. An interesting finding by Chongsathidkiet and colleagues was the accumulation of T cells in the bone marrow of patients with glioblastoma, in combination with low CD4 levels systemically [59]. This study raises the question if sequestration of T cells in bone marrow can be qualified as a T cell dysfunction, and therefore a possible target for therapy. Longitudinal flow cytometry analysis of blood/bone marrow and CSF will not only provide information on disease progression but could also be used as a tool in predicting immune treatment responsiveness. Multiple platforms can be used to design brain tumor specific flow cytometry panels, evaluating subsets and functionality of T/B/NK and myeloid cells.
Single cell sequencing
The unique advantage of single cell RNA sequencing (scRNA-seq) over bulk RNA-seq, is the ability to profile the transcriptome of thousands of individual cells. This technique has developed tremendously over recent years, resulting in single cell sequencing (SCS) “atlases” for several pediatric CNS tumors, and to a lesser extent, its microenvironment. Detailed scRNA-seq studies have been useful for appreciating the heterogeneity and functional differences in brain residing macrophages. A recent paper of Liu et al where they dissected a cohort of H3-K27M DMG patients by SCS, including the TME, revealed age dependent differences in the immune infiltrate of DMGs [60]. They found higher expression of the TAM-secreted ligand OSM in adult TAMs, with higher corresponding receptor OSMR in adult tumor cells, indicating immune-mediated engagement of a previously validated pathway [60]. The recent report on GD2-directed CAR T cell therapy for DMG performed scRNA-seq on blood and CSF of treated patients, where they identified distinct myeloid populations in both compartments, as well as during peak inflammation and late in course at the time of progression [25]. Moreover, scRNA-seq profiling has also been performed on CSF and blood to monitor the dynamic changes of leukocytes and myeloid cells during treatment for other brain metastases and non-oncologic disorders such as multiple sclerosis [61,62]. Proteomics will be the next step to validate the findings of SCS on a protein level. Further characterization of the TME, such as expression profiles and T cell surface receptors for various pediatric brain tumors, will aid in understanding the interaction between immune cells and tumors, paving the way for the development of novel immunotherapy strategies.
Tumor free markers of immunotherapy selection and response
Liquid biopsy
Considerable recent efforts have focused on the discovery and development of liquid biopsy-based techniques to identify biomarkers used to guide treatment recommendation and response. As it stands, circulating tumor-DNA (ctDNA) [63,64], methylated-ctDNA [65], circulating tumor micro-RNA (circulating miRNAs) [66,67], circulating tumor exosomal-DNA (exoDNA) [68], circulating tumor cells (CTCs) [69], and soluble proteins have been identified in the blood, CSF and urine of patients. While these have been studied to identify tumor markers, they are yet to be considered standard clinical approaches and are generally conducted as research. Below we describe tumor-related biomarkers that may aid in the development and optimization of immunotherapies for the treatment of CNS malignancies.
Tumor free nucleic acid biomarkers of pediatric CNS tumors
Analysis of circulating genomic material is an exciting frontier for the non-invasive tumor genotyping of pediatric CNS cancer. Targeted approaches such as nanopore sequencing of ultra-short ctDNA fragments and digital droplet PCR (ddPCR) are the most commonplace. These methods have been used to identify hallmark mutations in children with HGGs (such as H3K27M in DMG) [70], [71], [72], as predictive markers of disease response to investigational agents [73], and to track treatment response in medulloblastoma [74]. These properties make cfDNA biomarkers attractive for monitoring treatment response and potentially for the selection of the most appropriate immunooncology-based therapies. Next generation sequencing (FDA approved Memorial Sloan Kettering-Integrated Molecular Profiling of Actionable Cancer Targets – MSK-IMPACT) have also recently been used to detect somatic alterations in the CSF of 30/64 pediatric CNS cancer patients, and was found to be strongly positively associated with the presence of disseminated disease [75]. Although these studies did not inform treatment, they do highlight the potential of such approaches to be used to characterize the somatic alterations that give rise to these cancers, and, to stratify patients to relevant immunological treatments. Finally, low-coverage whole genome analysis from serially collected CSF of medulloblastoma patients was recently shown to identify measurable residual disease and predict patient outcome [64]. These techniques will continue to be evaluated across multiple tumor types and incorporated into upcoming clinical trials to evaluate applicability in discerning treatment response in the context of immune based therapies.
Protein inflammatory marker analysis in clinical trials
Plasma soluble tumor checkpoint proteins, including soluble PD-L1 (sPD-L1) [76], and soluble immune cell checkpoints such as soluble CTLA-4 from Tregs and soluble PD-1 from CD4+ T cells [77], have been identified in the blood and CSF of patients. This may act as a surrogate to genomics for the selection and/or monitoring of efficacy for pediatric brain tumor patients treated with immunotherapies. Serum and CSF levels of sPD-L1 in adults with HGG are significantly elevated compared to healthy controls, with CSF PD-L1 levels more indicative of aggressive tumor features [76].
As many ACT trials for children with CNS tumors incorporate locoregional delivery, enrolled patients on these studies have CNS catheters such as an Ommaya in place. Beyond allowing for a more localized delivery and the potential for less systemic toxicity, this neurosurgical technology also allows for frequent CSF sampling with minimal invasiveness. These precious biospecimens may be integral to future paradigms of disease assessment and tumor evolution, especially considering the known limitations of neuroimaging in the setting of possible pseudoprogression following immunotherapy [78]. Serial longitudinal chemokine/cytokine analysis has already been found to be feasible [23]. On Seattle Children's BrainChild-01 and -03 trials, delivering locoregional HER2 and B7-H3 CAR T cells, respectively, to children with recurrent/refractory CNS tumors and DIPG/DMG revealed elevations of critical analytes via multiplex assay [23,24]. For example, CXCL10, a type 1 CXC chemokine ligand that has been reported to improve effector cell migration into gliomas, was elevated post infusion in the CSF [23,24,79]. “IFN-γ” was also elevated, which is notable as IFN-γ can activate macrophages and promote cytotoxic T cell function [80], [81], [82]. However, there were only limited changes in serum chemokine/cytokine concentrations, correlating with the lack of systemic toxicities. Similar results were seen in Stanford's GD2-directed CAR initial report, where plasma cytokines such as IL-6 were elevated following intravenous delivery of CAR, but not after doses delivered into the CSF [25]. Similar to the Seattle trials, CSF levels of proinflammatory cytokines such as IFN-γ were elevated in the CSF following intracerebroventricular doses [25]. The understanding of the role of chemokines and cytokines of particular CAR T cell activity may fuel the next wave of cellular engineering, in which secretion of IL-12, IL-15, IL-18, and others may improve chemotaxis and cytotoxicity [83], [84], [85]. Beyond CAR T, other immune based clinical trials for pediatric CNS tumors have measured serum cytokines, with varied fluctuations depending on the type of therapy [86], [87], [88], and suggest continued monitoring in the serum will also be important for systemically delivered therapies.
Another emerging method of detection includes whole proteome analysis or targeted proteomics of patient biospecimens, such as CSF and serum. In BrainChild-03, serial targeted proteomics was piloted and showed feasibility in detecting the ACT target, B7-H3, in the CSF and serum of treated patients [24]. It was also possible to capture modulation of several other potentially important immunoregulatory peptides, such as BCL10, CXCL13, HAVCR2 (TIM-3), ICOSLG (ICOS ligand), PDCD1LG2 (PD-L2), TNF receptor super family member 14 (TNFRSF14), and VCAM-1 [24]. Using parallel platforms to validate findings of locoregionally treated patients, particularly in the CSF, may direct the field toward establishing early signs of treatment response and tumor evolution.
Future work will hopefully include both serial circulating tumor DNA and serial circulating proteomic assessments as they have already proven feasible in other clinical settings [64,89]. An ideal state may be that serial circulating chemokines/cytokines, genomics, and proteomics can be triangulated to ensure biospecimens are optimally used to craft the best potential treatment and allow iterations to optimize future therapies. Ultimately, immuno-neuro-oncology working groups should aim to align these assays to allow for analysis across trials and collaboratively build towards more effective immune based therapies.
Microbiome
The microbiome describes the totality of bacterial, fungal, and viral species in the body of which most reside in the gut [90]. The microbiome has a strong interaction with the immune system and can regulate T cell immunity in a variety of diseases, including malignancies. Although the composition of the microbiome varies greatly within individuals and among populations [91], its prognostic value and even therapeutic applications for patients with cancer has become evident over the last decade. In humans, profound correlations were found between immunotherapy responses and the microbiome composition, particularly for ICI treatment [92,93]. In addition, dysbiosis and treatment response was reported for a CAR T trial in adult B-cell malignancies, where the use of antibiotics four weeks before the start of treatment correlated negatively with outcome and positively with neurotoxicity [94]. As a next step, studies with feces transplantation for patients resistant to ICI are now well underway and have started to report positive results in a small cohort of patients [95].
Little is known about the relationship between the microbiome and tumor pathogenesis in pediatric brain tumors, or the predictive value of therapy response. However, it is thought that there is strong, two-way biochemical signaling between the microbiome and the CNS. In a group of meningioma and HGG patients, less diversity of the microbial ecosystem was found in brain tumor patients compared to healthy controls, but no significant differences between the meningioma and glioma groups [96]. Brain tumor groups lacked small chain fatty acids (SCFA)-producing probiotics. This metabolite is thought to play an important role in the so-called gut-brain-axis, reducing secretion of proinflammatory cytokines, and inducing FoxP3 regulation and IL-10 production [97]. Recently, the first data presented on DIPGs and the microbiome revealed a dysbiosis at diagnosis, where the Firmicutes/Bacteroidetes ratio identified certain bacterial family members were associated with reduced progression free survival [98].
Incorporating longitudinal feces collection, and ideally metabolites in plasma, into immunotherapy clinical trials for pediatric brain tumors can generate biomarkers to predict treatment response and provide possible targets for microbiome manipulation. One of the major challenges will be to build uniformity in terms of sample collection, sample processing, data analysis and data presentation [99]. We propose four time points for the collection of the microbiome: at diagnosis, during radiotherapy (if applicable), during chemotherapy (if applicable), and end of treatment/at time of progression. 16S RNA is the most common and least expensive mode of analysis, with a reliable reference library and therefore the most feasible. However, DNA metagenomics/metabolomics (shotgun) will generate more information and possibly new biomarkers to correlate to treatment effect or toxicity outcome.
Combination therapy and future studies
Despite the major advances over the past several decades, several hurdles still remain for effective immune activation in the CNS. Ongoing preclinical studies and upcoming clinical trials are aimed at overcoming these roadblocks. Here we review several of these roadblocks and active approaches toward them including specifically the cold TME, T cell exhaustion, and tumor antigen heterogeneity.
Warming the cold tumor microenvironment of pediatric CNS tumors
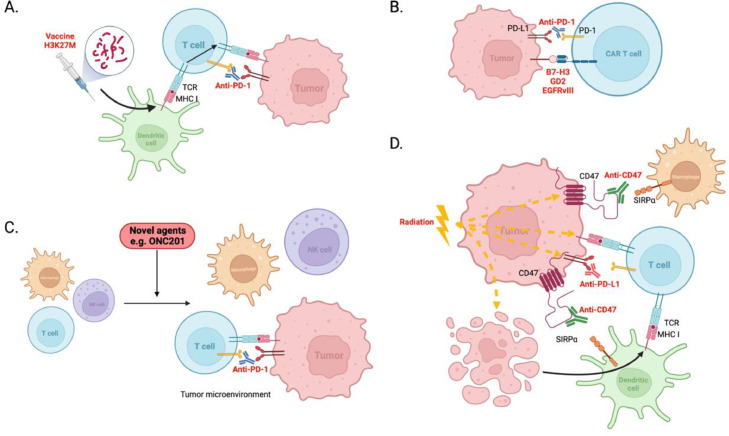
Many patients fail to respond to therapies designed to harness their own immune system in the fight against their cancer, particularly so for pediatric patients diagnosed with CNS malignancies. This is in part because CNS tumors harbor an immunologically cold TME, characterized by few tumor infiltrating lymphocytes (TILs), and featuring abundant immunosuppressive myeloid cells (such as microglia and macrophages) and secrete immunosuppressive cytokines [14,[100], [101], [102]]. To circumvent the cold TME, combination therapies that target the tumor and the TME is a potentially beneficial approach. As discussed above, ICIs can help promote an immune response in T cells, and have been actively studied as combinatorial approaches with vaccines [103,104] (PNOC007; NCT02960230, Table 1), and CAR T cells [105], (Fig. 2A and B).
Fig. 2.
Potential strategies to warm the cold TME in central nervous system tumors. A. Cancer vaccines, including H3K27M peptide vaccine, activates and guides immune cells to the tumor. Combination with ICIs prevent activated T cells from becoming exhausted and promotes their pro-tumor effects. B. CAR T cell therapy, such as B7-H3-, GD2- and EGFRvIII-directed CAR T cells in combination with ICI to reduce CAR T cell exhaustion and potentiate pro-tumor activity. C. Novel agents such as ONC201 recruit immune cells including T cells, NK cells and macrophages into the tumor microenvironment promoting immunogenic tumor cell death. Combination with ICIs may potentiate the immunological effects of ONC201. D. Radiation therapy upregulates surface expression of immune modulatory and checkpoint proteins including CD47 and PD-L1 on tumors, priming for combination with ICI and magrolimab. Radiation-induced cell death promotes the uptake of tumor-associated antigens by dendritic cells that are presented to T cells. Radiation therapy increases MHC I expression on tumor cells, further suggesting a benefit to combine with other immunotherapies. Created with BioRender.com.
Another way to warm the TME (and promote the activity of ICIs) is to use anticancer drugs that modulate the tumor and its surrounding TME (Fig. 2C). Preliminary results for the brain-penetrant, small molecule, ‘ONC201’, used in the treatment of DMG, shows that it is not only an anti-DMG therapy [106], but also promotes recruitment of immune cells into the tumor in other cancer types [107,108]. Immune cells shown to be recruited include CD3+ T cells, including CD8+ T cells in a patient with mantle cell lymphoma and murine colorectal cancers, and NK cells infiltrating in colorectal cancers [107,108], suggesting a combination with ICIs might be a promising approach. Indeed, combining ONC201 with an anti-PD-1 monoclonal antibody enhanced tumor regression in mice harboring colorectal carcinomas compared to anti-PD-1 monotherapy [108].
Radiotherapy is one of the primary treatments across different CNS tumors and has been shown to have both a proinflammatory effect and an immune suppressive effect, including release of immunosuppressive cytokines and direct toxicity to local lymphocytes [109]. Intriguingly, studies have shown damage caused by radiation treatment increases release of tumor-associated antigens that are then presented to T cells via antigen presenting cells (APCs) [110,111]. Thus radiotherapy unmasks the tumor to the immune system, providing a rationale for combining radiation with ICIs [110]. This has been investigated and proposed safe in the recently completed phase I trial in adults with newly diagnosed GBM [112]. Both PD-L1 and CD47 have been shown to be upregulated on cells post radiation [113]. Accordingly, radiation combined with dual PD-L1 and CD47 inhibition resulted in increased tumor regression in murine lung cancer models [113]. CD47 blockade (magrolimab) also augmented cross-priming between dendritic cells and T cells, promoting a more potent anti-tumor immune response [114]. Thus, radiation in combination with magrolimab will also be explored in children, adolescents, and adults with recurrent or progressive malignant brain tumors (Fig. 2D).
Combination strategies to reduce T cell exhaustion
Despite the success of ACT in hematologic malignancy, many solid tumor patients still fail to respond, even with the addition of ICIs. Thus, novel methods to enhance and sustain T cell activity are necessary. The PI3K pathway is one of the most overactivated oncopathways in pediatric CNS tumors [115,116], but also plays an important role in driving T cells to exhaustion [117]. Inhibition of PI3K-δ (PIK3CD) and PI3K-γ (PIK3CG) in T cells promotes the durability of activated T cells to drive sustained anticancer effects [117]. This suggests that combining PI3K inhibition and CAR T cell therapy, or cancer vaccine, could be a promising approach. Expanding CAR T cells with PI3K inhibitors ex vivo has shown to enhance their function in vivo [118]; however, studies investigating PI3K inhibitors and CAR T therapy both given in vivo are warranted. Further, combining an HPV16 E7(49–57) peptide vaccine with the PI3K inhibitor wortmannin resulted in an increase of E7-reactive T cells compared to the vaccine alone, leading to a greater reduction in tumor growth in a TC-1 murine model [119]. It must be noted, that the PI3K pathway is also necessary to activate T cells, thus the timing of treatment is an important consideration. In addition, PI3K-δ is important for sustained Treg function, which is known to suppress anticancer immunity. Inhibition of PI3K-δ (as well as PI3K-α/δ) led to a 50% reduction in Treg infiltration in mice harboring colorectal cancers [120]. Immunosuppressive macrophages are also known to reduce the anti-cancer benefits of T cells. Excitingly, pan-inhibition of PI3K with copanlisib used in combination with anti-PD-1 antibody decreased both Tregs and immunosuppressive macrophages, as well as MDSCs promoting an anticancer response [121].
The tyrosine kinase inhibitor dasatinib (targeting PDGFRA) in combination with the mTOR inhibitor everolimus, have been shown to extend survival in a DMG murine model, and importantly, everolimus improved retention of dasatinib in the CNS [122]. In addition to dasatinib's anti-tumor effect, Mestermann et al. have shown that dasatinib can inhibit CAR T cell activity [123], likely through its SFK inhibitor function without affecting the viability of the T cells [124]. Recent work by Weber et al. showed that transient rest of CAR T cells through exposure to dasatinib can improve exhaustion and prevent tonic signaling from the CAR domain to improve effector function [125]. Thus a combinatorial approach for DMG utilizing intermittent dasatinib and everolimus paired with CAR T cells may provide both an anti-tumor effect and boost to T cell function.
A myriad of additional strategies are also being studied to improve T cell exhaustion, in particular in the context of CAR T cells, and may also be important in CNS tumor targeting. These include targeting transcription factors such as Tox [126], epigenetic regulators such as TET2 [127] and DNMT3A [128], cytokine signaling such as dominant negative TGF-beta receptor [129], metabolic regulators [130,131], and adjustments in CAR engineering and manufacturing strategies [132].
Addressing tumor antigen heterogeneity
Pediatric CNS tumors represent a wide range of diagnoses, and specific histologies often harbor significant heterogeneity in their transcriptional and mutational profiles. As such, it may be difficult to target one single antigen without the risk of escape phenomenon from selective pressure or downregulation of the antigen. Prior immunotherapies targeting a single antigen such as EGFRvIII [133] and IL13RA2 [37] with CAR T cells in adult GBM have shown down regulation of the target antigen at the time of relapse. Thus, multiple strategies are being explored to overcome this roadblock.
As discussed above, mRNA vaccines offer the possibility of HLA independent multi-antigen targeting [134]. Due to their relatively nimble production, mRNA vaccines could be adjusted over time for an individual patient, offering the potential for real-time adjustments to evolving tumor landscapes.
Using T cells targeting multiple antigens is another potential mechanism to address heterogeneity, if multiple targets have been identified in the tumor population. As discussed above, multi-targeted CTLs are currently being studied in an ongoing clinical trial (REMIND trial, NCT03652545) [22]. Multi-antigen directed CAR T cells have also been studied pre-clinically in GBM models with advantages over single-antigen targeting CAR T cells [[135], [136], [137]]. Upcoming CAR studies will combine targets against B7-H3, IL13RA2, HER2, and EGFR (Brainchild-04, NCT05768880). As CAR T cells targeting intracellular proteins have now become possible [138], many additional oncogenic/tumor-dependent antigens may now also be potential targets to help overcome tumor heterogeneity.
Conclusion
The field of immunotherapy is a promising and rapidly evolving scientific arena with multiple technologies applicable and attractive for targeting pediatric CNS tumors. The unique aspects of igniting the immune system in the CNS bring both therapeutic challenges and distinctive potential toxicities, making preclinical studies and clinical trial design critical. With advances in non-invasive tumor monitoring techniques, there is potential to more clearly delineate efficacy and toxicity, as well as tailoring sequential therapies to overcome tumor evolution. Much work remains in fully engaging an immunotherapeutic response in children with CNS tumors, but - with coordinated efforts across pediatric medical centers and CNS focused clinical consortia - the initial signals of efficacy can hopefully blossom into long-standing cures.
CRediT authorship contribution statement
Jessica B. Foster: Conceptualization, Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Marta M. Alonso: Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Elias Sayour: Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Tom B. Davidson: Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Mika L. Persson: Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Matthew D. Dun: Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Cassie Kline: Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Sabine Mueller: Conceptualization, Investigation, Writing – review & editing. Nicholas A. Vitanza: Conceptualization, Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Jasper van der Lugt: Conceptualization, Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
We would like to acknowledge all the patients and families who have participated in clinical trials or donated their tumor tissue to help advance our knowledge of pediatric neuro-oncology and immunotherapy. Your generous participation and donations have helped propel science forward for generations to come.
References
- 1.Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, Delman KA, Spitler LE, Puzanov I, Agarwala SS, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J. Clin. Oncol. 2015;33:2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 2.Frampton JE. Teserpaturev/G47Delta: first approval. BioDrugs. 2022;36:667–672. doi: 10.1007/s40259-022-00553-7. [DOI] [PubMed] [Google Scholar]
- 3.Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat. Rev. Drug Discov. 2015;14:642–662. doi: 10.1038/nrd4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia-Moure M, Gonzalez-Huarriz M, Labiano S, Guruceaga E, Bandres E, Zalacain M, Marrodan L, de Andrea C, Villalba M, Martinez-Velez N, et al. Delta-24-RGD, an oncolytic adenovirus, increases survival and promotes proinflammatory immune landscape remodeling in models of AT/RT and CNS-PNET. Clin. Cancer Res. 2021;27:1807–1820. doi: 10.1158/1078-0432.CCR-20-3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Studebaker AW, Hutzen BJ, Pierson CR, Haworth KB, Cripe TP, Jackson EM, Leonard JR. Oncolytic herpes virus rRp450 shows efficacy in orthotopic xenograft group 3/4 medulloblastomas and atypical teratoid/rhabdoid tumors. Mol. Ther. Oncolyt. 2017;6:22–30. doi: 10.1016/j.omto.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez-Velez N, Garcia-Moure M, Marigil M, Gonzalez-Huarriz M, Puigdelloses M, Gallego Perez-Larraya J, Zalacain M, Marrodan L, Varela-Guruceaga M, Laspidea V, et al. The oncolytic virus Delta-24-RGD elicits an antitumor effect in pediatric glioma and DIPG mouse models. Nat. Commun. 2019;10:2235. doi: 10.1038/s41467-019-10043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pascual-Pasto G, Bazan-Peregrino M, Olaciregui NG, Restrepo-Perdomo CA, Mato-Berciano A, Ottaviani D, Weber K, Correa G, Paco S, Vila-Ubach M, et al. Therapeutic targeting of the RB1 pathway in retinoblastoma with the oncolytic adenovirus VCN-01. Sci. Transl. Med. 2019;11 doi: 10.1126/scitranslmed.aat9321. [DOI] [PubMed] [Google Scholar]
- 8.Chastkofsky MI, Pituch KC, Katagi H, Zannikou M, Ilut L, Xiao T, Han Y, Sonabend AM, Curiel DT, Bonner ER, et al. Mesenchymal stem cells successfully deliver oncolytic virotherapy to diffuse intrinsic pontine glioma. Clin. Cancer Res. 2021;27:1766–1777. doi: 10.1158/1078-0432.CCR-20-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman GK, Johnston JM, Bag AK, Bernstock JD, Li R, Aban I, Kachurak K, Nan L, Kang KD, Totsch S, et al. Oncolytic HSV-1 G207 immunovirotherapy for pediatric high-grade gliomas. N. Engl. J. Med. 2021;384:1613–1622. doi: 10.1056/NEJMoa2024947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallego Perez-Larraya J, Garcia-Moure M, Labiano S, Patino-Garcia A, Dobbs J, Gonzalez-Huarriz M, Zalacain M, Marrodan L, Martinez-Velez N, Puigdelloses M, et al. Oncolytic DNX-2401 virus for pediatric diffuse intrinsic pontine glioma. N. Engl. J. Med. 2022;386:2471–2481. doi: 10.1056/NEJMoa2202028. [DOI] [PubMed] [Google Scholar]
- 11.Laspidea V, Puigdelloses M, Labiano S, Marrodan L, Garcia-Moure M, Zalacain M, Gonzalez-Huarriz M, Martinez-Velez N, Ausejo-Mauleon I, de la Nava D, et al. Exploiting 4-1BB immune checkpoint to enhance the efficacy of oncolytic virotherapy for diffuse intrinsic pontine gliomas. JCI Insight. 2022;7 doi: 10.1172/jci.insight.154812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weller M, Butowski N, Tran DD, Recht LD, Lim M, Hirte H, Ashby L, Mechtler L, Goldlust SA, Iwamoto F, et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017;18:1373–1385. doi: 10.1016/S1470-2045(17)30517-X. [DOI] [PubMed] [Google Scholar]
- 13.Okada H, Kalinski P, Ueda R, Hoji A, Kohanbash G, Donegan TE, Mintz AH, Engh JA, Bartlett DL, Brown CK, et al. Induction of CD8+ T-cell responses against novel glioma–associated antigen peptides and clinical activity by vaccinations with α-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J. Clin. Oncol. 2011;29:330–336. doi: 10.1200/JCO.2010.30.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mueller S, Taitt JM, Villanueva-Meyer JE, Bonner ER, Nejo T, Lulla RR, Goldman S, Banerjee A, Chi SN, Whipple NS, et al. Mass cytometry detects H3.3K27M-specific vaccine responses in diffuse midline glioma. J. Clin. Investig. 2020;130:6325–6337. doi: 10.1172/JCI140378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okada H, Lieberman FS, Walter KA, Lunsford LD, Kondziolka DS, Bejjani GK, Hamilton RL, Torres-Trejo A, Kalinski P, Cai Q, et al. Autologous glioma cell vaccine admixed with interleukin-4 gene transfected fibroblasts in the treatment of patients with malignant gliomas. J. Transl. Med. 2007;5:67. doi: 10.1186/1479-5876-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prins RM, Soto H, Konkankit V, Odesa SK, Eskin A, Yong WH, Nelson SF, Liau LM. Gene expression profile correlates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clin. Cancer Res. 2011;17:1603–1615. doi: 10.1158/1078-0432.CCR-10-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nair SK, Driscoll T, Boczkowski D, Schmittling R, Reynolds R, Johnson LA, Grant G, Fuchs H, Bigner DD, Sampson JH, et al. Ex vivo generation of dendritic cells from cryopreserved, post-induction chemotherapy, mobilized leukapheresis from pediatric patients with medulloblastoma. J. Neurooncol. 2015;125:65–74. doi: 10.1007/s11060-015-1890-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laskowski TJ, Biederstadt A, Rezvani K. Natural killer cells in antitumour adoptive cell immunotherapy. Nat. Rev. Cancer. 2022 doi: 10.1038/s41568-022-00491-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson NR, Minutolo NG, Gill S, Klichinsky M. Macrophage-based approaches for cancer immunotherapy. Cancer Res. 2021;81:1201–1208. doi: 10.1158/0008-5472.CAN-20-2990. [DOI] [PubMed] [Google Scholar]
- 20.Okamoto Y, Shimizu K, Tamura K, Miyao Y, Yamada M, Matsui Y, Tsuda N, Takimoto H, Hayakawa T, Mogami H. An adoptive immunotherapy of patients with medulloblastoma by lymphokine-activated killer cells (LAK) Acta Neurochir. (Wien) 1988;94:47–52. doi: 10.1007/BF01406615. [DOI] [PubMed] [Google Scholar]
- 21.Khatua S, Cooper LJN, Sandberg DI, Ketonen L, Johnson JM, Rytting ME, Liu DD, Meador H, Trikha P, Nakkula RJ, et al. Phase I study of intraventricular infusions of autologous ex vivo expanded NK cells in children with recurrent medulloblastoma and ependymoma. Neuro. Oncol. 2020;22:1214–1225. doi: 10.1093/neuonc/noaa047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hwang E, Fortiz F, Cruz R, Geiger A, Grant M, Lang H, Shibli A, Burnett S, Lazarski C, Tanna J, et al. IMMU-19. Outcomes of Pediatric Patients with High-Risk CNS Tumors Treated with Multi-tumor associated antigen specific T cell (TAA-T) therapy: the ReMIND trial. Neuro-Oncol. 2022;24:i85–i86. [Google Scholar]
- 23.Vitanza NA, Johnson AJ, Wilson AL, Brown C, Yokoyama JK, Künkele A, Chang CA, Rawlings-Rhea S, Huang W, Seidel K, et al. Locoregional infusion of HER2-specific CAR T cells in children and young adults with recurrent or refractory CNS tumors: an interim analysis. Nat. Med. 2021 doi: 10.1038/s41591-021-01404-8. [DOI] [PubMed] [Google Scholar]
- 24.Vitanza NA, Wilson AL, Huang W, Seidel K, Brown C, Gustafson JA, Yokoyama JK, Johnson AJ, Baxter BA, Koning RW, et al. Intraventricular B7-H3 CAR T cells for diffuse intrinsic pontine glioma: preliminary first-in-human bioactivity and safety. Cancer Discov. 2022 doi: 10.1158/2159-8290.CD-22-0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Majzner RG, Ramakrishna S, Yeom KW, Patel S, Chinnasamy H, Schultz LM, Richards RM, Jiang L, Barsan V, Mancusi R, et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature. 2022;603:934–941. doi: 10.1038/s41586-022-04489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Theruvath J, Sotillo E, Mount CW, Graef CM, Delaidelli A, Heitzeneder S, Labanieh L, Dhingra S, Leruste A, Majzner RG, et al. Locoregionally administered B7-H3-targeted CAR T cells for treatment of atypical teratoid/rhabdoid tumors. Nat. Med. 2020 doi: 10.1038/s41591-020-0821-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, Lao CD, Wagstaff J, Schadendorf D, Ferrucci PF, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 2017;377:1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Motzer RJ, Tannir NM, McDermott DF, Aren Frontera O, Melichar B, Choueiri TK, Plimack ER, Barthelemy P, Porta C, George S, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N. Engl. J. Med. 2018;378:1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bouffet E, Larouche V, Campbell BB, Merico D, de Borja R, Aronson M, Durno C, Krueger J, Cabric V, Ramaswamy V, et al. Immune checkpoint inhibition for hypermutant glioblastoma multiforme resulting from germline biallelic mismatch repair deficiency. J. Clin. Oncol. 2016;34:2206–2211. doi: 10.1200/JCO.2016.66.6552. [DOI] [PubMed] [Google Scholar]
- 31.Das A, Sudhaman S, Morgenstern D, Coblentz A, Chung J, Stone SC, Alsafwani N, Liu ZA, Karsaneh OAA, Soleimani S, et al. Genomic predictors of response to PD-1 inhibition in children with germline DNA replication repair deficiency. Nat. Med. 2022;28:125–135. doi: 10.1038/s41591-021-01581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers GD, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lichtenstein DA, Schischlik F, Shao L, Steinberg SM, Yates B, Wang HW, Wang Y, Inglefield J, Dulau-Florea A, Ceppi F, et al. Characterization of HLH-like manifestations as a CRS variant in patients receiving CD22 CAR T cells. Blood. 2021;138:2469–2484. doi: 10.1182/blood.2021011898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gardner RA, Finney O, Annesley C, Brakke H, Summers C, Leger K, Bleakley M, Brown C, Mgebroff S, Kelly-Spratt KS, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129:3322–3331. doi: 10.1182/blood-2017-02-769208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santomasso BD, Nastoupil LJ, Adkins S, Lacchetti C, Schneider BJ, Anadkat M, Atkins MB, Brassil KJ, Caterino JM, Chau I, et al. Management of immune-related adverse events in patients treated with chimeric antigen receptor T-cell therapy: ASCO guideline. J. Clin. Oncol. 2021;39:3978–3992. doi: 10.1200/JCO.21.01992. [DOI] [PubMed] [Google Scholar]
- 36.Gust J, Finney OC, Li D, Brakke HM, Hicks RM, Futrell RB, Gamble DN, Rawlings-Rhea SD, Khalatbari HK, Ishak GE, et al. Glial injury in neurotoxicity after pediatric CD19-directed chimeric antigen receptor T cell therapy. Ann. Neurol. 2019;86:42–54. doi: 10.1002/ana.25502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown CE, Alizadeh D, Starr R, Weng L, Wagner JR, Naranjo A, Ostberg JR, Blanchard MS, Kilpatrick J, Simpson J, et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N. Engl. J. Med. 2016;375:2561–2569. doi: 10.1056/NEJMoa1610497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi J, Lee SY. Clinical characteristics and treatment of immune-related adverse events of immune checkpoint inhibitors. Immune Netw. 2020;20:e9. doi: 10.4110/in.2020.20.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ellithi M, Elnair R, Chang GV, Abdallah MA. Toxicities of immune checkpoint inhibitors: Itis-ending adverse reactions and more. Cureus. 2020;12:e6935. doi: 10.7759/cureus.6935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Durrechou Q, Domblides C, Sionneau B, Lefort F, Quivy A, Ravaud A, Gross-Goupil M, Daste A. Management of immune checkpoint inhibitor toxicities. Cancer Manag. Res. 2020;12:9139–9158. doi: 10.2147/CMAR.S218756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 2018;378:158–168. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 42.Marin-Acevedo JA, Chirila RM, Dronca RS. Immune checkpoint inhibitor toxicities. Mayo Clin. Proc. 2019;94:1321–1329. doi: 10.1016/j.mayocp.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 43.Conroy M, Naidoo J. Immune-related adverse events and the balancing act of immunotherapy. Nat. Commun. 2022;13:392. doi: 10.1038/s41467-022-27960-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mount CW, Majzner RG, Sundaresh S, Arnold EP, Kadapakkam M, Haile S, Labanieh L, Hulleman E, Woo PJ, Rietberg SP, et al. Potent antitumor efficacy of anti-GD2 CAR T cells in H3-K27M(+) diffuse midline gliomas. Nat. Med. 2018;24:572–579. doi: 10.1038/s41591-018-0006-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mahdi J, Dietrich J, Straathof K, Roddie C, Scott BJ, Davidson TB, Prolo LM, Batchelor TT, Campen CJ, Davis KL, et al. Tumor inflammation-associated neurotoxicity. Nat. Med. 2023;29:803–810. doi: 10.1038/s41591-023-02276-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Urban H, Steidl E, Hattingen E, Filipski K, Meissner M, Sebastian M, Koch A, Strzelczyk A, Forster MT, Baumgarten P, et al. Immune checkpoint inhibitor-induced cerebral pseudoprogression: patterns and categorization. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.798811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hwang E, Kilburn L, Onar A, Lin T, Young-Poussaint T, Mitchell D, Margol A, Dunkel I, Gilheeny S, Fouladi M. IMMU-09. Outcome of patients with recurrent diffuse intrinsic pontine glioma (DIPG) treated with pembrolizumab (anti-PD-1): a Pediatric Brain Tumor Consortium study (PBTC045) Neuro-Oncol. 2018;20 i100-i100. [Google Scholar]
- 48.Ayuketang FA, Jager U (2022). Management of cytokine release syndrome (CRS) and HLH. Kroger N, Gribben J, Chabannon C, Yakoub-Agha I and Einsele H (eds): Cham (CH), pp. 135-139. [PubMed]
- 49.Hay KA, Hanafi LA, Li D, Gust J, Liles WC, Wurfel MM, Lopez JA, Chen J, Chung D, Harju-Baker S, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood. 2017;130:2295–2306. doi: 10.1182/blood-2017-06-793141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gust J, Hay KA, Hanafi L-A, Li D, Myerson D, Gonzalez-Cuyar LF, Yeung C, Liles WC, Wurfel M, Lopez JA, et al. Endothelial activation and blood–brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017;7:1404–1419. doi: 10.1158/2159-8290.CD-17-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lowe KL, Mackall CL, Norry E, Amado R, Jakobsen BK, Binder G. Fludarabine and neurotoxicity in engineered T-cell therapy. Gene Ther. 2018;25:176–191. doi: 10.1038/s41434-018-0019-6. [DOI] [PubMed] [Google Scholar]
- 52.Hygino da Cruz LC, Rodriguez I, Domingues RC, Gasparetto EL, Sorensen AG. Pseudoprogression and pseudoresponse: imaging challenges in the assessment of posttreatment glioma. Am. J. Neuroradiol. 2011;32:1978–1985. doi: 10.3174/ajnr.A2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dromain C, Beigelman C, Pozzessere C, Duran R, Digklia A. Imaging of tumour response to immunotherapy. Eur. Radiol. Exp. 2020;4:2. doi: 10.1186/s41747-019-0134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kasten BB, Udayakumar N, Leavenworth JW, Wu AM, Lapi SE, McConathy JE, Sorace AG, Bag AK, Markert JM, Warram JM. Current and future imaging methods for evaluating response to immunotherapy in neuro-oncology. Theranostics. 2019;9:5085–5104. doi: 10.7150/thno.34415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garcia-Figueiras R, Baleato-Gonzalez S, Luna A, Munoz-Iglesias J, Oleaga L, Vallejo Casas JA, Martin-Noguerol T, Broncano J, Areses MC, Vilanova JC. Assessing immunotherapy with functional and molecular imaging and radiomics. Radiographics. 2020;40:1987–2010. doi: 10.1148/rg.2020200070. [DOI] [PubMed] [Google Scholar]
- 56.Griesinger AM, Birks DK, Donson AM, Amani V, Hoffman LM, Waziri A, Wang M, Handler MH, Foreman NK. Characterization of distinct immunophenotypes across pediatric brain tumor types. J. Immunol. 2013;191:4880–4888. doi: 10.4049/jimmunol.1301966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Plant AS, Koyama S, Sinai C, Solomon IH, Griffin GK, Ligon KL, Bandopadhayay P, Betensky R, Emerson R, Dranoff G, et al. Immunophenotyping of pediatric brain tumors: correlating immune infiltrate with histology, mutational load, and survival and assessing clonal T cell response. J. Neurooncol. 2018;137:269–278. doi: 10.1007/s11060-017-2737-9. [DOI] [PubMed] [Google Scholar]
- 58.Alban TJ, Alvarado AG, Sorensen MD, Bayik D, Volovetz J, Serbinowski E, Mulkearns-Hubert EE, Sinyuk M, Hale JS, Onzi GR, et al. Global immune fingerprinting in glioblastoma patient peripheral blood reveals immune-suppression signatures associated with prognosis. JCI Insight. 2018;3 doi: 10.1172/jci.insight.122264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chongsathidkiet P, Jackson C, Koyama S, Loebel F, Cui X, Farber SH, Woroniecka K, Elsamadicy AA, Dechant CA, Kemeny HR, et al. Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat. Med. 2018;24:1459–1468. doi: 10.1038/s41591-018-0135-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu I, Jiang L, Samuelsson ER, Marco Salas S, Beck A, Hack OA, Jeong D, Shaw ML, Englinger B, LaBelle J, et al. The landscape of tumor cell states and spatial organization in H3-K27M mutant diffuse midline glioma across age and location. Nat. Genet. 2022;54:1881–1894. doi: 10.1038/s41588-022-01236-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schafflick D, Xu CA, Hartlehnert M, Cole M, Schulte-Mecklenbeck A, Lautwein T, Wolbert J, Heming M, Meuth SG, Kuhlmann T, et al. Integrated single cell analysis of blood and cerebrospinal fluid leukocytes in multiple sclerosis. Nat. Commun. 2020;11:247. doi: 10.1038/s41467-019-14118-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rubio-Perez C, Planas-Rigol E, Trincado JL, Bonfill-Teixidor E, Arias A, Marchese D, Moutinho C, Serna G, Pedrosa L, Iurlaro R, et al. Immune cell profiling of the cerebrospinal fluid enables the characterization of the brain metastasis microenvironment. Nat. Commun. 2021;12:1503. doi: 10.1038/s41467-021-21789-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mouliere F, Smith CG, Heider K, Su J, van der Pol Y, Thompson M, Morris J, Wan JCM, Chandrananda D, Hadfield J, et al. Fragmentation patterns and personalized sequencing of cell-free DNA in urine and plasma of glioma patients. EMBO Mol. Med. 2021;13:e12881. doi: 10.15252/emmm.202012881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu APY, Smith KS, Kumar R, Paul L, Bihannic L, Lin T, Maass KK, Pajtler KW, Chintagumpala M, Su JM, et al. Serial assessment of measurable residual disease in medulloblastoma liquid biopsies. Cancer Cell. 2021;39:1519–1530. doi: 10.1016/j.ccell.2021.09.012. e1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nassiri F, Chakravarthy A, Feng S, Shen SY, Nejad R, Zuccato JA, Voisin MR, Patil V, Horbinski C, Aldape K, et al. Detection and discrimination of intracranial tumors using plasma cell-free DNA methylomes. Nat. Med. 2020;26:1044–1047. doi: 10.1038/s41591-020-0932-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang J, Che F, Zhang J, Zhang M, Xiao S, Liu Y, Zhou L, Su Q, You C, Lu Y, et al. Diagnostic and prognostic potential of serum cell-free microRNA-214 in glioma. World Neurosurg. 2019;125:e1217–e1225. doi: 10.1016/j.wneu.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 67.Bustos MA, Rahimzadeh N, Ryu S, Gross R, Tran LT, Renteria-Lopez VM, Ramos RI, Eisenberg A, Hothi P, Kesari S, et al. Cell-free plasma microRNAs that identify patients with glioblastoma. Lab. Invest. 2022;102:711–721. doi: 10.1038/s41374-021-00720-4. [DOI] [PubMed] [Google Scholar]
- 68.Piazza A, Rosa P, Ricciardi L, Mangraviti A, Pacini L, Calogero A, Raco A, Miscusi M. Circulating exosomal-DNA in glioma patients: a quantitative study and histopathological correlations-a preliminary study. Brain Sci. 2022;12 doi: 10.3390/brainsci12040500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Muller C, Holtschmidt J, Auer M, Heitzer E, Lamszus K, Schulte A, Matschke J, Langer-Freitag S, Gasch C, Stoupiec M, et al. Hematogenous dissemination of glioblastoma multiforme. Sci. Transl. Med. 2014;6 doi: 10.1126/scitranslmed.3009095. 247ra101. [DOI] [PubMed] [Google Scholar]
- 70.Mueller S, Jain P, Liang WS, Kilburn L, Kline C, Gupta N, Panditharatna E, Magge SN, Zhang B, Zhu Y, et al. A pilot precision medicine trial for children with diffuse intrinsic pontine glioma-PNOC003: a report from the pacific pediatric neuro-oncology consortium. Int. J. Cancer. 2019;145:1889–1901. doi: 10.1002/ijc.32258. [DOI] [PubMed] [Google Scholar]
- 71.Izquierdo E, Proszek P, Pericoli G, Temelso S, Clarke M, Carvalho DM, Mackay A, Marshall LV, Carceller F, Hargrave D, et al. Droplet digital PCR-based detection of circulating tumor DNA from pediatric high grade and diffuse midline glioma patients. Neurooncol. Adv. 2021;3 doi: 10.1093/noajnl/vdab013. vdab013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li D, Bonner ER, Wierzbicki K, Panditharatna E, Huang T, Lulla R, Mueller S, Koschmann C, Nazarian J, Saratsis AM. Standardization of the liquid biopsy for pediatric diffuse midline glioma using ddPCR. Sci. Rep. 2021;11:5098. doi: 10.1038/s41598-021-84513-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cantor E, Wierzbicki K, Tarapore RS, Ravi K, Thomas C, Cartaxo R, Nand Yadav V, Ravindran R, Bruzek AK, Wadden J, et al. Serial H3K27M cell-free tumor DNA (cf-tDNA) tracking predicts ONC201 treatment response and progression in diffuse midline glioma. Neuro. Oncol. 2022;24:1366–1374. doi: 10.1093/neuonc/noac030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Escudero L, Llort A, Arias A, Diaz-Navarro A, Martinez-Ricarte F, Rubio-Perez C, Mayor R, Caratu G, Martinez-Saez E, Vazquez-Mendez E, et al. Circulating tumour DNA from the cerebrospinal fluid allows the characterisation and monitoring of medulloblastoma. Nat. Commun. 2020;11:5376. doi: 10.1038/s41467-020-19175-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miller AM, Szalontay L, Bouvier N, Hill K, Ahmad H, Rafailov J, Lee AJ, Rodriguez-Sanchez MI, Yildirim O, Patel A, et al. Next-generation sequencing of cerebrospinal fluid for clinical molecular diagnostics in pediatric, adolescent and young adult (AYA) brain tumor patients. Neuro Oncol. 2022 doi: 10.1093/neuonc/noac035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu S, Zhu Y, Zhang C, Meng X, Sun B, Zhang G, Fan Y, Kang X. The clinical significance of soluble programmed cell death-ligand 1 (sPD-L1) in patients with gliomas. Front. Oncol. 2020;10:9. doi: 10.3389/fonc.2020.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gu D, Ao X, Yang Y, Chen Z, Xu X. Soluble immune checkpoints in cancer: production, function and biological significance. J. Immunother. Cancer. 2018;6:132. doi: 10.1186/s40425-018-0449-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Okada H, Weller M, Huang R, Finocchiaro G, Gilbert MR, Wick W, Ellingson BM, Hashimoto N, Pollack IF, Brandes AA, et al. Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol. 2015;16:e534–e542. doi: 10.1016/S1470-2045(15)00088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nishimura F, Dusak JE, Eguchi J, Zhu X, Gambotto A, Storkus WJ, Okada H. Adoptive transfer of type 1 CTL mediates effective anti-central nervous system tumor response: critical roles of IFN-inducible protein-10. Cancer Res. 2006;66:4478–4487. doi: 10.1158/0008-5472.CAN-05-3825. [DOI] [PubMed] [Google Scholar]
- 80.Duluc D, Corvaisier M, Blanchard S, Catala L, Descamps P, Gamelin E, Ponsoda S, Delneste Y, Hebbar M, Jeannin P. Interferon-gamma reverses the immunosuppressive and protumoral properties and prevents the generation of human tumor-associated macrophages. Int. J. Cancer. 2009;125:367–373. doi: 10.1002/ijc.24401. [DOI] [PubMed] [Google Scholar]
- 81.Bhat P, Leggatt G, Waterhouse N, Frazer IH. Interferon-gamma derived from cytotoxic lymphocytes directly enhances their motility and cytotoxicity. Cell Death. Dis. 2017;8:e2836. doi: 10.1038/cddis.2017.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat. Rev. Immunol. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 83.Hu B, Ren J, Luo Y, Keith B, Young RM, Scholler J, Zhao Y, June CH. Augmentation of antitumor immunity by human and mouse CAR T cells secreting IL-18. Cell Rep. 2017;20:3025–3033. doi: 10.1016/j.celrep.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chmielewski M, Kopecky C, Hombach AA, Abken H. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively Muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer research. 2011;71:5697–5706. doi: 10.1158/0008-5472.CAN-11-0103. [DOI] [PubMed] [Google Scholar]
- 85.Krenciute G, Prinzing BL, Yi Z, Wu MF, Liu H, Dotti G, Balyasnikova IV, Gottschalk S. Transgenic expression of IL15 improves antiglioma activity of IL13Ralpha2-CAR T cells but results in antigen loss variants. Cancer Immunol. Res. 2017;5:571–581. doi: 10.1158/2326-6066.CIR-16-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lasky JL, Panosyan EH, Plant A, DAVIDSON T, YONG WH, PRINS RM, LIAU LM, MOORE TB. Autologous tumor lysate-pulsed dendritic cell immunotherapy for pediatric patients with newly diagnosed or recurrent high-grade gliomas. Anticancer Research. 2013;33:2047–2056. [PMC free article] [PubMed] [Google Scholar]
- 87.Fangusaro J, Mitchell DA, Kocak M, Robinson GW, Baxter PA, Hwang EI, Huang J, Onar-Thomas A, Dunkel IJ, Fouladi M, et al. Phase 1 study of pomalidomide in children with recurrent, refractory, and progressive central nervous system tumors: a pediatric brain tumor consortium trial. Pediatr. Blood. Cancer. 2021;68:e28756. doi: 10.1002/pbc.28756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shevtsov MA, Kim AV, Samochernych KA, Romanova IV, Margulis BA, Guzhova IV, Yakovenko IV, Ischenko AM, Khachatryan WA. Pilot study of intratumoral injection of recombinant heat shock protein 70 in the treatment of malignant brain tumors in children. Onco. Targets Ther. 2014;7:1071–1081. doi: 10.2147/OTT.S62764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Whiteaker JR, Sharma K, Hoffman MA, Kuhn E, Zhao L, Cocco AR, Schoenherr RM, Kennedy JJ, Voytovich U, Lin C, et al. Targeted mass spectrometry-based assays enable multiplex quantification of receptor tyrosine kinase, MAP Kinase, and AKT signaling. Cell Rep. Method. 2021;1 doi: 10.1016/j.crmeth.2021.100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dzutsev A, Badger JH, Perez-Chanona E, Roy S, Salcedo R, Smith CK, Trinchieri G. Microbes and cancer. Annu. Rev. Immunol. 2017;35:199–228. doi: 10.1146/annurev-immunol-051116-052133. [DOI] [PubMed] [Google Scholar]
- 91.Human Microbiome Project C Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vetizou M, Pitt JM, Daillere R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CP, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Smith M, Dai A, Ghilardi G, Amelsberg KV, Devlin SM, Pajarillo R, Slingerland JB, Beghi S, Herrera PS, Giardina P, et al. Gut microbiome correlates of response and toxicity following anti-CD19 CAR T cell therapy. Nat. Med. 2022;28:713–723. doi: 10.1038/s41591-022-01702-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Davar D, Dzutsev AK, McCulloch JA, Rodrigues RR, Chauvin JM, Morrison RM, Deblasio RN, Menna C, Ding Q, Pagliano O, et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science. 2021;371:595–602. doi: 10.1126/science.abf3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jiang H, Zeng W, Zhang X, Pei Y, Zhang H, Li Y. The role of gut microbiota in patients with benign and malignant brain tumors: a pilot study. Bioengineered. 2022;13:7847–7859. doi: 10.1080/21655979.2022.2049959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sun M, Wu W, Liu Z, Cong Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J. Gastroenterol. 2017;52:1–8. doi: 10.1007/s00535-016-1242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.VB Loris De Cecco, Schiavello Elisabetta, Carenzo Andrea, Iannò Maria Federica, Licata Armando, Marra Manuela, Carollo Maria, Boschetti Luna, Massimino Maura. DIPG-36. The brain-gut-microbiota axis to predict outcome in pediatric diffuse intrinsic pontine glioma. Neuro-Oncol. 2022;24:i26. [Google Scholar]
- 99.Mirzayi C, Renson A, Genomic Standards C, Massive A, Quality Control S, Zohra F, Elsafoury S, Geistlinger L, Kasselman LJ, Eckenrode K, et al. Reporting guidelines for human microbiome research: the STORMS checklist. Nat. Med. 2021;27:1885–1892. doi: 10.1038/s41591-021-01552-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Persson ML, Douglas AM, Alvaro F, Faridi P, Larsen MR, Alonso MM, Vitanza NA, Dun MD. The intrinsic and microenvironmental features of diffuse midline glioma; implications for the development of effective immunotherapeutic treatment strategies. Neuro. Oncol. 2022 doi: 10.1093/neuonc/noac117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lieberman NAP, DeGolier K, Kovar HM, Davis A, Hoglund V, Stevens J, Winter C, Deutsch G, Furlan SN, Vitanza NA, et al. Characterization of the immune microenvironment of diffuse intrinsic pontine glioma: implications for development of immunotherapy. Neuro-Oncol. 2019;21:83–94. doi: 10.1093/neuonc/noy145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lin GL, Nagaraja S, Filbin MG, Suva ML, Vogel H, Monje M. Non-inflammatory tumor microenvironment of diffuse intrinsic pontine glioma. Acta Neuropathol. Commun. 2018;6:51. doi: 10.1186/s40478-018-0553-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kinkead HL, Hopkins A, Lutz E, Wu AA, Yarchoan M, Cruz K, Woolman S, Vithayathil T, Glickman LH, Ndubaku CO, et al. Combining STING-based neoantigen-targeted vaccine with checkpoint modulators enhances antitumor immunity in murine pancreatic cancer. JCI Insight. 2018;3 doi: 10.1172/jci.insight.122857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Antonios JP, Soto H, Everson RG, Orpilla J, Moughon D, Shin N, Sedighim S, Yong WH, Li G, Cloughesy TF, et al. PD-1 blockade enhances the vaccination-induced immune response in glioma. JCI Insight. 2016;1 doi: 10.1172/jci.insight.87059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Song Y, Liu Q, Zuo T, Wei G, Jiao S. Combined antitumor effects of anti-EGFR variant III CAR-T cell therapy and PD-1 checkpoint blockade on glioblastoma in mouse model. Cell. Immunol. 2020;352 doi: 10.1016/j.cellimm.2020.104112. [DOI] [PubMed] [Google Scholar]
- 106.Duchatel RJ, Mannan A, Woldu AS, Hawtrey T, Hindley PA, Douglas AM, Jackson ER, Findlay IJ, Germon ZP, Staudt D, et al. Preclinical and clinical evaluation of German-sourced ONC201 for the treatment of H3K27M-mutant diffuse intrinsic pontine glioma. Neurooncol. Adv. 2021;3:vdab169. doi: 10.1093/noajnl/vdab169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Romaguera JE, Lee HJ, Tarapore R, Prabhu V, Allen J, Schalop L, Zloza A, Ok CY, Sadimin ET, Schenkel J, et al. Integrated stress response and immune cell infiltration in an ibrutinib-refractory mantle cell lymphoma patient following ONC201 treatment. Br. J. Haematol. 2019;185:133–136. doi: 10.1111/bjh.15271. [DOI] [PubMed] [Google Scholar]
- 108.Wagner J, Kline CL, Zhou L, Campbell KS, MacFarlane AW, Olszanski AJ, Cai KQ, Hensley HH, Ross EA, Ralff MD, et al. Dose intensification of TRAIL-inducing ONC201 inhibits metastasis and promotes intratumoral NK cell recruitment. J. Clin. Invest. 2018;128:2325–2338. doi: 10.1172/JCI96711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ruckert M, Flohr AS, Hecht M, Gaipl US. Radiotherapy and the immune system: More than just immune suppression. Stem Cell. 2021;39:1155–1165. doi: 10.1002/stem.3391. [DOI] [PubMed] [Google Scholar]
- 110.Ahmed MM, Hodge JW, Guha C, Bernhard EJ, Vikram B, Coleman CN. Harnessing the potential of radiation-induced immune modulation for cancer therapy. Cancer Immunol. Res. 2013;1:280–284. doi: 10.1158/2326-6066.CIR-13-0141. [DOI] [PubMed] [Google Scholar]
- 111.Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, Camphausen K, Luiten RM, de Ru AH, Neijssen J, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J. Exp. Med. 2006;203:1259–1271. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Omuro A, Reardon DA, Sampson JH, Baehring J, Sahebjam S, Cloughesy TF, Chalamandaris A-G, Potter V, Butowski N, Lim M. Nivolumab plus radiotherapy with or without temozolomide in newly diagnosed glioblastoma: Results from exploratory phase I cohorts of CheckMate 143. Neuro-Oncol. Adv. 2022;4 doi: 10.1093/noajnl/vdac025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wan X, Fang M, Chen T, Wang H, Zhou Q, Wei Y, Zheng L, Zhou Y, Chen K. The mechanism of low-dose radiation-induced upregulation of immune checkpoint molecule expression in lung cancer cells. Biochem. Biophys. Res. Commun. 2022;608:102–107. doi: 10.1016/j.bbrc.2022.03.158. [DOI] [PubMed] [Google Scholar]
- 114.Liu X, Pu Y, Cron K, Deng L, Kline J, Frazier WA, Xu H, Peng H, Fu YX, Xu MM. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat. Med. 2015;21:1209–1215. doi: 10.1038/nm.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Duchatel RJ, Jackson ER, Alvaro F, Nixon B, Hondermarck H, Dun MD. Signal transduction in diffuse intrinsic pontine glioma. Proteomics. 2019;19 doi: 10.1002/pmic.201800479. [DOI] [PubMed] [Google Scholar]
- 116.Findlay IJ, De Iuliis GN, Duchatel RJ, Jackson ER, Vitanza NA, Cain JE, Waszak SM, Dun MD. Pharmaco-proteogenomic profiling of pediatric diffuse midline glioma to inform future treatment strategies. Oncogene. 2022;41:461–475. doi: 10.1038/s41388-021-02102-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chandrasekaran S, Funk CR, Kleber T, Paulos CM, Shanmugam M, Waller EK. Strategies to overcome failures in T-cell immunotherapies by targeting PI3K-delta and -gamma. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.718621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Funk CR, Wang S, Chen KZ, Waller A, Sharma A, Edgar CL, Gupta VA, Chandrakasan S, Zoine JT, Fedanov A, et al. PI3Kdelta/gamma inhibition promotes human CART cell epigenetic and metabolic reprogramming to enhance antitumor cytotoxicity. Blood. 2022;139:523–537. doi: 10.1182/blood.2021011597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Abu-Eid R, Samara RN, Ozbun L, Abdalla MY, Berzofsky JA, Friedman KM, Mkrtichyan M, Khleif SN. Selective inhibition of regulatory T cells by targeting the PI3K-Akt pathway. Cancer Immunol. Res. 2014;2:1080–1089. doi: 10.1158/2326-6066.CIR-14-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Carnevalli LS, Sinclair C, Taylor MA, Gutierrez PM, Langdon S, Coenen-Stass AML, Mooney L, Hughes A, Jarvis L, Staniszewska A, et al. PI3Kalpha/delta inhibition promotes anti-tumor immunity through direct enhancement of effector CD8(+) T-cell activity. J. Immunother. Cancer. 2018;6:158. doi: 10.1186/s40425-018-0457-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhu S, Ma AH, Zhu Z, Adib E, Rao T, Li N, Ni K, Chittepu V, Prabhala R, Garisto Risco J, et al. Synergistic antitumor activity of pan-PI3K inhibition and immune checkpoint blockade in bladder cancer. J. Immunother. Cancer. 2021;9 doi: 10.1136/jitc-2021-002917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Miklja Z, Yadav VN, Cartaxo RT, Siada R, Thomas CC, Cummings JR, Mullan B, Stallard S, Paul A, Bruzek AK, et al. Everolimus improves the efficacy of dasatinib in PDGFRalpha-driven glioma. J. Clin. Invest. 2020;130:5313–5325. doi: 10.1172/JCI133310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schade AE, Schieven GL, Townsend R, Jankowska AM, Susulic V, Zhang R, Szpurka H, Maciejewski JP. Dasatinib, a small-molecule protein tyrosine kinase inhibitor, inhibits T-cell activation and proliferation. Blood. 2008;111(3):1366–1377. doi: 10.1182/blood-2007-04-084814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mestermann K, Giavridis T, Weber J, Rydzek J, Frenz S, Nerreter T, Mades A, Sadelain M, Einsele H, Hudecek M. The tyrosine kinase inhibitor dasatinib acts as a pharmacologic on/off switch for CAR T cells. Sci. Transl. Med. 2019;11 doi: 10.1126/scitranslmed.aau5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Weber EW, Parker KR, Sotillo E, Lynn RC, Anbunathan H, Lattin J, Good Z, Belk JA, Daniel B, Klysz D, et al. Transient rest restores functionality in exhausted CAR-T cells through epigenetic remodeling. Science. 2021:372. doi: 10.1126/science.aba1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Khan O, Giles JR, McDonald S, Manne S, Ngiow SF, Patel KP, Werner MT, Huang AC, Alexander KA, Wu JE, et al. TOX transcriptionally and epigenetically programs CD8+ T cell exhaustion. Nature. 2019;571:211–218. doi: 10.1038/s41586-019-1325-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fraietta JA, Nobles CL, Sammons MA, Lundh S, Carty SA, Reich TJ, Cogdill AP, Morrissette JJD, DeNizio JE, Reddy S, et al. Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T cells. Nature. 2018;558:307–312. doi: 10.1038/s41586-018-0178-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Prinzing B, Zebley CC, Petersen CT, Fan Y, Anido AA, Yi Z, Nguyen P, Houke H, Bell M, Haydar D, et al. Deleting DNMT3A in CAR T cells prevents exhaustion and enhances antitumor activity. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.abh0272. eabh0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kloss CC, Lee J, Zhang A, Chen F, Melenhorst JJ, Lacey SF, Maus MV, Fraietta JA, Zhao Y, June CH. Dominant-negative TGF-beta receptor enhances PSMA-targeted human CAR T cell proliferation and augments prostate cancer eradication. Mol. Ther. 2018;26:1855–1866. doi: 10.1016/j.ymthe.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li X, Wenes M, Romero P, Huang SC, Fendt SM, Ho PC. Navigating metabolic pathways to enhance antitumour immunity and immunotherapy. Nat. Rev. Clin. Oncol. 2019;16:425–441. doi: 10.1038/s41571-019-0203-7. [DOI] [PubMed] [Google Scholar]
- 131.DePeaux K, Delgoffe GM. Metabolic barriers to cancer immunotherapy. Nat. Rev. Immunol. 2021;21:785–797. doi: 10.1038/s41577-021-00541-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Biederstadt A, Manzar GS, Daher M. Multiplexed engineering and precision gene editing in cellular immunotherapy. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.1063303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.O'Rourke DM, Nasrallah MP, Desai A, Melenhorst JJ, Mansfield K, Morrissette JJD, Martinez-Lage M, Brem S, Maloney E, Shen A, et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aaa0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hilf N, Kuttruff-Coqui S, Frenzel K, Bukur V, Stevanovic S, Gouttefangeas C, Platten M, Tabatabai G, Dutoit V, van der Burg SH, et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature. 2019;565:240–245. doi: 10.1038/s41586-018-0810-y. [DOI] [PubMed] [Google Scholar]
- 135.Hegde M, Corder A, Chow KKH, Mukherjee M, Ashoori A, Kew Y, Zhang YJ, Baskin DS, Merchant FA, Brawley VS, et al. Combinational targeting offsets antigen escape and enhances effector functions of adoptively transferred T cells in glioblastoma. Molecul. Ther. 2013;21:2087–2101. doi: 10.1038/mt.2013.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hegde M, Mukherjee M, Grada Z, Pignata A, Landi D, Navai SA, Wakefield A, Fousek K, Bielamowicz K, Chow KK, et al. Tandem CAR T cells targeting HER2 and IL13Ralpha2 mitigate tumor antigen escape. J. Clin. Invest. 2016;126:3036–3052. doi: 10.1172/JCI83416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bielamowicz K, Fousek K, Byrd TT, Samaha H, Mukherjee M, Aware N, Wu MF, Orange JS, Sumazin P, Man TK, et al. Trivalent CAR T cells overcome interpatient antigenic variability in glioblastoma. Neuro. Oncol. 2018;20:506–518. doi: 10.1093/neuonc/nox182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yarmarkovich M, Marshall QF, Warrington JM, Premaratne R, Farrel A, Groff D, Li W, di Marco M, Runbeck E, Truong H, et al. Cross-HLA targeting of intracellular oncoproteins with peptide-centric CARs. Nature. 2021 doi: 10.1038/s41586-021-04061-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]