Abstract
Background
The study aims to explore the relationship between obesity and serum uric acid in adolescents by combining body mass index and waist height ratio.
Methods
475 adolescents in our study were classified as normal weight without central obesity (NW), normal weight but central obesity (NWCO), overweight or obesity without central obesity (OB) and overweight or obesity with central obesity (OBCO). Odds ratios (OR) and 95% confidence intervals (CI) for hyperuricemia were calculated using a logistic regression model. The dose-response association between obesity indicators and serum uric acid were explored by restricted cubic spline model.
Results
The highest serum uric acid level and the OR for hyperuricemia were found in the OBCO group, regardless of sex. After controlling for waist height ratio, the risk of hyperuricemia increased with increasing body mass index in boys and girls. The restricted cubic spline model showed that boys had higher ORs for hyperuricemia at the 25th and 75th percentiles of body mass index than for waist height ratio and girls had a higher OR for hyperuricemia than waist height ratio at the 25th percentile of body mass index.
Conclusions
Hyperuricemia in adolescence was not only associated with the overweight or obesity in BMI, but with the combination of overweight or obesity in BMI and central obesity in WHtR. However, in boys and girls, the increased risk of hyperuricemia associated with elevated body mass index was significantly better than that of waist height ratio.
Keywords: serum uric acid, obesity, adolescent, body mass index, waist height ratio
Introduction
Serum uric acid (SUA) is an endogenous and dietary soluble metabolic end product, and after free filtration by the glomerulus, about 90% of filtered uric acid (UA) is reabsorbed at the renal tubules (1). Hyperuricemia is a metabolic disorder caused by a disorder of purine metabolism that results in excessive production and/or decreased excretion of UA (2). In addition to gout, SUA is also a clinical marker of other diseases, including hypertension, chronic kidney disease, hypertriglyceridemia, obesity, atherosclerotic heart disease and metabolic syndrome (MetS) (3, 4). A meta-analysis conducted among Chinese adults showed that from 2000 to 2014, the prevalence of hyperuricemia in mainland China was 13.3% (5). However, the prevalence of hyperuricemia in Chinese children and adolescents was not well characterized. A recent meta-analysis of children and adolescents showed that the total prevalence of hyperuricemia was estimated to be 23.3%, with 26.6% of boys and 19.8% of girls (6). During childhood and adolescence, SUA levels gradually increase from early childhood to physical development and plateau by age 15–17 (7). Hyperuricemia in adolescence is often thought to be associated with insulin resistance (IR) (8), MetS (3), and hypertension (4). Studies had shown that obesity, especially central obesity, increases xanthine oxidase activity in adipose tissue and leads to higher SUA production and lower SUA renal clearance (9), and the prevalence of hyperuricemia in adolescents with obesity was several times higher than in the general adolescents (10).
Body mass index (BMI) remains the most widely used indicator for assessing obesity in children and adults, but BMI does not reflect central obesity (11). It cannot separate muscle mass from bone and fat mass (12). Waist height ratio (WHtR) has been considered as an easily measured anthropometric indicator for detecting central obesity and assessing the relationship between cardiometabolic risk factor variables and central intraperitoneal obesity (13, 14). The results of a study conducted in Chinese adults have shown that WHtR is an independent and better predictor of hyperuricemia than BMI and WC (15). However, no study results have shown that WHtR is a stronger predictor of hyperuricemia than the other two indicators in children and adolescents. And, it has been suggested that single consideration of one of BMI, WC, WHtR provides limited information on fat distribution (16). Studies conducted in adults combined BMI and WHtR to study hyperuricemia in subjects with different body weights and different central obesity, thus taking into account both the weight and fat distribution of the subjects (17).
The aims of our study were to evaluate SUA levels in students aged 14–17 years and to explore the relationship between obesity and SUA in adolescents by combining BMI and WHtR.
Methods
Participants
This cross-sectional study was based on school and conducted in a high school randomly selected from Huanggu District, Shenyang City, Liaoning Province. A total of 481 14–17 years old high school students were included in this study by cluster random sampling initially, of whom 475 students (194 boys and 281 girls) remained in the sample for analysis in the current work after eliminating students with missing physical measurements and biochemical data records.
Anthropometric evaluation
Anthropometric measurement was carried out by trained professionals following the standard protocol. Referring to the equipment and methods of GB/T26343, fully automatic electronic scales were used to record anthropometric data. We uniformed and calibrated the instruments we used.
Participants were required to wear light clothes when WC (cm), height (cm) and weight (kg) were measured. A portable height measuring device (model TZG, Jiangyin Hongya Science and Education Equipment Co., Ltd.) was used to measure height with an accuracy of 0.1 cm. When measuring weight, the students stood in the central of the scale. Recorded the reading in “kg” to one decimal place. BMI was calculated as body weight (kg) divided by the square of height (m2). WC was measured with a tape measure at the midpoint between the iliac crest and lowest rib with 0.1 cm precision when the student was standing, with adequate exposure of the abdomen and spontaneous breathing. WHtR was calculated as WC (cm) divided by the height (cm). Blood pressure was measured using an auscultatory mercury sphygmomanometer (model XJ1ID, China) with a suitable cuff for children. Participants rested in a seated position for at least 5 min before having their blood pressure measured. The cuff was placed approximately 2 cm above the crease of the elbow on the students' right arm. Each student was measured 3 consecutive times with two intervals of no less than 30 s. The measurements were averaged after removing abnormal values and recorded in mmHg.
Biochemical measurements
Biochemical measurement analysis was performed by the Laboratory Department of Shengjing Hospital, China Medical University. SUA was measured using photoelectric colorimetry (Hitachi 7,600 clinical analyzer, Hitachi, Tokyo, Japan). Fasting plasma glucose (FPG), total cholesterol (TC), high-density lipoprotein (HDL), low density lipoprotein (LDL) and triglycerides (TG), were measured by using clinical methods (Hitachi 7,600 clinical analyzer, Hitachi, Tokyo, Japan). Automated particle-enhanced turbidimetric immunoassays were performed of cystatin C (CYSC) using an Architect I16200 automatic analyzer (Architect, Shandong, China). Furthermore, all the students' blood samples were taken early in the morning on an empty stomach.
Questionnaire survey
The questionnaire was required to be completed by the students and their parents in face-to-face interviews with well-trained personnel, and it was used to collect information about the population such as name, age, gender, ethnicity and address.
The Ethics Committee of Dalian Medical University gave its approval to these studies. We obtained informed consent from all young people and their parents.
Definitions of obesity and hyperuricemia
According to the BMI standards advocated by China for children and adolescents aged 3–18 years old by age and gender, students were classified as normal, overweight and obesity (18). In addition, the presence and absence of central obesity were defined as WHtR ≥0.48 and <0.48 (18). Based on BMI and central obesity status, students in this study were classified as normal weight without central obesity (NW), normal weight but central obesity (NWCO), overweight or obesity without central obesity (OB) and overweight or obesity with central obesity (OBCO).
Based on previous scholarly research on hyperuricemia in children and adolescents (19), in our present study, hyperuricemia was defined as SUA concentrations exceeding the normal range for age and sex: 111–353 µmol/L in children aged 14–15 years; 143–339 µmol/L for girls >15 years old and 202–416 µmol/L for boys >15 years old.
Statistical methods
For the description of study population characteristics: continuous variables were expressed as means and standard deviations (SD) or medians and interquartile ranges, and categorical variables were expressed as numbers and percentages. Students were analyzed stratified by sex. Comparisons were performed using t-tests (two-tailed), non-parametric statistical tests and chi-square tests for the two gender groups. We identified factors independently associated with hyperuricemia by univariate logistic regression. A trend test for the different types of obesity and the risk of hyperuricemia was also performed, using NW as reference, resulting in odds ratios (OR) and 95% confidence intervals (CI). We then further controlled for BMI or HWtR, using binary logistic regression to calculate ORs and 95% CIs for other types of obesity using a trend test for hyperuricemia in students with non-centric obesity or normal weight as a reference. To further explore the measured response relationships between SUA and BMI and between SUA and WHtR, we used a restricted cubic spline (RCS) model which nodes at the 10th, 50th and 90th percentiles to fit the linear regression model and the logistic regression model. In order to control the influence factor of age, we also converted BMI into BMI Z-score according to the BMI growth standards of WHO (20). In exploring the risk of hyperuricemia, we used the median of BMI and BMI Z-score and the WHtR cut-off value of 0.48 for boys and girls as reference values, combined with a binary logistic regression model to plot dose-response curves.
We performed two-sided statistical tests on all data and differences were considered statistically significant when the P-value was less than 0.05. SPSS 21.0 was used for comparison between groups and logistic regression analysis, and R (version 3.6.3) was used for RCS model.
Results
Baseline characteristics of study participants
Tables 1, 2 summarized the baseline characteristics of all boys and girls with different types of obesity. A total of 475 students aged 14–17 years (194 boys and 281 girls) were included in our analysis. For the four different obesity groups, the NW group accounted for the highest proportion in both boys and girls (51.55%; 51.60%) and the OB group accounted for the lowest proportion in both boys and girls (8.25%; 6.76%). Supplementary Table S1 compared the baseline characteristics between different genders. There was no significant difference in BMI levels between boys and girls (21.69; 21.56 kg/m2, respectively, P > 0.05), and no difference in WHtR between them (0.47; 0.46, respectively, P > 0.05). Boys had significantly higher SUA levels than girls, with a mean level of 428.12 ± 75.14 μmol/L in boys and 322.20 ± 60.50 µmol/L in girls (P < 0.05).
Table 1.
Characteristics of boys with different types of obesity.
| Obesity types | P | ||||
|---|---|---|---|---|---|
| NW | NWCO | OB | OBCO | ||
| N | 100 (51.55) | 21 (10.82) | 16 (8.25) | 57 (29.38) | |
| Age (years) | 16.00 (15.00–16.00) | 16.00 (16.00–17.00) | 16.00 (15.25–17.00) | 16.00 (16.00–16.00) | 0.015 |
| Height (cm) | 173.73 ± 6.44 | 170.21 ± 6.38 | 175.65 ± 6.44 | 171.63 ± 6.10 | 0.014 |
| Weight (kg) | 61.10 (56.53–65.73) | 61.90 (54.80–66.40) | 76.80 (73.23–80.70) | 81.30 (74.10–91.25) | 0.000 |
| BMI (kg/m2) | 20.14 ± 1.70 | 21.09 ± 1.27 | 24.77 ± 1.40 | 28.45 ± 3.85 | 0.000 |
| WC (cm) | 73.00 (70.00–77.00) | 85.00 (82.50–87.50) | 80.00 (78.25–83.00) | 91.00 (86.00–99.50) | 0.000 |
| WHtR | 0.42 (0.41–0.44) | 0.50 (0.49–0.51) | 0.46 (0.44–0.47) | 0.52 (0.50–0.57) | 0.000 |
| SBP (mmHg) | 125.00 (117.00–135.75) | 121.00 (120.00–133.00) | 128.50 (120.25–140.75) | 139.00 (125.50–150.50) | 0.000 |
| DBP (mmHg) | 69.50 (64.00–74.00) | 67.00 (55.50–73.50) | 70.00 (61.25–75.25) | 72.00 (64.50–79.00) | 0.214 |
| Hypertension | 35 (35.00) | 7 (33.33) | 6 (37.50) | 40 (70.18) | 0.000 |
| CYSC (mg/L) | 0.97 ± 0.10 | 0.96 ± 0.11 | 0.97 ± 0.06 | 0.97 ± 0.12 | 0.891 |
| TC (mmol/L) | 3.47 (3.13–3.90) | 3.45 (3.14–3.69) | 3.41 (3.08–4.06) | 3.73 (3.30–4.20) | 0.089 |
| TG (mmol/L) | 0.63 (0.51–0.85) | 0.70 (0.56–0.91) | 0.82 (0.54–0.99) | 0.91 (0.60–1.20) | 0.011 |
| HDL (mmol/L) | 1.26 (1.11–1.48) | 1.15 (1.02–1.40) | 1.14 (0.95–1.36) | 1.13 (0.97–1.26) | 0.005 |
| LDL (mmol/L) | 1.92 (1.55–2.09) | 1.81 (1.69–2.14) | 1.84 (1.56–2.51) | 2.22 (1.75–2.60) | 0.007 |
| FPG (mmol/L) | 4.71 (4.45–5.00) | 4.38 (4.21–4.62) | 4.58 (4.34–4.85) | 4.68 (4.43–4.96) | 0.007 |
Values are mean ± SD, n (%), or median (interquartile range).
BMI, body mass index; NW, normal weight without central obesity; NWCO, normal weight with central obesity; OB, obesity without central obesity; OBCO, obesity with central obesity; WC, waist circumference; WHtR, waist-to-height ratio; TG, triglyceride; HDL, high density lipoprotein; LDL, low density lipoprotein; FPG, fasting plasma glucose; CYSC, cystatin C; SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, total cholesterol; SUA, serum uric acid.
Table 2.
Characteristics of girls with different types of obesity.
| Obesity types | P | ||||
|---|---|---|---|---|---|
| NW | NWCO | OB | OBCO | ||
| N | 145 (51.60) | 59 (21.00) | 19 (6.76) | 58 (20.64) | |
| Age (years) | 16.00 (15.00–16.00) | 16.00 (16.00–17.00) | 15.00 (15.00–16.00) | 16.00 (15.00–16.00) | 0.000 |
| Height (cm) | 163.03 ± 5.65 | 159.30 ± 6.73 | 162.22 ± 4.93 | 160.90 ± 5.34 | 0.000 |
| Weight (kg) | 53.50 (49.20–58.00) | 53.30 (50.80–57.70) | 63.50 (60.40–67.40) | 68.55 (63.08–75.53) | 0.000 |
| BMI (kg/m2) | 20.22 (18.74–21.55) | 21.50 (20.35–22.69) | 24.22 (23.70–24.37) | 26.36 (24.82–28.63) | 0.000 |
| WC (cm) | 70.00 (67.00–74.00) | 82.00 (79.00–85.00) | 72.00 (70.00–77.00) | 87.00 (80.00–92.00) | 0.000 |
| WHtR | 0.43 (0.41–0.45) | 0.51 (0.49–0.53) | 0.45 (0.44–0.46) | 0.54 (0.50–0.56) | 0.000 |
| SBP (mmHg) | 112.00 (105.00–120.00) | 115.00 (109.00–125.00) | 120.00 (112.00–127.00) | 122.00 (116.00–132.50) | 0.000 |
| DBP (mmHg) | 68.12 ± 9.72 | 68.05 ± 12.85 | 68.26 ± 8.14 | 69.95 ± 11.29 | 0.714 |
| Hypertension | 30 (20.69) | 17 (28.81) | 8 (42.11) | 28 (48.26) | 0.001 |
| CYSC (mg/L) | 0.84 (0.79–0.91) | 0.85 (0.79–0.93) | 0.87 (0.84–0.95) | 0.88 (0.83–0.95) | 0.048 |
| TC (mmol/L) | 3.94 (3.62–4.39) | 3.72 (3.28–4.44) | 4.14 (3.56–4.86) | 3.92 (3.49–4.56) | 0.076 |
| TG (mmol/L) | 0.79 (0.63–1.02) | 0.72 (0.53–1.02) | 0.80 (0.62–1.13) | 0.90 (0.71–1.39) | 0.016 |
| HDL (mmol/L) | 1.51 (1.30–1.76) | 1.33 (1.22–1.54) | 1.50 (1.25–1.77) | 1.26 (1.09–1.51) | 0.000 |
| LDL (mmol/L) | 2.14 (1.80–2.40) | 2.07 (1.62–2.49) | 2.26 (1.93–2.65) | 2.22 (1.83–2.73) | 0.098 |
| FPG (mmol/L) | 4.50 (4.31–4.78) | 4.48 (4.25–4.58) | 4.58 (4.25–4.74) | 4.43 (4.30–4.77) | 0.205 |
Values are mean ± SD, n (%), or median (interquartile range).
BMI, body mass index; NW, normal weight without central obesity; NWCO, normal weight with central obesity; OB, obesity without central obesity; OBCO, obesity with central obesity; WC, waist circumference; WHtR, waist-to-height ratio; TG, triglyceride; HDL, high density lipoprotein; LDL, low density lipoprotein; FPG, fasting plasma glucose; CYSC, cystatin C; SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, total cholesterol; SUA, serum uric acid.
SUA levels in boys and girls based on different combinations of BMI with WHtR
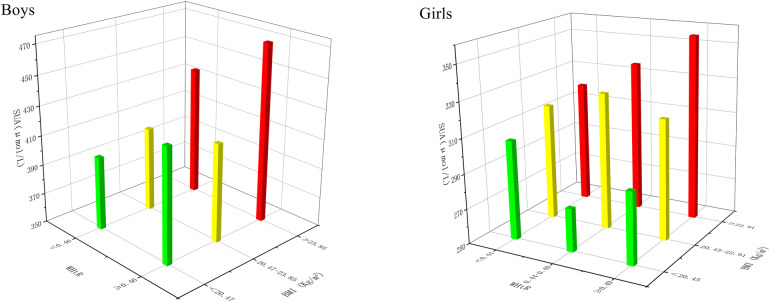
Table 3 showed the SUA levels of different types of obesity in girls and boys. Through comparison, it was found that the highest SUA level in boys and girls was in OBCO group (466.16; 359.79 µmol/L), while the lowest SUA level was in NWCO group (403.57; 310.42 µmol/L). Significant differences between groups occurred only when the NWCO group was compared with the OBCO group, and when the NW group was compared with the OBCO group (P < 0.05). In addition, as showed in Figure 1, we grouped the BMI of boys in tertiles, the WHtR in median, but girls' BMI and WHtR in their respective tertiles. In this grouping case, SUA levels were also the highest when both BMI and WHtR were at their highest levels, and SUA consistently increased with BMI in girls, regardless of WHtR.
Table 3.
SUA levels in boys and girls with different types of obesity.
| Types | Total | Boys | Girls | |||
|---|---|---|---|---|---|---|
| N | SUA | N | SUA | N | SUA | |
| NW | 245 | 351.57 ± 78.18 | 100 | 410.77 ± 66.72 | 145 | 310.74 ± 56.43 |
| NWCO | 80 | 334.88 ± 73.22 | 21 | 403.57 ± 72.28 | 59 | 310.42 ± 56.43 |
| OB | 35 | 378.00 ± 81.40 | 16 | 433.25 ± 71.88 | 19 | 331.47 ± 56.67 |
| OBCO | 115 | 412.51 ± 87.84 | 57 | 466.16 ± 78.06 | 58 | 359.79 ± 61.15 |
| P | P | P | ||||
| NW*NWCO | 0.093 | 0.659 | 0.971 | |||
| OB*OBCO | 0.040 | 0.134 | 0.079 | |||
| NW*OB | 0.064 | 0.218 | 0.134 | |||
| NWCO*OBCO | 0.000 | 0.002 | 0.000 | |||
| NW*OBCO | 0.000 | 0.000 | 0.000 | |||
Values are mean ± SD.
NW, normal weight without central obesity, NWCO, normal weight with central obesity; OB, obesity without central obesity; OBCO, obesity with central obesity; SUA, serum uric acid.
Figure 1.
SUA in boys and girls on different combinations of BMI and WHtR. SUA, serum uric acid; BMI, body mass index. WHtR, waist-to-height Ratio; Red: the highest level of BMI; Yellow: the middle level of BMI; Green: the lowest level of BM.
Blood biochemical indicators associated with hyperuricemia
We identified factors independently associated with hyperuricemia by univariate logistic regression. In boys, Age, TC and CYSC were factors associated with hyperuricemia. At the same time, in girls, SBP, TC, TG, LDL and CYSC were factors associated with hyperuricemia (P < 0.1) (Table 4).
Table 4.
Blood biochemical indicators associated with hyperuricemia.
| Variable | Boys | Girls | ||||
|---|---|---|---|---|---|---|
| OR | 95%CI | P | OR | 95%CI | P | |
| Age | 0.33 | (0.21–0.54) | 0.000 | 1.14 | (0.82–1.59) | 0.442 |
| SBP | 1.01 | (0.99–1.03) | 0.406 | 1.03 | (1.01–1.05) | 0.008 |
| DBP | 0.99 | (0.97–1.02) | 0.541 | 1.01 | (0.98–1.03) | 0.600 |
| TC | 1.49 | (0.93–2.39) | 0.097 | 1.60 | (1.10–2.33) | 0.014 |
| TG | 1.57 | (0.80–3.11) | 0.191 | 2.91 | (1.57–5.38) | 0.001 |
| HDL | 1.06 | (0.55–2.02) | 0.867 | 0.90 | (0.40–2.00) | 0.794 |
| LDL | 1.51 | (0.87–2.64) | 0.139 | 1.98 | (1.25–3.15) | 0.004 |
| FPG | 0.91 | (0.67–1.23) | 0.529 | 0.76 | (0.36–1.60) | 0.464 |
| CYSC | 28.86 | (1.57–530.49) | 0.024 | 664.89 | (28.85–15,325.56) | 0.000 |
OR, odds ratio; 95% CI, 95% confidence interval; TG, triglyceride; HDL, high density lipoprotein; LDL, low density lipoprotein; FPG, fasting plasma glucose; CYSC, cystatin C; SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, total cholesterol.
OR and 95% CI for hyperuricemia of different types of obesity in boys and girls
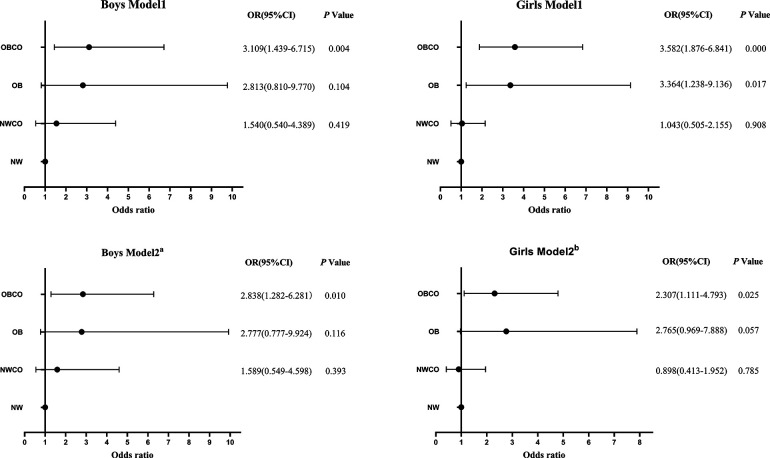
As shown in Figure 2, the risk of hyperuricemia in NWCO, OB and OBCO increased sequentially compared with NW in boys. In model 2, the risk of hyperuricemia was increased 59%-fold in the NWCO group compared to NW (OR = 1.59, 95% CI: 0.55–4.60, P = 0.393), and nearly 3-fold in both the OB and OBCO groups compared to NW (OR = 2.78, 95% CI: 0.78–9.92, P = 0.116; OR = 2.83, 95% CI: 1.28–6.28, P = 0.010). However, this trend is significant only for the OBCO group (P < 0.05).
Figure 2.
OR and 95% CI for hyperuricemia in different obesity types. Model 1: adjusted for age. Model 2a: adjusted for model 1 + TC + CYSC. Model 2b: adjusted for model 1+ TG + TC + LDL + CYSC and SBP. OR, odds ratio; 95% CI, 95% confidence interval, NW, normal weight without central obesity; NWCO, normal weight with central obesity; OB, obesity without central obesity OBCO, obesity with central obesity.
In girls, Model 1 was similar to that of boys, and the risk of hyperuricemia in the NWCO, OB, and OBCO groups was sequentially increased compared to NW. However, when adjusted factors were added, in Model 2, the highest risk of hyperuricemia compared with NW was observed in the OB group (OR = 2.77, 95% CI: 0.97–7.89, P = 0.057), followed by OBCO group (OR = 2.31, 95% CI: 1.11–4.79, P = 0.025), and finally NWCO group (OR = 0.90, 95% CI: 0.41–1.95, P = 0.79).
OR and 95% CI for hyperuricemia in boys and girls after control of BMI or WHtR
In Table 5, We controlled BMI to study the effects of WHtR on hyperuricemia. When students had severe central obesity, regardless of sex and BMI, the risk of hyperuricemia was increased compared with non-central obesity. But none of these trends were statistically significant (P > 0.05).
Table 5.
Or and 95% CI of hyperuricemia after control BMI and WHtR.
| Boys | Girls | |||||||
|---|---|---|---|---|---|---|---|---|
| N | hyperuricemia | OR (95%CI) | P | N | hyperuricemia | OR (95%CI) | P | |
| NW or NWCO | ||||||||
| Non-Central obesity | 100 | 56 (56.00) | 1.00 | — | 145 | 33 (22.76) | 1.00 | — |
| Central obesity | 13 | 8 (61.54) | 1.85 (0.52–6.59) | 0.345 | 20 | 2 (10.00) | 0.42 (0.09–1.95) | 0.269 |
| Severe Central obesity | 8 | 3 (37.50) | 1.08 (0.21–5.64) | 0.932 | 39 | 13 (33.33) | 1.42 (0.60–3.35) | 0.430 |
| OB or OBCO | ||||||||
| Non-Central obesity | 16 | 11 (68.75) | 1.00 | — | 19 | 9 (47.37) | 1.00 | — |
| Central obesity | 11 | 8 (72.73) | 0.68 (0.09–5.31) | 0.716 | 15 | 7 (46.67) | 0.91 (0.21–3.90) | 0.899 |
| Severe Central obesity | 46 | 35 (76.09) | 1.08 (0.23–4.98) | 0.927 | 43 | 23 (53.49) | 1.29 (0.39–4.24) | 0.679 |
| NW or OB | ||||||||
| Normal weight | 100 | 56 (56.00) | 1.00 | — | 144 | 32 (22.22) | 1.00 | — |
| Overweight | 15 | 10 (66.67) | 2.73 (0.76–9.73) | 0.123 | 17 | 7 (41.18) | 2.48 (0.80–7.67) | 0.115 |
| Obesity | — | — | — | — | 1 | 1 (100) | — | — |
| NWCO or OBCO | ||||||||
| Normal weight | 21 | 11 (52.38) | 1.00 | — | 60 | 16 (26.67) | 1.00 | — |
| Overweight | 25 | 17 (68.00) | 1.02 (0.24–4.39) | 0.981 | 37 | 15 (40.54) | 1.57 (0.61–4.05) | 0.347 |
| Obesity | 33 | 27 (81.82) | 2.41 (0.54–10.72) | 0.247 | 22 | 16(72.73) | 6.44(1.70–24.35) | 0.006 |
After controlling for WHtR, the risk of hyperuricemia increased progressively with increasing BMI in both boys and girls, regardless of WHtR. It was worth noting that among girls with abnormal WHtR, the risk of hyperuricemia was nearly 6.5 times higher in obesity girls than in those with normal BMI, and this risk was significant (OR = 6.44, 95% CI:1.70–24.35, P = 0.006).
Dose-response analysis of the association between SUA and obesity indicators
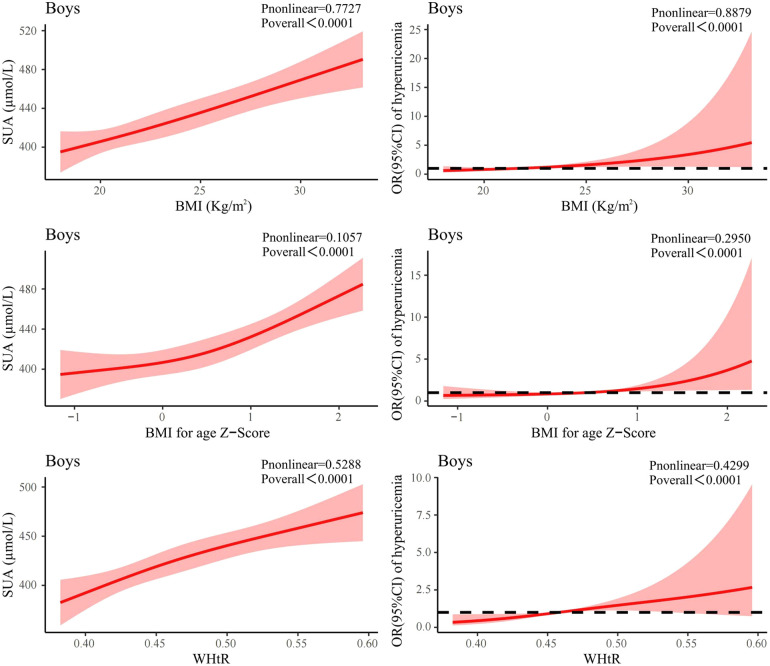
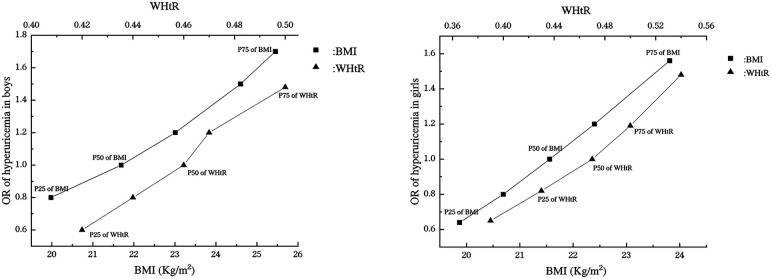
The dose-response association between obesity indicators (BMI, WHtR) and SUA was explored by RCS. As shown in Figure 3, SUA levels and the risk of hyperuricemia in boys were positively correlated with BMI (P nonlinear = 0.773, P nonlinear =0.888), BMI Z-score (P nonlinear = 0.106, P nonlinear = 0.295) and WHtR (P nonlinear = 0.529, P nonlinear = 0.430). In addition, when boys reached the 25th and 75th percentiles of BMI (19.98 kg/m2, 25.45 kg/m2), the OR for hyperuricemia were 0.80 (95% CI: 0.58–1.11) and 1.70(95% CI: 1.21–2.39). Besides, when WHtR reached the 25th and 75th percentile (0.42, 0.50), the OR were only 0.60 (95% CI: 0.40–0.90) and 1.48(95% CI: 1.13–1.94). The ORs for the 25th quartile and 75th quartile of BMI were all higher than the ORs for the 25th quartile and 75th quartile of WHtR (Figure 5).
Figure 3.
Association between obesity indicators and SUA and association between obesity indicators and risk of hyperuricemia using RCS regression model in boys. SUA, serum uric acid; OR, odds ratio; RCS, restricted cubic spline; BMI, body mass index; WHtR, waist-to-height Ratio.
Figure 5.
Association of BMI and WHtR with the risk of hyperuricemia in boys and girls. OR, odds ratio; BMI, body mass index; WHtR, waist-to-height Ratio; P25, the 25th percentile; P50, the 50th percentile; P75, the 75th percentile.
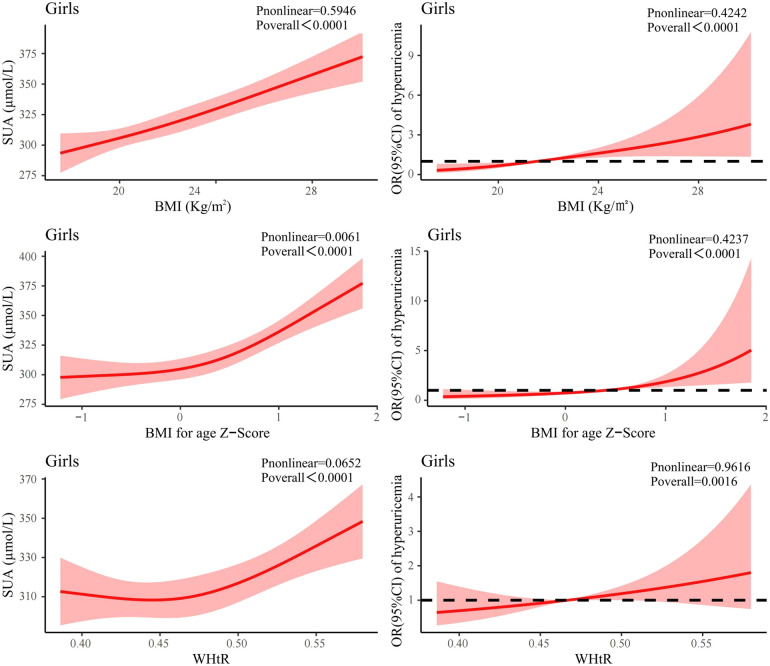
As shown in Figure 4, girls' BMI was similar to that of boys, with SUA level and the risk of hyperuricemia positively associated with BMI (P nonlinear = 0.595, P nonlinear = 0.424). There was also a positive association between BMI Z-score and SUA level in girls, but it was non-linear (P nonlinear = 0.0061). However, the association between WHtR and SUA level in girls was J-like shaped on a continuous scale with SUA reaching the lowest point at a WHtR of 0.44 (P nonlinear = 0.065). In addition, when girls reached the 25th and 75th percentiles of BMI (19.87 kg/m2, 23.81 kg/m2), the OR for hyperuricemia were 0.65 (95% CI: 0.47–0.90) and 1.54 (95% CI: 1.25–1.89). Moreover, when WHtR reached the 25th and 75th percentile (0.43, 0.50), the OR were 0.82 (95% CI: 0.59–1.13) and 1.20 (95% CI: 1.00–1.42). The OR for 25th quartile of BMI was lower than the OR for 25th quartile of WHtR, while the OR for 75th quartile of BMI was higher than the OR for 75th quartile of WHtR (Figure 5).
Figure 4.
Association between obesity indicators and SUA and association between obesity indicators and risk of hyperuricemia using RCS regression model in girls. SUA, serum uric acid; OR, odds ratio; RCS, restricted cubic spline; BMI, body mass index; WHtR, waist-to-height Ratio.
Discussion
This present study proposed that hyperuricemia in adolescence was not only associated with the overweight or obesity in BMI, but with the combination of overweight or obesity in BMI and central obesity in WHtR. In addition, we also found that the increased risk of hyperuricemia associated with elevated BMI was significantly better than that of WHtR in both boys and girls. SUA levels and the risk of hyperuricemia were all positively correlated with BMI and WHtR in boys. In girls, SUA levels and the risk of hyperuricemia were positively associated with BMI. However, the association between WHtR and SUA levels in girls on a continuous scale was J-like shaped, and as WHtR increased, SUA levels showed a trend of decreasing and then increasing.
In our study, boys and girls were 14–17 years old. The Tanner stage is used to divide the pubertal development internationally (21). Tanner 1 is the stage where secondary sexual characteristics do not appear, which is pre-puberty; Tanner 2∼3 is the stage when secondary sexual characteristics appear and sexual organs begin to develop, which is the early stage of puberty. Tanner 4–5 is the stage of rapid development of sexual organs and sexual maturity, which is the middle and late stage of puberty (22). A previous muti-center cross-sectional study showed that the median age and 95% CI of Chinese girls assessed by breasts and pubic hair at Tanner 4 were 14.21 (13.94–14.49) and 15.29 (14.99–15.62). Moreover, the median age and 95% CI of Chinese girls assessed by breasts and pubic hair at Tanner 5 were 17.39 (16.97–17.91) and 18.34 (17.88–18.91) (23). In addition, the cross-sectional study showed that the median age and 95% CI of Chinese boys assessed by genital, pubic hair and testicular volume at Tanner 4 were 14.39 (14.12–14.67), 14.76 (14.59–14.94) and 13.02 (12.84–13.21). Moreover, the median age and 95% CI of Chinese boys assessed by genital, pubic hair and testicular volume at Tanner 5 were 16.17 (15.93–16.45), 16.48 (16.24–16.75) and 15.78 (15.33–16.32) (24). Therefore, it can be considered that our subjects (14–17 years old) were in Tanner 4 and Tanner 5, which is the middle and late stage of puberty.
Our study found that boys' SUA levels were higher than girls', which is the same as other studies of children and adolescents (25). The reason for the gender difference in SUA between boys and girls during adolescence is that the SUA of boys increase sharply, while the SUA of girls remain relatively unchanged (26). Studies have shown that increased renal urate retention may be the main underlying mechanism of increased SUA (27). Adolescents have a progressive decline in renal fractional UA excretion and clearance, leading to increased UA retention (28). However, estrogen in girls can increase UA clearance, promote UA excretion, and reduce SUA levels (29), and estradiol, on the other hand, can inhibit isolated xanthine oxidase (UA-producing enzyme), and can also pharmacologically reduces circulating UA (30). In our study, girls aged 14–17 were in the pubertal developmental stage and had already secreted sex hormones in their bodies, so they had less UA retention and lower levels of SUA than boys. Another factor that contributes to higher SUA levels in boys than in girls was male testosterone. Testosterone has been reported to stimulate isolated xanthine oxidase to pharmacologically increase circulating UA, suggesting that testosterone may contribute to sex differences in UA (30, 31). Apart from this, some studies have found that higher SUA in adolescent boys is associated with lower sex hormone binding globulin (SHBG). The reason for this may be that SHBG can bind to testosterone, thereby reducing the level of free and bioavailable testosterone (26). It could also be due to some unknown mechanism. Considering the specificity of growth and development of adolescent children and the differences in the distribution of SUA between boys and girls, we conducted a stratified analysis by gender in the analysis.
Causes of elevated SUA or hyperuricemia in children and adolescents are similar to those in adults, however, secondary hyperuricemia due to other conditions is more common in adolescents, such as cyanotic congenital heart disease, kidney disease (32). Besides, elevated SUA or hyperuricemia in children and adolescents is also closely related to lifestyle factors such as obesity (33). Some scholars analyzed the carotid intima-media thickness (IMT), SUA, creatinine and serum triglyceride of 120 obese children and 50 healthy controls, and found that the concentration of SUA in children with obesity was higher than that in the healthy group significantly (34). This relationship between obesity and SUA levels was also shown in our study. This finding is similar to those of studies in Italy (35), Thailand (25) and Brazil (36). A recent follow-up study of children and adolescents aged 4–18 also found that 65 children who lost weight during follow-up had reduced SUA levels, while 23 children who gained weight during follow-up had increased SUA levels (37). Furthermore, Rocha EPAA and others suggested that SUA could be used to distinguish between metabolically healthy status and metabolically unhealthy status in children and adolescents with overweight and obesity, and that the higher the level of SUA, the higher the risk of metabolically unhealthy (38). Elevated SUA levels were associated with obesity, possibly due to IR (33). Gil-Campos and others studied the relationship between SUA and IR syndrome in children and concluded that elevated SUA levels in children with obesity during prepubertal compared to lean children appear to be an early metabolic change associated with other features of IR syndrome (39). The reason for this was that IR due to obesity reduced UA excretion by the kidneys over the proximal renal tubules, leading to hyperuricemia (40). In addition to IR, leptin was found to be a causative factor for hyperuricemia in obese patients and was hypothesized to be an association between the two (41). Leptin regulated fat mass and body weight by suppressing food intake and stimulating energy expenditure, and numerous adult studies have shown that leptin is a biomarker for obesity, IR and MetS (42). However, there have been no studies in children and adolescents to demonstrate an association between leptin and hyperuricemia. In addition, differences in SUA levels between subjects with normal BMI and subjects with overweight/obesity may be related to oxidative stress. UA is generally considered to be an important anti-oxidant in human body and it is responsible for 55% of the antioxidant scavenging of extracellular free radicals (43). However, from a biological perspective, UA is not only anti-oxidant, but also pro-oxidation (44). Extracellular UA acts primarily as an anti-oxidant, but pro-oxidation also occurs when UA levels are too high. At this point, the extracellular UA reacts with myeloperoxidase to form a compound with pro-oxidation properties called hydroperoxide urate, which plays a pro-oxidation role (45). Oxidative stress occurs when there is an imbalance between the production of reactive oxygen species (ROS) and cellular anti-oxidant factors (46). Supraphysiological production of ROS is one of the major determinants of overall obesity-related health deterioration, and oxidative stress has also been shown to be a significant feature of obesity (47). Therefore, this may be one of the reasons for the differences in SUA levels and prevalence of hyperuricemia between adolescents with overweight/obesity and adolescents with normal weight.
In the current study, BMI and WHtR were combined and the results showed that students had the highest SUA levels when both BMI overweight or obesity and central obesity as defined by WHtR were present. However, when BMI and WHtR were controlled separately, the increased risk of hyperuricemia due to increased BMI was more pronounced. This may indicate that there is a stronger association between BMI and hyperuricemia compared with the association between WHtR and hyperuricemia in adolescents. This is similar to the results of a longitudinal analysis on children and adolescents of APV registries in Germany/Austria/Switzerland (48) and a study of adults conducted in Liaoning province, China (16). However, a previous study of adults conducted in China indicated that WHtR is an independent and better predictor of hyperuricemia than BMI and WC (15). This phenomenon can be explained in two aspects. Firstly, leptin was found to be a causative factor for hyperuricemia in obese patients (41). Previous studies also showed a high correlation between leptin levels and BMI in both females and males and concluded that leptin production is proportional to adipose tissue mass (49). Another study also demonstrated a high association between leptin levels and overall adipose tissue depots rather than with fat depot in a certain body part (50). Secondly, as an indicator of central obesity, WHtR mainly reflects the accumulation of abdominal fat. However, adolescents are at a special stage of rapid physical growth and development, fat distribution and body shape may be changing rapidly. Therefore, the overall degree of obesity and the degree of obesity accumulation reflected by BMI may be more strongly associated with hyperuricemia in adolescence. This characteristic of adolescents in our study may also account for the lower SUA levels in the NWCO group than in the NW group. Compared with adolescents in the NW group, adolescents in the NWCO group only had more abdominal fat, but the general body weight was not higher and this may lead that the SUA level of adolescents in NWCO group not higher than that of the adolescents in NW group. In fact, NWCO is a new type of obesity that combined general obesity and central obesity, and has been used more in adult studies than in children and adolescents. As far as we know, our study is the first to use this type of obesity (NWCO) to assess the association between obesity and hyperuricemia in children and adolescents. Therefore, more studies in children and adolescents are needed to be conducted to explore the relationship between NWCO and SUA levels and the relationship between different indicators of obesity and hyperuricemia.
There were some other interesting findings in our study. Results of univariate Logistic regression in our study also showed that SBP, TG and LDL was associated with hyperuricemia in girls but not in boys. This phenomenon was consistent with a study of adolescents in America (51) and a study of adults using NHANES data from 2009 to 2018 (52). Potential mechanisms include the influence of sex hormones and genetic differences between the sexes (53, 54). Studies have shown a positive and independent correlation between xanthine oxidase (XO) levels and hypertension (55). XO is a UA-producing enzyme, and estradiol in women can inhibit isolated XO (30), resulting in a stronger association of SBP with hyperuricemia in girls. Regarding differences in the relationship between lipid profile and hyperuricemia in boys and girls, it has been suggested that estrogen can promote UA excretion and regulate lipid metabolism in the kidney (56). Therefore, reducing blood pressure and lipid profile levels in girls should be considered to control hyperuricemia.
A J-like shape association between WHtR and SUA in girls was found in our study, with a decreasing SUA before WHtR reached 0.44 and then increasing. However, this trend was not found among boys. Since the age distribution of our subjects was during puberty (14–17 years), this interesting difference may be due to the different levels of pubertal development in boys and girls. However, no other study found this J-like shape association between WHtR and girls' SUA. Thus, prospective studies with a large sample size should to be carried out to verify the differences between WHtR and SUA levels in boys and girls. According to the guidelines, adult hyperuricemia was defined as men whose SUA ≥ 416 μmol / L and women whose SUA ≥ 357 µmol/L (57). However, SUA levels change during development in children and adolescents. Thus, age—and sex-related SUA reference values should be taken into account when defining hyperuricemia in children and adolescents. In Masaru Kubota's report (58), two previous UA reference values for children and adolescents of different genders and ages have been summarized. In addition, for Chinese children and adolescents, the normal range of UA level was defined as 119–416 µmol/L, and hyperuricemia was defined as UA level > 416 µmol/L, according to Zhufutang Practical Pediatrics (59). Considering that the students in our study were 14–17 years old, we used the criteria in a previous study (19). The future need for separate reference values for hyperuricemia in adolescents remains to be explored.
There were several limitations in this analysis. First, the adolescents in our study were obtained by cluster random sampling in Shenyang, Liaoning Province, and were not nationally representative. Second, in the limited data available, our analysis did not take into account key negative factors such as family history, dietary status and IR levels. Therefore, further population-based prospective studies should be conducted to conquer these limitations and to investigate systematically the relationship between obesity and SUA and hyperuricemia in boys and girls during adolescence.
In conclusion, hyperuricemia in adolescence was not only associated with the overweight or obesity in BMI, but with the combination of overweight or obesity in BMI and central obesity in WHtR. However, in both boys and girls, the increased risk of hyperuricemia associated with elevated BMI was significantly better than that of WHtR. Prospective studies with larger sample sizes should be conducted to assess SUA levels in adolescents of different genders with different BMI and WHtR.
Acknowledgments
We thank all the participants for their support in this study.
Funding Statement
This work was supported by the National Natural Science Foundation of China [grant number 81602871 (to Xin Chen)].
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Dalian Medical University Ethics Committee. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
SL conceived data, wrote the original draft and performed the primary analysis. YL carried out the field data collection. WW, YC and JC extracted the data and helped with the analysis. ZW, MH, HZ and XL performed the formal analysis. XC provided design ideas, controlled the analytical methods, and edited the review & editing. All authors reviewed and approved the final manuscript. XC acts as guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1176897/full#supplementary-material.
References
- 1.Álvarez-Lario B, Macarrón-Vicente J. Uric acid and evolution. Rheumatology (Oxford, England). (2010) 49(11):2010–5. 10.1093/rheumatology/keq204 [DOI] [PubMed] [Google Scholar]
- 2.Zhao T, Lv X, Cao L, Guo M, Zheng S, Xue Y, et al. Renal excretion is a cause of decreased serum uric acid during acute gout. Int J Rheum Dis. (2018) 21(9):1723–7. 10.1111/1756-185X.13348 [DOI] [PubMed] [Google Scholar]
- 3.Feng X, Guo Y, Tu H, Li S, Chen C, Sun M, et al. Temporal changes in serum uric acid and risk for metabolic syndrome: a longitudinal cohort study. Diabetol Metab Syndr. (2022) 14(1):92. 10.1186/s13098-022-00861-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson RJ, Bakris GL, Borghi C, Chonchol MB, Feldman D, Lanaspa MA, et al. Hyperuricemia, acute and chronic kidney disease, hypertension, and cardiovascular disease: report of a scientific workshop organized by the national kidney foundation. Am J Kidney Dis. (2018) 71(6):851–65. 10.1053/j.ajkd.2017.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu R, Han C, Wu D, Xia X, Gu J, Guan H, et al. Prevalence of hyperuricemia and gout in mainland China from 2000 to 2014: a systematic review and meta-analysis. BioMed Res Int. (2015) 2015:762820. 10.1155/2015/762820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rao J, Ye P, Lu J, Chen B, Li N, Zhang H, et al. Prevalence and related factors of hyperuricaemia in Chinese children and adolescents: a pooled analysis of 11 population-based studies. Ann Med. (2022) 54(1):1608–15. 10.1080/07853890.2022.2083670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuo KL, Chen HM, Hsiao SH, Chu D, Huang SJ, Huang KC, et al. The relationship between anthropometric factors and hyperuricemia in adolescent athletes. Obes Res Clin Pract. (2021) 15(4):375–80. 10.1016/j.orcp.2021.03.012 [DOI] [PubMed] [Google Scholar]
- 8.Genoni G, Menegon V, Secco GG, Sonzini M, Martelli M, Castagno M, et al. Insulin resistance, serum uric acid and metabolic syndrome are linked to cardiovascular dysfunction in pediatric obesity. Int J Cardiol. (2017) 249:366–71. 10.1016/j.ijcard.2017.09.031 [DOI] [PubMed] [Google Scholar]
- 9.Goli P, Riahi R, Daniali SS, Pourmirzaei M, Kelishadi R. Association of serum uric acid concentration with components of pediatric metabolic syndrome: a systematic review and meta-analysis. J Res Med Sci. (2020) 25:43. 10.4103/jrms.JRMS_733_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ságodi L, Fehér V, Kiss-Tóth E, Almási A, Barkai L. Metabolic complications of obesity during adolescence, particularly regarding elevated uric acid levels. Orv Hetil. (2015) 156(22):888–95. 10.1556/650.2015.30140 [DOI] [PubMed] [Google Scholar]
- 11.Lam BC, Koh GC, Chen C, Wong MT, Fallows SJ. Comparison of Body Mass Index (BMI), Body Adiposity Index (BAI), Waist Circumference (WC), Waist-To-Hip Ratio (WHR) and Waist-To-Height Ratio (WHtR) as predictors of cardiovascular disease risk factors in an adult population in Singapore. PLoS One. (2015) 10(4):e0122985. 10.1371/journal.pone.0122985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Q, Guo H, Chen S, Ma J, Kim H. The association of body mass Index and fat mass with health-related physical fitness among Chinese school children: a study using a predictive model. Int J Environ Res Public Health. (2022) 20(1):355. 10.3390/ijerph20010355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tee JYH, Gan WY, Lim PY. Comparisons of body mass index, waist circumference, waist-to-height ratio and a body shape index (ABSI) in predicting high blood pressure among Malaysian adolescents: a cross-sectional study. BMJ open. (2020) 10(1):e032874. 10.1136/bmjopen-2019-032874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobylińska M, Antosik K, Decyk A, Kurowska K, Skiba D. Body composition and anthropometric indicators in children and adolescents 6-15 years old. Int J Environ Res Public Health. (2022) 19(18):11591. 10.3390/ijerph191811591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang ZP, Huang BX, Zhang H, Zhu MF, Zhu HL. Waist-to-Height ratio is a better predictor of hyperuricemia than body mass index and waist circumference in Chinese. Ann Nutr Metab. (2019) 75(3):187–94. 10.1159/000504282 [DOI] [PubMed] [Google Scholar]
- 16.Zhang N, Chang Y, Guo X, Chen Y, Ye N, Sun Y. A body shape index and body roundness index: two new body indices for detecting association between obesity and hyperuricemia in rural area of China. Eur J Intern Med. (2016) 29:32–6. 10.1016/j.ejim.2016.01.019 [DOI] [PubMed] [Google Scholar]
- 17.Shirasawa T, Ochiai H, Yoshimoto T, Nagahama S, Watanabe A, Yoshida R, et al. Cross-sectional study of associations between normal body weight with central obesity and hyperuricemia in Japan. BMC Endocr Disord. (2020) 20(1):2. 10.1186/s12902-019-0481-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang YF, Sun MX, Xue H, Zhao WH, Yang XG, Zhu XY, et al. Understanding the China blue paper on obesity prevention and control and policy implications and recommendations for obesity prevention and control in China. Zhonghua yu Fang yi xue za zhi. (2019) 53(9):875–84. 10.3760/cma.j.issn.0253-9624.2019.09.003. PMID: 31474067 [DOI] [PubMed] [Google Scholar]
- 19.Spartà G, Kemper MJ, Neuhaus TJ. Hyperuricemia and gout following pediatric renal transplantation. Pediatric nephrology (Berlin). Germany. (2006) 21(12):1884–8. 10.1007/s00467-006-0257-5 [DOI] [PubMed] [Google Scholar]
- 20.de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull WHO. (2007) 85(9):660–7. 10.2471/BLT.07.043497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emmanuel M, Bokor BR. Tanner stages. StatPearls. Treasure Island, FL: StatPearls Publishing Copyright © 2023, StatPearls Publishing LLC. (2023). [PubMed] [Google Scholar]
- 22.Wang Y, Chen X, Zhang F, Zhou X, Xu T. Effects of exercise on bone mineral content in children and adolescents at different growth stages: a meta-analysis. Wei Sheng Yan Jiu. (2023) 52(2):300–12. 10.19813/j.cnki.weishengyanjiu.2023.02.020 [DOI] [PubMed] [Google Scholar]
- 23.Sun Y, Tao FB, Su PY, Mai JC, Shi HJ, Han YT, et al. National estimates of the pubertal milestones among urban and rural Chinese girls. J Adolesc Health. (2012) 51(3):279–84. 10.1016/j.jadohealth.2011.12.019 [DOI] [PubMed] [Google Scholar]
- 24.Sun Y, Tao F, Su PY. National estimates of pubertal milestones among urban and rural Chinese boys. Ann Hum Biol. (2012) 39(6):461–7. 10.3109/03014460.2012.712156 [DOI] [PubMed] [Google Scholar]
- 25.Suttikomin W, Leelahagul P, Khamvang S, Chaimongkol C, Chaiwut N. Obesity and serum uric acid in secondary school-age students of srinagarindra the princess mother school, Phayao, Thailand. Indian J Public Health. (2018) 62(2):133–7. 10.4103/ijph.IJPH_117_17 [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Charchar FJ. Establishment of sex difference in circulating uric acid is associated with higher testosterone and lower sex hormone-binding globulin in adolescent boys. Sci Rep. (2021) 11(1):17323. 10.1038/s41598-021-96959-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Močnik M, Golob Jančič S, Filipič M, Marčun Varda N. The role of urate in cardiovascular risk in adolescents and young adults with hypertension, assessed by pulse wave velocity. Front Cardiovasc Med. (2022) 9:867428. 10.3389/fcvm.2022.867428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miao Y, Ottenbros SA, Laverman GD, Brenner BM, Cooper ME, Parving HH, et al. Effect of a reduction in uric acid on renal outcomes during losartan treatment: a post hoc analysis of the reduction of endpoints in non-insulin-dependent diabetes mellitus with the angiotensin II antagonist losartan trial. Hypertension (Dallas, Tex: 1979). (2011) 58(1):2–7. 10.1161/HYPERTENSIONAHA.111.171488 [DOI] [PubMed] [Google Scholar]
- 29.Alvim RO, Siqueira JH, Zaniqueli D, Dutra DM, Oliosa PR, Mill JG. Influence of muscle mass on the serum uric acid levels in children and adolescents. Nutr Metab Cardiovasc Dis. (2020) 30(2):300–5. 10.1016/j.numecd.2019.08.019 [DOI] [PubMed] [Google Scholar]
- 30.Yahyaoui R, Esteva I, Haro-Mora JJ, Almaraz MC, Morcillo S, Rojo-Martínez G, et al. Effect of long-term administration of cross-sex hormone therapy on serum and urinary uric acid in transsexual persons. J Clin Endocrinol Metab. (2008) 93(6):2230–3. 10.1210/jc.2007-2467 [DOI] [PubMed] [Google Scholar]
- 31.Kurahashi H, Watanabe M, Sugimoto M, Ariyoshi Y, Mahmood S, Araki M, et al. Testosterone replacement elevates the serum uric acid levels in patients with female to male gender identity disorder. Endocr J. (2013) 60(12):1321–7. 10.1507/endocrj.EJ13-0203 [DOI] [PubMed] [Google Scholar]
- 32.Yamanaka H. Gout and hyperuricemia in young people. Curr Opin Rheumatol. (2011) 23(2):156–60. 10.1097/BOR.0b013e3283432d35 [DOI] [PubMed] [Google Scholar]
- 33.Oyama C, Takahashi T, Oyamada M, Oyamada T, Ohno T, Miyashita M, et al. Serum uric acid as an obesity-related indicator in early adolescence. Tohoku J Exp Med. (2006) 209(3):257–62. 10.1620/tjem.209.257 [DOI] [PubMed] [Google Scholar]
- 34.Pacifico L, Cantisani V, Anania C, Bonaiuto E, Martino F, Pascone R, et al. Serum uric acid and its association with metabolic syndrome and carotid atherosclerosis in obese children. Eur J Endocrinol. (2009) 160(1):45–52. 10.1530/EJE-08-0618 [DOI] [PubMed] [Google Scholar]
- 35.Luciano R, Shashaj B, Spreghini M, Del Fattore A, Rustico C, Wietrzykowska Sforza R, et al. Percentiles of serum uric acid and cardiometabolic abnormalities in obese Italian children and adolescents. Ital J Pediatr. (2017) 43(1):3. 10.1186/s13052-016-0321-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomazini F, de Carvalho BS, de Araujo PX, Franco MDC. High uric acid levels in overweight and obese children and their relationship with cardiometabolic risk factors: what is missing in this puzzle? J Pediatr Endocrinol Metab. (2021) 34(11):1435–41. 10.1515/jpem-2021-0211 [DOI] [PubMed] [Google Scholar]
- 37.Jørgensen RM, Bøttger B, Vestergaard ET, Kremke B, Bahnsen RF, Nielsen BW, et al. Uric acid is elevated in children with obesity and decreases after weight loss. Front Pediatr. (2021) 9:814166. 10.3389/fped.2021.814166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rocha E, Vogel M, Stanik J, Pietzner D, Willenberg A, Körner A, et al. Serum uric acid levels as an indicator for metabolically unhealthy obesity in children and adolescents. Horm Res Paediatr. (2018) 90(1):19–27. 10.1159/000490113 [DOI] [PubMed] [Google Scholar]
- 39.Gil-Campos M, Aguilera CM, Cañete R, Gil A. Uric acid is associated with features of insulin resistance syndrome in obese children at prepubertal stage. Nutr Hosp. (2009) 24(5):607–13. [PubMed] [Google Scholar]
- 40.Li C, Hsieh MC, Chang SJ. Metabolic syndrome, diabetes, and hyperuricemia. Curr Opin Rheumatol. (2013) 25(2):210–6. 10.1097/BOR.0b013e32835d951e [DOI] [PubMed] [Google Scholar]
- 41.Bedir A, Topbas M, Tanyeri F, Alvur M, Arik N. Leptin might be a regulator of serum uric acid concentrations in humans. Jpn Heart J. (2003) 44(4):527–36. 10.1536/jhj.44.527 [DOI] [PubMed] [Google Scholar]
- 42.Moran O, Phillip M. Leptin: obesity, diabetes and other peripheral effects–a review. Pediatr Diabetes. (2003) 4(2):101–9. 10.1034/j.1399-5448.2003.00017.x [DOI] [PubMed] [Google Scholar]
- 43.Jakše B, Jakše B, Pajek M, Pajek J. Uric acid and plant-based nutrition. Nutrients. (2019) 11(8):1736. 10.3390/nu11081736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Isaka Y, Takabatake Y, Takahashi A, Saitoh T, Yoshimori T. Hyperuricemia-induced inflammasome and kidney diseases. Nephrol Dial Transplant. (2016) 31(6):890–6. 10.1093/ndt/gfv024 [DOI] [PubMed] [Google Scholar]
- 45.Gherghina ME, Peride I, Tiglis M, Neagu TP, Niculae A, Checherita IA. Uric acid and oxidative stress-relationship with cardiovascular, metabolic, and renal impairment. Int J Mol Sci. (2022) 23(6):3188. 10.3390/ijms23063188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pietrocola F, Bravo-San Pedro JM. Targeting autophagy to counteract obesity-associated oxidative stress. Antioxidants (Basel, Switzerland). (2021) 10(1):102. 10.3390/antiox10010102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gough DR, Cotter TG. Hydrogen peroxide: a Jekyll and Hyde signalling molecule. Cell Death Dis. (2011) 2(10):e213. 10.1038/cddis.2011.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weihrauch-Blüher S, Wiegand S, Weihe P, Prinz N, Weghuber D, Leipold G, et al. Uric acid and gamma-glutamyl-transferase in children and adolescents with obesity: association to anthropometric measures and cardiometabolic risk markers depending on pubertal stage, sex, degree of weight loss and type of patient care: evaluation of the adiposity patient follow-up registry. Pediatr Obes. (2023) 18(3):e12989. 10.1111/ijpo.12989 [DOI] [PubMed] [Google Scholar]
- 49.Zimmet P, Hodge A, Nicolson M, Staten M, de Courten M, Moore J, et al. Serum leptin concentration, obesity, and insulin resistance in Western Samoans: cross sectional study. BMJ (Clin Res). (1996) 313(7063):965–9. 10.1136/bmj.313.7063.965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng J, Luo Y, Li Y, Zhang F, Zhang X, Zhou X, et al. Sex- and body mass index-specific reference intervals for serum leptin: a population based study in China. Nutr Metab (Lond). (2022) 19(1):54. 10.1186/s12986-022-00689-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi Q, Wang R, Zhang H, Shan Y, Ye M, Jia B. Association between serum uric acid and cardiovascular disease risk factors in adolescents in America: 2001-2018. PLoS One. (2021) 16(8):e0254590. 10.1371/journal.pone.0254590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ding N, He L, Li C, Su Y. Uric acid and blood pressure in NHANES dated from 2009 to 2018: a cross-sectional research. Nutr Metab Cardiovasc Dis. (2022) 32(11):2568–78. 10.1016/j.numecd.2022.08.017 [DOI] [PubMed] [Google Scholar]
- 53.Antón FM, García Puig J, Ramos T, González P, Ordás J. Sex differences in uric acid metabolism in adults: evidence for a lack of influence of estradiol-17 beta (E2) on the renal handling of urate. Metabolism: Clinical and Experimental. (1986) 35(4):343–8. 10.1016/0026-0495(86)90152-6 [DOI] [PubMed] [Google Scholar]
- 54.Kolz M, Johnson T, Sanna S, Teumer A, Vitart V, Perola M, et al. Meta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genet. (2009) 5(6):e1000504. 10.1371/journal.pgen.1000504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miah R, Fariha KA, Sony SA, Ahmed S, Hasan M, Mou AD, et al. Association of serum xanthine oxidase levels with hypertension: a study on Bangladeshi adults. Sci Rep. (2022) 12(1):21727. 10.1038/s41598-022-26341-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ali N, Rahman S, Islam S, Haque T, Molla NH, Sumon AH, et al. The relationship between serum uric acid and lipid profile in Bangladeshi adults. BMC Cardiovasc Disord. (2019) 19(1):42. 10.1186/s12872-019-1026-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tian S, Liu Y, Xu Y, Feng A. Does obesity modify the epidemiological association between hyperuricemia and the prevalence of hypertension among northern Chinese community-dwelling people? A Chinese population-based study. BMJ open. (2019) 9(11):e031803. 10.1136/bmjopen-2019-031803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kubota M. Hyperuricemia in children and adolescents: present knowledge and future directions. J Nutr Metab. (2019) 2019:3480718. 10.1155/2019/3480718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hu YM, Jiang ZF, Shen KL, Shen Y. Zhufutang practical pediatrics (8th edition) Chin Med J. (2015) 50(03):48. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.