Introduction
Management of coronary artery disease (CAD) patients remains highly challenging, as underlined by the elevated residual risk of ischemic events,1 despite impressive progress during the last decades. Interventional cardiology has mainly been focused on research in the field of coronary stents, but since the advent of second-generation stents, recent advances have been reported mainly on target vessel revascularization (TVR). As CAD requires a more comprehensive approach, this article aims to provide an overview of several new broader strategies that have proven clinical impact by reducing major events in combination with percutaneous coronary interventions (PCI).
The mechanical approach
Over the years, PCI techniques have been refined to include newer generation stents with thinner struts, absorbable polymer coating, and open cell designs to treat coronary artery stenosis. In combination with other technologies that enable plaque modification, such as cutting balloons, rotational atherectomy, laser atherectomy, diamondback atherectomy, and more recently intracoronary lithotripsy, interventional cardiologists are able to treat more complex disease percutaneously. Such developments and tools have improved the effectiveness and safety profile of PCI significantly. However, ∼20–40% of patients have recurrent or persistent angina after PCI.1 Potential mechanisms include recurrent ischemic lesions due to stent thrombosis, in-stent restenosis (ISR), residual diffuse disease, myocardial bridging, coronary microvascular dysfunction, and more importantly, suboptimal revascularization. With newer-generation stents, rates of stent thrombosis are <1% and rates of clinical restenosis are about 5% for ISR at 1 year and 12.2% per lesion during follow-up, but these rates can vary based on coronary anatomy and risk factor (RF) control. After stenting with second-generation drug-eluting stents (DES), the incidence of target lesion revascularization (TLR) at 5 years and 10 years is estimated to be about 10% and 20% respectively.2,3
In addition, the rate of ISR remains high during follow-up with reported rates of 30.1% with bare metal stents, 14.6% with first-generation (DES), and annual ISR incidence remains at least 1–2% in contemporary analysis.4,5
Improvements in device technology
Since the advent in 1986 of the first coronary stent WALLSTENT® (Schneider AG), which was a self-expanding, stainless steel wire-mesh structure, many more contemporary iterations have revolutionized PCI.6 The most notable transformation was the development of stents that delivered an in situ drug reaching the atheromatous plaque by diffusion and not merely mechanically ‘crushing’ the plaque, i.e. DES. First-generation DES releasing sirolimus or paclitaxel significantly reduced neointimal hyperplasia but were hampered by a high rate of thrombotic events related to the stent.5 The 2010s saw the approval by the FDA of everolimus and zotarolimus-eluting stents.5 Second and latest generation DES have overcome of the disadvantages of earlier models, by integrating more efficient drug-elution mechanisms.5,7 Other modifications in the design of contemporary DES focused on the absorbable polymers that could be limited to an abluminal surface. Furthermore, the development of thinner struts reduced the intraluminal steric hindrance and improved the deliverability. For example, the BIOFLOW V study demonstrated a 40% relative reduction in target lesion failure, defined as the composite of cardiac death, target vessel-related myocardial infarction (MI), or ischemia-driven TLR, as well as significantly lower rates of target-vessel MI, ischemia-driven TLR, and late/very late stent thrombosis at 3 years with the bioresorbable-polymer ORSIRO DES stent.8,9 Despite technical improvements, the rate of ISR and need for TLR remains around 1% to 2% per year with contemporary DES technologies.5 Given the number of PCI procedures worldwide, this represents a major public health burden. Furthermore, it is estimated that PCI for ISR accounts for 10% of all PCI procedures in the USA, and the risk of major adverse cardiovascular events (MACE) is reportedly higher during procedures for ISR than in PCI procedures for de novo lesions.5
Improvements in revascularization technique with intracoronary physiology
Optimization of revascularization has witnessed progress as well. Today, operators have several tools at their disposal to evaluate a lesion more comprehensively. Intracoronary imaging with intravascular ultrasound (IVUS) or optical coherence tomography (OCT) and functional assessment of coronary physiology with fractional flow reserve (FFR) or instantaneous wave-free ratio (iFR) allow operators to determine the significance of a lesion, the need for atherectomy, the appropriate size of the DES, which optimizes the results.
FFR after stenting proved to be a strong and independent predictor for MACE in several studies whereby a post-PCI FFR of at least 0.90 is associated with a lower risk of repeat PCI and MACE.10,11 In the FFR-SEARCH study, post-PCI FFR assessment demonstrated that a functionally suboptimal result is frequent. After an angiographically successful PCI, the final FFR value in approximately half of the cases was below 0.90% and 10% under the ‘ischemic threshold’ of 0.80.12
Results of such physiology-based studies suggest suboptimal PCI. Reasons for suboptimal revascularization include under-expansion, malapposition, or edge dissections of the stent. Intracoronary imaging studies have made it possible to identify areas requiring further optimization. In the IVUS sub-analysis of the FFR-SEARCH, patients with an FFR ≤0.85, stent under-expansion was reported in 74% of the cases.13 Post-dilatation or further stenting was shown to reduce the ischemic burden.14 Diffuse coronary disease poses a particular challenge for PCI operators. The ‘pullback pressure gradient’ (PPG) enables better characterization of the pattern of distribution of disease, with such vessels often encountered in diabetic patients. A higher PPG index indicates a more focal pattern allowing operators to strategize accordingly and limit the stented segment.15 In this study, PCI in diffuse disease was associated with a higher release of markers of myocardial injury and longer stented segments, questioning the value of PCI in this setting. Recently, the DEFINE-PCI study reported fewer cardiac events after Optimization of PCI by using iFR guidance. At one year, patients with post-PCI iFR ≥0.95 with near complete restoration of coronary patency was associated with lower rates of cardiac death, spontaneous MI, or clinically driven TVR over the next year compared with patients with a lower residual iFR.16 The larger DEFINE-GPS trial is ongoing to validate this concept (NCT04451044).
Improvements in revascularization technique with intracoronary imaging
Similarly, several trials demonstrated the utility of intracoronary imaging with IVUS or OCT to optimize PCI and reduce MACE. Most notably, the IVUS XPL trial reported more adjunct post-dilation (76% vs. 57%) and a larger mean final balloon size (3.15 ± 0.43 vs. 3.05 ± 0.42 mm, P < 0.001) in the IVUS guided arm. Patients who underwent an IVUS-guided procedure had a higher minimum lumen diameter (2.64 ± 0.42 vs. 2.56 ± 0.4 mm, P = 0.001) and lower diameter stenosis (12.9 ± 8.6% vs. 13.5 ± 8.1, P = 0.216%) on post-intervention quantitative coronary angiography. The 12-month MACE (cardiac death, target lesion-related MI, or ischemia-driven TLR) occurred less frequently in the IVUS-guided arm (2.9% vs. 5.8%, P = 0.007) due to a reduction in ischemia-driven TLR. At 5-year follow-up, the cumulative incidence of MACE remained lower with IVUS guidance.17 These findings are in line with other data from ULTIMATE and ADAPT DES trials.18,19 Similarly, OCT data further confirms that intracoronary imaging facilitates procedural success, achieves a larger lumen and reduces MACE.20 The largest OCT trial, albeit observational, is the Pan London study where OCT was used in 1149 (1.3%) patients, IVUS was used in 10 971 (12.6%) patients, and angiography alone in 75 046 patients. OCT-guided procedures were associated with greater procedural success and reduced in-hospital MACE. A significant difference in mortality was observed between patients who underwent OCT-guided PCI (7.7%) compared with patients who underwent either IVUS-guided (12.2%) or angiography-guided (15.7%) PCI in both elective and acute coronary syndrome (ACS) subgroups.21 The use of intracoronary imaging to guide and optimize stent implantation may help to mitigate the stent-related factors that are associated with increased risk of ISR. Figure 1 illustrates OCT images of left main stenosis PCI guided by OCT. Randomized data from the Ilumien Trial series are still awaited. Finally, noninvasive evaluation of coronary arteries using cardiac computed tomography and CT-FFR is an emerging option, but requires further randomized assessment.
Figure 1.
OCT-guided PCI of left main pre (A) and post stent dilatation (B).
Overall, coronary revascularization cannot replace the need for guideline-directed medical therapies, cardiac rehabilitation, and lifestyle changes to reduce MACE and avoid repeat PCI.22,23 In addition, appropriately recognizing and treating ischemia or angina in non-obstructive disease is imperative.24
The lipid approach
The cardiovascular benefits of statins are well established, in terms of recurrent MI, cardiovascular mortality, and total mortality.25,26 The magnitude of benefits depends on the intensity of LDL-C decrease and time exposure under treatment.27 Additional benefits have been reported with ezetimibe in combination with statins in post ACS patients (IMPROVE-IT).28
Newer lipid-lowering treatments (LLT) that provide an intense and prolonged decrease in LDL-cholesterol or by modulation of omega 3 fatty acid blood levels have recently been evaluated in randomized clinical trials (RCTs) with cardiovascular outcomes. Results of key trials with lipid-lowering agents are summarized in Table 1.
Table 1.
Results of key studies of lipid-lowering therapies
| Study Acronym, Ref | Population | Treatment | Primary outcome | Key results |
|---|---|---|---|---|
| IMPROVE-IT28 | 18 144 patients hospitalized for ACS <10 days within the preceding 10 days and with LDL-c 50 to 100 mg/dL if receiving LLT, or 50 to 125 mg/dL if not receiving LLT | Simvastatin 40 mg plus ezetimibe 10 mg vs. simvastatin 40 mg and placebo | Composite of CV death, nonfatal MI, UA requiring rehospitalization, coronary revascularization (≥30 days), or nonfatal stroke | Mean LDL-c was 53.7 mg/dL in the simvastatin-ezetimibe group, vs. 69.5 mg/dL in the simvastatin-monotherapy group (P < 0.001). Primary endpoint event rate at 7 years was 32.7% vs. 34.7% respectively (absolute risk difference, 2.0%age points; HR 0.936; 95% CI, 0.89 to 0.99; P = 0.016). |
| FOURIER29 | 27 564 patients with atherosclerotic CV disease and LDL-c levels of ≥70 mg/dL who were receiving statin therapy | Evolocumab (either 140 mg every 2 weeks or 420 mg monthly) or matching placebo as subcutaneous injections. | Composite of CV death, MI, stroke, hospitalization for UA, or coronary revascularization | At 48 weeks, mean reduction in LDL-c with evolocumab was 59%, from a median baseline value of 92 to 30 mg/dL (P < 0.001). Evolocumab significantly reduced the risk of the primary endpoint vs. placebo (HR, 0.85; 95% CI 0.79 to 0.92; P < 0.001) |
| ODYSSEY OUTCOMES30 | 18 924 patients who had ACS 1 to 12 months earlier, had LDL-c of at least 70 mg/dL, a non-HDL-c level of at least 100 mg/dL, or apolipoprotein B level of at least 80 mg/dL, and were receiving statin therapy at high-intensity or maximum tolerated dose | Alirocumab subcutaneously at a dose of 75 mg or matching placebo every 2 weeks | Composite of death from coronary heart disease, nonfatal MI, fatal or nonfatal ischemic stroke, or UA requiring hospitalization | The primary endpoint occurred in 9.5% in the alirocumab group vs. 11.1% in the placebo group (HR, 0.85; 95% CI 0.78 to 0.93; P < 0.001). Mortality rate was 3.5% vs. 4.1% respectively (HR 0.85; 95% CI, 0.73 to 0.98). absolute benefit of alirocumab in terms of the primary composite endpoint was greater in patients with baseline LDL-c of 100 mg/dL or more compared to those with lower levels. |
| FOURIER OLE31 | 6635 patients who completed FOURIER at participating sites and were eligible to receive evolocumab in 2 open-label extension studies (FOURIER Open-Label Extension [OLE]) in the USA and Europe. | Evolocumab and placebo in the parent study | Incidence of adverse events. Lipid values and MACE were prospectively collected. |
Incidence of serious adverse events with long term evolocumab did not exceed those with placebo. Patients originally randomized to evolocumab had a 15% lower risk of CV death, MI, stroke, or hospitalization for UA or coronary revascularization vs. placebo (HR 0.85 [95% CI, 0.75–0.96]; P = 0.008); a 20% lower risk of CV death, MI or stroke (HR, 0.80 [95% CI, 0.68–0.93]; P = 0.003); and a 23% lower risk of CV death (HR, 0.77 [95% CI, 0.60–0.99]; P = 0.04). |
| ORION-4 (ongoing; NCT03705234) | Patients with prior MI or prior ischemic stroke or PAD | Inclisiran sodium 300 mg administered as a subcutaneous injection at randomization, 3 months and then every 6 months; or placebo. | Composite of coronary heart disease death, MI, fatal or non-fatal ischemic stroke or urgent coronary revascularization. | Study ongoing |
| VICTORION-2 PREVENT (ongoing, NCT05030428) | Patients with LDL-c ≥ 70 mg/dL and stable, well-tolerated LLT (i.e. high-intensity statin, with or without ezetimibe) and established CV disease. | Inclisiran sodium 300 mg administered as a subcutaneous injection on day 1, at 3 months and then every 6 months thereafter; or placebo. | Composite endpoint including CV death, non-fatal MI and non-fatal ischemic stroke | Study ongoing |
| Systematic Review by Di Minno et al32 | Seven studies were totalling 2767 BA-treated patients and 1469 controls | 180 mg daily | Efficacy outcome assessed by % changes in total cholesterol, LDL-c, triglycerides, HDL-c, apolipoprotein B, non-HDL-c, and hs-CRP in BA patients and controls | Significant reduction in LDL-c (MD, −17.5%; 95% CI, −22.9% to −12.0%), total cholesterol (MD, −10.9%; 95% CI, −13.3% to −8.5%), non–HDL-c (MD, −12.3%; 95% CI, −15.3% to −9.20%), apolipoprotein B (MD, −10.6%; 95% CI, −13.2% to −8.02%), and hs-CRP (MD, −13.2%; 95% CI, −16.7% to −9.79%) with BA vs. controls. |
| JELIS33 | 18 645 patients with total cholesterol of 6.5 mmol/L or greater recruited throughout Japan between 1996 and 1999. | 1800 mg of EPA daily with statin (EPA group) or statin only (control group) | Any major coronary event, including sudden cardiac death, fatal and non-fatal MI, and other non-fatal events including UA, angioplasty, stenting, or CABG | The primary endpoint occurred in 2.8% in the EPA group vs. 3.5% of controls, yielding a 19% relative reduction in major coronary events (P = 0.011). In EPA-treated patients with a history of CAD, major coronary events were reduced by 19% (8.7% in EPA vs. 10.7% in controls; P = 0.048). In patients with no history of CAD, there was non-significant reduction in major coronary events with EPA (1.4% vs. 1.7% in the control group; P = 0.132). |
| REDUCE-IT34 | 8179 patients with established CV disease or with diabetes plus other risk factors, receiving statin therapy and with fasting triglyceride level of 135–499 mg/dL and LDL-c of 41 to 100 mg/dL | 2 g of icosapent ethyl twice daily (total daily dose, 4 g) or placebo. | Composite of CV death, nonfatal MI, nonfatal stroke, coronary revascularization, or UA. | The primary endpoint occurred in 17.2% in the EPA group vs. 22.0% in the placebo group (HR, 0.75; 95% CI, 0.68 to 0.83; P < 0.001). Rates of additional ischemic endpoints were significantly lower in the EPA group vs. placebo, including CV death (4.3% vs. 5.2%; HR, 0.80; 95% CI, 0.66 to 0.98; P = 0.03). |
ACS, acute coronary syndrome; LDL-c, low density lipoprotein cholesterol; LLT, lipid-lowering therapy; CV, cardiovascular; MI, myocardial infarction; UA, unstable angina; HR, hazard ratio; 95% CI, 95% confidence interval; HDL-c, high density lipoprotein cholesterol; MACE, major adverse cardiovascular events; PAD, peripheral artery disease; BA, bempedoic acid; hs-CRP, high-sensitivity C-reactive protein; MD, mean difference; EPA, icosapent ethyl; CABG, coronary artery bypass graft;
Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors
These drugs act by reducing the density of LDL receptors on the cell membrane via inhibition of the PCSK9, which plays a key role in the regulation of the LDL-cholesterol level.
The two RCTs evaluating PCSK9 inhibitors (FOURIER and ODYSSEY OUTCOMES) reported cardiovascular benefits during a relatively short-term follow-up, one in patients with atherosclerotic cardiovascular disease (ASCVD), including chronic coronary stable patients, peripheral artery disease and prior stroke patients with a median follow-up of 2.2 years;29 and the other in post-ACS patients with a median follow up of 2.8 years.30 In patients with LDL-C levels of 70 mg/dL or higher on maximal tolerated dose of statin therapy, both trials demonstrated that PCSK9i reduced the primary combined endpoint (MI, stroke, cardiovascular death, hospitalisation, unstable angina, or coronary revascularisation) with a relative risk reduction of 15%, without safety issues even for very low LDL-C levels.29,30 As the cardiovascular risk was higher in patients with diabetes, the clinical benefits of PCSK9i were more pronounced, and glycemic levels remained stable under PCSK9i therapy during follow-up.29,30,35
Both PCSK9i were effective by reducing a composite primary end point by 15% with evolocumab [HR 0.85 (0.79–0.92), P < 0.001] and by 15% with alirocumab [HR 0.85 (0.78–0.93), P < 0.001].29,30 Furthermore, evolocumab reduced the need for repeat coronary revascularization by 22% (HR 0.78, 95% CI: 0.71–0.86; P = 0.01), either elective (HR 0.83, 95% CI: 0.73–0.95) or urgent (HR 0.73, 95% CI: 0.64–0.83).29,31 Alirocumab was also effective in reducing ischemia-driven coronary revascularizations by 12% (HR 0.88, CI: 0.79–0.97, P = 0.009), and unstable angina requiring hospitalizations [HR 0.61 (0.41–0.92)].30
The FOURIER OLE (Open Label Extension) has recently reported that a longer follow-up (median: 6 years with a maximum of 8 years) is also associated with a reduction in cardiovascular mortality compared with patients who started evolocumab later, advocating the concept ‘the earlier, the deeper, the longer, the better’ for the management of patients in secondary prevention, with no safety concern for a median LDL-cholesterol value of 30 mg/dL.
This study advocates the importance of time-exposure to LLT to better evaluate the magnitude of cardiovascular benefits and the safety.31
Inclisiran
Inclisiran is new drug to lower LDL and is also a PCSK9i, but works differently than the preceding forms. This novel drug is a small RNA molecule that targets the hepatic production of PCSK9 and induces gene silencing through an inhibition of transcription of specific genes. This regulation promotes cleavage and elimination of PCSK9, and therefore decreases the LDL-C level by 50–70% with an excellent clinical safety in phase II clinical trials, requiring biannual injection during its maintenance phase.36 The less frequent dosing schedule of inclisiran, compared to PCSK9 inhibitors, could be advantageous for patient compliance. Also, inclisiran inhibits the synthesis of PCSK9 in the liver, thus leading to a genuine reduction in protein production, whereas PCSK9i bind to extracellular PCSK9, preventing it from binding to the LDL receptor, and this situation may be reflected by increased levels of PCSK9 in the plasma.
The ongoing phase III randomized control trial ORION 4 is evaluating the clinical efficacy of inclisiran vs. placebo in secondary prevention atherosclerotic patients (ORION 4, NCT NCT03705234), with expected results in 2025. The VICTORION-2 PREVENT study, investigating the efficacy of inclisiran on a composite of CV death, non-fatal MI and non-fatal ischemic stroke (NCT05030428) is currently recruiting, with completion planned for 2027.
Bempedoic acid (BA)
BA (ETC-1002) (BA) is another new LLT that reduces cholesterol synthesis by inhibiting adenosine triphosphate citrate lyase.37 The decrease of cholesterol synthesis promotes an upregulation of LDL-receptors and decreases LDL-C.32 As BA is converted to its active moiety by the long-chain acyl-CoA synthetase-1, an enzyme found only in the liver, BA is believed not to be associated with myalgia, and may be well tolerated in patients with muscle symptoms encountered with statins.37 A decrease of 20% to 25% in LDL-C would be expected to provide benefits on coronary events, including PCI, and indeed, a signifcant reduction in coronary revascularization was recently seen in CLEAR Outcomes.32
Icosapent ethyl (EPA)
EPA is a purified eicosapentaenoic acid formulation, whose biological actions include vasodilation, reduction of platelet aggregation, and plaque stabilisation, underpinning potential to reduce cardiovascular events.33 The clinical efficacy of EPA has been demonstrated, first in the JELIS trial,33 then in REDUCE-IT,34 in which ASCVD patients (70.7%) or patients with diabetes and associated cardiovascular RFs were included. The triglyceride level at entry was between 135 to 499 mg/dL, and by protocol, all patients were to be treated by statin at inclusion. A total of 8179 patients were randomized to 2 g bid of EPA or placebo. The strategy based on EPA reduced the primary combined endpoint (CV death, nonfatal MI, nonfatal stroke, coronary revascularization, or unstable angina) by 25% [P < 0.0001)] at a median follow-up of 4.9 years. The clinical efficacy of EPA was more pronounced in populations with higher baseline risk, and the cardiovascular benefits were not related either to baseline triglyceride or LDL-C levels, suggesting other underlying mechanisms of action.34
EPA also reduced the rates of coronary revascularisations with a HR of 0.66 [95% CI: 0.58–0.76, P < 0.0001)] for first revascularisations and a HR of 0.64 [95% CI: 0.56–0.74, P < 0.0001)] for total (first and subsequent) procedures. EPA was effective in reducing PCI with a HR of 0.68 [95% CI: 0.59–0.79, P < 0.0001)] and also coronary artery bypass graft surgery, with a HR of 0.61 [95% CI: 0.45–0.81, P = 0.0005)].38 These data advocate that EPA may be part of the strategy to reduce residual risk in patients with established cardiovascular disease or diabetes. Regarding safety, a slightly increased risk of hospitalization for atrial fibrillation or flutter was reported (3.1% vs. 2.1%, P = 0.004).34 The 2019 EAS/ESC guidelines and the National Lipid Association have integrated this new option to decrease MACE in patients with established cardiovascular disease or diabetes.23
The glucometabolic approach
What we have recently learned about sodium-glucose cotransporter-2 inhibitors (SGLT2i) and Glucagon-like peptide 1 receptor agonists (GLP1-RA) in CAD patients treated with PCI?
New antidiabetic agents have been found to have specific cardiovascular benefits. Two new therapeutic classes are GLP1-RA and SGLT2i. Both classes have demonstrated benefits in RCTs that go beyond glycaemic control and could contribute to the reduction of primary or recurrent cardiovascular events. Current guidelines have changed to recommend GLP-1-RA or SGLT2i as the preferred therapy after metformin in patients with T2D with established cardiovascular disease.39
Glucagon-like peptide-1 (GLP-1) receptor agonists
A recent meta-analysis comparing GLP1-RA and SGLT2i to optimal medical treatment reported no difference between both classes in terms of MI, stroke, cardiovascular mortality 0.94; 95% CI 0.83–1.08), and total mortality.40 The underlying mechanisms promoting cardiovascular benefits are not yet fully explained.
Analogues of human GLP-1 provide higher and constant GLP1 levels with either daily or weekly injections controlling diabetes without severe hypoglycemia. In addition to the decrease in glycemia, GLP1-RA have pleiotropic actions with reductions in both weight and blood pressure.41
Several RCTs with GLP-1 receptor agonists reached their primary objective of a reduction in MACE in patients with type 2 diabetes, namely HARMONY (albiglutide) (HR 0.78, [CI], 0.68–0.90), P = 0.0006),42 LEADER (liraglutide) (HR, 0.87; [CI], 0.78–0.97; P = 0.001),43 SUSTAIN 6 (semaglutide) (HR 0.74; [CI], 0.58–0.95; P = 0.02),44 and REWIND (dulaglitide) (HR 0.88, [CI],0.79–0.99; P = 0·026).45 Conversely, the PIONEER-6 study did not find benefits in terms of MACE (HR 0.79; 95% CI 0.57–1.11; P < 0.001 for noninferiority; P = 0.17 for superiority).46
The heterogeneity of the clinical efficacy between GLP1 receptor agonists may be explained by the heterogeneity of pharmacological properties. There is no specific study to date evaluating the efficacy of GLP1 analogues on recurrent PCI, underlying the need for dedicated research in this field to better define the magnitude of specific benefits. In conclusion, GLP1-RA with proven clinical cardiovascular benefits should be started early in coronary patients.
SGLT2i and more
SGLT2i is a therapeutic class that decreases kidney glucose reabsorption, and increases glycosuria and natriuresis without potassium loss, which promotes lower systolic blood pressure. The decrease in glucose plasma levels is small and safe with rare severe hypoglycemia. Chronic administration is associated with weight loss, and better metabolic profile with increased lipolysis, increased glucagon secretion, improved lipid oxidation, and lower circulating insulin levels.47,48
For SGLT2i, protection from MI is also well documented; in all four major RCTs there were high percentages of participants with a history of cardiovascular disease: the EMPA-REG OUTCOME study (100%),49 CANVAS (65.6%),50 DECLARE-TIMI 58 (40.6%),51 CREDENCE (50.5%).52 SGLT2i reduced the risk of MI by 12% (HR, 0.88; 95% CI, 0.80–0.97) with an overall 17% relative reduction in cardiovascular death (HR, 0.83; 95% CI, 0.75–0.92), with major benefits in patients with a previous history of established cardiovascular disease.53
The effects of GLP-1 agonist treatment compared to SGLT2-inhibitor seem more pronounced on HbA1c and weight loss, whereas SGLT2i are more effective on kidney protection and heart failure (HF) reduction. Potential synergism in cardiovascular events and kidney protection is being evaluated by ongoing trials.54–56 A recent meta-analysis of SGLT-2i RCT confirmed the reduced risk of major ischemic CV events, in addition, with kidney protection and efficacy in patients with HF.57
Tirzepatide represents a novel dual GIP and GLP1 agonist that improved glycemic control and decreased weight without increasing the risk of hypoglycemia. A recent phase 3 RCT (SURPASS 1) has evaluated three doses (5, 10, and 15 mg) vs. placebo.58,59 The efficacy of tirzepatide compared to dulaglutide in terms of metabolic parameters is being confirmed by an ongoing cardiovascular outcomes trial in participants with type 2 diabetes and increased cardiovascular risk (NCT04255433).
Perspectives
All these new classes have proven clinical efficacy on various key outcomes, with no major safety signals. Nevertheless, further research is warranted to better define the high-risk coronary patients who will benefit most from these promising but currently expensive treatments. Indeed, cost effectiveness remains one of the major challenges, and finding innovative solutions to wider implementation of these drugs will probably be one of the major goals in the coming years to decrease recurrent ischemic events worldwide.
Conclusion
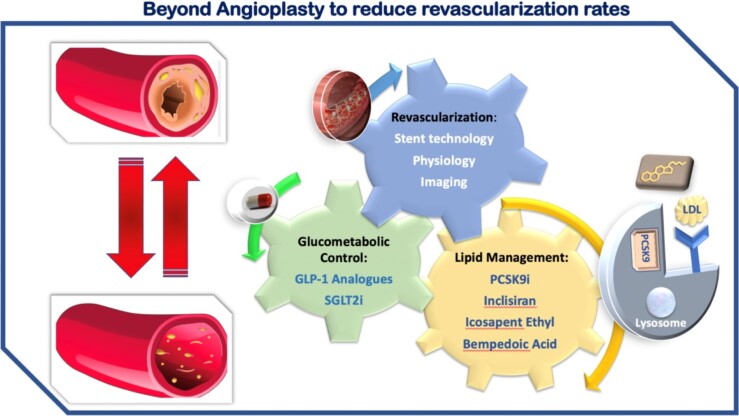
New strategies ‘outside of the stent’ yield a reduction in recurrent PCI and decrease MACE by potent modulation of the RFs contributing to recurrent events (Figure 2). The community of interventional cardiologists should be aware of the magnitude of cardiovascular benefits provided by these new approaches in order to improve the short- and long-term prognosis of their CAD patients and provide the optimal medical strategy in combination with coronary revascularisation. Indeed, prescribing optimal medical therapy, targeting not only lipid-lowering but also the metabolic pathway, and ensuring patient compliance, can go a long way towards reducing the residual risk, thereby having a downstream effect on the number of patients requiring repeat interventional procedures. Further efforts are mandatory regarding specificities of pathophysiology and management in special subgroups, such as women and ethnic minorities, who less often receive proven therapeutic strategies to reduce major cardiovascular events.
Figure 2.
Central illustration: beyond angioplasty to reduce revascularization.
Contributor Information
Pierre Sabouret, Heart Institute, ACTION Study Group-CHU Pitié-Salpétrière Paris, 47-83 Boulevard de l'Hôpital, 75005 Paris, France; Collège National des Cardiologues Français (CNCF), 13 rue Niepce, 75014 Paris, France.
Stéphane Manzo-Silberman, Heart Institute, ACTION Study Group-CHU Pitié-Salpétrière Paris, 47-83 Boulevard de l'Hôpital, 75005 Paris, France.
Mirvat Alasnag, Cardiac Center, King Fahd Armed Forces Hospital, Jeddah, Saudi Arabia.
Marinos Fysekidis, Department of endocrinology, Avicenne Hospital, AP-HP, 125, rue de Stalingrad, 93000 Bobigny, France.
Martha Gulati, Barbra Streisand Women’s Heart Center, Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, USA.
Giuseppe Galati, Heart Failure Unit, Division of Cardiology, Department of Cardiothoracic and Vascular, San Raffaele Hospital, Scientific Institute (IRCCS), Via Olgettina 60, 20132 Milan, Italy.
Luigi Spadafora, Department of Clinical, Internal Medicine, Anesthesiology and Cardiovascular Sciences, Sapienza University of Rome, Rome, Italy.
Maciej Banach, Department of Preventive Cardiology and Lipidology, Medical University of Lodz and Polish Mother's Memorial Hospital Research Institute, Lodz, Poland.
Giuseppe Biondi-Zoccai, Department of Medical-Surgical Sciences and Biotechnologies, Sapienza University of Rome, Latina, Italy; Mediterranea Cardiocentro, Napoli, Italy.
Deepak L Bhatt, Mount Sinai Heart, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Conflicts of interest
Dr Pierre Sabouret: lecture fees from Astra-Zeneca, Bayer, Les Laboratoires Servier, Novartis, Novonordisk, Recordati, Sanofi, Vifor, outside the submitted work.
Dr Stéphane Manzo-Silberman: consulting fees from Bayer, Organon, Exeltis, lecture fees from Bayer, BMS, Exeltis and Organon, has served in the adjudication board for a study for Biotronik.
Dr Maciej Banach: speakers bureau: Amgen, KRKA, Polpharma, Novartis, Sanofi-Aventis, Teva, and Zentiva; consultant to Amgen, Daiichi Sankyo, Esperion, Novartis, and Sanofi-Aventis; grants from Amgen, Sanofi, and Valeant, outside the submitted work.
Dr. Deepak L. Bhatt discloses the following relationships—Advisory Board: Boehringer Ingelheim, Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Janssen, Level Ex, Medscape Cardiology, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, Stasys; Board of Directors: Boston VA Research Institute, Bristol Myers Squibb (stock), DRS.LINQ (stock options), Society of Cardiovascular Patient Care, TobeSoft; Chair: Inaugural Chair, American Heart Association Quality Oversight Committee; Data Monitoring Committees: Acesion Pharma, Assistance Publique-Hôpitaux de Paris, Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Boston Scientific (Chair, PEITHO trial), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Novartis, Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Chair, ACC Accreditation Oversight Committee), Arnold and Porter law firm (work related to Sanofi/Bristol-Myers Squibb clopidogrel litigation), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Cowen and Company, Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Piper Sandler, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Afimmune, Aker Biomarine, Amarin, Amgen, AstraZeneca, Bayer, Beren, Boehringer Ingelheim, Bristol-Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CSL Behring, Eisai, Ethicon, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Javelin, Lexicon, Lilly, Medtronic, Moderna, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Owkin, Pfizer, PhaseBio, PLx Pharma, Recardio, Regeneron, Reid Hoffman Foundation, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, 89Bio; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Abbott, Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Philips, Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, Takeda.
The other coauthors have no disclosure to declare in relation with this article.
Authors’ contributions
P.S. conceived the article. P.S. and S.M.S. did the literary search and screened the articles. P.S., S.M.S., and M.A. wrote the first draft of the manuscript, PS wrote the other drafts.
All authors critically reviewed the manuscript. All authors read and approved the final version of the manuscript.
Data availability
All data is included in the submission/manuscript file.
References
- 1. Ben-Yehuda O, Kazi DS, Bonafede M, Wade SW, Machacz SF, Stephens LA, Hlatky MA, Hernandez JB. Angina and associated healthcare costs following percutaneous coronary intervention: A real-world analysis from a multi-payer database. Catheter Cardiovasc Interv 2016;88:1017–1024. [DOI] [PubMed] [Google Scholar]
- 2. Kufner S, Joner M, Thannheimer A, Hoppmann P, Ibrahim T, Mayer K, Cassese S, Laugwitz KL, Schunkert H, Kastrati A, Byrne RA. Ten-Year clinical outcomes from a trial of three limus-eluting stents with different polymer coatings in patients with coronary artery disease. Circulation 2019;139:325–333. [DOI] [PubMed] [Google Scholar]
- 3. Pilgrim T, Piccolo R, Heg D, Roffi M, Tuller D, Muller O, Moarof I, Siontis GCM, Cook S, Weilenmann D, Kaiser C, Cuculi F, Hunziker L, Eberli FR, Juni P, Windecker S. Ultrathin-strut, biodegradable-polymer, sirolimus-eluting stents versus thin-strut, durable-polymer, everolimus-eluting stents for percutaneous coronary revascularisation: 5-year outcomes of the BIOSCIENCE randomised trial. Lancet 2018;392:737–746. [DOI] [PubMed] [Google Scholar]
- 4. Cassese S, Byrne RA, Tada T, Pinieck S, Joner M, Ibrahim T, King LA, Fusaro M, Laugwitz KL, Kastrati A. Incidence and predictors of restenosis after coronary stenting in 10 004 patients with surveillance angiography. Heart 2014;100:153–159. [DOI] [PubMed] [Google Scholar]
- 5. Giustino G, Colombo A, Camaj A, Yasumura K, Mehran R, Stone GW, Kini A, Sharma SK. Coronary in-stent restenosis: JACC state-of-the-art review. J Am Coll Cardiol 2022;80:348–372. [DOI] [PubMed] [Google Scholar]
- 6. Sigwart U, Puel J, Mirkovitch V, Joffre F, Kappenberger L. Intravascular stents to prevent occlusion and restenosis after transluminal angioplasty. N Engl J Med 1987;316:701–706. [DOI] [PubMed] [Google Scholar]
- 7. Barreto Gameiro Silva G, de Ribamar Costa J Jr, Sousa A, Sousa JE, Abizaid A. Drug-eluting stents: state-of-the-art. Rev Soc Cardiol 2018;28:54–59. [Google Scholar]
- 8. Kandzari DE, Koolen JJ, Doros G, Garcia-Garcia HM, Bennett J, Roguin A, Gharib EG, Cutlip DE, Waksman R. Ultrathin bioresorbable-polymer sirolimus-eluting stents versus thin durable-polymer everolimus-eluting stents for coronary revascularization: 3-year outcomes from the randomized BIOFLOW V trial. JACC Cardiovasc Interv 2020;13:1343–1353. [DOI] [PubMed] [Google Scholar]
- 9. Kandzari DE, Koolen JJ, Doros G, Massaro JJ, Garcia-Garcia HM, Bennett J, Roguin A, Gharib EG, Cutlip DE, Waksman R, Investigators BV. Ultrathin bioresorbable polymer sirolimus-eluting stents versus thin durable polymer everolimus-eluting stents. J Am Coll Cardiol 2018;72:3287–3297. [DOI] [PubMed] [Google Scholar]
- 10. Johnson NP, Toth GG, Lai D, Zhu H, Acar G, Agostoni P, Appelman Y, Arslan F, Barbato E, Chen SL, Di Serafino L, Dominguez-Franco AJ, Dupouy P, Esen AM, Esen OB, Hamilos M, Iwasaki K, Jensen LO, Jimenez-Navarro MF, Katritsis DG, Kocaman SA, Koo BK, Lopez-Palop R, Lorin JD, Miller LH, Muller O, Nam CW, Oud N, Puymirat E, Rieber J, Rioufol G, Rodes-Cabau J, Sedlis SP, Takeishi Y, Tonino PA, Van Belle E, Verna E, Werner GS, Fearon WF, Pijls NH, De Bruyne B, Gould KL. Prognostic value of fractional flow reserve: linking physiologic severity to clinical outcomes. J Am Coll Cardiol 2014;64:1641–1654. [DOI] [PubMed] [Google Scholar]
- 11. Wolfrum M, Fahrni G, de Maria GL, Knapp G, Curzen N, Kharbanda RK, Frohlich GM, Banning AP. Impact of impaired fractional flow reserve after coronary interventions on outcomes: a systematic review and meta-analysis. BMC Cardiovasc Disord 2016;16:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van Bommel RJ, Masdjedi K, Diletti R, Lemmert ME, van Zandvoort L, Wilschut J, Zijlstra F, de Jaegere P, Daemen J, van Mieghem NM. Routine fractional flow reserve measurement after percutaneous coronary intervention. Circ Cardiovasc Interv 2019;12:e007428. [DOI] [PubMed] [Google Scholar]
- 13. van Zandvoort LJC, Masdjedi K, Witberg K, Ligthart J, Tovar Forero MN, Diletti R, Lemmert ME, Wilschut J, de Jaegere PPT, Boersma E, Zijlstra F, Van Mieghem NM, Daemen J. Explanation of postprocedural fractional flow reserve below 0.85. Circ Cardiovasc Interv 2019;12:e007030. [DOI] [PubMed] [Google Scholar]
- 14. Agarwal SK, Kasula S, Hacioglu Y, Ahmed Z, Uretsky BF, Hakeem A. Utilizing post-intervention fractional flow reserve to optimize acute results and the relationship to long-term outcomes. JACC Cardiovasc Interv 2016;9:1022–1031. [DOI] [PubMed] [Google Scholar]
- 15. Collet C, Sonck J, Vandeloo B, Mizukami T, Roosens B, Lochy S, Argacha JF, Schoors D, Colaiori I, Di Gioia G, Kodeboina M, Suzuki H, Van ‘t Veer M, Bartunek J, Barbato E, Cosyns B, De Bruyne B. . Measurement of hyperemic pullback pressure gradients to characterize patterns of coronary atherosclerosis. J Am Coll Cardiol 2019; 74:1772–1784. [DOI] [PubMed] [Google Scholar]
- 16. Jeremias A, Davies JE, Maehara A, Matsumura M, Schneider J, Tang K, Talwar S, Marques K, Shammas NW, Gruberg L, Seto A, Samady H, Sharp A, Ali ZA, Mintz G, Patel M, Stone GW. Blinded physiological assessment of residual ischemia after successful angiographic percutaneous coronary intervention: the DEFINE PCI study. JACC Cardiovasc Interv 2019;12:1991–2001. [DOI] [PubMed] [Google Scholar]
- 17. Hong SJ, Mintz GS, Ahn CM, Kim JS, Kim BK, Ko YG, Kang TS, Kang WC, Kim YH, Hur SH, Hong BK, Choi D, Kwon H, Jang Y, Hong MK. Effect of intravascular ultrasound-guided drug-eluting stent implantation: 5-year follow-up of the IVUS-XPL randomized trial. JACC Cardiovasc Interv 2020;13:62–71. [DOI] [PubMed] [Google Scholar]
- 18. Dohi T, Maehara A, Witzenbichler B, Rinaldi MJ, MazzaferriEL, Jr., Duffy PL, Weisz G, Neumann FJ, Henry TD, Cox DA, Stuckey TD, Brodie BR, Litherland C, Brener SJ, Kirtane AJ, Mintz GS, Stone GW. Etiology, frequency, and clinical outcomes of myocardial infarction after successful drug-eluting stent implantation: two-year follow-up from the ADAPT-DES study. Circ Cardiovasc Interv 2015;8:e002447. [DOI] [PubMed] [Google Scholar]
- 19. Zhang J, Gao X, Kan J, Ge Z, Han L, Lu S, Tian N, Lin S, Lu Q, Wu X, Li Q, Liu Z, Chen Y, Qian X, Wang J, Chai D, Chen C, Li X, Gogas BD, Pan T, Shan S, Ye F, Chen SL. Intravascular ultrasound versus angiography-guided drug-eluting stent implantation: the ULTIMATE trial. J Am Coll Cardiol 2018;72:3126–3137. [DOI] [PubMed] [Google Scholar]
- 20. Prati F, Di Vito L, Biondi-Zoccai G, Occhipinti M, La Manna A, Tamburino C, Burzotta F, Trani C, Porto I, Ramazzotti V, Imola F, Manzoli A, Materia L, Cremonesi A, Albertucci M. Angiography alone versus angiography plus optical coherence tomography to guide decision-making during percutaneous coronary intervention: the Centro per la Lotta contro l'Infarto-Optimisation of Percutaneous Coronary Intervention (CLI-OPCI) study. EuroIntervention 2012;8:823–829. [DOI] [PubMed] [Google Scholar]
- 21. Jones DA, Rathod KS, Koganti S, Hamshere S, Astroulakis Z, Lim P, Sirker A, O'Mahony C, Jain AK, Knight CJ, Dalby MC, Malik IS, Mathur A, Rakhit R, Lockie T, Redwood S, MacCarthy PA, Desilva R, Weerackody R, Wragg A, Smith EJ, Bourantas CV. Angiography alone versus angiography plus optical coherence tomography to guide percutaneous coronary intervention: outcomes from the pan-London PCI cohort. JACC Cardiovasc Interv 2018;11:1313–1321. [DOI] [PubMed] [Google Scholar]
- 22. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC Jr, Sperling L, Virani SS, Yeboah J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: A report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation 2018; 139:e1082–e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen MR, Tokgozoglu L, Wiklund O, Mueller C, Drexel H, Aboyans V, Corsini A, Doehner W, Farnier M, Gigante B, Kayikcioglu M, Krstacic G, Lambrinou E, Lewis BS, Masip J, Moulin P, Petersen S, Petronio AS, Piepoli MF, Pintó X, Räber L, Ray KK, Reiner Ž, Riesen WF, Roffi M, Schmid J-P, Shlyakhto E, Simpson IA, Stroes E, Sudano I, Tselepis AD, Viigimaa M, Vindis C, Vonbank A, Vrablik M, Vrsalovic M, Zamorano JL, Collet J-P, Koskinas KC, Casula M, Badimon L, John Chapman M, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen M-R, Tokgozoglu L, Wiklund O, Windecker S, Aboyans V, Baigent C, Collet J-P, Dean V, Delgado V, Fitzsimons D, Gale CP, Grobbee D, Halvorsen S, Hindricks G, Iung B, Jüni P, Katus HA, Landmesser U, Leclercq C, Lettino M, Lewis BS, Merkely B, Mueller C, Petersen S, Petronio AS, Richter DJ, Roffi M, Shlyakhto E, Simpson IA, Sousa-Uva M, Touyz RM, Nibouche D, Zelveian PH, Siostrzonek P, Najafov R, van de Borne P, Pojskic B, Postadzhiyan A, Kypris L, Špinar J, Larsen ML, Eldin HS, Viigimaa M, Strandberg TE, Ferrières J, Agladze R, Laufs U, Rallidis L, Bajnok L, Gudjónsson T, Maher V, Henkin Y, Gulizia MM, Mussagaliyeva A, Bajraktari G, Kerimkulova A, Latkovskis G, Hamoui O, Slapikas R, Visser L, Dingli P, Ivanov V, Boskovic A, Nazzi M, Visseren F, Mitevska I, Retterstøl K, Jankowski P, Fontes-Carvalho R, Gaita D, Ezhov M, Foscoli M, Giga V, Pella D, Fras Z, de Isla LP, Hagström E, Lehmann R, Abid L, Ozdogan O, Mitchenko O, Patel RS. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111–188. [DOI] [PubMed] [Google Scholar]
- 24. Gulati M, Levy PD, Mukherjee D, Amsterdam E, Bhatt DL, Birtcher KK, Blankstein R, Boyd J, Bullock-Palmer RP, Conejo T, Diercks DB, Gentile F, Greenwood JP, Hess EP, Hollenberg SM, Jaber WA, Jneid H, Joglar JA, Morrow DA, O'Connor RE, Ross MA, Shaw LJ. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: A report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation 2021;144:e368–e454. [DOI] [PubMed] [Google Scholar]
- 25. Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004;350:1495–1504. [DOI] [PubMed] [Google Scholar]
- 26. Cannon CP, Steinberg BA, Murphy SA, Mega JL, Braunwald E. Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J Am Coll Cardiol 2006;48:438–445. [DOI] [PubMed] [Google Scholar]
- 27. Sabatine MS, Wiviott SD, Im K, Murphy SA, Giugliano RP. Efficacy and safety of further lowering of low-density lipoprotein cholesterol in patients starting with very low levels: A meta-analysis. JAMA Cardiol 2018;3:823–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, De Ferrari GM, Ruzyllo W, De Lucca P, Im K, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387–2397. [DOI] [PubMed] [Google Scholar]
- 29. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 30. Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Lecorps G, Mahaffey KW, Moryusef A, Pordy R, Quintero K, Roe MT, Sasiela WJ, Tamby JF, Tricoci P, White HD, Zeiher AM. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med 2018;379:2097–2107. [DOI] [PubMed] [Google Scholar]
- 31. O’Donoghue ML, Giugliano RP, Wiviott SD, Atar D, Keech A, Kuder JF, Im K, Murphy SA, Flores-Arredondo JH, Lopez JAG, Elliott-Davey M, Wang B, Monsalvo ML, Abbasi S, Sabatine MS. Long-term evolocumab in patients with established atherosclerotic cardiovascular disease. Circulation 2022;146:1109–1119. [DOI] [PubMed] [Google Scholar]
- 32. Di Minno A, Lupoli R, Calcaterra I, Poggio P, Forte F, Spadarella G, Ambrosino P, Iannuzzo G, Di Minno MND. Efficacy and safety of bempedoic acid in patients with hypercholesterolemia: systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc 2020;9:e016262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet 2007;369:1090–1098. [DOI] [PubMed] [Google Scholar]
- 34. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT Jr, Juliano RA, Jiao L, Granowitz C, Tardif JC, Ballantyne CM. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019;380:11–22. [DOI] [PubMed] [Google Scholar]
- 35. Bonaca MP, Nault P, Giugliano RP, Keech AC, Pineda AL, Kanevsky E, Kuder J, Murphy SA, Jukema JW, Lewis BS, Tokgozoglu L, Somaratne R, Sever PS, Pedersen TR, Sabatine MS. Low-density lipoprotein cholesterol lowering with evolocumab and outcomes in patients with peripheral artery disease: insights from the FOURIER trial (further cardiovascular outcomes research with PCSK9 inhibition in subjects with elevated risk). Circulation 2018;137:338–350. [DOI] [PubMed] [Google Scholar]
- 36. Hardy J, Niman S, Pereira E, Lewis T, Reid J, Choksi R, Goldfaden RF. A critical review of the efficacy and safety of inclisiran. Am J Cardiovasc Drugs 2021;21:629–642. [DOI] [PubMed] [Google Scholar]
- 37. Zagelbaum NK, Yandrapalli S, Nabors C, Frishman WH. Bempedoic acid (ETC-1002): ATP citrate lyase inhibitor: review of a first-in-class medication with potential benefit in statin-refractory cases. Cardiol Rev 2019;27:49–56. [DOI] [PubMed] [Google Scholar]
- 38. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, DoyleRT, Jr., Juliano RA, Jiao L, Granowitz C, Tardif JC, Gregson J, Pocock SJ, Ballantyne CM. Effects of icosapent ethyl on total ischemic events: from REDUCE-IT. J Am Coll Cardiol 2019;73:2791–2802. [DOI] [PubMed] [Google Scholar]
- 39. Grant PJ, Cosentino F. The 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: new features and the ‘ten commandments’ of the 2019 guidelines are discussed by professor peter J. Grant and professor francesco cosentino, the task force chairmen. Eur Heart J 2019;40:3215–3217. [DOI] [PubMed] [Google Scholar]
- 40. Sabouret P, Bocchino PP, Angelini F, D'Ascenzo F, Galati G, Fysekidis M, DE Ferrari GM, Fischman DL, Bhatt DL, Biondi-Zoccai G. Comparing benefits from sodium-glucose cotransporter-2 inhibitors and glucagon-like peptide-1 receptor agonists in randomized clinical trials: a network meta-analysis. Minerva Cardiol Angiol 2022;71:199–207. [DOI] [PubMed] [Google Scholar]
- 41. Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev 2007;87:1409–1439. [DOI] [PubMed] [Google Scholar]
- 42. Hernandez AF, Green JB, Janmohamed S, D'Agostino RB Sr, Granger CB, Jones NP, Leiter LA, Rosenberg AE, Sigmon KN, Somerville MC, Thorpe KM, McMurray JJV, Del Prato S; Harmony Outcomes committees and investigators . Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (harmony outcomes): a double-blind, randomised placebo-controlled trial. Lancet 2018;392:1519–1529.Epub 2018 Oct 2. 2018. [DOI] [PubMed] [Google Scholar]
- 43. Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB; LEADER Steering Committee; LEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsbøll T; SUSTAIN-6 Investigators . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–1844. [DOI] [PubMed] [Google Scholar]
- 45. Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, Probstfield J, Riesmeyer JS, Riddle MC, Ryden L, Xavier D, Atisso CM, Dyal L, Hall S, Rao-Melacini P, Wong G, Avezum A, Basile J, Chung N, Conget I, Cushman WC, Franek E, Hancu N, Hanefeld M, Holt S, Jansky P, Keltai M, Lanas F, Leiter LA, Lopez-Jaramillo P, Cardona Munoz EG, Pirags V, Pogosova N, Raubenheimer PJ, Shaw JE, Sheu WH, Temelkova-Kurktschiev T; REWIND Investigators . Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 2019;394:121–130. [DOI] [PubMed] [Google Scholar]
- 46. Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, Jeppesen OK, Lingvay I, Mosenzon O, Pedersen SD, Tack CJ, Thomsen M, Vilsboll T, Warren ML, Bain SC. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2019;381:841–851. [DOI] [PubMed] [Google Scholar]
- 47. Ferrannini E. Sodium-glucose co-transporters and their inhibition: clinical physiology. Cell Metab 2017;26:27–38. [DOI] [PubMed] [Google Scholar]
- 48. Ferrannini E, Muscelli E, Frascerra S, Baldi S, Mari A, Heise T, Broedl UC, Woerle HJ. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest 2014;124:499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 50. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–657. [DOI] [PubMed] [Google Scholar]
- 51. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–357. [DOI] [PubMed] [Google Scholar]
- 52. Mahaffey KW, Jardine MJ, Bompoint S, Cannon CP, Neal B, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Capuano G, de Zeeuw D, Greene T, Levin A, Pollock C, Sun T, Wheeler DC, Yavin Y, Zhang H, Zinman B, Rosenthal N, Brenner BM, Perkovic V. Canagliflozin and cardiovascular and renal outcomes in type 2 diabetes mellitus and chronic kidney disease in primary and secondary cardiovascular prevention groups. Circulation 2019;140:739–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Arnott C, Li Q, Kang A, Neuen BL, Bompoint S, Lam CSP, Rodgers A, Mahaffey KW, Cannon CP, Perkovic V, Jardine MJ, Neal B. Sodium-glucose cotransporter 2 inhibition for the prevention of cardiovascular events in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. J Am Heart Assoc 2020;9:e014908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jabbour SA, Frias JP, Hardy E, Ahmed A, Wang H, Ohman P, Guja C. Safety and efficacy of exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy: 52-week results of the DURATION-8 randomized controlled trial. Diabetes Care 2018;41:2136–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ludvik B, Frias JP, Tinahones FJ, Wainstein J, Jiang H, Robertson KE, Garcia-Perez LE, Woodward DB, Milicevic Z. Dulaglutide as add-on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD-10): a 24-week, randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2018;6:370–381. [DOI] [PubMed] [Google Scholar]
- 56. Terauchi Y, Utsunomiya K, Yasui A, Seki T, Cheng G, Shiki K, Lee J. Safety and efficacy of empagliflozin as add-on therapy to GLP-1 receptor agonist (liraglutide) in Japanese patients with type 2 diabetes Mellitus: A randomised, double-blind, parallel-group phase 4 study. Diabetes Ther 2019;10:951–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. McGuire DK, Shih WJ, Cosentino F, Charbonnel B, Cherney DZI, Dagogo-Jack S, Pratley R, Greenberg M, Wang S, Huyck S, Gantz I, Terra SG, Masiukiewicz U, Cannon CP. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: A meta-analysis. JAMA Cardiol 2021;6:148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sattar N, McGuire DK, Pavo I, Weerakkody GJ, Nishiyama H, Wiese RJ, Zoungas S. Tirzepatide cardiovascular event risk assessment: a pre-specified meta-analysis. Nat Med 2022;28:591–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rosenstock J, Wysham C, Frias JP, Kaneko S, Lee CJ, Fernandez Lando L, Mao H, Cui X, Karanikas CA, Thieu VT. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet 2021;398:143–155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data is included in the submission/manuscript file.