Abstract
Background
The association between prior bevacizumab (BEV) therapy and ramucirumab (RAM)-induced proteinuria is not known. We aimed to investigate this association in patients with metastatic colorectal cancer (mCRC).
Methods
mCRC patients who received folinic acid, fluorouracil, and irinotecan (FOLFIRI) plus RAM were divided into with and without prior BEV treatment groups. The cumulative incidence of grade 2–3 proteinuria and rate of RAM discontinuation within 6 months (6M) after RAM initiation were compared between the two groups.
Results
We evaluated 245 patients. In the Fine-Gray subdistribution hazard model including prior BEV, age, sex, comorbidities, eGFR, proteinuria ≥ 2 + at baseline, and later line of RAM, prior BEV treatment contributed to proteinuria onset (P < 0.01). A shorter interval between final BEV and initial RAM increased the proteinuria risk; the adjusted odds ratios (95% confidence intervals) for the intervals of < 28 days, 28–55 days, and > 55 days (referring to prior BEV absence) were 2.60 (1.23–5.51), 1.51 (1.01–2.27), and 1.04 (0.76–1.44), respectively. The rate of RAM discontinuation for ≤ 6M due to anti-VEGF toxicities was significantly higher in the prior BEV treatment group compared with that in the no prior BEV treatment group (18% vs. 6%, P = 0.02). Second-line RAM discontinuation for ≤ 6M without progression resulted in shorter overall survival of 132 patients with prior BEV treatment (P < 0.01).
Conclusion
Sequential FOLFIRI plus RAM after BEV failure, especially within 55 days, may exacerbate proteinuria. Its escalated anti-VEGF toxicity may negatively impact the overall survival.
Keywords: Ramucirumab, Bevacizumab, Proteinuria, Colorectal cancer, FOLFIRI
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer-related deaths worldwide, and the 5-year relative survival rate of patients with metastatic disease is 15% [1, 2]. In the past two decades, remarkable progress has been made in the treatment of metastatic colorectal cancer (mCRC) using more effective agents, such as fluoropyrimidines, oxaliplatin, irinotecan, anti-vascular endothelial growth factor (VEGF) biologics, and anti-epidermal growth factor receptor monoclonal antibodies. In recent years, precision medicine has progressed further: encorafenib, binimetinib, and cetuximab for BRAF V600E-mutated CRC [3]; pertuzumab plus trastuzumab for HER2-amplified CRC [4]; and immunotherapy for deficient DNA mismatch repair CRC [5–7].
With regard to the anti-VEGF biologics used in treating mCRC, the anti-VEGF-A antibody bevacizumab (BEV), anti-VEGF receptor 2 (VEGFR2) antibody ramucirumab (RAM), and VEGF-trap aflibercept are available. In patients who receive oxaliplatin-based chemotherapy as first-line treatment, the three biologics are equally recommended in combination with fluorouracil and leucovorin plus irinotecan (FOLFIRI) as second-line treatment [8–10]. Nephrotoxicity is a well-known toxicity associated with the therapeutic inhibition of VEGF signaling, which is critical to the glomerular development and the maintenance of mature glomerular function during homeostasis and disease occurrence in the kidney [11]. The main clinical manifestation of nephrotoxicity is proteinuria, which sometimes results in nephrotic syndrome and renal-specific thrombotic microangiopathy (TMA) [11].
In terms of RAM-induced proteinuria, a previous meta-analysis of individual patient safety data from six randomized, placebo-controlled trials showed that patients with mCRC examined in the RAISE study were most likely to develop grade ≥ 3 proteinuria: the number need to harm (NNH) for grade ≥ 3 proteinuria was 1 in 35 patients [12]. Interestingly, previous case reports of nephrotic syndrome/glomerular microangiopathy associated with RAM showed that its onset was observed immediately after RAM initiation in patients who received prior BEV therapy [13–16]. In the abovementioned RAISE study, patients with mCRC who received BEV ≤ 28 days before randomization were considered ineligible [17]. Patients with mCRC will administered with anti-VEGF biologics during the period between first- and second-line chemotherapy owing to the overall survival (OS) benefit of sequential anti-VEGF biologics after receiving first-line BEV treatment [17–19]. Therefore, a safer treatment strategy for patients with mCRC receiving second-line RAM needs to be developed. Hence, this study aimed to investigate the impact of prior BEV on the incidence of RAM-induced proteinuria in patients with mCRC receiving FOLFIRI plus RAM.
Patients and methods
This retrospective, nineteen-institutional cohort study was conducted in patients with mCRC who received FOLFIRI plus RAM after first-line chemotherapy between June 1, 2015, and November 29, 2020, in Japan. Eligible patients were divided into two cohorts according to the status of prior BEV therapy. The exclusion criteria were as follows: (1) Patients who were previously treated with multi-kinase inhibitors, (2) post-transplant patients, (3) patients who died within 1 month after FOLFIRI plus RAM initiation, (4) patients with nephrotic syndrome as comorbidity, (5) patients who did not undergo dipstick proteinuria test at RAM initiation (baseline), and (6) patients who had a dipstick proteinuria score of 4 + at baseline. The primary endpoints were the cumulative incidence of grade 2–3 proteinuria (grade 2: dipstick proteinuria 2 + or 3 + ; grade 3: dipstick proteinuria 4 +) and worst random spot urine protein/creatinine (UPC) ratio between the two groups. The observation period for changes in proteinuria was set at 6 months after RAM initiation or the days of final RAM treatment plus 56 days (approximately 4 half-lives of RAM [20]), whichever came first. In patients with proteinuria at baseline, the outcome was considered in those with worsened proteinuria. The secondary endpoints were the incidence of UPC ratio of ≥ 3 and percentage of RAM discontinuation within 6 months between the two groups. We also investigated the percentage of eligible patients with kidney biopsy and biopsy-proven TMA. To evaluate the disadvantage of RAM discontinuation without progressive disease in patients with or without prior BEV, stratified OS was compared between patients with second-line RAM discontinuation within 6 months and those without discontinuation. In the time-to-event analysis, patients without grade 2–3 proteinuria or death were censored at their last patient record or at the end of the follow-up period (May 31, 2021), whichever occurred first.
Statistical analysis
Binary outcomes were compared using Fisher’s exact test, whereas continuous outcomes were compared using the unpaired Student’s t test or Mann–Whitney U test, as appropriate. For the primary endpoint analysis, the cumulative incidence of grade 2–3 proteinuria, accounting for death as a competing event between the two groups, was compared using Gray’s test. The Fine-Gray subdistribution hazard model was used to determine the cumulative incidence of grade 2–3 proteinuria in the multivariable analysis. The possible confounders were chosen to determine their potential association with grade 2–3 proteinuria onset based on the status of prior BEV, age (per 10 years), sex, eGFR at baseline (per 10 mL/min), comorbidities at baseline (diabetes and hypertension), dipstick proteinuria score of ≥ 2 + at baseline, and later (≥ 3) lines of FOLFIRI plus RAM. To evaluate the impact of the interval between the final BEV and initial RAM on grade 2–3 proteinuria onset, a logistic regression model was used to determine the adjusted odds ratio (OR), which referred to the absence of prior BEV. The intervals were divided into three categories: < 28 days, 28–55 days, and > 55 days. These intervals were based on the following concepts: an interval of ≤ 28 days was excluded from the RAISE study [17], the National Comprehensive Cancer Network guidelines recommended an interval of at least 6 weeks between the final BEV and any elective surgery [21], and a certain interval was selected as it can be easily integrated in clinical practice.
With regard to the OS between patients with second-line RAM discontinuation within 6 months and those without RAM discontinuation, we estimated the Kaplan–Meier curves and used the stratified log-rank test according to the status of prior BEV. All statistical analyses were performed using EZR version 1.55 (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface of R (The R Foundation for Statistical Computing, Vienna, Austria). EZR is a modified version of R commander designed to add statistical functions frequently used in biostatistics [22]. Statistical significance was set at a p value of < 0.05.
Ethical considerations and reporting guideline
This study was performed in accordance with the Declaration of Helsinki and its amendments, and the study protocol was approved by the Ethics Committee of each institution. Patients were allowed to opt out of this study at any time and were informed through the hospital website or onsite. This study was reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement [23].
Results
Patient’s characteristics
Two-hundred and fifty-three patients from 19 institutions met the inclusion criteria. Eight patients who did not undergo urinalysis after RAM initiation and whose BEV treatment period was unknown because of hospital transfer due to the coronavirus disease 2019 pandemic were excluded. Finally, 245 patients were assigned to two cohorts according to the absence (n = 64) or presence (n = 181) of prior BEV therapy. The patient’s baseline characteristics are shown in Table 1. Right-sided and RAS-mutated tumors were more frequent in patients with prior BEV treatment. Hypertension and dipstick proteinuria score of ≥ 2 + were also frequent in patients with prior BEV. With regard to the chemotherapy status, the presence of prior anti-EGFR antibody and later treatment with FOLFIRI plus RAM were frequent in patients without prior BEV. The duration of prior BEV treatment was approximately 7 months.
Table 1.
Characteristics at ramucirumab initiation between patients with prior bevacizumab treatment and those without prior bevacizumab treatment
| Variables | Prior bevacizumab | P value | |
|---|---|---|---|
| Absent N = 64 |
Present N = 181 |
||
| Median age [range]—yr | 68 [58–72] | 68 [59–73] | 0.91 |
| Male sex—no. (%) | 38 (59.4) | 93 (51.4) | 0.31 |
| ECOG PS—no. (%) | |||
| 0 or 1 | 46 (71.9) | 119 (65.7) | 0.75 |
| 2, 3, or 4 | 3 (4.7) | 11 (6.1) | |
| Missing | 15 (23.4) | 51 (28.2) | |
| Median eGFR [range]—mL/min/1.73m2 | 65.7 [54.1–76.7] | 63.8 [50.2–81.2] | 0.58 |
| Median diastolic BP [range]—mmHg | 76 [68–81] | 77 [68–86] | 0.40 |
| Median systolic BP [range]—mmHg | 127 [118–136] | 128 [116–140] | 0.98 |
| Right-sided tumors—no. (%) | 9 (14.1) | 53 (29.6) | 0.02 |
| BRAF V600E—no. (%) | |||
| Mutated | 1 (1.6) | 7 (3.9) | 0.14 |
| Not tested | 29 (45.3) | 103 (56.9) | |
| RAS mutated—no. (%) | 5 (7.8) | 135 (74.6) | < 0.001 |
| Median CEA [range]—ng/mL* | 12.5 [6.8–53.6] | 28.1 [9.3–171.0] | 0.06 |
| Dipstick proteinuria score of ≥ 2 + —no. (%) | 2 (3.1) | 21 (11.6) | 0.05 |
| Hypertension—no. (%) | 23 (35.9) | 101 (55.8) | 0.01 |
| Diabetes—no. (%) | 13 (20.3) | 21 (11.6) | 0.09 |
| Hyperlipidemia—no. (%) | 14 (21.9) | 31 (17.1) | 0.45 |
| Calcium channel blocker—no. (%) | |||
| Concomitant | 16 (25.0) | 69 (38.1) | 0.21 |
| Missing | 4 (6.2) | 9 (5.0) | |
| Renin-angiotensin system inhibitors—no. (%) | |||
| Concomitant | 13 (20.3) | 49 (27.1) | 0.28 |
| Missing | 5 (7.8) | 7 (3.9) | |
| Details of previous treatment—no. (%) | |||
| FOLFOXIRI based | 6 (9.4) | 14 (7.7) | < 0.01 |
| Irinotecan based | 12 (18.8) | 30 (16.6) | |
| Oxaliplatin based | 31 (48.4) | 127 (70.2) | |
| Others† | 15 (23.4) | 10 (5.5) | |
| Prior anti-EGFR antibody—no. (%) | 50 (78.1) | 0 (0.0) | < 0.001 |
| Later (> 2)-line of FOLFIRI + ramucirumab—no. (%) | 26 (40.6) | 49 (27.1) | 0.06 |
| Median line of FOLFIRI + ramucirumab [range] | 2 [2– 3] | 2 [2–3] | 0.06 |
| Median duration of bevacizumab administration [range]—day | 0 [0–0] | 204 [84–379] | < 0.001 |
| Median ramucirumab dosage [range]—mg/kg | 8.0 [7.9–8.0] | 8.0 [7.9–8.0] | 0.28 |
*Data on the CEA levels were missing in 2 of 64 patients without prior bevacizumab treatment and in 8 of 181 patients with prior bevacizumab treatment
†Other regimens included panitumumab monotherapy, capecitabine, trifluridine/tipiracil, and fluorouracil plus leucovorin
ECOG PS Eastern Cooperative Oncology Group Performance Status Scale, BP blood pressure, CEA carcinoembryonic antigen, FOLFOXIRI fluorouracil, leucovorin, oxaliplatin, and irinotecan, FOLFIRI fluorouracil, leucovorin, and irinotecan
Outcomes
During the follow-up period (median [interquartile range] follow-up days: absence of prior BEV treatment, 332 [204–448]; presence of prior BEV treatment, 250 [174–423]), the cumulative incidence of grade 2–3 proteinuria was significantly higher in patients with prior BEV treatment compared with that in patients without prior BEV treatment (P < 0.001) (Fig. 1). In the Fine-Gray subdistribution hazard model, the cumulative incidence of grade 2–3 proteinuria was only correlated with prior BEV treatment (sub-distribution hazard ratio [SHR]: 2.04, 95% confidence interval [CI]: 1.20–3.46, P < 0.01) (Table 2). Among the patients who had data on UPC ratio, the median (range) UPC ratio was significantly higher in patients with prior BEV treatment when compared with that in patients without prior BEV treatment:1.03 (0.08–10.2) vs. 0.27 (0.06–4.92), P < 0.001. Moreover, the percentage of patients with a UPC ratio of ≥ 3, which is the primary criterion for permanently discontinuing RAM, was higher in patients with prior BEV treatment than in those without BEV treatment (24.6% vs. 8.0%, P = 0.14) (Fig. 2). None of the patients underwent renal biopsy, although some patients, especially those with prior BEV treatment, had nephrotic-range proteinuria. Subsequently, the shorter interval between final BEV and initial RAM increased the incidence of grade 2–3 proteinuria (Table 3).
Fig. 1.
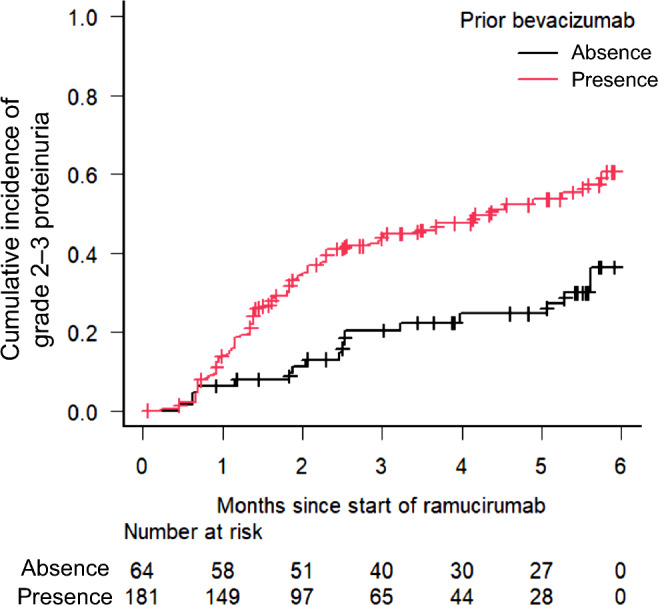
Cumulative incidence of grade 2–3 proteinuria between the two groups. The cumulative incidence of grade 2–3 proteinuria was significantly higher in patients with prior BEV treatment (P < 0.001, Gray’s test). Death as a competing risk event within 6 months was observed in 12 patients (nine with prior BEV treatment and three without prior BEV treatment). BEV bevacizumab
Table 2.
Multivariable analysis for cumulative incidence of grade 2–3 proteinuria using the Fine-Gray subdistribution hazard model
| Variables | SHR | 95% CI | P value |
|---|---|---|---|
| Age (per 10 year) | 1.03 | 0.93–1.14 | 0.64 |
| Female sex | 1.47 | 0.99–2.18 | 0.06 |
| eGFR at baseline (per 10 mL/min/1.73m2) | 0.90 | 0.80–1.03 | 0.12 |
| Diabetes at baseline | 1.05 | 0.54–2.02 | 0.90 |
| Hypertension at baseline | 1.21 | 0.79–1.86 | 0.38 |
| Dipstick proteinuria score of ≥ 2 + at baseline | 1.73 | 0.90–3.33 | 0.10 |
| Later-line of FOLFIRI + ramucirumab (ref. second-line) | 0.84 | 0.54–1.31 | 0.45 |
| Prior bevacizumab (ref. absent) | 2.04 | 1.20–3.46 | < 0.01 |
FOLFIRI fluorouracil, leucovorin, and irinotecan, ref reference, SHR sub-distribution hazard ratio, CI confidence interval
Fig. 2.
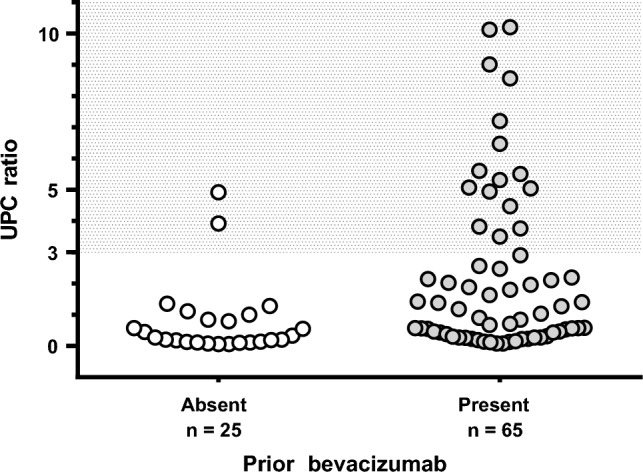
Maximum UPC ratio between the two groups. Each plot represents the worst UPC ratio for each patient during the observation period. The shaded areas represent the level of permanent RAM discontinuation. UPC urine protein/creatinine, RAM ramucirumab
Table 3.
Impact of the interval between final bevacizumab and initial ramucirumab on the onset of grade 2–3 proteinuria
| Interval between final bevacizumab and initial ramucirumab | N | No. of event | Adjusted OR* | 95% CI |
|---|---|---|---|---|
| Prior bevacizumab: absent | 64 | 17 (26.6%) | Ref | |
| < 28 days | 81 | 40 (49.4%) | 2.60 | 1.23–5.51 |
| 28–55 days | 64 | 32 (50.0%) | 1.51 | 1.01–2.27 |
| > 55 days | 36 | 13 (36.1%) | 1.04 | 0.76–1.44 |
*Covariates in the logistic regression model are as follows: age, sex, eGFR at baseline, diabetes at baseline, hypertension at baseline, and dipstick proteinuria score of ≥ 2 + at baseline
OR odds ratio, CI confidence interval, ref reference, eGFR estimated glomerular filtration rate
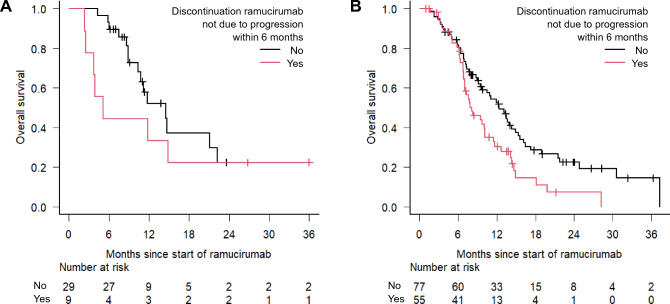
Then, we investigated the details of RAM therapy, including the treatment duration, the percentage of discontinuation within 6 months after initiation, and the reason for the difference between the two groups. Compared with patients without prior BEV treatment, a significantly shorter duration of RAM treatment and a higher RAM discontinuation rate were observed in patients with prior BEV treatment (Table 4). Interestingly, the RAM discontinuation rate due to anti-VEGF-related toxicities, such as proteinuria, bleeding, hypertension, and gastrointestinal perforation, was significantly higher in patients with prior BEV treatment than in those without prior BEV treatment (18% [32/181] vs. 6% [4/64], P = 0.02). Subsequently, stratified OS analysis was performed according to the status of prior BEV treatment among patients treated with second-line FOLFIRI plus RAM. RAM discontinuation not due to progressive disease within 6 months had a survival disadvantage in both groups (Fig. 3).
Table 4.
Details of ramucirumab treatment between the two groups
| Prior bevacizumab | P value | ||
|---|---|---|---|
| Absent N = 64 |
Present N = 181 |
||
| Median duration of ramucirumab administration [range]—day | 149 [14–679] | 80 [14–605] | < 0.001 |
| Ramucirumab discontinuation within 6 months—no. (%) | 41 (64.1%) | 151 (83.4%) | < 0.01 |
| Reasons—no. (%) | 0.42 | ||
| Best supportive care | 3 (7.3) | 19 (12.6) | |
| Progressive disease | 26 (63.4) | 77 (51.0) | |
| Bleeding | 1 (2.4) | 5 (3.3) | |
| Proteinuria | 2 (4.9) | 23 (15.2) | |
| Hypertension | 1 (2.4) | 2 (1.3) | |
| Gastrointestinal perforation | 0 (0.0) | 2 (1.3) | |
| Others | 8 (19.5) | 23 (15.2) | |
Fig. 3.
Stratified analysis according to the absence (A) or presence (B) of prior bevacizumab treatment in patients treated with second-line FOLFIRI plus ramucirumab: the impact of ramucirumab discontinuation not due to progressive disease within 6 months on overall survival. Among the patients with no prior BEV treatment (A), the 1-year survival rates (95% CI) were 33.3% (7.8–62.3) in patients with RAM discontinuation not due to progression within 6 months and 52.1% (29.5–70.6) in those without RAM discontinuation (P = 0.29 by log-rank test); among the patients with prior BEV treatment (B), the 1-year survival rates (95% CI) were 30.4% (18.0–43.7) in patients with RAM discontinuation not due to progression within 6 months and 52.7% (40.4–63.6) in those without RAM discontinuation (P < 0.01 by log-rank test). OS overall survival, CI confidence interval, BEV bevacizumab, RAM ramucirumab
Discussion
This study primarily aimed to determine whether sequential RAM after BEV treatment could exacerbate RAM-induced proteinuria in patients with mCRC receiving FOLFIRI plus RAM. The cumulative incidence of grade 2–3 proteinuria and the incidence of worst UPC ratio were significantly higher in patients with prior BEV treatment than in those without prior BEV treatment. Furthermore, a shorter interval between BEV and RAM exacerbated RAM-induced proteinuria; in particular, patients with an interval of < 28 days were at the greatest risk.
When administering sequential anti-VEGF agents after progression on first-line BEV, the following issues remains unresolved. Which strategy is the most appropriate: continuation of BEV after first-line BEV [19], sequential RAM after first-line BEV [17], or sequential AFL after first-line BEV? [18]. Two recent administrative claims database studies [24, 25] addressed this issue: among mCRC patients who failed first-line chemotherapy with fluoropyrimidines and oxaliplatin plus BEV [24], the time to treatment failure, defined as the time from initial second-line chemotherapy to treatment termination due to any cause or death, was significantly longer in the FOLFIRI plus BEV group compared with that in the FOLFIRI plus RAM (hazard ratio, 1.40; 95% CI, 1.26–1.56) and FOLFIRI plus AFL groups (hazard ratio, 1.34; 95% CI, 1.09–1.66). Among mCRC patients who were unresponsive to first-line FOLFOX plus anti-EGFR antibody [25], treatment durations of second-line irinotecan-based chemotherapy plus anti-VEGF agents were similar among the three groups (BEV, RAM, and AFL). As the duration of second-line chemotherapy concomitant with anti-VEGF agent could be affected by the presence or absence of prior BEV therapy, discontinuation of second-line anti-VEGF treatment may be due to the exacerbation of toxicities; RAM discontinuation due to the occurrence of anti-VEGF related toxicities, including proteinuria, within 6 months was likely to occur in patients who received sequential RAM after BEV. Another study also reported that the number of RAM toxicities increased in patients with advanced-stage hepatocellular carcinoma who received sequential RAM after BEV in combination with atezolizumab, compared with that reported in a pivotal phase III trial [26].
Then, with regard to the plausible mechanism of proteinuria exacerbation associated with sequential RAM after BEV, we focused on investigating the effects of dual anti-VEGF blockades, such as VEGF-A (BEV) and VEGFR-2 (RAM). In this study, patients whose interval between final BEV and initial RAM was longer than 55 days were not at risk of developing grade 2–3 proteinuria compared with patients without prior BEV treatment. Considering that the estimated half-life of BEV is 20 days [27], the pharmacological effects of BEV and RAM could overlap if RAM is initiated prior to the completion of BEV elimination, which corresponds to four-to-five half-lives. Since the level of VEGF-A increased after BEV therapy [28], this physiological response may help maintain renal homeostasis and may be strongly inhibited by treatment with dual anti-VEGF blockade.
Second, the negative impact of RAM discontinuation, not due to progressive disease, on OS was observed in patients who received second-line FOLFIRI plus RAM, especially in patients with prior BEV treatment. Although some studies have reported an association between proteinuria/hypertension onset and good prognosis among patients treated with BEV [29, 30], the avoidance/minimization of toxicities leading to treatment discontinuation is crucial. Patients with prior BEV treatment had a higher likelihood of developing anti-VEGF toxicities leading to RAM discontinuation than those without BEV treatment. Considering that East Asian patients are at a high risk of RAM-induced grade ≥ 3 proteinuria compared with non-East Asian patients [31], the interval between final BEV and initial RAM should be longer than 55 days to avoid RAM-induced proteinuria, potentially leading to treatment discontinuation.
The limitations of this study were intrinsic to its retrospective design. For the analysis of proteinuria, the unmeasured confounders included other medications potentially decreasing proteinuria, the cumulative dose of BEV, and the time-varying covariate included hypertension and antihypertensives during RAM treatment; there may be discrepancies in the frequency of performing urinalysis in each institution. For the survival analysis, we were unable to perform a multivariable analysis of subsequent therapy and the biology of colorectal cancer owing to the exploratory nature of the analysis. Although we cannot fully exclude these limitations, the results obtained from 19 institutions in real-world practice could have high external validity.
Conclusion
In conclusion, this multi-institutional cohort study focusing on RAM-induced proteinuria among mCRC patients receiving FOLFIRI plus RAM provides additional information on the high-risk population, that is, the presence of prior BEV and the interval between final BEV and initial RAM within 55 days. Clinicians should pay attention to the timing of providing sequential RAM after BEV. In the future, an optimal sequence of anti-VEGF agents should be developed throughout the course of first- and second-line chemotherapy to avoid the development of proteinuria leading to treatment discontinuation in patients with mCRC receiving FOLFIRI plus anti-VEGF agents.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Author contributions
SD: conceptualization, methodology, formal analysis, investigation, data curation, visualization, writing–original draft preparation, and project administration. ES, EK, RF, KM, NM, MY, NO, HI, MH, KT, MT, MT, SN, KI, AS, SI, YT, YN, MS, HW, DH, MN, MT, EG, NK, AY, AK, YN, MM, YH, YY, TH, KA, KM, RF, AT, KY, SH, YM: Investigation and writing-review. SS, DY, HM: writing-review. YK: supervision.
Declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tabernero J, Velez L, Trevino TL, Grothey A, Yaeger R, Van Cutsem E, Wasan H, Desai J, Ciardiello F, Yoshino T, Gollerkeri A, Maharry K, Christy-Bittel J, Kopetz S. Management of adverse events from the treatment of encorafenib plus cetuximab for patients with BRAF V600E-mutant metastatic colorectal cancer: insights from the BEACON CRC study. ESMO Open. 2021;6(6):100328. doi: 10.1016/j.esmoop.2021.100328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.SEER Cancer stat facts: colorectal cancer. https://seer.cancer.gov/statfacts/html/colorect.html. Accessed October 10, 2022
- 3.Kopetz S, Grothey A, Yaeger R, Van Cutsem E, Desai J, Yoshino T, Wasan H, Ciardiello F, Loupakis F, Hong YS, Steeghs N, Guren TK, Arkenau HT, Garcia-Alfonso P, Pfeiffer P, Orlov S, Lonardi S, Elez E, Kim TW, Schellens JHM, Guo C, Krishnan A, Dekervel J, Morris V, Calvo Ferrandiz A, Tarpgaard LS, Braun M, Gollerkeri A, Keir C, Maharry K, Pickard M, Christy-Bittel J, Anderson L, Sandor V, Tabernero J. Encorafenib, binimetinib, and cetuximab in BRAF V600E-mutated colorectal cancer. N Engl J Med. 2019;381(17):1632–1643. doi: 10.1056/NEJMoa1908075. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura Y, Okamoto W, Kato T, Esaki T, Kato K, Komatsu Y, Yuki S, Masuishi T, Nishina T, Ebi H, Sawada K, Taniguchi H, Fuse N, Nomura S, Fukui M, Matsuda S, Sakamoto Y, Uchigata H, Kitajima K, Kuramoto N, Asakawa T, Olsen S, Odegaard JI, Sato A, Fujii S, Ohtsu A, Yoshino T. Circulating tumor DNA-guided treatment with pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer: a phase 2 trial. Nat Med. 2021;27(11):1899–1903. doi: 10.1038/s41591-021-01553-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs P, de la Fouchardiere C, Rivera F, Elez E, Bendell J, Le DT, Yoshino T, Van Cutsem E, Yang P, Farooqui MZH, Marinello P, Diaz LA., Jr Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N Engl J Med. 2020;383(23):2207–2218. doi: 10.1056/NEJMoa2017699. [DOI] [PubMed] [Google Scholar]
- 6.Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, Desai J, Hill A, Axelson M, Moss RA, Goldberg MV, Cao ZA, Ledeine JM, Maglinte GA, Kopetz S, André T. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18(9):1182–1191. doi: 10.1016/s1470-2045(17)30422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, Morse MA, Van Cutsem E, McDermott R, Hill A, Sawyer MB, Hendlisz A, Neyns B, Svrcek M, Moss RA, Ledeine JM, Cao ZA, Kamble S, Kopetz S, André T. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol. 2018;36(8):773–779. doi: 10.1200/jco.2017.76.9901. [DOI] [PubMed] [Google Scholar]
- 8.Chiorean EG, Nandakumar G, Fadelu T, Temin S, Alarcon-Rozas AE, Bejarano S, Croitoru AE, Grover S, Lohar PV, Odhiambo A, Park SH, Garcia ER, Teh C, Rose A, Zaki B, Chamberlin MD. Treatment of patients with late-stage colorectal cancer: ASCO resource-stratified guideline. JCO Glob Oncol. 2020;6:414–438. doi: 10.1200/jgo.19.00367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshino T, Arnold D, Taniguchi H, Pentheroudakis G, Yamazaki K, Xu RH, Kim TW, Ismail F, Tan IB, Yeh KH, Grothey A, Zhang S, Ahn JB, Mastura MY, Chong D, Chen LT, Kopetz S, Eguchi-Nakajima T, Ebi H, Ohtsu A, Cervantes A, Muro K, Tabernero J, Minami H, Ciardiello F, Douillard JY. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO-ESMO initiative endorsed by CSCO, KACO, MOS SSO and TOS. Ann Oncol. 2018;29(1):44–70. doi: 10.1093/annonc/mdx738. [DOI] [PubMed] [Google Scholar]
- 10.Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, Hasegawa K, Hotta K, Ishida H, Ishiguro M, Ishihara S, Kanemitsu Y, Kinugasa Y, Murofushi K, Nakajima TE, Oka S, Tanaka T, Taniguchi H, Tsuji A, Uehara K, Ueno H, Yamanaka T, Yamazaki K, Yoshida M, Yoshino T, Itabashi M, Sakamaki K, Sano K, Shimada Y, Tanaka S, Uetake H, Yamaguchi S, Yamaguchi N, Kobayashi H, Matsuda K, Kotake K, Sugihara K. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020;25(1):1–42. doi: 10.1007/s10147-019-01485-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Estrada CC, Maldonado A, Mallipattu SK. Therapeutic inhibition of VEGF signaling and associated nephrotoxicities. J Am Soc Nephrol. 2019;30(2):187–200. doi: 10.1681/asn.2018080853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnold D, Fuchs CS, Tabernero J, Ohtsu A, Zhu AX, Garon EB, Mackey JR, Paz-Ares L, Baron AD, Okusaka T, Yoshino T, Yoon HH, Das M, Ferry D, Zhang Y, Lin Y, Binder P, Sashegyi A, Chau I. Meta-analysis of individual patient safety data from six randomized, placebo-controlled trials with the antiangiogenic VEGFR2-binding monoclonal antibody ramucirumab. Ann Oncol. 2017;28(12):2932–2942. doi: 10.1093/annonc/mdx514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujii T, Kawasoe K, Tonooka A, Ohta A, Nitta K. Nephrotic syndrome associated with ramucirumab therapy: a single-center case series and literature review. Medicine. 2019;98(27):e16236. doi: 10.1097/md.0000000000016236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamada R, Okawa T, Matsuo K, Suzuki M, Mori N, Mori K. Renal-limited thrombotic microangiopathy after switching from bevacizumab to ramucirumab: a case report. BMC Nephrol. 2019;20(1):14. doi: 10.1186/s12882-018-1194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozawa M, Ohtani H, Komatsuda A, Wakui H, Takahashi N. VEGF-VEGFR2 inhibitor-associated hyaline occlusive glomerular microangiopathy: a Japanese single-center experience. Clin Exp Nephrol. 2021;25(11):1193–1202. doi: 10.1007/s10157-021-02090-z. [DOI] [PubMed] [Google Scholar]
- 16.Yoshimura Y, Sawa N, Matsuoka S, Ikuma D, Oba Y, Sekine A, Hasegawa E, Mizuno H, Yamanouchi M, Suwabe T, Hoshino J, Kono K, Kinowaki K, Ohashi K, Toda S, Matoba S, Wakui H, Ubara Y. Glomerular microangiopathy with cellular crescent-like formation and endotheliopathy due to ramucirumab treatment for metastatic sigmoid colon cancer: a case report. Intern Med. 2022 doi: 10.2169/internalmedicine.9185-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tabernero J, Yoshino T, Cohn AL, Obermannova R, Bodoky G, Garcia-Carbonero R, Ciuleanu TE, Portnoy DC, Van Cutsem E, Grothey A, Prausová J, Garcia-Alfonso P, Yamazaki K, Clingan PR, Lonardi S, Kim TW, Simms L, Chang SC, Nasroulah F. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015;16(5):499–508. doi: 10.1016/s1470-2045(15)70127-0. [DOI] [PubMed] [Google Scholar]
- 18.Van Cutsem E, Tabernero J, Lakomy R, Prenen H, Prausová J, Macarulla T, Ruff P, van Hazel GA, Moiseyenko V, Ferry D, McKendrick J, Polikoff J, Tellier A, Castan R, Allegra C. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30(28):3499–3506. doi: 10.1200/jco.2012.42.8201. [DOI] [PubMed] [Google Scholar]
- 19.Bennouna J, Sastre J, Arnold D, Österlund P, Greil R, Van Cutsem E, von Moos R, Viéitez JM, Bouché O, Borg C, Steffens CC, Alonso-Orduña V, Schlichting C, Reyes-Rivera I, Bendahmane B, André T, Kubicka S. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14(1):29–37. doi: 10.1016/s1470-2045(12)70477-1. [DOI] [PubMed] [Google Scholar]
- 20.Cyramza [package insert], Indianapolis, Eli Lilly and Company. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/125477s002lbl.pdf. Accessed October 10, 2022
- 21.National Comprehensive Cancer Network. Colon cancer (Version 1.2022). https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Accessed October 10, 2022
- 22.Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Epidemiology. 2007;18(6):805–835. doi: 10.1097/EDE.0b013e3181577511. [DOI] [PubMed] [Google Scholar]
- 24.Yamazaki K, Yuki S, Oki E, Sano F, Makishima M, Aoki K, Hamano T, Yamanaka T. Real-world evidence on second-line treatment of metastatic colorectal cancer using fluoropyrimidine, irinotecan, and angiogenesis inhibitor. Clin Colorectal Cancer. 2021;20(3):e173–e184. doi: 10.1016/j.clcc.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Satake H, Kagawa Y, Shinozaki E, Tanizawa Y, Jin L, Cai Z, Makiyama A. Real-world data analysis of second-line antiangiogenic targeted treatments following anti-epidermal growth factor receptor monoclonal antibodies and first-line FOLFOX for patients with metastatic colorectal cancer. Adv Ther. 2022;39(6):2596–2613. doi: 10.1007/s12325-022-02122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuzuya T, Kawabe N, Hashimoto S, Funasaka K, Nagasaka M, Nakagawa Y, Miyahara R, Shibata T, Takahara T, Kato Y, Sugioka A, Hirooka Y. Clinical outcomes of ramucirumab as post-treatment following atezolizumab/bevacizumab combination therapy in advanced hepatocellular carcinoma. Anticancer Res. 2022;42(4):1905–1910. doi: 10.21873/anticanres.15667. [DOI] [PubMed] [Google Scholar]
- 27.Avastin [package insert], South San Francisco, Genentech Inc. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125085s336lbl.pdf. Accessed October 10, 2022
- 28.Van Cutsem E, Paccard C, Chiron M, Tabernero J. Impact of prior bevacizumab treatment on VEGF-A and PlGF levels and outcome following second-line aflibercept treatment: biomarker post hoc analysis of the VELOUR trial. Clin Cancer Res. 2020;26(3):717–725. doi: 10.1158/1078-0432.Ccr-19-1985. [DOI] [PubMed] [Google Scholar]
- 29.Carvalho B, Lopes RG, Linhares P, Costa A, Caeiro C, Fernandes AC, Tavares N, Osório L, Vaz R. Hypertension and proteinuria as clinical biomarkers of response to bevacizumab in glioblastoma patients. J Neurooncol. 2020;147(1):109–116. doi: 10.1007/s11060-020-03404-z. [DOI] [PubMed] [Google Scholar]
- 30.Moisuc DC, Marinca MV, Gafton B, Alexa-Stratulat T, Pavel-Tanasa M, Cianga P. Antiangiogenic drug-induced proteinuria as a prognostic factor in metastatic colorectal cancer. Curr Oncol. 2022;29(6):3996–4011. doi: 10.3390/curroncol29060319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yen CJ, Muro K, Kim TW, Kudo M, Shih JY, Lee KW, Chao Y, Kim SW, Yamazaki K, Sohn J, Cheng R, Zhang Y, Binder P, Mi G, Orlando M, Chung HC. Ramucirumab safety in east asian patients: a meta-analysis of six global, randomized, double-blind, placebo-controlled, phase III clinical trials. J Glob Oncol. 2018;4:1–12. doi: 10.1200/jgo.17.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]