Abstract
Background
Functional antagonism between transforming growth factor beta (TGF-β) and hyaluronidase has been demonstrated. For example, testicular hyaluronidase PH-20 counteracts TGF-β1-mediated growth inhibition of epithelial cells. PH-20 sensitizes various cancer cells to tumor necrosis factor (TNF) cytotoxicity by upregulating proapoptotic p53 and WW domain-containing oxidoreductase (WOX1). TGF-β1 blocks PH-20-increased TNF cytotoxicity. In the present study, the functional antagonism between TGF-β1 and lysosomal hyaluronidases Hyal-1 and Hyal-2 was examined.
Results
Murine L929 fibroblasts were engineered to stably express green-fluorescent protein (GFP)-tagged hyaluronidase (GFP-Hyal-1 or GFP-Hyal-2) or GFP alone. Compared to control cells, Hyal-2-expressing cells had a significantly increased sensitivity to TNF cytotoxicity (~60–110% increase), while Hyal-1-expressing cells were less sensitive to TNF (~20–90% increase). TNF activated NF-κB, along with IκBα degradation, occurred at 20 to 60 min in Hyal-2 cells post stimulation, but at the 20 min time point in both control and Hyal-1 cells. Hyal-2 cells, but not Hyal-1 and control cells, constitutively expressed WOX1, and transiently expressed Hyal-2 enhanced WOX1-mediated cell death. Unlike PH-20, Hyal-1 and Hyal-2 did not induce p53 expression. Hyal-2 translocated from the lysosome to the mitochondria during staurosporine-mediated apoptosis, suggesting that Hyal-2 may damage mitochondria. Finally, Hyal-1 and Hyal-2 blocked TGF-β1-enhanced L929 cell growth. In contrast, TGF-β1 inhibited Hyal-1- and Hyal-2-increased TNF cytotoxicity in L929 cells by 30–50%.
Conclusions
TGF-β1 limits the ability of Hyal-2 to induce TNF cytotoxicity in L929 cells. Hyal-2-increased TNF cytotoxicity in L929 cells appears to be correlated with upregulation of WOX1, a prolonged NF-κB activation, and Hyal-2 translocation to the mitochondria during apoptosis.
Background
Transforming growth factor beta (TGF-β) family proteins are multifunctional cytokines capable of regulating cell growth, extracellular matrix protein synthesis, and immune cell functions [1,2]. Hyaluronidase is an extracellular matrix-degrading enzyme. Interestingly, malignant and metastatic breast and prostate cancer cells frequently overexpress both hyaluronidase and TGF-β proteins [3-7]. Previously we have shown that bovine testicular hyaluronidase, also known as PH-20, increases the susceptibility of various cancer cells to tumor necrosis factor (TNF or TNF-α) cytotoxicity [[8], review]. PH-20 induces the expression of proapoptotic p53 [9] and WOX1 (WW domain-containing oxidoreductase; also known as WWOX or FOR) [10], which contributes to the increased TNF cytotoxic effect. PH-20-induced downregulation of the extracellular matrix inter-α-inhibitor is also associated with the enhanced TNF cytotoxicity [9].
TGF-β family proteins, including β1,β2 and β3, induce TNF-resistance and suppress the PH-20 effect of increasing TNF cytotoxicity in murine L929 fibroblasts [8,11]. TGF-β1 induces a novel extracellular matrix protein that prevents TNF-mediated cell death and blocks the activation of extracellular signal-regulated kinase (ERK; also known as p42/44 mitogen-activated protein kinase, p42/44 MAPK) in L929 cells [8,12]. Additionally, TGF-β1 induces the expression of TIAF1 (TGF-β-induced antiapoptotic factor) [13] and TIF2 (TGF-β-induced factor 2) [14] that inhibit TNF cytotoxicity. Whether these TGF-β1-induced proteins restrict the ability of PH-20 to increase TNF cytotoxicity is unknown.
PH-20 blocks TGF-β1-mediated growth suppression of epithelial cells [8,15]. Additionally, PH-20 rapidly activates ERK in L929 cells and TGF-β1 reduces the PH-20-induced ERK activation [16]. In contrast, TGF-β1 synergistically increases PH-20-mediated inhibition of staurosporine apoptosis [16]. Thus, PH-20 and TGF-β1 are physiological antagonists for only certain cellular responses.
Hyaluronidases Hyal-1 and Hyal-2 play a critical role in cancer invasion and metastasis [3-5], although inactivation of HYAL1 gene has been shown in head and neck squamous cell carcinomas [17]. Hyal-1, also known as Luca-1, is a lysosomal enzyme that is secreted from cells. Hyal-1 is a candidate tumor suppressor [5,18], although it enhances extravasation and metastasis of prostate cancer cells [3]. Also, mutations in Hyal-1 may contribute to the pathogenesis of a lysosomal disorder, mucopolysaccharidosis IX [19]. Hyal-2 is a lysosomal protein, which is active at low pH [20]. This protein is also known to be a glycosylphosphatidylinositol (GPI)-anchored cell-surface receptor for jaagsiekte sheep retrovirus [21].
Testicular hyaluronidase PH-20 counteracts TGF-β1-induced TNF-resistance in L929 cells [8]. The goal of the present study was to examine whether lysosomal Hyal-1 and Hyal-2 enhance TNF cytotoxic function and counteract TGF-β1-mediated TNF-resistance in L929 cells. It was determined that stable expression of murine Hyal-2 in L929 cells increased their sensitivity to TNF-mediated death, whereas Hyal-1 was somewhat less effective. TGF-β1 blocked this increased sensitivity. In contrast, Hyal-1 and Hyal-2 did not enhance L929 cell death by anticancer drugs such as daunorubicin, actinomycin D, staurosporine and camptothecin. Overexpressed Hyal-1 and Hyal-2 induced the expression of proapoptotic WOX1, but not p53, suggesting a role of WOX1 in the increased cellular susceptibility to TNF cytotoxicity. Additional mechanisms that are associated with the increased TNF cytotoxicity were also investigated.
Results
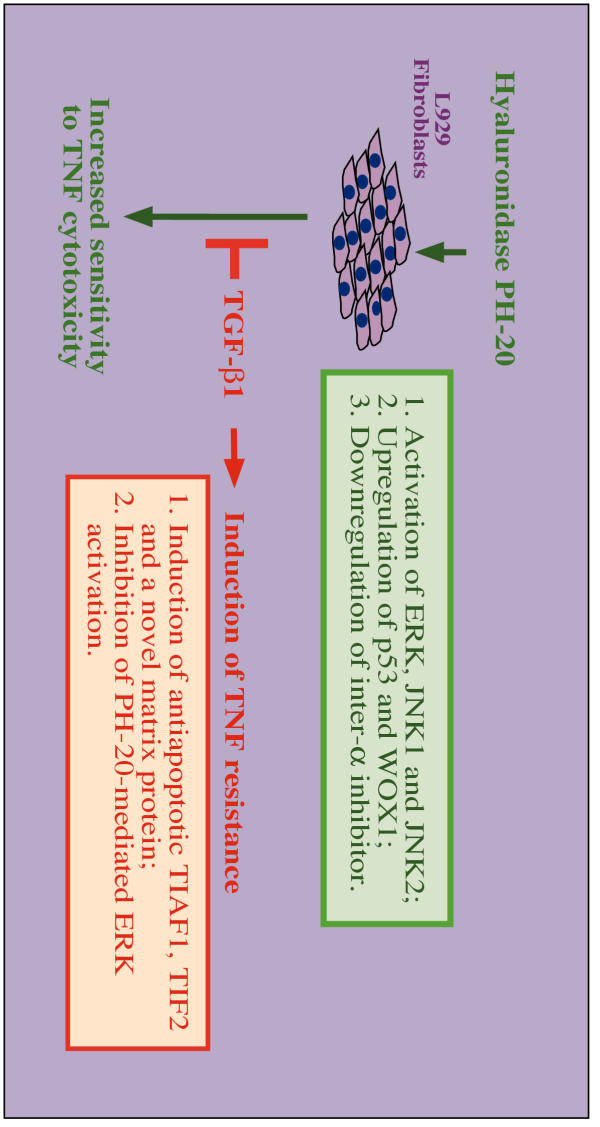
As summarized in Figure 1, PH-20 induces the expression of proapoptotic p53 and WOX1 [9,10] and downregulates antiapoptotic matrix inter-α-inhibitor [9], thereby enhancing TNF susceptibility in L929 cells. Also, PH-20 rapidly activates ERK and c-Jun N-terminal kinase (JNK1 and JNK2) [16]. Whether these signaling events lead to induction of p53 and WOX1 is unknown.
Figure 1.
Proposed basis for the antagonistic actions of PH-20 and TGF-β1 on L929 cell susceptibility to TNF cytotoxicity. Testicular hyaluronidase PH-20 enhances TNF cytotoxicity in L929 fibroblasts (see green), whereas TGF-β1 induces TNF-resistance in these cells (see red). Both upregulation of proapoptotic p53 and WOX1 and downregulation of antiapoptotic matrix inter-α-inhibitor are involved in the PH-20-increased TNF susceptibility in L929 cells [8-10]. Also, PH-20 rapidly activates ERK (or p42/44 MAPK) and c-Jun N-terminal kinases (JNK1 and JNK2) [16]. In contrast, TGF-β1 protects L929 cells from TNF cytotoxicity by upregulating antiapoptotic TIAF1, TIF2 and a novel matrix protein [11-14]. TGF-β1 counteracts PH-20-induced TNF-susceptibility in L929 cells [8]. TGF-β1 suppresses PH-20-mediated ERK activation [16], whereas ERK activation is not related with PH-20-increased TNF susceptibility in L929 cells [15]. Whether the proapoptotic p53 and WOX1 block the antiapoptotic function of TIAF1 and TIF2 is not known.
In contrast, TGF-β1 protects L929 cells from TNF cytotoxicity by upregulating the expression of antiapoptotic TIAF1, TIF2 and a novel matrix protein [11-14]. TGF-β1 blocks PH-20-increased TNF susceptibility [15]. Likewise, PH-20 inhibits TGF-β1-induced TNF-resistance [15]. TGF-β1 suppresses PH-20-mediated ERK activation [16]; however, the ERK activation is not related with PH-20-increased TNF susceptibility in L929 cells [15]. Whether the proapoptotic p53 and WOX1 block the function of antiapoptotic TIAF1 and TIF2 is not known. In this study, experiments were designed to determine whether lysosomal Hyal-1 and Hyal-2 enhance TNF cytotoxic function and counteract TGF-β1-mediated TNF-resistance in L929 cells.
The full-length murine hyaluronidase Hyal1 and Hyal2 cDNAs were found in the EST database (GenBank accession AA688635 and BF139787, respectively) and the determined DNA sequences have been deposited in the GenBank (Hyal1, AF422176; Hyal2, AF422177). The Hyal1 cDNA encodes a 463-amino-acid protein, which has 99.1% identity to a reported murine sequence (AF011567) [18]. Differences in the protein sequences are Gly-247, Gly-284 and Cys-450 in our Hyal-1 protein, compared to Arg-247, Glu-284 and Arg-450 in AF011567. Transitions in these amino acid residues are non-conservative.
The murine Hyal2 cDNA (AF422177) encodes a 473-amino-acid protein. The deduced protein sequence is identical to a reported murine sequence (AF302844), but has an Ile-355 to Val-355 transition compared to another clone (AF302843). Variation in the protein sequence is also found in a murine Hyal-2 homologue (NP_034619) [22], which possesses three unique 2-, 5- and 7-amino-acid segments, not identical to the above Hyal-2 proteins.
L929 cells were engineered to stably express GFP-Hyal-1 or GFP-Hyal-2 fusion proteins. The presence of these proteins in these cells was demonstrated in Western blotting using anti-GFP antibody (data not shown). Exogenous bovine testicular hyaluronidase PH-20 stimulates rapid activation of c-Jun N-terminal kinases (JNK1 and JNK2) in L929 cells [16]. In contrast, the stably expressed Hyal-1 and Hyal-2 could not mediate constitutive activation of JNK1 and JNK2 in L929 cells (data not shown).
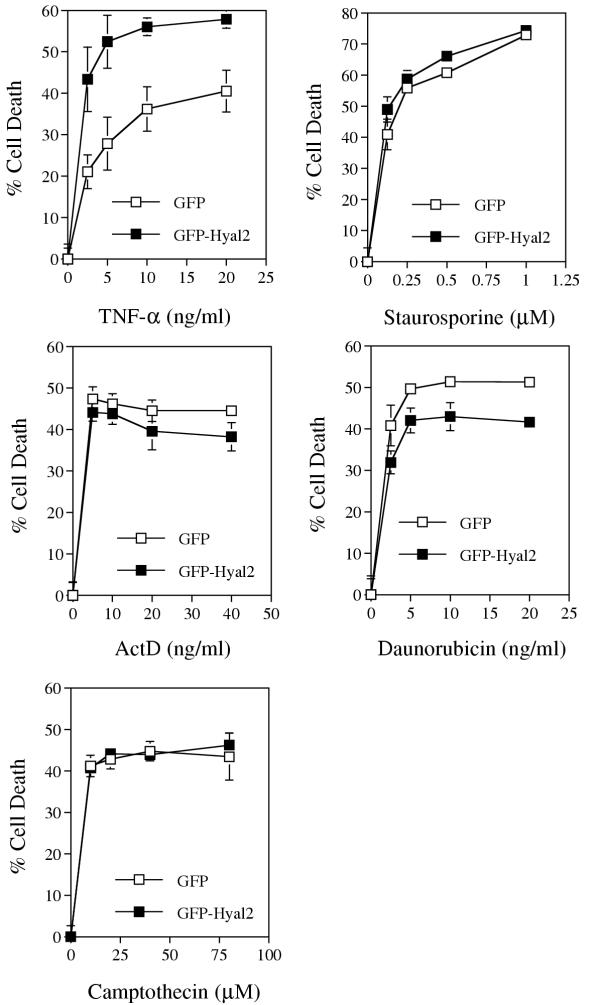
Exposure of the Hyal-2-expressing L929 cells to TNF for 16–24 hr resulted in increased cell death by ~60–110%, compared to the control GFP-expressing cells (Fig. 2). These results are similar to the bovine testicular PH-20-increased TNF cytotoxicity of L929 cells [15]. In contrast, there were no significant differences in the extent of cell death when GFP-Hyal-2 and control GFP-expressing cells were exposed to staurosporine, actinomycin D, daunorubicin, or camptothecin for 16–24 hr (Fig. 2).
Figure 2.
Hyal-2 increases L929 cell sensitivity to TNF cytotoxicity. L929 cell lines that stably expressed GFP and GFP-Hyal-2 were established (see Materials and Methods) and exposed to TNF-α, staurosporine, actinomycin D (ActD), daunorubicin, or camptothecin for 24 hr. The extent of cell death were measured by staining the cells with crystal violet as previously described (mean ± standard deviation; n = 8) [10].
There was also an increased susceptibility to TNF cytotoxicity in the Hyal-1-expressing L929 cells (approximately 20–90% increase; also see Fig. 7), compared to the GFP control cells. However, there were no significant differences among these cells in the extent of death caused by staurosporine, actinomycin D, daunorubicin and camptothecin (data not shown).
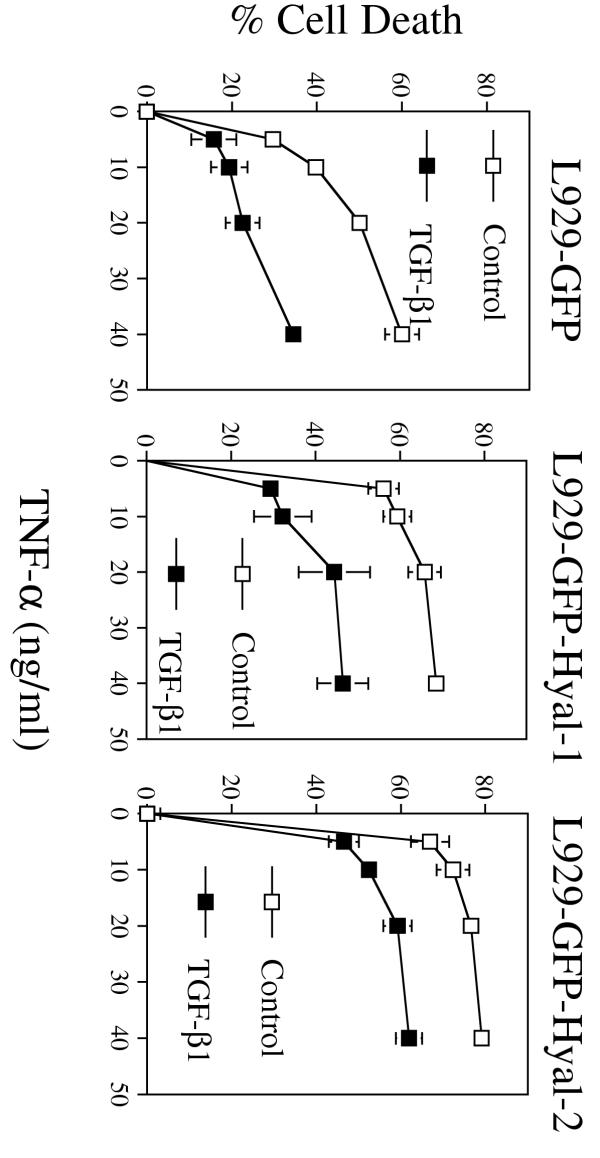
Figure 7.
TGF-β1 suppresses Hyal-1 and Hyal-2-increased TNF cytotoxicity in L929 cells. L929 cells stably expressing GFP, GFP-Hyal-1 or GFP-Hyal-2 were cultured in 96-well plates overnight, treated with TGF-β1 (2 ng/ml) for 2 hr, and then cotreated with TNF-α (5–40 ng/ml) for 24 hr. TGF-β1 inhibited TNF-α killing of L929 cells expressing GFP, and GFP-Hyal-1 or GFP-Hyal-2 (mean ± β1 inhibited TNF-α killing of the control GFP L929 cells to a greater extent (50–70%) than the GFP-Hyal-1 or GFP-Hyal-2 cells (30–50%).
In similar experiments, the effect of transient expression of Hyal-1 and Hyal-2 on cellular sensitivity to anticancer drugs was examined. L929 cells were electroporated with the GFP-Hyal-1 or GFP-Hyal-2 construct, cultured for 48 hr and then exposed to actinomycin D, daunorubicin or camptothecin for 24 hr. There were no differences in cell death in these Hyal-1 and Hyal-2-expressing cells, compared to control cells (data not shown). These findings are consistent with the results for stable transfectants presented above (Fig. 2).
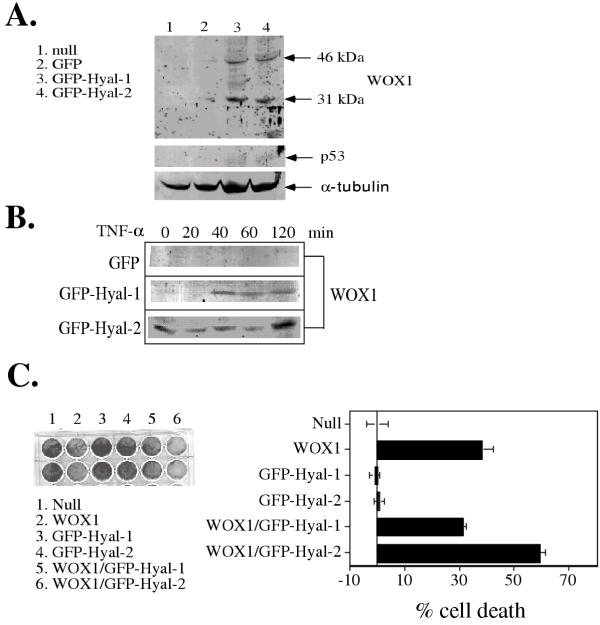
Bovine testicular PH-20 enhances TNF sensitivity in L929 cells by increasing the expression of proapoptotic p53 and WOX1 [9,10]. The ability of Hyal-1 and Hyal-2 to induce the expression of p53 and WOX1 in L929 cells was examined. L929 cells were electroporated with nothing (null), GFP, GFP-Hyal-1 or GFP-Hyal-2 constructs and cultured for 48 hr. Transient expression of GFP-Hyal-1 or GFP-Hyal-2 in L929 cells induced WOX1 expression, whereas no WOX1 expression was observed in non-transfected cells or control GFP-expressing cells (Fig. 3A). The 46-kDa band is full-length WOX1, while the 31-kDa band is a degraded WOX1 [10]. No detectable p53 expression was observed in these cells (Fig. 3A).
Figure 3.
Hyal-2 induces WOX1 expression in L929 cells. (A) L929 cells were electroporated with nothing (null), GFP, GFP-Hyal-1 or GFP-Hyal-2 construct (30 g DNA / 3 × 106 cells) and cultured for 48 hr. Transient expression of GFP-Hyal-1 and GFP-Hyal-2 in L929 cells induced WOX1 expression (46 and 31 kDa), whereas no WOX1 expression was observed in non-transfected cells or control GFP-expressing cells. The 46-kDa band is full-length WOX1, and the 31-kDa band is a degraded WOX1 [10]. No detectable p53 expression was observed in these cells. α-Tubulin was examined to confirm equal protein loading onto each lane of the gel. (B) In comparison, the GFP-Hyal-2 stable transfectant constitutively expressed cytosolic WOX1 (46 kDa) and TNF (50 ng/ml) reduced the WOX1 expression. The reduction is probably due to TNF-mediated WOX1 nuclear translocation from the mitochondria [10]. In contrast, WOX1 was not expressed in the stable GFP-Hyal-1 cells, and TNF induced the expression of WOX1 in a time-related manner. TNF could not induce WOX1 expression in the control GFP-expressing cells. (C) L929 cells were transfected with GFP-Hyal-2 and/or WOX1 constructs by electroporation. GFP-Hyal-2 enhanced WOX1-mediated L929 cell death, as determined 48 hr post-transfection using crystal violet staining (two representative rows of cell stains shown at left and the analyzed data shown at right; mean ± standard deviation, n = 4). cell death. In controls, GFP-Hyal-1 or GFP-Hyal-2 alone failed to induce cell death.
In comparison, the cytosolic WOX1 (46 kDa) was constitutively expressed in the L929 cells stably transfected with the GFP-Hyal-2 construct, while TNF reduced WOX1 expression in a time-dependent manner (Fig. 3B). This reduction is probably due to TNF-mediated WOX1 nuclear translocation from the mitochondria [10]. In contrast, WOX1 was not expressed in L929 cells stably transfected with the GFP-Hyal-1 construct, and TNF induced WOX1 expression in 40 min (Fig. 3B). TNF did not induce WOX1 expression in the control GFP-expressing cells (Fig. 3B).
Whether Hyal-2 enhances the apoptosis-inducing function of WOX1 was determined. In transient transfection experiments, L929 cells were electroporated with GFP-Hyal-2 and/or WOX1 constructs. GFP-Hyal-2 enhanced WOX1-mediated L929 cell death, as determined 48 hr post-transfection using crystal violet staining (Fig. 3C). In contrast, GFP-Hyal-1 did not enhance WOX1-mediated cell death (Fig. 3C). GFP-Hyal-1 and GFP-Hyal-2 alone did not mediate cell death (Fig. 3C).
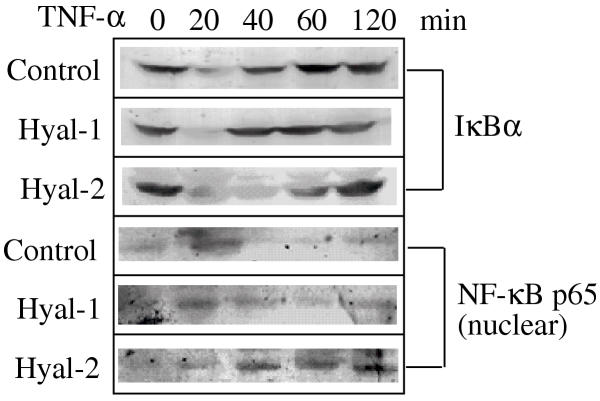
Next, the effect of TNF-mediated IκBα degradation and NF-κB activation was examined in both Hyal-1 and Hyal-2 cells. The GFP-Hyal-1 and GFP-Hyal-2 stable transfectants and control cells were exposed to TNF for various times. IκBα degradation and NF-κB activation (or NF-κB nuclear translocation) were observed at 20 to 60 min in Hyal-2 cells post TNF stimulation, but at the 20 min time point in both control and Hyal-1 cells (Fig. 4).
Figure 4.
TNF mediates a prolonged period of IκBα degradation and NF-κB activation in L929 cells stably expressing GFP-Hyal-2. The GFP-Hyal-1 and GFP-Hyal-2 stable transfectants, as well as control L929 cells, were exposed to TNF (50 ng/ml) for 0, 20, 40, 60 and 120 min, followed by analysis of protein expression by Western blotting. TNF mediated a prolonged cytosolic IκBα degradation, as well as a nuclear presence of NF-κB p65 (or NF-κB activation), over a 20 to 60 min time period in Hyal-2 cells. A similar effect was seen only at 20 min in Hyal-1 and control cells.
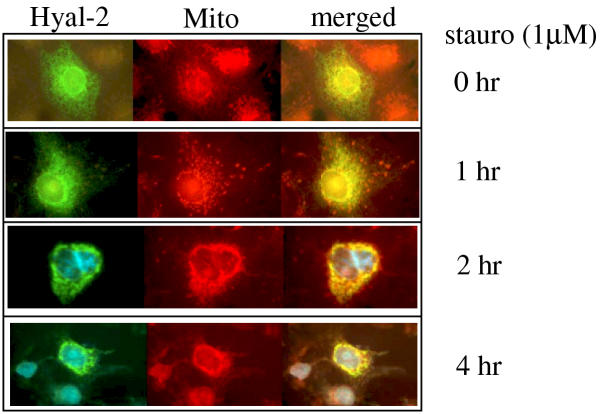
To further understand how Hyal-2 participates in the apoptosis pathway, the translocation of Hyal-2 to the mitochondria was examined during cell death. Transient expression of GFP-Hyal-2 in COS7 fibroblasts showed the localization of the expressed protein in the lysosome but not in the mitochondria, as determined by staining the cells using LysoTracker and Mitotracker Red (data not shown). These observations are consistent with other reports [5,20]. COS7 cells are TNF-resistant. The reasons for using this cell type are its large size (which provides better resolution under fluorescence microscopy) and the ease of transfection and gene expression using liposome-based reagents such as FuGene 6 and GeneFector. Exposure of the GFP-Hyal-2-expressing COS7 cells to staurosporine (for inducing apoptosis) resulted in a time-related increase in co-localization of GFP-Hyal-2 with the mitochondria (Fig. 5), suggesting that a portion of GFP-Hyal-2 migrates to the mitochondria during apoptosis.
Figure 5.
Migration of Hyal-2 to the mitochondria during staurosporine-mediated apoptosis. COS7 cells were transfected with the GFP-Hyal-2 construct using FuGene 6 (Roche) or GeneFector (Venn Nova). The cells were cultured for 24–48 hr and then treated with staurosporine (stauro). Mitochondria (Mito) were stained with Mitotracker Red and nuclei were stained with DAPI. Co-localization analysis showed that there was a time-related increase in co-localization of GFP-Hyal-2 with mitochondria (see merged), suggesting that a portion of GFP-Hyal-2 migrates to the mitochondria during apoptosis. The nuclear stain is shown at the 2- and 4-hr time points only.
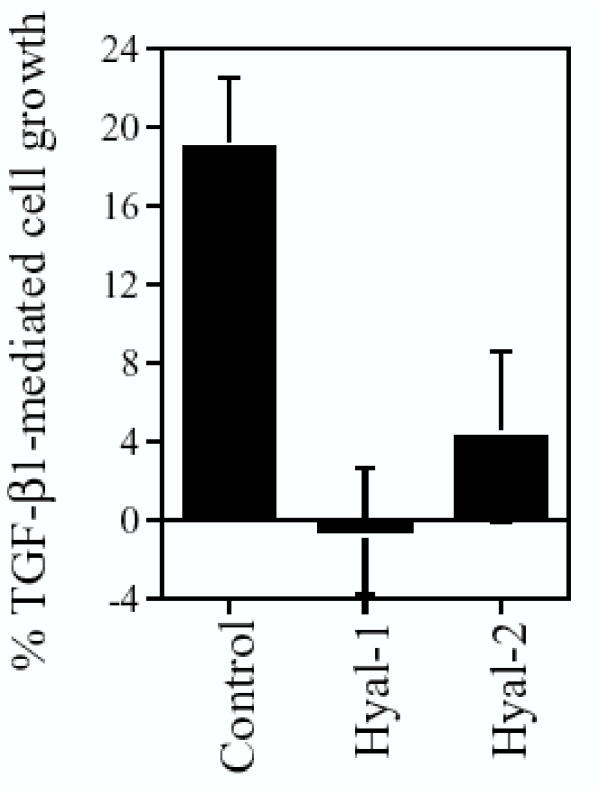
Next, whether TGF-β1 antagonizes the function of Hyal-1 and Hyal-2 in growth regulation was examined. TGF-β1 inhibits epithelial cell growth but promotes fibroblast growth [15]. TGF-β1 inhibition of epithelial cell growth is blocked by bovine testicular PH-20 [15]. In agreement with our previous observations [11], TGF-β1 promoted L929 cell growth by approximately 20% in 24–48 hr (Fig. 6). However, TGF-β1 did not promote the growth of L929 cells stably expressing Hyal-1 or Hyal-2 (Fig. 6).
Figure 6.
TGF-β1 promotes L929 cell growth but not the growth of Hyal-1 and Hyal-2-expressing L929 cells. Non-transfected L929 cells or the stable GFP-Hyal-1 and GFP-Hyal-2-expressing cells were treated with TGF-β1 (2 ng/ml) for 24 hr. The extent of cell growth was measured by staining the cells with crystal violet (mean ± standard deviation; n = 8).
Finally, the functional antagonism between TGF-β1 and Hyal-1 or Hyal-2 in regulating TNF susceptibility in L929 cells was examined. The Hyal-1 and Hyal-2 stable transfectants were pretreated with TGF-β1 for 1 hr and then treated with TNF overnight. Both Hyal-1- and Hyal-2-increased TNF cytotoxicity were suppressed by TGF-β1 (30–50%) (Fig. 7). However, TGF-β1 blocked TNF cytotoxicity of the GFP-expressing control cells to a greater extent (50–70%) (Fig. 7).
Discussion
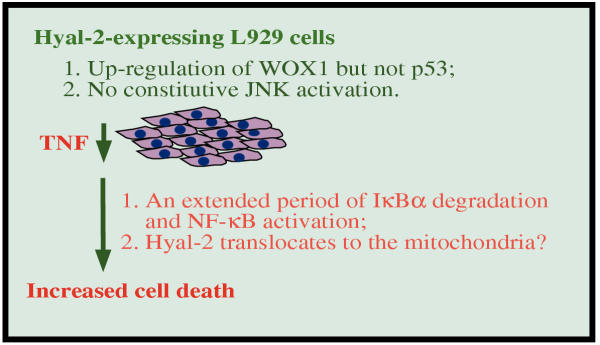
In this study, the novel function of the lysosomal hyaluronidases Hyal-1 and Hyal-2 in increasing cellular sensitivity to TNF cytotoxicity is reported. TGF-β1 counteracted Hyal-1- and Hyal-2-increased TNF killing of L929 fibroblasts. TGF-β1 increased L929 growth, while Hyal-1 and Hyal-2 blocked this enhancement of cell growth. These observations are consistent with our previous finding that exogenous bovine testicular hyaluronidase PH-20 enhances TNF cytotoxicity in various cancer cells, and that TGF-β1 blocks the increased TNF cytotoxicity [8,15]. As summarized in the Figure 8, the underlying mechanisms whereby Hyal-2 induces TNF cytotoxicity appear to be associated with 1) Hyal-2 induction of the proapoptotic WOX1, 2) Hyal-2 migration to the mitochondria during apoptosis, and 3) the prolonged period of IκBα degradation and NF-κB activation in TNF-treated Hyal-2-expressing cells.
Figure 8.
A summarized scheme for Hyal-2-induced TNF susceptibility in L929 cells. Upregulation of proapoptotic WOX1 but not p53 was observed in the Hyal-2-expressing cells. Unlike PH-20, Hyal-2 could not induce constitutive JNK activation. TNF cytotoxicity was enhanced in these Hyal-2-expressing cells. The enhancement of TNF function is likely due to 1) the induced proapoptotic WOX1, 2) the likely translocation of Hyal-2 to the mitochondria in response to TNF, and 3) the prolonged period of IκBα degradation and NF-κB activation in as mediated by TNF.
Upregulation of p53 and WOX1 contributes, in part, to the PH-20-enhanced cytotoxic function of TNF [9,10,23]. WOX1 is a p53-binding protein. Ectopic expression of both p53 and WOX1 cDNA constructs results in colocalization of these proteins in the mitochondria [23]. Increased synthesis and complex formation between p53 and WOX1 in the cytoplasm are observed during apoptosis [23]. Both p53 and WOX1 mediate apoptosis synergistically. Overexpressed WOX1 alone mediates apoptosis independently of p53 [10]. However, p53 apoptosis requires the participation of WOX1. Blocking WOX1 expression with antisense mRNA abolishes p53-mediated cell death, suggesting that WOX1 is a potential partner of p53 in apoptosis [10,23].
Both Hyal-1 and Hyal-2 induced WOX1 expression when transiently expressed in L929 cells. However, only Hyal-2 induced WOX1 expression following stable transfection of L929 cells. This correlates with the greater extent of Hyal-2 enhancement of TNF killing compared to Hyal-1. WOX1 expression was induced by TNF in the Hyal-1 stable transfectant. In transient transfection experiments, Hyal-2 enhanced WOX1-mediated L929 cell death, while Hyal-1 had no effect. Both Hyal-1 and Hyal-2 alone failed to induce cell death. Thus, induction of WOX1 expression contributes, in part, to Hyal-1- and Hyal-2-increased TNF sensitivity in L929 cells.
TNF rapidly induces phosphorylation of IκBα, which normally peaks at 5–10 min, followed by proteasome-dependent degradation (10–20 min) [10,24]. IκBα degradation and NF-κB activation occurred, as determined 20 min after exposure of control and Hyal-1 cells to TNF. However, there was an extended period of TNF-mediated IκBα degradation and NF-κB activation in Hyal-2 cells, ranging from 20–60 min. It is unclear whether this extend ed period of IκBα degradation and NF-κB activation directly contributes to the ability of Hyal-2 to enhance TNF-mediated apoptosis. IκBα is a physiological inhibitor of NF-κB activation; IκBα complexes with NF-κB and prevents its translocation to the nucleus [10,24]. NF-κB activation is believed to induce antiapoptotic proteins to block cell death [8]. However, in several studies activated NF-κB cannot protect cells from apoptosis [8]. Also, NF-κB appears to be essential in the p53-mediated apoptosis in tested cell lines [25].
The mitochondrion is a reservoir of proapoptotic proteins and plays a key role in apoptosis [[26,27]; reviews]. One of the pathways that leads to the release of proapoptotic proteins in the mitochondria has been determined. For example, the TNF/TNF receptor-signaling complex activates caspase 8, which leads to the activation and cleavage of Bid, a proapoptotic protein of the Bcl-2 family. Activated or truncated Bid (tBid) in turn activates Bak and Bax. Bak migrates to the mitochondrial outer membrane where it generates transmembrane pores; Bax induces the opening of the mitochondrial permeability transition pores. Cytochrome c is then released from the mitochondria into the cytosol, where it interacts with downstream proapoptotic proteins such as Apaf-1 and caspase 9. Activation of these proteins further activates nucleases, such as caspase-activated DNase (CAD), which cause DNA fragmentation.
During staurosporine-mediated apoptosis, a portion of the lysosomal Hyal-2 migrates to the mitochondria. Whether Hyal-2 causes mitochondrial membrane damage is unknown. Presumably, Hyal-2 interacts with a specific protein target on the mitochondrial surface that changes the membrane permeability. This notion is supported by our recent screening of Hyal-1 and Hyal-2 interacting proteins in yeast two-hybrid interactions (Chang et al, unpublished)
The functional property of Hyal-2 in degrading hyaluronic acid is controversial. Hyal-2 is found in the lysosome and is active under conditions of low pH [20]. Hyal-2 is also found on the cell surface as a glycosylphosphatidylinositol (GPI)-anchored receptor for jaagsiekte sheep retrovirus [21]. Nonetheless, the enzymatic activity of Hyal-2 has not been detected [21]. Whether the increased TNF cytotoxicity in L929 cells caused by Hyal-1 and Hyal-2 is associated with their enzymatic activities is being studied in this laboratory.
The TGF-β1 inhibition of Hyal-1 and Hyal-2 effects could be abolished by cycloheximide or actinomycin D (data not shown). That is, inhibition of protein synthesis or gene transcription reduced the inhibitory effect of TGF-β1 in the prevention of Hyal-1- or Hyal-2-increased TNF cytotoxicity. Whether TGF-β1-induced antiapoptotic proteins such as TIAF1 and TIF2 inhibit the function of Hyal-1 and Hyal-2 remains to be determined.
Conclusions
Like testicular PH-20, lysosomal Hyal-1 and Hyal-2 enhanced TNF susceptibility in L929 cells. TGF-β1 suppressed the induced TNF sensitivity. Furthermore, TGF-β1-mediated L929 cell proliferation was blocked by Hyal-1 and Hyal-2. These data indicate that there is a functional antagonism between TGF-β1 and Hyal-1 or Hyal-2. Hyal-2-increased TNF susceptibility in L929 cells appears to be associated with 1) Hyal-2 upregulation of the proapoptotic WOX1, 2) Hyal-2 migration to the mitochondria during apoptotic cell death, and 3) the prolonged IκBα degradation and NF-κB activation in Hyal-2-expressing cells stimulated with TNF.
Materials and Methods
cDNA constructs, transient expression and stable transfectants
The full-length murine hyaluronidase Hyal1 and Hyal2 cDNAs were found in the EST database (Genbank accession AA688635 and BF139787, respectively) and purchased from Incyte Genomics (St. Louis, MO). The determined DNA sequences have been deposited in the GenBank (Hyal1, AF422176; Hyal2, AF422177). The coding regions were tagged with a C-terminal green fluorescent protein (GFP) sequence by subcloning into the EcoR1 site of the pEGFP-N1 vector (Clontech, Palo Alto, CA). Murine L929 cells were transfected with the constructed Hyal-1-pEGFP-N1 or Hyal-2-pEGFP-N1 vector, or a control pEGFP-N1 vector by electroporation (40 μg DNA/ 5 × 106 cells; 250 volt and 960 Fd), as previously described [10]. The cells were cultured in the continuous presence of G418 (200 g/ml) for approximately 2 weeks, and the resulting total cell colonies were harvested. The presence of GFP-Hyal-1 and GFP-Hyal-2 proteins in these stable transfectants was examined by Western blotting using anti-GFP antibodies (Clontech). Where indicated, COS7 fibroblasts were transfected with the above constructs using a liposome-based reagent FuGene 6 (Roche, Indianapolis, IN) or GeneFector (Venn Nova, Pompano Beach, FL). Protein expression was visualized by fluorescence microscopy. Also, the cells were counterstained with LysoTracker or Mitotracker Red (Molecular Probes, Eugene, OR) to determine whether the expressed protein was located in the lysosome or mitochondria.
Cell lines and chemicals
Murine L929 fibroblasts and monkey kidney COS7 fibroblasts were cultured as previously described [10]. Staurosporine, daunorubicin, actinomycin D, camptothecin and DAPI were from Calbiochem (San Diego, CA), Sigma (St. Louis, MO) and Biomol (Plymouth Meeting, PA). Purified human platelet TGF-β1 was from R&D (Minneapolis, MN). Antisera against WOX1 were generated in rabbits against a synthetic peptide [10]. Antibodies against p53, IκBα, NF-κB p65 and α-tubulin were from Santa Cruz Biotechnology (Santa Cruz, CA) and Accurate Chemicals (Accurate Chemical, Westbury, NY).
TNF cytotoxicity assay and Western blotting
The above established cell lines were treated with TNF-α (2.5–20 ng/ml; Pepro-Tech, Rocky Hill, NJ) for 16–24 hr. The extent of cell death was determined by crystal violet staining [10]. Where indicated, the cells were also exposed to staurosporine, actinomycin D, daunorubicin, and camptothecin for 16–24 hr, before determining the extent of cell death. To determine whether TGF-β1 blocked Hyal-1 and Hyal-2-increased TNF killing, the cells were pretreated with TGF-β1 for 2 hr and then exposed to TNF-α in the continued presence of TGF-β1 for 16–24 hr. The extent of cell death was then determined. Where indicated, GFP-Hyal-1, GFP-Hyal-2 and GFP stable transfectants were treated with TNF (50 ng/ml) for 0, 20, 40, 60 and 120 minutes. The cells were extracted using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce, Rockford, IL) to separate the cytoplasmic and nuclear fractions. These preparations were subjected to SDS-PAGE and Western blotting using specific antibodies against IκBα, WOX1 and NF-κB.
Gene transfection and fluorescent microscopy
COS7 cells were transfected with the GFP-Hyal-2 vector using the liposome-based FuGene 6 or GeneFector, cultured for 24–48 hr, and treated with staurosporine (1 M) for 0, 1, 2 and 4 hr. Mitochondria were stained with the membrane potential sensitive Mitotracker Red. Nuclei were stained by DAPI. The cells were then examined by fluorescence microscopy.
Acknowledgments
Acknowledgment
Research was supported by the American Heart Association and the Guthrie Foundation for Education and Research. I thank Ms. Lori Schultz and Mr. John Heath for technical assistance, and Drs. Robert S. Aronstam, Margaret P. Quinlan and Victor Ruiz-Velasco for critically reviewing the manuscript.
References
- Sporn MB, Roberts AB. The transforming growth factor-betas: past, present, and future. New York Acad Sci. 1990;593:1–6. doi: 10.1111/j.1749-6632.1990.tb16095.x. [DOI] [PubMed] [Google Scholar]
- Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–161. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- Lokeshwar VB, Rubinowicz D, Schroeder GL, Forgacs E, Minna JD, Block NL, Nadji M, Lokeshwar BL. Stromal and epithelial expression of tumor markers hyaluronic acid and HYAL1 hyaluronidase in prostate cancer. J Biol Chem. 2001;276:11922–11932. doi: 10.1074/jbc.M008432200. [DOI] [PubMed] [Google Scholar]
- Novak U, Stylli SS, Kaye AH, Lepperdinger G. Hyaluronidase-2 overexpression accelerates intracerebral but not subcutaneous tumor formation of murine astrocytoma cells. Cancer Res. 1999;59:6246–6250. [PubMed] [Google Scholar]
- Patel S, Turner PR, Stubberfield C, Barry E, Rohlff CR, Stamps A, Tyson K, Terrett J, Box G, Eccles S, Page MJ. Hyaluronidase gene profiling and role of HYAL-1 overexpression in an orthotopic model of prostate cancer. Int J Cancer. 2002;97:416–424. doi: 10.1002/ijc.1638. [DOI] [PubMed] [Google Scholar]
- Donovan J, Slingerland J. Transforming growth factor-beta and breast cancer: Cell cycle arrest by transforming growth factor-beta and its disruption in cancer. Breast Cancer Res. 2000;2:116–124. doi: 10.1186/bcr43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikstrom P, Bergh A, Damber JE. Transforming growth factor-beta1 and prostate cancer. Scand J Urol Nephrol. 2000;34:85–94. doi: 10.1080/003655900750016689. [DOI] [PubMed] [Google Scholar]
- Chang N-S. Transforming growth factor-β protection of cancer cells against tumor necrosis factor cytotoxicity is counteracted by hyaluronidase (Review). Int J Mol Med. 1998;2:653–659. [PubMed] [Google Scholar]
- Chang N-S, Carey G, Pratt N, Chu E, Ou M. p53 overexpression and downregulation of inter-α-inhibitor are associated with hyaluronidase enhancement of TNF cytotoxicity in L929 fibroblasts. Cancer Lett. 1998;131:45–54. doi: 10.1016/S0304-3835(98)00200-6. [DOI] [PubMed] [Google Scholar]
- Chang N-S, Pratt N, Heath J, Schultz L, Sleve D, Carey GB, Zevotek N. Hyaluronidase induction of a WW domain-containing oxidoreductase that enhances tumor necrosis factor cytotoxicity. J Biol Chem. 2001;276:3361–3370. doi: 10.1074/jbc.M007140200. [DOI] [PubMed] [Google Scholar]
- Chang N-S. TGF-β1 induction of novel extracellular proteins that trigger resistance to TNF cytotoxicity in murine L929 fibroblasts. J Biol Chem. 1995;270:7765–7772. doi: 10.1074/jbc.270.13.7765. [DOI] [PubMed] [Google Scholar]
- Chang N-S. TGF-β-induced matrix proteins inhibit p42/44 MAPK and SAPK/JNK activation and suppress TNF-mediated IκBα degradation and NF-κB nuclear translocation in L929 fibroblasts. Biochem Biophys Res Commun. 2000;267:194–200. doi: 10.1006/bbrc.1999.1909. [DOI] [PubMed] [Google Scholar]
- Chang N-S, Mattison J, Cao H, Pratt N, Zhao Y, Lee C. Cloning and characterization of a novel transforming growth factor-beta1-induced TIAF1 protein that inhibits tumor necrosis factor cytotoxicity. Biochem Biophys Res Commun. 1998;253:743–749. doi: 10.1006/bbrc.1998.9846. [DOI] [PubMed] [Google Scholar]
- Carey GB, Chang N-S. Cloning and characterization of a transforming growth factor beta 1-induced antiapoptotic adhesion protein TIF2. Biochem Biophys Res Commun. 1998;249:283–286. doi: 10.1006/bbrc.1998.9132. [DOI] [PubMed] [Google Scholar]
- Chang N-S. Reversal of hyaluronidase enhancement of tumor necrosis factor-mediated cell death by transforming growth factor- Am J Physiol. 1997;273:C1987–C1994. doi: 10.1152/ajpcell.1997.273.6.C1987. [DOI] [PubMed] [Google Scholar]
- Chang N-S. Hyaluronidase activation of c-Jun N-terminal kinase is necessary for protection of L929 fibrosarcoma cells from staurosporine-mediated cell death. Biochem Biophys Res Commun. 2001;283:278–286. doi: 10.1006/bbrc.2001.4701. [DOI] [PubMed] [Google Scholar]
- Frost GI, Mohapatra G, Wong TM, Csoka AB, Gray JW, Stern R. HYAL1LUCA-1, a candidate tumor suppressor gene on chromosome 3p21.3, is inactivated in head and neck squamous cell carcinomas by aberrant splicing of pre-mRNA. Oncogene. 2000;19:870–877. doi: 10.1038/sj.onc.1203317. [DOI] [PubMed] [Google Scholar]
- Csoka TB, Frost GI, Heng HH, Scherer SW, Mohapatra G, Stern R. The hyaluronidase gene HYAL1 maps to chromosome 3p21.2-p21.3 in human and 9F1-F2 in mouse, a conserved candidate tumor suppressor locus. Genomics. 1998;48:63–70. doi: 10.1006/geno.1997.5158. [DOI] [PubMed] [Google Scholar]
- Triggs-Raine B, Salo TJ, Zhang H, Wicklow BA, Natowicz MR. Mutations in HYAL1, a member of a tandemly distributed multigene family encoding disparate hyaluronidase activities, cause a newly described lysosomal disorder, mucopolysaccharidosis IX. Proc Natl Acad Sci USA. 1999;96:6296–6300. doi: 10.1073/pnas.96.11.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepperdinger G, Strobl B, Kreil G. HYAL2, a human gene expressed in many cells, encodes a lysosomal hyaluronidase with a novel type of specificity. J Biol Chem. 1998;273:22466–22470. doi: 10.1074/jbc.273.35.22466. [DOI] [PubMed] [Google Scholar]
- Rai SK, Duh FM, Vigdorovich V, Danilkovitch-Miagkova A, Lerman MI, Miller AD. Candidate tumor suppressor HYAL2 is a glycosylphosphatidylinositol (GPI)-anchored cell-surface receptor for jaagsiekte sheep retrovirus, the envelope protein of which mediates oncogenic transformation. Proc Natl Acad Sci USA. 2001;98:4285–4287. doi: 10.1073/pnas.071572898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobl B, Wechselberger C, Beier DR, Lepperdinger G. Structural organization and chromosomal localization of Hyal2, a gene encoding a lysosomal hyaluronidase. Genomics. 1998;53:214–219. doi: 10.1006/geno.1998.5472. [DOI] [PubMed] [Google Scholar]
- Chang N-S. A potential role of p53 and WOX1 in the mitochondrial apoptosis (Review). Int J Mol Med. 2002;9:19–24. [PubMed] [Google Scholar]
- Chang NS. The non-ankyrin C-terminus of IκBα physically interacts with p53 in vivo and dissociates in response to apoptotic stress, hypoxia, DNA damage and transforming growth factor-β1-mediated growth suppression. J Biol Chem. 2002;277:10323–10331. doi: 10.1074/jbc.M106607200. [DOI] [PubMed] [Google Scholar]
- Ryan KM, Ernst MK, Rice NR, Vousden KH. Role of NF-kappaB in p53-mediated programmed cell death. Nature. 2000;404:892–897. doi: 10.1038/35009130. [DOI] [PubMed] [Google Scholar]
- Chang N-S. Mitochondria in programmed cell death: the role of apoptogenic proteins. Guthrie J. 1999;68:116–122. [Google Scholar]
- Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–2933. [PubMed] [Google Scholar]