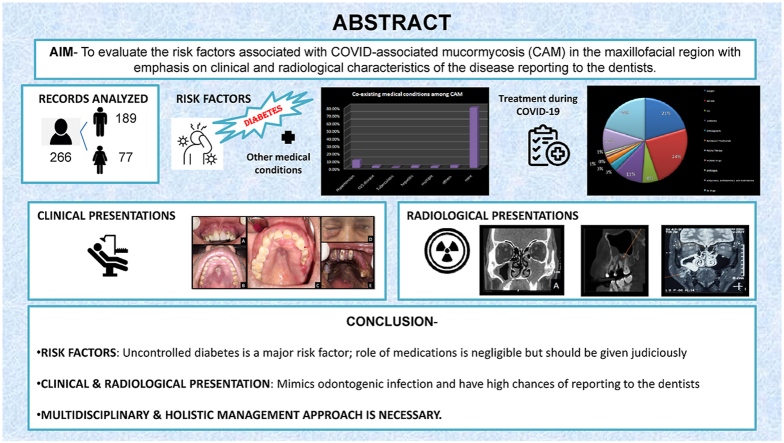
Abstract
Purpose
To evaluate the prevalence of risk factors associated with COVID-associated mucormycosis (CAM) in the maxillofacial region with emphasis on clinical and radiological characteristics of the disease reporting to the dentists.
Methods
Archival records of the patients diagnosed with rhino-cerebral mucormycosis through histopathology or culture, were screened and 266 records were included. These records were divided into three groups-previously diabetic (PD, n = 122), recently diagnosed diabetic (RD, n = 105) and non-diabetic (ND, n = 39). All the records were evaluated and compared among the three groups for the duration of presentation, history of co-existing medical conditions, the association of treatment given during COVID-19, and the clinical and radiographic presentations of the disease.
Results
The results confirmed uncontrolled diabetes mellitus as the major risk factor for the disease. The prevalence of steroid administration was lower in our study in contrast to previous literature. The risk factors and treatment rendered during COVID-19 did not differ significantly among the three groups (p > 0.05). The findings indicate that the disease was milder and progressed more slowly in the ND group, both clinically and radiographically, and it had close resemblance to odontogenic infection.
Conclusion
Patients with early CAM mimicked the odontogenic infection and were more likely to report in a dental setup. Hence, a multidisciplinary and holistic management approach is necessary.
Keywords: Mucormycosis, COVID-19, Invasive fungal infections, Mycoses, Diabetes
Graphical abstract
1. Introduction
COVID-19, a disease caused by SARS-CoV-2, is one of the worst disasters that humanity has ever faced. It has been associated with a multitude of opportunistic bacterial and fungal infections. Several cases of mucormycosis in people with COVID-19 have been reported across the world, notably in India.1,2
Mucormycosis is a rapidly progressing, opportunistic, angio-invasive fungal infection that is most common in immune-compromised people.3 It has six types of presentations which include: rhino-cerebral, pulmonary, cutaneous, gastrointestinal, disseminated, and miscellaneous.4 Rhino-orbito-cerebral form is frequently associated with uncontrolled diabetes and diabetic ketoacidosis, pulmonary involvement is frequently seen in patients with neutropenia, bone marrow and organ transplantation, diabetes, COVID-19 and hematological malignancies, and GIT involvement is more common in malnourished people.1,5
The early symptoms of the rhino-cerebral variant include facial pain and swelling, proptosis, and blurry vision, followed by, palatal mucosal necrosis, loss of vision, and in later stages may lead to cavernous sinus thrombosis and eventually death.4 It may also manifest as toothache and mobility of maxillary teeth, as well as abscess formation and restriction of jaw movement, which were rarely observed prior to COVID-19.6,7
Magnetic resonance imaging (MRI) and computed tomography (CT) are the most common types of diagnostic imaging used in CAM. MRI has an advantage over CT in that it can evaluate the degree of fungal invasion, whereas CT is better at detecting the bone erosion that occurs in the later stages of infection.7 Owing to its high resolution, CBCT may be reserved for accurate visualization of slight osseous and trabecular changes in the maxillary alveolus and adjacent regions that are likely to be missed on soft tissue algorithm of CT and MRI.8 Microbiological and/or histopathological analysis of tissues taken from various lesions can be used to provide a definitive diagnosis.7
The global fatality rate of mucormycosis is 46%. So, an early diagnosis and prompt treatment are necessary. A delay of even six days is associated with a twofold increase of 30- day mortality from 35% to 66%. Therefore, a high index of suspicion is fundamental to rapidly diagnose and treat this devastating disease.9
Some of the hypotheses put forth in the literature for the pathogenesis of COVID-19-associated mucormycosis (CAM) include immune dysregulation caused by COVID-19, similarity in biological behavior to SARS-CoV, the virus's propensity for ACE-2 receptors in the nasal mucosa, overexpression of pro-thrombotic CX3CL1 marker, the virus' propensity to initiate diabetes, the medications given during COVID-19 (glucocorticoids, remdesivir, tocilizumab, baricitinib zinc supplements, antibacterial agents, and antifungal therapy), or the continued use of the same mask.10,11
Most of the current literature available has succeeded in suggesting an association of mucormycosis with COVID-19, uncontrolled diabetes mellitus (DM), and inappropriate steroid administration. Also, a few recent literature analyzed the effect of other co-existing medical conditions and their correlation with the severity of COVID-19 and the treatment given.10,12,13
So, the present study was designed to retrospectively evaluate the prevalence of risk factors associated with COVID-associated Mucormycosis (CAM) in the maxillofacial region. The objectives were to study the effect of coexisting medical conditions on CAM, the association of medication consumed during the COVID infectivity period, and the clinical and imaging features of the disease.
2. Methods
This retrospective observational study was conducted at a tertiary care dental facility from July 2020 to December 2021. The study design was approved and ethically cleared by the Bio-medical & Health Research Ethics Committee (PGIDS/BHRC/21/45).
Complete records of the CAM patients who reported to the outpatient and emergency department during this duration were retrieved for the study. A case was defined as CAM, only if it was clinically, radiologically, and histopathologically compatible with the diagnosis. The diagnosis of COVID-19 in all the selected cases was confirmed by either reverse transcription polymerase chain reaction (RT-PCR) or by serological testing previously. The history of pre-existing DM and HbA1c levels at the time of reporting for mucormycosis was analyzed, and the patients were further categorized as PD (previously diabetic), RD (recently diagnosed diabetic), and ND (non-diabetic). Incomplete records and the patients who did not complete the treatment/follow-up were excluded. The records were analyzed in each group for the demographic status, duration of presentation of CAM post COVID-19, co-existing medical conditions, history of treatment at the time of COVID-19, clinical signs and symptoms of the disease, and the radiographic investigations performed at the time of presentation.
2.1. Statistical method
Based on a pilot study done on 139 CAM patients, a prevalence rate of 79% diabetic population was calculated. This was used to calculate a sample size of 266 at a 5% relative error, using the formula sample size = 4PQ/l2. The data were tabulated in a Microsoft excel sheet. All analyses were performed by Statistical package for social sciences (SPSS v 21.0, IBM) Software. Normality of data was checked using Shapiro-Wilk test and showed that the parameters were not normally distributed. Hence, chi square test was used for comparison of the variables and p < 0.05 was considered to be statistically significant. Mean age distribution and duration of presentation was calculated using descriptive analysis (compare means). Frequency (%) for the variables of gender distribution, diabetic status, other co-existing medical conditions, evaluation of risk factors and medications administered during the COVID-19 period, clinical features, and radiological features of CAM were derived using a frequency table.
3. Results
The mean age for the participants was 50.16 ± 13.127 years with 71.05% males and 28.95% females. Table 1 depicts the distribution of CAM cases according to their diabetic status along with the mean age and the time of presentation to the clinics in the three groups. All the patients included in the study had time duration of presentation ranging from 3 days to 40 days after COVID positivity. The mean duration of presentation was the highest in the ND group and the least in the RD group, however, the difference was not significant statistically (p = 0.321).
Table 1.
Diabetic status among the CAM patients with mean age and mean duration of presentation of disease among the three groups.
| PD* | RD* | ND* | |
|---|---|---|---|
| N | 45.86% (n = 122) | 39.47% (n = 105) | 14.66% (n = 39) |
| Mean age (days) | 53.66 ± 13.150 | 47.27 ± 11.534 | 47 ± 14.643 |
| Mean DOP (days)** | 19.28 ± 16.458 | 14.78 ± 5.289 | 15.58 ± 3.241 |
(*PD- Previously diagnosed diabetic, RD- Recently diagnosed diabetic, ND- Non diabetic **DOP- Duration of presentation) (Frequency (%)).
Apart from diabetes, other co-existing medical conditions among the three groups- PD, RD & ND are depicted in Table 2. The most common drugs administered during the COVID-19 treatment in the study groups were steroids (39.10%, n = 104) and oxygen administration (33.08%, n = 88) as depicted in Table 2. However, a significant number of patients (53%, n = 141) did not receive any medical therapy or only symptomatic therapy in form of antipyretics, anti-histaminics, and multivitamins. The results showed no significant difference among the three groups in context with co-existing medical conditions and the treatment rendered during COVID-19.
Table 2.
Frequency of other co-existing medical conditions and evaluation of treatment given during COVID-19 as risk factors among the three groups in CAM patients.
| OTHER CO-EXISTING MEDICAL CONDITION | |||||
|---|---|---|---|---|---|
| PD% (n) | RD% (n) | ND% (n) | TOTAL% (n) | p VALUE | |
| Hypertension | 13.93 (17) | 8.579 | 2.561 | 10.15 (27) | 0.097 |
| Cardiovascular disease | 2.46 (3) | 0.951 | 5.132 | 2.266 | 0.318 |
| Tuberculosis (old) | 0.82 (1) | 0 (0) | 2.561 | 0.752 | 0.284 |
| Hepatitis | 2.46 (3) | 2.863 | 2.561 | 2.637 | 0.982 |
| Multiple co-existing conditions | 3.28 (4) | 0 (0) | 2.561 | 1.885 | 0.182 |
| Others (Paralysis, Asthma) | 3.28 (4) | 2.863 | 2.561 | 3.018 | 0.968 |
| None |
73.77 (90) |
84.76 (89) |
82.05 (32) |
79.32 (211) |
0.057 |
| TREATMENT GIVEN DURING COVID-19 | |||||
|
PD% (n) |
RD% (n) |
ND% (n) |
TOTAL% (n) |
p VALUE |
|
| Oxygen | 30.33 (37) | 36.19 (38) | 33.3313 | 33.08 (88) | 0.645 |
| Steroids | 37.70 (46) | 38.10 (40) | 46.1518 | 39.10 (104) | 0.619 |
| ICU | 9.0211 | 12.3813 | 7.693 | 10.15 (27) | 0.606 |
| Antibiotics | 16.3920 | 18.1019 | 23.08 (9) | 18.04 (48) | 0.640 |
| Anticoagulants | 4.105 | 5.716 | 7.693 | 5.2614 | 0.658 |
| Remdesivir/Tocilizumab | 4.105 | 6.677 | 0 (0) | 4.5112 | 0.221 |
| Plasma Therapy | 0.821 | 0 (0) | 0 (0) | 0.381 | 0.553 |
| Multiple Drugs | 2.463 | 0.951 | 0 (0) | 1.504 | 0.458 |
| Antifungals | 0.821 | 0.951 | 5.132 | 1.504 | 0.131 |
| Antipyretics, Antihistaminics and multivitamins | 15.5719 | 16.1917 | 10.264 | 15.03 (40) | 0.659 |
| No drugs | 45.08 (55) | 30.48 (32) | 35.9014 | 37.97 (101) | |
(Frequency (%), Chi square test).
The percentage and number of cases in each group of CAM with their corresponding clinical and radiological presentations have been summarized in Table 3. The intergroup comparison for clinical presentation revealed significantly higher tooth mobility (p < 0.05) and significantly lesser orbital involvement in the ND group (p < 0.005). The difference in other parameters was however nonsignificant. The intergroup comparison for radiological presentation revealed significantly less involvement of ethmoid, sphenoid and frontal sinus; and less orbital involvement in the ND group. Also, there was less cranial involvement, however, the difference was not statistically significant. Maxillary sinus (MS) involvement and osteolytic changes were consistently present in all the groups.
Table 3.
Clinical and radiological presentations of CAM.
| CLINICAL PRESENTATIONS | |||||
|---|---|---|---|---|---|
| PD% (n) | RD% (n) | ND% (n) | TOTAL% (n) | p VALUE | |
| Sinus/Abscess formation | 34.43 (42) | 29.52 (31) | 38.4615 | 33.08 (88) | 0.546 |
| Palatal Ulceration | 42.62 (52) | 35.24 (37) | 25.6410 | 37.22 (99) | 0.140 |
| Mucosal Erythema | 63.11 (77) | 56.19 (59) | 66.6726 | 60.90 (162) | 0.412 |
| Tooth Pain/Mobility | 52.46 (64) | 54.29 (57) | 79.49 (31) | 57.14 (152) | 0.009* |
| Mucosal Edema | 46.72 (57) | 40 (42) | 51.2820 | 44.74 (119) | 0.402 |
| Bone Necrosis | 41.80 (51) | 35.24 (37) | 33.3313 | 37.97 (101) | 0.484 |
| Facial Swelling | 66.39 (81) | 71.43 (75) | 51.2820 | 66.16 (176) | 0.076 |
| Paresthesia | 90.98 (111) | 90.48 (95) | 89.74 (35) | 90.60 (241) | 0.972 |
| Nasal Discharge | 25.41 (31) | 21.9023 | 20.518 | 23.31 (62) | 0.745 |
| Orbital Involvement | 50.82 (62) | 45.71 (48) | 17.957 | 43.98 (117) | 0.001* |
| Cranial Involvement |
1.642 |
1.902 |
0 (0) |
1.504 |
0.696 |
| RADIOLOGICAL PRESENTATIONS | |||||
|
PD% (n) |
RD% (n) |
ND% (n) |
TOTAL% (n) |
p VALUE |
|
| Maxillary sinus involvement | 99.18 (121) | 100 (105) | 100 (39) | 99.62 (265) | 0.553 |
| Ethmoid sinus involvement | 83.61 (102) | 82.86 (87) | 48.7219 | 78.19 (208) | 0.000* |
| Sphenoid sinus involvement | 66.39 (81) | 60 (63) | 33.3313 | 59.02 (157) | 0.001* |
| Frontal sinus involvement | 48.36 (59) | 45.71 (48) | 17.957 | 42.86 (114) | 0.003* |
| Orbital involvement without optic nerve | 45.90 (56) | 45.71 (48) | 17.957 | 41.73 (111) | 0.005* |
| Optic nerve involvement | 5.747 | 4.765 | 0 (0) | 4.5112 | 0.319 |
| Cranial involvement | 10.6613 | 10.4811 | 2.561 | 9.0224 | 0.285 |
| Osteolysis | 100 (122) | 100 (105) | 100 (39) | 100 (266) | – |
| Osteosclerosis | 11.4814 | 6.677 | 2.561 | 8.2722 | 0.159 |
| Soft tissue and spaces involvement | 41.80 (51) | 44.76 (47) | 41.0316 | 42.86 (114) | 0.876 |
(*p < 0.05).
(Frequency (%), Chi square test).
4. Discussion
Most of the studies and reviews done previously show that the age range of CAM is 10–86 years, with a median age of nearly 55 years.1,6,14,15 Similarly, in our study, we found that the mean age of the study population was 50.16 ± 13.127 years with an age range of 20–85 years. Patel A. et al. also reported that the patients with CAM (mean age = 56.9 years) were slightly older than those with non-CAM (mean age = 46.9 years) which could predispose them to a higher risk of mortality.6,16 Among the three groups, the mean age and mean duration of presentation of the disease was higher in the PD group.
Most of the studies report that CAM is more prevalent in the male population.1,6,15, 16, 17 The similar trend of gender distribution was seen in our study. Also, a higher occurrence of mucormycosis in males has been reported in non-CAM population.6,16 DM is an independent risk factor for mucormycosis and some studies suggest that middle-aged men are at a significantly higher risk for type 2 DM than women.18,19 This could be one possible reason for this observation. It may also be attributed to higher outdoor activity in males as compared to females in the Indian population that are predisposed to inhalation of the fungal spores. This may also be explained in part by hormonal and sexual variations in immune response, which manifest as more effective cell-mediated and humoral pro-inflammatory responses in females than in males.20
The mean DOP in our study, ranged from 14.8 days in the RD group to 19.3 days in the ND group. This was in consistent with other studies done by Hoenigl et al. with a range of 0–90 days and Patel et al. with a range of 7–30 days.16,17 RD group had the least DOP, followed by PD group and then the ND group, however the difference was not statistically significant.
According to data from a global fungal infection registry, the most common underlying disease for mucormycosis prior to COVID-19 was hematological malignancy (63%).21 In the current investigation, however, uncontrolled diabetes was the greatest predisposing factor, accounting for 85.34% (n = 227) cases, similar to global guideline for mucormycosis by Cornely et al.22 One explanation could be, India a country with one of the highest loads of diabetes and a pre-diabetic population, is at risk of secondary infections after the COVID-19 pandemic.15 Secondly, according to the evidence, SARS CoV-1 causes pancreatic islet destruction, culminating in acute hyperglycemia and DKA. This could explain the "diabetogenic state" in SARS CoV-2 infection because pancreatic islets have increased angiotensin-converting enzyme 2 receptor expression and enhanced insulin resistance as a result of the cytokine storm. Thirdly, frequent corticosteroid administration, which exacerbated glucose homeostasis can be the reason that made COVID-19 patients susceptible to diabetes.15 Given that a sizable portion (39.47% (n = 105)) of the study group was recently diagnosed as diabetics, it is possible that they were already pre-diabetic but undiagnosed at the time COVID was infectious, and the disease was sparked by COVID-19's impact on the pancreas or by the COVID-19 therapy. Hence, DM is a ‘classic’ independent risk factor for CAM.
As per the review done by Hoenigl et al. for CAM, the second most common underlying ailment was hypertension, which was found in 18.8% (15/80) of patients, followed by chronic renal disease (5/80, 6.3%) and hematological malignancies (5/80, 6.3%).17 As per the study done by Patel A. et al., in 37.6% (175/465) of the patients, medical co-morbid disorders such as chronic kidney disease (93/465, 20.0%) and cardiovascular diseases (67/465, 14.4%) were detected in CAM patients.16 In this study too, there was a similar pattern of co-morbid conditions including, hypertension, hepatitis, cardiovascular disease, and tuberculosis which showed no difference among the three groups statistically. These conditions contributed to the immune-compromised status which predisposed the patients to develop CAM.
This study evaluated the administration of steroids and oxygen, along with intensive care unit (ICU) stay in CAM patients and observed these in decreasing frequency: steroids (39.10%, n = 104), oxygen administration (33.08%, n = 88) and ICU stay (10.15%, n = 27). The intergroup comparison did not reveal any difference statistically. The prevalence of steroid administration among CAM cases was lower in our study. This was in contrast to previous case control study by Muthu V. et al. which suggest glucocorticoid use in treatment of COVID-19 was significantly associated with CAM.10 However, lower prevalence of steroid intake during COVID management in the present study does not nullify the role of steroids as risk factor. According to Arora U. et al., patients in the CAM group had a 7.7 times higher likelihood of steroid administration when compared to COVID-19 patients who did not develop mucormycosis.23 A substantial portion of the study group (53% cases, n = 141) were administered either no drugs or drugs like antipyretics, anti-histaminics and multivitamins. Also, a significant proportion of CAM are observed in the mild disease category who were never administered steroids, and in non-diabetics, so it may be postulated that rather than the factors like diabetes, steroids or ICU stays, it is the COVID-19 virus itself that is responsible for mucormycosis. This effect is probably mediated through inflammation and microvascular thrombosis that occurred in COVID-19.24 Nonetheless, steroids should be used very judiciously in the right doses and for an appropriate duration in COVID-19 patients.
In this study, records revealed the administration of steroids (39.10%, n = 104), antibiotics (18.04%, n = 48), antipyretics, antihistaminics, and multivitamins (15.03%, n = 40), anticoagulants (5.26%, n = 14), remdesivir/tocilizumab (4.51%, n = 12), antifungals (1.50%, n = 4), multiple drugs (1.50%, n = 4), and plasma therapy (0.38%, n = 1) for the management of COVID-19. It has been reported that the dysbiosis caused by antibiotic treatment, combined with extensive epithelial damage, creates an ideal setting for the development of invasive fungal infections, such as mucormycosis.17 Therefore, the use of broad-spectrum antibiotics should be reconsidered, especially in the absence of infection. Also, the possible association of zinc, a component of multivitamins cannot be excluded.25 The patients were treated for COVID-19 at different hospitals. So, the constituents of multivitamin administered varied among the patients. Hence, it is difficult to infer the role of zinc as a risk factor in the present study. Some of the patients also received antifungal drugs like fluconazole and itraconazole at the time of COVID-19, which do not have much role in the management/prevention of mucormycosis. Primary antifungal prophylaxis is not recommended. Also, voriconazole prophylaxis may predispose an individual to develop mucormycosis.7
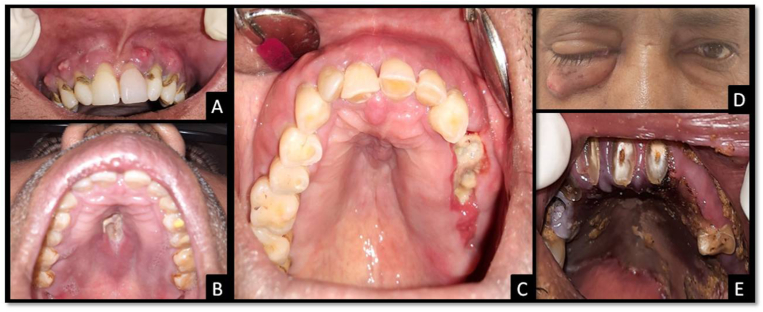
In the present study, the clinical presentations were classified into eleven categories, of which six were intra-oral namely, sinus/abscess formation, palatal ulceration, mucosal erythema, tooth pain/mobility, mucosal edema, and bone necrosis; and the remaining five were extra-oral namely, facial swelling, paresthesia, nasal discharge, orbital involvement, and cranial involvement. Almost 90% of the patients presented with paresthesia, followed by, facial swelling, mucosal erythema, tooth pain/mobility, mucosal edema, orbital involvement, bone necrosis, palatal ulceration, sinus/abscess formation, nasal discharge, cranial involvement. The findings of our study correlate with Motevasseli S. et al., Sharma S. et al.; who also reported that intra-orbital involvement is common while the intra-cranial involvement is rare.8,26, In the study by Sen M. et al. intra-cranial involvement was reported in 16% rhino-orbital mucormycosis cases.12 As the present study was done on patients reporting to a dental setup, cranial involvement was less represented. Some patients with early presentation had features like tooth pain/mobility, mucosal erythema and edema, and abscesses involving the maxillary alveolus, which could be easily mistaken for odontogenic infection. These findings were reported more in the ND group, however, the result was statistically significant only for tooth mobility (p = 0.009). Also, the findings such as orbital involvement, cranial involvement, palatal ulceration, bone necrosis and facial swelling were less reported in ND group (p > 0.05) suggesting slowly progressing disease, contained in the maxillary region. There were other cases with unhealed extraction sockets in the maxillary region resembling dry socket or osteomyelitic changes as depicted in Fig. 1. These findings suggest that patients with early presentation of CAM are expected to report more frequently to a dentist. So, it becomes empirical for the dental surgeon to diagnose and differentiate CAM in such patients as the initial sign and symptoms resemble odontogenic infections. Any delay in diagnosis may lead to the spread of infection with further deterioration and poor prognosis.
Fig. 1.
Clinical presentations of CAM. (A) Multiple abscesses involving the maxillary alveolus. (B) Palatal ulceration. (C) Exposed necrotic bone at the site of recent extraction along with mucosal edema. (D) Right side eye involvement with a draining sinus. (E) Palatal necrosis.
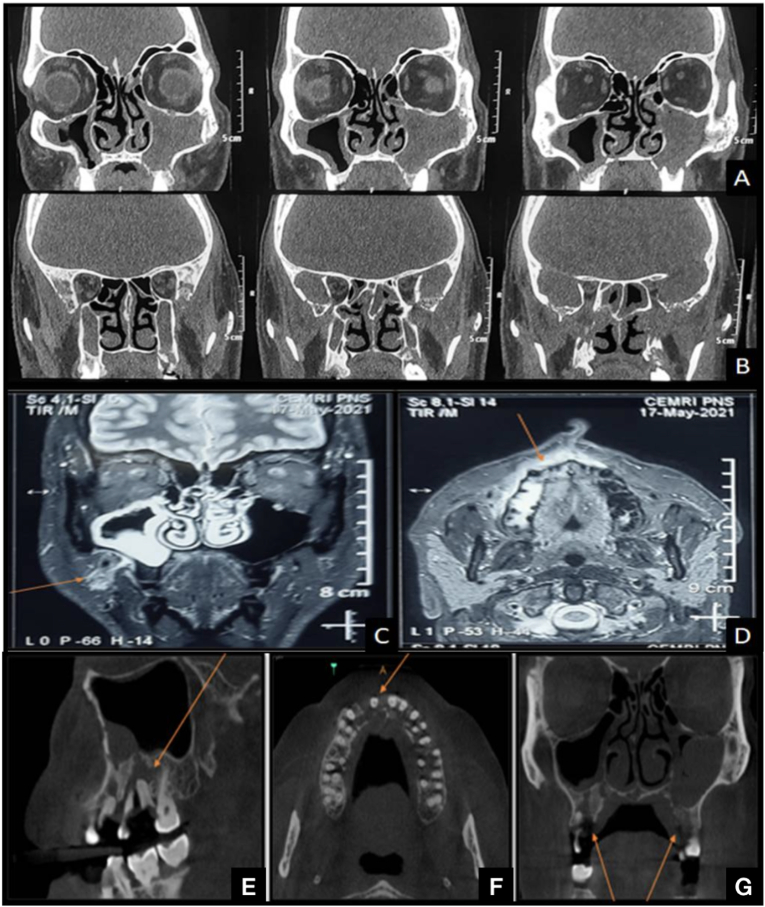
Radiologically, rhino-orbito-cerebral mucormycosis is characterized by mucosal thickening and opacification of the sinuses, edema, inflammation, or infarction of the brain.7 In this study, 9 radiographic parameters were considered, namely, maxillary sinus, ethmoidal sinus, sphenoid sinus, frontal sinus, orbital involvement without/with the optic nerve, cranial involvement, osteolysis, and osteosclerosis. It was noted that the ND group had significantly lesser ethmoid, sphenoid, frontal sinus and orbital involvement (p < 0.05); along with least cranial involvement (p > 0.05). These findings further support the clinical finding that the disease was milder and slowly progressing in the ND group. Almost all the patients in this study presented with maxillary sinus involvement along with osteomyelitic changes. The other common radiological presentations noted are described in Fig. 2 and Table 3. This is in contrast to a study done by Sharma S et al., who noted that ethmoidal sinus involvement was present in all cases followed by maxillary sinus, sphenoid sinus, and frontal sinus.26 Additionally, there was subcutaneous soft tissue and fascial space involvement in 42.86% (n = 114) cases. Thorough evaluation of these may help in understanding the pathway of the spread of the disease.
Fig. 2.
Radiological presentations of CAM. (A–B) Coronal sections of CT scan showing soft tissue densities in bilateral maxillary sinus, sphenoid sinus and left ethmoid sinus with osteolytic changes extending cranially. (C–D) Coronal and axial section of MRI showing early medullary bone changes as marked. (E–F) Sagittal, axial and coronal sections of CBCT of a CAM patient showing osteomyelitic changes involving the maxillary alveolus.
However, patients with early rhinocerebral mucormycosis may have normal MRI and CT scans, and in high-risk patients, surgical exploration with a biopsy of suspected infection sites should always be performed. Due to the limits of imaging methods, histological confirmation of fungal invasion of the tissues is nearly always required to diagnose mucormycosis.27 Mucormycosis management is a collaborative endeavor that varies based on the presentation and level of involvement. Infectious disease specialists, microbiologists, radiologists, maxillofacial surgeons, ENT surgeons, general surgeons, intensivists, ophthalmologists, neurologists, histopathologists, and pharmacologists are among the members of the team.7 For CAM patients, early diagnosis is critical for a better outcome. Clinicians must counsel all patients, especially diabetics, about the early symptoms and indicators of CAM while released from COVID wards.
This study had a large sample size and cases with histological evidence. It highlighted the clinical, and radiological presentation of CAM in the craniofacial region, not much discussed previously. However, it was an observational study and the treatment provided as well as its outcome and follow-up were not monitored. Because it was an outpatient-based study, several critically ill COVID-19 patients who were either bedridden or died may have been missed.
5. Conclusion
Based on the findings of the present study, it can be concluded that COVID-19 is associated with an increased incidence of invasive mycosis, owing to its immune dysregulation, diabetogenic action, and probable microvascular thrombotic action. Optimization of co-morbidities like DM is another important factor, which needs to be assessed promptly. All the physicians involved in the care of COVID-infected patients must pay attention to the high probability of secondary mucormycosis in the post-recovery phase even in those suffering from mild or moderate symptoms. The use of high-dose of corticosteroids and antibiotics should be reduced or eliminated.
The findings of the present study emphasized on the fact that patients with early CAM specially the ND group, are expected to report more frequently in dental setup, rather than to the Otolaryngology and Ophthalmology departments due to oral presentation of the disease. As the first signs and symptoms mirror odontogenic illnesses, it becomes empirical for the dental surgeon to identify and differentiate CAM in such patients. Hence, dentists should be well aware of the possibility of secondary invasive mycosis post-COVID-19 infection, for early diagnosis and prompt treatment, thereby reducing the associated morbidity and mortality.
Grants
None.
Funding
None.
Ethical approval
It was obtained from the Institutional ethical committee-Biomedical & Health Research Committee [Study protocol number- PGIDS/BHRC/21/45].
Patient consent
This was a record-analyzing study. Consent of patients who were traceable through contact numbers was obtained. However, it was not possible to get informed consent from all the patients, so confidentiality of the records was maintained throughout the study.
Author contributions
All authors contributed significantly to the conception, design, data collection, and writing the study.
Declaration of competing interest
None.
Acknowledgements
None.
References
- 1.Singh A.K., Singh R., Joshi S.R., Misra A. Mucormycosis in COVID-19: a systematic review of cases reported worldwide and in India. Diabetes Metabol Syndr: Clin Res Rev. 2021;15(4) doi: 10.1016/j.dsx.2021.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Popli H., Gupta A., Singh V., Agarwal V., Akilan R., Kumar A. Are low serum vitamin D levels a risk factor for advent of COVID-19 associated rhinocerebral mucormycosis: a preliminary case control study. Indian J Otolaryngol Head Neck Surg. 2022;74(2):3529–3533. doi: 10.1007/s12070-022-03080-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agarwal V., Gupta A., Singh V., Jajodia N., Popli H., Akilan R. Association of COVID-19 with rhino-cerebral mucormycosis: an observational study. J. Maxillofac. Oral Surg. 2021;21(3):990–994. doi: 10.1007/s12663-021-01665-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rapidis A.D. Orbitomaxillary mucormycosis (zygomycosis) and the surgical approach to treatment: perspectives from a maxillofacial surgeon. Clin Microbiol Infection. 2009;15(5):98–102. doi: 10.1111/j.1469-0691.2009.02989.x. [DOI] [PubMed] [Google Scholar]
- 5.Muthu V., Agarwal R., Patel A., et al. Definition, diagnosis, and management of COVID-19-associated pulmonary mucormycosis: delphi consensus statement from the fungal infection study forum and academy of pulmonary sciences, India. Lancet Infect Dis. 2022;22(9):e240–e253. doi: 10.1016/S1473-3099(22)00124-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel A., Agarwal R., Rudramurthy S.M., et al. Multicenter epidemiologic study of coronavirus disease–associated mucormycosis, India. Emerg Infect Dis. 2021;27(9):2349–2359. doi: 10.3201/eid2709.210934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rudramurthy S.M., Hoenigl M., Meis J.F., et al. ISHAM ECMM/ISHAM recommendations for clinical management of COVID‐19 associated mucormycosis in low‐and middle‐income countries. Mycoses. 2021;64(9):1028–1037. doi: 10.1111/myc.13335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Motevasseli S., Nazarpour A., Dalili Kajan Z., et al. Post-COVID mucormycosis osteomyelitis and its imaging manifestations in the North of Iran: case series. Oral Radiol. 2022:1–2. doi: 10.1007/s11282-022-00650-x. https://doi: 10.1007/s11282-022-00650-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Werthman-Ehrenreich A. Mucormycosis with orbital compartment syndrome in a patient with COVID-19. Am J Emerg Med. 2021;42:264. doi: 10.1016/j.ajem.2020.09.032. e5-264.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muthu V., Agarwal R., Rudramurthy S.M., et al. Multicenter case–control study of COVID-19–associated mucormycosis outbreak, India. Emerg Infect Dis. 2023;29(1):8–19. doi: 10.3201/eid2901.220926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agarwal V., Gupta A., Singh V., Jajodia N. Mucormycosis: a post covid-19 enigma. IJCMCR. 2021;10(4) doi: 10.46998/IJCMCR.2021.10.000241. [DOI] [Google Scholar]
- 12.Sen M., Honavar S.G., Bansal R., et al. Epidemiology, clinical profile, management, and outcome of COVID-19-associated rhino-orbital-cerebral mucormycosis in 2826 patients in India–Collaborative OPAI-IJO Study on Mucormycosis in COVID-19 (COSMIC), Report 1. Indian J Ophthalmol. 2021;69(7):1670–1692. doi: 10.4103/ijo.IJO_1565_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ponnaiah M., Ganesan S., Bhatnagar T., et al. Hyperglycemia and steroid use increase the risk of rhino-orbito-cerebral mucormycosis regardless of COVID-19 hospitalization: case-control study, India. PLoS One. 2022;17(8) doi: 10.1371/journal.pone.0272042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garg D., Muthu V., Sehgal I.S., et al. Coronavirus disease (Covid-19) associated mucormycosis (CAM): case report and systematic review of literature. Mycopathologia. 2021;186(2):289–298. doi: 10.1007/s11046-021-00528-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.John T.M., Jacob C.N., Kontoyiannis D.P. When uncontrolled diabetes mellitus and severe COVID-19 converge: the perfect storm for mucormycosis. J. Fungi. 2021;7(4):298. doi: 10.3390/jof7040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel A., Kaur H., Xess I., et al. A multicentre observational study on the epidemiology, risk factors, management and outcomes of mucormycosis in India. Clin Microbiol Infection. 2020;26(7) doi: 10.1016/j.cmi.2019.11.021. 944.e9-944.e15. [DOI] [PubMed] [Google Scholar]
- 17.Hoenigl M., Seidel D., Carvalho A., et al. The emergence of COVID-19 associated mucormycosis. analysis of cases from 18 countries. 2022;3(7):e543–e552. doi: 10.1016/S2666-5247(21)00237-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sattar N. Gender aspects in type 2 diabetes mellitus and cardiometabolic risk. Best Pract Res Clin Endocrinol Metabol. 2013;27(4):501–507. doi: 10.1016/j.beem.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Huebschmann A.G., Huxley R.R., Kohrt W.M., Zeitler P., Regensteiner J.G., Reusch J.E. Sex differences in the burden of type 2 diabetes and cardiovascular risk across the life course. Diabetologia. 2019;62(10):1761–1772. doi: 10.1007/s00125-019-4939-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egger M., Hoenigl M., Thompson G.R., III, Carvalho A., Jenks J.D. Let's talk about sex characteristics—as a risk factor for invasive fungal diseases. Mycoses. 2022;65(6):599–612. doi: 10.1111/myc.13449. [DOI] [PubMed] [Google Scholar]
- 21.Rueping M.J., Heinz W.J., Kindo A.J., et al. Forty-one recent cases of invasive zygomycosis from a global clinical registry. J Antimicrob Chemother. 2010;65(2):296–302. doi: 10.1093/jac/dkp430. [DOI] [PubMed] [Google Scholar]
- 22.Cornely O.A., Alastruey-Izquierdo A., Arenz D., et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European confederation of medical mycology in cooperation with the mycoses study group education and Research consortium. Lancet Infect Dis. 2019;19(12):e405–e421. doi: 10.1016/S1473-3099(19)30312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arora U., Priyadarshi M., Katiyar V., et al. Risk factors for Coronavirus disease-associated mucormycosis. J Infect. 2022;84(3):383–390. doi: 10.1016/j.jinf.2021.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Talasaz A.H., Sadeghipour P., Kakavand H., et al. Recent randomized trials of antithrombotic therapy for patients with COVID-19: JACC state-of-the-art review. J Am Coll Cardiol. 2021;77(15):1903–1921. doi: 10.1016/j.jacc.2021.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muthu V., Kumar M., Paul R.A., et al. Is there an association between zinc and COVID‐19–associated mucormycosis? Results of an experimental and clinical study. Mycoses. 2021;64(10):1291–1297. doi: 10.1111/myc.13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma S., Grover M., Bhargava S., Samdani S., Kataria T. Post coronavirus disease mucormycosis: a deadly addition to the pandemic spectrum. J Laryngol Otol. 2021;135(5):442–447. doi: 10.1017/S0022215121000992. [DOI] [PMC free article] [PubMed] [Google Scholar]