Abstract
Axial loading in rodents provides a controlled setting for mechanical loading, because load and subsequent strain, frequency, number of cycles and rest insertion between cycles, are precisely defined. These methodological aspects as well as factors, such as ovariectomy, aging, and disuse may affect the outcome of the loading test, including bone mass, structure, and bone mineral density. This review aims to overview methodological aspects and modifying factors in axial loading on bone outcomes. A systematic literature search was performed in bibliographic databases until December 2021, which resulted in 2183 articles. A total of 144 articles were selected for this review: 23 rat studies, 74 mouse studies, and 47 knock out (KO) mouse studies. Results indicated that peak load, frequency, and number of loading cycles mainly affected the outcomes of bone mass, structure, and density in both rat and mouse studies. It is crucial to consider methodological parameters and modifying factors such as age, sex-steroid deficiency, and disuse in loading protocols for the prediction of loading-related bone outcomes.
Keywords: Axial Loading, Bone, Rats, Mice, Mechanical Stress
Introduction
Bone tissue adapts to mechanical forces endured during growth, locomotion, and physical activities[1]. High impact loading activities such as weightlifting, tennis, squash or badminton are more osteogenic than swimming, cycling or running[2], because bone cells respond better to high strain changes at fast rates with an unusual distribution. This can be explained by the mechanostat-theory proposed by Frost[3-6], who postulated that several mechanical thresholds determine whether old bone is resorbed or new bone is formed[4]. Mechano-adaptation is considered as an important function of bone and is therefore used as an outcome in several studies. Several invasive[7], in vitro[8], and non-invasive in vivo[7] mechanical loading models have been reported previously to investigate mechano-adaptation. Non-invasive loading is preferred because it reduces surgical artifact[7]. These non-invasive models include axial compression of the ulna or tibia, four-point bending of the tibia[9], and the cantilever bending of the tibia[10]. Of the different models, axial compression of rodent long bones best simulates locomotor bone loading patterns, engenders physiologically relevant strains during short bouts of loading, and allows assessment of the loading-related response throughout the whole bone[11,12]. Moreover, it enables the study of mechanical loading in a controlled setting because load, frequency, duration of the loading, and rest period can be defined.
Each laboratory designs its own protocol, with reference to the choice of species, preparation of custom-designed molds for holding the bone in the load cell, the appropriate peak load, frequency, number of cycles, rest insertion between cycles, and the duration of the loading sessions. All these methodological aspects of loading protocols can influence outcomes of bone mass, structure, and density and thereby potentially determine the conclusion on mechano-adaptation. Axial loading has been widely used in studies with knock out mouse models. This makes the impact of different aspects of a loading regime on bone outcome indices highly relevant. There are several overviews of non-invasive loading models and their implications on aging, mechanosensitivity and subsequent bone adaptation. However, these have not taken the different methodological aspects of loading into account with respect to bone outcomes[7,11-13].
The main objective of this systematic review was to study the effects of methodological aspects of non-invasive in vivo axial compression loading in rat, mouse and knock out mouse studies on bone mass, structure, and density. In addition, we aimed to examine the influence of modifying factors like age, sex-steroid deficiency and disuse.
Methods
Search strategy
This systematic review used the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) checklist[14]. The literature search was performed in the bibliographic databases PubMed, Embase, and SPORTdiscus (via EBSCO) from the start of these databases to Dec 31, 2021. The following keyword search was used: “mechanical loading” AND “ulna” or “tibia” AND “rats” or “mice” AND “bone density.” Search terms included controlled terms (e.g. MeSH in PubMed) as well as free text terms for all six concepts. Only non-invasive in vivo axial loading studies in rats and mice and knock-out mouse models that studied bone outcomes (bone mass, structure, and density) were included. The flow chart for the selection steps of the articles is presented in Figure 1.
Figure 1.
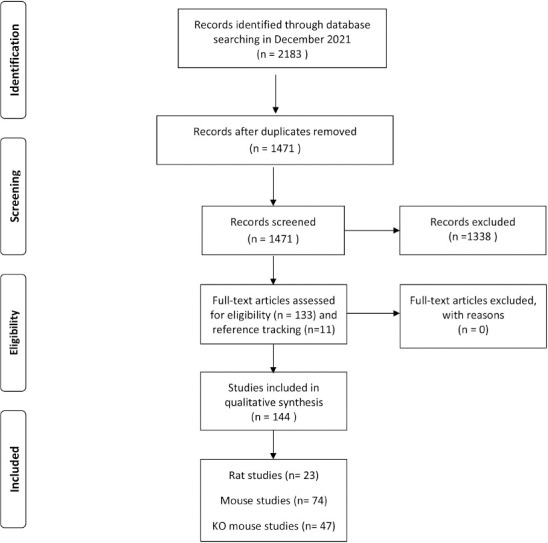
. Prisma flow diagram for systematic review search of in vivo axial loading and bone outcomes in rat, mouse and knock out mouse studies.
Study selection
Two authors (N.B. and H.E.) independently selected relevant titles from the electronic databases. The discrepancies that arose from the two independent authors were resolved by consensus after reading the abstracts and full text of selected titles. The following inclusion criteria were used: (a) intervention: in vivo axial mechanical loading, (b) outcome measurements: bone mass, bone volume, bone mineral density, and (c) rats or mice (d) tibia or ulna.
Experimental animals
The search regarded axial loading studies in the following rat species: Sprague Dawley, Wistar, Fischer, and mouse species: C57BL/6, BALB/c, DBA/2, C3H/He, CD1. Knock-out mouse model studies selected in this review were used to examine the effect of axial loading on genetically modified mice.
Loading protocols
We searched papers on the axial loading of ulna or tibia. Although most investigators used in house developed methods, in general, load was applied using a dynamic loading device: Instron (Instron, Norwood, MA USA), MTS (MTS, Eden Prairie, MN USA), Electroforce (TA Instruments, New Castle, DE USA) or Zwick testing device (ZwickRoell LP, Kennesaw, GA USA), connected to a computer in which the loading cycles are electronically recorded. Custom-made cups are adapted to hold ulna or tibia on the loading device for in vivo loading experiments. An example of rat ulna loaded in custom made cups on the Instron device is presented in Figure 2. A single loading cycle is the duration required for the load to ascend, reach its peak, and slowly descend to its initial level. A single loading bout consists of several loading cycles. In a typical axial loading experiment, rats or mice are anesthetized, ulna or tibia are held in custom loading cups, peak load is applied at a certain frequency, number of cycles in a single bout or multiple loading sessions. The variables used in the in vivo axial loading experiment include the peak load, strain rate, loading waveforms, frequency, number of loading cycles, rest period between cycles, and the duration of loading sessions. Loading protocols are designed by customizing the parameters to deliver the desired loading regimen, which differs according to the study rationale. The animals are anesthetized just before the loading experiment, and remain sedated until completion. Both inhalation-based anesthesia, such as 1-2% isoflurane or 1.5-3% halothane, and injectable anesthesia: a combination of ketamine 60-150 mg/kg body weight and α-2 adrenoreceptor agonists such as xylazine 7.5-20 mg/kg body weight or medetomidine 0.4-1 mg/kg body weight can be used in mouse or rat studies[15]. The latter is commonly used because inhalation-based anesthesia requires specialized equipment and training. For quick recovery from sedation after the loading experiment, α-2-adrenocortical antagonist atipamezole (1 mg/kg) is injected[15]. After applying sedation, first the tibia or ulna of the rodent is placed in the custom-made cup to stabilize it with a 0.5 N-1 N preload. This is recommended for proper stabilization of the bone in the loading apparatus[7,16]. The peak load corresponding to the peak compressive strain can be calculated by ex vivo load-strain calibration in cadaveric bones. The load is applied between the flexed carpus and olecranon joints in the ulna, and between the knee and ankle joints in the tibia. The peak load results in peak compressive strain, and can be applied cyclically to ensure maximum bone formation. For optimum bone formation, the peak compressive strain should not exceed the yield point at which damage occurs to the bone, and should be lower than the fatigue load which causes microdamage to the bone[7,11,13]. Strain rate is the change in strain per unit time[17]. Strain varies at different bone locations in both rat and mouse tibia and ulna[18-20]. Site-specific measurement of strain in proximal, medial, and lateral sites of the tibia using in vivo micro CT and finite element models, and digital image correlations has been performed[18,21]. These methods have shown to be more reproducible than using strain gauges and eliminates the sacrifice of animals for this measurement. The axial load is applied for a specific duration which consists of multiple loading cycles on the same day[22,23], or it is repeated for three to five days a week for two to six weeks[16,24,25]. Multiple loading waveforms are used, such as sinusoidal or haversine, and non-sinusoidal: trapezoid, triangular, or sawtooth waveform[26]. The loading frequency usually corresponds to the normal stride frequency during locomotion, also represented as the number of cycles per unit time[17]. A rest period between loading cycles can be applied in loading protocols. Dynamic load is mostly applied in a sinusoidal manner on the tibia or the ulna, while the contralateral bone is kept as control. Static loading applied at low compressive strain suppresses bone formation, and is therefore not often used[27]. An example of the rat ulna axial loading experiment with 14 N peak load and 10s rest periods between cycles for 40 cycles is presented in Figure 3.
Figure 2.
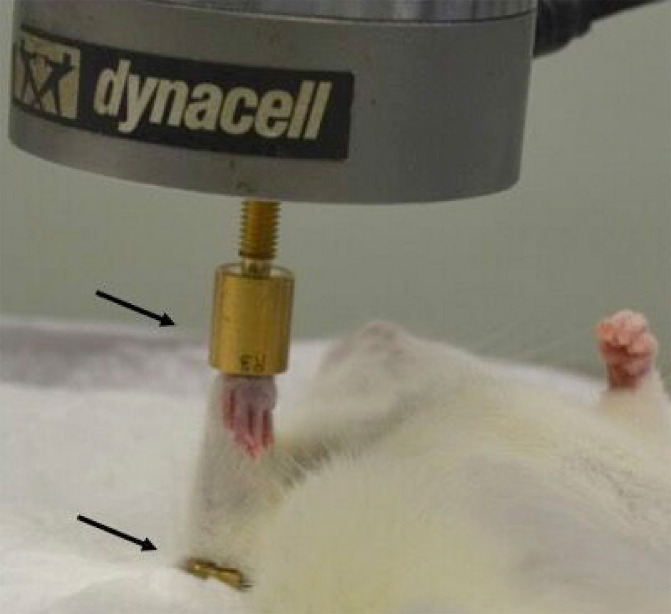
. Rat ulna held in custom-made cups (arrows) during an in vivo axial loading experiment on the Instron device. The two arrows represent upper and lower custom-made cups.
Figure 3.
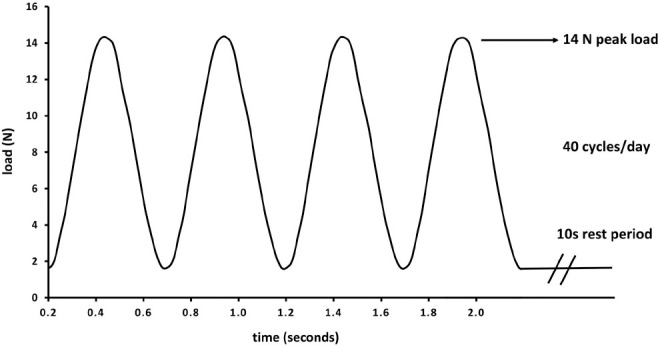
An example of axial loading protocol in rat ulna with 14 N peak load and 10s rest periods between cycles for 40 cycles.
Bone outcomes
This review restricted the outcome parameters to bone histomorphometry and micro CT, DXA, or peripheral quantitative computed tomography (pQCT).
Modifying factors
In this review, papers that possibly describe factors that might affect load induced bone formation are included. Several of these factors were investigated as potential modifying factors which include: age, sex steroids, osteoporosis treatment, and prior disuse. The effect of sex steroids was studied by ovariectomy (OVX) or orchiectomy (ORX), with or without treatment with sex-steroid hormones. Tamoxifen an estrogen receptor modulator that couples agonistic effects on bone with antagonistic effects on other organs was also tested for affecting mechano-adaptation[28]. Osteoporotic bone loss was often treated with a bone sparing medication. Bisphosphonates[29], and intermittent parathyroid hormone (iPTH) were included to test the effect on load induced bone formation[30]. Ultrasound exposure improves fracture healing by affecting cellular mechanisms such as inflammatory responses involved in the fracture healing process. Therefore, ultrasound exposure was also included as a potential modifying factor[31]. Disuse was induced by sciatic neurectomy, causing paralysis and immobilization of the limb[32].
Results
The systematic search resulted in 2183 potentially eligible articles. The search included 905 articles from Pubmed, 1207 from Embase, and 71 from SPORTdiscus (via EBSCO). After duplicates were removed, 1471 articles remained. Among the search results, 1222 articles were excluded by both reviewers, since they did not meet the inclusion criteria resulting in 249 articles. Since consensus on 59 articles was missing, the abstract and the full texts were read for 249 articles, resulting in another exclusion of 116 articles and the inclusion of 133 articles (Figure 1). Reference tracking led to the inclusion of 11 additional articles, resulting in a total of 144 articles for final analysis. The agreement between the two reviewers was good (Cohen’s Kappa=0.76). Of the 144 articles selected for the review, 23 articles concerned rat studies, 74 concerned mouse studies, and 47 concerned KO mouse studies (Figure 1).
Mechanical loading protocols and modifying factors affecting bone outcomes in rats
Twenty-three articles concerning rat studies are summarized in Table 1. In rat ulna axial loading studies, peak load ranged from 4.3 to 22.5 N and strain ranged from 360 µε to 4680 µε. One rat study applied a peak load on the tibia of 45 N, corresponding to a strain of 1500 µε[33]. In all rat studies, load-strain was calibrated using a strain gauge to calculate the strain. Frequencies and number of cycles ranged from 1.5 Hz to 15 Hz and 40 to 1500 cycles per day. A loading session that was repeated three times a week for two to five weeks was reported frequently[20,25,31,34-37], but two studies performed a single duration loading experiment[38,39]. A rest period of 10s between loading cycles was applied in one rat axial study[31]. One study compared rats of 3 groups, i) loaded 1st session of 5 weeks followed by 10 week rest (1x5), ii) loaded 1st and 3rd sessions of 5 weeks each with 5 weeks recovery period (2x5), and iii) loaded all 3 sessions of 5 weeks each (3x5) without recovery period with two control groups: one age-matched control group reeived no loading or anesthesia, and one control group sacrificed at baseline. This study reported an increase in bone formation parameters: mineralizing surface (MS/BS), bone formation rate (BFR/BS) and mineral apposition rate (MAR) for all three groups in the first five weeks as compared to controls[35]. However, only the 2x5 group showed improved bone formation as compared to controls after 15 weeks[35]. Two studies tested axial loading with increasing peak loads of 6.5-18.5 N[20] and 4.3-18.0 N[34] and showed that with increasing peak loads periosteal bone formation occurred in a dose-dependent manner[20,34]. The compressive strain varied with the diaphyseal location, which increased from proximal to distal region in the tibia, and periosteal bone formation also increased distally[34]. However, a clear dose-response was not observed on the endocortical surface[34]. The strain threshold, when peak strain magnitude attained during the loading session exceeds that of habitual activity and subsequently bone formation occurs, is also referred as the minimal effective strain (MES)[4-6]. Dynamic load, like a cyclic load improved bone outcomes, more than a static load, or constant load. This is true especially for the histomorphometric bone formation parameters BFR/BS, MS/BS and MAR, which, compared to controls, were suppressed or unaffected in periosteal and endocortical surfaces after static load but increased after dynamic load in both periosteal and endocortical surfaces[27]. The frequency of axial loading was shown to be an important determinant, for bone mineral density (BMD). A frequency of 10 Hz and 15 Hz showed a mechanical loading-related increase in ulnar BMD after two weeks while 5 Hz did not[37]. However, their study did not compare BMD in frequencies above 10 Hz and 15 Hz, so it could not be concluded whether frequencies above 10-15 Hz affected BMD. A rat axial loading study that used 1, 5 and 10 Hz frequency at peak loads of 4-18 N showed that increased loading frequency increased the slopes of peak strain versus rBFR/BS and rMS/BS curves, indicating that the increase in loading frequency enhanced loading-induced bone formation[34].
Table 1.
Summary of rat axial loading studies.
| Author/Year | Animals/ age/ bone type | Interventions | Peak load (N) | Strain (µε) | loading cycles (per day) | Frequency (Hz)/waveform | Rest periods (seconds) | Duration of loading experiment (days or week) | Outcomes parameters |
|---|---|---|---|---|---|---|---|---|---|
| Chen 2008[37] | Female Sprague Dawley rats, 12 weeks, left ulna | N/A | N/A | 2000 and 3000 | N/A | 5, 10 and 15 | N/A | 2 weeks | DEXA: BMD, histomorphometry: MS/BS, MAR, BFR/BS |
| Feher 2010[42] | Female Sprague Dawley rats | OVX, bisphosphonates | 15 | 3000 | 360 | 2 | N/A | 1 week | Ηistomorphometry: MS/BS, MAR, BFR/BS |
| Hsieh 2001[20] | Female Sprague Dawley rats, 28-32 weeks, right ulna | N/A | 6.5, 10.5, 14.5, 18.5, 22.5 | 1343, 2284 and 3074 | 360 | 2, haversine | N/A | 2 weeks | Ηistomorphometry: MS/BS, MAR, BFR/BS |
| Hsieh 2001[34] | Female Sprague Dawley rats, 28-32 weeks, right ulna | N/A | 4.3-18 | 360-4680 | 360 | 1, 5 and 10 | N/A | 2 weeks | Ηistomorphometry: MS/BS, MAR, BFR/BS |
| Ko 2012[43] | Female Sprague Dawley, 12 weeks, right tibia | OVX & SHAM | N/A | 2000 | 1500 | 2 | N/A | 2 weeks | Micro CT: trabecular bone; BV/TV |
| Li 2002[38] | Female Sprague Dawley rats, 28 weeks, right ulna | Vehicle and oral indomethacin or NS-398 | 17 | 3600 | 360 | 2 | N/A | N/A | Ηistomorphometry: MS/BS, MAR and BFR/BS |
| Li 2003[40] | Female Sprague Dawley, 28 weeks, right ulna | Vehicle treated, verapamil and PTH | 16.5 | 3600 | 360 | 2, haversine | N/A | N/A | histomorphometry: MS/BS, MAR, BFR/BS |
| Li 2021[33] | Female Sprague Dawley rats, 26 weeks, left tibia | Pregnancy, lactation, weaning and virgin | 45 | 1500 | N/A | 2 | N/A | 5 times a week for 2 weeks | Ιn vivo micro CT: trabecular bone; BV/TV, Tb.N, Tb.Th, Tb.Sp, Conn.D, SMI, cortical bone; Ct.Ar, Ct.Th, polar moment of inertia, histomorphometry: MS/BS, MAR, BFR/BS |
| Mosley 1998[17] | Male Sprague Dawley, 6 weeks, left ulna | N/A | 1-20 | 4000 | 1200 | 2, trapezoidal | N/A | 2 weeks | Ηistomorphometry: MS/BS, MAR, BFR/BS |
| Mustafy 2020[97] | Male Sprague Dawley, 4 weeks, tibia | N/A | N/A | 450, 850, and 1250 | 1200 | 2, haversine waveform | 0.10 | 5 days/week for 8 weeks | Ιn vivo micro CT: trabecular bone; BMD, BV/TV, Tb.Th, Tb.N, Tb.Sp, cortical bone; TMD, T.Ar, Ct.Ar, Ct.Th, Ma.Ar, Ps.Pm, Ec.Pm, mean eccentricity, polar moment of inertia |
| Noble 2003[98] | Sprague Dawley rats, left ulna | N/A | N/A | 4000 | 1200 | 2 | N/A | 1-5 and 8-12 days inclusive | Ηistomorphometry: calcein labelled surface |
| Perry 2009[31] | Female Wistar, left ulna | Ultrasound | 7 | 4000 and 4500 | 40 | 10 | 10 | two weeks | Ηistomorphometry: MS/BS, MAR, BFR/BS |
| Robling 2001[27] | Male Sprague Dawley, right ulna | N/A | 17 | 3500 | 1200 | 2 | N/A | 1-5 and 8-12 days inclusive | Ηistomorphometry: MS/BS, MAR, BFR/BS |
| Robling 2002[24] | Female Sprague Dawley | N/A | 17 | 3600 | 360 | 2, haversine | N/A | 16 weeks | DEXA: BA, BMC, aBMD, pQCT: cortical bone; CSA, Cortical vBMD, minimum and maximum moment of inertia |
| Saxon 2005[35] | Female Sprague Dawley, 12 weeks, right ulna | N/A | 15 | 3288 | 360 | 2, haversine | N/A | 15 weeks | Ηistomorphometry: MS/BS, MAR, BFR/BS pQCT: Ct.Ar, T.Ar, Cortical vBMD, second moment of inertia (Imin and Imax) |
| Saxon 2006[36] | Male Sprague Dawley, 8 weeks, right ulna | N/A | 17 | 4094, 4277 | N/A | N/A | N/A | 5 weeks | Ηistomorphometry: MS/BS, MAR, BFR/BS, pQCT: vBMD, second moment of inertia (Imin), BMC, Ct.Ar, periosteal and endocortical circumference |
| Schriefer 2005[99] | Female Sprague Dawley rats, right ulna | N/A | 9.0 , 11.3 and 13.5 | N/A | 360 | 2, haversine | N/A | 5 weeks | pQCT: BMC, T.Ar, second moment of inertia (Imin and Imax), histomorphometry: BFR/BS |
| Tomlinson 2014[39] | Male Fischer, 13-14 weeks, right ulna | αβ3 targetted nano-particle and vehicle treatment | 15 or 18 | N/A | 100 | 0.1 | N/A | N/A | Ηistomorphometry: MS/BS, MAR, BFR/BS, micro CT: BV/TV, BMD |
| Torrance 1994[9] | Male Sprague Dawley, left ulna | N/A | 15 or 20 | 1828 (proximal medial), 1700 (proximal lateral), 3750 (mid shaft medial), 3125 ( medial lateral) | 1200 | 10 or 20 | N/A | on days 5, 6, 8 11, 12, 13, 14 and 15 | Ηistomorphometry: periosteal new bone formation |
| Warden 2005[25] | Female Sprague Dawley, 18-20 weeks, right ulna | N/A | 17 | 3600 | 360 | 2, haversine | N/A | 3 days/week for 5 weeks | DXA: BMC, aBMD, pQCT: BMC, vBMD and micro CT: Ct.Ar, second moment of inertial (Imin and Imax) |
| Warden 2007[41] | Female Sprague Dawley, 5 weeks, right ulna, | N/A | 8.5 | 3500 | 360 | 2, haversine | N/A | 3 days/week for 7 weeks | DXA: aBMD, BMC, pQCT: Ct.Ar, second moment of inertia (Imin and Imax) |
| Warden 2013[44] | Female Sprague Dawley, right ulna | OVX, sham | 8.5 | 3500 | 360 | 2 | N/A | 3 days/week for 6 weeks | pQCT: vBMD, Ct.Ar, BMC, second moment of inertia (Imin and Imax), micro CT: Ct.Ar, Tt.Ar, Me.Ar, Ct.Th, Imin |
| Yang 2018[45] | Sprague Dawley rats, 20 weeks | Hindlimb unloading and controls | 20 | 800 | 600 | 1 | N/A | 5 days/week for 4 weeks | Μicro CT: trabecular bone; BV/TV, Tb.Sp, Tb.Th, Tb.N, bone volume surface ration (BSV/BV) cortical bone; Ct.Th, DEXA: BMD and BMC |
Although the choice of rat species, mostly female Sprague Dawley (SD) rats[20,24,25,34,35,37,40-43], varied with the design of in vivo axial loading, it did not seem to affect the bone outcomes. One study reported that load induced bone formation was not different in six-month old rats as compared to ten-month old rats after two weeks of axial loading[35]. Axial loading related bone formation response was not different in bisphosphonate-treated OVX rats, as compared to OVX alone[42]. Another OVX rat axial loading study reported that load-related skeletal maintenance was not affected by OVX, and no significant interactions were observed between OVX and loading[44]. Axial loading was also studied during disuse, using a hind-limb unloading model, where axial loading did not affect BMD as compared to age-matched controls after 21 days and 28 days[45]. Drug treatment with verapamil or prednisolone inhibited bone formation[40,46], whereas, PTH supplementation[40] and ultrasound exposure[31] increased bone formation. The different characteristics and protocols of studies with rat axial loading are summarized in Table 1.
Mechanical loading protocols and modifying factors affecting loading related bone outcomes in mouse and knock-out mouse models
Seventy-four articles which concerned mouse studies and 47 articles which concerned knock out mouse studies are summarized in Tables 2 and 3. Peak load ranged from 1.5 to 3.5 N in mouse ulna and from 1 to 17 N in mouse tibia studies. In most mice studies, strain gauge was used to calculate strain magnitudes. Strain magnitudes ranged from 500 to 4000 µε in mouse ulna studies, and 500 to 5081 µε in mouse tibia studies. Two studies used micro CT derived simulated strain measurement at the mid-shaft[21,22]; this technique allows the estimation of strain in proximal, medial, and distal sites of the tibia, without the need for strain gauge attachment at the bone sites and eliminates the sacrifice of the animals for this process[21,22]. The computational method of strain determination provides a suitable alternative to strain gauge, as it provides more accurate measurement of peak strain and eliminates the large variability of strain due to placement of strain gauge in small curved mouse bones[21]. Frequency and number of cycles in mouse ulna studies ranged from 2 to 15 Hz and 40 to 9000 cycles per day, while in mouse tibia studies it ranged from 1 to 51 Hz and from 40 to 1200 cycles per day. Mouse axial loading studies generally used loading sessions that were repeated five times a week for one week up to six weeks[16,19,47-49], and only two mouse studies reported a single loading session[50,51]. One mouse axial loading study reported that a loading session of five days per week for two weeks showed a better response in bone formation as compared to a three alternate days per week loading for two weeks[52]. The loading session of five consecutive days showed a larger increase in MS/BS (+38%) as compared to a three days per week loading session, which showed only 15% increase in periosteal MS/BS[52]. Moreover, there was no difference in bone formation between the loading session of five days per week for one week or five days per week for two weeks. The periosteal MS/BS increased 42.2% in one week and 38.1% is two weeks[52]. In the same study, no differences in MS/BS were observed in between three loading cycles of 60, 300 and 1200 when a 1800 µε strain was applied[52].
Table 2.
Summary of mouse axial loading studies.
| Author/Year | Animals/age/ loaded bone | Interventions | Peak loads (N) | Strain (µε) | loading cycles (per day) | Frequency (Hz)/waveform | Rest periods (seconds) | Duration of loading experiment (days or week) | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Bergstrom 2018[46] | Female C57BL/6J mice, 12 weeks old, right tibiae | Prednisolone treatment and vehicle | 13 | 3091 | 40 | Trapezoid waveform | 10 | 3 days/week for 2 weeks | pQCT: trabecular bone: Tb.vBMD, cortical bone; cortical thickness, Ct.BMC, periosteal perimeter, endocortical perimeter, moment of inertia, moment of resistance |
| Berman 2015[48] | Female C57BL/6 mice, 12 weeks, right tibiae | N/A | 8.8, 10.6 or 12.4 | 1700, 2050 and 2400 | 220 | N/A | N/A | 2 weeks | Micro CT: trabecular bone;BV/TV, Tb.Th, Tb.N, Tb.Sp, SMI, cortical bone: Ct.Ar, Ct.Th, Ma.Ar, Ec.Pm.moment of inertia |
| Berman 2019[100] | Male C57BL/6J mice, 3 months old, right tibiae | N/A | 11.9 | N/A | 220 | 4 | 1 | 3 weeks | Micro CT: trabecular bone; BV/TV, BMD, Tb.Th, Tb.N, Tb.Sp, cortical bone; T.Ar, Ct.Ar, Ma.Ar, Ct.Ar/Tt.Ar, TMD Ct.Th, Ps.Pm, Ec.Pm, principal moment of inertia, TMD |
| Bouchard 2021[79] | Female C57BL/6J mice, 10 weeks old, left tibiae | N/A | 11.0 | 1200 | 216 | 4 | N/A | 5 days per week for 2 weeks | Ηistomorphometry: MS/BS, MAR, BFR/BS, micro CT: cortical bone; Ct.Ar, Tt.Ar, Ct.Ar/Tt.Ar, Ct.Th, Ct.vTMD, trabecular bone: BV/TV, Tb.Th, Tb.N, Tb.Sp, Tb.vTMD |
| Cheong 2021[101] | Female C57BL6/J mice, 14 weeks, right tibiae | OVX | 12 | N/A | 40 | N/A | N/A | 3 days/ per week for 3 weeks | Μicro CT: BV, BMC, BV/TV, BMD |
| Cheong 2020[102] | Female C57BL6/J mice, 14 weeks, right tibiae | OVX | 2-12 | 1500 | 40 | Τrapezoid waveform | 10 | 3 days/ per week for 3 weeks | Μicro CT: BMC , BMD |
| Cheong 2021[103] | Female C57BL6/J mice, 13 weeks, right tibiae | OVX, PTH | 2-12 | 1500-2000 | 40 | Τrapezoid waveform | 10 | 3 days/ per week for 3 weeks | Μicro CT: BV, BV/TV, BMC, BMD |
| DeLong 2020[104] | Male C57BL/6J mice, 16 weeks, right tibiae | N/A | 9 | N/A | 1200 | 4 | N/A | 4 days/ week for 21 weeks | Micro CT: trabecular bone: TV, BV/TV, TbN, Tb.Th, Tb.Sp, Conn.D, SMI, and BMD, cortical bone: TV, total BV, CtBV/TV, Ct.Th, Ma.Ar, Ct.Po, TMD |
| Fioravanti 2021[105] | C57BL/6J mice, right tibia | Gambogic amide or VEH | 3 | N/A | 100 | 2 | N/A | N/A | Ηistomorphometry: MS/BS, MAR, BFR/BS |
| Fritton 2005[62] | Male C57BL/J | N/A | 3 | 800 | 1200 | N/A | 0.1 (every 4 cycles) | 2 weeks to 6 weeks | Ηistomorphometry: MS/BS, MAR, BFR/BS, micro CT: BMC, BV, TV, BV/TV, Tb.Th, Tb.Sp |
| Galea 2020[78] | Female and male C57BL/6J mice, 19 weeks and 19 month, right tibia | Aging, sciatic neurectomy | 14.5 | 2270 | 40 | Τrapezoid waveform | 10 | 3 alternate days for 2 weeks | Micro CT: trabecular bone: BV/TV, Tb.Th, Tb.N, Tb.Sp, Tb.Pf, SMI cortical bone: T.Ar, Ct.Ar, B.Ar/T.Ar, Ma.Ar, Cs.Th, Ct.Po, polar moment of inertia |
| Gohin 2020[106] | Male C57BL/6 mice, 10-12 weeks, right tibia | N/A | 12 | N/A | 40 | 2 | 10 | 3 days/ week for 2 weeks | micro CT: trabecular bone: BV/TV, Tb.Th, Tb.Sp, Tb.N, Tb.Pf, SMI, cortical bone: Tt.Ar, Tt.Pm, Ct.Ar, Ct.Th, polar moment of inertia |
| Holguin 2013[49] | Female C57BL/6, BALB/c, 16 weeks, right tibiae | N/A | 10 | 2800 (C57BL/6 ), 2350 (BALB/c), | WashU: 60, Cornell/HSS: 1200 | N/A | WashU:10s Cornell/HSS: 0.1s 0.1s rest insertion, | 3 days/week for 6 weeks | Ιn vivo micro CT: cortical bone; BV, Tt.Ar, Ma.Ar, Ct.Th, andTMD), trabecular bone; BV/TV, Tb.Th, Tb.N, and vBMD |
| Ko 2016[107] | Male C57BL/6, BALB/c, 26 weeks, left tibia | NA | 9 | 800 | 1200 | 4 | N/A | 3 times per week for 2 weeks | Μicro CT: trabecular bone: BV/TV, Tb.Th, and Tb.Sp |
| Kuruvilla 2008[19] | Female C57BL/6[B6], DBA/2[D2] and C3H/He[C3], 16 weeks, left tibiae | N/A | 1.5(DBA, 2(C57BL/6J and C3H/HeJ) | 2000 | 99 | 2 | N/A | 3 days/week for 3 weeks | Ηistomorphometry: MS/BS, MAR, BFR/BS |
| Krause 2020[108] | Male C57BL/ J6 nice, 15 week, right tibiae | Unloading | 9 | 1400 | 1200 | 4, sawtooth waveform | N/A | 4 times per week for 6 weeks | Μicro CT trabecular bone: BV/TV, Tb.N, Tb.Th, Tb.Sp, Conn.D, BMD, Cortical bone: Ct.TV, Ct.BV, Ct.BV/TV, Ct.Th, Ct.Po, Ct.BMD |
| Lee 2002[109] | Female CD1 mice, 17 weeks, left ulnae | NA | 3 and 4 | 2000 and 3000 | NA | 4 | N/A | 5 days/week for 2 weeks | Ηistomorphometry: MS/BS, MAR, BFR/BS |
| Lionikaite 2019[110] | Female C57BL/6N mice, 13 weeks, right tibiae | Vitamin A and vehicle | N/A | N/A | 40 | N/A | 10 | 3 times per week for 2 weeks | Ηistomorphometry: MS/BS, MAR and BFR/BS, micro CT: trabecular bone; BV/TV, Tb.Th, Tb.N, Tb.Sp, cortical bone; Ct.Ar, Ct.Th, Ma.Ar periosteal and endocortical perimeter |
| Lynch 2010[111] | Male & Female C57Bl/6 mice, 9 weeks, left tibia, | NA | 11.5 | 1300 | NA | 4 | NA | 5 times per week for 2 weeks | Ηistomorphometry: MS/BS, MAR and BFR/BS, micro CT: BV/TV, Tb.Th, and tBMD, TbSp |
| Lynch 2011[112] | Female C57Bl/6 mice, 26 weeks, left tibiae | N/A | 11.5 and 5.9 | 2100 and 1200 | 1200 | 4 | N/A | 5 times per week for 2 weeks | Μicro CT: trabecular bone: BV/TV, cnTMD, Tb.Th, Tb.Sp, cortical bone: ct.TMD, Ct.Ar, Ma.Ar, principal moment of inertia |
| Meakin, 2013[16] | Male and female C57BL/6, 16 weeks, right tibiae | NA | 13.3 | 2200 | 40 | N/A | 10 | 3 times per week for 2 weeks | Μicro CT: trabecular bone: BV/TV, Tb.Th, Tb.Sp, Tb.N, cortical bone: Ct.Ar, T.Ar, Ma.Ar, Ct.Ar/T.Ar, Ct.Th, polar moment of inertia |
| Miller 2021[113] | Female C57BL/6 mice, right tibiae | neurectomy | 6 | N/A | 40 | N/A | 10 | 2 weeks | Μicro CT: cortical bone, Ct.Th |
| Moustafa 2012[47] | Female C57BL/6, 19 weeks, right tibiae | NA | 13.5 | 1800 | 40 | N/A | 10 | 3 days/week for 2 weeks | Ηistomorphometry: MS/BS, MAR, BFR/BS, micro CT: trabecular bone: Tb.BV/TV, cortical bone: Ct.BV |
| Norman 2015[21] | Female C57BL/6, 16 weeks, right ulna | NA | 0.5, 1.4, 2.0, 2.6, 3.1 | 500, 1750, 2500, 3250, 4000 | 60 | 2 | N/A | 3 days/week for 2 weeks | Ηistomorphometry: MS/BS, MAR, BFR/BS |
| Park 2019[114] | Female C57BL/6J mice, right tibiae | Vehicle, aspirin, naproxen | 3 | 3000 | 100 | 2, sinusoidal waveform | N/A | 2 weeks | Ηistomorphometry: MS/BS, MAR, BFR/BS, trabecular bone: BV, BMD, cortical bone: Ct.Th, T.Ar, Ct.Ar, Ma.Ar |
| Roberts 2020[115] | Female C57BL/6 mice, 13 weeks | OVX, PTH | 12 | N/A | 40 | Τrapezoid waveform | 10 | 3 times per week for 2 weeks | Μicro CT: trabecular bone: Tb. BV/TV, Tb.Th, Tb.Sp, Tb.N, cortical bone: T.Ar, Ct.Ar, Ct.Ar/T.Ar, Ct.Th, moment of inertia and eccentricity |
| Robling 2002[70] | Female C3/He, C57BL/6 and DBA/2, 20 weeks, right ulna | NA | C3H/He (2.20, 2.75 & 3.30), C57BL/6(1.85, 2.30, 2.75), DBA/2(1.55, 1.90, 2.25 ) | C3H/He:2392, C57BL/6:1769, DBA/2:1860 | 60 | 2 | N/A | 3 days | Histomorphometry: MS/BS, MAR, BFR/BS, fluorochrome histomorphometry: T.Ar, Ct.Ar, maximum second moment of inertia |
| Sugiyama 2010[63] | Female C57BL/6, 19 weeks, right tibiae | NA | 11.5 | 1400 | 40 | N/A | 10 | 2 weeks | Micro CT: Ct.BV, BV/TV, Tb.N, and Tb.Th |
| Warden, 2004[57] | Female C57BL/6, 8-12 weeks, right ulna | NA | 1.5 or 2 | 1750 or 2566 | 120 | 1, 5, 10, 20 and 30 | N/A | 3 days | Histomorphometry: MS/BS, MAR, BFR/BS, and micro CT: Ct.Ar, moment of inertia (Imax, Imin) |
| Weatherholt 2013[116] | Female C57BL/6, 16 weeks, right tibiae | NA | 7 and 9 | 1833 | 360 | 2 | N/A | 3 days/week for 4 consecutive weeks | Histomorphometry: MS/BS, MAR, BFR/BS, in vivo pQCT: cortical bone: BMC, T.Ar, Ct.Ar., Ma.Ar, micro CT: BV/TV, Tb.N, Tb.Th and Tb.Sp |
| Birkhold 2016[53] | Female C57Bl/6J, 26 weeks, left tibiae | NA | 11 | 1200 | 216 | 4 | N/A | 5 days/week for 2 weeks | Histomorphometry: MS/BS, newly mineralized bone volume (MV/BV), mineralization thickness (M.Th), micro CT: cortical bone; Ct.BV, Ct.Th, Ct.Ar |
| Zhao 2014[23] | Male C57BL/6, right tibiae | NA | 7 | N/A | 200 | 1-17 (low), 18-34 (medium), 35-51 (high frequency) | N/A | N/A | Histomorphometry: MS/BS, MAR, BFR/BS, and micro CT |
| Sun 2018[52] | Female C57BL/6, 12 weeks, right tibiae | NA | 4.2, -5.5, -7 and -8 | 1000, 1,400, 1,800 and 2,200 | 60, 300 or 1200 | 4 | N/A | 5 times or 3 times per week for 2 weeks | Histomorphometry: MS/BS, MAR, BFR/BS |
| Castillo 2006[117] | Female C57BL/6, 12 weeks, right ulna | NA | 2 | N/A | 60 | 2 | N/A | 3 times/week for 4 weeks | Histomorphometry: MS/BS, MAR, BFR/BS |
| Yang 2017[69] | C57BL/6, left tibia | NA | 9 | N/A | 36, 216, 1200 | NA | 10 (between 4 cycles) | Micro CT: Ct.Ar, Ct.Th, TMD | |
| Galea 2015[64] | Female C57B/6J, 17-19 weeks, right tibia | OVX, aging | 13.5 | 1250 | 40 | N/A | N/A | 3 times a week for 2 weeks | Micro CT: Ct.Ar, Ct.Th and Tt.Ar |
| Li 2013[22] | Female KM mice, 8 weeks, right tibia | OVX and SHAM | N/A | 1000-3000 | N/A | 15 | N/A | 3 times a weeks for 4 weeks | Micro CT: B.Ar/T.Ar, Tb.Th, Tb.Sp, Tb.N , Tb.Th, Tb.Sp |
| Warden 2014[92] | Female C57BL/6J, 16 weeks, right tibia | OVX & SHAM, aging | 9 | 1833 | 360 | 2 | N/A | 3 times a weeks for 4 weeks | Histomorphometry: MS/BS, MAR, BFR/BS, micro CT: cortical bone; BMC, Ct.Ar and Ct.Th, trabecular bone;BV/TV, Tb.Th, and Tb.N and Tb.Sp |
| Fritton 2008[65] | Male C57BL/6J, 10 months, left tibia | ORX, SHAM | 4.6 | 1200 | N/A | N/A | 0.1 every 4 cycles | N/A | Histomorphometry: MS/BS, MAR, BFR/BS. micro CT BV/T, Tb.Th , Tb.Sp, Tb.N and BMC |
| Aido 2015[71] | Female C57Bl/6J mice, 10, 26 and 78 weeks, left tibia | Aging | 9 or 11 | 1200 | 216 | 4 | N/A | 5 days/week for 4 weeks | Histomorphometry: MS/BS, MAR, BFR/BS |
| Birkhold 2014[93] | Female C57BL/6 mice, 10, 26 and 78 weeks, left tibia | Aging | 9 | 1200 | 216 | 4 | N/A | 5days/week for 4 weeks. | Histomorphometry: MS/BS, MAR, BFR/BS. micro CT: Ct.BV |
| Brodt 2010[118] | Male BALB/c mice, 28 or 84 weeks, right tibiae | Aging | 8, 10 or 12 | 900, 1290 or 1670 | 60 | N/A | N/A | 5 days | Histomorphometry: MS/BS, MAR, BFR/BS, micro CT: trabecular bone, BV/TV, Tb.N, Tb.Th, vBMD, cortical bone: M.Ar, B.Ar, Ct.Wi |
| Checa 2015[74] | Female C57B1/6J, 10 and 26 weeks, left tibia | Aging | 11 | 1200 | 216 | 4 | N/A | 5 days/week for 2 weeks | histomorphometry: MS/BS, MAR, BFR/BS |
| De Souza 2005[66] | Female C57BL/J6, 8, 12, 20 weeks, right tibia | Aging | 2-13 | 500-3000 | 40 | 2 | 10 | 3 times/ week 2 weeks. | Histomorphometry: endosteal inter-label area, periosteal inter-label area, total inter-label area, micro CT: trabecular bone, BV/TV, Tb.N, Tb.Th, Tb.Sp and SMI |
| Holguin 2014[67] | Female C57BL/6J mice, 20, 48 and 88 weeks, right tibia | Aging | N/A | 2000-3000 | 1200 | 4 | 0.1 | 5 days/week for 2 weeks | Histomorphometry: MS/BS, MAR and BFR/BS, in vivo µCT: vBMD |
| Main 2014[119] | Female C57Bl/6 mice, 6, 10 and 16 weeks, left tibia | Aging | 8.8 or 9.1 | 1200 | 1200 | 4 | NA | 5 days/week for 2 weeks | Micro CT: trabecular bone, Tb. BV/TV, Tb.Th, cnTMD, and Tb.Sp, cortical bone, Ct.Ar |
| Meakin 2014[68] | Male and Female C57BL/6, 16 and 19 weeks, right tibiae | Aging | 5-17 | 500-2500 | 40 | N/A | 10 | 3 days/week for 2 weeks | Micro CT: trabecular bone, Tb.Th, cortical bone, Ct.Ar, T.Ar, Ct.Th |
| Silva 2012[120] | Female BALB/cBy, 8, 16, 28, 48 weeks, right tibiae | Aging | 7.5-11 | 1300 or 2350 | 60 | N/A | 10 | 3 days per week, for 6 weeks | Histomorphometry, MS/BS, MAR, BFR/BS, micro CT, Ct.BV, BV/TV |
| Willie 2013[72] | Female C57BL/6J, 10 and 26 weeks, left tibiae | Aging | 10, 26 | 1200 | 216 | N/A | 5 (every 4 cycles) | 5 days per week for 2 weeks. | Histomorphometry: MS/BS, MAR, BFR/BS, micro CT: BV/TV, Tb.Th |
| De Souza 2005[32] | Female C57BL/J6, 10 and 20 weeks, right tibiae | Aging, sciatic neurectomy | 12 | 2000 | 40 | 2 | N/A | 3 days/week for 2 weeks | Histomorphometry: MS/BS, MAR, BFR/BS |
| Meakin 2015[121] | Female C57BL/6 mice, 17 and 76 weeks, right tibiae | aging, sciatic neurectomy, sham | 13 or 13.3 N | 500-2500 | 40 | N/A | 10 | 8 loading sessions on alternate days | Histomorphometry: MS/BS, MAR, BFR/BS, micro CT: Tt.Ar, Ct.Ar, Ma.Ar |
| De Souza 2017[77] | Female C57BL/J6, 8, 14, 20 and 72 weeks, right tibiae | Aging, sciatic neurectomy (5 days and 100 days period) | 2-13 | 2000 | 40 | 2 | 10 | six alternate days for 2 weeks | Histomorphometry: MS/BS, MAR, BFR/BS, micro CT: T.Ar, Ct.Ar, trabecular bone, BV/TV, Tb.N, Tb.Th, Tb.Sp and SMI |
| Shirazi-Fard 2015[122] | Μale C57BL/6J mice, 16 weeks, right tibiae | Total body irradiation with high LET iron ions | 9 | N/A | 60 | N/A | 9.75 | 3 day/week for 4 weeks | Histomorphometry: MS/BS, MAR, BFR/BS, micro CT: trabecular bone, BV/TV, Tb.Th, Conn.D, TMD, cortical bone: Ct.Th, CSA and Ct.BV |
| Sugiyama, 2012[123] | Female C67BL/6, 17 weeks, right tibiae | Right sciatic neurectomy | 14 | 5000 | 40 | N/A | 10 | on alternate days for 2 weeks | Micro CT: BV/TV |
| Rapp 2015[124] | C57BL/6J, 18 weeks, right ulna | Mesenchymal stem cell or vehicle | 1.5 | N/A | N/A | 2 | N/A | 5 times a week for 2 weeks | Histomorphometry: MS/BS, MAR, BFR/BS, micro CT: Ct.BV |
| Leucht 2013[50] | C57BL/6 mice, SRXG mice, 16 weeks, right ulna | AMD3100 (CXCR4 receptor antagonist) & vehicle | 2.8 | 3550 | 120 | 2 | N/A | N/A | Histomorphometry: MS/BS, MAR, BFR/BS |
| Marenzana 2007[125] | Female C57Bl/J6 mice, 9 weeks, right tibia | β-adrenergic antagonist (Propranolol) & | 12.5 | 1500 | 40 | N/A | 10 | 3 alternate days per week for 2 weeks | Micro CT: trabecular bone;Tb.BV/TV and Tb.Th, cortical thickness: Ct.Th, B.Ar, T.Ar and Ma.Ar |
| McAteer 2010[126] | Female C57BL/6J mice, 10 weeks, right ulna | Low dose PTH, high dose PTH, vehicle | 1.95 or 2.25 | 1800-2200 | N/A | 2 | N/A | 3 days | Histomorphometry: MS/BS, MAR, BFR/BS, DXA: aBMD and BMC |
| Moustafa 2009[127] | Female C57BL/6 mice, 19 weeks, right tibiae | iPTH & vehicle | 13.5 | 1400 | N/A | N/A | N/A | 3 alternate days per week for 2 weeks | Histomorphometry: MS/BS, MAR, BFR/BS, micro CT: Ct.BV |
| Sugiyama, 2008[30] | Female C57BL/6, 7 weeks, right tibiae and ulna | human iPTH low, medium or high dose & vehicle | 2.5 (ulna), 12 (tibia) | 1200 (tibia), 1350 (ulna) | 40 | N/A | 10 | 3 days /week for 2 weeks. | Histomorphometry: MS/BS, MAR, BFR/BS, micro CT: trabecular bone, BV/TV, Tb.N and Tb.Th, cortical bone, Ct.BV |
| Sugiyama, 2013[85] | Female C57BL/6, 19 weeks, right tibiae | COX-2 inhibitor (NS-398) & vehicle | 13.5 | 1800 | 40 | N/A | 10 | 3 days /week for 2 weeks | Micro CT: trabecular bone; BV/TV, Tb.Th, Tb.N, cortical bone; Ct.BV |
| Stadelmann 2011[128] | Male C57BL/6, 17 weeks, left tibiae | Bisphosphonate (zoledronate) treatment & saline | 8 | 1800 at postero-tibial crest 1940 at anterodistal tibia | N/A | 2 | N/A | on day 1, 3, 5, 8 and 10 | In vivo micro CT: B.Pm, B.Ar, Ct.Th |
| Sugiyama, 2010[28] | Female C57BL/6, 17 weeks, right tibiae | OVX & SHAM, tamoxifen low, medium, high & vehicle | 10 | 1200 | 40 | N/A | 10 | 3 days/week for 2 weeks | Micro CT: trabecular bone; BV/TV, Tb.Th, cortical bone; Ct. BV |
| Sugiyama, 2011[29] | Female C57BL/6, 17 weeks, right tibia | Bisphosphonate (residronate) treatment & vehicle | 11.5 | 1200 | 40 | N/A | 10 | 3 days/week for 2 weeks | Micro CT: trabecular bone BV/TV, Tb.Th and cortical bone: Ct.BV |
| Borg 2018[129] | Female C57BL/6, 10 and 18 weeks, left tibiae | Vitamin D supplemented diet or vitamin D free diet | 10.5 | N/A | 40 | N/A | 10 | 3 times per week for 2 weeks | Histomorphometry: MS/BS, MAR, BFR/BS, micro CT: trabecular bone: TV, BV, BV/TV, cortical bone; Ct.Th, Ct.Po |
| Govey 2016[130] | Female C57BL/6J, 16 weeks, right tibiae | Total body irradiation (67.5 CgY/min), bone marrow transplantation | 10 | N/A | 1200 | 4 | N/A | 5 days per week for 3 weeks | Micro CT: trabecular bone; BV/TV, Tb.Th, ConnD, Cortical bone; Ma.Ar, Ct.Ar/T.Ar, Ct.Th, Ct.BMD, TMD |
| Meakin 2017[131] | Female C57BL/6, 76 weeks, right tibiae | Aging, PTH treatment | 12.6 | N/A | 40 | N/A | N/A | 3 times per week for 6 weeks | Histomorphometry: MS/BS, MAR, BFR/BS, micro CT: trabecular bone: BV/TV, cortical bone: Ct.Ar and T.Ar, Ct.Th |
| Heffner 2017[132] | Female C57BL/6 | Neonatal capsaicin and vehicle treatment | 3 and 7 | N/A | 1200 | N/A | N/A | 5 days/week for 2 weeks | Histomorphometry: MS/BS, MAR, BFR/BS, micro CT: cortical bone, T.Ar, BMC |
| Wang 2018[133] | Female ICR mice, 8 weeks, | OVX- icarine vehicle, | N/A | 2500 | N/A | 15 | N/A | 3 times per week for 4 weeks | Micro CT: trabecular bone, BV/TV, BMD, Tb.Th, Tb.N, Tb.Sp, Tb.Pf |
| Wang 2021[134] | Female C57BL/6J | N/A | 4.5 or 8 | N/A | 300 | 4 | N/A | 5 days per week for 4 weeks | Trabecular bone:Tb.BV/TV, Tb.Th, Tb.Sp, Tb.BMD, Tb.TMD. cortical bone:Ct.BMD, Ct.TMD, polar moment of inertia |
| Yang 2021[135] | Female C57BL/6 mice, 15 weeks, left tibia | N/A | 3.5, 5.2 or 7.5 | 694, 1034 or 1389 | 216 | 4 | 5 | 3 days or 10 days session over 2 weeks | Cortical bone: Ct.Ar, T.Ar, Ma.Ar, Ct.Th, moment of inertia, trabecular bone: BV/TV, Tb.Th, Tb.Sp, histomorphometry: MS/BS, MAR, BFR/BS |
| Lu 2019[136] | C57BL6/J mice, 8 and 16 weeks, | N/A | 10.5 | N/A | 40 | Trapezoid waveform | N/A | 3 times per week for 2 weeks | Trabecular bone: BV.TV, Tb.N, Tb.Sp, Tb.Th, cortical bone: Ct.Th, Ma.Ar |
| Zouti 2019[137] | BALB/c mice, 10 weeks, right tibia | N/A | 10 | 2000 | 216 | 4 | 5 (every 4 cycles) | 5 days per week for 3 weeks | Cortical bone: Ct.Ar, Ct.At/T.Ar, Ct.Th, Ct.vTMD, PoV, Ct. Po, trabecular bone: BV/TV, Tb.Th, Tb.N, Tb.Sp, Tb.vTMD, histomorphometry: MS/BS, MAR, BFR/BS |
| Javaheri 2020[138] | Female C57BL/J6 mice, 12 weeks, | N/A | 12 | N/A | 40 | 2 | 10 | 3 days per week for 2 weeks | Cortical bone: cross-sectional area, cortical thickness, histomorphometry: MAR |
Table 3.
Summary of knock out mouse axial loading studies.
| Author/Year | Mice studies | Interventions | Peak load (N) | Strain (µε) | loading cycles (per day) | Frequency (Hz)/waveform | Rest periods (seconds) | Duration of loading experiment (days or week) | Loading induced outcomes in bone |
|---|---|---|---|---|---|---|---|---|---|
| Alam 2005[139] | Female COX-2+/+, COX-2-/-, 16 weeks, right ulna | NA | 2.1 | 2580 | 120 | 2 | N/A | 2 days | Histomorphometry: MS/BS, MAR, BFR/BS, micro CT |
| Bivi 2013[55] | Female CX43Δot , CX43fl/fl, 17 weeks, right ulnae | NA | 2.3, 2.5 and 2.8 (CX43fl/fl mice), 2.8 , 3.1 and 3.5 (CX43Δot mice) | N/A | 120 | N/A | N/A | 3 days | Histomorphometry: MS/BS, MAR, BFR/BS |
| Bonnet 2009[58] | Postn -/-, Postn+/+, and Post+/-, 14 weeks, left tibiae | NA | 12 | 1500 | 40 | 0.1 | 10 | 3 days/week for 2 weeks | Micro CT: BV/TV, Tb.N, Tb.Th, TV, Ct. BV, Ct.Th, Histomorphometry: MS/BS, MAR, BFR/BS |
| Callewaert 2010[59] | Male AR-ERα KO, ERα KO, AR KO, and WT, and female WT, ERα KO, 20-22 weeks, left ulnae | NA | 2.5 | 1560-1740 | 40 | N/A | 14.9 | 3 alternate days for 2 weeks | Histomorphometry: MS/BS, MAR, BFR/BS |
| Castillo 2012[140] | Male and female FAK-KO and WT, 16 weeks, right ulnae | NA | 3.0 (males), 2.8 (females) | 3500 | 60 | 2 | N/A | 3 days | Histomorphometry: MS/BS, MAR, BFR/BS, micro CT: trabecular bone, BV/TV, Tb.N, Tb.Th, Tb.Sp, ConnD, SMI, cortical bone, Ct.Ar, Ct.Th |
| Grimston 2012[54] | WT and cKO (DM1Cre;Gja1flox/2), 8 weeks, right tibia | NA | 8 | 1200 | 60 | N/A | 10 | 5 days | Histomorphometry: MS/BS, MAR, BFR/BS, micro CT: Ct.BV, M.Ar, T.Ar |
| Javaheri 2014[141] | Male and female HET cKO & WT, 18-24 weeks, right ulna | NA | 18-24 | 2500 | 100 | 2 | N/A | 3 days/week for 3 weeks | Histomorphometry: MS/BS, MAR, BFR/BS, micro CT: trabecular bone: BV/TV, Tb.Th, BMD, cortical bone: Ct.BMD, Ct.Th |
| Kesavan 2011[60] | Female IGF-I KO and WT, 6 weeks, right tibiae | NA | 6-12 (female), 6.5-12 (male) | 745 and 780 | 40 | N/A | 10 | 3 days/week for 2 weeks | Micro CT: trabecular bone; Tb.BV/TV, Tb.BMD , cortical bone; Ct.TV, TMD, and Ct.Th |
| Lee 2004[82] | ERα-/- & ERβ-/- and ERα+/+ and ERβ+/+ mice, 20-24 weeks, left ulnae | NA | 3.4 | 2800 | 40 | N/A | 14.9 | 3 alternate days each week for 2 weeks | Histomorphometry: MS/BS, MAR, BFR/BS, micro CT:Ct.Ar |
| Liedert 2011[142] | Mdk-deficient mice and WT, 51-52 weeks, right ulnae | NA | 2.5 | 1825-1920 | 120 | 2 | N/A | 5 consecutive days for 2 weeks | Histomorphometry: MS/BS, MAR, BFR/BS, micro CT: cortical bone, Ct.Th, T.Ar, Ma.Ar, Ct.Ar, moment of inertial Imin and Imax |
| Litzenberger 2009[143] | Female ColI(α1) cKO and WT, 16 weeks, right ulna | NA | 3 | 3000 | 120 | 2 | N/A | 3 consecutive days | Histomorphometry: MS/BS, MAR, and BFR/BS, in vivo micro CT: trabecular bone, BV/TV, Tb.N, Tb.Sp, Tb.Th, ConnD, cortical bone, T.Ar, minimum and maximum moment of inertia (Imin and Imax) |
| Melville 2015[81] | Male and female pOC-ERαKO mice and Littermate control (LC), 10 weeks, left tibiae | NA | 9.0 | 1200 | 1200 | 4 | N/A | 5 days per week for 2 weeks | Histomorphometry: MS/BS, MAR, and BFR/BS, micro CT: BV/TV, Tb.Th, Ct.Ar |
| Morse 2014[144] | Female Sost -/- and WT, 10 weeks, left tibia | NA | 9 or 12.5 | 1200 | 1200 | N/A | N/A | 5 days/week for 2 weeks | Histomorphometry: MS/BS, MAR, and BFR/BS, micro CT: BV/TV, DXA: BMD |
| Niziolek 2012[145] | Male LRP5 (G171V & A214V) and WT, 18 weeks, right tibia | NA | 9, 9.8 and 14.4 | 2120 | 120 | 2 | N/A | 3 bouts at alternate days | Histomorphometry: MS/BS, MAR, and BFR/BS |
| Parajuli 2015[56] | Male and female heterozygous C57BL/6-Ins2Akita/J (Akita), 28 weeks, right ulna | NA | 2.7 N Akita females, 2.2 N Akita males | 3500 | N/A | 2 | N/A | 5 consecutive days | Histomorphometry: MS/BS, MAR, and BFR/BS, micro CT: Ct.T.Ar, Ct.B.Ar and Ct.Th |
| Pierroz 2012[146] | Adrb1-/-, Adrb2-/- and WT, 16 weeks, left tibiae | NA | 12 | 1500 | 40 | 0.1 | N/A | 3 days/week for 2 weeks | Histomorphometry: MS/BS, MAR, and BFR/BS, micro CT: Tb.BV/TV and Ct.BV, Ct.Th, DXA: BMD |
| Robling 2007[147] | Male and female B6C3H-1T(1T), B6.C3H-8T (8T), B6.C3GH-13B, C57BL/6J, 13 weeks, right ulna | NA | N/A | N/A | 60 | 2 | NA | 3 consecutive days | Histomorphometry: MS/BS, MAR, and BFR/BS |
| Saxon 2007[75] | Male and female, ER-β+/-, ER-β+/+, ER-β-/-, 16 weeks, right ulna | NA | N/A | 1400 | N/A | 2 | NA | Daily for 3 consecutive days | Histomorphometry: MS/BS, MAR, and BFR/BS, DXA: BMD, BMC, pQCT : BMC, B.Ar, vBMD |
| Sinnesael 2012[61] | Male AR(ocy-ARKO), WT, 18 weeks, right tibia | NA | 16.5 | N/A | 40 | N/A | 10 | 3 times a week for 2 weeks | Histomorphometry: MS/BS, MAR, and BFR/BS, micro CT, DEXA: BMD |
| Tomlinson 2015[148] | Female ΔHIF-1α mice and WT, 18-22 weeks, right ulna | NA | 3.5 | N/A | 100 | 2 | N/A | NA | Histomorphometry: MS/BS, MAR, and BFR/BS |
| Tu 2012[86] | Female hemizygous Dmp1-Sost transgenic mice and WT, 4, 8 and 16 weeks, right ulna | NA | 1.90 (low), 2.20, (medium) and 2.50 (high) | 2460, 2850, 3240 | 120 | N/A | N/A | 3 consecutive days | Histomorphometry: MS/BS, MAR, and BFR/BS |
| Wang 2013[149] | P2Y13R-/- and WT, 16 weeks, left tibia | NA | 14.5 | 5048 | 40 | N/A | 10 | 3 times a week for 2 weeks | Histomorphometry: MS/BS, MAR, and BFR/BS, micro CT: trabecular bone, BV/TV, Tb.Th, Tb.N, cortical bone, Ct.BV |
| Zhao 2013[150] | Dmp1-Cre;Lrpf/f (cKO) and WT, 13 weeks, right ulna | NA | 2.65 | N/A | 360 | 2 | N/A | 3 consecutive days. | Histomorphometry: MS/BS, MAR, BFR/BS, pQCT: BMC, vBMD , DXA: BMD |
| Svensson 2016[151] | IGF-I KO mice and Controls, 24 and 76 weeks, right tibiae | NA | 11 and 13.5 | 3500 and 2800 | 40 | N/A | 10 | 3 times per week for 2 weeks | Micro CT: BV/TV |
| Tomlinson 2017[87] | TrKAF592A mice, Thy1-YFP mice, 16-20 weeks, right ulna | NA | 3 | N/A | 100 | 0.1 | N/A | 3 days | Histomorphometry: MS/BS, MAR, and BFR/BS |
| Xiao 2011[152] | Dmp1-Cre;Pkd1flox/mlBei, Pkd1flox/mlBei and Dmp1-Cre; Pkd1flox/+, 16 weeks, right ulna | NA | 3 | N/A | 180 | 2 | N/A | 3 days per week for 3 weeks | Histomorphometry: MS/BS, MAR, and BFR/BS |
| Lories 2007[153] | Frzb-/- mice and WT, 17 weeks, left ulna | NA | 4 | N/A | N/A | 4 | N/A | 10 | pQCT: Ct.BMC, Ct.BMD, Ct.Th |
| Mohan 2014[154] | female IGF-I KO or WT, 10 weeks, right tibiae | OVX & SHAM | 10 | 745 and 780 | 40 | N/A | 10 | 3 alternate days/week for 2 weeks | Micro CT: TV, BV/TV, Ct.Th, BMD, Tb.N, Tb.Th, Tb.Sp |
| Windahl 2013[80] | Female ERα-/-, ERαAF-1°, ERαAF-2° and WT(sham ovx), 17 weeks, right tibiae | OVX & SHAM | 6-14 | 3050 | 40 | N/A | 10 | 3 alternate days per week for 2 weeks | Histomorphometry: MS/BS, MAR, and BFR/BS, micro CT:Tb.BV/TV, BMD, Tb.N, Tb.Th, pQCT: BMD, Ct.Ar |
| Saxon 2011[155] | Male and female Lrp5-/- mice, Lrp5HBM+ mice and WT, 17 weeks, right tibia | Sciatic neurectomy | N/A | 1500, 2400 and 3000 | 40 | N/A | 14.9 | 3 alternate days per week for 2 weeks | Micro CT : BV/TV, Tb.Th, Ct.Ar, T.Ar, Ma.Ar |
| Kang 2016[51] | Male, βCatf/f, Dmp1-CreERt2- or CreERt2+& WT, 18 weeks, right ulna | Tamoxifen or oil | 2.85 | 2740-2980 | 180 | 2 | N/A | Histomorphometry: MS/BS, MAR or BFR/BS, DEXA: BMD | |
| Pflanz 2017[156] | Sost KO and LC mice, 10 and 26 weeks, left tibiae | Aging | N/A | 900 | 216 | N/A | 0.1 (between cycles), 5 (every four cycles) | 5 days/week for 2 weeks | Histomorphometry: MS/BS, MAR or BFR/BS |
| Moore 2018[157] | Prx1CreER-GFP; ROSA26DTA mutant and ROSA26DTA controls, 16 weeks, right ulna | Tamoxifen treatment | 3 | 120 | 120 | 2 | N/A | 3 days | Histomorphometry: MS/BS, MAR or BFR/BS, micro CT: BV/TV, BMD, Ct.Th, the polar moment of inertia (J), and the minimum and maximum second moments of inertia (Imin and Imax) |
| Robling 2016[158] | Sost-/- mice, ECR5-/- mice and controls, 16 weeks, right ulna | Hind limb suspension | N/A | 1800, 2300 or 2800 | 180 | 2 | N/A | 3 days/week for 2 weeks | Histomorphometry: MS/BS, MAR or BFR/BS, micro CT: BV/TV, Tb.Th, DXA: BMC |
| Sawakami 2006[159] | Male and female Lrp5-/- | PTH | N/A | N/A | 60 | 2 | N/A | 3 days | Histomorphometry (calcein): MS/BS, MAR or BFR/BS micro CT: cortical bone, Ct.Ar, polar moment of inertia, trabecular bone: BV, BV/TV, Tb.N, Tb.Th, Tb.Sp, Conn.D, pQCT: BMC, DEXA: vBMD |
| Lee 2014[160] | AC6-KO mice and WT, 16 weeks, right ulna | PTH | 3 | N/A | 10 | 2 | N/A | 3 days | Histomorphometry: MS/BS, MAR or BFR/BS, micro CT: cortical bone, T.Ar, Ct.Ar, Ct.Th, and minimum and maximum second moments of inertia (Imin and Imax), trabecular bone, BV/TV, Conn D, Tb. N,Tb.Th, and Tb. Sp |
| Sample 2014[161] | CGRPα and CGRPβ KO and WT, 19-21 weeks, right ulna | Brachial Plexus Anesthesia (BPA) | 2.49 | 3500 | 800 | 2 | N/A | 3 days | Histomorphometry: MS/BS, MAR or BFR/BS, DEXA: BMD |
| Albiol 2020[162] | Sost KO and LC, age, left tibia | Sost KO | N/A | 900 | 216 | 4, triangular waveform | 0.1 (every load cycle) and 5 (every four cycles) | 5 days per week for 2 weeks | Micro CT: Tb.BV/TV, Tb.Th, Tb.V TMD and bone histomorphometry: Tb. MS/BS, Tb.MAR and Tb. BFR/BS |
| Davis 2019[163] | Osx-Cre;NT3 (NT3-Cre+) mice and Cre-negative Controls 16 weeks old, right tibiae | Osx-Cre;NT3 (NT3-Cre+) | 8.7 or 10 | 2000 | 1200 | 4, triangular waveform | 0.1 | 9 days | Histomorphometry: MS/BS, MAR and BFR/BS |
| Gerbaix 2021[164] | Periostin KO mice and controls, Left tibiae | Anti-sclerostin antibody (Scl-Ab) treatment and VEH | 12 or16 | 1500 | 40 | trapezoid waveform | 10 | Micro CT: trabecular bone: BV/TV, Tb.N, Tb.Th, Tb.Sp. Conn.D and SMI, cortical bone: Ct.Th, Ct.BV, Ct.TV, TND, Ct.Po, Ma.V | |
| Lawson 2021[165] | OsxCreERT2; WlsF/F experimental and WlsF/F controls, 5 months old | N/A | 8 or12 | 2200 | 60 | 4 | N/A | 5 days | Histomorphometry: MS/BS, MAR and BFR/BS |
| Lewis 2020[166] | Dmp1-Cre transgenic mice and Cre-negative mice, 16 weeks, right tibiae | N/A | 8.0 – 9.4 | 2250 | 180 | 2 | N/A | 3 bouts of load on 5 days | Histomorphometry: MS/BS, MAR, BFR/BS, DEXA: BMD |
| Moore 2018[157] | Prx1CreER-GFP; Rosa26DTA(-/+) and Rosa26DTA(-/+) | N/A | 3 | N/A | 120 | 2, sine wave | N/A | 3 days | Micro CT: trabecular bone volume: BV/TV, BMD, cortical bone: Ct.Th, mean polar moment of inertia, minimum and maximum second moment of inertia, histomorphometry: MS/BS, MAR, BFR/BS |
| Moore 2019[167] | Prx1CreER-GFP; Kif3afl/flc and controls | N/A | 3 | N/A | 120 | 2 | N/A | 3 days | Histomorphometry: MS/BS, MAR, BFR/BS |
| Morse 2020[168] | Dkk1 KO mice, Wnt3+/- and wild type | N/A | 7 or 12 | 1200 | N/A | N/A | N/A | 5 days per week for 2 weeks | Micro CT: trabecular bone: TV, BV/TV, TMD, Tb.Th, Tb.N, Tb.Sp, cortical bone: Ct.BV, Ct.Th, TMD, polar moment of inertia, histomorphometry: MS/BS, MAR and BFR/BS |
| Yang 2021[135] | Sost KO and WT, 10, 26 and 52 weeks, left tibiae | N/A | N/A | 900 | 216 | 4, triangular waveform | 5 (every four cycles) | 5 days per week for 2 weeks | Micro CT: cortical bone: Ct.Ar, T.Ar, Ct.Ar/T.Ar, Ct.Th, Ct.TMD, histomorphometry: MS/BS, MAR, BFR/BS. |
| Zannit 2020[169] | 3.6Colla1-tk mice and controls, 5 month and 12 months, right tibiae | N/A | 7 or 11 | 800 and 1400 | 1200 | 4, triangle waveform | 0.1 | 5 days | Histomorphometry: MS/BS, MAR, BFR/BS |
| Zannit 2019[170] | Male and female Osx-Cre-ERT2+/-; Ai9+/+ (iOsx-Ai9) and iOsx-Ai9 controls, 5 and 12 months | N/A | 11 or 14 | N/A | 1200 | 4, triangle waveform | 0.1 | 5 days | Histomorphometry: MS/BS, MAR, BFR/BS |
Mechanical loading increased BV/TV, Tb.Th, tissue mineral density (TMD) in trabecular bone, cortical area, and cortical thickness in cortical bone. These effects became more pronounced with an increase in strain levels of 1700 µε (low), 2050 µε (medium) and 2400 µε (high)[48]. While loading at a strain level of 2050 µε (compressive force 10.6 N) caused a strong formation response, a lower strain of 1700 µε (compressive force 8.8 N) had a smaller effect, and a higher strain of 2400 µε (compressive force 12.4 N) caused rapid bone formation, but led to the formation of woven bone in half of the animals. This suggests that the effective strain window for bone formation may be rather small[48]. Mechanical loading significantly increased the bone formation parameters, increasing newly mineralized volume by 134% and mineralizing surface by 50%. It decreased the bone resorption parameters (resorbed bone volume and eroded surface) at the metaphyseal periosteal region of bone, while diaphyseal remodeling was more pronounced at the endosteal surface. Setpoints and slopes for strain-mediated responses were different for the endosteal and periosteal surfaces in the metaphyseal and diaphyseal regions, suggesting that the bone formation response is site and region specific[53]. In KO mouse models, separate measurements of strain were performed with a strain gauge in both KO mice and controls[54-56]. In Connexin 43 deficient mice, peak loads of 3.1 N and 3.5 N increased MAR, MS/BS, and BFR/BS, but 2.8 N did not[55]. A mouse study that applied 1.6 N at different frequencies of 1, 5, 10, 20, or 30 Hz, reported significant influence of loading frequency in cortical bone geometry. Loading at 5 Hz frequency showed significantly greater changes in cortical area (Ct.Ar) as compared with either 1 or 20 Hz, loading at 30 Hz resulted in significantly greater Ct.Ar than 1 Hz, but no other statistical differences were reported among the frequencies[57]. In their short term loading experiment for 3 consecutive days, a load of 2.0 N increased rMAR, rMS/BS and rBFR/BS with a frequency of 10 Hz as compared to frequencies of 1, 2, and 5 Hz, but there were no differences in rBFR/BS between 10 and 30 Hz, as the cortical bone adaptation plateaued out above frequencies of 10 Hz[57]. Similarly, their long-term loading experiment for 4 weeks with a peak load of 1.6 N showed that cortical bone adaptation increased with loading of 5-10 Hz and plateaued out beyond frequencies of 10 Hz[57].
Rest periods between loading cycles were used in thirty-five mouse studies[16,28,47,49,54,58-68], but none of them were compared with a non-rest period control[16,28,47,49]. In one of these studies, a loading protocol of peak load 10 N, 60 loading cycles, 2 Hz frequency, and 10s rest period, 3 days a week for 6 weeks, was compared with a loading protocol of peak load 10 N, 1200 loading cycles, 6.7 Hz frequency and 0.1s rest period, which could be considered no rest, 3 days a week for 6 weeks[49]. The loading protocol with 0.1s rest actually showed a more rapid and greater response in bone formation than the protocol with 10s rest after the first three weeks of loading. However, after six weeks, similar bone formation was reported in the two loading protocols[49]. Another study compared 10s rest inserted after 4 cycles each for 216 cycles compared with 216 continuous cycles with no rest period using 9.0 N peak load and 4 Hz frequency in both groups and found similar increases in cortical bone parameters (cortical area (Ct.Ar), cortical thickness (Ct.Th), Total cross-sectional area (Tt.Ar), marrow area (MaAr) and maximum moment of inertia (Imax)) and metaphyseal cancellous bone parameters (BV/TV, Tv.Th and tissue mineral density (TMD))[69]. Addition of a rest period between cycles was not beneficial in improving bone outcomes in their study as compared to a continuous loading session with no rest period[69].
Axial loading was mostly carried out in female C57BL/6 mice[19,21,28,47,49] though strain specific responses in bone formation were observed for C3H/He, C57BL/6 and DBA/2 mice[70]. Aged mice were less responsive to load-induced periosteal bone formation, measured as MS/BS, MAR and BFR/BS[71-74], as compared to young mice, and aged mice showed diminished microstructural changes in both trabecular[71-73] and cortical regions[50,54,73,75] of bone, as compared to young mice. OVX mice showed deteriorated B.Ar/T.Ar, Tb.Th and increased Tb.Sp as compared to SHAM but loading increased B.Ar/T.Ar and Tb.Th, but decreased Tb.Sp in loaded OVX mice as compared to non-loaded OVX mice[22]. Tamoxifen treatment in the loaded OVX mice increased BV/TV, Tb.Th, Ct.BV, and periosteally-enclosed volume, as compared to vehicle-receiving sham and OVX groups, indicating a positive effect of tamoxifen in loading-related gain in both trabecular and cortical bone mass[28]. In orchiectomized mice (ORX), mechanical loading did not prevent cancellous bone loss associated with ORX[65]. Disuse induced by sciatic neurectomy resulted cortical bone loss in mice[32]. Axial loading increased cortical bone formation restoring the bone loss in mice immobilized by sciatic neurectomy for 4[76], 5[32,77] or 100 days[77]. Similarly, loading significantly increased cortical bone formation in mice immobilized by neurectomy for 5 days as compared to SHAM controls in both growing and adult mice. One study in 19 months old mice reported that prior and concurrent sciatic neurectomy significantly enlarged loading-induced increases in cortical bone total cross-sectional area (T.Ar) and trabecular bone thickness (Tb.Th), and structural model index (SMI). Mechanical loading after prior and current disuse resulted in site-specific rescue of age-related loss of mechanoresponsiveness. This occurred mainly in the 20% of cortical bone sites where there was greatest age-related decline in responsiveness[78]. One recent study in mice reported that the time of day at which loading was administered influenced the bone formation in a site-specific manner[79]. Mice loaded at 8:00 pm showed more cortical bone formation response: newly mineralized volume fraction (MV/BV), mineralizing bone surface (MS/BS), and mineralizing thickness (MTh) at the endocortical surface than mice loaded at 8:00 am, but these changes were not observed in the periosteal surface. Similarly, loading induced higher increases in cortical bone: Ct.Ar/T.Ar in the mice loaded at 8:00 pm (11% increase as compared to control) as compared to mice loaded at 8:00 am (8% increase). Similarly, Ct.Th was increased and Ma.Ar was decreased in load versus control limbs in both morning and evening groups[79].
From all publications on KO mice, four articles concerned ER-α KO mice[59,80-82], two articles concerned ER-β KO mice[83,84], and two articles concerned AR-KO mice[59,61]. These studies consistently indicated that ER-α KO mice showed reduced osteogenic responses to loading with reduced cortical bone area[80,84] and bone formation rates[59,80,81,84] as compared to wild type mice. Effects of drug treatment[28,29,85], PTH[30], and KO models[81,83,86,87] on mice axial loading-related bone outcomes are also outlined in Table 4.
Table 4.
Frequently used parameters and suggestions for rats and mice axial loading protocols.
| Frequently used parameters/factors affecting axial loading | Frequently used in rat studies | Frequently used in mouse studies | General suggestions for axial loading protocols |
|---|---|---|---|
| Peak load | 17N ulna[24,25,27,36,38] | 13.5 N tibia[47,64,85,127], 2.5 N ulna[30,126] | Peak loads are determined with load- strain calibration using a strain gage. However, strain gauges have some limitations, as glueing of the strain gauge on small mouse ulna is likely to alter the mechanical properties and measured strain. Alternatively, micro CT derived finite element models can be used to calculate strain. |
| Strain | 3600µε ulna[24,25,38,40] | 1200 µε tibia[28,30,65,71,93] 2000 µε ulna[19,126] | Site-wise determination of strain may be performed using in vivo micro CT and finite element analysis. |
| Frequency/ Number of cycles/loading waveforms | 2 Hz[17,24,25,27,38,40,41,43], 360 cycles/day[20,25,34,35,38,41,42,44] | 2 Hz[19,32,116,158], 40 cycles per day in tibia[16,28,32,47,64,85,121,125,139], [120] cycles per day in ulna[86,139,142] | Frequency and number of cycles per day should be adjusted with regard to strain/peak loads. Sine, trapezoid, triangular, or haversine waveforms may be used. |
| Rest period | 10s[31] | 10s[16,28,47,49,63,120,123,125] | Rest periods of 7-14s between loading cycles improved bone formation response. Optimal thresholds of rest periods should be determined for both mouse and rats studies. |
| Duration of loading | Multiple[17,20,34,37] | Multiple[19,24,47-50] | Single duration axial loading protocols were used to study acute response such as gene expression and histomorphometric changes. Multiple duration axial loading protocols applied three times a week for two weeks allow sufficient changes in bone mass and structure that can be studied with high resolution techniques such as micro CT. |
| Age | Young versus aged[35] | Young versus aged[67,71,72,74,118,156] | Aged mice were less mechanoresponsive than young mice, as loading-induced bone formation was lower in aged mice than in young mice. |
| Ovariectomy (OVX) | OVX versus sham[42,44] | OVX versus sham[22,28,92] | Axial loading did not prevent OVX induced bone loss in both rats and mice. Loading combined with tamoxifen treatment improved bone mass in mice studies. The effect of axial loading should be studied in conditions of sex-steroid treatment in OVX rats and mice. |
| Sciatic Neurectomy (SN) | NA | SN versus controls[32,77] | Disuse or sciatic neurectomy increased mechanosensitivity as compared to controls, suggesting bone mass improvement with loading in space flights or during long-term disuse. |
| Interventions | Bisphosphonates[42], COX-2 inhibitor NS398[38], verapamil[40], PTH[40], ultrasound[31] | Tamoxifen[28], CXCR4 antagonist AMD3100[50], PTH[126,127,131], Prednisolone[46], Gambogic amide[105] | Bisphosphonate treatment improved mechanoresponse in both OVX rats and mice. Axial loading induced bone formation was suppressed by COX-2 inhibitor indomethacin or NS398 treatment in rats. Verapamil treatment suppressed loading-induced bone formation. PTH treatment improved loading-induced bone formation in both rats and mice. Axial loading-induced bone formation was improved by ultrasound in rats. CCRX4 agonist AMD3100 suppressed axial loading-induced bone formation in mice. Gambogic amide was associated with load-induced increase in periosteal bone formation rate. |
Discussion
This review examines the effect of methodological aspects such as peak load and strain, frequency, number of cycles, duration of the loading sessions and rest periods independently and in conjunction with modifying factors such as age, sex-steroid deficiency and disuse in axial loading protocols, on bone outcomes. Method variations in mentioned studies are large, and the rationale for the chosen methodology in studies is not always clear. Peak load and consequently strain, frequency, number of loading cycles per day and rest period mostly varied among the selected rat and mouse axial loading studies, which was mainly due to variations in instrumental setup, loading cups, and adjustment of these methodological aspects. The effective window of strain magnitude that causes lamellar bone formation but not woven bone formation was rather small. Site-wise differences in strain were reported in proximal, medial, and lateral ulnar and tibial sites, which resulted in variation in strain at different sites of bone after axial loading. Axial loading-induced bone formation was affected by modifying factors such as age, ovariectomy and disuse. The variation in these methodological aspects and modifying factors affected bone outcomes of mass, structure, and bone mineral density.
Mechanical loading protocols and modifying factors affecting bone outcomes in rats
This review revealed that axial loading-related bone outcomes were mainly affected by load, strain, number of cycles, frequency, duration of loading sessions and possibly rest period between cycles. The load applied is usually based on strain, or strain-rate, required for bone formation. This was determined in each loading protocol using strain gauges for load-strain calibration. However, non-invasive computational methods of strain determination such as finite element analysis and digital image correlation have been reported, which detect strain on different sites of bone. These methods have shown similar or improved reproducibility in strain determination as compared to strain gauges[18,88]. Remarkably, a change in frequency or number of cycles increased bone mineral density when peak loads were kept constant in rat studies[9,37]. Number of cycles and frequency should be carefully adjusted in the loading protocols, as the change in these parameters in loading protocols affects bone formation[9,37]. A frequency of 2 Hz, which was a common frequency reported in the articles from this review was shown to be equivalent to a normal stride frequency observed during locomotion[17], although a frequency of 5-10 Hz was shown to have a larger effect on bone formation, but bone formation plateaued above 10 Hz frequency[57]. Strain must reach a certain magnitude above MES for bone formation[3-6]. The difference between the MES and a strain causing negative effects, such as woven bone formation, may be rather small and should be noticed[48]. Rest periods between loading cycles were also thought to enhance bone formation[31], but evidence using controlled axial loading experiments is lacking.
Other factors have been examined to determine the influence on mechano-adaptation. Axial loading studies commonly used Sprague Dawley rats. Species-related differences in axial loading-related bone outcomes were not reported, suggesting similarity in bone mass and structure among the different rat species. Additionally, aging did not seem to affect mechanoresponse to axial loading in one rat study[35]. Though, mechanoresponse decreased in aged rats as compared to younger rats using a four-point bending model[89]. Age is therefore still considered an important modifying factor that may influence the bone outcomes. The relationship between age and mechanoresponsiveness can likely be explained by the age-related decline in certain cell types—osteoblasts, osteocytes and lining cells—that sense mechanical signals[90], thereby reducing the mechanosensing ability of bone. Age related decline was reported in IGF-1 and binding proteins[91]. IGF-1 levels are associated with bone formation response, as IGF-1 stimulates osteoblastic activity[91]. Sex-steroid deficient rat models (OVX, ORX) were often used since they induce osteoporotic conditions and axial loading was investigated for prevention of bone loss or restoration of bone mass[42,43]. Although ovariectomized rats did not show overall differences in bone mass increase in response to loading as compared to controls[42,92], axial loading may be beneficial for OVX-induced osteoporosis in cases of low bone volume[43].
Mechanical loading protocols and modifying factors affecting loading related bone outcomes in mouse and knock out mouse models
In mouse studies, peak load, loading cycles, frequency, and rest periods were the main methodological determinants of bone outcomes. Load was mostly calculated on the basis of induced strain, which was determined through load-strain calibration using strain gauges or using a micro CT-derived computational method to determine site-specific strain in mouse tibia[21,22]. Additionally, axial loading of a mouse tibia or ulna also caused bending of the bone which is different on lateral and medial or anterior and posterior sides, leading to different strains on periosteal and endocortical surfaces[48]. Strain requirements for KO mice were always different from control mice due to bone phenotype with altered bone strength. Change in frequency or number of cycles applied with the different peak loads influenced outcomes of bone mass, structure, and density in the different loading protocols[52,57]. This indicates that from the methodological aspects, both number of loading cycles and peak loads are important parameters in the loading protocols that affect bone outcomes. However, a minimal number of loading cycles should be used, as a range of loading cycles from 60-1200 showed a similar bone formation response to loading[52], and a large number of load cycles may unnecessarily prolong loading experiments causing anesthesia related side-effects[52]. A rest period of ten seconds between loading cycles was sometimes applied to improve bone outcomes in mice. Evidence for a beneficial effect of including rest periods and the optimal duration of a rest period is lacking, since none of these studies were done with appropriate non-rest controls.
Aged mice showed reduced mechanoresponsiveness, as compared to young mice[71,93]. Although mechanoresponsiveness is diminished in aged mice, mechanical loading increased cortical bone volume in both young mice, adult mice, and older mice[93]. Thus, axial loading in adult mice may be a suitable model to study whether mechanical loading or exercise can prevent age-related bone loss. OVX mice responded to loading similarly as controls, whereas tamoxifen-treated OVX mice showed improved bone formation after loading as compared to non-tamoxifen-treated OVX mice. This agrees with previous studies in rodent models[94] and also in postmenopausal women[95], where bone mineral density improved with a combination of exercise and hormonal replacement. Tamoxifen is also used in conditional gene inactivation, (based on the DNA recombinase Cre and its recognition (loxP) sites), in mice to switch on or off the gene of interest[96]. It is in these animal models important to realize that tamoxifen might act as a confounder when investigating bone mechanoresponse[96]. Taken together, this implies that both age and estrogen status are important modifying factors in mice axial loading. Axial loading restored the cortical bone loss induced by sciatic neurectomy by increasing bone formation both in both short or long-term[32], which implies that loading after a period of disuse is not only useful to increase the effect size but may also be helpful to investigate conditions of disuse osteoporosis[32,76,77]. Sciatic neurectomy reduced bone mass by decreasing osteoblasts activity through adrenergic receptors present in these cells[32]. However, the mechanism of disuse-related rejuvenation in neurectomized aged rats is not clear. Neurectomy-related muscle catabolism which activates growth factors such as latent TGF-β may stimulate bone formation in aged-mice and disuse may activate periosteal and endosteal osteoblast function[78].
Since different studies have different objectives, a variety of loading protocols, outcome parameters and modifying factors will always be practiced. This review puts the methodological aspects of loading parameters and factors that can influence the different bone outcomes into perspective. This may help the interpretation of the many papers that published data on mechano-adaptation. The papers on methodological aspects affecting mechanical loading-related bone outcomes outlined in this study can also be helpful for future investigators when designing their loading protocols. Therefore, in this review, we summarized some general suggestions for rat and mouse axial loading protocols in Table 4. Frequently reported values of loading parameters including: peak load, strain, frequency, number of cycles and rest periods in the selected rat ulna, mice ulna and tibia studies, and effects of commonly used modifying factors, such as age, sex-steroid deficiency, and disuse are summarized in Table 4.
Conclusion
In conclusion, this review shows that peak load, loading cycles, loading frequency are the major methodological determinants of loading-induced outcomes of bone mass, structure, and density in rat and mouse axial loading studies. The effective window between MES and the strain that causes side effects such as woven bone due to rapid bone formation may be rather small. Therefore, the peak strain magnitude should be chosen carefully to avoid negative effects such as woven bone formation. Application of rest periods in loading protocols should be further investigated by introducing appropriate controls without rest insertion between loading cycles. In addition, micro CT- derived computational techniques have shown to accurately calculate optimal strain for bone formation at different sites in rat and mouse axial loading studies. These techniques are preferred since they reduce animal discomfort. In order to compare data on mechanoresponse and predict effects on bone outcomes, it is important to account for the methodological aspects of loading including load, strain, frequency, and number of cycles, especially in the context of modifying factors which may alter bone formation, such as age, sex-steroid deficiency and antecedent periods of disuse.
Funding
This study was funded by the European Commission through MOVE-AGE, an Erasmus Mundus Joint Doctorate program (2011–2015).
Authors’ contributions
AKN, NB and PL did the conception or design of the work. AKN, HWE, NB and RO did the acquisition, analysis, or interpretation of data. AKN drafted the manuscript, HWE, RJ, NS, RO, DV, PL and NB revising it critically for important intellectual content; AND AKN, HWE, RJ, NS, RO, DV, PL and NB approved the final version to be published.
Footnotes
The authors have no conflict of interest.
Edited by: G. Lyritis
References
- 1.Lanyon LE. Using functional loading to influence bone mass and architecture:objectives, mechanisms, and relationship with estrogen of the mechanically adaptive process in bone. Bone. 1996;18(1 Suppl):37s–43s. doi: 10.1016/8756-3282(95)00378-9. [DOI] [PubMed] [Google Scholar]
- 2.Ehrlich PJ, Lanyon LE. Mechanical strain and bone cell function:a review. Osteoporos Int. 2002;13(9):688–700. doi: 10.1007/s001980200095. [DOI] [PubMed] [Google Scholar]
- 3.Frost HM. Defining osteopenias and osteoporoses:another view (with insights from a new paradigm) Bone. 1997;20(5):385–91. doi: 10.1016/s8756-3282(97)00019-7. [DOI] [PubMed] [Google Scholar]
- 4.Frost HM. The Utah paradigm of skeletal physiology:an overview of its insights for bone, cartilage and collagenous tissue organs. J Bone Miner Metab. 2000;18(6):305–16. doi: 10.1007/s007740070001. [DOI] [PubMed] [Google Scholar]
- 5.Frost HM. The Utah paradigm of Skeletal Physiology. Bone and Bones and Associated Problems. ISMNI. 2004;1 [Google Scholar]
- 6.Frost HM. The Utah paradigm of Skeletal Physiology. Bone and Bones and Associated Problems. ISMNI. 2004;2 [Google Scholar]
- 7.Meakin LB, Price JS, Lanyon LE. The Contribution of Experimental in vivo Models to Understanding the Mechanisms of Adaptation to Mechanical Loading in Bone. Front Endocrinol (Lausanne) 2014;5:154. doi: 10.3389/fendo.2014.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wittkowske C, Reilly GC, Lacroix D, Perrault CM. In Vitro Bone Cell Models:Impact of Fluid Shear Stress on Bone Formation. Front Bioeng Biotechnol. 2016;4:87. doi: 10.3389/fbioe.2016.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torrance AG, Mosley JR, Suswillo RF, Lanyon LE. Noninvasive loading of the rat ulna in vivo induces a strain-related modeling response uncomplicated by trauma or periostal pressure. Calcif Tissue Int. 1994;54(3):241–7. doi: 10.1007/BF00301686. [DOI] [PubMed] [Google Scholar]
- 10.Srinivasan S, Weimer DA, Agans SC, Bain SD, Gross TS. Low-magnitude mechanical loading becomes osteogenic when rest is inserted between each load cycle. J Bone Miner Res. 2002;17(9):1613–20. doi: 10.1359/jbmr.2002.17.9.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stadelmann VA, Brun J, Bonnet N. Preclinical mouse models for assessing axial compression of long bones during exercise. Bonekey Rep. 2015;4:768. doi: 10.1038/bonekey.2015.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McBride SH, Silva MJ. Adaptive and Injury Response of Bone to Mechanical Loading. Bonekey Osteovision. 2012;1:192. doi: 10.1038/bonekey.2012.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melville KM, Robling AG, van der Meulen MC. In vivo axial loading of the mouse tibia. Methods Mol Biol. 2015;1226:99–115. doi: 10.1007/978-1-4939-1619-1_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses:the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sophocleous A, Idris AI. Rodent models of osteoporosis. Bonekey Rep. 2014;3:614. doi: 10.1038/bonekey.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meakin LB, Sugiyama T, Galea GL, Browne WJ, Lanyon LE, Price JS. Male mice housed in groups engage in frequent fighting and show a lower response to additional bone loading than females or individually housed males that do not fight. Bone. 2013;54(1):113–7. doi: 10.1016/j.bone.2013.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mosley JR, Lanyon LE. Strain rate as a controlling influence on adaptive modeling in response to dynamic loading of the ulna in growing male rats. Bone. 1998;23(4):313–8. doi: 10.1016/s8756-3282(98)00113-6. [DOI] [PubMed] [Google Scholar]
- 18.Begonia M, Dallas M, Johnson ML, Thiagarajan G. Comparison of strain measurement in the mouse forearm using subject-specific finite element models, strain gaging, and digital image correlation. Biomechanics and modeling in mechanobiology. 2017;16(4):1243–1253. doi: 10.1007/s10237-017-0885-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuruvilla SJ, Fox SD, Cullen DM, Akhter MP. Site specific bone adaptation response to mechanical loading. J Musculoskelet Neuronal Interact. 2008;8(1):71–8. [PubMed] [Google Scholar]
- 20.Hsieh YF, Robling AG, Ambrosius WT, Burr DB, Turner CH. Mechanical loading of diaphyseal bone in vivo:the strain threshold for an osteogenic response varies with location. J Bone Miner Res. 2001;16(12):2291–7. doi: 10.1359/jbmr.2001.16.12.2291. [DOI] [PubMed] [Google Scholar]
- 21.Norman SC, Wagner DW, Beaupre GS, Castillo AB. Comparison of three methods of calculating strain in the mouse ulna in exogenous loading studies. J Biomech. 2015;48(1):53–8. doi: 10.1016/j.jbiomech.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Li H, Li RX, Wan ZM, et al. Counter-effect of constrained dynamic loading on osteoporosis in ovariectomized mice. J Biomech. 2013;46(7):1242–7. doi: 10.1016/j.jbiomech.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 23.Zhao L, Dodge T, Nemani A, Yokota H. Resonance in the mouse tibia as a predictor of frequencies and locations of loading-induced bone formation. Biomech Model Mechanobiol. 2014;13(1):141–51. doi: 10.1007/s10237-013-0491-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robling AG, Hinant FM, Burr DB, Turner CH. Shorter, more frequent mechanical loading sessions enhance bone mass. Medicine and science in sports and exercise. 2002;34(2):196–202. doi: 10.1097/00005768-200202000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Warden SJ, Hurst JA, Sanders MS, Turner CH, Burr DB, Li J. Bone adaptation to a mechanical loading program significantly increases skeletal fatigue resistance. J Bone Miner Res. 2005;20(5):809–16. doi: 10.1359/JBMR.041222. [DOI] [PubMed] [Google Scholar]
- 26.Kumar R, Tiwari AK, Tripathi D, Shrivas NV, Nizam F. Canalicular fluid flow induced by loading waveforms:A comparative analysis. J Theor Biol. 2019;471:59–73. doi: 10.1016/j.jtbi.2019.03.023. [DOI] [PubMed] [Google Scholar]
- 27.Robling AG, Duijvelaar KM, Geevers JV, Ohashi N, Turner CH. Modulation of appositional and longitudinal bone growth in the rat ulna by applied static and dynamic force. Bone. 2001;29(2):105–13. doi: 10.1016/s8756-3282(01)00488-4. [DOI] [PubMed] [Google Scholar]
- 28.Sugiyama T, Galea GL, Lanyon LE, Price JS. Mechanical loading-related bone gain is enhanced by tamoxifen but unaffected by fulvestrant in female mice. Endocrinology. 2010;151(12):5582–90. doi: 10.1210/en.2010-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sugiyama T, Meakin LB, Galea GL, et al. Risedronate does not reduce mechanical loading-related increases in cortical and trabecular bone mass in mice. Bone. 2011;49(1):133–9. doi: 10.1016/j.bone.2011.03.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sugiyama T, Saxon LK, Zaman G, et al. Mechanical loading enhances the anabolic effects of intermittent parathyroid hormone (1-34) on trabecular and cortical bone in mice. Bone. 2008;43(2):238–48. doi: 10.1016/j.bone.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 31.Perry MJ, Parry LK, Burton VJ, et al. Ultrasound mimics the effect of mechanical loading on bone formation in vivo on rat ulnae. Med Eng Phys. 2009;31(1):42–7. doi: 10.1016/j.medengphy.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 32.de Souza RL, Pitsillides AA, Lanyon LE, Skerry TM, Chenu C. Sympathetic nervous system does not mediate the load-induced cortical new bone formation. J Bone Miner Res. 2005;20(12):2159–68. doi: 10.1359/JBMR.050812. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, de Bakker CMJ, Lai X, et al. Maternal bone adaptation to mechanical loading during pregnancy, lactation, and post-weaning recovery. Bone. 2021;151:116031. doi: 10.1016/j.bone.2021.116031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsieh YF, Turner CH. Effects of loading frequency on mechanically induced bone formation. J Bone Miner Res. 2001;16(5):918–24. doi: 10.1359/jbmr.2001.16.5.918. [DOI] [PubMed] [Google Scholar]
- 35.Saxon LK, Robling AG, Alam I, Turner CH. Mechanosensitivity of the rat skeleton decreases after a long period of loading, but is improved with time off. Bone. 2005;36(3):454–64. doi: 10.1016/j.bone.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 36.Saxon LK, Turner CH. Low-dose estrogen treatment suppresses periosteal bone formation in response to mechanical loading. Bone. 2006;39(6):1261–7. doi: 10.1016/j.bone.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 37.Chen Xy, Zhang Xz, Guo Y, Li Rx, Lin Jj, Wei Y. The establishment of a mechanobiology model of bone and functional adaptation in response to mechanical loading. Clinical Biomechanics. 2008;23(SUPLL.1):S88–S95. doi: 10.1016/j.clinbiomech.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 38.Li J, Burr DB, Turner CH. Suppression of prostaglandin synthesis with NS-398 has different effects on endocortical and periosteal bone formation induced by mechanical loading. Calcif Tissue Int. 2002;70(4):320–9. doi: 10.1007/s00223-001-1025-y. [DOI] [PubMed] [Google Scholar]
- 39.Tomlinson RE, Schmieder AH, Quirk JD, Lanza GM, Silva MJ. Antagonizing the αvβ3 integrin inhibits angiogenesis and impairs woven but not lamellar bone formation induced by mechanical loading. Journal of Bone and Mineral Research. 29(9):1970–1980. doi: 10.1002/jbmr.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li J, Duncan RL, Burr DB, Gattone VH, Turner CH. Parathyroid hormone enhances mechanically induced bone formation, possibly involving L-type voltage-sensitive calcium channels. Endocrinology. 2003;144(4):1226–33. doi: 10.1210/en.2002-220821. [DOI] [PubMed] [Google Scholar]
- 41.Warden SJ, Fuchs RK, Castillo AB, Nelson IR, Turner CH. Exercise when young provides lifelong benefits to bone structure and strength. J Bone Miner Res. 2007;22(2):251–9. doi: 10.1359/jbmr.061107. [DOI] [PubMed] [Google Scholar]
- 42.Feher A, Koivunemi A, Koivunemi M, et al. Bisphosphonates do not inhibit periosteal bone formation in estrogen deficient animals and allow enhanced bone modeling in response to mechanical loading. Bone. 2010;46(1):203–7. doi: 10.1016/j.bone.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 43.Ko CY, Jung YJ, Park JH, et al. Trabecular bone response to mechanical loading in ovariectomized Sprague-Dawley rats depends on baseline bone quantity. J Biomech. 2012;45(11):2046–9. doi: 10.1016/j.jbiomech.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 44.Warden SJ, Galley MR, Hurd AL, et al. Elevated mechanical loading when young provides lifelong benefits to cortical bone properties in female rats independent of a surgically induced menopause. Endocrinology. 2013;154(9):3178–87. doi: 10.1210/en.2013-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang PF, Huang LW, Nie XT, et al. Moderate tibia axial loading promotes discordant response of bone composition parameters and mechanical properties in a hindlimb unloading rat model. J Musculoskelet Neuronal Interact. 2018;18(2):152–164. [PMC free article] [PubMed] [Google Scholar]
- 46.Bergstrom I, Isaksson H, Koskela A, et al. Prednisolone treatment reduces the osteogenic effects of loading in mice. Bone. 2018;112:10–18. doi: 10.1016/j.bone.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 47.Moustafa A, Sugiyama T, Prasad J, et al. Mechanical loading-related changes in osteocyte sclerostin expression in mice are more closely associated with the subsequent osteogenic response than the peak strains engendered. Osteoporos Int. 2012;23(4):1225–34. doi: 10.1007/s00198-011-1656-4. doi:10.1007/s00198-011-1656-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berman AG, Clauser CA, Wunderlin C, Hammond MA, Wallace JM. Structural and Mechanical Improvements to Bone Are Strain Dependent with Axial Compression of the Tibia in Female C57BL/6 Mice. PLoS One. 2015;10(6):e0130504. doi: 10.1371/journal.pone.0130504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holguin N, Brodt MD, Sanchez ME, Kotiya AA, Silva MJ. Adaptation of tibial structure and strength to axial compression depends on loading history in both C57BL/6 and BALB/c mice. Calcif Tissue Int. 2013;93(3):211–21. doi: 10.1007/s00223-013-9744-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leucht P, Temiyasathit S, Russell A, et al. CXCR4 antagonism attenuates load-induced periosteal bone formation in mice. J Orthop Res. 2013;31(11):1828–38. doi: 10.1002/jor.22440. [DOI] [PubMed] [Google Scholar]
- 51.Kang KS, Hong JM, Robling AG. Postnatal beta-catenin deletion from Dmp1-expressing osteocytes/osteoblasts reduces structural adaptation to loading, but not periosteal load-induced bone formation. Bone. 2016;88:138–45. doi: 10.1016/j.bone.2016.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun D, Brodt MD, Zannit HM, Holguin N, Silva MJ. Evaluation of loading parameters for murine axial tibial loading:Stimulating cortical bone formation while reducing loading duration. J Orthop Res. 2018;36(2):682–691. doi: 10.1002/jor.23727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Birkhold AI, Razi H, Duda GN, Checa S, Willie BM. Tomography-Based Quantification of Regional Differences in Cortical Bone Surface Remodeling and Mechano-Response. Calcified tissue international. 2016;100(3):255–270. doi: 10.1007/s00223-016-0217-4. [DOI] [PubMed] [Google Scholar]
- 54.Grimston SK, Watkins MP, Brodt MD, Silva MJ, Civitelli R. Enhanced periosteal and endocortical responses to axial tibial compression loading in conditional connexin43 deficient mice. PLoS One. 2012;7(9):e44222. doi: 10.1371/journal.pone.0044222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bivi N, Pacheco-Costa R, Brun LR, et al. Absence of Cx43 selectively from osteocytes enhances responsiveness to mechanical force in mice. J Orthop Res. 2013;31(7):1075–81. doi: 10.1002/jor.22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parajuli A, Liu C, Li W, et al. Bone's responses to mechanical loading are impaired in type 1 diabetes. Bone. 2015;81:152–60. doi: 10.1016/j.bone.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Warden SJ, Turner CH. Mechanotransduction in the cortical bone is most efficient at loading frequencies of 5-10 Hz. Bone. 2004;34(2):261–70. doi: 10.1016/j.bone.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 58.Bonnet N, Standley KN, Bianchi EN, et al. The matricellular protein periostin is required for sost inhibition and the anabolic response to mechanical loading and physical activity. J Biol Chem. 2009;284(51):35939–50. doi: 10.1074/jbc.M109.060335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Callewaert F, Bakker A, Schrooten J, et al. Androgen Receptor Disruption Increases the Osteogenic Response to Mechanical Loading in Male Mice. Journal of Bone &Mineral Research. 2010;25(1):124–131. doi: 10.1359/jbmr.091001. [DOI] [PubMed] [Google Scholar]
- 60.Kesavan C, Wergedal JE, William Lau KH, Mohan S. Conditional disruption of IGF-I gene in type 1(alpha) collagen-expressing cells shows an essential role of IGF-I in skeletal anabolic response to loading. American Journal of Physiology - Endocrinology and Metabolism. 2011;301(6):E1191–E1197. doi: 10.1152/ajpendo.00440.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sinnesael M, Claessens F, Laurent M, et al. Androgen receptor (AR) in osteocytes is important for the maintenance of male skeletal integrity:evidence from targeted AR disruption in mouse osteocytes. J Bone Miner Res. 2012;27(12):2535–43. doi: 10.1002/jbmr.1713. [DOI] [PubMed] [Google Scholar]
- 62.Fritton JC, Myers ER, Wright TM, van der Meulen MC. Loading induces site-specific increases in mineral content assessed by microcomputed tomography of the mouse tibia. Bone. 2005;36(6):1030–8. doi: 10.1016/j.bone.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 63.Sugiyama T, Price JS, Lanyon LE. Functional adaptation to mechanical loading in both cortical and cancellous bone is controlled locally and is confined to the loaded bones. Bone. 2010;46(2):314–21. doi: 10.1016/j.bone.2009.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Galea GL, Hannuna S, Meakin LB, Delisser PJ, Lanyon LE, Price JS. Quantification of Alterations in Cortical Bone Geometry Using Site Specificity Software in Mouse models of Aging and the Responses to Ovariectomy and Altered Loading. Front Endocrinol (Lausanne) 2015;6:52. doi: 10.3389/fendo.2015.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fritton JC, Myers ER, Wright TM, van der Meulen MC. Bone mass is preserved and cancellous architecture altered due to cyclic loading of the mouse tibia after orchidectomy. J Bone Miner Res. 2008;23(5):663–71. doi: 10.1359/JBMR.080104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.De Souza RL, Matsuura M, Eckstein F, Rawlinson SC, Lanyon LE, Pitsillides AA. Non-invasive axial loading of mouse tibiae increases cortical bone formation and modifies trabecular organization:a new model to study cortical and cancellous compartments in a single loaded element. Bone. 2005;37(6):810–8. doi: 10.1016/j.bone.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 67.Holguin N, Brodt MD, Sanchez ME, Silva MJ. Aging diminishes lamellar and woven bone formation induced by tibial compression in adult C57BL/6. Bone. 2014;65:83–91. doi: 10.1016/j.bone.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meakin LB, Galea GL, Sugiyama T, Lanyon LE, Price JS. Age-related impairment of bones'adaptive response to loading in mice is associated with sex-related deficiencies in osteoblasts but no change in osteocytes. J Bone Miner Res. 2014;29(8):1859–71. doi: 10.1002/jbmr.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang H, Embry RE, Main RP. Effects of Loading Duration and Short Rest Insertion on Cancellous and Cortical Bone Adaptation in the Mouse Tibia. PLoS One. 2017;12(1):e0169519. doi: 10.1371/journal.pone.0169519. doi:10.1371/journal.pone.0169519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Robling AG, Turner CH. Mechanotransduction in bone:genetic effects on mechanosensitivity in mice. Bone. 2002;31(5):562–9. doi: 10.1016/s8756-3282(02)00871-2. [DOI] [PubMed] [Google Scholar]
- 71.Aido M, Kerschnitzki M, Hoerth R, et al. Effect of in vivo loading on bone composition varies with animal age. Exp Gerontol. 2015;63:48–58. doi: 10.1016/j.exger.2015.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Willie BM, Birkhold AI, Razi H, et al. Diminished response to in vivo mechanical loading in trabecular and not cortical bone in adulthood of female C57Bl/6 mice coincides with a reduction in deformation to load. Bone. 2013;55(2):335–46. doi: 10.1016/j.bone.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 73.Main RP. Load-induced changes in bone stiffness and cancellous and cortical bone mass following tibial compression diminish with age in female mice. The Journal of experimental biology. 2014;217:1775–1783. doi: 10.1242/jeb.085522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Checa S, Hesse B, Roschger P, et al. Skeletal maturation substantially affects elastic tissue properties in the endosteal and periosteal regions of loaded mice tibiae. Acta Biomater. 2015;21:154–164. doi: 10.1016/j.actbio.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 75.Saxon LK, Robling AG, Castillo AB, Mohan S, Turner CH. The skeletal responsiveness to mechanical loading is enhanced in mice with a null mutation in estrogen receptor-(beta) American Journal of Physiology - Endocrinology and Metabolism. 2007;293(2):E484–E491. doi: 10.1152/ajpendo.00189.2007. [DOI] [PubMed] [Google Scholar]
- 76.Meakin LB, Delisser PJ, Galea GL, Lanyon LE, Price JS. Disuse rescues the age-impaired adaptive response to external loading in mice. Osteoporos Int. 2015;26(11):2703–8. doi: 10.1007/s00198-015-3142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.DeSouza R, Javaheri B, Collinson RS, et al. Prolonging disuse in aged mice amplifies cortical but not trabecular bones'response to mechanical loading. J Musculoskelet Neuronal Interact. 2017;17(3):218–225. [PMC free article] [PubMed] [Google Scholar]
- 78.Galea GL, Delisser PJ, Meakin L, Price JS, Windahl SH. Bone gain following loading is site-specifically enhanced by prior and concurrent disuse in aged male mice. Bone. 2020;133:115255. doi: 10.1016/j.bone.2020.115255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bouchard AL, Dsouza C, Julien C, et al. Bone adaptation to mechanical loading in mice is affected by circadian rhythms. Bone. 2022;154:116218. doi: 10.1016/j.bone.2021.116218. [DOI] [PubMed] [Google Scholar]
- 80.Windahl SH, Borjesson AE, Farman HH, et al. Estrogen receptor-alpha in osteocytes is important for trabecular bone formation in male mice. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(6):2294–9. doi: 10.1073/pnas.1220811110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Melville KM, Kelly NH, Surita G, et al. Effects of Deletion of ERalpha in Osteoblast-Lineage Cells on Bone Mass and Adaptation to Mechanical Loading Differ in Female and Male Mice. J Bone Miner Res. 2015;30(8):1468–80. doi: 10.1002/jbmr.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee KCL, Jessop H, Suswillo R, Zaman G, Lanyon LE. The adaptive response of bone to mechanical loading in female transgenic mice is deficient in the absence of oestrogen receptor-(alpha) and -(beta) Journal of Endocrinology. 2004;182(2):193–201. doi: 10.1677/joe.0.1820193. [DOI] [PubMed] [Google Scholar]
- 83.Saxon LK, Robling AG, Castillo AB, Mohan S, Turner CH. The skeletal responsiveness to mechanical loading is enhanced in mice with a null mutation in estrogen receptor-beta. Am J Physiol Endocrinol Metab. 2007;293(2):E484–91. doi: 10.1152/ajpendo.00189.2007. [DOI] [PubMed] [Google Scholar]
- 84.Lee KC, Jessop H, Suswillo R, Zaman G, Lanyon LE. The adaptive response of bone to mechanical loading in female transgenic mice is deficient in the absence of oestrogen receptor-alpha and -beta. J Endocrinol. 2004;182(2):193–201. doi: 10.1677/joe.0.1820193. [DOI] [PubMed] [Google Scholar]
- 85.Sugiyama T, Meakin LB, Galea GL, Lanyon LE, Price JS. The cyclooxygenase-2 selective inhibitor NS-398 does not influence trabecular or cortical bone gain resulting from repeated mechanical loading in female mice. Osteoporos Int. 2013;24(1):383–8. doi: 10.1007/s00198-012-1922-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tu X, Rhee Y, Condon KW, et al. Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone. 2012;50(1):209–17. doi: 10.1016/j.bone.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tomlinson RE, Li Z, Li Z, et al. NGF-TrkA signaling in sensory nerves is required for skeletal adaptation to mechanical loads in mice. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(18):E3632–E3641. doi: 10.1073/pnas.1701054114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kotha SP, Hsieh YF, Strigel RM, Muller R, Silva MJ. Experimental and finite element analysis of the rat ulnar loading model-correlations between strain and bone formation following fatigue loading. J Biomech. 2004;37(4):541–8. doi: 10.1016/j.jbiomech.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 89.Turner CH, Takano Y, Owan I. Aging changes mechanical loading thresholds for bone formation in rats. J Bone Miner Res. 1995;10(10):1544–9. doi: 10.1002/jbmr.5650101016. [DOI] [PubMed] [Google Scholar]
- 90.Kita K, Kawai K, Hirohata K. Changes in bone marrow blood flow with aging. J Orthop Res. 1987;5(4):569–75. doi: 10.1002/jor.1100050412. [DOI] [PubMed] [Google Scholar]
- 91.Benedict MR, Adiyaman S, Ayers DC, et al. Dissociation of bone mineral density from age-related decreases in insulin-like growth factor-I and its binding proteins in the male rat. J Gerontol. 1994;49(5):B224–30. doi: 10.1093/geronj/49.5.b224. [DOI] [PubMed] [Google Scholar]
- 92.Warden SJ, Galley MR, Hurd AL, et al. Cortical and trabecular bone benefits of mechanical loading are maintained long term in mice independent of ovariectomy. J Bone Miner Res. 2014;29(5):1131–40. doi: 10.1002/jbmr.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Birkhold AI, Razi H, Duda GN, Weinkamer R, Checa S, Willie BM. Mineralizing surface is the main target of mechanical stimulation independent of age:3D dynamic in vivo morphometry. Bone. 2014;66:15–25. doi: 10.1016/j.bone.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 94.Tromp AM, Bravenboer N, Tanck E, et al. Additional weight bearing during exercise and estrogen in the rat:the effect on bone mass, turnover, and structure. Calcif Tissue Int. 2006;79(6):404–15. doi: 10.1007/s00223-006-0045-z. [DOI] [PubMed] [Google Scholar]
- 95.Kohrt WM, Snead DB, Slatopolsky E, Birge SJ., Jr Additive effects of weight-bearing exercise and estrogen on bone mineral density in older women. J Bone Miner Res. 1995;10(9):1303–11. doi: 10.1002/jbmr.5650100906. [DOI] [PubMed] [Google Scholar]
- 96.Friedel RH, Wurst W, Wefers B, Kühn R. Generating conditional knockout mice. Methods Mol Biol. 2011;693:205–31. doi: 10.1007/978-1-60761-974-1_12. [DOI] [PubMed] [Google Scholar]
- 97.Mustafy T, Londono I, Moldovan F, Villemure I. Isolated Cyclic Loading During Adolescence Improves Tibial Bone Microstructure and Strength at Adulthood. JBMR Plus. 2020;4(4):e10349. doi: 10.1002/jbm4.10349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Noble BS, Peet N, Stevens HY, et al. Mechanical loading:biphasic osteocyte survival and targeting of osteoclasts for bone destruction in rat cortical bone. Am J Physiol Cell Physiol. 2003;284(4):C934–43. doi: 10.1152/ajpcell.00234.2002. [DOI] [PubMed] [Google Scholar]
- 99.Schriefer JL, Robling AG, Warden SJ, Fournier AJ, Mason JJ, Turner CH. A comparison of mechanical properties derived from multiple skeletal sites in mice. Journal of Biomechanics. 2005;38(3):467–475. doi: 10.1016/j.jbiomech.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 100.Berman AG, Hinton MJ, Wallace JM. Treadmill running and targeted tibial loading differentially improve bone mass in mice. Bone Rep. 2019;10:100195. doi: 10.1016/j.bonr.2019.100195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cheong VS, Kadirkamanathan V, Dall'Ara E. The Role of the Loading Condition in Predictions of Bone Adaptation in a Mouse Tibial Loading Model. Front Bioeng Biotechnol. 2021;9:676867. doi: 10.3389/fbioe.2021.676867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cheong VS, Roberts BC, Kadirkamanathan V, Dall'Ara E. Bone remodelling in the mouse tibia is spatio-temporally modulated by oestrogen deficiency and external mechanical loading:A combined in vivo/in silico study. Acta Biomater. 2020;116:302–317. doi: 10.1016/j.actbio.2020.09.011. [DOI] [PubMed] [Google Scholar]
- 103.Cheong VS, Roberts BC, Kadirkamanathan V, Dall'Ara E. Positive interactions of mechanical loading and PTH treatments on spatio-temporal bone remodelling. Acta Biomater. 2021;136:291–305. doi: 10.1016/j.actbio.2021.09.035. [DOI] [PubMed] [Google Scholar]
- 104.DeLong A, Friedman MA, Tucker SM, et al. Protective Effects of Controlled Mechanical Loading of Bone in C57BL6/J Mice Subject to Disuse. JBMR Plus. 2020;4(3):e10322. doi: 10.1002/jbm4.10322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fioravanti G, Hua PQ, Tomlinson RE. The TrkA agonist gambogic amide augments skeletal adaptation to mechanical loading. Bone. 2021;147:115908. doi: 10.1016/j.bone.2021.115908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gohin S, Javaheri B, Hopkinson M, Pitsillides AA, Arnett TR, Chenu C. Applied mechanical loading to mouse hindlimb acutely increases skeletal perfusion and chronically enhanced vascular porosity. J Appl Physiol (1985) 2020;128(4):838–846. doi: 10.1152/japplphysiol.00416.2019. [DOI] [PubMed] [Google Scholar]
- 107.Ko FC, Dragomir CL, Plumb DA, et al. Progressive cell-mediated changes in articular cartilage and bone in mice are initiated by a single session of controlled cyclic compressive loading. J Orthop Res. 2016;34(11):1941–1949. doi: 10.1002/jor.23204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Krause AR, Speacht TA, Steiner JL, Lang CH, Donahue HJ. Mechanical loading recovers bone but not muscle lost during unloading. NPJ Microgravity. 2020;6(1):36. doi: 10.1038/s41526-020-00126-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee KC, Maxwell A, Lanyon LE. Validation of a technique for studying functional adaptation of the mouse ulna in response to mechanical loading. Bone. 2002;31(3):407–12. doi: 10.1016/s8756-3282(02)00842-6. [DOI] [PubMed] [Google Scholar]
- 110.Lionikaite V, Henning P, Drevinge C, et al. Vitamin A decreases the anabolic bone response to mechanical loading by suppressing bone formation. FASEB J. 2019;33(4):5237–5247. doi: 10.1096/fj.201802040R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lynch ME, Main RP, Xu Q, et al. Cancellous bone adaptation to tibial compression is not sex dependent in growing mice. Journal of Applied Physiology. 2010;109(3):685–691. doi: 10.1152/japplphysiol.00210.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lynch MA, Brodt MD, Stephens AL, Civitelli R, Silva MJ. Low-magnitude whole-body vibration does not enhance the anabolic skeletal effects of intermittent PTH in adult mice. J Orthop Res. 2011;29(4):465–72. doi: 10.1002/jor.21280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Miller CJ, Trichilo S, Pickering E, et al. Cortical Thickness Adaptive Response to Mechanical Loading Depends on Periosteal Position and Varies Linearly With Loading Magnitude. Front Bioeng Biotechnol. 2021;9:671606. doi: 10.3389/fbioe.2021.671606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Park J, Fertala A, Tomlinson RE. Naproxen impairs load-induced bone formation, reduces bone toughness, and diminishes woven bone formation following stress fracture in mice. Bone. 2019;124:22–32. doi: 10.1016/j.bone.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 115.Roberts BC, Arredondo Carrera HM, Zanjani-Pour S, et al. PTH(1-34) treatment and/or mechanical loading have different osteogenic effects on the trabecular and cortical bone in the ovariectomized C57BL/6 mouse. Sci Rep. 2020;10(1):8889. doi: 10.1038/s41598-020-65921-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Weatherholt AM, Fuchs RK, Warden SJ. Cortical and trabecular bone adaptation to incremental load magnitudes using the mouse tibial axial compression loading model. Bone. 2013;52(1):372–9. doi: 10.1016/j.bone.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Castillo AB, Alam I, Tanaka SM, et al. Low-amplitude, broad-frequency vibration effects on cortical bone formation in mice. Bone. 2006;39(5):1087–96. doi: 10.1016/j.bone.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 118.Brodt MD, Silva MJ. Aged mice have enhanced endocortical response and normal periosteal response compared with young-adult mice following 1 week of axial tibial compression. J Bone Miner Res. 2010;25(9):2006–15. doi: 10.1002/jbmr.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Main RP, Lynch ME, van der Meulen MC. Load-induced changes in bone stiffness and cancellous and cortical bone mass following tibial compression diminish with age in female mice. J Exp Biol. 2014;217(Pt 10):1775–83. doi: 10.1242/jeb.085522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Silva MJ, Brodt MD, Lynch MA, Stephens AL, Wood DJ, Civitelli R. Tibial loading increases osteogenic gene expression and cortical bone volume in mature and middle-aged mice. PLoS One. 2012;7(4):e34980. doi: 10.1371/journal.pone.0034980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Meakin LB, Udeh C, Galea GL, Lanyon LE, Price JS. Exercise does not enhance aged bone's impaired response to artificial loading in C57Bl/6 mice. Bone. 2015;81:47–52. doi: 10.1016/j.bone.2015.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shirazi-Fard Y, Alwood JS, Schreurs AS, Castillo AB, Globus RK. Mechanical loading causes site-specific anabolic effects on bone following exposure to ionizing radiation. Bone. 2015;81:260–9. doi: 10.1016/j.bone.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 123.Sugiyama T, Meakin LB, Browne WJ, Galea GL, Price JS, Lanyon LE. Bones'adaptive response to mechanical loading is essentially linear between the low strains associated with disuse and the high strains associated with the lamellar/woven bone transition. J Bone Miner Res. 2012;27(8):1784–93. doi: 10.1002/jbmr.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rapp AE, Bindl R, Heilmann A, et al. Systemic mesenchymal stem cell administration enhances bone formation in fracture repair but not load-induced bone formation. Eur Cell Mater. 2015;29:22–34. doi: 10.22203/ecm.v029a02. [DOI] [PubMed] [Google Scholar]
- 125.Marenzana M, De Souza RL, Chenu C. Blockade of beta-adrenergic signaling does not influence the bone mechano-adaptive response in mice. Bone. 2007;41(2):206–15. doi: 10.1016/j.bone.2007.04.184. [DOI] [PubMed] [Google Scholar]
- 126.McAteer ME, Niziolek PJ, Ellis SN, Alge DL, Rob AG. Mechanical stimulation and intermittent parathyroid hormone treatment induce disproportional osteogenic, geometric, and biomechanical effects in growing mouse bone. Calcified Tissue International. 2010;86(5):389–396. doi: 10.1007/s00223-010-9348-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Moustafa A, Sugiyama T, Saxon LK, et al. The mouse fibula as a suitable bone for the study of functional adaptation to mechanical loading. Bone. 2009;44(5):930–5. doi: 10.1016/j.bone.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stadelmann VA, Bonnet N, Pioletti DP. Combined effects of zoledronate and mechanical stimulation on bone adaptation in an axially loaded mouse tibia. Clin Biomech (Bristol, Avon) 2011;26(1):101–5. doi: 10.1016/j.clinbiomech.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 129.Borg SA, Buckley H, Owen R, et al. Early life vitamin D depletion alters the postnatal response to skeletal loading in growing and mature bone. PLoS One. 2018;13(1):e0190675. doi: 10.1371/journal.pone.0190675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Govey PM, Zhang Y, Donahue HJ. Mechanical Loading Attenuates Radiation-Induced Bone Loss in Bone Marrow Transplanted Mice. PLoS One. 2016;11(12):e0167673. doi: 10.1371/journal.pone.0167673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Meakin LB, Todd H, Delisser PJ, et al. Parathyroid hormone's enhancement of bones'osteogenic response to loading is affected by ageing in a dose- and time-dependent manner. Bone. 2017;98:59–67. doi: 10.1016/j.bone.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Heffner MA, Genetos DC, Christiansen BA. Bone adaptation to mechanical loading in a mouse model of reduced peripheral sensory nerve function. PLoS One. 2017;12(10):e0187354. doi: 10.1371/journal.pone.0187354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang QS, Wang GF, Lu YR, et al. The Combination of icariin and constrained dynamic loading stimulation attenuates bone loss in ovariectomy-induced osteoporotic mice. J Orthop Res. 2018;36(5):1415–1424. doi: 10.1002/jor.23777. [DOI] [PubMed] [Google Scholar]
- 134.Wang S, Pei S, Wasi M, et al. Moderate tibial loading and treadmill running, but not overloading, protect adult murine bone from destruction by metastasized breast cancer. Bone. 2021;153:116100. doi: 10.1016/j.bone.2021.116100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yang H, Bullock WA, Myhal A, DeShield P, Duffy D, Main RP. Cancellous Bone May Have a Greater Adaptive Strain Threshold Than Cortical Bone. JBMR Plus. 2021;5(5):e10489. doi: 10.1002/jbm4.10489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lu Y, Zuo D, Li J, He Y. Stochastic analysis of a heterogeneous micro-finite element model of a mouse tibia. Med Eng Phys. 2019;63:50–56. doi: 10.1016/j.medengphy.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 137.Ziouti F, Ebert R, Rummler M, et al. NOTCH Signaling Is Activated through Mechanical Strain in Human Bone Marrow-Derived Mesenchymal Stromal Cells. Stem Cells Int. 2019;2019:5150634. doi: 10.1155/2019/5150634. doi:10.1155/2019/5150634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Javaheri B, Razi H, Gohin S, et al. Lasting organ-level bone mechanoadaptation is unrelated to local strain. Sci Adv. 2020;6(10):eaax8301. doi: 10.1126/sciadv.aax8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Alam I, Warden SJ, Robling AG, Turner CH. Mechanotransduction in bone does not require a functional cyclooxygenase-2 (COX-2) gene. J Bone Miner Res. 2005;20(3):438–46. doi: 10.1359/JBMR.041124. [DOI] [PubMed] [Google Scholar]
- 140.Castillo A, Mahaffey I, Cole W. Aging mice exhibit reduced periosteal and greater endosteal mechanoresponsiveness following two weeks of axial compressive loading. Journal of Bone and Mineral Research. 2012:27. [Google Scholar]
- 141.Javaheri B, Stern AR, Lara N, et al. Deletion of a single beta-catenin allele in osteocytes abolishes the bone anabolic response to loading. J Bone Miner Res. Mar. 2014;29(3):705–15. doi: 10.1002/jbmr.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liedert A, Mattausch L, Rontgen V, et al. Midkine-deficiency increases the anabolic response of cortical bone to mechanical loading. Bone. 2011;48(4):945–51. doi: 10.1016/j.bone.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 143.Litzenberger JB, Tang WJ, Castillo AB, Jacobs CR. Deletion of (beta)1 integrins from cortical osteocytes reduces load-induced bone formation. Cellular and Molecular Bioengineering. 2009;2(3):416–424. [Google Scholar]
- 144.Morse A, McDonald MM, Kelly NH, et al. Mechanical load increases in bone formation via a sclerostin-independent pathway. J Bone Miner Res. 2014;29(11):2456–67. doi: 10.1002/jbmr.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Niziolek PJ, Warman ML, Robling AG. Mechanotransduction in bone tissue:The A214V and G171V mutations in Lrp5 enhance load-induced osteogenesis in a surface-selective manner. Bone. 2012;51(3):459–65. doi: 10.1016/j.bone.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pierroz DD, Bonnet N, Bianchi EN, et al. Deletion of (beta)-adrenergic receptor 1, 2, or both leads to different bone phenotypes and response to mechanical stimulation. Journal of Bone and Mineral Research. 2012;27(6):1252–1262. doi: 10.1002/jbmr.1594. [DOI] [PubMed] [Google Scholar]
- 147.Robling AG, Warden SJ, Shultz KL, Beamer WG, Turner CH. Genetic effects on bone mechanotransduction in congenic mice harboring bone size and strength quantitative trait loci. J Bone Miner Res. 2007;22(7):984–91. doi: 10.1359/jbmr.070327. [DOI] [PubMed] [Google Scholar]
- 148.Tomlinson RE, Silva MJ. HIF-1(alpha) regulates bone formation after osteogenic mechanical loading. Bone. 2015;73:98–104. doi: 10.1016/j.bone.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang N, Rumney RM, Yang L, et al. The P2Y(13) receptor regulates extracellular ATP metabolism and the osteogenic response to mechanical loading. J Bone Miner Res. 2013;28(6):1446–56. doi: 10.1002/jbmr.1877. doi:10.1002/jbmr.1877. [DOI] [PubMed] [Google Scholar]
- 150.Zhao L, Shim JW, Dodge TR, Robling AG, Yokota H. Inactivation of Lrp5 in osteocytes reduces young's modulus and responsiveness to the mechanical loading. Bone. 2013;54(1):35–43. doi: 10.1016/j.bone.2013.01.033. doi:10.1016/j.bone.2013.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Svensson J, Windahl SH, Saxon L, et al. Liver-derived IGF-I regulates cortical bone mass but is dispensable for the osteogenic response to mechanical loading in female mice. Am J Physiol Endocrinol Metab. 2016;311(1):E138–44. doi: 10.1152/ajpendo.00107.2016. [DOI] [PubMed] [Google Scholar]
- 152.Xiao Z, Dallas M, Qiu N, et al. Conditional deletion of Pkd1 in osteocytes disrupts skeletal mechanosensing in mice. Faseb j. 2011;25(7):2418–32. doi: 10.1096/fj.10-180299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lories RJ, Peeters J, Bakker A, et al. Articular cartilage and biomechanical properties of the long bones in Frzb-knockout mice. Arthritis Rheum. 2007;56(12):4095–103. doi: 10.1002/art.23137. [DOI] [PubMed] [Google Scholar]
- 154.Mohan S, Bhat CG, Wergedal JE, Kesavan C. In vivo evidence of IGF-I-estrogen crosstalk in mediating the cortical bone response to mechanical strain. Bone Res. 2014;2:14007. doi: 10.1038/boneres.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Saxon LK, Jackson BF, Sugiyama T, Lanyon LE, Price JS. Analysis of multiple bone responses to graded strains above functional levels, and to disuse, in mice in vivo show that the human Lrp5 G171V High Bone Mass mutation increases the osteogenic response to loading but that lack of Lrp5 activity reduces it. Bone. 2011;49(2):184–93. doi: 10.1016/j.bone.2011.03.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pflanz D, Birkhold AI, Albiol L, et al. Sost deficiency led to a greater cortical bone formation response to mechanical loading and altered gene expression. Sci Rep 25. 2017;7(1):9435. doi: 10.1038/s41598-017-09653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Moore ER, Zhu YX, Ryu HS, Jacobs CR. Periosteal progenitors contribute to load-induced bone formation in adult mice and require primary cilia to sense mechanical stimulation. Stem Cell Res Ther. 2018;9(1):190. doi: 10.1186/s13287-018-0930-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Robling AG, Kang KS, Bullock WA, et al. Sost, independent of the non-coding enhancer ECR5, is required for bone mechanoadaptation. Bone. 2016;92:180–188. doi: 10.1016/j.bone.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sawakami K, Robling AG, Ai M, et al. The Wnt co-receptor LRP5 is essential for skeletal mechanotransduction but not for the anabolic bone response to parathyroid hormone treatment. J Biol Chem. 2006;281(33):23698–711. doi: 10.1074/jbc.M601000200. [DOI] [PubMed] [Google Scholar]
- 160.Lee KL, Hoey DA, Spasic M, Tang T, Hammond HK, Jacobs CR. Adenylyl cyclase 6 mediates loading-induced bone adaptation in vivo. Faseb j. 2014;28(3):1157–65. doi: 10.1096/fj.13-240432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sample SJ, Heaton CM, Behan M, et al. Role of calcitonin gene-related Peptide in functional adaptation of the skeleton. PLoS One. 2014;9(12):e113959. doi: 10.1371/journal.pone.0113959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Albiol L, Büttner A, Pflanz D, et al. Effects of Long-Term Sclerostin Deficiency on Trabecular Bone Mass and Adaption to Limb Loading Differ in Male and Female Mice. Calcif Tissue Int. 2020;106(4):415–430. doi: 10.1007/s00223-019-00648-4. [DOI] [PubMed] [Google Scholar]
- 163.Davis JL, Cox L, Shao C, et al. Conditional Activation of NF-κB Inducing Kinase (NIK) in the Osteolineage Enhances Both Basal and Loading-Induced Bone Formation. J Bone Miner Res. 2019;34(11):2087–2100. doi: 10.1002/jbmr.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gerbaix M, Ammann P, Ferrari S. Mechanically Driven Counter-Regulation of Cortical Bone Formation in Response to Sclerostin-Neutralizing Antibodies. J Bone Miner Res. 2021;36(2):385–399. doi: 10.1002/jbmr.4193. [DOI] [PubMed] [Google Scholar]
- 165.Lawson LY, Brodt MD, Migotsky N, Chermside-Scabbo CJ, Palaniappan R, Silva MJ. Osteoblast-Specific Wnt Secretion Is Required for Skeletal Homeostasis and Loading-Induced Bone Formation in Adult Mice. J Bone Miner Res. 2022;37(1):108–120. doi: 10.1002/jbmr.4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lewis KJ, Yi X, Wright CS, et al. The mTORC2 Component Rictor Is Required for Load-Induced Bone Formation in Late-Stage Skeletal Cells. JBMR Plus. 2020;4(7):e10366. doi: 10.1002/jbm4.10366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Moore ER, Chen JC, Jacobs CR. Prx1-Expressing Progenitor Primary Cilia Mediate Bone Formation in response to Mechanical Loading in Mice. Stem Cells Int. 2019;2019:3094154. doi: 10.1155/2019/3094154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Morse A, Ko FC, McDonald MM, et al. Increased anabolic bone response in Dkk1 KO mice following tibial compressive loading. Bone. 2020;131:115054. doi: 10.1016/j.bone.2019.115054. [DOI] [PubMed] [Google Scholar]
- 169.Zannit HM, Brodt MD, Silva MJ. Proliferating osteoblasts are necessary for maximal bone anabolic response to loading in mice. FASEB J. 2020;34(9):12739–12750. doi: 10.1096/fj.202000614R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zannit HM, Silva MJ. Proliferation and Activation of Osterix-Lineage Cells Contribute to Loading-Induced Periosteal Bone Formation in Mice. JBMR Plus. 2019;3(11):e10227. doi: 10.1002/jbm4.10227. [DOI] [PMC free article] [PubMed] [Google Scholar]


