Abstract
Objectives:
Balance disorders and falls are common in the elderly and have a multifactorial etiology. The purpose of the present cross-sectional study is to evaluate a possible association between vitamins D3 and B12 and impaired balance and falls.
Methods:
Ninety patients, females and males, were evaluated, from December 2019 to December 2020 during their first ambulatory visit at the Prevention of Falls Clinic of the General University Hospital of Patras. Vitamins B12 and D3 levels were measured. The number of falls during the last 12 months was recorded and patients were assessed using Mini-Balance Evaluation Systems Test (Mini-BESTest), Fried Phenotype, Walking Speed, Hand Grip Strength, Short Physical Performance Battery.
Results:
A multiple linear regression analysis showed that Mini-BESTest are statistically significantly predicted, F(10,79)=18.734, p<0.001, adj. R2=0.70 from Vit-B12 and FRIED Phenotype (pre-frail vs non-frail). Similarly, in the multiple binary logistic regression analysis, falls were statistically significantly predicted from FRIED Phenotype (pre-frail vs non-frail) χ2(5)=63.918, p<0.001, Nagelkerke R Squared=0.68.
Conclusions:
Higher levels of vitamins B12 but not of D3 are associated with better balance but not with less falls in a sample of community-dwelling older people.
Keywords: Balance, Falls, Mini-BESTest, Vitamin B12, Vitamin D
Introduction
Falls are a common occurrence in the elderly and they affect almost 30% of the people over 65 annually, a rate that reaches 50% for people over 80, with serious consequences regarding both the physical and mental health of those people[1]. At the same time, they cause serious social-financial problem because of the impact they have on the health systems and the environment in which the elderly person who has fallen lives[2,3].
Falls have a multifactorial etiology, constituting more a geriatric syndrome rather than an isolated pathological entity of particular etiopathology. That is the reason that the fall rates among the older population are differentiated from country to country and place to place, depending on the cultural differences and lifestyles, or particular living arrangements such as homes for the elderly or long-term-care. Their treatment and prevention demand, thus, a holistic and interdisciplinary approach.
Among the factors that cause falls, are the disorders of gait and balance which in turn have multiple causes. It appears that vitamins D3 and B12 can affect both balance and falls with various mechanisms. More specifically, vitamin D deficiency has been associated with reduced muscular performance and loss/atrophy of fast-twitch type II muscular fibers. The mechanism responsible for this is dependent on the non-genomic action of vitamin D through its receptors in the cell layer (VDR), that control the flow of calcium into the sarcoplasm and the subsequent rapid contraction or relaxation of the muscles, as well as the synthesis of muscular proteins that are involved in the cellular multiplication and differentiation of myocytes. Vitamin D may also be important for balance, as the VDR receptors exist in the nervous system as well, and the age-related reduction in their number may be responsible for the reducing the transmission speed of the nerve impulses with consequent slower reactions and poorer balance[4,5].
Body posture depends closely on proprioception, vestibular and visual function and muscle tone, all parameters that are affected by vitamin B12 deficiency. Vitamin B12 is important for the trofism of the neural cells and its deficiency may manifest as spinal cord neuropathy, peripheral neuropathy, and subsequent loss/reduction of proprioception, especially of the lower extremities, of deep and superficial sensibility, due to damage of the large sensitive fibers, paresthesia, loss/reduction in the deep osteomuscular reflexes, thus increasing sensitivity in body posture disorders[6,7].
B12 deficiency may also affect the autonomic nerve system, as in related studies demyelination damages have been found to cause dysautonomic dysfunction such as orthostatic hypotension[8]. Older people often develop nutritional deficiencies due to malabsorption, malnutrition, medication, and various pathological conditions, as well as limited exposure to sunlight, particularly when considering institutionalized and frail patients with mobility issues and reduced autonomy.
Among these nutritional deficiencies, are vitamins B12 and D3. There are no sources in the bibliographic databases, which posit a minimum limit in vitamin D3 and its impact on falls and balance, which may be different to the one concerning its impact on bones[5]. Likewise, concerning vitamin B12 no clear limits have been set, beyond which its deficiency manifests itself with neural or hematological symptoms[9]. Despite the fact that the impact mechanisms of vitamins D and B12 on falls and balance are known[3,6] related studies on the general population have resulted in inconclusive results[3], while studies on the Greek population are scant. Despite the large number of publications on vitamin D, in the recent worldwide guidelines for the prevention and management of falls for the elderly by Montero-Odasso M. et al.[3], regarding the assessment and management of nutrition and vitamin D, even though the recommendation of grade 1 is strong, the quality of evidence is grade B (intermediate), meaning that further research is likely to have an important impact in elucidating the effect of vitamin D status and supplementation on falls. Similarly, as regards vitamin B12 in the same guidelines, this is considered during nutritional assistance, without however referring to cut-offs under which its integration must begin and there are no clear indications regarding its use in the prevention of falls and balance disorders. The purpose of this study for the first time on a sample of Greek elderly patients, who live in the community and visited the Outpatient clinic for Falls or Balance Problems at the Rehabilitation Clinic of Spinal Cord Injury of the General University Hospital of Patras is to evaluate the relationship between those two vitamins, falls and balance and confirm the hypothesis that these two vitamins reduce the incidence of falls and that their deficiency negatively affects balance.
Materials and methods
Study population and data collection
Sample
A total 77 women and 13 men aged 65 and above who applied to our outpatient clinic were enrolled into the study between December 2019 and December 2020.
Exclusion criteria
A history of intake vitamin D and/or B12 supplement and/or medications that affect the central nervous system and/or bone metabolism; the presence of a disease that affects postural stability and gait such as Parkinson’s disease, stroke, dementia; the presence of any neurological motor deficit such as paresis or orthopedic problems such as previous arthroplasty, amputation or severe osteoarthritis in the lower extremities. In addition, patients who had severe liver disease, cardiac failure, other severe metabolic diseases, and mental disorders were also excluded. The patients’ cognitive status was measured by the Mini Mental State Examination (MMSE)[10,11]. If the patient had a score <24 in the MMSE, they were excluded. The patients’ depression level was measured by the Geriatric Depression Scale (GDS)[12]. If the patient had a score >5 in the GDS, they were excluded.
Procedures
After the socio-demographic characteristics were recorded, a detailed medical history was taken. We received a history of falls during the previous 12 months, after which patients were allocated into fallers or non-fallers. To define falls, we adopted the World Health Organization definition: “a fall is an event which results in a person coming to rest inadvertently on the ground or floor or other lower level. Falls, trips and slips can occur on one level or from a height”[13].
Socio-demographic and assessment data
The data collected include age, gender, Body Mass Index (BMI), vitamin B12, vitamin D3, Mini-Balance Evaluation Systems Test (Mini-BESTest), Fried Phenotype (FP), Walking Speed (WS), Hand Grip Max (HGM), Short Physical Performance Battery (SPPB), Mini-Mental State Examination (MMSE), and Geriatric Depression Scale (GDS).
Haematological measurements
Collection of serum venous blood samples were taken at the hospital phlebotomy unit. For the measurement of serum total 25-OH-vit. D electrochemiluminescence, Roche reagents (Elecsys V D Total II) were used for a cobas 6000 analyzer in units of ng/ml. Τhe limit of quantitation was ≤5 ng/mL (≤12.5 nmol/L). The Endocrine Society has defined serum 25(OH)D of 50 nmol/L (20 ng/mL) as the threshold for deficiency and 75 nmol/L (30 ng/mL) as the threshold for sufficiency[14]. Τhe kit used to measure vit. B12 was the SimulTRAC-S Vitamin B12/Folate RIA Kit, in units of pg/ml. The sensitivity as defined by the concentration at 90% trace binding was 75 pg/mL. Serum vit. B12 levels can be interpreted, as follows: >300 pg/mL: normal result; vit. B12 deficiency is unlikely (i.e., probability of 1-5%). 200-300 pg/mL: borderline result; vit. B12 deficiency possible. <200 pg/mL low result; consistent with vit.B12 deficiency (specificity of 95-100%)[15].
Anthropometric assessment
At the time of the clinical measurements, the study participant came to the clinic with an carer or alone. The following anthropometric parameters were measured: body weight in kg units (a digital scale model of Omron Karada Scan was used), height in meters units and BMI was calculated. To evaluate Hand Grip strength of the dominant hand the digital JAMAR Plus Hydraulic Hand Dynamometer was used, which has proven to be a reliable tool for measuring handgrip strength[16]. Participants were seated upright with the feet flat on the ground, shoulders in a neutral position, the elbow against the side and 90° flexed, and the wrist in a neutral position. Next, participants were asked to perform a maximal isometric contraction at a distance of 1 minute from each other. The highest value (in kg) of three attempts was recorded. Α cut-off <27 kg points for men and <16 kg for women was indicative of possible sarcopenia according to revised European consensus on definition and diagnosis agreed by Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2)[17].
Then the patient was subjected to the tests of the “Short Physical Performance Battery (SPPB)”[18]. The SPPB has 3 variables: balance, walking speed, standing up 5 times from a chair. The total sum of these three tasks ranges from a minimum score of 0 to a maximum score of 12. A cut-off ≤8 point score according to EWGSOP2 is indicative of the low physical performance[17]. To measure walking speed, the patient was asked to cross a distance of 4 meters with a normal gait. The process was repeated 2 times and the best performance was recorded. A Gait speed ≤0.8 m/s is indicative of low physical performance[17].
Frailty
The Fried Frail Phenotype (FP), also commonly known as phenotype-based Cardiovascular Health Study (CHS) frailty scale[19], was used to determine physical frailty. Five FP components: unintentional weight loss, weakness, exhaustion, reduced walking speed, and reduced physical activity were evaluated as follows: 1) Weight loss: was defined a unintentional weight loss of 4.5 kg or more kg in the previous year or the 5% weight loss during the last year. 2) Weakness: To evaluate Hand Grip strength of the dominate hand the digital JAMAR Plus Hydraulic Hand Dynamometer was used and a Cut-off <27 kg points for men and <16 kg for women was indicative of Frailty. 3) Poor endurance and energy: was indicated by self-report of exhaustion. Self-reported exhaustion: identified by two positively responded questions: “Did you experience difficulties in doing about everything?” or “Did you feel that you can’t do anything?” a score of 1 point was provided if the participant experienced exhaustion 3 or more days per week, while 0 point if it was less than that. 4) Slowness: defined a walking speed less of 0,8 m/s. 5) Low physical activity level: Sedentariness was assessed by administering the following question to the patient: “What is your current level of physical activity?”. Possible patient answers: no physical activity (confined to bed); rather sedentary, some short walks or other exercise of very light intensity; light intensity exercise (walking, dancing, fishing or shooting, shopping on foot) at least 2 to 4 hours a week; Moderate intensity exercise (running, walking uphill, swimming, gardening, cycling) for 1 to 2 hours a week, or light intensity exercise (walking, dancing, fishing or shooting) for more than 4 hours a week; Moderate intensity exercise more than 3 hours a week; Vigorous exercise several times a week. by answering the question, the participant was instructed that light intensity exercise does not cause sweating and does not prevent conversation, moderate intensity exercise causes sweating and conversation is not possible, and vigorous exercise involves maximum effort[20]. Based on the CHS frailty scale score based on summing up each component, those who scored 0 points were classified as fit (or robust), those who scored 1-2 points were classified as pre-frail, and those who scored 3-5 points, were classified as frail.
Mini-Balance Evaluation Systems Test (Mini-BESTest)
The mini-Bestest was used to assess balance and includes four subscales: transitions/anticipatory postural control, reactive postural control, sensory orientation and stability in gait. Clinical measurements were completed with the Mini-BESTest validated in Greek. Subject Conditions: Patients were tested with flat shoes, or no shoes and no socks. Equipment: Presence of Temper®foam (also called T-foamTM, 10 cm thick foam, medium density, hardness scale T41), chair without arms or wheels, inclined plane (ramp), timer, a box (height 23 cm), and a distance of 3 meters measured and marked on the ground (from the chair) with tape. There are a total of 14 items in the Mini-BESTest and each of them is rated from 0 to 2 depending on the perfection of their execution. Mini-BESTest scores can range from 0 to 28[21-23].
Statistical analysis
We performed the statistical analysis using SPSS version 25.0 software (SPSS Inc., Chicago, IL, USA)[24]. Continuous variables were expressed as mean±standard deviation (SD) and categorical variables were expressed as absolute numbers (N) and percentages (%). Normality (normal distribution) of the variables was tested using the Shapiro-Wilks test, and through the study “Normal Q-Q plot”, “Detrended Normal Q-Q plot”, and “Box Plot”. For all tests, statistical differences were determined to be significant at the p<0.05 level. We used the independent samples t-test when sought to compare two groups means, and One-way ANOVA to compare the means of three or more group. To correlate two continuous variables, we used the Pearson correlation coefficient (Pearson’s r). A multiple linear regression analysis is used to predict a continuous dependent variable based on multiple independent variables. A multiple binary logistic regression analysis is used to predict a binary dependent variable based on multiple independent variables.
Results
The study sample consists of 90 participants (77 women and 13 men), mean age of 72.6 years (±6.2) and average Body Mass Index (BMI) 27.6 kg/m2 (±4.2). The mean values of vitamins B12 and D3 are 400.8 pg/mL (±162.6) and 27.5 ng/mL (±9.6), respectively. Forty-four (48.9%) participants reported having at least one fall in the previous 12 months. The mean Mini-BESTest score is 14.8 (±5.9). The average walking speed is 0.8 m/s (±0.2). The mean value of Hand Grip Max is 23 kg (±5.6) and the mean value of SPPB total is 9.7 (±2.6). Based on the FRIED Frail Phenotype (CHS), 14 (15.6%) participants are classified as “frail”, 26 (28.9%) as “pre-frail” and 50 (55.6 %) as “non-frail” (also referred to as fit/robust in CHS reporting).
An independent-samples t-test was run to determine if there were differences in level of vitamin B12 between participants reported having, and not having, at least one fall in the previous 12 months (Table 1). The level of vitamin B12 is statistically significant lower to participants reported having at least one fall in the previous 12 months (M=346.9, SD=128.5) than participants reported not having (M=452.3, SD=176.0), t(88)=3.235, p=0.002. An independent-samples t-test was run to determine if there were differences in level of vitamin D3 between participants reported having, and not having, at least one fall in the previous 12 months (Table 1). The level of vitamin D3 is statistically significant lower to participants reported having at least one fall in the previous 12 months (M=24.3, SD=8.9) than participants reported not having (M=30.6, SD=9.3), t(88)=3.285, p=0.001. The results are shown in Figure 1.
Table 1.
Comparison of the level of vitamin B12 and D3 for biological sex, falls and FRIED phenotype.
| Vitamin B12 (pg/mL) | Vitamin D3 (ng/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| n | n % | Mean (sd) | p-value | n | n % | Mean (sd) | p-value | ||
| Gender | Male | 13 | 14.4% | 395.8 (168.5) | 0.906 | 13 | 14.4% | 27.4 (8.7) | 0.964 |
| Female | 77 | 85.6% | 401.6 (162.7) | 77 | 85.6% | 27.5 (9.8) | |||
| Falls | No | 46 | 51.1% | 452.3 (176.0) | 0.002 | 46 | 51.1% | 30.6 (9.3) | 0.001 |
| Yes | 44 | 48.9% | 346.9 (128.5) | 44 | 48.9% | 24.3 (8.9) | |||
| FRIED Phenotype | Non-frail | 50 | 55.6% | 454.4 (167.2) | 0.001 | 50 | 55.6% | 30.6 (8.7) | 0.002 |
| Pre-frail | 26 | 28.9% | 345.2 (142.2) | 26 | 28.9% | 24.1 (9.8) | |||
| Frail | 14 | 15.6% | 312.6 (105.7) | 14 | 15.6% | 22.9 (8.6) | |||
Figure 1.
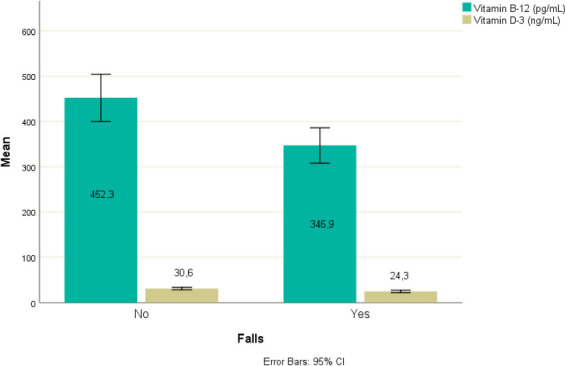
Level of vitamin B12 and D3 for falls.
An independent-samples t-test was run to determine if there were differences in level of vitamin B12 between male and female participants (Table 1). The level of vitamin B12 is not statistically significant different between males (M=395.8, SD=168.5) females (M=401.6, SD=162.7), t(88)= -0.119, p=0.906. An independent-samples t-test was run to determine if there were differences in level of vitamin D3 between male and female participants (Table 1). The level of vitamin D3 is not statistically significant different between males (M=27.4, SD=8.7) and females (M=27.5, SD=9.8), t(88)=-0.045, p=0.964.
A one-way ANOVA was conducted to determine if the level of vitamin B12 was different for groups with different FRIED Phenotype. Participants were classified into three groups: frail (n=50), pre-frail (n=26), non-frail levels of FRIED Phenotype (n=14). Bonferroni post hoc analysis revealed that level of vitamin B12 increased from the frail (M=312.6, SD=105.7), to pre-frail (M=345.2, SD=142.2), to non-frail (M=454.4, SD=167.2) FRIED Phenotype groups, in that order, and the differences between these FRIED Phenotype groups is statistically significant (F(2,87)=7.169, p=0.001). Similarly, a one-way ANOVA was conducted to determine if the level of vitamin D3 was different for groups with different FRIED Phenotype levels. Participants were classified into three groups: frail (n=50), pre-frail (n=26), non-frail levels of FRIED Phenotype (n=14). Bonferroni post hoc analysis revealed that level vitamin D3 increased from the frail (M=22.9, SD=8.6), to pre-frail (M= 24.1, SD=9.8), to non-frail (M=30.6, SD=8.7) FRIED Phenotype groups, in that order, and the differences between these FRIED Phenotype groups is statistically significant (F(2,87)=6.732, p=0.002).
The results of Pearson correlation tests are shown in Table 2. There is statistically significant negative correlation between level of vitamin B12 and participant’s age, r(90)= -0.871, p<0.001. There was statistically significant negative correlation between level of vitamin D3 and age, r(90)= -0.940, p<0.001. Older ages correspond to lower values of vitamin B12 and D3.
Table 2.
Pearson correlation tests.
| Age | ΒΜΙ (kg/m^2) | Mini-BESTest | Hand Grip Max (kg) | Walking Speed (m/s) | SPPB total score | ||
|---|---|---|---|---|---|---|---|
| Vitamin B-12 (pg/mL) | Pearson Correlation | -0.871 | 0.078 | 0.816 | 0.643 | 0.466 | 0.799 |
| Sig. (2-tailed) | <0.001 | 0.464 | <0.001 | <0.001 | <0.001 | <0.001 | |
| N | 90 | 90 | 90 | 90 | 90 | 90 | |
| Vitamin D-3 (ng/mL) | Pearson Correlation | -0.940 | 0.100 | 0.785 | 0.708 | 0.469 | 0.895 |
| Sig. (2-tailed) | <0.001 | 0.348 | <0.001 | <0.001 | <0.001 | <0.001 | |
| N | 90 | 90 | 90 | 90 | 90 | 90 | |
There is not statistically significant correlation between level of vitamin B12 and ΒΜΙ, r(90)=0.078, p=0.464. Similarly, there is not statistically significant correlation between level of vitamin D3 and BMI, r(90)=0.100, p=0.348.
There is statistically significant positive correlation between level of vitamin B12 and Mini-BESTest, r(90)=0.816, p<0.001. There is statistically significant positive correlation between level of vitamin D3 and Mini-BESTest, r(90)=0.785, p<0.001. Higher scores on the Mini-BESTest correspond to higher values of vitamin B12 and D3.
There is statistically significant positive correlation between level of vitamin B12 and Hand Grip Max, r(90)=0.643, p<0.001. There is statistically significant positive correlation between level of vitamin D3 and Hand Grip Max, r(90)=0.708, p<0.001. Higher scores on the Hand Grip Max correspond to higher values of vitamin B12 and D3.
There is statistically significant positive correlation between level of vitamin B12 and Walking Speed, r(90)= 0.466, p<0.001. There is statistically significant positive correlation between level of vitamin D3 and Walking Speed, r(90)=0.469, p<0.001. Higher values on the Walking Speed correspond to higher values of vitamin B12 and D3.
There is statistically significant positive correlation between level of vitamin B12 and SPPB total, r(90)=0.799, p<0.001. There is statistically significant positive correlation between level of vitamin D3 and SPPB total, r(90)=0.895, p<0.001. Higher scores on the SPPB total correspond to higher values of vitamin B12 and D3.
A multiple linear regression was run to predict Mini-BESTest from age, biological sex, ΒΜΙ (kg/m^2), FRIED Phenotype, Walk Speed (m/s), Hand Grip Max, SPPB total, vit-B12, and vit-D3. The multiple regression model statistically significantly predicted Mini-BESTest, F(10,79)=18.734, p<0.001, adj. R2=0.70. Vit-B12 and FRIED Phenotype (pre-frail vs non-frail) added statistically significantly to the prediction, p<0.05. Regression coefficients and standard errors can be found in Table 3.
Table 3.
Coefficientsa.
| Model | Unstandardized Coefficients | Standardized Coefficients | t | Sig. | 95.0% Confidence Interval for B | ||
|---|---|---|---|---|---|---|---|
| B | Std. Error | Beta | Lower Bound | Upper Bound | |||
| (Constant) | 57,360 | 49,521 | 1,158 | ,250 | -41,209 | 155,930 | |
| Age | -,566 | ,517 | -,595 | -1,094 | ,277 | -1,596 | ,464 |
| Biological sex | -1,202 | 1,415 | -,072 | -,849 | ,398 | -4,018 | 1,614 |
| Hand Grip Max | ,155 | 1,491 | ,010 | ,104 | ,917 | -2,813 | 3,124 |
| ΒΜΙ (kg/m^2) | ,065 | ,091 | ,046 | ,711 | ,479 | -,117 | ,247 |
| Walk Speed (m/s) | 1,575 | 2,056 | ,065 | ,766 | ,446 | -2,518 | 5,668 |
| SPPB total | -1,442 | 1,006 | -,627 | -1,433 | ,156 | -3,444 | ,560 |
| Vitamin B-12 (pg/mL) | ,019 | ,008 | ,512 | 2,230 | ,029 | ,002 | ,035 |
| Vitamin D-3 (ng/mL) | ,136 | ,180 | ,221 | ,756 | ,452 | -,222 | ,495 |
| FriedPhenotype=Pre-frail | -2,261 | ,894 | -,175 | -2,530 | ,013 | -4,040 | -,482 |
| FriedPhenotype=Frail | -,609 | 1,283 | -,038 | -,475 | ,636 | -3,164 | 1,945 |
a. Dependent Variable: Mini-BESTest. Multiple linear regression analysis to predict Mini-BESTest from age, biological sex, ΒΜΙ (kg/m^2), FRIED Phenotype, Walk Speed (m/s), Hand Grip Max, SPPB total, vit-B12, and vit-D3. S.E: standard error around the co-efficient for the constant; Wald chi square statistics; df: degree of freedom for Wald chi square statistics; Exp(B): exponentiation of B co-efficient which is an odds ratio (OR); 95% CI: Confidence interval for the odds ratio with its upper and lower limits.
A multiple binary logistic regression was run to predict falls from age, biological sex, ΒΜΙ (kg/m^2), FRIED Phenotype, Walk Speed (m/s), Hand Grip Max, SPPB total, vit-B12, and vit-D3. The multiple binary logistic regression model statistically significantly predicted falls, χ2(5)=63.918, p<0.001, Nagelkerke R Squared=0.68. FRIED Phenotype (pre-frail vs non-frail) added statistically significantly to the prediction, p<0.05. Regression coefficients and standard errors can be found in Table 4.
Table 4.
Variables in the Equation.
| B | S.E. | Wald | df | Sig. | Exp(B) | 95% C.I.for EXP(B) | |||
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||||
| Step 1a | Age | ,672 | ,543 | 1,531 | 1 | ,216 | 1,958 | ,675 | 5,680 |
| Biological sex(1) | -,468 | 1,248 | ,141 | 1 | ,708 | ,626 | ,054 | 7,230 | |
| Hand Grip Max(1) | -2,262 | 1,447 | 2,443 | 1 | ,118 | ,104 | ,006 | 1,777 | |
| ΒΜΙ (kg/m^2) | ,085 | ,085 | ,986 | 1 | ,321 | 1,088 | ,921 | 1,286 | |
| Walk Speed (m/s) | -1,201 | 1,773 | ,459 | 1 | ,498 | ,301 | ,009 | 9,723 | |
| SPPB total | 1,414 | 1,002 | 1,992 | 1 | ,158 | 4,112 | ,577 | 29,304 | |
| Vitamin B-12 (pg/mL) | ,010 | ,008 | 1,532 | 1 | ,216 | 1,010 | ,994 | 1,026 | |
| Vitamin D-3 (ng/mL) | -,157 | ,142 | 1,224 | 1 | ,269 | ,854 | ,647 | 1,129 | |
| FriedPhenotype=Pre-frail | 3,647 | ,834 | 19,140 | 1 | ,000 | 38,377 | 7,489 | 196,669 | |
| FriedPhenotype=Frail | 22,539 | 10349,143 | ,000 | 1 | ,998 | 6144620267,020 | ,000 | . | |
| Constant | -64,498 | 51,847 | 1,548 | 1 | ,213 | ,000 | |||
a. Variable(s) entered on step 1: Age, Biological sex, Hand Grip Max, ΒΜΙ (kg/m^2), Walk Speed (m/s), SPPB total, Vitamin B-12 (pg/mL), Vitamin D-3 (ng/mL), FriedPhenotype=Pre-frail, FriedPhenotype=Frail. Multiple binary logistic regression analysis to predict falls from age, biological sex, ΒΜΙ (kg/m^2), FRIED Phenotype, Walk Speed (m/s), Hand Grip Max, SPPB total, vit-B12, and vit-D3. S.E: standard error around the co-efficient for the constant; Wald chi square statistics; df: degree of freedom for Wald chi square statistics; Exp(B): exponentiation of B co-efficient which is an odds ratio (OR); 95% CI: Confidence interval for the odds ratio with its upper and lower limits.
Discussion
Vitamins B12-D3 and falls
Although the effect and importance of vitamin B12 for the nervous system and the adverse effects that it lacks can cause are known, there are not enough studies to establish its relationship with falls and balance, nor clear thresholds of its blood levels below which these disorders and events may occur. In our study we found that the levels of vitamin B12 and D3 was statistically significant lower in participants reported having at least one fall in the previous 12 months than participants reported not having any. But in the multiple binary logistic regression analysis, only FRIED Phenotype (pre-frail vs non-frail) added statistically significantly to the prediction, p<0.05. Vitamins D3 and B12 did not add statistically significantly to the prediction, (p=0.269 & 0.216 respectively). These results are partially in agreement with an Irish observational study in a sample of 4939 older adults where a significant association was found between falls and Holo-transcobalamin but not vitamin B12[25]. On the other hand, a randomized controlled trial to examine the effect of 2-Year Vitamin B12 and Folic Acid supplementation on Physical Performance, Strength, and Falling held in the contexts of the B-PROOF Study, concludes that two-year supplementation of vitamin B12 and folic acid was neither effective in reducing the age-related decline in physical performance and handgrip strength, nor in the prevention of falling in elderly persons[26]. In this study, however, patients were selected according to blood levels of homocysteine and not based on levels of vitamin B12 and folate, not knowing for sure whether these patients were deficient in vitamin B12 or not.
Similarly, like vitamin B12, studies regarding the relationship between vitamin D3 and falls are ambiguous. According to the study by Menant JC et al. Vitamin D insufficiency in men but not women, significantly increased the rate of falls[27]. Considering that in our study the sample is not homogeneous, with the prevalence of it being made up of women (85.6%), it is in agreement with the study by Menant JC et al. And this could explain the lack of association found between vitamin D3 and falls. Sim M. et al. in their study found that compared to low vitamin D3 levels, women in high vitamin D3 levels had significantly lower hazards for a falls-related hospitalization[28]. In contrast to our study were the results of a prospective cohort study[29] where low 25(OH)D (10 ng/ml) was associated with an increased risk of falling, in the elderly, particularly in those aged 65-75 yr. However, the vitamin D3 level threshold of 10 ng/mL below which the risk of falling was increased, is significantly lower than the mean of 24.3 ng/mL in our sample. In another multi-center study among 9,526 community-dwelling women enrolled in the Study of Osteoporotic Fractures, higher 1,25(OH)2D3 concentration was associated with lower fall rates (p=0.039)[30]. Similarly, a study in older women in residential care in Australia, serum 25OHD less than 20 ng/mL (50 nmol/L) were independently related to the incidence of first fall[31]. In the present study it is noteworthy that, for fallers, the mean values for vitamins B12 and D3 (346.9±128.5 pg/mL and 24.3±8.9 ng/mL respectively) are much higher than the deficiency limits for B12 and insufficiency for D3. This finding obviously requires special management regarding the clinical approach to prescribing supplements of these vitamins in patients with falls.
Vitamins B12-D3 and balance
In the present study we found that there is a statistically significant relationship between vitamins B12 and D3 and balance. This result was also confirmed in the case of vitamin B12 but not of D3, after a multiple linear regression analysis was performed, to predict Mini-BESTest from vitamins B12 and D3. This finding seems to disagree with a large cross-sectional cohort study of the older Korean population[32] where in Activities-specific Balance Confidence (ABC) scale - which measures a person’s confidence in their balance ability to perform various activities, did not have a statistically significant relationship with the portion of the sample designated as vitamin B12 deficient (<350 pg/mL). Granted, the ABC is a 16-item tool that measures the individual’s ability to perform functional tasks and uses a question rated scale and not a balance assessment tool through physical tests such as the Mini BESTest. On the other hand, a longitudinal cross-sectional study in older adults, Ng TP. et al. showed significant negative associations between POMA balance score and homocysteine, but no significant independent associations for vitamin B-12 and falls were observed[33]. Regarding vitamin D and balance, a study of elderly people who had a fall, those with vitamin D levels lower than 12 mcg/L showed to have the greatest degree of postural sway and the weakest quadriceps strength compared to people who had vitamin D levels higher than these levels, although this difference was not statistically significant[34]. And this is in agreement with our findings where in the multiple regression analysis model, the Mini-BESTest was not statistically significantly predicted by vitamin D3. Menant JC et al. partially contrast our study, concluding in their study that among community dwellers aged 70-90 years, those with vitamin D insufficiency (<20 ng/L) showed slower simple finger press and choice stepping reaction time, poorer leaning balance and slower gait speed and men but not women, had a significantly increased rate of falls[27]. Our study is one of the few that specifically examines the relationship between vitamins D and B12 and balance and uses the Mini-BESTest as a tool to assess it. The reliability of the Mini-BESTest is excellent to good, with ICC values >0.90 for people with mixed diagnoses and is reported to have high content validity[22]. These properties make it both a reliable and valid assessment tool in establishing if exist a relationship between vitamins B12 and D3 and balance. Relationship which is confirmed by the statistically significant positive correlation found between them; higher scores on the Mini-BESTest corresponded to higher values of vitamin B12 and D3.
However, only in the case of vitamin B12 can the multiple regression model predict the Mini-BESTest from it in a statistically significant way. This finding verifies the association between plasma levels of vitamin B12 - but not of D3 - and balance disorders and confirms in part our hypothesis that the deficiency of these two vitamins affects postural balance negatively. And this is of importance in identifying patients who should be screened for possible vitamin B12 deficiency and who are at high risk to have balance disorders or to have a fall. Age is a risk factor associated with both falls and various nutritional deficiencies. Low values at the Mini-BESTest should direct clinicians in search of nutritional deficiencies and evaluation of the vitamins B12 and D3 levels.
Vitamins B12-D3 and frailty
Frailty is a contributor to functional decline and early mortality in older adults. Physical frailty is also related to sarcopenia and poor physical performance, a fact that can negatively affect balance and increase the risk of falls. The lack of vitamins B12 and D3 can either contribute to the mechanisms that promote physical frailty or be a consequence of it. Nutritional assessment is fundamental in people with frailty and among the nutritional factors that are assessed in order to make the appropriate intervention in case of their deficiency are included vitamins B12 and D3. In our study people with frailty (15,6%) had demonstrated lower levels of vitamins B12 and D3 (312,6±105,7 and 22,9±8.6 respectively). The corresponding limits for fallers were 346.9±128.5 pg/mL and 24.3±8.9 ng/mL respectively. This finding could be interpreted in such a way that frail individuals are more likely to fall if they have lower levels of vitamin B12 and D3 than a threshold of <312-346 pg/mL and <22-24 ng/mL respectively. In the study by Soh Y and Won CW[32], in the unadjusted model, frailty was highly prevalent in the B12 insufficient group (defined here as <350 pg/mL) (odds ratio=1.298). However, when the model was fully adjusted to control for demographic data and comorbidities, these associations were attenuated.
In another study[35] no correlation was found between vitamin B12 levels and frailty. But in this instance, the patients were divided into 2 groups: vitamin B12 levels above and below 400 pg/mL. A threshold much higher than was set in our study. In contrast, a statistically significant negative correlation was found between vitamin D levels and frailty in concordance with our results. A probable explanation of the threshold variability between vitamin B12 levels and the correlation with frailty can be that Transcobalamin-II (TCN2) variants decrease vitamin B12 availability leading to reduced energy metabolism, ultimately contributing to frailty pathology[36]. Concerning vitamin D and its relationship with frailty in a systematic review and meta-analysis, Zhou J. et al. found that the pooled odds ratio (OR) of frailty for the lowest versus the highest level of vitamin D was 1.27 (95% CI=1.17-1.38, I2=59%), suggesting that low level of vitamin D was significantly associated with the risk of frailty[37]. However in our study in regression analysis, balance - represented by mini-BESTest - and falls are significantly statistically predicted by frailty regarding the pre-frailty group (p=0,013) but not the frail group (p=0,636). That result probably can be attributed to the limitations of the present study regarding the small sample of people with frailty (n=14) and the unequal distribution between both sexes.
Vitamins B12-D3 AND Hand Grip, Walking Speed and SPPB
In terms of a possible association between physical performance and vitamins B12 and D levels, we found that the highest values on Hand Grip Max and score in SPPB and higher Walking Speed correspond to higher values of vitamin B12 and D3 respectively. In current literature the findings are contentious. Our results are in accordance with the findings of Soh Y. and Won CW[32] for the SPPB, where the B12 sufficiency group showed better total SPPB and TUG test scores, even though they were not statistically significant in the fully adjusted model. Wolffenbuttel BHR. et al found that serum B12 concentration was not significantly associated with muscle strength (coefficient -0.279, SE 0.82, p=0.737), while serum Methylmalonic Acid (MMA) - another marker of vitamin B12 deficiency - was significantly associated with muscle strength (coefficient -6.90, SE 0.79, p<0.001)[38]. In contrast, regarding the relationship of vitamin D with physical performance, the results of our study clearly align with the bibliographic references that are more conclusive on the specific subject. In Houston DK et al’s in CHIANTI Study vitamin D status was inversely associated with poor physical performance[39]. Moreover handgrip strength was higher in people with serum 25OHD higher than 20 ng/mL and subjects with levels lower than 10 ng/mL performed lower on a short physical performance battery test (chair rises, balance) compared with those with higher 25OHD level[39]. However, in our study, the multiple regression model fails to statistically significantly predict falls and Mini-BESTest from walking speed, maximal hand grip, and total SPPB. And consequently, this finding further reinforces the importance of vitamin B12 and frailty as causative factors of poor balance and falls in our study sample.
Strengths and limitations
Our study contains some strengths as well as limitations. The key strength is that this is the first study in a Greek population that attempts to correlate balance and falls with vitamin B12 and D. Another strength is that the sample has been selected (via exclusion criteria) in such a way as to limit confounding factors such as motor problems, neurodegenerative or severe neuropsychological diseases. Additionally, the selection and use of the Mini-BESTest as a reliable and sensitive instrument for measuring balance to highlight the effect of vitamins B12 and D3 on it, reinforces the importance of the results.
On the other hand, the study was cross sectional, and there was a potential for coding errors and missing data. For instance, we cannot exclude the possibility that individuals started taking supplements months before the ambulatory visit. We do not know the exact values of D3 and B12 at the time the falls occurred. In order to limit possible confounding variables in our study, we set a series of exclusion criteria for our sample for those factors that could influence the incidence of falls and determine predisposing factors for various balance disorders. Among these confounding factors, we have not considered the pharmacological regimens of each patient who is part of our sample. The small sample with an unequal distribution between men and women imparts statistical limitations to the data analysis. The levels of vitamin B12 (cyanocobalamin) in the blood are not always a reliable indicator to assess its deficiency, since it is stored in the various tissues and its reserves differ in each of them. Other markers such as Methylmalonic Acid (MMA) and co-measurement of vitamin B12 levels simultaneously with folic acid and homocysteine appear to be more closely related and better identify a potential vitamin B12 deficiency. A similar limitation applies to the determination of vitamin D3 levels, as its measurement may vary from laboratory to laboratory[14].
Conclusions
In our study, 48.9% of the sample had at least one fall. In these patients, the levels of vitamins B12 and D were statistically significantly lower than in participants who reported not having fallen. The fallers had a mean value of vitamin B12 at 346.9±128.5 pg/mL and 24.3±8.9 ng/mL of vitamin D3. These cut-offs - should be taken into consideration at a clinical level - especially for vitamin B12 where most laboratories impose a lower physiological limit of around 200 pg/mL - as values to intervene with the integration of these vitamins in patients with falls, especially in older adults. In conclusion, after the multiple regression analysis model was performed, our initial hypothesis that vitamins B12 and D3 may positively affect balance and reduce the incidence of falls was partially confirmed, and that only in the case of vitamin B12 regarding balance but not falls. We have not found any statistically significant associations of falls and Mini-BESTest with vitamin D3 in the multiple regression model. Also, frailty seems to be another determining co-factor for falls and balance impairment, even though we have not found a statistically significant association for frail people but only for the pre–frail group. The fact that the Mini-BESTest can be predicted by the vitamin B12 levels can also constitute a clinical indicator for screening patients with vitamin B12 deficiency for balance problems. However, we are not able yet to fix a clear cut-off, under which to proceed to the screening. Moreover, the small sample of this study, and some possible methodological and data collection biases, do not allow for definitive conclusions to be drawn. For that reason, further investigation in larger population samples is required, especially in the Greek population where this first study was conducted.
Ethics approval
The study was approved by the institutional review board of the General University Hospital of Patras (EC number 733/10/12/2019).
Consent to participate
All participants provided written informed consent.
Acknowledgements
We would like to thank and acknowledge Dr Demi Patsios (University of Bristol) for reviewing and commenting on an earlier draft of this paper.
Footnotes
The authors have no conflict of interest.
Edited by: G. Lyritis
References
- 1.Todd C, Skelton D. What are the main risk factors for falls amongst older people and what are the most effective interventions to prevent these falls? 2004. [[Accessed 13th January 2023]]. March. [Internet] Available from:URL: http://www.euro.who.int/document/E82552.pdf .
- 2.Khow KSF, Visvanathan R. Falls in the Aging Population. Clin Geriatr Med. 2017;33(3):357–68. doi: 10.1016/j.cger.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Montero-Odasso M, van der Velde N, Martin FC, Petrovic M, Tan MP, Ryg J, et al. World guidelines for falls prevention and management for older adults:a global initiative. Age Ageing. 2022;51(9):afac205. doi: 10.1093/ageing/afac205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouillon R, Marcocci C, Carmeliet G, Bikle D, White JH, Dawson-Hughes B, et al. Skeletal and Extraskeletal Actions of Vitamin D:Current Evidence and Outstanding Questions. Endocr Rev. 2019;40(4):1109–51. doi: 10.1210/er.2018-00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhaliwal R, Aloia JF. Effect of Vitamin D on Falls and Physical. Performance. Endocrinol Metab Clin North Am. 2017;46(4):919–33. doi: 10.1016/j.ecl.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Diagne NS. Postural Disorders in Neuromyelopathy by Deficiency in Vitamin B12. Phys Med Rehabil Disabil. 2016;2(1):1–3. [Google Scholar]
- 7.Egger NG, Anderson KE, Lindenbaum J, Healton EB, Savage DG, Brust JCM, et al. Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. N Engl J Med. 1988;318(26):180–2. doi: 10.1056/NEJM198806303182604. [DOI] [PubMed] [Google Scholar]
- 8.Moore A, Ryan J, Watts M, Pillay I, Clinch D, Lyons D. Orthostatic tolerance in older patients with vitamin B12 deficiency before and after vitamin B12 replacement. Clin Auton Res. 2004;14(2):67–71. doi: 10.1007/s10286-004-0142-x. [DOI] [PubMed] [Google Scholar]
- 9.Shipton MJ, Thachil J. Vitamin B12 deficiency - A 21st century perspective. Clin Med (Lond) 2015;15(2):145–50. doi: 10.7861/clinmedicine.15-2-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fountoulakis KN, Tsolaki M, Chantzi H, Kazis A. Mini Mental State Examination (MMSE):A validation study in Greece. Am J Alzheimers Dis Other Demen. 2000;15(6):342–5. [Google Scholar]
- 11.Tafiadis D, Ziavra N, Prentza A, Siafaka V, Zarokanellou V, Voniati L, et al. Validation of the Greek version of the Abbreviated Mental Test Score:Preliminary findings for cognitively impaired patients of different etiology. Appl Neuropsychol Adult. 2022;29(5):1003–14. doi: 10.1080/23279095.2020.1835915. [DOI] [PubMed] [Google Scholar]
- 12.Fountoulakis KN, Tsolaki M, Iacovides A, Yesavage J, O'Hara R, Kazis A, et al. The validation of the short form of the Geriatric Depression Scale (GDS) in Greece. Aging (Milano) 1999;11(6):367–72. doi: 10.1007/BF03339814. [DOI] [PubMed] [Google Scholar]
- 13.Step Safely:Strategies for preventing and managing falls across the life-course [Internet]. Who.int. World Health Organization. 2021. [cited 2023 Jan 10] Available from: https://www.who.int/publications/i/item/978924002191-4 .
- 14.Lips P, Cashman KD, Lamberg-Allardt C, Bischoff-Ferrari HA, Obermayer-Pietsch B, Bianchi ML, et al. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency:a position statement of the European Calcified Tissue Society. Eur J Endocrinol. 2019;180(4):P23–54. doi: 10.1530/EJE-18-0736. [DOI] [PubMed] [Google Scholar]
- 15.Mazokopakis EE, Starakis IK. Recommendations for diagnosis and management of metformin-induced vitamin B12 (Cbl) deficiency. Diabetes Res Clin Pract. 2012;97(3):359–67. doi: 10.1016/j.diabres.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Massy-Westropp NM, Gill TK, Taylor AW, Bohannon RW, Hill CL. Hand Grip Strength:age and gender stratified normative data in a population-based study. BMC Res Notes. 2011:4. doi: 10.1186/1756-0500-4-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia:revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function:association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 19.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults:evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 20.Subra J, Gillette-Guyonnet S, Cesari M, Oustric S, VellaS B. The integration of frailty into clinical practice:preliminary results from the Gérontopôle. J Nutr Health Aging. 2012;16(8):714–20. doi: 10.1007/s12603-012-0391-7. [DOI] [PubMed] [Google Scholar]
- 21.Anson E, Thompson E, Ma L, Jeka J. Reliability and Fall Risk Detection for the BESTest and Mini-BESTest in Older Adults. J Geriatr Phys Ther. 2019;42(2):81–5. doi: 10.1519/JPT.0000000000000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Potter K, Brandfass K. The Mini-Balance Evaluation Systems Test (Mini-BESTest) J Physiother. 2015;61(4):225. doi: 10.1016/j.jphys.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Lampropoulou S, Gedikoglou I, Michailidou C, Billis E. Cross Cultural Validation of the Mini-BESTest into Greek. World J Res Rev. 2016;3(3):61–7. [Google Scholar]
- 24.Field A. Discovering statistics using IBM SPSS statistics. 4th ed. Thousand Oaks CA: SAGE Publications; 2013. [Google Scholar]
- 25.Laird E, Hoey L, Healy M, Casey M, Cunningham C, Chong W, et al. Is Vitamin B12 status a risk factor for falling in older adults (>60 yrs)? Proc Nutr Soc. 2013;72(OCE4):E248. [Google Scholar]
- 26.Swart KMA, Ham AC, van Wijngaarden JP, Enneman AW, van Dijk SC, Sohl E, et al. A Randomized Controlled Trial to Examine the Effect of 2-Year Vitamin B12 and Folic Acid Supplementation on Physical Performance, Strength, and Falling:Additional Findings from the B-PROOF Study. Calcif Tissue Int. 2016;98(1):18–27. doi: 10.1007/s00223-015-0059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menant JC, Close JCT, Delbaere K, Sturnieks DL, Trollor J, Sachdev PS, et al. Relationships between serum vitamin D levels, neuromuscular and neuropsychological function and falls in older men and women. Osteoporos Int. 2012;23(3):981–9. doi: 10.1007/s00198-011-1637-7. [DOI] [PubMed] [Google Scholar]
- 28.Sim M, Zhu K, Lewis JR, Hodgson JM, Prince RL. Association between vitamin D status and long-term falls-related hospitalization risk in older women. J Am Geriatr Soc. 2021;69(11):3114–23. doi: 10.1111/jgs.17442. [DOI] [PubMed] [Google Scholar]
- 29.Snijder MB, Van Schoor NM, Pluijm SMF, Van Dam RM, Visser M, Lips P. Vitamin D status in relation to one-year risk of recurrent falling in older men and women. J Clin Endocrinol Metab. 2006;91(8):2980–5. doi: 10.1210/jc.2006-0510. [DOI] [PubMed] [Google Scholar]
- 30.Faulkner KA, Cauley JA, Zmuda JM, Landsittel DP, Newman AB, Studenski SA, et al. Higher 1,25-dihydroxyvitamin D3 concentrations associated with lower fall rates in older community-dwelling women. Osteoporos Int. 2006;17(9):1318–28. doi: 10.1007/s00198-006-0071-8. [DOI] [PubMed] [Google Scholar]
- 31.Flicker L, Mead K, MacInnis RJ, Nowson C, Scherer S, Stein MS, et al. Serum vitamin D and falls in older women in residential care in Australia. J Am Geriatr Soc. 2003;51(11):1533–8. doi: 10.1046/j.1532-5415.2003.51510.x. [DOI] [PubMed] [Google Scholar]
- 32.Soh Y, Won CW. Association between frailty and vitamin B12 in the older Korean population. Medicine (Baltimore) 2020;99(43):e22327. doi: 10.1097/MD.0000000000022327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ng TP, Aung KCY, Feng L, Scherer SC, Yap KB. Homocysteine, folate, vitamin B-12, and physical function in older adults:cross-sectional findings from the Singapore Longitudinal Ageing Study. Am J Clin Nutr. 2012;96(6):1362–8. doi: 10.3945/ajcn.112.035741. [DOI] [PubMed] [Google Scholar]
- 34.Dhesi JK, Bearne LM, Moniz C, Hurley M V, Jackson SHD, Swift CG, et al. Neuromuscular and psychomotor function in elderly subjects who fall and the relationship with vitamin D status. J Bone Miner Res. 2002;17(5):891–7. doi: 10.1359/jbmr.2002.17.5.891. [DOI] [PubMed] [Google Scholar]
- 35.Dokuzlar O, Soysal P, Isik AT. Association between serum vitamin B12 level and frailty in older adults. North Clin Istanbul. 2017;4(1):22. doi: 10.14744/nci.2017.82787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matteini AM, Walston JD, Bandeen-Roche K, Arking DE, Allen RH, Fried LP, et al. Transcobalamin-II variants, decreased vitamin B12 availability and increased risk of frailty. J Nutr Health Aging. 2010;14(1):73–7. doi: 10.1007/s12603-010-0013-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou J, Huang P, Liu P, Hao Q, Chen S, Dong B, et al. Association of vitamin D deficiency and frailty:A systematic review and meta-analysis. Maturitas. 2016;94:70–6. doi: 10.1016/j.maturitas.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 38.Wolffenbuttel BHR, Wouters HJCM, De Jong WHA, Huls G, Van Der Klauw MM. Association of vitamin B12, methylmalonic acid, and functional parameters. Neth J Med. 2020;78(1):10–24. [PubMed] [Google Scholar]
- 39.Houston DK, Cesari M, Ferrucci L, Cherubini A, Maggio D, Bartali B, et al. Association between vitamin D status and physical performance:the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2007;62(4):440–6. doi: 10.1093/gerona/62.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]


