Abstract
Alzheimer’s disease is initiated by the toxic aggregation of amyloid-β. Immunotherapeutics aimed at reducing amyloid beta are in clinical trials but with very limited success to date. Identification of orthogonal approaches for clearing amyloid beta may complement these approaches for treating Alzheimer’s disease. In the brain, the astrocytic water channel Aquaporin 4 is involved in clearance of amyloid beta, and the fraction of Aquaporin 4 found perivascularly is decreased in Alzheimer’s disease. Further, an unusual stop codon readthrough event generates a conserved C-terminally elongated variant of Aquaporin 4 (AQP4X), which is exclusively perivascular. However, it is unclear whether the AQP4X variant specifically mediates amyloid beta clearance.
Here, using Aquaporin 4 readthrough-specific knockout mice that still express normal Aquaporin 4, we determine that this isoform indeed mediates amyloid beta clearance. Further, with high-throughput screening and counterscreening, we identify small molecule compounds that enhance readthrough of the Aquaporin 4 sequence and validate a subset on endogenous astrocyte Aquaporin 4. Finally, we demonstrate these compounds enhance brain amyloid-β clearance in vivo, which depends on AQP4X.
This suggests derivatives of these compounds may provide a viable pharmaceutical approach to enhance clearance of amyloid beta and potentially other aggregating proteins in neurodegenerative disease.
Keywords: Aqp4, glymphatic, readthrough, amyloid beta, Alzheimer’s disease
Sapkota et al. show that a perivascular isoform of the water channel Aquaporin 4 (AQP4), generated via an unusual stop codon readthrough event, mediates Aβ clearance from the brain. Compounds that enhance readthrough of the AQP4 mRNA also enhance Aβ clearance, suggesting a new therapeutic strategy for Alzheimer’s disease.
See Giannetto and Hablitz (https://doi.org/10.1093/brain/awac289) for a scientific commentary on this article.
See Giannetto and Hablitz (https://doi.org/10.1093/brain/awac289) for a scientific commentary on this article.
Introduction
The astrocyte-specific water channel protein Aquaporin 4 (AQP4) is a key mediator of the glymphatic system, which clears amyloid-β (Aβ) and other waste products from the brain. In Aqp4 global knockout mice, intrastriatally infused Aβ was cleared at 50% reduced efficiency, and crossing these mice to APP/PS1 Alzheimer's disease models exacerbated Aβ accumulation and memory deficits.1–3 Thus, drugs that enhance clearance functions of AQP4 may expedite the removal of Aβ and other by-products from the brain.
Translation termination determines the length and fate of proteins. This final step of translation is enabled by release factors eRF1 and eRF3, which outcompete amino acyl-tRNAs for the A site of 80S ribosomes that have encountered a stop codon, i.e. UGA, UAG or UAA.4 This results in the hydrolysis of the nascent polypeptide chain from the P site followed by the dissociation of the two ribosomal subunits from the mRNA. A skipped termination, and hence the addition of extra amino acids to the growing polypeptide chain, can result in a protein product that becomes rapidly degraded or acquires untoward functions. It also dissipates a cell’s resources as translation is among the most energy-consuming processes. Evolutionarily and thermodynamically, termination is therefore an essential step.
Yet, translation past a stop codon is desirable under certain conditions. Viruses use stop codon readthrough to increase their protein repertoire from a limited amount of genetic material.5 Higher-order organisms, from yeasts to humans, also use a programmed readthrough in a subset of transcripts.6–8 Studies on a few of these transcripts have shown that the C-terminally extended protein variants arising from readthrough can gain new properties, including a distinct subcellular localization.9,10 In the case of Aqp4, for example, we and others have shown that the readthrough-extended version (AQP4X) becomes exclusively perivascular within astrocytic endfeet in the brain whereas the normal-length AQP4 is localized to parenchyma away from blood vessels.8,11 However, it has not yet been established whether it is the perivascular AQP4 that specifically clears endogenous Aβ, and/or whether the AQP4X isoform mediates this function. Furthermore, perivascular AQP4 is reduced in Alzheimer's disease, with the extent of reduction being proportional to Aβ levels.12,13 Thus, it is possible that enhancing readthrough might enhance clearance functions of APQ4, thereby slowing or preventing interstitial accumulation of Aβ.
Here, we first establish an AQP4X-specific knockout (Aqp4No_X) mouse line, replicating that this results in a loss of perivascular AQP4.14,15 Using this mouse, we demonstrate that loss of AQP4X reduces Aβ clearance and thus elevates baseline brain interstitial fluid (ISF) Aβ levels. Next, we adapt a dual-luciferase assay previously used to quantify stop codon readthrough7,8 to screen compounds for their ability to modulate Aqp4 readthrough. We then validate hits using orthogonal approaches independent of luciferase. Finally, we deliver these compounds to the mouse brain and show an enhanced clearance of Aβ, and demonstrate this enhanced clearance activity requires AQP4X. These results identify a new approach for reducing central Aβ levels that could form the basis for the future development of therapeutics for neurodegenerative disease.
Materials and methods
Materials and methods are available at Brain online and describe the following: generation of Aqp4No_X mouse, cell culture and transfection, vectors for readthrough assay, high-throughput screening, validation using dual-luciferase and fluorescence assays, immunofluorescence staining, primary astrocyte culture and drug treatment, dot blot and western blot, in vivo microdialysis to assess ISF Aβ, Aβ elimination half-life, Aβ sandwich enzyme-linked immunosorbent assay and MRI.
Results
AQP4X regulates clearance of Aβ
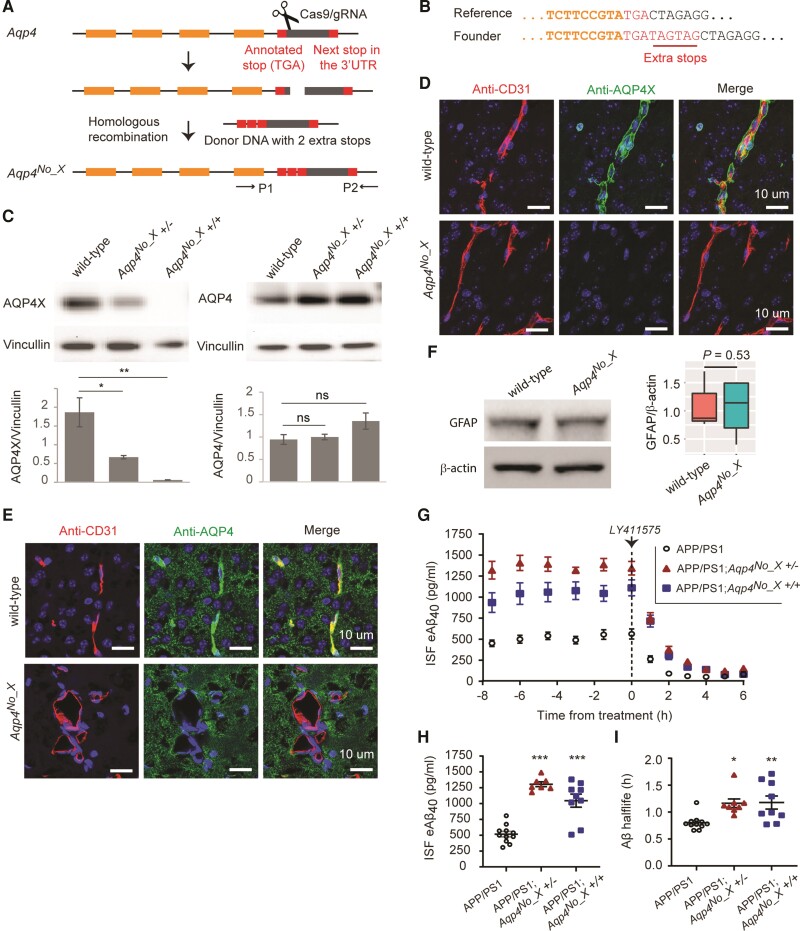
Using CRISPR–Cas9, we inserted two additional stop codons in frame downstream of the canonical stop codon in Aqp4 (Fig. 1A and B), generating Aqp4No_X mice that lost immunogenicity for the readthrough peptide-specific antibody but retained that for the pan-AQP4 antibody (Fig. 1C). As expected, Aqp4No_X mice lacked perivascular AQP4 (Fig. 1D and E), although pan-AQP4 antibody, which labels membranes of astrocytes,16 showed no significant change in total AQP4 levels (Fig. 1E). Otherwise, the brains appeared structurally normal by MRI (Supplementary Fig. 1A and B), with no apparent gliosis by GFAP western blot (Fig. 1F). Mutant mice had normal weight at weaning and were grossly indistinguishable from wildtype littermates. Aqp4No_X mice were then crossed to APP/PS1 transgenic mice carrying a human APP with a Swedish mutation and presenilin-1 with a ΔE9 mutation,17 resulting in APP/PS1; Aqp4No_X mice. We conducted in vivo microdialysis to assess steady-state levels of soluble ISF Aβ and its clearance rate using our standard protocol.18,19 Young mice (2–4 months old) were used to be before plaque formation. At baseline, both homozygote and heterozygote AQP4No_X mutant mice showed significantly elevated brain ISF Aβ (Fig. 1G and H, see also Supplementary Table 1 for statistical details). Next, we blocked new synthesis of Aβ with a potent γ-secretase inhibitor and measured clearance rates of existing Aβ to calculate elimination half-life in the brain. Both AQP4No_X mutant genotypes showed a prolonged half-life, consistent with decreased clearance, suggesting this is the mechanism of elevated baseline Aβ (Fig. 1I). The results in the homozygote indicated that the AQP4X isoform is required for normal Aβ clearance rates. The equally substantial alterations in the heterozygote mice further show that modulation of AQP4X levels can have a non-linear impact on Aβ levels and clearance.
Figure 1.
AQPX regulates clearance of Aβ. (A) CRISPR–Cas9 for generating the Aqp4No_X mouse. A double-strand break is made using a gRNA and two extra stop codons are introduced using a donor oligo for homologous recombination. P1 and P2 indicate primers designed to screen the founders. (B) Genotyping of founder mice. Illumina sequencing read from a founder is shown. (C) Western blot to validate mice. Note that anti-AQP4 recognizes both normal and readthrough AQP4s and hence continues to generate signal in the Aqp4No_X mouse. n = 3, t-test. (D and E) Characterization of Aqp4No_X mice. Perivascular AQP4, i.e. AQP4X, is lost while parenchymal AQP4 is intact in mutant mice. CD31 marks endothelial cells. Blue = nuclear staining with DAPI. (F) No gliosis is observed in mutant mice, as apparent from GFAP western blot. n = 3, t-test. (G) Steady-state exchangeable hippocampal ISF Aβ (eAβ) was measured longitudinally using in vivo microdialysis for 7.5 h followed by administration of a γ-secretase inhibitor (LY411575, 3 mg/kg intraperitoneally) then Aβ measured hourly for an additional 6 h. (H) Steady-state ISF eAβ concentration in APP/PS1 Aqp4 wild-type mice is 516.0 ± 42.8 pg/ml and significantly elevated to 1305 ± 38.82 pg/ml and 1045 ± 102.9 in Aqp4No_X +/− and +/+, respectively. Concentrations are not significantly different between +/− and +/+. (I) ISF eAβ elimination half-life in APP/PS1 Aqp4 wild-type mice is 0.80 + 0.04 h and significantly prolonged to 1.16 ± 0.08 and 1.18 ± 0.12 h in Aqp4No_X +/− and +/+ mice, respectively. Data presented as mean ± SEM. See Supplementary Table 1 for n and statistical tests. See Supplementary Fig. 4 for full images of blots used to prepare C and F. ***P ≤ 0.0001; **P ≤ 0.01; *P ≤ 0.05.
Aged APP/PS1 mice show disruption of AQP4X
A previous human study suggested that although total AQP4 levels increase with gliosis, the perivascular localization, specifically, is disrupted in Alzheimer’s disease.13 However, the existence of a specific perivascular (AQP4X) isoform was unknown at that time. Furthermore, our prior work showed a decrease of AQP4X/AQP4 ratio following a variety of gliotic insults.8 Therefore, we also tested whether gliosis secondary to Alzheimer’s-like pathology results in a disrupted AQP4X/AQP4 ratio in aged tissue. APP/PS1 Alzheimer’s model mice, with the expected Aβ plaques, showed a decreased AQP4X/AQP4 ratio (Supplementary Fig. 2). This indicates that not only is AQP4X required for efficient clearance of Aβ (Fig 1), but as disease progresses AQP4X is relatively lost, suggesting a feedforward loop that may further exacerbate Aβ accumulation. This also suggests that treatments that enhance AQP4X levels may be of some benefit both in early and late stages of disease.
High-throughput screening identifies compounds modulating readthrough of Aqp4 sequence
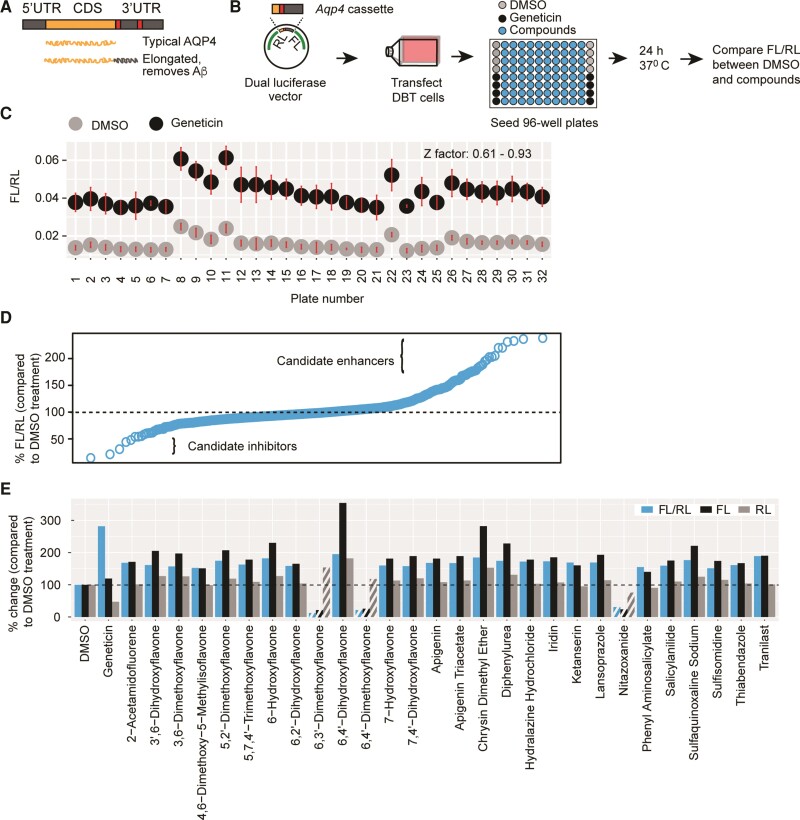
The fact that readthrough generates an elongated AQP4 variant that is required for efficient removal of Aβ suggests that enhancing readthrough can be of translational significance. We therefore sought to screen compounds for their ability to enhance Aqp4 readthrough using a fragment of the transcript we previously demonstrated as supporting readthrough activity (Fig. 2A). We focused on compounds likely to be potential drug candidates, surveying a collection of 2560 compounds, nearly half of which have reached clinical trials in the USA. The remainder are either drugs sold in Asia and Europe or natural products and their derivatives.
Figure 2.
High-throughput screening identifies readthrough modulators. (A) Schematic illustration of Aqp4 readthrough. (B) Screening procedure. Transfected cells were seeded into wells pre-spotted with compounds, DMSO (no-treatment control) or Geneticin (positive control). The last column received control cells in which the Aqp4 stop in the vector was mutated to a sense codon. (C) Separation bands between the no-treatment and positive controls and a summary of Z-factors for the plates used in screening. Dots and error bars represent means and 3 SD, respectively. (D) Quantile analysis of the screening results. (E) Compounds enhancing (solid bars) or inhibiting (patterned bars) FL/RL values by at least 50%. The change in FL/RL is largely due to the modulation of FL, not RL.
We began by evaluating a dual-luciferase assay that we have previously used to quantify readthrough8 for its suitability as a screening approach. This assay uses a dual-luciferase vector with an Aqp4 test cassette cloned in between Renilla (RL) and Firefly luciferases (FL) in such a way that the former is expressed constitutively, but the latter only if the Aqp4 stop codon is read past by ribosomes. Readthrough rate is then quantified as the ratio of Firefly activity to Renilla activity. We transfected delayed brain tumour cells, an astrocyte-like tumour cell line, with this vector and subsequently treated the cells with a series of concentrations of Geneticin, which is an antibiotic known to enhance stop codon readthrough. We found that FL/RL values are highly responsive to Geneticin concentrations (R2 = 0.99). Furthermore, the Z-factor, which measures the separation band between the treated cells and untreated cells, was well within the acceptable range of 0.5 and 1 as described,20 thus indicating that the assay is suitable for identifying readthrough modulators with confidence.
We then screened the 2600 compounds by spotting 96-well plates with 10 µM of each compound, and Geneticin and no-treatment [dimethyl sulphoxide (DMSO)] controls; seeding the plates with cells pre-transfected with dual-luciferase vector; and measuring FL and RL after 24 h (Fig. 2B). We found that the Z-factor was at least 0.9 for most of the plates and never <0.6, demonstrating the robustness of the screening (Fig. 2C). A quartile analysis revealed that most of the compounds did not alter the FL/RL ratio when compared to untreated wells, whereas a few clearly did so (Fig. 2D). Twenty-eight compounds altered the ratio by at least 50%, with 25 increasing and three decreasing it (Fig. 2E). Importantly, all 28 hits acted by inducing pronounced changes in FL, which depends on readthrough, but not RL, which is independent of readthrough (Fig. 2E). Therefore, we decided to further validate a set of these hits, which included antiparasitics (thiabendazole, sulphaquinoxaline), an antihypertensive (ketanserin), a common dietary flavonoid (apigenin) and a variety of other flavones.
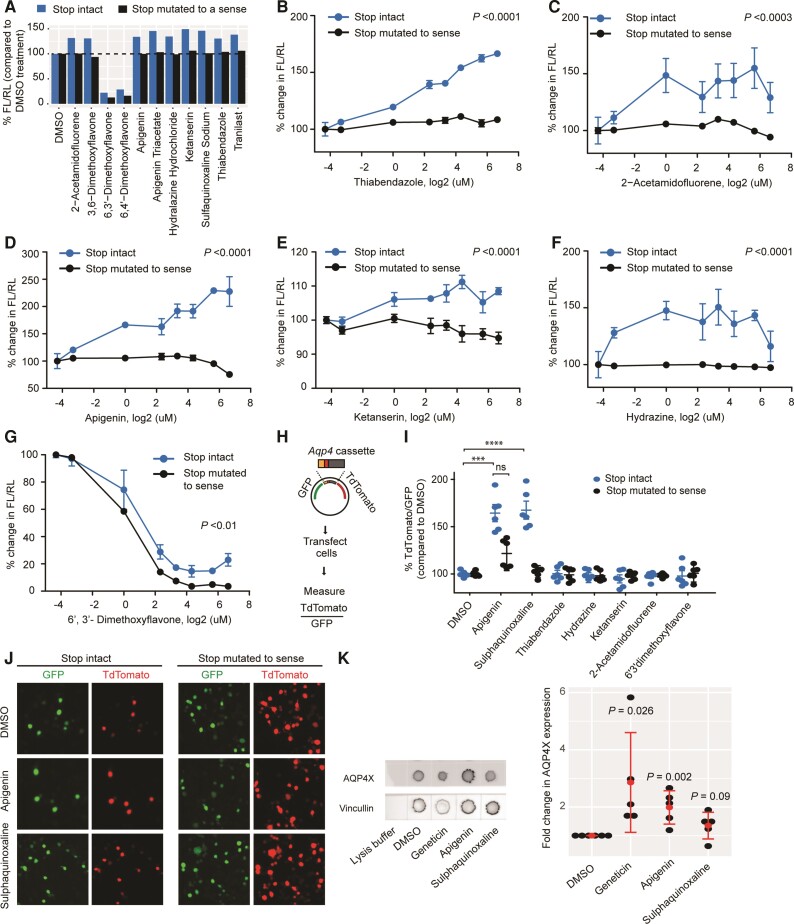
Independent assays confirm two compounds as genuine modulators of Aqp4 readthrough
Although Gentamicin and PTC124 are purported to modulate readthrough of all transcripts, the former does not exert the desired effect at subtoxic doses,21 and the latter appears to modulate the Firefly luciferase used in the original screening and hence not to be a genuine modulator at all.22,23 Therefore, there is a need for additional secondary screens and rigorous validation of hits in readthrough assays.
We started the validation experiments by repeating the dual-luciferase assay with 10 µM of the 28 hits in independent cultures. Most of these compounds caused expected changes in FL/RL, although the magnitudes of changes were smaller than in the primary screening, possibly due to differences in liquid handling and luciferase detection systems in the two experiments. As in screening, these changes were largely due to the modulation of FL but not RL. In total, 11 compounds altered FL/RL by at least 30% when compared to DMSO controls, with nine enhancing and two inhibiting it (Fig. 3A). In parallel, we tested the hypothesis that compounds should cease to exert their effect if FL is already translated at 100%. For this purpose, we mutated the TGA stop codon of the Aqp4 test cassette cloned in the dual-luciferase vector to a sense codon (TGG). We found that all 25 putative enhancers stopped exhibiting their effect (Fig. 3A). The three putative inhibitors, however, continued to reduce FL/RL, suggesting they might be impacting translation in general or FL activity independently of readthrough. They were thus excluded as candidates.
Figure 3.
Validation of candidate compounds. (A) Retesting with dual-luciferase vectors in which the Aqp4 stop codon is either intact (blue) or mutated to a sense codon (black). Compounds altering readthrough by at least 30% are shown. Enhancers cease activity in the absence of stop codon. (B–G) Dose-response curves for candidate compounds. Concentrations are log2-transformed. For log transformation of 0 µM (DMSO alone), a value of 0.05 µM was used. Enhancers cease to be active (B–F), whereas an inhibitor continues to be active (G) in the absence of stop codon. n = 3. ANOVA followed by a post hoc Tukey was used to compare the means for the two constructs across concentrations. (H) Dual-fluorescence assay. Readthrough is measured as TdTomato/GFP (I) dual-fluorescence assays in which the Aqp4 stop codon is either intact (blue) or mutated to a sense codon (black). ****P ≤ 0.0001; ***P ≤ 0.001, n = 3, t-test. (J) Representative images for dual-fluorescence assay with apigenin and sulphaquinoxaline. (K) Dot blot demonstrates apigenin enhances readthrough of endogenous Aqp4 transcript in cultured astrocytes. AQP4X expression is normalized to Vincullin. Red dots represent means and bars represents standard deviations. t-test comparing DMSO and compounds. n = 3 **P ≤ 0.01; *P ≤ 0.05.
We also performed a dose-response experiment and, as expected, found the tested enhancers act dose-dependently when the test cassette is used but not when the stop-to-sense positive control is used (Fig. 3B–F). In contrast, a tested inhibitor acted dose-dependently with both constructs, with a greater magnitude on the stop-to-sense control, again suggesting an impact on overall translation (Fig. 3G) or other readthrough-independent mechanisms. Nonetheless, the results suggested that the putative enhancers were likely to be genuine modulators of readthrough.
Next, we wanted to further exclude any candidates that altered the FL/RL ratio due to direct activity on the luciferases (i.e. modulating the enzymes rather than actual readthrough). Indeed, some candidates (e.g. sulphaquinoxaline) appear structurally similar to luciferin. We therefore developed an orthogonal system using a fluorescence-based assay wherein TdTomato/green fluorescent protein (GFP) is equivalent to FL/RL (Fig. 3H). Unlike the dual-luciferase assay, however, the fluorescence assay was not sufficiently sensitive for use in delayed brain tumour cells and required a more easily transfectable N2a cell line. Thus, we treated transfected N2a cells with the minimum effective doses of candidate enhancers, as identified from the dose-response experiment, and quantified TdTomato and GFP intensities by plate scanning. We found that two candidate enhancers, apigenin and sulphaquinoxaline, significantly increase the TdTomato/GFP ratio when compared to DMSO controls, as expected (Fig. 3I and J). The remaining inhibitor (6′3′-dimethoxyflavone, 10 µM) did not alter this ratio, indicating it probably inhibits FL directly. We also designed a stop-to-sense positive control fluorescence vector to test whether apigenin and sulphaquinoxaline will show activity in a vector that already allows 100% ‘readthrough’ as had been done with the luciferase constructs. Sulphaquinoxaline ceased its activity (Fig. 3I), suggesting it specifically modulates readthrough. Unlike on the luciferase construct, apigenin showed partial activity on the stop-to-sense construct, (Fig. 3I), indicating while it modulates readthrough it possibly also has a degree of non-specific action on TdTomato or GFP intensity. The activity on this mutated construct, however, was lower than that on the ‘stop intact’ construct; thus, we carried through both compounds to the final steps of screening.
To evaluate the specificity of apigenin and sulphaquinoxaline to Aqp4 readthrough, we next tested whether these drugs affected readthrough of Mdh1 (which also shows endogenous readthrough) and Actr2 (which does not),8 using TdTomato/GFP reporter constructs, measured by fluorescence-activated cell sorting. Comparison of the apigenin (20 µM) treatment to DMSO controls showed significant and relatively larger increase in the TdTomato/GFP ratio for Aqp4 and Mdh1. Actr2 also showed a very small but significant effect, which suggests this dose of apigenin enhances readthrough of multiple constructs, with a magnitude dependent on sequence (Supplementary Fig. 3A–C). Because the sulphaquinoxaline effect was only trending at 10 µM in the fluorescence-activated cell sorting assay and had not been yet evaluated across doses, we examined concentrations of 2 µM, 10 µM, 50 µM, 250 µM, 500 µM and 1 mM. We saw increased readthrough in all constructs starting from concentrations between 10 and 250 µM, which suggests sulphaquinoxaline may enhance readthrough at all constructs, but the effective dose depends on sequence (Supplementary Fig. 3D–F).
A bona fide modulator should work not just on the cloned fragments of Aqp4 in our reporters, but also on the endogenous transcript. Thus, we treated primary mouse astrocytes with compounds and quantified the levels of C-terminally extended AQP4 (AQP4X), using a dot blot assay with an antibody that specifically recognizes this variant.8 We observed that apigenin significantly upregulated the expression of AQP4X, and sulphaquinoxaline showed a similar but non-significant trend (Fig. 3K). These results confirm that apigenin, and possibly sulphaquinoxaline, increases the biosynthesis of readthrough version of endogenous AQP4 in live cells and is a strong candidate for to enhance AQP4 readthrough in vivo.
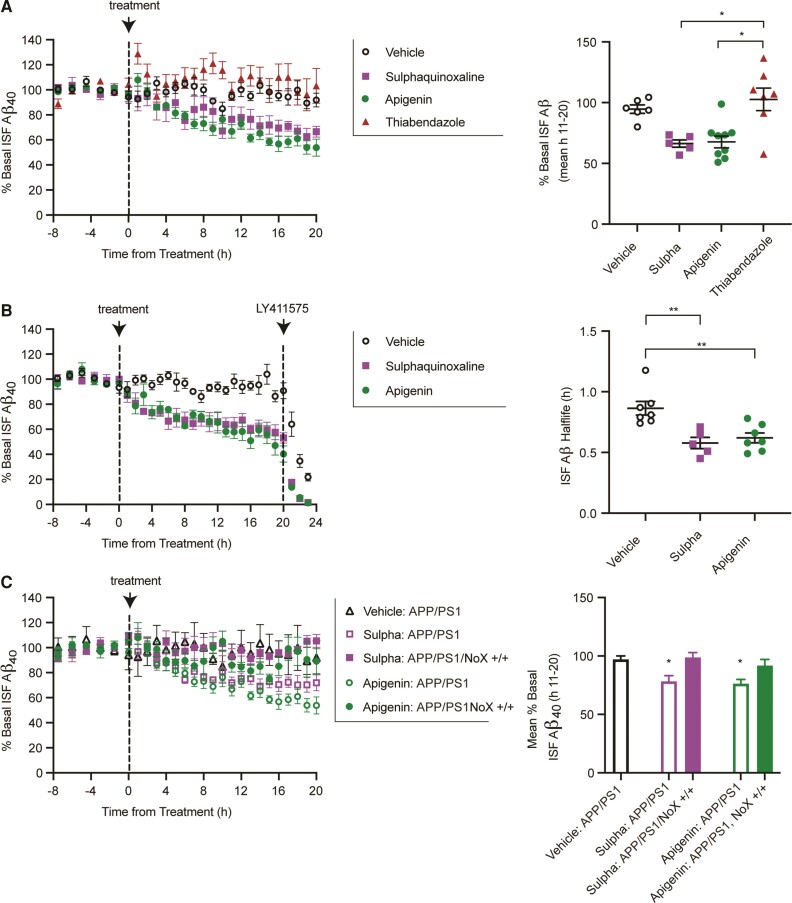
Readthrough enhancers promote decrease Aβ in vivo in a AQP4X dependent manner
Finally, we infused both compounds, as well as vehicle and a negative control Thiabendazole (a candidate ruled out in the first counterscreen) directly and continuously into the hippocampus of APP/PS1 mice by reverse microdialysis. Brain ISF Aβ levels were measured every 60–90 min for 7.5 h before drug and 20 h during drug administration. Over the trial, we observed a steady decrease in Aβ in the apigenin and sulphaquinoxaline treated mice (Fig. 4A). Thiabendazol, however, was not significantly different from vehicle treatment. While this steady decrease was consistent with increased clearance via increased readthrough, we could not rule out that the drugs were instead influencing production somehow. Therefore, we replicated drug administration in a separate set of animals. After 20 h of treatment with apigenin, sulphaquinoxaline or vehicle, mice were administered a γ-secretase inhibitor to block Aβ generation, thus allowing assessment of the elimination half-life of existing ISF Aβ. Mice treated with apigenin or sulphaquinoxaline had a significantly faster elimination half-life of Aβ than mice treated with vehicle, suggesting the drugs indeed worked by increasing clearance (Fig. 4B). Finally, we repeated drug administration in in APP/PS1; Aqp4No_X mice. Importantly, the drugs completely lost effect in mutants without the ability to produce AQP4X (Fig. 4C), indicating the ability of the drugs to alter Aβ levels required AQP4 readthrough in vivo.
Figure 4.
Compounds lower Aβ in a readthrough dependent manner. (A) Continuous reverse microdialysis administration of sulphaquinoxaline (200 μM) and apigenin (100 μM) reduces hippocampal ISF Aβ in APP/PS1 mice over a 20 h period, while a non-readthrough promoting compound, thiabendazole (400 μM), does not (left). Concentrations vary based on in vitro potency. Sulphaquinoxaline and apigenin reduce ISF Aβ by 29.9 ± 3.2 and 27.3 ± 5.2%, respectively, compared to vehicle-treated mice (n = 6) (right). In contrast, thiabendazole was not significantly different from vehicle, but significantly higher than the other treatment groups (mean over hours 11–20 of administration; Kruskal–Wallis test). (B) A separate cohort of APP/PS1 mice was treated with apigenin, sulphaquinoxaline or vehicle by reverse microdialysis for 20 h following by LY411575 (3 mg/kg intraperitoneally). The ISF Aβ elimination half-lives in apigenin and sulphaquinoxaline treated mice were 0.62 ± 0.04 and 0.57 ± 0.47 h, respectively, compared to vehicle-treated mice at 0.86 ± 0.56 h. (C) Sulphaquinoxaline and apigenin do not alter interstitial Aβ in APP/PS1 mice deficient in Aqp4 readthrough. Data presented as mean ± SEM. See Supplementary Table 1 for n and statistical tests. *P = 0.01, **P = 0.001.
Discussion
AQP4 was previously shown to be essential for efficient clearance of Aβ1,3 and presumably other waste products as well.2 Here, using mice that specifically lacked perivascular AQP4X isoform without altering the parenchymal AQP4, we have shown that it is this isoform mediates efficient Aβ clearance. Depending on different experimental approaches, mouse ages and Aβ species, from 50% to a >100% increase in Aβ accumulation is reported in AQP4 global knockout mice in previous studie.1,3 Because we observed a ∼100% increase in soluble Aβ in AQP4X knockout mice, we think that AQP4X is the major, if not the sole, AQP4 variant that removes Aβ, although this should be confirmed with directly comparable approaches. Interestingly, heterozygous mutants also had reduced clearance and elevated baselines, suggesting the relative decrease in perivascular AQP4 (presumably AQP4X) in Alzheimer's disease patients may create a feedforward loop that further exacerbates disease progression. Indeed, APP/PS1 mice showed a similarly disrupted AQP4X/AQP4 ratio. As decreasing AQP4X genetically reduced clearance rates and elevated baseline Aβ, we suspected that instead enhancing programmed stop codon readthrough in Aqp4 would offer an alluring opportunity to enhance clearance. Through a series of screens and counterscreens using two orthogonal reporters and a readthrough-specific antibody, we identified modulators of stop codon readthrough for AQP4 in living cells. While these compounds may serve as useful tools for investigating the molecular mechanisms behind readthrough in Aqp4, more importantly, we demonstrated that they can enhance Aβ clearance in the brain highlighting potential translational applications. Indeed, apigenin has been shown to reduce plaque burden and improve memory function in a preclinical model of Alzheimer's disease,24 although any relationship between this compound and stop codon readthrough was not suspected at that time. Revisiting these studies in the context of Aqp4No_X mice will be essential to determine whether apigenin, sulphaquinoxaline or derivatives thereof can reduce Alzheimer’s pathology in the brain through enhancing AQP4 readthrough. Likewise, it will be important to determine whether these compounds are enhancing AQP4X levels specifically in the perivascular space, or also shifting the localization of AQP4X. Regardless, enhancing readthrough to promote clearance (whether through glymphatics or another mechanism) provides an orthogonal approach to other therapeutic avenues under investigation for Alzheimer's disease. Although these specific compounds may or may not be suitable for therapeutics (sulphaquinoxaline, an antiparasitic in chickens, can crystallize in primate kidney25), they indicate this is a modulatable pathway in vivo. Further, while we have focused here on Aβ clearance, it is possible that enhancing readthrough might equally promote clearance of other proteins involved in neurodegenerative diseases (e.g. tau, synuclein), thus providing a singular broadly applicable therapeutic strategy for diseases involving aggregation in the brain.
Supplementary Material
Acknowledgements
We thank Maxene Ilagan at the High-Throughput Screening Center at Washington University in St. Louis for her technical support with screening; Renate Lewis for genome editing; Hannah Edwards, Chengran Yang, Shayna Mueller and Justine Garin for technical assistance; Gary Loughran for the dual-luciferase plasmid; and members of the J.D.D. laboratory for scientific discussions.
Abbreviations
- Aβ
amyloid beta
- FL =
firefly luciferase
- GFP
green fluorescent protein
- ISF
interstitial fluid
- RL =
Renilla luciferase
Contributor Information
Darshan Sapkota, Department of Psychiatry, Washington University School of Medicine, St. Louis, MO 63110, USA; Department of Genetics, Washington University School of Medicine, St. Louis, MO 63110, USA; Department of Biological Sciences, The University of Texas at Dallas, Richardson, TX 75080, USA.
Colin Florian, Department of Psychiatry, Washington University School of Medicine, St. Louis, MO 63110, USA; Department of Genetics, Washington University School of Medicine, St. Louis, MO 63110, USA.
Brookelyn M Doherty, Department of Neurology, Washington University School of Medicine, St. Louis, MO 63110, USA.
Kelli M White, Department of Psychiatry, Washington University School of Medicine, St. Louis, MO 63110, USA; Department of Genetics, Washington University School of Medicine, St. Louis, MO 63110, USA.
Kate M Reardon, Department of Neurology, Washington University School of Medicine, St. Louis, MO 63110, USA.
Xia Ge, Department of Radiology, Washington University School of Medicine, St. Louis, MO 63110, USA; Intellectual and Developmental Disabilities Research Center, Washington University School of Medicine, St. Louis, MO 63110, USA.
Joel R Garbow, Department of Radiology, Washington University School of Medicine, St. Louis, MO 63110, USA; Intellectual and Developmental Disabilities Research Center, Washington University School of Medicine, St. Louis, MO 63110, USA; Alvin J Siteman Cancer Center, Washington University School of Medicine, St. Louis, MO 63110, USA.
Carla M Yuede, Department of Psychiatry, Washington University School of Medicine, St. Louis, MO 63110, USA.
John R Cirrito, Department of Neurology, Washington University School of Medicine, St. Louis, MO 63110, USA.
Joseph D Dougherty, Department of Psychiatry, Washington University School of Medicine, St. Louis, MO 63110, USA; Department of Genetics, Washington University School of Medicine, St. Louis, MO 63110, USA.
Funding
This work was supported by the NINDS (1R01NS102272), the Mallinckrodt Institute of Radiology, and the Hope Center to J.D.D./J.R.C., NIA (K99AG061231) to D.S., COINS for Alzheimer’s Research, Rotary Club International to J.R.C. and NIA (R01AG064902) to J.R.C.
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4:147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mestre H, Hablitz LM, Xavier AL, et al. Aquaporin-4-dependent glymphatic solute transport in the rodent brain. eLife. 2018;7:e40070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xu Z, Xiao N, Chen Y, et al. Deletion of aquaporin-4 in APP/PS1 mice exacerbates brain Aβ accumulation and memory deficits. Mol Neurodegener. 2015;10:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alkalaeva EZ, Pisarev AV, Frolova LY, Kisselev LL, Pestova TV. In vitro reconstitution of eukaryotic translation reveals cooperativity between release factors eRF1 and eRF3. Cell. 2006;125:1125–1136. [DOI] [PubMed] [Google Scholar]
- 5. Firth AE, Brierley I. Non-canonical translation in RNA viruses. J Gen Virol. 2012;93:1385–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dunn JG, Foo CK, Belletier NG, Gavis ER, Weissman JS. Ribosome profiling reveals pervasive and regulated stop codon readthrough in Drosophila melanogaster. eLife. 2013;2:e01179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Loughran G, Chou MY, Ivanov IP, et al. Evidence of efficient stop codon readthrough in four mammalian genes. Nucleic Acids Res. 2014;42:8928–8938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sapkota D, Lake AM, Yang W, et al. Cell-type-specific profiling of alternative translation identifies regulated protein isoform variation in the mouse brain. Cell Rep. 2019;26:594–607.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Loughran G, Jungreis I, Tzani I, et al. Stop codon readthrough generates a C-terminally extended variant of the human vitamin D receptor with reduced calcitriol response. J Biol Chem. 2018;293:4434–4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schueren F, Lingner T, George R, et al. Peroxisomal lactate dehydrogenase is generated by translational readthrough in mammals. eLife. 2014;3:e03640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Bellis M, Pisani F, Mola MG, et al. Translational readthrough generates new astrocyte AQP4 isoforms that modulate supramolecular clustering, glial endfeet localization, and water transport. Glia. 2017;65:790–803. [DOI] [PubMed] [Google Scholar]
- 12. Yang J, Lunde LK, Nuntagij P, et al. Loss of astrocyte polarization in the tg-ArcSwe mouse model of Alzheimer’s disease. J Alzheimers Dis. 2011;27:711–722. [DOI] [PubMed] [Google Scholar]
- 13. Zeppenfeld DM, Simon M, Haswell JD, et al. Association of perivascular localization of aquaporin-4 with cognition and Alzheimer disease in aging brains. JAMA Neurol. 2017;74:91. [DOI] [PubMed] [Google Scholar]
- 14. Palazzo C, Buccoliero C, Mola MG, et al. AQP4ex is crucial for the anchoring of AQP4 at the astrocyte end-feet and for neuromyelitis optica antibody binding. Acta Neuropathol Commun. 2019;7:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Palazzo C, Abbrescia P, Valente O, et al. Tissue distribution of the readthrough isoform of AQP4 reveals a dual role of AQP4ex limited to CNS. Int J Mol Sci. 2020;21:1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sakers K, Lake AM, Khazanchi R, et al. Astrocytes locally translate transcripts in their peripheral processes. Proc Natl Acad Sci USA. 2017;114:E3830–E3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Savonenko AV, Xu GM, Price DL, Borchelt DR, Markowska AL. Normal cognitive behavior in two distinct congenic lines of transgenic mice hyperexpressing mutant APPSWE. Neurobiol Dis. 2003;12:194–211. [DOI] [PubMed] [Google Scholar]
- 18. Cirrito JR, May PC, O’Dell MA, et al. In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-β metabolism and half-life. J Neurosci. 2003;23:8844–8853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yuede CM, Wallace CE, Davis TA, et al. Pimavanserin, a 5HT2A receptor inverse agonist, rapidly suppresses Aβ production and related pathology in a mouse model of Alzheimer’s disease. J Neurochem. 2021;156:658–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang JH, Chung TDY, Oldenburg KR. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen. 1999;4:67–73. [DOI] [PubMed] [Google Scholar]
- 21. Linde L, Kerem B. Introducing sense into nonsense in treatments of human genetic diseases. Trends Genet. 2008;24:552–563. [DOI] [PubMed] [Google Scholar]
- 22. Auld DS, Thorne N, Maguire WF, Inglese J. Mechanism of PTC124 activity in cell-based luciferase assays of nonsense codon suppression. Proc Natl Acad Sci USA. 2009;106:3585–3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chowdhury HM, Siddiqui MA, Kanneganti S, Sharmin N, Chowdhury MW, Nasim MT. Aminoglycoside-mediated promotion of translation readthrough occurs through a non-stochastic mechanism that competes with translation termination. Hum Mol Genet. 2018;27:373–384. [DOI] [PubMed] [Google Scholar]
- 24. Zhao L, Wang JL, Liu R, Li XX, Li JF, Zhang L. Neuroprotective, anti-amyloidogenic and neurotrophic effects of apigenin in an Alzheimer’s disease mouse model. Molecules. 2013;18:9949–9965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Seeler AO, Mushett CW, Graessle O, Silber RH. Pharmacological studies on sulfaquinoxaline. J Pharmacol Exp Ther. 1944;82:357–363. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.