Variants in PRDX3 have recently been described to cause autosomal-recessive cerebellar ataxia.1 However, confirmation by an independent second report is still lacking and information on phenotypic variety (in particular, distribution of age of onset) and MRI findings is limited. Here we leverage an updated screening of >3500 ataxia exomes and genomes to identify two novel autosomal-recessive PRDX3 families. Our findings represent an independent confirmation of this novel ataxia gene, suggest a neurodevelopmental component of PRDX3 disease, and a broad range of striking MRI signal changes, which indicate damage also to non-cerebellar neuronal systems in PRDX3 disease.
Genetics
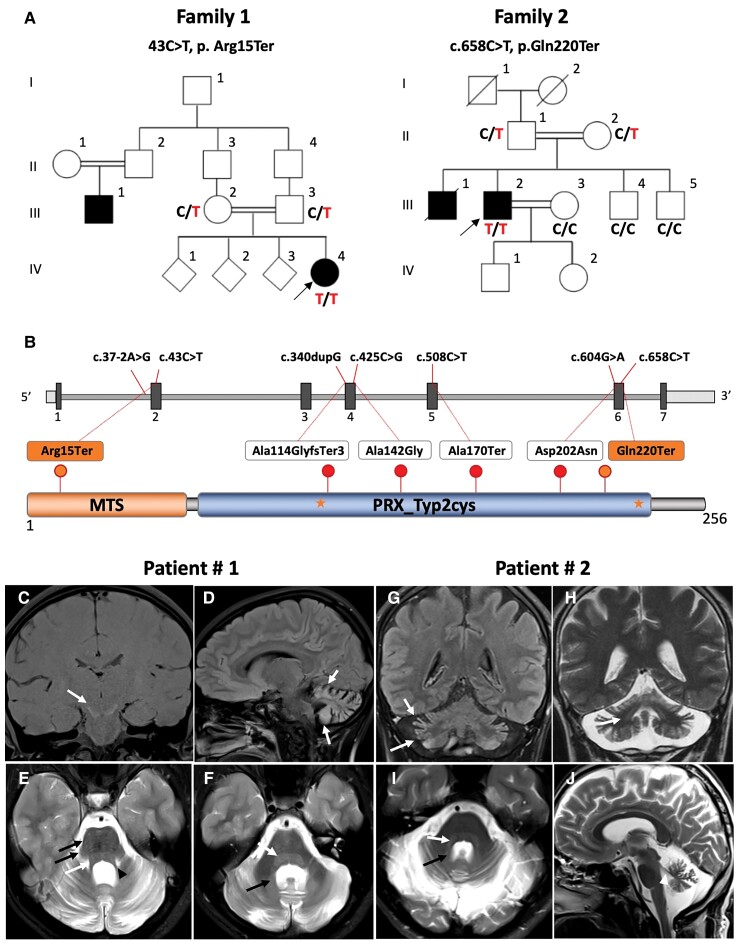
Whole-exome (WES) or whole genome (WGS) sequencing was performed in 3500 families diagnosed with cerebellar ataxia and >20 000 individuals with other, unrelated phenotypes. Sequencing data was analysed with bioinformatics tools provided by the GENESIS genomics platform (PMID: 26173844) and the IMGAG GSvar pipeline (https://github.com/imgag/ngs-bits/tree/master/doc/GSvar).2 Recessive missense variants were analysed using strict filters for allele frequency [minimum allele frequency (MAF)gnomAD < 0.01], high functional prediction [Combined Annotation Dependent Depletion (CADD) > 20] and conservation [Genomic Evolutionary Rate Profiling (GERP) > 3] scores; loss-of-function variants were analysed irrespective of functional and conservation scores. Beyond the previously identified PRDX3 patients,1 we identified two novel unrelated recessive families with novel PRDX3 loss-of-function variants: c.43C>T, p.Arg15Ter, homozygous in a Syrian patient from a consanguineous family with infantile onset cerebellar ataxia; and c.658C<T, Gln220Ter (Fig. 1A and B and Table 1), homozygous in a Turkish patient from a consanguineous family with mid-adult onset (35 years) cerebellar ataxia. PRDX3 variants and segregation analysis were confirmed by Sanger sequencing (Fig. 1A). Both variants are predicted to be ‘pathogenic’ according to the ACMG (American College of Medical Genetics) criteria, including the very strong PVS1 criterium for null variants and the strong PM2 criterium for extreme low allele frequency in population databases. No biallelic PRDX3 variants were observed in any of the other probands’ WES/WGS datasets with unrelated phenotypes, indicating the specificity of the finding of damaging PRDX3 variants to ataxia and thus further supporting their pathogenic relevance.
Figure 1.
Cerebellar ataxia families with novel homozygous loss-of-function variants in PRDX3. (A) Pedigree of families with infantile-onset (Family 1) and mid-adult onset (Family 2) cerebellar ataxia. The index patient of Family 1 is homozygous for PRDX3 variant c.43C>T, p.Arg15Ter. The index patient of Family 2 is homozygous for the variant c.658C<T, Gln220Ter. (B) Diagram illustrates the two novel variants (orange) and the previously reported variants (red) in PRDX3. cDNA variants positions are shown on top and their respective protein change below in the protein domain diagram. Stars represent active site resolving cysteines. (C–J) MRI signal changes in PRDX3 disease. MRI images from the index patient Family 1 (C–F) and index patient Family 2 (G–J). Patient 1: (C) Coronal T1-weighted image shows hyperintensity of the substantia nigra (white arrow). Atrophy and T2-hyperintensity of the cortical grey matter in the cerebellum seen on sagittal T2-fluid-attenuated inversion recovery (FLAIR) (D, white arrows) and on the axial T2-weighted scan (E and F). Also, the superior cerebellar peduncle shows a volume reduction (arrowhead, E). T2 hyperintensity of the formatio reticularis (white arrows in E and F), transversal stripes of the pons (black arrows, E) and T2 hyperintensity of the dentate nucleus (black arrow, I). Patient 2: Coronal T2-FLAIR-weighted image (G) shows hyperintensity of the cortical grey matter in the cerebellum and on the coronal and axial T2-weighted scans (F and G). T2 hyperintensity of the formatio reticularis (white arrow, G) and T2 hyperintensity of the dentate nucleus (white arrow H and I). The superior cerebellar peduncle shows a volume reduction (arrowhead, J).
Table 1.
Phenotypic features and evolution of the PRDX3 patients
| Patient 1 | Patient 2 | |
|---|---|---|
| Demographics | ||
| Current age, years | 18 | 51 |
| Sex | Female | Male |
| Ethnicity | Syrian (Kurdish) | Turkish (nomads; Antalya) |
| Consanguinity | Yes (first degree) | Yes (third degree) |
| Family history | Cousin, ataxia | Brother: cerebellar ataxia with dysarthria (starting at age 30 years), liver cirrhosis (starting at age 39 years; no history of alcohol, negative for Wilson’s disease), death at age 52 years from liver cirrhosis |
| Mode of inheritance | Autosomal recessive | Autosomal recessive |
| Allele 1 | c.43C>T (p.Arg15Ter) | c.658C>T (Gln220Ter) |
| Allele 2 | c.43C>T (p.Arg15Ter) | c.658C>T (Gln220Ter) |
| Chromosome | Chr10:119177147 | Chr10:119169236 |
| Transcript | NM_006793.5 | NM_006793.5 |
| Delayed motor milestones | Yes, first walking age 2 years | Normal |
| Gait ataxia, age in years | 2 | 35 |
| Upper limb ataxia, age in years | 3 | 36 |
| Dysarthria | None | 35 |
| Dysphagia, age in years | 16 | 35 |
| SARA (age in years) | 7.5 (18) | 12 (51) |
| Overall disease severity trajectory (by history) | Improving (slowly) | Worsening (slowly) |
| Age at last exam, years | 18 | 51 |
| Oculomotor signs | No saccadic pursuit, hypometric saccades | Saccadic pursuit, hypermetric saccades |
| Cerebellar dysarthria | No | Yes |
| Hypokinetic features | No | Hypomimia |
| Hyperkinetic features | No | Spontaneous startling reactions |
| Pyramidal signs | No | No |
| Muscle weakness | No | No |
| Sensory impairment | No | No |
| Cognitive impairment | No | No |
| MRI | Cerebellar atrophy, T2 hyperintense signals cerebellum and brainstem | Cerebellar atrophy, T2 hyperintense signals cerebellum and brainstem |
No = absent; SARA = Scale for the Assessment and Rating of Ataxia; Yes = present.
Phenotypic spectrum
Patient 1 (Family 1, IV-4) is a female born in Syria from first degree consanguineous parents. She showed congenital, infantile onset, cerebellar ataxia with motor developmental delay, with independent walking starting not before age 2 years. Cerebellar ataxia was manifest by gait ataxia from early on (i.e. from age 2 years onwards) and progressed to upper limb ataxia from age 3 years and to dysphagia from age 16 years onwards, suggesting a caudal-to-rostral spatial spread of disease. She does not show cognitive impairment, cerebellar dysarthria or neither hypokinetic or hyperkinetic features at current age of 18 years [current score Scale for the Assessment and Rating of Ataxia (SARA): 7.5/40 points]. One distant cousin (Family 1, III-1)—also from consanguineous parents—likewise showed a childhood-onset ataxia, with moderate disability at the current age of 40 years, but no DNA was available from this person for segregation analysis.
Patient 2 (Family 2, III-2) was born in a Turkish family of nomadic origin in a village of Antalya. The parents are from the same village and remotely related (third degree cousins). Development was normal, and patient undertook coordinatively highly demanding military service at the age of 20–22 years. He developed gait ataxia at age 35 years, followed by cerebellar dysarthria at age 36 years, with a SARA score of 13/40 points at the current age of 51 years. Neither of the two index cases showed clinical signs of upper or lower motor neuron involvement or typical mitochondrial disease features like e.g. sensory neuropathy, ptosis, hypoacusis, epilepsy, diabetes or muscle weakness.
The older brother of Patient 2 (Family 2, III-1) likewise showed a mid-adult cerebellar ataxia, including ataxic gait and cerebellar dysarthria, starting at age 30 years. He died at age 52 years due to kryptogenic (non-ethyltoxic, non-Wilson’s disease) liver cirrhosis, which had started at age 39 years, raising the possibility that liver dysfunction might be part of the disease spectrum, but no DNA was available from this individual for segregation analysis.
MRI spectrum
MRI images showed a remarkable broad range of signal changes in both patients (Fig. 1C–J), comprising T2-hyperintense cortical grey matter in the cerebellum, T1-hyperintense substantia nigra (e.g. from iron or calcification), T2-hyperintense formatio reticularis and dentate nucleus in both patients, plus transversal stripes of the pons in Patient 1. All of these changes were in addition to cerebellar atrophy, including a marked volume reduction of the superior cerebellar peduncle in both patients (Fig. 1C–J).
Our findings not only confirm PRDX3 mutations as a recurrent, albeit rare, cause of cerebellar ataxia, but expand the mutational landscape, clinical phenotype and MRI spectrum of PRDX3-associated ataxia, providing new insights into PRDX3 disease. In particular, they suggest that PRDX3 disease can not only have a ‘neurodegenerative’ component (as reported by Rebelo et al.1) but also a ‘neurodevelopmental’ component, observed in Family 1, which is highly relevant for both the neurobiological understanding of the disease as well as developing and initiating future mechanistic disease-modifying treatments. This is of high importance, as a series of treatment options are on the horizon for such mitochondrial diseases with decreased mitochondrial respiratory capacity and increased reactive oxygen species (as shown for PRDX31), e.g. neuronally targeted antioxidant treatments.3
Our study substantially extends the MRI spectrum of PRDX3 disease. While a previous study had already indicated that symmetric T2-hyperintense signal changes might be observed in PRDX3 disease, these had only been found in two out of five individuals and were limited to the medullary olives.1 Our findings now demonstrate that symmetric T2-hyperintense signal changes are indeed a recurrent feature of PRDX3 disease and can include the substantia nigra, formatio reticularis, dentate nucleus and cerebellar cortex. While the T2 signal changes in the cerebellar cortex and dentate nucleus might be related to atrophy, a striking hyperintense T2 signal is not a common finding in hereditary degenerative ataxias.4 Also, the T2 signal changes in the substantia nigra and formation reticular might be more related to changes secondary to metabolic changes, as often seen in mitochondrial diseases, in particular Leigh and Leigh-like syndromes.
These MRI findings suggest that neuronal damage in PRDX3 disease also extends to extra-cerebellar neuronal systems. In line with previous findings,1 our report confirms, however, that classical ‘clinical’ signs of a mitochondrial disease are rare in PRDX3 disease (as often the case in nuclear-encoded mitochondrial diseases). While it remains speculative whether fatal liver disease—a recurrent feature in both mtDNA and nuclear-encoded mitochondrial diseases5–7—in the affected family member of Family 2 was due to PRDX3 variants, future studies should look deeper into liver functioning in PRDX3 patients and disease models to test for potential PRDX3-related liver dysfunction.
The novel null (nonsense) variants we report in PRDX3 support protein loss-of-function as a mechanism of the disease as reported in our previous work.1 All PRDX3 variants reported to date act via loss-of-function, including the missense variants, since the mutant proteins are absent in patients’ fibroblasts, leading to complete loss of the enzyme. Thus, the phenotypic variability, including the broad age of onset as observed here (from 1 to 35 years), cannot be explained by the patients’ genotypes. Given that PRDX3 functions exclusively in the mitochondria, this clinical variability might be attributed to the heterogeneous nature of mitochondrial composition in different tissues and individuals.
Acknowledgements
We thank Rebecca Buchert, Institute of Medical Genetics and Applied Genomics, University of Tübingen, for technical execution of the Sanger segregation analysis in Family 1.
Contributor Information
Adriana P Rebelo, Dr. John T. Macdonald Foundation Department of Human Genetics, John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, Miami, USA.
Benjamin Bender, Department of Diagnostic and Interventional Neuroradiology, University of Tübingen, Tübingen, Germany.
Tobias B Haack, Institute of Medical Genetics and Applied Genomics, University of Tübingen, Tübingen, Germany; Centre for Rare Diseases, University of Tübingen, Tübingen, Germany.
Stephan Zuchner, Dr. John T. Macdonald Foundation Department of Human Genetics, John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, Miami, USA.
A Nazli Basak, Koc University, School of Medicine, Department of Molecular Biology, KUTTAM-NDAL, Istanbul, Turkey.
Matthis Synofzik, Research Division Translational Genomics of Neurodegenerative Diseases, Hertie-Institute for Clinical Brain Research and Center of Neurology, University of Tübingen, Tübingen, Germany; German Center for Neurodegenerative Diseases (DZNE), University of Tübingen, Tübingen, Germany.
Data availability
Data underlying this study are available upon reasonable request.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) No 441409627, as part of the PROSPAX consortium under the frame of EJP RD, by the European Joint Programme on Rare Diseases, under the EJP RD COFUND-EJP No. 825575 (to M.S., N.B, and as an associated partner to S.Z.) as well as to T.B.H. (418081722, 433158657); by the Clinician Scientist programme ‘PRECISE.net’ funded by the Else Kröner-Fresenius-Stiftung (to M.S. and T.H.); the European Union’s Horizon 2020 research and innovation program grant 779257 ‘Solve-RD’ (M.S); and NINDS (2R01NS072248-11A1 to S.Z.).
Competing interests
The authors report no competing interests.
References
- 1. Rebelo AP, Eidhof I, Cintra VP, et al. Biallelic loss-of-function variations in PRDX3 cause cerebellar ataxia. Brain. 2021;144:1467–1481. [DOI] [PubMed] [Google Scholar]
- 2. Falb RJ, Muller AJ, Klein W, et al. Bi-allelic loss-of-function variants in KIF21A cause severe fetal akinesia with arthrogryposis multiplex. J Med Genet. Published online 5 November 2021. doi: 10.1136/jmedgenet-2021-108064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jiang Q, Yin J, Chen J, et al. Mitochondria-targeted antioxidants: a step towards disease treatment. Oxid Med Cell Longev. 2020;2020:8837893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cocozza S, Pontillo G, De Michele G, et al. Conventional MRI findings in hereditary degenerative ataxias: a pictorial review. Neuroradiology. 2021;63:983–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee WS, Sokol RJ. Liver disease in mitochondrial disorders. Semin Liver Dis. 2007;27:259–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gopan A, Sarma MS. Mitochondrial hepatopathy: respiratory chain disorders- ‘breathing in and out of the liver’. World J Hepatol. 2021;13:1707–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stewart JD, Horvath R, Baruffini E, et al. Polymerase gamma gene POLG determines the risk of sodium valproate-induced liver toxicity. Hepatology. 2010;52:1791–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data underlying this study are available upon reasonable request.