Abstract
Background
This study quantifies the mortality attributable to Candida bloodstream infections (BSI) in the modern era of echinocandins.
Methods
We conducted a retrospective cohort study of adult patients admitted to Barnes Jewish Hospital, a 1368-bed tertiary care academic hospital, in Saint Louis, Missouri, from 1 February 2012 to 30 April 2019. We identified 626 adult patients with Candida BSI that were frequency-matched with 6269 control patients that had similar Candida BSI risk-factors. The 90-day all-cause mortality attributable to Candida BSI was calculated using three methods—propensity score matching, matching by inverse weighting of propensity score, and stratified analysis by quintile.
Results
The 90-day crude mortality was 42.4% (269 patients) for Candida BSI cases and 17.1% (1083 patients) for frequency-matched controls. Following propensity score-matching, the attributable risk difference for 90-day mortality was 28.4% with hazard ratio (HR) of 2.12 (95% confidence interval [CI], 1.98–2.25, P < .001). In the stratified analysis, the risk for mortality at 90 days was highest in patients in the lowest risk quintile to develop Candida BSI (hazard ratio [HR] 3.13 (95% CI, 2.33–4.19). Patients in this lowest risk quintile accounted for 81(61%) of the 130 untreated patients with Candida BSI. Sixty-nine percent of untreated patients (57/83) died versus 35% of (49/127) of treated patients (P < .001).
Conclusions
Patients with Candida BSI continue to experience high mortality. Mortality attributable to Candida BSI was more pronounced in patients at lowest risk to develop Candida BSI. A higher proportion of these low-risk patients went untreated, experienced higher mortality, and should be the target of aggressive interventions to ensure timely, effective treatment.
Keywords: Candida, mortality, fluconazole, amphotericin, echinocandin
In the modern era of echinocandins, this study found the crude and attributable mortality of Candidabloodstream infections remain high—42% and 28%, respectively. Patients at the lowest risk of developing infection were found to have the highest attributable mortality.∗
Candida species are the second most common group of pathogens causing healthcare-associated bloodstream infections (BSI) and the most common etiology of fungal BSI in the United States [1]. The crude mortality of Candida BSI has been cited as high as 70%, but crude mortality is a poor measurement of the impact of Candida BSI on mortality. Patients at highest risk to develop Candida BSI frequently have comorbid medical conditions that are also associated with high mortality rates and poor long-term survival [2–4].
Several studies have quantified mortality attributable to Candida BSI with estimates ranging from 15% to 49% [5–8]. These prior studies, conducted before the development of echinocandins, provided useful insights but were limited by sample size (88–108 cases) [5–7] or by a larger encounter-level data set that, by design, would underestimate attributable mortality [8]. The wide range of estimates suggests this debate over the mortality attributable to Candida BSI remains unsettled. Our study aims to provide additional data and perspective to the mortality attributable to Candida BSI using a rich, clinical dataset and propensity score methodology to minimize the confounding that is inherent to observational studies.
Methods
Study Population
This study was conducted at Barnes-Jewish Hospital (BJH), a 1368-bed tertiary care academic hospital located in St. Louis, Missouri, and approved by the Washington University School of Medicine Research Protection Office with a waiver of informed consent. All patients 18 years of age or older with Candida spp. isolated from a blood culture from 1 February 2012 through 30 April 2019 were eligible for inclusion in the study as Candida BSI case patients. The first positive blood culture with Candida spp. was deemed the index blood culture. The time of collection of the index blood culture was considered the index time for all time-related variables and outcomes for Candida BSI cases.
Candidates for the control comparison group were selected from the population of inpatient and observation patients from 1 February 2012 through 30 April 2019 who did not develop Candida BSI. The control subjects were identified as having at least one factor associated with increased risk of Candida BSI identified by ICD-9/10 diagnosis or procedure codes (Table 1, codes in Supplementary Table 1). The conditions imparting similar risk to develop Candida BSI were arranged to form 10 categorical groups. These well-established risk factors included diagnosis of hematologic malignancy, myelodysplastic syndrome (MDS), and/or BMT recipient; metastatic cancer; presence of a central venous catheters (CVC) and/or hemodialysis (HD) catheters; SOT recipient; pancreatitis, abdominal fistulas, or abdominal surgery; cardiac surgery; chronic liver disease; drug abuse; acute renal failure; and bacteremia [2, 4]. Other classic risk factors such as intensive care unit (ICU) admission and antibiotic exposure were not considered in favor of conditions that impart risk to develop Candida BSI in the population (eg, presence of CVC in ICU patients) and underlying diagnoses resulting in antibiotic exposure (eg, bacteremia, immunosuppression), and our selected conditions are present in > 95% of cases, adequately capturing the at risk group.
Table 1.
Demographics and Medical Comorbidities of Candida BSI Case Patients and Frequency-Matched Control Patients
| Characteristic | Candida Cases N = 626 n (%) | Matched Controls N = 6269 n (%) |
|---|---|---|
| Age, median (IQR) years | 58 (46–66) | 61 (52–70) |
| Sex | ||
| Female | 268 (42.8) | 2952 (47.1) |
| Race | ||
| White | 437 (69.8) | 4603 (73.4) |
| Black | 146 (23.3) | 1343 (21.4) |
| Other | 43 (6.9) | 323 (5.2) |
| Hierarchical categoriesa of medical conditions (hierarchical rank) | ||
| Leukemia, myelodysplastic syndrome, bone marrow transplant (1) | 143 (22.8) | 1416 (22.6) |
| Central venous catheter, dialysis catheter (2) | 338 (54.0) | 3379 (53.9) |
| Solid organ transplant (3) | 3 (0.5) | 30 (0.5) |
| Lymphoma, metastatic cancer (4) | 40 (6.4) | 408 (6.5) |
| Fistula, pancreatitis, abdominal surgery (5) | 35 (5.6) | 368 (5.8) |
| Bacteremia (6) | 24 (3.8) | 233 (3.7) |
| Cardiac surgery (7) | 8 (1.3) | 80 (1.3) |
| Chronic liver disease (8) | 18 (2.9) | 189 (3.0) |
| Drug abuse (9) | 6 (1.0) | 60 (1.0) |
| Acute renal failure (10) | 11 (1.7) | 107 (1.7) |
The 1:10 frequency-match distribution of the hierarchical categories is not exact due to patients excluded due death occurring within 24 hours of admission.
Abbreviations: BSI, bloodstream infection; IQR, interquartile range.
Candida BSI cases and frequency-matched controls were placed into the first risk factor category for which they had a qualifying diagnosis. They remained in that category without regards to subsequent risk factors. Finally, for the control candidates, if they did not match with a Candida BSI case patient, they could not be bumped to a risk factor category lower in the hierarchy.
Candida BSI case patients and control candidates were assigned to the first category in the hierarchical order for which they had a compatible diagnosis. A frequency match was then performed using a ratio of 1:10 (cases:controls) to create the pool of control candidates for subsequent analyses. For each control patient, a random admission meeting the hierarchical category assignment was selected as the index hospitalization. Within that index hospitalization, a reference date was randomly chosen to replicate the distribution of first positive culture dates of the Candida BSI cases using a negative binomial distribution. (Supplementary Figure 1) If the date fell outside a control patient’s hospitalization, the negative binomial distribution was resampled until a date within the hospitalization occurred. Finally, cases and control patients who died within 24 hours of admission of index hospitalization were excluded due to missing important predictor variables.
Covariates
Demographic characteristics, comorbidities, surgical history, microbiological data, vital signs, laboratory values, and antimicrobial administration data were obtained from the BJH Clinical Data Repository and explored for potential covariates. The complete list of covariates is listed in Supplementary Table 2.
Comorbidities were identified during the index hospitalization or in the preceding year. ICD-9/10-CM diagnosis codes were used to identify standard comorbidities [9], malignancies, receipt of SOT, receipt of BMT, concomitant infectious diseases, and National Health Safety Network (NHSN) surgical codes (codes provided in Supplementary Table 1).
Laboratory values and vital signs measured during the 24 hours preceding the index time (collection time of the index blood culture or derived control index time) considered for potential covariates included: parameters from complete blood counts (CBC), complete metabolic panels (CMP) and the extremes of the following vital signs: highest temperature, highest respiratory rate, highest heart rate, and lowest mean-arterial blood pressure. Cases with untreated Candida BSI were identified by medical record review.
Outcomes
Ninety-day all-cause mortality was the primary outcome. Mortality beyond 90 days is unlikely to be related to the index Candida BSI, whereas shorter durations (eg, 30 days) miss related mortality events. Thirty-day all-cause mortality was a secondary outcome to allow for comparison to previously published literature. The date of death was obtained from the medical record. Those who did not have a death date and did not have a follow-up in our electronic medical record after 90 days were supplemented with data from the Social Security Death Index [10].
Statistical Analysis
For bivariate analyses, categorical variables were evaluated with χ2 tests; continuous variables were evaluated with Student tor Mann-Whitney U tests, as appropriate.
To determine the attributable mortality of Candida BSI, a propensity score for the risk to develop Candida BSI was developed using multivariable logistic regression with Candida BSI as the dependent variable and all potential predictors for developing Candida BSI or mortality included in the model as independent variables, regardless of significance [11]. Continuous variables were explored with splines. The logit of the propensity score was used to match cases and controls in a 1:1 ratio, using a greedy algorithm without replacement and caliper of 0.2 times the standard deviation (SD) of the logit of the propensity score [12]. Balance of covariates was assessed using standardized differences (SDs) before and after matching (Supplementary Table 2), with SDs of 0.1 or greater indicating imbalance [13].
McNemar’s test was used to compare mortality in the propensity score-matched pairs. Hazard ratios (HR) for mortality were calculated for Candida BSI cases, and their propensity score-matched controls using a Cox-proportional-hazards model stratified by the matched pairs, with Candida BSI as the only independent variable. The mortality attributable to Candida BSI was calculated by subtraction of the mortality rate of the propensity score-matched controls from the mortality rate of the cases. Cox proportional hazards models were performed with a robust variance estimator to account for the matching [14], with calculation of 95% confidence intervals (CI).
A second analysis was performed using a Cox proportional-hazards model with inverse weighting by the propensity score and Candida BSI as the sole variable [14]. The population was trimmed by removing the top and bottom 2% of the propensity score distribution to account for instability of weights at the ends of the distribution.
A third analysis was performed by stratification by the propensity score. The quintiles of the propensity score for the Candida BSI cases were used and applied to the controls, resulting in variable numbers of control patients per stratum. Analysis of mortality in the five strata was performed using Cox proportional-hazards models with Candida BSI as the sole independent variable. A Cox proportional-hazards model was created for each quintile and hazard ratios were reported for each quintile separately. A sensitivity analysis was conducted using adjusted models that included covariates with the largest standardized differences in each model (Supplementary Table 2).
All analyses were performed using SAS, version 9.4 Software (SAS Institute. Cary, North Carolina, USA). All statistical tests were 2-tailed, and P < .05 was considered statistically significant.
Results
In total, 634 patients with Candida BSI were identified with 6340 frequency-matched controls. Eight cases (1%) and 71 controls (1%) died within 24 hours of admission to the index hospitalization and were excluded from analysis. Age, sex, and race were similar between both groups (Table 1).
Table 1 shows the distribution of Candida BSI case patients with frequency-matched control patients amongst the hierarchical categories. The first 2 hierarchical categories accounted for the majority of patients for both cases and controls (Leukemia/MDS/BMT 22.8% and 22.6%, CVC/HD 54% and 53.9%, respectively). The distribution of other medical conditions identified as risk factors for Candida BSI are shown in Supplementary Table 3.
In total, 83 additional risk factors were identified and used in the propensity score analysis. The standardized differences of covariates and distribution of propensity scores before and after weighting are shown in Supplementary Table 2 and Supplementary Figure 2. The propensity score model had good balance of covariates; there was a single covariate out of 93 with an SD > .1 (SOT recipient, SD = .1275), suggesting a well-balanced model.
Mortality
The 90-day crude all-cause mortality for patients with Candida BSI was 269/626 (42.4%) compared to 1083/6269 (17.1%) for the frequency-matched controls (P < .001) with a crude mortality rate difference of 25.3%. The median time to death was 41 days for cases with Candida BSI vs 64 days for controls (P < .001). The 30-day crude mortality for patients with Candida BSI was 156/626 (24.6%) compared to 729/6269 (11.5%) for the control group (P < .001) with a crude mortality rate difference of 13.1% (Table 2). Crude morality of Candida BSI case patients by Candida species and antifungal class is shown in Supplementary Tables 4 and 5.
Table 2.
Crude Mortality of Candida BSI Case Patients and Frequency-Matched Control Patients
| Mortality | Candida BSI Cases N = 626 N (%) | Controls N = 6269 N (%) | Crude Mortality Difference (%) |
|---|---|---|---|
| 30 days | 156 (24.6) | 729 (11.5) | 13.1 |
| 90 days | 269 (42.4) | 1083 (17.1) | 25.3 |
Abbreviation: BSI, bloodstream infection.
In the propensity score-matched pairs analysis, the matching was successful in 528 of 626 Candida BSI cases (84.4%). The hazard ratio (HR) for 90-day all-cause mortality associated with Candida BSI was 2.1 (95% CI, 2.0–2.3) with an attributable risk difference of 28.4% compared to the propensity score-matched controls. The 30-day all-cause mortality HR associated with Candida BSI was 1.7 (95% CI, 1.6–1.8) with an attributable risk difference of 20.6% compared to the propensity score-matched controls (Table 3).
Table 3.
Mortality Attributable to Candida BSI From Propensity Score-Matched Analysis
| Propensity-Matched Pairs | ||
|---|---|---|
| Mortality | Hazard Ratio (95% CI) | Attributable Risk Difference, % |
| 30 Days | 1.68 (1.56–1.80) | 20.64 |
| 90 Days | 2.12 (1.98–2.25) | 28.44 |
Abbreviations: BSI, bloodstream infection; CI, confidence interval.
In the analysis using inverse weighting by the propensity score, the HR for 90-day all-cause mortality associated with Candida BSI was 2.1 (95% CI, 2.0–2.3, P < .001) compared to the control population.
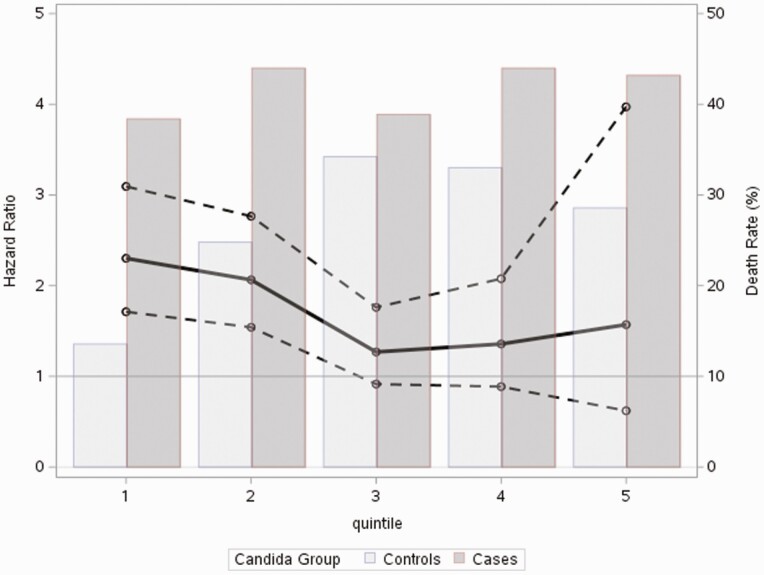
In the stratified analysis, 90-day all-cause mortality for Candida BSI cases was stable across quintiles of risk for developing Candida BSI (range 38.3% to 44%) (Figure 1). Conversely, the 90-day mortality of the control patients increased from 13.6% for the first quintile group to 34.2%, 33%, and 28.6% in quintiles 3 through 5, respectively. The pattern of high crude mortality across all quintiles of risk to develop Candida BSI in Candida case subjects and more variable crude mortality in the control population was also present in the 30-day all-cause mortality analysis (Table 4). The increase in mortality risk at 90 days was highest in patients in the two lowest-risk quintiles (HR 3.13 [95% CI 2.33–4.19] and HR 1.97 [95% CI, 1.46–2.67). The hazard ratios for the third through fifth quintiles were 1.15 (95% CI, .83–1.61), 1.42 (95% CI, .93–2.18), and 1.63 (95% CI, .70–3.79), respectively (Figure 1). Similarly, 30-day mortality was significantly higher in patients with Candida BSI from the 2 lowest-risk quintiles (Table 4). The distribution of hierarchical medical conditions in each quintile is available in Supplementary Table 6.
Figure 1.
Hazard ratio for 90-day all-cause mortality superimposed on death rate (%) per propensity score quintile of Candida BSI risk. Abbreviation: BSI, bloodstream infection.
Table 4.
Comparison of Mortality Rates and Hazard Ratios of 30-day and 90-day Mortality by Propensity Score Quintile With Candida BSI as Only Covariate
| Candida BSI Cases | Control Cases | |||||
|---|---|---|---|---|---|---|
| Baseline Rrisk of Developing Candidemia | Total No. of patients | Mortality | Total No. of Patients | Mortality | Mortality Difference (%) | Hazard Ratio (95% CI) |
| 30-day | ||||||
| First quintile (lowest risk) | 125 | 19.2% | 5088 | 8.7% | 11.0% | 2.35 (1.75–3.15) |
| Second quintile | 125 | 32.0% | 718 | 17.7% | 14.0% | 1.78 (1.32–2.41) |
| Third quintile | 126 | 22.2% | 339 | 23.0% | 0.8% | 1.09 (.78–1.52) |
| Fourth quintile | 125 | 23.2% | 103 | 16.5% | 6.7% | 1.25 (.81–1.91) |
| Fifth quintile (highest risk) | 125 | 21.6% | 21 | 19.1% | 2.3% | 1.25 (.54–2.92) |
| 90-day | ||||||
| First quintile (lowest risk) | 125 | 38.4% | 5088 | 13.6% | 24.8% | 3.13 (2.33–4.19) |
| Second quintile | 125 | 44.0% | 718 | 24.8% | 19.2% | 1.97 (1.46–2.67) |
| Third quintile | 126 | 38.3% | 339 | 34.2% | 4.1% | 1.15 (.83–1.61) |
| Fourth quintile | 125 | 44.0% | 103 | 33.0% | 11.0% | 1.42 (.93–2.18) |
| Fifth quintile (highest risk) | 125 | 43.2% | 21 | 28.6% | 14.6% | 1.63 (.70–3.79) |
Abbreviations: BSI, bloodstream infection; CI, confidence interval.
The same pattern (highest HRs in the lowest propensity score quintiles) was also present in the sensitivity analysis when the covariates with the highest standardized differences were included as covariates in the adjusted model (Supplementary Table 7). Candida BSI was associated with 2.3-fold higher 90-day mortality (95% CI 1.71–3.10) and 1.7-fold higher 30-day mortality (95% CI 1.28–2.34) in the first risk quintile. Similarly, in the second quintile, the 90-day mortality was 2.07 (95% CI 1.52–2.80) and 1.81 (95% CI 1.33–2.45) for 30-day mortality. Candida BSI was not associated with significantly increased risk of 30-day or 90-day all-cause mortality in the upper 3 quintiles in the sensitivity analysis (Table 4, Supplementary Table 7).
Of the 626 Candida BSI cases, there were 32 patients were not treated; 22/32 (68.7%) were in the lowest 2 quintiles of risk to develop Candida BSI (Supplementary Table 8). We analyzed this further using an expanded dataset available from a prior study [15]. There were 130 patients with untreated Candida BSI in the expanded data set out of 1820 cases total. Untreated Candida BSI cases were predominately (81/130, 62%) in the lowest risk quintile for development of Candida BSI (Supplementary Table 8). In the first quintile, 56/81 (69.1%) untreated patients died compared to 364/540 (40.3%) of treated patients (P < .001); the remaining quintiles are presented in Supplementary Table 9. The odds ratio (OR) for mortality of untreated Candida BSI in the first quintile was 3.3 (95% CI 2.0–5.4).
Discussion
Compared with propensity score-matched controls, patients with Candida BSI had 2.1-fold higher 90-day all-cause mortality; the mortality attributable to Candida BSI was 28.4%. This finding was consistent over 3 separate analyses, and similar for 30-day mortality.
Our study, starting with data collected in 2012, was conducted entirely during the era of echinocandin empiric therapy, distinguishing it from most of the other studies conducted [7, 8, 16]. The crude mortality in our study (42.4%) was lower than the mortality found in the landmark University of Iowa studies in 1988 (57%) [7] and 2003 (61%) [6]. These studies were conducted when amphotericin deoxycholate and fluconazole, respectively, were the recommended empiric antifungals. The estimated attributable mortality from the 1988 and 2003 studies were higher (38% and 49%) compared to our study (28.4%) [6, 7]. The decrement observed in attributable mortality is likely multifactorial with potential contributions from the widespread use of echinocandins, more rapid diagnoses and improved supportive care.
Zaoutis et al conducted a propensity score analysis of the 2000 Nationwide Inpatient Sample (NIS), a nationwide sampling of administrative hospital data. They reported a crude mortality of 30.6% and attributable mortality of 14.5% [8]. Both are lower than our study and others [3, 7, 8, 17, 18]. The NIS data set, predictably, underestimates attributable mortality because it does not allow identification of individual patients and recurrent hospitalizations of a single patient are treated as new “patients” [19]. Subsequently, a patient with Candida BSI transferred to a higher level of care or discharged on hospice is counted as having survived. This systematically biases the NIS data set to underreporting mortality.
Similarly, the clinical trials used to obtain Food and Drug Administration (FDA) approval for the echinocandins had relatively low mortality [18, 20–22]. The trials likely underestimated the mortality as they excluded patients at risk for poor outcomes and those who died prior to having an opportunity to be screened for study randomization. Examples include exclusion of neutropenic patients [22], endocarditis/meningitis [18], life expectancy <5 days at randomization [20], and refractory Candida infection [21]. Excluding patients based on these criteria would underestimate both crude and attributable mortality of Candida BSI. Furthermore, the strict inclusion and exclusion criteria of clinical trials are often not reflective of real-world clinical practice, limiting generalizability.
Our study is unique in that we focused on 90-day all-cause mortality, not previously used in studies quantifying mortality attributable to Candida BSI [5–8, 17, 23–26]. The median time to death in Candida BSI cases in our study was 41 days. Protracted hospitalizations observed in Candida BSI patients demonstrate a 30-day endpoint would underestimate mortality. Using in-hospital mortality as a primary endpoint can also underestimate mortality, as demonstrated by the NIS database [19]. Furthermore, using in-hospital mortality limits comparisons between studies as hospitalization and discharge practices vary across medical institutions and healthcare systems. We advocate for use of 90-day all-cause mortality as a primary endpoint use in future studies because it more accurately captures short- and long-term mortality.
Interestingly, the stratified analysis demonstrating the mortality attributable to Candida BSI was highest in those at lowest risk of developing Candida BSI. Of the 130 untreated Candida BSI cases identified in our cohort, 75% (97/130) were in the lowest 2 quintiles of risk with a significantly higher proportion of death. Mischaracterization of Candida BSI as contaminants and unawareness by the treating clinician of the Candida BSI accounted for 91% of untreated cases. We hypothesize that treating clinicians incorrectly adjudicated the risk of Candida BSI in these patients due to fewer traditional risk factors, resulting in more instances of untreated Candida BSI. Predictably, our data showed that there was a significant increase in mortality in patients with untreated Candida BSI, particularly in those at lowest risk. Appropriate and timely treatment of all Candida BSI, even in those deemed low risk, is imperative to lowering mortality. We believe the lowest risk groups should be the focus of future study and subsequent interventions as they likely have the highest potential for mortality reduction. For instance, obtaining Infectious Disease consultation for all patients has been associated with fewer untreated patients and lower mortality [15, 27].
Our study had several limitations. Patients expiring within 24 hours of index blood culture were excluded to avoid deaths occurring in the emergency room or very early in a hospitalization before initiation of treatment. We favored this more conservative approach and subsequent estimation of attributable mortality. These patients accounted for only 1% of cases and controls. Unmeasured confounding is an issue with all observational studies. However, the propensity score was developed from an extensive list of predisposing risk factors for the development of Candida BSI and prognostic comorbidities for death [28, 29], with good balance of the covariates, ensuring comparable case-control matches for the matched-pairs analysis. To adjust for residual confounding, a sensitivity analysis was done by adding variables into the models, with similar results. Finally, the data used in this study was from a single tertiary referral center, limiting the generalizability of our findings.
Overall, Candida BSI was associated with 2.3-fold increased risk of 90-day all-cause mortality. High mortality observed has persisted over decades despite treatment advances, though mortality attributable to Candida BSI has decreased compared to most historical studies. The increased mortality attributable to Candida BSI was more pronounced in patients at low baseline risk to develop Candida BSI, possibly due to lower proportion receiving treatment. It is imperative that clinicians maximize efforts to adhere to guideline recommendations [30, 31] and seek Infectious Disease consultation to assist in timely, efficacious treatment of Candida BSI [15].
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Dr. Carlos Mejia-Chew for his contribution to the collection of the data used in this manuscript.
Financial support. This work was supported by Astellas Global Development Pharma, through an investigator-sponsored grant (grant number MYCA-15L03) to the hosting institution; Washington University Institute of Clinical and Translational Sciences grant from the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1 grant number TR002345); and the Agency for Healthcare Research and Quality (grant number R24 HS19455).
Contributor Information
Patrick B Mazi, Division of Infectious Diseases, Department of Medicine, Washington University in St Louis School of Medicine, St Louis, Missouri, USA.
Margaret A Olsen, Division of Infectious Diseases, Department of Medicine, Washington University in St Louis School of Medicine, St Louis, Missouri, USA.
Dustin Stwalley, Division of Infectious Diseases, Department of Medicine, Washington University in St Louis School of Medicine, St Louis, Missouri, USA.
Adriana M Rauseo, Division of Infectious Diseases, Department of Medicine, Washington University in St Louis School of Medicine, St Louis, Missouri, USA.
Chapelle Ayres, Division of Infectious Diseases, Department of Medicine, Washington University in St Louis School of Medicine, St Louis, Missouri, USA.
William G Powderly, Division of Infectious Diseases, Department of Medicine, Washington University in St Louis School of Medicine, St Louis, Missouri, USA.
Andrej Spec, Division of Infectious Diseases, Department of Medicine, Washington University in St Louis School of Medicine, St Louis, Missouri, USA.
References
- 1. Magill SS, O’Leary E, Janelle SJ, et al. Changes in prevalence of health care-associated infections in U.S. hospitals. N Engl J Med 2018; 379:1732–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kullberg BJ, Arendrup MC. Invasive candidiasis. N Engl J Med 2015; 373:1445–56. [DOI] [PubMed] [Google Scholar]
- 3. Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 62:e1–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pappas PG, Lionakis MS, Arendrup MC, Ostrosky-Zeichner L, Kullberg BJ. Invasive candidiasis. Nat Rev Dis Primers 2018; 4:18026. [DOI] [PubMed] [Google Scholar]
- 5. Cornely FB, Cornely OA, Salmanton-Garcia J, et al. Attributable mortality of candidemia after introduction of echinocandins. Mycoses 2020; 63:1373–81. [DOI] [PubMed] [Google Scholar]
- 6. Gudlaugsson O, Gillespie S, Lee K, et al. Attributable mortality of nosocomial candidemia, revisited. Clin Infect Dis 2003; 37:1172–7. [DOI] [PubMed] [Google Scholar]
- 7. Wey SB, Mori M, Pfaller MA, Woolson RF, Wenzel RP. Hospital-acquired candidemia. The attributable mortality and excess length of stay. Arch Intern Med 1988; 148:2642–5. [DOI] [PubMed] [Google Scholar]
- 8. Zaoutis TE, Argon J, Chu J, Berlin JA, Walsh TJ, Feudtner C. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin Infect Dis 2005; 41:1232–9. [DOI] [PubMed] [Google Scholar]
- 9. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care 1998; 36:8–27. [DOI] [PubMed] [Google Scholar]
- 10. Quinn J, Kramer N, McDermott D. Validation of the social security death index (SSDI): an important readily-available outcomes database for researchers. West J Emerg Med 2008; 9:6–8. [PMC free article] [PubMed] [Google Scholar]
- 11. Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Sturmer T. Variable selection for propensity score models. Am J Epidemiol 2006; 163:1149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 2011; 10:150–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009; 28:3083–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med 2014; 33:1242–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mejia-Chew C, O’Halloran JA, Olsen MA, et al. Effect of infectious disease consultation on mortality and treatment of patients with Candida bloodstream infections: a retrospective, cohort study. Lancet Infect Dis 2019; 19:1336–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anne MR, Halpern MT, Raleigh B. The impact of candidemia on length of hospital stay, outcome, and overall cost of illness. Clin Infect Dis 1998; 27:781–8. [DOI] [PubMed] [Google Scholar]
- 17. Blot SI, Vandewoude KH, Hoste EA, Colardyn FA. Effects of nosocomial candidemia on outcomes of critically ill patients. Am J Med 2002; 113:480–5. [DOI] [PubMed] [Google Scholar]
- 18. Mora-Duarte J, Betts R, Rotstein C, et al. Comparison of caspofungin and amphotericin B for invasive candidiasis. N Engl J Med 2002; 347:2020–9. [DOI] [PubMed] [Google Scholar]
- 19. Khera R, Krumholz HM. With great power comes great responsibility: big data research from the national inpatient sample. Circ Cardiovasc Qual Outcomes 2017; 10:e003846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pappas PG, Rotstein CM, Betts RF, et al. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin Infect Dis 2007; 45:883–93. [DOI] [PubMed] [Google Scholar]
- 21. Reboli AC, Rotstein C, Pappas PG, et al. Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med 2007; 356:2472–82. [DOI] [PubMed] [Google Scholar]
- 22. Rex JH, Bennett JE, Sugar AM, et al. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. Candidemia study group and the national institute. N Engl J Med 1994; 331:1325–30. [DOI] [PubMed] [Google Scholar]
- 23. Hassan I, Powell G, Sidhu M, Hart WM, Denning DW. Excess mortality, length of stay and cost attributable to candidaemia. J Infect 2009; 59:360–5. [DOI] [PubMed] [Google Scholar]
- 24. Gonzalez de Molina FJ, Leon C, Ruiz-Santana S, Saavedra P, CAVA I Study Group. Assessment of candidemia-attributable mortality in critically ill patients using propensity score matching analysis. Crit Care 2012; 16:R105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morgan J, Meltzer MI, Plikaytis BD, et al. Excess mortality, hospital stay, and cost due to candidemia: a case-control study using data from population-based candidemia surveillance. Infect Cont Hosp Epidemiol 2005; 26:540–7. [DOI] [PubMed] [Google Scholar]
- 26. Rentz AM, Halpern MT, Bowden R. The impact of candidemia on length of hospital stay, outcome, and overall cost of illness. Clin Infect Dis 1998; 27:781–8. [DOI] [PubMed] [Google Scholar]
- 27. Lee RA, Zurko JC, Camins BC, et al. Impact of infectious disease consultation on clinical management and mortality in patients with candidemia. Clin Infect Dis 2019; 68:1585–7. [DOI] [PubMed] [Google Scholar]
- 28. Johnston JA, Wagner DP, Timmons S, Welsh D, Tsevat J, Render ML. Impact of different measures of comorbid disease on predicted mortality of intensive care unit patients. Med Care 2002; 40:929–40. [DOI] [PubMed] [Google Scholar]
- 29. Suleyman G, Alangaden GJ. Nosocomial fungal infections: epidemiology, infection control, and prevention. Infect Dis Clin North Am 2016; 30:1023–52. [DOI] [PubMed] [Google Scholar]
- 30. Mellinghoff SC, Hoenigl M, Koehler P, et al. EQUAL candida score: An ECMM score derived from current guidelines to measure quality of clinical candidaemia management. Mycoses 2018; 61:326–30. [DOI] [PubMed] [Google Scholar]
- 31. Nemer S, Imtiaz T, Varikkara M, Collier A, Bal AM. Management of candidaemia with reference to the European confederation of medical mycology quality indicators. Infect Dis 2019; 51:527–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.