Abstract
Classic pharmacological studies suggested that endogenous dynorphin-KOR signaling is important for reproductive neuroendocrine regulation. With the seminal discovery of an interconnected network of hypothalamic arcuate neurons co-expressing kisspeptin, neurokinin B, and dynorphin (KNDy neurons), the KNDy hypothesis was developed to explain how gonadotropin-releasing hormone (GnRH) and luteinizing hormone (LH) pulses are generated. Key to this hypothesis is dynorphin released from KNDy neurons acting in a paracrine manner on other KNDy neurons via kappa opioid receptor (KOR) signaling to terminate neural “pulse” events. While in vitro evidence supports this aspect of the KNDy hypothesis, a direct in vivo test of the necessity of KOR signaling in kisspeptin neurons for proper LH secretion has been lacking. We therefore conditionally knocked out KOR selectively from kisspeptin neurons of male and female mice and tested numerous reproductive measures, including in vivo LH pulse secretion. Surprisingly, despite validating successful knockout of KOR in kisspeptin neurons, we found no significant effect of kisspeptin cell-specific deletion of KOR on any measure of puberty, LH pulse parameters, LH surges, follicle-stimulating hormone (FSH) levels, estrous cycles, or fertility. These outcomes suggest that the KNDy hypothesis, while sufficient normally, may not be the only neural mechanism for sculpting GnRH and LH pulses, supported by recent findings in humans and mice. Thus, besides normally acting via KOR in KNDy neurons, endogenous dynorphin and other opioids may, under some conditions, regulate LH and FSH secretion via KOR in non-kisspeptin cells or perhaps via non-KOR pathways. The current models for GnRH and LH pulse generation should be expanded to consider such alternate mechanisms.
Keywords: kisspeptin, Kiss1, GnRH, KNDy, dynorphin, opioid
In the late 2000s, a number of labs collectively made the seminal discovery that hypothalamic arcuate (ARC) neurons expressing kisspeptin (encoded by Kiss1) also co-express the neuropeptides neurokinin B (NKB) and dynorphin (encoded by Tac2 and Pdyn, respectively). First reported in sheep by the group led by Goodman, Coolen, and Lehman (1), and subsequently followed up by others studying rodents and other species (2–8), these ARC neurons were henceforth termed KNDy (kisspeptin/NKB/dynorphin) neurons (9–11). This fundamental discovery soon gave rise to the KNDy hypothesis of gonadotropin-releasing hormone (GnRH) pulse generation (9, 12), which models how GnRH, and hence luteinizing hormone (LH), pulses are generated by an upstream pulse generator neural mechanism comprised of the KNDy neurons (discussed more below). As a key component of the KNDy hypothesis, neural dynorphin signaling has become regarded as a critical regulator of reproductive neuroendocrine function (2, 3, 13–16). However, even before the KNDy hypothesis, multiple lines of evidence suggested endogenous opioids, including dynorphin, could modulate LH secretion, pubertal development, and reproductive physiology. Early pharmacological studies in rats and sheep demonstrated that blockade of endogenous opioid signaling with receptor antagonists could alter in vivo LH secretion (reviewed in (17)). For example, in prepubertal female rats, peripheral injection of naloxone, an opioid receptor antagonist for the kappa opioid receptor (KOR) and mu opioid receptor (MOR) subtypes, increased circulating LH levels (18), an effect replicated in adult female rats (19), prepubertal and adult sheep (20–23), monkeys (24) and humans (25, 26) These findings were extended in other studies administrating KOR-specific antagonists, such as nor-BNI, peripherally or centrally in different species and eliciting increased LH pulse secretion, suggesting a role for KOR and its primary ligand dynorphin (13–15, 27). This was supported by evidence that exogenous dynorphin treatment is sufficient to suppress in vivo LH pulse secretion (3).
Despite the pharmacological evidence that endogenous dynorphin signaling might act to inhibit LH secretion, the neuroanatomical source of the dynorphin, as well as the specific targets cells expressing KOR that mediate the effect, have not been definitively determined, as both dynorphin and KOR are widely expressed in many hypothalamic nuclei. However, this issue took a step forward with the discovery of KNDy neurons in the ARC. The co-expression of dynorphin with the potent stimulatory reproductive neuropeptides kisspeptin and NKB was even more intriguing owing to the fact that KNDy neurons were found to send reciprocal neuronal projections to each other (28, 29). In support of this, it was shown in both rodents and sheep that KNDy neurons also express the receptors for both NKB and dynorphin (NK3R and KOR, respectively), but not MOR, which could theoretically permit paracrine dynorphin-KOR and NKB-NK3R signaling among KNDy neurons (1, 2, 11, 30–39). These critical findings gave birth to the KNDy hypothesis of GnRH pulse generation which posits that dynorphin and NKB act in a paracrine manner to synchronize KNDy neuronal activity and kisspeptin release that drives downstream GnRH pulses. In this model, NKB is thought to initiate KNDy neuronal activity and kisspeptin secretion via stimulatory NK3R signaling, after which dynorphin terminates KNDy neuronal activity via inhibitory KOR signaling (reviewed in (9, 10, 12, 40, 41)). Each synchronized burst of kisspeptin secretion from KNDy neurons then stimulates GnRH neurons to release a pulse of GnRH that triggers an LH pulse (41–43).
The KNDy hypothesis posits that dynorphin acts on KOR specifically in KNDy neurons to help terminate each kisspeptin, and hence GnRH, pulse. Indirect evidence for this hypothesis comes from classic findings in sheep in which an implant of naloxone localized to the mediobasal hypothalamus, which contains KNDy neurons, facilitated pulsatile LH release (44). Likewise, in goats, dynorphin treatment inhibited and KOR antagonists stimulated volleys of multiunit electrical activity in the mediobasal hypothalamus where KNDy neurons reside; these volleys were suggested to reflect the rhythmic discharge of the neural GnRH pulse generator which is now known to be the KNDy neuron population (3, 45). Currently there is no direct in vivo evidence for a necessary role of dynorphin action on KNDy neurons for LH pulse termination, although in vitro electrophysiology studies in mice show that dynorphin or a KOR agonist can inhibit spontaneous KNDy cell firing in ARC slices from male mice (46, 47), as well as decrease NKB agonist-induced firing of KNDy neurons in some conditions (46). A subsequent in vitro study found that a KOR antagonist increased KNDy neuron electrical activity and indicated that dynorphin may act presynaptically on KNDy neurons to inhibit NKB release (28). A recent study in sheep, studying different stages of LH pulse generation induced by NKB injections, reported increased cellular internalization of KOR in KNDy neurons during both pulse onset and termination. This indirectly suggests that endogenous dynorphin signals via KOR on KNDy neurons during pulse events (48). Collectively, these studies suggest that endogenous dynorphin-KOR signaling in KNDy neurons is involved in the paracrine sculpting of synchronized electrical activity of these neurons to ultimately drive pulsatile GnRH and LH secretion. However, an in vivo functional test of the necessity of KOR signaling in kisspeptin neurons for proper LH secretion has not yet been demonstrated.
In addition to playing a role in GnRH pulse generation, dynorphin-KOR signaling may also modulate the preovulatory GnRH and LH surges that trigger ovulation in females. Classic pharmacological studies using opioid receptor antagonists in rodents and humans suggest that a transient decrease in the endogenous opioid tone may facilitate the generation of the LH surge. This was evidenced based on observations that naloxone treatment both advances the onset of the LH surge and magnifies the surge amplitude (49–51). A more specific treatment with central infusion of KOR antagonist similarly potentiated the LH surge initiation and amplitude in rats (52, 53), and immunological neutralization of endogenous dynorphin action in the preoptic area tended to advance the LH surge (54). These findings suggest that endogenous dynorphin may modulate (inhibit) LH surge generation or magnitude. This is supported by the ability of exogenous dynorphin or a KOR agonist treatment to block the LH surge (55). Where in the brain the dynorphin signal arises from, and on which KOR-expressing target cells it acts to alter the surge, remain to be determined. In rodents, the preoptic kisspeptin population in the rostral periventricular nucleus of the third ventricle (RP3V; also termed the anteroventral periventricular nucleus [AVPV]), is thought to mediate estrogen positive feedback induction of the LH surge (12, 56–58). Interestingly, RP3V kisspeptin neurons receive direct neural input from KNDy neurons, suggesting there might be some dynorphin released by the latter onto the former (56). Moreover, we recently reported that female RP3V kisspeptin neurons themselves express both dynorphin (Pdyn) and KOR (Oprk1) (59). Based on this finding, it has been speculated that dynorphin might possibly suppress the onset of the LH surge in a paracrine manner, similar to how dynorphin-KOR operates in KNDy neurons for terminating LH pulses (17), but this has not yet been tested.
The findings discussed above suggest that endogenous dynorphin-KOR signaling in KNDy neurons—and possibly in RP3V kisspeptin neurons—is involved in the neural regulation of pulsatile and perhaps surge modes of GnRH and LH secretion. However, a direct functional test of the necessity of KOR signaling in kisspeptin neurons for proper in vivo LH secretion and reproductive function has not been reported. In the present study, we address this issue by selectively knocking out KOR from kisspeptin neurons in mice, permitting in vivo functional evaluation of the KOR component of the KNDy hypothesis of LH pulse generation, as well as effects on puberty, the LH surge, and fertility.
Materials and Methods
Animals
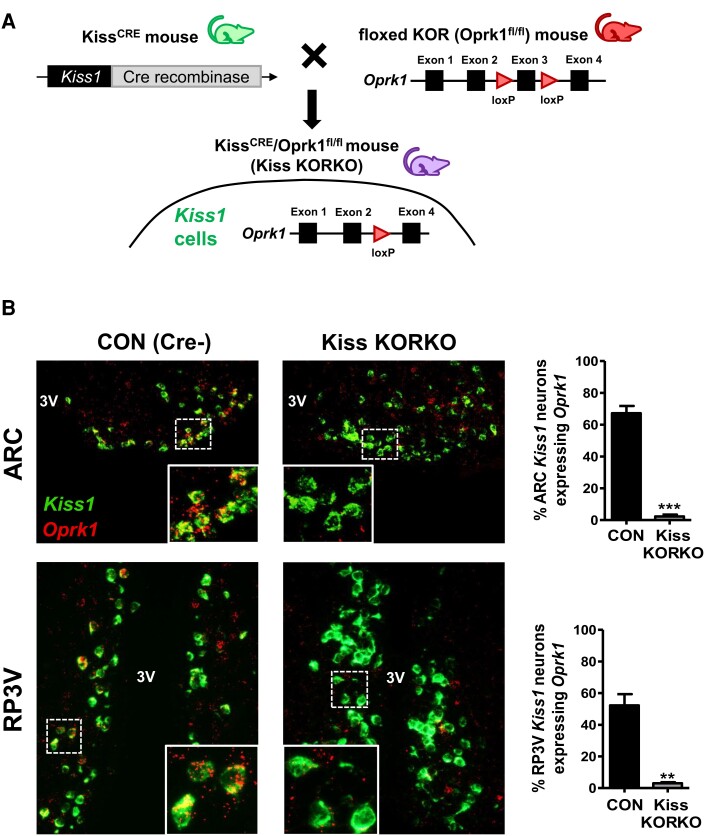
We conditionally deleted KOR from kisspeptin neurons in mice. To do so, we generated KissCre/Oprk1fl/fl mice by crossing a commonly-used Kiss1 Cre mouse line (60–62) (KissCre+; provided by Dr. Carol Elias, University of Michigan) with an established “floxed” KOR mouse line (63, 64) (B6; 129-Oprk1 < tm2.1Kff>/J; Jackson Labs, Stock 030 076) and then backcrossing offspring to produce mice homozygous for the floxed KOR allele (Fig. 1A). The resulting KissCre+/Oprk1fl/fl males and females (termed “Kiss KORKO”) and their KissCre-/Oprk1fl/fl control littermates (termed CON) were used for experiments. Kiss KORKOs have KOR conditionally deleted from Kiss1 cells owing to the expression of Cre recombinase in these cells, whereas CON mice retrain normal KOR expression due to the absence of Cre. All mice were housed in a 12:12 light-dark cycle (lights off at 18:00 hours) with ad libitum access to food and water. Mice were housed in cages of 2 to 3 animals for males and 2 to 4 for females, depending on the experiment. All mice were weaned and weighed on postnatal day (PND) 21, with PND 1 being the day of birth. All mice were genotyped for KissCre and KORflox status via PCR of tail DNA collected at weaning; occasional mice harboring unwanted germline recombination were excluded from study. All animal experiments were approved by the IACUC of the University of California San Diego.
Figure 1.
Generation and validation of transgenic mice with conditional KOR deletion in Kiss1 cells. (A) Schematic representation of crossing KissCRE mice with “floxed” KOR (Oprk1fl/fl) mice to obtain KissCRE/KORfl/fl mice (Kiss KORKO) and control (CON) littermates. (B) Microscope images of Kiss1 (green) and Oprk1 (red) gene expression in the ARC and RP3V brain regions of CON and Kiss KORKO mice. White bordered insets in each image show a magnified zoom of the dotted white box on the main image. Bar graphs on the right depict mean % of Kiss1 neurons co-expressing Oprk1 in the ARC and RP3V for the 2 genotypes. **P < 0.01; ***P < 0.001.
RNAscope In Situ Hybridization Validation of KOR Deletion From Kisspeptin Neurons
RNAscope fluorescent in situ hybridization was used to assess successful deletion of KOR from kisspeptin neurons. Brains were collected fresh frozen from adult Kiss KORKO and CON female mice (n = 3/genotype) and stored at −80 °C. Additional brains from 1 adult male of each genotype were similarly collected. Frozen brains were cut on a cryostat directly onto Superfrost plus microscope slides in 5 alternating sets of 20 µm. Brian sections comprising the ARC and RP3V regions (where kisspeptin neurons are located) was assayed via double-label in situ hybridization for Kiss1 mRNA and Oprk1 mRNA using the RNAscope Multiplex Fluorescent Reagent Kit v2 (Catalog No. 323100, Advanced Cell Diagnostics) and following the manufacturer's protocol. For this assay, we used a standard Kiss1 probe (Cat. No. 500141, Advanced Cell Diagnostics) and a custom designed probe targeting bp 427-857 in exon 3 of the murine Oprk1 gene (Advanced Cell Diagnostics). This permitted analysis of whether exon 3 of the Oprk1 gene was successfully knocked out in kisspeptin cells of KissCre+ mice compared to CON mice with KOR still expressed in kisspeptin cells. After the assay was completed, slides were analyzed under fluorescent microscopy for co-expression of Opkr1 in KNDy and RP3V kisspeptin neurons by an observer blind to genotype. The percentage of Kiss1 neurons co-expressing Oprk1 in each region was determined by adapting a validated computer-assisted microscopy-linked counting software (GRAINS) (65–67), which quantifies cell number, mRNA staining intensity, threshold, and background in each image to objectively identify co-labeled cells, defined as having signal-to background ratios and grains per cell values >5. Data are presented as the mean percentage of identifiable Kiss1 neurons in each brain region that positively co-express Oprk1.
Puberty Analyses
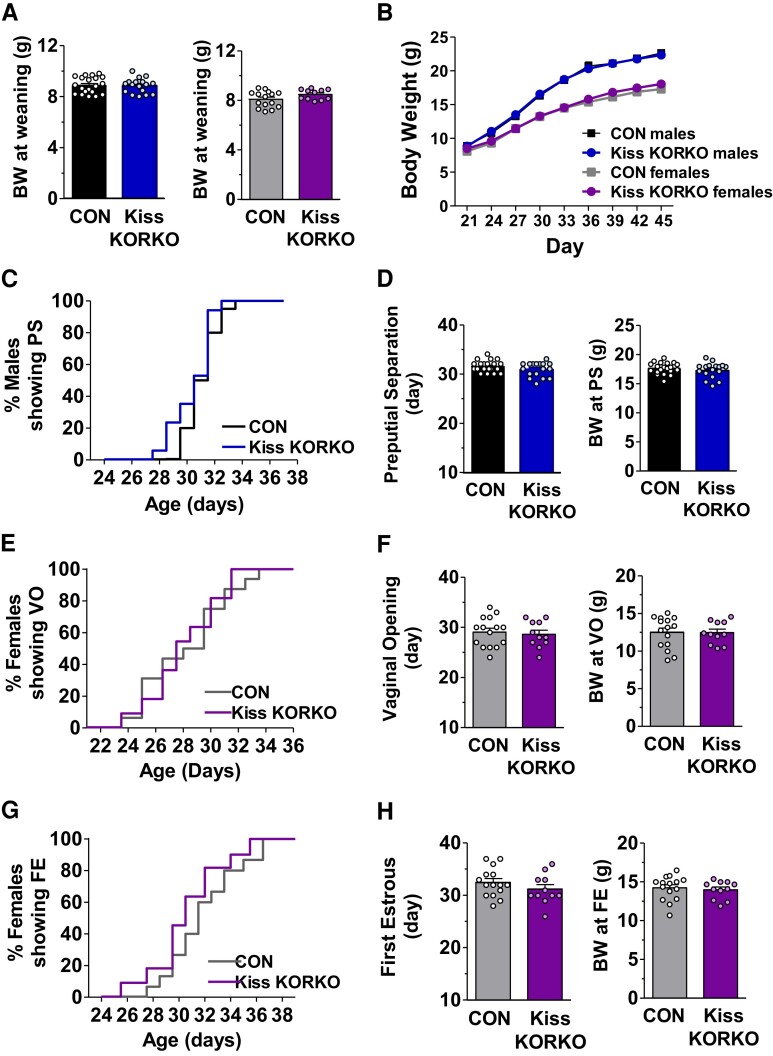
A hallmark of puberty onset is the emergence of GnRH pulse secretion that drives LH and follicle-stimulating hormone (FSH) secretion to govern the gonads. Since kisspeptin neurons, and KNDy neurons in particular, are thought to play an important role in the timing of puberty onset, not to mention comprising the GnRH pulse generator, we determined whether absent KOR signaling selectively in kisspeptin cells affects puberty onset in either sex. Puberty onset was determined by monitoring and recording the day of preputial separation (PS) for males (n = 17-20/genotype) and vaginal opening (VO) for females (n = 11-15/genotype). For females, vaginal smears were taken daily starting that day after VO to analyze cytology and determine the day of first estrus (FE; marked by presence of cornified epithelial cells), a common marker of late puberty typically occurring after the first ovulatory event. Since body weight and metabolism can alter pubertal tempo, body weights were recorded every third day from PND 21 to PND 45 as well as at the time of PS, VO, and FE. Moreover, as litter size can affect body weight and pubertal timing, we avoided the influence of widely divergent litter sizes by only using litters within a “normal” range of 7 to 9 pups and excluded any infrequent runts of either genotype from the study.
Castrations and Ovariectomies
In adulthood, some cohorts of mice of each genotype were gonadectomized to permit studying of LH secretion under different conditions. To measure postcastration LH pulse secretion, adult Kiss KORKO and CON male littermates (7-8 weeks old) were anesthetized using 4% isoflurane and bilateral castrations were performed via a single mid-line incision of the abdomen. To measure estradiol (E2)-induced LH surges, adult Kiss KORKO and CON female littermates (age 9-10 weeks old) were anesthetized with 4% isoflurane and bilateral ovariectomies likewise performed via a single mid-line incision of the abdomen. At the time of ovariectomy (OVX), an additional small incision was made above the dorsal interscapular region and a Silastic capsule containing an elevated “proestrus” dose of E2 in oil was implanted under the skin, as in prior studies (67–71). All skin incisions were closed with surgical wound clips, and all mice were given subcutaneous injections of buprenorphine for pain management.
Adult Reproductive Hormone Measures
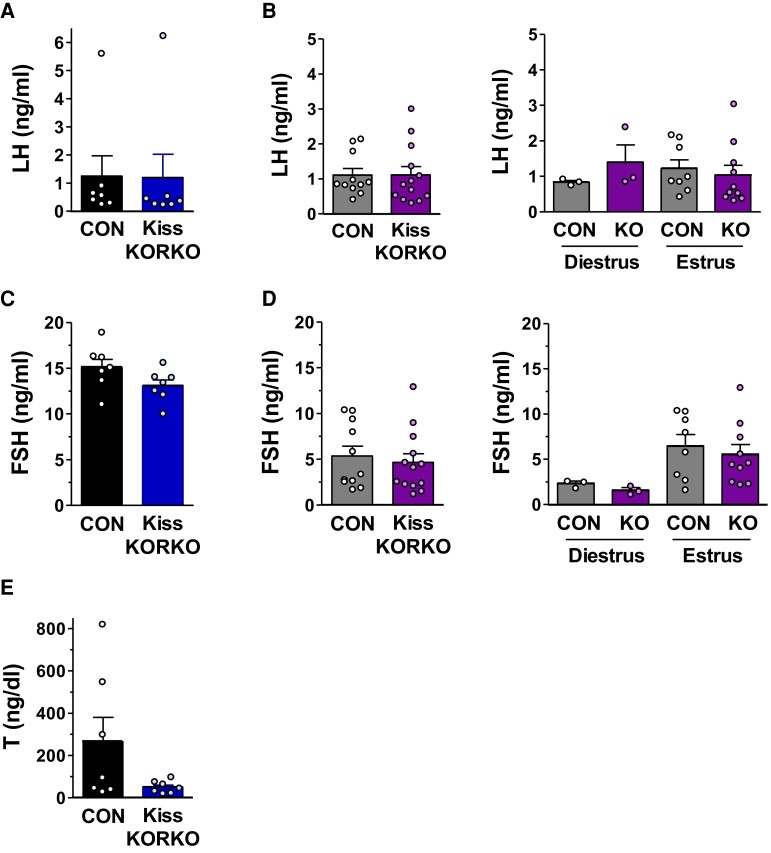
Circulating reproductive hormone levels were analyzed in adult Kiss KORKO and CON littermates. For both males (n = 7/genotype) and females (n = 11-13/genotype), a single, survival retro-orbital blood sample was collected at 7 to 8 weeks of age, along with a vaginal smear in the females to determine the estrous cycle stage. All females were in estrous (n = 8-10/genotype) or diestrus (n = 3/genotype). Blood was allowed to clot at room temperature for 90 minutes and then centrifuged to isolate the serum. Serum was stored at −20 °C until assaying by the University of Virginia Ligand Assay Core for LH and FSH using murine ultrasensitive-LH and murine ultrasensitive-FSH assays, respectively. The ultrasensitive-LH assay (72) used a capture monoclonal antibody (anti-bovine LH-beta subunit, 518B7; RRID: AB_2665514) and detection polyclonal antibody (rabbit LH antiserum, AFP240580Rb; RRID: AB_2665533) and was run on 5 µL serum diluted in 55 µL assay buffer. The murine ultrasensitive-FSH assay (73) used a capture antibody (guinea pig anti-mouse-FSH antibody, AFP-176019; RRID: AB_2665512) and detection antibody (rabbit anti-rat-FSH antiserum FSH-S-11, AFP-C0972881; RRID: AB_2687903) and was run on 6 µL serum diluted in 54 µL assay buffer. Based on their respective dilution factors, the ultrasensitive-LH assay had a reportable range of 0.192 to 48.0 ng/mL and the ultrasensitive-FSH assay had a reportable range of 0.16 to 80 ng/mL. Testosterone (T) in males was also assayed from 35 µL serum by the UVA Ligand Assay Core using an established rodent testosterone ELISA (RRID: AB_2814981) with an assay reportable range of 10.0 to 1600 ng/dL.
In Vivo LH Pulse Secretion Dynamics
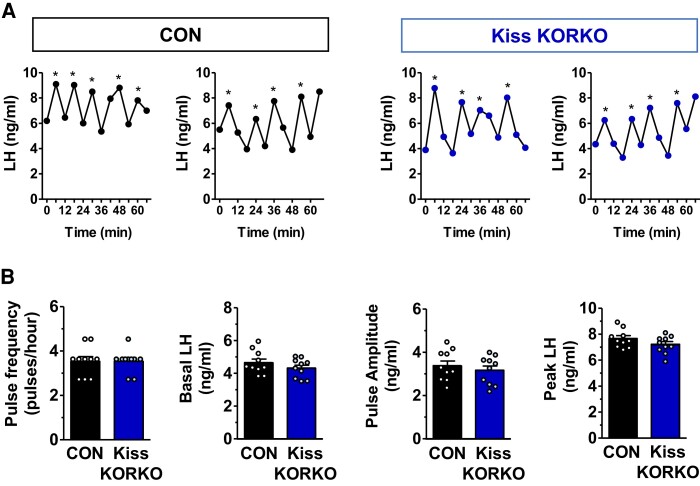
Dynorphin-KOR signaling is proposed to play an important role in sculpting GnRH and LH pulse secretion and is hypothesized to be involved specifically in pulse termination via paracrine/autocrine signaling on KNDy neurons (48). Furthermore, specific ablation of KOR-expressing cells in the arcuate nucleus led to increased LH pulse frequency in rats (74). We therefore tested if LH pulse secretion parameters would be altered in mice lacking KOR selectively in kisspeptin neurons. To investigate in vivo pulsatile LH secretion, we performed serial tail-tip blood sampling in adult castrated Kiss KORKO and CON male mice (n = 10/genotype). Beginning 1 week after castration, all mice were handled daily for 3 weeks to acclimatize them to the tail-tip blood sampling procedure, a common practice for mouse serial bleeding (66, 75). Whole blood samples (3 μL) were collected from the tip of the tail every 6 minutes for 72 minutes, immediately diluted in 57 μL of assay buffer, and stored on ice before long term storage at −20 °C. Serial blood samples were assayed for LH by the UVA Ligand Assay Core using the murine ultrasensitive-LH assay (72) noted above, as in prior LH pulse studies (66, 76–78). Based on the 20× dilution factor of whole blood in buffer, this ultrasensitive-LH assay had a reportable range of 0.32 to 80.0 ng/mL. LH pulse parameters were analyzed with the PULSAR Otago software program using the following validated parameters outlined for OVX mice in the report by Porteous and colleagues (76): smoothing 0.7, peak split 2.5, level of detection 0.32, amplitude distance 3, assay variability 0 2.5 3.3, and G values of 2.2, 2.7, 1.9, 1.5, and 1.2.
E2-induced LH Surge (Positive Feedback)
RP3V kisspeptin neurons are thought to govern the preovulatory GnRH/LH surge, which in female rodents occurs on a circadian basis in the early evening under the influence of elevated E2 (58, 71, 79). Conversely, the role of KNDy neurons in the rodent LH surge mechanism is less clear, with most studies suggesting a primary role instead in LH pulse generation but a few studies suggesting a possible modulatory role of KNDy neurons on the surge (reviewed in (56)). To directly test whether KOR deletion from kisspeptin neurons alters the LH surge, we used a well-established E2-induced LH surge paradigm (67, 69–71). After the conclusion of estrous cycle assessments, female Kiss KORKO and CON mice (9-11 weeks old) were OVX and implanted subcutaneously with a Silastic E2 capsule (0.75 µg E2 in oil) which reliably elicits early evening LH surges 2 days later (68–70). Two days after E2 implantation, a small subset of mice in each genotype was euthanized in the morning (Am groups; a non-surge control time; n = 3-4/genotype) and the remaining females were euthanized in the early evening right before lights off (Pm groups; time of the LH surge; n = 6-10/genotype). Blood was collected and left to clot at room temperature for 90 minutes and then centrifuged to obtain serum. Serum LH levels were assayed by the UVA Ligand Assay Core using a well-established, sensitive murine LH sandwich radioimmunoassay (RIA) with antibodies against bovine LH and human LH-beta (RRID: AB_2665514, RRID: AB_2665513; assay sensitivity: 0.04 ng/mL; reportable range: 0.04-75.0 ng/mL). With this specific murine LH RIA, an LH surge was defined as a Pm value > 0.70 ng/mL, similar to our previous surge studies (68).
Estrous Cyclicity
Kisspeptin neurons regulate both pulsatile and surge LH secretion in females. To ascertain whether dynorphin-KOR signaling in kisspeptin neurons is involved in modulating hormonal control of estrous cyclicity, daily vaginal smears were collected in the morning from 7- to 8-week-old female Kiss KORKO and CON mice for 2 weeks to monitor estrous cyclicity (n = 10-16/genotype). Vaginal smears were stained with 0.01% methylene blue, and cytology was studied under a light microscope to determine the cycle stage. Proestrus was scored by the presence of nucleated cells while presence of cornified cells indicated estrous and nucleated cells along with leucocytes indicated diestrus. Cycle length for each animal was calculated by the number of days from the first day of diestrus of one cycle to the first day of diestrus of the next cycle.
Fertility Assessment
Adult Kiss KORKO and CON females (2-3 months old) were paired with CON breeder partner males and tracked for fertility and fecundity for the next 75 days. Cages were examined daily for new litters and the following parameters recorded for analysis: time to first litter, number of litters generated during the 75-day period, and number of pups per litter. Means were calculated and compared between genotypes (n = 6/genotype).
Statistical Analysis
All data were graphed and analyzed using Prism software (GraphPad, San Diego, USA). Data are represented as mean ± SEM. Data for 2 groups were analyzed using unpaired t tests. More than 2 groups were analyzed using ANOVA. P < 0.05 was considered statistically significant.
Results
Validation of KOR Deletion in Kisspeptin Neurons of Kiss KORKO Mice
We first validated that our breeding strategy of crossing KissCre+ and KORfl/fl mice would successfully delete the Oprk1 gene (KOR) from kisspeptin neurons in the resulting Kiss KORKOs (Fig. 1A). Double-label RNAscope in situ hybridization with a custom designed Oprk1 probe was used on brain sections comprising the ARC and RP3V regions to determine whether exon 3 was successfully knocked out in kisspeptin neurons of Kiss KORKOs (Cre+) compared to CON (Cre−) mice. Fig. 1B shows representative photomicrographs of Kiss1 and Oprk1 gene expression in both the ARC and RP3V regions of Kiss KORKO and CON littermates. CON females had high occurrence of co-expression of Oprk1 in Kiss1 neurons in both the ARC and RP3V, with ∼65% and 50% of ARC and RP3V kisspeptin neurons, respectively, clearly co-expressing Oprk1 in control females (Fig. 1B). By contrast, the occurrence of Oprk1 co-expression in Kiss1 neurons was virtually absent in Kiss KORKO females, in both the ARC and RP3V (Fig. 1B). Given space restrictions on the maximum number of slides in an RNAscope assay, there was only enough room in this validation assay for 1 male of each genotype, but these males showed the same outcome as females, with only ∼2% of KNDy neurons expressing Oprk1 in the Cre + male vs ∼43% in the CON male. Collectively, these data confirm that KOR had been successfully deleted from kisspeptin neurons of Kiss KORKOs.
Absence of KOR Signaling in Kiss1 Neurons Does Not Alter Puberty Onset
Puberty is driven by the onset of adult-like GnRH pulse secretion that stimulates reproductive maturation. It was recently reported that chronic KOR antagonist treatment in peri-pubertal female rats advanced puberty onset (males were not studied) (13). Thus, endogenous dynorphin-KOR signaling may play a key role in timing sexual maturation, but the neural pathways by which this occurs remain unknown. Because kisspeptin neurons, and ARC KNDy neurons in particular, play important roles in both puberty onset and GnRH pulse generation, we determined whether absent KOR signaling selectively in kisspeptin cells alters puberty. For both sexes, there was no significant genotype difference in mean body weight at weaning (Fig. 2A) or during the pubertal and early adulthood periods (PND 21 to 45; Fig. 2B). With regard to puberty onset in males, there was no significant genotype difference between Kiss KORKOs and CON in the day of PS (Fig. 2C and 2D) or the mean body weight on the day of PS (Fig. 2D, right). Like males, Kiss KORKO females demonstrated normal puberty onset, with no significant genotype difference in the timing of VO (Fig. 2E and 2F) or the day of FE (Fig. 2G and 2H). Likewise, there was no difference between Kiss KORKO and CON females in body weight at VO (Fig. 2F, right) or FE (Fig. 2H, right). Collectively, these results indicate that pubertal timing and body weights are normal in male and female mice lacking KOR in kisspeptin neurons.
Figure 2.
Absence of dynorphin-KOR signaling in kisspeptin cells does not alter body weight or puberty onset in either sex. Mean body weights at weaning for male (n = 17-20/genotype) and female mice (n = 11-15/genotype) (A) and from PND 21 to PND 45 for both sexes (B) did not differ between genotypes. Day of preputial separation (PS) and mean body weight at PS did not differ between CON and Kiss KORKO males (C, D). Day of vaginal opening (VO) and mean body weight at VO did not differ between CON and Kiss KORKO females (E, F) nor did day of first estrus (FE) or mean body weight at FE (G, H).
Reproductive Hormone Levels Are Normal in Adult Kiss KORKO Mice
Circulating serum reproductive hormone levels were analyzed in adult male and female Kiss KORKO and CON littermates. Serum LH levels were not significantly different between genotypes in either sex (Fig. 3A and 3B). Moreover, when LH levels in females were analyzed separately for diestrus and estrous cycles stages, there was no statistical difference between Kiss KORKOs and CONs at either stage (Fig. 3B, right). As with LH, serum FSH levels were not statistically different between Kiss KORKO and CON mice in either sex, although levels were generally higher in males than females as expected (Fig. 3C and 3D). Likewise, when female FSH levels were analyzed separately for diestrus and estrous stages, there was no statistical difference between genotypes at either stage (Fig. 3D, right). Finally, mean serum T levels were compared in Kiss KORKO and CON males; while mean T levels trended higher in CON than Kiss KORKOs, this was not statistically significant (P = 0.09; Fig. 3E). Indeed, the higher mean value of T in CON males was strongly driven by a few very high values, a common outcome when measuring male mouse T levels owing to infrequent “T pulses” induced by occasional LH pulses. All of the Kiss KORKO T values were in the normal male range and similar to many of the CON males’ T levels, as shown in Fig. 3E. Collectively, these findings indicate that despite the knockout of KOR in kisspeptin neurons, circulating reproductive hormone levels of adult Kiss KORKO males and females are in the normal physiological range and not significantly different from CON levels.
Figure 3.
Adult levels of circulating reproductive hormones are not altered by deletion of KOR in kisspeptin neurons. Mean serum LH levels in CON and Kiss KORKO males (n = 7/genotype) (A) and females (n = 11-13/genotype) (B) were not different between genotypes. The single lone high LH values in both the CON and Kiss KORKO males likely represent an LH pulse peak at the time of blood collection. Female LH levels broken down by cycle stage also did not differ between genotypes (n = 3/diestrus groups; n = 8-10/estrus groups) (B, right). Mean serum FSH levels in CON and Kiss KORKO males (C) and females (D) were not different between genotypes. Female FSH levels based on cycle stage also did not differ between genotypes (D, right). Mean serum T levels in CON and Kiss KORKO males (E) were not statistically different, despite a non-significant trend (P = 0.09) toward higher levels in CON males which was due to 2 very high values in that group.
In Vivo Pulsatile LH Secretion Parameters Are Unaffected by KOR Knockout in Kiss1 Neurons
Dynorphin-KOR signaling is proposed to play a fundamental role in sculpting GnRH and LH pulse secretion. According to the KNDy hypothesis of GnRH pulse generation, dynorphin released from KNDy neurons acts in a paracrine or autocrine manner on KNDy neurons to terminate the neuronal activation that was induced by similar paracrine/autocrine NKB action. Thus, dynorphin-KOR signaling on KNDy neurons is proposed to terminate each pulse (48). We therefore tested if in vivo LH pulses would be impaired or altered in mice lacking KOR selectively in kisspeptin neurons. Adult Kiss KORKO and CON mice both displayed high LH levels, as expected owing to their gonadectomized status (Fig. 4A). However, there was surprisingly no significant difference between genotypes in any parameter of LH pulse secretion (Fig. 4A and 4B). Indeed, mean pulse frequency, basal LH, pulse amplitude, and pulse peak were all identical between KORKOs and CONs (Fig. 4B). Thus, despite the absence of KOR in KNDy neurons, there was no disruption or change in LH pulse frequency or other LH secretion parameters.
Figure 4.
In vivo pulsatile LH secretion is unaffected by selective KOR knockout in kisspeptin neurons. (A) Representative examples of LH pulse profiles in castrated CON and Kiss KORKO males. (B) Mean values of LH pulse frequency, basal LH, pulse amplitude, and pulse peak did not differ between CON and Kiss KORKO mice (n = 10/genotype). Pulses were identified by the PULSAR Otago software program.
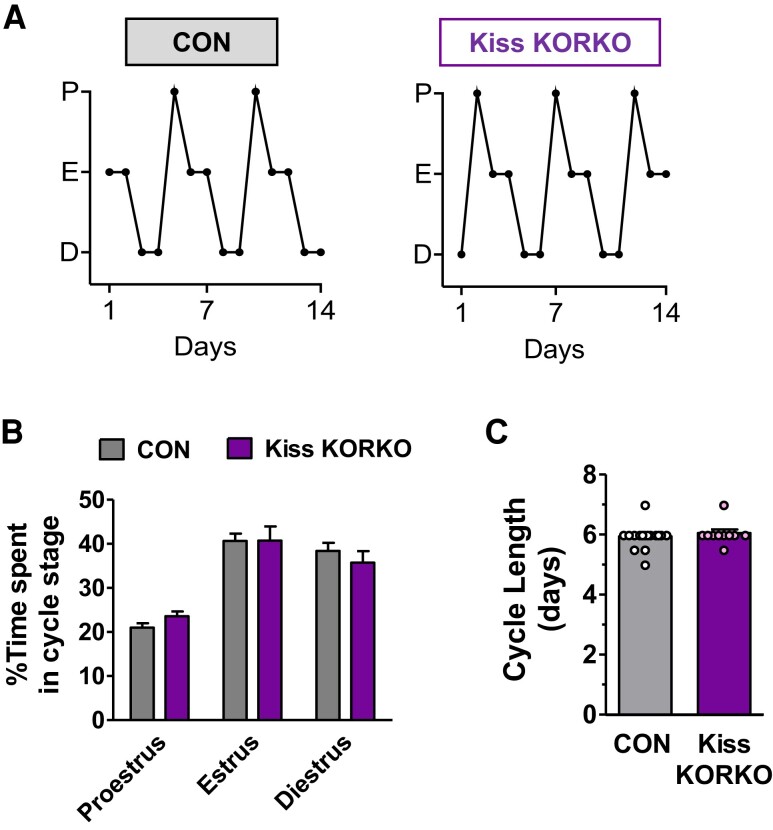
Absent KOR in Kiss1 Cells Does Not Affect Estrous Cyclicity
To determine whether KOR signaling directly in kisspeptin neurons influences the adult neuroendocrine reproductive axis control of female estrous cyclicity, daily vaginal smears were collected from Kiss KORKO and CON females for 2 weeks to monitor estrous cycles. Females from both genotypes showed regular occurring estrous cycles with all 3 cycle stages present (Fig. 5). There was no significant difference between Kiss KORKO and CON females in the time spent in each specific cycle stage (Fig. 5B) or the mean cycle length (Fig. 5C).
Figure 5.
Absent KOR in kisspeptin neurons does not affect estrous cyclicity. (A) Examples of estrous cycles in CON and Kiss KORKO females. (B) Percentage of time spent in each cycle stage and (C) mean cycle length did not differ for CON and Kiss KORKO females (n = 10-16/genotype).
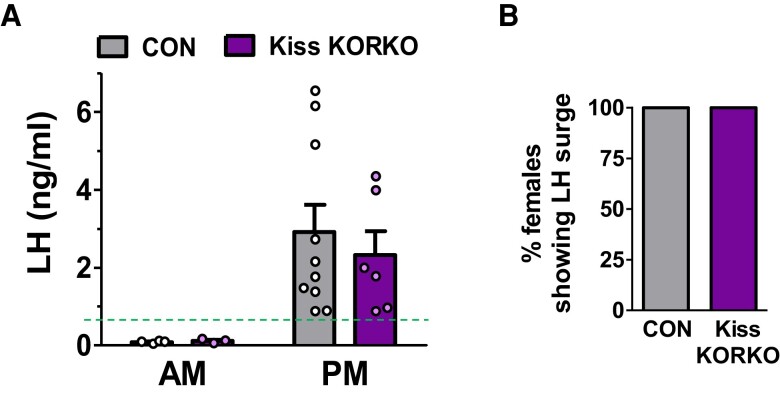
Absent KOR in Kisspeptin Neurons Does Not Affect the LH Surge
RP3V kisspeptin neurons express KOR (59) and participate in the E2-induced LH surge (56, 68), which in female rodents is circadian and occurs in the early evening (58, 69, 71, 79). Conversely, the role of KNDy neurons, which also express KOR, in the rodent LH surge mechanism is currently less clear (56, 80, 81) though it is possible that alteration in KNDy neuron function due to the loss of KOR mice might, in theory, influence the LH surge circuitry. We therefore tested whether KOR deletion from kisspeptin neurons affects the occurrence or magnitude of the LH surge in females given a validated E2-induced LH surge paradigm (67–71). E2-treated females of both genotypes had very low mean LH levels at the Am control time, as expected (Fig. 6A). In the Pm, both Kiss KORKO and CON females demonstrated clear LH surges (Fig. 6A). 100% of females in each genotype showed an LH surge (Fig. 6B), and there was no genotype difference in the magnitude of LH during the LH surge (Fig. 6A). Thus, the LH surge is not altered by the deletion of KOR signaling in RP3V and ARC kisspeptin neurons.
Figure 6.
Conditional deletion of KOR in kisspeptin neurons does not alter LH surge occurrence or magnitude. (A) Mean LH levels in the AM (non-surge control time; n = 3-4/genotype) and PM (time of LH surge; n = 6-10/genotype) for OVX + E2-treated CON and Kiss KORKO females. The dotted green line denotes the threshold for a value being considered an LH surge. (B) Percentage of CON and Kiss KORKO females in the PM groups showing an LH surge.
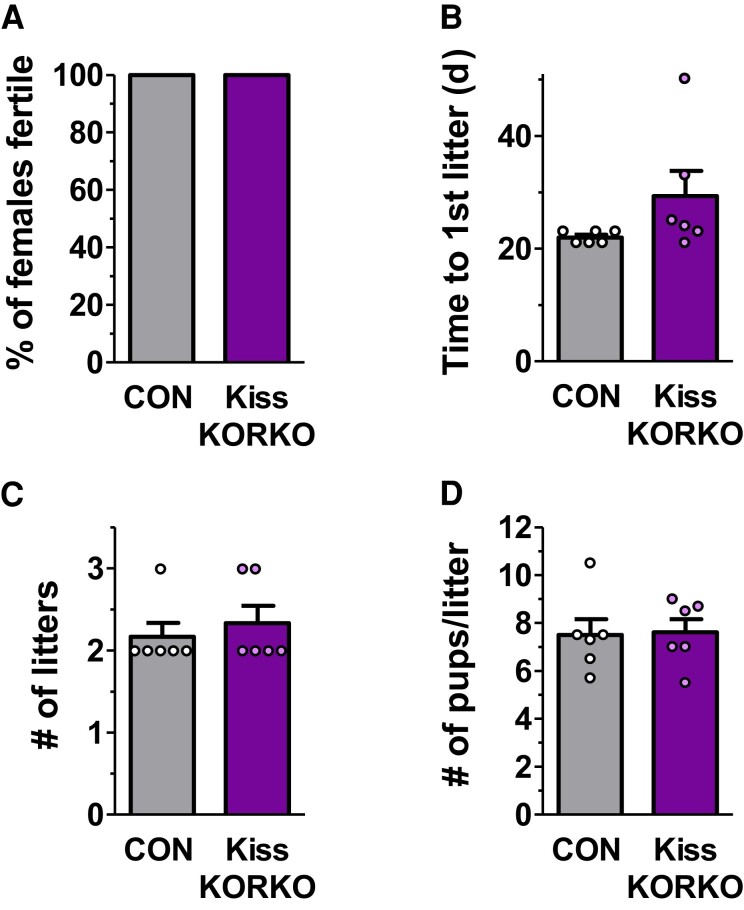
Absent KOR Signaling in Kisspeptin Neurons Does Not Alter Fertility
Adult Kiss KORKO and CON females paired with CON male partners were evaluated for fertility and fecundity during a 75-day breeding assessment. All (100%) females in each genotype produced offspring and were fertile (Fig. 7A). Although the time to first litter trended toward being a few days later in Kiss KORKOs, this was not statistically different between genotypes (P = 0.13; Fig. 7B). The mean number of litters in the 75-day breeding period and the mean litter size (pups per litter) were also not significantly different between Kiss KORKO and CON mice (Fig. 7C and 7D). Collectively, this indicates that both fertility and fecundity are unaltered by deletion of KOR from kisspeptin neurons.
Figure 7.
Absence of dynorphin-KOR signaling in kisspeptin cells does not impact fertility. (A) The percent of CON and Kiss KORKO females (n = 6/genotype) that were fertile did not differ, with all females of both genotypes producing litters. The mean time to first litter (B), mean number of litters in the 75-day assessment period (C), and mean number of pups per litter (D) also did not differ significantly between the 2 genotypes.
Discussion
Classic findings in the 1980s and 1990s leading into more recent findings in the “Kisspeptin Era” have suggested that endogenous dynorphin-KOR signaling in the brain plays important roles in reproductive neuroendocrine regulation. With the discovery of an interconnected network of KNDy neurons expressing KOR and NK3R, the KNDy hypothesis was put forth by several labs to explain how GnRH and LH pulses might be generated. Key to this model is dynorphin released from KNDy neurons acting in a paracrine manner on other KNDy neurons via KOR signaling to terminate these neurons’ synchronous “pulse” activity. While several anatomical, in vitro, and indirect lines of evidence support this aspect of the KNDy hypothesis, a functional in vivo test of the necessity of KOR signaling in kisspeptin neurons for proper LH secretion and fertility was lacking. Here, we addressed this issue by knocking out KOR selectively from kisspeptin neurons of male and female mice. Surprisingly, despite validation of successful conditional knockout, we found no effects of kisspeptin cell-specific deletion of KOR on any measure of puberty, reproductive hormone levels, LH pulse parameters, LH surge, cycles, or fertility. This outcome suggests that the KNDy hypothesis, while still likely sufficient under normal conditions, may not be the only neural mechanism capable of sculpting LH pulses or tonic gonadotropin secretion. Moreover, our findings suggest that previously reported effects of KOR signaling on inhibiting either pubertal onset or the LH surge may not occur via KOR in kisspeptin neurons. Collectively, these findings suggest that, along with acting via KOR in kisspeptin neurons, dynorphin and other opioids may sometimes also alter reproductive hormones via KOR in non-kisspeptin cells or possibly via non-KOR mediated pathways, the mechanisms of which remain to be determined.
Increased GnRH pulse secretion is a key process driving normal puberty onset (82, 83), thereby stimulating gonadotropin secretion and gonadal function. Kisspeptin signaling and KNDy neurons in the ARC have both been implicated in the sexual maturation process, as evidenced from absent puberty in individuals with mutations in the Kiss1 or Kiss1r genes (84–86), impaired puberty in female mice with postnatal ablation of all kisspeptin neurons (both RP3V and ARC) (87), advanced puberty onset in females lacking ERα in kisspeptin neurons (88), altered puberty in rodent models with manipulated levels of transcription factors that regulate the ARC Kiss1 gene (89–91), peri-pubertal increases in kisspeptin release in the primate brain (92), and developmental increases in ARC and RP3V Kiss1 levels during puberty onset in mice (93, 94). Studies have demonstrated that blocking endogenous opioid signaling, including KOR-specific action, can both increase LH secretion in peri-pubertal animals and advance the onset of puberty, suggesting that endogenous dynorphin signaling via KOR acts to keep the reproductive axis in check before puberty begins (13, 18, 20, 21). Because of these findings, and the proposed role of KOR in KNDy neurons for GnRH pulse secretion (which drives pubertal progression), we hypothesized that deletion of KOR from kisspeptin neurons would hasten puberty onset. However, puberty timing was normal in both male and female Kiss KORKOs, indicating that the KOR signaling in kisspeptin cells is not required for normal puberty onset. This also suggests that the prior KOR antagonist studies that increased LH or puberty onset may have achieved such effects by blocking KOR action in non-kisspeptin cells, the identity of which is unknown.
The KNDy hypothesis of GnRH pulse generation proposes that dynorphin and NKB act in a paracrine manner to synchronize KNDy neuronal activity and kisspeptin release to drive GnRH pulse secretion (9, 10, 40, 95). In this model, dynorphin is considered the “brake” on a pulse event, acting via KOR to terminate KNDy neuronal activity (and hence, terminate pulsatile kisspeptin release onto GnRH neurons). There is some in vitro evidence for direct dynorphin-KOR inhibitory action on KNDy neurons based on electrophysiology studies showing that (i) dynorphin or KOR agonist treatment can inhibit KNDy cell firing in murine ARC slices (46, 47); (ii) dynorphin can decrease NKB agonist-induced firing of murine KNDy neurons under some steroid conditions (46); and (iii) a KOR antagonist treatment can increase murine KNDy neuron electrical activity (28). While these electrophysiology studies support the KNDy hypothesis, in vivo functional tests of the necessity of KOR action in KNDy neurons for proper LH pulse secretion were lacking until the present study. Here, we found that both basal LH and FSH levels measured in single blood draws and LH pulse secretion parameters assessed via serial blood sampling were all normal in Kiss KORKO mice, being no different in any measure from CON littermates. This was unexpected to us given that the KNDy hypothesis would predict that LH pulses (and, correspondingly, single LH and FSH measures) would be greatly altered if KOR was not present in kisspeptin neurons, as KNDy neuron pulse termination would be impaired. Indeed, if KOR action in KNDy neurons is essential for LH pulse termination, one might predict the pulse duration to be lengthened (wider pulses and/or a much slower return to basal levels after each peak). While we did not quantitatively measure these aspects, visual comparison of the pulse patterns of the 2 genotypes (eg, Fig. 4) obtained with our 6-minute sampling rate does not suggest a major widening or greatly slowed decay rate of each pulse in the Kiss KORKOs compared to controls. Moreover, if pulse termination rate was greatly slowed, we might predict elevated basal levels due to the onset of the next pulse beginning before the prior pulse fully declined; however, basal levels were similar between genotypes. A more frequent sampling rate might better identify a possible widening effect on pulse shape that is more subtle, though ultimately there appears to be no consequence on reproductive physiology and function in our present model.
Matching the normal gonadotropin secretion results, estrous cycles and fertility were also not compromised by removing KOR from kisspeptin neurons, which would not be expected if pulsatile LH secretion was impaired. Moreover, despite prior studies suggesting that endogenous dynorphin signaling may alter the timing or magnitude of the LH surge (49–55), and the fact that RP3V kisspeptin neurons (the “neural surge generator”) express KOR (59) and are contacted by dynorphin-expressing neurons (including KNDy neurons) (28, 80), E2-induced LH surges were completely normal in Kiss KORKO females. Indeed, 100% of Kiss KORKO females produced an LH surge, with normal magnitude similar to that in CON females. This ability of Kiss KORKO females to mount a normal LH surge suggests triggering ovulation would be unaffected in these mice, which was confirmed by our fertility assessment showing normal fertility and fecundity in Kiss KORKO, including normal number of pups per litter. Collectively, these findings indicate that KOR in kisspeptin neurons is not required in mice for normal neuroendocrine reproductive function, including tonic or surge modes of LH secretion, FSH secretion, normal estrous cyclicity, and ultimately, normal fertility.
Our present data reinforce the proposed notion (96) that, while dynorphin-KOR signaling may be important for GnRH pulse termination under normal conditions, the KOR component of the KNDy model is not the only manner in which pulses can be regulated. Indeed, KOR action in KNDy neurons is not needed for normal LH pulse pattern, FSH and LH secretion, and reproductive function in Kiss KORKOs. Apart from being in KNDy neurons, dynorphin and KOR are also both expressed in numerous other brain nuclei and cell types (including RP3V kisspeptin neurons, which also lacked KOR in our Kiss KORKOs; Fig. 1B). Thus, it is possible that KOR in other cells in the ARC or elsewhere in the hypothalamus can relay dynorphin signals to the reproductive axis, but this remains to be tested. It is also possible that dynorphin released from KNDy neurons acts on other non-KNDy inter-neurons in the ARC or elsewhere which then feed back to KNDy neurons in an indirect relay circuit. Interestingly, a recent study selectively ablated KOR cells in the greater ARC region using a dynorphin-saporin conjugate and found altered LH pulse secretion, despite the fact that KNDy neurons were apparently still intact (not ablated) (74). That study suggested that (i) KNDy neurons do not readily express KOR (or do not express high enough levels to bind sufficient levels of saporin conjugate), which our present study and others do not corroborate; and (ii) non-KNDy cells expressing KOR in the ARC may be important for relaying dynorphin signaling directly or indirectly to GnRH neurons. The phenotype and identity of such non-KNDy KOR cells is unknown and could possibly include neurons or glia. In sheep, there appears to be some direct action of dynorphin-KOR signaling on GnRH neurons which has been implicated in the pulse termination phase (35, 48), but whether murine GnRH neurons are directly regulated by dynorphin-KOR signaling is not well understood.
Several other studies suggest that the KNDy hypothesis is either species specific or a work in progress that still needs refining or expanding. First, unlike other species, humans appear to lack colocalization of dynorphin in ARC kisspeptin/NKB neurons (97–99), which, if true, suggests that any dynorphin signal involved in pulse generation in humans arises from non-ARC kisspeptin/NKB cells. As human brain histology studies are limited and can be hard to control for cofounding variables that could affect dynorphin levels, more studies are needed to confirm this intriguing finding. However, recent physiological clinical data in both humans and mice suggest that some LH pulse secretion can be observed in individuals lacking both endogenous NKB signaling (due to genetic Tac2 mutation) and endogenous dynorphin signaling (due to opioid receptor antagonism treatment) (96). That study concluded that the preservation of LH pulses in the absence of both NKB and dynorphin signaling suggests that both peptides are dispensable for GnRH pulse generation, a concept supported by another study in mice lacking NKB receptor signaling (100); however, the former study did not rule out possible compensatory action achieved by cross talk between various tachykinin and opioid ligands and their different receptor subtypes. Indeed, in our current study it is possible that dynorphin action on KNDy or RP3V neurons was still achieved via non-KOR pathways. However, MOR is reportedly not highly expressed in kisspeptin neurons, DOR is not readily expressed in the brain, and DOR antagonists do not alter LH levels [reviewed in (17)], leaving KOR as the only known receptor in KNDy neurons to receive dynorphin signals.
A caveat to our study is that we cannot rule out the occurrence of developmental compensation owing to embryonic or early postnatal deletion of KOR in the Kiss KORKOs. At present, a validated inducible KissCre mouse has not been reported and AAV methods to lower gene expression in adulthood often result in only partial knockdowns. This currently limits the technical ability to temporally induce complete KOR deletions in kisspeptin neurons that avoid possible developmental compensation. However, it should not go unappreciated that if there was developmental compensation, it would further emphasize that, under certain conditions, other mechanisms in the brain can sculpt normal LH pulse patterns in the absence of KOR signaling in kisspeptin neurons. Regardless, it remains possible that developmental compensation did not occur in the present Kiss KORKO mice, but this requires future investigation with temporally controlled knockout models. Finally, we note that a very tiny percentage (∼2%) of kisspeptin cells in the ARC and RP3V still had Oprk1. While we cannot rule out that retained KOR signaling in this very small number of kisspeptin neurons is itself sufficient to impart normal pulse generation dynamics and LH secretion, we view this as unlikely given that other studies suggest a much higher percentage of kisspeptin neurons (∼20%) is needed for proper reproductive neuroendocrine function (101).
In conclusion, we tested the functional necessity of KOR in kisspeptin neurons by conditionally knocking out KOR selectively from kisspeptin cells of male and female mice. Surprising to us, we found no significant effects of such selective KOR deletion on any measure of puberty, reproductive hormone levels, LH pulse parameters, LH surge occurrence or magnitude, or fertility. Our findings suggest that the KNDy hypothesis, while still viable and likely operating under normal conditions, may not be the only neural mechanism for governing GnRH and LH pulses. Moreover, our results suggest the possibility that some of the previously reported alterations in pubertal onset, LH pulses, or the LH surge by blocking endogenous KOR signaling might occur independently of KOR specifically in kisspeptin neurons, although direct action on kisspeptin neurons still remains possible. Thus, in addition to the mechanism outlined by the KNDy hypothesis, endogenous dynorphin and other opioids may, under some circumstances, regulate LH secretion via additional mechanisms, possibly via KOR in non-kisspeptin cells and/or via non-KOR mediated pathways. Revisiting or expanding the current KNDy hypothesis could be useful to incorporate such possibilities and understand how they affect the pulse generator on a mechanistic level.
Abbreviations
- ARC
arcuate
- CON
KissCre−/Oprk1fl/fl mice
- E2
estradiol
- FE
first estrus
- FSH
follicle-stimulating hormone
- GnRH
gonadotropin-releasing hormone
- Kiss KORKO
KissCre+/Oprk1fl/fl mice
- KNDy neurons
kisspeptin/neurokinin B/dynorphin neurons
- KOR
kappa opioid receptor
- LH
luteinizing hormone
- MOR
mu opioid receptor
- NKB
neurokinin B
- NK3R
neurokinin-3 receptor
- OVX
ovariectomy
- PND
postnatal day
- PS
preputial separation
- RP3V
rostral periventricular nucleus of the third ventricle
- T
testosterone
- VO
vaginal opening
Contributor Information
Eulalia A Coutinho, Department of OBGYN and Reproductive Sciences, University of California San Diego, La Jolla, CA 92093, USA.
Lourdes A Esparza, Department of OBGYN and Reproductive Sciences, University of California San Diego, La Jolla, CA 92093, USA.
Alexandra D Hudson, Department of OBGYN and Reproductive Sciences, University of California San Diego, La Jolla, CA 92093, USA.
Nathanael Rizo, Department of OBGYN and Reproductive Sciences, University of California San Diego, La Jolla, CA 92093, USA.
Paige Steffen, Department of OBGYN and Reproductive Sciences, University of California San Diego, La Jolla, CA 92093, USA.
Alexander S Kauffman, Department of OBGYN and Reproductive Sciences, University of California San Diego, La Jolla, CA 92093, USA.
Funding
This research was supported by NIH grants R01 HD090161, R01 HD100580, and P50 HD012303. Additional funding provided by NIH HD007203 and NIH R24 HD1020614 (University of Virginia Ligand Core).
Disclosures
The authors declare no competing financial interests.
Data Availability
Some datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Goodman RL, Lehman MN, Smith JT, et al. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148(12):5752–5760. [DOI] [PubMed] [Google Scholar]
- 2. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of GnRH secretion by Kiss1/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29(38):11859–11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wakabayashi Y, Nakada T, Murata K, et al. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010;30(8):3124–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Murakawa H, Iwata K, Takeshita T, Ozawa H. Immunoelectron microscopic observation of the subcellular localization of kisspeptin, neurokinin B and dynorphin A in KNDy neurons in the arcuate nucleus of the female rat. Neurosci Lett. 2016;612:161–166. [DOI] [PubMed] [Google Scholar]
- 5. True C, Kirigiti M, Ciofi P, Grove KL, Smith MS. Characterisation of arcuate nucleus kisspeptin/neurokinin B neuronal projections and regulation during lactation in the rat. J Neuroendocrinol. 2011;23(1):52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moore AM, Lucas KA, Goodman RL, Coolen LM, Lehman MN. Three-dimensional imaging of KNDy neurons in the mammalian brain using optical tissue clearing and multiple-label immunocytochemistry. Sci Rep. 2018;8(1):2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moore AM, Lohr DB, Coolen LM, Lehman MN. Prenatal androgen exposure alters KNDy neurons and their afferent network in a model of polycystic ovarian syndrome. Endocrinology. 2021;162(11):bqab158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology. 2010;151(9):4494–4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lehman MN. Origins of the ‘KNDy hypothesis’ of GnRH pulse generation. Nat Rev Endocrinol. 2022;18(9):521. [DOI] [PubMed] [Google Scholar]
- 10. Moore AM, Coolen LM, Porter DT, Goodman RL, Lehman MN. KNDy cells revisited. Endocrinology. 2018;159(9):3219–3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cheng G, Coolen LM, Padmanabhan V, Goodman RL, Lehman MN. The kisspeptin/neurokinin B/dynorphin (KNDy) cell population of the arcuate nucleus: sex differences and effects of prenatal testosterone in the sheep. Endocrinology. 2010;151(1):301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goodman RL, Herbison AE, Lehman MN, Navarro VM. Neuroendocrine control of gonadotropin-releasing hormone: pulsatile and surge modes of secretion. J Neuroendocrinol. 2022;34(5):e13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nakahara T, Uenoyama Y, Iwase A, et al. Chronic peripheral administration of kappa-opioid receptor antagonist advances puberty onset associated with acceleration of pulsatile luteinizing hormone secretion in female rats. J Reprod Dev. 2013;59(5):479–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mostari P, Ieda N, Deura C, et al. dynorphin-kappa opioid receptor signaling partly mediates estrogen negative feedback effect on LH pulses in female rats. J Reprod Dev. 2013;59(3):266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goodman RL, Hileman SM, Nestor CC, et al. Kisspeptin, neurokinin B, and dynorphin act in the arcuate nucleus to control activity of the GnRH pulse generator in ewes. Endocrinology. 2013;154(11):4259–4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goodman RL, Holaskova I, Nestor CC, et al. Evidence that the arcuate nucleus is an important site of progesterone negative feedback in the ewe. Endocrinology. 2011;152(9):3451–3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Uenoyama Y, Tsuchida H, Nagae M, Inoue N, Tsukamura H. Opioidergic pathways and kisspeptin in the regulation of female reproduction in mammals. Front Neurosci. 2022;16:958377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ieiri T, Chen HT, Meites J. Effects of morphine and naloxone on serum levels of luteinizing hormone and prolactin in prepubertal male and female rats. Neuroendocrinology. 1979;29(4):288–292. [DOI] [PubMed] [Google Scholar]
- 19. Petraglia F, Locatelli V, Penalva A, Cocchi D, Genazzani AR, Muller EE. Gonadal steroid modulation of naloxone-induced LH secretion in the rat. J Endocrinol. 1984;101(1):33–39. [DOI] [PubMed] [Google Scholar]
- 20. Wood RI, I'Anson H, Ebling FJ, Foster DL. Opioid inhibition of luteinizing hormone secretion compared in developing male and female sheep. Neuroendocrinology. 1992;56(6):822–830. [DOI] [PubMed] [Google Scholar]
- 21. Ebling FJ, Schwartz ML, Foster DL. Endogenous opioid regulation of pulsatile luteinizing hormone secretion during sexual maturation in the female sheep. Endocrinology. 1989;125(1):369–383. [DOI] [PubMed] [Google Scholar]
- 22. Lincoln GA, Ebling FJ, Martin GB. Endogenous opioid control of pulsatile LH secretion in rams: modulation by photoperiod and gonadal steroids. J Endocrinol. 1987;115(3):425–438. [DOI] [PubMed] [Google Scholar]
- 23. Brooks AN, Lamming GE, Lees PD, Haynes NB. Opioid modulation of LH secretion in the ewe. J Reprod Fertil. 1986;76(2):693–708. [DOI] [PubMed] [Google Scholar]
- 24. Van Vugt DA, Bakst G, Dyrenfurth I, Ferin M. Naloxone stimulation of luteinizing hormone secretion in the female monkey: influence of endocrine and experimental conditions. Endocrinology. 1983;113(5):1858–1864. [DOI] [PubMed] [Google Scholar]
- 25. Ropert JF, Quigley ME, Yen SS. Endogenous opiates modulate pulsatile luteinizing hormone release in humans. J Clin Endocrinol Metab. 1981;52(3):583–585. [DOI] [PubMed] [Google Scholar]
- 26. Quigley ME, Yen SS. The role of endogenous opiates in LH secretion during the menstrual cycle. J Clin Endocrinol Metab. 1980;51(1):179–181. [DOI] [PubMed] [Google Scholar]
- 27. Sasaki T, Ito D, Sonoda T, et al. Peripheral administration of κ-opioid receptor antagonist stimulates gonadotropin-releasing hormone pulse generator activity in ovariectomized, estrogen-treated female goats. Domest Anim Endocrinol. 2019;68:83–91. [DOI] [PubMed] [Google Scholar]
- 28. Qiu J, Nestor CC, Zhang C, et al. High-frequency stimulation-induced peptide release synchronizes arcuate kisspeptin neurons and excites GnRH neurons. eLife. 2016;5:e16246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Burke MC, Letts PA, Krajewski SJ, Rance NE. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol. 2006;498(5):712–726. [DOI] [PubMed] [Google Scholar]
- 30. Cholanian M, Krajewski-Hall SJ, Levine RB, McMullen NT, Rance NE. Electrophysiology of arcuate neurokinin B neurons in female Tac2-EGFP transgenic mice. Endocrinology. 2014;155(7):2555–2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, et al. Arcuate kisspeptin/neurokinin B/dynorphin (KNDy) neurons mediate the estrogen suppression of gonadotropin secretion and body weight. Endocrinology. 2012;153(6):2800–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rometo AM, Rance NE. Changes in prodynorphin gene expression and neuronal morphology in the hypothalamus of postmenopausal women. J Neuroendocrinol. 2008;20(12):1376–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Navarro VM. Interactions between kisspeptins and neurokinin B. Adv Exp Med Biol. 2013;784:325–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gottsch ML, Navarro VM, Zhao Z, et al. Regulation of Kiss1 and dynorphin gene expression in the murine brain by classical and nonclassical estrogen receptor pathways. J Neurosci. 2009;29(29):9390–9395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Weems PW, Witty CF, Amstalden M, Coolen LM, Goodman RL, Lehman MN. κ-Opioid receptor is colocalized in GnRH and KNDy cells in the female ovine and rat brain. Endocrinology. 2016;157(6):2367–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tsuchida H, Kawai N, Yamada K, et al. Central µ-opioid receptor antagonism blocks glucoprivic LH pulse suppression and gluconeogenesis/feeding in female rats. Endocrinology. 2021:162(10):bqab140. [DOI] [PubMed] [Google Scholar]
- 37. Tsuchida H, Mostari P, Yamada K, et al. Paraventricular dynorphin A neurons mediate LH pulse suppression induced by hindbrain glucoprivation in female rats. Endocrinology. 2020;161(11):bqaa161. [DOI] [PubMed] [Google Scholar]
- 38. Ikegami K, Minabe S, Ieda N, et al. Evidence of involvement of neurone-glia/neurone-neurone communications via gap junctions in synchronised activity of KNDy neurones. J Neuroendocrinol. 2017;29(6). doi: 10.1111/jne.12480 [DOI] [PubMed] [Google Scholar]
- 39. Amstalden M, Coolen LM, Hemmerle AM, et al. Neurokinin 3 receptor immunoreactivity in the septal region, preoptic area and hypothalamus of the female sheep: colocalisation in neurokinin B cells of the arcuate nucleus but not in gonadotrophin-releasing hormone neurones. J Neuroendocrinol. 2010;22(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Uenoyama Y, Nagae M, Tsuchida H, Inoue N, Tsukamura H. Role of KNDy neurons expressing kisspeptin, neurokinin B, and dynorphin A as a GnRH pulse generator controlling mammalian reproduction. Front Endocrinol (Lausanne). 2021;12:724632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Herbison AE. The gonadotropin-releasing hormone pulse generator. Endocrinology. 2018;159(11):3723–3736. [DOI] [PubMed] [Google Scholar]
- 42. Han SY, Kane G, Cheong I, Herbison AE. Characterization of GnRH pulse generator activity in Male mice using GCaMP fiber photometry. Endocrinology. 2019;160(3):557–567. [DOI] [PubMed] [Google Scholar]
- 43. Clarkson J, Han SY, Piet R, et al. Definition of the hypothalamic GnRH pulse generator in mice. Proc Natl Acad Sci U S A. 2017;114(47):E10216–E10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Goodman RL, Coolen LM, Anderson GM, et al. Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology. 2004;145(6):2959–2967. [DOI] [PubMed] [Google Scholar]
- 45. Ohkura S, Takase K, Matsuyama S, et al. Gonadotrophin-releasing hormone pulse generator activity in the hypothalamus of the goat. J Neuroendocrinol. 2009;21(10):813–821. [DOI] [PubMed] [Google Scholar]
- 46. Ruka KA, Burger LL, Moenter SM. Regulation of arcuate neurons coexpressing kisspeptin, neurokinin B, and dynorphin by modulators of neurokinin 3 and kappa-opioid receptors in adult male mice. Endocrinology. 2013;154(8):2761–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. de Croft S, Boehm U, Herbison AE. Neurokinin B activates arcuate kisspeptin neurons through multiple tachykinin receptors in the male mouse. Endocrinology. 2013;154(8):2750–2760. [DOI] [PubMed] [Google Scholar]
- 48. Weems PW, Coolen LM, Hileman SM, et al. Evidence that dynorphin acts upon KNDy and GnRH neurons during GnRH pulse termination in the Ewe. Endocrinology. 2018;159(9):3187–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Allen LG, Kalra SP. Evidence that a decrease in opioid tone may evoke preovulatory luteinizing hormone release in the rat. Endocrinology. 1986;118(6):2375–2381. [DOI] [PubMed] [Google Scholar]
- 50. Gabriel SM, Simpkins JW, Kalra SP. Modulation of endogenous opioid influence on luteinizing hormone secretion by progesterone and estrogen. Endocrinology. 1983;113(5):1806–1811. [DOI] [PubMed] [Google Scholar]
- 51. Rossmanith WG, Mortola JF, Yen SS. Role of endogenous opioid peptides in the initiation of the midcycle luteinizing hormone surge in Normal cycling women. J Clin Endocrinol Metab. 1988;67(4):695–700. [DOI] [PubMed] [Google Scholar]
- 52. Zhang Q, Gallo RV. Presence of kappa-opioid tone at the onset of the ovulatory luteinizing hormone surge in the proestrous rat. Brain Res. 2003;980(1):135–139. [DOI] [PubMed] [Google Scholar]
- 53. Smith MJ, Gallo RV. The effect of blockade of kappa-opioid receptors in the medial preoptic area on the luteinizing hormone surge in the proestrous rat. Brain Res. 1997;768(1-2):111–119. [DOI] [PubMed] [Google Scholar]
- 54. Zhang Q, McCoy JM, Gallo RV. Further studies on possible dynorphin involvement in the ovulatory luteinizing hormone surge in the proestrous rat. Endocrine. 2002;18(3):231–238. [DOI] [PubMed] [Google Scholar]
- 55. Zhang Q, Gallo RV. Effect of prodynorphin-derived opioid peptides on the ovulatory luteinizing hormone surge in the proestrous rat. Endocrine. 2002;18(1):27–32. [DOI] [PubMed] [Google Scholar]
- 56. Kauffman AS. Neuroendocrine mechanisms underlying estrogen positive feedback and the LH surge. Front Neurosci. 2022;16:953252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Herbison AE. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V). Brain Res Rev. 2008;57(2):277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Khan AR, Kauffman AS. The role of kisspeptin and RFamide-related peptide-3 neurones in the circadian-timed preovulatory luteinising hormone surge. J Neuroendocrinol. 2012;24(1):131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Stephens SBZ, Kauffman AS. Estrogen regulation of the molecular phenotype and active translatome of AVPV kisspeptin neurons. Endocrinology. 2021;162(9):bqab080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gal A, Lin PC, Cacioppo JA, et al. Loss of fertility in the absence of progesterone receptor expression in kisspeptin neurons of female mice. PLoS One. 2016;11(7):e0159534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Qiu X, Dowling AR, Marino JS, et al. Delayed puberty but normal fertility in mice with selective deletion of insulin receptors from Kiss1 cells. Endocrinology. 2013;154(3):1337–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cravo RM, Margatho LO, Osborne-Lawrence S, et al. Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience. 2011;173:37–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Liu SS, Pickens S, Burma NE, et al. Kappa opioid receptors drive a tonic aversive component of chronic pain. J Neurosci. 2019;39(21):4162–4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ehrich JM, Messinger DI, Knakal CR, et al. Kappa opioid receptor-induced aversion requires p38 MAPK activation in VTA dopamine neurons. J Neurosci. 2015;35(37):12917–12931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chowen JA, Argente J, Vician L, Clifton DK, Steiner RA. Pro-opiomelanocortin messenger RNA in hypothalamic neurons is increased by testosterone through aromatization to estradiol. Neuroendocrinology. 1990;52(6):581–588. [DOI] [PubMed] [Google Scholar]
- 66. Yang JA, Hughes JK, Parra RA, Volk KM, Kauffman AS. Stress rapidly suppresses in vivo LH pulses and increases activation of RFRP-3 neurons in male mice. J Endocrinol. 2018;239(3):339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Stephens SBZ, Rouse ML, Tolson KP, et al. Effects of selective deletion of tyrosine hydroxylase from kisspeptin cells on puberty and reproduction in male and female mice. eNeuro. 2017;4(3):ENEURO.0150-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mohr MA, Esparza LA, Steffen P, Micevych PE, Kauffman AS. Progesterone receptors in AVPV kisspeptin neurons are sufficient for positive feedback induction of the LH surge. Endocrinology. 2021;162(11):bqab161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Poling MC, Luo EY, Kauffman AS. Sex differences in steroid receptor coexpression and circadian-timed activation of kisspeptin and RFRP-3 neurons may contribute to the sexually dimorphic basis of the LH surge. Endocrinology. 2017;158(10):3565–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Stephens SBZ, Tolson KP, Rouse ML Jr., et al. Absent progesterone signaling in kisspeptin neurons disrupts the LH surge and impairs fertility in female mice. Endocrinology. 2015;156(9):3091–3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Dror T, Franks J, Kauffman AS. Analysis of multiple positive feedback paradigms demonstrates a complete absence of LH surges and GnRH activation in mice lacking kisspeptin signaling. Biol Reprod. 2013;88(6):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Steyn FJ, Wan Y, Clarkson J, Veldhuis JD, Herbison AE, Chen C. Development of a methodology for and assessment of pulsatile luteinizing hormone secretion in juvenile and adult male mice. Endocrinology. 2013;154(12):4939–4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ongaro L, Alonso CAI, Zhou X, et al. Development of a highly sensitive ELISA for measurement of FSH in serum, plasma, and whole blood in mice. Endocrinology. 2021;162(4):bqab014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Dai M, Nakamura S, Takahashi C, et al. Reduction of arcuate kappa-opioid receptor-expressing cells increased luteinizing hormone pulse frequency in female rats. Endocr J. 2021;68(8):933–941. [DOI] [PubMed] [Google Scholar]
- 75. Coutinho EA, Prescott M, Hessler S, Marshall CJ, Herbison AE, Campbell RE. Activation of a classic hunger circuit slows luteinizing hormone pulsatility. Neuroendocrinology. 2020;110(7-8):671–687. [DOI] [PubMed] [Google Scholar]
- 76. Porteous R, Haden P, Hackwell ECR, et al. Reformulation of PULSAR for analysis of pulsatile LH secretion and a revised model of estrogen-negative feedback in mice. Endocrinology. 2021;162(11):bqab165. [DOI] [PubMed] [Google Scholar]
- 77. Czieselsky K, Prescott M, Porteous R, et al. Pulse and surge profiles of luteinizing hormone secretion in the mouse. Endocrinology. 2016;157(12):4794–4802. [DOI] [PubMed] [Google Scholar]
- 78. Esparza LA, Schafer D, Ho BS, Thackray VG, Kauffman AS. Hyperactive LH pulses and elevated kisspeptin and NKB gene expression in the arcuate nucleus of a PCOS mouse model. Endocrinology. 2020;161(4):bqaa018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Robertson JL, Clifton DK, de la Iglesia HO, Steiner RA, Kauffman AS. Circadian regulation of Kiss1 neurons: implications for timing the preovulatory GnRH/LH surge. Endocrinology. 2009;150(8):3664–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Helena CV, Toporikova N, Kalil B, et al. KNDy neurons modulate the magnitude of the steroid-induced luteinizing hormone surges in ovariectomized rats. Endocrinology. 2015;156(11):4200–4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Mittelman-Smith MA, Krajewski-Hall SJ, McMullen NT, Rance NE. Ablation of KNDy neurons results in hypogonadotropic hypogonadism and amplifies the steroid-induced LH surge in female rats. Endocrinology. 2016;157(5):2015–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sisk CL, Richardson HN, Chappell PE, Levine JE. In vivo gonadotropin-releasing hormone secretion in female rats during peripubertal development and on proestrus. Endocrinology. 2001;142(7):2929–2936. [DOI] [PubMed] [Google Scholar]
- 83. Watanabe G, Terasawa E. In vivo release of luteinizing hormone releasing hormone increases with puberty in the female rhesus monkey. Endocrinology. 1989;125(1):92–99. [DOI] [PubMed] [Google Scholar]
- 84. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100(19):10972–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Funes S, Hedrick JA, Vassileva G, et al. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun. 2003;312(4):1357–1363. [DOI] [PubMed] [Google Scholar]
- 86. Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614–1627. [DOI] [PubMed] [Google Scholar]
- 87. Mayer C, Boehm U. Female reproductive maturation in the absence of kisspeptin/GPR54 signaling. Nat Neurosci. 2011;14(6):704–710. [DOI] [PubMed] [Google Scholar]
- 88. Mayer C, Acosta-Martinez M, Dubois SL, et al. Timing and completion of puberty in female mice depend on estrogen receptor alpha-signaling in kisspeptin neurons. Proc Natl Acad Sci USA. 2010;107(52):22693–22698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wright H, Aylwin CF, Toro CA, Ojeda SR, Lomniczi A. Polycomb represses a gene network controlling puberty via modulation of histone demethylase Kdm6b expression. Sci Rep. 2021;11(1):1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Vazquez MJ, Toro CA, Castellano JM, et al. SIRT1 Mediates obesity- and nutrient-dependent perturbation of pubertal timing by epigenetically controlling Kiss1 expression. Nat Commun. 2018;9(1):4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Toro CA, Wright H, Aylwin CF, Ojeda SR, Lomniczi A. Trithorax dependent changes in chromatin landscape at enhancer and promoter regions drive female puberty. Nat Commun. 2018;9(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology. 2008;149(8):4151–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Semaan SJ, Kauffman AS. Developmental sex differences in the peri-pubertal pattern of hypothalamic reproductive gene expression, including Kiss1 and Tac2, may contribute to sex differences in puberty onset. Mol Cell Endocrinol. 2022;551:111654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Semaan SJ, Kauffman AS. Daily successive changes in reproductive gene expression and neuronal activation in the brains of pubertal female mice. Mol Cell Endocrinol. 2015;401:84–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Navarro VM. New insights into the control of pulsatile GnRH release: the role of Kiss1/neurokinin B neurons. Front Endocrinol (Lausanne). 2012;3:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lippincott MF, León S, Chan YM, et al. Hypothalamic reproductive endocrine pulse generator activity independent of neurokinin B and dynorphin signaling. J Clin Endocrinol Metab. 2019;104(10):4304–4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Hrabovszky E, Takacs S, Rumpler E, Skrapits K. The human hypothalamic kisspeptin system: functional neuroanatomy and clinical perspectives. Handb Clin Neurol. 2021;180:275–296. [DOI] [PubMed] [Google Scholar]
- 98. Hrabovszky E. Neuroanatomy of the human hypothalamic kisspeptin system. Neuroendocrinology. 2014;99(1):33–48. [DOI] [PubMed] [Google Scholar]
- 99. Hrabovszky E, Sipos MT, Molnar CS, et al. Low degree of overlap between kisspeptin, neurokinin B, and dynorphin immunoreactivities in the infundibular nucleus of young male human subjects challenges the KNDy neuron concept. Endocrinology. 2012;153(10):4978–4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Talbi R, Ferrari K, Choi JH, et al. Characterization of the action of tachykinin signaling on pulsatile LH secretion in Male mice. Endocrinology. 2021;162(8):bqab074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Nagae M, Uenoyama Y, Okamoto S, et al. Direct evidence that KNDy neurons maintain gonadotropin pulses and folliculogenesis as the GnRH pulse generator. Proc Natl Acad Sci U S A. 2021:118(5):e2009156118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.