Abstract
The human brain is active at rest, and spontaneous fluctuations in functional MRI BOLD signals reveal an intrinsic functional architecture. During childhood and adolescence, functional networks undergo varying patterns of maturation, and measures of functional connectivity within and between networks differ as a function of age. However, many aspects of these developmental patterns (e.g. trajectory shape and directionality) remain unresolved. In the present study, we characterised age-related differences in within- and between-network resting-state functional connectivity (rsFC) and integration (i.e. participation coefficient, PC) in a large cross-sectional sample of children and adolescents (n = 628) aged 8–21 years from the Lifespan Human Connectome Project in Development. We found evidence for both linear and non-linear differences in cortical, subcortical, and cerebellar rsFC, as well as integration, that varied by age. Additionally, we found that sex moderated the relationship between age and putamen integration where males displayed significant age-related increases in putamen PC compared with females. Taken together, these results provide evidence for complex, non-linear differences in some brain systems during development.
Keywords: brain development, generalised additive models, participation coefficient, resting-state functional connectivity, sex differences
Introduction
The human brain at rest (i.e. in the absence of a specific task) exhibits spontaneous activity. This activity, however, is not random, as blood oxygen level dependent (BOLD) signal fluctuations reflect the brain’s functional topography (Fox and Raichle 2007), with patterns of BOLD changes across brain regions cohering within identifiable brain networks. Recent resting-state functional connectivity (rsFC) studies have described a high degree of similarity between network rsFC at rest and during task performance, suggesting the presence of an “intrinsic” functional architecture that underlies most (if not all) brain activity (Cole et al. 2014; Gratton et al. 2018; Kraus et al. 2021; Krienen et al. 2014; Seitzman et al. 2019). The development of intrinsic functional networks is, therefore, of vital importance to understanding myriad neural processes. Although many studies have investigated age-related differences in rsFC, the literature contains key gaps, including the need to better characterise linear versus non-linear associations with age of within- and between-network connectivity and network integration. In the present study, we aimed to characterise both linear and non-linear age-related differences in functional network connectivity and integration using a cross-sectional sample diverse in race, ethnicity, and SES from the Lifespan Human Connectome Project in Development (HCP-D).
Much of the fundamental organisation of functional brain networks is largely established at birth with brain regions organised into larger-scale networks including the default mode network (DMN), the frontal parietal network (FPN), and visual and auditory networks (Grayson and Fair 2017). However, the development of individual networks is a continual process as topography changes across childhood and adolescence (Thomason et al. 2015; Keunen et al. 2017; Gilmore et al. 2018). Research characterising these changes has pointed to a shift from a local to a more distributed organisation, where functional connectivity increases between broadly distributed brain regions throughout childhood, adolescence, and into adulthood (Fair et al. 2009; Supekar et al. 2009; Menon 2013; Marek et al. 2015). Yet, there are inconsistencies in the literature regarding the direction of these changes (Stevens 2016). For example, although some reports found that between-network connectivity (e.g. DMN to FPN) increased with age during adolescence (Baker et al. 2015; Marek et al. 2015; Fan et al. 2021), others have found decreases with age in between-network connectivity during adolescence (Bernard et al. 2016; Stevens 2016; Long et al. 2017; Wig 2017). Both positive and negative rsFC relationships with age have also been reported between subcortical regions such as the dorsal striatum and various cortical networks (Supekar et al. 2009; van Duijvenvoorde et al. 2016). Similarly, contrasting associations between age and rsFC between the amygdala and cortical, subcortical, and paralimbic regions have also been found (Gabard-Durnam et al. 2014; Alarcón et al. 2015). These inconsistencies highlight an important problem in developmental science: How researchers theoretically model developmental change.
Adolph and Robinson (2008) argued that a contributing approach to such problems is the hyperfocus on (perhaps arbitrary) starting and ending points. This practice imposes a linear theoretical framework, where development is stable and constant. However, development is a dynamic system, and the shape of its change elucidates the process. Incongruities in developmental research, therefore, may be due in part to the assumption of linear change or difference over development, where age-related change was only modeled linearly without considering non-linear possibilities (Faghiri et al. 2019). Still, non-linear tests that assume a basis function (e.g. binning, polynomials) can mask the true pattern of change by imposing a pre-specified set of functional forms. These predictor functions are not defined by the data and therefore lack the flexibility to properly identify periods of significant change. Generalised additive modeling (GAM), which we used in the present study, avoids this by using local penalised spline bases that flexibly adapt to data. Other recent studies have also adopted these modeling techniques, such as work by Pines et al. (2022), who used GAMs in their investigation of between-network development. GAMs do not impose a particular pre-defined relationship function (e.g. cubic, quadratic) and are not restricted to global polynomial bases (Wood 2004, 2006). They are, therefore, uniquely advantageous to the characterisation of non-linear change and difference.
Another critical source of discrepant findings faced in developmental neuroimaging studies is motion. Motion confounds differ as a function of age, whereby children exhibit an increased tendency for head movement during scan sessions (Satterthwaite et al. 2019). Advancing methods to counteract motion have resulted in a re-evaluation of earlier reported developmental rsFC results, given the previously unrecognised influence of small, spurious movement (Power et al. 2012, 2013; Satterthwaite et al. 2012; Van Dijk et al. 2012). These considerations suggest that implementing rigorous motion control and evaluating non-linear age-related differences in rsFC might elucidate current disagreement in the literature and provide insight into the patterns of distinct developmental stages in rsFC brain connectivity.
Although rsFC suggests how within- and between-network connectivity differs over the course of development, the degree of network integration informs how nodes within a network connect with other brain circuits (Rubinov and Sporns 2010). In other words, the extent to which a node establishes links between its own network (indicating segregation) versus a different network (indicating integration; Marek et al. 2015) is considered to reflect integration of brain networks. This can be measured by the participation coefficient (PC), which provides insight into how brain networks are integrated with one another. PC is a graph theoretical construct that quantifies a node’s connections to networks other than its own network versus its total number of connections (Guimerà and Amaral 2005). In a cross-sectional sample of 195 participants aged 10-26 years, Marek et al. (2015) found that PC displayed non-linear age-related differences in the cingulo-opercular/salience network (CON/SN), DMN, visual network (VIS), and FPN. Specifically, PC in the CON/SN and FPN increased from late childhood to middle adolescence, increased into late adolescence in the VIS, and decreased throughout adolescence in the DMN. Lopez et al. (2020) also found non-linear age-related decreases in PC of frontal regions that were a part of the FPN and DMN as well as dorsal attention (DAN) and ventral attention (VAN) frontal regions but did not find evidence of increases in PC in CON/SN frontal regions in a sample of 615 participants aged 8–21 years. Taken together, both studies show increasing segregation (i.e. decreasing PC) with age in control networks (e.g. brain networks involved in attentional enhancement, goal-appropriate behaviors, response inhibition, etc.), consistent with prior reports of age-related increases in within-network rsFC of such networks (Stevens et al. 2009; Supekar et al. 2010; Sherman et al. 2014; Farrant and Uddin 2015; Solé-Padullés et al. 2016). This may reflect functional specialisation, whereby neural information is primarily exchanged within a network ultimately reducing potential interference from other brain regions (Fornito et al. 2012; Baum et al. 2017). Alternatively, others have reported increases in DMN and CON/SN PC, particularly during late adolescence, in relation to increasing cognitive demands (Marek et al. 2015; Finc et al. 2017). However, the literature describing both linear and non-linear age-related differences in PC at the network level during development remains sparse and in need of replication and extension.
An important caveat to consider when utilising resting-state fMRI is how to properly interpret “connectivity” in the context of what is known about the biological origin of these correlations. rsFC measures are derived from regional correlations in BOLD signals but may have contributions from physiological and neurovascular processes such as cardiac and respiratory oscillations (Birn et al. 2006; Shmueli et al. 2007; Liu 2013; Archila-Meléndez et al. 2020). A recent editorial in Frontiers in Neuroscience by Chen et al. (2020) outlined disadvantages related to the sensitivity and specificity of rsfMRI, namely vascular, arousal, and physiological oscillations that contribute to rsfMRI metrics. Acknowledging that challenges persist, advanced variance removal methods (such as those implemented in the present study) that target signals of non-neuronal origin can improve upon these limitations. Although we refer to rsFC measures in terms of connectivity (or co-activation) in the present manuscript, we remind the reader that rsFC measures represent statistical correlations in BOLD patterns and should not be conflated with a measure of the strength of direct anatomical connections.
The goal of the present study was to characterise age-related differences in within- and between-network rsFC and PC in a large cross-sectional sample of children and adolescents aged 8–21 years from the HCP-D. Given mixed findings in the literature, we evaluated both linear and non-linear differences in rsFC. Although most prior rsFC studies have modeled non-linearity with polynomials, we used GAMs. With the use of local penalised spline bases, GAMs flexibly adapt to data and are not restricted to global polynomial bases which can be too inflexible for modeling non-linearity (Wood 2001). Given the noted sex differences in brain development during our sampled age range, we also investigated sex differences in the association between age and functional network connectivity. Previous studies report decreased putamen-frontal medial cortex activity in boys (van Duijvenvoorde et al. 2019), greater BOLD response in the left putamen in girls to a categorical choice task (Korucuoglu et al. 2020), and volumetric differences such as larger putamen, insula, and amygdalae volumes in boys (Peper et al. 2009). We therefore assessed whether sex moderated any age-related differences in rsFC and PC.
Materials and methods
Sample
The present study included 628 typically developing children, adolescents, and young adults aged 8–21 years from the HCP-D (Harms et al. 2018; Somerville et al. 2018) (Table 1). Participants were recruited across four sites: Harvard University, University of California-Los Angeles, University of Minnesota and Washington University in St Louis. Exclusion criteria for recruitment included (i) premature birth (< 37 weeks gestation); (ii) serious neurological condition (e.g. stroke, cerebral palsy); (iii) serious endocrine condition (e.g. precocious puberty, untreated growth hormone deficiency); (iv) long-term use of immunosuppressants or steroids; (v) any history of serious head injury; (vi) hospitalisation > 2 days for certain physical or psychiatric conditions or substance use; (vii) treatment > 12 months for psychiatric conditions; (viii) claustrophobia; or (ix) pregnancy. Participants provided written informed consent and assent, and parents of participants under 18 years provided written informed consent for their child’s participation. All methods were approved by the Institutional Review Board at Washington University in St Louis (IRB #201603135).
Table 1.
Demographic and acquisition site characteristics of the sample. SD, standard deviation; IQR, interquartile range; SES, socioeconomic status; UCLA, University of California, Los Angeles; UMinn, University of Minnesota; WUSTL, Washington University in St Louis.
| Participants (n = 628) | |
|---|---|
| Mean age in years (SD) | 14.7 (3.9) |
| Range (years) | 8.1–21.9 |
| Sex (% female) | 52.7 |
| Race (%) | |
| Native American/Alaska native | 1.8 |
| Asian | 10.3 |
| Black/African American | 18.3 |
| Native Hawaiian/Pacific Islander | 0.5 |
| White | 58.2 |
| More than one race | 8.9 |
| Unknown | 2.0 |
| Ethnicity (%) | |
| Hispanic | 16.9 |
| Median income ($) | 110,000 |
| IQR ($) | 100,000 |
| SES bracket (%) | |
| Low | 23.4 |
| Medium | 33.5 |
| High | 43.0 |
| Acquisition site (%) | |
| Harvard | 34.0 |
| UCLA | 18.3 |
| UMinn | 26.3 |
| WUSTL | 21.3 |
Image acquisition
Across all four acquisition sites, participants were scanned using a Siemens 3 T Prisma whole-body scanner with a 32-channel head coil and completed T1w (multi-echo 3D MPRAGE sequence; 0.8 mm isotropic voxels, TR/TI = 2500/1000 ms, TE = 1.8/3.6/5.4/7.2 ms, flip angle = 8°, FOV = 256 × 240 × 166 mm3, matrix size = 320 × 300, 208 sagittal slices, in-plane (iPAT) acceleration factor of 2; Mugler et al. 1990; van der Kouwe et al. 2008) and T2w (same spatial resolution using the variable-flip-angle turbo-spin-echo 3D SPACE sequence; TR/TE = 3200/564 ms; same FOV, matrix, and in-plane acceleration; Mugler et al. 2000) structural scans.
Resting-state functional MRI data were acquired using T2*-weighted scans sensitive to the BOLD contrast with a 2D multiband (MB) gradient-recalled echo echo-planar imaging sequence (MB8, TR/TE = 800/37 ms, flip angle = 52°) and 2.0-mm isotropic voxels covering the whole brain (72 oblique-axial slices). For all participants included in the current study, 26 minutes of resting-state scanning were acquired in four runs consisting of 6.5 minutes each. Participants viewed a small, white crosshair fixation on a black screen and were instructed to remain still, remain awake, and blink normally while viewing the fixation crosshair (see Harms et al. 2018 for a detailed protocol description).
Image processing
The HCP processing pipeline has previously been described and used extensively in the literature. Briefly, the fMRI timeseries images were processed in the fMRI-Volume pipeline (Glasser et al. 2013), which concatenates a set of transformations to register the fMRI timeseries to MNI152 standard space and apply them in a one-step spline resampling (i.e. gradient non-linearity and susceptibility distortion corrections, rigid body motion correction, two-step registration to the T1w anatomical with FSL FLIRT+BBR and FreeSurfer’s BBRegister, and non-linear T1w-to-MNI registration). Surfaces were registered using a joint multi-modal registration including information from areal features derived from myelin, resting-state networks, and rfMRI visuotopic maps (Robinson et al. 2014, 2018). The ultimate output was a dense “CIFTI” timeseries file containing the timeseries for cortical surface vertices from both hemispheres and the timeseries for subcortical voxels constrained to gray matter parcels (Glasser et al. 2013).
Due to the shorter duration of resting-state scans compared with the HCP-YA dataset (Harms et al. 2018), rest and task datasets were concatenated, then run through the Multi-run sICA+FIX pipeline (Glasser et al. 2018) for the purposes of denoising. For this analysis, in addition to structured noise removal, we included motion regression and mean grayordinate timeseries regression to address global epochs contaminated by motion and/or respiration (Burgess et al. 2016, Siegel 2017). Timeseries were processed by aggressively removing all variance due to motion from the timeseries, followed by unaggressive removal of variance due to spatial noise as detected by FIX. The mean grayordinate timeseries was computed and removed to generate the final timeseries. For computing correlations, timepoints with filtered frame displacement (FD) > 0.2 mm were excluded from calculations. Additionally, time points were removed if the filtered FD was ≥0.2 mm (Power 2019). Following Multi-run sICA+FIX, the resting-state data was dissociated from the task data and parcellated, and the Fisher z-transformed Pearson correlation (z(r)) between the timeseries of all parcels (nodes) was computed for all participants using a previously defined parcellation scheme (161 and 172 distinct parcels for the left and right hemisphere, respectively; Gordon et al. 2016) along with 19 subcortical structures (9 bilateral structures per hemisphere and 1 brainstem parcel) forming a 352 × 352 connectivity matrix, using a combination of “Connectome Workbench” (Marcus et al. 2011) and custom Matlab code.
Connectivity matrices were further processed using the Brain Connectivity Toolbox (Rubinov and Sporns 2010). The PC (Guimera and Amaral, 2005) is the ratio of the number of above-threshold connections that a given node (parcel) has to nodes of other networks (between-network connectivity) relative to its affiliated network (within-network connectivity; Rubinov and Sporns 2010; Lopez et al. 2020). Per subject, each correlation matrix was thresholded from 1 to 25% of the top edges in 1% increments. The thresholded graphs were then used to compute the PC for each node, where community assignment was defined by the original parcellation.
Statistical analyses
We provide an overview of our general approach, followed by a more detailed description. First, generalised additive models (GAM) were conducted for all within- and between-network connectivity and PC age-related differences based on our assumption that differences may be non-linear. Next, smooth terms from the GAMs were tested for non-linearity. Age-related differences that were determined to be linear were subsequently also fit using linear modeling. For non-linear differences, periods of significant difference in network connectivity and PC slopes were identified. Analyses were false discovery rate (FDR) corrected for multiple comparisons. Scatterplots were created using the R package “ggplot2” (Wickham 2016) and illustrate differences in rsFC as a function of age, not accounting for covariates.
We analyzed the associations between age and (i) within-network rsFC of the 12 networks in the Gordon parcellation [default (DMN), parieto-occipital (PON), frontoparietal (FPN), salience (SAL), cingulo-opercular (CON), medial parietal (MPN), dorsal attention (DAN), ventral attention (VAN), visual (VIS), somatomotor body (SmB), somatomotor head (SmH), and auditory (AUD)]; (ii) between-network rsFC of 12 cortical networks; (iii) between-network rsFC of 12 cortical networks to 7 subcortical regions [amygdala (AMYG), hippocampus (HIP), nucleus accumbens (NAc), caudate nucleus (CN), pallidum (PAL), putamen (PUT), thalamus (THA)], and the cerebellum (CB); (iv) between-network rsFC of those same 7 subcortical structures to the cerebellum; and (v) PC for the 12 cortical networks, 7 subcortical structures and the cerebellum. In total, rsFC was FDR-corrected for 210 comparisons, and PC was FDR-corrected for 20 comparisons. rsFC for subcortical left and right hemispheres were averaged to produce one total rsFC metric for each subcortical structure.
Network rsFC and PC age-related differences
All age-related differences were evaluated for non-linearity using GAMs in the R package “mgcv” version 1.8-38. With the use of penalised splines, GAMs can flexibly model non-linear relationships. Non-linearities are estimated using restricted maximum likelihood (REML), where smooth terms are penalised for increasing complexity (Wood 2001). Default “mgcv” smoothing parameters were used, where k (i.e. knots) was set to 10, indicating the maximum effective degrees of freedom (EDF) the model smooth could use. For k = 10, the EDF for the smooth term “age” was < 3.9 for all rsFC and PC models, suggesting that this function space was large enough for the expected wiggliness of the true function to be accommodated (Wood 2017). rsFC was calculated as the average of the (Fisher Z-transformed) pairwise correlations within (e.g. DMN, FPN, CON) and between (e.g. DMN-FPN, DMN-CON) each Gordon cortical network, subcortical region, and cerebellum. For PC (calculations are described in the Image Processing section), nodes (parcels) were grouped by network, and the mean network PC was calculated for each subject. Age was entered into the GAM as a smooth factor to capture any important non-linear patterns over development. Covariates included sex, mean framewise displacement (FD), and acquisition site. Example R syntax modeling non-linear difference is as follows:
gam(Network ~ s(Age) + Sex + Mean FD + Acquisition Site).
Next, a restricted likelihood ratio test was used to determine whether the non-linearity of the GAMs was a significantly better fit than a linear model. This was accomplished with the R package “RLRsim” (Scheipl et al. 2008). Using the function “exactRLRT,” non-linearity was determined by examining if the variance of the random effect was equal to zero. If the test for non-linearity returned a value of P < 0.05, the non-linear GAM was determined to be the appropriate fit (Larsen et al. 2020; Vandekar et al. 2015). This test was not adjusted for multiple comparisons.
For models where the smooth term was determined to be non-linear, posterior simulations of the first derivative of the spline term were generated to identify periods of change in the slope between age and rsFC and age and PC. This was accomplished with the “confint.fderiv” function in the R package “gratia” (Simpson & Singmann 2018). This function produces plots of the first derivative of the splines (slopes) along with the 95% point-wise confidence intervals. Periods where the confidence interval around the first derivative excludes 0 represent the developmental periods with the strongest evidence for age-related differences (i.e. increasing or decreasing slope).
If the GAM model was rejected by the test for non-linearity, linear models examining age-related differences in connectivity and PC were fit using the “lm” function in the R package “stats” (R Development Core Team, 2020). Linear model estimated beta values and associated P-values were investigated to determine if the regression slopes were significantly different from zero. Specifically, if FDR q-values (e.g. p-values corrected for multiple comparisons) were q < 0.05, the regression slope was determined to be significantly different from zero, indicating a difference in connectivity over age. Standardised beta values were used to examine the rate of change in connectivity strength and PC over our age span.
Sex as a moderator of age-related differences in rsFC and PC
To investigate if sex moderated the relationship between age and rsFC and PC, an interaction term of age by sex was entered into the model. Results were FDR-corrected for multiple comparisons (see above for correction breakdown). Significant interaction effects were probed via simple slopes with the “probe_interaction” function in the R package “interactions” (Long 2019).
Results
Age-related differences in within- and between-network rsFC
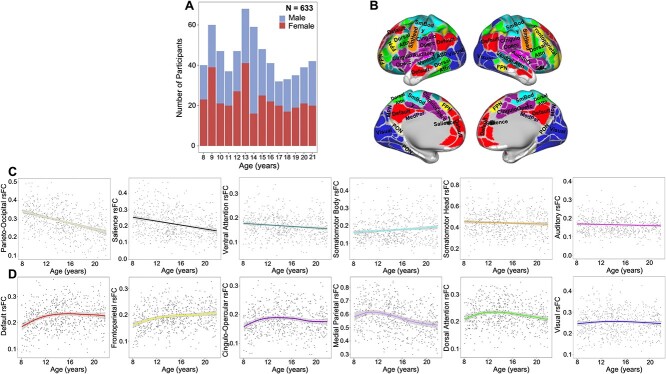
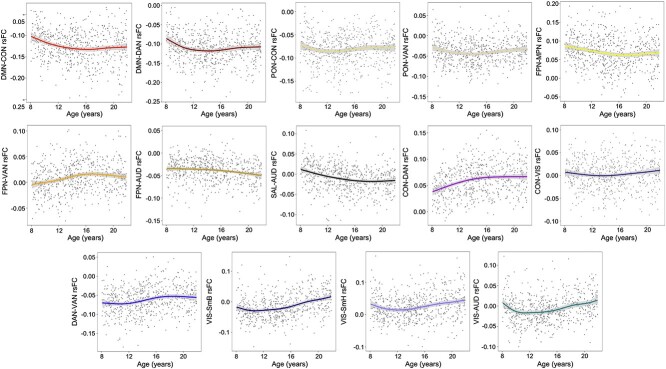
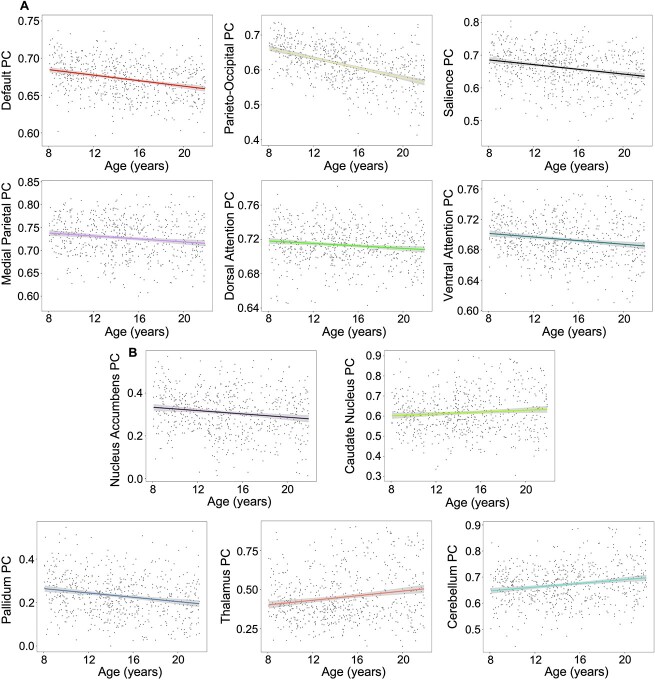
Age-related differences for within- and between-network connectivity were estimated for the 12 Gordon networks. Many of these age-related differences were best captured by linear relationships (Fig. 1). In sum, 5 within- and 14 between-cortical network connectivity patterns displayed significant non-linear age-related differences (Figs 2D and 3) that passed FDR correction. Most non-linear within-network differences with age followed an inverted U-shaped curve where rsFC increased significantly during childhood and then either remained relatively stable or even decreased in adolescence. In contrast, within the medial parietal network, rsFC decreased significantly during middle adolescence (Supplementary Fig. S1). With the exception of the visual network, all other sensory networks followed linear within-network rsFC patterns. Linear within-network age-related differences in rsFC mostly decreased, whereas somatomotor body rsFC significantly increased from ages 8–21 (Fig. 2C). Non-linear between-network rsFC age-related differences occurred between higher-order cognitive, attention, and sensorimotor networks. Specifically, CON-DAN and FPN-VAN rsFC increased significantly from childhood to middle adolescence and remained stable into late adolescence. The DMN displayed a period of decreasing rsFC with both the CON and DAN from childhood to early adolescence, followed by a period of stability into late adolescence. The visual network exhibited periods of increasing and decreasing rsFC with other sensory and motor networks during childhood (decreasing) and late adolescence (increasing) (Fig. 3 and Supplementary Fig. S2). Linearly, rsFC increased between sensory/motor networks and the PON, DMN, CON, DAN, and VAN, but decreased between sensory/motor networks and the FPN and SAL. However, the CON and DAN also displayed decreasing rsFC with other primary sensory networks (i.e. CON-AUD and DAN-VIS). rsFC between higher order/associative networks linearly increased and decreased (Fig. 1), whereas linear rsFC differences between sensory/motor networks with age all displayed increasing connectivity.
Fig. 1.
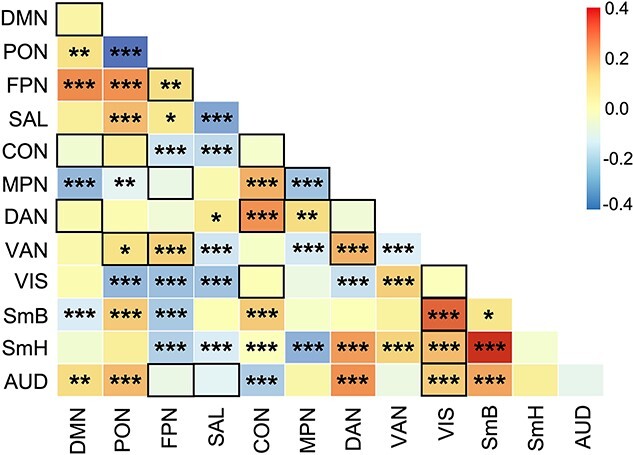
Linear correlation matrix of age with within- and between-cortical network resting state functional connectivity (main and below diagonal, respectively). Colored boxes and legend represent standardised beta values estimated from models that included covariates. Black outlined boxes represent non-linear relationships with age, which were modeled linearly here for comparison of overall magnitude of age-related differences. DMN, default mode network; PON, parieto-occipital network; FPN, frontoparietal network; SAL, salience network; CON, cingulo-opercular network; MPN, medial parietal network; DAN, dorsal attention network; VAN, ventral attention network; VIS, visual network; SmB, somatomotor body network; SmH, somatomotor head network; AUD, auditory network; *FDR-corrected q-values, q < 0.05; **q < 0.01; ***q < 0.001.
Fig. 2.
(A) Age by sex sample distribution. (B) Gordon 333 parcellation adapted from Gordon et al. (2016). (C) Linear within-network age-related differences in rsFC of the six networks with a statistically significant (FDR-corrected) linear relationship with age. (D) Non-linear within cortical network age-related differences in rsFC for the 6 networks with a statistically significant non-linear relationship with age, based on a restricted likelihood ratio test of the GAM. rsFC, resting state functional connectivity. Shaded bands around lines represent 95% confidence interval. Scatterplots represent differences in rsFC as a function of age and do not account for covariates.
Fig. 3.
Non-linear between cortical network rsFC. DMN, default mode network; PON, parieto-occipital network; FPN, frontoparietal network; SAL, salience network; CON, cingulo-opercular network; MPN, medial parietal network; DAN, dorsal attention network; VAN, ventral attention network; VIS, visual network; SmH, somatomotor hand network; SmM somatomotor mouth network; AUD, auditory network; rsFC, resting state functional connectivity. Shaded bands around lines represent 95% confidence interval. Scatterplots represent differences in rsFC as a function of age and do not account for covariates.
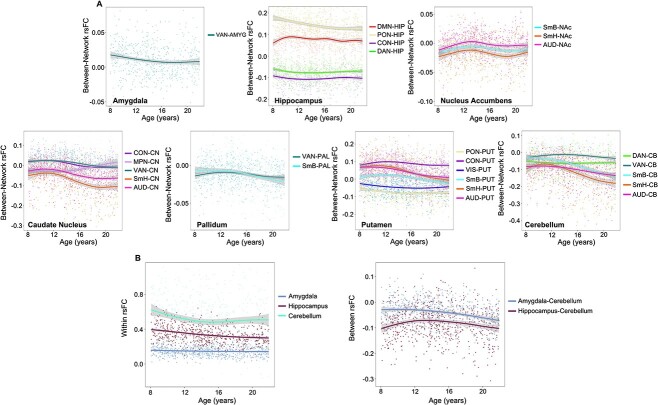
As with age-related differences in cortical connectivity, many age-related differences in connectivity between cortical networks and subcortical and cerebellar regions were best characterised by linear relationships (see Fig. 4 for indication of those that passed FDR correction). Results revealed significant linear declines in rsFC between sensory/motor networks and both the PAL and THA, whereas significant linear increases in rsFC were observed between sensory/motor networks and both the AMYG and HIP (Fig. 4A).
Fig. 4.
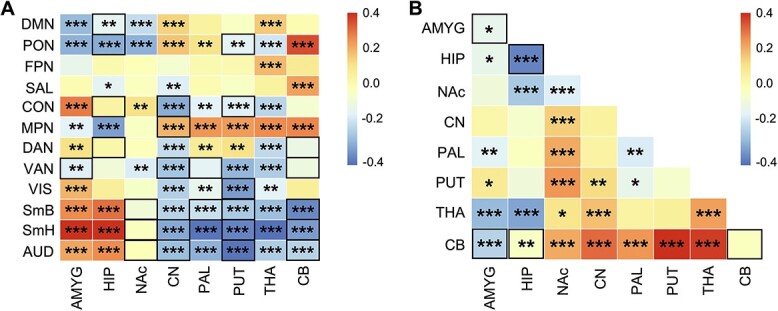
(A) Linear correlation matrix of age with between cortico-subcortical rsFC. (B) Linear correlation matrix of age with within- (main diagonal) and between-subcortical network (below diagonal) rsFC. Colored boxes and legend represent standardised beta values. Black outlined boxes represent non-linear relationships with age, which were modeled linearly here for comparison. *FDR-corrected q-values, q < 0.05; **q < 0.01; ***q < 0.001. DMN, default mode network; PON, parieto-occipital network; FPN, frontoparietal network; SAL, salience network; CON, cingulo-opercular network; MPN, medial parietal network; DAN, dorsal attention network; VAN, ventral attention network; VIS, visual network; SmB, somatomotor body network; SmH, somatomotor head network; AUD, auditory network; AMYG, amygdala; HIP, hippocampus; NAc, nucleus accumbens; CN, caudate nucleus; PAL, pallidum; PUT, putamen; THA, thalamus; CB, cerebellum.
Cerebello-striatal-thalamic circuits linearly increased in rsFC with higher-order cognitive and association networks. Interestingly, cortical-thalamic rsFC all displayed linear differences, where rsFC decreased between the thalamus and both cortical attention and sensory/motor networks with increasing age. Subcortically, between cerebello-striatal-thalamic circuits largely increased in rsFC with increasing age (Fig. 4B). Additionally, 26 connectivities between cortical networks, subcortical structures, and cerebellum, and 5 within and between subcortical structures and cerebellum displayed significant non-linear age-related differences (Fig. 4A). Non-linear between cortico-subcortical rsFC largely occurred between subcortical structures and cortical sensory, motor, and attention networks with periods of significantly decreasing connectivity (Supplementary Fig. S3). In contrast, the nucleus accumbens exhibited periods of both increasing rsFC with sensory/motor networks from childhood to early adolescence and decreasing rsFC with sensory/motor networks for a short period during middle adolescence. Non-linear age-related differences within subcortical rsFC were exclusive to within the AMYG, HIP, and CB, as well as between the AMGY-CB and HIP-CB (Fig. 5B and Supplementary Fig. S4).
Fig. 5.
(A) Non-linear differences between cortico-subcortical and cortico-cerebellum rsFC. Plots are aggregated by subcortical region. (B) Non-linear differences within and between subcortico-cerebellum rsFC. rsFC, resting-state functional connectivity. Shaded bands around lines represent 95% confidence interval.
To illustrate connectivity differences by discrete developmental stage, we performed a series of supplementary analyses. See Supplementary Materials for complimentary results and associated figures (Supplementary Statistical Analyses and Results and Supplementary Figs. S5A–C and S6).
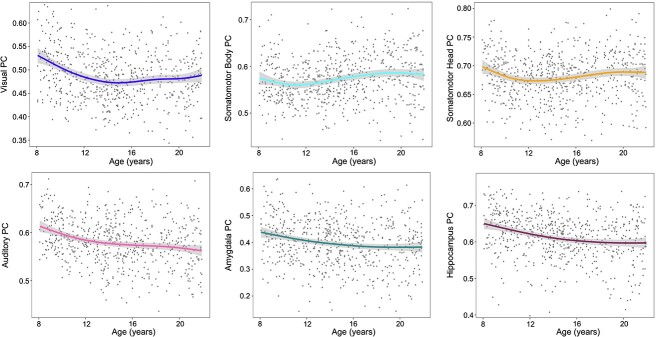
Age-related differences in PC
PC associations with age were estimated for the 12 Gordon networks, 7 subcortical structures, and the cerebellum. Four network PCs (VIS, SmB, SmH, and AUD) displayed non-linear differences (Fig. 6). Amygdala and hippocampal PC age-related differences were also non-linear. Periods of significant change identified from posterior simulations of the first derivative of the spline term “age” are displayed in Supplementary Fig. S7 and show a significant decrease in rsFC between AMYG-CB from 12 to 21 years. Additionally, between HIP-CB rsFC displayed an initial period of increasing connectivity from 8 to 12 years followed by a period of decreasing connectivity from 18 to 20 years.
Fig. 6.
Non-linear differences in cortical and subcortical PC. PC, participation coefficient. Shaded bands around lines represent the 95% confidence interval.
Significant linear decreases in network PC across our sampled age span were seen in the DMN, PON, SAL, MPN, DAN, and VAN (Fig. 7). Subcortically, NAc and PAL PCs displayed significant linear decreases with age. In contrast, CN, THA, and CB PCs displayed significant linear increases with age (Fig. 7B). Results were FDR-corrected for multiple comparisons.
Fig. 7.
(A) Linear differences in cortical PC. All plots represent significant linear differences that passed FDR correction. (B) Linear differences in subcortical PC. All plots represent significant linear differences that passed FDR correction. PC, participation coefficient; shaded bands around lines represent the 95% confidence interval.
Sex moderates the relationship between age and putamen PC
There was a significant interaction between age and sex for putamen PC (β = −0.60, q = 0.002.) To decompose this interaction, simple slopes were examined. Males displayed a significant increase in putamen integration (i.e. increasing PC) as they aged (t = 3.25, P < 0.001). There was no significant difference in putamen PC with age for females (t = −1.95, p = 0.05) (Fig. 8). There were no other significant age by sex interactions for rsFC or PC.
Fig. 8.
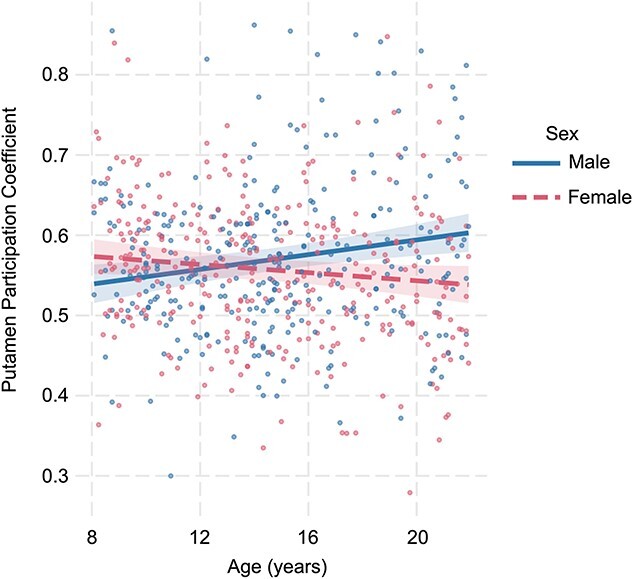
The moderating role of sex in the relationship between putamen PC and age.
Discussion
The present study evaluated age-related differences in within- and between-network rsFC and network-level PC. Current literature reports conflicting results regarding the directionality of functional connectivity developmental differences (Hwang et al. 2013; Satterthwaite et al. 2013; Gu et al. 2015; Gracia-Tabuenca et al. 2021). Although sample composition and other methodological differences may influence these diverging results, testing brain maturation trajectories for non-linearity can potentially reduce these biases (Zuo et al. 2017). Development is complex and does not unfold in fixed, uniform epochs. With this knowledge, it is important that we statistically treat these processes as such and acknowledge that patterns of age-related difference may reveal significant mechanistic information (e.g. plasticity of network malleability with increasing age; Ailion et al. 2022). Therefore, we estimated linear and non-linear age-related differences in rsFC and integration. Six main observations emerged from this study: (i) within- and between-network connectivity displayed both linear and non-linear age-related differences; (ii) within-network rsFC in cognitive control networks (i.e. DMN, FPN, and CON) increased from childhood to early adolescence, whereas from middle to late adolescence, within-network rsFC for many association networks (i.e. PON, MPN, SAL, CON, and DAN) decreased; (iii) between-network rsFC of cortical networks and subcortical regions varied significantly by developmental stage, where sensorimotor networks increased in rsFC with the amygdala and hippocampus at each stage of development but decreased in rsFC with cerebellar-striatal-thalamic circuits from early to late adolescence; (iv) network-level PC largely displayed linear age-related differences, with the exception of non-linear differences in the visual, somatomotor body, somatomotor head, and auditory networks and subcortically in the hippocampus and amygdala; (v) differences in PC mostly occurred between childhood to early adolescence; and (vi) males displayed a significant increase in putamen integration over our sampled age span.
Age-related differences in rsFC
Our results show both linear and non-linear age-related differences in rsFC. Non-linear within-network differences were observed in cognitive control and visual networks and included the default, frontoparietal, cingulo-opercular, medial parietal, dorsal attention, and visual networks. The default, frontoparietal, cingulo-opercular, dorsal attention, and visual networks increased significantly in within-network rsFC from our earliest sampled age of 8 to 11–13 years (Fig. 2). Similar increases in within-network rsFC have been reported previously through the early adolescent period (van Duijvenvoorde et al. 2016; Jiang et al. 2018; Fan et al. 2021) and might reflect enhanced specialisation and segregation of neural circuits that facilitate internal cognition, such as working memory, and control of executive functions. During childhood and adolescence, the ability to exercise cognitive control increases, though its development is highly protracted and continues into adulthood (Somerville and Casey 2010). Our results support the current literature regarding observed increases in connectivity within control networks during this developmental period. However, we found that these age-related patterns were non-linear in nature, potentially reflecting more complex age-related periods of functional refinement. As participants reached late adolescence, we found evidence for decreased within-network rsFC by the developmental stage. These differences were exclusive to executive and attentional control networks. This pattern has previously been documented in other developmental rsFC studies and has been hypothesised to mirror the pruning and refinement of signal transmission (Stevens et al. 2009; Uddin et al. 2011; Marek et al. 2015).
Cortically, non-linear age-related differences in connectivity were observed between higher-order cognitive networks and between primary sensory and motor networks (Fig. 3). The human brain is a complex non-linear system in which age-related changes are adaptive, dynamic, and variable (Bullmore and Sporns 2009; Hutka et al. 2013). Connectivity between spatially distinct networks may provide the foundation for information processing. These complex systems and their functions are ultimately shaped by interactions between their component parts, which modify with age as a product of experience. In the present study, non-linear and linear cortico-cortical connectivity differences observed between cognitive networks varied by developmental stage, largely decreasing in strength. This pattern might be indicative of age-related increases in connection efficiency (Cherniak 1994; Achard and Bullmore 2007). However, between sensory, motor, and attention network connectivity displayed a generally increasing trend, both linearly and non-linearly, over our age span (Figs. 1 and 3). This was particularly apparent from middle to late adolescence (Fig. 3). Significant non-linear rsFC increases between primary networks during this developmental period were exclusive to between VIS and SmH, SmB, and AUD networks (Supplementary Fig. S2). This suggests increased differentiation of a sensory and motor circuit during the latter part of adolescence. Váša et al. (2020) found that sensory and motor regions were strongly connected by age 14 but continued to increase into early adulthood, ultimately reflecting a conservative (e.g. prolonged) maturation.
Cortico-subcortical age-related differences in rsFC also displayed variable patterns (Figs. 4 and 5). Interestingly, many non-linear cortical–subcortical connectivity differences were observed between subcortical regions and primary sensorimotor and attention networks. This suggests that some long-range connections between primary cortical networks and subcortical regions mature non-incrementally, with variable periods of significant increases and decreases in connectivity. Significant linear age-related differences in rsFC also occurred largely between cortical primary sensorimotor and attention networks and subcortical regions. From childhood to early adolescence, sensorimotor and auditory networks increased in connectivity with the amygdala, hippocampus, and nucleus accumbens (Fig. 5A and Supplementary Fig. S5B). With the exception of the nucleus accumbens, these connections continued to increase from early to middle adolescence and again from middle to late adolescence. Communication between the hippocampus and amygdala with sensory and motor regions is hypothesised to support the modulation of subjective sensorial experiences induced by emotions (Toschi et al. 2017). These networks co-activate during emotion processing, potentially facilitating action readiness and adaptive responses to affective stimuli (de Gelder et al. 2004; Pichon et al. 2009; Schürmann et al. 2011; Grèzes et al. 2014). Connectivity between these networks starts to mature prior to childhood and shapes social-emotional behaviors (Gabard-Durnam et al. 2018). Our results suggest that communication between these circuits continues to strengthen into late adolescence, reflecting developmental stimulus-response learning that guides appropriate behavioral reactivity to environmental conditions. Conversely, we found decreased rsFC between sensory and motor networks and cerebellar-striatal-thalamic circuits. This was particularly evident from early to middle adolescence and again from middle to late adolescence. Collectively, these networks communicate to support goal-directed behaviors, movement execution, and habit formation (Doyon et al. 2003; Rădulescu et al. 2017). The observed developmental stage-dependent decreases in rsFC between these networks in the present study suggest significant connection refinement, where communication becomes increasingly efficient with age.
Subcortically and in the cerebellum, rsFC displayed non-linear age-related differences between the hippocampus and cerebellum, and between the amygdala and cerebellum (Fig. 5B). From middle to late adolescence, rsFC between the amygdala and cerebellum decreased significantly. A small (n = 15) study published in 2018 by Habas described an intrinsically connected network between the cerebellum and amygdala in adults, and suggested that this circuit may be implicated in sensorimotor, emotional, and motivational assimilation of somatosensory stimuli (Habas 2018). Therefore, our results suggest that circuits involved in such integrations mature into late adolescence, supporting cognitive processes involved in information processing. Striatal-thalamic regions increased in connectivity with the cerebellum at each stage of development, particularly from early to middle adolescence and from middle to late adolescence (Fig. 4B and Supplementary Fig. S5B). Animal models have provided support for the idea that cerebellar-striatal circuitry is mediated by nuclei in the thalamus (Ichinohe et al. 2000). While regions involved in these circuits support sensory and motor control, they are also critical for cognitive functions, language processing, and affective control (Doya 2000; Laforce and Doyon 2001; Booth et al. 2007; Ide and Li 2011). These processes are developmentally complex and display increased efficiency through adolescence and early adulthood (Kail and Ferrer 2007; Luna et al. 2010; Kar et al. 2013). Taken together, the observed increases in cerebellar-striatal-thalamic rsFC complement the developmental maturation of behaviors associated with these circuits.
Age-related differences in network integration
Cortical and subcortical PCs displayed both linear and non-linear differences over our age span. Cortically, non-linear age-related differences included visual, somatomotor head, somatomotor body, and auditory networks (Fig. 6). The non-linear nature of cortical sensory and motor network integration (as measured by PC) was similarly seen in their between-network rsFC strength. These results point to periods of visual, auditory, and somatomotor head network segregation (e.g. network nodes linking primarily to other nodes within their home network) and a period of somatomotor body integration (e.g. network nodes establishing links to other networks). Visual, auditory, and somatomotor head network segregation largely occurred from childhood to early adolescence and suggests that this developmental period is characterised by sensorimotor circuit modularisation (Supplementary Fig. S7). These results are supported by the current literature, which indicates that primary sensory and motor regions are relatively specialised prior to childhood (Lyall et al. 2015; Gao et al. 2017, 2019; Grayson and Fair 2017). Significant linear age-related decreases in cortical PC were observed in the default, parieto-occipital, salience, medial parietal, and ventral attention networks (Fig. 7A). These cortical networks appear to become increasingly segregated from childhood through late adolescence, potentially reflecting within-network fine-tuning of specialised functions.
Subcortically, non-linear PC differences were observed in the amygdala and hippocampus (Fig. 6). Significant decreases in PC for these areas were mainly observed during the transition from childhood to early adolescence—a pattern similarly noted in cortical networks. This early segregation coupled with the observed non-linear age-related differences in PC suggests a pattern by which primary sensory and motor networks, hippocampus, and amygdala increase nodal connectivity within their home networks but vary in their integration with outside networks through adolescence. Alternatively, the caudate, thalamus, and cerebellum displayed linearly increasing integration with remote networks (Fig. 7B). These results are consistent with prior studies showing that both subcortical structures and the cerebellum contain integrative hubs (Hwang et al. 2017; Garrett et al. 2018; Greene et al. 2020). Greene et al. (2020) found that the caudate and thalamus were highly integrated with the default and control networks and suggested that this integration may occur via cortico-striato-thalamo-cortical loops. Supporting this hypothesis, we found that cerebellar-striato-thalamic circuits increased in connectivity with the default, parieto-occipital, and medial parietal networks from early to middle adolescence and again from middle to late adolescence. These hubs are ultimately integral for large-scale systems that support stimulus feedback and higher-order cognition.
Finally, we found a significant moderating effect of sex in the relationship between age and putamen PC. Specifically, putamen integration significantly increased with age for males. There was no significant difference in putamen PC over the age span for females. The extant literature points to differential trajectories of putamen development by sex, though these findings are primarily volumetric in nature. Wierenga et al. (2018) found that in a sample aged 3–21 years, males showed greater variability in putamen volume than did females. They postulated that these differences may relate to male-biased disorders, such as schizophrenia. Taken together, these results provide support for male-specific differences in putamen development warranting further research into both mechanisms by which this may occur and behavioral and/or clinical relevance.
Regarding statistical versus clinical significance, most of our findings are small in effect size (see Supplementary Fig. S1 and S2). Although results reported here are statistically significant and survived correction for multiple comparisons, further investigation is necessary to evaluate whether these differences have behavioral or cognitive implications.
rsFC and PC—incongruencies?
Readers might wonder why some networks, such as the parieto-occipital, displayed age-related decreases (or increases) in both within-network rsFC and PC. Although this could be interpreted as inconsistent, it is important to consider certain confounds and mathematical properties inherent to such measures. First, PC considers both the between and within network weights in its calculation. One can have both decreasing within-network rsFC and decreasing between-network PC if the decreases between networks are larger than the decreases within. Additionally, while rsFC quantifies the magnitude of the node’s correlation, PC measures the diversity/distribution of the node’s connections (Power et al. 2013). Second, rsFC is strongly related to a network’s community size (Bertolero et al. 2017). The parieto-occipital network, for example, is comprised of eight parcels in total, making it among the smaller Gordon networks. These factors can result in (i) a weak or non-significant correlation between network rsFC and PC, and (ii) a correlation that is positive or negative. We therefore argue that these considerations (among others) make the seemingly contradictory rsFC and PC results for some communities possible and, in some cases, likely.
Limitations
A primary limitation of the present study is its cross-sectional design. This precluded us from analyzing within-person changes in rsFC and PC across our age span, which is necessary for capturing true developmental change. However, longitudinal data collection is now complete in the HCP-D and thus a longitudinal analysis will be possible in the future once all imaging sessions are processed. A second limitation is that participants younger than 8 years of age were excluded from the present study due to differences in collection protocol (Harms et al. 2018). Furthermore, some primary sensory and motor regions reach maturity during the first 2 years of life (Lyall 2014; Gao et al. 2019). Thus, we were unable to characterise developmental differences prior to age 8 that likely contribute to maturational differences. Third, children have a higher propensity for movement while in the scanner, sometimes resulting in spurious functional connectivity effects. Although we implemented rigorous motion control, motion-related effects on rsFC might still impact the observed age-related differences in functional connectivity.
Conclusions
The results presented here illustrate unique information about linear and non-linear differences within- and between-network resting-state connectivity and network integration. Prior work characterising these age-related modifications has largely either assumed a linear change over development or modeled non-linear change with polynomials. Here, we used GAM, which uses local penalised spline bases to flexibly adapt to data. These are not restricted to global polynomial bases which can be too inflexible for modeling non-linearity (Wood 2001). We found evidence for both linear and non-linear differences in connectivity cortically, subcortically and in the cerebellum from ages 8 to 21 years. Cortically, within-network rsFC increased in higher-order cognitive networks from childhood to early adolescence and decreased from middle to late adolescence, whereas between-network connectivity showed variable patterns across the developmental period. Primary sensory networks increased in rsFC with the amygdala and hippocampus at each developmental stage and decreased in rsFC with cerebellar-striatal-thalamic circuits from early to late adolescence. Subcortically, rsFC between the cerebellum and striatal-thalamic networks increased at each stage of development.
Supplementary Material
Acknowledgments
We wish to thank Randy L. Buckner, PhD, for his conceptual help during the revision process
Contributor Information
Ashley F P Sanders, Department of Psychiatry, Washington University School of Medicine, St Louis, MO 63110, USA.
Michael P Harms, Department of Psychiatry, Washington University School of Medicine, St Louis, MO 63110, USA.
Sridhar Kandala, Department of Psychiatry, Washington University School of Medicine, St Louis, MO 63110, USA.
Scott Marek, Department of Radiology, Washington University School of Medicine, St Louis, MO 63119, USA.
Leah H Somerville, Department of Psychology and Center for Brain Science, Harvard University, Cambridge, MA 02138, USA.
Susan Y Bookheimer, Department of Psychiatry and Biobehavioral Sciences, University of California Los Angeles School of Medicine, Los Angeles, CA 90095, USA.
Mirella Dapretto, Department of Psychiatry and Biobehavioral Sciences, University of California Los Angeles School of Medicine, Los Angeles, CA 90095, USA.
Kathleen M Thomas, Institute of Child Development, University of Minnesota, Minneapolis, MN 55455, USA.
David C Van Essen, Department of Neuroscience, Washington University School of Medicine, St Louis, MO 63110, USA.
Essa Yacoub, Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN 55455, USA.
Deanna M Barch, Department of Psychiatry, Washington University School of Medicine, St Louis, MO 63110, USA; Department of Psychological and Brain Sciences, Washington University, St Louis, MO 63130, USA.
CRediT authors statement
Ashley Sanders (Conceptualisation, Formal analysis, Investigation, Methodology, Visualisation, Writing—original draft, Writing—review and editing), Michael P. Harms (Conceptualisation, Data curation, Investigation, Methodology, Project administration, Supervision, Writing—original draft, Writing—review and editing), Sridhar Kandala (Data curation, Formal analysis, Methodology, Writing—original draft, Writing—review and editing), Scott Marek (Conceptualisation, Methodology, Writing—review & editing), Leah Somerville (Conceptualisation, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing—original draft, Writing—review and editing), Susan Y. Bookheimer (Conceptualisation, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Writing—original draft, Writing—review and editing), Mirella Dapretto (Conceptualisation, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Writing—original draft, Writing—review and editing), Kathleen Thomas (Conceptualisation, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Writing—original draft, Writing—review and editing), David Van Essen (Conceptualisation, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Writing—original draft, Writing—review and editing), Essa Yacoub (Conceptualisation, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Writing—original draft, Writing—review and editing), Deanna M. Barch (Conceptualisation, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing—original draft, Writing—review and editing.
Funding
This work was supported by HCP-D: Mapping the Human Connectome During Typical Development (U01MH109589), Connectome Coordination Facility I (5R24MH108315-05), Connectome Coordination Facility II (1R24MH122820-01), the McDonnel Center for Systems Neuroscience at Washington University and the Office of the Provost at Washington University. A.F.P.S. was supported by the National Institutes of Health grants T32 MH100019 (PIs: Joan L. Luby and Deanna M. Barch at Washington University in St Louis).
Conflict of interest statement: None declared.
Data availability
The HCP-Development Lifespan 2.0 Release is available for download from the HCP website at: https://www.humanconnectome.org/study/hcp-lifespan-development/data-releases. This website also contains detailed release notes: https://www.humanconnectome.org/study/hcp-lifespan-development/document/hcp-development-20-release. This release includes cross-sectional visit 1 (V1) preprocessed structural and functional imaging data, unprocessed V1 imaging data for all included modalities (structural, resting state fMRI, task fMRI, diffusion, and ASL), and non-imaging demographic and behavioral assessment data from 652 HCP-Development (HCP-D, ages 5–21) healthy participants. This manuscript used data from 628 HCP-D, some of which are not currently available for download.
References
- Achard S, Bullmore E. Efficiency and cost of economical brain functional networks. PLoS Comput Biol. 2007:3(2):e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolph KE, Robinson SR. In defense of change processes. Child Dev. 2008:79(6):1648–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ailion AS, You X, Mbwana JS, Fanto EJ, Krishnamurthy M, Vaidya CJ, Sepeta LN, Gaillard WD, Berl MM. Functional connectivity as a potential mechanism for language plasticity. Neurology. 2022:98(3):e249–e259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcón G, Cservenka A, Rudolph MD, Fair DA, Nagel BJ. Developmental sex differences in resting state functional connectivity of amygdala sub-regions. NeuroImage. 2015:115:235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archila-Meléndez ME, Sorg C, Preibisch C. Modeling the impact of neurovascular coupling impairments on BOLD-based functional connectivity at rest. NeuroImage. 2020:218:116871. 10.1016/j.neuroimage.2020.116871. [DOI] [PubMed] [Google Scholar]
- Baker ST, Lubman DI, Yucel M, Allen NB, Whittle S, Fulcher BD, Zalesky A, Fornito A. Developmental changes in brain network hub connectivity in late adolescence. J Neurosci. 2015:35(24):9078–9087. 10.1523/JNEUROSCI.5043-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum GL, Ciric R, Roalf DR, Betzel RF, Moore TM, Shinohara RT, Kahn AE, Vandekar SN, Rupert PE, Quarmley M, et al. Modular segregation of structural brain networks supports the development of executive function in youth. Curr Biol. 2017:27(11):1561–1572.e1568. 10.1016/j.cub.2017.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Orr JM, Mittal VA. Differential motor and prefrontal cerebello-cortical network development: evidence from multimodal neuroimaging. NeuroImage. 2016:124(Pt A):591–601. 10.1016/j.neuroimage.2015.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolero MA, Yeo BTT, D'Esposito M. The diverse club. Nat Commun. 2017:8(1):1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Diamond JB, Smith MA, Bandettini PA. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. NeuroImage. 2006:31(4):1536–1548. 10.1016/j.neuroimage.2006.02.048. [DOI] [PubMed] [Google Scholar]
- Booth JR, Wood L, Lu D, Houk JC, Bitan T. The role of the basal ganglia and cerebellum in language processing. Brain Res. 2007:1133(1):136–144. 10.1016/j.brainres.2006.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009:10(3):186–198. 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Burgess GC, Kandala S, Nolan D, Laumann TO, Power JD, Adeyemo B, Harms MP, Petersen SE, Barch DM. Evaluation of denoising strategies to address motion-correlated artifacts in resting-state functional magnetic resonance imaging data from the human connectome project. Brain Connect. 2016:6(9):669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, Herman P, Keilholz S, Thompson GJ. Origins of the resting-state fMRI signal. Front Neurosci. 2020:14:594990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherniak C. Component placement optimization in the brain. J Neurosci. 1994:14(4):2418–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Bassett DS, Power JD, Braver TS, Petersen SE. Intrinsic and task-evoked network architectures of the human brain. Neuron. 2014:83(1):238–251. 10.1016/j.neuron.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gelder B, Snyder J, Greve D, Gerard G, Hadjikhani N. Fear fosters flight: a mechanism for fear contagion when perceiving emotion expressed by a whole body. Proc Natl Acad Sci USA. 2004:101(47):16701–16706. 10.1073/pnas.0407042101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doya K. Complementary roles of basal ganglia and cerebellum in learning and motor control. Curr Opin Neurobiol. 2000:10(6):732–739. 10.1016/s0959-4388(00)00153-7. [DOI] [PubMed] [Google Scholar]
- Doyon J, Penhune V, Ungerleider LG. Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia. 2003:41(3):252–262. 10.1016/s0028-3932(02)00158-6. [DOI] [PubMed] [Google Scholar]
- Faghiri A, Stephen JM, Wang YP, Wilson TW, Calhoun VD. Brain development includes linear and multiple nonlinear trajectories: a cross-sectional resting-state functional magnetic resonance imaging study. Brain Connect. 2019:9(10):777–788. 10.1089/brain.2018.0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, Schlaggar BL, Petersen SE. Functional brain networks develop from a "local to distributed" organization. PLoS Comput Biol. 2009:5(5):e1000381. 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F, Liao X, Lei T, Zhao T, Xia M, Men W, Wang Y, Hu M, Liu J, Qin S, et al. Development of the default-mode network during childhood and adolescence: a longitudinal resting-state fMRI study. NeuroImage. 2021:226:117581. 10.1016/j.neuroimage.2020.117581. [DOI] [PubMed] [Google Scholar]
- Farrant K, Uddin LQ. Asymmetric development of dorsal and ventral attention networks in the human brain. Dev Cogn Neurosci. 2015:12:165–174. 10.1016/j.dcn.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finc K, Bonna K, Lewandowska M, Wolak T, Nikadon J, Dreszer J, Duch W, Kühn S. Transition of the functional brain network related to increasing cognitive demands. Hum Brain Mapp. 2017:38(7):3659–3674. 10.1002/hbm.23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A, Harrison BJ, Zalesky A, Simons JS. Competitive and cooperative dynamics of large-scale brain functional networks supporting recollection. Proc Natl Acad Sci USA. 2012:109(31):12788–12793. 10.1073/pnas.1204185109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007:8(9):700–711. 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Gabard-Durnam LJ, Flannery J, Goff B, Gee DG, Humphreys KL, Telzer E, Hare T, Tottenham N. The development of human amygdala functional connectivity at rest from 4 to 23 years: a cross-sectional study. NeuroImage. 2014:95:193–207. 10.1016/j.neuroimage.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabard-Durnam LJ, O'Muircheartaigh J, Dirks H, Dean DC, Tottenham N, Deoni S. Human amygdala functional network development: a cross-sectional study from 3 months to 5 years of age. Dev Cogn Neurosci. 2018:34:63–74. 10.1016/j.dcn.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Lin W, Grewen K, Gilmore JH. Functional connectivity of the infant human brain: plastic and modifiable. Neuroscientist. 2017:23(2):169–184. 10.1177/1073858416635986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Grewen K, Knickmeyer RC, Qiu A, Salzwedel A, Lin W, Gilmore JH. A review on neuroimaging studies of genetic and environmental influences on early brain development. NeuroImage. 2019:185:802–812. 10.1016/j.neuroimage.2018.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett DD, Epp SM, Perry A, Lindenberger U. Local temporal variability reflects functional integration in the human brain. NeuroImage. 2018:183:776–787. 10.1016/j.neuroimage.2018.08.019. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Knickmeyer RC, Gao W. Imaging structural and functional brain development in early childhood. Nat Rev Neurosci. 2018:19(3):123–137. 10.1038/nrn.2018.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, Xu J, Jbabdi S, Webster M, Polimeni JR et al. The minimal preprocessing pipelines for the human connectome project. Neuroimage. 2013:80:105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Coalson TS, Bijsterbosch JD, Harrison SJ, Harms MP, Anticevic A, Van Essen DC, Smith SM. Using temporal ica to selectively remove global noise while preserving global signal in functional mri data. Neuroimage. 2018:181:692–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon EM, Laumann TO, Adeyemo B, Huckins JF, Kelley WM, Petersen SE. Generation and evaluation of a cortical area parcellation from resting-state correlations. Cereb Cortex. 2016:26(1):288–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracia-Tabuenca Z, Moreno MB, Barrios FA, Alcauter S. Development of the brain functional connectome follows puberty-dependent nonlinear trajectories. NeuroImage. 2021:229:117769. 10.1016/j.neuroimage.2021.117769. [DOI] [PubMed] [Google Scholar]
- Gratton C, Laumann TO, Nielsen AN, Greene DJ, Gordon EM, Gilmore AW, Nelson SM, Coalson RS, Snyder AZ, Schlaggar BL, et al. Functional brain networks are dominated by stable group and individual factors, not cognitive or daily variation. Neuron. 2018:98(2):439–452.e435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson DS, Fair DA. Development of large-scale functional networks from birth to adulthood: a guide to the neuroimaging literature. NeuroImage. 2017:160:15–31. 10.1016/j.neuroimage.2017.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene DJ, Marek S, Gordon EM, Siegel JS, Gratton C, Laumann TO, Gilmore AW, Berg JJ, Nguyen AL, Dierker D, et al. Integrative and network-specific connectivity of the basal ganglia and thalamus defined in individuals. Neuron. 2020:105(4):742–758.e746. 10.1016/j.neuron.2019.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grèzes J, Valabrègue R, Gholipour B, Chevallier C. A direct amygdala-motor pathway for emotional displays to influence action: a diffusion tensor imaging study. Hum Brain Mapp. 2014:35(12):5974–5983. 10.1002/hbm.22598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu S, Satterthwaite TD, Medaglia JD, Yang M, Gur RE, Gur RC, Bassett DS. Emergence of system roles in normative neurodevelopment. Proc Natl Acad Sci USA. 2015:112(44):13681–13686. 10.1073/pnas.1502829112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimerà R, Amaral LA. Cartography of complex networks: Modules and universal roles. J Stat Mech. 2005:2005(P02001):nihpa35573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas C. Research note: a resting-state, cerebello-amygdaloid intrinsically connected network. Cerebellum Ataxias. 2018:5:4. 10.1186/s40673-018-0083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms MP, Somerville LH, Ances BM, Andersson J, Barch DM, Bastiani M, Bookheimer SY, Brown TB, Buckner RL, Burgess GC, et al. Extending the human connectome project across ages: imaging protocols for the lifespan development and aging projects. NeuroImage. 2018:183:972–984. 10.1016/j.neuroimage.2018.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutka S, Bidelman GM, Moreno S. Brain signal variability as a window into the bidirectionality between music and language processing: moving from a linear to a nonlinear model. Front Psychol. 2013:4:984. 10.3389/fpsyg.2013.00984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang K, Hallquist MN, Luna B. The development of hub architecture in the human functional brain network. Cereb Cortex. 2013:23(10):2380–2393. 10.1093/cercor/bhs227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang K, Bertolero MA, Liu WB, D'Esposito M. The human thalamus is an integrative hub for functional brain networks. J Neurosci. 2017:37(23):5594–5607. 10.1523/JNEUROSCI.0067-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinohe N, Mori F, Shoumura K. A di-synaptic projection from the lateral cerebellar nucleus to the laterodorsal part of the striatum via the central lateral nucleus of the thalamus in the rat. Brain Res. 2000:880(1–2):191–197. 10.1016/s0006-8993(00)02744-x. [DOI] [PubMed] [Google Scholar]
- Ide JS, Li CS. A cerebellar thalamic cortical circuit for error-related cognitive control. NeuroImage. 2011:54(1):455–464. 10.1016/j.neuroimage.2010.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang P, Vuontela V, Tokariev M, Lin H, Aronen ET, Ma Y, Carlson S. Functional connectivity of intrinsic cognitive networks during resting state and task performance in preadolescent children. PLoS One. 2018:13(10):e0205690. 10.1371/journal.pone.0205690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kail RV, Ferrer E. Processing speed in childhood and adolescence: longitudinal models for examining developmental change. Child Dev. 2007:78(6):1760–1770. 10.1111/j.1467-8624.2007.01088.x. [DOI] [PubMed] [Google Scholar]
- Kar BR, Vijay N, Mishra S. Development of cognitive and affective control networks and decision making. Prog Brain Res. 2013:202:347–368. 10.1016/B978-0-444-62604-2.00018-6. [DOI] [PubMed] [Google Scholar]
- Keunen K, Counsell SJ, Benders MJNL. The emergence of functional architecture during early brain development. NeuroImage. 2017:160:2–14. 10.1016/j.neuroimage.2017.01.047. [DOI] [PubMed] [Google Scholar]
- Korucuoglu O, Harms MP, Kennedy JT, Golosheykin S, Astafiev SV, Barch DM, Anokhin AP. Adolescent decision-making under risk: Neural correlates and sex differences. Cereb Cortex. 2020:30(4):2690–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus BT, Perez D, Ladwig Z, Seitzman BA, Dworetsky A, Petersen SE, Gratton C. Network variants are similar between task and rest states. Neuroimage. 2021:229:117743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krienen FM, Yeo BT, Buckner RL. Reconfigurable task-dependent functional coupling modes cluster around a core functional architecture. Philos Trans R Soc Lond B Biol Sci. 2014:369(1653). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laforce R, Doyon J. Distinct contribution of the striatum and cerebellum to motor learning. Brain Cogn. 2001:45(2):189–211. 10.1006/brcg.2000.1237. [DOI] [PubMed] [Google Scholar]
- Larsen B, Bourque J, Moore TM, Adebimpe A, Calkins ME, Elliott MA, Gur RC, Gur RE, Moberg PJ, Roalf DR et al. Longitudinal development of brain iron is linked to cognition in youth. J Neurosci. 2020:40(9):1810–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TT. Neurovascular factors in resting-state functional MRI. NeuroImage. 2013:80:339–348. 10.1016/j.neuroimage.2013.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long X, Benischek A, Dewey D, Lebel C. Age-related functional brain changes in young children. NeuroImage. 2017:155:322–330. 10.1016/j.neuroimage.2017.04.059. [DOI] [PubMed] [Google Scholar]
- Long JA. Interactions: Comprehensive, user-friendly toolkit for probing interactions. R package version 1.1.5. The Comprehensive R Archive Network. 2019. [Google Scholar]
- Lopez KC, Kandala S, Marek S, Barch DM. Development of network topology and functional connectivity of the prefrontal cortex. Cereb Cortex. 2020:30(4):2489–2505. 10.1093/cercor/bhz255. [DOI] [PubMed] [Google Scholar]
- Luna B, Padmanabhan A, O'Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain Cogn. 2010:72(1):101–113. 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall AE. Trajectories of early cortical development in healthy and at-risk children. United States, North Carolina: Doctoral dissertation, University of North Carolina at Chapel Hill; 2014. [Google Scholar]
- Lyall AE, Shi F, Geng X, Woolson S, Li G, Wang L, Hamer RM, Shen D, Gilmore JH. Dynamic development of regional cortical thickness and surface area in early childhood. Cereb Cortex. 2015:25(8):2204–2212. 10.1093/cercor/bhu027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus DS, Harwell J, Olsen T, Hodge M, Glasser MF, Prior F, Jenkinson M, Laumann T, Curtiss SW, van Essen DC. Informatics and data mining tools and strategies for the human connectome project. Frontiers Neuroinform. 2011:5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek S, Hwang K, Foran W, Hallquist MN, Luna B. The contribution of network organization and integration to the development of cognitive control. PLoS Biol. 2015:13(12):e1002328. 10.1371/journal.pbio.1002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. Developmental pathways to functional brain networks: emerging principles. Trends Cogn Sci. 2013:17(12):627–640. 10.1016/j.tics.2013.09.015. [DOI] [PubMed] [Google Scholar]
- Mugler JP, Brookeman JR. Three-dimensional magnetization-prepared rapid gradient-echo imaging (3d mp rage). Magn Reson Med. 1990:15(1):152–157. [DOI] [PubMed] [Google Scholar]
- Mugler JP, Bao S, Mulkern RV, Guttmann CR, Robertson RL, Jolesz FA, Brookeman JR. Optimized single-slab three-dimensional spin-echo mr imaging of the brain. Radiology. 2000:216(3):891–899. [DOI] [PubMed] [Google Scholar]
- Peper JS, Brouwer RM, Schnack HG, van Baal GC, van Leeuwen M, van den Berg SM, Delemarre-Van de Waal HA, Boomsma DI, Kahn RS, Hulshoff Pol HE. Sex steroids and brain structure in pubertal boys and girls. Psychoneuroendocrinology. 2009:34(3):332–342. [DOI] [PubMed] [Google Scholar]
- Pichon S, de Gelder B, Grèzes J. Two different faces of threat. Comparing the neural systems for recognizing fear and anger in dynamic body expressions. NeuroImage. 2009:47(4):1873–1883. 10.1016/j.neuroimage.2009.03.084. [DOI] [PubMed] [Google Scholar]
- Pines AR, Larsen B, Cui Z, Sydnor VJ, Bertolero MA, Adebimpe A, Alexander-Bloch AF, Davatzikos C, Fair DA, Gur RC et al. Dissociable multi-scale patterns of development in personalized brain networks. Nat Commun. 2022:13(1):2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity mri networks arise from subject motion. Neuroimage. 2012:59(3):2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Schlaggar BL, Lessov-Schlaggar CN, Petersen SE. Evidence for hubs in human functional brain networks. Neuron. 2013:79(4):798–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Silver BM, Silverman MR, Ajodan EL, Bos DJ, Jones RM. Customized head molds reduce motion during resting state fmri scans. Neuroimage. 2019:189:141–149. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical programming. 3.6.2 ed. Vienna, Austria: R Foundation for Statistical Programming, 2020. [Google Scholar]
- Rădulescu A, Herron J, Kennedy C, Scimemi A. Global and local excitation and inhibition shape the dynamics of the cortico-striatal-thalamo-cortical pathway. Sci Rep. 2017:7(1):7608. 10.1038/s41598-017-07527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson EC, Jbabdi S, Glasser MF, Andersson J, Burgess GC, Harms MP, Smith SM, Van Essen DC, Jenkinson M. Msm: A new flexible framework for multimodal surface matching. Neuroimage. 2014:100:414–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson EC, Garcia K, Glasser MF, Chen Z, Coalson TS, Makropoulos A, Bozek J, Wright R, Schuh A, Webster M et al. Multimodal surface matching with higher-order smoothness constraints. Neuroimage. 2018:167:453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. NeuroImage. 2010:52(3):1059–1069. 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Loughead J, Ruparel K, Elliott MA, Hakonarson H, Gur RC, Gur RE. Impact of in-scanner head motion on multiple measures of functional connectivity: Relevance for studies of neurodevelopment in youth. Neuroimage. 2012:60(1):623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Ruparel K, Erus G, Elliott MA, Eickhoff SB, Gennatas ED, Jackson C, Prabhakaran K, Smith A, et al. Heterogeneous impact of motion on fundamental patterns of developmental changes in functional connectivity during youth. NeuroImage. 2013:83:45–57. 10.1016/j.neuroimage.2013.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Ciric R, Roalf DR, Davatzikos C, Bassett DS, Wolf DH. Motion artifact in studies of functional connectivity: Characteristics and mitigation strategies. Hum Brain Mapp. 2019:40(7):2033–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheipl F, Greven S, Küchenhoff H. Size and power of tests for a zero random effect variance or polynomial regression in additive and linear mixed models. Computational statistics & data analysis. 2008:52(7):3283–3299. [Google Scholar]
- Schürmann M, Hlushchuk Y, Hari R. Embodied visual perception of distorted finger postures. Hum Brain Mapp. 2011:32(4):612–623. 10.1002/hbm.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitzman BA, Gratton C, Laumann TO, Gordon EM, Adeyemo B, Dworetsky A, Kraus BT, Gilmore AW, Berg JJ, Ortega M et al. Trait-like variants in human functional brain networks. Proc Natl Acad Sci U S A. 2019:116(45):22851–22861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman LE, Rudie JD, Pfeifer JH, Masten CL, McNealy K, Dapretto M. Development of the default mode and central executive networks across early adolescence: a longitudinal study. Dev Cogn Neurosci. 2014:10:148–159. 10.1016/j.dcn.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmueli K, van Gelderen P, de Zwart JA, Horovitz SG, Fukunaga M, Jansma JM, Duyn JH. Low-frequency fluctuations in the cardiac rate as a source of variance in the resting-state fMRI BOLD signal. NeuroImage. 2007:38(2):306–320. 10.1016/j.neuroimage.2007.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JS, Mitra A, Laumann TO, Seitzman BA, Raichle M, Corbetta M, Snyder AZ. Data quality influences observed links between functional connectivity and behavior. Cereb Cortex. 2017:27(9):4492–4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson GL, Singmann H. Package gratia. Ggplot‐based graphics and other useful functions for GAMs fitted using Mgcv, 01‐0 (Ggplot‐based graphics and utility functions for working with GAMs fitted using the mgcv package). 2018. [Google Scholar]
- Solé-Padullés C, Castro-Fornieles J, de la Serna E, Calvo R, Baeza I, Moya J, Lázaro L, Rosa M, Bargalló N, Sugranyes G. Intrinsic connectivity networks from childhood to late adolescence: effects of age and sex. Dev Cogn Neurosci. 2016:17:35–44. 10.1016/j.dcn.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Casey BJ. Developmental neurobiology of cognitive control and motivational systems. Curr Opin Neurobiol. 2010:20(2):236–241. 10.1016/j.conb.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Bookheimer SY, Buckner RL, Burgess GC, Curtiss SW, Dapretto M, Elam JS, Gaffrey MS, Harms MP, Hodge C et al. The lifespan human connectome project in development: A large-scale study of brain connectivity development in 5-21 year olds. Neuroimage. 2018:183:456–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MC. The contributions of resting state and task-based functional connectivity studies to our understanding of adolescent brain network maturation. Neurosci Biobehav Rev. 2016:70:13–32. 10.1016/j.neubiorev.2016.07.027. [DOI] [PubMed] [Google Scholar]
- Stevens MC, Pearlson GD, Calhoun VD. Changes in the interaction of resting-state neural networks from adolescence to adulthood. Hum Brain Mapp. 2009:30(8):2356–2366. 10.1002/hbm.20673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Musen M, Menon V. Development of large-scale functional brain networks in children. PLoS Biol. 2009:7(7):e1000157. 10.1371/journal.pbio.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Uddin LQ, Prater K, Amin H, Greicius MD, Menon V. Development of functional and structural connectivity within the default mode network in young children. NeuroImage. 2010:52(1):290–301. 10.1016/j.neuroimage.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME, Grove LE, Lozon TA, Vila AM, Ye Y, Nye MJ, Manning JH, Pappas A, Hernandez-Andrade E, Yeo L, et al. Age-related increases in long-range connectivity in fetal functional neural connectivity networks in utero. Dev Cogn Neurosci. 2015:11:96–104. 10.1016/j.dcn.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toschi N, Duggento A, Passamonti L. Functional connectivity in amygdalar-sensory/(pre)motor networks at rest: new evidence from the human connectome project. Eur J Neurosci. 2017:45(9):1224–1229. 10.1111/ejn.13544. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Supekar KS, Ryali S, Menon V. Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. J Neurosci. 2011:31(50):18578–18589. 10.1523/JNEUROSCI.4465-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kouwe AJW, Benner T, Salat DH, Fischl B. Brain morphometry with multiecho mprage. Neuroimage. 2008:40(2):559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity mri. Neuroimage. 2012:59(1):431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duijvenvoorde ACK, Achterberg M, Braams BR, Peters S, Crone EA. Testing a dual-systems model of adolescent brain development using resting-state connectivity analyses. NeuroImage. 2016:124(Pt A):409–420. 10.1016/j.neuroimage.2015.04.069. [DOI] [PubMed] [Google Scholar]
- van Duijvenvoorde ACK, Westhoff B, de Vos F, Wierenga LM, Crone EA. A three-wave longitudinal study of subcortical-cortical resting-state connectivity in adolescence: Testing age- and puberty-related changes. Hum Brain Mapp. 2019:40(13):3769–3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandekar SN, Shinohara RT, Raznahan A, Roalf DR, Ross M, DeLeo N, Ruparel K, Verma R, Wolf DH, Gur RC et al. Topologically dissociable patterns of development of the human cerebral cortex. J Neurosci. 2015:35(2):599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Váša F, Romero-Garcia R, Kitzbichler MG, Seidlitz J, Whitaker KJ, Vaghi MM, Kundu P, Patel AX, Fonagy P, Dolan RJ, et al. Conservative and disruptive modes of adolescent change in human brain functional connectivity. Proc Natl Acad Sci USA. 2020:117(6):3248–3253. 10.1073/pnas.1906144117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H. ggplot2: elegant graphics for data analysis. New York, NY: Springer-Verlag; 2016. ISBN 978-3-319-24277-4, https://ggplot2.tidyverse.org. [Google Scholar]
- Wierenga LM, Sexton JA, Laake P, Giedd JN, Tamnes CK. Pediatric Imaging Nu, and Genetics Study. A key characteristic of sex differences in the developing brain: Greater variability in brain structure of boys than girls. Cereb Cortex. 2018:28(8):2741–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wig GS. Segregated Systems of Human Brain Networks. Trends Cogn Sci. 2017:21(12):981–996. 10.1016/j.tics.2017.09.006. [DOI] [PubMed] [Google Scholar]
- Wood SN. Mgcv: GAMs and generalized ridge regression for R. R news. 2001:1(2):20–25. [Google Scholar]
- Wood SN. Stable and efficient multiple smoothing parameter estimation for generalized additive models. J Am Stat Assoc. 2004:99(467):673–686. [Google Scholar]
- Wood SN. Generalized additive models: an introduction with R. Boca Raton, London, New York: Chapman and Hall/CRC; 2006. [Google Scholar]
- Wood S. Generalized additive models: An introduction with r. Generalized Additive Models. 2017:10:9781315370279. [Google Scholar]
- Zuo XN, He Y, Betzel RF, Colcombe S, Sporns O, Milham MP. Human connectomics across the life span. Trends Cogn Sci. 2017:21(1):32–45. 10.1016/j.tics.2016.10.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The HCP-Development Lifespan 2.0 Release is available for download from the HCP website at: https://www.humanconnectome.org/study/hcp-lifespan-development/data-releases. This website also contains detailed release notes: https://www.humanconnectome.org/study/hcp-lifespan-development/document/hcp-development-20-release. This release includes cross-sectional visit 1 (V1) preprocessed structural and functional imaging data, unprocessed V1 imaging data for all included modalities (structural, resting state fMRI, task fMRI, diffusion, and ASL), and non-imaging demographic and behavioral assessment data from 652 HCP-Development (HCP-D, ages 5–21) healthy participants. This manuscript used data from 628 HCP-D, some of which are not currently available for download.