Abstract
Background
Under pathological conditions, tissue factor (TF)-positive extracellular vesicles (EVs) are released into the circulation and activate coagulation. Therefore, it is important to identify methods that accurately quantitate levels of TF in plasma. Enzyme-linked immunosorbent assays (ELISAs) are a fast and simple method to quantitate levels of proteins. However, there are several specific challenges with measuring TF antigen in plasma including its low concentration and the complexity of plasma.
Objectives
We aimed to evaluate the ability of 4 commercial ELISAs to measure TF in human plasma.
Methods
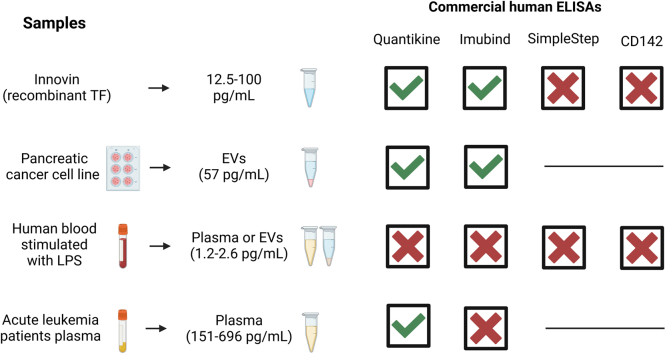
We determined the ability of 4 commercial ELISAs (Imubind, Quantikine, Human SimpleStep, and CD142 Human) to detect recombinant human TF (Innovin) (12.5-100 pg/mL), TF-positive EVs isolated from the culture supernatant from a human pancreatic cancer cell line (57 pg/mL), TF in plasma containing low levels of EV TF activity (1.2-2.6 pg/mL) from lipopolysaccharide-stimulated whole blood, and plasma containing high levels of EV TF activity (151-696 pg/mL) from patients with acute leukemia.
Results
The CD142 Human ELISA could not detect recombinant TF. Imubind and Quantikine but not Human SimpleStep detected recombinant TF spiked into plasma and TF-positive EVs isolated from the culture supernatant of a human pancreatic cancer cell line. Quantikine and Imubind could not detect low levels of TF in plasma from lipopolysaccharide-stimulated whole blood. However, Quantikine but not Imubind detected TF in plasma from acute leukemia patients with high levels of EV TF activity.
Conclusion
Our results indicate that commercial ELISAs have different abilities to detect TF. Quantikine and Imubind could not detect low levels of TF in plasma, but Quantikine detected TF in plasma with high levels of TF.
Keywords: acute leukemia, coagulation, enzyme-linked immunosorbent assay, extracellular vesicles, plasma, tissue factor
Graphical abstract
Essentials
-
•
Tissue factor (TF) in human plasma can be measured using activity- or antigen-based assays.
-
•
We evaluated the ability of 4 commercial ELISAs to measure TF in human plasma.
-
•
Two of the 4 ELISAs detected recombinant TF spiked into human plasma.
-
•
One ELISA detected TF in plasma from leukemia patients with high levels of extracellular vesicle TF activity.
1. Introduction
Tissue factor (TF) is the transmembrane receptor for factor (F) VII/VIIa [1]. The TF-FVIIa complex is a potent activator of the coagulation protease cascade. TF is essential for hemostasis but can also cause pathologic activation of coagulation. TF is not normally present in blood. However, TF-positive extracellular vesicles (EVs) can be released into the circulation from various cells, such as activated monocytes and cancer cells, and can contribute to thrombosis and disseminated intravascular coagulation [2,3]. Therefore, it is important to establish assays that reliably and accurately quantitate levels of TF in plasma. Activity-based assays are more sensitive than antigen-based assays for measuring TF in plasma [4]. Another challenge with antigen-based assays is that TF can be present in both active and inactive forms [5]. Although the majority of TF in plasma and cell culture supernatants is associated with EVs [6,7], an alternatively spliced form of TF (asTF) can be expressed that is soluble but has low procoagulant activity compared to full-length TF [[8], [9], [10]].
Enzyme-linked immunosorbent assays (ELISAs) are commonly used to measure levels of protein in a variety of samples, including plasma. In general, ELISAs that use monoclonal antibodies for capture and detection are more specific than those that use a monoclonal antibody for capture and a polyclonal antibody for detection. There are several specific challenges with immunological quantitation of TF in human plasma. These include the low concentration of TF, the different forms of TF (full-length TF and asTF), use of an appropriate standard, the specificity of the anti-TF antibodies used in the ELISAs, and the presence of multivalent substances, such as heterophilic antibodies, in plasma that can generate immunoassay signals independent of the target protein by simply bridging assay antibodies [[11], [12], [13], [14]].
The reproducibility of research is a concern. The National Institutes of Health prepared a document describing the principles and guidelines for reporting preclinical research (https://www.nih.gov/research-training/rigor-reproducibility//principles-guidelines-reporting-preclincal-research). It was recommended that for antibodies, the dilution, source, and how they were validated should be included. The International Working Group for Antibody Validation highlighted the need for validating antibodies in common research applications [15]. Unfortunately, companies do not provide information on the antibodies used in commercial ELISAs.
Several groups have developed in-house TF ELISAs to measure TF antigen in plasma [[16], [17], [18], [19], [20], [21], [22]]. However, because these ELISAs are not widely available, most investigators use commercial TF ELISAs to quantitate levels of TF antigen in human plasma. Many studies have used commercial TF ELISAs to quantitate TF-like antigen in plasma from patients with a variety of diseases, including cardiovascular disease, infection, inflammation, and various malignancies [20,[23], [24], [25], [26], [27]]. The most popular TF ELISAs are Imubind and Quantikine.
We and others have raised concerns about the ability of commercial ELISAs to accurately quantitate levels of TF antigen in human plasma [21,[28], [29], [30], [31], [32]]. For instance, the Zymutest TF ELISA and Quantikine ELISA failed to detect TF-positive EVs in plasma from either lipopolysaccharide (LPS)-treated whole blood of healthy donors or ovarian cancer patients that contained EV TF activity [28,29].
In this study, we evaluated the ability of 4 commercial TF ELISAs to measure various forms of TF: recombinant human TF (Innovin), TF in EVs isolated from the culture supernatant of a human pancreatic cancer cell line, TF in plasma containing low levels of EV TF activity (1.2-2.6 pg/mL), and plasma containing high levels of EV TF activity (151-696 pg/mL).
2. Methods
2.1. Commercial TF ELISAs
We tested 4 commercial ELISAs that have previously been used by investigators to measure TF in human plasma: IMUBIND Tissue Factor ELISA (BioMedica Diagnostics, Cat. #845, lot #210726), Tissue Factor Quantikine ELISA (R&D Systems, Cat. #DCF300, lot #P299971), Human Tissue Factor SimpleStep ELISA (Abcam, Cat. #ab220653, lot #70850C0014), and Tissue Factor (CD142) Human ELISA (Abcam, Cat. #ab108903, lot #GR3410631-3). ELISAs were used according to the manufacturer’s instructions. The standards provided in the kits were used as calibrators for each kit. All ELISA kits list plasma as a sample type. Imubind and Quantikine use a monoclonal antibody for capture (precoated on the ELISA plates) and a polyclonal antibody for detection. SimpleStep uses a new technology developed to make ELISAs faster and more sensitive. Antibodies and sample were added in solution and tagged-antibody-antigen complexes were captured onto an ELISA plate using an antitag antibody. Imubind targets TF, lipidated TF, and TF-FVII. Quantikine and SimpleStep target full-length TF (extracellular domain). CD142 does not specify the types of antibodies and specific targets. Imubind states that “plasma samples may have a higher background signal than other samples and that a normal range for human plasma is not well-established yet.”
Innovin was diluted in sample diluent from the kits. Cancer cell-derived EVs and EV-free supernatant were diluted 1:5 (for Imubind) and 1:2 (for Quantikine) in sample diluent from the kits. Plasma samples, EV-free plasma, and plasma-derived EVs were diluted to the minimum amount listed in the manufacturer’s directions (1:4 for Imubind, and 1:2 for Quantikine, SimpleStep, and CD142). Samples were measured in duplicate for Innovin, cell-derived EVs, and for plasma from LPS-stimulated whole blood. Samples from patients with acute leukemia were measured in a singlet because of the small amount of sample available.
2.2. Measurement of the concentration of TF in Innovin
The concentration of TF in Innovin was determined by titrating known concentrations of FVIIa into a fixed concentration of Innovin. The rate of substrate cleavage was plotted against the concentration of FVIIa. The TF-FVIIa complex cleaves the substrate more efficiently than FVIIa alone. When every FVIIa molecule is bound to a TF molecule, the rate of substrate cleavage stops increasing and the substrate curve shows an inflection. The TF concentration is equal to the FVIIa concentration at the inflection point.
2.3. Cell Culture
The human pancreatic cancer cell line HPAF-II expresses high levels of TF [33] and was cultured in Minimum Essential Medium (Thermo Fisher Scientific, Cat. #11095-080). The human acute leukemia cell lines NB4 and HL-60 express high and low levels of TF, respectively [34,35] (Hisada Y, May 2022, unpublished data). They were cultured in Roswell Park Memorial Institute 1640 medium (Thermo Fisher Scientific, Cat. #11875-093) and Iscove’s modified Dulbecco’s medium (Thermo Fisher Scientific, Cat. # 12440053), respectively. Media contained 10% (for NB4) and 20% (for HL-60) fetal bovine serum (Omega Scientific, Cat. #FB-02) and 1% antibiotics-antimycotic (Thermo Fisher Scientific, Cat. #15240-062).
2.4. Plasma preparation
Whole blood was collected from 5 healthy volunteers from the antecubital vein into sodium citrate tubes (BD, Cat. #366560) with a 21G Safety-Lok Blood Collection Set (BD, Cat. #367281). The first 3 mL was discarded to prevent contamination with TF from the vessel wall. All donors gave consent for the collection according to a protocol (14-2108) approved by the Institutional Review Board of the University of North Carolina at Chapel Hill. Blood was centrifuged twice at 2500 g for 15 minutes at room temperature to obtain TF-negative platelet-low plasma samples following the protocol created by the International Society on Thrombosis and Hemostasis workshop [36]. A recent study showed residual levels of platelets (5.1 × 105−2.8 × 107 per mL) in plasma prepared using this protocol [37]. A different aliquot of blood from the same donors was stimulated with LPS (10 μg/mL, Sigma-Aldrich, Cat. no. L2630) for 5 hours at 37 °C to obtain TF-positive samples. Platelet-low plasma was prepared as described above.
Whole blood was collected from 6 acute leukemia patients into EDTA at the time of diagnosis. Blood was centrifuged at 626 g for 5 minutes to generate platelet-poor plasma. All donors gave consent for the collection according to a protocol (30008180) approved by the Institutional Review Board at the University of Alabama at Birmingham. The transfer to the University of North Carolina at Chapel Hill and measurement of TF was approved by the Institutional Review Board of the University of North Carolina at Chapel Hill (21-0554).
2.5. Isolation of EVs
HPAF-II cells were cultured in serum-free media for 24 hours and the culture supernatant was centrifuged at 3220 g for 5 minutes at room temperature to preclear apoptotic bodies and cell debris. The supernatant was added to a new tube and used for EV isolation. EVs were isolated by centrifugation of 100 μL of cell supernatant at 20,000 g for 15 minutes at 4 °C. The EV pellets were washed in 1 mL of 1X phosphate-buffered saline (PBS), centrifuged once more, and resuspended in 100 μL of PBS. The supernatant was removed after the first centrifugation and placed in a separate tube and centrifuged at 100,000 g for 70 minutes to pellet the residual EVs. The supernatant was removed, placed in a separate tube, and designated EV-free supernatant.
Plasma EVs were isolated by centrifugation of 100 μL of plasma at 20,000 g for 15 minutes at 4 °C. The EV pellet was washed in 1 mL of 1X PBS, centrifuged once more, and resuspended in 100 μL using each specific ELISA kit sample buffer. The supernatant was removed and placed in a separate tube and centrifuged at 100,000 g for 70 minutes to pellet the residual EVs. The supernatant was removed, placed in a separate tube and designated EV-free plasma. It should be noted that we did not measure levels of EVs in either EV-free culture supernatant or EV-free plasma. However, double centrifugation of samples at 100,000 g for 70 minutes is a standard method for isolating EVs [38,39].
2.6. Western blotting
Western blotting for TF was performed as described previously [33]. Innovin (Dade Innovin, Siemens) was diluted in PBS. SDS sample buffer (GenScript, Cat. #M00676-250) and β-mercaptoethanol were added to Innovin (2.5% final concentration of β-mercaptoethanol). Samples were heated at 95 °C for 10 minutes and loaded on gels (4%-20% gradient, Mini-PROTEAN TGX gels, BioRad, Cat. #456-1093). Innovin was added to 1, 2, 4, 8, 17, 35, 70, 140, 280, 560, and 1120 pg/well. NB4 and HL60 cells were lysed in RIPA lysis buffer (MilliporeSigma, Cat. #20-188) containing a protease inhibitor cocktail (Roche, Cat. 11697498001) and incubated on ice for 30 minutes. Protein concentrations in the cell lysates were determined using the bicinchoninic acid (BCA) assay (Thermo Fisher Scientific, Cat. #23227). SDS sample buffer and β-mercaptoethanol were added to the cell lysates and 40 μg of protein were added per well. SDS sample buffer and β-mercaptoethanol were added to 100 μL of EVs isolated from the cell culture supernatant of NB4 and HL-60 cells. After running the gels, proteins were transferred to polyvinylidene difluoride membranes (MilliporeSigma, Cat. #IPFL00010) and the membrane was blocked with Pierce Protein-Free (PBS) Blocking Buffer (Thermo Fisher Scientific, Cat. #37572) for 1 hour at room temperature. The membrane was incubated with an anti-TF antibody as the primary antibody (human anti-TF produced in goat, R&D Systems, Cat. #AF2339, 1:1000 in blocking buffer) and subsequently with an antigoat IgG-HRP as a secondary antibody (antigoat IgG produced in donkey conjugated with HRP, Jackson ImmunoResearch, Cat. #705-035-14, 1:5000 in blocking buffer). Membranes were developed using chemiluminescence (SuperSignal West Pico PLUS Chemiluminescent Substrate (Thermo Fisher Scientific, Cat. #34577) and proteins detected using a ChemiDoc MP Imaging System (BioRad) and analyzed using the software Image Lab 6.1 (BioRad).
2.7. EV TF activity assay
EV TF activity was measured using an in-house FXa generation assay [40]. Briefly, EVs were isolated from 100 μL of cell culture supernatants or plasma by centrifugation at 20,000 g for 15 minutes at 4 °C, washed, centrifuged again, and resuspended in 100 μL of assay buffer. Samples were incubated with an inhibitory antibody against TF (HTF-1, BD Biosciences, Cat. #550252) or IgG control antibody (anti-IgG, Sigma, Cat. #I5381) to distinguish TF-dependent and TF-independent FXa generation. After incubation with FVIIa and FX, FXa generation was measured using an FXa chromogenic substrate, Pefachrome FXa 8595 (DSM Cat. #085-27).
2.8. Statistical analysis
Data are shown as mean ± SD or individual values. The data were analyzed using Prism version 9.4 (GraphPad Software, USA).
3. Results
3.1. Measurement of recombinant TF using commercial ELISAs
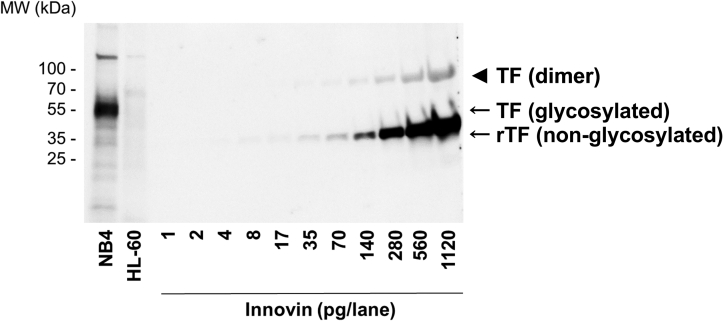
We determined the ability of commercial TF ELISAs to detect recombinant TF (Innovin). Innovin is a well-defined product and is used for prothrombin time assays and as a standard in TF activity assays [40,41]. First, we analyzed Innovin by western blotting. We observed a strong ∼40-kDa band (Figure 1), which is the expected molecular weight of nonglycosylated TF [33]. We also observed a faint band at ∼80 kDa, which is likely to be a TF dimer. TF is known to form dimers in reconstituted lipid vesicles [[42], [43], [44]]. The limit of detection of Innovin by western blotting was 2 pg from the scanned blot (Figure 1). The high TF-expressing human acute leukemia cell line NB4 and the low TF-expressing human acute leukemia cell line HL-60 were used as controls in the western blot. A band of ∼50 kDa was detected in the NB4 cell lysate, which corresponds to the size of glycosylated TF (Figure 1). No TF was detected in the HL-60 cell lysate.
Figure 1.
Analysis of Innovin by western blotting. Recombinant (r) tissue factor (TF) (Innovin) was analyzed by western blot analysis (1-1120 pg/lane). Cell lysates from a high TF leukemia cell line (NB4, 40 μg/lane) and a low TF leukemia cell line (HL-60, 40 μg/lane) and were used as controls. An antihuman TF polyclonal antibody from R&D Systems (#AF2339) was used (1:1000 dilution in blocking buffer, final concentration 0.2 μg/mL). The secondary antibody was used at a 1:5000 dilution in blocking buffer. Molecular weight (MW) standards are shown. The position of rTF (non-glycosylated, ∼40kDa) and TF (glycosylated, ∼50kDa) are shown. The arrowhead indicates the position of a TF dimer.
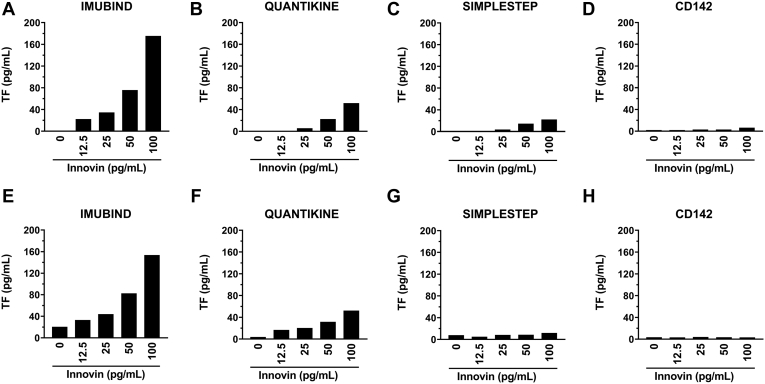
Next, we evaluated the ability of 4 commercial TF ELISAs to detect different amounts of Innovin (12.5-100 pg/mL). Three of the ELISAs (Imubind, Quantikine, and SimpleStep) detected Innovin, whereas the TF CD142 ELISA did not (Figure 2A-D). Interestingly, the different ELISAs gave different values for a given amount of Innovin. For Imubind, the amount of Innovin was higher than expected (∼175%), whereas Quantikine and SimpleStep only detected 50% and 10% of the Innovin, respectively (Figure 2A-D). Imubind and Quantikine but not SimpleStep or CD142 also detected Innovin spiked in human plasma (Figure 2E-H).
Figure 2.
Measurement of recombinant tissue factor using different ELISAs. Different amounts of Innovin (12.5-100 pg/mL) were measured using (A) Imubind ELISA, (B) Quantikine ELISA, (C) SimpleStep ELISA, and (D) CD142 ELISA. Different amounts of Innovin (12.5-100 pg/mL) were spiked into pooled human plasma and measured using (E) Imubind ELISA, (F) Quantikine ELISA, (G) SimpleStep ELISA, and (H) CD142 ELISA.
3.2. Measurement of TF-positive EVs isolated from the cell supernatant of a human pancreatic cancer cell line using commercial ELISAs
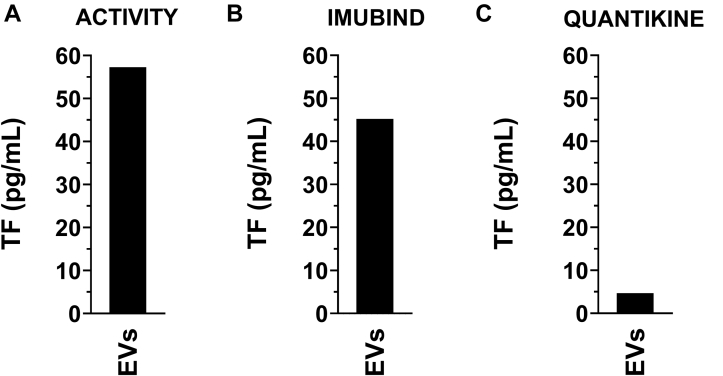
We have previously shown that the human pancreatic cancer cell line HPAF-II expresses high levels of TF [7,33]. Therefore, we used HPAF-II as TF-positive cell line. As expected, EVs isolated from the culture supernatant of HPAF-II cells contained a high level of TF activity (57 pg/mL) (Figure 3A).
Figure 3.
Measurement of tissue factor in extracellular vesicles isolated from cancer cell culture supernatant. Extracellular vesicles (EVs) were isolated from a tissue factor (TF)-positive pancreatic cancer cell line (HPAF-II) by centrifuging 100 μL of cell culture supernatant at 20,000 g for 15 minutes at 4 °C. The pellet was resuspended in 100 μL of PBS. (A) TF activity was determined using a FXa generation assay. EV TF antigen was determined using (B) Imubind ELISA, and (C) Quantikine ELISA.
Next, we determined the ability of Imubind and Quantikine to detect TF in EVs isolated from HPAF-II cell culture supernatant. We and others have shown that TF in cancer cell culture supernatants is because of the presence of TF on EVs [3,7]. Both ELISAs detected TF in EVs isolated from the culture supernatant of HPAF-II cells (Figure 3B and C). The different ELISAs gave different values for the HPAF-II EVs: 45 pg/mL for Imubind and 4.7 pg/mL for Quantikine (Figure 3B and C). No signal was detected in EV-free culture supernatant using Imubind or Quantikine (data not shown). These data show that 2 commercial TF ELISAs can detect TF in EVs isolated from cell culture supernatant from a human pancreatic cell line that expresses a high level of TF.
3.3. Commercial ELISAs cannot detect low levels of TF in plasma derived from LPS-stimulated whole blood
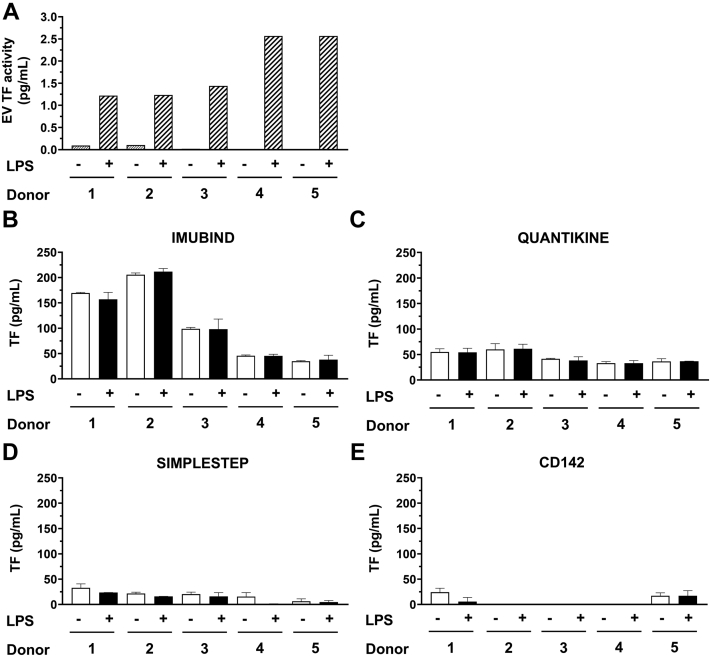
We have previously shown that LPS stimulation of human whole blood from healthy donors induces TF expression in monocytes and the release of TF-positive EVs [28,29,31]. We prepared plasma from LPS-stimulated whole blood that contained TF-positive EVs and plasma from whole blood without LPS stimulation as a negative control from 5 healthy donors. As expected, TF activity in EVs isolated from plasma from LPS-stimulated whole blood was significantly higher than the respective negative controls for each donor (Figure 4A). The EV TF activity ranged between 1.2 and 2.6 pg/mL for the different donors (Figure 4A).
Figure 4.
Measurement of tissue factor activity and antigen in human plasma from whole blood with or without lipopolysaccharide stimulation. Whole blood from 5 healthy donors was left untreated or stimulated with lipopolysaccharide (LPS) and plasma prepared. (A) Extracellular vesicles (EVs) were isolated from plasma by centrifuging 100 μL of plasma and resuspended in 100 μL of assay buffer. Tissue factor (TF) activity was determined using a FXa generation assay. TF antigen in plasma from unstimulated or LPS-stimulated whole blood was determined using (B) Imubind ELISA, (C) Quantikine ELISA, (D) SimpleStep ELISA, and (E) CD142 ELISA.
Notably, none of the ELISAs detected a difference between samples with or without TF-positive EVs from the 5 donors (Figure 4B-E). We observed a large difference in background values for the different ELISAs (Figure 4B-E). The background of Imubind was highly variable in the different donors and ranged between 35 and 212 pg/mL, whereas Quantikine gave much lower background values (range 32-68 pg/mL). These results indicate that the ELISAs cannot detect low levels of TF-positive EVs in plasma.
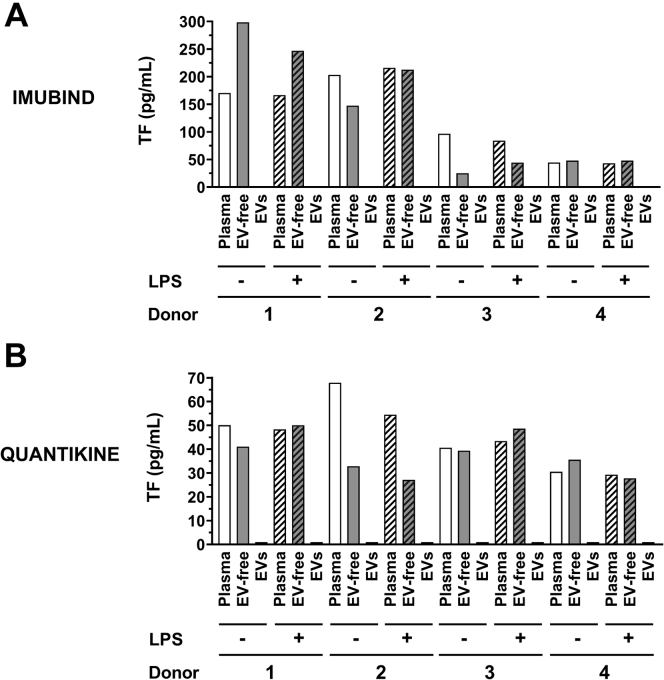
TF activity in plasma is mainly because of the presence of TF on EVs [10,45]. Therefore, we determined whether the Imubind and Quantikine ELISAs could detect TF in EVs isolated from plasma isolated from LPS-treated whole blood. However, the 2 ELISAs failed to detect TF in EVs isolated from plasma derived from LPS-stimulated whole blood (Figure 5). Furthermore, removal of EVs from plasma did not decrease the signal, suggesting that the signal was not specific for TF (Figure 5).
Figure 5.
Measurement of tissue factor antigen in plasma, extracellular vesicle-free plasma, and extracellular vesicles from whole blood with or without lipopolysaccharide stimulation. Whole blood from 4 healthy donors was left untreated or stimulated with lipopolysaccharide (LPS) and plasma was prepared. Extracellular vesicles (EVs) were isolated from plasma by centrifuging 100 μL of plasma at 20,000 g for 15 minutes at 4 °C. The pellet was resuspended in 100 μL of sample buffer. EV-free plasma was generated by centrifuging the supernatant at 100,000 g for 70 minutes at 4 °C. Tissue factor (TF) antigen in plasma, EV-free plasma (EV-free) and EVs was determined using (A) Imubind ELISA, and (B) Quantikine ELISA.
3.4. Evaluation of the ability of commercial ELISAs to detect TF in plasma from patients with acute leukemia
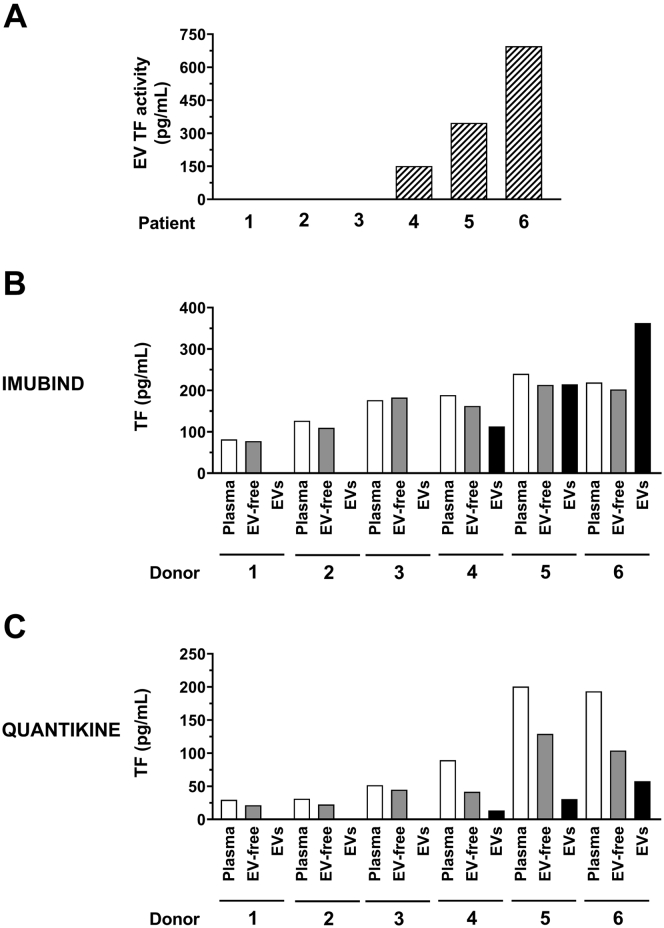
We determined the ability of 2 commercial TF ELISAs (Imubind and Quantikine) to detect TF using clinical samples from patients with acute leukemia with very low (0.14-0.17 pg/mL, patients 1-3) or very high (151-696 pg/mL, patients 4-6) levels of EV TF activity (Figure 6A). For Imubind, we did not observe a difference in signal between plasma and EV-free plasma for all the patients (Figure 6B). However, we did observe a signal for isolated EVs in the 3 patients with high levels of EV TF activity with a pattern that was similar to the EV TF activity (Figure 6B). For Quantikine, we observed a higher signal for plasma compared with EV-free plasma for the 3 patients with high levels of EV TF activity but not for the 3 patients with low levels of EV TF activity (Figure 6C). In addition, we observed a signal for isolated EVs in the 3 patients with high levels of EV TF activity (Figure 6C).
Figure 6.
Measurement of tissue factor in plasma, extracellular vesicle-free plasma, and extracellular vesicles isolated from plasma from acute leukemia patients. Extracellular vesicles (EVs) were isolated from 100 μL of plasma from 6 acute leukemia patients by centrifuging at 20,000 g for 15 minutes at 4 °C. The pellet was resuspended in 100 μL of sample buffer. EV-free plasma was generated by centrifuging the supernatant at 100,000 g for 70 minutes at 4 °C. (A) EV TF activity was determined using a FXa generation. TF antigen in plasma, EV-free plasma, and EVs were determined using (B) Imubind ELISA, and (C) Quantikine ELISA.
4. Discussion
In this study, we evaluated the ability of 4 different commercial ELISAs to detect various forms of TF including TF in plasma. We found that 3 of the 4 ELISAs detected recombinant TF in a dose-dependent manner. However, the ELISAs gave very different values for a given amount of recombinant TF (Imubind > Quantikine > SimpleStep). Only Imubind and Quantikine detected recombinant TF spiked in human plasma. Imubind and Quantikine also detected TF on EVs isolated from culture supernatant from a TF-expressing human pancreatic cancer cell line HPAF-II. Again, Imubind gave much higher values compared with Quantikine. A recent study evaluated the ability of 4 commercial TF ELISAs (Imubind, Quantikine, TF CD142 ELISA, Zymutest TF ELISAs) to detect TF in lysates of a variety of tumor cell lines [46]. Consistent with our observations, the ELISAs gave different values for TF in cell lysates (Imubind> Zymutest> Quantikine> TF CD142). Imubind also gave a much higher background and range (38-212 pg/mL) in plasma from healthy donors compared to Quantikine (32-68 pg/mL). These ranges are consistent with values reported in the literature for healthy controls (Table). One study reported that the background value of healthy donor plasma in 14 different studies using Imubind was 61 to 187 pg/mL [21]. We also found that Imubind gave higher values for plasma of healthy donors compared with Zymutest [28]. These differences are likely because of the use of different standards in the different ELISAs. Parhami-Seren et al. [21] found that the Imubind ELISA had a ∼3-fold higher background compared to their in-house ELISA. They compared their in-house calibrator and the Imubind standard and observed that the Imubind standard had less TF than the in-house calibrator, which explained the higher TF-like signals from the Imubind ELISA.
Table.
Clinical studies measuring TF antigen in plasma using Imubind and Quantikine ELISAs.
| Kit | Healthy group TF antigen (pg/mL) | Disease group TF antigen (pg/mL) | Disease | Data type | Sample type | P value | Ref. |
|---|---|---|---|---|---|---|---|
| Imubind | 117 ± 19.2 | 273 ± 90 | Leukemia (DIC) | Mean ± SD | Plasma | <.01 | 1997 [47] |
| Imubind | 187.3 ± 108.7 | 303.6 ± 134.1 | Coronary artery disease | Mean ± SD | Heparin PPP | <.05 | 2000 [48] |
| Imubind | 158 ± 60 | 275 ± 122 | Peripheral artery disease | Mean ± SD | Citrated PPP | <.001 | 2000 [49] |
| Imubind | 142 (18–262) | 179.2 (51.2–380) (stable) | Ischemic heart disease | Median (IQR) | Citrated PPP | <.0001 | 2000 [50] |
| 215 (56.8–834.3) (unstable) | <.0001 | ||||||
| Imubind | 76 (32–108) | 116 (92–150) (stable) | Coronary artery disease | Median (IQR) | Citrated PPP | <.01 | 2001 [51] |
| 146 (104–188) (unstable) | <.001 | ||||||
| Imubind | 144 ± 73.9 | 283 ± 145 | Glomerulonephritis | Mean ± SD | PPP | .009 | 2006 [52] |
| Imubind | 124.1 ± 14.79 | 334.9 ± 95.44 | Nonsmall-cell lung cancer | Mean ± SD | PPP | .02 | 2008 [53] |
| Imubind | 42.83 ± 14.18 | 66.78 ± 41.59 | Obstructive sleep apnea | Mean ± SD | PPP | .005 | 2008 [54] |
| Imubind | 62 ± 20 | 134 ± 54 (AAA) | Aneurysm | Mean ± SD | Citrated | <.001 | 2013 [55] |
| 91 ± 30 (IAA/ atherosclerotic lesion) | PPP | <.008 | |||||
| Imubind | 215.6 ± 55.1 | 265.2 ± 124.3 | Cancer | Mean ± SD | Citrated PPP | .014 | 2013 [56] |
| Imubind | 115.35 (92.65–166.81) | 539.93 (400.94–710.40) | Breast cancer | Median (IQR) | Citrated PPP | .0001 | 2018 [57] |
| Quantikine | 52.4 (28.4–72.4) | 63.8 (32.7–112) (stages I–III) | Pancreatic adenocarcinoma | Mean (range) | EDTA PPP | NS | 2020 [58] |
| 82.8 (45.6–141) (metastatic) | <.001 | ||||||
| Quantikine | 39.1 (16.8–87.3) | 47.2 (0.0–224.8) (without CAT) | Pancreatic adenocarcinoma | Median (range) | PFP | .009 | 2021 [46] |
| 56.0 (37.6–318.7) (with CAT) | .002 | ||||||
| Quantikine | 23 ± 5 | 20 ± 2 (mild) | COVID-19 | Mean ± SD | Citrated PPP | <.05 | 2022 [59] |
| 2000 ± 500 (moderate) |
AAA, abdominal aortic aneurysm; CAT, cancer-associated thrombosis; DIC, disseminated intravascular coagulation; IAA, Inflammatory aortic aneurysm; NS, non-significant; PFP, platelet-free plasma; PPP, platelet-poor plasma; TF, tissue factor.
Kobayashi et al. [46] compared the signal from the different ELISAs with the TF antigen signal measured by western blotting of cell lysates from different human cancer cell lines. Linear regression analysis showed that the R2 ranged from 0.345 to 0.930 for the different ELISAs. The TF CD142 ELISA had the highest R2 but had low sensitivity. Therefore, the Quantikine ELISA was selected to measure TF-like antigen levels in plasma from patients with pancreatic cancer and healthy controls. However, measuring TF in cell lysates is very different from measuring TF in plasma. The main differences are the low concentration of TF in plasma and the complexity of plasma.
Several studies have reported that disease groups, such as cancer, bacterial, and viral infections, and cardiovascular diseases had increased levels of TF-like antigen measured by Imubind [[47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57]] or Quantikine [46,58,59] compared with healthy controls (Table). These studies report statistically significant increases in TF-like antigen in different disease groups using the Imubind and Quantikine ELISAs. However, in most cases values for the disease group overlap with values from the healthy controls. In addition, there were no consistent patterns to allow us to conclude that levels of TF-like antigen in the plasma are increased in most of the disease groups. One exception is a study that reported high TF-like antigen (2000 pg/mL) measured using Quantikine in COVID-19 patients with moderate disease [59]. This is surprising because we and others have found that levels of EV TF activity are increased in patients with severe COVID-19 but not in moderate COVID-19 patients [26,27].
TF activity assays are more sensitive compared with TF antigen assays [4]. The sensitivity of our EV TF activity assay is ∼0.5 pg/mL for EVs isolated from human plasma. In this study, we found that commercial ELISAs could not detect TF antigen in plasma or isolated EVs from plasma containing up to 2.6 pg/mL of EV TF activity. This is more than 5-fold higher than the activity assay. In a previous study, we evaluated the ability of the Imubind and Zymutest ELISAs to detect TF in plasma from LPS-treated whole blood from 6 healthy donors. Interestingly, the range of EV TF activity was 2 to 14 pg/mL. In the 5 donors with EV TF activity in the LPS-treated samples of >4 pg/mL but not the donor with a value of 2 pg/mL, we observed an increase in the TF-like values for the LPS samples compared with the controls for Imubind but not Zymutest [28]. We did not measure the levels of TF antigen in isolated EVs in this study.
One study found that a patient with giant-cell lung carcinoma had very high levels of TF-like antigen in plasma (3764 pg/mL) measured using Imubind [6]. Importantly, 94% of the TF-like signal was associated with EVs. We found that Quantikine but not Imubind detected a TF-like signal in plasma from 3 patients (#4-6) with acute leukemia with high levels of EV TF activity (151-696 pg/mL). There was a higher signal in plasma compared with EV-free plasma with Quantikine but not Imubind. TF was detected in EVs isolated from the plasma of these 3 patients (#4-6) using Quantikine and Imubind. In a previous study, we analyzed TF protein in EVs and culture supernatant from human pancreatic cell lines using the Imubind ELISA and by western blotting [7]. The majority of the signal from Imubind was associated with EVs, but there was a residual signal in the EV-free culture supernatant. Importantly, western blotting revealed that full-length TF was associated with EVs, whereas degraded TF was observed in the EV-free culture supernatant. By analogy, we speculate that the TF-like signal detected in EV-free plasma by Quantikine represents degraded TF. Moreover, the plasma TF-like signal detected by Quantikine appears to represent the combination of the full-length TF in EVs and degraded TF in EV-free plasma.
A major challenge with detecting proteins in plasma is the presence of heterophilic antibodies. These natural antibodies exhibit multi-species reactivity and can make a bridge between immunoassay antibodies, producing a false-positive signal [11,14,60]. The validation of TF antibodies is essential to ensure rigor and reproducibility of data. Investigators and companies are responsible for assuring the specificity of the antibodies and assays used.
It should be noted that the blood from the leukemia patients was collected in EDTA. A previous study showed that there was 5 times more LPS-induced TF activity in monocytes when citrate was used as an anticoagulant compared with EDTA [61]. Similarly, we found that the level of EV TF activity in LPS-treated blood was 3 to 10 times higher with citrated compared with EDTA (K Tatsumi and N Mackman, unpublished data, 2017). Therefore, we do not believe that the high levels of EV TF activity in the leukemia patients are because of the use of EDTA. We did not collect information about race/ethnicity of the healthy controls and patients used in this study. We believe that this does not impact the conclusions of the study.
In summary, our study indicates that commercial TF ELISAs could not detect TF in human plasma containing 1.2 to 2.6 pg/mL of EV TF activity in part because of a high non-specific background from the plasma. We believe that the level of TF in plasma from LPS-stimulated blood is similar or even higher than the levels in most diseases and therefore is a good positive control for the study. One exception is patients with acute leukemia where very high levels of EV TF activity (151-696 pg/mL) are observed (Hisada Y, manuscript in preparation). In this case, the Quantikine ELISA was able to detect a TF signal in plasma. In conclusion, our study showed that commercial ELISAs are unable to detect TF in human plasma apart from rare instances where there are very high levels of TF.
Acknowledgments
Funding
This work was supported by the NIH NHLBI R35HL155657 (N.M) and the John C. Parker professorship (N.M).
Ethics statement
All healthy donors gave consent for the collection of blood according to a protocol (14-2108) approved by the Institutional Review Board of the University of North Carolina at Chapel Hill. All leukemia patients gave consent for the collection of blood according to a protocol (30008180) approved by the Institutional Review Board at the University of Alabama at Birmingham. The transfer to the University of North Carolina at Chapel Hill and measurement of TF was approved by the Institutional Review Board of the University of North Carolina at Chapel Hill (21-0554).
Author contributions
A.T.A.S. and N.M. designed experiments, interpreted data, and edited the manuscript. A.T.A.S and S.A. conducted experiments, analyzed data, and wrote the manuscript. R.B. collected acute leukemia patient plasma samples. Y.H. and D.M. edited the manuscript. All the authors have read and approved the final manuscript.
Relationship Disclosure
The authors have no relevant conflicts of interest to disclose.
Informed patient consent
All donors gave consent for the collection of blood according to the protocols approved by the Institutional Review Boards of the Universities.
Footnotes
Handling Editor: Henri Spronk
References
- 1.Grover S.P., Mackman N. Tissue factor: an essential mediator of hemostasis and trigger of thrombosis. Arterioscler Thromb Vasc Biol. 2018;38:709–725. doi: 10.1161/ATVBAHA.117.309846. [DOI] [PubMed] [Google Scholar]
- 2.Basavaraj M.G., Olsen J.O., Østerud B., Hansen J.-B. Differential ability of tissue factor antibody clones on detection of tissue factor in blood cells and microparticles. Thromb Res. 2012;130:538–546. doi: 10.1016/j.thromres.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Yu J.L., Rak J.W. Shedding of tissue factor (TF)-containing microparticles rather than alternatively spliced TF is the main source of TF activity released from human cancer cells. J Thromb Haemost. 2004;2:2065–2067. doi: 10.1111/j.1538-7836.2004.00972.x. [DOI] [PubMed] [Google Scholar]
- 4.Key N., Mackman N. Tissue factor and its measurement in whole blood, plasma, and microparticles. Sem Thromb Hemost. 2010;36:865–875. doi: 10.1055/s-0030-1267040. [DOI] [PubMed] [Google Scholar]
- 5.Ansari S.A., Pendurthi U.R., Rao L.V.M. Role of cell surface lipids and thiol-disulphide exchange pathways in regulating the encryption and decryption of tissue factor. Thromb Haemost. 2019;119:860–870. doi: 10.1055/s-0039-1681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Del Conde I., Bharwani L.D., Dietzen D.J., Pendurthi U., Thiagarajan P., López J.A. Microvesicle-associated tissue factor and Trousseau's syndrome. J Thromb Haemost. 2007;5:70–74. doi: 10.1111/j.1538-7836.2006.02301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J.-G., Geddings J.E., Aleman M.M., Cardenas J.C., Chantrathammachart P., Williams J.C., et al. Tumor-derived tissue factor activates coagulation and enhances thrombosis in a mouse xenograft model of human pancreatic cancer. Blood. 2012;119:5543–5552. doi: 10.1182/blood-2012-01-402156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matiash K., Lewis C.S., Bogdanov V.Y. Functional characteristics and regulated expression of alternatively spliced tissue factor: an update. Cancers. 2021;13:4652. doi: 10.3390/cancers13184652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mackman N. Alternatively spliced tissue factor – one cut too many? Thromb Haemost. 2007;97:5–8. doi: 10.1160/th06-11-0670. [DOI] [PubMed] [Google Scholar]
- 10.Censarek P., Bobbe A., Grandoch M., Schrör K., Weber A.A. Alternatively spliced human tissue factor (asHTF) is not pro-coagulant. Thromb Haemost. 2007;97:11–14. [PubMed] [Google Scholar]
- 11.Levinson S.S., Miller J.J. Towards a better understanding of heterophile (and the like) antibody interference with modern immunoassays. Clin Chim Acta. 2002;325:1–15. doi: 10.1016/s0009-8981(02)00275-9. [DOI] [PubMed] [Google Scholar]
- 12.Levinson S.S. Antibody multispecificity in immunoassay interference. Clin Biochem. 1992;25:77–87. doi: 10.1016/0009-9120(92)80048-l. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan I.V., Levinson S.S. When is a heterophile antibody not a heterophile antibody? When it is an antibody against a specific immunogen. Clin Chem. 1999;45:616–618. [PubMed] [Google Scholar]
- 14.Tate J., Ward G. Interferences in immunoassay. Clin Biochem Rev. 2004;25:105–120. [PMC free article] [PubMed] [Google Scholar]
- 15.Uhlen M., Bandrowski A., Carr S., Edwards A., Ellenberg J., Lundberg E., et al. A proposal for validation of antibodies. Nat Methods. 2016;13:823–827. doi: 10.1038/nmeth.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koyama T., Nishida K., Ohdama S., Sawada M., Murakami N., Hirosawa S., et al. Determination of plasma tissue factor antigen and its clinical significance. Br J Haematol. 1994;87:343–347. doi: 10.1111/j.1365-2141.1994.tb04919.x. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi H., Sato N., Shibata A. Plasma tissue factor pathway inhibitor in disseminated intravascular coagulation: comparison of its behavior with plasma tissue factor. Thromb Res. 1995;80:339–348. doi: 10.1016/0049-3848(95)00185-t. [DOI] [PubMed] [Google Scholar]
- 18.Tay S.P., Cheong S.K. An in-house ELISA method for quantification of circulating tissue factor. Malays J Pathol. 2002;24:45–51. [PubMed] [Google Scholar]
- 19.Der Putten R.F.M., Velthuis H.T., Zwaan C., Aarden L.A., Glatz J.F.C., Hermens W.T. State and diagnostic value of plasma tissue factor in early-hospitalised patients with chest pain. Br J Haematol. 2005;131:91–99. doi: 10.1111/j.1365-2141.2005.05722.x. [DOI] [PubMed] [Google Scholar]
- 20.Khorana A.A., Francis C.W., Menzies K.E., Wang J.-G., Hyrien O., Hathcock J., Mackman N., Taubman M.B. Plasma tissue factor may be predictive of venous thromboembolism in pancreatic cancer. J Thromb Haemost. 2008;6:1983. doi: 10.1111/j.1538-7836.2008.03156.x. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parhami-Seren B., Butenas S., Krudysz-Amblo J., Mann K.G. Immunologic quantitation of tissue factors. J Thromb Haemost. 2006;4:1747–1755. doi: 10.1111/j.1538-7836.2006.02000.x. [DOI] [PubMed] [Google Scholar]
- 22.Albrecht S., Luther T., Grossmann H., Flössel C., Kotzsch M., Müller M. An ELISA for tissue factor using monoclonal antibodies. Blood Coagul Fibrinolysis. 1992;3:263–270. doi: 10.1097/00001721-199206000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Hisada Y., Sachetto A.T.A., Mackman N. Circulating tissue factor-positive extracellular vesicles and their association with thrombosis in different diseases. Immunol Rev. 2022;312:61–75. doi: 10.1111/imr.13106. [DOI] [PubMed] [Google Scholar]
- 24.Khorana A.A., Kamphuisen P.W., Meyer G., Bauersachs R., Janas M.S., Jarner M.F., et al. Tissue factor as a predictor of recurrent venous thromboembolism in malignancy: biomarker analyses of the CATCH trial. J Clin Oncol. 2017;35:1078–1085. doi: 10.1200/JCO.2016.67.4564. [DOI] [PubMed] [Google Scholar]
- 25.Meng S., Kang K., Fei D., Yang S., Gu Q., Pan S., Zhao M. Preliminary study of microparticle coagulation properties in septic patients with disseminated intravascular coagulation. J Int Med Res. 2021;49 doi: 10.1177/03000605211014094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosell A., Havervall S., Von Meijenfeldt F., Hisada Y., Aguilera K., Grover S.P., et al. Patients with COVID-19 have elevated levels of circulating extracellular vesicle tissue factor activity that is associated with severity and mortality—brief report. Arterioscler Thromb Vasc Biol. 2021;41:878–882. doi: 10.1161/ATVBAHA.120.315547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guervilly C., Bonifay A., Burtey S., Sabatier F., Cauchois R., Abdili E., et al. Dissemination of extreme levels of extracellular vesicles: tissue factor activity in patients with severe COVID-19. Blood Adv. 2021;5:628–634. doi: 10.1182/bloodadvances.2020003308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee R.D., Barcel D.A., Williams J.C., Wang J.G., Boles J.C., Manly D.A., et al. Pre-analytical and analytical variables affecting the measurement of plasma-derived microparticle tissue factor activity. Thromb Res. 2012;129:80–85. doi: 10.1016/j.thromres.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Claussen C., Rausch A.-V., Lezius S., Amirkhosravi A., Davila M., Francis J.L., et al. Microvesicle-associated tissue factor procoagulant activity for the preoperative diagnosis of ovarian cancer. Thromb Res. 2016;141:39–48. doi: 10.1016/j.thromres.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Ay C., Mackman N. Tissue Factor: Catch Me If You Can. J Clin Oncol. 2017;35:1128–1130. doi: 10.1200/JCO.2016.70.6788. [DOI] [PubMed] [Google Scholar]
- 31.Mackman N., Hisada Y., Archibald S.J. Tissue factor and its procoagulant activity on cancer-associated thromboembolism in pancreatic cancer: comment by Mackman et al. Cancer Sci. 2022;113:1885. doi: 10.1111/cas.15276. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mackman N., Sachetto A.T.A., Hisada Y. Measurement of tissue factor-positive extracellular vesicles in plasma: strengths and weaknesses of current methods. Curr Opin Hematol. 2022;29:266–274. doi: 10.1097/MOH.0000000000000730. [DOI] [PubMed] [Google Scholar]
- 33.Rosell A., Moser B., Hisada Y., Chinthapatla R., Lian G., Yang Y., et al. Evaluation of different commercial antibodies for their ability to detect human and mouse tissue factor by western blotting. Res Pract Thromb Haemost. 2020;4:1013–1023. doi: 10.1002/rth2.12363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marchetti M., Diani E., Ten Cate H., Falanga A. Characterization of the thrombin generation potential of leukemic and solid tumor cells by calibrated automated thrombography. Haematologica. 2012;97:1173–1180. doi: 10.3324/haematol.2011.055343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Falanga A., Consonni R., Marchetti M., Locatelli G., Garattini E., Passerini C.G., et al. Cancer procoagulant and tissue factor are differently modulated by all-trans-retinoic acid in acute promyelocytic leukemia cells. Blood. 1998;92:143–151. [PubMed] [Google Scholar]
- 36.Lacroix R., Judicone C., Mooberry M., Boucekine M., Key N.S., Dignat-George F. Standardization of pre-analytical variables in plasma microparticle determination: results of the International Society on Thrombosis and Haemostasis SSC Collaborative workshop. J Thromb Haemost. 2013;11:1190–1193. doi: 10.1111/jth.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bettin B., Gasecka A., Li B., Dhondt B., Hendrix A., Nieuwland R., Van Der Pol E. Removal of platelets from blood plasma to improve the quality of extracellular vesicle research. J Thromb Haemost. 2022;20:2679–2685. doi: 10.1111/jth.15867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Théry C., Amigorena S., Raposo G., Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Current Protocols Cell Biol. 2006;30 doi: 10.1002/0471143030.cb0322s30. 3.22.1-3.9. [DOI] [PubMed] [Google Scholar]
- 39.Krishnamachary B., Cook C., Kumar A., Spikes L., Chalise P., Dhillon N.K. Extracellular vesicle-mediated endothelial apoptosis and EV-associated proteins correlate with COVID-19 disease severity. J Extracellular Vesicles. 2021;10 doi: 10.1002/jev2.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hisada Y., Mackman N. Measurement of tissue factor activity in extracellular vesicles from human plasma samples. Res Pract Thromb Haemost. 2019;3:44–48. doi: 10.1002/rth2.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hisada Y., Alexander W., Kasthuri R., Voorhees P., Mobarrez F., Taylor A., et al. Measurement of microparticle tissue factor activity in clinical samples: a summary of two tissue factor-dependent FXa generation assays. Thrombosis Res. 2016;139:90–97. doi: 10.1016/j.thromres.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bach R., Gentry R., Nemerson Y. Factor VII binding to tissue factor in reconstituted phospholipid vesicles: induction of cooperativity by phosphatidylserine. Biochemistry. 1986;25:4007–4020. doi: 10.1021/bi00362a005. [DOI] [PubMed] [Google Scholar]
- 43.Fair D.S., MacDonald M.J. Cooperative interaction between factor VII and cell surface-expressed tissue factor. J Biol Chem. 1987;262:11692–11698. [PubMed] [Google Scholar]
- 44.Roy S., Paborsky L.R., Vehar G.A. Self-association of tissue factor as revealed by chemical crosslinking. J Biol Chem. 1991;266:4665–4668. [PubMed] [Google Scholar]
- 45.Tripisciano C., Weiss R., Eichhorn T., Spittler A., Heuser T., Fischer M.B., Weber V. Different potential of extracellular vesicles to support thrombin generation: contributions of phosphatidylserine, tissue factor, and cellular origin. Sci Rep. 2017;7 doi: 10.1038/s41598-017-03262-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kobayashi S., Koizume S., Takahashi T., Ueno M., Oishi R., Nagashima S., et al. Tissue factor and its procoagulant activity on cancer-associated thromboembolism in pancreatic cancer. Cancer Sci. 2021;112:4679–4691. doi: 10.1111/cas.15106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shimura M., Wada H., Wakita Y., Nakase T., Hiyoyama K., Nagaya S., Mori Y., Shiku H. Plasma tissue factor and tissue factor pathway inhibitor levels in patients with disseminated intravascular coagulation. Am J Hematol. 1997;55:169–174. doi: 10.1002/(sici)1096-8652(199707)55:4<169::aid-ajh1>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 48.Kim H.K., Song K.S., Park Y.S., Yun Y.-S., Shim W.H. Changes of plasma tissue factor and tissue factor pathway inhibitor antigen levels and induction of tissue factor expression on the monocytes in coronary artery disease. Cardiology. 2000;93:31–36. doi: 10.1159/000006999. [DOI] [PubMed] [Google Scholar]
- 49.Blann A.D., Amiral J., McCollum C.N., Lip G.Y. Differences in free and total tissue factor pathway inhibitor, and tissue factor in peripheral artery disease compared to healthy controls. Atherosclerosis. 2000;152:29–34. doi: 10.1016/s0021-9150(99)00444-x. [DOI] [PubMed] [Google Scholar]
- 50.Marcucci R., Prisco D., Brunelli T., Pepe G., Gori A.M., Fedi S., et al. Tissue factor and homocysteine levels in ischemic heart disease are associated with angiographically documented clinical recurrences after coronary angioplasty. Thromb Haemost. 2000;83:826–832. [PubMed] [Google Scholar]
- 51.Lindmark E., Wallentin L., Siegbahn A. Blood cell activation, coagulation, and inflammation in men and women with coronary artery disease. Thromb Res. 2001;103:249–259. doi: 10.1016/s0049-3848(01)00313-9. [DOI] [PubMed] [Google Scholar]
- 52.Naumnik B., Borawski J., Chyczewski L., Pawlak K., Mysliwiec M. Tissue factor and its inhibitor in human non-crescentic glomerulonephritis--immunostaining vs plasma and urinary levels. Nephrol Dial Transpl. 2006;21:3450–3457. doi: 10.1093/ndt/gfl365. [DOI] [PubMed] [Google Scholar]
- 53.Goldin-Lang P., Tran Q.-V., Fichtner I., Eisenreich A., Antoniak S., Schulze K., et al. Tissue factor expression pattern in human non-small cell lung cancer tissues indicate increased blood thrombogenicity and tumor metastasis. Oncol Rep. 2008;20:123–128. [PubMed] [Google Scholar]
- 54.El Solh A.A., Akinnusi M.E., Berim I.G., Peter A.M., Paasch L.L., Szarpa K.R. Hemostatic implications of endothelial cell apoptosis in obstructive sleep apnea. Sleep Breath. 2008;12:331–337. doi: 10.1007/s11325-008-0182-x. [DOI] [PubMed] [Google Scholar]
- 55.Skóra J., Dawiskiba T., Zaleska P., Kurcz J., Mastalerz-Migas A., Adamiec R., Gosk-Bierska I. Prognostic value of tissue factor in patients with abdominal aortic and iliac arterial aneurysms – preliminary study. Arch Med Sci. 2013;6:1071–1077. doi: 10.5114/aoms.2013.39795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hernández C., Orbe J., Roncal C., Alvarez-Hernandez M., De Lizarrondo S.M., Alves M.T., Mata J.G., Páramo J.A. Tissue factor expressed by microparticles is associated with mortality but not with thrombosis in cancer patients. Thromb Haemost. 2013;110:598–608. doi: 10.1160/TH13-02-0122. [DOI] [PubMed] [Google Scholar]
- 57.Zarychta E., Rhone P., Bielawski K., Rosc D., Szot K., Zdunska M., Ruszkowska-Ciastek B. Elevated plasma levels of tissue factor as a valuable diagnostic biomarker with relevant efficacy for prediction of breast cancer morbidity. J Physiol Pharmacol. 2018;69 doi: 10.26402/jpp.2018.6.06. [DOI] [PubMed] [Google Scholar]
- 58.Talar-Wojnarowska R., Woźniak M., Borkowska A., Cypryk K., Olakowski M., Małecka-Panas E. Procoagulant disorders in patients with newly diagnosed pancreatic adenocarcinoma. Medicina. 2020;56:677. doi: 10.3390/medicina56120677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cacciola R., Gentilini Cacciola E., Vecchio V., Cacciola E. Cellular and molecular mechanisms in COVID-19 coagulopathy: role of inflammation and endotheliopathy. J Thromb Thromb. 2022;53:282–290. doi: 10.1007/s11239-021-02583-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hayden H., Ibrahim N., Klopf J., Zagrapan B., Mauracher L.-M., Hell L., et al. ELISA detection of MPO-DNA complexes in human plasma is error-prone and yields limited information on neutrophil extracellular traps formed in vivo. PLOS ONE. 2021;16 doi: 10.1371/journal.pone.0250265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Engstad C.S., Gutteberg T.J., Osterud B. Modulation of blood cell activation by four commonly used anticoagulants. Thromb Haemost. 1997;77:690–696. [PubMed] [Google Scholar]