Abstract
Aims
We developed a novel mouse model with increased skeletal muscle estrogen content via inducible, skeletal-muscle–specific aromatase overexpression (SkM-Arom↑). We proposed to examine the effect that increased skeletal muscle estrogen both in gonadally intact and ovariectomized (OVX) female mice has on preventing or rescuing high-fat diet (HFD)-induced obesity.
Methods
In the prevention experiment, gonadally intact and OVX SkM-Arom↑ mice and littermate controls were fed a low-fat diet (LFD) or HFD for 13 weeks. SkM-Arom↑ was induced at the initiation of dietary treatment. In the intervention experiment, gonadally intact and OVX SkM-Arom↑ mice and littermate controls were fed an HFD for 14 weeks before induction of SkM-Arom↑ for 6 weeks. Glucose tolerance, insulin action, adipose tissue inflammation, and body composition were assessed. Liquid chromatography–mass spectrometry was used to determine circulating and skeletal muscle steroid content.
Results
SkM-Arom↑ significantly increased skeletal muscle 17β-estradiol (E2) and estrone (E1) in both experiments. Interestingly, this resulted in leakage of estrogens into circulation, producing a physiologically relevant E2 concentration. Consequently, bone mineral density (BMD) was enhanced and adipose tissue inflammation was reduced in the prevention experiment only. However, no benefits were seen with respect to changes in adiposity or metabolic outcomes.
Conclusion
We show that increasing skeletal muscle estrogen content does not provide a metabolic benefit in gonadally intact and OVX female mice in the setting of obesity. However, a chronic physiological concentration of circulating E2 can improve BMD and reduce adipose tissue inflammation independently of a metabolic benefit or changes in adiposity.
Keywords: skeletal muscle, estrogen, obesity, metabolism, aromatase
17β-estradiol (E2) is the most abundant circulating estrogen in females during the premenopausal period. The effect that E2 has on metabolic processes is an ongoing field of study given the hormone’s broad role in regulating energy balance and metabolic homeostasis (1). As evident during postmenopause in humans and ovariectomy (OVX) in rodents, E2 deficiency is linked to increased adiposity as well as impaired glucose metabolism and insulin sensitivity, which can be rescued with E2 treatment (2–5). Skeletal muscle is the main source of insulin-stimulated glucose metabolism and thus plays a central role in maintaining a healthy metabolic phenotype (6). Previous in vivo studies have established that E2 has a role in regulating mitochondrial bioenergetics and insulin sensitivity in skeletal muscle (3, 7). However, the primary limitation of previous in vivo research using E2 supplementation is the lack of measurement of circulating E2 to determine if the dose of E2 used is physiologically relevant. This is important because different modes of E2 delivery have been shown to elicit supraphysiological circulating E2 levels (8). Additionally, E2 has been shown to act within the central nervous system to regulate food intake, which makes it difficult to interrogate the peripheral effect of E2 (9). Given these previous limitations, we developed a novel mouse model using the reverse tetracycline transactivator (rtTA) and tet-on system to inducibly and specifically increase E2 production in skeletal muscle via overexpression of aromatase—the enzyme necessary to convert androgens into estrogens. This model was applied to high-fat-diet (HFD)-induced obesity to determine if increased skeletal muscle estrogen content both in gonadally intact and OVX female mice could beneficially affect HFD-induced metabolic dysregulation.
Materials and Methods
Animals
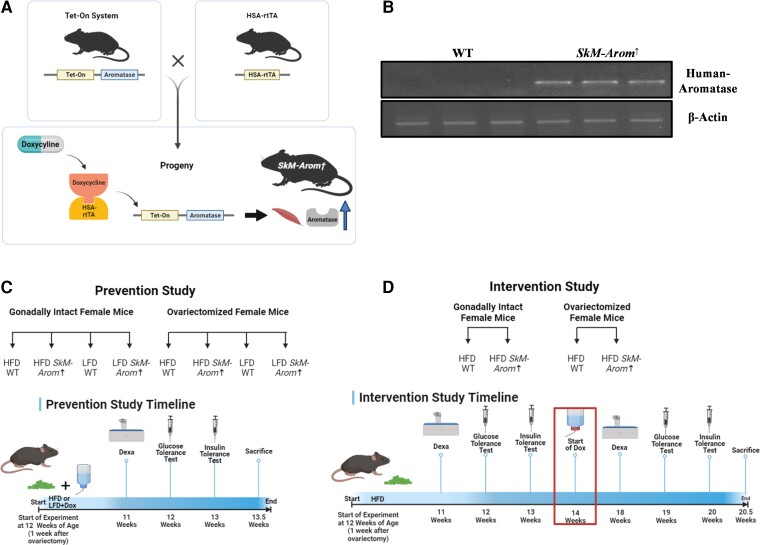
HSA-rtTA+/+ (10) and tet-Aromatase+/− mice (11) on a C57BL/6 background were bred to generate a novel mouse model that allows for inducible overexpression of aromatase specifically in skeletal muscle on doxycycline (DOX) treatment (HSA-rtTA+/−, tet-Aromatase+/−) (Fig. 1A and 1B). We have named this novel mouse model the SkM-Arom↑ mouse. Littermate controls (HSA-rtTA+/−, tet-Aromatase−/−) were used for all experiments. Inducible overexpression of aromatase in SkM-Arom↑ mice was achieved by providing 0.1 mg/mL DOX in drinking water. To account for any potential side effects of DOX treatment, all groups received DOX-supplemented water. Drinking water was changed on a weekly basis. Female mice were used for all experiments.
Figure 1.
Schematic of the skeletal-muscle–specific aromatase overexpression (SkM-Arom↑) model and experimental design. A, Schematic of the SkM-Arom↑ mouse model; B, validation of SkM-Arom↑ overexpression via polymerase chain reaction and Northern blot; C, experimental design of the prevention experiment; and D, experimental design of the intervention experiment.
Diets and Ovariectomy
Two different experiments were performed as illustrated in Fig. 1C and 1D. In the first experiment, SkM-Arom↑ was induced simultaneously with the administration of an HFD as a preventive measure against obesity development. In the second experiment, mice were fed an HFD to induce obesity before inducing SkM-Arom↑ as an interventive therapy.
For the prevention experiment, 10-week-old SkM-Arom↑ and littermate control mice were randomly assigned to 1 of 4 groups: wild-type (WT) low-fat diet (LFD), SkM-Arom↑-LFD, WT-HFD, and SkM-Arom↑-HFD groups. A second cohort of 4 groups was also included in which mice were OVX to examine the effect of skeletal muscle aromatase overexpression in an estrogen-deficient state. All OVX surgeries were performed at approximately age 11 weeks when female mice were sexually mature. Mice were given 1 week of recovery before the initiation of dietary treatment. Diets were administered for 13 weeks. In previous unpublished work from our laboratory, we have found this length of feeding to be sufficient to induce HFD increases in adiposity, adipose tissue inflammation, and mildly impaired glucose tolerance. The LFD used in this experiment was the open-source, purified AIN-76A diet (3.77 kcal/g). The HFD (4.57 kcal/g) was a purified diet composed of 47%, 40%, and 13% of total calories from carbohydrate, fat, and protein, respectively, with saturated fat making up 12% of total calories to mimic the standard American diet (BioServ). Details and previous use of this diet are provided elsewhere (12–20).
For the intervention experiment, gonadally intact and OVX SkM-Arom↑ and littermate control mice were fed an HFD for 14 weeks before administration of DOX-supplemented water for an additional 6 weeks (20 weeks of dietary treatment in total). The sample size for all experimental groups in both the prevention and intervention experiments was n = 10 to 20 mice/group. Mice were housed, 3 to 5/cage, maintained on a 12:12-hour light-dark cycle in a low-stress environment (22 °C, 50% humidity, low noise), and given food and water ad libitum. All methods were in accordance with the American Association for Laboratory Animal Science, and the Institutional Animal Care and Usage Committee of the University of South Carolina approved all experiments.
Body Weight and Body Composition
Body weight was monitored on a weekly basis throughout the study. Body composition was assessed after 11 weeks of diet to use lean mass as the basis for the dose of glucose and insulin administration for glucose (GTTs) and insulin tolerance tests (ITTs), respectively. For the intervention experiment, body composition was assessed after 11 weeks of diet and after 5 weeks of DOX treatment (18 weeks of diet). For this procedure, mice were briefly anesthetized via isoflurane inhalation, and lean mass, fat mass, percentage of body fat, and bone mineral density (BMD) were assessed by dual-energy x-ray absorptiometry (Lunar PIXImus). BMD analysis of the lumbar vertebrae as well as the proximal, medial, and distal femur was performed as previously described (21).
Metabolic Assessment
Fasting blood glucose and insulin levels were assessed after 12 weeks of dietary treatment. After a 5-hour fast, blood samples were collected from the tip of the tail. A glucometer (Bayer Contour) was used to determine blood glucose concentrations in whole blood. Collected blood was centrifuged at 4000 rpm for 10 minutes at 4 °C. Plasma insulin concentrations were analyzed according to the manufacturer’s instructions using a mouse insulin enzyme-linked immunosorbent assay (ELISA) kit (Mercodia) (antibody ID: AB_2783837). GTTs and ITTs were performed after 12 and 13 weeks of dietary treatment, respectively. For these procedures, mice were fasted for 5 hours, and glucose or insulin was administered intraperitoneally at 2 g/kg or 0.75 U/kg lean mass, respectively. A glucometer (Bayer Contour) was used to measure blood glucose concentrations (tail sampling) intermittently over a 2-hour period (0, 15, 30, 60, 90, and 120 minutes) for GTTs and intermittently over a 1-hour period (0, 15, 30, 45, and 60 minutes) for ITTs. Baseline glucose levels were subtracted accordingly, and area of the curve was generated as previously described (22). Area under the curve of the area of the curve was calculated using the trapezoidal rule. Blood was collected from the tip of the tail during the GTT (0, 15, 30, and 60 minutes) to assess the insulin response to the GTT for a subset of mice from each group. Fasting serum was collected (using nonheparinized capillary tubes) for free fatty acid (FFA) analysis at the 0- and 30-minute time points of the ITT to assess insulin’s ability to inhibit lipolysis. FFAs were analyzed using a commercially available kit according to the manufacturer’s instructions (Wako Diagnostics). For the intervention experiment, baseline GTTs and ITTs were performed after 12 and 13 weeks of dietary treatment, respectively, as well as after 5 and 6 weeks of DOX treatment (19 and 20 weeks of dietary treatment).
Tissue Collection
At the termination of each experiment, mice were euthanized via isoflurane inhalation for tissue collection. Blood was collected from the inferior vena cava using heparinized syringes and subsequently centrifuged to isolate plasma. Gonadal, mesenteric, perirenal fat pads, uterus, and skeletal muscle (gastrocnemius and quadriceps) were removed and immediately snap-frozen in liquid nitrogen and stored at −80 °C until analysis.
Quantitative Real-time Polymerase Chain Reaction
An EZNA Total RNA Kit (Omega Bio-Tek) was used to isolate RNA from gonadal adipose tissue. Bio-Rad reverse transcription reagents and probe assays (Bio-Rad) were used to reverse transcribe and analyze the expression of the following genes in adipose tissue: CD68, CD11c, CD206, MCP-1, tumor necrosis factor α (TNFα), and toll-like receptor 2 (TLR2). Potential reference genes (HPRT, 18s, GAPDH, β-Actin, HMBS, TBP, H2AFV, RPLPO, and B2M) were analyzed for stability using qBase+ software (Biogazelle) for each tissue analyzed. The optimal number of reference genes was determined by qBase+, and the geometric mean of these genes was used as the normalization factor for each analysis. Gene expression was quantified using the ΔΔCT method and qBase+ software (23).
Extraction of Steroids
Before processing samples, internal standards (Sigma Aldrich) for testosterone (T) (-23,4-13C3), E1 (-23,4-13C3), and E2 (D5) were added to each tissue and plasma sample (E1 and E2). Calibration curves using T (Sigma Aldrich, catalog No. T037), E1 (Sigma Aldrich, catalog No. E-075), and E2 (Sigma Aldrich, catalog No. E-060) were used to determine the quantity of each steroid. Steroids were extracted from approximately 150 to 200 μL of plasma via vortex using 500 mL of MTBE (×2). The resulting upper phase was removed and placed into a glass vial where it was dried down using N2. The dried-down steroids were derivatized as previously described using 1-methylimidazole-2-sulfonyl chloride (24). For tissue samples, approximately 150 mg of skeletal muscle (1.5 gastrocnemii) was cut into sections with a razor blade, weighed, and homogenized in 1 mL of acetonitrile for 3 minutes using a bead beater (Biospec Products). The samples were centrifuged for 5 minutes × 12 000g and the resulting supernatant was removed and placed into glass vials. Subsequently, the tissue samples were resuspended in 1 mL of acetonitrile, homogenized, centrifuged, and the supernatant collected for a second time. The supernatants were dried down using N2 and were resuspended in 200 mM sodium acetate. MTBE was added to the sample for liquid-liquid extraction of the steroids (×2). The MTBE layer was removed and dried under nitrogen gas before derivatization as previously described (24). After derivatization, the samples were placed in 0.2 poly(vinylidene fluoride) micro spin filters (Fisher Scientific) and centrifuged to remove any insoluble material before mass spectrometry analysis. Analyte recovery for plasma and tissue was greater than 80%.
Chromatography and Mass Spectrometry
As we did not have the mass spectrometry run set up to measure T initially, plasma samples were analyzed only for E1 and E2. After further method development, T was added to the mass spectrometry run to analyze skeletal muscle T in addition to E1 and E2. Only HFD samples from each respective group were analyzed for plasma and skeletal muscle steroid content for consistency between the prevention and intervention experiments. The analysis was carried out on a Q Exactive HF-X hybrid quadrupole-orbitrap mass spectrometer with a Vanquish HPLC on the front end (Thermo Electron). A total of 10 μL of the derivatized sample was injected onto a Waters Xbridge C18 column (2.1 mm × 100 mm, 3.5 μm particles; Waters Corp) using a mobile phase consisting of 0.1% formic acid in water (A) and 0.1% formic acid in methanol (B). The estrogen derivatives and T were separated using the following linear solvent gradient at a flow rate of 200 μL/min: initial conditions 30% B ramping to 65% B over 6 minutes followed by a shallow ramp to 73% B ending at 20 minutes. At 20.1 minutes a quick ramp to 95% B holding until 23 minutes. Finally, a return to starting conditions of 30% B at 23.1 minute ending at 26 minutes.
Mass analysis was carried out using electrospray ionization in positive ion mode. The HESI ion source settings were as follows: sheath gas flow, 45 units; aux gas flow, 10 units; sweep gas flow, 2 units; electrospray voltage, 4 kV; capillary temperature, 350 °C; funnel RF, 40; aux gas heater, 400 °C. The estrogen derivatives were analyzed using targeted selected ion monitoring using the M + H ion of each derivative (for E1 M + H = 415.1686; for E2 M + H = 417.1843); simultaneously, the heavy isotopically labeled internal standards were similarly monitored (for 13C3 E1 M + H = 418.1787; d5 E2 M + H = 422.2156). The targeted selected ion monitoring settings were: microscans, 2; resolution, 60K; AGC target, 1E6; maximum IT, 200 ms; isolation window, 4 m/z; data type, profile. T and its stable isotope labeled internal standard were analyzed using parallel reaction monitoring mode fragmenting the M + H ion in the HCD collision cell and measuring the fragments in the orbitrap analyzer (for T, M + H precursor ion = 289.2162 with quantifying product ion 109 m/z; for 13C3 T, M + H precursor ion = 292.2263 with quantifying product ion 100 m/z). The parallel reaction monitoring settings were: microscans, 1; resolution, 30K; AGC target, 2E5; maximum IT, 100 ms; isolation window, 2.0 m/z; stepped NCE, 34,45,55; data type, profile. The mass spectrometer was mass calibrated before each batch of runs and the data were collected and processed using the vendor-provided Xcalibur software with mass error filter set at 5 ppm. Peak area ratios of analyte and internal standard were used to determine steroid concentrations. With respect to sensitivity, we were successfully able to detect a statistically significant difference (P < .05, one-tailed t test) using a minimum of 3 replicates in the peak area of 0.1 pg for E1 and E2 and 0.5 pg for T compared to a method blank. For the E1 and E2 calibration curves, a sensitive calibration curve (0.1, 0.25, 0.5, 0.75, 1.0, and 1.5 pg) and a more robust calibration curve (1, 3, 5, 10, and 50 pg) yielded r2 values greater than 0.98. For T, the sensitive calibration curve (0.5, 1, 2, 3, and 5 pg) and the robust calibration curve (5, 10, 20, 100, 200, and 1000 pg) also yielded r2 values greater than 0.98.
Statistical Analysis
For the prevention experiment, data were analyzed using commercially available statistical software: Prism 9 (GraphPad Software). A 2-way analysis of variance (ANOVA) (diet × genotype) followed by a Newman-Keuls post hoc test was used to assess differences between gonadally intact LFD-WT, LFD-SkM-Arom↑, HFD-WT, and HFD-SkM-Arom↑ groups. Additionally, a 2-way ANOVA (diet × genotype) followed by a Newman-Keuls post hoc test was used to assess differences between OVX LFD-WT, OVX LFD-SkM-Arom↑, OVX HFD-WT, and OVX HFD-SkM-Arom↑. For the intervention experiment, a 2-way ANOVA (gonadal status × genotype) followed by a Newman-Keuls post hoc test was used to assess differences between HFD-WT, OVX HFD-WT, HFD-SkM-Arom↑, and OVX HFD-SkM-Arom↑ groups. For metabolic assessment over time, a 2-way ANOVA (diet × genotype) followed by a Newman-Keuls post hoc test was run within each time point. For steroid analysis, if any steroid was not detected because of low abundance for all samples in the group, a 2-way ANOVA was performed to assess main effects (gonadal status × genotype) only. Any statistical test that did not pass the equal-variance test (Bartlett test for equal variances) was log-transformed and then reanalyzed. Data are presented as means ± SE, and the level of statistical significance was set at P less than .05.
Results
Prevention Experiment
Skeletal-muscle–specific aromatase overexpression genotype increases skeletal muscle and circulating estrogen content after 13 weeks of doxycycline treatment
Skeletal muscle (E1, E2, and T) and plasma (E1 and E2) steroid content were measured after 13 weeks of DOX treatment (Table 1). Both intact and OVX SkM-Arom↑ mice presented increased skeletal muscle E1 and E2 content. The OVX mice displayed significantly lower skeletal muscle E2 content only in the WT genotype (P < .05). With respect to skeletal muscle T, the OVX SkM-Arom↑ displayed the lowest level of skeletal muscle T compared to all other groups (P < .05).
Table 1.
Skeletal muscle and plasma steroid concentrations
| Prevention experiment | |||||
|---|---|---|---|---|---|
| Intact | OVX | Main effect statistical analysis | |||
| WT | SkM-Arom↑ | WT | SkM-Arom↑ | ||
| Skeletal muscle | |||||
| E1 (pg/g tissue) | ND | 27 ± 5 | ND | 30 ± 2 | *Genotype |
| E2 (pg/g tissue) | 12 ± 3a | 38 ± 11b | 2 ± 1c | 40 ± 7b | *Genotype, gonadal status, interaction |
| T (pg/g tissue) | 38 ± 10a | 20 ± 3a | 44 ± 14a | 10 ± 3b | *Genotype |
| Plasma | |||||
| E1 (pg/mL) | ND | 11 ± 4 | ND | 10 ± 2 | *Genotype |
| E2 (pg/mL) | 18 ± 3a | 24 ± 2a | 9 ± 1b | 26 ± 5a | *Genotype, gonadal status, interaction |
Tissue and circulating steroids were analyzed after 13 weeks of dietary and doxycycline treatment in HFD gonadally intact and OVX female WT and SkM-Arom↑ mice (n = 8-15/group). Data are presented as mean ± SE. Letters that do not match are statistically different from one another (P < .05). *Signifies statistically significant main effect.
Abbreviations: E1, estrone; E2, 17β-estradiol; HFD, high-fat diet; ND, not detected; OVX, ovariectomized; SkM-Arom↑, skeletal-muscle–specific aromatase overexpression; WT, wild-type.
Regarding plasma steroid analysis, both the intact and OVX SkM-Arom↑ mice displayed an increase in circulating E1 (P < .05). The plasma E2 concentration was decreased only in WT OVX mice compared to all other groups (P < .05). These data suggest that estrogens from skeletal muscle leaked into circulation for the SkM-Arom↑ mice.
In general, increased skeletal muscle estrogen content and physiological levels of circulating 17β-estradiol do not affect body weight or body composition in gonadally intact and ovariectomized mice
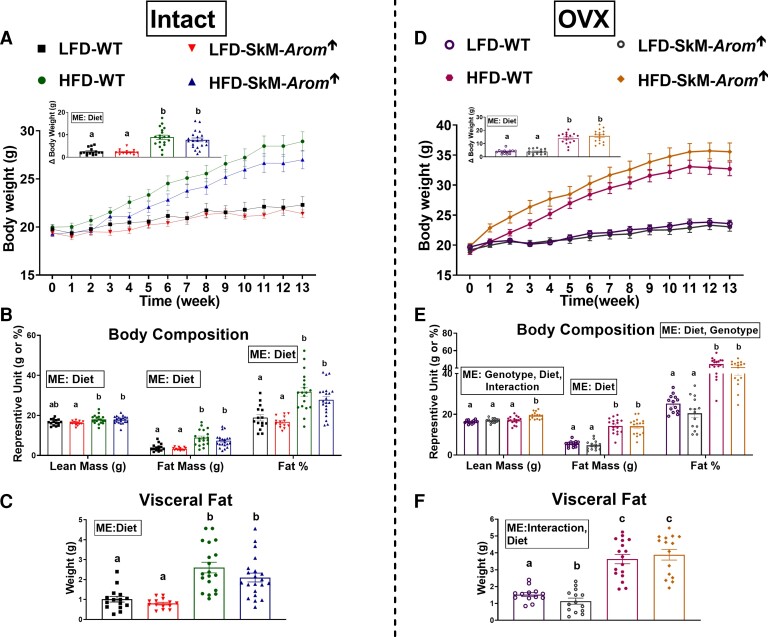
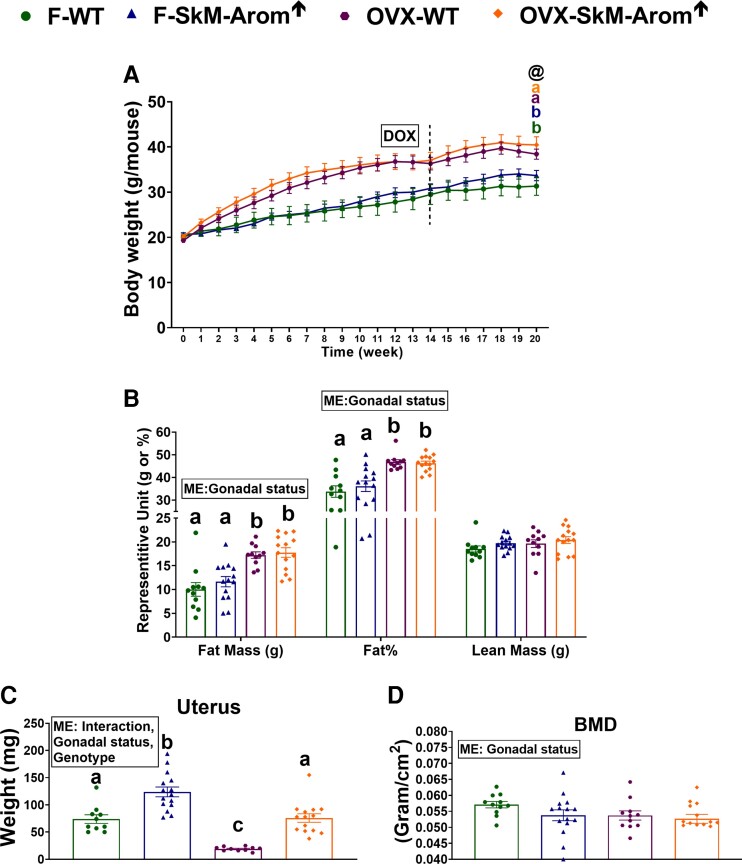
The HFD increased body weight (Fig. 2A and 2D), body composition (Fig. 2B and 2E), and visceral fat (Fig. 2C and 2F) both in gonadally intact and OVX mice (P < .05). Within the OVX groups, the OVX HFD SkM-Arom↑ mice displayed an increase in lean mass (P < .05). Additionally, visceral fat was reduced in the OVX LFD SkM-Arom↑ mice compared to all other groups (P < .05).
Figure 2.
Body weight and body composition are largely unaffected by increased tissue and circulating estrogens. A and D, body weight; B and E, body composition; and C and F, visceral fat were assessed after 13 weeks of dietary and doxycycline treatment (low-fat diet [LFD] or high-fat diet [HFD]) in gonadally intact and ovariectomized (OVX) female wild-type (WT) and skeletal-muscle–specific aromatase overexpression (SkM-Arom↑) mice (n = 13-20). Data are presented as mean ± SE. Bar graphs not sharing a common letter are statistically significantly different from one another (P < .05). ME, main effect.
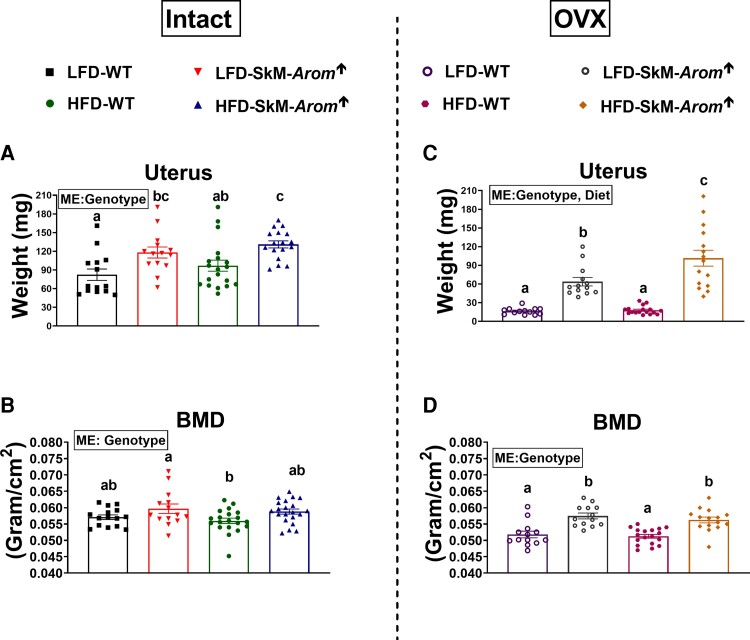
Skeletal-muscle–specific aromatase overexpression mice display increased uterus weight and bone mineral density
The SkM-Arom↑ genotype increased uterus weight as well as BMD independent of gonadal status (Fig. 3A-3D) (P < .05). In the intact mice, BMD analysis of the lumbar vertebrae was found to be decreased in the WT HFD mice compared to all other groups (Table 2) (P < .05). No differences across groups were found with respect to BMD of the femur. Regarding the OVX mice, the SkM-Arom↑ genotype increased BMD of the lumbar vertebrae independent of diet (P < .05). BMD of the proximal and medial femur was increased in the SkM-Arom↑ mice compared to their WT counterparts and when comparing between the LFD and HFD SkM-Arom↑ mice, the HFD SkM-Arom↑ mice displayed the greatest BMD in the proximal femur only (P < .05). WT HFD mice displayed a lower medial and distal femur BMD compared to all other groups (P < .05).
Figure 3.
Uterus size and bone mineral density (BMD) are affected by physiological levels of circulating estrogens. A and C, Uterus weight, and B and D, BMD, were assessed after 13 and 11 weeks of dietary treatment (low-fat diet [LFD] or high-fat diet [HFD]), respectively, in gonadally intact and ovariectomized (OVX) female wild-type (WT) and skeletal-muscle–specific aromatase overexpression (SkM-Arom↑) mice (n = 13-20). Data are presented as mean ± SE. Bar graphs not sharing a common letter are statistically significantly different from one another (P < .05). ME, main effect.
Table 2.
Bone mineral density analysis
| Prevention experiment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intact | Main effect statical analysis | OVX | Main effect statical analysis | |||||||
| LFD | HFD | LFD | HFD | |||||||
| WT | SkM-Arom↑ | WT | SkM-Arom↑ | WT | SkM-Arom↑ | WT | SkM-Arom↑ | |||
| Lumbar (g/cm2) | 0.073 ± 0.003a | 0.078 ± 0.002a | 0.062 ± 0.002b | 0.074 ± 0.003a | *Diet, genotype | 0.048 ± 0.003a | 0.072 ± 0.003b | 0.048 ± 0.003a | 0.074 ± 0.003b | *Genotype |
| Femur (g/cm2) | 0.088 ± 0.003 | 0.089 ± 0.002 | 0.091 ± 0.002 | 0.091 ± 0.001 | 0.076 ± 0.002a | 0.083 ± 0.002b | 0.076 ± 0.002a | 0.091 ± 0.001c | *Genotype, diet, interaction | |
| Proximal | ||||||||||
| Medial | 0.065 ± 0.002 | 0.066 ± 0.001 | 0.065 ± 0.001 | 0.067 ± 0.001 | 0.059 ± 0.001a | 0.062 ± 0.001c | 0.054 ± 0.002b | 0.067 ± 0.002c | *Genotype, interaction | |
| Distal | 0.066 ± 0.002 | 0.073 ± 0.002 | 0.068 ± 0.001 | 0.070 ± 0.002 | *Genotype | 0.058 ± 0.001a | 0.063 ± 0.001a | 0.052 ± 0.002b | 0.064 ± 0.002a | *Genotype, interaction |
BMD of the lumbar vertebrae and the proximal, medial, and distal regions of the femur were assessed after 11 weeks of dietary (LFD or HFD and doxycycline treatment in gonadally intact and OVX female WT and SkM-Arom↑ mice (n = 13-20). Data are presented as mean ± SE. Letters that do not match are statistically different from one another (P < .05). *Signifies statistically significant main effect.
Abbreviations: BMD, bone mineral density; HFD, high-fat diet; LFD, low-fat diet; OVX, ovariectomized; SkM-Arom↑, skeletal-muscle–specific aromatase overexpression; WT, wild-type.
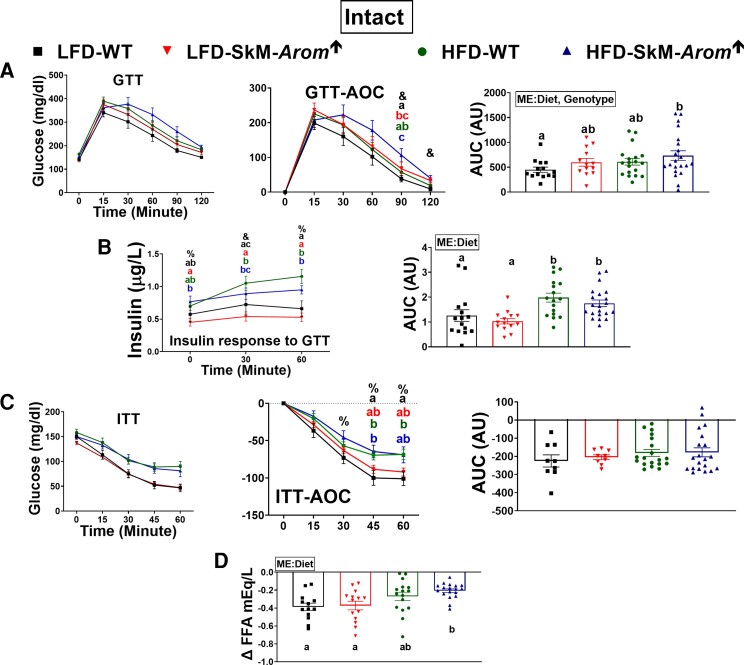
Increased skeletal muscle estrogen content does not hinder the development of obesity-associated metabolic dysfunction in gonadally intact or high-fat diet ovariectomized mice, but does improve glucose tolerance in low-fat diet ovariectomized mice
In the gonadally intact mice, glucose tolerance was impaired in the HFD SkM-Arom↑ mice relative to the LFD WT mice only (Fig. 4A) (P < .05). However, as evidenced by the insulin response to the GTT it took more insulin in both HFD groups to lower blood glucose levels to a similar degree as the LFD groups (Fig. 4B) (P < .05). With respect to insulin action, although no statistical difference was found in the overall ITT area under the curve, both HFD groups presented higher blood glucose levels at 45 and 60 minutes after insulin administration compared to the LFD WT group (Fig. 4C) (P < .05). Additionally, there was evidence of impaired adipose tissue insulin action in the HFD-SkM-Arom↑ mice relative to both LFD groups as evidenced by a minimal change in plasma FFAs after insulin administration (Fig. 4D) (P < .05).
Figure 4.
Glucose tolerance is not beneficially affected by increased skeletal muscle estrogen content in gonadally intact female mice. After approximately 12 weeks of dietary and doxycycline treatment (low-fat diet [LFD] or high-fat diet [HFD]), gonadally intact female mice were metabolically assessed by examining an A, glucose tolerance test (GTT); B, insulin response to the GTT; a C, insulin tolerance test (ITT); and D, Δ plasma free fatty acid (FFA) in response to insulin administration (n = 9-20). Bar graphs not sharing a common letter are statistically significantly different from one another (P < .05) (n = 9-20). %, main effect of diet; &, main effect of genotype; #, main effect for interaction; ME, main effect.
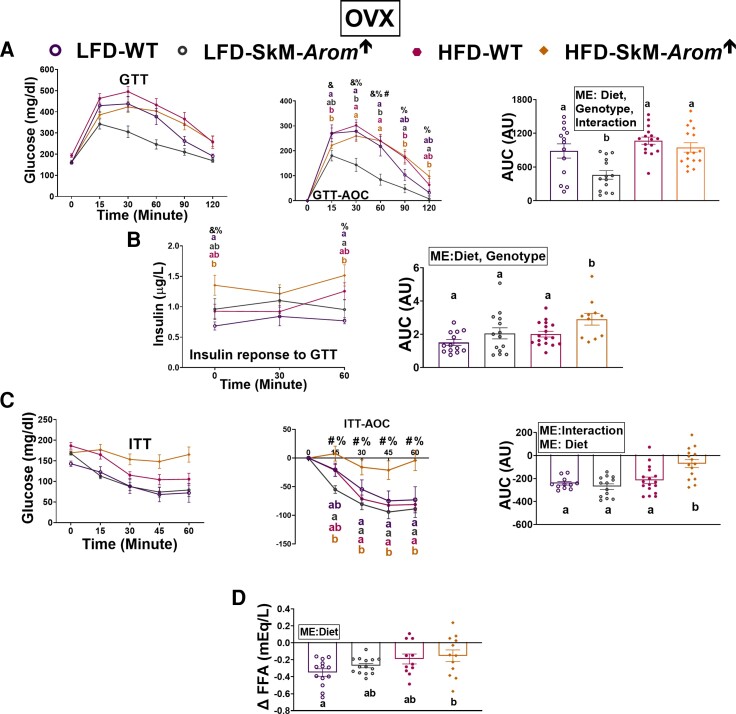
In the OVX mice, the SkM-Arom↑ genotype improved glucose tolerance only within the LFD groups (Fig. 5A) (P < .05). Fasting plasma insulin and circulating insulin over the course of the GTT was greatest in the OVX HFD-SkM-Arom↑ mice (Fig. 5B) (P < .05). For the ITT, impaired insulin action was evident only in the OVX HFD-SkM-Arom↑ mice (Fig. 5C) (P < .05). With respect to ability of insulin to hinder FFA release from adipose tissue, the OVX HFD-SkM-Arom↑ mice displayed the greatest degree of adipose tissue insulin resistance (Fig. 5D) (P < .05).
Figure 5.
Glucose tolerance is enhanced only under low-fat diet (LFD) conditions in ovariectomized (OVX) skeletal-muscle–specific aromatase overexpression (SkM-Arom↑) mice. After approximately 12 weeks of dietary and doxycycline treatment (LFD or high-fat diet [HFD]), OVX female mice were metabolically assessed by examining an A, glucose tolerance test (GTT); B, insulin response to the GTT; a C, insulin tolerance test (ITT); and D, Δ plasma free fatty acid (FFA) in response to insulin administration (n = 13-20). Bar graphs not sharing a common letter are statistically significantly different from one another (P < .05). %, main effect of diet; &, main effect of genotype; #, main effect for interaction; ME, main effect.
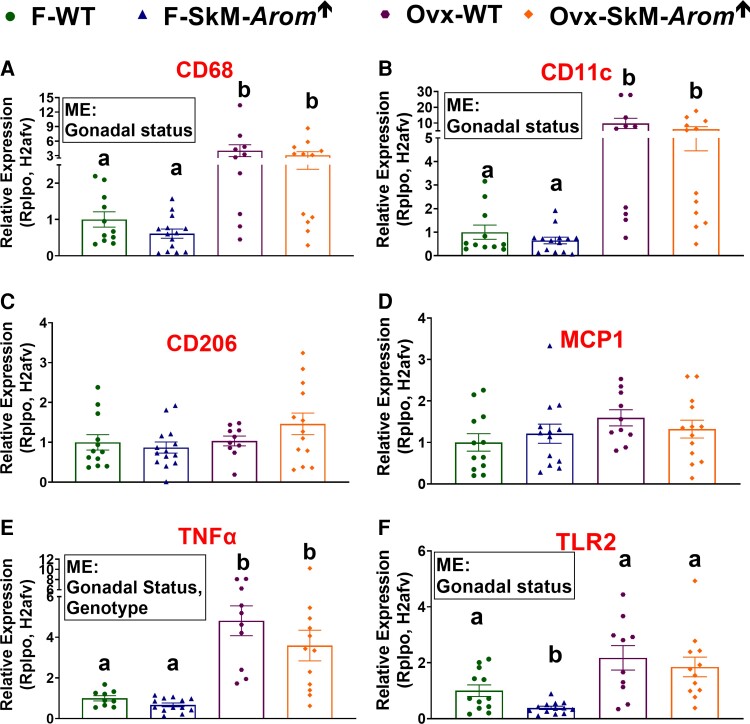
Skeletal-muscle–specific aromatase overexpression mice present decreased adipose tissue inflammation with high-fat diet feeding
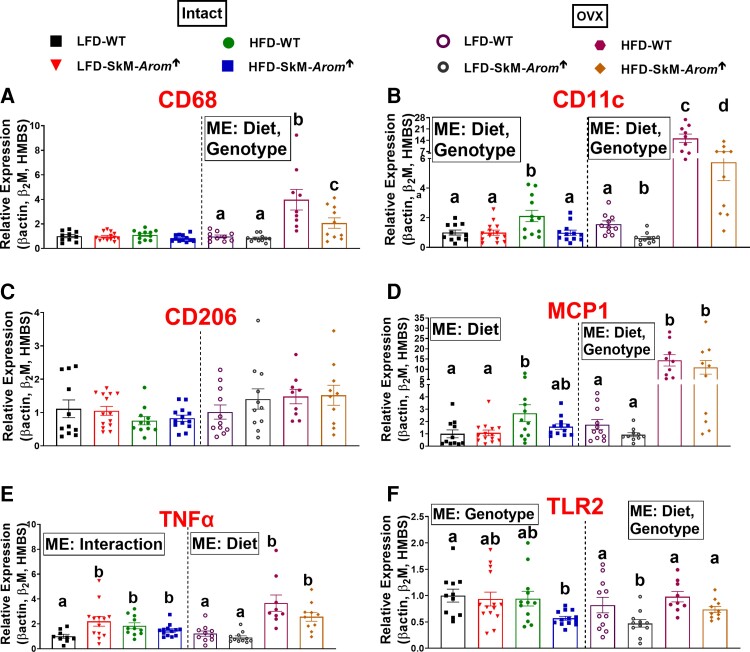
Adipose tissue inflammation was assessed by examining gene expression of macrophage markers (CD68 [pan macrophage marker], CD11c [proinflammatory macrophage marker], and CD206 [anti-inflammatory marker]) and inflammatory mediators (MCP1, TNFα, and TLR2) (Fig. 6). With respect to the gonadally intact mice, no difference was found regarding gene expression of CD68 (Fig. 6A) or CD206 between groups (Fig. 6C). However, CD11C and MCP1 expression were significantly elevated in the HFD-WT mice compared to LFD mice, whereas HFD-SkM-Arom↑ mice did not exhibit a statistically significant increase in expression of these genes relative to the LFD groups (P < .05). Regarding TNFα, all groups displayed higher expression of this cytokine relative to the LFD-WT group (Fig. 6E) (P < .05). For TLR2, the HFD-SkM-Arom↑ mice possessed less TLR2 gene expression than the LFD-WT mice (P < .05).
Figure 6.
The skeletal-muscle–specific aromatase overexpression (SkM-Arom↑) phenotype is characterized by reduced adipose tissue inflammation with high-fat diet (HFD) consumption. After 13 weeks of dietary and doxycycline treatment (low-fat diet [LFD] or HFD) in gonadally intact and ovariectomized (OVX) female wild-type (WT) and SkM-Arom↑ mice, adipose inflammation was assessed via quantitative reverse transcription–polymerase chain reaction for messenger RNA expression of A, CD68; B, CD11c; C, CD206; D, MCP1; E, tumor necrosis factor α (TNFα); and F, toll-like receptor 2 (TLR2) (n = 9-14). Bar graphs not sharing a common letter are statistically significantly different from one another (P < .05).
For the OVX mice, the HFD increased gene expression of all inflammatory markers analyzed, except for CD206, in which no difference across groups was found (see Fig. 6A) (P < .05). Post hoc analysis uncovered a statistically significant difference in CD68 and CD11c in the HFD-SkM-Arom↑ mice relative to their WT counterparts, whereas CD11c and TLR2 were downregulated in the LFD HFD-SkM-Arom↑ mice relative to their WT counterparts (P < .05).
Intervention Experiment
Six weeks of skeletal-muscle–specific aromatase overexpression used as an intervention for high-fat diet–induced obesity increases skeletal muscle and circulating estrogen levels, but does not rescue obesity in gonadally intact and ovariectomized mice
Both SkM-Arom↑ intact and OVX groups presented greater skeletal muscle E1 content relative to their WT counterparts (Table 3) (P < .05). Additionally, an interaction was found with respect to skeletal muscle E2 values with the OVX mice displaying significantly lower skeletal muscle E2 content only in the WT genotype (P < .05). Regarding plasma estrogen levels, the SkM-Arom↑ mice displayed an increase in the plasma E1 concentration irrespective of gonadal status and E2 was found to be lowest in the WT OVX mice (P < .05).
Table 3.
Skeletal muscle and plasma steroid concentrations
| Intervention experiment | |||||
|---|---|---|---|---|---|
| Intact | OVX | Main effect statical analysis | |||
| WT | SkM-Arom↑ | WT | SkM-Arom↑ | ||
| Skeletal muscle | |||||
| E1 (pg/g tissue) | ND | 12 ± 4 | ND | 13 ± 2 | *Genotype |
| E2 (pg/g tissue) | 15 ± 7a | 24 ± 10a | 0.1 ± 0.3b | 21 ± 4a | *Genotype, gonadal status, interaction |
| T (pg/g tissue) | 68 ± 9 | 47 ± 2 | 83 ± 7 | 47 ± 1 | *Genotype |
| Plasma | |||||
| E1 (pg/mL) | ND | 5 ± 1 | ND | 8 ± 2 | *Genotype |
| E2 (pg/mL) | 14 ± 7a | 10 ± 2a | 1 ± 1b | 12 ± 2a | *Interaction |
Gonadally intact and OVX female WT and SkM-Arom↑ mice (n = 10-15) were fed an HFD for 14 weeks before the administration of doxycycline in the drinking water to induce SkM-Arom↑ for 6 weeks. Tissue and circulating steroids were analyzed (n = 5-8/group). Data are presented as mean ± SE. Letters that do not match are statistically different from one another (P < .05). *Signifies statistically significant main effect.
Abbreviations: E1, estrone; E2, 17β-estradiol; HFD, high-fat diet; LFD, low-fat diet; ND, not detected; OVX, ovariectomized; SkM-Arom↑, skeletal-muscle–specific aromatase overexpression; WT, wild-type.
Despite changes in tissue and circulating steroid levels, no difference was found with respect to body weight or body composition when comparing gonadally intact and OVX mice (Fig. 7A and 7B). There was, however, an obvious main effect of gonadal status, with the OVX mice displaying a greater body weight, fat mass, and percentage of body fat than the intact mice (P < .05). The SkM-Arom↑ mice displayed a larger uterus weight compared to their WT counterparts (Fig. 7C). A main effect of the OVX gonadal status was found to decrease BMD (Fig. 7D), but no effect of the SkM-Arom↑ genotype to affect BMD of the lumbar vertebrae or femur was found (data not shown).
Figure 7.
Six weeks of skeletal-muscle–specific aromatase overexpression (SkM-Arom↑) used as an intervention therapy does not affect body weight, body composition, or body mineral density (BMD), but does increase uterus size. Gonadally intact and ovariectomized (OVX) female wild-type (WT) and SkM-Arom↑ mice (n = 10-15) were fed a high-fat-diet (HFD) for 14 weeks before the administration of doxycycline in the drinking water to induce SkM-Arom↑ for 6 weeks. A, Body weight; B, body composition; C, uterus weight; and D, BMD were assessed. Data are presented as mean ± SE. Letters that do not match are statistically significantly different from one another (P < .05). @, gonadal status; ME, main effect.
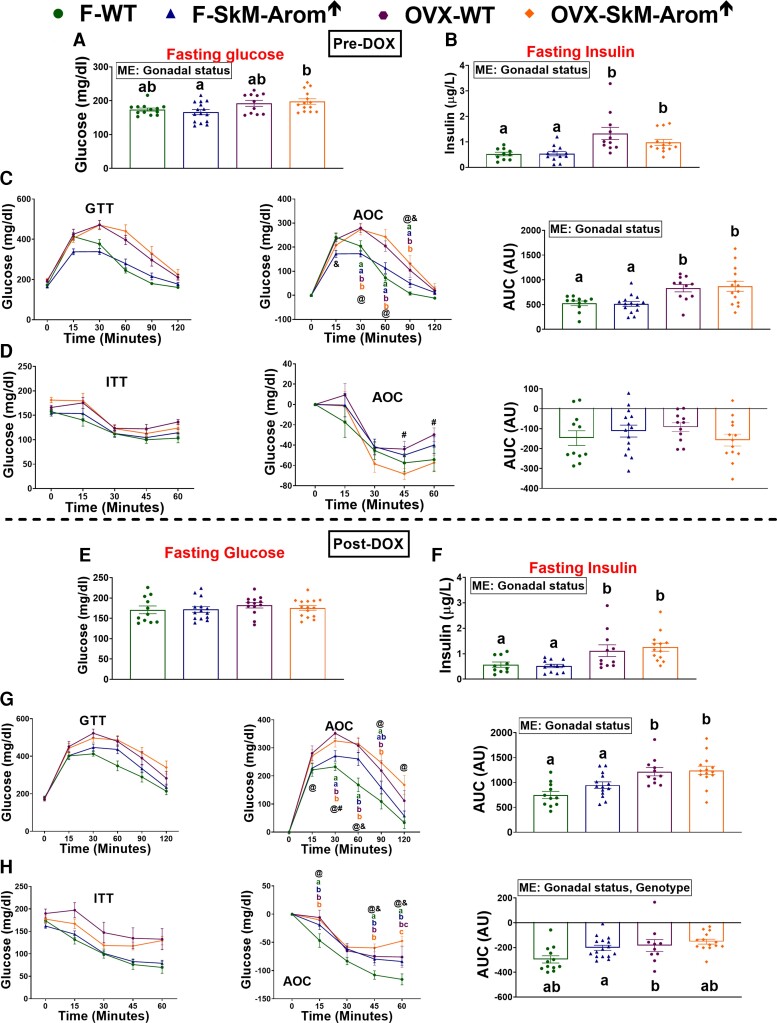
Regarding metabolic outcomes, before DOX treatment, the general pattern was for the OVX status to impair metabolic outcomes independent of genotype (Fig. 8A-8D) (P < .05). Six weeks of SkM-Arom↑ did not beneficially affect any metabolic outcome (Fig. 8E-8H).
Figure 8.
Six weeks of skeletal-muscle–specific aromatase overexpression (SkM-Arom↑) used as an intervention therapy does not rescue obesity-associated metabolic dysfunction. Gonadally intact and ovariectomized (OVX) female wild-type (WT) and SkM-Arom↑ mice (n = 10-15) were fed a high-fat diet (HFD) for 14 weeks before the administration of doxycycline (DOX) in the drinking water to induce SkM-Arom↑ for 6 weeks. Metabolic outcomes were assessed A to D, pre DOX, and E to H, at the termination of the study (post DOX). Data are presented as mean ± SE. Letters that do not match are statistically significantly different from one another (P < .05). @, main effect of gonadal status; &, main effect of genotype; ME, main effect.
With respect to adipose tissue inflammation, OVX increased gene expression of CD68, CD11c, TNFα, and TLR2 regardless of genotype (Fig. 9) (P < .05). TLR2 expression was found to be decreased in the gonadally intact SkM-Arom↑ mice relative to all other groups (P < .05). However, the general consensus is that SkM-Arom↑ did not have a statistically significant beneficial effect on adipose tissue inflammation.
Figure 9.
Six weeks of skeletal-muscle–specific aromatase overexpression (SkM-Arom↑) used as an intervention therapy does not rescue adipose tissue inflammation. Gonadally intact and ovariectomized (OVX) female wild-type (WT) and SkM-Arom↑ mice (n = 10-15) were fed a high-fat diet (HFD) for 14 weeks before the administration of doxycycline (DOX) in the drinking water to induce SkM-Arom↑ for 6 weeks. Adipose inflammation was assessed via quantitative reverse transcription–polymerase chain reaction for messenger RNA expression of A, CD68; B, CD11c; C, CD206; D, MCP1; E, tumor necrosis factor α (TNFα); and F, toll-like receptor 2 (TLR2). Data are presented as mean ± SE. Letters that do not match are statistically significantly different from one another (P < .05). ME, main effect.
Discussion
It is well established that estrogens, E2 in particular, play an integral role in regulating metabolic processes both peripherally and centrally (1). However, it is difficult to discern the tissue-specific effects of estrogens in vivo because estrogen supplementation is typically provided subcutaneously in basic science research, which ultimately affects all organ systems. Given these limitations, we designed a novel mouse model to determine if increased skeletal muscle estrogen content both in an estrogen-sufficient and estrogen-deficient state could hinder or rescue the metabolic dysfunction linked to HFD-induced obesity in vivo. We found that despite an augmentation in skeletal muscle estrogen content, HFD-induced impairments in glucose tolerance and insulin action were unaltered or aggravated suggesting that restoration or amplification of skeletal muscle estrogen content does not provide any metabolic benefits in an obese setting.
Using a liquid chromatography–mass spectrometry methodology coupled with estrogen derivatization, we determined that our mouse model was effective at increasing skeletal muscle estrogen content after 6 (intervention experiment) and 13 weeks (prevention experiment) of SkM-Arom↑. However, of interest was the finding that the increased local production of estrogens resulted in a leakage of E2 into the circulation at a physiological level (25, 26). Previous research using mass spectrometry has found circulating E2 levels to range from undetectable to approximately 60 pg/mL depending on the stage of the estrus cycle in gonadally intact adult female mice (25, 26). Although we cannot rule out the role of E1, it is likely that this physiological level of circulating E2 in the prevention experiment is largely responsible for the increase in uterus size and BMD, particularly evident in the lumbar region, as well as the decrease in adipose tissue inflammation as E2 has previously been shown to regulate these biological processes (27, 28). Furthermore, given that the 6-week overexpression model yielded skeletal muscle and circulating E2 concentrations that were physiological, yet less than the 13-week overexpression model, we can surmise that this overexpression model produced a slow, chronic increase in tissue and circulating E2 over time. A limitation of our experiment is we did not determine if the increased circulating E2 exhibited by the gonadally intact SkM-Arom↑ mice resulted in acyclic estrous cycling. However, we can infer that the expected chronic rather than cyclical circulating E2 levels exhibited by the intact SkM-Arom↑ mice is responsible for the increased uterus mass, BMD, and reduced adipose tissue CD11c gene expression relative to the gonadally intact WT littermates. This same effect of increased BMD and decreased adipose tissue inflammation was not elicited in the intervention experiment, suggesting that 1) the uterus is more responsive to circulating E2 levels, and 2) a longer duration of exposure and/or a higher circulating E2 concentration is necessary to elicit such physiological effects.
The fact that SkM-Arom↑ reduced adipose tissue inflammation, but this did not beneficially affect metabolic processes, is not surprising. We have previously shown that neither macrophage depletion nor macrophage TNFα deletion protect against obesity-associated metabolic dysfunction (16, 20). Furthermore, others have shown that reductions in adipose tissue inflammation do not necessarily improve insulin sensitivity (29). Moreover, it has been elucidated that ectopic lipid accumulation and specific lipid species are the driver behind obesity-induced metabolic impairments rather than adipose tissue inflammation (30). We are uncertain as to why insulin action was significantly impaired with SkM-Arom↑ only in the OVX HFD mice given that the OVX HFD and intact SkM-Arom↑ presented similar concentrations of skeletal muscle and circulating E1 and E2. It may be that an interaction between estrogen deficiency and HFD feeding negatively regulates the effect of skeletal muscle E2 on insulin action. However, this is merely speculative and provides an impetus for more research to be conducted to fully understand the in vivo action of estrogen in skeletal muscle when estrogen status and diet are taken into account.
Given that SkM-Arom↑ induced physiological levels of circulating E2, it was surprising that this had no effect on body weight as E2 has been suggested to play a role in regulating physical activity and suppressing food intake, which would ultimately affect body weight (31–33). However, when examining previous literature it is clear is that a limitation of the majority of the basic science studies examining E2 replacement therapy is the failure to assess circulating E2 levels (5, 32, 34–42). The lack of E2 measurement is largely due to the fact that measuring E2 levels is an extremely difficult task as commercially available ELISAs have been found to be inaccurate (43, 44). Accurate E2 quantification requires an ultra-sensitive methodology such as mass spectrometry coupled with gas or liquid chromatography and chemical derivatization of E2 to enhance sensitivity (25). We have applied a previously published liquid chromatography–mass spectrometry methodology used for the sensitive assessment of estrogens in human plasma samples (24) to the assessment of circulating and tissue E2 levels in mice. Previous studies using E2 therapy in which circulating E2 is not assessed is problematic as the methods of delivery (45) and dose of E2 administered have been shown to produce varying levels of circulating E2 that are supraphysiological (8). The commonly used matrix pellet from Innovative Research of America used by the majority of researchers for E2 give-back has been shown to produce a circulating E2 concentration 13 to 44 times elevated than what was expected with significant variability (45). For instance, the 1.8 mg E2, 60-day release pellet used by many researchers (7, 32, 34, 42, 46, 47) has been shown to produce supraphysiological levels of E2 as high as 500 pg/mL for 3 weeks before reaching physiological levels (8). These supraphysiological E2 concentrations make it impossible to ascertain the true biological effects of E2 therapy in a physiological range. Ultimately, the effect that chronic E2 replacement therapy has on physical activity remains controversial. The majority of studies that have examined the influence of E2 supplementation to affect physical activity have used E2 pellets from Innovative Research of America, eliciting supraphysiological doses that have produced results that contradict one another (32, 34, 46–48). Furthermore, other studies using silastic tubing as a means of E2 replacement have found inconclusive results or no effect of E2 supplementation on ambulatory activity (35, 36, 49). With respect to eating behavior, changes in food intake have been reported to occur during the ovarian cycle, with reduced food intake during proestrus in rodents when the concentration of circulating E2 is at its highest (33). This is also true of humans as a decrease of 250 to 600 kcal/day is evident during the periovulatory phase of the menstrual cycle (33). Thus, it is our belief that a chronic circulating E2 concentration within the low or mid-range of physiological levels is not sufficient to regulate eating behavior, physical activity, or glucose metabolism. Rather, we hypothesize that a circulating E2 concentration at the higher end of the physiological range or the pulsatile nature of the rodent estrous and human menstrual cycle resulting in acute elevated circulating E2 levels is responsible for regulating these physiological outcomes. This hypothesis is supported by the fact that despite an approximately 3-fold increase in plasma E2 relative to the OVX HFD WT mice in the prevention experiment, the OVX HFD SkM-Arom↑ did not present any statistically significant differences in adiposity or metabolic outcomes. However, future studies are needed to corroborate this hypothesis.
It should also be noted that we used a custom HFD composed of 40% of total kcals from fat designed to mimic the standard American diet. Consumption of this diet elicited a mildly impaired metabolic phenotype. If we had administered the diet for a longer period, or used a diet composed of 60% of total kcals from fat, as is typically used by obesity researchers, we may have induced a more pronounced deleterious metabolic phenotype to better tease out the potentially beneficial effects of increased SkM-Arom↑. Additionally, despite the fact that in general we did not see any changes with respect to body composition with SkM-Arom↑, it may be possible that SkM-Arom↑ may have affected skeletal muscle fiber type distribution as has been shown with E2 deficiency in certain skeletal muscles (50).
In summary, this is the first experiment to determine if increased skeletal muscle estrogen content could hinder or rescue the metabolic dysfunction linked to HFD-induced obesity. We found that increasing local estrogen production in skeletal muscle does not beneficially affect insulin resistance or glucose tolerance under HFD conditions both in gonadally intact and OVX mice. However, we did observe a beneficial effect of physiological levels of circulating E2 on BMD and adipose tissue inflammation independent of changes to body composition. Given these outcomes, and the fact that alterations in circulating levels of E2 differentially affect biological outcomes, future studies are needed to determine the physiological circulating and tissue concentration of E2 that regulates physiological processes to better understand the pleiotropic effects of E2 within the body.
Acknowledgments
We would like to thank Dr John McCarthy (University of Kentucky) and the Center for Muscle Biology at the University of Kentucky for kindly providing the HSA-rtTA animals as well as Dr Priscilla Furth (Georgetown University) for kindly providing the tet-Aromatase animals.
Abbreviations
- ANOVA
analysis of variance
- BMD
bone mineral density
- DOX
doxycycline
- E1
estrone
- E2
17β-estradiol
- ELISA
enzyme-linked immunosorbent assay
- FFA
free fatty acid
- GTT
glucose tolerance test
- HFD
high-fat diet
- ITT
insulin tolerance test
- LFD
low-fat diet
- OVX
ovariectomized
- rtTA
reverse tetracycline transactivator
- SkM-Arom↑
skeletal-muscle–specific aromatase overexpression
- T
testosterone
- TLR2
toll-like receptor 2
- TNFα
tumor necrosis factor α
- WT
wild-type
Contributor Information
Ahmed K Aladhami, Department of Pathology, Microbiology, and Immunology, University of South Carolina School of Medicine, Columbia, South Carolina 29209, USA; University of Baghdad, Nursing College, Baghdad, Iraq.
Christian A Unger, Department of Pathology, Microbiology, and Immunology, University of South Carolina School of Medicine, Columbia, South Carolina 29209, USA.
Marion C Hope, III, Department of Pathology, Microbiology, and Immunology, University of South Carolina School of Medicine, Columbia, South Carolina 29209, USA.
William E Cotham, Department of Chemistry and Biochemistry, College of Arts and Science, University of South Carolina, Columbia, South Carolina 29208, USA.
Kandy T Velázquez, Department of Pathology, Microbiology, and Immunology, University of South Carolina School of Medicine, Columbia, South Carolina 29209, USA.
Reilly T Enos, Department of Pathology, Microbiology, and Immunology, University of South Carolina School of Medicine, Columbia, South Carolina 29209, USA.
Financial Support
This work was supported by the National Institutes of Health (grant Nos. K01-AT010348 to R.T.E. and R00-AT009206 to K.T.V.).
Disclosures
The authors have nothing to disclose.
Data Availability
Original data generated and analyzed during this study are included in this published article.
References
- 1. Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev. 2013;34(3):309–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond). 2008;32(6):949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Camporez JPG, Jornayvaz FR, Lee HY, et al. Cellular mechanism by which estradiol protects female ovariectomized mice from high-fat diet-induced hepatic and muscle insulin resistance. Endocrinology. 2013;154(3):1021–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bader J, Carson M, Enos R, et al. High-fat diet-fed ovariectomized mice are susceptible to accelerated subcutaneous tumor growth potentially through adipose tissue inflammation, local insulin-like growth factor release, and tumor associated macrophages. Oncotarget. 2020;11(49):4554–4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Riant E, Waget A, Cogo H, Arnal JF, Burcelin R, Gourdy P. Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology. 2009;150(5):2109–2117. [DOI] [PubMed] [Google Scholar]
- 6. Baron AD, Brechtel G, Wallace P, Edelman SV. Rates and tissue sites of non-insulin- and insulin-mediated glucose uptake in humans. Am J Physiol. 1988;255(6 Pt 1):E769–E774. [DOI] [PubMed] [Google Scholar]
- 7. Torres MJ, Kew KA, Ryan TE, et al. 17β-Estradiol directly lowers mitochondrial membrane microviscosity and improves bioenergetic function in skeletal muscle. Cell Metab. 2018;27(1):167–179.e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ingberg E, Theodorsson A, Theodorsson E, Strom JO. Methods for long-term 17β-estradiol administration to mice. Gen Comp Endocrinol. 2012;175(1):188–193. [DOI] [PubMed] [Google Scholar]
- 9. Rivera HM, Stincic TL. Estradiol and the control of feeding behavior. Steroids. 2018;133:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iwata M, Englund DA, Wen Y, et al. A novel tetracycline-responsive transgenic mouse strain for skeletal muscle-specific gene expression. Skelet Muscle. 2018;8(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Díaz-Cruz ES, Sugimoto Y, Gallicano GI, Brueggemeier RW, Furth PA. Comparison of increased aromatase versus ERα in the generation of mammary hyperplasia and cancer. Cancer Res. 2011;71(16):5477–5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Enos RT, Velázquez KT, McClellan JL, Cranford TL, Walla MD, Murphy EA. Lowering the dietary omega-6: omega-3 does not hinder nonalcoholic fatty-liver disease development in a murine model. Nutr Res. 2015;35(5):449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Enos RT, Velázquez KT, Murphy EA. Insight into the impact of dietary saturated fat on tissue-specific cellular processes underlying obesity-related diseases. J Nutr Biochem. 2014;25(6):600–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Enos RT, Velázquez KT, McClellan JL, et al. High-fat diets rich in saturated fat protect against azoxymethane/dextran sulfate sodium-induced colon cancer. Am J Physiol Gastrointest Liver Physiol. 2016;310(11):G906–G919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Velázquez KT, Enos RT, Carson MS, et al. Mir155 deficiency aggravates high-fat diet-induced adipose tissue fibrosis in male mice. Physiol Rep. 2017;5(18):e13412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bader JE, Enos RT, Velázquez KT, et al. Repeated clodronate-liposome treatment results in neutrophilia and is not effective in limiting obesity-linked metabolic impairments. Am J Physiol Endocrinol Metab. 2019;316(3):E358–E372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cranford TL, Enos RT, Velázquez KT, et al. Role of MCP-1 on inflammatory processes and metabolic dysfunction following high-fat feedings in the FVB/N strain. Int J Obes (Lond). 2016;40(5):844–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Enos RT, Davis JM, Velázquez KT, et al. Influence of dietary saturated fat content on adiposity, macrophage behavior, inflammation, and metabolism: composition matters. J Lipid Res. 2013;54(1):152–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Enos RT, Velázquez KT, Carson MS, et al. A low dose of dietary quercetin fails to protect against the development of an obese phenotype in mice. PLoS One. 2016;11(12):e0167979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aladhami AK, Unger CA, Ennis SL, et al. Macrophage tumor necrosis factor-alpha deletion does not protect against obesity-associated metabolic dysfunction. FASEB J. 2021;35(7):e21665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shi J, Lee S, Uyeda M, et al. Guidelines for dual energy X-ray absorptiometry analysis of trabecular bone-rich regions in mice: improved precision, accuracy, and sensitivity for assessing longitudinal bone changes. Tissue Eng Part C Methods. 2016;22(5):451–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Virtue S, Vidal-Puig A. GTTs and ITTs in mice: simple tests, complex answers. Nat Metab. 2021;3(7):883–886. [DOI] [PubMed] [Google Scholar]
- 23. Vandesompele J, De Preter K, Pattyn F, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):research0034.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li X, Franke AA. Improved profiling of estrogen metabolites by orbitrap LC/MS. Steroids. 2015;99(Pt A):84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Handelsman DJ, Gibson E, Davis S, Golebiowski B, Walters KA, Desai R. Ultrasensitive serum estradiol measurement by liquid chromatography-mass spectrometry in postmenopausal women and mice. J Endocr Soc. 2020;4(9):bvaa086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nilsson ME, Vandenput L, Tivesten Å, et al. Measurement of a comprehensive sex steroid profile in rodent serum by high-sensitive gas chromatography-tandem mass spectrometry. Endocrinology. 2015;156(7):2492–2502. [DOI] [PubMed] [Google Scholar]
- 27. Noirrit-Esclassan E, Valera MC, Tremollieres F, et al. Critical role of estrogens on bone homeostasis in both male and female: from physiology to medical implications. Int J Mol Sci. 2021;22(4):1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28(5):521–574. [DOI] [PubMed] [Google Scholar]
- 29. Skurski J, Penniman CM, Geesala R, et al. Loss of iRhom2 accelerates fat gain and insulin resistance in diet-induced obesity despite reduced adipose tissue inflammation. Metabolism. 2020;106:154194. [DOI] [PubMed] [Google Scholar]
- 30. Turner N, Kowalski GM, Leslie SJ, et al. Distinct patterns of tissue-specific lipid accumulation during the induction of insulin resistance in mice by high-fat feeding. Diabetologia. 2013;56(7):1638–1648. [DOI] [PubMed] [Google Scholar]
- 31. Shay DA, Welly RJ, Givan SA, et al. Changes in nucleus accumbens gene expression accompany sex-specific suppression of spontaneous physical activity in aromatase knockout mice. Horm Behav. 2020;121:104719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gorzek JF, Hendrickson KC, Forstner JP, Rixen JL, Moran AL, Lowe DA. Estradiol and tamoxifen reverse ovariectomy-induced physical inactivity in mice. Med Sci Sports Exerc. 2007;39(2):248–256. [DOI] [PubMed] [Google Scholar]
- 33. Butera PC. Estradiol and the control of food intake. Physiol Behav. 2010;99(2):175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cabelka CA, Baumann CW, Collins BC, et al. Effects of ovarian hormones and estrogen receptor α on physical activity and skeletal muscle fatigue in female mice. Exp Gerontol. 2019;115:155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fuller KNZ, McCoin CS, Von Schulze AT, Houchen CJ, Choi MA, Thyfault JP. Estradiol treatment or modest exercise improves hepatic health and mitochondrial outcomes in female mice following ovariectomy. Am J Physiol Endocrinol Metab. 2021;320(6):E1020–E1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Morgan MA, Pfaff DW. Effects of estrogen on activity and fear-related behaviors in mice. Horm Behav. 2001;40(4):472–482. [DOI] [PubMed] [Google Scholar]
- 37. Rodier WI III, Segal S. The effect of progesterone on the activity-wheel running of ovariectomized rats. Horm Behav. 1977;9(3):214–221. [DOI] [PubMed] [Google Scholar]
- 38. Stern JJ, Murphy M. The effects of thyroxine and estradiol benzoate on wheel running activity in female rats. Physiol Behav. 1972;9(1):79–82. [DOI] [PubMed] [Google Scholar]
- 39. Hertrampf T, Degen GH, Kaid AA, et al. Combined effects of physical activity, dietary isoflavones and 17β-estradiol on movement drive, body weight and bone mineral density in ovariectomized female rats. Planta Med. 2006;72(6):484–487. [DOI] [PubMed] [Google Scholar]
- 40. Ogawa S, Chan J, Gustafsson JA, Korach KS, Pfaff DW. Estrogen increases locomotor activity in mice through estrogen receptor alpha: specificity for the type of activity. Endocrinology. 2003;144(1):230–239. [DOI] [PubMed] [Google Scholar]
- 41. Golub MS, Germann SL, Mercer M, et al. Behavioral consequences of ovarian atrophy and estrogen replacement in the APPswe mouse. Neurobiol Aging. 2008;29(10):1512–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Heikkinen T, Kalesnykas G, Rissanen A, et al. Estrogen treatment improves spatial learning in APP + PS1 mice but does not affect beta amyloid accumulation and plaque formation. Exp Neurol. 2004;187(1):105–117. [DOI] [PubMed] [Google Scholar]
- 43. Haisenleder DJ, Schoenfelder AH, Marcinko ES, Geddis LM, Marshall JC. Estimation of estradiol in mouse serum samples: evaluation of commercial estradiol immunoassays. Endocrinology. 2011;152(11):4443–4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Handelsman DJ, Newman JD, Jimenez M, McLachlan R, Sartorius G, Jones GRD. Performance of direct estradiol immunoassays with human male serum samples. Clin Chem. 2014;60(3):510–517. [DOI] [PubMed] [Google Scholar]
- 45. Gérard C, Gallez A, Dubois C, et al. Accurate control of 17β-estradiol long-term release increases reliability and reproducibility of preclinical animal studies. J Mammary Gland Biol Neoplasia. 2017;22(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Greising SM, Carey RS, Blackford JE, Dalton LE, Kosir AM, Lowe DA. Estradiol treatment, physical activity, and muscle function in ovarian-senescent mice. Exp Gerontol. 2011;46(8):685–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Moran AL, Nelson SA, Landisch RM, Warren GL, Lowe DA. Estradiol replacement reverses ovariectomy-induced muscle contractile and myosin dysfunction in mature female mice. J Appl Physiol (1985). 2007;102(4):1387–1393. [DOI] [PubMed] [Google Scholar]
- 48. Greising SM, Baltgalvis KA, Kosir AM, Moran AL, Warren GL, Lowe DA. Estradiol's beneficial effect on murine muscle function is independent of muscle activity. J Appl Physiol (1985). 2011;110(1):109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mosquera L, Shepherd L, Torrado AI, Torres-Diaz YM, Miranda JD, Segarra AC. Comparison of two methods of estradiol replacement: their physiological and behavioral outcomes. J Vet Sci Technol. 2015;6(6):276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Haizlip KM, Harrison BC, Leinwand LA. Sex-based differences in skeletal muscle kinetics and fiber-type composition. Physiology (Bethesda). 2015;30(1):30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original data generated and analyzed during this study are included in this published article.