Abstract
Cerebral white matter hyperintensities are an important contributor to ageing brain pathology. Progression in white matter hyperintensity volume is associated with cognitive decline and gait impairment. Understanding the factors associated with white matter hyperintensity progression provides insight into pathogenesis and may identify novel treatment targets to improve cognitive health. We postulated that the immune system interaction with cerebral vessels and tissue may be associated with disease progression, and thus evaluated the relationship of blood leucocyte gene expression to progression of cerebral white matter hyperintensities.
A brain MRI was obtained at baseline in 166 patients assessed for a cognitive complaint, and then repeated at regular intervals over a median of 5.9 years (interquartile range 3.5–8.2 years). White matter hyperintensity volumes were measured by semi-automated segmentation and percentage change in white matter hyperintensity per year calculated. A venous blood sample obtained at baseline was used to measure whole-genome expression by RNA sequencing. The relationship between change in white matter hyperintensity volumes over time and baseline leucocyte gene expression was analysed.
The mean age was 77.8 (SD 7.5) years and 60.2% of participants were female. The median white matter hyperintensity volume was 13.4 ml (SD 17.4 ml). The mean change in white matter hyperintensity volume was 12% per year. Patients were divided in quartiles by percentage change in white matter hyperintensity volume, which was: −3.5% per year in quartile 1, 7.4% per year in quartile 2, 11.7% in quartile 3 and 33.6% per year in quartile 4. There were 148 genes associated with changing white matter hyperintensity volumes over time (P < 0.05 r > |0.2|). Genes and pathways identified have roles in endothelial dysfunction, extracellular matrix remodelling, altered remyelination, inflammation and response to ischaemia. ADAM8, CFD, EPHB4, FPR2, Wnt-B-catenin, focal adhesion kinase and SIGLEC1 were among the identified genes.
The progression of white matter hyperintensity volumes over time is associated with genes involved in endothelial dysfunction, extracellular matrix remodelling, altered remyelination, inflammation and response to ischaemia. Further studies are needed to evaluate the role of peripheral inflammation in relation to rate of white matter hyperintensity progression and the contribution to cognitive decline.
Keywords: white matter hyperintensity, leucocyte, gene expression, mRNA, cognitive decline
Jickling et al. identify differences in leucocyte gene expression associated with the rate of white matter hyperintensity progression over an average of 5.9 years. The genes and pathways identified are involved in endothelial dysfunction, extracellular matrix remodelling, remyelination, inflammation and response to ischaemia.
Introduction
Cerebral white matter hyperintensities (WMH) are believed to be a manifestation of cerebral small vessel disease. They increase with advancing age and are associated with cognitive decline, dementia and stroke.1–3 With vascular dementia as an important cause of cognitive decline, improved understanding of the factors that promote progression of WMH could lead to novel strategies to reduce age-related brain injury and improve cognitive health. In this study, we evaluated the relationship of leucocyte gene expression in blood at baseline to progression of cerebral WMH volumes over time.
The pathogenesis of WMH remains unknown. However, chronic cerebral hypoperfusion,4–6 inflammation, endothelial dysfunction, altered blood–brain barrier permeability6–9 and degeneration of myelin sheaths with axonal loss may all contribute.10 Progression of WMH is associated with age, hypertension and initial severity of WMH.11,12 No specific therapies exist to slow the progression WMH. While antiplatelet therapies are commonly prescribed for persons with WMH, in the absence of clinical stroke the practice is not supported by either American Heart Association or European Stroke Organisation guidelines given a lack of clear benefit and concern over increased risk of intracerebral haemorrhage.13,14 Blood pressure control has slowed the development of WMH in some studies.15–18 In the SPRINT-MIND trial targeting a systolic blood pressure ≤120 mmHg in patients with hypertension slowed accumulation of WMH (0.16 ml/year reduction) and lowered risk of cognitive decline.16,19 Control of other risk factors such as diabetes and hyperlipidaemia have not been clearly demonstrated to prevent or slow WMH. Improved understanding of the factors that promote progression of WMH over time is needed to develop therapies to slow its development and improve cognitive function.
Small vessel endothelial injury elicits a response from the immune system. This response can be identified by changes in gene expression in circulating leucocytes. We previously found changes in RNA expression in cells of whole blood in patients with lacunar small vessel stroke as well as patients with WMH.20–23 Furthermore, inflammation and associated oxidative stress may contribute to WMH through effects on the blood–brain barrier and myelin. In this study, we sought to evaluate the relationship of leucocyte gene expression to changes in WMH volumes over time. We propose that patients who are going to have the greatest progression in cerebral WMH volumes on MRI may have specific differences in leucocyte gene expression, reflecting early or ongoing small vessel injury. These features may reveal novel targets to treat and slow the development of cerebral WMH and thus improve cognitive health.
Materials and methods
Patients
Patients were recruited prospectively from the University of California, Davis. Brain MRIs were acquired at baseline and at regular intervals over the course of patient follow-up. Patients were followed for a mean of 5.9 years (range 2–12.1 years) from initial enrolment into the study. A standardized protocol was used to record patient characteristics at baseline, study follow-up appointments, demographics, cognitive testing, blood draws and brain MRIs.
MRI protocol
Participants underwent serial MRI scans that were conducted and analysed using a standardized automated segmentation protocol that is independent of demographic, clinical and blood sample information. This WHM segmentation method has high inter-rater reliability (ICC > 0.94, P-values < 0.001), and agreement between test and retest sessions (ICC > 0.98, P < 0.001) and very consistent across different scanners (ICC > 0.98, P < 0.001).24 The algorithm used to assess WMH is based on a Bayesian approach to segmentation of high-resolution 3D T1 and FLAIR sequences. In brief, non-brain structures are excluded from the 3D T1 images befire measurement using an automated atlas-based method. The FLAIR image is transformed to a 3D T1 image using the FLIRT method from the FSL toolbox, with error estimation based on correlation ratio. Inhomogeneity correction of the 3D T1 is performed using interleaved bias estimation and B-spline deformation with a template.25 This multiple iteration method updates a B-spline intensity deformation between an unbiased template image and the subject image, with an estimation of a bias field based on the current templatetoimage alignment. The bias field is modelled using a spatially smooth thin-plate spline interpolation based on ratios of local image patch intensity means between the deformed template and subject images. FLAIR inhomogeneity corrections after coregistration of the FLAIR to the 3D T1 image is based on a previously published local histogram normalization method.26 Prior to WMH calculation, each 3D T1 image is non-linearly aligned to a common template atlas and each of the accompanying images are transformed onto the same atlas using the same transformation parameters. Estimation of WMH is then performed using a modified Bayesian probability structure based on a previously published method of histogram fitting.27 Prior probability maps for WMH were created from >700 individuals with semi-automatic detection of WMH followed by manual editing. Likelihood estimates of the native image are calculated through histogram segmentation and thresholding. All segmentation is initially performed in standard space resulting in probability likelihood values of WMH at each voxel in the white matter. These probabilities are then thresholded at 3.5 standard deviations above the mean to create a binary WMH mask. Segmentation is based on a modified Bayesian approach that combines image likelihood estimates, spatial priors and tissue class constraints. The segmented WMH masks are then transformed back to the native space for tissue volume calculation.
White matter hyperintensity progression rate
The rate of change in WMH volume per year was determined. For each patient, WMH volume was measured and compared to subsequent MRI. Every patient had an MRI at time of enrolment, and this was then repeated 1–5 times over the course of study. The mean number of MRIs obtained per patient was 3 (range 2–6) over a mean of 5.9 years (range 2–12.1 years). A linear model of WMH volume change per year was determined for each patient. In cases where the blood draw was not acquired at the time of the MRI, the rate of WMH change over time was used to estimate the WMH at the time of blood draw. Because larger volumes of WMH tend to have larger increases in WMH volume over time, the rate of progression was assessed as a proportion of baseline WMH (percentage change in WMH).
Leucocyte gene expression
Blood samples were collected into PAXgene tubes at baseline at or near the time of initial MRI of the brain. Samples were stored at −80°C until the time of RNA isolation. All samples were isolated at the same time to reduce variance related to batch effect using a QIAgen PAXgene kit for total RNA. RNA quality was assessed by Agilent Bioanalyzer and quantity by Nanodrop and Qubit. Samples required a RIN >7.0 and a A260/280 > 1.8 to proceed. RNA expression was measured by 3′ focused RNA sequencing. mRNA libraries were prepared using the Lexogen QuantSeq FWD 3′ mRNA-Seq Library Prep Kit. Multiplexed samples were sequenced as 100 bp single end reads to a depth of 11.0 ± 0.4 million reads with an Illumina HiSeq 4000 Sequencer.
Statistics and data analysis
Patient characteristics between quartiles were compared by chi-square, Mann–Whitney or ANOVA as appropriate. Distribution was assessed by Kolmogorov–Smirnoff. Analysis was performed using Partek Flow, Partek Genomics Suite (St. Louis, MO, USA) and Stata V16.1 (College Station, TX, USA). RNA-sequenced demultiplexed fastq files were processed using the Lexogen QuantSeq pipeline in Partek Flow. In brief, raw reads were trimmed for adapters and quality thresholds, then aligned to the human genome (hg38) using STAR 2.5.3a. Reads at the gene level were quantified and annotated with GENCODE 25. A low variance filter of 10% was applied. The relationship of gene expression to percentage change in WMH was analysed by analysis of covariance, adjusted for age and sex. Patients were divided into quartiles of percentage change in WMH as the data were not normally distributed. Genes associated with percentage change in WMH were identified on the basis of r > |0.2| and corrected P < 0.05. Functional analysis of genes associated with WMH progression was performed using Ingenuity Pathway Analysis (Qiagen, LLC.) and literature review.
Data availability
The data that support the findings of this study are available from the authors upon reasonable request.
Results
The demographics of the 166 patients studied are shown in Table 1. The mean age was 77.8 years (SD 7.5), and 60.2% were female. Vascular risk factors were common, with 75% having hypertension, 77.2% hyperlipidaemia, 33.7% diabetes and 8.3% smokers. Patients were followed over a mean of 5.9 years (interquartile range 2.2–9.5 years).
Table 1.
Characteristics of patients divided in quartiles by percentage change in WMH volume per year over a mean of 5.9 years
| Quartile 1 (n = 42) | Quartile 2 (n = 41) | Quartile 3 (n = 41) | Quartile 4 (n = 42) | Total (n = 166) | P-value | |
|---|---|---|---|---|---|---|
| Age years (SD) | 76.9 (7.3) | 79.8 (5.9) | 76.7 (6.6) | 77.7 (9.7) | 77.8 (7.5) | 0.38 |
| Female, n (%) | 25 (59.5%) | 28 (68.3%) | 25 (61.0%) | 22 (52.4%) | 100 (60.2%) | 0.53 |
| Percentage change in WMH volume per year mean (SD) | −4.2% (4.9%) | 7.4% (1.3%) | 11.7% (1.3%) | 33.6% (28.2%) | 12.1% (19.8%) | 0.001 |
| WMH baseline ml (SD) | 4.7 (5.2) | 19.8 (19.6) | 10.5 (8.6) | 18.6 (24.2) | 13.4 (17.4) | 0.0001 |
| Systolic BP mmHg (SD) | 134.6 (13.2) | 143.6 (16.9) | 144.2 (20.2) | 144.1 (21.1) | 141.6 (18.4) | 0.043 |
| Diastolic BP mmHg (SD) | 68.6 (10.0) | 76.4 (11.2) | 74.1 (12.2) | 74.9 (12.2) | 73.5 (11.7) | 0.013 |
| Hypertension n (%) | 10 (45.5%) | 19 (73.1%) | 20 (76.9%) | 26 (89.7%) | 75 (72.8%) | 0.005 |
| Diabetes n (%) | 14(33.3%) | 14 (35.8%) | 15 (38.5%) | 11 (27.5%) | 54 (33.7%) | 0.85 |
| Hyperlipidaemia n (%) | 28 (70.0%) | 26 (63.4%) | 34 (81.0%) | 28 (66.6%) | 116 (77.2%) | 0.63 |
| Caucasian n (%) | 25 (59.5%) | 21 (51.2%) | 27 (65.9%) | 20 (47.6%) | 93 (56.0%) | 0.33 |
| African-American n (%) | 4 (9.5%) | 10 (24.4%) | 6 (14.6%) | 6 (14.3%) | 26 (15.7%) | 0.30 |
| Latino/Hispanic n (%) | 5 (11.9%) | 10 (24.4%) | 8 (19.5%) | 12 (28.6%) | 35 (21.1%) | 0.28 |
| Asian n (%) | 8 (19.0%) | 0 (0%) | 0 (0%) | 2 (4.8%) | 10 (6.0%) | 0.20 |
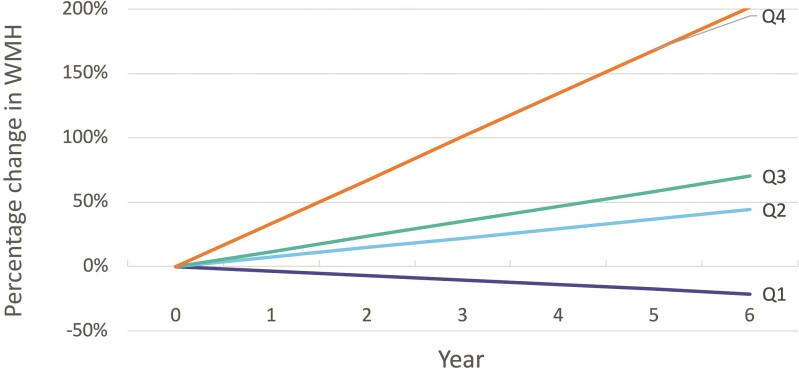
The median baseline WMH volume was 13.4 cm3 (SD 17.4 cm3). Between baseline and final MRI (mean 5.9 years) there was variability in progression of WMH volume. The mean percentage change in WMH volume was 12% per year. The cohort was divided into quartiles of percentage change in WMH volume (Table 1). In quartile 1 the mean percentage change in WMH volume was −3.5% per year, in quartile 2 it was 7.4% per year, in quartile 3 it was 11.7% per year and in quartile 4 it was 33.6% per year (Fig. 1). Patients in quartile 1 with little change or regression in WMH had less hypertension, and less baseline WMH. Patients in quartiles 2–4 where WMH increased over time were of similar age, sex and vascular risk factor status. Quartile 1 and 4 subjects were termed ‘slow progressors’ and ‘fast progressors’, respectively.
Figure 1.
Progression rate of WMH over time. Patients are divided into quartiles by percentage change in WMH volume. Change in WMH was determined from MRI of the brain at enrolment compared to subsequent MRIs performed over a mean of 5.9 years. A mean of three MRIs of the brain were performed per patient over this time (range 2–6). The mean percentage change in WMH volume in was −3.5% per year in quartile 1 (Q1), 7.4% per year in quartile 2 (Q2), 11.7% in quartile 3 (Q3) and 33.6% per year in quartile 4 (Q4).
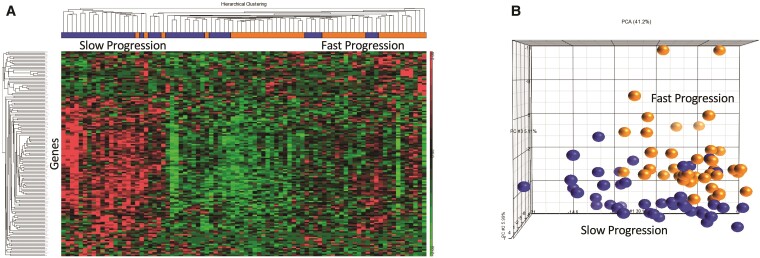
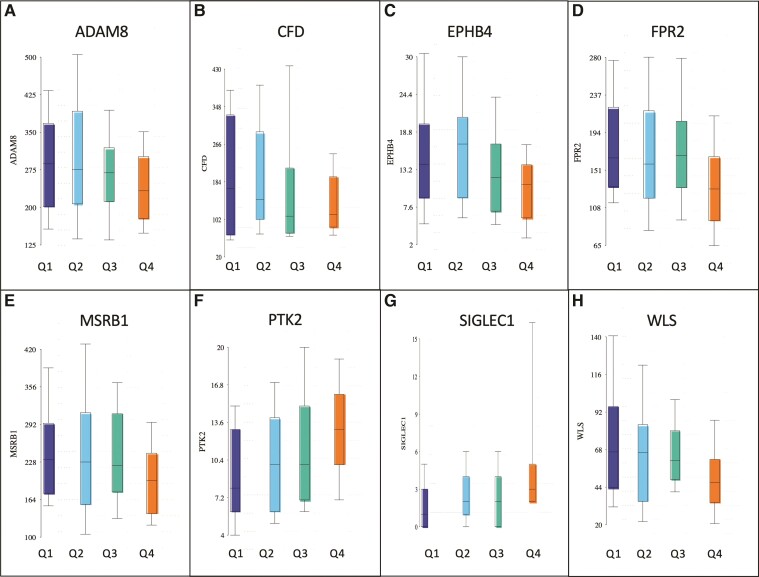
Genes associated with percentage change in WMH volume over time were identified by analysis of covariance comparing fast progressors to slow progressors adjusted for age and sex. There were 148 genes associated with change of WMH volumes over time (P < 0.05, r > |0.2|) (Supplementary Table 1). The differences in gene expression between patients with fast and slow change in WMH are shown in a hierarchical cluster plot and principal components analysis in Fig. 2. Among the genes identified were several that may have potential as therapeutic targets including ADAM metallopeptidase domain 8 (ADAM8), complement factor D (CFD), ephrin type-B receptor 4 (EPHB4), formyl peptide receptor 2 (FPR2), Wnt-B-catenin, focal adhesion kinase (FAK) and sialoadhesin (SIGLEC1) (Fig. 3).
Figure 2.
Genes associated with change in WMH volume. (A) Hierarchical cluster plot of the 148 genes associated with change in WMH volume over time. Patients are shown on the x-axis, with slow WMH progression in blue and fast WMH progression in orange. Genes are shown on the y-axis, with higher relative expression shown in red and lower relative expression in green. (B) Principal components analysis of the 148 genes associated with change in WMH volume over time. The ability of the identified genes to separate fast (fourth quartile, orange) from slow progressors (first quartile, blue) is depicted.
Figure 3.
Representative genes associated with change in WMH over time (P < 0.05). Patients are divided by quartiles of change in WMH, with quartile 1 (dark blue) being slow progression and quartile 4 (orange) fast progression. Genes shown include: (A) ADAM8, (B) CFD, (C) EPHB4, (D) FPR2; FAK, (E) MSRB1, (F) PTK2 (protein tyrosine kinase), (G) SIGLEC1 and (H) WLS (Wnt ligand secretion mediator).
Functional analysis of genes associated with WMH progression was performed to identify pathways over-represented greater than expected by chance. Identified pathways are summarized in Table 2. Among the top canonical pathways identified were endothelial dysfunction, extracellular matrix remodelling, altered remyelination, inflammation and response to ischaemia/hypoxia.
Table 2.
Pathways from the 148 genes associated with change in WMH volume over time
| Genes | P-value | |
|---|---|---|
| Canonical pathways | ||
| Rho signalling | ACTA1, ANLN, CDH7, GNB2, MYL12B, PIP4K2C, PTK2, RHOB | 0.0010 |
| Integrin signalling | ACTA1, CAPN5, MYL12B, PTK2, RHOB, TSPAN2 | 0.0020 |
| Ephrin B signalling | EPHB4, GNB2, PTK2, SDCBP | 0.0100 |
| CXCR4 signalling | GNB2, MYL12B, PTK2, RHOB | 0.0195 |
| Interferon signalling | IFNGR2, MX1 | 0.0204 |
| FAK signalling | ACTA1, CAPN5, PTK2 | 0.0263 |
| Thrombin signalling | GNB2, MYL12B, PTK2, RHOB | 0.0398 |
| Functional pathways | ||
| Degranulation of phagocytes | ADAM8, CD9, CFD, CYSTM1, DUSP1, DYNC1LI1, FPR2, GOLGA7, JUNB, PGM2, QPCT, S100P, SDCBP | 0.0002 |
| Chemotaxis of macrophages | FPR2, MTUS1, PTK2, RHOB | 0.0072 |
| Cell movement of phagocytes | ADAM8, CD9, CFD, DUSP1, FPR2, MTUS1, PROK2, PTK2, QPCT, RHOB, SCN9A, WLS | 0.0101 |
| Infiltration by neutrophils | ADAM8, CFD, DUSP1, FPR2, WLS | 0.0177 |
| Acute inflammation of tissue | FPR2, TRADD | 0.0239 |
| Complement activation | CFD | 0.0303 |
Discussion
With an ageing population, strategies to improve cognitive health are of great importance. Cerebral WMH contribute to cognitive decline, thus understanding the factors associated with WMH progression may identify novel strategies to slow their development and improve cognition. We identified differences in leucocyte gene expression associated with the progression of cerebral WMH over the course of a mean of 5.9 years. Genes and pathways identified have roles in endothelial dysfunction, extracellular matrix remodelling, altered remyelination, inflammation and response to ischaemia. Differences in these genes and associated pathways provide insight to the progression of WMH and may have potential as novel targets to slow accumulation of WMH.
WMH on brain MRI are believed to be a manifestation of cerebral small vessel disease. The diseased cerebral small vessels and injured white matter interact with circulating leucocytes. Indeed, the involvement of immune cells is observed in pathological studies of WMH.5,28,29 Leucocyte interactions with diseased cerebral small vessels and WMH alter gene expression. Among the genes we identified were several that may have potential as therapeutic targets to slow the progression of WMH including extracellular matrix (ADAM8, EPHB4, Wnt-B-catenin) myelination [CFD, methionine sulphoxide reductase B1 (MSRB1)] and inflammation (SIGLEC1, FPR2 and FAK).
Extracellular matrix/cerebrovascular matrisome
Perturbation of the cerebrovascular extracellular matrix (ECM) and matrisome plays a role in pathogenesis of WMH.30–34 Genetic variants in the ECM associated with WMH include the glycoprotein NID2,35,36 basement membrane collagen COL4A1/2,37 VCAN (versican) involved in cell adhesion and ECM assembly38 and fibulin 3 (EFEMP1), a glycoprotein in the basement membrane.39 Consistent with these results, we identified several genes (ADAM8, EPHB4, WNT) that have effects on the ECM that may contribute to WMH progression. ADAM8 is a disintegrin matrix metalloproteinase that disrupts the extracellular matrix by cleaving chondroitin sulphate proteoglycan and promoting barrier disruption.40 ADAM8 also cleaves several leucocyte adhesion molecules (L-selectin, P-selectin glycoprotein ligand-1 and vascular cell adhesion molecule 1) to alter the ECM and dynamics of leucocyte adhesion to cerebral endothelium in a manner that may promote WMH.41 EPHB4 is an endothelial cell receptor for ephrin-B2, which acts through Rho-Src signalling to affect integrins and vascular barrier integrity.42–46 Both EPHB4 and Rho signalling were associated with progression of WMH. Wnt/β-catenin pathway is essential for blood–brain barrier maintenance and plays an important role in endothelial response to hypertension.47,48 Therapies to prevent disruption of the matrisome may have potential to slow the progression of WMH. Targeting ADAM8 with batimatstat (BB94), EPHB8 with tesevatinib or use of Wnt with inhibitors49 may warrant further study as treatments to slow the progression of WMH.
Myelination
An impairment in myelination and oligodendrocyte function may also contribute to the progression of WMH. Indeed, early dysfunction of the endothelium can impair oligodendrocyte function and promote white matter disease.50 GPR126 is gene variant associated with WMH and lacunar stroke that may contribute to small vessel disease by inhibiting myelin repair.32 Two genes we identified that may contribute to demyelination are CFD and MSRB1. CFD is a trypsin peptidase involved in the alternative complement pathway, which has roles in demyelination and synaptic pruning of the ageing brain.51,52 MSRB1 has antioxidant effects, reducing methionine sulphoxides produced from reactive oxygen species. An impairment in MSRB1 could enhance oxidative and inflammatory injury of oligodendrocytes and promote WMH.53,54 Preventing myelin injury by targeting complement activity with therapies such as eculizumab and ACH-4471, or enhancing antioxidant defence by activating MSRB1 with agents such as fusaricidin55 may be strategies worth evaluating to slow progression of WMH.
Inflammation
Inflammation has also been implicated in the pathogenesis of small vessel disease and may contribute to the progression of WMH.32,56 Inflammatory markers are present in regions of WMH in both animal models57 and human neuropathological studies.58,59 Inflammatory markers in plasma are increased in SVD, and related to WMH progression.41,60 HLA variants are associated with mean diffusivity and fractional anisotropy.33 Neuroinflammation measured by PET imaging is increased in patients with WMH, as is an increase in blood–brain barrier permeability and microglial activation.57,61 We found SIGLEC1, FPR2 and FAK to be associated with progression of WMH. SIGLEC1 (CD169) is a leucocyte adhesion molecule that binds sialic acids to promote inflammation. In multiple sclerosis, SIGLEC1 contributes to inflammatory injury and demyelination.62 FPR2 is a leucocyte receptor with both pro-inflammatory and anti-inflammatory activities.63,64 A shift towards pro-inflammatory effects may occur that may promote WMH progression. FAK is a focal adhesion kinase that enhances leucocyte attachment to endothelium and promotes inflammation.65,66 Targeting inflammation may be a strategy to slow progression of WMH. Indeed, minocycline has been found to reduce white matter damage in an animal model.57 Whether WMH progression can be slowed by targeting SIGLEC1,67 FPR2 (e.g. barbadin)68 or FAK (e.g. defactinib, PF-573 228) will be of interest to study.
We evaluated the progression of WMH over time due to a stronger relationship to cognitive decline. It is important to note that, while related, WMH progression is distinct from total WMH volume. While larger WMH volume is associated with progression of WMH over time, there are patients with small volume WMH who experience fast progression, and likewise patients with a large volume of WMH with slow progression. Genes associated with WMH volume progression in the present study differed from genes differentially expressed in blood of patients with large volume WMH compared to low volume WMH.23 Ephrin signalling was common to both studies. Thus, the immune response that may promote WMH progression has differences from the immune response related to different WMH volumes. This is supported by previous reports noting the clinical factors associated with WMH volume differ from those associated with progression of WMH. While age and hypertension are among the most consistent factors associated with WMH volume, for WMH progression the baseline WMH volume is the most consistent associated factor. We observed a similar relationship in our cohort, with baseline WMH progression being associated with baseline WMH progression more so than age or hypertension. Furthermore, the identified genes were more strongly associated with WMH progression than baseline WMH volume.
Strengths of this study include follow-up of patients over a course of 5.9 years, baseline blood draw in RNA-stabilizing PAXgene tubes and standardized imaging and rating of WMH by MRI-segmentation. However, there are limitations. This was a single cohort study and, thus, further evaluation in larger cohorts is required to confirm identified findings. Whole blood was analysed, which limits our ability to specifically assess individual leucocyte subsets and their contribution to WMH. The progression of WMH over time was assumed to be linear. While this is consistent with previous studies of WMH progression, there may be periods when WMH progresses faster than others that were not captured in the current study. Similarly, we were not able to assess the effect of genetic variation or other environmental factors. While the relationship of WMH progression to cognitive decline has been reported in previous studies, we did not specifically examine the relationship of WMH progression and gene expression changes to cognitive decline in this study. Further evaluation regarding the relationship of identified genes and pathways to cognitive decline will be of interest.
In conclusion, we identified differences in leucocyte gene expression associated with a percentage change in WMH over a course of 5.9 years. Aspects of leucocyte activation and endothelial dysfunction associated with WMH progression were identified. With further study, the impact of these targets on the blood–brain barrier, extracellular matrix, endothelium and myelination may help better understand how WMH develop in humans and potentially guide novel therapeutic strategies.
Supplementary Material
Abbreviations
- FAK =
focal adhesion kinase
- WMH =
white matter hyperintensity(ies)
Contributor Information
Glen C Jickling, Department of Neurology, University of California Davis School of Medicine, Sacramento, CA, USA; Department of Medicine, Division of Neurology, University of Alberta, Edmonton, Alberta, Canada.
Bradley P Ander, Department of Neurology, University of California Davis School of Medicine, Sacramento, CA, USA.
Xinhua Zhan, Department of Neurology, University of California Davis School of Medicine, Sacramento, CA, USA.
Boryana Stamova, Department of Neurology, University of California Davis School of Medicine, Sacramento, CA, USA.
Heather Hull, Department of Neurology, University of California Davis School of Medicine, Sacramento, CA, USA.
Charles DeCarli, Department of Neurology, University of California Davis School of Medicine, Sacramento, CA, USA.
Frank R Sharp, Department of Neurology, University of California Davis School of Medicine, Sacramento, CA, USA.
Funding
This research was supported by the grants AG042292 (F.R.S., C.D., B.S.S., B.P.A., G.C.J.) AG010129 (C.D.) from the National Institute on Aging. G.C.J. receives research funding from CIHR, Heart and Stroke Foundation of Canada, University Hospital Foundation and Canada Research Chair.
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Callisaya ML, Beare R, Phan T, et al. Progression of white matter hyperintensities of presumed vascular origin increases the risk of falls in older people. J Gerontol A Biol Sci Med Sci. 2015;70:360–366. [DOI] [PubMed] [Google Scholar]
- 2. Xu X, Gao Y, Liu R, et al. Progression of white matter hyperintensities contributes to lacunar infarction. Aging Dis. 2018;9:444–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ye S, Dong S, Tan J, et al. White-matter hyperintensities and lacunar infarcts are associated with an increased risk of Alzheimer's disease in the elderly in China. J Clin Neurol. 2019;15:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tomimoto H, Ihara M, Wakita H, et al. Chronic cerebral hypoperfusion induces white matter lesions and loss of oligodendroglia with DNA fragmentation in the rat. Acta Neuropathol. 2003;106:527–534. [DOI] [PubMed] [Google Scholar]
- 5. Farkas E, Donka G, de Vos RAI, Mihaly A, Bari F, Luiten PGM. Experimental cerebral hypoperfusion induces white matter injury and microglial activation in the rat brain. Acta Neuropathol. 2004;108:57–64. [DOI] [PubMed] [Google Scholar]
- 6. Qiao M, Malisza KL, Del Bigio MR, Tuor UI. Correlation of cerebral hypoxic-ischemic T2 changes with tissue alterations in water content and protein extravasation. Stroke. 2001;32:958–963. [DOI] [PubMed] [Google Scholar]
- 7. Fernando MS, Simpson JE, Matthews F, et al. White matter lesions in an unselected cohort of the elderly: Molecular pathology suggests origin from chronic hypoperfusion injury. Stroke. 2006;37:1391–1398. [DOI] [PubMed] [Google Scholar]
- 8. Topakian R, Barrick TR, Howe FA, Markus HS. Blood-brain barrier permeability is increased in normal appearing white matter in patients with lacunar stroke and leukoaraiosis. J Neurol Neurosurg Psychiatry. 2009;81:192–197. [DOI] [PubMed] [Google Scholar]
- 9. Farrall AJ, Wardlaw JM. Blood-brain barrier: Ageing and microvascular disease–systematic review and meta-analysis. Neurobiol Aging. 2009;30:337–352. [DOI] [PubMed] [Google Scholar]
- 10. Peters A. The effects of normal aging on myelin and nerve fibers: A review. J Neurocytol. 2002;31:581–593. [DOI] [PubMed] [Google Scholar]
- 11. Burton EJ, McKeith IG, Burn DJ, Firbank MJ, O'Brien JT. Progression of white matter hyperintensities in Alzheimer disease, dementia with Lewy bodies, and Parkinson disease dementia: A comparison with normal aging. Am J Geriatr Psychiatry. 2006;14:842–849. [DOI] [PubMed] [Google Scholar]
- 12. Sachdev P, Wen W, Chen X, Brodaty H. Progression of white matter hyperintensities in elderly individuals over 3 years. Neurology. 2007;68:214–222. [DOI] [PubMed] [Google Scholar]
- 13. Smith EE, Saposnik G, Biessels GJ, et al. Prevention of stroke in patients with silent cerebrovascular disease: A scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2017;48:e44–e71. [DOI] [PubMed] [Google Scholar]
- 14. Wardlaw JM, Debette S, Jokinen H, et al. ESO Guideline on covert cerebral small vessel disease. Eur Stroke J. 2021;6:IV. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15. Yoon CW, Choi Y, Jeon S, et al. Is antiplatelet treatment effective at attenuating the progression of white matter hyperintensities? PLoS One. 2017;12:e0176300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. SPRINT MIND Investigators for the SPRINT Research Group; Nasrallah IM, Pajewski NM, et al. Association of intensive vs standard blood pressure control with cerebral white matter lesions. JAMA. 2019;322:524–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dickie DA, Ritchie SJ, Cox SR, et al. Vascular risk factors and progression of white matter hyperintensities in the Lothian birth cohort 1936. Neurobiol Aging. 2016;42:116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lai Y, Jiang C, Du X, et al. Effect of intensive blood pressure control on the prevention of white matter hyperintensity: Systematic review and meta-analysis of randomized trials. J Clin Hypertens (Greenwich). 2020;22:1968–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kjeldsen SE, Narkiewicz K, Burnier M, Oparil S. Intensive blood pressure lowering prevents mild cognitive impairment and possible dementia and slows development of white matter lesions in brain: The SPRINT Memory and Cognition IN Decreased Hypertension (SPRINT MIND) study. Blood Press. 2018;27:247–248. [DOI] [PubMed] [Google Scholar]
- 20. Bai Z, Stamova B, Xu H, et al. Distinctive RNA expression profiles in blood associated with Alzheimer disease after accounting for white matter hyperintensities. Alzheimer Dis Assoc Disord. 2014;28:226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jickling G, Salam A, Mohammad A, et al. Circulating endothelial progenitor cells and age-related white matter changes. Stroke. 2009;40:3191–3196. [DOI] [PubMed] [Google Scholar]
- 22. Jickling GC, Stamova B, Ander BP, et al. Profiles of lacunar and nonlacunar stroke. Ann Neurol. 2011;70:477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu H, Stamova B, Jickling G, et al. Distinctive RNA expression profiles in blood associated with white matter hyperintensities in brain. Stroke. 2010;41:2744–2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maillard P, Lu H, Arfanakis K, et al. Instrumental validation of free water, peak-width of skeletonized mean diffusivity and white matter hyperintensities: MarkVCID neuroimaging kits. Alzheimers Dement. 2022;14(1):e12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fletcher E, Carmichael O, Decarli C. MRI non-uniformity correction through interleaved bias estimation and B-spline deformation with a template. In: Conference proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Conference. IEEE. 2012:106–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. DeCarli C, Murphy DG, Teichberg D, Campbell G, Sobering GS. Local histogram correction of MRI spatially dependent image pixel intensity nonuniformity. J Magn Reson Imaging. 1996;6:519–528. [DOI] [PubMed] [Google Scholar]
- 27. DeCarli C, Miller BL, Swan GE, et al. Predictors of brain morphology for the men of the NHLBI twin study. Stroke. 1999;30:529–536. [DOI] [PubMed] [Google Scholar]
- 28. Noz MP, Ter Telgte A, Wiegertjes K, et al. Pro-inflammatory monocyte phenotype during acute progression of cerebral small vessel disease. Front Cardiovasc Med. 2021;8:639361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rosenberg GA. Extracellular matrix inflammation in vascular cognitive impairment and dementia. Clin Sci (Lond). 2017;131:425–437. [DOI] [PubMed] [Google Scholar]
- 30. Joutel A, Haddad I, Ratelade J, Nelson MT. Perturbations of the cerebrovascular matrisome: A convergent mechanism in small vessel disease of the brain? J Cereb Blood Flow Metab. 2016;36:143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sargurupremraj M, Suzuki H, Jian X, et al. Cerebral small vessel disease genomics and its implications across the lifespan. Nat Commun. 2020;11:6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Traylor M, Persyn E, Tomppo L, et al. Genetic basis of lacunar stroke: A pooled analysis of individual patient data and genome-wide association studies. Lancet Neurol. 2021;20:351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Persyn E, Hanscombe KB, Howson JMM, Lewis CM, Traylor M, Markus HS. Genome-wide association study of MRI markers of cerebral small vessel disease in 42,310 participants. Nat Commun. 2020;11:2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Malik R, Beaufort N, Frerich S, et al. Whole-exome sequencing reveals a role of HTRA1 and EGFL8 in brain white matter hyperintensities. Brain. 2021;144:2670–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vasudevan A, Ho MS, Weiergraber M, et al. Basement membrane protein nidogen-1 shapes hippocampal synaptic plasticity and excitability. Hippocampus. 2010;20:608–620. [DOI] [PubMed] [Google Scholar]
- 36. Buga AM, Margaritescu C, Scholz CJ, Radu E, Zelenak C, Popa-Wagner A. Transcriptomics of post-stroke angiogenesis in the aged brain. Front Aging Neurosci. 2014;6:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gould DB, Phalan FC, van Mil SE, et al. Role of COL4A1 in small-vessel disease and hemorrhagic stroke. N Engl J Med. 2006;354:1489–1496. [DOI] [PubMed] [Google Scholar]
- 38. Leonardo CC, Eakin AK, Ajmo JM, Gottschall PE. Versican and brevican are expressed with distinct pathology in neonatal hypoxic-ischemic injury. J Neurosci Res. 2008;86:1106–1114. [DOI] [PubMed] [Google Scholar]
- 39. Lin MK, Yang J, Hsu CW, et al. HTRA1, an age-related macular degeneration protease, processes extracellular matrix proteins EFEMP1 and TSP1. Aging Cell. 2018;17:e12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pu A, Mishra MK, Dong Y, et al. The glycosyltransferase EXTL2 promotes proteoglycan deposition and injurious neuroinflammation following demyelination. J Neuroinflammation. 2020;17:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Markus HS, Hunt B, Palmer K, Enzinger C, Schmidt H, Schmidt R. Markers of endothelial and hemostatic activation and progression of cerebral white matter hyperintensities: Longitudinal results of the Austrian Stroke Prevention Study. Stroke. 2005;36:1410–1414. [DOI] [PubMed] [Google Scholar]
- 42. Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52. [DOI] [PubMed] [Google Scholar]
- 43. Ivanov AI, Romanovsky AA. Putative dual role of ephrin-Eph receptor interactions in inflammation. IUBMB Life. 2006;58:389–394. [DOI] [PubMed] [Google Scholar]
- 44. Larson J, Schomberg S, Schroeder W, Carpenter TC. Endothelial EphA receptor stimulation increases lung vascular permeability. Am J Physiol Lung Cell Mol Physiol. 2008;295:L431–L439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vihanto MM, Plock J, Erni D, Frey BM, Frey FJ, Huynh-Do U. Hypoxia up-regulates expression of Eph receptors and ephrins in mouse skin. FASEB J. 2005;19:1689–1691. [DOI] [PubMed] [Google Scholar]
- 46. Sohl M, Lanner F, Farnebo F. Sp1 mediate hypoxia induced ephrinB2 expression via a hypoxia-inducible factor independent mechanism. Biochem Biophys Res Commun. 2010;391:24–27. [DOI] [PubMed] [Google Scholar]
- 47. Manukjan N, Ahmed Z, Fulton D, Blankesteijn WM, Foulquier S. A systematic review of WNT signaling in endothelial cell oligodendrocyte interactions: Potential relevance to cerebral small vessel disease. Cells. 2020;9:1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lengfeld JE, Lutz SE, Smith JR, et al. Endothelial Wnt/beta-catenin signaling reduces immune cell infiltration in multiple sclerosis. Proc Natl Acad Sci USA. 2017;114:E1168–E1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang Y, Wang X. Targeting the Wnt/β-catenin signaling pathway in cancer. J Hematol Oncol. 2020;13:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rajani RM, Quick S, Ruigrok SR, et al. Reversal of endothelial dysfunction reduces white matter vulnerability in cerebral small vessel disease in rats. Sci Transl Med. 2018;10:eaam9507. [DOI] [PubMed] [Google Scholar]
- 51. Barnett MH, Parratt JD, Cho ES, Prineas JW. Immunoglobulins and complement in postmortem multiple sclerosis tissue. Ann Neurol. 2009;65:32–46. [DOI] [PubMed] [Google Scholar]
- 52. Werneburg S, Jung J, Kunjamma RB, et al. Targeted complement inhibition at synapses prevents microglial synaptic engulfment and synapse loss in demyelinating disease. Immunity. 2020;52:167–182.e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lee BC, Lee SG, Choo MK, et al. Selenoprotein MsrB1 promotes anti-inflammatory cytokine gene expression in macrophages and controls immune response in vivo. Sci Rep. 2017;7:5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Xu Y, Li F, Zhao X, et al. Methionine sulfoxide reductase A attenuates atherosclerosis via repairing dysfunctional HDL in scavenger receptor class B type I deficient mice. FASEB J. 2020;34:3805–3819. [DOI] [PubMed] [Google Scholar]
- 55. Cudic P, Joshi N, Sagher D, Williams BT, Stawikowski MJ, Weissbach H. Identification of activators of methionine sulfoxide reductases A and B. Biochem Biophys Res Commun. 2016;469:863–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Low A, Mak E, Rowe JB, Markus HS, O'Brien JT. Inflammation and cerebral small vessel disease: A systematic review. Ageing Res Rev. 2019;53:100916. [DOI] [PubMed] [Google Scholar]
- 57. Jalal FY, Yang Y, Thompson JF, Roitbak T, Rosenberg GA. Hypoxia-induced neuroinflammatory white-matter injury reduced by minocycline in SHR/SP. J Cereb Blood Flow Metab. 2015;35:1145–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Akiguchi I, Tomimoto H, Suenaga T, Wakita H, Budka H. Blood-brain barrier dysfunction in Binswanger's disease; An immunohistochemical study. Acta Neuropathol. 1998;95:78–84. [DOI] [PubMed] [Google Scholar]
- 59. Wharton SB, Simpson JE, Brayne C, Ince PG. Age-associated white matter lesions: The MRC Cognitive Function and Ageing Study. Brain Pathol. 2015;25:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Poggesi A, Pasi M, Pescini F, Pantoni L, Inzitari D. Circulating biologic markers of endothelial dysfunction in cerebral small vessel disease: A review. J Cereb Blood Flow Metab. 2016;36:72–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Walsh J, Tozer DJ, Sari H, et al. Microglial activation and blood-brain barrier permeability in cerebral small vessel disease. Brain. 2021;144:1361–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bogie JF, Boelen E, Louagie E, et al. CD169 is a marker for highly pathogenic phagocytes in multiple sclerosis. Mult Scler J. 2018;24:290–300. [DOI] [PubMed] [Google Scholar]
- 63. Dahlgren C, Gabl M, Holdfeldt A, Winther M, Forsman H. Basic characteristics of the neutrophil receptors that recognize formylated peptides, a danger-associated molecular pattern generated by bacteria and mitochondria. Biochem Pharmacol. 2016;114:22–39. [DOI] [PubMed] [Google Scholar]
- 64. Ge Y, Zhang S, Wang J, et al. Dual modulation of formyl peptide receptor 2 by aspirin-triggered lipoxin contributes to its anti-inflammatory activity. FASEB J. 2020;34:6920–6933. [DOI] [PubMed] [Google Scholar]
- 65. Murphy JM, Jeong K, Lim SS. FAK family kinases in vascular diseases. Int J Mol Sci. 2020;21:3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Malinin NL, Pluskota E, Byzova TV. Integrin signaling in vascular function. Curr Opin Hematol. 2012;19:206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Laubli H, Kawanishi K, George Vazhappilly C, Matar R, Merheb M, Sarwar Siddiqui S. Tools to study and target the Siglec-sialic acid axis in cancer. FEBS J. 2020;288:6206–6225. [DOI] [PubMed] [Google Scholar]
- 68. Sundqvist M, Holdfeldt A, Wright SC, et al. Barbadin selectively modulates FPR2-mediated neutrophil functions independent of receptor endocytosis. Biochim Biophys Acta Mol Cell Res. 2020;1867:118849. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the authors upon reasonable request.