Abstract
Blood-based biomarkers such as tau phosphorylated at threonine 181 (phosphorylated-tau181) represent an accessible, cost-effective and scalable approach for the in vivo detection of Alzheimer’s disease pathophysiology. Plasma-pathological correlation studies are needed to validate plasma phosphorylated-tau181 as an accurate and reliable biomarker of Alzheimer’s disease neuropathological changes.
This plasma-to-autopsy correlation study included participants from the Boston University Alzheimer’s Disease Research Center who had a plasma sample analysed for phosphorylated-tau181 between 2008 and 2018 and donated their brain for neuropathological examination. Plasma phosphorelated-tau181 was measured with single molecule array technology.
Of 103 participants, 62 (60.2%) had autopsy-confirmed Alzheimer’s disease. Average time between blood draw and death was 5.6 years (standard deviation = 3.1 years). Multivariable analyses showed higher plasma phosphorylated-tau181 concentrations were associated with increased odds for having autopsy-confirmed Alzheimer’s disease [AUC = 0.82, OR = 1.07, 95% CI = 1.03–1.11, P < 0.01; phosphorylated-tau standardized (z-transformed): OR = 2.98, 95% CI = 1.50–5.93, P < 0.01]. Higher plasma phosphorylated-tau181 levels were associated with increased odds for having a higher Braak stage (OR = 1.06, 95% CI = 1.02–1.09, P < 0.01) and more severe phosphorylated-tau across six cortical and subcortical brain regions (ORs = 1.03–1.06, P < 0.05). The association between plasma phosphorylated-tau181 and Alzheimer’s disease was strongest in those who were demented at time of blood draw (OR = 1.25, 95%CI = 1.02–1.53), but an effect existed among the non-demented (OR = 1.05, 95% CI = 1.01–1.10). There was higher discrimination accuracy for Alzheimer’s disease when blood draw occurred in years closer to death; however, higher plasma phosphorylated-tau181 levels were associated with Alzheimer’s disease even when blood draw occurred >5 years from death.
Ante-mortem plasma phosphorylated-tau181 concentrations were associated with Alzheimer’s disease neuropathology and accurately differentiated brain donors with and without autopsy-confirmed Alzheimer’s disease. These findings support plasma phosphorylated-tau181 as a scalable biomarker for the detection of Alzheimer’s disease.
Keywords: plasma p-tau181, Alzheimer’s disease, autopsy, biomarkers, tau
In one of the largest studies of its kind, Morrison et al. show that ante-mortem plasma phosphorylated-tau181 concentrations accurately differentiate brain donors with and without autopsy-confirmed Alzheimer‘s disease. Blood tests could be a minimally invasive, cost-effective tool for the detection and monitoring of Alzheimer’s disease.
Introduction
Alzheimer’s disease is characterized by the extracellular accumulation of the amyloid-β peptide and intracellular aggregation of hyperphosphorylated tau (p-tau) protein.1 In the National Institute on Aging (NIA) and Alzheimer’s Association framework,2 it is possible to detect preclinical Alzheimer’s disease neuropathological changes using in vivo biomarkers, allowing for early disease detection and timely therapeutic intervention.2 Lumbar puncture for analysis of CSF and PET ligands for amyloid-β and p-tau have revolutionized our ability to detect Alzheimer’s disease pathology.2 However, lumbar puncture is viewed as invasive and PET scans are expensive, not covered by medical insurance and involve exposure to radiation. They have limited scalability and are often unavailable in non-specialized clinics and in low- and middle-income countries.
It is now possible to detect low abundant proteins associated with Alzheimer’s disease neuropathology in the blood, including p-tau.3 Recent studies demonstrate plasma p-tau181 is associated with CSF levels of p-tau181 and tau and amyloid uptake on PET.4–7 Higher plasma p-tau concentrations (including at 181 and 217 phosphorylation sites) can accurately differentiate mild cognitive impairment (MCI) and Alzheimer’s disease dementia participants from those with normal cognition.4–6,8 Research on the validity of plasma p-tau in Alzheimer’s disease is nascent and the extent to which proteins in the blood reflect the CNS environment is emerging.
Clinical–pathological correlation studies are the gold standard for the development and validation of in vivo biomarkers.9–15 There have been a few plasma-to-autopsy correlation studies in Alzheimer’s disease. Brickman et al.4 showed higher ante-mortem plasma p-tau217 and p-tau181 in 33 brain donors with high Alzheimer’s disease neuropathological changes compared to 80 donors who had low Alzheimer’s disease. Among 115 individuals with longitudinal blood samples, plasma p-tau181 accurately discriminated Alzheimer’s disease from non-Alzheimer’s disease neuropathological diagnoses as long as 8 years before death (AUC = 0.97).16 Smirnov et al.17 also demonstrated strong sensitivity and specificity of plasma p-tau181 in predicting Alzheimer’s disease neuropathology among 312 brain donors (AUC = 0.856). Furthermore, plasma p-tau181 accurately discriminated (AUC = 0.88) 15 participants with autopsy-confirmed cases of Alzheimer’s disease from 67 brain donors with frontotemporal lobar degeneration (FTLD).7 A recent study found plasma p-tau181 accurately discriminated (AUC = 0.91) 14 cases of autopsy-confirmed Alzheimer’s disease from amyloid-β-negative controls, as well as Alzheimer’s disease from non-Alzheimer’s disease autopsy cases (n = 4).18 Plasma p-tau181 levels also correlated with Braak stage and neuritic amyloid plaque scores.7,16,18
Additional large-scale plasma-pathological correlation studies are needed to validate plasma p-tau181 as an accurate and reliable biomarker of Alzheimer’s disease neuropathological changes. In addition, no study has examined the association between plasma p-tau181 and regional p-tau aggregation, which is an important validation step as it will provide insight on the association between plasma p-tau181 and tau in regions classically affected by Alzheimer’s disease (e.g. the hippocampus). This study examined the ability of ante-mortem plasma p-tau181 levels to accurately differentiate brain donors with and without autopsy-confirmed Alzheimer’s disease. We tested the association between ante-mortem plasma p-tau181 and p-tau aggregation across six cortical and subcortical brain regions. We hypothesized that ante-mortem p-tau181 levels would accurately discriminate between brain donors with and without Alzheimer’s disease neuropathology and be associated with p-tau severity at autopsy.
Materials and methods
Study design and brain donors
This study included participants from the NIA-funded Boston University Alzheimer’s Disease Research Center (BU ADRC) Clinical Core who donated their brain to the BU ADRC Neuropathology Core for neuropathological examination. The BU ADRC is one of more than 30 centres funded by the NIA that provides standardized data to the National Alzheimer’s Coordinating Center (NACC) to promote collaborative research on Alzheimer’s disease and related dementias. The BU ADRC follows older adults with and without cognitive impairment from the Boston neighbourhoods surrounding Boston Medical Center and the Greater Boston area. All participants are English-speaking older adults with adequate visual acuity and hearing. Participants are excluded for a history of a serious mental illness (e.g. bipolar disorder, schizophrenia, etc.), non-Alzheimer’s disease or related dementias neurological disorders (e.g. brain tumour, multiple sclerosis) or medical conditions that preclude study participation. The BU ADRC protocol involves an annual NACC Uniform Data Set evaluation that includes neurological examination, a clinical and medical interview, neuropsychological testing and other procedures. Participants are asked to donate their brain following death to the BU ADRC brain bank for comprehensive neuropathological processing and examination.
Beginning in 2008, voluntary annual blood draws were initiated at the BU ADRC. Blood samples collected through 2018 were analysed for plasma p-tau181 as part of a separate published study that examined the ability of plasma p-tau181 to discriminate participants with cognitive impairment from normal cognition.19 We leveraged p-tau181 data from that study. We included participants from that sample who had p-tau181 and who donated their brain for neuropathological examination. If multiple blood draws were performed, the most recent was used. Because p-tau181 data were acquired from a study focused on clinical outcomes, the visit of the plasma sample did not necessarily correspond to the visit proximate to death. This resulted in a sample size of 103 after exclusion for missing data on primary study variables and exclusion of one brain donor with p-tau concentration level below the lower limit of quantification (Supplementary Fig. 1). Procedures including brain donation were approved by the BU Medical Campus Institutional Review Board (BUMC IRB). Participants (or their legally authorized representatives) provided written informed consent prior to participation in the BU ADRC protocol. Approval for neuropathological evaluation was obtained through the BUMC IRB. Next of kin provided written informed consent if written informed consent from the participant was obtained more than three years prior to death.
Plasma biomarker collection and analysis
Blood collection, processing and storage followed standard operating procedures that adhere to those set forth by the National Centralized Repository for Alzheimer’s Disease and Related Dementias. Non-fasting blood samples were collected into plastic dipotassium EDTA tubes and processed with plasma aliquoted and frozen at −80°C. Frozen plasma aliquots were shipped on dry ice to the University of Gothenburg (Sweden) for batch analysis. Plasma p-tau181 concentration was measured using an in-house single molecule array method on an HD-X analyser (Quanterix), as previously described in detail.20 The lower limit of quantification was 1.0 pg/ml, with a dynamic range of 1.0–128.0 pg/ml. The measurements were performed in one round of experiments, using one batch of reagents. Intra-assay coefficients of variation were below 10%.
Neuropathological evaluation
Neuropathological processing and evaluation were conducted using published methodology21,22 and following procedures described in the NACC standardized Neuropathology Form and Coding Guidebook.23–26 Ratings of Thal phase were added later to the BU ADRC and available on 56 of 103 brain donors. The NIA–Reagan Institute criteria utilizing Braak stage and Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) scores were thus used for the neuropathological diagnosis of Alzheimer’s disease.27 Brain donors who had no or low Alzheimer’s disease neuropathological changes were combined (non-Alzheimer’s disease). The Alzheimer’s disease group included brain donors who had intermediate or high likelihood of Alzheimer’s disease. Established criteria were used for other neuropathological diagnosis of neurodegenerative diseases.28–32 Semiquantitative scales [0 (none)–3 (severe)] were used to rate severity of cerebral amyloid angiopathy, atherosclerosis and arteriolosclerosis.
The CERAD score was used to rate the presence and severity of neuritic amyloid-β plaques.33 Braak staging of neurofibrillary degeneration was rated on a scale from 0 (no degeneration) to VI (widespread degeneration that has spread to the neocortex) based on Bielchowsky silver staining.34 Independent assessments of the density of AT8-positive p-tau pathology were performed by study neuropathologists using semiquantitative rating scales (0–3 scale; 0 = none, 3 = severe) in various cortical and subcortical brain regions. AT8-immunostained, 10-µm thick paraffin-embedded sections of the following regions were examined in this study: inferior parietal cortex, superior temporal cortex, CA1-hippocampus, CA2-hippocampus, entorhinal cortex and the amygdala. These regions were a priori selected due to their involvement in Alzheimer’s disease.34
Dementia severity
Dementia severity was rated using the global score from the Clinical Dementia Rating (CDR®) Dementia Staging Instrument.35,36 An algorithm is used to calculate a global severity rating designated as: 0 (no dementia), 0.5 (MCI), 1.0 (mild dementia), 2.0 (moderate dementia) and 3.0 (severe dementia). Global CDR score at the time of blood draw was included in statistical models.
Statistical analytic plan
All analyses were conducted using SPSS statistical software version 27. A P-value < 0.05 was considered statistically significant. Plasma p-tau181 served as the independent variable. Three binary logistic regression models were performed to examine the association between plasma p-tau181 and Alzheimer’s disease neuropathological diagnosis: Model 1: unadjusted (i.e. plasma p-tau181 alone); Model 2: controlling for age at death, years between last blood draw and death, sex (1 = female, 0 = male) and APOE ε4 status (1 = ε4 carrier, 0 = non-carrier); and Model 3: Model 2 covariates in addition to global CDR score at the time of blood draw to account for differences in disease severity.7 CDR scores were stratified by <1 and 1 or higher (i.e. dementia versus no dementia). For each model, discrimination accuracy for Alzheimer’s disease neuropathological diagnosis was evaluated using the area under the receiver operating curve (AUC) statistic. AUC statistic was calculated based on p-tau181 alone (Model 1) and using predicted probabilities from the multivariable logistic regression that included the aforementioned covariates (Models 2 and 3). Note that the AUC statistic was also calculated for a covariate-only model (i.e. Model 2 without plasma p-tau181) as reference for Models 1–3. Discrimination accuracy was categorized based on guidelines suggested in Hosmer and Lemeshow (AUC =0.50: no discrimination; AUC = 0.70–0.80: acceptable discrimination; AUC = 0.80–0.90: excellent discrimination; AUC ≥ 0.90: outstanding discrimination).37
In the entire sample, multivariable ordinal logistic regressions tested the associations between plasma p-tau181 and Braak NFT stage (stage 0, I/II, III/IV, V/VI), CERAD neuritic plaque score, and semiquantitative ratings of p-tau severity for the inferior parietal cortex, superior temporal cortex, entorhinal cortex, amygdala, CA1-hippocampus and CA2-hippocampus. Sample size for the semiquantitative ratings of regional p-tau severity was reduced to 90 due to missingness. Covariates included age at death, years between last blood draw and death, sex and APOE ε4 status. Due to the number of analyses performed for the semiquantitative ratings of regional p-tau severity (six total outcomes), p-values were false discovery rate-adjusted using the Benjamini–Hochberg procedure.
As sensitivity analyses, the logistic regression was repeated with a p-tau181 × CDR score (at time of blood draw; Model 3 repeated) and a p-tau181 × years between blood draw and death interaction term included (Model 2 repeated), in separate models. These models tested whether (i) dementia severity; and (ii) the time between blood draw and neuropathological examination moderated the association between plasma p-tau181 levels and Alzheimer’s disease neuropathological diagnosis. We examined the accuracy of plasma p-tau181 in discriminating Alzheimer’s disease and non-Alzheimer’s disease brain donors, using the AUC statistic, stratified by CDR scores (<1 and 1 or higher) and by who those who had a blood draw greater than or equal to and less than 5 years prior to death.
Data availability
All uniform and neuropathology data set evaluation data are shared with the NACC and are publicly available. Data are also available upon reasonable request to the BU ADRC.
Results
Sample characteristics
Tables 1 and 2 present sample characteristics of the 103 brain donors. The mean (standard deviation, SD) time between blood draw and death was 5.6 (3.1) years with a median of 5.0 and range of 0.0 (blood draw done same month of death) to 12.0 years. Sixty-two (60.2%) had Alzheimer’s disease at autopsy. Compared to those without autopsy-confirmed Alzheimer’s disease, those with Alzheimer’s disease were more likely to have an APOE ε4 allele (P = 0.06) and a higher global CDR score at time of death and blood draw (P < 0.01). There were no statistically significant differences between the donors with and without Alzheimer’s disease in terms of age at death, race, ethnicity, sex, years between blood draw and death or self-reported vascular risk factors. Donors with Alzheimer’s disease had more severe ratings of cerebral amyloid angiopathy and regional p-tau than the non-Alzheimer’s disease donors (P-values < 0.01). There were no statistically significant differences between Alzheimer’s disease and non-Alzheimer’s disease on neuropathological diagnosis of Lewy body disease, FTLD, arteriolosclerosis or atherosclerosis.
Table 1.
Sample characteristics
| Total sample (n = 103) | Alzheimer’s disease pathology (n = 62) | Non-Alzheimer’s disease pathology (n = 41) | P-value (effect size) | |
|---|---|---|---|---|
| Demographics | ||||
| Sex, n (%) female | 47 (45.6) | 28 (45.2) | 19 (46.3) | 0.91 |
| Age at blood draw, mean (SD) | 78.77 (8.21) | 77.97 (8.46) | 79.98 (7.76) | 0.23 |
| Age at death, mean (SD) | 84.40 (8.25) | 83.27 (8.26) | 86.10 (8.02) | 0.09 |
| Race, n (%) | 0.19 | |||
| American Indian/Alaska Native | 2 (1.9) | 2 (3.2) | 0 | |
| Asian | 1 (1.0) | 0 | 1 (2.4) | |
| Black or African American | 4 (3.9) | 1 (1.6) | 3 (7.3) | |
| White | 95 (92.2) | 59 (95.2) | 36 (87.8) | |
| Other | 1 (1.0) | 0 | 1 (2.4) | |
| Ethnicity, n (%) | – | |||
| Hispanic | 0 | 0 | 0 | |
| Diagnosis at death, n (%) | <0.01 (OR = 7.53) |
|||
| Normal cognition | 10 (9.7) | 0 (0) | 10 (24.4) | |
| MCI/non-MCI cognitively impaired | 20 (19.4) | 7 (11.3) | 13 (31.7) | |
| Dementia | 73 (70.9) | 55 (88.7) | 18 (43.9) | |
| Dementia severity | ||||
| Global CDR score at death, mean (SD) | 1.22 (1.13) | 1.59 (1.14) | 0.66 (0.85) | <0.01 (d = 0.90) |
| Global CDR score at death, n (%) | <0.01 (OR = 4.91) | |||
| <1 | 55 (53.4) | 24 (38.7) | 31 (75.6) | |
| ≥1 | 48 (46.6) | 38 (61.3) | 10 (24.4) | |
| Global CDR score at blood draw, mean (SD) | 0.83 (0.93) | 1.11 (0.97) | 0.39 (0.68) | <0.01 (d = 0.84) |
| Global CDR score at blood draw, n (%) | <0.01 (OR = 5.26) | |||
| <1 | 57 (55.3) | 25 (40.3) | 32 (78.0) | |
| ≥1 | 46 (44.7) | 37 (59.7) | 9 (22.0) | |
| Vascular risk factors, n (%) | ||||
| Hypertension | 61 (59.2) | 34 (54.8) | 27 (65.8) | 0.27 |
| Diabetes | 13 (12.6) | 8 (12.9) | 5 (12.2) | 0.92 |
| Obstructive sleep apnoea | 9 (8.7) | 3 (4.8) | 6 (14.6) | 0.10 |
| Genetic | ||||
| APOE ε 4 allele status, n (%) carrier | 47 (45.6) | 33 (53.2) | 14 (34.1) | 0.06 (OR = 2.20) |
| Plasma biomarker | ||||
| P-tau181, mean (SD)/range, pg/ml | 27.19 (16.52)/3–95 | 31.28 (15.67)/9–90 | 20.99 (16.00)/3–95 | <0.01 (d = 0.65) |
The 1997 NIA–Reagan criteria were used for the neuropathological diagnosis of Alzheimer’s disease and those with sparse neuritic plaques and Braak stage 5 or 6 were classified as Alzheimer’s disease. Binary logistic regression was used to compare donors with and without autopsy-confirmed Alzheimer’s disease on binary outcomes; independent samples t-test was used for continuous outcomes. For race, White and non-White were compared and coded as 1 (White) and 0 (non-White). Sex was coded as 0 (male) and 1 (female). Sample size for ethnicity was 101 as two were unknown.
Table 2.
Neuropathology characteristics
| Total sample (n = 103) | Alzheimer’s disease pathology (n = 62) | Non- Alzheimer’s disease pathology (n = 41) | P-value (effect size) | |
|---|---|---|---|---|
| Braak stage, n (%) | – | |||
| Stage 0 | 4 (3.9) | 0 (0) | 4 (9.8) | |
| Stage I/II | 15 (14.6) | 0 (0) | 15 (36.6) | |
| Stage III/IV | 30 (29.1) | 8 (12.9) | 22 (53.7) | |
| Stage V/VI | 54 (52.4) | 54 (87.1) | 0 (0) | |
| Semiquantitative ratings of regional p-tau severity, n (%) moderate–severe | ||||
| Inferior parietal cortex | 48 (53.3) | 48 (88.9) | 0 | – |
| Superior temporal cortex | 56 (62.2) | 50 (92.6) | 6 (16.7) | <0.01 (OR = 62.50) |
| Entorhinal cortex | 74 (82.2) | 52 (96.3) | 22 (61.1) | <0.01 (OR = 16.55) |
| Amygdala | 63 (70.0) | 51 (94.4) | 12 (33.3) | <0.01 (OR = 34.00) |
| CA1-hippocampus | 67 (74.0) | 50 (92.6) | 17 (47.2) | <0.01 (OR = 13.97) |
| CA2-hippocampus | 53 (58.9) | 40 (74.1) | 13 (36.1) | <0.01 (OR = 5.06) |
| CERAD neuritic plaque score, n (%) | – | |||
| None | 23 (22.3) | 0 (0) | 23 (56.1) | |
| Sparse | 20 (19.4) | 6 (9.7) | 14 (34.1) | |
| Moderate | 27 (26.2) | 23 (37.1) | 4 (9.8) | |
| Frequent | 33 (32.0) | 33 (53.2) | 0 | |
| Lewy body disease, n (%) | 0.16 | |||
| Brainstem predominant | 4 (4.0) | 3 (4.8) | 1 (2.4) | |
| Limbic (transitional) | 8 (8.1) | 4 (6.5) | 4 (9.8) | |
| Neocortical (diffuse) | 18 (18.2) | 11 (17.7) | 7 (17.1) | |
| Amygdala predominant | 4 (4.0) | 4 (6.5) | 0 (0) | |
| Olfactory bulb | 3 (3.0) | 3 (4.8) | 0 (0) | |
| Frontotemporal lobar degeneration, n (%) | 11 (10.7) | 4 (6.5) | 7 (17.1) | 0.10 |
| Chronic traumatic encephalopathy, n (%) | 2 (2.0) | 2 (3.4) | 0 | – |
| Cerebral amyloid angiopathy, n (%) moderate–severe | 45 (43.7) | 35 (56.5) | 10 (24.4) | <0.01 (OR = 4.02) |
| Arteriosclerosis, n (%) moderate–severe | 84 (81.6) | 51 (82.3) | 33 (80.5) | 0.82 |
| Atherosclerosis, n (%) moderate–severe | 38 (36.9) | 23 (37.1) | 15 (36.6) | 0.96 |
The 1997 NIA–Reagan criteria were used for the neuropathological diagnosis of Alzheimer’s disease and those with sparse neuritic plaques and Braak stage 5 or 6 were classified as Alzheimer’s disease. Binary logistic regression was used to compare donors with and without autopsy-confirmed Alzheimer’s disease on all outcomes. Braak and CERAD were not compared because they were used to define the Alzheimer’s disease groups. Note analyses were not performed for those with insufficient cell sizes. For semiquantitative ratings of regional p-tau, cerebral amyloid angiopathy, arteriolosclerosis and atherosclerosis, donors with moderate to severe ratings were grouped compared with donors who had no or mild severity ratings. Lewy body disease was examined as absent/present. Sample size for Lewy body disease was 99 because it was not assessed for four donors. Three brain donors had missingness for chronic traumatic encephalopathy. Sample sizes for the semiquantitative ratings of p-tau severity was 90 (sample restricted to donors who had complete ratings for all regions). CERAD = Consortium to Establish a Registry for Alzheimer’s Disease.
Plasma p-tau181 associations with Alzheimer’s disease neuropathology and p-tau
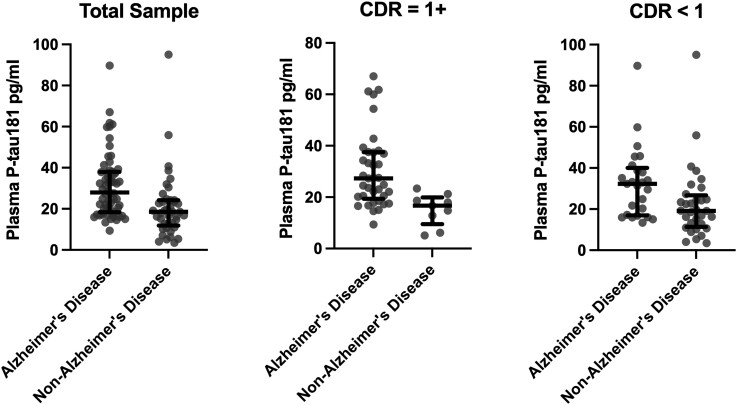
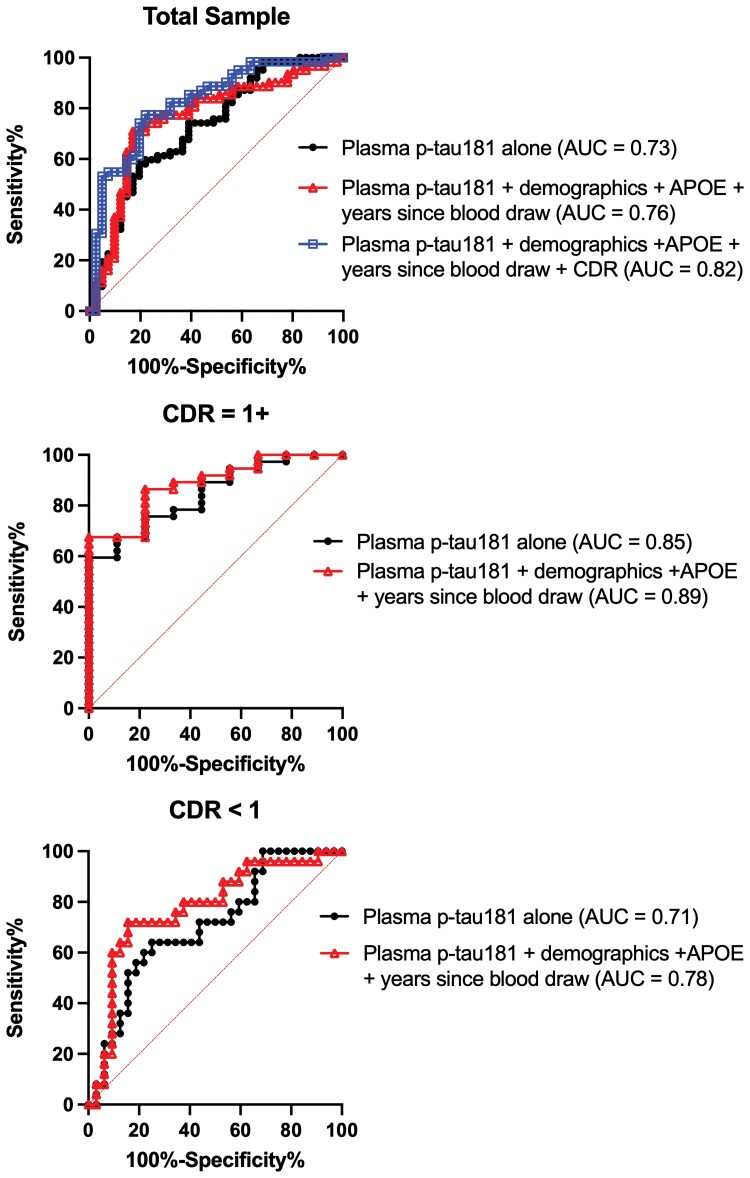
Statistical models are summarized in Table 3. Figure 1 shows the distribution of plasma p-tau181 concentrations by Alzheimer’s disease status. A covariate-only model (i.e. age at death, years between last blood draw and death, sex, and APOE ε4 status) had an AUC of 0.65 (95% CI = 0.54–0.76) for discriminating between brain donors with and without Alzheimer’s disease. In a plasma p-tau181-only model, higher plasma p-tau181 concentrations were associated with increased odds of having Alzheimer’s disease neuropathological changes (OR = 1.05, 95% CI = 1.02–1.09) with an AUC of 0.73 (95% CI = 0.63–0.83). This association remained after controlling for age at death, years between blood draw and death, sex and APOE ε4 status (OR = 1.06, 95% CI = 1.02–1.10) with an AUC of 0.76 (95% CI = 0.67–0.86), as well as when global CDR score was included as a covariate (OR = 1.07, 95% CI = 1.03–1.11). The full multivariable model that included global CDR discriminated Alzheimer’s disease from non-Alzheimer’s disease with excellent accuracy (AUC = 0.82, 95% CI = 0.74–0.91). Figure 2 shows the ROC curves for each model. We repeated the fully adjusted Model 3 with p-tau181 standardized (z-transformed) to facilitate interpretation of its association with Alzheimer’s disease status in this sample. The OR for the association between standardized p-tau181 levels and Alzheimer’s disease status at autopsy was 2.98 (95% CI = 1.50–5.93, P < 0.01).
Table 3.
Association between plasma p-tau181, Alzheimer’s disease neuropathology and regional p-tau severity
| OR | 95% CI | P-value | AUC (95% CI), P-value | |
|---|---|---|---|---|
| Autopsy-confirmed Alzheimer’s disease (n = 62 versus n = 41 non-Alzheimer’s disease) | ||||
| Model 1 | 1.05 | 1.02–1.09 | <0.01 | 0.73 (0.63–0.83), <0.01 |
| Model 2 | 1.06 | 1.02–1.10 | <0.01 | 0.76 (0.67–0.86), <0.01 |
| Model 3 | 1.07 | 1.03–1.11 | <0.01 | 0.82 (0.74–0.91), <0.01 |
| Autopsy-confirmed Alzheimer’s disease, CDR <1.0 (n = 25 Alzheimer’s disease versus n = 32 non-Alzheimer’s disease) | ||||
| Model 1 | 1.04 | 0.99–1.08 | 0.05 | 0.71 (0.57–0.84), <0.01 |
| Model 2 | 1.05 | 1.01–1.10 | 0.02 | 0.78 (0.65–0.91), <0.01 |
| Autopsy-confirmed Alzheimer’s disease, CDR ≥1.0 (n = 37 Alzheimer’s disease versus n = 9 non-Alzheimer’s disease) | ||||
| Model 1 | 1.23 | 1.05–1.45 | 0.01 | 0.85 (0.73–0.97), <0.01 |
| Model 2 | 1.25 | 1.02–1.53 | 0.03 | 0.89 (0.78–0.99), <0.01 |
| Braak stage (n = 103) | 1.06 | 1.02–1.09 | <0.01 | – |
| CERAD neuritic plaque score (n = 103) | 1.05 | 1.02–1.08 | <0.01 | – |
| Regional p-tau severity (n = 90) | ||||
| Superior temporal cortex | 1.06 | 1.02–1.09 | <0.01 | – |
| Inferior parietal cortex | 1.04 | 1.01–1.07 | <0.01 | – |
| Entorhinal cortex | 1.06 | 1.02–1.10 | <0.01 | – |
| Amygdala | 1.04 | 1.01–1.07 | 0.03 | – |
| CA1-hippocampus | 1.06 | 1.02–1.10 | <0.01 | – |
| CA2-hippocampus | 1.03 | 1.01–1.06 | 0.02 | – |
Binary logistic regression examined the association between plasma p-tau181 levels and Alzheimer’s disease neuropathologic changes (per NIA–Reagan criteria). Model 1 examined plasma p-tau181 alone. Model 2 controlled for age at death, years between last blood draw and death, sex and APOE ε4 status. Model 3 controlled for Model 2 covariates in addition to global CDR (<1 and 1 or higher) score at time of blood draw. The AUC statistics for Models 2 and 3 were calculated using predicted probabilities from the binary logistic regression. P-values that examined the semiquantitative ratings of regional p-tau severity as outcomes (six total outcomes) were false discovery rate-adjusted using the Benjamini–Hochberg procedure and covariates included age at death, years between last blood draw and death, sex and APOE ε4 status.
Figure 1.
Distribution of plasma p-tau181 concentrations between brain donors with and without autopsy-confirmed Alzheimer’s disease. NIA–Reagan Institute criteria were used for the neuropathological diagnosis of Alzheimer’s disease. Analyses were done in the entire sample (n = 62 Alzheimer’s disease versus 41 non-Alzheimer’s disease) and stratified by CDR score ≥1 (n = 37 Alzheimer’s disease versus n = 9 non-Alzheimer’s disease) and CDR score <1 (n = 25 Alzheimer’s disease versus n = 32 non-Alzheimer’s disease). Figure shows the median (bar) and interquartile range (whiskers) as well as the individual data points. Results of the binary logistic regression models that tested the association between plasma p-tau181 and Alzheimer’s disease status in the entire sample and stratified by CDR are shown in Table 3. p-tau = phosphorylated-tau.
Figure 2.
Accuracy of plasma p-tau181 in discriminating brain donors with and without autopsy-confirmed Alzheimer’s disease. NIA–Reagan Institute criteria were used for the neuropathological diagnosis of Alzheimer’s disease. Analyses were done in the entire sample and stratified by CDR score ≥1 and CDR score <1. AUC statistic was calculated based on p-tau181 alone (Model 1) and using predicted probabilities from multivariable binary logistic regression that included age at death, years between last blood draw and death, sex (1 = female, 0 = male) and APOE ε4 status (1 = ε4 carrier, 0 = non-carrier; Model 2). For the entire sample, a third model was done that included Model 2 covariates in addition to inclusion of global CDR score at the time of blood draw (Model 3). This model was not done in those stratified by CDR score. p-tau = phosphorylated-tau.
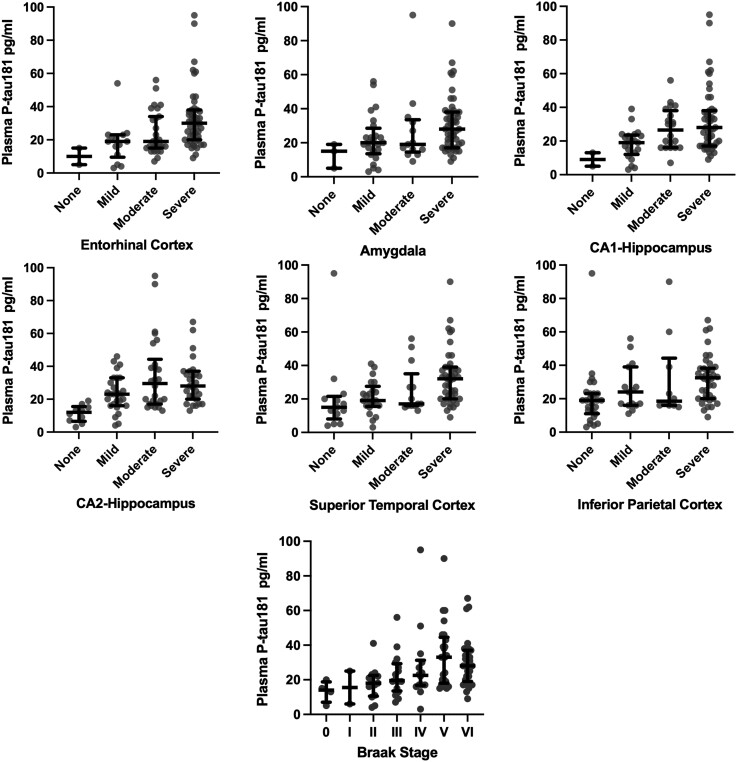
Higher levels of plasma p-tau181 were associated with Braak stage (OR = 1.06, 95% CI = 1.02–1.09) and CERAD neuritic plaque score (OR = 1.05, 95% CI = 1.02–1.08). Higher plasma p-tau181 concentrations corresponded to higher odds for having more severe p-tau in the superior temporal cortex, inferior parietal cortex, entorhinal cortex, amygdala, CA1-hippocampus and CA2-hippocampus (OR = 1.03–1.06, false discovery rate-adjusted P-values < 0.05; Table 3). Figure 3 shows the associations.
Figure 3.
Distribution of plasma p-tau181 by p-tau severity ratings at autopsy. Figure shows the median (bar) and interquartile range (whiskers) as well as the individual data points for plasma p-tau181 levels by Braak staging of neurofibrillary tangles, as well as hyperphosphorylated tau severity across six cortical and subcortical brain regions rated at autopsy using a 0 (none) to 3 (severe) scale. As shown in Table 3, ordinal logistic regression controlling for age at death, years between last blood draw and death, sex and APOE ε4 status showed higher plasma p-tau181 levels were associated with increased odds for more severe Braak stage and p-tau severity across all of the regions (Ps < 0.05). p-tau = phosphorylated-tau.
Stratified by global CDR score at blood draw
Corresponding to global CDR scores at time of blood draw of 0, 0.5, 1.0, 2.0 and 3.0, there were 39 (37.9%), 18 (17.5%), 25 (24.3%), 12 (11.7%) and 9 (8.7%) participants, respectively. See Supplementary Table 1 for sample characteristics by CDR score. Figure 1 also shows distribution of plasma ptau181 concentrations by CDR score. Those who had a higher CDR score were more likely to have Alzheimer’s disease neuropathology and have higher p-tau severity ratings. There was a significant plasma p-tau181 × CDR interaction effect on Alzheimer’s disease neuropathological diagnosis (OR = 1.22, 95% CI = 1.01–1.48, P = 0.04). Plasma p-tau181 levels had better discrimination accuracy among those with high CDR scores compared with low. Discrimination for Alzheimer’s disease neuropathological diagnosis was good for both the participants with CDR scores ≥1.0 (AUC = 0.89, 95% CI = 0.78–0.99, P < 0.01) and those who had a CDR score <1.0 (AUC = 0.78, 95% CI = 0.65–0.91, P < 0.01), for models that included p-tau181, age at death, years between last blood draw and death, sex and APOE ε4 status (Fig. 2).
Stratified by blood draw greater than and less than 5 years before death
In the sample stratified by donors who had a blood draw <5 (n = 45) or ≥5 years (n = 58) prior to death, 29 (64.4%) and 33 (56.9%) had autopsy-confirmed Alzheimer’s disease, respectively. Those who had a blood draw within 5 years were older at the time of blood draw by approximately 4 years (P = 0.01), had a higher global CDR score (P < 0.001) and had a higher Braak stage (P < 0.01). There were no other differences between the groups (Ps > 0.05; Supplementary Table 2). As shown in Table 4 and Supplementary Fig. 2, all three models had excellent discrimination accuracy among brain donors who had a blood draw <5 years from death with AUCs ranging from 0.83–0.91 (Ps < 0.01). Discrimination accuracy for plasma p-tau181 was worse in brain donors who had a blood draw ≥5 years from death, particularly for plasma p-tau181 alone (AUC = 0.65, P = 0.049). Discrimination accuracy for the adjusted models remained acceptable with an AUC of 0.71 (P = 0.006) for Model 2 and AUC of 0.77 (P < 0.001) for Model 3. There was a statistical trend for plasma p-tau181 × years between blood draw and death interaction effect on Alzheimer’s disease neuropathological diagnosis in Model 3 (P = 0.099).
Table 4.
Accuracy of plasma p-tau181 in discriminating donors with and without autopsy-confirmed Alzheimer’s disease stratified by blood draw greater than and less than 5 years before death
| AUC | 95% CI | P-value | |
|---|---|---|---|
| Blood draw less than 5 years before death (n = 45) | |||
| Autopsy-confirmed Alzheimer’s disease | |||
| Model 1 | 0.83 | 0.71–0.95 | <0.01 |
| Model 2 | 0.85 | 0.74–0.97 | <0.01 |
| Model 3 | 0.91 | 0.83–1.00 | <0.01 |
| Blood draw greater than 5 years before death, n = 58 | |||
| Autopsy-confirmed Alzheimer’s disease | |||
| Model 1 | 0.65 | 0.51–0.80 | 0.049 |
| Model 2 | 0.71 | 0.58–0.85 | <0.01 |
| Model 3 | 0.77 | 0.65–0.90 | <0.01 |
Model 1 examined plasma p-tau181 alone. Model 2 was based on predicted probabilities from binary logistic regression that included plasma p-tau181, age at death, years between last blood draw and death, sex and APOE ε4 status. Model 3 was also based on predicted probabilities from binary logistic regression that included Model 2 covariates in addition to global CDR score at time of blood draw.
Discussion
In this sample of 103 brain donors (62 with autopsy-confirmed Alzheimer’s disease), ante-mortem plasma p-tau181 concentrations were associated with Alzheimer’s disease neuropathological changes at autopsy, including NIA–Reagan Alzheimer’s disease neuropathological diagnosis, Braak stage, CERAD neuritic plaque score and semiquantitative ratings of cortical and subcortical p-tau severity. Higher plasma p-tau181 levels accurately differentiated donors with and without autopsy-confirmed Alzheimer’s disease, including among a subgroup who were cognitively unimpaired or had MCI at the time of blood sampling. Discrimination accuracy across all models was superior when plasma p-tau181 was examined jointly with demographics, APOE ε4 status and global CDR score. Discrimination accuracy was optimal when blood draw was within 5 years of death; however, plasma p-tau181 levels from ≥5 years before death also accurately discriminated—albeit to a lesser extent—between Alzheimer’s disease and non-Alzheimer’s disease neuropathological diagnoses. These findings support plasma p-tau181 as a biomarker for the accurate and early detection of underlying Alzheimer’s disease neuropathology.
The development and validation of plasma biomarkers for Alzheimer’s disease and related dementias has been the focus of research in recent years.38 Clinical–pathological correlation studies are the gold standard, but there are few plasma-to-autopsy studies and existing ones are limited by smaller sample sizes. The present findings are consistent with previous studies that show an association between ante-mortem plasma p-tau181 and Alzheimer’s disease neuropathological changes at autopsy. Jointly published results from two independent neuropathology cohorts replicated and cross-validated the finding that plasma p-tau181 differentiates autopsy-proven Alzheimer’s disease in small samples (n = 15 and n = 16, respectively5,7). This compares with a similarly sized sample (n = 14) from the Alzheimer’s Disease Neuroimaging Initiative that associated cerebrospinal fluid levels of p-tau181 with autopsy-confirmed Alzheimer’s disease neuropathological changes.18 Plasma p-tau181 correlated with CSF p-tau181 levels in that study, and higher plasma p-tau181 levels had similar pathologic specificity as CSF p-tau181 for Braak stage and neuritic amyloid-β plaques.18
Recent studies and the present one support the utility of plasma p-tau181 in larger samples. A UK clinical registry cohort associated elevated plasma p-tau181, measured by single molecular array, with Alzheimer’s disease neuropathological diagnosis and higher Braak stage among 111 brain donors (67 with autopsy-confirmed Alzheimer’s disease).16 Similar results associating elevated plasma p-tau181 with neuropathological diagnosis of Alzheimer’s disease were demonstrated in a sample of 312 brain donors.17 In the present sample of 103 brain donors (62 with Alzheimer’s disease), we observed similar associations and, for the first time, show that plasma p-tau181 levels signalled regional p-tau aggregation in areas such as the entorhinal cortex, hippocampus, amygdala, inferior parietal cortex and superior temporal cortex. These are important regions of neuropathological changes in Alzheimer’s disease and are affected early in the disease.2,34,39 This finding highlights the utility of plasma p-tau181 for early disease detection, which is necessary if blood-based biomarkers are to be used in clinical trials for primary prevention of Alzheimer’s disease.
Additional data from the present study and others suggest that plasma p-tau181 has potential use as a biomarker for the early detection of Alzheimer’s disease.4,5,7,15–18,40 As many studies show that amyloid deposits precede tauopathy in the brain for Alzheimer’s disease,2,41 our study shows that plasma p-tau181 was associated with CERAD neuritic plaque scores. Plasma p-tau181 discriminated Alzheimer’s disease from non-Alzheimer’s disease in participants who were either cognitively unimpaired or were rated as having MCI based on global CDR score at the time of the blood draw. Plasma p-tau181 prediction was superior in those who had a CDR of 1 or higher at the time of blood draw. However, AUC for those with a low CDR was still of acceptable discrimination. Supporting these findings, the Washington Heights–Inwood Columbia Aging Project showed that higher plasma p-tau181 values improved prediction of future clinical Alzheimer’s disease among participants without dementia at the time of first blood draw.4 Mielke et al.6 associated plasma p-tau181 with tau (on PET) in cognitively unimpaired participants or participants with only MCI. Plasma p-tau181 was recently shown to accurately discriminate Alzheimer’s disease from non-Alzheimer’s disease pathology from blood drawn 7.9 years prior to autopsy (mean ± SD 7.9 ± 1.2, range 6.3–9.4).16 Biomarker levels in that study increased across time points from 8 to 4 years before death, providing information on the longitudinal trajectory of plasma p-tau181 levels and demonstrating how the biomarker could be used to track progression of Alzheimer’s disease. In our sensitivity analysis, discrimination accuracy of p-tau181 for Alzheimer’s disease pathology was acceptable among brain donors who had a blood draw greater than 5 years before death, although there was higher discrimination accuracy among participants with blood draw less than 5 years from death and this might have been because these individuals had a higher CDR.
The present findings add to the literature for plasma p-tau181 as a putative risk biomarker to screen for Alzheimer’s disease and to enrich clinical trials for participants at high risk for Alzheimer’s disease. A potential use of biomarkers is to select for and enrol clinical trial participants that have no or subtle symptoms and are at a stage before pathology has advanced, where an early intervention may be more effective. In our models that included age, sex, APOE ε4 status and global CDR rating score, which are commonly collected and measured in clinic, plasma p-tau181 measurement greatly improved prediction of Alzheimer’s disease. This observation underscores the potential utility of measuring a putative Alzheimer’s disease blood biomarker, both for clinical trials and in the clinic, to better estimate risk.
There are limitations to the present findings. We did not explore trends in plasma p-tau181 levels longitudinally. Although plasma p-tau181 accurately detects Alzheimer’s disease at autopsy, the clinical meaning of a unit increase in raw pg/ml values of plasma p-tau181 is unclear. When plasma p-tau181 was standardized, the odds ratio for Alzheimer’s disease status substantially increased (OR = 2.98). Although standardizing plasma p-tau181 can facilitate interpretation and clarify the true association in this sample, raw plasma p-tau181 values were of the primary focus to facilitate comparison across studies and generalizability to the clinic. Non-fasting blood samples were collected. At this time, there are no formal recommendations to require fasting blood samples for plasma biomarker analysis of neurodegenerative disease proteins given the insufficient evidence to support its superiority. Additional research is needed to compare fasting and non-fasting samples on plasma biomarker assay analysis. The findings are limited to participants from a single clinical cohort, which introduces the potential for selection bias. The present sample is from a NIA-funded ADRC and is most representative of individuals who present to a clinic with concerns regarding their cognitive functioning. This population allows for development and validation of biomarkers, but inferences regarding risk and screening for Alzheimer’s disease in the general population cannot be made. The sample was demographically homogenous and a majority identified as white. Prospective population-based studies are needed to address these knowledge gaps and identify generalizable cut-off values that optimize sensitivity and specificity for the detection of Alzheimer’s disease.
Conclusion
The results of this study show an association between plasma p-tau181 levels and Alzheimer’s disease neuropathological changes at autopsy. With millions of individuals living with or at risk of developing Alzheimer’s disease, an increased understanding of accessible, cost-effective tools for evaluating disease diagnosis, including plasma p-tau181, is essential.
Supplementary Material
Abbreviations
- BU ADRC
Boston University Alzheimer’s Disease Research Center
- CDR
Clinical Dementia Rating
- CERAD
Consortium to Establish a Registry for Alzheimer’s Disease
- MCI
mild cognitive impairment
- NACC
National Alzheimer’s Coordinating Center
- p-tau181
phosphorylated-tau181
Contributor Information
Madeline S Morrison, Boston University Alzheimer’s Disease Research Center and CTE Center, Boston University School of Medicine, Boston, MA 02118, USA.
Hugo J Aparicio, Boston University Alzheimer’s Disease Research Center and CTE Center, Boston University School of Medicine, Boston, MA 02118, USA; Department of Neurology, Boston University School of Medicine, Boston, MA 02118, USA; Framingham Heart Study, Boston University School of Medicine, Boston, MA 02118, USA.
Kaj Blennow, Clinical Neurochemistry Laboratory, Sahlgrenska University Hospital, 413 45 Mölndal, Sweden; Department of Psychiatry and Neurochemistry, Institute of Neuroscience and Physiology, Sahlgrenska Academy at the University of Gothenburg, 413 90 Gothenburg, Sweden.
Henrik Zetterberg, Clinical Neurochemistry Laboratory, Sahlgrenska University Hospital, 413 45 Mölndal, Sweden; Department of Psychiatry and Neurochemistry, Institute of Neuroscience and Physiology, Sahlgrenska Academy at the University of Gothenburg, 413 90 Gothenburg, Sweden; Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London WC1N 3BG, UK; UK Dementia Research Institute at UCL, London WC1N 3BG, UK.
Nicholas J Ashton, Clinical Neurochemistry Laboratory, Sahlgrenska University Hospital, 413 45 Mölndal, Sweden; Department of Psychiatry and Neurochemistry, Institute of Neuroscience and Physiology, Sahlgrenska Academy at the University of Gothenburg, 413 90 Gothenburg, Sweden.
Thomas K Karikari, Clinical Neurochemistry Laboratory, Sahlgrenska University Hospital, 413 45 Mölndal, Sweden; Department of Psychiatry and Neurochemistry, Institute of Neuroscience and Physiology, Sahlgrenska Academy at the University of Gothenburg, 413 90 Gothenburg, Sweden.
Yorghos Tripodis, Boston University Alzheimer’s Disease Research Center and CTE Center, Boston University School of Medicine, Boston, MA 02118, USA; Department of Biostatistics, Boston University School of Public Health, Boston, MA 02118, USA.
Brett Martin, Boston University Alzheimer’s Disease Research Center and CTE Center, Boston University School of Medicine, Boston, MA 02118, USA; Biostatistics and Epidemiology Data Analytics Center, Boston University School of Public Health, Boston, MA 02118, USA.
Joseph N Palmisano, Boston University Alzheimer’s Disease Research Center and CTE Center, Boston University School of Medicine, Boston, MA 02118, USA; Biostatistics and Epidemiology Data Analytics Center, Boston University School of Public Health, Boston, MA 02118, USA.
Michael A Sugarman, Department of Neurology, Medical University of South Carolina, Charleston, SC 29425, USA.
Brandon Frank, Boston University Alzheimer’s Disease Research Center and CTE Center, Boston University School of Medicine, Boston, MA 02118, USA.
Eric G Steinberg, Boston University Alzheimer’s Disease Research Center and CTE Center, Boston University School of Medicine, Boston, MA 02118, USA.
Katherine W Turk, Boston University Alzheimer’s Disease Research Center and CTE Center, Boston University School of Medicine, Boston, MA 02118, USA; Department of Neurology, Boston University School of Medicine, Boston, MA 02118, USA; VA Boston Healthcare System, U.S. Department of Veteran Affairs, Jamaica Plain, MA 02130, USA.
Andrew E Budson, Boston University Alzheimer’s Disease Research Center and CTE Center, Boston University School of Medicine, Boston, MA 02118, USA; Department of Neurology, Boston University School of Medicine, Boston, MA 02118, USA; VA Boston Healthcare System, U.S. Department of Veteran Affairs, Jamaica Plain, MA 02130, USA.
Rhoda Au, Boston University Alzheimer’s Disease Research Center and CTE Center, Boston University School of Medicine, Boston, MA 02118, USA; Department of Neurology, Boston University School of Medicine, Boston, MA 02118, USA; Framingham Heart Study, Boston University School of Medicine, Boston, MA 02118, USA; Department of Anatomy & Neurobiology, Boston University School of Medicine, Boston, MA 02118, USA; Department of Epidemiology, Boston University School of Public Health, Boston, MA 02118, USA.
Lee E Goldstein, Boston University Alzheimer’s Disease Research Center and CTE Center, Boston University School of Medicine, Boston, MA 02118, USA; Department of Pathology and Laboratory Medicine, Boston University School of Medicine, Boston, MA 02118, USA; Department of Psychiatry, Boston University School of Medicine, Boston, MA 02118, USA; Department of Ophthalmology, Boston University School of Medicine, Boston, MA 02118, USA; Department of Biomedical Engineering, Boston University College of Engineering, Boston, MA 02215, USA; Department of Electrical and Computer Engineering, Boston University College of Engineering, Boston, MA 02215, USA.
Gyungah R Jun, Department of Medicine, Boston University School of Medicine, Boston, MA 02118, USA.
Neil W Kowall, Boston University Alzheimer’s Disease Research Center and CTE Center, Boston University School of Medicine, Boston, MA 02118, USA; Department of Neurology, Boston University School of Medicine, Boston, MA 02118, USA; VA Boston Healthcare System, U.S. Department of Veteran Affairs, Jamaica Plain, MA 02130, USA; Department of Pathology and Laboratory Medicine, Boston University School of Medicine, Boston, MA 02118, USA.
Ronald Killiany, Boston University Alzheimer’s Disease Research Center and CTE Center, Boston University School of Medicine, Boston, MA 02118, USA; Department of Neurology, Boston University School of Medicine, Boston, MA 02118, USA; Department of Anatomy & Neurobiology, Boston University School of Medicine, Boston, MA 02118, USA; Center for Biomedical Imaging, Boston University School of Medicine, Boston, MA 02118, USA.
Wei Qiao Qiu, Boston University Alzheimer’s Disease Research Center and CTE Center, Boston University School of Medicine, Boston, MA 02118, USA; Department of Psychiatry, Boston University School of Medicine, Boston, MA 02118, USA; Department of Pharmacology and Experimental Therapeutics, Boston University School of Medicine, Boston, MA 02118, USA.
Robert A Stern, Boston University Alzheimer’s Disease Research Center and CTE Center, Boston University School of Medicine, Boston, MA 02118, USA; Department of Neurology, Boston University School of Medicine, Boston, MA 02118, USA; Department of Anatomy & Neurobiology, Boston University School of Medicine, Boston, MA 02118, USA; Department of Neurosurgery, Boston University School of Medicine, Boston, MA 02118, USA.
Jesse Mez, Boston University Alzheimer’s Disease Research Center and CTE Center, Boston University School of Medicine, Boston, MA 02118, USA; Department of Neurology, Boston University School of Medicine, Boston, MA 02118, USA; Framingham Heart Study, Boston University School of Medicine, Boston, MA 02118, USA.
Ann C McKee, Boston University Alzheimer’s Disease Research Center and CTE Center, Boston University School of Medicine, Boston, MA 02118, USA; Department of Neurology, Boston University School of Medicine, Boston, MA 02118, USA; VA Boston Healthcare System, U.S. Department of Veteran Affairs, Jamaica Plain, MA 02130, USA; Department of Pathology and Laboratory Medicine, Boston University School of Medicine, Boston, MA 02118, USA; VA Bedford Healthcare System, U.S. Department of Veteran Affairs, Bedford, MA 01730, USA.
Thor D Stein, Boston University Alzheimer’s Disease Research Center and CTE Center, Boston University School of Medicine, Boston, MA 02118, USA; VA Boston Healthcare System, U.S. Department of Veteran Affairs, Jamaica Plain, MA 02130, USA; Department of Pathology and Laboratory Medicine, Boston University School of Medicine, Boston, MA 02118, USA; VA Bedford Healthcare System, U.S. Department of Veteran Affairs, Bedford, MA 01730, USA.
Michael L Alosco, Boston University Alzheimer’s Disease Research Center and CTE Center, Boston University School of Medicine, Boston, MA 02118, USA; Department of Neurology, Boston University School of Medicine, Boston, MA 02118, USA.
Funding
This work is supported by the NIA (P30AG072978), NINDS (U54NS115266) and through the BU-CTSI Grant Number 1UL1TR001430. M.L.A. is supported by the NINDS (K23NS102399). T.K.K. was funded by the Swedish Research Council (Vetenskåpradet), the Alzheimer’s Association Research Fellowship (#850325), the BrightFocus Foundation (#A2020812F), the International Society for Neurochemistry’s Career Development Grant, the Swedish Alzheimer Foundation (Alzheimerfonden; #AF-930627), the Swedish Brain Foundation (Hjärnfonden; #FO2020-0240), the Swedish Dementia Foundation (Demensförbundet), the Swedish Parkinson Foundation (Parkinsonfonden), Gamla Tjänarinnor Foundation, the Aina (Ann) Wallströms and Mary-Ann Sjöbloms Foundation, the Agneta Prytz-Folkes & Gösta Folkes Foundation (#2020-00124), the Gun and Bertil Stohnes Foundation and the Anna Lisa and Brother Björnsson’s Foundation. K.J. is supported by the Swedish Research Council (#2017-00915), the Alzheimer Drug Discovery Foundation (ADDF), USA (#RDAPB-201809-2016615), the Swedish Alzheimer Foundation (#AF-742881), Hjärnfonden, Sweden (#FO2017-0243), the Swedish state under the agreement between the Swedish government and the County Councils, the ALF-agreement (#ALFGBG-715986), the European Union Joint Program for Neurodegenerative Disorders (JPND2019-466-236), the National Institute of Health (NIH), USA (grant #1R01AG068398-01) and the Alzheimer’s Association 2021 Zenith Award (ZEN-21-848495). H.Z. is a Wallenberg Scholar supported by grants from the Swedish Research Council (#2018-02532), the European Research Council (#681712), Swedish State Support for Clinical Research (#ALFGBG-720931), the Alzheimer Drug Discovery Foundation (ADDF), USA (#201809-2016862), the AD Strategic Fund and the Alzheimer‘s Association (#ADSF-21-831376-C, #ADSF-21-831381-C and #ADSF-21-831377-C), the Olav Thon Foundation, the Erling-Persson Family Foundation, Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden (#FO2019-0228), the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 860197 (MIRIADE), European Union Joint Program for Neurodegenerative Disorders (JPND2021-00694) and the UK Dementia Research Institute at UCL. A.E.B. is supported by a Veterans Affairs Merit Award (CX001698) and National Institutes of Health grant P30 (AG072978). H.J.A. is supported by an American Academy of Neurology Career Development Award, Alzheimer’s Association (AARGD-20-685362), and National Institutes of Health (R01AG066524, NS017950, L30 NS093634). K.T. is supported by the United States Department of Veterans Affairs (#IK2 CX002065). R.A. is supported by the NIA (AG062109, AG063635, AG068753). T.S. is supported by the United States Department of Veterans Affairs Merit Award (I01-CX001038).
Competing interests
K.J. has served as a consultant, at advisory boards, or at data monitoring committees for Abcam, Axon, BioArctic, Biogen, JOMDD/Shimadzu, Julius Clinical, Lilly, MagQu, Novartis, Pharmatrophix, Prothena, Roche Diagnostics and Siemens Healthineers, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program, outside the work presented in this paper. H.K. has served at scientific advisory boards and/or as a consultant for Abbvie, Alector, Annexon, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Pinteon Therapeutics, Red Abbey Labs, Passage Bio, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics and Wave; has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen and Roche; and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. A.E.B. has served on consultant or advisory boards for Sage Pharmaceuticals and Cognito Therapeutics and has received grant monies from Biogen, Bristol Myers Squibb and Cyclerion. He receives publishing royalties from Elsevier and Oxford University Press. R.A. serves on the scientific advisory board of Signant Health, as consultant to Biogen and has given a lecture in a symposia sponsored by Eisai. R.A.S. has served as a consultant to Biogen and Lundbeck. He receives royalties for published neuropsychological tests from Psychological Assessment Resources, Inc.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging–Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jack CR, Bennett DA, Blennow K, et al. NIA-AA Research framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zetterberg H, Blennow K. Blood biomarkers: Democratizing Alzheimer’s diagnostics. Neuron. 2020;106:881–883. [DOI] [PubMed] [Google Scholar]
- 4. Brickman AM, Manly JJ, Honig LS, et al. Plasma p-tau181, p-tau217, and other blood-based Alzheimer’s disease biomarkers in a multi-ethnic, community study. Alzheimers Dement. 2021;17:1353–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Janelidze S, Mattsson N, Palmqvist S, et al. Plasma P-tau181 in Alzheimer’s disease: Relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat Med. 2020;26:379–386. [DOI] [PubMed] [Google Scholar]
- 6. Mielke MM, Frank RD, Dage JL, et al. Comparison of plasma phosphorylated tau species with amyloid and tau positron emission tomography, neurodegeneration, vascular pathology, and cognitive outcomes. JAMA Neurol. 2021;78:1108–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thijssen EH, La Joie R, Wolf A, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat Med. 2020;26:387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bayoumy S, Verberk IMW, den Dulk B, et al. Clinical and analytical comparison of six Simoa assays for plasma P-tau isoforms P-tau181, P-tau217, and P-tau231. Alzheimers Res Ther. 2021;13:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Blennow K, Mattsson N, Schöll M, Hansson O, Zetterberg H. Amyloid biomarkers in Alzheimer’s disease. Trends Pharmacol Sci. 2015;36:297–309. [DOI] [PubMed] [Google Scholar]
- 10. Clark CM, Pontecorvo MJ, Beach TG, et al. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: A prospective cohort study. Lancet Neurol. 2012;11:669–678. [DOI] [PubMed] [Google Scholar]
- 11. Fleisher AS, Pontecorvo MJ, Devous MD Sr, et al. Positron emission tomography imaging with [18F]flortaucipir and postmortem assessment of Alzheimer disease neuropathologic changes. JAMA Neurol. 2020;77:829–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ikonomovic MD, Klunk WE, Abrahamson EE, et al. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain. 2008;131:1630–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thal DR, Beach TG, Zanette M, et al. [18F]flutemetamol amyloid positron emission tomography in preclinical and symptomatic Alzheimer’s disease: Specific detection of advanced phases of amyloid-β pathology. Alzheimers Dement. 2015;11:975–985. [DOI] [PubMed] [Google Scholar]
- 14. Olsson B, Lautner R, Andreasson U, et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: A systematic review and meta-analysis. Lancet Neurol. 2016;15:673–684. [DOI] [PubMed] [Google Scholar]
- 15. Ashton NJ, Pascoal TA, Karikari TK, et al. Plasma p-tau231: A new biomarker for incipient Alzheimer’s disease pathology. Acta Neuropathol. 2021;141:709–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lantero Rodriguez J, Karikari TK, Suárez-Calvet M, et al. Plasma p-tau181 accurately predicts Alzheimer’s disease pathology at least 8 years prior to post-mortem and improves the clinical characterisation of cognitive decline. Acta Neuropathol. 2020;140:267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smirnov DS, Ashton NJ, Blennow K, et al. Plasma biomarkers for Alzheimer’s Disease in relation to neuropathology and cognitive change. Acta Neuropathol. 2022;143:487–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grothe MJ, Moscoso A, Ashton NJ, et al. Associations of fully automated CSF and novel plasma biomarkers with Alzheimer disease neuropathology at autopsy. Neurology. 2021;97:e1229–e1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Frank B, Ally M, Brekke B, et al. Plasma p-tau181 shows stronger network association to Alzheimer’s disease dementia than neurofilament light and total tau. Alzheimers Dement. Published online 2 December 2021. doi:10.1002/alz.12508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Karikari TK, Pascoal TA, Ashton NJ, et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: A diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 2020;19:422–433. [DOI] [PubMed] [Google Scholar]
- 21. Vonsattel JPG, Aizawa H, Ge P, et al. An improved approach to prepare human brains for research. J Neuropathol Exp Neurol. 1995;54:42–56. [DOI] [PubMed] [Google Scholar]
- 22. Vonsattel JPG, del Amaya MP, Keller CE. Twenty-first century brain banking. Processing brains for research: The Columbia University methods. Acta Neuropathol. 2008;115:509–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Besser LM, Kukull WA, Teylan MA, et al. The revised National Alzheimer’s Coordinating Center’s neuropathology form—Available data and new analyses. J Neuropathol Exp Neurol. 2018;77:717–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mock C, Teylan M, Beecham G, et al. The utility of the National Alzheimer’s Coordinating Center’s database for the rapid assessment of evolving neuropathologic conditions. Alzheimer Dis Assoc Disord. 2020;34:105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Beekly DL, Ramos EM, van Belle G, et al. The National Alzheimer’s Coordinating Center (NACC) database: An Alzheimer disease database. Alzheimer Dis Assoc Disord. 2004;18:270–277. [PubMed] [Google Scholar]
- 26. NACC Researchers Data Dictionary—The Neuropathology (NP) Data Set. 2016. Accessed 9 July 2018. https://www.alz.washington.edu/NONMEMBER/NP/rdd_np.pdf
- 27. Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease . The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease. Neurobiol Aging. 1997;18:S1–2. [PubMed] [Google Scholar]
- 28. Bigio EH. Update on recent molecular and genetic advances in frontotemporal lobar degeneration. J Neuropathol Exp Neurol. 2008;67:635–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cairns NJ, Neumann M, Bigio EH, et al. TDP-43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. Am J Pathol. 2007;171:227–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dickson DW. Neuropathology of non-Alzheimer degenerative disorders. Int J Clin Exp Pathol. 2009;3:1–23. [PMC free article] [PubMed] [Google Scholar]
- 31. Litvan I, Hauw JJ, Bartko JJ, et al. Validity and reliability of the preliminary NINDS neuropathologic criteria for progressive supranuclear palsy and related disorders. J Neuropathol Exp Neurol. 1996;55:97–105. [DOI] [PubMed] [Google Scholar]
- 32. Mackenzie IRA, Neumann M, Bigio EH, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: An update. Acta Neuropathol. 2010;119:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. [DOI] [PubMed] [Google Scholar]
- 34. Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. [DOI] [PubMed] [Google Scholar]
- 35. Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry J Ment Sci. 1982;140:566–572. [DOI] [PubMed] [Google Scholar]
- 36. Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2412-a. [DOI] [PubMed] [Google Scholar]
- 37. Hosmer DW, Lemeshow S, Sturdivant RX. Applied logistic regression: Wiley & Sons; 2000. [Google Scholar]
- 38. Teunissen CE, Verberk IMW, Thijssen EH, et al. Blood-based biomarkers for Alzheimer’s disease: Towards clinical implementation. Lancet Neurol. 2021;21:66–77. [DOI] [PubMed] [Google Scholar]
- 39. Marks SM, Lockhart SN, Baker SL, Jagust WJ. Tau and β-amyloid are associated with medial temporal lobe structure, function, and memory encoding in normal aging. J Neurosci. 2017;37:3192–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Palmqvist S, Janelidze S, Quiroz YT, et al. Discriminative accuracy of plasma phospho-tau217 for Alzheimer disease vs other neurodegenerative disorders. JAMA. 2020;324:772–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging–Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All uniform and neuropathology data set evaluation data are shared with the NACC and are publicly available. Data are also available upon reasonable request to the BU ADRC.