Abstract
The common intersection of autism and transgender identities has been described in clinical and community contexts. This study investigates autism-related neurophenotypes among transgender youth. Forty-five transgender youth, evenly balanced across non-autistic, slightly subclinically autistic, and full-criteria autistic subgroupings, completed resting-state functional magnetic resonance imaging to examine functional connectivity. Results confirmed hypothesized default mode network (DMN) hub hyperconnectivity with visual and motor networks in autism, partially replicating previous studies comparing cisgender autistic and non-autistic adolescents. The slightly subclinically autistic group differed from both non-autistic and full-criteria autistic groups in DMN hub connectivity to ventral attention and sensorimotor networks, falling between non-autistic and full-criteria autistic groups. Autism traits showed a similar pattern to autism-related group analytics, and also related to hyperconnectivity between DMN hub and dorsal attention network. Internalizing, gender dysphoria, and gender minority-related stigma did not show connectivity differences. Connectivity differences within DMN followed previously reported patterns by designated sex at birth (i.e. female birth designation showing greater within-DMN connectivity). Overall, findings suggest behavioral diagnostics and autism traits in transgender youth correspond to observable differences in DMN hub connectivity. Further, this study reveals novel neurophenotypic characteristics associated with slightly subthreshold autism, highlighting the importance of research attention to this group.
Keywords: Autistic, Gender diverse, Resting state
Introduction
The common overlap between autism, elevated autistic traits, and gender identity diversity has been reported in both clinical (de Vries et al. 2010; Hisle-Gorman et al. 2019) and community (Strauss et al. 2017; Walsh et al. 2018) samples. In the largest study to date, the odds of an autism diagnosis were more than six times greater among transgender (or broadly gender-diverse) individuals than among those who were cisgender (Warrier et al. 2020). A recent metanalysis of all available studies estimated that approximately 11% of transgender and gender diverse individuals are autistic (Kallitsounaki and Williams 2022). Further, up to 13% of autistic adults identify as a gender different than their designated sex at birth (Walsh et al. 2018). Hypotheses regarding factors underpinning the common intersection of gender diversity and autism range from potential prenatal sex hormone pathways (Bejerot et al. 2012) to autistic thinking styles less swayed by socialized gender expectations (Furlong 2021). Given the clinical complexity of supporting autistic transgender individuals (Strang et al. 2016), as well as increased acknowledgment of the importance of parsing the heterogeneity of autism according to key subgroups (Eckerd 2020; Strang et al. 2021a), investigations into autistic transgender phenotypes and endophenotypes are needed. Initial behavioral work has identified common patterns of: (1) highly increased mental health risks in autistic transgender individuals (George and Stokes 2018; Strang et al. 2021a) and (2) experience of specific, profound and impairing barriers to receiving appropriate supports and care (Strang et al. 2018; Strang et al. 2021a). The research and clinical communities currently lack any information regarding autistic gender-diverse neurophenotypes. Characterizing these neurophenotypes is essential given that: (1) autism and gender diversity often co-occur, (2) there is widespread interest in employing neuroimaging approaches to advance targeted supports for autistic people (Chen and Van Horn 2017; Loth et al. 2017) and (3) appropriate inclusion and modeling of autistic gender-diverse people in neuroimaging will require knowledge of autistic transgender neurophenotypes.
In this study, we begin to address this critical gap by examining neurophenotypic characteristics of autistic transgender individuals, focusing on functional connectivity (FC) of two hubs of the default mode network (DMN) in autistic and non-autistic transgender youth via resting-state functional magnetic resonance imaging (rsfMRI). Network FC during rsfMRI captures spontaneous low-frequency (< 0.1 Hz) fluctuations in the blood-oxygen-level-dependent signal in the absence of a task (Fox and Raichle 2007). Spatially distributed brain regions that demonstrate correlated patterns of activity over the fMRI time-course are considered functionally connected networks (Biswal et al. 1995). Studies of resting-state FC have identified key brain networks, where regions that are implicated in related functions and are active during tasks probing those functions also show co-activation at rest and are therefore understood to form a network and represent the intrinsic brain functional architecture (Fox and Raichle 2007). The default mode network (DMN) is one of the most prominent of the FC networks (Raichle et al. 2001; Greicius et al. 2003). The DMN has been referred to as a “task-negative network,” demonstrating decreased activity during cognitively demanding tasks, but increased activation in the absence of a task, where activation at rest is associated with internally-oriented processing, or the integration of information about the self with information of stimulus-related or externally-oriented processing (Yeshurun et al. 2021). Key regions of the DMN include the medial prefrontal cortex (MPFC; including ventromedial and dorsomedial PFC) and posterior cortex (including posterior cingulate cortex [PCC] and lateral parietal cortex) (Buckner and DiNicola 2019). Importantly, it is not clear whether systematic differences in DMN-related FC may reflect functionally meaningful and/or experiential differences between minds at rest (Cole et al. 2010).
Amid the rich and often contradictory literature on FC in autism, the DMN, internally and as connected to other brain networks, has been frequently and consistently implicated (e.g. Haghighat et al. 2021; Monk et al. 2009; Wang et al. 2021; Wang et al. 2017; Washington et al. 2014; Weng et al. 2010; for review, see Padmanabhan et al. 2017), although the exact nature of autistic versus non-autistic group differences in DMN coupling varies across reports. This variability appears related to demographic, phenotypic, and developmental factors; for instance, studies at various developmental stages show a range of specific patterns (Weng et al. 2010; Washington et al. 2014; Lawrence et al. 2019). Overall, DMN FC differences by designated sex at birth appear reduced in studies of autistic people (Ypma et al. 2016; Olson et al. 2020), though there is evidence that pre-adolescent and adolescent autistic youth assigned female at birth may demonstrate stronger FC between DMN and the central executive network than those assigned male at birth (Lawrence et al. 2020a). Across non-autistic samples, there is also evidence for possible transgender-specific DMN FC differences (Nota et al. 2017a; Nota et al. 2017b; Uribe et al. 2020; Skorska et al. 2022). Thus, the DMN is a promising candidate for a neurofunctional system to help parse heterogeneity within the autism spectrum, and for autistic transgender people specifically.
The study has four aims and primary hypotheses driven by essential research questions related to this population. Specifically, there is emerging evidence for at least three salient autism-related classifications within transgender youth: those who are non-autistic; those who are slightly subclinically autistic (i.e. highly elevated autistic traits/symptoms, but slightly subthreshold in terms of autism diagnosis); and those who meet full criteria for autism (Ruzich et al. 2016; Strang et al. 2021a). Therefore, Aim 1 investigates DMN FC in youth carefully characterized and balanced across these three autism-related statuses. Further, given evidence of elevations of autism traits, apart from a categorical autism diagnosis, in transgender populations (Akgül et al. 2018), Aim 2 uses a dimensional approach to investigate how DMN FC relates to individual autistic trait level. In response to previous postulations that autistic-like traits in transgender people are driven by underlying internalizing psychopathology or gender diversity-related stress (Turban and van Schalkwyk 2018; Leef et al. 2019), Aim 3 explores associations between DMN FC and internalizing psychopathology, gender dysphoria, and perceived sexual/gender-minority (SGM)-related stigma. Finally, given preliminary findings of possible differentiation of DMN FC by gender within transgender youth (Nota et al. 2017b), gender-related DMN FC is explored in Aim 4. Considering that previous work has found autism-related differences both in within-network DMN FC, and between DMN hubs and other FC networks (e.g. Lawrence et al. 2019; Floris et al. 2020; Haghighat et al. 2021), we investigate DMN hub to whole-brain FC across the four aims:
DMN-to-whole-brain FC by autism diagnostic status (Aim 1)
Autistic versus non-autistic FC differences have been reported between DMN hubs and visual, executive control, and motor/sensorimotor networks during mid-late adolescence (Washington et al. 2014; Abbott et al. 2016; Mash et al. 2019). These FC differences have been in the direction of hyperconnectivity in autism compared to neurotypical controls. Some findings of autism-related hypoconnectivity have also been reported between DMN hubs and non-DMN regions, but these findings were in a younger age group (Olson et al. 2020). Therefore, in the present study of transgender youth, we hypothesize hyperconnectivity in autism between DMN hubs and regions within the visual, executive control, and motor/sensorimotor networks. Specifically, we predict that the full-criteria autistic group will show greater DMN-based whole-brain FC than the slightly subclinical group, and the slightly subclinical group will show greater FC than the non-autistic group.
Although not fully consistent across the literature, a pattern has emerged suggesting hyperconnectivity within DMN for younger autistic children followed by a developmental transition away from hyperconnectivity in autistic adolescents and adults (Nomi and Uddin 2015; Ypma et al. 2016; Haghighat et al. 2021). Therefore, given the adolescent to young adult age range of our sample, we hypothesize no, or relatively few, within-DMN FC differences based on autism-related status.
Relationships between DMN-to-whole-brain FC and autism trait level (Aim 2)
To our knowledge, two studies have found relationships between measures of autism traits and DMN FC (Lynch et al. 2013; Yerys et al. 2015). Extrapolating from these studies to the current study presents some challenges, as the existing studies were conducted with younger samples and only included autism trait analyses for individuals diagnosed with autism. The current study includes a sample reflecting a broad range of autistic trait levels (i.e. across both non-autistic and autistic adolescents). We hypothesize that in transgender youth, higher autism trait levels will be related to increased FC between DMN hubs and temporal, occipital, and parahippocampal regions (based on prior DMN hub-to-whole-brain findings; Lynch et al. 2013). We also hypothesize overlap with regions identified in the analyses described above in Aim 1. Finally, as in Aim 1, we hypothesize no, or relatively few, within-DMN FC differences based on level of autism traits.
DMN FC by internalizing, gender dysphoria, and SGM-related stigma (Aim 3—exploratory)
This aim serves as a corollary to Aims 1 and 2. Given the principle of equifinality, behavioral autistic-like traits in transgender people could theoretically stem from underlying internalizing psychopathology or gender diversity-related stress instead of autism, per se (Cicchetti and Rogosch 1996; Turban and van Schalkwyk 2018; Leef et al. 2019). Therefore, this exploratory aim investigates—in a parallel manner to Aims 1 and 2—DMN hub-to-whole-brain FC associations with each of the following: emotional internalizing psychopathology, gender dysphoria, and perceived SGM-related stigma.
DMN FC by gender identity (Aim 4—exploratory)
If transgender youth follow DMN-related FC patterns typical of their designated sex, youth assigned female at birth will show greater within-DMN FC than youth assigned male at birth (Bluhm et al. 2008; Tomasi and Volkow 2012; Olson et al. 2020). There is some evidence from studies in younger youth that there may be greater FC from key DMN regions to primary visual, primary sensorimotor, and secondary sensorimotor regions in individuals assigned male at birth (Olson et al. 2020). It is unclear whether these patterns are present in older adolescent populations or in transgender youth. One existing resting-state FC study of transgender adolescents (Nota et al. 2017b) reported that transgender adolescent young women showed lower within-DMN FC than cisgender adolescent young men. Interestingly, this difference was not observed in adult transgender women compared to adult transgender men (Bluhm et al. 2008; Nota et al. 2017a; Uribe et al. 2020). In a different line of research, autism has been associated with reduced DMN FC sex-based differences compared to non-autistic samples (Olson et al. 2020; Ypma et al. 2016; though it must be noted that Lawrence and colleagues found otherwise; Lawrence et al. 2020b). Thus, accounting for autistic traits may allow gender-related differences to be more clearly observed.
Given gaps in the literature and the research novelty of this study population, we make no specific hypothesis regarding gender-based differences (female versus male) in DMN hub to whole-brain FC among transgender youth. However, we hypothesize that including level of autism traits in the model will reveal greater gender-related differences in DMN FC as compared to a model not accounting for autism traits.
Materials and methods
Participants and characterization
As is the standard for respectful communication regarding transgender persons, when we use the term gender, we refer to the participant’s affirmed gender identity, not their designated sex at birth (American Psychological Association 2015). This was a two-stage study employing targeted sampling to achieve an even distribution of autism symptomatology within the sample ranging from an absence of autism symptoms to high levels of autism symptoms. Sixty-six transgender binary youth (i.e. trans young men or trans young women), aged 13–21 years, were enrolled through clinic and community recruitments (see Strang et al. 2021a for greater detail). Nonbinary transgender youth were not included given the limits of sample size for the recruitment; including a nonbinary comparison group would have required a significantly larger sample. This study was conducted in accordance with the Declaration of Helsinki. According to the IRB protocol for the study, approved by Children’s National Hospital, youth age 13–17 years provided written assent and their parent/guardian provided written consent; youth age 18–21 years provided written consent. A combination of clinic and community recruitment approaches aimed to increase sample representativeness; previous studies employing gold-standard ASD diagnostics in gender-diverse youth have been limited to recruitment through clinic referrals only (Strang et al. 2018; Strang et al. 2020). The age range was selected in accordance with reports that transgender youth, and especially autistic transgender youth, often do not communicate their gender diversity to others until their adolescent years (Grossman and D'Augelli 2006) and often not until their late adolescent years (Strang et al. 2018). Two separate recruitment criteria were employed to diversify the sample by autism status: (1) transgender binary youth with no suspected autism and (2) transgender binary youth with suspected or confirmed autism.
Within this recruitment approach, targeted sampling was used to recruit equal numbers of youth in each of three categories: transgender binary youth with confirmed autism, transgender binary youth with suspected autism, and transgender binary youth with no suspected autism. Intellectual ability falling in the range of intellectual disability (ID; i.e. IQ scores falling below 70) was an exclusion criterion, given that the self-report measures in the study have been developed for and validated in samples without an ID. Sixty-six transgender binary youth without ID were enrolled through the above-described recruitment approach.
Following comprehensive autism clinical assessments, which included the Autism Diagnostic Observation Schedule 2nd edition Module 4 (Lord et al. 2012), the Autism Diagnostic Interview-Revised (conducted with parents/caregivers) (Lord et al. 1994), and clinical DSM-5 autism-based interviewing, the 66 enrolled youth were categorized based on categorical autism status: no autism; slightly subclinical autism; and full-criteria autism. Importantly, previous work in the field of intersectional gender diversity and autism has described the common profile of transgender youth who are slightly subclinical (i.e. slightly below autism diagnostic cutoffs) (Strang et al. 2018). The broader literature highlights the intersection of autism and gender diversity at the continuous trait level (Nabbijohn et al. 2019; Kallitsounaki and Williams 2020a, 2020b; Kallitsounaki et al. 2021), suggesting that the two characteristics are linked in the population even for those who do not meet full diagnostic criteria for ASD. The slightly subclinical autistic group, hereafter referred to as “subclinically autistic,” was defined as meeting all of the following conditions: (1) clinically suspected of being autistic but not meeting full DSM-5 ASD criteria (American Psychiatric Association 2022); (2) meeting criteria for at least two DSM-5 ASD symptoms, including at least one in the social-communication domain; and (3) current ASD symptoms were not better explained by another condition.
Following the comprehensive ASD diagnostic procedures, 46 of the youth then completed the neuroimaging protocol. These youth participated in neuroimaging based on the following sequence of determinations: (1) exclusion based on presence of dental work or unremovable metal jewelry (n = 8 of the 66 clinically assessed); (2) exclusion based on lack of interest in completing an MRI scan due to personal reasons (e.g. claustrophobia; n = 3); (3) optimization of imaging sample to balance gender identity (i.e. as equal as possible numbers of trans young men and trans young women); and (4) optimization of imaging sample to balance the three autism-related groupings (i.e. non-autistic; subclinically autistic; full-criteria autistic). Of the 46 attempted scans, 45 were successfully completed; one participant (an autistic trans young woman) terminated the scan after approximately 5 minutes in the scanner due to claustrophobia. See Table 1 for demographic characteristics of the imaged sample. See Supplementary Table 1 for diagnostic characteristics: https://osf.io/tcqex/?view_only=e7330358ecec49e391f3ff28fcd37f4e. The sample was slightly uneven in its distribution by gender (i.e. more trans young men than trans young women), a common feature in transgender youth recruitments (Cooper et al. 2018). It was nearly even in terms of categorical autism-related groupings.
Table 1.
Sample characterization.
| Non-autistic n = 16 | Sub-clinical autistic n = 14 | Autistic n = 15 | Test of differences | |
|---|---|---|---|---|
| Age (years) M (SD) | 17.65 (1.97) | 18.10 (2.20) | 18.02 (2.13) | F(2,42) = 0.21 P = 0.81 |
| IQ estimate (Full scale IQ) M (SD) | 115.88 (15.71) | 119.79 (19.14) | 116.13 (11.91) | F(2,42) = 0.28 P = 0.76 |
| Gender (% female/male) | 25/75 (n = 4/12) | 43/57 (n = 6/8) | 60/40 (n = 9/6) | χ2(2) = 3.89 P = 0.14 |
| Race (%) | ||||
| Asian | 6.3 (n = 1) | 0 (n = 0) | 20.0 (n = 3) | χ2(2) = 3.79 P = 0.15 |
| Black or African American | 0 (n = 0) | 7.1 (n = 1) | 6.7 (n = 1) | χ2(2) = 1.16 P = 0.56 |
| White | 93.8 (n = 15) | 92.9 (n = 13) | 86.7 (n = 13) | χ2(2) = 0.56 P = 0.76 |
| More than one reported | 6.3 (n = 1) | 0 (n = 0) | 13.3 (n = 2) | χ2(2) = 2.03 P = 0.36 |
| Ethnicity (% Hispanic or Latina,o,e) | 6.3 (n = 1) | 0 (n = 0) | 0 (n = 0) | χ2(2) = 1.79 P = 0.41 |
| Parent education (years) M (SD) | 16.41 (2.53) | 17.46 (2.50) | 16.80 (2.44) | F(2,42) = 0.68 P = 0.51 |
To reduce recruitment bias, participants were not selected based on gender-affirming medication status. International guidance recommends a slower, more cautious approach for gender-related medical decision-making in autistic gender-diverse youth (Strang et al. 2016); recruitment of a sample based on whether a young person had been approved for, or was receiving, gender-affirming hormones (GAH) would likely introduce an age and developmental bias into the sample (i.e. older autistic transgender youth compared to younger non-autistic transgender youth). Because the focus of this study was resting-state FC with DMN hubs, which varies with age (Nomi and Uddin 2015; Ypma et al. 2016; Haghighat et al. 2021), but not by GAH status (Nota et al. 2017b), recruitment was intentionally agnostic to GAH treatment status. To confirm a lack of FC variation based on GAH treatment status, testosterone treatment status (for trans young men) and estrogen treatment status (for trans young women) were compared by FC (see Data analytic plan below).
Measures
This study employed autism diagnostic measures, autism trait-related measures, and measures of mental health and gender-related experience. A composite score for overall multi-modal, multi-informant autistic traits was derived from the autism diagnostic and autism trait-related instruments (see Data analytic plan below).
Autism diagnostic measures
The Autism Diagnostic Observation Schedule 2nd edition (ADOS-2) is a gold-standard autism diagnostic measure. It includes semi-structured tasks and interview questions which are coded across a range of behavioral indicators on a three-point scale. Employing the algorithm updated by Hus and colleagues, the measure produces two total severity scores: Social Communication and Restricted and Repetitive Behaviors and Interests (Lord et al. 2012; Hus and Lord 2014). In this study, the ADOS-2 was used as part of the autism-diagnostic procedures, as well as in the composite autism traits score.
The Autism Diagnostic Interview-Revised (ADI-R; Lord et al. 1994) is a gold-standard parent/caregiver interview of autism symptoms and developmental history assessing key autism-related developmental characteristics from childhood, including communication, social development, and repetitive and restrictive behaviors. In this study, all ADI-R Domains (i.e. A Domain [Qualitative Abnormalities in Reciprocal Social Interaction], B Domain [Qualitative Abnormalities in Communication], C Domain [Restricted, Repetitive, and Stereotyped Patterns of Behavior], and D Domain [Abnormality of Development Evident at or Before 36 months]) were used as part of the autism-diagnostic procedures, as well as in the composite autism traits score.
Autistic trait-related measures
The Social Responsiveness Scale-2 (SRS-2; Constantino and Gruber 2012) is a parent-report scale assessing the severity of current real-world autism-related symptoms. Responses are tallied as a Total Score, where higher scores indicate higher level of autism characteristics. The SRS-2 calibration reports good reliability, validity, and internal consistency for the Total Score (Constantino and Gruber 2012); the Total Score is strongly correlated with autism diagnosis. The SRS-2 Total raw score was included in the composite autism traits score; members of our team have previously employed the SRS-2 raw score, instead of the standard score, to avoid the sex-based autism norms which are not validated in transgender populations (Grannis et al. 2021).
The Broad Autism Phenotype Questionnaire (BAPQ; Hurley et al. 2007) is a self-report scale assessing autism-related traits. The total BAPQ score is created by summing the scores across all 36 items. The BAPQ has demonstrated good sensitivity and specificity in detecting broad autism phenotypic characteristics (Hurley et al. 2007). The BAPQ total score was used as part of the composite autism traits score. The BAPQ has been employed with children, adolescents, and adults (Hurley et al. 2007; Suh et al. 2016; Sabatino DiCriscio and Troiani 2018).
The Reading the Mind in the Eyes Test (RMET) is a task designed to assess mental state recognition; the version employed here was designed for children and adolescents (Baron-Cohen et al. 2001a) and has previously been used in neuroimaging studies with adolescents (e.g. Baribeau et al. 2019). The participant views gray-scale photos of the eye region of various people’s faces, one face at a time, with each image surrounded by four words describing mental states; they are asked to select the word that describes what the person in the photo is thinking or feeling. The RMET score is computed by calculating the percent correct out of 28, the total number of questions. A higher score indicates a greater ability for mental state recognition. RMET scores relate to categorical autism diagnosis (Baron-Cohen et al. 2001a; Brent et al. 2004) and autistic traits (Baron-Cohen et al. 2001b) and were used as part of the composite autism trait score.
Emotional internalizing psychopathology measure
The Achenbach System of Empirically Based Assessment (ASEBA) Self Report (YSR/ASR; Achenbach 2009) is a well-validated standardized self-report questionnaire that measures broad emotional and behavioral problems. Summary scores are generated based on standardization samples, with higher scores representing more problems. In the current study, we employed the summary score for Internalizing Problems, reflecting broad experiences of internalized emotional problems, including symptoms of both anxiety and depression.
Gender dysphoria measure
The Utrecht Gender Dysphoria Scale (UGDS) assesses the experience of gender dysphoria (i.e. incongruence and distress regarding an inconsistency between one’s experienced gender identity and assigned sex at birth), including physical and identity-related aspects of dysphoria (Cohen-Kettenis and van Goozen 1997). Scores range from 12–60, with higher scores related to greater gender dysphoria. Using a cut-point of 40, sensitivity is high for identifying gender-referred youth (88.3%) and differentiating non-referred youth (specificity = 99.5%; Steensma et al. 2013).
Sex and gender minority stigma measure
The LGBT Stigma Scale (LGBTSS) is a self-report scale that evaluates the level of behaviors and interactions involving LGBT rejection and stigma experienced by an individual at the family and community levels (Weinhardt et al. 2017). Higher scores on the Perceived Stigma subscale reflect greater perceived stigma, including perceptions of feeling unsafe due to one’s LGBT identity. The Perceived Stigma scale has been validated in transgender youth and demonstrates strong internal reliability (Cronbach’s α = 0.84); specifically, higher scores are strongly related to everyday experiences of gender diversity-related stigma and bias (Weinhardt et al. 2017).
Confirmation of absence of intellectual disability
The Wechsler Abbreviated Scale of Intelligence, Second Edition (WASI-II), an IQ assessment measure, confirmed the absence of ID (Wechsler and Zhou 2011). The Vocabulary and Matrix Reasoning subtests were administered to calculate a two-subtest Full-Scale IQ (FSIQ) for all participants.
Imaging procedures
Images were acquired using the 3T Magnetom Prisma (Siemens, Erlangen, Germany) scanner at Georgetown University in Washington, D.C. Using a gradient echo pulse sequence, 346 volumes were acquired in a single run with a repetition time (TR) = 1390 ms, echo time (TE) = 30 ms, flip angle = 70°, FOV = 205 mm, voxel size 2.5x2.5x2.5 mm, 48 transverse slices and 2.5 mm isotropic resolution, for a total of 8 minutes. A high-resolution T1-weighted structural magnetization-prepared rapid-acquisition gradient echo scan for normalization purposes was also acquired (voxel size 1 × 1 × 1 mm, TR = 2530 ms, TE = 3.34 ms, inversion time = 1100 ms, flip angle = 7°, total scan time 7 min 26 s). Participants were instructed to remain awake and relaxed with their eyes open and to focus on a white fixation point on a projection screen. Resting-state fMRI data were preprocessed through afni_proc.py using a 4 mm full-width-half-maximum smoothing kernel (Taylor et al. 2018). A seed-based analytic approach was used with two canonical DMN regions of interest (ROIs) previously identified as differentiating autistic versus non-autistic youth in resting-state fMRI (Yerys et al. 2015). Two DMN seeds were defined (via AFNI’s 3dUndump) as 4 mm radius spheres centered around MNI coordinates identified in a large N study that established reliability of DMN FC (Van Dijk et al. 2009); specifically, the PCC (0, −53, 6) and MPFC (0, 52, −6). The average time course of the seed was then obtained. Frames with framewise displacement (FD) > 0.5 mm and outliers greater than 5% of the brain mask were censored (Salek-Haddadi et al. 2006); all participants had at least 310 volumes. Pearson’s correlations were calculated between the seed time course and the timeseries of other voxels in the brain in a voxel-wise manner. Seed-based analysis identifies the most intensely altered FC patterns for each seed (i.e. differences which survive multiple comparison corrections), and facilitates cross-study comparison (Yerys et al. 2015).
Data analytic plan
Resting-state data from completed scans (n = 45) were analyzed as follows. For each seed (i.e. MPFC and PCC), whole-brain FC was computed separately with 3dClustSim correction by AFNI to account for multiple comparisons (voxel P < 0.001, k > 31). GLMFlex was used for statistical analyses (Schultz 2020). Schaefer’s seven network atlas was used to determine network labeling for each cluster (Schaefer et al. 2018). Aim 1 employed one-way ANCOVA on whole-brain FC, with age, gender, and head motion (mean framewise displacement) as covariates; post hoc comparisons examined differences in autism-related groupings. Global signal was not regressed.
Aim 2 employed multiple regression analysis of whole-brain FC, with FC regressed onto the composite autism trait score; age, gender, and head motion were included as covariates. A multi-informant, multi-measure autism trait level composite has been employed previously in studies of autism to merge complementary multi-modal measurements of autism into a continuous gestalt metric (Lopez et al. 2005; Black et al. 2009; Kenworthy et al. 2009). The composite autism trait score combines the ADI-R Domain value (i.e. developmental signals of autism), ADOS-2 Severity scores (i.e. in vivo coded behavioral characteristics of autism), DSM-5 ASD symptom count (i.e. expert clinician global assessment of autism symptoms based on all available information), SRS-2 Total score (i.e. parent report of real-world autism-related youth behaviors), BAPQ (i.e. self-report of autistic features), and RMET child/adolescent scores (i.e. phenotypic social cognition performance task related to autism) through principal component factor analytics with mean-centered scores. See the study’s supplement for details: https://osf.io/9c87e/?view_only=6250c84e45f6455da2c44253bd5d9663. Prior to running Aim 2 regression analytics, we examined the composite autism trait score by Aim 1 autism-related grouping with ANOVA and post hoc probing via Tukey’s honestly significant difference procedure.
Aim 3 mirrored Aim 2 analytics, with multiple regression analysis of whole-brain FC. Three separate multiple linear regressions were conducted. FC was regressed onto: (1) self-reported internalizing symptoms, (2) gender dysphoria, and (3) SGM-related stigma, independently, with age, gender, and head motion included as covariates for each of the three analyses.
Aim 4 employed multiple regression analysis of whole-brain FC, with two separate models: (1) FC regressed onto gender (female vs. male), with age and head motion included as covariates, but without accounting for autism trait level and (2) the same analysis, but with autism trait level as an additional covariate. The interaction of gender and autism trait level was not included in the data analytics given insufficient sample size to examine potential interaction effects; power analysis of two predictors with a sample of n = 45 indicated the current study was underpowered to examine interaction terms, even if interaction effects were of large effect size (1-beta <.8) (Statistics Kingdom 2017).
To explore potential relationships between GAH treatment status and FC findings, a series of analyses were performed. Given: (1) expected autism-related differences between those receiving and not receiving GAH (i.e. fewer autism traits among those receiving treatment; Strang et al. 2016) and (2) possible differences in age by GAH treatment status (Strang et al. 2021b), analytics began with t-tests comparing GAH treated and untreated youth by autism trait level and age. Any resulting differences in composite autism trait level or age were controlled for in ANCOVAs examining differences in FC findings based on GAH status. These analytics were conducted separately for testosterone (in trans young men) and estrogen (in trans young women). Because gender-affirming testosterone was only given in trans young men and gender-affirming estrogen only in trans young women, hormone-based exploratory analytics (i.e. multiple regression models) were planned if any significant hormone-based FC differences were observed by autism status and/or internalizing symptoms, gender dysphoria, or SGM stigma (Aims 1–3), but not for any FC differences identified based on binary gender (Aim 4).
Results
Effect sizes for the results are available on the study’s technical website: https://osf.io/t7jfh/?view_only=863dc720dfc743b4a9e8e464d8665811. We encourage caution in the interpretation of the effect sizes, however, given previous evidence of overestimation in neuroimaging studies (Reddan et al. 2017).
Aim 1: Autism diagnostic group-based findings
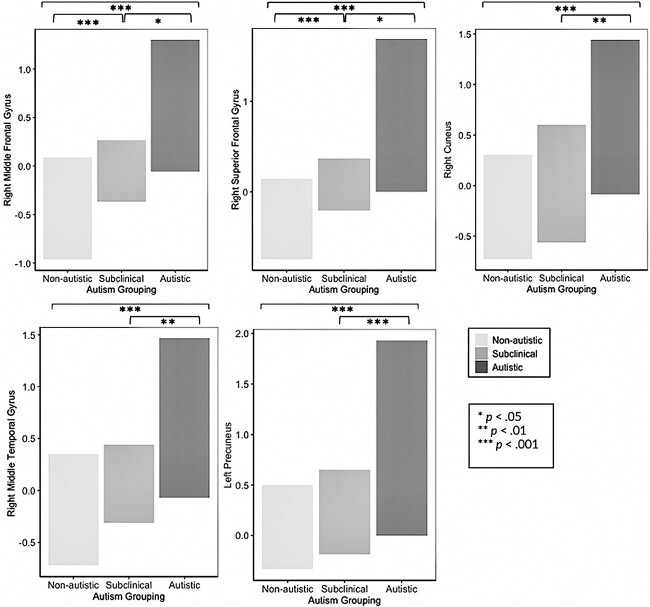
In MPFC seed to whole-brain analyses, there was a significant main effect of grouping with increased FC between the MPFC seed and five regions (see Table 2 and Figs. 1 and 2). The five regions were, in order of cluster size: 1) a right premotor cluster in the middle frontal gyrus (MFG) close to the medial eye field (ventral attention network); 2) a cluster in the right premotor planning area of the superior frontal gyrus (SFG; sensorimotor network); 3) a cluster in the cuneus (DMN and visual networks [V1/V2]); 4) a cluster in the right middle temporal gyrus (MTG) within the visual cortex (visual network); and 5) a cluster in the precuneus (salience and ventral attention networks). Post hoc tests (Fischer’s LSD) showed significant FC differences between all autism-related groupings (non-autistic vs. subclinically autistic vs. autistic) for the first two clusters: right MFG and right SFG. The remaining clusters were significantly different between the autistic as compared to the subclinically autistic and non-autistic groups, but not between the subclinically autistic and non-autistic groups (see Table 2). All differences were in the direction of greater FC for autistic youth (i.e. autistic > subclincally autistic > non-autistic for the first two regions and autistic > subclinically autistic and non-autistic for the remaining regions). As predicted based on previous studies in this age range, all of these FC differences were in the direction of hyperconnectivity related to autism. There were no significant FC findings observed in PCC-seed analyses. Overall, our predictions regarding FC differences by autism status between DMN hubs and visual and sensorimotor networks were supported, though we did not find predicted DMN-to-executive control network FC differences. We hypothesized that within-DMN FC differences would not be a primary finding; however, we did identify one significant cluster of within-DMN hyperconnectivity for the autistic group (MPFC to cuneus).
Table 2.
Significant autism-based FC differences.
| Region | BA | Network | Side | peak MNI | Z | k | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Autism Related Grouping | ||||||||
| Autistic > subclinically-autistic > non-autistic | ||||||||
| Seed: MPFC | ||||||||
| MFG/Premotor region/medial eye field (a) | 8 | VAN | R | 50 | 8.25 | 43 | 5.13 | 140 |
| SFG | 6 | Sensorimotor | R | 5 | 10.75 | 68 | 6.14 | 79 |
| Autistic > subclinically autistic and non-autistic | ||||||||
| Cuneus (b) | 30 | DMN/Visual | R | 5 | −66.75 | 10.5 | 4.20 | 48 |
| MTG (c) | 19 | Visual | R | 45 | −76.75 | 13 | 5.18 | 37 |
| Precuneus | 5 | Salience/VAN | L | −15 | −36.75 | 48 | 5.06 | 35 |
| Composite Autism Trait Score | ||||||||
| Higher composite autism trait score > Lower composite autism trait score | ||||||||
| Seed: MPFC | ||||||||
| MFG (a) | 6 | DAN | R | 37.5 | −1.75 | 53 | 4.84 | 109 |
| MTG (c) | 19 | Visual | R | 45 | −76.75 | 13 | 5.33 | 53 |
| Cuneus/primary visual cortex/PCC (b) | 31 | Visual | R | 5 | −66.75 | 13 | 4.74 | 48 |
| Insula | 13 | Salience/VAN | R | 37.5 | 3.25 | 3 | 5.40 | 41 |
| Seed: PCC | ||||||||
| Precuneus | 31 | DMN/Visual | L | −17.5 | −76.75 | 28 | 4.48 | 41 |
Note: k = Cluster size in voxels; BA = Brodmann Area; L = left; R = right; IH = interhemispheric; DMN = default mode network; VAN = ventral attention network; DAN = dorsal attention network; PCC = posterior cingulate cortex; MTG = medial temporal gyrus; MFG = medial frontal gyrus; IFG = inferior frontal gyrus; SFG = superior frontal gyrus; MPFC = medial prefrontal cortex. Superscript letter indicates overlap in regions between analyses. Regions with the same letter had substantially overlapped regions.
Fig. 1.
Significance of between-group functional connectivity differences.
Fig. 2.
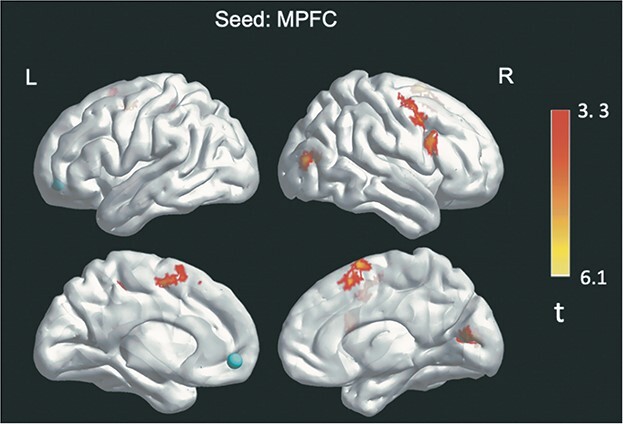
Functional connectivity differences by autism-related grouping. Note. Circle represents seed region. Heat map represents t values.
Aim 2: Autism trait level findings
In the preliminary ANOVA, composite autism trait level was different between the three Aim 1 autism-related groupings (F(2,42) = 74.37, P < 0.001). Tukey post hoc tests were significant for every group comparison (all p’s < 0.001). See Fig. 3.
Fig. 3.
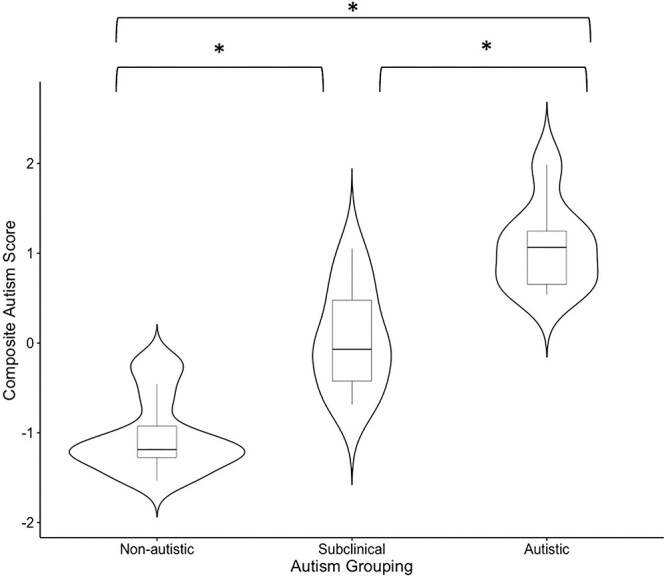
Autism trait level by autistic grouping.
Note. Tukey’s post hocs indicated by brackets, *P < 0.001.
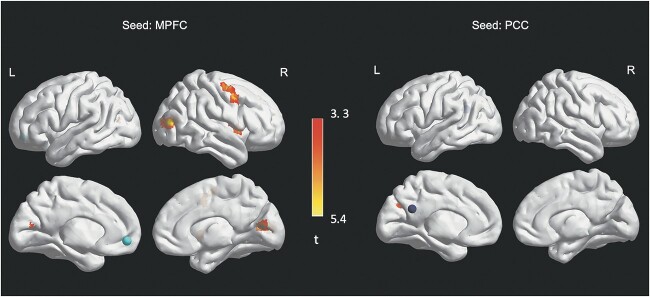
For neuroimaging analytics, composite autism trait level was related to increased FC between the MPFC seed and four regions: 1) a cluster in the MFG (dorsal attention network); 2) a cluster in the right MTG within the associative visual cortex (visual network [V3, V4, V5]); 3) a cluster within the PCC extending to the cuneus/primary visual cortex (visual network [V3, V4, V5]); and 4) a cluster within the middle right insula (salience and ventral attention networks). Autism trait level was also related to increased FC between the PCC seed and a cluster in the left precuneus (DMN and visual networks). Findings align with our predictions of autism-trait-related hyperconnectivity between DMN hubs and occipital/temporal regions. Contrary to our hypothesis, autism traits were not related to FC between DMN seeds and parahippocampal regions. As with the Aim 1 autism related grouping-based analyses, we did find evidence for some within-DMN hyperconnectivity (i.e. PCC to left precuneus) associated with elevated autistic traits. See Table 2 and Fig. 4.
Fig. 4.
Autism trait level-based findings (higher autism trait level > lower autism trait level).
Note. Circles represent seed regions. Heat map represents t values.
As predicted, there was overlap between findings generated using a group-differences approach with categorical autism-related groupings, and those generated using a dimensional approach based on autism trait levels. Specifically, as illustrated in Fig. 5, we find that elevated FC between the MPFC seed and MFG, PCC/precuneus/cuneus, and MTG clusters is related to autism status both when modeled categorically and dimensionally.
Fig. 5.
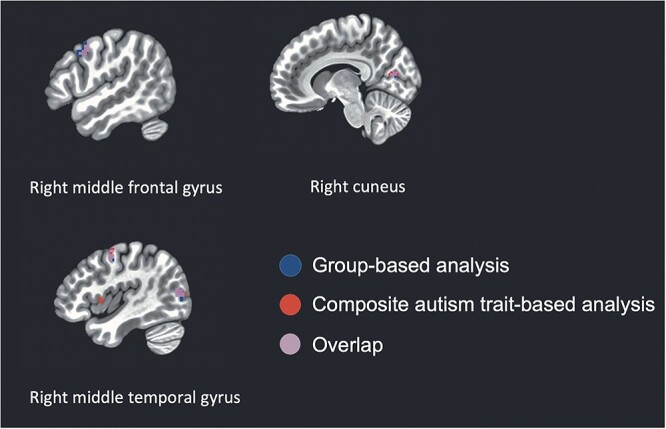
Overlap between group-based and composite autism trait score-based findings.
Aim 3 (exploratory): Internalizing, gender dysphoria, and sex and gender minority-related stigma findings
There were no significant DMN hub-to-whole-brain findings by self-reported internalizing symptoms, gender dysphoria, or SGM-related stigma.
Aim 4 (exploratory): Gender identity findings
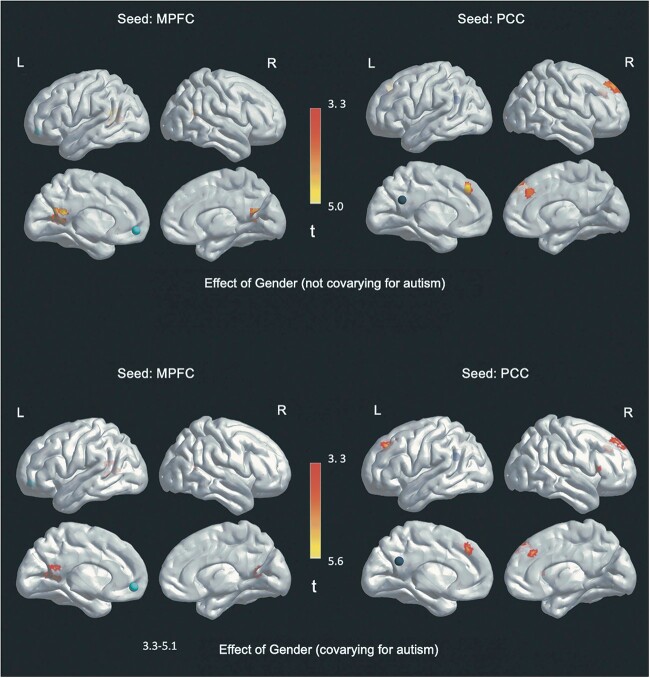
Within-DMN FC was significantly greater in trans young men (i.e. those designated female at birth) than trans young women (i.e. those designated male at birth). This pattern of greater FC for trans males compared to trans females holds true across all of the following findings. As hypothesized, when accounting for the level of autism traits, more FC differences by gender were identified than when not accounting for autism traits. Specifically, when autism trait level was not included in the model, there were two significant FC differences by gender from the PCC seed: 1) a cluster in the right SFG (DMN) and 2) a cluster in the left MPFC/MFG (control network). For the MPFC analysis not accounting for autism trait level, one significant FC difference by gender was found, also in the DMN: a cluster in the left PCC. In contrast, when autism trait level was included in the model, four regions showed significant FC differences by gender from the PCC seed and two regions from the MPFC seed (see Table 3 and Fig. 6).
Table 3.
Significant gender-based FC differences.
| Region | BA | Network | Side | Peak MNI | Z | k | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Gender Identity (Not controlling for autism) | ||||||||
| Trans young men > Trans young women | ||||||||
| Seed: PCC | ||||||||
| SFG (a) | 8/9 | DMN | R | 30 | 40.75 | 45.5 | 4.80 | 52 |
| MPFC/MFG (b) | 8/9 | Control | L | −2.5 | 35.75 | 43 | 4.77 | 33 |
| Seed: MPFC | ||||||||
| PCC (left) (c) | 30 | DMN | L | −15 | −56.75 | 13 | 4.99 | 122 |
| Gender Identity (Controlling for autism) | ||||||||
| Trans young men > Trans young women | ||||||||
| Seed: PCC | ||||||||
| Left medial frontal gyrus | 8/9 | DMN | L | −17.5 | 28.25 | 40.5 | 4.73 | 73 |
| SFG (a) | 8 | DMN | R | 30 | 40.75 | 45.5 | 5.60 | 55 |
| MPFC/MFG (b) | 8/9 | DMN | IH | 0 | 35.75 | 43 | 4.70 | 37 |
| IFG (pars triangularis) | 45 | DMN | R | 50 | 28.25 | 8 | 4.53 | 34 |
| Seed: MPFC | ||||||||
| PCC (left) (c) | 30 | DMN | L | −15 | −56.75 | 13 | 5.13 | 87 |
| PCC (left extending into right) | 30 | DMN | L | −5 | −46.75 | 18 | 4.96 | 45 |
Note: k = Cluster size in voxels; BA = Brodmann Area; L = left; R = right; IH = interhemispheric; DMN = default mode network; MPFC = medial prefrontal cortex; PCC = posterior cingulate cortex; MFG = medial frontal gyrus; IFG = inferior frontal gyrus. Regions with the same letter had substantially overlapped regions.
Fig. 6.
Gender-based findings (trans young men > trans young women).
Note. Circles represent seed regions. Heat map represents t values.
The four regions of greater FC from the PCC seed were all in the DMN: In addition to clusters in the right SFG and interhemispheric MPFC/MFC (which overlapped with results from the analysis that did not account for autism trait level), two additional clusters were observed in the left SFG and right inferior frontal gyrus (IFG), pars triangularis. The two regions of greater FC from the MPFC seed were also in the DMN: a cluster in the left PCC (which overlapped with the cluster identified in the analysis that did not control for autism trait level), and an additional PCC cluster that extended from the left into the right hemisphere.
Gender affirming hormones relationships with autism-related functional connectivity findings
As expected based on previous findings suggesting relatively later referrals for autistic transgender youth for gender affirming care (Strang et al. 2018) as well as international clinical guidance recommending a more extended approach for medical gender-related decision making with autistic adolescents (Strang et al. 2016), there was a significant relationship between GAH treatment status and autism; participants receiving GAH had fewer autistic traits: t(43) = −2.46, P < 0.018. There were no differences in age by GAH treatment status (P = 0.349). Autism traits were therefore controlled for in ANCOVAs examining potential differences in FC based on GAH treatment status. There were no significant FC differences based on GAH treatment status for trans young women or trans young men after controlling for autism trait level (all p’s > 0.20).
Discussion
This default mode network (DMN) functional connectivity (FC) study of the autism spectrum among transgender youth is novel in five ways. (1) It is the first study to present findings of autistic transgender as compared to non-autistic transgender neurophenotypes. (2) It captures through careful gold-standard autism-related phenotyping three levels of autism-related behavioral phenotypes and demonstrates their relatedness to DMN FC neurophenotypes. (3) In addition to autism group-based analyses, this study employs a composite autism trait approach, previously used in studies of autistic youth alone, and extends this approach to the broad autism phenotypes present among transgender youth. (4) This is the first brain-based study to address theoretical controversies regarding whether autistic features in transgender youth are “true” developmental autism versus a manifestation of sex and gender minority (SGM) stress. (5) The study investigates DMN FC by gender identity in transgender youth to understand whether FC patterns generally follow those associated with assigned sex at birth, affirmed gender, or a different pattern. Increasing novelty, the impact of autistic traits on gender-based analyses is also investigated.
Previous studies of DMN hub FC have fairly consistently reported autistic vs. non-autistic differences, though no studies have focused on autistic transgender individuals. The present study finds significant DMN hub-based FC differences by categorical autism diagnostic grouping as well as composite autism trait level. We did not find DMN hub-based FC differences by levels of internalizing symptoms, gender dysphoria, or SGM-related stigma. The autism-based FC differences largely followed expected patterns based on the extant autism FC research in this age range, including: (1) direction of FC differences (i.e. hyperconnectivity in autism) and (2) the specific regions and networks showing significant FC differences with DMN hubs. Overall, the autism-related findings from this study suggest that the autism behavioral phenotype measured in transgender young people, when obtained through gold-standard ASD diagnostic procedures, is associated with DMN-based FC patterns that would be generally expected in comparing autistic vs. non-autistic cisgender youth.
As hypothesized, autism status was associated with DMN seed-based FC differences between DMN hubs and visual and sensorimotor networks. Relative to their non-autistic transgender peers, autistic transgender youth showed hyperconnectivity across all findings, with subclinically autistic youth falling in between the autistic and non-autistic youth in terms of hyperconnectivity. Our findings are consistent with previous literature reporting hyperconnectivity between the DMN and visual and sensorimotor networks in autistic relative to non-autistic youth of approximately the same age-range (Washington et al. 2014; Mash et al. 2019). We did not find hypothesized FC differences between DMN hubs and the executive control network but did observe autism-related hyperconnectivity between the DMN and ventral and dorsal attention networks; one previous study reported autism-related differences in the ventral attention network, specifically (Yerys et al. 2015). Only one within-DMN FC difference was observed by autism status, which accords to some degree with existing literature suggesting that within-DMN FC differences by autism status may become less prominent as young people move toward adulthood (Nomi and Uddin 2015; Ypma et al. 2016).
Notably, a slightly subclinical autistic phenotype has been previously described as clinically relevant by specialists in autism and gender as well as clinician specialists in transgender care (Eckerd 2020; Strang et al. 2021a). In our sample, this subclinical autistic subgroup shows a DMN neurophenotype intermediate to that of the non-autistic and autistic groups, reinforcing the potential importance of this subgroup, both clinically and in designing future research. The two ROIs showing the greatest FC differences with DMN hubs varied in level of FC between all three autism-related groupings (i.e. non-autistic, subclinically autistic, autistic), with the autistic group greater than the subclinically autistic group, and the subclinically autistic group greater than the non-autistic group. These between-group differences provide the first evidence of dimensional differences in neurophenotypes associated with clinically-described transgender autism-based phenotypes.
Autism trait level FC findings showed considerable overlap with categorical group-based findings. This provides further support for the use of composite autism metrics for continuous characterization of autistic traits (Lopez et al. 2005; Black et al. 2009; Kenworthy et al. 2009). As with the group-based findings, all findings were, as predicted, in the direction of hyperconnectivity in autism. Differences in DMN hub to whole brain FC were somewhat consistent with available literature for this age range (Lynch et al. 2013), with two of three predicted neural regions (i.e. DMN to temporal and occipital regions) showing increased FC with greater autism traits. Hypothesized DMN hub-to-parahippocampal differences were not observed.
Some have suggested that behavioral autism trait elevations in transgender youth may reflect underlying internalizing symptomatology or gender-related concerns (e.g. gender dysphoria or SGM-related stigma) instead of “true” developmental autism (Turban and van Schalkwyk 2018). To explore this speculation, this study probed DMN hub FC differences by autism diagnostic grouping and autism trait level and level of emotional internalizing, gender dysphoria, and SGM-related stigma. With findings of significant autism-based DMN FC differences at both the level of categorical diagnostic autism-related grouping and trait level, but no significant influence of internalizing symptoms, gender dysphoria, or SGM-related stigma, this study provides the first multi-modal support (i.e. autism behavioral diagnostic indicators linked to autism-associated neurophenotypes) for the presence of “true” developmental autism among a subset of transgender youth. Notably, this study employed gold-standard comprehensive ASD assessment approaches, and not simply the autism screeners that have been used in some studies of the autism and gender diversity co-occurrence (VanderLaan et al. 2015; Shumer et al. 2016).
There is limited available information on FC patterns in transgender individuals. Therefore, this study included analytics by gender identity in this transgender binary sample and found substantial evidence for gender-based FC differences within the DMN, especially when controlling for level of autism traits. Specifically, male gender (i.e. female designation at birth) was associated with greater within-DMN FC, akin to patterns often observed in cisgender females vs. cisgender males (Bluhm et al. 2008; Tomasi and Volkow 2012; Ypma et al. 2016; Olson et al. 2020). In this way, the current study suggests that within-DMN FC for transgender young people may follow patterns associated with their designated sex at birth, which is in-line with recent findings from a Canadian study of transgender young men, cisgender young men, and cisgender young women (Skorska et al. 2022).
As hypothesized (in Aim 4), controlling for autism traits increased observed gender-related differences (i.e. greater number of significant voxels in DMN, and significant findings with the IFG). As noted in Aim 4, evidence suggests autism diagnosis is associated with reduced sex-based DMN connectivity differences as compared to the sex-based DMN connectivity differences observed in non-autistic individuals (Ypma et al. 2016). This study provides further evidence that elevated autistic traits are associated with attenuated DMN-hub-based FC differences by gender.
Of note, autism-related FC differences were identified almost exclusively with the MPFC DMN seed; only one autism trait level finding difference was from the PCC DMN seed (i.e. PCC to within-DMN left precuneus structures). In contrast, most gender-related findings were from the PCC seed (i.e. 4 of 6). This differentiation of DMN-based hub connectivity where autism differences are observed primarily in MPFC connectivity and gender differences observed primarily in PCC connectivity may indicate initial neurophenotypic markers relevant to transgender and autistic transgender populations.
Limitations and future directions
Future studies should include larger samples to allow for consideration of potential interaction effects, such as by autism, gender, and designated sex. Future recruitments will need to dedicate significant resources to obtain more balanced samples across gender, given the naturally uneven gender ratios in autism, gender diversity, and the intersection of autism and gender diversity (Aitken et al. 2015; Lai and Szatmari 2020; Strang et al. 2021a). Although we controlled for gender in the analyses, an important limitation for generalizability in the current study is the relatively uneven gender ratio between the three autism-related subgroupings. The sample size of the current study was too small to adequately examine potential interactions between autism trait level and gender, though we were able to compare findings controlling for, versus not controlling for, autism traits. A critical next step for this research is including autistic cisgender individuals as an additional comparison group to probe and compare connectivity differences by autism status, gender diversity status (cisgender vs. gender-diverse), gender, designated sex, and their interactions. Due to the decision within this study to recruit binary transgender youth exclusively—to minimize the number of comparison groups and increase power—this study did not include nonbinary genders. Yet, autism appears to be over-represented in nonbinary populations as well (Stagg and Vincent 2019), so future work should intentionally recruit within additional gender identity categories.
Conclusions
This study provides a first look at aspects of the autistic transgender neurophenotype, key, given the common intersection of autism and gender diversity. Studies of this kind are needed, not only to inform the nature of intersecting autism and gender diversity, but also to provide information for large-scale studies of autistic people and transgender people independently, where the overlap of autism and gender diversity may be common, but not adequately modeled. This study also provides multi-modal evidence contradicting the supposition that autistic features in transgender youth are driven primarily by SGM-related stress and internalizing problems. Finally, the study provides initial neurophenotypic support for a slightly subclinical autism phenotype, commonly reported by gender development specialists.
Acknowledgments
We would like to thank the youth participants who made this study possible.
Contributor Information
John F Strang, Gender and Autism Program, Children’s National Hospital, 15245 Shady Grove Road, Suite 350, Rockville, MD 20850, USA; Departments of Pediatrics, Psychiatry, and Behavioral Sciences, George Washington University School of Medicine, Washington, DC, USA; Division of Neuropsychology, Children’s National Hospital, Washington, DC, USA.
Lucy S McClellan, Division of Neuropsychology, Children’s National Hospital, Washington, DC, USA.
Sufang Li, Department of Psychology, Georgetown University, Washington, DC, USA.
Allison E Jack, Department of Psychology, George Mason University, Fairfax, VA, USA.
Gregory L Wallace, Department of Speech, Language, & Hearing Sciences, George Washington University, Washington, DC, USA.
Goldie A McQuaid, Department of Psychology, George Mason University, Fairfax, VA, USA.
Lauren Kenworthy, Departments of Pediatrics, Psychiatry, and Behavioral Sciences, George Washington University School of Medicine, Washington, DC, USA; Division of Neuropsychology, Children’s National Hospital, Washington, DC, USA.
Laura G Anthony, Department of Psychiatry and Behavioral Sciences, University of Colorado School of Medicine, Aurora, CO, USA.
Meng-Chuan Lai, Child and Youth Mental Health Collaborative at the Centre for Addiction and Mental Health, The Hospital for Sick Children, Toronto, Ontario, Canada; Department of Psychiatry, Temerty Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada.
Kevin A Pelphrey, Department of Neurology, University of Virginia Medical School, Charlottesville, VA, USA.
Alexandra E Thalberg, Department of Biology, Georgetown University, Washington, DC, USA.
Eric E Nelson, Center for Biobehavioral Health, The Research Institute at Nationwide Children’s Hospital, Columbus, OH, USA.
Jenny M Phan, Division of Neuropsychology, Children’s National Hospital, Washington, DC, USA.
Eleonora Sadikova, School of Education and Human Development, University of Virginia, Charlottesville, VA, USA.
Abigail L Fischbach, Division of Neuropsychology, Children’s National Hospital, Washington, DC, USA.
John Thomas, Falls Church Counseling, VA, USA.
Chandan J Vaidya, Department of Psychology, Georgetown University, Washington, DC, USA.
Funding
This work was supported by the National Institutes of Health (grant numbers CTSI-CN UL1TR001876, CTSA KL2TR001877 to JFS).
Conflict of interest statement: The authors have no conflicts of interest to declare.
Data availability statement
The data that support the findings of this study are not publicly available due to privacy restrictions.
References
- Abbott AE, Nair A, Keown CL, Datko M, Jahedi A, Fishman I, Müller RA. Patterns of atypical functional connectivity and Behavioral links in autism differ between default, salience, and executive networks. Cereb Cortex. 2016:26(10):4034–4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achenbach TM. The Achenbach system of empirically based sssessment (ASEBA): development, findings, theory, and applications. Burlington (VT): University of Vermont Research Center for Children, Youth, & Families; 2009 [Google Scholar]
- Aitken M, Steensma TD, Blanchard R, VanderLaan DP, Wood H, Fuentes A, Fitzsimmons CL, Leef JH, Lishak V, Reim E et al. Evidence for an altered sex ratio in clinic-referred adolescents with gender dysphoria. J Sex Med. 2015:12(3):756–763. [DOI] [PubMed] [Google Scholar]
- Akgül GY, Ayaz AB, Yildirim B, Fis NP. Autistic traits and executive functions in children and adolescents with gender dysphoria. J Sex Marital Ther. 2018:44(7):619–626. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. Washington, DC: American Psychiatric Association; 2022. Fifth Edition
- American Psychological Association . Guidelines for psychological practice with transgender and gender nonconforming people. Am Psychol. 2015:70(9):832–864. [DOI] [PubMed] [Google Scholar]
- Baribeau DA, Dupuis A, Paton TA, Hammill C, Scherer SW, Schachar RJ, Arnold PD, Szatmari P, Nicolson R, Georgiades S et al. Structural neuroimaging correlates of social deficits are similar in autism spectrum disorder and attention-deficit/hyperactivity disorder: analysis from the POND network. Transl Psychiatry. 2019:9(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Spong A, Scahill V, Lawson J. Are intuitive physics and intuitive psychology independent? A test with children with Asperger syndrome. J Dev Learn Disord. 2001a:5:47–78. [Google Scholar]
- Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The "reading the mind in the eyes" test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatry. 2001b:42(2):241–251. [PubMed] [Google Scholar]
- Bejerot S, Eriksson JM, Bonde S, Carlström K, Humble MB, Eriksson E. The extreme male brain revisited: gender coherence in adults with autism spectrum disorder. The British Journal of Psychiatry: J Ment Sci. 2012:201(2):116–123. [DOI] [PubMed] [Google Scholar]
- Biswal B, Zerrin Yetkin F, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar mri. Magn Reson Med. 1995:34(4):537–541. [DOI] [PubMed] [Google Scholar]
- Black DO, Wallace GL, Sokoloff JL, Kenworthy L. Brief report: IQ split predicts social symptoms and communication abilities in high-functioning children with autism spectrum disorders. J Autism Dev Disord. 2009:39(11):1613–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluhm RL, Osuch EA, Lanius RA, Boksman K, Neufeld RW, Theberge J, Williamson P. Default mode network connectivity: effects of age, sex, and analytic approach. Neuroreport. 2008:19(8):887–891. [DOI] [PubMed] [Google Scholar]
- Brent E, Rios P, Happé F, Charman T. Performance of children with autism spectrum disorder on advanced theory of mind tasks. Autism. 2004:8(3):283–299. [DOI] [PubMed] [Google Scholar]
- Buckner RL, DiNicola LM. The brain’s default network: updated anatomy, physiology and evolving insights. Nat Rev Neurosci. 2019:20(10):593–608. [DOI] [PubMed] [Google Scholar]
- Chen C, Van Horn JD. Developmental neurogenetics and multimodal neuroimaging of sex differences in autism. Brain Imaging Behav. 2017:11(1):38–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Equifinality and multifinality in developmental psychopathology. Dev Psychopathol. 1996:8(4):597–600. [Google Scholar]
- Cohen-Kettenis PT, van Goozen SH. Sex reassignment of adolescent transsexuals: a follow-up study. J Am Acad Child Adolesc Psychiatry. 1997:36(2):263–271. [DOI] [PubMed] [Google Scholar]
- Cole D, Smith S, Beckmann C. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front Syst Neurosci. 2010:4(8):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP. Social responsiveness scale – second edition (SRS-2). Los Angeles (CA): Western Psychological Services; 2012 [Google Scholar]
- Cooper K, Smith LGE, Russell AJ. Gender identity in autism: sex differences in social affiliation with gender groups. J Autism Dev Disord. 2018:48(12):3995–4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries ALC, Noens ILJ, Cohen-Kettenis PT, van Berckelaer-Onnes IA, Doreleijers TA. Autism spectrum disorders in gender dysphoric children and adolescents. J Autism Dev Disord. 2010:40(8):930–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckerd M. Detection and diagnosis of ASD in females. J Health Serv Psychol. 2020:46(1):37–47. [Google Scholar]
- Floris DL, Filho JOA, Lai M-C, Giavasis S, Oldehinkel M, Mennes M, Charman T, Tillmann J, Dumas G, Ecker C. Towards robust and replicable sex differences in the intrinsic brain function of autism. Mol Autism. 2020:12(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007:8(9):700–711. [DOI] [PubMed] [Google Scholar]
- Furlong Y. Autism and gender identity. In: Fitzgerald M, editors. Autism spectrum disorder - profile, heterogeneity, neurobiology and intervention. InTechOpen; 2021. pp. 39–58 [Google Scholar]
- George R, Stokes MA. A quantitative analysis of mental health among sexual and gender minority groups in ASD. J Autism Dev Disord. 2018:48(6):2052–2063. [DOI] [PubMed] [Google Scholar]
- Grannis C, Leibowitz SF, Gahn S, Nahata L, Morningstar M, Mattson WI, Chen D, Strang JF, Nelson EE. Testosterone treatment, internalizing symptoms, and body image dissatisfaction in transgender boys. Psychoneuroendocrino. 2021:132:105358. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003:100(1):253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman AH, D'Augelli AR. Transgender youth. J Homosex. 2006:51(1):111–128. [DOI] [PubMed] [Google Scholar]
- Haghighat H, Mirzarezaee M, Araabi BN, Khadem A. Functional networks abnormalities in autism spectrum disorder: age-related hypo and hyper connectivity. Brain Topogr. 2021:34(3):306–322. [DOI] [PubMed] [Google Scholar]
- Hisle-Gorman E, Landis CA, Susi A, Schvey NA, Gorman GH, Nylund CM, Klein DA. Gender dysphoria in children with autism spectrum disorder. LGBT Health. 2019:6(3):95–100. [DOI] [PubMed] [Google Scholar]
- Hurley RS, Losh M, Parlier M, Reznick JS, Piven J. The broad autism phenotype questionnaire. J Autism Dev Disord. 2007:37(9):1679–1690. [DOI] [PubMed] [Google Scholar]
- Hus V, Lord C. The autism diagnostic observation schedule, module 4: revised algorithm and standardized severity scores. J Autism Dev Disord. 2014:44(8):1996–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallitsounaki A, Williams D. Mentalising moderates the link between autism traits and current gender dysphoric features in primarily non-autistic, cisgender individuals. J Autism Dev Disord. 2020a:50(11):4148–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallitsounaki A, Williams D. A relation between autism traits and gender self-concept: evidence from explicit and implicit measures. J Autism Dev Disord. 2020b:50(2):429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallitsounaki A, Williams DM. Autism spectrum disorder and gender dysphoria/incongruence. A systematic literature review and meta-analysis. J Autism Dev Disord. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallitsounaki A, Williams DM, Lind SE. Links between autistic traits, feelings of gender dysphoria, and mentalising ability: replication and extension of previous findings from the general population. J Autism Dev Disord. 2021:51(5):1458–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenworthy L, Black DO, Harrison B, della Rosa A, Wallace GL. Are executive control functions related to autism symptoms in high-functioning children? Child Neuropsychol. 2009:15(5):425–440. [DOI] [PubMed] [Google Scholar]
- Lai M-C, Szatmari P. Sex and gender impacts on the behavioural presentation and recognition of autism. Curr Opin Psychiatry. 2020:33(2):117–123. [DOI] [PubMed] [Google Scholar]
- Lawrence KE, Hernandez LM, Bookheimer SY, Dapretto M. Atypical longitudinal development of functional connectivity in adolescents with autism spectrum disorder. Autism Res. 2019:12(1):53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence KE, Hernandez LM, Bowman HC, Padgaonkar NT, Fuster E, Jack A, Aylward E, Gaab N, Van Horn JD, Bernier RA et al. Sex differences in functional connectivity of the salience, default mode, and central executive networks in youth with ASD. Cereb Cortex. 2020a:30(9):5107–5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence KE, Hernandez LM, Eilbott J, Jack A, Aylward E, Gaab N, Van Horn JD, Bernier RA, Geschwind DH, JC MP et al. Neural responsivity to social rewards in autistic female youth. Transl Psychiatry. 2020b:10(1):178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leef JH, Brian J, VanderLaan DP, Wood H, Scott K, Lai M-C, Bradley SJ, Zucker KJ. Traits of autism spectrum disorder in school-aged children with gender dysphoria: a comparison to clinical controls. Clin Pract Pediatr Psychol. 2019:7(4):383–395. [Google Scholar]
- Lopez BR, Lincoln AJ, Ozonoff S, Lai Z. Examining the relationship between executive functions and restricted, repetitive symptoms of autistic disorder. J Autism Dev Disord. 2005:35(4):445–460. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994:24(5):659–685. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop SL. ADOS-2: autism diagnostic observation schedule, second edition (ADOS-2). Torrance (CA): Western Psychological Services; 2012 [Google Scholar]
- Loth E, Charman T, Mason L, Tillmann J, Jones EJH, Wooldridge C, Ahmad J, Auyeung B, Brogna C, Ambrosino S et al. The EU-AIMS longitudinal European autism project (LEAP): design and methodologies to identify and validate stratification biomarkers for autism spectrum disorders. Mol Autism. 2017:8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch CJ, Uddin LQ, Supekar K, Khouzam A, Phillips J, Menon V. Default mode network in childhood autism: posteromedial cortex heterogeneity and relationship with social deficits. Biol Psychiatry. 2013:74(3):212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann Whitney U. test calculator calculator [internet]. Statistics Kingdom. 2017. Available from: http://www.statskingdom.com/170median_mann_whitney.html. [Google Scholar]
- Mash LE, Linke AC, Olson LA, Fishman I, Liu TT, Müller RA. Transient states of network connectivity are atypical in autism: a dynamic functional connectivity study. Hum Brain Mapp. 2019:40(8):2377–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Peltier SJ, Wiggins JL, Weng S-J, Carrasco M, Risi S, Lord C. Abnormalities of intrinsic functional connectivity in autism spectrum disorders. NeuroImage. 2009:47(2):764–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabbijohn AN, van der Miesen AIR, Santarossa A, Peragine D, de Vries AL, Popma A, Lai M-C, VanderLaan DP. Gender variance and the autism spectrum: an examination of children ages 6–12 years. J Autism Dev Disord. 2019:49(4):1570–1585. [DOI] [PubMed] [Google Scholar]
- Nomi JS, Uddin LQ. Developmental changes in large-scale network connectivity in autism. Neuroimage Clin. 2015:7:732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nota NM, Burke SM, den Heijer M, Soleman RS, Lambalk CB, Cohen-Kettenis PT, Veltman DJ, Kreukels BP. Brain sexual differentiation and effects of cross-sex hormone therapy in transpeople: a resting-state functional magnetic resonance study. Neurophysiol Clin. 2017a:47(5–6):361–370. [DOI] [PubMed] [Google Scholar]
- Nota NM, Kreukels BPC, den Heijer M, Veltman DJ, Cohen-Kettenis PT, Burke SM, Bakker J. Brain functional connectivity patterns in children and adolescents with gender dysphoria: sex-atypical or not? Psychoneuroendocrino. 2017b:86:187–195. [DOI] [PubMed] [Google Scholar]
- Olson LA, Mash LE, Linke A, Fong CH, Muller RA, Fishman I. Sex-related patterns of intrinsic functional connectivity in children and adolescents with autism spectrum disorders. Autism. 2020:24(8):2190–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan A, Lynch CJ, Schaer M, Menon V. The default mode network in autism. Biol Psychiatry: Cogn Neurosci. 2017:2(6):476–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001:98(2):676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddan MC, Lindquist MA, Wager TD. Effect size estimation in neuroimaging. JAMA Psychiatry. 2017:74(3). [DOI] [PubMed] [Google Scholar]
- Ruzich E, Allison C, Smith P, Watson P, Auyeung B, Ring H, Baron-Cohen S. Subgrouping siblings of people with autism: identifying the broader autism phenotype. Autism Res. 2016:9(6):658–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatino DiCriscio A, Troiani V. The broader autism phenotype and visual perception in children. J Autism Dev Disord. 2018:48(8):2809–2820. [DOI] [PubMed] [Google Scholar]
- Salek-Haddadi A, Diehl B, Hamandi K, Merschhemke M, Liston A, Friston K, Duncan JS, Fish DR, Lemieux L. Hemodynamic correlates of epileptiform discharges: an EEG-fMRI study of 63 patients with focal epilepsy. Brain Res. 2006:1088(1):148–166. [DOI] [PubMed] [Google Scholar]
- Schaefer A, Kong R, Gordon EM, Laumann TO, Zuo XN, Holmes AJ, Eickhoff SB, Yeo BTT. Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cereb Cortex. 2018:28(9):3095–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz A. GLM flex Fast2 [Internet]. 2020. Available from: https://habs.mgh.harvard.edu/researchers/data-tools/glm-flex-fast2/
- Shumer DE, Reisner SL, Edwards-Leeper L, Tishelman A. Evaluation of Asperger syndrome in youth presenting to a gender dysphoria clinic. LGBT health. 2016:3(5):387–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorska MN, Lobaugh NJ, Lombardo MV, van Bruggen N, Chavez S, Thurston LT, Aitken M, Zucker KJ, Chakravarty MM, Lai MC et al. Inter-network brain functional connectivity in adolescents assigned female at birth who experience gender dysphoria. Front Endocrinol. 2022:13:903058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg SD, Vincent J. Autistic traits in individuals self-defining as transgender or nonbinary. Eur Psychiatry. 2019:61:17–22. [DOI] [PubMed] [Google Scholar]
- Steensma TD, Kreukels BP, Jürgensen M, Thyen U, de Vries ALC, Cohen-Kettenis P. The Utrecht gender dysphoria scale: a validation study. Archives of sexual behavior. 2013:903058.
- Strang JF, Meagher H, Kenworthy L, de Vries ALC, Menvielle E, Leibowitz S, Janssen A, Cohen-Kettenis P, Shumer DE, Edwards-Leeper L et al. Initial clinical guidelines for co-occurring autism spectrum disorder and gender dysphoria or incongruence in adolescents. J Clin Child Adolesc Psychol. 2016:47(1):1–11. [DOI] [PubMed] [Google Scholar]
- Strang JF, Powers MD, Knauss M, Sibarium E, Leibowitz SF, Kenworthy L, Sadikova E, Wyss S, Willing L, Caplan R et al. "They thought it was an obsession": Trajectories and perspectives of autistic transgender and gender-diverse adolescents. J Autism Dev Disord. 2018:48(12):4039–4055. [DOI] [PubMed] [Google Scholar]
- Strang JF, Knauss M, van der Miesen AIR, McGuire JK, Kenworthy L, Caplan R, Freeman A, Sadikova E, Zaks Z, Pervez N et al. A clinical program for transgender and gender-diverse neurodiverse/autistic adolescents developed through community-based participatory design. J Clin Child Adolesc Psychol. 2020:50(6):730–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang JF, Anthony LG, Song A, Lai M-C, Knauss M, Sadikova E, Graham E, Zaks Z, Wimms H, Willing L et al. In addition to stigma: cognitive and autism-related predictors of mental health in transgender adolescents. J Clin Child Adolesc Psychol. 2021a:53:1–18. [DOI] [PubMed] [Google Scholar]
- Strang JF, Chen D, Nelson E, Leibowitz SF, Nahata L, Anthony LG, Song A, Grannis C, Graham E, Henise S et al. Transgender youth executive functioning: relationships with anxiety symptoms, autism spectrum disorder, and gender-affirming medical treatment status. Child Psychiatry Hum Dev. 2021b:53(6):1252–1265. [DOI] [PubMed] [Google Scholar]
- Strauss P, Cook A, Winter S, Watson V, Wright Toussaint D, Lin A. Trans pathways: the mental health experiences and care pathways of trans young people. Perth: Telethon Kids Inst; 2017 [Google Scholar]
- Suh J, Orinstein A, Barton M, Chen C-M, Eigsti I-M, Ramirez-Esparza N, Fein D. Ratings of broader autism phenotype and personality traits in optimal outcomes from autism spectrum disorder. J Autism Dev Disord. 2016:46(11):3505–3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PA, Chen G, Glen DR, Rajendra JK, Reynolds RC, Cox RW. FMRI processing with AFNI: some comments and corrections on “exploring the impact of analysis software on task fMRI results”. bioRxiv. 2018:308643. [Google Scholar]
- Tomasi D, Volkow ND. Aging and functional brain networks. Mol Psychiatry. 2012:17(5):471, 549–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turban JL, van Schalkwyk GI. ``Gender dysphoria'' and autism spectrum disorder: is the link real? J Am Acad Child Adolesc Psychiatry. 2018:57(1):8–9.e2. [DOI] [PubMed] [Google Scholar]
- Uribe C, Junque C, Gomez-Gil E, Abos A, Mueller SC, Guillamon A. Brain network interactions in transgender individuals with gender incongruence. NeuroImage. 2020:211:116613. [DOI] [PubMed] [Google Scholar]
- Van Dijk KRA, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol. 2009:103(1):297–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderLaan DP, Leef JH, Wood H, Hughes SK, Zucker KJ. Autism spectrum disorder risk factors and autistic traits in gender dysphoric children. J Autism Dev Disord. 2015:45(6):1742–1750. [DOI] [PubMed] [Google Scholar]
- Walsh RJ, Krabbendam L, Dewinter J, Begeer S. Brief report: gender identity differences in autistic adults: associations with perceptual and socio-cognitive profiles. J Autism Dev Disord. 2018:48(12):4070–4078. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang Y-B, Liu L-L, Cui J-F, Wang J, Shum DHK, van Amelsvoort T, Chan RCK. A meta-analysis of working memory impairments in autism spectrum disorders. Neuropsychol Rev. 2017:27(1):46–61. [DOI] [PubMed] [Google Scholar]
- Wang Q, Li H-Y, Li Y-D, Lv Y-T, Ma H-B, Xiang A-F, Jia X-Z, Liu D-Q. Resting-state abnormalities in functional connectivity of the default mode network in autism spectrum disorder: a meta-analysis. Brain Imaging Behav. 2021:15(5):2583–2592. [DOI] [PubMed] [Google Scholar]
- Warrier V, Greenberg DM, Weir E, Buckingham C, Smith P, Lai M-C, Allison C, Baron-Cohen S. Elevated rates of autism, other neurodevelopmental and psychiatric diagnoses, and autistic traits in transgender and gender-diverse individuals. Nat Commun. 2020:11(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington SD, Gordon EM, Brar J, Warburton S, Sawyer AT, Wolfe A, Mease-Ference ER, Girton L, Hailu A, Mbwana J et al. Dysmaturation of the default mode network in autism. Hum Brain Mapp. 2014:35(4):1284–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D, Zhou X. Wechsler abbreviated scale of intelligence II. New York (NY): The Psychological Corporation: Harcourt Brace & Company; 2011 [Google Scholar]
- Weinhardt LS, Stevens P, Xie H, Wesp LM, John SA, Apchemengich I, Kioko D, Chavez-Korell S, Cochran KM, Watjen JM et al. Transgender and gender nonconforming youths' public facilities use and psychological well-being: a mixed-method study. Transgender Health. 2017:2(1):140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng SJ, Wiggins JL, Peltier SJ, Carrasco M, Risi S, Lord C, Monk CS. Alterations of resting state functional connectivity in the default network in adolescents with autism spectrum disorders. Brain Res. 2010:1313:202–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerys BE, Gordon EM, Abrams DN, Satterthwaite TD, Weinblatt R, Jankowski KF, Strang J, Kenworthy L, Gaillard WD, Vaidya CJ. Default mode network segregation and social deficits in autism spectrum disorder: evidence from non-medicated children. Neuroimage Clin. 2015:9:223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeshurun Y, Nguyen M, Hasson U. The default mode network: where the idiosyncratic self meets the shared social world. Nat Rev Neurosci. 2021:22(3):181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ypma RJ, Moseley RL, Holt RJ, Rughooputh N, Floris DL, Chura LR, Spencer MD, Baron-Cohen S, Suckling J, Bullmore ET et al. Default mode hypoconnectivity underlies a sex-related autism spectrum. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016:1(4):364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are not publicly available due to privacy restrictions.