Abstract
Malignant brain tumours are the cause of a disproportionate level of morbidity and mortality among cancer patients, an unfortunate statistic that has remained constant for decades. Despite considerable advances in the molecular characterization of these tumours, targeting the cancer cells has yet to produce significant advances in treatment. An alternative strategy is to target cells in the glioblastoma microenvironment, such as tumour-associated astrocytes. Astrocytes control multiple processes in health and disease, ranging from maintaining the brain’s metabolic homeostasis, to modulating neuroinflammation. However, their role in glioblastoma pathogenicity is not well understood. Here we report that depletion of reactive astrocytes regresses glioblastoma and prolongs mouse survival. Analysis of the tumour-associated astrocyte translatome revealed astrocytes initiate transcriptional programmes that shape the immune and metabolic compartments in the glioma microenvironment. Specifically, their expression of CCL2 and CSF1 governs the recruitment of tumour-associated macrophages and promotes a pro-tumourigenic macrophage phenotype. Concomitantly, we demonstrate that astrocyte-derived cholesterol is key to glioma cell survival, and that targeting astrocytic cholesterol efflux, via ABCA1, halts tumour progression. In summary, astrocytes control glioblastoma pathogenicity by reprogramming the immunological properties of the tumour microenvironment and supporting the non-oncogenic metabolic dependency of glioblastoma on cholesterol. These findings suggest that targeting astrocyte immunometabolic signalling may be useful in treating this uniformly lethal brain tumour.
Keywords: glioma, cholesterol, astrocytes
Perelroizen et al. report that depletion of reactive astrocytes leads to regression of glioblastoma and improved survival in mice via regulation of tumour-associated macrophages. They show too that glioma cells depend on astrocyte-derived cholesterol for survival, and that targeting astrocytic cholesterol efflux halts tumour growth.
See Murk and Hülse (https://doi.org/10.1093/brain/awac302) for a scientific commentary on this article.
See Murk and Hülse (https://doi.org/10.1093/brain/awac302) for a scientific commentary on this article.
Introduction
Cancers develop in complex tissue-dependent environments, on which they rely for sustained growth, response to therapy and regulate of the immune response to the tumours.1 Unlike the tumour cells, non-neoplastic cells in the tumour microenvironment (TME) are genetically stable and thus represent an attractive therapeutic target, with a lower risk of resistance and tumour recurrence. Accumulating data suggest that modulation of the bioenergetic,2–4 metabolic5,6 or immunological7–11 properties of the TME can regulate tumour progression in the CNS. However, the mechanisms that shape the immunometabolic landscape of the TME, and thus control brain cancer pathogenesis, are not well understood.
Glioblastoma multiforme (GBM) is the most common primary malignant brain tumour and carries an abysmal 5-year survival rate of just 5.6%, a statistic that, regrettably, has shown little change over decades.12,13 Despite significant advances in our understanding of this disease, the translation to improved treatment has been quite disappointing. As a result, surgery, radiation and cytotoxic chemotherapy remain the mainstays of therapy, although immune checkpoint therapy has recently been suggested as a potentially efficacious approach.10,14 Astrocytes are the most abundant cells in the CNS. They perform essential functions during development and homeostasis, such as participating in the maintenance of the blood–brain barrier, storing and distributing energetic substrates to neurons and supporting the development of neural cells and synaptogenesis.15,16 Astrocytes can also control CNS inflammation and neurodegeneration through multiple mechanisms, including neurotoxicity, modulation of microglial activities, recruitment of inflammatory cells into the CNS and even via their metabolic cascade.17–23 Tumour-associated astrocytes (TAAs) were recently suggested to participate in shaping the TME of primary and secondary brain tumours.24,25 However, the role of reactive astrocytes in GBM pathogenicity is poorly understood.
Here we report that depletion of reactive TAAs regresses GBM progression in mice and prolongs animal survival. In addition, we found that astrocytes support the tumour by regulating the recruitment of tumour-associated macrophages (TAMs) to the TME via CCL2, and by promoting a pro-tumourigenic phenotype in the TAMs, partially via the release of CSF1. We also found that glioma cells depend on astrocyte-derived cholesterol for survival and, accordingly, targeting the cholesterol efflux from TAAs halts tumour growth. These findings explain the role of astrocytes in GBM pathogenesis and define the molecular circuits by which the astrocytes shape the immunometabolic landscape of the TME and control tumour progression, thereby identifying potential druggable candidates for therapeutic intervention.
Materials and methods
Animals
C57BL/6J, Gfap-TK [B6.Cg-Tg(Gfap-TK)7.1Mvs/J], Gfap-CRE [B6.Cg-Tg(Gfap-cre)77.6Mvs/2J, from founder line 77.6, stock no 024098], induced diphtheria toxin (iDTR) [Gt(ROSA)26Sortm1(HBEGF)Awai] and RiboTag (B6N.129-Rpl22tm1.1Psam/J) mice were purchased from Jackson Laboratory (ME, USA). Female Gfap-TK+/− mice were crossed with male C57BL/6J to generate Gfap-TK+/− or wild-type (WT) mice. Female Gfap-Cre+/− were crossed with male RiboTagfl/fl or iDTRfl/fl to generate F1 littermates of GfapCre+/−-RiboTagfl/− or Gfap-Cre+/−-iDTRfl/− or GfapCre−/−-iDTRfl/− mice. All animals were kept in a pathogen-free facility at the Tel Aviv University Faculty of Medicine and were housed five animals per cage under a standard light cycle (12 h:12 h light:dark) with ad libitum access to water and food. All experiments were carried out in accordance with guidelines prescribed by the Institutional Animal Care and Use Committee of Tel Aviv University.
Cell lines
GL261-Luc2 cells (#9361, Caliper), CT-2A-Luc2 (#SCC195, Merck Ltd), U87EGFRvIII26 (kindly provided by Dr Paul S. Mischel, Stanford University, CA, USA) and 293T AAVpro cells (#632273, Takara) were grown in Dulbecco’s modified Eagle medium (DMEM) (Gibco #41965-039) with 10% foetal bovine serum (Gibco #12657-029) and 1% penicillin-streptomycin (Gibco #15140-122). The 293T medium was further supplemented with non-essential amino acids (BI, #01-340-1B) and 1% sodium pyruvate (BI, #03-042-1B). Cells were maintained free of mycoplasma contamination; routine mycoplasma analysis was performed using the EZ-PCR™ Mycoplasma Detection Kit (BI #2-700-20). In lipoprotein starvation experiments, normal foetal calf serum (FCS) was replaced with lipoprotein-deficient serum (LPDS, Sigma-Aldrich, #S5394).
Primary glial cultures
Primary murine mixed glia (astrocytes and microglial cells) and astrocytes were prepared as we previously described.19 Primary astrocytes cultures were found to be >99% GFAP-positive by immunofluorescent staining (Supplementary Fig. 5E). Microglial cultures were isolated by subjecting confluent mixed glial cultures to mild trypsinization (0.05% Trypsin in DMEM) according to previously published protocols.27 This results in the detachment of an intact layer of cells containing virtually all the astrocytes, leaving undisturbed a population of firmly attached cells. Primary microglial cultures were identified as >99% IBA1+ by immunofluorescent staining, and ∼97.8% positive for the microglial marker CD11b (1:50; M1/70, BioLegend #101251) and negative for the astrocyte marker GLAST (1:11, ACSA-1, Miltenyi Biotec, #130-095-821) by fluorescence-activated cell sorting (FACS) analysis (Supplementary Fig. 5E and F). The following reagents were used for analysis: anti-IBA1 (1:500, Wako Chem, #019-19741), anti-GFAP (1:500, 4A11, 1B4, 2E1, BD Pharmingen #556330), anti-CD11b (1:50; M1/70, BioLegend #101251) and anti-GLAST (1:11, ACSA-1, Miltenyi Biotec, #130-095-821). For microglial proliferation analysis, pure microglial cultures were stained with CellTrace™ Violet Cell Proliferation Kit (Invitrogen, #C34517), according to the manufacturer’s instructions. Human astrocytes (ScienceCell, #1800) were grown according to the manufacturer’s instructions. In some studies, cells were treated with 2 mM N-nitro-L-arginine methyl ester hydrochloride (L-NAME, Sigma, N5751), or anti-mouse CSF1R monocloncal antibody (mAb) (BioXcell, clone AFS98, #BE0213), anti-mouse TGFβ mAb (BioXcell, clone 1D11.16.8, #BE0057), anti-mouse IL10R mAb (BioXcell, clone 1B1.3A, #BP0050), anti-mouse IFNγ mAb (BioLegend, clone XMG1.2, 505834), anti-mouse IL6R mAb (BioXcell, clone 15A7, #BE0047) or appropriate isotype control IgG (BioXcell, #BE0090, BE0090). All mAbs were used at a final concentration of 25 µg/ml.
Preparation of glioblastoma-conditioned medium
A total of 2 × 106 GL261 cells were cultivated in a 10 cm culture plate for 48 h, media was removed and 8 ml of fresh medium, containing DMEM with 1% FCS and 1% penicillin-streptomycin was added. The supernatant was collected after 24 h, centrifuged at 500g for 10 min at 4°C, sterile filtered using a 22-μm filter and stored at −80°C. For microglial stimulation, glioblastoma-conditioned medium (GBM-CM) was diluted 2:1 with DMEM.
Preparation of astrocyte-conditioned medium
A total of 5 × 106 primary astrocytes were cultivated in a 10 cm culture plate, and stimulated for 12 h with DMEM containing 1% FCS and 1% penicillin-streptomycin [to generate astrocyte-conditioned medium (ACM)] or GBM-CM (to generate T-ACM). Media was removed and 8 ml of fresh medium, containing DMEM with 0.5% BSA (Millipore, #810683) and 10 mM HEPES, was added. The supernatant was collected after 24 h, centrifuged at 500g for 10 min at 4°C, sterile filtered using a 22-μm filter and stored at −80°C.
Chemotaxis assay
Spleen monocytes (CD11b+/CD3−/CD45R−/CD117−/Ly-6G−/NK1.1−/SiglecF−/SSClow) were isolated from C57BL/6 mice using EasySep Mouse Monocyte Isolation Kit (Stemcell, #19861). Monocytes were stained with CellTrace CFSE Cell Proliferation (Thermo Fisher Scientific, #C34554) and a total of 2 × 103 monocytes per well were plated in IncuCyte ClearView 96 well Cell Migration Plate (Sartorius, #4582), which was precoated with 50 μg/ml Matrigel® (Corning, #FAL356237). ACM or T-ACM were added to the lower chamber and monocytes migration to the lower chamber was measured following 2 h incubation at 37°C with IncuCyte ZOOM (v.2020C). The chemotaxis index was defined as the percentage of monocytes that infiltrated the lower chamber. In some experiments, the T-ACM was supplemented with 30 μg/ml of anti-mouse CCL2 neutralizing mAb (BioXcell, clone 2H5, #BE0185) or isotype control (BioXcell, #BE0091).
Tumour model and treatments
For intracranial mouse glioma, mice were anaesthetized, positioned in a Stereotaxic Alignment System and injected with 1.5 × 104 GL261 or CT-2A cells in 2 μl of DMEM. Injections were made to the right frontal lobe, ∼2.5 mm lateral and 0.1 mm caudal from bregma at a depth of 3 mm. In vivo bioluminescence imaging, following XenoLight D-luciferin potassium salt (150 mg/kg) i.p. administration, was determined using the IVIS Spectrum system (PerkinElmer). Ganciclovir (GCV, Cymevene, Roche #SAP-10051872; 25 mg/kg), diphtheria toxin (DT, Sigma-Aldrich, #D0564; 1100 ng/mice nasally) or vehicle control (PBS) were administered daily following tumour establishment (Day 10), as previously described.19,28 CD8+ T-cell depletion was performed using an anti-CD8 monoclonal antibody (53.6-7, Bioxcell) or an isotype control monoclonal antibody (2A3, Bioxcell), as previously described.9 GBM-bearing mice were intraperitoneally injected (0.1 mg/mouse) with an anti-CD8 or an isotype control mAbs, 1 day before and 7 days after, GCV administration. CD8 T-cell depletion was validated by FACS analysis using a Sony SH800 FACS instrument (Sony Biotechnology). Data analysis was performed using FlowJo v.10 (TreeStar, USA). The following reagents were used for analysis: anti-CD45 (1:100; 30-F11, BioLegend, #103106), anti-CD3ε (1:50; 145-2C11, BioLegend #100335) and anti-CD8a (1:100; 53-6.7, BioLegend, #100765).
Immunofluorescence
Animals were perfused with ice-cold PBS, followed by 4% paraformaldehyde in 0.1 M PBS. Tissues were cryoprotected in 0.1 M PBS plus 30% sucrose and cut with cryostat into 10-μm thick sections. Sections were blocked in 5% goat serum and 5% donkey serum containing 0.3% Triton™ X-100 (Sigma-Aldrich, #9002-93-1) and 0.3 M Glycine (Holland Moran, #BP381-1), and incubated overnight at 4°C with following antibodies: GFAP (chicken, 1:1000, Abcam, #ab4674), IBA1 (rabbit, 1:1000, Wako Chem, #019-19741), HA (rat, 1:300, Roche, #11-867-423-001), MBP (chicken, 1:50, Chemicon #AB9348), NeuN (mouse, 1:100, EMD Millipore, #MAB377) DRAQ7 (1:500 BioLegend, #424001), Cleaved Caspase 3 (rabbit, 1:500, Cell Signaling Technology, #9664S), ABCA1 (rabbit, 1:200, Novusbio, #NB400-105), Annexin A2 (rabbit, 1:500, proteintech, #11256-1-AP), Ki67 (Rat, 1:500, Thermo Fisher Scientific, #14-6698-82), PD-L1 (Rat, 1:500, BioXcell, #BE0101) or CD74 (Rabbit. Clone EPR25399-94, 1:500, Abcam, # ab289885). The next day sections were washed five times and incubated with an appropriate fluorophore-conjugated goat secondary antibodies (1:500) from Abcam (#ab150167 or ab150176) or Thermo Fisher scientific (#A11037, A11020 or A32733) for 1 h at room temperature. Sections were mounted in Prolong™ Gold containing DAPI (Invitrogen, P36935). Images were acquired using a Leica SP8 confocal microscope and Leica LAS AF software and processed using Fiji and LAS X. All settings were kept the same during image acquisition of comparable images including magnification, laser intensity and optical configuration.
Analysis of TAMs
GL261-implanted mice were killed 17 days after implantation and single-cell suspension was prepared as we previously described.19 In brief, mice were anaesthetized and perfused with 10 ml of PBS and the brain was isolated, enzymatically dissociated using collagenase type III (Worthington Biochemical, #LS004182) and DispaseII (Roche, #04942078001), and mechanically dissociated using gentleMACS™ Dissociator. Myelin was removed by resuspending the homogenate in 25% Percoll solution, underlaid by 75% Percoll and overlaid with 5 ml HBSS. Centrifugation at 1000g for 30 min with slow acceleration and without breaks created a gradient that separated the cell pellet on the bottom from the myelin, which was carefully aspirated. Cells were carefully collected from the 75–25% interphase. TAMs (CD11b+CD45+) or microglial cells (CD11b+CD45dim) were analysed or sorted into TRIzol LS reagent (Invitrogen, #10296028) for RNA purification using Sony SH800 FACS (Sony Biotechnology). Data analysis was performed using FlowJo v.10 (TreeStar, USA). The following reagents were used for analysis: anti-CD11b (1:50; M1/70, eBioscience, #12-0112-82, BioLegend #101251), anti-CD45 (1:100; 30-F11, BioLegend, #103116) and anti-PD-L1 (1:50; 10F.9G2, BioLegend, #124314).
Filipin III staining
Filipin III was used to evaluate cholesterol content in the cells using FACS and immunofluorescence staining. Filipin III (Cayman Chemical Company, #70440) staining was performed at 100 µg/ml for 25 min at room temperature, as previously described.29 For FACS analysis cells were first fixated with 0.5% in paraformaldehyde (Electron Microscopy Sciences, #15710; dissolved in PBS), stained and then analysed by CytoFLEX LX flow cytometer (Beckman Coulter) equipped with a 355 nm (UV) laser.
Metabolic flux assays
Real-time extracellular acidification rate (ECAR) and OCR measurements were made with an XF-96 Extracellular Flux Analyzer (Seahorse Bioscience). 1 × 105 cells were plated into each well of Seahorse X96 cell culture microplates and preincubated at 37°C for 24 h in 5% CO2 in either FCS or LPDS supplemented media. The sensor cartridge for the Xfe96 analyser was hydrated in a 37°C non-CO2 incubator a day before the experiment. OCR and ECAR were measured under basal conditions and after the addition of the following compounds: 1.5 μM oligomycin, 2 μM FCCP [carbonyl cyanide4-(trifluoromethoxy)phenylhydrazone], 0.5 μM rotenone and 0.5 μM antimycin, 10 mM glucose and 50 mM 2-deoxy-D-glucose (all obtained from Sigma) as indicated. Data were expressed as the rate of oxygen consumption in pmol/min or the rate of extracellular acidification in mpH/min, normalized to DNA labelling in individual wells determined by the Hoechst 33342 staining. Results were collected with Wave software v.2.4 (Agilent).
Cell viability assay
GL261, CT-2A or astrocytes were seeded at a density of 1.5 × 105 cells per well in a six-well plate. Cells were treated for 72 h FCS- or LPDS- supplemented media for 5 days. In some studies, cells were treated with lovastatin (Thermo Scientific, #PH1285R) at 2.5 or 5 μM (GL261, CT-2A, astrocytes), 24(S)-hydroxy-cholesterol (Cayman Chemical Company, #10009931) at 2.5, 5 or 10 μM (GL261 cells) or at 1.25, 2.5, 5 μM (CT-2A cells) for 72 h, or 250 ng/ml cholesterol (Sigma-Aldrich, #C4951). Cell death was analysed by FACS analysis based using Annexin-V assay according to the manufacturer’s instructions (BioLegend, #640951, 640941). Microglial cell death was determined by LDH Assay (Sigma-Aldrich, #4744934001) according to the manufacturer’s instructions.
Astrocyte-specific lentivirus
pLenti-GFAP-EGFP-mir30-sh Abca1 (Fig. 6F) harbouring shRNA sequences against Abca1 was cloned into the pLenti-GFAP-EGFP-mir30-shB4galt6 vector backbone19 and replaced by validated Abca1-targeting shRNA sequence (MISSION® TRC shRNA clone #TRCN0000271860, Sigma-Aldrich), as we previously described.19 Non-targeting shRNA vector (pLenti-GFAP-EGFP-mir30-shNT) was previously described.19 pLenti-GFAP-CRISPRv2GFP-sgRNA vectors (Supplementary Fig. 8B), were generated by cloning the relevant single-guide RNA (sgRNA) to pLentiCRISPRv2GFP (Addgene #82416) as previously described.30 The EF-1α promoter, which drives the expression of the polyprotein Cas9-P2A-GFP, was then replaced with the gfaABC1D promoter (GeneArt, Thermo Fisher Scientific) using XbaI and EcoRI restriction enzymes (#R3101 and #R0145, New England Biolabs). CRISPR–Cas9 sgRNA sequences were designed using the Broad Institute’s sgRNA GPP Web Portal (https://portals.broadinstitute.org/gpp/public/analysis-tools/sgrna-design). All sequences are detailed in Supplementary Table 1. Lentivirus particles were then generated by transfecting 3.9 × 106 293T cells using 24.4 µg PEI MAX (Polysciences, #24765-1) with 6.1 µg pLenti-GFAP-EGFP-mir30-shRNA or pLenti-GFAP-CRISPRv2GFP-sgRNA vectors with the packing plasmids [4.6 µg psPAX2 (Addgene, #12260) and 1.5 µg pMD2.G (Addgene, #12259)]. Forty-eight hours after transfection supernatant was concentrated using Lenti-X Concentrator (Takara, #631232) and stored at −80°C until use. The viral titrate was determined using the quantitative real-time polymerase chain reaction (qPCR) Lentivirus titration kit (ABM, #LV900) according to the manufacturer’s instructions.
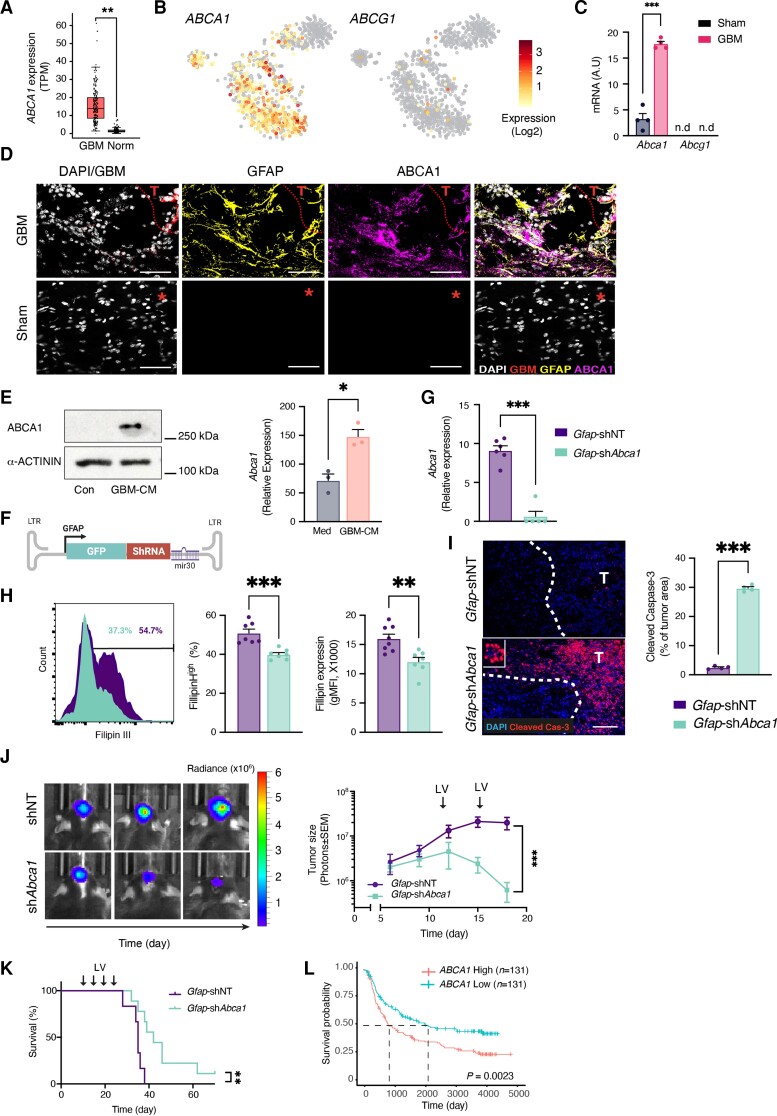
Figure 6.
Astrocytic expression of ABCA1 regulates glioma cholesterol levels and tumour progression. (A) Box plot analysis of TCGA gene expression for ABCA1 in normal (Norm; n = 207) or GBM patients (n = 163). n represents the number of patients per group. (B) Heat map overly of the scRNAseq gene expression intensity of ABCA1, and ABCG1 in TAAs from GBM patients42 (astrocyte cluster as in Supplementary Fig. 3A). (C) qPCR analysis of Abca1 expression in Ribotag-isolated astrocytes GBM-bearing mice, as in Fig. 2. (D) Representative immunofluorescence images of sham-injected or GL261-bearing mice stained for ABCA1 (purple), GFAP (reactive astrocytes, yellow), GBM, (GFP+-GL261, red) and nuclei (DAPI, white); asterisk indicates the injection coordinates in sham, scale bars = 35 μm (n = 2 biologically independent experiments, four mice per group). (E) Representative immunoblot (left) and quantitative reverse transcription (right) analyses comparing ABCA1 levels in primary astrocytes treated with GBM-CM for 24 h (immunoblot) or 6 h (quantitative reverse transcription; expression normalized to Ppia) (n = 3 biologically independent experiments). (F) Schematic map of the astrocyte-specific shRNA lentiviral vector. (G–K) Intracranially injection of astrocyte-specific shAbca1 lentivirus attenuates GBM progressions. Non-targeting (Gfap-shNT) or Abca1-targeting (Gfap-shAbca1) astrocyte-specific lentiviruses were injected into the TME of GL261-bearing mice every 5 days (as indicated) starting 9 days after tumour implantation. (G) Quantitative reverse transcription analysis of FACS-sorted GFP+-astrocytes for Abca1 on Day 18; expression normalized to Ppia. Data are representative of two independent experiments (n = 4 biologically independent samples). (H) Representative flow cytometry plots of Filipin III staining in tdTomato+-expression GL261 cells from each group are shown on the left and quantification analyses of the percentage of Filipin+ GL261 cells and filipin intensity (gMFI) are on the right. Data are representative of two independent experiments (n = 4 biologically independent samples per group). (I) Representative immunofluorescence images of cleaved caspase-3 (red) and nuclei (DAPI, blue) 10 days after lentivirus injection are shown on the left and quantification is on the right. T indicates the tumour. Data are representative of two independent experiments (n = 4 biologically independent samples per group). (J) Representative images of GL261-derived bioluminescence from each group are shown on the left and quantification of tumour size on the right. Data are representative of three independent experiments with n = 6 mice/group. (K) Kaplan–Meier curves assessing overall survival of these groups. Data are representative of two independent experiments with n = 6 mice/group. (L) Kaplan–Meier curves assess the overall survival of GBM patients on the basis of ABCA1 expression; n represents the number of patients per group. Data are shown as mean ± SEM. P-values were determined by two-sided Student’s t-tests (A, C, E, G, H and I), two-way ANOVA (J) or log rank (Mantel–Cox) test (K and L). *P < 0.05, **P < 0.01, ***P < 0.001. n.d. = not detected.
Immunoblotting
Cells were lysed in RIPA buffer supplemented with protease inhibitors (Cell Signaling). A total of 35 µg of sample was separated by 7.5, 10 or 12% Tris-Glycine gels, transferred to nitrocellulose membranes (Millipore) and developed with antibodies against anti-ABCA1 (rabbit, 1:1000; Novus Biologicals, #NB400-105), anti-low-density lipoprotein receptor (LDLR) (rabbit, 1:500, ProteinTech, #10785-1-AP), anti-ACTIN (mouse, 1:1000-30 000; MP Biomedicals, #08691001), anti-a-ACTININ (mouse, 1:1000; Cell Signaling Technology, #69758S) and anti-VINCULIN (rabbit, 1:1000, Proteintech, #26520-1-AP). Blots were developed using a Clarity Western ECL kit (Bio-Rad, #1705061) on Amersham Imager 600 (GE Healthcare). Expression levels were normalized to ACTIN, α-ACTININ or VINCULIN. Quantification was done using Image Studio Lite software v.5.2 (LI-COR Biosciences).
Metabolic pathway analysis
iMAT31 was used to incorporate gene expression levels into the metabolic model to predict a set of high and low activity reactions. Network integration was determined by mapping the genes to the reactions according to the metabolic model, and by solving constraint-based modelling optimization to find a steady-state metabolic flux distribution.32 By using the constraint-based modelling approach, we assign permissible flux ranges to all the reactions in the network, in a way that satisfies the stoichiometric and thermodynamic constraints embedded in the model and maximizes the number of reactions whose activity is consistent with their expression state. The pathway enrichment analysis was carried out by a hypergeometric test where the background is the number of reactions found in the human model, and the overlap of each metabolic pathway with the set of active (top 20%) and inactive reactions (low 20%) is then examined via hypergeometric test.
nCounter gene expression
Total RNA (100 ng) was analysed using the nCounter Mouse Immunology V1 Panel according to the manufacturer’s instructions (NanoString Technologies). Data were analysed using nSolver Analysis software. Functional enrichment analysis was performed using the Expander33 and g:Profiler34 platforms.
RiboTag
Sham or tumour-bearing mice were sacrificed and were subjected to perfusion through the left ventricle with ice-cold PBS, followed by perfusion with 10 ml ice-cold 1% PFA (Electron Microscopy Sciences, #15710; dissolved PBS). Brains were harvested, and the right hemisphere was homogenized as previously described.35 Briefly, samples were homogenized using 7 ml Dounce homogenizer in 10% w/v supplemented homogenization buffer. Homogenate was transferred to an Eppendorf tube and centrifuged for 15 min at 15 000g at 4°C. The supernatant was transferred to a new tube and 5 µl of mouse monoclonal anti-HA antibody was added (BioLegend, #901515). Samples were placed on a rotator for 6 h at 4°C. Pierce beads (200 µl) (Pierce, #88803) or 70 µl of Dynabeads (Invitrogen, #10004D) were washed with 800 μl homogenization buffer and coupled with the tissue-antibody homogenate for an overnight rotation at 4°C. The following day, samples were placed on a magnet and the supernatant was removed before washing the pellets three times for 10 min in a high salt buffer. RLT lysis buffer (350 µl) was added to the beads and RNA was extracted using the RNeasy Mini kit (Qiagen, #74104) per the manufacturer’s instructions and quantified with a Qubit RNA HS Assay Kit (Thermo Fisher, #Q32852). RNA was kept at −80°C until use.
RNA-sequencing and processing
RNA extracted from immunoprecipitated polyribosomes was used to prepare libraries by NEBNext® rRNA depletion kit (#E6310), NEBNext® Ultra™ II RNA Library Prep Kit for Illumina (#E7770G) and NEBNext® Multiplex Oligos for Illumina® (#E7335G) kit, according to the manufacturers’ instructions. Libraries were normalized, pooled and sequenced on the Illumina NextSeq 500 with the NextSeq 500/550 Mid Output Kit v.2.5 (150 Cycles) (#20024904), according to the manufacturers’ instructions. RNA-sequencing (RNA-seq) reads were aligned using Kallisto36 to mouse genome v.mm10, and expression levels were calculated using RSEM.37 The data were normalized using TMM normalization and differentially expressed genes were defined using the differential expression pipeline on the raw counts with a single call to the function Bioconductor package DESeq2 v.1.24.0 in R38 (FDR-adjusted P-value <0.05). Heat map figures were generated using the pheatmap package39 and clustered using Euclidian distance. Functional enrichment analysis was performed using the Expander33 and g:Profiler34 platforms.
Human gene expression and survival analysis data
Bulk gene expression of matched GBM patients and normal brain tissue was analysed using the GEPIA portal40 based on The Cancer Genome Atlas (TCGA) and Genotype-Tissue Expression (GTEx) datasets. P < 0.01 was considered statistically significant. Survival analysis was performed using the Chinese Glioma Genome Atlas (CGGA),41 P < 0.05 was considered statistically significant. Single-cell analysis was performed on data of GBM patients [isocitrate dehydrogenase 1 (IDH1)-negative, grade IV] reported by Darmanis et al.42 using Bbrowser43 (v.2.10.40; BioTuring Inc.). Cell-linage clusters were defined on the basis of the expression of known markers44,45 (TAMs, PTPRC, C1QC and TMEM119; OPCs, PDGFRA, GPR17, OLIGO1 and AC058822.1; TAAs, SLC7A10, GJA1 and AQP4; Oligodendrocytes, MOBP and MOG; endothelial cells, CD34 and PECAM1).
Statistical analysis
Samples were randomly allocated into experimental groups at the start of each individual experiment. Genetically identical mice were randomly allocated to experimental groups at the start of the experiments. For in vitro experiments, biological samples were randomly allocated into experimental groups at the beginning of the experiment. Statistical analyses were performed with Prism v.9.3 software (GraphPad), and the statistical tests used are indicated in the individual figure legends. P < 0.05 was considered statistically significant. All error bars represent SEM. Box-and-whisker plots show median, interquartile interval, minimum and maximum values.
Data availability
RNA-seq data that support the findings of this study have been deposited in the Gene Expression Omnibus GSE193526. All other data supporting the findings of this study are available from the corresponding authors on request.
Results
Tumour-associated astrocytes promote glioblastoma pathogenicity
Reactive astrocytes, characterized by elevated expression of glial fibrillary acidic protein (GFAP), have a considerable impact on the course of traumatic, ischaemic, inflammatory and degenerative diseases of the CNS.17,18 Astrocytes are one of the most abundant non-cancerous cell types in glioblastoma,42 and reactive astrocytes are present around the tumour margins (Fig. 1A). Accumulating ex vivo data concerning the cross-talk between astrocytes and glioma cells,24 and specific inhibition of astrocytic signalling in medulloblastoma and brain metastasis tumour models,25,46,47 suggest that astrocytes might play a role in glioblastoma progression. However, the role reactive astrocytes play in glioblastoma pathogenicity in vivo is not well understood.
Figure 1.
TAA depletion regress GBM progression. (A) Representative immunofluorescence images of reactive TAAs stained for GFAP (cyan) crowning a glioblastoma (GBM) tumour (GFP+-GL261, white), and nuclei (DAPI, yellow). The right image is an expansion of the area marked by the white box. (n = 3 biologically independent experiments, three mice per group). Scale bar = 1000 μm. (B–G) Wild-type (WT), or Gfap-TK GBM-bearing mice were treated daily with GCV (25 mg/kg) from Day 10 until the experimental end point as illustrated in B. (C) representative immunofluorescence images of reactive-astrocyte depletion at the tumour margins (GFP+-GL261, white), as detected by GFAP (cyan) and nuclei staining (DAPI, yellow). Scale bars = 500 μm. Data are representative of three independent experiments with n = 4 mice/group. (D and E) Tumour size in GCV-treated wild-type or Gfap-TK GBM-bearing littermates. (D) Representative images of GL261-derived bioluminescence from each group are shown on the left and quantification of tumour size on the right. Data are representative of five independent experiments with n = 6 mice/group. (E) Representative images from each group, 17 days after GL261 cell implantation, are shown on the left with quantification of tumour size on the right, tumour (GL261 cells in purple) and nuclei (DAPI; white). Scale bar = 1000 μm. Data are representative of three independent experiments with n = 5 mice/group. (F) Bodyweight assessment of mice from D. (G) Kaplan–Meier curves assessing overall survival. Data are representative of three independent experiments with n = 8 mice/group. Data in D–F are shown as mean ± SEM. P-values were determined by two-way ANOVA (D–F) or log rank (Mantel–Cox) test (G). *P < 0.05, **P < 0.01, ***P < 0.001, n.s. = not significant.
To study the role of TAAs in glioblastoma, we analysed the course of tumour progression in mice expressing the herpes simplex virus thymidine kinase (HSVtk) under the Gfap promotor (Gfap-TK), in which GCV administration can deplete reactive and proliferating astrocytes.19,48,49 For this purpose, we intracranially injected GFP+GL26l-Luc (GL261) glioma cells, into syngeneic C57Bl/6 wild-type or Gfap-TK littermates. Ten days after tumour implantation, once the tumours were established, the mice were treated with GCV for 7 days, before an examination of the presence of GFAPhigh reactive astrocytes at the tumour margins (Fig. 1B). In line with previous reports of the specificity and efficiency of the Gfap-TK model,19,48,49 we found that GCV administration to GBM-bearing mice results in specific ablation of reactive astrocytes around the tumour in the Gfap-TK mice but not in wild-type mice (Fig. 1C). To test the functional contribution of reactive astrocytes to GBM progression, we repeated the same experimental paradigm, as in Fig. 1B, and evaluated the tumour growth by bioluminescence imaging and histology (Fig. 1D and E). While neither GCV treatment of wild-type mice nor genetic insertion of the HSVtk into Gfap-TK mice, affected GBM progression (Supplementary Fig. 1A and B), GCV treatment of GL261-bearing Gfap-TK mice did result in dramatic tumour regression (Fig. 1D and E). Of note, clinical trials aimed at over-expressing HSVtk in high-grade gliomas have shown minimal to no improvement in tumour burden and survival.50 Collectively, these data suggest that depletion of TAAs halts glioma growth. To further validate these findings, we used an alternative astrocyte-depleting transgenic mice model, in which the DT receptor is expressed under the control of the murine Gfap promoter (GfapCRE:iDTR, Supplementary Fig. 1C). Astrocytes are ablated in the Gfap CRE:iDTR mice by administration of diphtheria toxinA (DT-A). We, therefore, crossed heterozygote GfapCRE with homozygotes mice harbouring a Cre-inducible expression of DTR (iDTR). GfapCRE:iDTR and iDTR littermates were intracranially implanted with GL261 gliomas, and were treated with DT-A once the tumours were established. The results indicate a significant regression in tumour size in the GL261-bearing GfapCRE:iDTR mice, compared to their iDTR tumour-bearing littermates (Supplementary Fig. 1D), which is in agreement with the data obtained from the Gfap-TK astrocyte-depleting model (Fig. 1D).
Next, we examined the effect of astrocyte depletion on the disease pathophysiology. To this end, we intracranially implanted GL261 cells into wild-type or Gfap-TK littermates. The mice were treated with GCV (as in Fig. 1D) and the weight and survival were monitored. In accordance with the observed tumour regression, we found that astrocyte ablation significantly attenuates the weight loss in GL261-bearing Gfap-TK mice compared to their tumour-bearing wild-type littermates and dramatically improves their survival (Fig. 1F and G, respectively). In contrast, GCV administration to GL261-bearing wild-type mice, or expression of the HSVtk transgene without GCV treatment, had no effect on survival (Supplementary Fig. 1E and F). Interestingly, the high survival rate of the GL261-implanted Gfap-TK mice persisted even after GCV administration was terminated following the death of all the mice in the wild-type group (Fig. 1G). It should be noted that prolonged treatment (3–4 weeks) of the GfapCRE:iDTR mice with DT-A led to lethal bowel inflammation, presumably due to ablation of GFAP+ enteric glia in the distal small intestine. This was unrelated to tumour burden (data not shown), but as a result the GfapCRE:iDTR model was only used to monitor tumour growth for a short regimen of DT-A treatment (7 days), well before the appearance of any DT-dependent clinical phenotype, and was not used in the survival studies. Importantly, our observations that astrocytes support tumour pathogenicity were validated by the finding of similar results in an alternative syngeneic GBM model (CT-2A glioma cells) (Supplementary Fig. 1G–I). Thus, these data suggest that reactive TAAs play a pivotal role in supporting glioma progression and tumour pathogenicity.
Tumour-associated astrocytes acquire a pro-tumourigenic phenotype
To investigate the mechanisms by which reactive astrocytes support GBM pathogenicity, we first analysed their transcriptional programme using the RiboTag strategy, in which the expression of a haemagglutinin (HA)-tagged ribosome subunit, under the control of a CRE recombinase, allows for the analysis of cell-specific ribosome-associated mRNA. Mice carrying the floxed Rpl22-HA allele35 (Ribotag mice) were crossed with GfapCRE mice to generate GfapCRE:Rpl22HA mice. To implement the RiboTag method for the study of TAAs, GfapCRE:Rpl22HA mice were intracranially implanted with GL261 glioma cells (GBM) or injected with PBS (sham). Seventeen days later, RNA was retrieved from mouse brain extract (input) by anti-HA immunoprecipitation (IP-HA, Fig. 2A). First, we examined the co-localization of the HA tag specifically with astrocytes in the GfapCRE:Rpl22HA mice and the cell-type specificity of the obtained translatomes. Analysis of astrocyte-enriched RNAs (immunoprecipitated by an anti-HA antibody) confirmed enrichment of astrocyte-specific gene expression, and concomitant depletion of neuronal, oligodendroglial and macrophage-specific gene expression (Fig. 2B). This was further validated by immunostaining (Fig. 2C). As the next step, we examined the transcriptional phenotype of the reactive astrocytes in the GBM microenvironment. Principal component analysis demonstrated distinct differences between the TAAs (GBM) and the astrocytes isolated from the sham control group (Supplementary Fig. 2A). Indeed, we found that 3884 genes were differentially expressed (FDR-adjusted P-value <0.01, fold change >2) between the two groups of astrocytes (Fig. 2D). These included an increased expression of immune-associated genes (Chi3l1, Cd74),24 complement components (C1s, C3),24,51 chemokines (Ccl2)52 and proliferation (Mki67, Anxa2),24 transcription factors associated with glial support of brain tumours (Stat1, Stat3, Ahr),9,53 as well as genes associated with astrocyte cross-talk with microglial cells (Csf1, Cd44)7,8,54 or immunosuppression (e.g. Cd274, Gpnmb)24,55,56 (Fig. 2E and Supplementary Fig. 2B and C). In line with our findings, analysis of single-cell RNA-sequencing data (scRNAseq) released by Darmanis et al.42 of the TME of GBM patients (IDH1-negative, grade IV) identified a similar expression pattern in TAAs (Supplementary Figs 3A and B, and 4D). Functional analysis34 of the differentially expressed genes, identified enrichments in three main categories, namely perturbation of metabolic process, immune regulation, and cell proliferation (Fig. 2F and Supplementary Table 2). Notably, the astrocyte immunosuppressive activity in the TME is not dependent on the CD8+ T-cell response, as the latter depletion did not modulate astrocyte-driven GBM pathogenicity (Supplementary Fig. 2D and E). Thus, these data identify a distinct phenotype of TAAs in the glioblastoma microenvironment, whose transcriptomic characteristics resemble those of astrocytes from GBM patients. These results suggest that astrocytes might support glioma pathogenicity by directly promoting immunosuppression, regulating the neighbouring immune cells and contributing to the metabolic landscape of the TME.
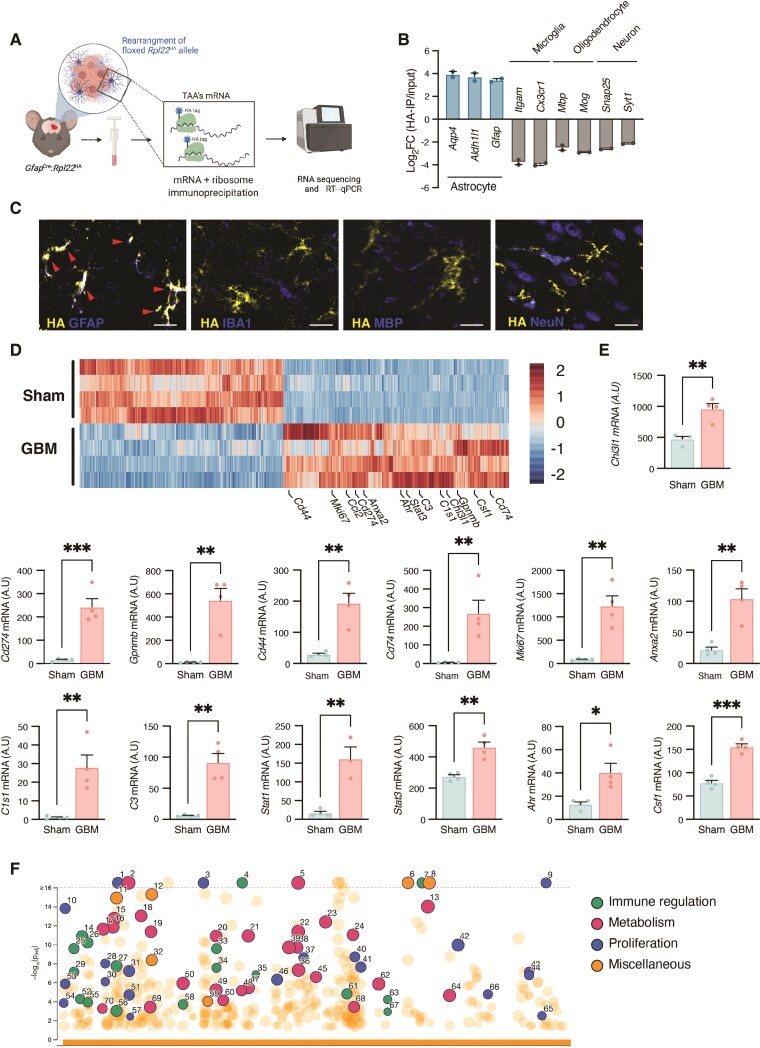
Figure 2.
RiboTag analysis of TAAs reveals activation of immunoregulatory pathways and perturbation of metabolic circuits. (A) Illustration of the RiboTag workflow. (B) Enrichment of astrocyte-specific gene expression and de-enrichment of neuronal, oligodendroglial and TAMs gene expression, shown as the log fold change calculated between astrocyte RNAs immunoprecipitated by anti-HA antibody versus brain total cell RNAs from the original homogenate (including astrocytes). (C) Representative immunofluorescence images of GfapCRE:Rpl22HA mice demonstrating co-localization of ribosome-associated HA Tag (yellow) with specific cell-lineage markers of astrocytes, TAMs, oligodendrocytes or neurons cell-linage specific markers (GFAP, IBA1, MBP or NeuN, respectively; blue). Co-localization (white) is identified by red arrowheads. Scale bars = 20 μm. (D) Heat map of differently expressed genes (at least 2-fold, Padj < 0.01) of astrocytes derived from sham-injected or GBM-bearing brain hemisphere specimens. (E) RiboTag-isolated mRNA expression in astrocytes from GL261-bearing (GBM) or PBS-injected (Sham) GfapCre:Rpl22HA mice; 17 days after intracranial injection. Data are representative of three independent experiments with n = 4 biologically independent samples, pool of two mice per sample. (F) Manhattan plot of gene ontology (GO) of upregulated in TAAs. Similar pathways are colour-coded: Immune regulation (green), metabolism (pink), proliferation (blue), miscellaneous (orange). Highlighted GO are numbered and detailed in Supplementary Table 2. Data in B and E are shown as mean ± SEM. P-values were determined by one-way ANOVA (B) or two-sided Student’s t-tests (E). *P < 0.05, **P < 0.01, ***P < 0.001.
Astrocytes modulate the recruitment of GBM-infiltrating macrophages
The population of TAMs is made up of brain-resident microglia and monocyte-derived macrophages from the periphery.42,57,58 Together, they account for 30–50% of the tumour mass, and their number is positively correlated with the tumour malignancy grade and inversely correlated with overall survival in patients with recurrent glioblastoma.57 Astrocytes regulate leukocyte infiltration to the CNS through several mechanisms that range from the regulation of blood–brain barrier permeability to the secretion of chemokines.17,18 Indeed, we found that TAAs significantly upregulate their chemoattractant profile, compared to astrocytes isolated from the sham control (Fig. 3A), of which CCL2 and CXCL16, chemokines associated with TAMs tumour-promoting activity,52,59 were significantly expressed in astrocytes from GBM patients42 (Fig. 3B). There are many factors that may mediate TAM chemoattraction to the GBM,57 but the main pathway for the recruitment of peripheral macrophages to the TAM compartment is considered to be via CCR2 signalling, which is triggered by CCL2 and CCL7. Accordingly, our analysis of the GBM Genome Atlas data revealed high levels of expression of CCR2, CCL2 and CCL7, which were associated with decreased survival in glioblastoma patients (Supplementary Fig. 4A–C). Under neuroinflammatory conditions, astrocytes regulate leucocyte infiltration to the CNS through several mechanisms, including the secretion of CCL2.19,23,60 However, the contribution of TAAs to the recruitment of TAMs during GBM progression is not well understood. To address this question, we first screened scRNAseq data from GBM patients42 for astrocytic expression of CCL2 and CCL7. The results indicated that while astrocytes express high levels of CCL2, CCL7 was barely detectable (Supplementary Fig. 4D). Similarly, the levels of Ccl2 were elevated in astrocytes isolated from GL261-bearing mice using the RiboTag system (Fig. 3C) and primary astrocytes treated with tumour-conditioned media generated using murine GL261 glioma (GBM-CM) (Fig. 3D). Interestingly, Ccl7 expression was not detected in Ribotag-isolated astrocytes, or GBM-CM treated primary astrocytes. Accordingly, we found that ACM, harvested from GBM-treated astrocytes (T-ACM), was significantly more efficient in inducing monocyte migration than control ACM (Fig. 3E). Importantly, the use of anti-CCL2 neutralizing antibodies to block CCL2 signalling, inhibited this increase in monocyte migration, implicating CCL2 as the main chemoattractant in GBM-induced astrocyte recruitment of the monocytes (Fig. 3E). To investigate whether these observations indicate that TAAs regulate TAM recruitment to the TME, we monitored the recruitment of TAMs into the TME of GL261-bearing wild-type and Gfap-TK mice treated with GCV (as in Fig. 1B). We found that depletion of reactive astrocytes with GCV significantly reduces the accumulation of IBA-1+ TAMs in the TME (Fig. 3F). Similar results were obtained by FACS analysis of the frequency of TAMs (CD11b+CD45+) cells in the TME (Fig. 3G), suggesting that reactive astrocytes directly control the recruitment of TAMs to the GBM TME and that this process is predominantly mediated by CCL2.
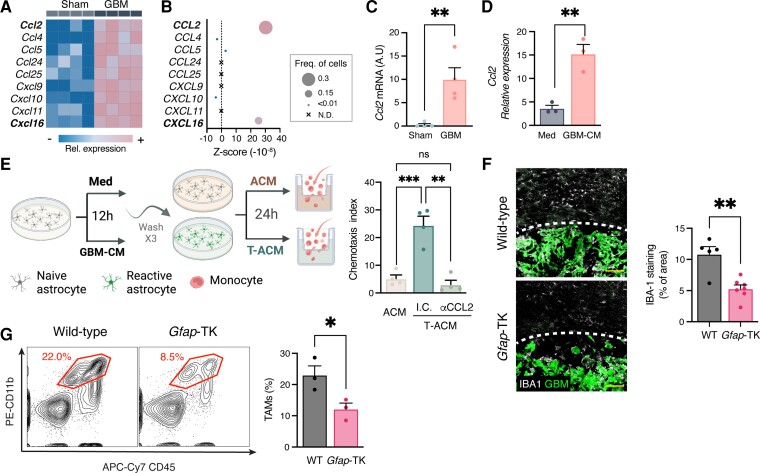
Figure 3.
TAA depletion attenuates TAM recruitment. (A–C) Chemokine expression in TAA. (A) Heat map of differently expressed chemokines (at least 2-fold, Padj < 0.01) (A) and Ccl2 mRNA expression (C) in astrocytes derived from sham-injected or GBM-bearing mice, as in Fig. 2E. Data are representative of three independent experiments with n = 4 biologically independent samples. (B) scRNAseq analysis of chemokine expression intensity (colour-coded), frequency (dot size) and Z-score in TAAs from GBM patients42 (astrocyte cluster as in Supplementary Fig. 2A). Expression levels (A and B) are defined by colour-coded expression as indicated (from blue to pink). (D) Primary astrocytes were stimulated with complete medium (Med) or GBM-CM from GL261 cells for 6 h. qPCR analysis of Ccl2 expression normalized to Ppia (n = 4 biologically independent experiments). (E) Astrocytes were stimulated with complete medium or GBM-CM for 12 h, extensively washed and used to prepare ACM or tumour cell-induced ACM (T-ACM), which was tested in an in vitro monocyte migration assay. (F and G) Analysis of TAMs in the TME. GFP+-GL261 glioma cells were intracranially injected into wild-type (WT) or Gfap-TK mice. Following tumour establishment (Day 9 after tumour implantation), mice were treated daily with GCV (as in Fig. 1); TAMs recruitment to the tumour was examined 17 days after tumour implantation. (F) Representative immunofluorescence images TAMs stained for IBA-1 (white) in the tumour (GBM, green) microenvironment (n = 2 biologically independent experiments, four mice per group). Scale bars = 100 μm. (G) Percentage of TAMs (CD11b+ CD45+) gated from GCV-treated GL261-bearing wild-type and Gfap-TK mice. Representative flow cytometry plots from each group are shown on the left and quantification analyses are on the right (n = 3 independent experiments, six mice per experiment). Data are shown as mean ± SEM. P-values were determined by one-way ANOVA, followed by Fisher’s LSD post hoc analysis (E) or two-sided Student’s t-tests (C, D, F and G). *P < 0.05, **P < 0.01, ***P < 0.001.
Tumour-associated astrocytes direct TAMs towards an immunosuppressive phenotype
TAMs support glioblastoma pathogenicity, by promoting tumour growth, immunosuppression and resistance to therapy.9,57,61–65 They acquire this distinct pro-tumour phenotype as a result of direct interactions with the tumour and the TME,57,66,67 although the mechanisms concerned are not entirely understood. We have previously demonstrated that astrocytes regulate the immunological profile of resident microglial cells and CNS-infiltrating monocytes during neuroinflammation19,23 and Henrik Heiland et al.24 reported a similar cross-talk between astrocyte and microglial cells in in vitro models of glioma. Thus, to investigate whether reactive astrocytes also control TAMs activity in the context of tumour pathology, we analysed the transcriptome from TAMs harvested from GL261-bearing wild-type and Gfap-TK mice that were treated with GCV (as in Fig. 1D) to deplete their reactive astrocytes (Fig. 4A–C and Supplementary Fig. 5A). We found that astrocyte depletion was accompanied by significant differences in the mRNA expression profile of TAMs. These changes were identified as factors associated with the functional enrichment of immune regulation pathways, TAM metabolome and regulation of cell death (Fig. 4A and Supplementary Fig. 5A). Notably, astrocyte depletion reduced the expression of a number of the hallmark genes associated with the tumour-promoting TAM phenotype,57,61–65 including Arg1, Mmp14, Stat3, Irf7, Gpnmb, Vegfa and aryl hydrocarbon receptor (Ahr), which was recently shown to regulate TAMs activity in GBM9 (Fig. 4B). Promoting immunosuppression is one of the mechanisms by which TAMs are known to contribute to GBM progression and resistance to emerging immunotherapies.57,68 Programmed death-ligand 1 (PD-L1), encoded by CD274, is among the prominent members of the checkpoint inhibitor family and has shown promising therapeutic potential in a variety of different neuropathologies, including GBM.10,14,69 Analysis of the scRNAseq data42 derived from the TME of GBM patients reveals that the high expression of CD274 seen by TCGA analysis in GBM patients can be attributed predominantly to TAMs and astrocytes. Importantly, a reduction in the expression of CD274 is associated with improved survival (Supplementary Fig. 5B–D). We found that depletion of reactive astrocytes significantly attenuated Cd274 induction and PD-L1 expression by TAMs (Fig. 4C and D), suggesting that TAAs program the TAMs to support GBM pathogenicity and induce an immunosuppressive environment. To test this hypothesis, we studied the effects of the presence or absence of astrocytes on the microglial expression of PD-L1 in response to GBM-CM stimulation (Fig. 4E). In line with our in vivo analysis (Fig. 4D), the expression of PD-L1 was inhibited in the absence of astrocytes (Fig. 4F). These data therefore support the notion that astrocytes can directly reprogramme the microglial cells to promote the immunosuppressive profile of the TME.
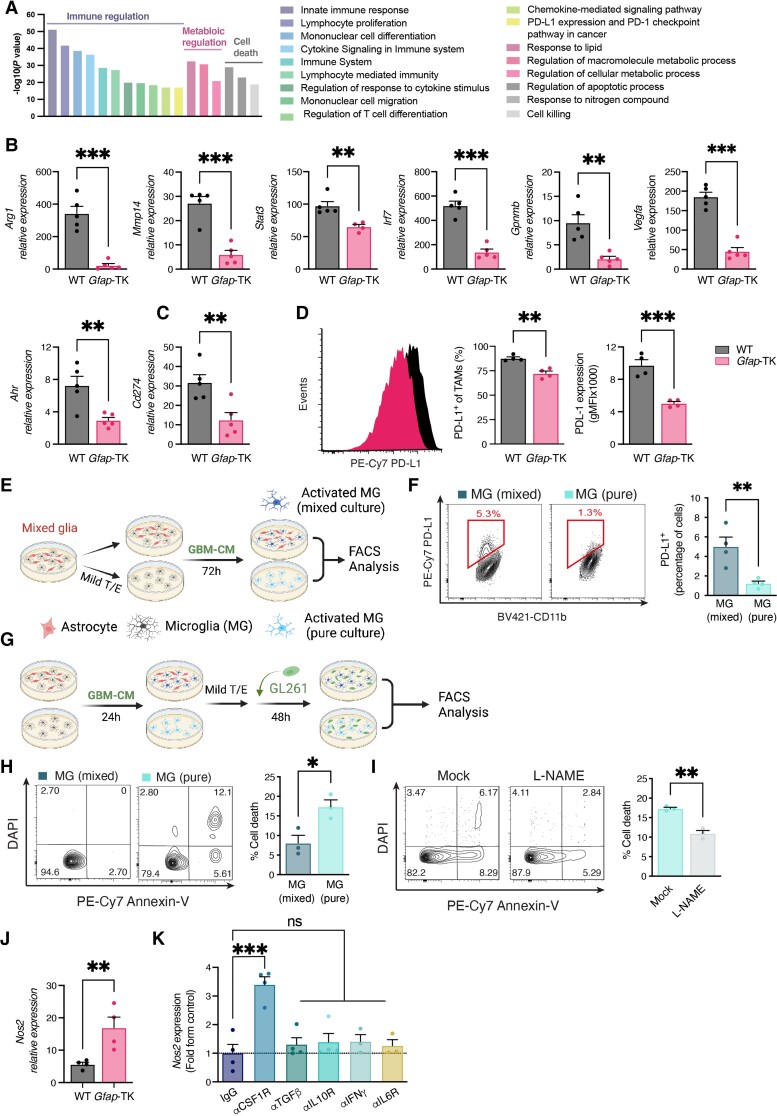
Figure 4.
TAA ablation attenuates TAM activation. (A–D) Functional analysis of TAMs (CD11b+CD45+) isolated from GCV-treated wild-type (WT) and Gfap-TK GL261-implanted mice (as in Fig. 3) 17 days after tumour implantation. (A) Pathway enrichment analysis of differentially expressed genes (at least 2-fold, Padj < 0.05) as detected by Nanostring (Supplementary Fig. 5A). (B and C) qPCR analysis of Arg1, Ahr, Stat3, Irf7, Gpnmb, Vegfa, Mmp14 and Cd274 expression in FACS-sorted TAMs; expression normalized to Ppia. Data are representative of three independent experiments (n = 4 biologically independent samples, pool of two mice per sample). (D) PD-L1 expression in TAMs. Representative flow cytometry plots from each group are shown on the left and quantification analyses of the percentage of PD-L1+ TAMs and PD-L1 expression (geometric mean fluorescence intensity, gMFI) is on the right. Data are representative of three independent experiments (n = 4 biologically independent samples, pool of two mice per sample). (E and F) Mixed glial cultures were treated with mild trypsin/EDTA to remove the astrocyte monolayer leaving only the microglia attached to the plate, or were left untreated. Cultures were then treated with GL261 conditioned media (GBM-CM) for 72 h. (F) Representative flow cytometry plots of microglial (gated as CD11b+ cells) PD-L1 expression from each group are shown on the left, and quantification analyses of the percentage of PD-L1+ microglial cells are on the right (n = 4 biologically independent experiments). (G) Mixed glia and microglial cultures were prepared as in E and treated with GBM-CM for 24 h. Microglial cultures were then isolated with mild trypsin/EDTA and co-cultured with GFP+-GL261 cells for 48 h. The viability of GFP-gated GL261 cells was then determined by Annexin-V assay. (H) Representative flow cytometry plots of GFP-gated GL261 cells from each group are shown on the left, and quantification analyses of cell death are on the right (n = 4 biologically independent experiments). (I) Microglial cultures were pretreated with L-NAME (2 mM) before co-incubation with GL261 cells, and glioma viability was analysed as in H. n = 3 biologically independent experiments. (J) qPCR analysis of Nos2 expression in FACS-sorted microglial cells (CD11b+CD45dim) isolated from GCV-treated wild-type and Gfap-TK GBM-bearing mice, as in Fig. 1; expression normalized to Ppia. Data are representative of three independent experiments (n = 3 biologically independent samples, pool of two mice per sample). (K) Mixed glial cells were treated with the indicated blocking antibodies or appropriate isotype controls (25 μg/ml), and then activated with GBM-CM. Microglia were isolated as in E, and microglial expression of Nos2 was determined by qPCR relative to Ppia (n = 5 biologically independent experiments). Data are shown as mean ± SEM. P-values were determined by two-sided Student’s t-tests (A–J) and one-way ANOVA, followed by Fisher’s LSD post hoc analysis (K) or *P < 0.05, **P < 0.01, ***P < 0.001; ns = not significant.
Most microglial cells in non-malignant or regressing tumours have a pro-inflammatory activity that may promote tumour lysis. Accordingly, pro-inflammatory microglial cells, which express the inducible nitric oxide synthase (iNOS), have been shown to induce glioma cell death.57,70,71 Our transcriptomic analysis suggests that astrocyte depletion regulates the TAMs cytotoxic potential and can modulate nitric oxide (NO) metabolism in the TME (Figs 2F and 4A and Supplementary Table 2). As the first step to confirm this possibility, we examined the ability of astrocytes to regulate the microglial-mediated glioma cytotoxicity (Fig. 4G and H). For this purpose, microglial cells were pre-activated with GBM-CM in the presence or absence of astrocytes for 24 h, to allow for astrocyte modulation of microglial activity. The astrocytes were then removed by mild trypsin/EDTA treatment, and the microglial cells were co-incubated with GFP+-GL261 cells for 48 h. Glioma cells were then isolated on the basis of their green fluorescent protein (GFP) expression, and their viability was determined (as illustrated in Fig. 4G). We found that astrocytes attenuate the microglial-dependent killing of glioma cells by 48 ± 12.6% (Fig. 4H). Next, we investigated whether this immunosuppressive function on microglial cell cytotoxicity towards glioma cells is a specific immunosuppression response or a general response to the withdrawal of trophic support by astrocytes. To this end, microglial cells were treated with ACM, which was generated from astrocyte cultures without previous contact with neither glioblastoma nor microglial cells, or control medium (Med) and co-incubated with GFP+-GL261 cells as in Fig. 4H. We found that ACM did not significantly regulate the microglial-dependent killing of glioma cells (P = 0.678 by two-sided Student’s t-test, Supplementary Fig. 5G). Suggesting that astrocytes play a direct and specific immunosuppressive function in regulating the microglial response to glioma cells. To continue and explore this hypothesis, we then repeated in the presence of the NO synthase inhibitor, L-NAME, which has been shown to inhibit iNOS-depend neuroinflammation and microglial-induced cell death.72,73 We observed that inhibition of NO synthesis also attenuates microglial-induced killing in our system, suggesting that it is at least partially dependent on NO production (Fig. 4I). Accordingly, we could also detect a significant upregulation in Nos2 (the transcript encoding iNOS) expression in microglial cells isolated from GCV-treated GL261-bearing Gfap-TK, compared to wild-type mice (Fig. 4J). Next, we studied the signalling cascade by which astrocytes govern microglial reprogramming. Treating mixed glia cultures (containing microglial cells and astrocytes) with neutralizing antibodies to CSF1, TGFβ, IL-10, IFNγ or IL-6 signalling, and analysing GBM-CM stimulated microglial induction of Nos2, indicated that blockade of CSF-1R induced its expression. Suggesting that astrocyte-driven CSF1 inhibits microglial Nos2 induction (Fig. 4K). Of note, blocking CSF-1R signalling did not significantly regulate microglial viability or microglial proliferation (Supplementary Fig. 5H and I). Indeed, analysis of TCGA data, and scRNAseq data of the GBM TME,42 identify the upregulation of CSF1 and CSF-1R expression (mainly in astrocytes and TAMs, respectively), which is correlated with lower patient survival (Supplementary Fig. 5J–N). Taken together, the data indicate that astrocytes reprogramme the microglial cells to promote immunosuppression and support tumour survival, a process that is partly mediated by CSF1 signalling and iNOS expression.
Astrocyte-derived cholesterol supports glioma survival
The metabolism of cancer cells adapts during transformation, and the altered cellular metabolism that is a hallmark of gliomas, may be a promising source of druggable targets for therapy.2,74 This is particularly pertinent for glioblastoma progression, as the CNS is isolated from the circulation by the blood–brain barrier and depends heavily on astrocytes for metabolic homeostasis. Our analysis of the tumour-associated astrocyte transcriptome suggests a significant perturbation in the metabolic network (Fig. 2F). We used genome-scale metabolic modelling31 to identify which of the astrocyte core metabolic pathways are regulated by the tumour, since this method was previously shown to model human brain metabolism in health and disease.75,76 For this purpose, we analysed the transcriptome data using the Integrative Metabolic Analysis Tool (iMAT31) to predict the metabolic flux activity (Table 1). Interestingly, the results reveal a significant enrichment of the cholesterol metabolic pathway (P = 1.22 × 10−15).
Table 1.
Metabolic pathway enrichment in glioblastoma-associated astrocytes
| Metabolic pathway | P-value |
|---|---|
| Bile acid biosynthesis | <10−16 |
| Hyaluronan metabolism | <10−16 |
| Stilbene, coumarine and lignin biosynthesis | <10−16 |
| Cholesterol metabolism | 1.22 × 10−15 |
| Folate metabolism | 4.04 × 10−7 |
| Ubiquinone biosynthesis | 3.88 × 10−6 |
| Carnitine shuttle | 1.83 × 10−5 |
| Eicosanoid metabolism | 7.21 × 10−5 |
| Transport, lysosomal | 1.13 × 10−4 |
| Transport, peroxisomal | 3.72 × 10−4 |
| Transport, endoplasmic reticular | 3.91 × 10−4 |
| Pentose and glucuronate interconversions | 3.85 × 10−3 |
| Nucleotide sugar metabolism | 6.69 × 10−3 |
| Glycosylphosphatidylinositol-anchor biosynthesis | 6.82 × 10−3 |
Cholesterol is predominantly localized to bilayer membranes such as the cell membrane and the mitochondrial membrane. It is essential for cellular biological functions ranging from signal transduction to modulation of critical mitochondrial-governed processes such as oxidative phosphorylation (OXPHOS) and regulation of apoptosis.77,78–79 Many cancers display a high cholesterol content, which is thought to support tumour growth29,79,80 and the viability and activity of cells in the TME,29 and targeted disruption of cholesterol metabolism was recently shown to be beneficial in adult and childhood brain tumours.26,81 Analysis of cholesterol distribution in the brain of GL261-bearing mice identified higher cholesterol levels in the tumour cells than in the surrounding brain tissue, which agrees with previous reports2,26 that glioma cells take up significantly more extracellular cholesterol than non-neoplastic cells (Supplementary Fig. 6A). Importantly, cholesterol synthesis in the brain is mainly dependent on astrocytes, as the blood–brain barrier effectively prevents the uptake of lipoprotein-bound cholesterol from circulation. Indeed, perturbations in astrocyte-derived cholesterol have been associated with a variety of neuropathologies.22,82 However, the role of astrocyte-derived cholesterol in GBM progression is unknown.
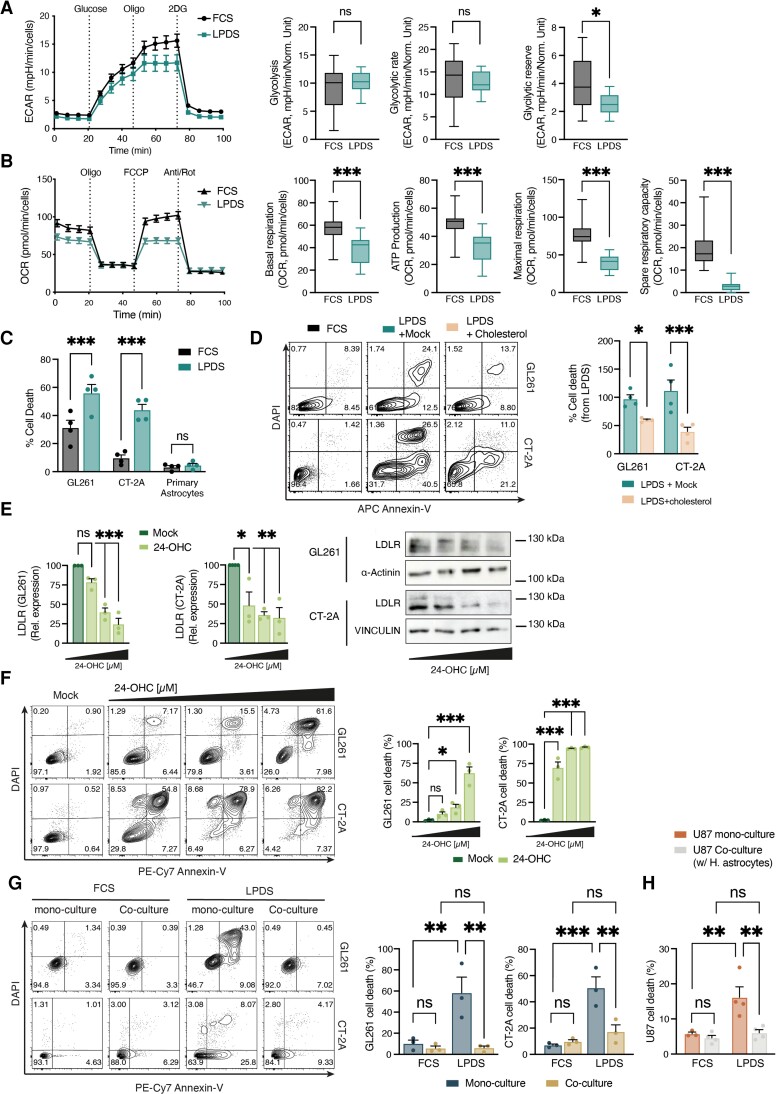
Since cholesterol has been found to be specifically enriched in the mitochondrial membrane of cancer cells where it decreases membrane fluidity and reduces the mitochondria sensitivity to stress,79,83 we hypothesized that cholesterol produced by the TAAs could be used to regulate GBM pathogenicity. To test this hypothesis, we first studied the dependency of glioma cells on exogenous cholesterol by removing the cholesterol-carrying lipoproteins from the culture media and assessing the mitochondrial bioenergetic potential as an indication of cellular stress. Interestingly, although like many other cancer cells glioma cells can use glycolysis for growth (Warburg effect), they rely on mitochondrial OXPHOS to support aggressive tumour growth.2,4 We therefore monitored the ECAR, indicative of aerobic glycolysis and oxygen consumption rate (OCR) as a readout of OXPHOS, in GL261 and CT-2A glioma cells cultured for 18 h in media supplemented with LPDS or normal serum (FCS) (Fig. 5A and B and Supplementary Fig. 6B and C). We found that lipoprotein depletion had little to no effect on ECAR, and the addition of glucose did not affect the cytosolic-based glycolysis rate or glycolytic capacity in the glioma cell lines, with only a minor effect on the GL261 glycolytic reserve (Fig. 5A and Supplementary Fig. 6B). However, in absence of lipoproteins, there was significant inhibition of the OCR potential as well as a decrease in basal respiration and ATP production by the glioma cells. Notably, OCR was attenuated when oxygen consumption was uncoupled from ATP production by FCCP [carbonyl cyanide4-(trifluoromethoxy)phenylhydrazone], demonstrating a significant reduction in the glioma cells’ maximal respiration and spare respiratory capacity. Thus, suggesting that the removal of exogenous cholesterol resulted in mitochondrial stress, specifically targeting the OCR activity of the glioma cells, which is required for the tumour progression.3,4
Figure 5.
Astrocyte-derived cholesterol support glioma survival. (A and B) Real-time changes in the ECAR (A) and OCR (B) of GL261 glioma cells, cultured in media supplemented with full serum (FCS) or lipoprotein-deprived serum (LPDS) for 18 h and measured using Seahorse. Oligo = oligomycin; FCCP = carbonyl cyanide4-(trifluoromethoxy) phenylhydrazone; R/A = rotenone plus antimycin A; 2-DG = 2-deoxy-D-glucose. Glycolysis, glycolytic capacity and glycolytic reserve were extracted from the ECAR reading, and basal respiration, ATP production, maximal respiration and spare respiratory capacity were determined on the basis of OCR. Data are representative of two independent experiments (n = 6 technical replicates per experiment). (C) Percentage of cell death of GL261 and CT-2A glioma cells, or primary astrocytes, cultured in FCS or LPDS for 5 days; as determined by Annexin-V assay (n = 4 independent experiments). (D) GL261 or CT-2A glioma cells were cultured in FCS or LPDS-media and treated with PBS (Mock) or cholesterol (250 ng/ml) for 5 days. Representative flow cytometry plots of Annexin-V/DAPI staining from each group are shown on the left and quantification analyses of cholesterol rescue are on the right (n = 4 biologically independent experiments). (E and F) Representative images and quantification of LDLR expression (Immunoblot, E) and cell death via FACS analysis of Annexin-V/DAPI staining (F) in response to endogenous LXR agonists (24-OHC) or Mock (DMSO) treatment in GL261 and CT-2A cells. (n = 3 biologically independent experiments). (G and H) Analysis of LPDS-induced glioma cell death, in the presence of absence of primary astrocytes, by Annexin-V assay. (G) Representative flow cytometry plots of murine GL261 and CT-2A glioma cells co-cultured for 5 days with primary mouse astrocytes are shown on the left and quantification analyses on the right (n = 4 biologically independent experiments). (H) quantification analyses of human U87EGFRvIII26 (U87) glioma cells co-cultured with human primary astrocytes for 5 days (n = 3 biologically independent experiments). Data are shown as mean ± SEM. P-values were determined by two-sided Student’s t-tests (B–D, G and H) or by two-way ANOVA (E and F). *P < 0.05, **P < 0.01, ***P < 0.001.
Mitochondrial stress is often associated with cell death, as is the reduction in cellular cholesterol levels.79,83 Therefore, we analysed the viability of the murine glioma cells and primary astrocytes subjected to prolonged deprivation of cholesterol. We reasoned that while astrocytes, which can de novo synthesize cholesterol, will resist exogenous cholesterol deprivation, the murine glioma cells will be highly vulnerable. To address this objective, we treated primary astrocytes and glioma cells with lovastatin, which inhibits the 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR), a rate-limiting enzyme in the cholesterol synthesis pathway. We found that suppressing endogenous cholesterol synthesis did not affect glioma viability, but led to significant cell death of primary astrocytes (Supplementary Fig. 6D and E). Conversely, depriving the murine glioma cells of lipoprotein-bound cholesterol resulted in substantial cell death while astrocyte viability remained unaffected (Fig. 5C), suggesting that the glioma cells are indeed dependent on exogenous cholesterol. Consistent with this interpretation, removing the cholesterol-carrying lipoproteins from the culture media increased the expression of the LDLR, which mediates cholesterol uptake by the cells, a finding we have also noted in GBM patients (Supplementary Fig. 6F and G). Furthermore, exogenous cholesterol overcame the deleterious effects of lipoprotein-bound cholesterol deprivation on glioma viability (Fig. 5D). Also, similarly, activating the liver X receptor (LXR) signalling pathway with the endogenous ligand 24(S)-hydroxycholesterol (24-OHC), which results in a 24-OHC dose-dependent LDLR degradation, thus limiting cholesterol uptake by the cells,26 led to dramatic cell death in both GL261 and CT-2A glioma cell lines (Fig. 5E and F). Collectively, these data demonstrate that glioma cells depend on exogenous cholesterol for their survival. Importantly, these findings align with similar data described recently by Villa et al.26 that demonstrate a dependency of human gliomas on LXR-depended cholesterol uptake.
Next, we investigated the dynamics of cholesterol metabolism in the astrocyte-glioma cross-talk. Treating astrocytes with GBM-CM, or co-culturing them with glioma cells, induces the expression of rate-limiting enzymes in the cholesterol de novo synthesis pathway, including Hmgcs1, Hmgcr and Dhcr24 (Supplementary Fig. 7A–C). Again, this increase is in line with the elevated expression levels of these genes seen in scRNAseq data of astrocytes from GBM patients42 (Supplementary Fig. 7D), suggesting that glioma cells may elicit astrocytes to support their metabolic requirement for cholesterol. Culturing glioma cells in the presence (co-culture) or absence (mono-culture) of astrocytes, and subjecting them to lipoprotein deprivation, revealed a dramatic rescue of the murine GL261 and CT-2A glioma cells by the astrocytes (Fig. 5G and Supplementary Fig. 7E). Moreover, we found a similar recovery from cholesterol starvation in human U87EGFRvIII26 glioma cells in the presence of human primary astrocytes (Fig. 5H and Supplementary Fig. 7F). Taken together, these data indicate that the glioma cells rely on the uptake of exogenous cholesterol for survival and that human and murine astrocytes can meet these metabolic requirements for cholesterol and support glioma cell survival.
Astrocytic ABCA1-driven cholesterol efflux promotes tumour progression
Intracellular cholesterol trafficking in the brain is mediated mainly by the sterol transporters ABCA1 (ATP binding cassette transporter A1) and ABCG1 (ATP binding cassette subfamily G member 1).84 Our analysis of TCGA data identified a high expression of both genes in the CNS of GBM patients (Fig. 6A and Supplementary Fig. 8A), although our analysis of scRNAseq of the GBM microenvironment42 identified that TAAs predominantly express ABCA1 (Fig. 6B). Transcriptomic analysis of the TAAs isolated from GBM-bearing mice transcriptome similarly detected high levels of Abca1 induction with an absence of Abcg1 transcripts (Fig. 6C). Concomitantly, we could detect the expression of ABCA1 in reactive astrocytes surrounding the GBM tumour in mice by immunofluorescence (Fig. 6D) and also show that exposure to GBM-CM directly induces ABCA1 expression in astrocytes (Fig. 6E), suggesting that ABCA1 plays a role in the astrocyte-mediated cholesterol shuttling to the tumour.
We, therefore, reasoned that knocking down astrocytic ABCA1 expression would limit the astrocyte-derived cholesterol available to the tumour cells and enable us to evaluate the extent to which it contributes to tumour pathology. To investigate this hypothesis, we generated ABCA1-targeting lentiviruses using RNA interference (RNAi) or the CRISPR–Cas9 systems (Fig. 6F and Supplementary Fig. 8B, respectively). We transduced primary astrocytes with our lentiviruses targeting ABCA1 [shAbca1, sgAbca1(#1) and sgAbca1(#2)] or appropriate controls [non-targeting shRNA (shNT) or sgRNA targeting the luciferase gene (sgLuc2), respectively]. We found that all Abca1-targeting sequences significantly knocked-down ABCA1 expression compared to their respective controls (Supplementary Fig. 8C). We next studied whether astrocytic ABCA1 knock-down would attenuate astrocyte-mediated rescue of glioblastoma cells from cholesterol depredation. To that end, the transduced primary astrocytes were co-cultured with glioblastoma cells in a cholesterol-free medium, and their survival was analysed (as in Fig. 5G). We found that all Abca1-targeting sequences significantly inhibited the astrocyte-mediated rescue of the glioma cells (Supplementary Fig. 8D). Thus, these data demonstrate that ABCA1 plays an important role in the astrocyte-mediated cholesterol shuttling to the glioma cells.
To further investigate our initial hypothesis and determine the role of astrocytic ABCA1 expression on tumour pathogenicity, we delivered the validated shRNAs (shAbca1 or shNT) to reactive astrocytes in the GBM TME. To this end, we used a previously established lentivirus-based system optimized for astrocyte-specific knock-down in vivo19,21,23 (Fig. 6F). In this system, the truncated GFAP promoter, GfaABC1D, drives the expression of a miR30-based shRNA and a GFP reporter. Intracranial injection of shRNA-encoding lentivirus to GL261-bearing mice indeed confirmed that the GFP reporter was detected exclusively in the GFAP+ astrocytes (Supplementary Fig. 8E and F), and significantly reduced astrocytic Abca1 expression (Fig. 6G).
As the next step, we investigated whether knock-down of Abca1 in astrocytes affects the cholesterol content of tumour cells. To address this objective, GL261-bearing mice were intracranially injected with the Gfap-shNT or Gfap-shAbca1 and we assessed cholesterol accumulation in the tumour cells using Filipin III staining.29 In accordance with our hypothesis, knock-down of astrocytic Abca1 led to a significant decrease in glioma cholesterol (Fig. 6H) and concomitant induction of apoptotic cell death (Fig. 6I). Moreover, inhibiting the efflux of cholesterol from the TAAs significantly regressed tumour growth and prolonged survival of the GL261-bearing mice (Fig. 6J and K). Notably, analysis of TCGA data indicated that reduced expression of ABCA1 was associated with increased survival in patients with GBM (Fig. 6L). Collectively, these data suggest that glioblastoma pathogenicity is dependent on astrocyte-derived cholesterol.
Discussion
Whereas it is becoming increasingly clear that astrocytes play an important role in a number of neurological disorders, little is known about the nature of astrocyte contribution to glioblastoma pathogenicity in vivo. Here, we first addressed this question by genetically depleting GFAPhigh reactive astrocytes in adult immunocompetent mice, a method that has proven valuable for understanding the role of astrocytes in various neuropathologies.19,28,48,49 Two models were used: the GfapCre:iDTR mice model, which depletes GFAPhigh astrocytes, and the Gfap-TK mice model,19,48,49 which also requires the astrocytes to be in a proliferative state [a characteristic feature of both human24 and murine TAAs (Fig. 2E and F)]. The results indicate that ablation of reactive glioma-associated astrocytes from the TME halts tumour progression, regresses established gliomas and markedly enhances survival of the animals in both GBM models (Fig. 1 and Supplementary Fig. 1), suggesting that TAAs play a pivotal role in controlling glioblastoma pathogenicity.
To unravel the molecular circuits by which astrocytes govern GBM pathogenicity, we performed RiboTag-based RNA-seq on TAAs. RiboTag restricts analysis to ribosome-associated mRNAs that are likely to be in active translation, thereby reflecting the cellular protein expression profile, while minimizing any bias resulting from cell isolation-based methods.85,86 Our results identify unique transcriptomic reprogramming of glioma-associated astrocytes, with the capacity to directly induce immunosuppression and control the immunological compartment and the metabolic landscape of the TME (Fig. 2 and Supplementary Fig. 2). Importantly, our astrocyte gene expression dataset is validated by broad agreement with other RNA-seq profiles of astrocytes from the GBM patients including the scRNAseq analysis of the GBM (IDH1-negative, grade IV) TME by Darmanis et al.42 and the analysis of GBM-associated astrocytes isolated by immunopanning by Henrik Heiland et al.24
TAMs, which possess both tumour-promoting and immunosuppressive capacities, are abundant in the TME. Since their accumulation has been shown to be reversely correlated with patient survival, and given the abysmal prognosis of GBM patients, there is growing interest in developing novel therapeutics to target TAM activity. However, whether these activities are predominantly shaped by the non-neoplastic cells in the TME milieu or by the malignancy itself is unknown.87 Here we demonstrate that astrocytes have an important role in reprogramming TAMs in the glioma microenvironment. We found that astrocytes regulate the recruitment of TAMs to the tumour (Fig. 3) and control various aspects of the TAM tumour-promoting and immunosuppressive phenotype (Fig. 4 and Supplementary Fig. 5). In addition, we implicate the CCL2–CCR2 and CSF1–CSF1R axes in the molecular mechanisms by which the astrocytes control the TAM compartment and thus shape tumour-specific immunity (Figs 3 and 4). We could also detect an increased expression of these genes in GBM patient-derived scRNAseq and in TCGA data, which was inversely correlated to patient survival (Supplementary Figs 4 and 5). Similar expression and survival patterns were also found for CD274, the transcript encoding PD-L1 (Supplementary Fig. 5). These findings are in agreement with previous reports showing the perturbations of CCL2, CSF1 or PD-L1 signalling are able to regulate the TAMs niche in the TME and affect tumour pathogenicity in mouse models of GBM.7,52,88 Regulating TAMs activity, for example, by using minocycline that blocks microglial activation or the specific CSF-1R inhibitor PLX3397, reduces glioma expansion in experimental glioma mouse models. Unfortunately, so far, the clinical application of these basic science advances has been disappointing, and strategies designed to silence TAM function have not translated well to human clinical trials.57,89 Immunotherapies have demonstrated limited efficacy in patients with glioblastoma. However, in recent early phase clinical trials, the use of nivolumab or pembrolizumab, which targets the PD-L1/PD-1 axis, has shown promising outcomes.10,14 Thus, suggesting that to overcome the tumour-supporting properties of the TAMs, it might not be enough to target specific aspects of TAM biology. Instead, there is a need to develop strategies to harness the astrocytes to reprogramme the TAMs and redirect them back to fight cancer.
Alterations in cellular metabolism, governed by the interaction between tumour genotype and its microenvironment, are a hallmark of many malignancies including brain gliomas.2 Cancer cells, due to their genetic abnormalities, aggressive proliferation rate and metabolic restrictions of their microenvironment, may become dependent on factors that are not themselves oncogenic. This process is known as non-oncogene addiction or non-oncogene co-dependency and opens up treatment possibilities. Accordingly, two of the main genetic abnormalities found in GBM tumours are the amplification of the gene encoding for epidermal growth factor receptor (EGFR), with nearly half of the cases bearing the gain-of-function EGFR variant III (EGFRvIII) alteration; And, the recurrence of mutations in the genes IDH1 and IDH2, which are components involved in the tricarboxylic acid cycle. Both EGFR and IDH have been associated with the regulation of glioma bioenergetics, which favours OXPHOS for aggressive growth, over glycolysis.2,4 Accordingly, treatment of patient-derived GBM xenograft mice with Gboxin, an OXPHOS inhibitor, suppresses the growth of tumours with EGFR or IDH1 mutations.3 In recent years, several important findings as to the role of IDH mutations in glioblastoma pathogenicity, have linked tumour metabolism and immune perturbations of T cells87,90 and TAMs58,87 in TME. Accordingly, Bunse et al.90 have elegantly demonstrated that the oncometabolite (R)-2-hydroxyglutarate, produced by IDH mutations, suppresses T-cell activity, while Klemm et al.58 found that IDH mutation status shapes TAM composition (favouring microglial recruitment over monocyte-derived macrophages) and phenotype (e.g. suppressing GPNMB induction) in TME, suggesting that understanding the immunometabolic cross-talk between the glioma cells and the non-neoplastic cells in the TME is becoming increasingly important for finding more effective ways to treat glioblastoma patients: specifically, by targeting the tumour altered cellular metabolism, and the unique metabolic vulnerabilities enforced by the blood–brain barrier. However, currently, the metabolic landscape of the GBM microenvironment and the mechanisms by which it is changed by the cross-talk between the glioma cells and the non-neoplastic cells in the TME, mainly the astrocytes, are not well understood.
We found that GBM modulates the metabolic activity of the TAAs and, using genome-scale metabolic network analysis, identified significant perturbations in several key metabolic pathways that might be associated with tumour progression. These include cholesterol metabolism,5,80 bile acid (synthesized from cholesterol) biosynthesis and hyaluronan metabolism, which was shown to maintain the glioblastoma stem-like cell tumourigenicity potential91 (Fig. 2F and Table 1). Given the unique role of astrocytes in controlling brain cholesterol homeostasis,2,92 here we decided to focus on studying the contribution of astrocyte-derived cholesterol to glioma pathogenicity. Our results indicate that glioma cells rely on exogenous cholesterol to maintain their energy metabolism and support viability. Accordingly, deprivation of cholesterol-carrying lipoproteins from their environment lowers the OXPHOS potential, which is important for tumour progression.3,4 Indeed, a variety of OXPHOS-targeting drugs, including metformin, Gboxin and IACS-010759, were shown to inhibit glioma proliferation and induce cell death. In this context, metformin combined with temozolomide (standard-of-care chemotherapy for GBM patients) or radiotherapy is currently in clinical trials for glioma (NCT02780024, NCT03243851).2 In addition, we demonstrate that both mouse and human glioma cells are dependent on cholesterol efflux from the astrocytes (Fig. 5 and Supplementary Figs 6 and 7), and that blocking the efflux of cholesterol from the astrocytes, by targeting astrocytic expression of the cholesterol transporter ABCA1, causes regression of tumour growth and prolongs mouse survival (Fig. 6). The physiological relevance of these findings to human disease is provided by scRNAseq data analysis of the TME of GBM patients, as well as TCGA data demonstrating an increased expression of astrocytic ABCA1 in the GBM TME and an inverse correlation with patient survival. Further support is provided by recent reports by Villa et al.26 that targeting the ABCA1–LDLR axis, using a brain-penetrant LXR agonist, kills glioma cells and attenuates tumour pathogenicity in a GBM xenograft model.
Our data demonstrate a tissue context in which reactive astrocytes in the TME supply the metabolic requirements of tumour cells, thereby exposing vulnerabilities that merit clinical exploitation and contributing to the recently emerging line of research that demonstrates a profound astrocyte-mediated metabolic-dependent regulation of neuro-pathologies.15,19–21–22 Notably, here we only addressed the cholesterol node in the astrocyte–tumour cell axis within the vast complexities of the metabolic network that governs GBM pathogenicity. Future studies should continue to unmask the role of additional metabolic circuits in the TME (Table 1), and expand our understanding of the wider role of astrocyte-derived cholesterol in the TME (Ma et al.29).
Astrocytes’ diverse functions in health and disease encompass a continuum of cellular states with the potential for plasticity and reprogramming.93,94 Recent scRNAseq studies had provided valuable insights into basal95 and reactive-astrocyte heterogeneity in neuroinflammation and neurodegeneration.96,97 However, little is known about astrocyte heterogeneity in the GBM TME, and the underlying mechanisms driving it. To address the question of astrocyte diversity, we analysed the astrocyte scRNAseq data from the GBM patients42 by means of differential expression, pathway analysis and transcription factors enrichment. We found that the astrocytes clustered into two main populations (Supplementary Fig. 3C–H and Supplementary Table 3). Cluster A (Blue, 599 cells), which is enriched with the immune response transcripts, and cluster B (Pink, 453 cells), enriched in genes associated with cholesterol synthesis and bioenergetics (Supplementary Fig. 3C–G). Interestingly, each cluster was also significantly (FDR < 0.001) enriched by a unique transcription factor signature. The transcripts encoding AHR, ATF3, SOCS3, PITX1, RELB and CRBPB were mainly associated with cluster A, while the transcripts encoding NFIA, HES6, HES1, SOX11, SOX15, TFDP2 and PPARGC1A were mainly associated with cluster B (Supplementary Fig. 3H). Notably, several transcripts encoding key transcription factors19,25,93 such as STAT1, STAT3, NFATC3, SOX9 and IRF1 displayed a pan-astrocyte activation pattern and were evenly expressed between the two clusters (Supplementary Fig. 3H). Suggesting a heterogeneity in the astrocyte response to the GBM TME. Interestingly, Kambach et al.98 suggested an inverse correlation between astrocyte cell density and their capacity for cholesterol synthesis. To study if the difference in astrocyte cell density may be related to the differential expression in cholesterol synthesis between the two clusters, we analysed the expression of fibroblast growth factor 2 (FGF2), whose expression is inhibited in high cell density in human astrocytes.99 We found that FGF2 expression was strongly associated with cluster A, and had no significant correlation with any of the key cholesterol synthesis transcripts that were shown to be associated with astrocyte density98 (Supplementary Fig. 3I and J, respectively). Suggesting that astrocyte cell density plays a role in TAAs responses in the TME. Indeed, with recent advances in the field of spatial transcriptomics, it will be interesting to further investigate astrocyte heterogeneity in the GBM to better target the astrocyte regulation of the TME.
In conclusion, we identified a mechanism by which astrocytes participate in the control of TAM immunity and sustain the metabolic landscape necessary for tumour survival. These findings shed new light on the pivotal role astrocytes play in promoting glioblastoma pathogenicity and the potential vulnerabilities caused by the tumour dependence on astrocyte immunometabolic support of the TME, thereby identifying candidate targets for therapeutic intervention.
Supplementary Material
Abbreviations
- ACM =
astrocyte-conditioned medium
- CM =
condition media
- DT-A =
diphtheria toxin A
- ECAR =
extracellular acidification rate
- FACS =
fluorescence-activated cell sorting
- FCS =
foetal calf serum
- GBM =
glioblastoma multiforme
- GCV =
ganciclovir
- GFP =
green fluorescent protein
- IDH =
isocitrate dehydrogenase
- iDTR =
induced diphtheria toxin
- iNOS =
inducible nitric oxide synthase
- LPDS =
lipoprotein-deficient serum
- OCR =
oxygen consumption rate
- OXPHOS =
oxidative phosphorylation
- TAA =
tumour-associated astrocytes
- TAM =
tumour-associated macrophages
- TCGA =
The Cancer Genome Atlas
- TME =
tumour microenvironment
Contributor Information
Rita Perelroizen, Sagol School of Neuroscience, Tel Aviv University, Tel Aviv, Israel.
Bar Philosof, Sagol School of Neuroscience, Tel Aviv University, Tel Aviv, Israel.
Noga Budick-Harmelin, Shmunis School of Biomedicine and Cancer Research, George S. Wise Faculty of Life Sciences, Tel Aviv University, Tel Aviv, Israel.
Tom Chernobylsky, Shmunis School of Biomedicine and Cancer Research, George S. Wise Faculty of Life Sciences, Tel Aviv University, Tel Aviv, Israel.
Ariel Ron, Sagol School of Neuroscience, Tel Aviv University, Tel Aviv, Israel.
Rotem Katzir, Cancer Data Science Laboratory (CDSL), National Cancer Institute (NCI), National Institutes of Health (NIH), Bethesda, MD, USA.
Dor Shimon, Shmunis School of Biomedicine and Cancer Research, George S. Wise Faculty of Life Sciences, Tel Aviv University, Tel Aviv, Israel.
Adi Tessler, Shmunis School of Biomedicine and Cancer Research, George S. Wise Faculty of Life Sciences, Tel Aviv University, Tel Aviv, Israel.
Orit Adir, Shmunis School of Biomedicine and Cancer Research, George S. Wise Faculty of Life Sciences, Tel Aviv University, Tel Aviv, Israel.
Anat Gaoni-Yogev, Shmunis School of Biomedicine and Cancer Research, George S. Wise Faculty of Life Sciences, Tel Aviv University, Tel Aviv, Israel.
Tom Meyer, Sagol School of Neuroscience, Tel Aviv University, Tel Aviv, Israel.
Avivit Krivitsky, Shmunis School of Biomedicine and Cancer Research, George S. Wise Faculty of Life Sciences, Tel Aviv University, Tel Aviv, Israel.
Nuphar Shidlovsky, Shmunis School of Biomedicine and Cancer Research, George S. Wise Faculty of Life Sciences, Tel Aviv University, Tel Aviv, Israel.
Asaf Madi, Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
Eytan Ruppin, Cancer Data Science Laboratory (CDSL), National Cancer Institute (NCI), National Institutes of Health (NIH), Bethesda, MD, USA.
Lior Mayo, Sagol School of Neuroscience, Tel Aviv University, Tel Aviv, Israel; Shmunis School of Biomedicine and Cancer Research, George S. Wise Faculty of Life Sciences, Tel Aviv University, Tel Aviv, Israel.
Funding
This work was supported by grants from the Israel Cancer Research Fund (ICRF, GA-16-1640), Uncle Kory Foundation, and the United States-Israel Binational Science Foundation (BSF, 2019197) to L.M.
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bi J, Chowdhry S, Wu S, Zhang W, Masui K, Mischel PS. Altered cellular metabolism in gliomas—An emerging landscape of actionable co-dependency targets. Nat Rev Cancer. 2020;20:57–70. [DOI] [PubMed] [Google Scholar]
- 3. Shi Y, Lim SK, Liang Q, et al. Gboxin is an oxidative phosphorylation inhibitor that targets glioblastoma. Nature. 2019;567:341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marin-Valencia I, Yang C, Mashimo T, et al. Analysis of tumor metabolism reveals mitochondrial glucose oxidation in genetically diverse human glioblastomas in the mouse brain in vivo. Cell Metab. 2012;15:827–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. An Z, Weiss WA. Cholesterol: An Achilles’ heel for glioblastoma? Cancer Cell. 2016;30:653–654. [DOI] [PubMed] [Google Scholar]
- 6. Kohanbash G, Carrera DA, Shrivastav S, et al. Isocitrate dehydrogenase mutations suppress STAT1 and CD8+ T cell accumulation in gliomas. J Clin Invest. 2017;127:1425–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pyonteck SM, Akkari L, Schuhmacher AJ, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19:1264–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Quail DF, Bowman RL, Akkari L, et al. The tumor microenvironment underlies acquired resistance to CSF-1R inhibition in gliomas. Science. 2016;352:aad3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Takenaka MC, Gabriely G, Rothhammer V, et al. Control of tumor-associated macrophages and T cells in glioblastoma via AHR and CD39. Nat Neurosci. 2019;22:729–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cloughesy TF, Mochizuki AY, Orpilla JR, et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med. 2019;25:477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sun C, Mezzadra R, Schumacher TN. Regulation and function of the PD-L1 checkpoint. Immunity. 2018;48:434–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015. Neuro Oncol. 2018;20:iv1–iv86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wen PY, Reardon DA. Neuro-oncology in 2015: Progress in glioma diagnosis, classification and treatment. Nat Rev Neurol. 2016;12:69–70. [DOI] [PubMed] [Google Scholar]
- 14. Zhao J, Chen AX, Gartrell RD, et al. Immune and genomic correlates of response to anti-PD-1 immunotherapy in glioblastoma. Nat Med. 2019;25:462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ferris HA, Perry RJ, Moreira GV, Shulman GI, Horton JD, Kahn CR. Loss of astrocyte cholesterol synthesis disrupts neuronal function and alters whole-body metabolism. Proc Natl Acad Sci U S A. 2017;114:1189–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Allen NJ, Eroglu C. Cell biology of astrocyte-synapse interactions. Neuron. 2017;96:697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Linnerbauer M, Wheeler MA, Quintana FJ. Astrocyte crosstalk in CNS inflammation. Neuron. 2020;108:608–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liddelow SA, Barres BA. Reactive astrocytes: Production, function, and therapeutic potential. Immunity. 2017;46:957–967. [DOI] [PubMed] [Google Scholar]
- 19. Mayo L, Trauger SA, Blain M, et al. Regulation of astrocyte activation by glycolipids drives chronic CNS inflammation. Nat Med. 2014;20:1147–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wheeler MA, Jaronen M, Covacu R, et al. Environmental control of astrocyte pathogenic activities in CNS inflammation. Cell. 2019;176:581–596.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chao CC, Gutierrez-Vazquez C, Rothhammer V, et al. Metabolic control of astrocyte pathogenic activity via cPLA2-MAVS. Cell. 2019;179:1483–1498.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang H, Kulas JA, Wang C, Holtzman DM, Ferris HA, Hansen SB. Regulation of beta-amyloid production in neurons by astrocyte-derived cholesterol. Proc Natl Acad Sci U S A. 2021;118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mayo L, Cunha AP, Madi A, et al. IL-10-dependent Tr1 cells attenuate astrocyte activation and ameliorate chronic central nervous system inflammation. Brain. 2016;139(Pt 7):1939–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Henrik Heiland D, Ravi VM, Behringer SP, et al. Tumor-associated reactive astrocytes aid the evolution of immunosuppressive environment in glioblastoma. Nat Commun. 2019;10:2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Priego N, Zhu L, Monteiro C, et al. STAT3 labels a subpopulation of reactive astrocytes required for brain metastasis. Nat Med. 2018;24:1024–1035. [DOI] [PubMed] [Google Scholar]
- 26. Villa GR, Hulce JJ, Zanca C, et al. An LXR-cholesterol axis creates a metabolic co-dependency for brain cancers. Cancer Cell. 2016;30:683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lin L, Desai R, Wang X, Lo EH, Xing C. Characteristics of primary rat microglia isolated from mixed cultures using two different methods. J Neuroinflammation. 2017;14:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schreiner B, Romanelli E, Liberski P, et al. Astrocyte depletion impairs redox homeostasis and triggers neuronal loss in the adult CNS. Cell Rep. 2015;12:1377–1384. [DOI] [PubMed] [Google Scholar]
- 29. Ma X, Bi E, Lu Y, et al. Cholesterol induces CD8+ T cell exhaustion in the tumor microenvironment. Cell Metab. 2019;30:143–156.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Walter DM, Venancio OS, Buza EL, et al. Systematic in vivo inactivation of chromatin-regulating enzymes identifies Setd2 as a potent tumor suppressor in lung adenocarcinoma. Cancer Res. 2017;77:1719–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zur H, Ruppin E, Shlomi T. iMAT: An integrative metabolic analysis tool. Bioinformatics. 2010;26:3140–3142. [DOI] [PubMed] [Google Scholar]
- 32. Bordbar A, Monk JM, King ZA, Palsson BO. Constraint-based models predict metabolic and associated cellular functions. Nat Rev Genet. 2014;15:107–120. [DOI] [PubMed] [Google Scholar]
- 33. Ulitsky I, Maron-Katz A, Shavit S, et al. Expander: From expression microarrays to networks and functions. Nat Protoc. 2010;5:303–322. [DOI] [PubMed] [Google Scholar]
- 34. Raudvere U, Kolberg L, Kuzmin I, et al. g:Profiler: A web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019;47(W1):W191–W198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sanz E, Yang L, Su T, Morris DR, McKnight GS, Amieux PS. Cell-type-specific isolation of ribosome-associated mRNA from complex tissues. Proc Natl Acad Sci U S A. 2009;106:13939–13944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bray NL, Pimentel H, Melsted P, Pachter L. Near-optimal probabilistic RNA-seq quantification. Nat Biotechnol. 2016;34:525–527. [DOI] [PubMed] [Google Scholar]
- 37. Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kolde R, Vilo J. GOsummaries: an R Package for visual functional annotation of experimental data. F1000Research 2015;4:574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45:W98–W102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhao Z, Zhang KN, Wang Q, et al. Chinese Glioma Genome Atlas (CGGA): A comprehensive resource with functional genomic data from Chinese glioma patients. Genom Proteom Bioinf. 2021;19:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Darmanis S, Sloan SA, Croote D, et al. Single-cell RNA-seq analysis of infiltrating neoplastic cells at the migrating front of human glioblastoma. Cell Rep. 2017;21:1399–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Le T, Phan T, Pham M, et al. BBrowser: Making single-cell data easily accessible. bioRxiv. [Preprint] 10.1101/2020.12.11.414136 [DOI]
- 44. Wu YE, Pan L, Zuo Y, Li X, Hong W. Detecting activated cell populations using single-cell RNA-seq. Neuron. 2017;96:313–329.e6. [DOI] [PubMed] [Google Scholar]
- 45. Zhong S, Zhang S, Fan X, et al. A single-cell RNA-seq survey of the developmental landscape of the human prefrontal cortex. Nature. 2018;555:524–528. [DOI] [PubMed] [Google Scholar]
- 46. Doron H, Amer M, Ershaid N, et al. Inflammatory activation of astrocytes facilitates melanoma brain tropism via the CXCL10-CXCR3 signaling axis. Cell Rep. 2019;28:1785–1798.e6. [DOI] [PubMed] [Google Scholar]
- 47. Liu Y, Yuelling LW, Wang Y, et al. Astrocytes promote medulloblastoma progression through hedgehog secretion. Cancer Res. 2017;77:6692–6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Anderson MA, Burda JE, Ren Y, et al. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532:195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bush TG, Puvanachandra N, Horner CH, et al. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23:297–308. [DOI] [PubMed] [Google Scholar]
- 50. Wang JL, Scheitler KM, Wenger NM, Elder JB. Viral therapies for glioblastoma and high-grade gliomas in adults: A systematic review. Neurosurg Focus. 2021;50:E2. [DOI] [PubMed] [Google Scholar]
- 51. Liddelow SA, Guttenplan KA, Clarke LE, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Flores-Toro JA, Luo D, Gopinath A, et al. CCR2 inhibition reduces tumor myeloid cells and unmasks a checkpoint inhibitor effect to slow progression of resistant murine gliomas. Proc Natl Acad Sci U S A. 2020;117:1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Piperi C, Papavassiliou KA, Papavassiliou AG. Pivotal role of STAT3 in shaping glioblastoma immune microenvironment. Cells. 2019;8:1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Akkari L, Bowman RL, Tessier J, et al. Dynamic changes in glioma macrophage populations after radiotherapy reveal CSF-1R inhibition as a strategy to overcome resistance. Sci Transl Med. 2020;12:eaaw7843. [DOI] [PubMed] [Google Scholar]
- 55. Neal ML, Boyle AM, Budge KM, Safadi FF, Richardson JR. The glycoprotein GPNMB attenuates astrocyte inflammatory responses through the CD44 receptor. J Neuroinflammation. 2018;15:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kuan CT, Wakiya K, Keir ST, et al. Affinity-matured anti-glycoprotein NMB recombinant immunotoxins targeting malignant gliomas and melanomas. Int J Cancer. 2011;129:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hambardzumyan D, Gutmann DH, Kettenmann H. The role of microglia and macrophages in glioma maintenance and progression. Nat Neurosci. 2016;19:20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Klemm F, Maas RR, Bowman RL, et al. Interrogation of the microenvironmental landscape in brain tumors reveals disease-specific alterations of immune cells. Cell. 2020;181:1643–1660.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lepore F, D’Alessandro G, Antonangeli F, et al. CXCL16/CXCR6 axis drives microglia/macrophages phenotype in physiological conditions and plays a crucial role in glioma. Front Immunol. 2018;9:2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Izikson L, Klein RS, Charo IF, Weiner HL, Luster AD. Resistance to experimental autoimmune encephalomyelitis in mice lacking the CC chemokine receptor (CCR)2. J Exp Med. 2000;192:1075–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Colegio OR, Chu NQ, Szabo AL, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Franklin RA, Liao W, Sarkar A, et al. The cellular and molecular origin of tumor-associated macrophages. Science. 2014;344:921–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Noy R, Pollard JW. Tumor-associated macrophages: From mechanisms to therapy. Immunity. 2014;41:49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hutter G, Theruvath J, Graef CM, et al. Microglia are effector cells of CD47-SIRPα antiphagocytic axis disruption against glioblastoma. Proc Natl Acad Sci U S A. 2019;116:997–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Abels ER, Maas SLN, Nieland L, et al. Glioblastoma-associated microglia reprogramming is mediated by functional transfer of extracellular miR-21. Cell Rep. 2019;28:3105–3119.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Saha D, Martuza RL, Rabkin SD. Macrophage polarization contributes to glioblastoma eradication by combination immunovirotherapy and immune checkpoint blockade. Cancer Cell. 2017;32:253–267.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rosenzweig N, Dvir-Szternfeld R, Tsitsou-Kampeli A, et al. PD-1/PD-L1 checkpoint blockade harnesses monocyte-derived macrophages to combat cognitive impairment in a tauopathy mouse model. Nat Commun. 2019;10:465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hwang SY, Yoo BC, Jung JW, et al. Induction of glioma apoptosis by microglia-secreted molecules: The role of nitric oxide and cathepsin B. Biochim Biophys Acta. 2009;1793:1656–1668. [DOI] [PubMed] [Google Scholar]
- 71. Xue N, Zhou Q, Ji M, et al. Chlorogenic acid inhibits glioblastoma growth through repolarizating macrophage from M2 to M1 phenotype. Sci Rep. 2017;7:39011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Casas AI, Hassan AA, Larsen SJ, et al. From single drug targets to synergistic network pharmacology in ischemic stroke. Proc Natl Acad Sci U S A. 2019;116:7129–7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mayo L, Stein R. Characterization of LPS and interferon-γ triggered activation-induced cell death in N9 and primary microglial cells: Induction of the mitochondrial gateway by nitric oxide. Cell Death Differ. 2007;14:183–186. [DOI] [PubMed] [Google Scholar]
- 74. Vander Heiden MG, DeBerardinis RJ. Understanding the intersections between metabolism and cancer biology. Cell. 2017;168:657–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Styr B, Gonen N, Zarhin D, et al. Mitochondrial regulation of the hippocampal firing rate set point and seizure susceptibility. Neuron. 2019;102:1009–1024.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lewis NE, Schramm G, Bordbar A, et al. Large-scale in silico modeling of metabolic interactions between cell types in the human brain. Nat Biotechnol. 2010;28:1279–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Maxfield FR, Tabas I. Role of cholesterol and lipid organization in disease. Nature. 2005;438:612–621. [DOI] [PubMed] [Google Scholar]
- 78. Dang EV, McDonald JG, Russell DW, Cyster JG. Oxysterol restraint of cholesterol synthesis prevents AIM2 inflammasome activation. Cell. 2017;171:1057–1071.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Smith B, Land H. Anticancer activity of the cholesterol exporter ABCA1 gene. Cell Rep. 2012;2:580–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kuzu OF, Noory MA, Robertson GP. The role of cholesterol in cancer. Cancer Res. 2016;76:2063–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Phillips RE, Yang Y, Smith RC, et al. Target identification reveals lanosterol synthase as a vulnerability in glioma. Proc Natl Acad Sci U S A. 2019;116:7957–7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Itoh N, Itoh Y, Tassoni A, et al. Cell-specific and region-specific transcriptomics in the multiple sclerosis model: Focus on astrocytes. Proc Natl Acad Sci U S A. 2018;115:E302–E309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Montero J, Morales A, Llacuna L, et al. Mitochondrial cholesterol contributes to chemotherapy resistance in hepatocellular carcinoma. Cancer Res. 2008;68:5246–5256. [DOI] [PubMed] [Google Scholar]
- 84. Courtney R, Landreth GE. LXR regulation of brain cholesterol: From development to disease. Trends Endocrinol Metab. 2016;27:404–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Haimon Z, Volaski A, Orthgiess J, et al. Re-evaluating microglia expression profiles using RiboTag and cell isolation strategies. Nat Immunol. 2018;19:636–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Boisvert MM, Erikson GA, Shokhirev MN, Allen NJ. The aging astrocyte transcriptome from multiple regions of the mouse brain. Cell Rep. 2018;22:269–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Friebel E, Kapolou K, Unger S, et al. Single-cell mapping of human brain cancer reveals tumor-specific instruction of tissue-invading leukocytes. Cell. 2020;181:1626–1642.e20. [DOI] [PubMed] [Google Scholar]
- 88. Chen Z, Feng X, Herting CJ, et al. Cellular and molecular identity of tumor-associated macrophages in glioblastoma. Cancer Res. 2017;77:2266–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Markovic DS, Vinnakota K, van Rooijen N, et al. Minocycline reduces glioma expansion and invasion by attenuating microglial MT1-MMP expression. Brain Behav Immun. 2011;25:624–628. [DOI] [PubMed] [Google Scholar]
- 90. Bunse L, Pusch S, Bunse T, et al. Suppression of antitumor T cell immunity by the oncometabolite (R)-2-hydroxyglutarate. Nat Med. 2018;24:1192–1203. [DOI] [PubMed] [Google Scholar]
- 91. Tilghman J, Wu H, Sang Y, et al. HMMR maintains the stemness and tumorigenicity of glioblastoma stem-like cells. Cancer Res. 2014;74:3168–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Li D, Zhang J, Liu Q. Brain cell type-specific cholesterol metabolism and implications for learning and memory. Trends Neurosci. 2022;45:401–414. [DOI] [PubMed] [Google Scholar]
- 93. Escartin C, Galea E, Lakatos A, et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat Neurosci. 2021;24:312–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Anderson MA, Ao Y, Sofroniew MV. Heterogeneity of reactive astrocytes. Neurosci Lett. 2014;565:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Batiuk MY, Martirosyan A, Wahis J, et al. Identification of region-specific astrocyte subtypes at single cell resolution. Nat Commun. 2020;11:1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wheeler MA, Clark IC, Tjon EC, et al. MAFG-driven astrocytes promote CNS inflammation. Nature. 2020;578:593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Habib N, McCabe C, Medina S, et al. Disease-associated astrocytes in Alzheimer’s disease and aging. Nat Neurosci. 2020;23:701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kambach DM, Halim AS, Cauer AG, et al. Disabled cell density sensing leads to dysregulated cholesterol synthesis in glioblastoma. Oncotarget. 2017;8:14860–14875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Moffett J, Kratz E, Florkiewicz R, Stachowiak MK. Promoter regions involved in density-dependent regulation of basic fibroblast growth factor gene expression in human astrocytic cells. Proc Natl Acad Sci U S A. 1996;93:2470–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA-seq data that support the findings of this study have been deposited in the Gene Expression Omnibus GSE193526. All other data supporting the findings of this study are available from the corresponding authors on request.