Abstract
Background:
Mortality from cervical cancer has declined steadily in the United States over the past several decades due to widespread screening for precancerous and early-stage cervical cancer (ECC), which are significantly easier to treat compared with late-stage cervical cancer (LCC). Unequal screening access continues to cause significant racial/ethnic disparities in cervical cancer diagnosis stage. This study examined the underlying role of neighborhood-level socioeconomic disadvantage as a potential mediator of the association between race/ethnicity and cervical cancer diagnosis stage.
Methods:
We analyzed Texas Cancer Registry data for cervical cancer cases diagnosed among women ages 18 or older from 2010 to 2018. We performed causal mediation analyses of the association between race/ethnicity and cervical cancer stage at diagnosis mediated by neighborhood-level socioeconomic disadvantage.
Results:
Of the 9,192 women with cervical cancer, 4,720 (51.3%) had LCC at diagnosis. Compared with non-Hispanic white (NHW) women (106.13, standard deviation (SD) = 13.32), non-Hispanic Black (NHB; 111.46, SD = 9.55) and Hispanic (112.32, SD = 9.42) women had higher area deprivation index (ADI) and had greater odds of LCC diagnosis [total effects: adjusted odds ratios (AOR) = 1.29 (95% CI, 1.11–1.46) and AOR 1.14 (95% CI, 1.03–1.25), respectively]. Approximately 34.7% and 71.6% of the disparity in LCC diagnosis were attributable to higher neighborhood socioeconomic disadvantage among NHB and Hispanic women, respectively.
Conclusions:
LCC disparity varied by race/ethnicity and was partly attributable to neighborhood disadvantage. The disparity among Hispanic women due to neighborhood deprivation was twice as high among NHB women.
Impact:
Findings may be used to develop targeted race- and place-specific interventions to improve cancer care equity.
Introduction
Despite overall decreasing incidence and mortality rates from cervical cancer due to population-based preventive efforts, the disease remains a substantial public health problem, with an estimated 604,000 new cases and 342,000 deaths worldwide in 2020 (1). The American Cancer Society projects that 14,100 women will be newly diagnosed with invasive cervical cancer and approximately 4,280 women will die from the disease in the United States in 2022 (2). According to the Surveillance Epidemiology and End Results (SEER) data, Texas ranked fourth nationally in cervical cancer incidence rate (9.2 per 100,000) from 2012 to 2016 [SEER (RRID: SCR_006902)]. Despite preventive and early detection efforts, recent studies have shown a stage shift in the trend of cervical cancer incidence, with increasing rates of late-stage cervical cancer (LCC) at diagnosis in the past two decades (3–5). This trend may be due to inequitable access to preventive resources, including vaccination, screening, and follow-up for abnormal screening results (6). Stage at diagnosis is strongly linked to prognosis (7, 8) such that individuals diagnosed with cervical cancer with distant spread have lower survival compared with those at an earlier stage [18% 5-year survival in the United States; National Cancer Institute (RRID:SCR_011403)].
Substantial disparities in stage at diagnosis and outcomes have been reported in terms of race/ethnicity. Non-Hispanic Black (NHB) and Hispanic women in the United States bear the greatest burden of delayed diagnosis (9, 10), primarily due to lower screening rates and follow-up for abnormal screening (11, 12). Compared with non-Hispanic white (NHW) women, women from all other racial/ethnic subpopulations have a higher incidence of LCC diagnosis (9). There is growing evidence of the indirect impact of social determinants of health (SDOH) on health outcomes, including across the cancer care continuum (13, 14). SDOH, according to the World Health Organization's (WHO) definition are, “the conditions in which people are born, grow, work, live, and age, and the wider set of forces and systems shaping the conditions of daily life” (15). Socioeconomic disadvantage is a measure of place-based stressors that encompass important aspects of the neighborhood in which someone lives (i.e., level of poverty, low educational attainment, substandard housing, and lack of employment; ref. 16). Given that the US racial/ethnic minority populations have disproportionate place-based social, economic, and environmental disadvantages, SDOH may contribute to disparities in cervical cancer screening and diagnosis.
Previous studies across the globe have demonstrated the associations between socioeconomic status (SES) and stage of cancer at diagnosis, including cervical cancer (17–21). Studies examining the role of place-based factors on the influence of race/ethnicity on cervical cancer stages at diagnosis are scarce, though racial/ethnic minority women and those living in neighborhoods with greater disadvantages have poorer cervical cancer outcomes (22). A previous study reported that neighborhood-level factors including SES predicted cervical cancer mortality disparity in Texas (23). Another study examining cancer stage at diagnosis found that individuals in neighborhoods with higher SES were more likely to present with localized cancers compared with low SES neighborhoods (24).
Here, we used the area deprivation index (ADI), a composite measure of neighborhood socioeconomic deprivation (25), to examine the role of neighborhood-level socioeconomic disadvantage as a potential mediator of the association between race/ethnicity and being diagnosed with LCC versus early-stage cervical cancer (ECC). We hypothesized that the racial/ethnic disparity in cervical cancer stage at diagnosis would be in part mediated by ADI. We chose Texas as the setting for our study because of the high rate of cervical cancer and the diversity of the minority–majority population in the state, allowing for a unique opportunity to explore the role of race/ethnicity on cervical cancer diagnosis and outcomes. Understanding the role of neighborhood-level socioeconomic deprivation in mediating the links between individual characteristics could provide greater insights that could help develop community-targeted race-specific interventions to bridge the gaps in cervical cancer outcomes and improve survival.
Materials and Methods
Study design, data sources, and study population
In this cross-sectional, population-based study, we analyzed data from the Texas Cancer Registry (TCR) and corresponding US Census Bureau data. The TCR, which is now a SEER Registry, is one of the largest US statewide population-based cancer registries and uses active and passive surveillance systems to collect data on all cancers diagnosed among Texas residents [SEER (RRID: SCR_006902)]. The database includes demographic and clinical data, such as diagnosis date, type of cancer, stage, and primary payer at diagnosis. The TCR codes cancer primary site and histology according to the International Classification of Diseases for Oncology, Third Edition (ICD-O-3). Stage at diagnosis is coded based on the National Cancer Institute (NCI) SEER summary stage. We retrieved all primary cervical cancer cases (ICO-O-3 codes C530, C531, C538, and C539) in the TCR, diagnosed among women ages 18 years or older who were Texas residents at the time of diagnosis from January 2010 to December 2018 (n = 11,560). This study follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Cases ascertained by autopsy report, death certificates, and without cancer staging (n = 1,706), missing XY coordinates or non-codable geographical identification (n = 39), duplicates (n = 3), and missing ADI (n = 287) were excluded. Also, we excluded non-Hispanic other races due to small sample size. A total of 9,192 women, ages 18 years or older, diagnosed with cervical cancer from 2010 to 2018, who resided in Texas at the time of their diagnosis were included in the final analysis.
Study variables
The primary outcome variable was cervical cancer stage at diagnosis derived from the SEER summary stage variable. We categorized the stage as ECC [SEER summary stage 0/1 (in situ/localized)] versus LCC [SEER Summary Stage 2–7 (regional to distant metastasis)] for this analysis. The main independent variable was race/ethnicity (derived from two variables—race and Hispanic origin and categorized as NHW, NHB, and Hispanic). Other covariates were age at diagnosis (categorized as <35, 35–44, 45–54, 55–64, and ≥65), health insurance coverage at diagnosis (yes, no, missing/unknown (due to high missing value (13%), and year of diagnosis (2010–2012, 2013–2015, and 2016–2018), since cervical cancer screening guidelines changed during our study period (26), and rural–urban status (yes, no). TCR does not provide information on individual SES, such as education, employment, and income. Thus, these were not included in the models.
Potential mediator variable: area deprivation index
Accompanying data matching the TCR cases were retrieved from the U.S. Census Bureau by linking the TCR-provided patient's primary address longitude and latitude (XY coordinates at diagnosis) to a census tract using the 2010 TIGER/Line census tract shapefile ArcGIS Pro (Esri). The 5-year estimates of the American Community Surveys (ACS) summarized for census tracts were used to calculate the ADI as described by Knighton and colleagues (25). ADI is a validated, composite measure of neighborhood socioeconomic disadvantage that uses 17 census tract indicators representing income, housing characteristics, employment, and education (25). ADI has been associated with disease risk factors (27), healthcare utilization (28), and disparities in healthcare (29). To properly match cases with neighborhood-level indicators over our study period, we followed the example from previous research (30) and used two nonoverlapping ACS 5-year estimates. Thus, cases diagnosed from 2010 to 2014 were assigned the 2010–2014 ACS data, and those diagnosed from 2015 to 2018 were given the 2015–2019 ACS data. The computed ADI scores were used as a continuous variable throughout the study.
This study was approved by the Institutional Review Board of Baylor College of Medicine and Texas Department of State Health Services.
Statistical analysis
Sample characteristics, frequencies (%) and means (SD), were compared by cancer stage using the χ2 test for categorical variables and independent t tests for continuous variables. Descriptive statistical analyses were conducted using Stata Software version 17.0 (StataCorp LLC).
Mediation analysis model
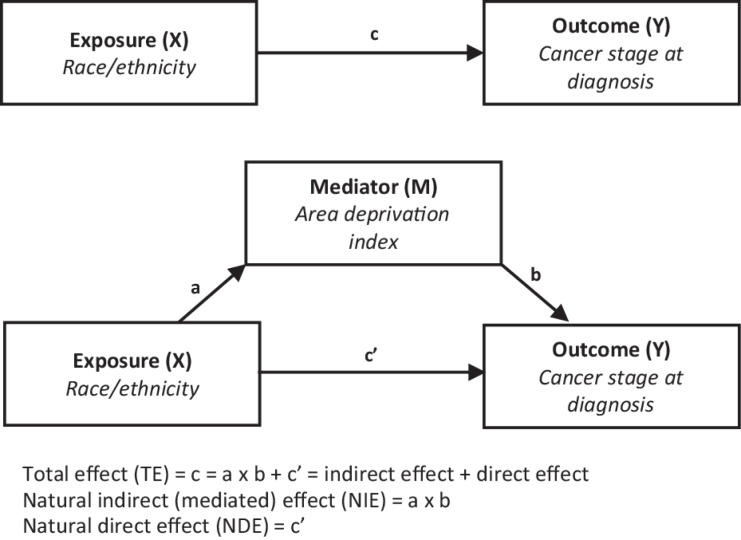
Causal mediation analysis allows the investigation of the potential effects of a third variable [mediator (M)] in a causal pathway between exposure (X) and outcome (Y) variables (31). It offers valuable insights for developing strategic interventions that account for the role of mediators. Figure 1 shows the two causal pathways of effects from the exposure variable—A direct pathway: X
 Y, and a mediated or indirect pathway: X
Y, and a mediated or indirect pathway: X
 M
M
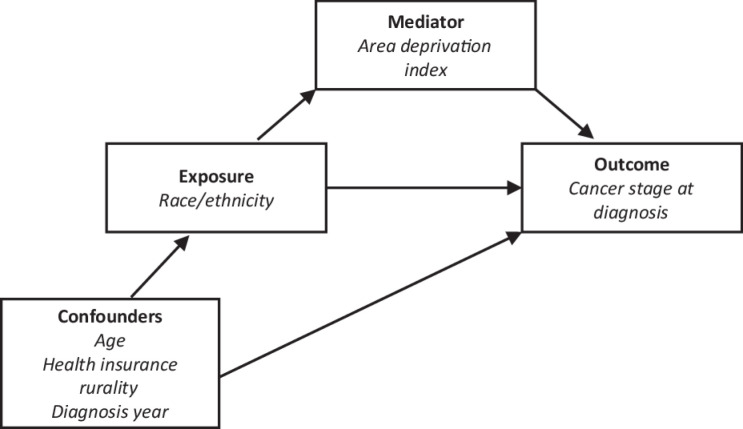
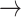 Y. Causal mediation analysis is based on the counterfactual framework (32, 33) and implements the regression adjustment method to estimate causal mediation effects (34). The PROC CAUSALMED in SAS Software has the advantage of supporting causal mediation analysis using continuous or binary exposure, outcome, and mediator variables. In addition, the model allows the inclusion of covariates (Fig. 2) and interaction terms in the analysis (31, 35, 36).
Y. Causal mediation analysis is based on the counterfactual framework (32, 33) and implements the regression adjustment method to estimate causal mediation effects (34). The PROC CAUSALMED in SAS Software has the advantage of supporting causal mediation analysis using continuous or binary exposure, outcome, and mediator variables. In addition, the model allows the inclusion of covariates (Fig. 2) and interaction terms in the analysis (31, 35, 36).
Figure 1.
Mediation analysis model. The figure shows the causal mediation analysis pathways, including the total effect, direct, and indirect effects.
Figure 2.
Components of the counterfactual framework-based model. The figure shows components of the causal mediation model using the counterfactual framework.
Prior to the mediation analyses, three sets of analyses were conducted to examine the interrelationship among exposure, outcome, and mediator variables. First, we assessed the relationship between race/ethnicity (exposure) and ADI (mediator) using a simple linear regression analysis. Second, to evaluate whether the potential mediator was linked to the outcome of interest, we performed a logistic regression analysis with cervical cancer stage at diagnosis as the outcome and ADI as the predictor (exposure) variable. Third, we fitted a logistic regression model to examine the associations between race/ethnicity (exposure) and stage at diagnosis. Then, we performed causal mediation analyses using the counterfactual framework approach (CAUSALMED procedure) described by Yung and colleagues (35) to estimate the total effect (TE), natural direct effect (NDE), natural indirect effect (NIE, also referred to as the mediated effect), and percent mediated. For this study, TE represents the total effect of race/ethnicity (exposure) on LCC diagnosis (outcome). At the same time, NDE is the effect of race/ethnicity on LCC diagnosis that is not mediated by ADI (mediator). In addition, NIE represents the evidence for the effect of race/ethnicity on LCC diagnosis mediated by ADI, and percent mediated depicts the percentage of NIE in relation to the TE (35). We adjusted for age, health insurance at diagnosis, rural–urban status, and diagnosis year, in the causal mediation analyses model. Mediation analyses were conducted using the SAS Statistical software, version 9.4 (SAS Institute Inc).
Data availability
Data are available at TCR https://www.dshs.texas.gov/texas-cancer-registry
Results
A total of 9,192 patients with cervical cancer were included in the analyses. Table 1 presents the individual-level and neighborhood-level sociodemographic characteristics overall and according to cervical cancer stage. Of the 9,192 patients, 4,472 (48.7%) had ECC (i.e., in situ/localized cancer), and 4,720 (51.3%) were diagnosed with LCC (i.e., regional/distant). The mean age and ADI for women with ECC diagnosis were 44.56 (SD 13.45) years and 107.82 (13.08), respectively. For LCC diagnosis, mean age and ADI were 51.59 (SD 14.20) years and 110.39 (SD 10.54), respectively. In addition, the proportions of NHB and Hispanic women in the LCC group (15.3% and 38.1%, respectively) were significantly higher than in the ECC group (11.6% and 35.8%). All individual variables used to derive ADI differed among women with ECC and LCC, except for percent housing units without complete plumbing (Table 1).
Table 1.
Characteristics of cervical cancer cases diagnosed in Texas from 2010 to 2018, overall and by cancer stage at diagnosis.
| Comparing early with late-stage cervical cancer | ||||
|---|---|---|---|---|
| Characteristic | Overall N = 9,192 | Early-stage cervical cancer n = 4,472 | Late-stage cervical cancer n = 4,720 | P |
| Age, mean (SD) | 48.17 (14.28) | 44.56 (13.45) | 51.59 (14.20) | <0.001 |
| Age, n (%) | <0.001 | |||
| <35 | 1,675 (18.2) | 1,125 (25.2) | 550 (11.7) | |
| 35–44 | 2,504 (27.2) | 1,440 (32.2) | 1,064 (22.5) | |
| 45–54 | 2,140 (23.3) | 898 (20.1) | 1,242 (26.3) | |
| 55–64 | 1,613 (17.6) | 591 (13.2) | 1,022 (21.7) | |
| ≥65 | 1,260 (13.7) | 418 (9.4) | 842 (17.8) | |
| Race/ethnicity, n (%) | <0.001 | |||
| Non-Hispanic white | 4,551 (49.51) | 2,351 (52.6) | 2,200 (46.6) | |
| Non-Hispanic Black | 1,238 (13.47) | 518 (11.6) | 720 (15.3) | |
| Hispanic | 3,403 (37.02) | 1,603 (35.8) | 1,800 (38.1) | |
| ADI; mean (SD) | 109.14 (11.91) | 107.82 (13.08) | 110.39 (10.54) | <0.001 |
| Health insurance coverage n (%) | <0.001 | |||
| Yes | 6,165 (67.1) | 3,149 (70.4) | 3,016 (63.9) | |
| No | 1,832 (19.9) | 711 (15.9) | 1,121 (23.8) | |
| Missing/unknown | 1,195 (13.0) | 612 (13.7) | 583 (12.3) | |
| Diagnosis year | 0.347 | |||
| 2010–2012 | 2,978 (32.40) | 1,479 (33.07) | 1,499 (31.76) | |
| 2013–2015 | 3,183 (34.63) | 1,544 (34.53) | 1,639 (34.72) | |
| 2016–2018 | 3,031 (32.97) | 1,449 (32.40) | 1,582 (33.52) | |
| Rural–urban status | 0.182 | |||
| Rural | 684 (7.4) | 316 (7.1) | 368 (7.8) | |
| Urban | 8,508 (92.6) | 4,156 (92.9) | 4,352 (92.2) | |
| Variables used for ADI, mean (SD) | ||||
| Median family income | $64,297.93 ($32,241.08) | $68,010.41 ($34,623.19) | $60,778.39 ($29,381.12) | <0.001 |
| Income disparity | 0.50 (1.07) | 0.46 (1.13) | 0.54 (1.02) | <0.001 |
| % Families below poverty level | 15.48 (11.83) | 14.40 (11.53) | 16.50 (12.01) | <0.001 |
| % Population below 150% poverty threshold | 30.35 (16.88) | 28.57 (16.82) | 32.03 (16.77) | <0.001 |
| % Single-parent households with dependent <18 | 36.96 (16.33) | 35.60 (16.28) | 38.25 (16.27) | <0.001 |
| % Households without a motor vehicle | 6.46 (6.47) | 5.94 (6.05) | 6.94 (6.81) | <0.001 |
| % Households without a telephone | 1.66 (1.81) | 1.59 (1.74) | 1.73 (1.87) | <0.001 |
| % Occupied housing units without complete plumbing | 0.58 (1.11) | 0.58 (1.13) | 0.59 (1.10) | 0.585 |
| % Owner occupied housing units | 62.67 (21.31) | 63.63 (21.45) | 61.76 (21.14) | <0.001 |
| % Households with >1 person per room | 5.92 (5.52) | 5.54 (5.38) | 6.28 (5.62) | <0.001 |
| Median selected monthly owner cost; Mortgage | $1,380.72 ($531.11) | $1,431.06 ($563.25) | $1,332.88 ($493.98) | <0.001 |
| Median gross rent | $960.45 ($330.65) | $992.40 ($349.29) | $930.17($308.96) | <0.001 |
| Median home value | $144,299.90 ($108,573.20) | $154,443.00 ($119,472.30) | $134,681.70 ($96,144.22) | <0.001 |
| % Employed person 16+ in white collar occupation | 30.29 (15.09) | 32.19 (15.72) | 28.48 (14.22) | <0.001 |
| % Civilian labor force unemployed (ages 16+) | 4.53 (2.64) | 4.44 (2.61) | 4.61 (2.65) | 0.003 |
| % Population 25+ year old; <9-year education | 10.80 (9.72) | 9.89 (9.42) | 11.66 (9.93) | <0.001 |
| % Population 25+ year old; at least a high school education | 78.75 (14.82) | 80.41 (14.63) | 77.18 (14.83) | <0.001 |
The three-step initial analyses showed evidence of relationships between (i) race/ethnicity [exposure] and cervical cancer diagnosis stage [outcome] (P < 0.001) [step 1 fulfilled], (ii) ADI [mediator] and cervical cancer diagnosis stage (P < 0.001) [step 2 fulfilled], and (iii) cervical cancer diagnosis stage and ADI (P < 0.001) [step 3 fulfilled]. Table 2 depicts the sample characteristics and cancer stage based on mean ADI among all women with cervical cancer. NHB and Hispanic women had higher ADI (111.46 and 112.32, respectively) compared with NHW women (106.13) P < 0.001. Table 3 shows the individual and area-level characteristics of women diagnosed with LCC stratified by race/ethnicity.
Table 2.
Sample characteristics by mean (SD) ADI among women with of cervical cancer cases diagnosed in Texas, 2010 to 2018.
| Characteristic | N | ADI (SD) | P value |
|---|---|---|---|
| Age, n (%) | <0.001 | ||
| <35 | 1,675 | 108.79 (11.90) | |
| 35–44 | 2,504 | 108.51 (12.31) | |
| 45–54 | 2,140 | 109.80 (10.86) | |
| 55–64 | 1,613 | 109.45 (11.40) | |
| ≥65 | 1,260 | 109.6 (13.32) | |
| Race/ethnicity | <0.001 | ||
| Non-Hispanic white | 4,551 | 106.13 (13.32) | |
| Non-Hispanic Black | 1,238 | 111.46 (9.55) | |
| Hispanic | 3,403 | 112.32 (9.42) | |
| Health insurance coverage, n (%) | <0.001 | ||
| Yes | 6,165 | 105.61 (13.74) | |
| No | 1,832 | 111.18 (10.00) | |
| Missing/unknown | 1,195 | 111.69 (9.74) | |
| Diagnosis year | 0.118 | ||
| 2010–2012 | 2,978 | 109.74 (12.09) | |
| 2013–2015 | 3,183 | 109.44 (11.95) | |
| 2016–2018 | 3,031 | 108.24 (11.65) | |
| Rural–urban status | <0.001 | ||
| Rural | 684 | 113.63 (4.60) | |
| Urban | 8,508 | 108.78 (12.24) | |
| Cervical cancer stage at diagnosis | <0.001 | ||
| Early (localized/in situ) | 4,472 | 107.82 (13.08) | |
| Late (regional/distant) | 4,720 | 110.39 (10.54) |
P values from two-sample t test with equal variance (binary variable) or one-way ANOVA (categorical variable more than two levels).
Table 3.
Individual- and census tract-level characteristics of LCC cases by race/ethnicity in Texas, 2010 to 2018.
| NHW (n = 2,200) | Non-Hispanic Black (n = 720) | Hispanic (n = 1,800) | P value | |
|---|---|---|---|---|
| Age; mean (SD) | 52.32 (14.0) | 53.35 (14.14) | 49.99 (14.31) | <0.001 |
| Age category, N (%) | <0.001 | |||
| <35 | 233 (10.6) | 66 (9.2) | 251 (13.9) | |
| 35–44 | 458 (20.8)) | 140 (19.4) | 466 (25.9) | |
| 45–54 | 572 (26.0) | 198 (27.5) | 472 (26.2) | |
| 55–64 | 527 (24.0) | 157 (21.8) | 338 (18.8) | |
| ≥65 | 410 (18.6) | 159 (22.08) | 273 (15.2) | |
| Area deprivation index (ADI); mean (SD) | 107.73 (11.68) | 111.95 (9.64) | 113.02 (8.43) | <0.001 |
| Health insurance coverage, n (%) | <0.001 | |||
| Yes | 1,593 (72.4) | 487 (67.6) | 936 (52.0) | |
| No | 330 (15.0) | 157 (21.8) | 634 (35.2) | |
| Missing/unknown | 277 (12.6) | 76 (10.6) | 230 (12.8) | |
| Diagnosis year | 0.138 | |||
| 2010–2012 | 671 (30.5) | 233 (32.4) | 595 (33.1) | |
| 2013–2015 | 764 (34.7) | 236 (32.8) | 639 (35.5) | |
| 2016–2018 | 765 (34.8) | 251 (34.9) | 566 (31.4) | |
| Rural–urban status | <0.001 | |||
| Rural | 251 (11.4) | 26 (3.6) | 91 (5.1) | |
| Urban | 1949 (88.6) | 694 (96.4) | 1,709 (94.9) | |
| Variables used for ADI, mean (SD) | ||||
| Median family income | $69,964.98 ($30,734.90) | $54,751.79 ($27,254.47) | $51,953.13 ($24,840.61) | <0.001 |
| Income disparity, mean (SD) | 0.31 (0.48) | 0.78 (1.29) | 0.73 (1.29) | <0.001 |
| % Families below poverty level | 11.93 (9.1) | 19.51 (12.92) | 20.90 (12.74) | <0.001 |
| % Population below 150% poverty threshold | 25.59 (13.89) | 36.46 (17.15) | 38.14 (16.99) | <0.001 |
| % Single-parent households with dependents <18 | 33.55 (15.22) | 46.54 (18.51) | 40.68 (14.62) | <0.001 |
| % Households without a motor vehicle | 4.99 (4.82) | 9.39 (8.18) | 8.35 (7.60) | <0.001 |
| % Households without a telephone | 1.42 (1.59) | 1.91 (1.99) | 2.04 (2.08) | <0.001 |
| % Occupied housing units without complete plumbing | 0.47 (0.87) | 0.60 (1.00) | 0.73 (1.35) | <0.001 |
| % Owner occupied housing units | 66.35 (20.18) | 55.45 (21.74) | 58.69 (20.85) | 0.039 |
| % Households with >1 person per room | 4.58 (4.34) | 6.01 (4.93) | 8.46 (6.47) | <0.001 |
| Median selected monthly owner costs; mortgage ($) | $1,428.51 ($516.33) | $1,309.78 ($509.31) | $1,224.47 ($432.67) | <0.001 |
| Median gross rent ($) | $980.58 ($331.25) | $935.59 ($275.63) | $866.50 ($280.62) | <0.001 |
| Median home value ($) | $153,569.90 ($106,271.50) | $125,656.00 ($92,497.53) | $115,189.70 ($78,569.97) | <0.001 |
| % Employed person 16+ in white collar occupation | 32.56 (14.08) | 26.62 (13.77) | 24.24 (13.15) | 0.010 |
| % Civilian labor force unemployed (ages 16+) | 4.05 (2.33) | 5.53 (3.12) | 4.92 (2.67) | <0.001 |
| % Population 25+ y.o.; <9-year education | 7.85 (7.28) | 10.81 (8.23) | 16.66 (11.12) | <0.001 |
| % Population 25+ y.o.; at least a high school edu. | 82.82 (11.95) | 76.97 (13.06) | 70.37 (15.77) | <0.001 |
Results of the mediation analyses (Table 4) showed that NHB women had significantly greater odds of being diagnosed with LCC than NHW women [TE: adjusted odds ratio (AOR) = 1.29 (95% CI, 1.11–1.46), P = 0.002], with 34.71% (95% CI, 5.01%–64.40%, P = 0.022) of the total effect was mediated by ADI. Also, Hispanic women were more likely to be diagnosed with LCC compared with NHW women [TE: AOR = 1.14 (95% CI, 1.03–1.25), P = 0.016] and nearly two thirds of the effect [71.61% (95% CI, 13.68%–129.53%), P = 0.015] was mediated by ADI. The ADI-mediated effect was 2-fold higher in Hispanic women than in NHB women.
Table 4.
Mediation effects of ADI on the association between race/ethnicity and LCC diagnosis in Texas, 2010 to 2018.
| Causal effects modelsa | ||||
|---|---|---|---|---|
| Adjusted odds ratio (95% CI) | P | % Mediated (95% CI) | % Due to interaction (95% CI) | |
| Non-Hispanic Black vs. NHW | 34.71 (5.01–64.40) | −3.85 (−42.40 to 34.71) | ||
| Total effect (TE) | 1.29 (1.11–1.46) | 0.002 | ||
| Natural direct effect (NDE) | 1.19 (1.02–1.35) | 0.029 | ||
| Natural indirect effect (NIE) | 1.08 (1.01–1.16) | 0.028 | ||
| Hispanic vs. NHW | 71.61 (13.68–129.53) | −10.28 (−55.46 to 34.90) | ||
| Total effect (TE) | 1.14 (1.03–1.25) | 0.016 | ||
| Natural direct effect (NDE) | 1.04 (0.93–1.15) | 0.473 | ||
| Natural indirect effect (NIE) | 1.10 (1.04–1.15) | <0.001 | ||
aModels included an interaction term between race/ethnicity and ADI, and adjusted for age, health insurance, rurality, and year of diagnosis.
Discussion
In this population-based study, cervical cancer stage at diagnosis varied by race/ethnicity and neighborhood SES. NHB and Hispanic women were more likely to be diagnosed with LCC, compared with NHW women, and greater neighborhood socioeconomic disadvantage was associated with LCC diagnosis. Importantly, neighborhood socioeconomic disadvantage (ADI) substantially mediated the racial/ethnic disparity in cervical cancer stage at diagnosis with percent effect mediated being 2-fold higher among Hispanic than NHB. Our study complements and extends prior work, underscoring the complex interplay between race/ethnicity, neighborhood disadvantage, and cervical cancer stage disparities. Additionally, our data highlight a potential mechanism of racial/ethnic differences in cervical cancer outcomes using mediation analyses.
Others have examined the mediation effect of ADI on race and some cancer outcomes. For example, Oluyomi and colleagues found that ADI mediation explained 13% of the disparities in hepatocellular cancer stage at diagnosis among Hispanics (30). In another US study investigating differences in childhood cancer survival by race/ethnicity, Zhao and colleagues found that social deprivation index contributed to 19% of variations among NHB versus NWH and 45% among NHB versus Hispanic (37). To our knowledge, the current analysis may be the first to assess the mediation effect of ADI on the relationship between race/ethnicity and cervical cancer stage at diagnosis.
NHB women had greater odds of being diagnosed with LCC than Hispanic women when compared with NHW in our study. However, ADI mediated twice more of the race/ethnicity effect on stage at diagnosis among Hispanic women than NHB women. Similar to what Oluyomi and colleagues and Zhao and colleagues observed (30, 37), our data support their hypotheses that although ADI may capture the socioeconomic disadvantages among racial minority subpopulations, it may not adequately cover all important social factors specific to NHBs. Moreover, past studies have suggested that residential segregation is linked to racial/ethnic cancer disparities (38) and may still be prevalent among NHBs. For example, Firebaugh and colleagues (39) found a slower decline in black residential segregation [geographic separation of racial/ethnic minorities (NHB) from whites in residential communities; ref. 40] compared with a faster decline in black neighborhood poverty disparity than among other racial minority subpopulations (39).
In addition, other social factors not covered by ADI, including racism, may be necessary for the mediation pathway for NHBs. Reactions to or perceptions about racial prejudice may downplay the mediation effect of ADI on the relationship between race and cervical cancer among NHB. Again, as previously noted (30), findings from the current study support the notion that complex relationships among social structure, racial prejudices, and socioeconomic factors underly disparities in cancer outcomes among racial/ethnic subgroups in the United States. More studies examining the mechanisms through which other social factors influence cervical cancer stage at diagnosis are needed, especially among NHB women.
The causal mediation analysis models allowed potential confounders to be controlled for, and results suggest that multilevel factors, including individual and place-based factors, intermingle at varying degrees of influence to produce cancer disparities among racial/ethnic subgroups. These findings are important because, despite advancements in cancer care, cervical cancer diagnosed after regional or distant spread has 58% and 18% 5-year survival rates, respectively, compared with 92% if detected early [SEER, RRID: SCR_006902]. There is a need to identify high-risk women and develop targeted and cost-effective interventions that address race-specific root causes, including SDOH, to reduce cervical cancer disparity.
Strengths of the current study include using population-based data that meet high-quality national and international standards. In addition, we analyzed the potential mediation effect of neighborhood disadvantage in the link between race/ethnicity and cervical cancer stage at diagnosis, unlike previous studies that examined only associations between race/ethnicity and neighborhood disadvantage and cervical cancer stage at diagnosis (21, 22, 41, 42). Nonetheless, there are important limitations to this study. First, we analyzed data from a cancer registry and assessed neighborhood-level socioeconomic disadvantage. Access to individual-level factors that could contribute to delayed cervical cancer diagnosis was limited. For example, education, family history of cancer, comorbid conditions, and previous preventive services utilization, including cervical cancer screening that could serve as predictors in the causal mediation models, were unavailable. Second, although ADI is a comprehensive composite measure of area-level socioeconomic deprivation, it may only represent some aspects of social deprivation. However, it is a validated and widely used proxy measure for assessing individual-level SES (25).
In conclusion, the findings of this study emphasize racial/ethnic disparities in cervical cancer staging at diagnosis, and neighborhood deprivation explains some of the variations. Cervical cancer prevention, including HPV vaccination, screening, and follow-up for abnormal screening results, could be affected by neighborhood-level disadvantage. Policies for long-term multilevel investments in low-resource neighborhoods and among racial minority populations are needed to reduce health disparities in cervical cancer stage at diagnosis and survival outcomes. In addition, our results can be used to develop neighborhood-level and race-specific interventions for early diagnosis and treatment of cervical cancer.
Acknowledgments
This work was funded by a research training award from the Cancer Prevention and Research Institute of Texas (CPRIT) for the Systems Epidemiology of Cancer Training (SECT) Program (RP210037; to A.P. Thrift and I.O. Sokale). This research was also supported by a grant from the National Institute on Minority Health and Health Disparities (NIMHD, R01MD01375; to J.R. Montealegre). This study was also supported by the facilities and resources of the Dan L Duncan Comprehensive Cancer Center (P30 CA125123; all authors). The funders had no role in the design of the study, the collection, analyses or interpretation of the data, the writing of the manuscript, or the decision to publish the results.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authors' Disclosures
A.P. Thrift reports grants from Cancer Prevention and Research Institute of Texas during the conduct of the study. No disclosures were reported by the other authors.
Authors' Contributions
I.O. Sokale: Conceptualization, data curation, formal analysis, investigation, methodology, writing–original draft. A.O. Oluyomi: Software, methodology, writing–review and editing. J.R. Montealegre: Funding acquisition, writing–review and editing. A.P. Thrift: Conceptualization, resources, software, supervision, funding acquisition, methodology, project administration, writing–review and editing.
References
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. [DOI] [PubMed] [Google Scholar]
- 2. American Cancer Society. Cancer facts and figures 2022. [cited 2022 Dec 02]. Available from: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2022.html.
- 3. Matsuo K, Chang EJ, Matsuzaki S, Shimada M, Ciccone MA, Roman LD. Recent changes in demographics and outcomes of cervical cancer in the United States. Arch Gynecol Obstet 2021;304:1–3. [DOI] [PubMed] [Google Scholar]
- 4. Islami F, Fedewa SA, Jemal A. Trends in cervical cancer incidence rates by age, race/ethnicity, histological subtype, and stage at diagnosis in the United States. Prev Med 2019;123:316–23. [DOI] [PubMed] [Google Scholar]
- 5. Francoeur AA, Liao CI, Caesar MA, Chan A, Kapp DS, Cohen JG, et al. The increasing incidence of stage IV cervical cancer in the USA: what factors are related? Int J Gynecol Cancer 2023;33:317–8. [DOI] [PubMed] [Google Scholar]
- 6. Tsu VD, Levin CE. Making the case for cervical cancer prevention: what about equity? Reprod Health Matters 2008;16:104–12. [DOI] [PubMed] [Google Scholar]
- 7. Patel A, West HJ. What does my stage of cancer mean? JAMA Oncol 2020;6:1308. [DOI] [PubMed] [Google Scholar]
- 8. McPhail S, Johnson S, Greenberg D, Peake M, Rous B. Stage at diagnosis and early mortality from cancer in England. Br J Cancer 2015;112Suppl 1(Suppl 1):S108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yu L, Sabatino SA, White MC. Rural-urban and racial/ethnic disparities in invasive cervical cancer incidence in the United States, 2010–2014. Prev Chronic Dis 2019;16:E70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ko NY, Hong S, Winn RA, Calip GS. Association of insurance status and racial disparities with the detection of early-stage breast cancer. JAMA Oncol 2020;6:385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sackey ENS, Pemmaraju M, Griffin MR, Castilho JL. Impact of prior underinsurance on cervical cancer screening among davidson county, tennessee, women diagnosed with invasive cervical cancer, 2008–2018. BMC Womens Health 2022;22:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sokale IO, Montealegre JR, Oluyomi AO, Thrift AP. Trends and racial/ethnic differences in predictors of cervical cancer screening among US women aged 30–64 years. Cancer Epidemiol Biomarkers Prev 2023;32:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Korn AR, Walsh-Bailey C, Pilar M, Sandler B, Bhattacharjee P, Moore WT, et al. Social determinants of health and cancer screening implementation and outcomes in the USA: a systematic review protocol. Syst Rev 2022;11:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hughes Halbert C. Social determinants of health and cancer care: where do we go from here? J Natl Cancer Inst 2022;114:1564–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. World Health Organization. Social determinants of health 2022. Assessed [cited 2022 Dec 8]. Available from: https://www.who.int/health-topics/social-determinants-of-health#tab=tab_1.
- 16. Shavers VL. Measurement of socioeconomic status in health disparities research. J Natl Med Assoc 2007;99:1013–23. [PMC free article] [PubMed] [Google Scholar]
- 17. Kweon SS, Kim MG, Kang MR, Shin MH, Choi JS. Difference of stage at cancer diagnosis by socioeconomic status for four target cancers of the national cancer screening program in Korea: results from the gwangju and jeonnam cancer registries. J Epidemiol 2017;27:299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mathew A, George PS, Ramadas K, Mathew BS, Kumar A, Roshni S, et al. Sociodemographic factors and stage of cancer at diagnosis: a population-based study in South India. J Glob Oncol 2019;5:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ibfelt EH, Kjær SK, Høgdall C, Steding-Jessen M, Kjær TK, Osler M, et al. Socioeconomic position and survival after cervical cancer: influence of cancer stage, comorbidity and smoking among Danish women diagnosed between 2005 and 2010. Br J Cancer 2013;109:2489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rosskamp M, Verbeeck J, Gadeyne S, Verdoodt F, De Schutter H. Socio-economic position, cancer incidence and stage at diagnosis: a nationwide cohort study in Belgium. Cancers (Basel) 2021;13:933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Breen N, Figueroa JB. Stage of breast and cervical cancer diagnosis in disadvantaged neighborhoods: a prevention policy perspective. Am J Prev Med 1996;12:319–26. [PubMed] [Google Scholar]
- 22. McCarthy AM, Dumanovsky T, Visvanathan K, Kahn AR, Schymura MJ. Racial/ethnic and socioeconomic disparities in mortality among women diagnosed with cervical cancer in New York City, 1995–2006. Cancer Causes Control 2010;21:1645–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lin Y, Zhan FB. Geographic variations of racial/ethnic disparities in cervical cancer mortality in Texas. South Med J 2014;107:281–8. [DOI] [PubMed] [Google Scholar]
- 24. Schwartz KL, Crossley-May H, Vigneau FD, Brown K, Banerjee M. Race, socioeconomic status and stage at diagnosis for five common malignancies. Cancer Causes Control 2003;14:761–6. [DOI] [PubMed] [Google Scholar]
- 25. Knighton AJ, Savitz L, Belnap T, Stephenson B, VanDerslice J. Introduction of an area deprivation index measuring patient socioeconomic status in an integrated health system: implications for population health. EGEMS (Wash DC) 2016;4:1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. National Cancer Institute. ACS's updated cervical cancer screening guidelines explained. How have the cervical cancer screening recommendations changed? [cited 2022 Dec 12]. Available from: https://www.cancer.gov/news-events/cancer-currents-blog/2020/cervical-cancer-screening-hpv-test-guideline. [Google Scholar]
- 27. Wardle J, Jarvis MJ, Steggles N, Sutton S, Williamson S, Farrimond H, et al. Socioeconomic disparities in cancer-risk behaviors in adolescence: baseline results from the Health and Behaviour in Teenagers Study (HABITS). Prev Med 2003;36:721–30. [DOI] [PubMed] [Google Scholar]
- 28. Phillips RL, Liaw W, Crampton P, Exeter DJ, Bazemore A, Vickery KD, et al. How other countries use deprivation indices—and why the United States desperately needs one. Health Aff 2016;35:1991–8. [DOI] [PubMed] [Google Scholar]
- 29. Singh GK, Jemal A. Socioeconomic and racial/ethnic disparities in cancer mortality, incidence, and survival in the United States, 1950–2014: over six decades of changing patterns and widening inequalities. J Environ Public Health 2017;2017:2819372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oluyomi AO, Mohammadi KA, El-Serag HB, Thrift AP. Mediating effects of neighborhood-level socioeconomic deprivation on the association between race/ethnicity and advanced hepatocellular carcinoma. Cancer Epidemiol Biomarkers Prev 2022;31:1402–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods 2013;18:137–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Robins JM, Greenland S. Identifiability and exchangeability for direct and indirect effects. Epidemiology 1992;3:143–55. [DOI] [PubMed] [Google Scholar]
- 33. Pearl J. Direct and indirect effects. Probabilistic and causal inference: the works of Judea Pearl 2022.373–92. [Google Scholar]
- 34. VanderWeele TJ. A unification of mediation and interaction: a 4-way decomposition. Epidemiology 2014;749–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yung You-FaiLamm M, Zhang W, . SAS Institute. Causal mediation analysis with the CAUSALMED procedure [cited 2022 Nov 05]. Available from: https://www.sas.com/content/dam/SAS/support/en/sas-global-forum-proceedings/2018/1991-2018.pdf.
- 36. Valente MJ, Rijnhart JJM, Smyth HL, Muniz FB, MacKinnon DP. Causal mediation programs in R, Mplus, SAS, SPSS, and Stata. Struct Equ Modeling 2020;27:975–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhao J, Han X, Zheng Z, Nogueira LM, Nathan PC, Lu A, et al. Racial/ethnic disparities in childhood cancer survival in the United States: mediation effects of health insurance coverage and area-level social deprivation. J Clin Oncol 2019;143. [Google Scholar]
- 38. Pruitt SL, Lee SJ, Tiro JA, Xuan L, Ruiz JM, Inrig S. Residential racial segregation and mortality among black, white, and Hispanic urban breast cancer patients in Texas, 1995 to 2009. Cancer 2015;121:1845–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Firebaugh G, Acciai F. For blacks in America, the gap in neighborhood poverty has declined faster than segregation. Proc Natl Acad Sci 2016;113:13372–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Landrine H, Corral I, Lee JGL, Efird JT, Hall MB, Bess JJ. Residential segregation and racial cancer disparities: a systematic review. J Racial Ethn Health Disparities 2017;4:1195–205. [DOI] [PubMed] [Google Scholar]
- 41. Gauri A, Messiah SE, Bouzoubaa LA, Moore KJ, Koru-Sengul T. Cervical cancer sociodemographic and diagnostic disparities in Florida: a population-based study (1981–2013) by stage at presentation. Ethn Health 2020;25:995–1003. [DOI] [PubMed] [Google Scholar]
- 42. Zhan FB, Lin Y. Racial/Ethnic, socioeconomic, and geographic disparities of cervical cancer advanced-stage diagnosis in Texas. Women's Health Issues 2014;24:519–27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available at TCR https://www.dshs.texas.gov/texas-cancer-registry