Abstract
Purpose:
Biomarkers that predict response to immune checkpoint inhibitors (ICI) in recurrent or metastatic head and neck squamous cell carcinoma (R/M HNSCC) are needed. This retrospective study assessed tumor mutational burden (TMB) and outcomes in the phase II HAWK and CONDOR and phase III EAGLE studies of durvalumab with or without tremelimumab in platinum-resistant R/M HNSCC.
Patients and Methods:
Tumor samples from HAWK/CONDOR (N = 153) and blood samples from EAGLE (N = 247) were analyzed for TMB. Associations with survival were evaluated for tissue TMB (tTMB) at cutoffs from 10 to 20 mutations/megabase (mut/Mb) and for blood plasma TMB (bTMB) at cutoffs from 8 to 24 mut/Mb.
Results:
In HAWK/CONDOR, overall survival (OS) with durvalumab with or without tremelimumab was longer for high versus low tTMB: statistically significant differences were observed with durvalumab plus tremelimumab at tTMB ≥ 10 mut/Mb [HR, 0.52 (95% confidence interval, CI, 0.28–0.98)] and tTMB ≥ 12 mut/Mb [HR, 0.46 (95% CI, 0.24–0.86)]. In EAGLE, a significant OS benefit versus chemotherapy was observed with durvalumab and durvalumab plus tremelimumab at bTMB≥16 mut/Mb [HR, 0.39 (95% CI, 0.20–0.76) and 0.38 (95% CI, 0.19–0.78), respectively] but not bTMB < 16 mut/Mb [HR, 0.92 (0.61–1.37) and 0.92 (95% CI, 0.62–1.36), respectively]. A significant progression-free survival benefit was also observed in the ICI arms versus chemotherapy at bTMB ≥ 16 mut/Mb.
Conclusions:
Findings support TMB as a biomarker for predicting survival in patients with platinum-resistant R/M HNSCC treated with ICIs. The analysis of EAGLE demonstrated that bTMB was predictive of survival with ICI treatment versus chemotherapy in a large, randomized controlled study population.
Translational Relevance.
There is an ongoing need for improved strategies to select patients for treatment with immune checkpoint inhibitors (ICI) in recurrent or metastatic head and neck squamous cell carcinoma (R/M HNSCC). This study analyzed data from three studies of durvalumab (a PD-L1 inhibitor) with or without tremelimumab (a CTLA-4 inhibitor) to evaluate tumor mutational burden (TMB) as a predictive biomarker for ICI treatment outcomes in R/M HNSCC. In the phase II HAWK and CONDOR studies, patients with TMB-high tumors, determined at cutoffs of TMB ≥ 10 mutations (mut)/megabase (Mb) or TMB ≥ 12 mut/Mb, had longer overall survival (OS) than patients with TMB-low tumors. In the phase III EAGLE study, significant OS and progression-free survival benefit with durvalumab or durvalumab plus tremelimumab versus chemotherapy was observed in patients with high blood plasma TMB (bTMB), determined at a cutoff of TMB ≥ 16 mut/Mb, but not in patients with low bTMB. These findings demonstrate that TMB, including bTMB that can be measured noninvasively, is predictive of efficacy outcomes with ICIs and thus could be used to select patients with R/M HNSCC most likely to benefit from treatment with ICIs.
Introduction
Patients with recurrent or metastatic head and neck squamous cell carcinoma (R/M HNSCC) have a poor prognosis (1, 2). Immune checkpoint inhibitors (ICI) targeting CTL antigen 4 (CTLA-4), programmed cell death-1 (PD-1), or programmed cell death-ligand 1 (PD-L1) have been evaluated for second-line treatment of R/M HNSCC (3–5). The anti-PD-1 antibodies nivolumab and pembrolizumab demonstrated significantly improved overall survival (OS) versus standard of care (SoC; methotrexate, docetaxel, or cetuximab) in phase III studies (3, 4). In CheckMate 141, high PD-L1 expression was associated with higher objective response rates but was not significantly associated with longer OS for nivolumab versus single-agent SoC (3). In KEYNOTE-040, a greater OS benefit for pembrolizumab versus SoC was observed in the PD-L1–high subgroup than in the PD-L1–low subgroup (4). In the phase III EAGLE study, durvalumab (anti-PD-L1) with or without tremelimumab (anti-CTLA-4) demonstrated antitumor activity, but neither treatment regimen showed a significant OS benefit compared with investigator's choice of SoC chemotherapy (5). While median OS for durvalumab was longer in patients with tumor cell (TC) PD-L1 expression ≥25% versus TC < 25% (9.8 vs. 7.6 months), OS in a small population of patients with TC < 1% was longer for durvalumab versus SoC (5).
On the basis of the results of the CheckMate 141 study, nivolumab monotherapy is approved by the FDA and European Medicines Agency (EMA) for the second-line treatment of R/M HNSCC (6, 7). Pembrolizumab in combination with platinum and 5-flurouracil chemotherapy is approved by the FDA for the first-line treatment of R/M HNSCC. Pembrolizumab plus platinum and 5-flurouracil chemotherapy is approved by the EMA, and pembrolizumab monotherapy is approved by the FDA and EMA, for first-line treatment of patients with R/M HNSCC whose tumors express PD-L1 with a combined positive score of ≥1 (8, 9). Pembrolizumab monotherapy is also approved by the FDA for the second-line treatment of R/M HNSCC and, based on the results of the KEYNOTE-040 study, by the EMA for the second-line treatment of adults whose tumors express PD-L1 with a ≥50% tumor proportion score (8, 9).
Generally, high PD-L1 tumor expression correlates with improved responses to ICIs (10). However, some patients who are PD-L1–low respond to ICIs, whereas some patients who are PD-L1–high do not (11). These findings suggest that PD-L1 is an imperfect biomarker and that there is an ongoing need for improved patient-selection biomarkers for ICI treatment in R/M HNSCC (10).
In recent years, observational or retrospective studies have found that TMB, defined as the total number of somatic mutations per coding region of a tumor genome (12), may be associated with outcomes with ICIs in HNSCC (13–15). A combined analysis of data from the KEYNOTE-012 and KEYNOTE-55 studies showed that high TMB was significantly associated with better overall responses and prolonged progression-free survival (PFS) in 261 patients with HNSCC treated with pembrolizumab (16). On the basis of results from the nonrandomized, phase II KEYNOTE-158 study, which did not include patients with HNSCC, pembrolizumab was granted accelerated approval in the United States for treatment of patients with unresectable or metastatic solid tumors who had tissue TMB (tTMB) ≥ 10 mut/Mb and no other treatment options, and the FoundationOne CDx assay (Foundation Medicine) was coapproved as a diagnostic (17, 18).
Prognostic scores and nomograms have been investigated in patients with R/M HNSCC treated with ICIs (19, 20). A frequent component of prognostic algorithms in other tumor types, such as gastric cancer and non–small cell lung cancer (NSCLC), is the neutrophil-to-lymphocyte ratio (NLR), which is an index of systemic inflammation (21, 22). Though NLR is generally considered prognostic for clinical outcome in HNSCC (23), the efficacy of PD-1 and PD-L1 inhibitors are contingent on immune competence. Therefore, NLR could provide a valuable tool to help identify patients who would derive the most benefit from ICI therapy.
We analyzed data from the phase II HAWK and CONDOR studies of durvalumab with or without tremelimumab in platinum-resistant R/M HNSCC (24, 25) to evaluate baseline tTMB as a biomarker for treatment with ICIs. Data from the phase III EAGLE study were analyzed to evaluate the predictive value of blood plasma TMB (bTMB), PD-L1, and NLR for outcomes with treatment with ICIs compared with SoC.
Patients and Methods
Patients
Patients with evaluable samples for biomarker analysis from the HAWK, CONDOR, and EAGLE trials were included. Patients were adults (ages ≥18 years) with histologically confirmed R/M HNSCC of the oral cavity, oropharynx, larynx, or hypopharynx, and an Eastern Cooperative Oncology Group performance status of 0 or 1. Patients had tumor progression or recurrence during or after treatment with one systemic platinum-based regimen for R/M disease (HAWK/CONDOR/EAGLE) or progression within 6 months of the last dose of platinum given as part of multimodality therapy with curative intent (EAGLE).
HAWK, CONDOR, and EAGLE were performed in accordance with ethical principles that have their origin in the Declaration of Helsinki and are consistent with International Conference on Harmonisation/Good Clinical Practice guidelines, applicable regulatory requirements, and the AstraZeneca policy on Bioethics and Human Biological Samples. Each patient provided informed written consent before conducting any procedure specifically for HAWK, CONDOR, or EAGLE. All patients gave informed consent for the use of biological samples for biomarker analysis. Investigations were performed after approval by an Institutional Review Board or Ethics Committee at participating sites.
Study designs and treatment
HAWK was an international, multicenter, single-arm phase II study of durvalumab involving patients with platinum-refractory R/M HNSCC tumors with high PD-L1 expression (24). CONDOR was an international, multicenter, open-label, phase II study in which patients with PD-L1–low tumors were randomized 1:1:2 to receive durvalumab, tremelimumab, or the combination of durvalumab plus tremelimumab; randomization was stratified according to human papillomavirus (HPV) status and smoking status (25). EAGLE was an international, multicenter, open-label, phase III trial in which patients (regardless of PD-L1 expression) were randomized 1:1:1 to receive durvalumab, durvalumab plus tremelimumab, or SoC; randomization was stratified according to PD-L1 expression, tumor location, HPV status, and smoking status (5).
TMB analysis datasets
TMB analysis was performed on tumor samples collected from the patients enrolled in the HAWK and CONDOR studies. Baseline samples were up to 3 years old. Data from the durvalumab monotherapy arm of CONDOR were combined with data from HAWK for this analysis. The durvalumab plus tremelimumab arm from CONDOR was evaluated separately. Analysis of TMB was performed on plasma samples collected at baseline from patients in the EAGLE study until protocol version 6.
Evaluation of TMB and determination of cutoffs
For tTMB, whole-exome sequencing (WES) with 100 million reads at 200X coverage was conducted on archival formalin-fixed, paraffin-embedded tumor and patient-matched blood samples using the HiSeq 4000 System (Illumina Inc.) for 2 × 100 paired end reads. Somatic alterations were identified using VarDict (26). Only single-nucleotide variants and small insertions and deletions (<20 bp) with an allele frequency between 5% and 80% and were retained. tTMB was calculated as the number of nonsynonymous somatic alterations divided by the WES panel size of 30 Mb.
For bTMB, circulating tumor DNA was evaluated in blood plasma samples using the Guardant Health OMNI platform, a targeted next-generation sequencing platform using a 500-gene panel with a 2-Mb DNA footprint (1 Mb coding regions only). The Guardant Health bTMB algorithm incorporated somatic single-nucleotide variants and insertions/deletions and accounted for low tumor shedding or low cell-free DNA input.
A series of tTMB cutoff values ranging from 10 to 20 mut/Mb were assessed to determine the optimal HR for OS between tTMB-high and tTMB-low for each treatment group. A series of bTMB cutoff values ranging from 8 to 24 mut/Mb were assessed to determine the optimal HR for survival endpoints with durvalumab and durvalumab plus tremelimumab compared with SoC. Two-fold cross-validation analyses were performed, and cutoffs were selected using a minimum P-value approach based on a Cox proportional hazards model. The most frequently selected cutoffs in the Cox proportional hazards model training sets were considered as potential optimal cutoffs. The predictivity of these cutoffs was then assessed on the basis of HR distribution for OS and PFS (EAGLE only). Associations of TMB with patient demographics and baseline disease characteristics were assessed using cutoffs of 10 mut/Mb (tTMB) and 16 mut/Mb (bTMB) in the HAWK/CONDOR and EAGLE datasets, respectively.
Evaluation of other biomarkers
PD-L1 expression status in tumor tissue samples was determined using the VENTANA PD-L1 (SP263) Assay (Ventana Medical Systems), with high PD-L1 expression defined as ≥25% of TCs with membrane staining and low PD-L1 expression defined as <25% of TCs with membrane staining. Absolute neutrophil count and absolute lymphocyte count were assessed according to local standard to derive NLRs.
Statistical analyses
The Kaplan–Meier method was used to calculate univariate survival estimates. Two-sided P values were assessed using the log-rank test. The Wilcoxon rank-sum test and Kruskal–Wallis test were used to compare continuous variables. Analyses were performed using SAS version 9.4 (SAS Institute) and R version 4.0.0 (R Foundation).
Data availability
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Anonymized datasets may be available on request. Data for studies directly listed on Vivli can be requested through Vivli at https://search.vivli.org/. Data for studies not listed on Vivli may be requested through Vivli at https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/. The request will undergo an internal review process, and if approved, data will be prepared and shared with specified accessors named on the request form for 12 months via Vivli Secure Research Environment. AstraZeneca Vivli member page is also available outlining further details: https://vivli.org/ourmember/astrazeneca/.
Results
Patient demographic and disease characteristics
tTMB was evaluable in tumor samples from 153 patients in HAWK/CONDOR, including 78 patients who received durvalumab monotherapy and 48 patients who received durvalumab plus tremelimumab. The distribution of tTMB was similar between the HAWK and CONDOR datasets (Supplementary Fig. S1A). Patient demographics and baseline disease characteristics of the TMB-evaluable populations of HAWK/CONDOR were generally representative of the intent-to-treat (ITT) populations of these studies (Supplementary Table S1). The tTMB-high subgroup was enriched for patients who had ever smoked, and the proportion of patients who had ever smoked was significantly higher in the tTMB-high subgroup versus the tTMB-low subgroup (83.3% vs. 65.2%; P = 0.017; Supplementary Table S2). No significant difference in HPV status between the tTMB subgroups was observed.
In EAGLE, bTMB was evaluable in 247 patients, evenly distributed among the three treatment arms. The bTMB distributions were similar among the three treatment arms of the EAGLE dataset (Supplementary Fig. S1B). The patient demographics and disease characteristics of the bTMB-evaluable population were generally representative of the ITT population, except for a higher proportion of Asian patients and a lower proportion of White patients (Supplementary Table S1). The bTMB ≥ 16 mut/Mb subgroup included a significantly higher proportion of patients of a race other than White or Asian compared with the bTMB < 16 mut/Mb subgroup, though the number of patients in this race category was low (n = 10; Supplementary Table S3).
Association of tTMB and OS in HAWK/CONDOR
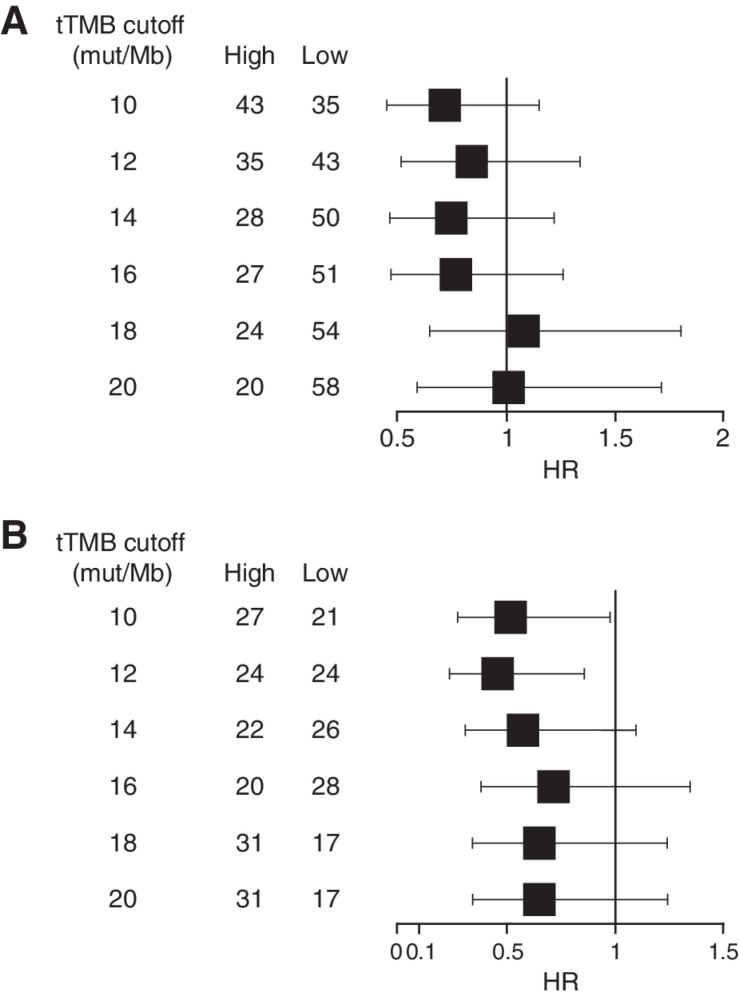
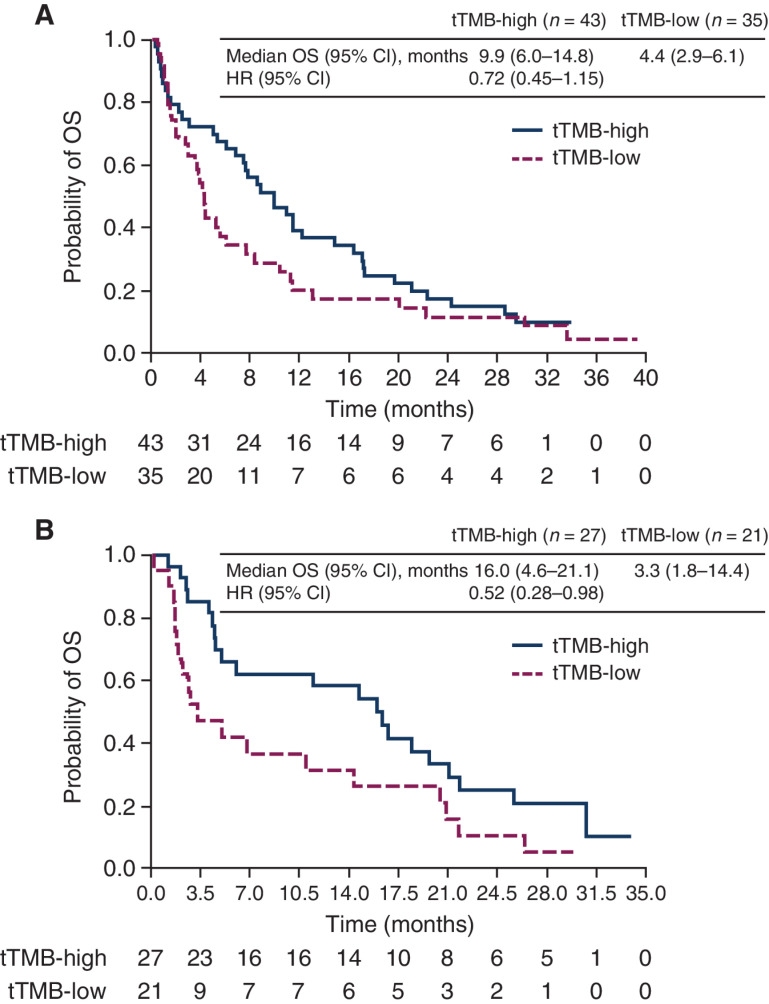
In the HAWK/CONDOR dataset, OS was compared between subgroups of patients with high- versus low-tTMB in the durvalumab monotherapy and durvalumab plus tremelimumab treatment arms. For both treatment arms, the HRs of the tTMB-high versus tTMB-low subgroups generally increased with progressively higher tTMB cutoffs (Fig. 1). For durvalumab monotherapy, the 10-mut/Mb cutoff showed the best discrimination in OS between the tTMB subgroups, with high-TMB associated with prolonged OS versus low-TMB [HR, 0.72 (95% confidence interval, CI, 0.45–1.15); Fig. 1A], although differences were not significant at any of the cutoffs evaluated. For durvalumab plus tremelimumab, significant differences in OS, favoring the high-tTMB subgroup, were observed at the 10 mut/Mb [HR, 0.52 (95% CI, 0.28–0.98)] and 12 mut/Mb [HR, 0.46 (95% CI, 0.24–0.86)] cutoffs (Fig. 1B). Using the 10 mut/Mb cutoff, median OS (95% CI) in patients with high- versus low-tTMB was 9.9 (6.0–14.8) months versus 4.4 (2.9–6.1) months, respectively, with durvalumab and 16.0 (4.6–21.1) months versus 3.3 (1.8–14.4) months, respectively, with durvalumab plus tremelimumab (Fig. 2).
Figure 1.
Forest plot of OS in the tTMB-high versus tTMB-low subgroups by tTMB cutoff with durvalumab monotherapy (A) or with durvalumab plus tremelimumab (B) in HAWK/CONDOR. HRs were calculated as tTMB-high versus -low for each treatment group. HR, hazard ratio; OS, overall survival; tTMB, tissue tumor mutational burden.
Figure 2.
Kaplan–Meier plot of OS in the tTMB-high (≥10 mut/Mb) and tTMB-low (<10 mut/Mb) subgroups for patients treated with durvalumab monotherapy (A) or with durvalumab plus tremelimumab (B) in HAWK/CONDOR. HRs were calculated as tTMB-high versus -low for each treatment group. CI, confidence interval; HR, hazard ratio; OS, overall survival; mut/Mb, mutations/megabase; tTMB, tissue tumor mutational burden
Association of bTMB with survival benefit in EAGLE
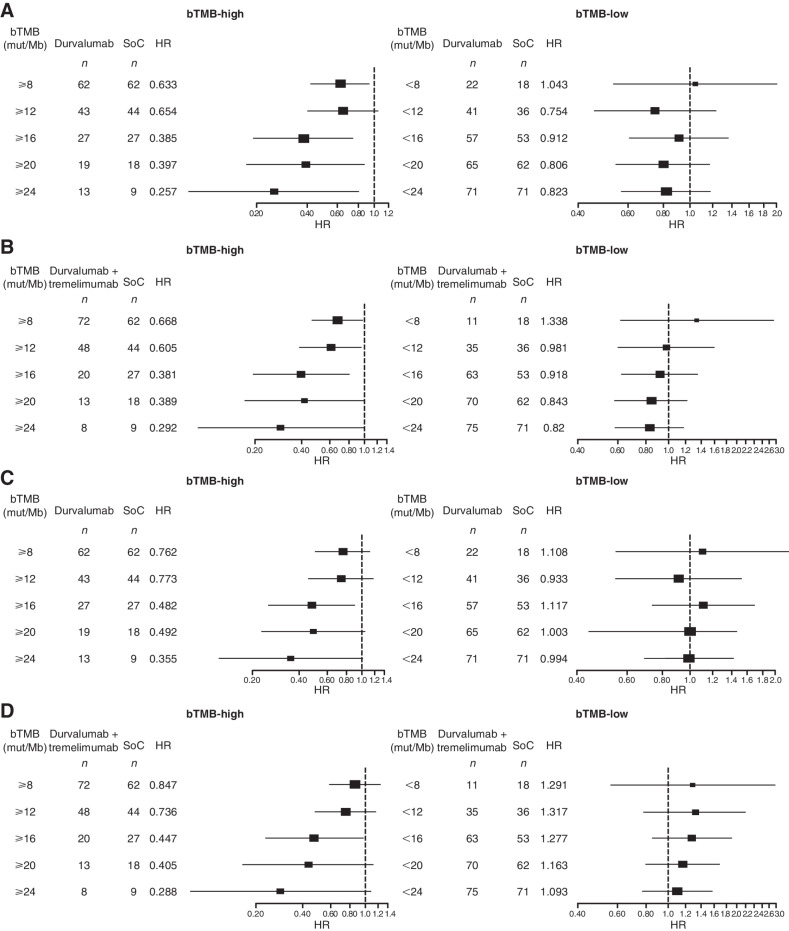
In the EAGLE dataset, OS and PFS were compared between patients who received durvalumab monotherapy or durvalumab plus tremelimumab versus SoC in the bTMB-high and bTMB-low subgroups. Across the evaluated cutoffs, patients in the bTMB-high subgroups had prolonged OS with durvalumab or durvalumab plus tremelimumab compared with SoC, and this OS benefit generally improved with progressively higher bTMB cutoffs (Fig. 3). In the bTMB ≥16 mut/Mb subgroup, OS and PFS were statistically significantly longer for durvalumab versus SoC (OS HR, 0.39; PFS HR, 0.48) and for durvalumab plus tremelimumab versus SoC (OS HR, 0.38; PFS HR, 0.45). The 16 mut/Mb cutoff was the only cutoff at which there was a statistically significant benefit for OS and PFS in the investigational arms compared with SoC.
Figure 3.
Forest plot of OS in the bTMB-high and bTMB-low subgroups by bTMB cutoff for durvalumab monotherapy versus SoC (A) and for durvalumab plus tremelimumab versus SoC (B) and PFS in the bTMB-high and bTMB-low subgroups by bTMB cutoff for durvalumab versus SoC (C) and for durvalumab plus tremelimumab versus SoC (D) in EAGLE. HRs were calculated as durvalumab with/without tremelimumab versus chemotherapy. bTMB, blood plasma tumor mutational burden; D, durvalumab; HR, hazard ratio; OS, overall survival; PFS, progression-free survival; SoC, standard of care; tTMB, tissue tumor mutational burden.
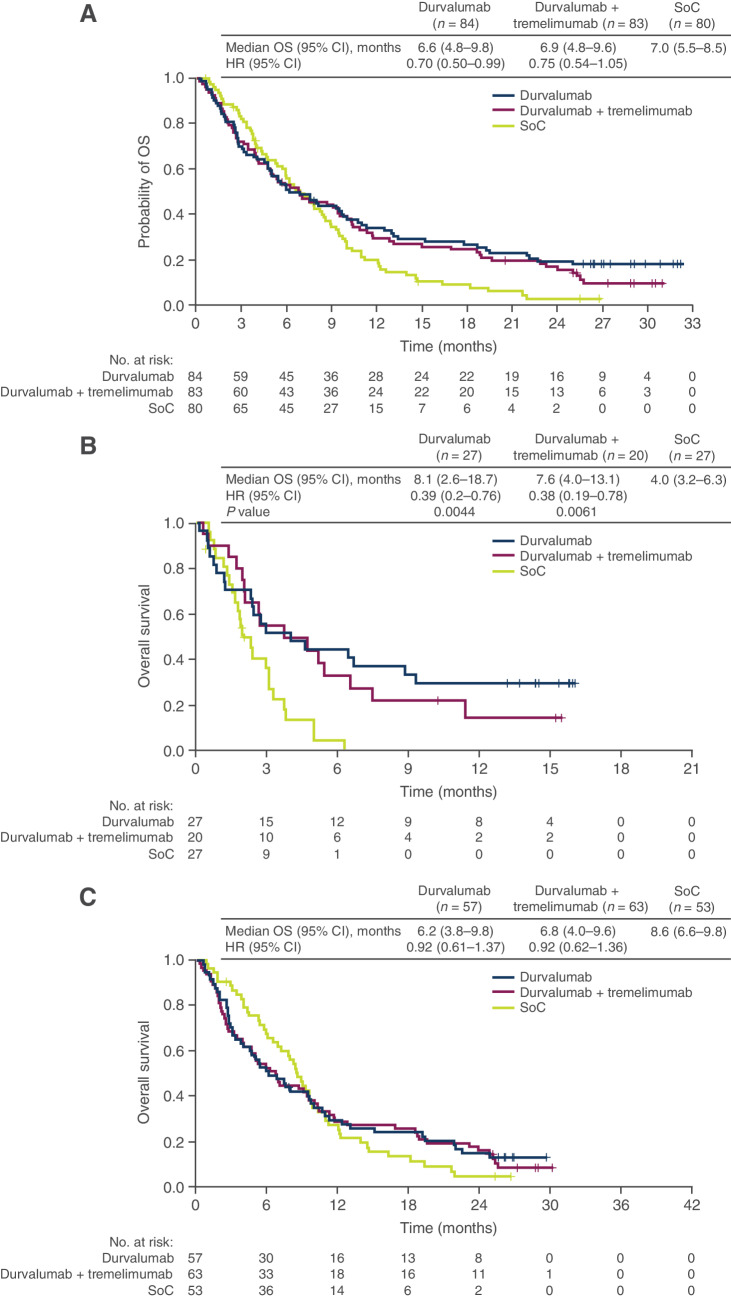
The OS HRs favored the durvalumab and durvalumab plus tremelimumab arms over the SoC arm in biomarker-evaluable population (0.70 and 0.75, respectively; Fig. 4A) relative to the ITT population (0.88 and 1.04, respectively). Nevertheless, the OS benefits of durvalumab and durvalumab plus tremelimumab versus SoC were substantially improved in the bTMB-high subgroup (HRs, 0.39 and 0.38, respectively; Fig. 4B). In this subgroup, significantly longer OS was observed for durvalumab and durvalumab plus tremelimumab versus SoC (median OS, 8.1 and 7.6 vs. 4.0 months, respectively), and shorter survival was observed for the SoC arm than in the biomarker-evaluable population (4.0 vs. 7.0 months). OS was not prolonged with durvalumab or durvalumab plus tremelimumab versus SoC in the TMB-low group (Fig. 4C).
Figure 4.
Kaplan–Meier plot of OS in the durvalumab monotherapy, durvalumab plus tremelimumab, and SoC treatment arms in the bTMB-evaluable population (A), bTMB-high subgroup (B), and bTMB-low subgroup (C). HRs were calculated as durvalumab with/without tremelimumab versus chemotherapy. bTMB, blood plasma tumor mutational burden; CI, confidence interval; HR, hazard ratio; OS, overall survival; SoC, standard of care.
Association of bTMB and PD-L1 together with survival benefit in EAGLE
OS for durvalumab with or without tremelimumab versus SoC was assessed in subgroups of patients in the EAGLE study categorized by bTMB and PD-L1 together (Supplementary Table S4). The small subgroup of patients with both high tumor PD-L1 expression and high TMB (n = 17) had the most OS benefit with durvalumab versus SoC [HR, 0.25 (95% CI, 0.06–1.03)] and durvalumab plus tremelimumab versus SoC [HR, 0.21 (95% CI, 0.04–1.16)].
Association of NLR with survival benefit in EAGLE
Baseline NLR results were available for the entire ITT population of EAGLE. A cutoff of 7 was used, based on unpublished results from a previous analysis of HAWK/CONDOR. Within the pooled ITT population, median OS was longer in patients with NLR ≤ 7 and shorter in patients with NLR > 7, irrespective of treatment [HR, 0.57 (95% CI, 0.48–0.69)]. In this analysis, there was a statistically significant survival benefit in the NLR ≤ 7 subgroup treated with durvalumab versus SoC [HR, 0.75 (95% CI, 0.57–0.97)], which was not seen in patients with NLR > 7 [HR, 1.07 (95% CI, 0.76–1.51); Supplementary Fig. S2]. No correlation was observed between NLR and bTMB (Spearman rho = 0.08).
Discussion
Findings from these analyses support the clinical utility of TMB in HNSCC. High tTMB was associated with longer OS versus low tTMB in patients who received either durvalumab monotherapy or durvalumab plus tremelimumab in HAWK/CONDOR. While no statistical difference was seen, this trend appeared to be greater with durvalumab plus tremelimumab, with numerical improvements in median OS observed between the high- and low-tTMB subgroups when comparing durvalumab plus tremelimumab with durvalumab monotherapy. In EAGLE, durvalumab monotherapy and durvalumab plus tremelimumab demonstrated prolonged OS versus SoC in patients with high bTMB, but not in those with low bTMB. TMB was not associated with prolonged OS in the SoC group, consistent with the proposed mechanism that TMB increases neoantigen load, thus potentiating T-cell responses to ICIs (27). The higher survival for patients with high bTMB treated with ICI therapy and relatively poor survival in the SoC arm support the predictive value of bTMB for ICI treatment rather than prognosis.
Our findings are consistent with published studies that have shown associations between high TMB and improved outcomes with ICI treatment in HNSCC (13–16). A limitation of published studies and this analysis of HAWK/CONDOR is the lack of a comparator group, thus making it impossible to rule out that TMB is a prognostic indicator. To our knowledge, this analysis of EAGLE provides the first evidence that TMB was predictive of survival with ICI treatment versus SoC.
The predictive value of bTMB for ICI treatment has also been shown in NSCLC. A study using the same hybridization-capture methodology as the FoundationOne CDx assay found that bTMB ≥ 16 mut/Mb selected for patients with NSCLC who derived a significant improvement in PFS with atezolizumab (anti-PD-L1) versus docetaxel (28). Another study using the NCC-GP150 panel, designed using WES data from The Cancer Genome Atlas, identified 6 mut/Mb as the optimal cutoff to discriminate PFS with ICIs in NSCLC (29). An analysis of the MYSTIC study using the Guardant Health OMNI platform showed prolonged OS for durvalumab plus tremelimumab versus SoC in patients with bTMB ≥ 20 mut/Mb, but not <20 mut/Mb, with a trend toward longer OS at ≥10 mut/Mb (30). Thus, clinically meaningful cutoffs for bTMB appear to vary by tumor type and methodology.
Our findings suggest that TMB may predict survival with ICIs, whether measured in tissue or blood. Measuring TMB in blood overcomes technical challenges with tissue samples and offers several advantages, including a less invasive sampling technique. Blood samples are more easily taken proximal to treatment and may better reflect current disease status compared with archived tissue. Results are less affected by intratumor heterogeneity and may represent multiple tumors simultaneously. However, tumors must be shedding a detectable level of ctDNA to analyze bTMB, and the current sensitivity of most platforms is likely to limit its use to patients with advanced or metastatic disease.
High tTMB was significantly associated with tobacco smoking in the HAWK/CONDOR dataset. This finding is consistent with a previous study showing that smoking caused specific mutational signatures in tumors, associated with increased overall mutation count (31). Infection with HPV has been shown to be an independent risk factor for HNSCC, and HPV-positive HNSCCs represent a distinct genetic subset of tumors (32). We did not find an association between tTMB or bTMB and HPV status.
Although no statistical testing was performed, the OS benefit of durvalumab or durvalumab plus tremelimumab versus SoC for patients with high bTMB appeared to be numerically higher than for those with high PD-L1 in EAGLE (5). The predictive value of bTMB also compares favorably to PD-L1 in other clinical trials of ICIs in platinum-resistant R/M HNSCC. In CheckMate 141, the OS HR for nivolumab versus SoC in the PD-L1–high subgroup was 0.55 (3). A similar HR of 0.53 was observed for pembrolizumab versus SoC in patients with a PD-L1 tumor proportion score ≥50% in KEYNOTE-040 (4). In KEYNOTE-040, the prevalence of high PD-L1 expression was 26% (4), similar to the prevalence of high bTMB in EAGLE. Consistent with findings in other tumor types (33), our analysis showed that bTMB and PD-L1 were independent. Furthermore, selecting for high TMB and PD-L1 together resulted in improved OS HRs for durvalumab and durvalumab plus tremelimumab versus SoC, compared with either biomarker alone, although this population was small and results were not significant.
Analysis of OS in NLR subgroups demonstrated that NLR was prognostic across the entire population and predictive of OS benefit for durvalumab monotherapy versus SoC. Other studies have reported predictive value for NLR for ICI treatment in HNSCC (34–36). Using a cutoff of 8.77, Foster and colleagues observed that NLR was more strongly associated with outcome than PD-L1 in patients receiving anti-PD-1 therapy. NLR measurement in the clinical setting is routine and may offer useful information for the selection of patients for ICI treatment. However, an optimal threshold is yet to be determined.
Limitations of the study included that it was retrospective and the incomplete ascertainment of samples. Archival tumor samples up to 3 years old from HAWK/CONDOR were used for the tTMB analysis and may have not been representative of tTMB at the time of treatment. The bTMB analysis was performed on plasma samples collected at baseline from patients in the EAGLE study and, compared with archival tumor samples for tTMB analysis, may more accurately reflect the disease stage at the time of treatment. A direct comparison of tTMB and bTMB analyses in the same study population was not performed and would have enabled a direct comparison of the two methods. The number of samples included in the analyses was relatively small, particularly for tTMB. This precluded the assessment of the impact of any imbalances in baseline characteristics, such as smoking status, and may explain why a trend, but no significant difference, in OS was seen in patients that were tTMB-high and treated with durvalumab monotherapy in HAWK/CONDOR when a significant difference was observed in EAGLE. Another limitation of the tTMB analysis was that it was performed using samples from two studies with distinct patient populations. For example, HAWK was restricted to PD-L1–high patients, whereas CONDOR was restricted to PD-L1–low patients. Taking this into account, there is a risk that selection bias may have influenced outcomes. However, this limitation did not apply to the bTMB analysis as it used samples from EAGLE, an unselected, randomized study with a chemotherapy control arm.
While definitive data on the clinical utility of TMB are lacking, our findings suggest that TMB is a promising biomarker for predicting survival with ICI treatment in R/M HNSCC, particularly for the combination of immunotherapy plus chemotherapy. Cutoffs need to be optimized for methodologies used. This analysis provides evidence that 16 mut/Mb is the optimal cutoff for bTMB using the Guardant Health OMNI platform to identify patients with HNSCC who have hypermutated tumors and may benefit from ICI treatment. Prospective evaluation of TMB and validation of the high-bTMB (16 mut/Mb) cutoff in large, randomized clinical trials is warranted. Whether these findings can be translated to the first-line setting for patients with HNSCC remains to be determined.
Supplementary Material
Supplementary Data
FIG S1. TMB distribution in samples from HAWK and CONDOR (A) and EAGLE (B).
FIG S2. Kaplan-Meier plot of OS in the durvalumab monotherapy, durvalumab plus tremelimumab and SoC treatment arms in the NLR ≤ 7 subgroup (A) and NLR > 7 subgroup (B) in EAGLE.
Acknowledgments
The authors thank the patients who volunteered to participate in the HAWK, CONDOR, and EAGLE studies and their families, as well as all of the investigators and study site personnel. We thank Ward A. Pedersen and Anne-Marie Manwaring of Parexel for medical writing and editorial assistance, and Sara Gibson of CMC Connect, a division of IPG Health Medical Communications for editorial assistance, which were funded by AstraZeneca. This study was funded by AstraZeneca.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
S. Wildsmith reports personal fees from AstraZeneca during the conduct of the study, as well as personal fees from AstraZeneca outside the submitted work; in addition, S. Wildsmith has a patent for WO2021/228988 pending. W. Li reports other support from AstraZeneca during the conduct of the study, as well as other support from AstraZeneca outside the submitted work; in addition, W. Li has a patent for Biomarkers for Predicting OS in Recurrent Metastatic Head and Neck Squamous Cell Carcinoma pending to AstraZeneca. R. Stewart reports other support from AstraZeneca during the conduct of the study, as well as other support from Pfizer Inc outside the submitted work. N. Morsli reports other support from AstraZeneca during the conduct of the study. R. Raja reports other support from AstraZeneca outside the submitted work; in addition, R. Raja has a patent for tumor mutational burden pending. Q. Zhang reports personal fees from GSK outside the submitted work; in addition, Q. Zhang has a patent for WO2021/228988 A1 pending. P. He was an AstraZeneca employee at the time of the article preparation. A. Yovine reports other support from AstraZeneca outside the submitted work.N. Holoweckyj reports personal fees (employment) from AstraZeneca. J. Walker reports personal fees from AstraZeneca during the conduct of the study, as well as personal fees from AstraZeneca outside the submitted work; in addition, J. Walker is an employee and shareholder of AstraZeneca. M. de los Reyes reports other support from AstraZeneca outside the submitted work. C. Barker reports other support from AstraZeneca outside the submitted work. R. Haddad reports personal fees from Merck, BMS, Pfizer, GSK, Merck Sorono, Eisai, Bayer, AstraZeneca, Kura, NCCN, Nanobiotix, ISA, Mirati, and Boehringer Ingelheim, as well as grants from Pfizer, Kura, Merck, Merck Serono, and BMS outside the submitted work. L. Licitra reports grants from Adlai Nortye, AstraZeneca, BMS, Debiopharm International SA, Eisai, Eli Lilly and Company, Exelixis, Hoffmann-La Roche ltd, Isa Therapeutics, Kura Oncology, Merck-Serono, MSD, Merck Sharp / Dohme Corp, Nektar Therapeutics, Novartis, Regeneron, Roche, Sanofi, Syneos, and Sun Pharmaceutical during the conduct of the study, as well as personal fees from Adlai Nortye, AstraZeneca, Bicara Therapeutics, DRG (part of Clarivate), Genmab US, GlaxoSmithKline, Bayer, Mirati Therapeutics, MSD, Merck-Serono, Merck Healthcare KGaA, Neutron Therapeutics Inc, and Seagen International BmbH outside the submitted work. R. Ferris reports personal fees from Adagene Incorporated, Aduro Biotech, Brooklyn Immunotherapeutics LLC, Catenion, EMD Serono, Everest Clinical Research Corp, F. Hoffman-La Roche Ltd, Federation Bio, Inc, Genocea Biosciences, Inc, Kowa Research Institute, Inc, Mirati Therapeutics, Inc, Nanobiotix, Novartis Pharmaceutical Inc, PPD Development LP, Sanofi, and Zymeworks, Inc; grants from AstraZeneca/Medimmune, Bicara Therapeutics Inc, and Tesaro; grants and other support from Bristol Myers Squibb and Merck; other support from Coherus BioSciences, Eisai Europe Limited, Genmab, Hookipa Biotech GmbH, Instil Bio, Inc, Lifescience Dynamics Limited, MacroGenics Inc, MeiraGTx LLC, Mirror Biologics Inc, Numab Therapeutics AG, Oncocyte Corporation, Pfizer, Raukaten Medical Inc, Seagen, Inc, SIRPant Immunotherapeutics, and Vir Biotechnology Inc; and grants, personal fees, and other support from Novasenta outside the submitted work. J. Fayette reports grants and personal fees from BMS; personal fees and non-financial support from MSD; grants, personal fees, and non-financial support from AstraZeneca; and personal fees from Innate Pharma, Roche, and Merck Serono during the conduct of the study. D.P. Zandberg reports personal fees from Merck, Macrogenics, Blueprint Medicines, and Prelude Therapeutics, as well as grants from Merck, BMS, AstraZeneca, GlaxoSmithKline, BICARA, Novasenta, and Macrogenics during the conduct of the study. L.L. Siu reports personal fees from AstraZeneca, as well as grants from AstraZeneca during the conduct of the study; in addition, L.L. Siu reports personal fees from Merck, Pfizer, Roche-Genentech, GlaxoSmithKline, Voronoi, Arvinas, Tessa, Navire, Relay, Daiichi Sankyo, Amgen, Marengo, Medicenna, Tubulis, LTZ Therapeutics, Agios, and Treadwell Therapeutics, as well as grants from Novartis, Bristol Myers Squibb, Pfizer, Boehringer Ingelheim, GlaxoSmithKline, Roche-Genentech, Merck, Bayer, AbbVie, Amgen, Symphogen, Intensity Therapeutics, Mirati, Shattucks Laboratory, BioNTech, 23Me, and EMD Serono outside the submitted work. R. Mesía reports other support from Merck KGaA, MSD, Pierre-Fabre, Bayer, Roche, Gemlab, Nanobiotics, and Boehringer outside the submitted work. No disclosures were reported by the other authors.
Authors' Contributions
S. Wildsmith: Conceptualization, data curation, formal analysis, supervision, methodology, project administration, writing–review and editing. W. Li: Conceptualization, data curation, formal analysis, supervision, methodology, writing–review and editing. S. Wu: Formal analysis, writing–review and editing. R. Stewart: Formal analysis, project administration, writing–review and editing. N. Morsli: Supervision, writing–review and editing. R. Raja: Formal analysis, supervision, writing–review and editing. Q. Zhang: Formal analysis, project administration, writing–review and editing. J. Ye: Formal analysis, writing–review and editing. P. He: Formal analysis, writing–review and editing. J. Shetty: Data curation, project administration, writing–review and editing. A. Yovine: Supervision, writing–review and editing. N. Holoweckyj: Project administration, writing–review and editing. K. Real: Data curation, project administration, writing–review and editing. J. Walker: Conceptualization, formal analysis, methodology, writing–review and editing. M. Wrona: Data curation, project administration, writing–review and editing. M. de los Reyes: Data curation, project administration, writing–review and editing. C. Barker: Data curation, project administration, writing–review and editing. J. Whiteley: Data curation, project administration, writing–review and editing. R. Haddad: Data curation, formal analysis, writing–review and editing. L. Licitra: Data curation, formal analysis, writing–review and editing. R. Ferris: Data curation, formal analysis, writing–review and editing. J. Fayette: Data curation, formal analysis, writing–review and editing. D.P. Zandberg: Data curation, formal analysis, writing–review and editing. L.L. Siu: Data curation, formal analysis, writing–review and editing. R. Mesía: Data curation, formal analysis, writing–review and editing.
References
- 1. Lala M, Chirovsky D, Cheng JD, Mayawala K. Clinical outcomes with therapies for previously treated recurrent/metastatic head-and-neck squamous cell carcinoma (R/M HNSCC): a systematic literature review. Oral Oncol 2018;84:108–20. [DOI] [PubMed] [Google Scholar]
- 2. Lasinska I, Kolenda T, Teresiak A, Lamperska KM, Galus L, Mackiewicz J. Immunotherapy in patients with recurrent and metastatic squamous cell carcinoma of the head and neck. Anticancer Agents Med Chem 2019;19:290–303. [DOI] [PubMed] [Google Scholar]
- 3. Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 2016;375:1856–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cohen EEW, Soulières D, Le Tourneau C, Dinis J, Licitra L, Ahn MJ, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet 2019;393:156–67. [DOI] [PubMed] [Google Scholar]
- 5. Ferris RL, Haddad R, Even C, Tahara M, Dvorkin M, Ciuleanu TE, et al. Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study. Ann Oncol 2020;31:942–50. [DOI] [PubMed] [Google Scholar]
- 6. US Food and Drug Administration. Opdivo (nivolumab) prescribing information. Available from:https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/125554s106lbl.pdf.
- 7. European Medicines Agency. Opdivo (nivolumab) summary of product characteristics. Available from:https://www.ema.europa.eu/en/documents/product-information/opdivo-epar-product-information_en.pdf.
- 8. US Food and Drug Administration. Keytruda (pembrolizumab) prescribing information. Available from:https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/125514s110lbl.pdf.
- 9. Merck. KEYTRUDA summary of product characteristics. Available from:https://www.ema.europa.eu/en/documents/product-information/keytruda-epar-product-information_en.pdf.
- 10. Napolitano M, Schipilliti FM, Trudu L, Bertolini F. Immunotherapy in head and neck cancer: the great challenge of patient selection. Crit Rev Oncol Hematol 2019;144:102829. [DOI] [PubMed] [Google Scholar]
- 11. Ulrich BC, Guibert N. Non-invasive assessment of tumor PD-L1 status with circulating tumor cells. Ann Transl Med 2018;6:S48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Melendez B, Van Campenhout C, Rorive S, Remmelink M, Salmon I, D'Haene N. Methods of measurement for tumor mutational burden in tumor tissue. Transl Lung Cancer Res 2018;7:661–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hanna GJ, Lizotte P, Cavanaugh M, Kuo FC, Shivdasani P, Frieden A, et al. Frameshift events predict anti-PD-1/L1 response in head and neck cancer. JCI Insight 2018;3:e98811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Samstein RM, Lee CH, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet 2019;51:202–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang Y, Lin A, Li Y, Ding W, Meng H, Luo P, et al. Age and mutations as predictors of the response to immunotherapy in head and neck squamous cell cancer. Front Cell Dev Biol 2020;8:608969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Seiwert TY, Haddad R, Baumi J, Weiss J, Pfister D, Gupta S, et al. Biomarkers predictive of response to pembrolizumab in head and neck cancer (HNSCC). Cancer Res 2018;78:Abs LB–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol 2020;21:1353–65. [DOI] [PubMed] [Google Scholar]
- 18. US Food and Drug Administration. FDA approves pembrolizumab for adults and children with TMB-H solid tumors. Available from:https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pembrolizumab-adults-and-children-tmb-h-solid-tumors.
- 19. Herrera Gomez RG, Mezquita L, Auclin E, Saleh K, Baste Rotllan N, Iacobs M, et al. Association of LIPI score with immune checkpoint inhibitors (ICI) outcomes in recurrent or metastatic squamous cell carcinoma of the head and neck (R/M SCCHN) patients (pts). Ann Oncol 2018;29:VIII378. [Google Scholar]
- 20. Mousa L, Issa M, Klamer B, Pan J, Old M, Kang S, et al. A nomogram based prognostic score to predict overall survival (OS) in recurrent-metastatic (R/M) head and neck squamous cell carcinoma (HNSCC) patients (pts) treated with immune checkpoint inhibitors (ICI). Ann Oncol 2019;30:V470–1. [Google Scholar]
- 21. Choi JH, Suh YS, Choi Y, Han J, Kim TH, Park SH, et al. Comprehensive analysis of the neutrophil-to-lymphocyte ratio for preoperative prognostic prediction nomogram in gastric cancer. World J Surg 2018;42:2530–41. [DOI] [PubMed] [Google Scholar]
- 22. Wang Y, Qu X, Kam NW, Wang K, Shen H, Liu Q, et al. An inflammation-related nomogram for predicting the survival of patients with non-small cell lung cancer after pulmonary lobectomy. BMC Cancer 2018;18:692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Takenaka Y, Oya R, Kitamiura T, Ashida N, Shimizu K, Takemura K, et al. Prognostic role of neutrophil-to-lymphocyte ratio in head and neck cancer: a meta-analysis. Head Neck 2018;40:647–55. [DOI] [PubMed] [Google Scholar]
- 24. Zandberg DP, Algazi AP, Jimeno A, Good JS, Fayette J, Bouganim N, et al. Durvalumab for recurrent or metastatic head and neck squamous cell carcinoma: results from a single-arm, phase II study in patients with ≥25% tumour cell PD-L1 expression who have progressed on platinum-based chemotherapy. Eur J Cancer 2019;107:142–52. [DOI] [PubMed] [Google Scholar]
- 25. Siu LL, Even C, Mesía R, Remenar E, Daste A, Delord JP, et al. Safety and efficacy of durvalumab with or without tremelimumab in patients with PD-L1-low/negative recurrent or metastatic HNSCC: the phase 2 CONDOR randomized clinical trial. JAMA Oncol 2019;5:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lai Z, Markovets A, Ahdesmaki M, Chapman B, Hofmann O, McEwen R, et al. VarDict: a novel and versatile variant caller for next-generation sequencing in cancer research. Nucleic Acids Res 2016;44:e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gandara DR, Paul SM, Kowanetz M, Schleifman E, Zou W, Li Y, et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med 2018;24:1441–8. [DOI] [PubMed] [Google Scholar]
- 29. Wang Z, Duan J, Cai S, Han M, Dong H, Zhao J, et al. Assessment of blood tumor mutational burden as a potential biomarker for immunotherapy in patients with non-small cell lung cancer with use of a next-generation sequencing cancer gene panel. JAMA Oncol 2019;5:696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rizvi NA, Cho BC, Reinmuth N, Lee KH, Luft A, Ahn M-J, et al. Durvalumab with or without tremelimumab vs standard chemotherapy in first-line treatment of metastatic non-small cell lung cancer: the MYSTIC phase 3 randomized clinical trial. JAMA Oncol 2020;6:661–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alexandrov LB, Ju YS, Haase K, Van Loo P, Martincorena I, Nik-Zainal S, et al. Mutational signatures associated with tobacco smoking in human cancer. Science 2016;354:618–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015;517:576–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yarchoan M, Albacker LA, Hopkins AC, Montesion M, Murugesan K, Vithayathil TT, et al. PD-L1 expression and tumor mutational burden are independent biomarkers in most cancers. JCI Insight 2019;4:e126908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Foster CC, Kochanny S, Khattri A, Acharya R, Dekker A, Tan YHC, et al. Association of a baseline neutrophil-to-lymphocyte ratio (NLR) with progression-free and overall survival in head and neck cancer patients receiving anti-PD-1 therapy. J Clin Oncol 36:15s, 2018. (suppl; abstr 6038). [Google Scholar]
- 35. Ueda T, Chikuie N, Takumida M, Furuie H, Kono T, Taruya T, et al. Baseline neutrophil-to-lymphocyte ratio (NLR) is associated with clinical outcome in recurrent or metastatic head and neck cancer patients treated with nivolumab. Acta Otolaryngol 2020;140:181–7. [DOI] [PubMed] [Google Scholar]
- 36. Nenclares P, Gunn L, Soliman H, Bover M, Trinh A, Leslie I, et al. On-treatment immune prognostic score for patients with relapsed and/or metastatic head and neck squamous cell carcinoma treated with immunotherapy. J Immunother Cancer 2021;9:e002718. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data
FIG S1. TMB distribution in samples from HAWK and CONDOR (A) and EAGLE (B).
FIG S2. Kaplan-Meier plot of OS in the durvalumab monotherapy, durvalumab plus tremelimumab and SoC treatment arms in the NLR ≤ 7 subgroup (A) and NLR > 7 subgroup (B) in EAGLE.
Data Availability Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Anonymized datasets may be available on request. Data for studies directly listed on Vivli can be requested through Vivli at https://search.vivli.org/. Data for studies not listed on Vivli may be requested through Vivli at https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/. The request will undergo an internal review process, and if approved, data will be prepared and shared with specified accessors named on the request form for 12 months via Vivli Secure Research Environment. AstraZeneca Vivli member page is also available outlining further details: https://vivli.org/ourmember/astrazeneca/.