Abstract
Radiotherapy is a pillar of cancer treatment, which has historically been used primarily to treat localized disease with curative intent. With the increasing role of radiotherapy for metastatic disease and rapid integration of immunotherapy into the standard of care for various cancers, it has been observed that local radiation to one malignant site can lead to shrinkage of tumors at other sites, a phenomenon termed the “abscopal effect.” Historically, there was little mechanistic elucidation as to how this phenomenon occurs. However, multiple groups have recently identified associated immuno-prognostic factors, such as high post-radiotherapy absolute lymphocyte count, neoantigens, myeloid-derived suppressor cells, and NK cells. The concomitant use of immunotherapy with radiotherapy has been documented to induce the abscopal effect. As immunotherapies continue to be incorporated into most cancer treatment approaches, understanding which patients are more likely to benefit from an abscopal effect may allow for optimization of both systemic and radiotherapeutic strategies. This review highlights the tumor histologic subtypes and biomarkers of the greatest utility for the recognition and identification of patients likely to benefit from the abscopal effect.
Introduction
R.H. Mole devised the word “abscopal” (“ab” - away from; “scopus” - target) in 1953 to describe radiation effects “at a distance from the irradiated volume but within the same organism” (1). Historically deemed a rare, dramatic, and unexpected clinical phenomenon, the abscopal effect (AE) is defined as regression of distant nontreated sites following primary irradiation to a specific site. However, with the advent of immunotherapy synergizing with targeted radiotherapy (RT), the incidence of AEs has sharply risen. Mechanistically, it has been demonstrated that radiation induces cellular damage, resulting in the exposure of tumor antigens, which in turn stimulates the release of inflammatory cytokines. Downstream, this leads to suppression of regulatory T-cell (Treg) activity and promotion of PD-L1 expression, thereby turning the irradiated lesion into an “in situ vaccine” (2). Essentially, when combined with immunotherapy, radiation, via antigen exposure, provides a connection between innate and adaptive immunity, thus enhancing the immune elimination of tumor cells. What was once regarded as an isolated, sporadic phenomenon is now under consideration for prospective clinical evaluation. Furthermore, active investigation into the role of neoantigens, damage-associated molecular pattern (DAMP) molecules NK cells, lymphocytes, and cytokines as biomarkers and related molecular mechanisms associated with abscopal effect is underway and will be explored in this review.
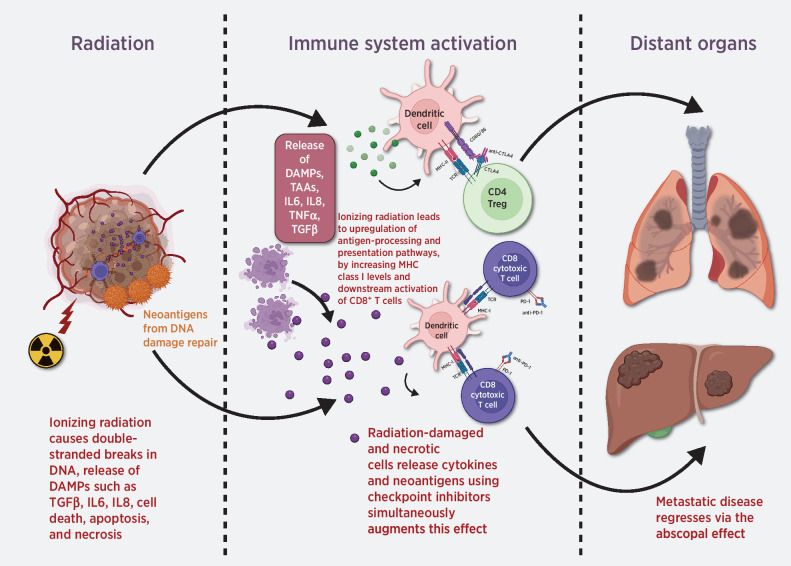
It is well established that the AE is dependent on immune priming against tumor antigens but is often hampered by tumor microenvironmental factors and lymphodepletion. These attenuating variables are conceivably why radiation monotherapy may only rarely and unreliably elicit AEs (3). More generally, the efficacy of single-agent immunotherapy is also limited by similar variables, including maximum tolerated immune activation, poor antigenicity, and negative immune regulation (4). The addition of immunotherapy to RT could ameliorate some of these issues. Specifically, combination therapy could elicit a synergism between the downstream molecular effects of both modalities, as depicted in Fig. 1. Although one should note that synergy between radiation and immunotherapy is predominantly locally radiosensitizing due to the factors above, increasing abscopal responses of distant nonirradiated lesions have been seen and active investigation into the underlying mechanisms is underway.
Figure 1.
Potential abscopal effects resulting from combined RT and immunotherapy. TAA, tumor-associated antigen.
Although AEs have been documented in a diverse spectrum of cancers, including melanoma, breast cancer, lung cancer, liver cancer, sarcoma, mesothelioma, and thymic cancer, a unifying molecular mechanism as to why some types of tumors elicit this effect more readily than others do has not been well defined. In this review, we explore the clinical literature suggestive of histologically based patterns of response, as well as discuss preclinical data suggestive of possible biomarkers and related molecular mechanisms for prognostication of AEs.
Tumor Histologic Subtypes Associated with Abscopal Effects
Hatten and colleagues (5) reported the first patient-level data meta-analysis of the AE, which found that more than 60% were reported in immunogenic tumors, such as lung cancer, renal cancer, and melanoma. Similarly, our literature review suggests that AEs predominantly occur in highly immunogenic tumors, i.e., renal cell carcinoma (RCC), non–small cell lung cancer (NSCLC), head and neck squamous cell carcinoma, melanoma, and hepatocellular carcinoma. For an exhaustive list of abscopal cases reported in the literature, we would like to direct the reader to Dagoglu and colleagues’ recent publication (6). Here, we will focus on tumor types where abscopal effects have been demonstrated.
Metastatic melanoma
A seminal case report by Postow and colleagues (7) in 2012 introduced the role of AEs in metastatic melanoma. The patient received surgery for an upper-back primary lesion but had malignant spread to her right hilar lymph node. She subsequently received 1 year of treatment with the immune checkpoint inhibitor (ICI) ipilimumab, after which she developed progression with new pleural-based paraspinal mass and splenic lesions. She subsequently received palliative RT to the paraspinal mass. Within 4 months of RT completion, a decrease in size was seen not only in the primary irradiated paraspinal lesion but also in the right hilar lymph nodes and splenic lesions, demonstrating a profound AE. Humoral studies showed presence of NY-ESO-1 antibodies and high CD4+ ICOS cell levels correlated with treatment response (7).
In a retrospective study from Italy of 21 patients with metastatic melanoma with predominantly cutaneous subtype, AEs were seen in 52% of patients. Median time to AEs after RT exposure was 1 month, and the overall survival duration for patients who experienced AEs was 22.4 months, compared with 13 months for patients without AEs. Interestingly, 2 of the patients in the trial who did not have AEs had the mucosal melanoma subtype with BRAF–wild-type tumor profiles (8).
Congruently, another retrospective cohort study in patient with mucosal melanoma identified 11 patients who were treated with RT alone and 12 patients with RT and the ICI pembrolizumab. Although the primary tumor control rate was higher in the combination arm, no AEs were noted in this histologic subtype (9).
D'Andrea and colleagues (10) summarized 19 cases of malignant melanoma in which AEs were noted with radiation. Patients had either received whole-body RT, stereotactic radiosurgery, or stereotactic body RT (SBRT) to metastatic lesions in the brain, skin, lung, lymph nodes, or liver. AEs were noted in the distant lung, liver, chest, renal, pelvic, and skin lesions, with a range of 2 weeks to 9 months for development.
Finally, in one prospective study, 26 patients were enrolled to evaluate for AEs with hypofractionated RT after their disease progressed during anti–PD-L1 therapy (11) AEs were noted in 38% (n = 10) of patients. This study was particularly notable, given that AEs could be elicited even when resistance to immune stimulation with systemic therapy had developed.
Metastatic non–small cell lung cancer
Multiple preclinical and clinical phase I and II studies have been conducted in NSCLC, with promising AEs. The earliest case reports for this histology and for a case of esophageal carcinoma metastatic to the lung were published in 1983 (12). D'Andrea and Reddy (13) summarized 19 cases with metastatic NSCLC who received radiation to metastatic sites in the lung, brain, mediastinum, bone, lymph nodes, and liver. Some patients also received the immune-active component of Bacillus Calmette-Guerin (cell wall skeleton; BCG-CWS) or GM-CSF. Time to AE varied from 3 weeks to 10 months.
Later, Shao and colleagues (14) reported a case of NSCLC with squamous histology; after developing progression on camrelizumab (an ICI) and apatinib (anti-VEGFR2), the patient received microwave ablation to the primary lesion and eventually demonstrated a durable AE (1 year at the time of the report).
In a retrospective study of 7 patients with NSCLC who received RT and an ICI (pembrolizumab or nivolumab) concurrently, 43% (n = 3) of patients had AEs (15). In a prospective study, 16 patients with metastatic lung cancer received RT and GM-CSF, and 12.5% (n = 2) of these patients had AEs (16). Similarly, in a prospective study with 6 patients with NSCLC, combined stereotactic RT with nivolumab resulted in a 67% (n = 4) AE rate (17).
A pooled analysis of two randomized trials in patients with metastatic NSCLC, the PEMBRO-RT (phase II) and MD Anderson Cancer Center (phase I/II) trials, examined the role of radiation, when combined with immunotherapy, in amplifying AEs (18). In the PEMBRO-RT trial, patients received pembrolizumab with or without RT, with the first dose of pembrolizumab delivered at the end of RT (24 Gy/3 fractions or fx). In the MD Anderson trial, patients received pembrolizumab with or without concurrent RT (50 Gy/4 fx or 45 Gy/15 fx). The abscopal response rate (ARR) for patients receiving pembrolizumab monotherapy was 19.7%, compared with 41.7% for the pembrolizumab plus RT combination (P = 0.0039). The abscopal disease control rate (ACR) in unirradiated lesions for patients receiving pembrolizumab monotherapy was 43.4%, compared with 65.3% for the pembrolizumab plus RT combination (P = 0.0071). The median overall survival duration was 8.7 months for patients receiving pembrolizumab monotherapy, compared with 19.2 months for the combination (P = 0·0004). The median progression-free survival durations were 4.4 and 9.0 months, respectively (P = 0.045; ref. 18).
Clinical outcomes improved with synergy with ICI and RT which was demonstrated in a systematic review and meta-analysis of patients with NSCLC who received an ICI plus RT by Fiorica and colleagues (19) who concluded that the combination globally decreased the risk of death at 1 year by 24% and at 3 years by 15% compared with either ICI or RT monotherapy. It should be noted that abscopal responses were not examined in the above study.
Metastatic renal cell carcinoma
A 1996 article described reports of spontaneous tumor regression for unknown reasons at distant sites, occurring at a 3-fold higher frequency in males than in females (20). After primary site radiation, the incidence of spontaneous regression of tumor at distant sites spanned between 1% and 7%, most commonly reported with lung metastases. Numerous reports of spontaneous regression had been seen after a primary or debulking nephrectomy, but abscopal regression reports occurring after ionizing radiation have also been seen as below (20).
Wersäll and colleagues (21) reported the presence of abscopal responses in 4 of 28 patients with metastatic RCC who were treated with stereotactic RT. In two cases, the primary renal masses were irradiated (32 Gy/8 fx), causing AEs in the lungs and regional lymph nodes, with durability for 2 years in one case and 9 months in the other. The other 2 patients received radiation to their lung metastases and had AEs at other sites, with durability for 2 years in one case and 4 years in the other.
Other histologic subtypes
A review of the literature from 1969 to 2014 by Abuodeh and colleagues (22) summarized case reports on AEs of durable nature. Among 46 cases, the following histologic subtypes were not discussed above: 14 cases of diverse types of lymphoma (follicular; Hodgkin; non-Hodgkin), 4 cases of hepatocellular carcinoma, 3 cases of chronic lymphocytic leukemia, 2 cases of sarcoma, 1 case of esophageal adenocarcinoma, 1 case of medullary thyroid carcinoma, 1 case of Merkel cell carcinoma, and 1 case of cervical carcinoma.
Another systematic review in 2019 reported 94 cases of AEs after RT with or without immunotherapy. Among these, 1 patient with pancreatic cancer and gastric cancer experienced AEs (6).
AEs lasting 24 months were seen in a patient with malignant pleural mesothelioma treated with a combination of pembrolizumab and palliative doses of radiation (23). AEs were also reported in the first reported case of a patient with a metastatic functioning pulmonary carcinoid tumor who received SBRT (40 Gy/5 fx) to the primary tumor, with a shrinkage greater than 50% noted in the adjacent lung metastasis, lasting 12 months at the time of the report (24). Another series reported 3 patients with metastatic intrahepatic cholangiocarcinoma who received upfront SBRT to the hepatic lesions along with immunotherapy and were noted to have AEs in regional nodal basins and the retroperitoneum (25).
A phase II trial by Golden and colleagues (26) further demonstrated the clinical relevance of sustained AEs in patients with lung, breast, and thymic cancer. GM-CSF was administered subcutaneously with conventional radiation (35Gy/10 fx) along with the investigator's choice of chemotherapy. Among 41 patients, a 27% AE rate was noted. Improved median overall survival was seen in patients who had AE (21 months compared with 8 months for nonresponders).
Although, AE seems to be an elusive phenomenon, multiple phase II/III clinical trials have demonstrated AE-related efficacy as compiled in Table 1. ARRs ranged from 8.3% to 53% regardless of the sequential or concurrent administration of immunotherapy and radiation modality with median overall survival from 6.1 months to not reached at the time of publication. These studies continue to drive the impetus in warranting further clinical exploration of harnessing the abscopal phenomenon in diverse tumor histologies.
Table 1.
Clinical trials showing abscopal effect in tumor subtypes.
| Trial | Tumor type (n) | Best abscopal CR/PR RECIST responses | Radiation | Sequence | Systemic agent | Median overall survival (months) | Unirradiated sites assessed | ARR (%) |
|---|---|---|---|---|---|---|---|---|
| Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumors: a proof-of-principle trial (26) | Non–small cell lung cancer (n = 13) | 2 PR; 2 CR | Conventional 35 Gy/10 fx | Sequential GM-CSF and concurrent chemotherapy | Chemotherapy + GM-CSF | 20.98 | Lung, liver, lymph nodes | 27 |
| Breast cancer (n = 11) | 4 PR | |||||||
| Thymic cancer (n = 2) | 2 PR | |||||||
| Nivolumab and hypofractionated radiotherapy in patients with advanced lung cancer: ABSCOPAL-1 clinical trial (75) | Non–small cell lung cancer (n = 23) | 3 CR | SBRT | Sequential | Nivolumab | NR | Unknown | 45.2 |
| 6 PR | 40–50 Gy/5 fx and hypofractionated brachytherapy (30 Gy/1 fx) | |||||||
| Potentiation of the abscopal effect by modulated electro-hyperthermia in locally advanced cervical cancer patients (76) | Cervical cancer (n = 108) | 13 CMR | Conventional | Concurrent | Cisplatin | Not assessed | Lymph nodes | 24.1a |
| 50 Gy/25 fx and HDR (24 Gy/3 fx) ± electro hypothermia | ||||||||
| Phase II trial of ipilimumab with stereotactic radiation therapy for metastatic disease: outcomes, toxicities, and low-dose radiation -related abscopal responses (77) | Multiple tumor types (n = 106) | NSCLC: 1 CR; | SBRT | Concurrent vs. sequential | Ipilimumab | NR | Liver, lung, adrenal glands | 26 |
| 3 PR | 50–60 Gy/4–10 fx | |||||||
| Cervical adenocarcinoma: 1 CR | ||||||||
| RCC: 1 PR | ||||||||
| NET: 1 PR | ||||||||
| Phase II trial of combined durvalumab plus tremelimumab with proton therapy to boost the abscopal effect for recurrent or metastatic head and neck squamous cell carcinoma (78) | Head and neck squamous cell carcinoma (n = 31) | 1 CR | Proton therapy | Concurrent | Durvalumab and tremelimumab | 6.4 | Unknown | 27.3 |
| 5 PR | 25 Gy/5 fx | |||||||
| ARRs after salvage radiation in patients with progressive disease on immunotherapy (79) | Non–small cell lung cancer (n = 17) Melanoma (n = 5) | Unknown | Conventional or SBRT | Concurrent | Immunotherapy | Not assessed | Liver, lung, adrenal glands | 11 |
| Intratumoral tavokinogene telseplasmid induces abscopal clinical responses in metastatic melanoma patients (80) | Melanoma (n = 28) | 5 CR | Electroporation | Sequential | Intratumorally with IL-12 | NR | Subcutaneous satellite nodules | 29.2 |
| 6 PR | D1,5,8 Q90D | |||||||
| Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: a pooled analysis of two randomised trials (18) | Lung cancer (n = 148) | 30 CR+PR | Conventional or SBRT | Concurrent vs. sequential | Pembrolizumab | 19.2 | Lung, liver, lymph nodes, adrenal glands | 41.7 |
| 24Gy/3 fx; 50Gy/4 fx; 45Gy/15 fx | ||||||||
| Phase 1/2 study of intratumoral G100 (TLR4 agonist) with or without pembrolizumab in follicular lymphoma (81) | Follicular lymphoma (n = 51) | 6 CR+PR | Low-dose radiation (4Gy/2 fx) | Sequential | Intratumoral G100 (TLR4 agonist) ± pembrolizumab | NR | Lymph nodes | 16.2 |
| Nivolumab in Combination with Stereotactic Body Radiotherapy in Pretreated Patients with Metastatic Renal Cell Carcinoma. Results of the Phase II NIVES study (82) | Renal cell carcinoma (n = 69) | 2 CR | SBRT 30Gy/3 fx | Sequential | Nivolumab | 20 | Lungs, liver, lymph nodes, bones | 12 |
| 6 PR | ||||||||
| A stratified phase I dose escalation trial of hypofractionated radiotherapy followed by ipilimumab in metastatic melanoma: long-term follow-up and final outcomes (83) | Melanoma (n = 22) | 5 PR | SBRT 6 or 8 Gy (2–3 fx) | Sequential | Ipilimumab | 10.7 | Lung, liver, subcutaneous nodules | 22.7 |
| A prospective trial evaluating the safety and systemic response from the concurrent use of radiation therapy with checkpoint inhibitor immunotherapy in metastatic non–small cell lung cancer (84) | Non–small cell lung cancer (n = 34) | 2 CR | SBRT | Concurrent | PD-1/PD-L1 inhibitor ± chemotherapy | 15 | Lungs, liver, lymph nodes, bones, adrenal glands | 53 |
| 16 PR | 48Gy/3–5 fx | |||||||
| Phase II single-arm study of durvalumab and tremelimumab with concurrent radiotherapy in patients with mismatch repair–proficient metastatic colorectal cancer (85) | Colorectal cancer | 2 PR | Conventional or SBRT | Sequential | Durvalumab and tremelimumab | 11.4 | Liver, lung, bone, soft tissue, peritoneum, spleen, lymph nodes | 8.3 |
| (n = 24) | 27–70Gy/3–25 fx | |||||||
| Radiotherapy enhances immunotherapy response in microsatellite stable colorectal and pancreatic adenocarcinoma in a phase II trial (86) | Colorectal cancer (n = 40) | 1 CR | SBRT | Concurrent | Ipilimumab + nivolumab | 7.1 | Unknown | 10 |
| 3 PR | 24Gy/3 fx | |||||||
| Pancreatic cancer (n = 25) | 1 CR | 4.2 | 12 | |||||
| 2 PR |
Abbreviations: CMR, complete metabolic resolution (SUV <2.5) on 18F-FDG PET/CT; CR, complete response; HDR, high-dose brachytherapy; PR, partial response.
aARR in this study includes resolution of tumor within and outside the radiation field.
Biomarkers and Related Molecular Mechanisms Associated With Abscopal Effect
A considerable number of publications have proposed biomarkers associated with preclinical and clinical abscopal trends, both describing how and predicting when the effect occurs. However, given the poor reproducibility of the AE, there is an inherent challenge in accounting for the degree of stochasticism. Here we review biomarkers and related molecular mechanisms that have been investigated for their association with AEs.
Mismatch repair and tumor neoantigens
Tumor neoantigens are defined as immune-recognizable upregulated tumor proteins against which antitumor responses are directed. The induction of DNA damage is the rate-limiting step for generating an effective burden of neoantigens. Neoantigens can arise from SNVs, insertion–deletion mutations, RNA transcription and splicing errors, gene fusions, and proteasome-generated spliced peptides (27, 28).
Neoantigens are known to develop primarily due to changes in peptide sequence and inactivating mutations in canonical DNA damage repair (DDR) pathways (29). Mismatch repair (MMR) deficiency is associated with potentiating immunotherapy (ICI) effects, which has led to the first-ever FDA-approved histology-agnostic cancer therapy, an ICI for patients with microsatellite instability. In such patients, frameshift mutations are predictive of response, suggesting that this form of genome instability is particularly active in generating neoantigens (30). Specific associated MMR pathway factors predictive of antitumor immune response with ICI treatment include the MSH2 and MSH6 MMR enzymes (31). Although earlier, we had explored histologies that could be prognostic to being able to predict response, predictive biomarkers such as neoantigens despite tumor histology could be employed. One preclinical study explored this question by using poorly immunogenic sarcoma cell line through mechanisms involving the de novo generation of tumor-specific mutant neoantigens and render noncurative doses of RT to induce tumor-specific mutant MHC-I neoantigens capable of eliciting functional CD8+ T-cell responses which may enhance the synergy of immunotherapy with RT but further evidence is required to elucidate the role of neoantigens in eliciting AEs distantly (32).
Infiltrating lymphocytes in the tumor microenvironment
Radiation induces T helper (Th) cell differentiation, with dose-variable effects on eventual Th1, Th2, and Treg immune response balance (33). Specifically, animal data suggest that whole body low-dose radiation (0.075 Gy in 1 fx) tends to bias a more Th1 response phenotype, whereas whole body high-dose radiation (2 Gy in 1 fx) favors a more Th2 and Treg response phenotype (33). Th1 cells produce IL2 and IFNγ, which stimulate the cytotoxic effects of NK and T cells. This process in turn further promotes macrophagic activation, thereby activating the adaptive immune process. On the other hand, Treg cells, via TGFβ, promote immune downregulation [25]. Multiple preclinical models have investigated the possibility of using either ICI or cytokine therapy for increasing infiltrating lymphocytes to augment tumor cell killing.
Twyman-Saint Victor and colleagues (34) showed a symbiotic relationship between ionizing radiation and both anti–PD-1/PD-L1 and anti–CTLA-4 blockade in a subset of melanoma cell lines. Specifically, radiation caused T-cell repertoire expansion. Anti–CTLA-4 inhibited Tregs, and anti–PD-L1 therapy increased CD8 tumor-infiltrating lymphocytes (TIL). Together, these effects enhanced tumor-cell killing. Dual therapy with ICI and radiation demonstrated superiority over either therapy alone.
Another in vitro model demonstrated similar immune-system activation with ionizing radiation but via a different mechanism: upregulation of antigen-processing and presentation pathways. Increases in MHC class I levels led to greater subsequent downstream activation of CD8+ T cells and increased tumoricidality (35, 36).
In an in vivo study, Kamensek and colleagues (37) demonstrated that after in situ vaccination with TNFα and IL12 gene therapy, their mouse tumor model exhibited greater tumor mutational burden, immune infiltration, and expression of MHC class I on tumor cells. All these factors were associated with a more robust AE.
Murakami and colleagues (34), in a mesothelioma mouse-model, successfully demonstrated increased reproducibility of an AE by targeted alteration of radiation-induced immunosuppressive changes. Their efficacious triple-therapy approach consisted of radiation, an IL15 super-agonist [IL15 is a known activator of CD8+ cells and NK and T cells], and a glucocorticoid-induced TNF receptor–related protein (GITR) agonist. RT initially induced CD8+ T cells via tumor antigen exposure. IL15 agonism subsequently augmented the expansion of these specific antitumor memory CD8 T cells, whereas targeted GITR agonism caused selective intratumoral depletion of immune-inhibitory Treg cells (38).
Prospective clinical trials can consider a similar approach of initially inducing cytotoxic immune induction via radiation, coupled with both targeted sustained ICI-induced immune enhancement and pharmacologic downregulation of immunosuppressive elements. The complex interplay between RT and ICI agents in the tumor microenvironment—specifically, the ability to affect immunosuppressive modulation to enhance tumor cell killing—is under tremendous exploration (39).
Lymphocyte count
Lymphopenia secondary to radiation correlates with poor tumor outcomes in several malignancies (40). Given how many studies have shown how lymphocytes mediate AEs, using RT strategies that minimize lymphocyte death may play a critical role in increasing the probability of the effect occurring (41).
In one analysis of three phase I/II trials of patients receiving combined immunotherapy and radiation, it was demonstrated that increased levels of post-RT absolute lymphocyte counts (ALC) correlated with an increased incidence of AEs. Dividing the pooled cohort into two groups using the median ALC as a threshold, the analysis showed an abscopal incidence of 34.2% in the ALC-high arm and only 3.9% in the ALC-low arm (41).
Further supportive of the possible role of lymphocytes in the AE, pre-irradiation lymphocyte counts also appear to play a role. In a study of 10 patients with liver cancer, Gustafson and colleagues (42) showed that elevated pre-irradiation neutrophil-to-lymphocyte ratios (NLR) and lymphocytopenia were both negatively correlated with survival outcomes. This study also investigated whether peripheral lymphocyte count could be a good surrogate marker for lymphocytes elsewhere. Circulating T-cell counts decreased by 50% at the end of RT and took approximately 3 months to normalize. These trends directly correlated with lymphocyte counts in adjacent lymph nodes and the tumor microenvironment, both at the primary lesion and distant metastatic locations. This information, when combined with the hypothesized mechanistic role of lymphocytes in the AE, supports a potential role for peripheral lymphocyte counts as prognosticators (42).
In addition, it is also important to note that radiation has a promoting role in increasing Treg cells, myeloid-derived suppressor cells (MDSC), and M2 tumor-associated macrophages. These immune-regulatory elements can cause a deleterious immune-suppressive environment, leading to lymphocyte depletion and inactivation. Therefore, studying their role further may help to increasingly clarify the prognostic context of ALC (43).
Finally, with respect to treatment strategies, given lymphocytes likely play a key role in mediating AEs, using RT strategies that minimize lymphocyte death may be worth pursuing. Hypofractionation could be one such strategy. Hypofractionated radiation refers to treatment with greater than 2 Gy per fraction, usually in fewer number and an overall shorter treatment period, compared with conventional radiation fractionation. One prospective study examined the role of a conventional RT regimen (50.4 Gy/28 fx) versus a hypofractionated regimen (30 Gy/3 fx) on T lymphocytes in 20 patients with advanced pancreatic cancer (44). Compared with the hypofractionated-regimen cohort, the conventional-RT cohort suffered from greater prolonged T lymphocyte suppression (50 days vs. 272 days, respectively). Specifically, conventional fractionated radiation resulted in a dramatic reduction of naïve, effector, and memory T-cell subtypes equally, without concurrent myeloid suppression and with up to 2 years’ time for count recovery. Interestingly, in these depleted lymphocyte populations, there was no difference in functional response to cytokines nor any rapid homeostatic cytokine-driven repopulation of cells, as typically seen in transient lymphopenia. In terms of cytokine levels, the only significant difference between patients treated with conventional and hypofractionated RT was a relative greater increase in serum IL15 in the conventional group at the post-RT lymphocyte nadir time point. Overall, cytokine levels and signaling were not sufficient to explain the difference in lymphodepletion seen between the two radiation-fractionation modalities (44).
MDSCs
MDSCs also play an immunosuppressive role in the tumor microenvironment. In preclinical tumor models, fractionated radiation downregulated the effect of MDSCs by inducing molecular restructuring, such that antigen recognition increased. One study, in the context of MDSCs, explored the synergistic effect of anti–PD-L1 and radiation in mammary and colon adenocarcinoma cell lines. The combined approach led to a decreased MDSC population via upregulation of self-death in the tumor microenvironment, which led to relative augmentation of the effector functions of the CD8+ T cells. In turn, this led to a form of positive feedback, with further destruction of the MDSC population and thus an overall upregulation in immunogenicity of the tumor microenvironment (45). This was clinically demonstrated in the translational work reported by Postow and colleagues (7), where congruent depreciation of MDSC levels were seen with abscopal effects with exposure to ipilimumab and radiation in melanoma.
Damage-associated molecular patterns
Radiation is known to initiate “immunogenic cell death,” which leads to increases in DAMPs. DAMPs include molecules, such as high-mobility group box 1 protein (HMGB1), adenosine triphosphate (ATP), and calreticulin, that play a role in immune priming and neoantigen presentation. Higher DAMP concentration led to downstream T-cell activation and proliferation, both critical to the proposed synergistic mechanism of action of immune modulation and radiation on AE development (46). Specifically, toll-like receptor 4 (TLR-4) on dendritic cells, via the HMGB1 pathway, stimulates monocytes, which subsequently increase the production and release of IL1, IL6, IL8, and TNF. This interaction leads to inhibition of intracellular antigen degradation, thus facilitating greater antigen presentation and thereby enhancing immune priming (46). To stimulate antigen-presenting cell aggregation, ATP upregulates their localization to tumors via P2×7 signaling and cytokines, such as IL1β and IL18 (24). In addition, further maturation, activation, and aggregation of dendritic cells occurs, leading to helper T-cell activation and downstream increased cytotoxic T-cell–mediated tumoricidality (35).
NK cells
NK cells constitute a special subtype of lymphocytes, with a unique immunologic niche, that are also affected by radiation and thus may also contribute to the AE. NK cells are large granular lymphocytes with a distinctive cytotoxicity profile. NK cells do not require antigen-specific presentation nor possess MHC limitations and thus can target cells spontaneously (47). Operating via the secretion of proinflammatory cytokines and promoting antigen-presenting cell differentiation, they also activate adaptive cell-mediated immunity to target tumor cells (47).
Low-dose ionizing radiation has been shown to upregulate NK-cell function in vitro; on the other hand, high-dose ionizing radiation tended to do the opposite, although with demonstrable effect attenuation with IL2 pretreatment (48). Several competing effects underlie this difference. On one hand, radiation induces antigen-presenting cells to produce stimulatory cytokines, including IL2 and IFNγ, that upregulate NK-cell function. However, radiation also activates immunosuppressive cells, such as Tregs and alternatively activated macrophages, which leads to downstream secretion of TGFβ and subsequent impaired NK-cell activity. Further downregulation of NK-cell activity also occurs, albeit indirectly, with radiation-induced PD-L1 and classical HLA class I upregulation, leading to increased immunogenicity and thus NK-cell recognition of tumor cells. Conventional fractionated radiation in breast and prostate have demonstrated increased NK-cell proliferation. But results have been mixed with SBRT, with increased numbers seen after radiation in the breast but no change or decreased numbers seen after radiation in the lung (48).
Direct killing, via transmembrane protein interactions, mediates NK-cell activity against cancer cells. An area of potential related targeted immunomodulation in both NK- and tumor-cell membrane-specific agents. In one preclinical model, using NK-cell membrane-cloaked, TCPP-loaded nanoparticles increased tumor therapeutic response in both primary and distant lesions (49). Similarly, in another report, when radiation was combined with matrix metalloproteinase inhibitors, tumor cell membrane expression of NKG2D ligands (NK-cell receptor activators) increased, which led to greater tumoral NK cell–mediated cytotoxicity (50).
Exosomes
Exosomes are a family of extracellular vesicles, approximately 30 to 100 nm, in the trans-Golgi network. Tumor-derived exosomes (TEX) transmit immunostimulatory and immunosuppressing data based on the particles carried and the tumor microenvironment. Immunostimulatory molecules include the immunoglobulin (Ig) superfamily, the TNF receptor superfamily, the T-cell Ig and mucin (TIM) domain, and MHC class I and II molecules. Immunosuppressive molecules include tumor-associated antigens, such as TGFβ, PD-1, PD-L1, TRAIL, IL10, and Fas ligands. Collectively, these have a diverse set of downstream inhibitory actions, such as inactivation of dendritic cells, effector T-cell destruction, and proliferation of other immunoinhibitory entities, such as Tregs and MDSCs (51).
Even outside of immunomodulation, these exosomes possess both pro- and anti-oncogenic properties. They can activate miRNAs, which help mediate many of the antitumor effects of ionizing radiation, including inducing tumor-cell attack, limiting tumor-cell movement, and promoting tumor-cell apoptosis (52). At the same time, TEXs are important mediators of pro-oncogenic drivers, promoting neoplasia, and augmenting tumor resistance via upregulation of angiogenic signals (53). Exosomes are generated in the tumor microenvironment and play a key role in modulating it. They stimulate antigen-presenting cell activity, thereby leading to downstream cytotoxic T-cell activation. Therefore, harnessing their ability could potentially increase the probability of AEs, especially in the context of radiation (54).
It has been demonstrated that TEXs carrying heat shock protein 70 also have the ability to stimulate NK cells (55). NK cells can also transport exosomes. In one preclinical study in melanoma cell lines, NK-cell exosome delivery led to an upregulation of FAS ligand and TNFs, enhancing tumoricidal effects (56).
TEXs may also intracellularly affect the tumor microenvironment. TEXs are effective communicators of the Golgi apparatus, thus giving them the ability to affect the secretion and localization of various types of molecules intracellularly. This can have various downstream stimulatory, inhibitory, and transcriptional influences in the cellular system of tumor cells (56). In the context of RT, preclinical studies show exosomal miR-21 and miR-7–5p are markedly upregulated in primary irradiated and bystander cells. Even when transferred to specific target cells, TEXs cause DNA damage, with subsequent autophagy induced via modulation of the EGFR/AKT/mTOR pathways. This initially localized and subsequent nonlocalized effect could help explain AEs (57, 58). Finally, it should be noted that immune cell–derived exosome (IEX) subtypes can elicit effector T-cell responses based on specific antigen presentation, another possible mediator of AEs. Despite robust preclinical models and sustained interest in investigating exosomes as biomarkers to predict AEs and radioresistance, no promising specific biomarkers for clinical prognostication have emerged.
AE-upregulating cytokine factors
Another potential area of exploration of the underlying mechanism of molecular synergism between radiation and immunotherapy, in the context of the AE, is cytokine factors. In preclinical models, secondary to ionizing radiation, tumor microenvironments promote the secretion of pro-inflammatory cytokines, such as IFNγ and CXCL9/10/16, which lead to recruitment of T cells to the tumor bed (59). In addition, ionizing radiation exposure leads to the upregulation of NKG2D ligand, CD80 molecule, Fas receptor, and ICAM-1 on cancer cell membranes, thereby elevating their susceptibility to the cytotoxic effects of cytotoxic T lymphocytes (60). Ionizing radiation leads to the upregulation of the STING and cyclic guanosine monophosphate-adenosine monophosphate synthase (cGAS) pathways, which subsequently increases type I IFN levels (61). Coupled with IL12 and TNF secretion by activated M1 macrophages, this mediates further cytotoxic response (62).
Translational work by Lai and colleagues (63) supports the above-described findings; highly immunogenic murine models, MC-38 and E.G7-OVA, showed increased IFNγ levels and AEs after radiation in distant lesions. Dose dependency was also demonstrated, with greater effects with a single dose of 15 Gy compared with three 5-Gy fractions.
AE-downregulating cytokine factors
Although we have thus far discussed synergistic interactions, there are some interactions between radiation and the immune system that may attenuate AEs. Although these markers have not been directly demonstrated to affect AEs, they have been shown to influence the immunogenicity of radiation. Exposure to ionizing radiation mediates a crucial immunosuppressive protein, TGFβ, a known inhibitor of the immunologic tumor microenvironment. Specifically, TGFβ downregulates dendritic cells and therefore dysregulates eventual cytotoxic T-cell activity against tumor cells (64).
As previously discussed, radiation can enhance downstream immune cancer cytotoxicity through type I IFN. However, radiation also induces the release of Trex-1, a DNA nuclease that clears the byproducts of cancer cells required to activate type I IFN (65). Radiation also activates M2 macrophages in the tumor environment, which are known secretors of T-cell inhibitory cytokines, such as TGFβ and IL10 (62).
Continuing to identify radiation-associated downregulators of immunogenicity in the tumor microenvironment, both within and outside the context of radiation, will be important going forward to identify negative prognosticators of the AE. Some examples of other markers under active exploration are KRAS and FLT3 mutations, which were associated with poorer local control in sequential radiation phase II trials (66, 67).
A retrospective analysis of 74 patients with early-stage NSCLC treated with conventional SBRT found worse freedom from recurrence and cancer-specific survival associated with presence of KRAS mutation (68). Similarly, a phase II study of 89 patients with various GI malignancies, investigating the efficacy of proton-based SBRT administration to limited liver metastases, found worse local control in patients with mutant KRAS (69).
FLT3 ligand is a known stimulator of dendritic cells, which act as primers of T-cell antitumor responses (70). Two ongoing phase I/II trials are investigating the potential for FTL3 ligand to enhance radiation-induced AEs. One trial (NCT03789097) is investigating the combination of FLT3 ligand, radiation, poly-ICLC (polyinosinic-polycytidilic acid, an immune cell–activating factor), and pembrolizumab in non-Hodgkin lymphoma, metastatic breast cancer, and head and neck squamous cell carcinoma. Another trial (NCT04491084) is investigating the possible therapeutic benefits of combining FLT3 ligand, CD40 agonist antibody, and SBRT in patients with NSCLC.
Future Directions: Radiomics and the Radscopal Technique
Exciting developments meriting future investigation in the abscopal space are underway. Radiomics is a potentially useful and innovative means to better predict AEs. In a seminal preclinical investigation, conducted by Mihaylov and colleagues (71) 15 lung cancer murine models were studied for the presence of AEs after treatment with hypofractionated RT (8 Gy/1fx) followed by anti–PD-1 therapy. Approximately 27% of the treated animals experienced an AE. Using CT and MRI, more than 90 radiomic features were analyzed. Pretreatment neutrophil-to-lymphocyte (N:L) ratios were also documented. Subsequent binary logistic regression of pretreatment imaging features and N:L ratios was conducted, with an AUC measurement greater than 0.84, indicating a high AE predictive power for the approach. It should be noted, however, that this study included a single tumor model with a small sample size, suggesting a strong need for further preclinical and clinical inquiry.
Although AEs have been traditionally associated with high-dose radiation, many pioneering preclinical studies are under way to evaluate the role of low-dose RT (LDRT) in mediating AEs. In a lung adenocarcinoma tumor model study by Barsoumian and colleagues (72), the addition of LDRT (1 Gy/2 fx) was effective in extending mouse survival by controlling tumor growth. Mechanistically, antitumor immunity was boosted through activation of T cells, infiltration of NK cells, polarization of M1 macrophages, and reduction of TGFβ cytokines, leading to tumor stromal modulation.
Furthermore, optimal antitumor control was achieved by treating the primary tumor with high-dose radiation (HDRT), using a schedule of 36 Gy in three fractions, while concurrently treating the secondary tumor with LDRT, in combination with an anti–PD-1 ICI. The technique of combining HDRT to the primary tumor, LDRT to the secondary tumor, and an ICI was coined the “RadScopal” concept, a potentially novel means of using ionizing radiation for systemic disease control (72). Although this approach is not classically aligned with the traditional understanding of abscopal effect, it has and will continue to be relevant as further studies are underway. This triple-therapy combination led to a 90% mouse survival rate over the 50-day observation period, significant control of both primary and secondary tumors, and reduction of TGFβ, a known tumor microenvironment immunosuppressor, in the TILs of secondary tumors (72).
Similar translational findings were observed with the combination of HDRT, LDRT, and anti-PD-1 therapy in preclinical colon adenocarcinoma murine models and in NSCLC patients (73). In a preclinical study, authors found that the approach resulted in 55% of treated mice experiencing complete AEs. In a clinical study in patients with metastatic NSCLC (n = 9), the triple-therapy combination induced partial response in 3 patients (33%), and six of nine LDRT-treated large lesions decreased in size by an average of 28% (73).
From a molecular targeting perspective, moving beyond anti-CTLA and anti-PD-1 and anti-PD-L1 agents, AEs have also been elicited by addition of an anti-TIGIT checkpoint inhibitor to therapy with HDRT, LDRT, and an anti–PD-1 agent. In aggressive 344SQ-P lung adenocarcinoma murine models, when the four agents were combined, the resulting median survival observed at 50 days was higher than that seen for the combination without anti-TIGIT (74). Given the promising data, both the triple- and quadruple-therapy approaches warrant further investigation.
Conclusion
With the prodigious rise of AE reports in the literature, what was once considered an exceedingly rare, elusive, and random phenomenon is now becoming more mainstream. Technologic advancements allowing for improved side effect profile of RT as well as better understanding of potential systemic benefits of localized RT have led to increased use of RT in the metastatic setting. This, along with rapid adoption of immunotherapy, has led to increased occurrence of AE, and better understanding of how to utilize this phenomenon clinically. However, despite our advances, much remains to be discovered about the complex intricacies of these beneficial off-target effects of local therapy. As seen throughout our review, a delicate balance is present with every situation of molecular cross-talk, constantly modulated by dynamic tumor microenvironmental and other systemic interactions. This complexity is underscored by the stochastic-appearing nature of AEs. But the scientific consensus has established a few notable trends. AEs tend to be seen in immunogenic tumors; thus, stratifying individual patients’ tumor histologic subtypes and understanding their individual immune systems—accounting for their unique tumor spread, site permeability, and predilection—will all be crucial factors for predicting and affecting AEs. To maximize clinical utility, prospective validation of biomarkers in the trial setting, along with a holistic understanding of molecular mechanisms will be essential to establishing predictability and reproducibility of AEs.
Acknowledgments
V. Subbiah is an Andrew Sabin Family Foundation Fellow at the University of Texas MD Anderson Cancer Center, acknowledges support of the Jacquelyn A. Brady Fund, and has been supported by NIH grants (nos. R01CA242845 and R01CA273168). The MD Anderson Cancer Center's Department of Investigational Cancer Therapeutics is supported by the Cancer Prevention and Research Institute of Texas (no. RP1100584) and by the NIH through the MD Anderson Sheikh Khalifa Bin Zayed Al Nahyan Institute for Personalized Cancer Therapy (no. 1U01CA180964), the Center for Clinical and Translational Sciences (no. UL1TR000371), and the MD Anderson Cancer Center Support Grant (no. P30CA016672). The authors would like to thank Yimin Geng and Sunita Patterson in the Research Medical Library at the MD Anderson Cancer Center for assistance with the literature review and scientific editing.
This article is featured in Highlights of This Issue, p. 691
Authors' Disclosures
J.J. Adashek reports nonfinancial support from CureMatch during the conduct of the study. V. Subbiah reports grants from Eli Lilly/LOXO Oncology, Blueprint Medicines Corporation, Turning Point Therapeutics, Boston Pharmaceuticals, and Helsinn Pharmaceuticals, and Roche/Genentech, Bayer, GlaxoSmithKline, Nanocarrier, Vegenics, Celgene, Northwest Biotherapeutics, Berghealth, Incyte, Fujifilm, D3, Pfizer, Multivir, Amgen, AbbVie, Alfa-sigma, Agensys, Boston Biomedical, Idera Pharma, Inhibrx, Exelixis, Blueprint Medicines, Altum, Dragonfly Therapeutics, Takeda, National Comprehensive Cancer Network, NCI-CTEP, the University of Texas MD Anderson Cancer Center, Turning Point Therapeutics, Boston Pharmaceuticals, Novartis, Pharmamar, and MedImmune outside the submitted work; and nonfinancial support from Eli Lilly/Loxo Oncology. No disclosures were reported by the other authors.
Authors' Contributions
B.E. Nelson: Study conception and design, data collection, analysis and interpretation of results, draft manuscript preparation. J.J. Adashek: Draft manuscript preparation. A.A. Sheth: Draft manuscript preparation. V. Subbiah: Study conception and design, critical review and intellectual supervision.
References
- 1. Mole R. Whole body irradiation—radiobiology or medicine? Br J Radiol 1953;26:234–41. [DOI] [PubMed] [Google Scholar]
- 2. Kalbasi A, June CH, Haas N, Vapiwala N. Radiation and immunotherapy: a synergistic combination. J Clin Invest 2013;123:2756–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Order SE. The effects of therapeutic irradiation on lymphocytes and immunity. Cancer 1977;39:737–43. [DOI] [PubMed] [Google Scholar]
- 4. Dobosz P, Stępień M, Golke A, Dzieciątkowski T. Challenges of the immunotherapy: perspectives and limitations of the immune checkpoint inhibitor treatment. Int J Mol Sci 2022;23:2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hatten SJ, Lehrer EJ, Liao J, Sha CM, Trifiletti DM, Siva S, et al. A patient-level data meta-analysis of the abscopal effect. Adv Radiat Oncol 2022;7:100909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dagoglu N, Karaman S, Caglar HB, Oral EN. Abscopal effect of radiotherapy in the immunotherapy era: systematic review of reported cases. Cureus 2019;11:e4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012;366:925–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grimaldi AM, Simeone E, Giannarelli D, Muto P, Falivene S, Borzillo V, et al. Abscopal effects of radiotherapy on advanced melanoma patients who progressed after ipilimumab immunotherapy. Oncoimmunology 2014;3:e28780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim HJ, Chang JS, Roh MR, Oh BH, Chung K-Y, Shin SJ, et al. Effect of radiotherapy combined with pembrolizumab on local tumor control in mucosal melanoma patients. Front Oncol 2019;9:835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. D'Andrea MA, Reddy GK. Systemic antitumor effects and abscopal responses in melanoma patients receiving radiation therapy. Oncology 2020;98:202–15. [DOI] [PubMed] [Google Scholar]
- 11. Saiag P, Baghad B, Fort M, Aouidadd I, Roger A, Mazeron JJ, et al. Efficacy of hypofractionated radiotherapy (Rx) in melanoma patients who failed anti-PD-1 monotherapy: assessing the abscopal effect. J Clin Oncol 2019;37Suppl 15:9537. [Google Scholar]
- 12. Rees GJ, Ross CM. Abscopal regression following radiotherapy for adenocarcinoma. Br J Radiol 1983;56:63–6. [DOI] [PubMed] [Google Scholar]
- 13. D'Andrea MA, Reddy GK. Systemic effects of radiation therapy-induced abscopal responses in patients with advanced lung cancer. Oncology 2021;99:1–14. [DOI] [PubMed] [Google Scholar]
- 14. Shao C, Yang M, Pan Y, Xie D, Chen B, Ren S, et al. Case report: abscopal effect of microwave ablation in a patient with advanced squamous NSCLC and resistance to immunotherapy. Front Immunol 2021;12:696749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Trommer M, Yeo SY, Persigehl T, Bunck A, Grull H, Schlaak M, et al. Abscopal effects in radio-immunotherapy-response analysis of metastatic cancer patients with progressive disease under anti-PD-1 immune checkpoint inhibition. Front Pharmacol 2019;10:511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu M, Cai X, Zeng Y. EP1.04–28 the abscopal effects of the combination of radiotherapy and GM-CSF for patients with metastatic thoracic cancers. J Thorac Oncol 2019;14:S980. [Google Scholar]
- 17. Miyamoto S, Nomura R, Sato K, Awano N, Kuse N, Inomata M, et al. Nivolumab and stereotactic radiation therapy for the treatment of patients with stage IV non-small-cell lung cancer. Jpn J Clin Oncol 2019;49:160–4. [DOI] [PubMed] [Google Scholar]
- 18. Theelen WSME, Chen D, Verma V, Hobbs BP, Peulen HMU, Aerts JGJV, et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Respir Med 2021;9:467–75. [DOI] [PubMed] [Google Scholar]
- 19. Fiorica F, Tebano U, Gabbani M, Perrone M, Missiroli S, Berretta M, et al. Beyond abscopal effect: a meta-analysis of immune checkpoint inhibitors and radiotherapy in advanced non-small cell lung cancer. Cancers 2021;13:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Papac RJ. Spontaneous regression of cancer. Cancer Treat Rev 1996;22:395–423. [DOI] [PubMed] [Google Scholar]
- 21. Wersäll PJ, Blomgren H, Pisa P, Lax I, Kälkner K-M, Svedman C. Regression of non-irradiated metastases after extracranial stereotactic radiotherapy in metastatic renal cell carcinoma. Acta Oncol 2006;45:493–7. [DOI] [PubMed] [Google Scholar]
- 22. Abuodeh Y, Venkat P, Kim S. Systematic review of case reports on the abscopal effect. Curr Probl Cancer 2016;40:25–37. [DOI] [PubMed] [Google Scholar]
- 23. Mampuya WA, Bouchaab H, Schaefer N, Kinj R, La Rosa S, Letovanec I, et al. Abscopal effect in a patient with malignant pleural mesothelioma treated with palliative radiotherapy and pembrolizumab. Clin Transl Radiat Oncol 2021;27:85–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kareff SA, Lischalk JW, Krochmal R, Kim C. Abscopal effect in pulmonary carcinoid tumor following ablative stereotactic body radiation therapy: a case report. J Med Case Rep 2020;14:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu X, Yao J, Song L, Zhang S, Huang T, Li Y. Local and abscopal responses in advanced intrahepatic cholangiocarcinoma with low TMB, MSS, pMMR and negative PD-L1 expression following combined therapy of SBRT with PD-1 blockade. J Immunother Cancer 2019;7:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Golden EB, Chhabra A, Chachoua A, Adams S, Donach M, Fenton-Kerimian M, et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol 2015;16:795–803. [DOI] [PubMed] [Google Scholar]
- 27. Gopanenko AV, Kosobokova EN, Kosorukov VS. Main strategies for the identification of neoantigens. Cancers 2020;12:2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hicks JK, Howard R, Reisman P, Adashek JJ, Fields KK, Gray JE, et al. Integrating somatic and germline next-generation sequencing into routine clinical oncology practice. JCO Precis Oncol 2021;5:PO.20.00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kakoti S, Sato H, Laskar S, Yasuhara T, Shibata A. DNA repair and signaling in immune-related cancer therapy. Front Mol Biosci 2020;7:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sena LA, Fountain J, Isaacsson Velho P, Lim SJ, Wang H, Nizialek E, et al. Tumor frameshift mutation proportion predicts response to immunotherapy in mismatch repair-deficient prostate cancer. Oncologist 2021;26:e270–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhao P, Li L, Jiang X, Li Q. Mismatch repair deficiency/microsatellite instability-high as a predictor for anti-PD-1/PD-L1 immunotherapy efficacy. J Hematol Oncol 2019;12:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lussier DM, Alspach E, Ward JP, Miceli AP, Runci D, White JM, et al. Radiation-induced neoantigens broaden the immunotherapeutic window of cancers with low mutational loads. Proc Natl Acad Sci 2021;118:e2102611118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gao H, Dong Z, Gong X, Dong J, Zhang Y, Wei W, et al. Effects of various radiation doses on induced T-helper cell differentiation and related cytokine secretion. J Radiat Res 2018;59:395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015;520:373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Janopaul-Naylor JR, Shen Y, Qian DC, Buchwald ZS. The abscopal effect: a review of pre-clinical and clinical advances. Int J Mol Sci 2021;22:11061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Burnette BC, Liang H, Lee Y, Chlewicki L, Khodarev NN, Weichselbaum RR, et al. The efficacy of radiotherapy relies upon induction of type I interferon–dependent innate and adaptive immunity. Cancer Res 2011;71:2488–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kamensek U, Ursic K, Markelc B, Cemazar M, Setrajcic Dragos V, Sersa G. Mutational burden, MHC-I expression and immune infiltration as limiting factors for in situ vaccination by TNFα and IL-12 gene electrotransfer. Bioelectrochemistry 2021;140:107831. [DOI] [PubMed] [Google Scholar]
- 38. Murakami J, Wu L, Kohno M, Chan M-L, Zhao Y, Yun Z, et al. Triple-modality therapy maximizes antitumor immune responses in a mouse model of mesothelioma. Sci Transl Med 2021;13:eabd9882. [DOI] [PubMed] [Google Scholar]
- 39. Elbanna M, Chowdhury NN, Rhome R, Fishel ML. Clinical and preclinical outcomes of combining targeted therapy with radiotherapy. Front Oncol 2021;11:749496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Venkatesulu BP, Mallick S, Lin SH, Krishnan S. A systematic review of the influence of radiation-induced lymphopenia on survival outcomes in solid tumors. Crit Rev Oncol Hematol 2018;123:42–51. [DOI] [PubMed] [Google Scholar]
- 41. Chen D, Verma V, Patel RR, Barsoumian HB, Cortez MA, Welsh JW. Absolute lymphocyte count predicts abscopal responses and outcomes in patients receiving combined immunotherapy and radiation therapy: analysis of 3 phase 1/2 trials. Int J Radiat Oncol Biol Phys 2020;108:196–203. [DOI] [PubMed] [Google Scholar]
- 42. Gustafson MP, Bornschlegl S, Park SS, Gastineau DA, Roberts LR, Dietz AB, et al. Comprehensive assessment of circulating immune cell populations in response to stereotactic body radiation therapy in patients with liver cancer. Adv Radiat Oncol 2017;2:540–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Menon H, Ramapriyan R, Cushman TR, Verma V, Kim HH, Schoenhals JE, et al. Role of radiation therapy in modulation of the tumor stroma and microenvironment. Front Immunol 2019;10:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Crocenzi T, Cottam B, Newell P, Wolf RF, Hansen PD, Hammill C, et al. A hypofractionated radiation regimen avoids the lymphopenia associated with neoadjuvant chemoradiation therapy of borderline resectable and locally advanced pancreatic adenocarcinoma. J Immunother Cancer 2016;4:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti–PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 2014;124:687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rodriguez-Ruiz ME, Vanpouille-Box C, Melero I, Formenti SC, Demaria S. Immunological mechanisms responsible for radiation-induced abscopal effect. Trends Immunol 2018;39:644–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ljunggren H-G, Kärre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today 1990;11:237–44. [DOI] [PubMed] [Google Scholar]
- 48. Chen J, Liu X, Zeng Z, Li J, Luo Y, Sun W, et al. Immunomodulation of NK cells by ionizing radiation. Front Oncol 2020;10:874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Deng G, Sun Z, Li S, Peng X, Li W, Zhou L, et al. Cell-membrane immunotherapy based on natural killer cell membrane coated nanoparticles for the effective inhibition of primary and abscopal tumor growth. ACS Nano 2018;12:12096–108. [DOI] [PubMed] [Google Scholar]
- 50. Heo W, Lee YS, Son CH, Yang K, Park YS, Bae J. Radiation-induced matrix metalloproteinases limit natural killer cell-mediated anticancer immunity in NCI-H23 lung cancer cells. Mol Med Rep 2015;11:1800–6. [DOI] [PubMed] [Google Scholar]
- 51. Whiteside TL. The potential of tumor-derived exosomes for noninvasive cancer monitoring: an update. Expert Rev Mol Diagn 2018;18:1029–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gao Y, Ma H, Lv C, Lan F, Wang Y, Deng Y. Exosomes and exosomal microRNA in non-targeted radiation bystander and abscopal effects in the central nervous system. Cancer Lett 2021;499:73–84. [DOI] [PubMed] [Google Scholar]
- 53. Saleem SN, Abdel-Mageed AB. Tumor-derived exosomes in oncogenic reprogramming and cancer progression. Cell Mol Life Sci 2015;72:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yin T, Xin H, Yu J, Teng F. The role of exosomes in tumour immunity under radiotherapy: eliciting abscopal effects? Biomark Res 2021;9:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Li X-B, Zhang Z-R, Schluesener HJ, Xu S-Q. Role of exosomes in immune regulation. J Cell Mol Med 2006;10:364–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhu L, Kalimuthu S, Gangadaran P, Oh JM, Lee HW, Baek SH, et al. Exosomes derived from natural killer cells exert therapeutic effect in melanoma. Theranostics 2017;7:2732–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Song M, Wang Y, Shang Z-F, Liu X-D, Xie D-F, Wang Q, et al. Bystander autophagy mediated by radiation-induced exosomal miR-7–5p in non-targeted human bronchial epithelial cells. Sci Rep 2016;6:30165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Xu S, Ding N, Pei H, Hu W, Wei W, Zhang X, et al. MiR-21 is involved in radiation-induced bystander effects. RNA Biology 2014;11:1161–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Matsumura S, Demaria S. Up-regulation of the pro-inflammatory chemokine CXCL16 is a common response of tumor cells to ionizing radiation. Radiat Res 2010;173:418–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chakraborty M, Abrams SI, Camphausen K, Liu K, Scott T, Coleman CN, et al. Irradiation of tumor cells up-regulates fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol 2003;170:6338–47. [DOI] [PubMed] [Google Scholar]
- 61. Craig DJ, Nanavaty NS, Devanaboyina M, Stanbery L, Hamouda D, Edelman G, et al. The abscopal effect of radiation therapy. Fut Oncol 2021;17:1683–94. [DOI] [PubMed] [Google Scholar]
- 62. Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 2002;23:549–55. [DOI] [PubMed] [Google Scholar]
- 63. Lai JZ, Zhu YY, Liu Y, Zhou LL, Hu L, Chen L, et al. Abscopal effects of local radiotherapy are dependent on tumor immunogenicity. Front Oncol 2021;11:690188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Vanpouille-Box C, Diamond JM, Pilones KA, Zavadil J, Babb JS, Formenti SC, et al. TGFβ is a master regulator of radiation therapy-induced antitumor immunity. Cancer Res 2015;75:2232–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Liu Y, Dong Y, Kong L, Shi F, Zhu H, Yu J. Abscopal effect of radiotherapy combined with immune checkpoint inhibitors. J Hematol Oncol 2018;11:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lin SH, Mok I, Leos D, Pasia MG, Thall PF, Lin HY, et al. NCI 9448: phase I study of trametinib in combination with chemoradiation for KRAS-mutant non-small cell lung cancer. J Clin Oncol 2015;33Suppl 15:TPS7585–TPS. [Google Scholar]
- 67. Hong TS, Wo JY-L, Ryan DP, Zheng H, Borger DR, Kwak EL, et al. Phase Ib study of neoadjuvant chemoradiation (CRT) with midostaurin, 5-fluorouracil (5-FU) and radiation (XRT) for locally advanced rectal cancer: sensitization of RAS mutant tumors. J Clin Oncol 2018;36Suppl 15:e15674. [Google Scholar]
- 68. Mak RH, Hermann G, Lewis JH, Aerts HJ, Baldini EH, Chen AB, et al. Outcomes by tumor histology and KRAS mutation status after lung stereotactic body radiation therapy for early-stage non-small-cell lung cancer. Clin Lung Cancer 2015;16:24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hong TS, Wo JY, Borger DR, Yeap BY, McDonnell EI, Willers H, et al. Phase II study of proton-based stereotactic body radiation therapy for liver metastases: importance of tumor genotype. J Natl Cancer Inst 2017;109. [DOI] [PubMed] [Google Scholar]
- 70. Cueto FJ, Sancho D. The Flt3L/Flt3 axis in dendritic cell biology and cancer immunotherapy. Cancers (Basel) 2021;13:1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mihaylov IB, Totiger TM, Giret TM, Wang D, Spieler B, Welford S. Toward prediction of abscopal effect in radioimmunotherapy: pre-clinical investigation. PLoS One 2021;16:e0255923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Barsoumian HB, Ramapriyan R, Younes AI, Caetano MS, Menon H, Comeaux NI, et al. Low-dose radiation treatment enhances systemic antitumor immune responses by overcoming the inhibitory stroma. J Immunother Cancer 2020;8:e000537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yin L, Xue J, Li R, Zhou L, Deng L, Chen L, et al. Effect of low-dose radiation therapy on abscopal responses to hypofractionated radiation therapy and anti-PD1 in mice and patients with non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2020;108:212–24. [DOI] [PubMed] [Google Scholar]
- 74. Barsoumian HB, Sezen D, Menon H, Younes AI, Hu Y, He K, et al. High plus low dose radiation strategy in combination with TIGIT and PD1 blockade to promote systemic antitumor responses. Cancers 2022;14:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ye H, Pang H, Shi X, Ren P, Huang S, Yu H, et al. Nivolumab and hypofractionated radiotherapy in patients with advanced lung cancer: ABSCOPAL-1 clinical trial. Front Oncol 2021;11:657024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Minnaar CA, Kotzen JA, Ayeni OA, Vangu MD, Baeyens A. Potentiation of the abscopal effect by modulated electro-hyperthermia in locally advanced cervical cancer patients. Front Oncol 2020;10:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Welsh JW, Tang C, de Groot P, Naing A, Hess KR, Heymach JV, et al. Phase II trial of ipilimumab with stereotactic radiation therapy for metastatic disease: outcomes, toxicities, and low-dose radiation-related abscopal responses. Cancer Immunol Res 2019;7:1903–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kim H, Ahn MJ, Oh D, Park S, Jung HA, Lee SH, et al. Phase II trial of combined durvalumab plus tremelimumab with proton therapy to boost the abscopal effect for recurrent or metastatic head and neck squamous cell carcinoma. J Clin Oncol 2021;39Suppl 15:6034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Patel RR, Guo C, Hong DS, Chang JY, Altan M, Chun SG, et al. Abscopal response rates after salvage radiation in patients with progressive disease on immunotherapy. Int J Radiat Oncol Biol Phys 2020;108Suppl 3:e120–e1. [Google Scholar]
- 80. Algazi A, Bhatia S, Tsai KK, Gargosky S, Bannavong D, Talia R, et al. Intratumoral tavokinogene telseplasmid induces abscopal clinical responses in metastatic melanoma patients. Ann Oncol 2020;31:532–40. [DOI] [PubMed] [Google Scholar]
- 81. Halwani AS, Panizo C, Isufi I, Herrera AF, Okada CY, Cull EH, et al. Phase 1/2 study of intratumoral G100 (TLR4 agonist) with or without pembrolizumab in follicular lymphoma. Leuk Lymphoma 2021:1–13. [DOI] [PubMed] [Google Scholar]
- 82. Masini C, Iotti C, De Giorgi U, Bellia RS, Buti S, Salaroli F, et al. Nivolumab in combination with stereotactic body radiotherapy in pretreated patients with metastatic renal cell carcinoma. results of the phase II NIVES study. Eur Urol 2022;81:274–82. [DOI] [PubMed] [Google Scholar]
- 83. Maity A, Mick R, Rengan R, Mitchell TC, Amaravadi RK, Schuchter LM, et al. A stratified phase I dose escalation trial of hypofractionated radiotherapy followed by ipilimumab in metastatic melanoma: long-term follow-up and final outcomes. Oncoimmunology 2021;10:1863631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Mattes MD, Eubank TD, Almubarak M, Wen S, Marano GD, Jacobson GM, et al. A prospective trial evaluating the safety and systemic response from the concurrent use of radiation therapy with checkpoint inhibitor immunotherapy in metastatic non–small cell lung cancer. Clin Lung Cancer 2021;22:268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Segal NH, Cercek A, Ku G, Wu AJ, Rimner A, Khalil DN, et al. Phase II single-arm study of durvalumab and tremelimumab with concurrent radiotherapy in patients with mismatch repair–proficient metastatic colorectal cancer. Clin Cancer Res 2021;27:2200–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Parikh AR, Szabolcs A, Allen JN, Clark JW, Wo JY, Raabe M, et al. Radiation therapy enhances immunotherapy response in microsatellite stable colorectal and pancreatic adenocarcinoma in a phase II trial. Nature Cancer 2021;2:1124–35. [DOI] [PMC free article] [PubMed] [Google Scholar]