Plain language summary
How mitochondrial shape and substrate‐specific metabolism are related has been a difficult question to address. Here, new work by Ngo et al (2023) reports that mitochondrial shape—long versus fragmented—determines the activity of β‐oxidation of long‐chain fatty acids, supporting a novel role for mitochondrial fission products as β‐oxidation hubs.
Subject Categories: Autophagy & Cell Death, Cancer, Metabolism
Recent work links changes in mitochondrial morphology to fatty acid utilization via dynamic control of CPT1 activity.
That mitochondria generate a tremendous amount of ATP and can adopt a variety of shapes, including a diversity of intra‐mitochondrial organization, have long been known. But how mitochondrial metabolism and shape are related has been difficult to investigate. New opportunities to study this relationship were created by the discovery of the mitochondrial shaping proteins, the mitofusins (MFN1/2) and Drp1 that, respectively, drive outer mitochondrial membrane (OMM) fusion and fission to dictate overall mitochondrial architecture, and OPA1 that shapes the inner mitochondrial membrane (IMM; Quintana‐Cabrera & Scorrano, 2023). Furthermore, OPA1 assists MICOS to bridge apposed IMMs to physically protect the cristae compartment, that is, from diffusional losses. ATP synthase dimers also create membrane curvature and are enriched in the cristae together with the oxidative phosphorylation complexes and supercomplexes to provide for ATP generation (Cogliati et al, 2016; Kondadi et al, 2020).
Genetic perturbation of any of the three major fusion/fission proteins revealed a bioenergetics deficit (Mishra & Chan, 2016). However, the relationship between mitochondrial shape and substrate‐specific metabolism has remained an unanswered question. In the context of mitochondrial β‐oxidation (hereafter referred to as “β‐oxidation”) of long‐chain fatty acids, CPT1 activity was less sensitive to its major allosteric inhibitor, malonyl‐CoA, when CPT1 localized to OMM‐IMM contact regions versus to the bulk OMM (Fraser et al, 2001). A similar outcome was obtained from simulation studies that compared curved vs. planar lipid bilayers (Frigini et al, 2020). Whether membrane curvature influences CPT1 activity in this way in a cellular context, and has any relationship with β‐oxidation flux, remained unclear, however. Using intact systems, substrate type and abundance have been linked to mitochondrial shape and the expression of fusion/fission proteins (Mishra & Chan, 2016 and references in Ngo et al, 2023). Furthermore, scattered evidence from MFN1/2 or Drp1 genetic models has suggested that fragmented mitochondria more capably perform β‐oxidation compared to elongated mitochondria (Wikstrom et al, 2014; Kulkarni et al, 2016). However, complexities in data interpretation can arise from manipulating the expression of the fusion/fission proteins. In particular, their genetic perturbation leads to multiple organism‐, organ‐, and cellular‐level changes, such as stress responses and mtDNA depletion, which can confound attempts to test the basic question of how mitochondrial metabolism and shape are related. Another complication is that the shaping proteins might have additional roles such as a role of MFN2 in mitochondria‐ER tethering. Separating the relevance of shape and dynamics from other factors in substrate‐specific mitochondrial metabolism, and the cell‐type specific from generalizable impacts, also remains difficult.
A cleverly designed new study by Ngo et al (2023) adopts an approach that confronts many of these complexities to test for a causal relationship between mitochondrial shape and β‐oxidation. First, a striking reverse correlation was demonstrated between mitochondrial elongation and palmitoyl‐CoA oxidation rate, measured as etomoxir‐sensitive O2 consumption (JO2), using four genetic perturbations and/or excess nutrient supply in cell culture models. Etomoxir was used at a low concentration (3 μM) to ensure specificity for CPT1 inhibition, and use of permeabilized cells restricted the substrate catabolic pathways to those associated with mitochondria. To complement this approach, TCA cycle activity, measured as citrate labeled by U‐13C‐palmitate or as 14CO2 from U‐14C‐palmitate, was greater in cells with fragmented mitochondria as investigated using genetic/nutrient models in four cell types including primary hepatocytes and islets that normally have a high β‐oxidation capacity. Furthermore, in naturally occurring subtypes of lymphoma cells that differ in their ability to oxidize fatty acids, a more fragmented population of mitochondria was correlated with a higher β‐oxidation rate, and this was despite the wider variation of mitochondrial length in these cells compared to the genetic models.
Having established that β‐oxidation is higher when mitochondria are less elongated, the next series of studies by Ngo et al (2023) aimed for mechanistic insight. First, oxidation of a long‐chain fatty acid (palmitate), which requires CPT1 for transport into mitochondria, was compared to oxidation of a medium‐chain fatty acid (hexanoate) which does not. Genetic perturbations of mitochondrial length affected β‐oxidation only when palmitate was used. This raised the possibility that CPT1 could integrate information about mitochondrial shape. Building on previous studies showing a lower sensitivity of CPT1 to its endogenous inhibitor, malonyl‐CoA, in a curved versus a planar lipid bilayer (Frigini et al, 2020), Ngo et al (2023) reasoned that mitochondrial elongation results in fewer regions of high curvature within the mitochondrial network. Testing the sensitivity of malonyl‐CoA inhibition of CPT1, they found that genetic approaches or excess nutrients that elongate or fragment mitochondria, respectively, increased or lessened malonyl‐CoA's inhibition of β‐oxidation. Collectively, these results suggest that mitochondrial length and dynamics alterations can affect the CPT1 activity to control β‐oxidation. It remains possible that mitochondrial fusion–fission and shape are integrated with β‐oxidation beyond CPT1's sensitivity to membrane curvature. An example of such integration could be from the direct regulation of fusion/fission proteins by fatty acids or their derivatives; to this end, the recent development of a discovery pipeline for metabolite–protein interactions (Hicks et al, 2023) could be useful.
Testing the physiological relevance of mitochondrial shape, Ngo et al observed lower gluconeogenesis in hepatocytes with elongated mitochondria, and greater insulin secretion in primary β‐cells with fragmented mitochondria. Both outcomes are consistent with established roles for β‐oxidation in those processes, but also raise the question of how mitochondrial shape would influence metabolism under long‐term lipid oversupply, often accompanied by insulin resistance and eventually diabetes. A fragmented mitochondrial network in the liver would be expected to counter insulin resistance, as shown in mice with hepatocyte‐specific MFN1 knockdown (Kulkarni et al, 2016). On the other hand, prolonged fragmentation of β‐cell mitochondria that enhances β‐oxidation of long‐chain fatty acids might lead to uncontrolled insulin secretion, unrestricted insulin signaling, and deleterious outcomes. Hence, any benefit of mitochondrial fission‐induced β‐oxidation for insulin signaling is expected to be tissue‐specific, and with an impact on the whole organism that depends on which tissues are most affected. Similarly, if fragmentation of liver mitochondria were to increase ketosis in the setting of lipid overload, the impact to the organism would be context‐dependent: beneficial in the case of therapeutic ketosis (e.g., with ketogenic diet), but harmful in the setting of insulin resistance (e.g., in type 2 diabetes).
Fission has commonly been understood as a mechanism to segregate damaged mitochondrial components and enable their removal by mitophagy (Twig et al, 2008), but increased fission does not always result in increased mitophagy (references in Ngo et al, 2023). The present data from Ngo et al (2023) advocate for a new role for mitochondrial fission products: as β‐oxidation specialists. These assignments seem to be mutually exclusive and so at least two different populations of fission products likely co‐exist in each cell. Discriminating between these populations of fragmented mitochondria seems to be important but might not be trivial with current mitochondrial activity reporters. Mitophagy candidate fission products were described to have a relatively low IMM potential (Twig et al, 2008), whereas β‐oxidation specialists might have either comparatively lower or higher IMM potential depending on their ATP synthase activity. However, etomoxir likely causes depolarization only in the β‐oxidation specialists. Additional distinction between mitophagy candidate and β‐oxidation specialist mitochondria might come from reporters of redox homeostasis (NAD(P)H/NAD(P), reactive oxygen species) or ATP/ADP. As to transitions between the different fragmented mitochondria, it is unlikely that mitochondria assigned to recycle would convert to β‐oxidation specialists but direct transition in the opposite direction might be envisioned. Furthermore, in the context of maintaining β‐oxidation specialist mitochondria, rapid fusion–fission cycles (kiss‐and‐run) that allow renewal of some mitochondrial components without altering the overall shape (Liu et al, 2009) might be of special relevance. What we can speculate on the dynamics of β‐oxidation specialist mitochondria is depicted in a scheme (Fig 1) but advances to measure metabolism at single mitochondrial resolution will be needed to sort out many important questions of the mitochondrial shape–metabolism relationship.
Figure 1. Dynamics of mitochondria specialized for β‐oxidation.
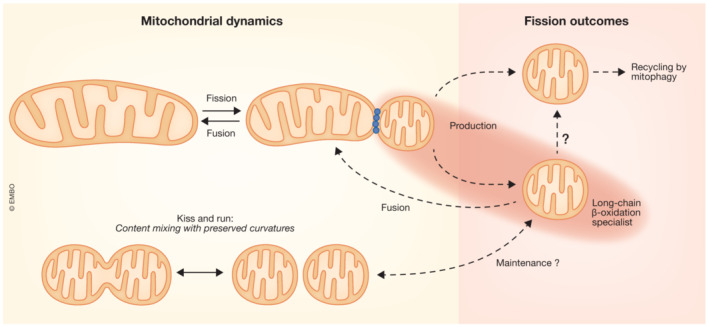
Red spotlight puts emphasis on the findings of Ngo et al. “?”, to be investigated.
The EMBO Journal (2023) 42: e114129
See also: J Ngo et al (June 2023)
Contributor Information
Erin L Seifert, Email: erin.seifert@jefferson.edu.
György Hajnóczky, Email: gyorgy.hajnoczky@jefferson.edu.
References
- Cogliati S, Enriquez JA, Scorrano L (2016) Mitochondrial cristae: where beauty meets functionality. Trends Biochem Sci 41: 261–273 [DOI] [PubMed] [Google Scholar]
- Fraser F, Padovese R, Zammit VA (2001) Distinct kinetics of carnitine palmitoyltransferase I in contact sites and outer membranes of rat liver mitochondria. J Biol Chem 276: 20182–20185 [DOI] [PubMed] [Google Scholar]
- Frigini EN, Barrera EE, Pantano S, Porasso RD (2020) Role of membrane curvature on the activation/deactivation of carnitine Palmitoyltransferase 1A: a coarse grain molecular dynamic study. Biochim Biophys Acta Biomembr 1862: 183094 [DOI] [PubMed] [Google Scholar]
- Hicks KG, Cluntun AA, Schubert HL, Hackett SR, Berg JA, Leonard PG, Ajalla Aleixo MA, Zhou Y, Bott AJ, Salvatore SR et al (2023) Protein‐metabolite interactomics of carbohydrate metabolism reveal regulation of lactate dehydrogenase. Science 379: 996–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondadi AK, Anand R, Reichert AS (2020) Cristae membrane dynamics – a paradigm change. Trends Cell Biol 30: 923–936 [DOI] [PubMed] [Google Scholar]
- Kulkarni SS, Joffraud M, Boutant M, Ratajczak J, Gao AW, Maclachlan C, Hernandez‐Alvarez MI, Raymond F, Metairon S, Descombes P et al (2016) Mfn1 deficiency in the liver protects against diet‐induced insulin resistance and enhances the hypoglycemic effect of metformin. Diabetes 65: 3552–3560 [DOI] [PubMed] [Google Scholar]
- Liu X, Weaver D, Shirihai O, Hajnoczky G (2009) Mitochondrial ‘kiss‐and‐run’: interplay between mitochondrial motility and fusion‐fission dynamics. EMBO J 28: 3074–3089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra P, Chan DC (2016) Metabolic regulation of mitochondrial dynamics. J Cell Biol 212: 379–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo J, Choi DW, Stanley IA, Stiles L, Molina AJA, Chen PH, Lako A, Sung ICH, Goswami R, Kim MY et al (2023) Mitochondrial morphology controls fatty acid utilization by changing CPT1 sensitivity to malonyl‐CoA. EMBO J 42: e111901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana‐Cabrera R, Scorrano L (2023) Determinants and outcomes of mitochondrial dynamics. Mol Cell 83: 857–876 [DOI] [PubMed] [Google Scholar]
- Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G et al (2008) Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J 27: 433–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikstrom JD, Mahdaviani K, Liesa M, Sereda SB, Si Y, Las G, Twig G, Petrovic N, Zingaretti C, Graham A et al (2014) Hormone‐induced mitochondrial fission is utilized by brown adipocytes as an amplification pathway for energy expenditure. EMBO J 33: 418–436 [DOI] [PMC free article] [PubMed] [Google Scholar]


