Key Points
Question
Does proactive specialist palliative care improve patient-reported outcomes in patients receiving curative-intent surgeries for high morbidity and mortality upper gastrointestinal cancers?
Findings
In this randomized clinical trial across 5 geographically diverse cancer centers that included 356 adults randomized to either surgeon alone or surgeon–palliative care team comanagement, there was no significant difference in postoperative patient-reported outcomes, such as quality of life or mental health. Palliative care was well tolerated by study patient and practitioner participants with no associated harms.
Meaning
Data from this study do not suggest benefits with palliative care for surgical oncology patients with newly diagnosed cancers who were pursuing curative-intent operations; integration of palliative care specialists could possibly be more effectively targeted toward patients with poorer baseline quality of life and functionality.
Abstract
Importance
Involvement of palliative care specialists in the care of medical oncology patients has been repeatedly observed to improve patient-reported outcomes, but there is no analogous research in surgical oncology populations.
Objective
To determine whether surgeon–palliative care team comanagement, compared with surgeon team alone management, improves patient-reported perioperative outcomes among patients pursuing curative-intent surgery for high morbidity and mortality upper gastrointestinal (GI) cancers.
Design, Setting, and Participants
From October 20, 2018, to March 31, 2022, a patient-randomized clinical trial was conducted with patients and clinicians nonblinded but the analysis team blinded to allocation. The trial was conducted in 5 geographically diverse academic medical centers in the US. Individuals pursuing curative-intent surgery for an upper GI cancer who had received no previous specialist palliative care were eligible. Surgeons were encouraged to offer participation to all eligible patients.
Intervention
Surgeon–palliative care comanagement patients met with palliative care either in person or via telephone before surgery, 1 week after surgery, and 1, 2, and 3 months after surgery. For patients in the surgeon-alone group, surgeons were encouraged to follow National Comprehensive Cancer Network–recommended triggers for palliative care consultation.
Main Outcomes and Measures
The primary outcome of the trial was patient-reported health-related quality of life at 3 months following the operation. Secondary outcomes were patient-reported mental and physical distress. Intention-to-treat analysis was performed.
Results
In total, 359 patients (175 [48.7%] men; mean [SD] age, 64.6 [10.7] years) were randomized to surgeon-alone (n = 177) or surgeon–palliative care comanagement (n = 182), with most patients (206 [57.4%]) undergoing pancreatic cancer surgery. No adverse events were associated with the intervention, and 11% of patients in the surgeon-alone and 90% in the surgeon–palliative care comanagement groups received palliative care consultation. There was no significant difference between study arms in outcomes at 3 months following the operation in patient-reported health-related quality of life (mean [SD], 138.54 [28.28] vs 136.90 [28.96]; P = .62), mental health (mean [SD], −0.07 [0.87] vs −0.07 [0.84]; P = .98), or overall number of deaths (6 [3.7%] vs 7 [4.1%]; P > .99).
Conclusions and Relevance
To date, this is the first multisite randomized clinical trial to evaluate perioperative palliative care and the earliest integration of palliative care into cancer care. Unlike in medical oncology practice, the data from this trial do not suggest palliative care–associated improvements in patient-reported outcomes among patients pursuing curative-intent surgeries for upper GI cancers.
Trial Registration
ClinicalTrials.gov Identifier: NCT03611309
This randomized clinical trial evaluates the use of surgeon–palliative care team comanagement to improve health-related quality of life among individuals undergoing curative-intent surgery for upper gastrointestinal cancers.
Introduction
Although major surgery is well documented to be safe, perioperative morbidity and mortality are not inconsequential and patients can develop psychological and physical symptoms.1,2,3,4,5,6,7 Upper gastrointestinal (GI) cancers typically require extensive surgeries with increased cancer-related mortality and persistent pain, decreased quality of life, poorer global health, diminished appetite, and decreased emotional and social functioning for months to years.8,9 Patients pursuing pancreaticoduodenectomy surgery for pancreatic adenocarcinoma have a median survival of 18 months, a greater than 85% 5-year mortality, and postoperative morbidities including delayed gastric emptying, pancreatic fistulae, bilomas, and biliary strictures.8,10,11 Esophagectomy surgery may have a perioperative mortality rate of less than 5% but moderate morbidity ranges from 40% to 60%, and 24% of patients experience major morbidity including postoperative bleeding, anastomotic leak, pneumonia, or prolonged postoperative intubation.3,7 Curative-intent surgical resections for upper GI cancers are extensive and frequently require postoperative admission to an intensive care unit. Palliative care is patient-centered care that symptomatically and psychosocially supports patients with serious illness and optimizes quality of life, regardless of diagnosis, prognosis, or care goals.12 Multiple studies among medical oncology patients support that palliative care improves diverse patient-reported outcomes,13,14,15,16,17,18 but few to no studies have evaluated proactive palliative care among a surgical oncologic patient population.19 Indeed, multiple studies document surgical culture resistance to palliative and end-of-life care.20,21,22,23,24,25,26,27,28,29
Through past research work, some members of our research team identified that patients with upper GI cancers pursuing curative-intent surgeries are a population likely to benefit from proactive palliative care.30,31,32,33,34,35 In this population, our trial goal was to evaluate the effect of palliative care integrated with standard surgical oncology care on patient-reported outcomes. We hypothesized that, analogous to studies in medical oncology, proactive palliative care among surgical oncology patients would be associated with better quality of life and mood symptoms.
Methods
Study Design
From October 20, 2018, to March 31, 2022, we enrolled ambulatory patients with newly diagnosed upper GI cancers in a nonblinded pragmatic randomized clinical trial to receive either surgeon-alone management (enhanced usual care) or surgeon–palliative care comanagement (intervention). The study was performed at 5 geographically diverse sites including The Johns Hopkins Hospital in Baltimore, Maryland; the Dana-Farber Cancer Institute in Boston, Massachusetts; Stanford University Medical Center in Stanford, California; the University of New Mexico Medical Center in Albuquerque; and The Ohio State University Wexner Medical Center in Columbus. Eligible patients were enrolled when scheduled for surgery and computer randomized in a 1:1 ratio in blocks of 4 and stratified by enrollment site with allocation provided at the time of randomization. Patients assigned to the intervention met either in person or via telephone with a member of an interprofessional palliative care specialist team, which consisted of board-certified palliative care physicians, advanced-practice nurses, social workers, pharmacists, and/or chaplains. Palliative care visits occurred before surgery, 1 week after surgery, and 1, 2, and 3 months after surgery, with additional visits scheduled at the discretion of the patient, surgery team, or palliative care team. The protocol (Supplement 1) was approved by the institutional review board at each institution and all participants provided verbal informed and written consent was later requested at some study sites. Participation in the trial was not compensated but participants would receive a $20 gift card for completion of services (maximum, $100). The full study protocol was previously published.36 The study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline for randomized studies.
Guidelines for palliative care visits (Table 1) were adapted from the National Consensus Project for Quality Palliative Care37 based on interventions used in previous palliative care clinical trials among medical oncology patients13,14 and included in the study protocol.36 Palliative care clinicians received training materials about upper GI cancers and operations prepared by a study member board certified in both surgery and palliative care and with content approved by key study team surgeons. Intervention group patients also received routine surgical oncologic care. As consistent with standard practice and adherent to an intention-to-treat analysis, patients in the surgeon-only group could receive palliative care consultation if requested by their surgical team. The surgeon-alone group was considered enhanced usual care as participating surgeons were encouraged to follow the National Comprehensive Cancer Network–recommended triggers for when to involve palliative care consultants38 although, as consistent with standard practice, the surgical team decided whether or when to consult palliative care consultants. To facilitate intervention implementation and fidelity, site leadership teams met weekly to discuss study events and one of us (R.A.A.) periodically checked for intervention fidelity through review of deidentified palliative care notes from patients in the intervention arm.
Table 1. Components of Surgeon–Palliative Care Team Comanagement.
| Team element | Description |
|---|---|
| Time | A goal of at least 60 min/mo (per patient and caregiver preference) devoted to palliative care treatment |
| Education | Patients and family members, per their wishes, counseled and educated about the disease, including self-management of symptoms, prognosis, and treatment options |
| Assessment | Formal assessment of symptoms, including pain, dyspnea, constipation/diarrhea, anxiety/depression, fatigue, and nausea |
| Multidisciplinary | Access to a multidisciplinary palliative care team composed of nurse, physician, social worker, pharmacist, and/or chaplain team members |
Patients
Patients who presented to outpatient hepatobiliary surgery clinics were invited by their surgical oncologist to enroll. Surgeons were encouraged but not required to offer participation to all eligible patients; no additional screening or recruitment measures were used. If a patient declined to participate, study coordinators recorded the reason. Patients were eligible if they were being scheduled for a curative-intent operation for an upper GI cancer and had not previously received specialist palliative care. Patients who did not speak English participated in study activities using a translator service and study materials and activities were available in both English and Spanish.
Patient-Reported Measures
Health-related quality of life (HRQOL) was measured with the Functional Assessment of Chronic Illness Therapy–Palliative Care (FACIT-Pal) Subscale (46-item metric with scores ranging from 0 to 184; higher scores indicate higher HRQOL),39 which includes all elements of the Functional Assessment of Cancer Therapy–General (FACT-G) metric (27-item metric with scores ranging from 0 to 108; higher scores indicate higher HRQOL),40,41 which has been used extensively as a quality-of-life outcome in previous medical oncology studies. Patient-Reported Outcomes Measurement Information System (PROMIS) profile summary mental health and physical health scores were calculated from the 29-item (PROMIS-29) tool.42 The PROMIS-Preference (PROPr) summary score was developed as a societal-preference–based health-related quality-of-life instrument and is a summary measure of 7 PROMIS-29 domains: cognition, depression, fatigue, pain, physical, sleep, and social; PROPr scores were calculated.43
Data Collection
Participants completed baseline questionnaires at the time of randomization with follow-up questionnaires at 1 week, 1 month, 3 months, and 6 months following the operation. Questionnaires could be completed electronically, on paper, or verbally and could be completed within 2 weeks of the specified date; participants were contacted by email or telephone a maximum of 3 times for survey completion. After each visit, palliative care clinicians completed a survey documenting visit length and content (eAppendix in Supplement 2). Adverse events were patient-, family-, or clinician-reported detrimental events deemed due to involvement of the palliative care specialists; potential adverse events were discussed at weekly site meetings.
Statistical Analysis
Analysis team members and the study principal investigator (R.A.A.) were blinded to allocation and the analytic data set included data obtained through September 14, 2022. The primary study outcome was group differences in patient-reported HRQOL at 3 months after the operation. Estimated sample size was based on an unpaired, 2-sample t test to detect a small to moderate effect size of 0.4 with 90% power and a probability of type I error of .05 (2-sided). We incorporated a variance inflation rate of 20% to account for potential within-site association and assumed a completion rate of 0.86; this attrition rate was based on previously published perioperative mortality rates and past study team experience. Under these assumptions, the estimated sample size was 186 patients per arm for a total of 372 patients. We planned exploratory subgroup analyses among race and ethnicity, and study site subgroups. Hypothesizing a potential association between study outcomes and patient race and ethnicity and/or study site, we planned exploratory subgroup analyses among race and ethnicity and study site groups.
We conducted statistical analyses using R, version 4.2.2 software (R Foundation for Statistical Computing) and used descriptive statistics to estimate the frequencies and means (SDs) of the study variables stratified by study group. We imputed missing outcomes data at 3 months with the nearest postoperative survey. We used t tests to compare numeric patient and outcomes variables and χ2 tests of association to compare categorical variables between treatment groups. We conducted 2 sensitivity analyses to compare the subset of patients who completed a survey at 3 months postoperatively (no imputation of outcomes) using t tests and used linear mixed models to compare the mean treatment effect across all time points for the primary outcome. These models included a binary indicator of treatment group and a random effect to account for within-patient variation across time. To score the PROMIS mental and physical profile scores, we followed the code provided by Spritzer and Hays44 to estimate z scores.44 To score the PROMIS PROPr scores, we applied the R function propr.maut.function.201709 of Hanmer and Dewitt.45 Side-by-side boxplots of patient-reported outcome scores were compared by treatment group at each survey time point.
Results
Baseline Characteristics of the Patients
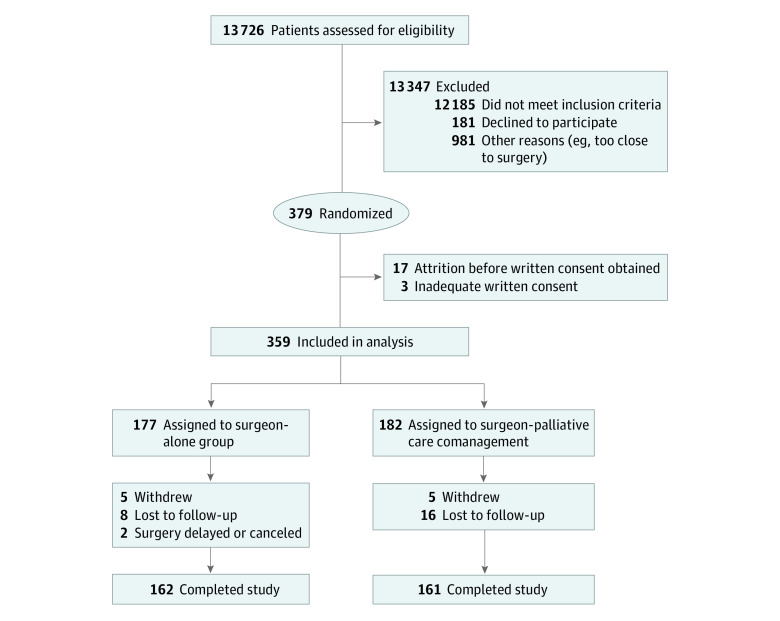
A total of 379 patients were enrolled in the study and verbally consented (Figure 1); 359 patients (175 [48.7%] men; mean [SD] age, 64.6 [10.7] years) were randomized, including 177 participants in the enhanced control arm (mean [SD] age, 64.7 [10.4] years; 90 women [51.4%] and 85 men [51.4%]). Self-reported race and ethnicity categories included American Indian, 1.1%; Asian, 5.1%; Black or African American, 9.1%; Latino or Latina, 10.3%; White or Caucasian, 77.7%; multirace, 0.6%; and other unlisted race, 6.3%. In this cohort, 69.2% individuals had a college degree or higher; cancer types included pancreatic, 61.4%; gastric, 15.2%; esophageal, 6.4%; cholangiocarcinoma, 8.8%; hepatocellular, 4.7%; and other, 3.5%. A total of 182 participants were included in the intervention arm (mean [SD] age, 64.6 [10.9]; 73 women [40.1%]; 109 men [59.9%]). Race and ethnicity categories included American Indian, 2.7%; Asian, 2.2%; Black or African American, 6.0%; Hawaiian or Pacific Islander, 0.5%; Latino or Latina, 12.6%; White or Caucasian, 79.7%; multirace, 1.1%; and other unlisted race, 7.7%. In this cohort, 59.0% of the participants had a college degree or higher; cancer types included pancreatic, 55.8%; gastric, 18.2%; esophageal, 12.2%; cholangiocarcinoma, 7.7%; hepatocellular, 2.2%; and other, 3.9%.
Figure 1. Patient Flowchart.
There were no significant changes in methods or collected study outcomes after trial commencement. All patients provided verbal consent, but with changes in written consent practices, particularly surrounding the COVID-19 pandemic, 17 patients could not be contacted for follow-up written consent and 3 had inadequate written consent; data from these 20 patients were excluded. Data from 359 patients were analyzed. Baseline characteristics were similar (Table 2). Consistent with study site variations in surgical volume, enrollment varied by study site. Enrolled patients predominantly underwent operations for 1 of 5 upper GI cancers, with most undergoing curative-intent resection of pancreatic cancer (206 [57.4%] of patients whose malignancy type was known). Loss to follow-up was 8 of 177 patients (4.5%) in the enhanced control arm and 16 of 182 (8.7%) in the intervention arm. A total of 119 eligible patients declined to participate with the most common reasons (patients could select more than 1 reason) being too overwhelmed/stressed by diagnosis/generally tired (n = 64), surgery too soon/don’t want to deal with anything extra (n = 31), and not interested in palliative care/do not see how it would be beneficial/do not want to deviate from current care plan (n = 25).
Table 2. Patient Characteristics.
| Characteristic | No. (%) | |
|---|---|---|
| Enhanced control (n = 177) | Intervention (n = 182) | |
| Study site | ||
| Dana-Farber | 47 (26.6) | 47 (25.8) |
| Johns Hopkins | 80 (45.2) | 83 (45.6) |
| New Mexico | 18 (10.2) | 19 (10.4) |
| Ohio State | 3 (1.7) | 3 (1.6) |
| Stanford | 29 (16.4) | 30 (16.5) |
| Age, y | ||
| Missing | 1 | 1 |
| Mean (SD) | 64.72 (10.40) | 64.57 (10.90) |
| Sex | ||
| Male | 85 (48.6) | 109 (59.9) |
| Female | 90 (51.4) | 73 (40.1) |
| Missing | 2 | 0 |
| Race | ||
| American Indian or Alaskan Native | 2 (1.1) | 5 (2.7) |
| Asian | 9 (5.1) | 4 (2.2) |
| Black or African American | 16 (9.1) | 11 (6.0) |
| Hawaiian or Pacific Islander | 0 | 1 (0.5) |
| White or Caucasian | 136 (77.7) | 145 (79.7) |
| Multirace | 1 (0.6) | 2 (1.1) |
| Other unlisted race | 11 (6.3) | 14 (7.7) |
| Missing | 2 | 0 |
| Ethnicity | ||
| Hispanic (Latino or Latina) | 18 (10.3) | 23 (12.6) |
| Non-Hispanic | 157 (89.7) | 159 (87.4) |
| Missing | 2 | 0 |
| Highest educational level completed | ||
| <8 y | 1 (0.7) | 2 (1.4) |
| 8-11 y (without graduating high school) | 4 (2.8) | 4 (2.8) |
| High school graduation or GED, vocational, or technical school | 35 (24.1) | 52 (35.9) |
| College degree | 49 (33.8) | 53 (36.6) |
| Graduate, postgraduate, or professional degree | 52 (35.9) | 31 (21.4) |
| Prefer not to answer | 4 (2.8) | 3 (2.1) |
| Missing | 32 | 37 |
| Marital status | ||
| Never married | 13 (7.7) | 17 (9.8) |
| Married | 122 (72.2) | 120 (69.4) |
| Divorced | 16 (9.5) | 18 (10.4) |
| Domestic partnership/living together | 2 (1.2) | 4 (2.3) |
| Separated | 1 (0.6) | 1 (0.6) |
| Widowed | 14 (8.3) | 12 (6.9) |
| Other | 1 (0.6) | 1 (0.6) |
| Missing | 8 | 9 |
| Caregiver | ||
| No | 111 (65.7) | 117 (67.2) |
| Yes | 58 (34.3) | 57 (32.8) |
| Missing | 8 | 8 |
| Malignancy type | ||
| Pancreatic | 105 (61.4) | 101 (55.8) |
| Gastric | 26 (15.2) | 33 (18.2) |
| Esophageal | 11 (6.4) | 22 (12.2) |
| Cholangiocarcinoma | 15 (8.8) | 14 (7.7) |
| Hepatocellular | 8 (4.7) | 4 (2.2) |
| Other | 6 (3.5) | 7 (3.9) |
| Missing | 6 | 1 |
| Baseline patient HRQOL | ||
| FACT-G | ||
| Missing | 30 | 30 |
| Mean (SD) | 81.2 (16.3) | 80.7 (17.8) |
| FACIT-Pal total | ||
| Missing | 31 | 30 |
| Mean (SD) | 142.0 (26.1) | 140.3 (28.0) |
| PROMIS-29: physical health | ||
| Missing | 44 | 48 |
| Mean (SD) | −0.05 (0.9) | −0.1 (0.9) |
| PROMIS-29: mental health | ||
| Missing | 44 | 48 |
| Mean (SD) | 0.1 (0.8) | 0.1 (0.9) |
| PROPr PROMIS summary | ||
| Missing | 44 | 48 |
| Mean (SD) | 0.5 (0.2) | 0.5 (0.2) |
Abbreviations: FACIT, Functional Assessment of Chronic Illness Therapy; FACIT-Pal, FACIT–Palliative Care; FACT-G, Functional Assessment of Cancer Therapy–General; GED, general educational development; HRQOL, health-related quality of life; PROMIS-29, Patient-Reported Outcomes Measurement Information System; PROPr, PROMIS-Preference.
Palliative Care Visits in Both Groups
Among 182 patients randomized to the intervention, 164 individuals (90%) received at least 1 palliative care team visit with a mean (SD) of 3.43 (1.70) of the 5 total possible visits during the first 3 months following the operation; mean visit time was 30 (14.2) minutes (range, 5-75 minutes) and the most common visit content involved relationship/rapport building (71.2%), symptom management (66.7%), illness understanding/education (62.4%), and coping with serious illness (61.3%). No harms associated with the inclusion of palliative care specialists were reported by intervention patients, family members, or clinicians. Among 177 patients randomized to the enhanced control group, 20 (11.3%) had a palliative care consultation during the first 3 months following the operation and 5 were referred for hospice care; 1 study site accounted for 17 of these 20 consultations with 21.3% (17 of 80) of enhanced control arm patients at that site receiving palliative care consultation. Other sites had 4.2% (2 of 47), 3.4% (1 of 29), and 0% (0 of 18 and 0 of 3) of enhanced control arm patients receiving palliative care consultation.
HRQOL Outcomes
A comparison of measures of HRQOL at 3 months following the operation did not show any significant differences between study groups (mean [SD], FACIT-Pal, 138.54 [28.28] vs 136.90 [28.96]; P = .62; FACT-G, 79.90 [17.14] vs 79.40 [17.45]; P = .80; PROMIS-29 physical health, −0.43 [0.89] vs −0.50 [1.01]; P = .56; PROMIS-29 mental health, −0.07 [0.87] vs −0.07 [0.84]; P = .98; and PROPr PROMIS, 0.40 [0.19] vs 0.40 [0.20]; P = .83) (Table 3; Figure 2). There was also no significant difference in mortality during the study between the groups (deaths from enrollment until 3 months postoperatively: enhanced control, 6 of 177 [3.7%]; intervention, 7 of 182 [4.1%]; P > .99).
Table 3. Patient-Reported Outcomes at 3 Months After Surgery.
| Outcome | Enhanced control (n = 177) | Intervention (n = 182) | P value |
|---|---|---|---|
| FACT-G | |||
| Missing, No. | 30 | 30 | .80 |
| Mean (SD) | 79.90 (17.14) | 79.40 (17.45) | |
| FACIT-Pal total | |||
| Missing, No. | 30 | 30 | .62 |
| Mean (SD) | 138.54 (28.28) | 136.90 (28.96) | |
| PROMIS-29: physical health | |||
| Missing, No. | 32 | 38 | .56 |
| Mean (SD) | −0.43 (0.89) | −0.50 (1.01) | |
| PROMIS-29: mental health | |||
| Missing, No. | 32 | 38 | .98 |
| Mean (SD) | −0.07 (0.87) | −0.07 (0.84) | |
| PROPr PROMIS summary | |||
| Missing, No. | 32 | 38 | .83 |
| Mean (SD) | 0.40 (0.19) | 0.40 (0.20) | |
| Died during study, No. (%) | |||
| Alive | 156 (96.3) | 164 (95.9) | >.99 |
| Died | 6 (3.7) | 7 (4.1) | |
| Missing | 15 | 11 |
Abbreviations: FACIT-Pal, Functional Assessment of Chronic Illness Therapy–Palliative Care; FACT-G, Functional Assessment of Cancer Therapy–General; PROMIS-29, Patient-Reported Outcomes Measurement Information System; PROPr, PROMIS-Preference.
Figure 2. Comparisons of Patient-Reported Outcomes Across Time and Treatment Group.
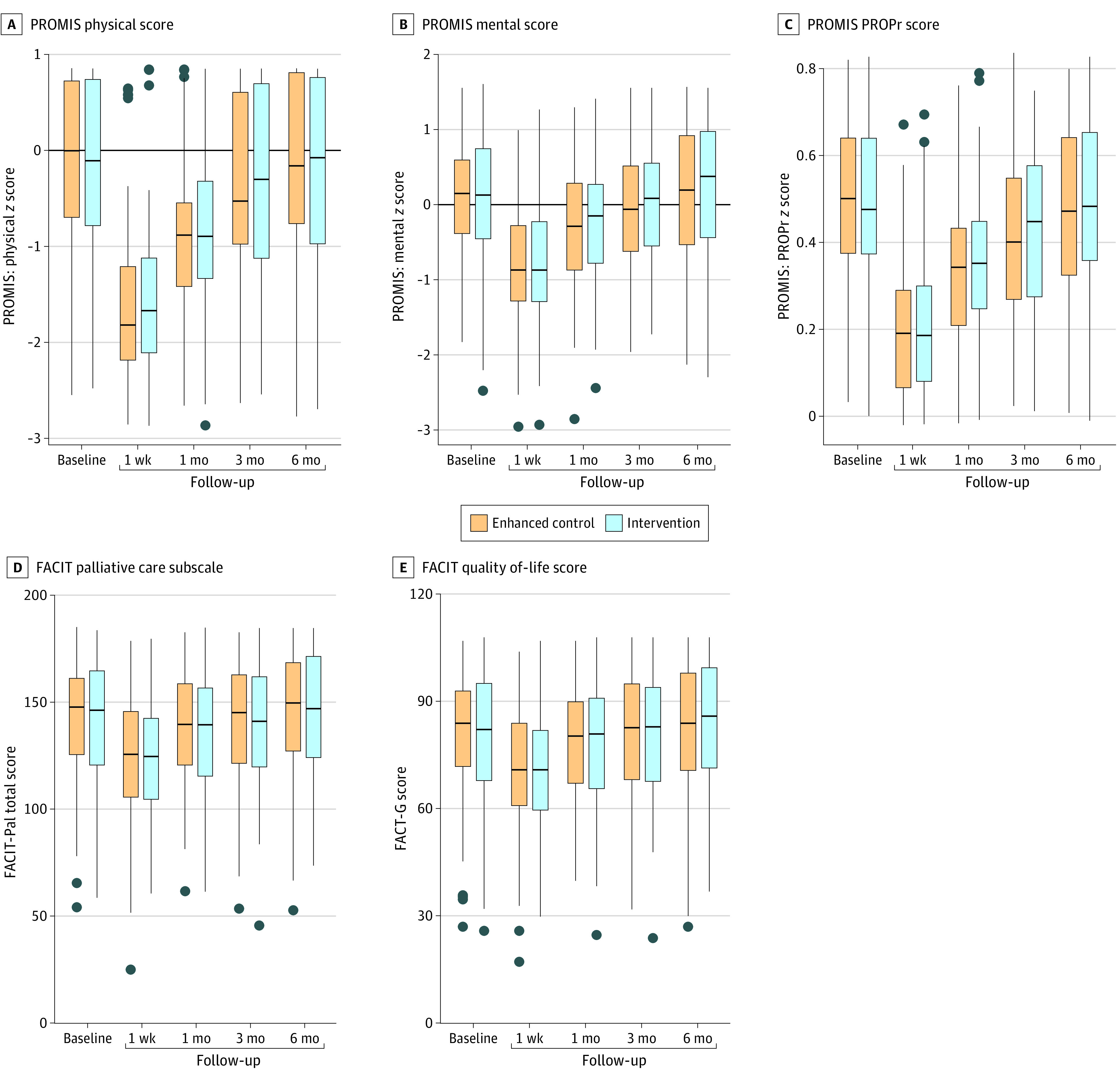
Boxes indicate the IQR, black lines within the boxes indicate the medians, lines indicate quartile 1 − 1.5 × IQR (lower) and quartile 3 + 1.5 × IQR (upper). Dots represent potential outliers. FACIT indicates Functional Assessment of Chronic Illness Therapy; FACIT-Pal, FACIT–Palliative Care; FACT-G, Functional Assessment of Cancer Therapy–General; PROMIS, Patient-Reported Outcomes Measurement Information System; PROPr, PROMIS-Preference.
Missing Data and Sensitivity Analyses
Of the 128 enhanced control patients who completed a 3-month postoperative survey, 118 completed the FACIT questionnaire and 109 completed PROMIS-29. Among the 141 intervention patients who completed the 3-month postoperative survey, 104 completed the FACIT questionnaire and 87 completed the PROMIS-29. When outcomes were imputed at 3 months, 147 enhanced control patients completed at least 1 patient-reported HRQOL survey and 152 intervention patients completed at least 1 patient-reported HRQOL survey postoperatively. For the sensitivity analysis comparing patient-reported HRQOL at 3 months postoperatively between the 2 arms, among only patients who answered the 3-month survey (ie, no imputed data: enhanced control, 128; intervention, 141), the results were nearly identical with no significant differences between arms. For the sensitivity analysis using linear mixed models, there were again no significant differences between the 2 arms for FACIT-Pal (−1.79; 95% CI, −7.12 to 3.55; P = .50), FACT-G (−0.74; 95%, CI, −3.99 to 2.51; P = .70), PROMIS physical (0.001; 95% CI, −0.16 to 0.16; P > .90), PROMIS mental (0.03; 95% CI, −0.12 to 0.19; P = .70), and PROPr (0.001; 95% CI, −0.03 to 0.03; P > .90).
Discussion
To our knowledge, this is the first multisite randomized clinical trial of palliative care in a surgery patient population and includes the earliest reported integration of palliative care in oncologic care. Despite previously discussed surgical cultural barriers to palliative care, this study was completed without any reported palliative care–related harms and was well received by both patients and participating surgeons. Our results do not support that early integration of palliative care among patients with upper GI cancers pursuing curative-intent surgeries improves patient-reported HRQOL or mood symptoms.
Studies support that early integration of palliative care improves patient-reported HRQOL and mood symptoms, particularly among medical oncology patients with metastatic disease with little chance of cure.46 Data also support benefits associated with palliative care among medical oncology patients receiving curative-intent treatments,15,47 and in observational studies using administrative data sets of surgery patients.48 Our data suggest a more nuanced and context-sensitive ecosystem. The surgical oncology clinical milieu may differ from a medical one with more self-limited patient symptoms surrounding the time of surgery. The potentially curative operation may also elevate hope and lessen existential distress. Moreover, while existing data support that palliative care is not harmful, perhaps there is a time when palliative care—particularly provided by specialists—is less likely to be as helpful. It is plausible that patients with newly diagnosed cancer pursuing curative-intent surgery were too early in their cancer trajectory for specialist palliative care to have a measurable benefit. Palliative care also may be less helpful among patients with good functional status, even if they have a serious illness. Compared with our study patients, those in other palliative care clinical trials frequently had metastatic and/or terminal disease at the time of study enrollment and poorer baseline quality-of-life scores. Palliative care delivery in cancer care has also substantially changed over the past decade with increased quality and quantity of palliative care integrated within standard oncologic treatment, even when palliative care specialists may not be explicitly involved. Being less depressed at this early stage of their cancer journey, the patients could have been less likely to have an appreciable improvement in mood symptoms similar to that found in previous palliative care studies.14
Existing data support a current, widespread, persistent, and substantial shortage of palliative care specialists.49,50 Given this work force limitation, our data suggest that nonspecialist palliative care resources may be sufficient for some populations. Our data also suggest variation in existing palliative care consultation practices for surgical oncology patients and that surgical oncology teams may already be selective in determining when to involve palliative care specialists. Moreover, palliative care, particularly when provided by specialists, may be more influential when delivered closer to patient death. Our limited perioperative study timeline may not yet reveal a palliative care–associated benefit precisely because of relatively few patient deaths during the study period.51,52
Limitations
Study limitations include that patient attrition was higher than anticipated and exacerbated by both direct and downstream consequences of the COVID-19 pandemic, although attrition was similar between the 2 groups. While one could hypothesize pandemic-related impacts to study protocols and procedures, these impacts were likely to be similar across study arms and, even before the pandemic and consistent with previous trials with palliative care delivered by telephone,13,18,53 our study always offered a telephonic option for palliative care that was highly used and preferred. While our study incorporated patients from diverse geographic areas, most were White, highly educated, and received care from urban and suburban tertiary or quaternary medical centers; study results may be less generalizable among patients who are more ethnically and racially diverse, less educated, and/or who reside in rural areas. Patients in the intervention arm also overwhelming preferred telephone-based palliative care and, after March 2020 and due to the COVID-19 pandemic, all outpatient palliative care was delivered via telephone; the “dose” of palliative care delivered by telephone could have been lower and less likely to impact outcomes. The study patients also started with high HRQOL compared with patients with cancer in many medical oncology studies, which highlights a relative lack of symptoms that palliative care specialists could potentially ameliorate. In addition, this was a pragmatic trial and, while intervention fidelity was monitored, provision of palliative care in the intervention arm was not protocolized and perhaps could have been stronger in effect if more prescriptively delivered.
Conclusions
Existing data support that proactive palliative care is safe and likely to confer benefit, particularly among patients who have complex symptoms and/or who are approaching the end of life; our data support that palliative care is feasible and safe in surgical patient populations. Given improved acceptance and delivery of palliative care practices and principles within general medical practice, particularly within cancer care, palliative care provided by nonspecialists may be sufficient for some patients, particularly when a serious illness is at an early stage and symptomatic care is straightforward. Specialist palliative care resources are often limited and can likely be targeted toward patients with a more complex status, such as those with more advanced disease, those closer to the time of death, and/or those with complex physical, psychological, and/or social symptoms that may merit involvement of trained and experienced specialists.
Trial Protocol
eAppendix. Patient Questionnaire
Data Sharing Statement
References
- 1.Schwarze ML, Shen Y, Hemmerich J, Dale W. Age-related trends in utilization and outcome of open and endovascular repair for abdominal aortic aneurysm in the United States, 2001-2006. J Vasc Surg. 2009;50(4):722-729.e2. doi: 10.1016/j.jvs.2009.05.010 [DOI] [PubMed] [Google Scholar]
- 2.Finlayson E, Fan Z, Birkmeyer JD. Outcomes in octogenarians undergoing high-risk cancer operation: a national study. J Am Coll Surg. 2007;205(6):729-734. doi: 10.1016/j.jamcollsurg.2007.06.307 [DOI] [PubMed] [Google Scholar]
- 3.Paul S, Altorki N. Outcomes in the management of esophageal cancer. J Surg Oncol. 2014;110(5):599-610. doi: 10.1002/jso.23759 [DOI] [PubMed] [Google Scholar]
- 4.Kneuertz PJ, Pitt HA, Bilimoria KY, et al. Risk of morbidity and mortality following hepato-pancreato-biliary surgery. J Gastrointest Surg. 2012;16(9):1727-1735. doi: 10.1007/s11605-012-1938-y [DOI] [PubMed] [Google Scholar]
- 5.Pezzilli R, Falconi M, Zerbi A, et al. Clinical and patient-reported outcomes after pancreatoduodenectomy for different diseases: a follow-up study. Pancreas. 2011;40(6):938-945. doi: 10.1097/MPA.0b013e318216f693 [DOI] [PubMed] [Google Scholar]
- 6.Bouras G, Markar SR, Burns EM, et al. The psychological impact of symptoms related to esophagogastric cancer resection presenting in primary care: A national linked database study. Eur J Surg Oncol. 2017;43(2):454-460. doi: 10.1016/j.ejso.2016.10.010 [DOI] [PubMed] [Google Scholar]
- 7.Dhungel B, Diggs BS, Hunter JG, Sheppard BC, Vetto JT, Dolan JP. Patient and peri-operative predictors of morbidity and mortality after esophagectomy: American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP), 2005-2008. J Gastrointest Surg. 2010;14(10):1492-1501. doi: 10.1007/s11605-010-1328-2 [DOI] [PubMed] [Google Scholar]
- 8.McEvoy SH, Lavelle LP, Hoare SM, et al. Pancreaticoduodenectomy: expected post-operative anatomy and complications. Br J Radiol. 2014;87(1041):20140050. doi: 10.1259/bjr.20140050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cameron JL, He J. Two thousand consecutive pancreaticoduodenectomies. J Am Coll Surg. 2015;220(4):530-536. doi: 10.1016/j.jamcollsurg.2014.12.031 [DOI] [PubMed] [Google Scholar]
- 10.Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371(11):1039-1049. doi: 10.1056/NEJMra1404198 [DOI] [PubMed] [Google Scholar]
- 11.American Cancer Society . Cancer statistics. Accessed February 5, 2019. http://www.cancer.org
- 12.Kelley AS, Morrison RS. Palliative care for the seriously ill. N Engl J Med. 2015;373(8):747-755. doi: 10.1056/NEJMra1404684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bakitas M, Lyons KD, Hegel MT, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009;302(7):741-749. doi: 10.1001/jama.2009.1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733-742. doi: 10.1056/NEJMoa1000678 [DOI] [PubMed] [Google Scholar]
- 15.El-Jawahri A, Traeger L, Greer JA, et al. Effect of inpatient palliative care during hematopoietic stem-cell transplant on psychological distress 6 months after transplant: results of a randomized clinical trial. J Clin Oncol. 2017;35(32):3714-3721. doi: 10.1200/JCO.2017.73.2800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Jawahri A, LeBlanc T, VanDusen H, et al. Effect of inpatient palliative care on quality of life 2 weeks after hematopoietic stem cell transplantation: a randomized clinical trial. JAMA. 2016;316(20):2094-2103. doi: 10.1001/jama.2016.16786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greer JA, Pirl WF, Jackson VA, et al. Effect of early palliative care on chemotherapy use and end-of-life care in patients with metastatic non–small-cell lung cancer. J Clin Oncol. 2012;30(4):394-400. doi: 10.1200/JCO.2011.35.7996 [DOI] [PubMed] [Google Scholar]
- 18.Bakitas MA, Tosteson TD, Li Z, et al. Early versus delayed initiation of concurrent palliative oncology care: patient outcomes in the ENABLE III randomized controlled trial. J Clin Oncol. 2015;33(13):1438-1445. doi: 10.1200/JCO.2014.58.6362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lilley EJ, Khan KT, Johnston FM, et al. Palliative care interventions for surgical patients: a systematic review. JAMA Surg. 2016;151(2):172-183. doi: 10.1001/jamasurg.2015.3625 [DOI] [PubMed] [Google Scholar]
- 20.Buchman TG, Cassell J, Ray SE, Wax ML. Who should manage the dying patient? Rescue, shame, and the surgical ICU dilemma. J Am Coll Surg. 2002;194(5):665-673. doi: 10.1016/S1072-7515(02)01157-2 [DOI] [PubMed] [Google Scholar]
- 21.Cassell J, Buchman TG, Streat S, Stewart RM. Surgeons, intensivists, and the covenant of care: administrative models and values affecting care at the end of life—updated. Crit Care Med. 2003;31(5):1551-1557. doi: 10.1097/00003246-200305000-00039 [DOI] [PubMed] [Google Scholar]
- 22.Cassell J. Life and Death in Intensive Care. Temple University Press; 2005. [Google Scholar]
- 23.Kruser JM, Pecanac KE, Brasel KJ, et al. “And I think that we can fix it”: mental models used in high-risk surgical decision making. Ann Surg. 2015;261(4):678-684. doi: 10.1097/SLA.0000000000000714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pecanac KE, Kehler JM, Brasel KJ, et al. It’s big surgery: preoperative expressions of risk, responsibility, and commitment to treatment after high-risk operations. Ann Surg. 2014;259(3):458-463. doi: 10.1097/SLA.0000000000000314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwarze ML, Redmann AJ, Alexander GC, Brasel KJ. Surgeons expect patients to buy-in to postoperative life support preoperatively: results of a national survey. Crit Care Med. 2013;41(1):1-8. doi: 10.1097/CCM.0b013e31826a4650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paul Olson TJ, Brasel KJ, Redmann AJ, Alexander GC, Schwarze ML. Surgeon-reported conflict with intensivists about postoperative goals of care. JAMA Surg. 2013;148(1):29-35. doi: 10.1001/jamasurgery.2013.403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwarze ML, Bradley CT, Brasel KJ. Surgical “buy-in”: the contractual relationship between surgeons and patients that influences decisions regarding life-supporting therapy. Crit Care Med. 2010;38(3):843-848. doi: 10.1097/CCM.0b013e3181cc466b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bradley CT, Brasel KJ, Schwarze ML. Physician attitudes regarding advance directives for high-risk surgical patients: a qualitative analysis. Surgery. 2010;148(2):209-216. doi: 10.1016/j.surg.2010.05.020 [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez R, Marr L, Rajput A, Fahy BN. Utilization of palliative care consultation service by surgical services. Ann Palliat Med. 2015;4(4):194-199. [DOI] [PubMed] [Google Scholar]
- 30.Aslakson RA, Isenberg SR, Crossnohere NL, et al. Integrating advance care planning videos into surgical oncologic care: a randomized clinical trial. J Palliat Med. 2019;22(7):764-772. doi: 10.1089/jpm.2018.0209 [DOI] [PubMed] [Google Scholar]
- 31.Bridges JF, Crossnohere NL, Schuster AL, Miller JA, Pastorini C, Aslakson RA. A patient and community-centered approach selecting endpoints for a randomized trial of a novel advance care planning tool. Patient Prefer Adherence. 2018;12:241-249. doi: 10.2147/PPA.S150663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aslakson RA, Schuster ALR, Lynch TJ, et al. Developing the storyline for an advance care planning video for surgery patients: patient-centered outcomes research engagement from stakeholder summit to state fair. J Palliat Med. 2018;21(1):89-94. doi: 10.1089/jpm.2017.0106 [DOI] [PubMed] [Google Scholar]
- 33.Isenberg SR, Crossnohere NL, Patel MI, et al. An advance care plan decision support video before major surgery: a patient- and family-centred approach. BMJ Support Palliat Care. 2018;8(2):229-236. doi: 10.1136/bmjspcare-2017-001449 [DOI] [PubMed] [Google Scholar]
- 34.Isenberg SR, Aslakson RA, Dionne-Odom JN, et al. Family companions’ involvement during pre-surgical consent visits for major cancer surgery and its relationship to visit communication and satisfaction. Patient Educ Couns. 2018;101(6):1066-1074. doi: 10.1016/j.pec.2018.01.011 [DOI] [PubMed] [Google Scholar]
- 35.Mitchell IA, Schuster ALR, Lynch T, Smith KC, Bridges JFP, Aslakson RA. Why don’t end-of-life conversations go viral? A review of videos on YouTube. BMJ Support Palliat Care. 2017;7(2):197-204. doi: 10.1136/bmjspcare-2014-000805 [DOI] [PubMed] [Google Scholar]
- 36.Aslakson RA, Chandrashekaran SV, Rickerson E, et al. A multicenter, randomized controlled trial of perioperative palliative care surrounding cancer surgery for patients and their family members (PERIOP-PC). J Palliat Med. 2019;22(S1):44-57. doi: 10.1089/jpm.2019.0130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferrell BR, Twaddle ML, Melnick A, Meier DE. National Consensus Project clinical practice guidelines for quality palliative care guidelines, 4th edition. J Palliat Med. 2018;21(12):1684-1689. doi: 10.1089/jpm.2018.0431 [DOI] [PubMed] [Google Scholar]
- 38.Dans M, Smith R, Back A, et al. Palliative care, version 2.2017: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2017;15(8):989-997. doi: 10.6004/jnccn.2017.0132 [DOI] [PubMed] [Google Scholar]
- 39.Lyons KD, Bakitas M, Hegel MT, Hanscom B, Hull J, Ahles TA. Reliability and validity of the Functional Assessment of Chronic Illness Therapy–Palliative Care (FACIT-Pal) scale. J Pain Symptom Manage. 2009;37(1):23-32. doi: 10.1016/j.jpainsymman.2007.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11(3):570-579. doi: 10.1200/JCO.1993.11.3.570 [DOI] [PubMed] [Google Scholar]
- 41.King MT, Agar M, Currow DC, Hardy J, Fazekas B, McCaffrey N. Assessing quality of life in palliative care settings: head-to-head comparison of four patient-reported outcome measures (EORTC QLQ-C15-PAL, FACT-Pal, FACT-Pal-14, FACT-G7). Support Care Cancer. 2020;28(1):141-153. doi: 10.1007/s00520-019-04754-9 [DOI] [PubMed] [Google Scholar]
- 42.Hays RD, Spritzer KL, Schalet BD, Cella D. PROMIS-29 v2.0 profile physical and mental health summary scores. Qual Life Res. 2018;27(7):1885-1891. doi: 10.1007/s11136-018-1842-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dewitt B, Jalal H, Hanmer J. Computing PROPr utility scores for PROMIS profile instruments. Value Health. 2020;23(3):370-378. doi: 10.1016/j.jval.2019.09.2752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spritzer KL, Hays RD. Calculating physical and mental health summary scores for PROMIS-29 v2.0 and v2.1. HealthMeasures.net. 2018. Accessed September 13, 2022. https://drhays.dgsom.ucla.edu/files/view/docs/programs-utilities/prom29/PROMIS29_Scoring_08082018.pdf
- 45.PROPr/Generic MAUT code 2017_09_02.R. 2017. Accessed September 13, 2022. https://github.com/janelhanmer/PROPr/blob/master/Generic%20MAUT%20code%202017_09_02.R
- 46.Kavalieratos D, Corbelli J, Zhang D, et al. Association between palliative care and patient and caregiver outcomes: a systematic review and meta-analysis. JAMA. 2016;316(20):2104-2114. doi: 10.1001/jama.2016.16840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.El-Jawahri A, LeBlanc TW, Temel JS. Integrated palliative and oncology care for patients with acute myeloid leukemia—moving from evidence to practice: reply. JAMA Oncol. 2021;7(6):943-944. doi: 10.1001/jamaoncol.2021.0709 [DOI] [PubMed] [Google Scholar]
- 48.Yefimova M, Aslakson RA, Yang L, et al. Palliative care and end-of-life outcomes following high-risk surgery. JAMA Surg. 2020;155(2):138-146. doi: 10.1001/jamasurg.2019.5083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lupu D, Salsberg E, Quigley L, Wu X. The 2015 class of hospice and palliative medicine fellows—from training to practice: implications for HPM workforce supply. J Pain Symptom Manage. 2017;53(5):944-951. doi: 10.1016/j.jpainsymman.2017.01.005 [DOI] [PubMed] [Google Scholar]
- 50.Lupu D; American Academy of Hospice and Palliative Medicine Workforce Task Force . Estimate of current hospice and palliative medicine physician workforce shortage. J Pain Symptom Manage. 2010;40(6):899-911. doi: 10.1016/j.jpainsymman.2010.07.004 [DOI] [PubMed] [Google Scholar]
- 51.Conroy T, Castan F, Lopez A, et al. ; Canadian Cancer Trials Group and the Unicancer-GI–PRODIGE Group . Five-year outcomes of FOLFIRINOX vs gemcitabine as adjuvant therapy for pancreatic cancer: a randomized clinical trial. JAMA Oncol. 2022;8(11):1571-1578. doi: 10.1001/jamaoncol.2022.3829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Conroy T, Hammel P, Hebbar M, et al. ; Canadian Cancer Trials Group and the Unicancer-GI–PRODIGE Group . FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379(25):2395-2406. doi: 10.1056/NEJMoa1809775 [DOI] [PubMed] [Google Scholar]
- 53.Bakitas M, Stevens M, Ahles T, et al. ; Project Enable Co-Investigators . Project ENABLE: a palliative care demonstration project for advanced cancer patients in three settings. J Palliat Med. 2004;7(2):363-372. doi: 10.1089/109662104773709530 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix. Patient Questionnaire
Data Sharing Statement