Key Points
Question
Are preterm birth, socioeconomic status (SES), and neonatal brain structure associated?
Findings
In this cohort study of 261 infants, after mutual adjustment, both low birth gestational age (GA) and SES were associated with brain structure. The nature of SES–brain structure associations varied depending how SES was operationalized; there were interactions between GA and measures of family-level SES on brain structure.
Meaning
In this study, low birth GA, and to a lesser extent SES, were associated with neonatal brain structure; further work is required to elucidate potential mechanisms underlying this association.
This cohort study investigates associations of gestational age at birth and socioeconomic status with neonatal brain morphology in a cohort of preterm and full-term infants in the United Kingdom.
Abstract
Importance
Preterm birth and socioeconomic status (SES) are associated with brain structure in childhood, but the relative contributions of each during the neonatal period are unknown.
Objective
To investigate associations of birth gestational age (GA) and SES with neonatal brain morphology by testing 3 hypotheses: GA and SES are associated with brain morphology; associations between SES and brain morphology vary with GA; and associations between SES and brain structure and morphology depend on how SES is operationalized.
Design, Setting, and Participants
This cohort study recruited participants from November 2016 to September 2021 at a single center in the United Kingdom. Participants were 170 extremely and very preterm infants and 91 full-term or near-term infants. Exclusion criteria were major congenital malformation, chromosomal abnormality, congenital infection, cystic periventricular leukomalacia, hemorrhagic parenchymal infarction, and posthemorrhagic ventricular dilatation.
Exposures
Birth GA and SES, operationalized at the neighborhood level (using the Scottish Index of Multiple Deprivation), the family level (using parental education and occupation), and subjectively (World Health Organization Quality of Life measure).
Main Outcomes and Measures
Brain volume (85 parcels) and 5 whole-brain cortical morphology measures (gyrification index, thickness, sulcal depth, curvature, surface area) at term-equivalent age (median [range] age, 40 weeks, 5 days [36 weeks, 2 days to 45 weeks, 6 days] and 42 weeks [38 weeks, 2 days to 46 weeks, 1 day] for preterm and full-term infants, respectively).
Results
Participants were 170 extremely and very preterm infants (95 [55.9%] male; 4 of 166 [2.4%] Asian, 145 of 166 [87.3%] White) and 91 full-term or near-term infants (50 [54.9%] male; 3 of 86 [3.5%] Asian, 78 of 86 [90.7%] White infants) with median (range) birth GAs of 30 weeks, 0 days (22 weeks, 1 day, to 32 weeks, 6 days) and 39 weeks, 4 days (36 weeks, 3 days, to 42 weeks, 1 day), respectively. In fully adjusted models, birth GA was associated with a higher proportion of brain volumes (27 of 85 parcels [31.8%]; β range, −0.20 to 0.24) than neighborhood-level SES (1 of 85 parcels [1.2%]; β = 0.17 [95% CI, −0.16 to 0.50]) or family-level SES (maternal education: 4 of 85 parcels [4.7%]; β range, 0.09 to 0.15; maternal occupation: 1 of 85 parcels [1.2%]; β = 0.06 [95% CI, 0.02 to 0.11] respectively). There were interactions between GA and both family-level and subjective SES measures on regional brain volumes. Birth GA was associated with cortical surface area (β = 0.10 [95% CI, 0.02 to 0.18]) and gyrification index (β = 0.16 [95% CI, 0.07 to 0.25]); no SES measure was associated with cortical measures.
Conclusions and Relevance
In this cohort study of UK infants, birth GA and SES were associated with neonatal brain morphology, but low GA had more widely distributed associations with neonatal brain structure than SES. Further work is warranted to elucidate the mechanisms underlying the association of both GA and SES with early brain development.
Introduction
Preterm birth, defined as birth at less than 37 weeks of gestation, affects 11% of births globally and is a leading cause of atypical brain development, underpinning long-term motor, cognitive, and behavioral problems.1,2 There is a dose-dependent association between low gestational age (GA) and increased likelihood of difficulties, with those born extremely (<28 weeks) and very (28 to 32 weeks) preterm being at greatest risk for cerebral palsy, cognitive impairment, lower educational attainment, visual or hearing impairment, attention-deficit/hyperactivity disorder, autism spectrum disorder, and mental health diagnoses across the life course.2,3,4
Preterm birth is associated with structural brain changes apparent by term-equivalent age, including global and regional tissue volume reduction, altered cortical configuration, and enlargement of cerebrospinal fluid (CSF) spaces, although smaller tissue volumes are not inevitable.5,6,7,8 Changes in neonatal morphology are associated with later functional impairment, highlighting the importance of elucidating factors contributing to early brain development.9,10,11,12
In the general population, brain structure, cognition, educational attainment, and adult income are patterned by socioeconomic status (SES) in childhood.13 Children living in poverty are more likely to experience difficulties with memory, language, self-regulation, and socioemotional processing and are more likely to receive behavioral and mental health diagnoses.14,15,16,17,18,19,20,21 Indeed, mediation analyses suggest a causal pathway between SES and cognitive development in childhood via changes in brain anatomy and function.13,22,23,24,25 SES is inherently a multifaceted construct and can be operationalized using neighborhood-level, family-level, or subjective measures.14,21 These capture different phenomena, and the extent to which they correlate with one another depends on setting and population. Understanding the relative associations of socioeconomic disadvantage, operationalized in different ways, and low GA with neonatal brain development is important for designing rational therapies and support strategies for children born preterm.
To investigate associations between birth GA and SES with neonatal brain development, we combined high-resolution brain magnetic resonance imaging (MRI) from infants born extremely preterm, very preterm, and at full term, with neighborhood-level, family-level, and subjective SES measures. We investigated associations of GA and SES with brain structure by testing hypotheses that (1) GA and SES are associated with neonatal brain structure in mutually adjusted models; (2) associations between SES and brain structure vary with GA at birth; and (3) associations between SES and brain morphology depend on how SES is operationalized.
Methods
Study Participants
Participants were extremely or very preterm infants (birth <33 weeks’ gestation [n = 170]) and full-term or near-term control infants (n = 91), recruited to a longitudinal study investigating the association of preterm birth with brain development and outcomes26 (eFigure 1 in Supplement 1). The cohort is focused on very and extremely preterm infants due to their increased likelihood of atypical brain development.2,4
Exclusion criteria were major congenital malformation, chromosomal abnormality, congenital infection, cystic periventricular leukomalacia, hemorrhagic parenchymal infarction, and posthemorrhagic ventricular dilatation. We excluded infants with significant parenchymal brain injury to be representative of most survivors of modern intensive care practices.27
Recruitment was conducted at the Royal Infirmary of Edinburgh, United Kingdom, between November 2016 and September 2021. Ethical approval was obtained from the UK National Research Ethics Service, and parents provided written consent. As per COVID-19 policies, recruitment and MRI scans were paused from March to June 2020. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Demographic Variables
We assigned preterm infants into 4 categories with similar numbers of participants based on birth GA: 22 weeks, 0 days, to 26 weeks, 6 days; 27 weeks, 0 days, to 28 weeks, 6 days; 29 weeks, 0 days, to 30 weeks, 6 days; and 31 weeks, 0 days, to 32 weeks, 6 days, of gestation. SES was measured in 3 ways: neighborhood-level, defined by the Scottish Index of Multiple Deprivation 2016 (SIMD), derived from the family’s postal code at birth28; family-level, defined as parental education (highest educational qualification and age leaving education) and parental occupation (current or most recent job); and subjective SES provided by parental self-report using the environment domain of the World Health Organization Quality of Life (WHO QoL) assessment.29 SIMD rank was chosen as the primary measure of SES because it correlates with child development30 and is a tractable tool for policy makers.28 Other SES measures were investigated in exploratory analyses, as specified in our preregistered statistical plan31: maternal and paternal education, maternal and paternal occupation, and subjective SES (eMethods in Supplement 1). Ethnicity data were collected, as there are relationships between ethnicity and SES.32 Ethnicity (Asian, Black, mixed ethnicity, White, and other ethnic group [Arab, Iraqi, Bulgarian/Turkish, and Fijian]) was determined by self-report through questionnaire (eMethods in Supplement 1).
Selection of Covariates
Covariate selection was based on associations with brain structure in prior research: birth weight z score,33,34 birth head circumference z score,35 age at MRI, infant sex,36 smoking in pregnancy,37,38 and any breast milk at discharge.39,40 Definitions appear in the eMethods in Supplement 1, and the conceptual model appears in eFigure 2 in Supplement 1.
MRI Data Acquisition
MRI scans were performed at term-corrected gestation according to a published protocol.26 In summary, a MAGNETOM Prisma 3T MRI clinical scanner (Siemens Healthcare) and 16-channel phased-array pediatric head receive coil were used to acquire a 3-dimensional T1-weighted magnetization-prepared rapid acquisition with gradient echo structural volume scan (voxel size, 1 mm isotropic); and a 3-dimensional T2-weighted (T2w) sampling scheme with application-optimized contrasts using flip angle evolution structural scan (voxel size, 1 mm isotropic).
Infants were fed, wrapped, and slept naturally. Flexible earplugs and neonatal earmuffs (MiniMuffs) were used for acoustic protection. Infants were monitored throughout, and scans were supervised by a doctor or nurse trained in neonatal resuscitation.
MRI Data Analysis
Structural images were reported by a radiologist with experience in neonatal MRI (A.J.Q.). The developing Human Connectome Project (dHCP) minimal processing pipeline for neonatal data41 was used to preprocess T2w images, allowing surface reconstruction from tissue segmentation. We obtained bias field–corrected T2w, brain masks, tissue segmentation, label parcellation, and surface reconstruction. We then calculated tissue volumes, gyrification index (GI), cortical thickness, sulcal depth, cortical curvature, and cortical surface area (SA).41 Visual inspection and quality control were performed by experienced neuroscientists (M.B.-C. and K.V.).
Selection of Image Features
We included 85 individual regional brain parcels (including white matter, gray matter, and CSF spaces) and 5 whole-cortex measures (GI, thickness, sulcal depth, curvature, and SA), as defined by the dHCP.41 This was due to previous literature providing evidence for regional volumetric changes in association with SES in childhood.31,42,43,44,45,46,47,48,49 Based on reports of SES–brain structure correlations when the brain is characterized using larger parcellations, we analyzed associations of GA and SES with whole brain volume, lobar regional volumes, and lobar cortical measures.
Statistical Analysis
Statistical analyses were preregistered31 and conducted in R version 4.2.1 (R Project for Statistical Computing). We compared demographic data and SES measures across preterm and full-term groups. We compared categorical variables using χ2 tests (significance threshold, P < .05). For continuous variables, we used Mann-Whitney U tests. We assessed statistical relationships between SES measures using Spearman correlation, with strength of correlation classified as very weak (r = 0-0.19), weak (r = 0.20-0.39), moderate (r = 0.40-0.59), strong (r = 0.60-0.79), or very strong (r = 0.80-1).
Because the sample included twins (25 pairs) and siblings (5 groups), which violates the assumption of nonindependence among data points, we repeated analyses after random removal of all but 1 sibling or twin per family. To investigate associations between SES and birth GA with regional brain volumes and cortical measures, we developed regression models. Model 1 was a baseline linear regression model, including GA at birth, SES (neighborhood-level, family-level, or subjective), gestation at MRI, and a product interaction term (GA at birth × SES, if significant). Model 2 was a linear ridge regression model50 including all covariates: GA at birth, SES (neighborhood-level, family-level, or subjective), gestation at MRI, the product interaction term (if significant), birth weight z score, birth head circumference z score, sex, smoking in pregnancy, and breast milk at discharge. Ridge regression aims to partially mitigate potential multicollinearity among factors. We ran 85 regression models testing the association between each SES measure and GA and each regional brain volume, and 5 additional regression models testing the association between each SES measure and GA and each whole-brain cortical measure. Results are reported as standardized β values, classified as small (β < 0.20), medium (β = 0.20-0.49), and large (β ≥ 0.50), with 95% CIs. To correct for multiple comparisons for each measure, we used Benjamini-Hochberg correction51 across the 85 parcels for each SES measure at adjusted and unadjusted levels separately, with the threshold of statistical significance set at P < .05. We compared the frequency of brain volume associations with GA and SES variables using McNemar tests.
As described in the statistical analysis plan,31 SIMD was the primary SES measure in analyses, and maternal and paternal education, maternal and paternal occupation, and subjective SES were investigated in exploratory analyses. For these, the same statistical thresholds used in the primary analyses were applied.
Results
Participant Characteristics
Participants were 170 extremely and very preterm (95 [55.9%] male; 4 of 166 [2.4%] Asian and 145 of 166 [87.3%] White) and 91 full-term or near-term (50 [54.9%] male; 3 of 86 [3.5%] Asian, 76 of 86 [90.7%] White) infants, with median (range) birth GAs of 30 weeks, 0 days (22 weeks, 1 day, to 32 weeks, 6 days) and 39 weeks, 4 days (36 weeks, 3 days, to 42 weeks, 1 day), respectively; their demographic characteristics are shown in Table 1. There was no difference in sex distribution across groups. Ethnicity did not differ between groups and is representative of Edinburgh.52 All SES measures, smoking prevalence, and multiple pregnancy differed between groups, and the SES measure spread is representative of Edinburgh.52,53
Table 1. Participant Characteristics.
| Characteristic | Participants, No./total No. (%)a | P valueb | |
|---|---|---|---|
| Preterm (n = 170) | Term (n = 91) | ||
| Gestational age at birth, median (range), wk + d | 30 + 0 (22 + 1 to 32 + 6) | 39 + 4 (36 + 3 to 42 + 1) | <.001 |
| Gestational age at MRI, median (range), wk + d | 40 + 5 (36 + 2 to 45 + 6) | 42 + 0 (38 + 2 to 46 + 1) | <.001 |
| Birth weight, median (range), g | 1315 (370 to 2510) | 3460 (2410 to 4560) | <.001 |
| Birthweight z score, median (range) | 0.13 (−3.13 to 2.14) | 0.45 (−2.30 to 2.57) | <.001 |
| Head circumference, median (range), cmc | 27.5 (17.5 to 33.8) | 35 (32 to 39) | <.001 |
| Head circumference z score, median (range)c | −0.12 (−3.13 to 5.31) | 0.99 (−1.54 to 3.73) | <.001 |
| Sex | |||
| Male | 95/170 (55.9) | 50/91 (54.9) | .21 |
| Female | 75/170 (44.1) | 41/91 (45.1) | |
| Maternal smoking during pregnancy | 30/167 (18.0) | 3/91 (3.3) | <.001 |
| Multiple pregnancy | 51/170 (30.0) | 2/91 (2.2) | <.001 |
| Any breast milk at discharge | 122/167 (73.1) | 85/91 (93.4) | <.001 |
| Child ethnicity | |||
| Asian | 4/166 (2.4) | 3/86 (3.5) | .22 |
| Black | 2/166 (1.2) | 0/86 | |
| Mixed ethnicity | 9/166 (5.4) | 5/86 (5.8) | |
| White | 145/166 (87.3) | 76/86 (90.7) | |
| Other ethnic groupd | 5/166 (3.0) | 0/86 | |
| SIMD rank, median (range) | 3913 (6-6929) | 5502 (727-6967) | <.001 |
| Mother age leaving education, median (range), ye | 20 (14-33) | 23 (16-36) | <.001 |
| Father age leaving education, median (range), yf | 18 (14-34) | 22 (16-36) | <.001 |
| Mother highest educational qualification | |||
| None | 5/165 (3.0) | 0/91 | <.001 |
| Basic high school qualification | 18/165 (10.9) | 5/91 (5.5) | |
| Advanced high school qualification | 13/165 (7.9) | 2/91 (2.2) | |
| College qualification | 42/165 (25.5) | 9/91 (9.9) | |
| University undergraduate degree | 44/165 (26.7) | 40/91 (44.0) | |
| University postgraduate degree | 34/165 (20.6) | 35/91 (38.5) | |
| Not applicable | 9/165 (5.5) | 0/91 | |
| Father highest educational qualification | |||
| None | 4/148 (2.7) | 0/90 | <.001 |
| Basic high school qualification | 33/148 (22.3) | 7/90 (7.8) | |
| Advanced high school qualification | 13/148 (8.8) | 5/90 (5.6) | |
| College qualification | 33/148 (22.3) | 12/90 (13.3) | |
| University undergraduate degree | 33/148 (22.3) | 41/90 (45.6) | |
| University postgraduate degree | 22/148 (14.9) | 25/90 (27.8) | |
| Not applicable | 10/148 (6.8) | 0/90 | |
| Mother current or recent job | |||
| Professional | 75/166 (45.2) | 76/91 (83.5) | <.001 |
| Nonmanual skilled | 32/166 (19.3) | 6/91 (6.6) | |
| Manual skilled | 16/166 (9.6) | 1/91 (1.1) | |
| Partly skilled | 8/166 (4.8) | 4/91 (4.4) | |
| Unskilled | 14/166 (8.4) | 2/91 (2.2) | |
| Unemployed | 3/166 (1.8) | 1/91 (1.1) | |
| Homemaker | 10/166 (6.0) | 0/91 | |
| Still in full-time education | 7/166 (4.2) | 1/91 (1.1) | |
| Father current or recent job | |||
| Professional | 59/157 (37.6) | 63/91 (69.2) | <.001 |
| Nonmanual skilled | 23/157 (14.6) | 6/91 (6.6) | |
| Manual skilled | 37/157 (23.6) | 7/91 (7.7) | |
| Partly skilled | 22/157 (14.0) | 6/91 (6.6) | |
| Unskilled | 6/157 (3.8) | 3/91 (3.3) | |
| Unemployed | 3/157 (1.9) | 0/91 | |
| Homemaker | 3/157 (1.9) | 0/91 | |
| Still in full time education | 3/157 (1.9) | 6/91 (6.6) | |
| WHO QoL environment score, median (range) | 75.0 (21.9 to 100.0) | 84.4 (40.6 to 100.0) | <.001 |
Abbreviations: MRI, magnetic resonance imaging; SIMD, Scottish Index of Multiple Deprivation; WHO QoL, World Health Organization Quality of Life assessment.
Case definitions are available in eMethods in Supplement 1.
For binary data, the χ2 test was used for P values. For continuous data, Mann-Whitney U test was used.
For head circumference and head circumference z score, there were data for 157 preterm infants and 83 full-term infants.
This included Arab, Iraqi, Bulgarian/Turkish, and Fijian.
For maternal age leaving education, data were available for 158 preterm and 85 full-term infants.
For partner age leaving education, data were available for 139 preterm and 79 full-term infants.
There were no significant demographic differences between included and excluded infants (eTable 1 in Supplement 1). The major exposures and comorbidities of the preterm group are shown in eTable 2 in Supplement 1. Removal of all but 1 sibling per family from the sample and rerunning analyses yielded a similar pattern of results, so the whole sample is included in reported analyses.
Associations Between Regional Brain Volumes, Prematurity, and Neighborhood Deprivation
Birth GA was associated with more regional brain volumes than SIMD (McNemar test comparing frequency of associations: P < .001) (Table 2; eTable 3 in Supplement 1). After Benjamini-Hochberg correction, GA correlated with the volume of 22 of 85 parcels (26%) (range: subthalamic nucleus left, β = −0.13 [95% CI, −0.23 to −0.03] to medial and inferior temporal gyri anterior part right gray matter, β = 0.22 [95% CI, 0.16 to 0.29]) compared with 1 of 85 (1.2%) for SIMD (medial and inferior temporal gyri anterior part right white matter: β = 0.17 [95% CI, −0.16 to 0.50]). GA-associated parcels were within gray and white matter, often bilaterally, and predominantly had a positive association (17 of 19 tissue [non-CSF] regions [89.5%]) meaning that higher GA at birth was associated with increased tissue volume. There were negative associations between GA and volumes of CSF spaces (bilateral lateral ventricles and extracerebral CSF). There were no interactions between GA and SIMD for any brain region.
Table 2. Regional Brain Volumes With Significant Associations With Either Gestational Age or the SIMDa.
| Regional volume | Standardized β coefficient (95% CI) | P value | |
|---|---|---|---|
| Raw | BH corrected | ||
| Gestational age | |||
| Anterior temporal lobe lateral part left gray matter | 0.14 (0.06 to 0.23) | <.001 | .003 |
| Caudate nucleus left | 0.16 (0.07 to 0.25) | <.001 | .001 |
| Caudate nucleus right | 0.13 (0.04 to 0.22) | <.001 | .01 |
| Cerebrospinal fluid | −0.11 (−0.21 to −0.005) | <.001 | <.001 |
| Cingulate gyrus posterior part right gray matter | −0.12 (−0.21 to −0.02) | .01 | .047 |
| Frontal lobe left white matter | 0.20 (0.12 to 0.28) | <.001 | <.001 |
| Frontal lobe right white matter | 0.16 (0.08 to 0.25) | <.001 | <.001 |
| Gyri parahippocampalis et ambiens anterior part left white matter | 0.16 (0.06 to 0.26) | <.001 | .001 |
| Gyri parahippocampalis et ambiens posterior part right white matter | 0.14 (0.04 to 0.24) | <.001 | .01 |
| Insula left white matter | 0.17 (0.08 to 0.26) | <.001 | <.001 |
| Insula right white matter | 0.16 (0.08 to 0.25) | <.001 | .001 |
| Lateral occipitotemporal gyrus gyrus fusiformis anterior part left white matter | 0.20 (0.12 to 0.29) | <.001 | <.001 |
| Lateral occipitotemporal gyrus gyrus fusiformis anterior part right gray matter | 0.15 (0.07 to 0.24) | <.001 | .001 |
| Lateral ventricle left | −0.12 (−0.23 to −0.02) | <.001 | .001 |
| Lateral ventricle right | −0.10 (−0.20 to 0.004) | <.001 | .002 |
| Medial and inferior temporal gyri anterior part left gray matter | 0.22 (0.15 to 0.29) | <.001 | <.001 |
| Medial and inferior temporal gyri anterior part right gray matter | 0.22 (0.16 to 0.29) | <.001 | <.001 |
| Medial and inferior temporal gyri posterior part left gray matter | 0.11 (0.05 to 0.18) | <.001 | .005 |
| Medial and inferior temporal gyri posterior part right gray matter | 0.14 (0.07 to 0.20) | <.001 | .001 |
| Parietal lobe right gray matter | 0.09 (0.03 to 0.15) | .001 | .01 |
| Subthalamic nucleus left | −0.13 (−0.23 to −0.03) | .003 | .03 |
| Superior temporal gyrus middle part right gray matter | 0.11 (0.0 to 0.18) | .001 | .01 |
| SIMD | |||
| Medial and inferior temporal gyri anterior part right white matter | 0.17 (−0.16 to 0.50) | .004 | .03 |
Abbreviations: BH, Benjamini Hochberg correction; SIMD, Scottish Index of Multiple Deprivation.
Fully adjusted ridge regression model, including gestation at birth, SIMD, gestation at magnetic resonance imaging scan, the interaction term (when significant), birth weight z score, birth head circumference z score, sex, smoking in pregnancy, and breast milk at discharge. Corrected for false discovery rate. Results for all parcels are available in eTable 3 in Supplement 1.
Fully adjusted models showed a similar profile of results to baseline models (eTable 4 in Supplement 1), with GA-parcel volume associations more widely distributed than SIMD-parcel volume associations. The number of associations was higher for GA than SIMD: 50 of 85 (58.8%) and 5 of 85 (5.9%), respectively.
Associations Between Global Cortical Measures, Prematurity, and Neighborhood Deprivation
Birth GA was associated with cortical SA (β = 0.10 [95% CI, 0.02-0.18]; P = .03) and GI (β = 0.16 [95% CI, 0.07-0.25]; P < .001), whereas SIMD was not associated with any measure of cortical morphology (Table 3). There were no interactions between GA and SIMD for any global cortical measure.
Table 3. Association of Cortical Measures With Gestational Age and the SIMDa.
| Cortical measure | Standardized β coefficient (95% CI) | P value | |
|---|---|---|---|
| Raw | BH corrected | ||
| Gestational age | |||
| Mean cortical curvature | 0.01 (−0.07 to 0.09) | .75 | .75 |
| Mean cortical surface area | 0.10 (0.02 to 0.18) | .01 | .03 |
| Mean cortical thickness | −0.03 (−0.12 to 0.06) | .43 | .48 |
| Mean gyrification index | 0.16 (0.07 to 0.25) | <.001 | <.001 |
| Mean sulcal depth | 0.03 (−0.08-0.14) | .04 | .15 |
| SIMD | |||
| Mean cortical curvature | −0.05 (−0.15 to 0.06) | .37 | .46 |
| Mean cortical surface area | 0.05 (−0.05 to 0.16) | .29 | .46 |
| Mean cortical thickness | −0.05 (−0.18 to 0.08) | .36 | .46 |
| Mean gyrification index | 0.07 (−0.05 to 0.19) | .20 | .40 |
| Mean sulcal depth | 0.02 (−0.12 to 0.17) | .19 | .40 |
Abbreviations: BH, Benjamini Hochberg correction; SIMD, Scottish Index of Multiple Deprivation.
Fully adjusted ridge regression model, including gestation at birth, SIMD, gestation at magnetic resonance imaging scan, the interaction term (if applicable), birth weight z score, birth head circumference z score, sex, smoking in pregnancy, and breast milk at discharge. Corrected for false discovery rate.
Fully adjusted models revealed a similar profile of results to the baseline linear regression models. There were GA associations in 2 of 5 cortical measures (40.0%) and no associations with SIMD (eTable 5 in Supplement 1).
Correlations Between Neighborhood-Level, Family-Level, and Subjective SES Measures
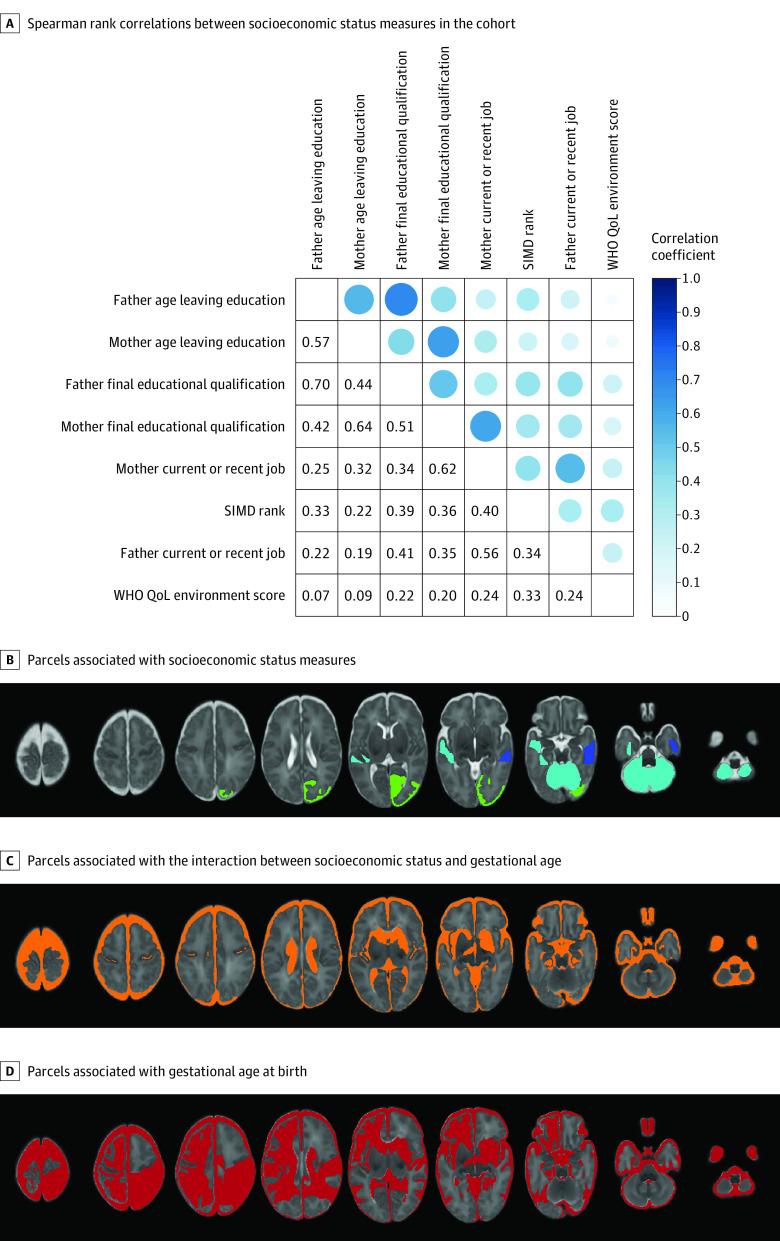
Figure, A shows that correlations between SES measures ranged from very weak (eg, between age of father leaving education and the WHO QoL environment score, r = 0.07) to strong (eg, age of father and mother leaving education, r = 0.70; final education qualification, r = 0.64). Correlations between SIMD rank and parental education and occupation were weak to moderate (r = 0.22-0.40).
Figure. Socioeconomic Status Measures.
A, Coefficients are shown when P < .05. B-D, Panels show all regions and parcels that were significantly associated with respective measures after Benjamini-Hochberg correction in fully adjusted ridge regression models. B, Blue indicates SIMD (1 parcel); turquoise, maternal education (4 parcels); green, maternal occupation (1 parcel). C, Orange indicates socioeconomic status × gestational age interaction (12 parcels). D, Red indicates gestational age at birth (27 parcels).
Associations Between Brain Features and Family-Level and Subjective SES Measures
In exploratory analyses defining SES by parental education and occupation and subjective measures, a similar pattern of results was obtained as with SIMD: a greater proportion of brain volumes were associated with birth GA than SES (McNemar test comparing frequency of associations: P < .001 for all SES measures) (Table 4; eTables 6-10 in Supplement 1; Figure, B-D). However, there were differences in associations between SES and brain structure depending on the SES measure used. Specifically, maternal education was associated with the volume of 4 parcels (left and right cerebella, left middle superior temporal gyrus, and left anterior lateral occipitotemporal gyrus/gyrus fusiformis; β range, 0.09 to 0.15) (eTable 6 in Supplement 1), whereas neighborhood SES (Table 2) and maternal occupation (eTable 8 in Supplement 1) were each associated with 1 parcel volume: right anterior medial and inferior temporal gyri (white matter) (β = 0.17 [95% CI, −0.16 to 0.50], P = .03), and right occipital lobe (gray matter) (β = 0.06 [95% CI, 0.02 to 0.11]; P = .049), respectively. Associations between parcel volumes and family-level SES were positive, meaning that higher family-level SES was associated with increased regional tissue volume.
Table 4. Summary of Associations Between GA and Neighborhood-Level, Family-Level, and Subjective Measures of SES With Brain Featuresa.
| Brain Features | Parcels, No./total No. (%) | ||
|---|---|---|---|
| GA | SES | Interaction effect | |
| Proportion of brain volumes associated with GA and SES | |||
| SIMD | 22/85 (25.9) | 1/85 (1.2) | 0/85 |
| Maternal education | 15/85 (17.6) | 4/85 (4.7) | 5/85 (5.9) |
| Paternal education | 22/85 (25.9) | 0/85 | 7/85 (8.2) |
| Maternal occupation | 12/85 (14.1) | 1/85 (1.2) | 10/85 (11.8) |
| Paternal occupation | 21/85 (24.7) | 0/85 | 5/85 (5.9) |
| Subjective socioeconomic status | 17/85 (20.0) | 0/85 | 4/85 (4.7) |
| Proportion of cortical features associated with GA and SES | |||
| SIMD | 2/5 (40.0) | 0/5 | 0/5 |
| Maternal education | 0/5 | 0/5 | 0/5 |
| Paternal education | 0/5 | 0/5 | 0/5 |
| Maternal occupation | 0/5 | 0/5 | 0/5 |
| Paternal occupation | 0/5 | 0/5 | 0/5 |
| Subjective socioeconomic status | 1/5 (20.0) | 0/5 | 0/5 |
Abbreviations: GA, gestational age; SES, socioeconomic status; SIMD, Scottish Index of Multiple Deprivation.
Results summary from ridge regression models for each socioeconomic status measure, for the 85 regional brain volumes and 5 mean cortical measures. Significance threshold is P < .05 following Benjamini-Hochberg correction. Full results are available in Table 3; eTables 3 and 6 to 15 in Supplement 1.
GA-volume associations for paternal education and occupation were found in 22 of 85 parcels (25.9%) and 21 of 85 parcels (24.7%), respectively. For maternal education and occupation, GA-volume associations were found in 15 of 85 parcels (17.6%) and 12 of 85 parcels (14.1%), respectively. Associations were observed for subjective SES in 17 of 85 parcels (20.0%) (Table 4).
In contrast to SIMD, there were significant interactions between family-level and subjective SES measures and GA for several brain structures (eTables 6-15 in Supplement 1; Figure, C). For family-level and subjective SES measures, the association with SES was smaller in children with higher GA for extracerebral CSF and left and right lateral ventricular volumes (β range, −0.05 to −0.001). The direction of the interaction varied for those involving white and gray tissue parcels, such as left anterior gyri parahippocampalis et ambiens (white matter), left caudate nucleus, and left subthalamic nucleus. For the 5 global cortical measures (eTables 11-15 in Supplement 1), there were no associations with family-level or subjective SES in the fully adjusted model.
Associations Between GA and SES Measures With Whole Brain and Lobar Volumes and Lobar Cortical Measures
GA was associated with whole brain volume (β range, 0.05-0.09), left frontal lobe SA (β range, 0.14-0.19), right frontal lobe volume (β range, 0.10-0.13) and SA (β range, 0.13-0.18), left parietal lobe volume (β range, 0.09-0.11) and SA (β range, 0.14-0.17), right parietal lobe volume (β range, 0.09-0.10) and SA (β range, 0.10-0.12), left temporal lobe volume (β range, 0.10-0.11) and SA (β range 0.11-0.14), right temporal lobe volume (β range, 0.09-0.10) and SA (β range, 0.09-0.13), and left occipital lobe thickness (β = −0.02) (eTables 16-27 in Supplement 1). Maternal education was associated with right frontal lobe sulcal depth (β = 0.14), and paternal occupation was associated with left occipital lobe GI (β = −0.09) (eTables 16-27 in Supplement 1).
There was an interaction between GA and maternal education in association with left and right frontal lobe thickness (β = −0.02). An interaction with GA and maternal occupation was seen with left and right frontal lobe thickness (β = −0.01) and left occipital lobe curvature (β = −0.01) and GI (β = −0.01). There was an interaction between GA and paternal education in association with left frontal lobe thickness (β = −0.01) and between GA and SIMD with right parietal lobe sulcal depth (β = −0.03) (eTables 16-27 in Supplement 1).
Discussion
In a high-income setting, low GA at birth and SES were both associated with regional differences in brain structure that were apparent at the end of neonatal care. Low GA was associated with widely distributed differences in brain structure and cortical morphology, whereas differences associated with SES were less widely distributed. Standardized β values showed small to medium associations between brain structure and low GA and small associations with SES measures. We found that neighborhood-level, family-level, and subjective measures of SES were only weakly to moderately correlated; of these, family-level measures (parental education and occupation) were associated with more differences in brain structure than subjective SES and neighborhood deprivation. Furthermore, there was an interaction between low GA and family-level and subjective SES measures. These results suggest that atypical brain development seen in preterm infants is associated predominantly with GA at birth, but prematurity does not override SES-brain structure patterning, so interventions designed to attenuate family-level socioeconomic disadvantage in the perinatal period54 could promote healthier brain development in preterm infants.
Our results are consistent with studies suggesting that SES may modify the association between preterm birth and neurodevelopmental and educational outcomes, especially in the context of preterm brain injury.55,56,57,58,59 Although few studies have reported the impact of SES on brain morphology of preterm infants, there is some consensus that socioeconomic factors play a role. In a large study of US infants with GA range of 27 to 42 weeks who were scanned between term and age 4 months, higher SES, indexed by parental education, had marginal associations with brain volume in infancy, and paternal education was associated with gray matter volume after accounting for birth weight. However, extremely preterm infants were not represented in this study, and findings could have been confounded by post–neonatal intensive care unit (NICU) exposures.57 In an Australian cohort, a multidimensional measure of social risk was associated with global and regional brain volumes in a group of preterm infants at term-equivalent age, but the strength of association varied by GA category (strongest for late preterm [34-36 weeks of gestation] and full-term infants compared with moderate or very preterm infants [<34 weeks of gestation]) and was diminished in multivariable models adjusting for poor intrauterine growth, multiple birth, and male sex.59 Our results indicate that although low GA was associated with the widest distribution of parcels, SES associations were detectable at the end of NICU care after adjustment for GA at birth, age at MRI, birth weight z score, birth head circumference z score, sex, smoking in pregnancy, and nutrition during NICU care.
The regional volumes associated with low birth GA are in white and gray matter bilaterally, with concurrent increases in extra-axial and lateral ventricular CSF spaces. This pattern is consistent with the cerebral signature of preterm birth, which includes altered cortical morphology, focal white matter volume loss, and reduced deep gray matter volume.5 By using a contemporary atlas,60 we have added anatomic granularity to the preterm brain structural phenotype. In adjusted models, regions associated with SES were right anterior medial and inferior temporal gyri (white matter) (SIMD); right occipital lobe (gray matter) (maternal occupation); left and right cerebella, left middle superior temporal gyrus, and left anterior lateral occipitotemporal gyrus/gyrus fusiformis (maternal education). SES measures have previously shown variable associations in childhood with the cerebellum,25,45,56 occipital lobe,25,61 and temporal lobe,24,25,61 and the middle temporal gyri and occipitotemporal regions specifically have been positively associated with a composite SES of maternal education and occupation.49 The possible functions of these SES-associated regions include memory, visual information processing, speech, motor control, balance, and cognition.62
Most studies examining associations between SES and brain morphology have used individual or family measures of SES (parental education or occupation, or family income)46,56,57,58,61,63,64 or a composite,59,65 and only 1 included neighborhood deprivation.65 We found that family-level SES measures were most closely associated with brain structure. This could be explained by family-level measures capturing shared genetic determinants of brain anatomy and exposures to stress, nutrition, or smoking that influence brain development,38,66,67 which are not fully captured by neighborhood deprivation. Neighborhood-level SES is associated with functional changes in later infancy within this cohort, including preference to view social stimuli68 and emotional regulation and cortisol response,69 suggesting that neighborhood deprivation could have a greater impact after discharge from hospital.
Strengths and Limitations
The study has the following strengths. First, the preterm infants did not have focal parenchymal brain injuries so are representative of most survivors of modern intensive care in high-income countries. Second, we assessed whole brain anatomy and global cortical structure using an open-source age-specific atlas. Third, we adjusted for real and potential variables associated with neonatal brain structure and used ridge regression to mitigate the problem of multicollinearity. Fourth, we explored SES-brain associations with SES operationalized at different levels, showing that family and subjective measures interact with the associations of GA with the brain. Finally, we used a preregistered analysis plan to reduce the risk of false-positive results.
The study also has some limitations. Although the study population was comparable with neonatal populations in high-income, majority-White settings, the results may not be generalizable to settings with different socioeconomic or ethnicity profiles. We studied a range of measures of SES but did not include all that could be relevant, such as household income. Our preterm cohort was designed to assess only those born extremely or very preterm; further work would be required to determine whether the associations we observed are present in moderate to late preterm infants.26 Our choice to study morphology was based on existing literature. We did not investigate possible SES associations with structural or functional connectivity, or other metrics of early brain development such as network complexity,70 brain age,71 voxel-based morphometry,72 tensor-based morphometry,73 or relative brain volumes; future research could investigate whether these techniques uncover additional insight about SES and preterm brain development. SES and preterm birth were correlated (Table 1), which makes the disambiguation of effects challenging. The statistical mitigations we used to address this issue included ridge regression to reduce impacts of colinearity and inclusion of interaction terms (GA × SES) in models. The results imply that GA leads to more widely distributed associations across the brain than SES. However, associations with SES might be underestimated, for example, if atypical fetal brain development begins in utero, before the event of preterm birth, for some women living in deprived circumstances. Prospective fetal studies using MRI and/or large-scale epidemiological studies that include neurobehavioral outcomes could provide additional information about the role of social determinants on the outcomes of children born preterm. A larger sample would be required to investigate whether comorbidities of preterm birth, such as sepsis, necrotizing enterocolitis or bronchopulmonary dysplasia, modify the brain-SES associations we observed.
We cannot comment on whether SES patterning of brain structure is dynamic through childhood after preterm birth. The consensus for associations between SES and brain structure in older children and adolescents is much greater47,74 than it is for neonates.46,56,57,58,59,61,63,64,65 By inference, SES associations, which were relatively modest in neonates in comparison with low GA, might accumulate through childhood. Of note, functional outcome data from preterm infants suggest the importance of SES increases after the age of 5 years while the importance of birth events as determinants diminishes.75 To address this question, longitudinal imaging and neurodevelopmental assessment of participants is planned.26
Conclusions
In comparison with socioeconomic factors, low birth GA was associated with more widely distributed measures of brain structure in preterm infants at term-equivalent age. However, family-level SES measures of parental education and occupation were associated with neonatal brain development, and they interacted with low GA. This suggests that strategies designed to mitigate the adverse effects of family-level disadvantage during neonatal intensive care could improve the brain development of preterm infants. Further research is warranted to understand the biological mechanisms that underlie associations of preterm birth and level-specific social disadvantage with brain development.
eMethods. Case Definitions and Sources
eFigure 1. Flow Diagram of Participants
eFigure 2. Diagram of Variables and Covariates in Models
eTable 1. Comparison of Included and Excluded Participants
eTable 2. Clinical Features of the Preterm Sample
eTable 3. Regional Brain Volumes: Association With Gestational Age, the Scottish Index of Multiple Deprivation, and Interaction Effect (Fully Adjusted Ridge Regression Model)
eTable 4. Regional Brain Volumes: Association With Gestational Age, the Scottish Index of Multiple Deprivation, and Interaction Effect (Baseline Unadjusted Linear Regression Model)
eTable 5. Cortical Measures: Association With Gestational Age and the Scottish Index of Multiple Deprivation (Baseline Unadjusted Linear Regression Model)
eTable 6. Regional Brain Volumes: Association With Gestational Age, Maternal Final Educational Qualification, and Interaction Effect (Fully Adjusted Ridge Regression Model)
eTable 7. Regional Brain Volumes: Association With Gestational Age, Paternal Final Educational Qualification, and Interaction Effect (Fully Adjusted Ridge Regression Model)
eTable 8. Regional Brain Volumes: Association With Gestational Age, Maternal Occupation, and Interaction Effect (Fully Adjusted Ridge Regression Model)
eTable 9. Regional Brain Volumes: Association With Gestational Age, Paternal Occupation, and Interaction Effect (Fully Adjusted Ridge Regression Model)
eTable 10. Regional Brain Volumes: Association With Gestational Age, Subjective Socioeconomic Status, and Interaction Effect (Fully Adjusted Ridge Regression Model)
eTable 11. Cortical Measures: Association With Gestational Age and Maternal Final Educational Qualification (Fully Adjusted Ridge Regression Model)
eTable 12. Cortical Measures: Association With Gestational Age and Paternal Final Educational Qualification (Fully Adjusted Ridge Regression Model)
eTable 13. Cortical Measures: Association With Gestational Age, Maternal Occupation, and Interaction Effect (Fully Adjusted Ridge Regression Model)
eTable 14. Cortical Measures: Association With Gestational Age and Paternal Occupation (Fully Adjusted Ridge Regression Model)
eTable 15. Cortical Measures: Association With Gestational Age, Subjective Socioeconomic Status, and Interaction Effect (Fully Adjusted Ridge Regression Model)
eTable 16. Lobar Volumes: Association With Gestational Age and the Scottish Index of Multiple Deprivation (Fully Adjusted Ridge Regression Models)
eTable 17. Lobar Volumes: Association With Gestational Age and Maternal Education (Fully Adjusted Ridge Regression Models)
eTable 18. Lobar Volumes: Association With Gestational Age and Paternal Education (Fully Adjusted Ridge Regression Models)
eTable 19. Lobar Volumes: Association With Gestational Age and Maternal Occupation (Fully Adjusted Ridge Regression Models)
eTable 20. Lobar Volumes: Association With Gestational Age and Paternal Occupation (Fully Adjusted Ridge Regression Models)
eTable 21. Lobar Volumes: Association With Gestational Age and Subjective Socioeconomic Status (Fully Adjusted Ridge Regression Models)
eTable 22. Cortical Measures: Association With Gestational Age, the Scottish Index of Multiple Deprivation, and Interaction Effect (Fully Adjusted Ridge Regression Models)
eTable 23. Cortical Measures: Association With Gestational Age, Maternal Education, and Interaction Effect (Fully Adjusted Ridge Regression Models)
eTable 24. Cortical Measures: Association With Gestational Age, Paternal Education, and Interaction Effect (Fully Adjusted Ridge Regression Models)
eTable 25. Cortical Measures: Association With Gestational Age, Maternal Occupation, and Interaction Effect (Fully Adjusted Ridge Regression Models)
eTable 26. Cortical Measures: Association With Gestational Age, Paternal Occupation, and Interaction Effect (Fully Adjusted Ridge Regression Models)
eTable 27. Cortical Measures: Association With Gestational Age, Subjective Socioeconomic Status, and Interaction Effect (Fully Adjusted Ridge Regression Models)
eReferences.
Data Sharing Statement
References
- 1.Chawanpaiboon S, Vogel JP, Moller AB, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. 2019;7(1):e37-e46. doi: 10.1016/S2214-109X(18)30451-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson S, Marlow N. Early and long-term outcome of infants born extremely preterm. Arch Dis Child. 2017;102(1):97-102. doi: 10.1136/archdischild-2015-309581 [DOI] [PubMed] [Google Scholar]
- 3.Agrawal S, Rao SC, Bulsara MK, Patole SK. Prevalence of autism spectrum disorder in preterm infants: a meta-analysis. Pediatrics. 2018;142(3):1-14. doi: 10.1542/peds.2018-0134 [DOI] [PubMed] [Google Scholar]
- 4.Twilhaar ES, Wade RM, de Kieviet JF, van Goudoever JB, van Elburg RM, Oosterlaan J. Cognitive outcomes of children born extremely or very preterm since the 1990s and associated risk factors: a meta-analysis and meta-regression. JAMA Pediatr. 2018;172(4):361-367. doi: 10.1001/jamapediatrics.2017.5323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batalle D, Edwards AD, O’Muircheartaigh J. Annual research review: not just a small adult brain: understanding later neurodevelopment through imaging the neonatal brain. J Child Psychol Psychiatry. 2018;59(4):350-371. doi: 10.1111/jcpp.12838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boardman JP, Counsell SJ, Rueckert D, et al. Early growth in brain volume is preserved in the majority of preterm infants. Ann Neurol. 2007;62(2):185-192. doi: 10.1002/ana.21171 [DOI] [PubMed] [Google Scholar]
- 7.Romberg J, Wilke M, Allgaier C, et al. MRI-based brain volumes of preterm infants at term: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2022;107(5):520-526. doi: 10.1136/archdischild-2021-322846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dimitrova R, Pietsch M, Ciarrusta J, et al. Preterm birth alters the development of cortical microstructure and morphology at term-equivalent age. Neuroimage. 2021;243:118488. doi: 10.1016/j.neuroimage.2021.118488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson DK, Matthews LG, Alexander B, et al. Tracking regional brain growth up to age 13 in children born term and very preterm. Nat Commun. 2020;11(1):696. doi: 10.1038/s41467-020-14334-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boardman JP, Craven C, Valappil S, et al. A common neonatal image phenotype predicts adverse neurodevelopmental outcome in children born preterm. Neuroimage. 2010;52(2):409-414. doi: 10.1016/j.neuroimage.2010.04.261 [DOI] [PubMed] [Google Scholar]
- 11.Dubois J, Benders M, Borradori-Tolsa C, et al. Primary cortical folding in the human newborn: an early marker of later functional development. Brain. 2008;131(Pt 8):2028-2041. doi: 10.1093/brain/awn137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kapellou O, Counsell SJ, Kennea N, et al. Abnormal cortical development after premature birth shown by altered allometric scaling of brain growth. PLoS Med. 2006;3(8):e265. doi: 10.1371/journal.pmed.0030265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farah MJ. The neuroscience of socioeconomic status: correlates, causes, and consequences. Neuron. 2017;96(1):56-71. doi: 10.1016/j.neuron.2017.08.034 [DOI] [PubMed] [Google Scholar]
- 14.Johnson SB, Riis JL, Noble KG. State of the art review: poverty and the developing brain. Pediatrics. 2016;137(4):e20153075. doi: 10.1542/peds.2015-3075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Stumm S, Plomin R. Socioeconomic status and the growth of intelligence from infancy through adolescence. Intelligence. 2015;48:30-36. doi: 10.1016/j.intell.2014.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farah MJ, Shera DM, Savage JH, et al. Childhood poverty: specific associations with neurocognitive development. Brain Res. 2006;1110(1):166-174. doi: 10.1016/j.brainres.2006.06.072 [DOI] [PubMed] [Google Scholar]
- 17.Noble KG, Norman MF, Farah MJ. Neurocognitive correlates of socioeconomic status in kindergarten children. Dev Sci. 2005;8(1):74-87. doi: 10.1111/j.1467-7687.2005.00394.x [DOI] [PubMed] [Google Scholar]
- 18.McLoyd VC. Socioeconomic disadvantage and child development. Am Psychol. 1998;53(2):185-204. doi: 10.1037/0003-066X.53.2.185 [DOI] [PubMed] [Google Scholar]
- 19.Duncan GJ, Yeung WJ, Brooks-Gunn J, Smith JR. How much does childhood poverty affect the life chances of children? Am Sociol Rev. 1998;63(3):406-423. doi: 10.2307/2657556 [DOI] [Google Scholar]
- 20.Duncan GJ, Ziol-Guest KM, Kalil A. Early-childhood poverty and adult attainment, behavior, and health. Child Dev. 2010;81(1):306-325. doi: 10.1111/j.1467-8624.2009.01396.x [DOI] [PubMed] [Google Scholar]
- 21.Pillas D, Marmot M, Naicker K, Goldblatt P, Morrison J, Pikhart H. Social inequalities in early childhood health and development: a European-wide systematic review. Pediatr Res. 2014;76(5):418-424. doi: 10.1038/pr.2014.122 [DOI] [PubMed] [Google Scholar]
- 22.Noble KG, Engelhardt LE, Brito NH, et al. ; PASS Network . Socioeconomic disparities in neurocognitive development in the first two years of life. Dev Psychobiol. 2015;57(5):535-551. doi: 10.1002/dev.21303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mackey AP, Finn AS, Leonard JA, et al. Neuroanatomical correlates of the income-achievement gap. Psychol Sci. 2015;26(6):925-933. doi: 10.1177/0956797615572233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hair NL, Hanson JL, Wolfe BL, Pollak SD. Association of child poverty, brain development, and academic achievement. JAMA Pediatr. 2015;169(9):822-829. doi: 10.1001/jamapediatrics.2015.1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gur RE, Moore TM, Rosen AFG, et al. Burden of environmental adversity associated with psychopathology, maturation, and brain behavior parameters in youths. JAMA Psychiatry. 2019;76(9):966-975. doi: 10.1001/jamapsychiatry.2019.0943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boardman JP, Hall J, Thrippleton MJ, et al. Impact of preterm birth on brain development and long-term outcome: protocol for a cohort study in Scotland. BMJ Open. 2020;10(3):e035854. doi: 10.1136/bmjopen-2019-035854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gale C, Statnikov Y, Jawad S, Uthaya SN, Modi N; Brain Injuries expert working group . Neonatal brain injuries in England: population-based incidence derived from routinely recorded clinical data held in the National Neonatal Research Database. Arch Dis Child Fetal Neonatal Ed. 2018;103(4):F301-F306. doi: 10.1136/archdischild-2017-313707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scottish National Statistics . SIMD—Scottish Index of Multiple Deprivation 2016: introductory booklet. Accessed April 25, 2023. https://www.gov.scot/publications/scottish-index-multiple-deprivation-2016/
- 29.World Health Organization. WHOQOL: measuring quality of life. Accessed April 25, 2023. https://www.who.int/tools/whoqol/whoqol-bref
- 30.Minh A, Muhajarine N, Janus M, Brownell M, Guhn M. A review of neighborhood effects and early child development: how, where, and for whom, do neighborhoods matter? Health Place. 2017;46:155-174. doi: 10.1016/j.healthplace.2017.04.012 [DOI] [PubMed] [Google Scholar]
- 31.Mckinnon K, Galdi P, Blesa-Cábez M, et al. The impact of preterm birth and socioeconomic status on neonatal brain morphology. OSF. 2022. Accessed April 25, 2023. https://osf.io/ftv2s [DOI] [PMC free article] [PubMed]
- 32.Cebula C, Evans J. Ethnicity, Poverty and the Data in Scotland. Joseph Rowntree Foundation; 2021. [Google Scholar]
- 33.Miller SL, Huppi PS, Mallard C. The consequences of fetal growth restriction on brain structure and neurodevelopmental outcome. J Physiol. 2016;594(4):807-823. doi: 10.1113/JP271402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson DK, Warfield SK, Carlin JB, et al. Perinatal risk factors altering regional brain structure in the preterm infant. Brain. 2007;130(Pt 3):667-677. doi: 10.1093/brain/awl277 [DOI] [PubMed] [Google Scholar]
- 35.Selvanathan T, Guo T, Kwan E, et al. Head circumference, total cerebral volume and neurodevelopment in preterm neonates. Arch Dis Child Fetal Neonatal Ed. 2022;107(2):181-187. doi: 10.1136/archdischild-2020-321397 [DOI] [PubMed] [Google Scholar]
- 36.Lehtola SJ, Tuulari JJ, Karlsson L, et al. Associations of age and sex with brain volumes and asymmetry in 2-5-week-old infants. Brain Struct Funct. 2019;224(1):501-513. doi: 10.1007/s00429-018-1787-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roustaei Z, Räisänen S, Gissler M, Heinonen S. Associations between maternal age and socioeconomic status with smoking during the second and third trimesters of pregnancy: a register-based study of 932 671 women in Finland from 2000 to 2015. BMJ Open. 2020;10(8):e034839. doi: 10.1136/bmjopen-2019-034839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ekblad M, Korkeila J, Lehtonen L. Smoking during pregnancy affects foetal brain development. Acta Paediatr. 2015;104(1):12-18. doi: 10.1111/apa.12791 [DOI] [PubMed] [Google Scholar]
- 39.Blesa M, Sullivan G, Anblagan D, et al. Early breast milk exposure modifies brain connectivity in preterm infants. Neuroimage. 2019;184:431-439. doi: 10.1016/j.neuroimage.2018.09.045 [DOI] [PubMed] [Google Scholar]
- 40.Sullivan G, Vaher K, Blesa M, et al. Breast milk exposure is associated with cortical maturation in preterm infants. Ann Neurol. 2023;93(3):591-603. doi: 10.1002/ana.26559 [DOI] [PubMed] [Google Scholar]
- 41.Makropoulos A, Robinson EC, Schuh A, et al. The developing human connectome project: a minimal processing pipeline for neonatal cortical surface reconstruction. Neuroimage. 2018;173:88-112. doi: 10.1016/j.neuroimage.2018.01.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noble KG, Houston SM, Brito NH, et al. Family income, parental education and brain structure in children and adolescents. Nat Neurosci. 2015;18(5):773-778. doi: 10.1038/nn.3983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Assari S, Boyce S, Bazargan M, et al. Parental educational attainment, the superior temporal cortical surface area, and reading ability among American children: a test of marginalization-related diminished returns. Children (Basel). 2021;8(5):412. doi: 10.3390/children8050412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu YC, Kapse K, Andersen N, et al. Association between socioeconomic status and in utero fetal brain development. JAMA Netw Open. 2021;4(3):e213526. doi: 10.1001/jamanetworkopen.2021.3526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Machlin L, McLaughlin KA, Sheridan MA. Brain structure mediates the association between socioeconomic status and attention-deficit/hyperactivity disorder. Dev Sci. 2020;23(1):e12844. doi: 10.1111/desc.12844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jha SC, Xia K, Ahn M, et al. Environmental influences on infant cortical thickness and surface area. Cereb Cortex. 2019;29(3):1139-1149. doi: 10.1093/cercor/bhy020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rakesh D, Whittle S. Socioeconomic status and the developing brain: a systematic review of neuroimaging findings in youth. Neurosci Biobehav Rev. 2021;130:379-407. doi: 10.1016/j.neubiorev.2021.08.027 [DOI] [PubMed] [Google Scholar]
- 48.Taylor RL, Cooper SR, Jackson JJ, Barch DM. Assessment of neighborhood poverty, cognitive function, and prefrontal and hippocampal volumes in children. JAMA Netw Open. 2020;3(11):e2023774. doi: 10.1001/jamanetworkopen.2020.23774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jednoróg K, Altarelli I, Monzalvo K, et al. The influence of socioeconomic status on children’s brain structure. PLoS One. 2012;7(8):e42486. doi: 10.1371/journal.pone.0042486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hastie T, Tibshirani R, Friedman J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. 2nd ed. Springer; 2009. doi: 10.1007/978-0-387-84858-7 [DOI] [Google Scholar]
- 51.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser A Stat Soc. 1995;57(1):289-300. doi: 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 52.National Records of Scotland . Scotland’s Census. Published 2020. Accessed December 2, 2021. https://www.scotlandscensus.gov.uk/
- 53.Ene D, Der G, Fletcher-Watson S, et al. Associations of socioeconomic deprivation and preterm birth with speech, language, and communication concerns among children aged 27 to 30 months. JAMA Netw Open. 2019;2(9):e1911027. doi: 10.1001/jamanetworkopen.2019.11027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Noble KG, Magnuson K, Gennetian LA, et al. Baby’s first years: design of a randomized controlled trial of poverty reduction in the United States. Pediatrics. 2021;148(4):e2020049702. doi: 10.1542/peds.2020-049702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Benavente-Fernández I, Siddiqi A, Miller SP. Socioeconomic status and brain injury in children born preterm: modifying neurodevelopmental outcome. Pediatr Res. 2020;87(2):391-398. doi: 10.1038/s41390-019-0646-7 [DOI] [PubMed] [Google Scholar]
- 56.Stiver ML, Kamino D, Guo T, et al. Maternal postsecondary education associated with improved cerebellar growth after preterm birth. J Child Neurol. 2015;30(12):1633-1639. doi: 10.1177/0883073815576790 [DOI] [PubMed] [Google Scholar]
- 57.Knickmeyer RC, Xia K, Lu Z, et al. Impact of demographic and obstetric factors on infant brain volumes: a population neuroscience study. Cereb Cortex. 2017;27(12):5616-5625. doi: 10.1093/cercor/bhw331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gui L, Loukas S, Lazeyras F, Hüppi PS, Meskaldji DE, Borradori Tolsa C. Longitudinal study of neonatal brain tissue volumes in preterm infants and their ability to predict neurodevelopmental outcome. Neuroimage. 2019;185:728-741. doi: 10.1016/j.neuroimage.2018.06.034 [DOI] [PubMed] [Google Scholar]
- 59.Thompson DK, Kelly CE, Chen J, et al. Early life predictors of brain development at term-equivalent age in infants born across the gestational age spectrum. Neuroimage. 2019;185:813-824. doi: 10.1016/j.neuroimage.2018.04.031 [DOI] [PubMed] [Google Scholar]
- 60.Makropoulos A, Aljabar P, Wright R, et al. Regional growth and atlasing of the developing human brain. Neuroimage. 2016;125:456-478. doi: 10.1016/j.neuroimage.2015.10.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Spann MN, Bansal R, Hao X, Rosen TS, Peterson BS. Prenatal socioeconomic status and social support are associated with neonatal brain morphology, toddler language and psychiatric symptoms. Child Neuropsychol. 2020;26(2):170-188. doi: 10.1080/09297049.2019.1648641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nieuwenhuys R, Voogd J, van Huijzen C. The Human Central Nervous System. 4th ed. Springer; 2008. doi: 10.1007/978-3-540-34686-9 [DOI] [Google Scholar]
- 63.Kersbergen KJ, Leroy F, Išgum I, et al. Relation between clinical risk factors, early cortical changes, and neurodevelopmental outcome in preterm infants. Neuroimage. 2016;142(C):301-310. doi: 10.1016/j.neuroimage.2016.07.010 [DOI] [PubMed] [Google Scholar]
- 64.Betancourt LM, Avants B, Farah MJ, et al. Effect of socioeconomic status (SES) disparity on neural development in female African-American infants at age 1 month. Dev Sci. 2016;19(6):947-956. doi: 10.1111/desc.12344 [DOI] [PubMed] [Google Scholar]
- 65.Triplett RL, Lean RE, Parikh A, et al. Association of prenatal exposure to early-life adversity with neonatal brain volumes at birth. JAMA Netw Open. 2022;5(4):e227045. doi: 10.1001/jamanetworkopen.2022.7045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dipietro JA. Maternal stress in pregnancy: considerations for fetal development. J Adolesc Health. 2012;51(2)(suppl):S3-S8. doi: 10.1016/j.jadohealth.2012.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baron R, Manniën J, te Velde SJ, Klomp T, Hutton EK, Brug J. Socio-demographic inequalities across a range of health status indicators and health behaviours among pregnant women in prenatal primary care: a cross-sectional study. BMC Pregnancy Childbirth. 2015;15(1):261. doi: 10.1186/s12884-015-0676-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dean B, Ginnell L, Ledsham V, et al. Eye-tracking for longitudinal assessment of social cognition in children born preterm. J Child Psychol Psychiatry. 2021;62(4):470-480. doi: 10.1111/jcpp.13304 [DOI] [PubMed] [Google Scholar]
- 69.Ginnell L, O’Carroll S, Ledsham V, et al. Emotion regulation and cortisol response to the still-face procedure in preterm and full-term infants. Psychoneuroendocrinology. 2022;141:105760. doi: 10.1016/j.psyneuen.2022.105760 [DOI] [PubMed] [Google Scholar]
- 70.Blesa M, Galdi P, Cox SR, et al. Hierarchical complexity of the macro-scale neonatal brain. Cereb Cortex. 2021;31(4):2071-2084. doi: 10.1093/cercor/bhaa345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Galdi P, Blesa M, Stoye DQ, et al. Neonatal morphometric similarity mapping for predicting brain age and characterizing neuroanatomic variation associated with preterm birth. Neuroimage Clin. 2020;25:102195. doi: 10.1016/j.nicl.2020.102195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ou X, Andres A, Pivik RT, et al. Voxel-based morphometry and fMRI revealed differences in brain gray matter in breastfed and milk formula-fed children. AJNR Am J Neuroradiol. 2016;37(4):713-719. doi: 10.3174/ajnr.A4593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ng IHX, Bonthrone AF, Kelly CJ, et al. Investigating altered brain development in infants with congenital heart disease using tensor-based morphometry. Sci Rep. 2020;10(1):14909. doi: 10.1038/s41598-020-72009-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gard AM, Maxwell AM, Shaw DS, et al. Beyond family-level adversities: exploring the developmental timing of neighborhood disadvantage effects on the brain. Dev Sci. 2021;24(1):e12985. doi: 10.1111/desc.12985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Linsell L, Malouf R, Morris J, Kurinczuk JJ, Marlow N. Prognostic factors for poor cognitive development in children born very preterm or with very low birth weight: a systematic review. JAMA Pediatr. 2015;169(12):1162-1172. doi: 10.1001/jamapediatrics.2015.2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Case Definitions and Sources
eFigure 1. Flow Diagram of Participants
eFigure 2. Diagram of Variables and Covariates in Models
eTable 1. Comparison of Included and Excluded Participants
eTable 2. Clinical Features of the Preterm Sample
eTable 3. Regional Brain Volumes: Association With Gestational Age, the Scottish Index of Multiple Deprivation, and Interaction Effect (Fully Adjusted Ridge Regression Model)
eTable 4. Regional Brain Volumes: Association With Gestational Age, the Scottish Index of Multiple Deprivation, and Interaction Effect (Baseline Unadjusted Linear Regression Model)
eTable 5. Cortical Measures: Association With Gestational Age and the Scottish Index of Multiple Deprivation (Baseline Unadjusted Linear Regression Model)
eTable 6. Regional Brain Volumes: Association With Gestational Age, Maternal Final Educational Qualification, and Interaction Effect (Fully Adjusted Ridge Regression Model)
eTable 7. Regional Brain Volumes: Association With Gestational Age, Paternal Final Educational Qualification, and Interaction Effect (Fully Adjusted Ridge Regression Model)
eTable 8. Regional Brain Volumes: Association With Gestational Age, Maternal Occupation, and Interaction Effect (Fully Adjusted Ridge Regression Model)
eTable 9. Regional Brain Volumes: Association With Gestational Age, Paternal Occupation, and Interaction Effect (Fully Adjusted Ridge Regression Model)
eTable 10. Regional Brain Volumes: Association With Gestational Age, Subjective Socioeconomic Status, and Interaction Effect (Fully Adjusted Ridge Regression Model)
eTable 11. Cortical Measures: Association With Gestational Age and Maternal Final Educational Qualification (Fully Adjusted Ridge Regression Model)
eTable 12. Cortical Measures: Association With Gestational Age and Paternal Final Educational Qualification (Fully Adjusted Ridge Regression Model)
eTable 13. Cortical Measures: Association With Gestational Age, Maternal Occupation, and Interaction Effect (Fully Adjusted Ridge Regression Model)
eTable 14. Cortical Measures: Association With Gestational Age and Paternal Occupation (Fully Adjusted Ridge Regression Model)
eTable 15. Cortical Measures: Association With Gestational Age, Subjective Socioeconomic Status, and Interaction Effect (Fully Adjusted Ridge Regression Model)
eTable 16. Lobar Volumes: Association With Gestational Age and the Scottish Index of Multiple Deprivation (Fully Adjusted Ridge Regression Models)
eTable 17. Lobar Volumes: Association With Gestational Age and Maternal Education (Fully Adjusted Ridge Regression Models)
eTable 18. Lobar Volumes: Association With Gestational Age and Paternal Education (Fully Adjusted Ridge Regression Models)
eTable 19. Lobar Volumes: Association With Gestational Age and Maternal Occupation (Fully Adjusted Ridge Regression Models)
eTable 20. Lobar Volumes: Association With Gestational Age and Paternal Occupation (Fully Adjusted Ridge Regression Models)
eTable 21. Lobar Volumes: Association With Gestational Age and Subjective Socioeconomic Status (Fully Adjusted Ridge Regression Models)
eTable 22. Cortical Measures: Association With Gestational Age, the Scottish Index of Multiple Deprivation, and Interaction Effect (Fully Adjusted Ridge Regression Models)
eTable 23. Cortical Measures: Association With Gestational Age, Maternal Education, and Interaction Effect (Fully Adjusted Ridge Regression Models)
eTable 24. Cortical Measures: Association With Gestational Age, Paternal Education, and Interaction Effect (Fully Adjusted Ridge Regression Models)
eTable 25. Cortical Measures: Association With Gestational Age, Maternal Occupation, and Interaction Effect (Fully Adjusted Ridge Regression Models)
eTable 26. Cortical Measures: Association With Gestational Age, Paternal Occupation, and Interaction Effect (Fully Adjusted Ridge Regression Models)
eTable 27. Cortical Measures: Association With Gestational Age, Subjective Socioeconomic Status, and Interaction Effect (Fully Adjusted Ridge Regression Models)
eReferences.
Data Sharing Statement