Key Points
Question
Do patients with atopic dermatitis have an increased risk for venous thromboembolism?
Findings
In this nationwide cohort study from Taiwan of 284 858 participants, adults with atopic dermatitis had a 1.28-fold hazard for incident venous thromboembolism compared with adults without atopic dermatitis. Individual outcome analyses suggested that atopic dermatitis was associated with elevated hazards for deep vein thrombosis and pulmonary embolism.
Meaning
The results of this cohort study suggest an increased risk of incident venous thromboembolism among adults with atopic dermatitis; dermatologists should be alert to relevant cardiovascular symptoms in such patients.
Abstract
Importance
The associations of atopic dermatitis (AD) with multiple cardiovascular comorbidities have been investigated because of its pathomechanisms regarding chronic systemic inflammation and potential vascular effects. Nevertheless, the association between AD and incident venous thromboembolism (VTE) in adulthood is largely unknown. This study aimed to investigate the association of AD with incident VTE.
Objective
To examine the risk of incident VTE among patients with AD.
Design, Setting, and Participants
This population-based nationwide cohort study included adults 20 years or older (adults with AD newly diagnosed between 2003 and 2017 and matched controls) from the National Health Insurance Research Database. Patients with AD were subgrouped according to the severity of the disease. A Cox regression model was used to estimate hazard ratios (HRs) for VTE. Stratified analyses according to age and sex, and a sensitivity analysis excluding systemic steroid users were performed.
Main Outcomes and Measures
Hazard ratios (HRs) for incident VTE associated with AD.
Results
This analysis included a total of 284 858 participants, with 142 429 participants each in the AD (mean [SD] age, 44.9 [18.3] years; 78 213 women) and non-AD cohorts (mean [SD] age, 44.1 [18.1] years; 79 636 women). During the follow-up, 1066 patients (0.7%) in the AD cohort and 829 patients (0.6%) in the non-AD cohort developed VTE, with incidence rates of 1.05 and 0.82 per 1000 person-years, respectively. Adults with AD had a significantly increased risk of incident VTE (HR, 1.28; 95% CI, 1.17-1.40) compared with adults without AD. Individual outcome analyses suggested that AD was associated with higher risks of deep vein thrombosis (HR, 1.26; 95% CI, 1.14-1.40) and pulmonary embolism (HR, 1.30; 95% CI, 1.08-1.57).
Conclusions and Relevance
The results of this cohort study suggest that AD in adulthood is associated with an increased risk of VTE; however, the absolute risk difference of VTE between adults with and without AD appears small. Nevertheless, cardiovascular examination and imperative management may be considered for adults with AD who present with symptoms suggestive of VTE. Future research is warranted to elucidate the pathophysiology underlying the association between AD and VTE.
This cohort study examines the risk of incident venous thromboembolism among Asian patients with atopic dermatitis.
Introduction
Atopic dermatitis (AD) is a chronic immune-mediated inflammatory dermatosis that affects up to 10% of adults globally.1 Adults with AD may experience relapsing skin symptoms, sleep disturbances, and reduced life quality.2,3 Atopic dermatitis is caused by skin-barrier defects and immune dysregulation, with increasing evidence suggesting that it is a systemic disorder.4,5 Atopic dermatitis in adulthood has been associated with an increased risk of cardiovascular diseases, including myocardial infarction and ischemic stroke.6,7,8
Venous thromboembolism (VTE), comprising deep vein thrombosis (DVT) and pulmonary embolism (PE), is a vascular disease involving blood clot formation.9 If DVT is left untreated, the subsequent development of PE could be life-threatening.10 Patients with AD were found to have elevated levels of inflammatory and prothrombotic markers,11 which are also involved in the pathophysiology of VTE.12,13,14 Previous studies have attempted to evaluate the risk of VTE in patients with AD, but the results remain controversial.15,16,17 These studies also seldom focused on adult populations with AD. Furthermore, the association of AD severity with the risk of VTE among adults with AD was unclear.
To our knowledge, to date, there has been insufficient evidence underlying the current guidelines to prevent or manage VTE among adults with AD.18 Given that AD is rarely associated with serious health-related outcomes, investigations on its associations with potentially life-threatening diseases, such as VTE, are valuable. In this study, we aimed to investigate whether AD in adulthood is associated with an increased risk of incident VTE.
Methods
Data Source
This nationwide retrospective cohort study used claim data from Taiwan's National Health Insurance Research Database (NHIRD). The NHIRD encompasses the health care data of approximately 23.6 million individuals, representing the entire population of Taiwan. A concise description of NHIRD can be found in the eNote in the Supplement, and further details have been reported previously.19,20 This study was reviewed and approved by the research ethics committee of Hualien Tzu Chi Hospital (Taiwan). The need for informed consent was waived as the anonymized data of the NHIRD guarantee participant confidentiality.
Study Population
Adults 20 years or older who contributed to the NHIRD between January 1, 2003, and December 31, 2017, were eligible for inclusion. The exposed cohort comprised all patients with AD with a new diagnosis from either dermatologists or rheumatologists. The definition of an AD case required an AD diagnosis confirmed 3 or more times in an outpatient department within 1 year or a discharge diagnosis using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code 691.8 and ICD-10-CM code L20. These diagnostic codes have been applied in previous studies using the NHIRD.21,22,23 We excluded patients who received a diagnosis of AD before 2003 to ensure that patients with a new diagnosis of AD were identified. The index date (when the follow-up started) for the patients in the exposed (AD) cohort was defined as the date of the third outpatient diagnosis of AD or the date of the first inpatient diagnosis, whichever came first.
The unexposed (non-AD) cohort comprised participants without any AD diagnosis during the entire period from 2000 to 2018 that was covered by the database. To construct the non-AD cohort as the control group, we randomly matched each patient with AD with a control without AD from the database by exact matching on age and sex. The index date for each non-AD participant was assigned to the same index date as the matched patient with AD. Patients with any record of VTE before the index date were excluded from the AD and non-AD cohorts.
Outcome Measures
The outcome of interest was incident VTE, including diagnoses of DVT (ICD-9-CM codes 453.2, 453.4, 453.5, 453.7, 453.8, and 453.9; ICD-10-CM codes I82.2, I82.4, I82.5, I82.60, I82.62, I82.89, and I82.9) and PE (ICD-9-CM code 415.1; ICD-10-CM code I26). The accuracy of the diagnostic codes for VTE has been validated with high positive predictive values.24 An outcome event was defined as a diagnosis made either during an outpatient or inpatient service. All participants were followed up from the index date until the occurrence of VTE, death, or December 31, 2018 (the last date in the database), whichever came first. Additionally, we performed individual analyses on DVT and PE. In the individual outcome analysis, patients were followed up until the desired outcome occurred, ie, a patient with DVT who later developed PE was also considered a case in the PE analysis.
Covariates and Confounders
The baseline characteristics of eligible participants were identified from the NHIRD. Preexisting comorbidities and baseline medication use (eTable 1 in Supplement 1), which were considered as potential confounders, were selected according to previous publications.9,14,25 Any preexisting comorbidity was defined as a discharge diagnosis or a diagnosis ascertained twice or more in an outpatient department within the year before the index date by using the ICD-9-CM, ICD-10-CM, and procedure codes. Medication use at baseline was defined as a drug prescribed for 30 days or longer within the year before the index date. Monthly income levels were evaluated based on income-related National Health Insurance premiums.
Subgroup, Stratified, and Sensitivity Analyses
The exposed cohort was divided according to severity into 2 subgroups (mild and severe AD). Patients were classified as having severe AD if they received any systemic therapy (methotrexate, cyclosporine, mycophenolate mofetil, azathioprine, systemic corticosteroids, and/or phototherapy) that was prescribed by dermatologists or rheumatologists after AD diagnosis; otherwise, they were considered as having mild AD. To avoid an immortal time bias, we adjusted the index date for patients with severe AD to the date of the first prescription of any systemic therapy (the index date of the matched controls was also adjusted). This adjustment was only made in the analysis of severe AD, not of mild AD. This classification of AD severity was adopted from previous studies6,25 and modified according to the AD guideline in Taiwan.26 The subgroup analyses compared patients with mild AD with those without AD, patients with severe AD with those without AD, and patients with severe AD with those with mild AD.
Age-stratified and sex-stratified analyses were performed. For the age-stratified analysis, we divided the study participants into subgroups of those 45 years or younger and those 45 years or older based on the overall mean age of the study population. To eliminate the confounding from systemic steroid use, which is potentially associated with an increased risk of VTE,27 we performed a sensitivity analysis by excluding patients who had used any systemic steroid during follow-up.
Statistical Analyses
To balance the systematic differences in baseline characteristics between the exposed and unexposed cohorts and control potential confounders, we further performed propensity score matching (PSM) after defining the AD and non-AD cohorts. A propensity score was calculated for each patient using multivariable logistic regression models based on the covariates listed in Table 1 to estimate the probability of exposure to AD. We used the nearest-neighbor matching algorithm without replacement, with a caliper width equal to 0.2 SDs of the logit of the propensity score.28,29 We performed PSM individually for each comparison set, including overall, subgroup, stratified, and sensitivity analyses, before conducting any data analysis. The standardized mean difference (SMD) was used to examine the differences in baseline characteristics between the study cohorts, with a value of less than 0.1 indicating a negligible difference.30 Kaplan-Meier methods were used to illustrate the cumulative incidence curves. Cox proportional hazards models were applied to estimate hazard ratios (HRs) for each outcome. A confidence interval not containing 1 was considered significant. All statistical analyses were performed using SAS, software version 9.4 (SAS Institute). A 2-sided P < .05 was considered significant.
Table 1. Baseline Characteristics of the Study Population After Propensity Score Matching.
| Characteristicsa | AD (n = 142 429) | Non-AD (n = 142 429) | SMDb |
|---|---|---|---|
| Age, mean (SD), y | 44.9 (18.3) | 44.1 (18.1) | 0.0440 |
| Sex, No. (%) | |||
| Female | 78 213 (54.91) | 79 636 (55.91) | 0.0201 |
| Male | 64 216 (45.09) | 62 793 (44.09) | 0.0201 |
| Income level in NTD (USD), No. (%) | |||
| Financially dependent | 26 222 (18.41) | 24 764 (17.39) | 0.0266 |
| 15 840 (519.2)-24 999 (819.4) | 68 451 (48.06) | 69 710 (48.94) | 0.0176 |
| 25 000 (819.5)-39 999 (1311.1) | 23 027 (16.17) | 23 215 (16.3) | 0.0035 |
| ≥40 000 (1311.2) | 24 729 (17.36) | 24 740 (17.37) | 0.0003 |
| Comorbidities, No. (%) | |||
| Hypertension | 24 400 (17.13) | 22 816 (16.02) | 0.0299 |
| Atrial fibrillation | 880 (0.62) | 708 (0.50) | 0.0161 |
| Stroke | 4873 (3.42) | 4184 (2.94) | 0.0274 |
| Heart failure | 1641 (1.15) | 1321 (0.93) | 0.0217 |
| Coronary artery disease | 7105 (4.99) | 6289 (4.42) | 0.0269 |
| Chronic obstructive pulmonary disease | 5388 (3.78) | 4253 (2.99) | 0.0437 |
| Chronic kidney disease | 2281 (1.60) | 1876 (1.32) | 0.0233 |
| Cirrhosis | 825 (0.58) | 685 (0.48) | 0.0138 |
| Hyperlipidemia | 13 473 (9.46) | 12 289 (8.63) | 0.0289 |
| Fracture of lower limbs | 1476 (1.04) | 1264 (0.89) | 0.0153 |
| Gout | 4418 (3.10) | 3875 (2.72) | 0.0226 |
| Cancer | 3678 (2.58) | 3307 (2.32) | 0.0168 |
| Pregnancy | 1557 (1.09) | 1580 (1.11) | 0.0019 |
| Thyroid dysfunction | 1871 (1.31) | 1569 (1.10) | 0.0192 |
| Allergic conjunctivitis | 2221 (1.56) | 2191 (1.54) | 0.0016 |
| Allergic rhinitis | 5086 (3.57) | 4794 (3.37) | 0.0109 |
| Asthma | 3731 (2.62) | 2718 (1.91) | 0.0477 |
| Food allergy | 426 (0.30) | 258 (0.18) | 0.0245 |
| Urticaria | 3499 (2.46) | 3400 (2.39) | 0.0046 |
| Autoimmune disease | 2700 (1.90) | 1501 (1.05) | 0.0706 |
| Bullous pemphigoid | 61 (0.04) | 26 (0.02) | 0.0115 |
| Pemphigus | 24 (0.02) | 11 (0.01) | 0.0082 |
| Psoriasis | 355 (0.25) | 318 (0.22) | 0.0062 |
| Baseline medication use, No. (%) | |||
| Hormone therapies | 1800 (1.26) | 1481 (1.04) | 0.0206 |
| Statins | 9552 (6.71) | 8697 (6.11) | 0.0245 |
| Antiplatelets | 11 108 (7.80) | 9851 (6.92) | 0.0337 |
Abbreviations: AD, atopic dermatitis; NTD, New Taiwan Dollar; SMD, standardized mean difference.
All listed covariates were used to calculate the propensity score.
A standardized mean difference of less than 0.1 indicated a negligible difference.
Results
The original study population included 284 858 adults. After PSM, the exposed cohort comprised 142 429 adults with AD, and the unexposed cohort comprised 142 429 adults without AD. All baseline characteristics were adequately balanced between the 2 cohorts after PSM, with all SMDs less than 0.1 (Table 1). The baseline characteristics of the study cohorts before PSM are outlined in eTable 2 in Supplement 1.
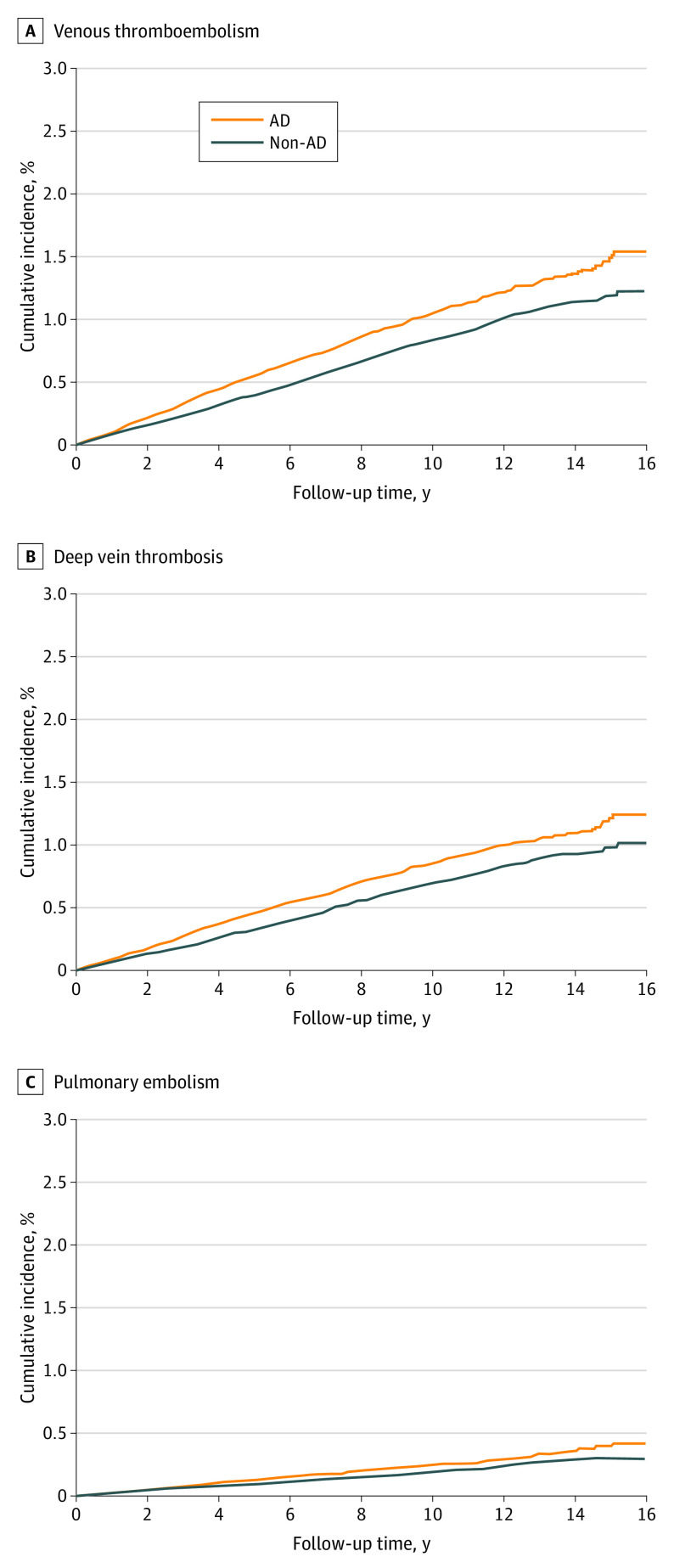
Overall, 1066 patients in the AD cohort and 829 participants in the non-AD cohort developed VTE, with incidence rates of 1.05 and 0.82 per 1000 person-years, respectively (Table 2). The mean (SD) follow-up for the AD and non-AD cohorts was 7.15 (4.4) and 7.09 (4.4) years, respectively (eTable 3 in Supplement 1 for detailed follow-up times). Adults with AD were at an increased risk of incident VTE (HR, 1.28; 95% CI, 1.17-1.40) compared with adults without AD. Regarding the individual outcome analyses, AD was associated with a higher risk of incident DVT (HR, 1.26; 95% CI, 1.14-1.40) and PE (HR, 1.30; 95% CI, 1.08-1.57). The cumulative incidence curves are presented in Figure 1.
Table 2. Risk of Incident Venous Thromboembolism Among Patients With AD.
| Outcomes/comparison | No. | Person-years at risk | Incidence rate per 1000 person-years | HR (95% CI)a | |
|---|---|---|---|---|---|
| Patients | Events | ||||
| Venous thromboembolism | |||||
| AD cohort | 142 429 | 1066 | 1 018 454 | 1.05 | 1.28 (1.17-1.40) |
| Non-AD cohort | 142 429 | 829 | 1 009 561 | 0.82 | 1 [Reference] |
| Deep vein thrombosis | |||||
| AD cohort | 142 429 | 869 | 1 018 962 | 0.85 | 1.26 (1.14-1.40) |
| Non-AD cohort | 142 429 | 682 | 1 009 916 | 0.68 | 1 [Reference] |
| Pulmonary embolism | |||||
| AD cohort | 142 429 | 255 | 1 021 500 | 0.25 | 1.30 (1.08-1.57) |
| Non-AD cohort | 142 429 | 194 | 1 012 036 | 0.19 | 1 [Reference] |
Abbreviations: AD, atopic dermatitis; HR, hazard ratio.
The HRs were calculated using a univariable Cox regression model with propensity score matching; the HR was calculated using the corresponding non-AD group as the reference.
Figure 1. Cumulative Incidence Curves for Venous Thromboembolism, Deep Vein Thrombosis, and Pulmonary Embolism.
AD indicates atopic dermatitis.
In the subgroup analysis according to AD severity, adults with mild AD (HR, 1.30; 95% CI, 1.12-1.51) and severe AD (HR, 1.35; 95% CI, 1.19-1.53) were at an increased risk of incident VTE compared with non-AD controls (Table 3). However, there was no significant difference in the risks of VTE between patients with severe and mild AD (HR, 1.04, 95% CI, 0.92-1.18; eTable 4 in Supplement 1). The risks of DVT and PE showed similar results.
Table 3. Subgroup Analyses According to Severity of AD.
| Outcomes | AD cohort | Non-AD cohort | HR (95% CI)a | ||
|---|---|---|---|---|---|
| Events, No. | IRb | Events, No. | IRb | ||
| Venous thromboembolism | |||||
| Mild AD vs non-AD | 393 | 1.17 | 304 | 0.90 | 1.30 (1.12-1.51) |
| Severe AD vs non-AD | 567 | 0.98 | 418 | 0.73 | 1.35 (1.19-1.53) |
| Deep vein thrombosis | |||||
| Mild AD vs non-AD | 308 | 0.92 | 245 | 0.72 | 1.27 (1.07-1.50) |
| Severe AD vs non-AD | 467 | 0.81 | 350 | 0.61 | 1.32 (1.17-1.48) |
| Pulmonary embolism | |||||
| Mild AD vs non-AD | 106 | 0.31 | 74 | 0.22 | 1.44 (1.07-1.94) |
| Severe AD vs non-AD | 133 | 0.23 | 95 | 0.17 | 1.39 (1.07-1.81) |
Abbreviations: AD, atopic dermatitis; HR, hazard ratio; IR, incidence rate.
The HRs were calculated using a univariable Cox regression model with propensity score matching; the HR was calculated using the corresponding non-AD group as the reference.
Incidence rate per 1000 person-years.
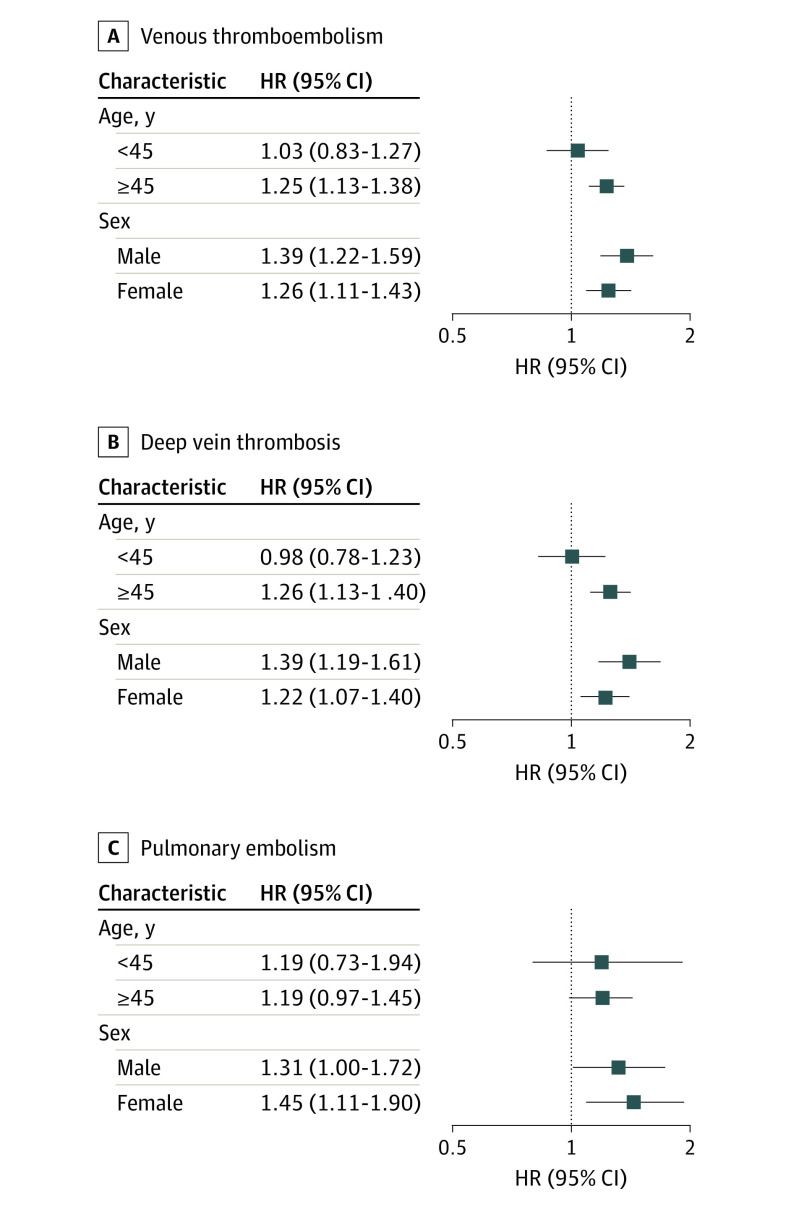
The results of the age-stratified and sex-stratified analyses are illustrated in Figure 2 (eTables 5-7 in Supplement 1). The increased risk of incident VTE associated with AD remained significant among people 45 years or older (HR, 1.25; 95% CI, 1.13-1.38) but not among those younger than 45 years (HR, 1.03; 95% CI, 0.83-1.27). When stratified by sex, the association between AD and increased VTE risk remained significant for both sexes. The analyses for DVT and PE demonstrated similar findings. In the sensitivity analysis excluding systemic steroid users (eTable 8 in Supplement 1), the risk for VTE remained significantly increased among patients with AD (HR, 1.45; 95% CI, 1.04-2.03).
Figure 2. Risk of Venous Thromboembolism in Patients With Atopic Dermatitis (AD) Compared With Controls as Stratified by Age and Sex.
HR indicates hazard ratio.
Discussion
This cohort study found an overall 1.28-fold increased risk of newly developed VTE among adults with AD. Individual outcome analyses suggested that AD was associated with increased risks for DVT and PE. However, the absolute risk difference of VTE between AD and non-AD adults appeared small.
Emerging evidence on the prothrombotic properties of AD supports this study’s findings. Animal models have revealed the role of prothrombotic factors, such as fibrinogen and thrombin, in the pathogenesis of AD and allergic sensitization.31,32 Enhanced platelet activation and prolonged fibrinolysis have also been observed among patients with AD.11,31 Additionally, systemic inflammation in AD is believed to promote vascular inflammation, which is associated with endothelial dysfunction and thrombosis.32 Atopic dermatitis is increasingly being recognized as a systemic disease with enhanced T-helper 2 inflammation, according to proteomic studies.33 These preclinical and translational findings may potentially explain the elevated risks of VTE associated with AD.
Previous observational studies evaluating the association between AD and VTE have had conflicting results. A cross-sectional survey of hospitalized adults reported a positive association between AD and VTE (adjusted odds ratio, 1.22; 95% CI, 1.17-1.27).15 However, the study was unable to clarify the temporal association between AD and VTE. Undas et al17 revealed that atopic diseases were more prevalent in patients with VTE aged 20 to 45 years.16 Nevertheless, this study did not consider important covariates, such as hormone therapies, which might lead to confounding bias. Furthermore, 2 retrospective cohort studies showed inconsistent observations. The study by Meyers et al16 revealed a reduced risk of VTE in adults with AD (adjusted HR, 0.77; 95% CI, 0.69-0.85), whereas Schneeweiss et al34 reported a trend toward an elevated VTE risk in patients with AD, although this was not significant (adjusted HR, 1.19; 95% CI, 0.95-1.48). Meyers et al16 identified patients with AD using a broad definition (≥1 outpatient or inpatient record of ICD codes), which might be insufficient to capture patients with AD according to a previous validation study from the US.35 The inclusion criteria in the present study were more rigorous because the study participants needed to have received an AD diagnosis 3 or more times in outpatient services within 1 year or a discharge diagnosis. In contrast to Schneeweiss et al,34 which included 30 418 patients with AD using US commercial insurance claims, the present study enrolled more patients with AD and complemented the prior studies with an Asian population. In general, the cohort analysis expanded on previous studies with rigorous methods to provide potentially reliable results. Further studies are needed to clarify the effects of race or genetics on the VTE risk associated with AD.
We found an increased risk of incident VTE and DVT in adults with AD 45 years or older but not in those younger than 45 years (Figure 2). Recent research has shown higher levels of systemic inflammatory markers in older patients with AD than in young adults.36 In the stratified analysis, the risk of VTE in men and women remained significantly increased. The incidence of VTE was slightly higher in men than in women. This result aligned with previous epidemiologic studies.37,38 Additionally, we performed a sensitivity analysis that excluded systemic steroid users, as steroid use has been considered a risk factor for VTE.27 This analysis demonstrated that the increased risk of VTE associated with AD remained when the confounding from systemic steroid use was eliminated.
As multiple biologics and small molecule inhibitors have been approved for treating AD, the safety profiles of these novel agents have been under investigation. Studies on dupilumab use among patients with AD did not detect serious cardiovascular adverse events.39,40 However, the US Food and Drug Administration issued a black-box warning about an increased risk of blood clots for tofacitinib, baricitinib, and upadacitinib, causing concerns from patients and physicians.41 The risk of VTE in patients with AD receiving abrocitinib has also been raised.42 Prior meta-analyses have indicated that Janus kinase (JAK) inhibitors may not increase VTE risk among several chronic inflammatory diseases.43,44 Nonetheless, whether an increased risk of VTE exists among patients with AD treated with JAK inhibitors remains debatable. Although a recent meta-analysis of randomized clinical trials demonstrated no difference in the VTE risks among patients with AD who were receiving treatment with abrocitinib, baricitinib, upadacitinib, and ivarmacitinib, long-term safety data in a clinical setting are warranted.45 The associations of dupilumab and JAK inhibitors with VTE risk were not examined in this study because these medicines were not yet reimbursed by Taiwan’s National Health Insurance during the study period. Considering the anti-inflammatory effects of biologics and small molecule inhibitors, future studies are warranted to explore their associations with VTE.
Strengths and Limitations
To our knowledge, only a few studies have investigated the association between AD and incident VTE; this study was designed to fill this knowledge gap. The main strength of this study is the large-scale nationwide analysis using data from routine clinical practice to provide empirical evidence. As comorbidities among adults with AD are not well established, this study’s findings potentially provide insights into the prevention and management of VTE in adults with AD. The implementation of risk prediction models could be considered within the current AD guideline. Additional diagnostic approaches, such as ultrasonography and computed tomography, may be helpful.
This study had several limitations. First, the study used a claim database that lacked direct data on physical activity, lifestyle, smoking history, body mass index, and laboratory results of coagulation profiles. Despite the PSM applied to mitigate potential confounding, unmeasured confounding might have existed. Second, the ascertainment of AD and VTE was based on ICD codes. Although the codes have been validated, we cannot completely rule out any misclassification. Third, the NHIRD did not provide sufficient information to define AD severity on the basis of indices used in clinical trials, such as total body surface area, Investigator Global Assessment score, and Eczema Area and Severity Index score.2 Instead, the severity of AD in this study was defined according to a treatment pattern that was adopted in previous studies but lacks validation in Taiwan’s NHIRD. Fourth, since we included newly diagnosed AD during the study period, patients with persistent AD were inevitably excluded. Moreover, the analyses focused on adults with AD, which may not represent the overall AD population because many patients with AD receive their diagnosis at a relatively young age. Since the civil law in Taiwan defined adult as a person aged 20 years or older, the association of AD with VTE among patients aged 18 to 19 years (who may be categorized as adults in other countries) was not investigated. Due to the aforementioned limitations, the study findings should be interpreted cautiously.
Conclusions
In this cohort study, AD in adulthood was associated with an increased risk of incident VTE. Although this was a small absolute risk of VTE, vascular examination and consultation with the emergency department, cardiologists, or pulmonologists are indicated for patients with AD who present with relevant symptoms (eg, unexplained dyspnea, chest tightness, and limb swelling). Prompt prophylaxis with anticoagulants may be imperative in patients with symptoms that suggest VTE. This study’s findings may also have implications for interpreting pharmacovigilance studies on JAK inhibitors.
eNote. A brief introduction to Taiwan's National Health Insurance Research Database (NHIRD)
eTable 1. Diagnostic and procedure codes for covariates
eTable 2. Demographic data of the study population before propensity score matching
eTable 3. Follow-up time (in years) of the study population
eTable 4. Risks of venous thromboembolism in patients with severe atopic dermatitis and those with mild atopic dermatitis
eTable 5. Risks of venous thromboembolism in stratified analyses by age and sex
eTable 6. Risks of deep vein thrombosis in stratified analyses by age and sex
eTable 7. Risks of pulmonary embolism in stratified analyses by age and sex
eTable 8. Risks of venous thromboembolism in the sensitivity analyses excluding systemic steroid users
Data sharing statement
References
- 1.Barbarot S, Auziere S, Gadkari A, et al. Epidemiology of atopic dermatitis in adults: results from an international survey. Allergy. 2018;73(6):1284-1293. doi: 10.1111/all.13401 [DOI] [PubMed] [Google Scholar]
- 2.Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70(2):338-351. doi: 10.1016/j.jaad.2013.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho YT, Chu CY. Advances in systemic treatment for adults with moderate-to-severe atopic dermatitis. Dermatol Sin. 2019;37(1):3-11. doi: 10.4103/ds.ds_48_18 [DOI] [Google Scholar]
- 4.Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396(10247):345-360. doi: 10.1016/S0140-6736(20)31286-1 [DOI] [PubMed] [Google Scholar]
- 5.Wang CH, Fu Y, Chi CC. Association of atopic dermatitis with inflammatory bowel disease: a systematic review and meta-analysis. Dermatol Sin. 2020;38(3):159-165. doi: 10.4103/ds.ds_20_20 [DOI] [Google Scholar]
- 6.Silverwood RJ, Forbes HJ, Abuabara K, et al. Severe and predominantly active atopic eczema in adulthood and long term risk of cardiovascular disease: population based cohort study. BMJ. 2018;361:k1786. doi: 10.1136/bmj.k1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu JJ, Amand C, No DJ, et al. The use of real-world data to evaluate the association between atopic dermatitis and cardiovascular disease: a retrospective claims analysis. Dermatol Ther (Heidelb). 2021;11(5):1707-1715. doi: 10.1007/s13555-021-00587-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su VY, Chen TJ, Yeh CM, et al. Atopic dermatitis and risk of ischemic stroke: a nationwide population-based study. Ann Med. 2014;46(2):84-89. doi: 10.3109/07853890.2013.870018 [DOI] [PubMed] [Google Scholar]
- 9.Goldhaber SZ. Risk factors for venous thromboembolism. J Am Coll Cardiol. 2010;56(1):1-7. doi: 10.1016/j.jacc.2010.01.057 [DOI] [PubMed] [Google Scholar]
- 10.Søgaard KK, Schmidt M, Pedersen L, Horváth-Puhó E, Sørensen HT. 30-year mortality after venous thromboembolism: a population-based cohort study. Circulation. 2014;130(10):829-836. doi: 10.1161/CIRCULATIONAHA.114.009107 [DOI] [PubMed] [Google Scholar]
- 11.Tamagawa-Mineoka R, Katoh N, Ueda E, Masuda K, Kishimoto S. Elevated platelet activation in patients with atopic dermatitis and psoriasis: increased plasma levels of beta-thromboglobulin and platelet factor 4. Allergol Int. 2008;57(4):391-396. doi: 10.2332/allergolint.O-08-537 [DOI] [PubMed] [Google Scholar]
- 12.Piazza G, Ridker PM. Is venous thromboembolism a chronic inflammatory disease? Clin Chem. 2015;61(2):313-316. doi: 10.1373/clinchem.2014.234088 [DOI] [PubMed] [Google Scholar]
- 13.Riva N, Donadini MP, Ageno W. Epidemiology and pathophysiology of venous thromboembolism: similarities with atherothrombosis and the role of inflammation. Thromb Haemost. 2015;113(6):1176-1183. doi: 10.1160/TH14-06-0563 [DOI] [PubMed] [Google Scholar]
- 14.Chen TL, Lee LL, Huang HK, et al. Association of psoriasis with incident venous thromboembolism and peripheral vascular disease: a systematic review and meta-analysis. JAMA Dermatol. 2022;158(1):59-67. doi: 10.1001/jamadermatol.2021.4918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaheen MS, Silverberg JI. Association of inflammatory skin diseases with venous thromboembolism in US adults. Arch Dermatol Res. 2021;313(4):281-289. doi: 10.1007/s00403-020-02099-6 [DOI] [PubMed] [Google Scholar]
- 16.Meyers KJ, Silverberg JI, Rueda MJ, et al. Risk of venous thromboembolism among patients with atopic dermatitis: a cohort study in a US administrative claims database. Dermatol Ther (Heidelb). 2021;11(3):1041-1052. doi: 10.1007/s13555-021-00538-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Undas A, Cieśla-Dul M, Drążkiewicz T, Potaczek DP, Sadowski J. Association between atopic diseases and venous thromboembolism: a case-control study in patients aged 45 years or less. J Thromb Haemost. 2011;9(4):870-873. doi: 10.1111/j.1538-7836.2011.04198.x [DOI] [PubMed] [Google Scholar]
- 18.Davis DMR, Drucker AM, Alikhan A, et al. American Academy of Dermatology guidelines: awareness of comorbidities associated with atopic dermatitis in adults. J Am Acad Dermatol. 2022;86(6):1335-1336.e18. doi: 10.1016/j.jaad.2022.01.009 [DOI] [PubMed] [Google Scholar]
- 19.Hsing AW, Ioannidis JP. Nationwide population science: lessons from the Taiwan National Health Insurance Research Database. JAMA Intern Med. 2015;175(9):1527-1529. doi: 10.1001/jamainternmed.2015.3540 [DOI] [PubMed] [Google Scholar]
- 20.Wang SH, Wang J, Lin YS, Tung TH, Chi CC. Increased risk for incident thyroid diseases in people with psoriatic disease: a cohort study. J Am Acad Dermatol. 2019;80(4):1006-1012. doi: 10.1016/j.jaad.2018.11.049 [DOI] [PubMed] [Google Scholar]
- 21.Dai YX, Tai YH, Chang YT, Chen TJ, Chen MH. Bidirectional association between psoriasis and atopic dermatitis: a nationwide population-based cohort study. Dermatology. 2021;237(4):521-527. doi: 10.1159/000514581 [DOI] [PubMed] [Google Scholar]
- 22.Lin TL, Fan YH, Chang YL, Ho HJ, Wu CY, Chen YJ. Early-life infections in association with the development of atopic dermatitis in infancy and early childhood: a nationwide nested case-control study. J Eur Acad Dermatol Venereol. 2022;36(4):615-622. doi: 10.1111/jdv.17908 [DOI] [PubMed] [Google Scholar]
- 23.Hu JM, Lin CS, Chen SJ, Chen CY, Lin CL, Kao CH. Association between obstructive sleep apnea and atopic dermatitis in children: a nationwide, population-based cohort study. Pediatr Allergy Immunol. 2018;29(3):260-266. doi: 10.1111/pai.12853 [DOI] [PubMed] [Google Scholar]
- 24.Ng KJ, Lee YK, Huang MY, Hsu CY, Su YC. Risks of venous thromboembolism in patients with liver cirrhosis: a nationwide cohort study in Taiwan. J Thromb Haemost. 2015;13(2):206-213. doi: 10.1111/jth.12805 [DOI] [PubMed] [Google Scholar]
- 25.Ivert LU, Johansson EK, Dal H, Lindelöf B, Wahlgren CF, Bradley M. Association between atopic dermatitis and cardiovascular disease: a nationwide register-based case-control study from Sweden. Acta Derm Venereol. 2019;99(10):865-870. doi: 10.2340/00015555-3235 [DOI] [PubMed] [Google Scholar]
- 26.Lee EM, Cho YT, Hsieh WT, et al. Healthcare utilization and costs of atopic dermatitis in Taiwan. J Formos Med Assoc. 2022;121(10):1963-1971. doi: 10.1016/j.jfma.2022.01.028 [DOI] [PubMed] [Google Scholar]
- 27.Johannesdottir SA, Horváth-Puhó E, Dekkers OM, et al. Use of glucocorticoids and risk of venous thromboembolism: a nationwide population-based case-control study. JAMA Intern Med. 2013;173(9):743-752. doi: 10.1001/jamainternmed.2013.122 [DOI] [PubMed] [Google Scholar]
- 28.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424. doi: 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10(2):150-161. doi: 10.1002/pst.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083-3107. doi: 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nastałek M, Wojas-Pelc A, Undas A. Plasma fibrin clot properties in atopic dermatitis: links between thrombosis and atopy. J Thromb Thrombolysis. 2010;30(2):121-126. doi: 10.1007/s11239-010-0478-0 [DOI] [PubMed] [Google Scholar]
- 32.Brunner PM, Suárez-Fariñas M, He H, et al. The atopic dermatitis blood signature is characterized by increases in inflammatory and cardiovascular risk proteins. Sci Rep. 2017;7(1):8707. doi: 10.1038/s41598-017-09207-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pavel AB, Zhou L, Diaz A, et al. The proteomic skin profile of moderate-to-severe atopic dermatitis patients shows an inflammatory signature. J Am Acad Dermatol. 2020;82(3):690-699. doi: 10.1016/j.jaad.2019.10.039 [DOI] [PubMed] [Google Scholar]
- 34.Schneeweiss MC, Kim SC, Wyss R, et al. Incidence of venous thromboembolism in patients with dermatologist-diagnosed chronic inflammatory skin diseases. JAMA Dermatol. 2021;157(7):805-816. doi: 10.1001/jamadermatol.2021.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsu DY, Dalal P, Sable KA, et al. Validation of International Classification of Disease Ninth Revision codes for atopic dermatitis. Allergy. 2017;72(7):1091-1095. doi: 10.1111/all.13113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He H, Li R, Choi S, et al. Increased cardiovascular and atherosclerosis markers in blood of older patients with atopic dermatitis. Ann Allergy Asthma Immunol. 2020;124(1):70-78. doi: 10.1016/j.anai.2019.10.013 [DOI] [PubMed] [Google Scholar]
- 37.Roach RE, Cannegieter SC, Lijfering WM. Differential risks in men and women for first and recurrent venous thrombosis: the role of genes and environment. J Thromb Haemost. 2014;12(10):1593-1600. doi: 10.1111/jth.12678 [DOI] [PubMed] [Google Scholar]
- 38.Heit JA. Epidemiology of venous thromboembolism. Nat Rev Cardiol. 2015;12(8):464-474. doi: 10.1038/nrcardio.2015.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Halling AS, Loft N, Silverberg JI, Guttman-Yassky E, Thyssen JP. Real-world evidence of dupilumab efficacy and risk of adverse events: a systematic review and meta-analysis. J Am Acad Dermatol. 2021;84(1):139-147. doi: 10.1016/j.jaad.2020.08.051 [DOI] [PubMed] [Google Scholar]
- 40.Bettuzzi T, Drucker A, Staumont-Sallé D, Bihan K, Lebrun-Vignes B, Sbidian E. Adverse events associated with dupilumab in the World Health Organization pharmacovigilance database. J Am Acad Dermatol. 2022;86(2):431-433. doi: 10.1016/j.jaad.2021.09.050 [DOI] [PubMed] [Google Scholar]
- 41.US Food and Drug Administration . FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. Accessed April 10, 2022. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- 42.Samuel C, Cornman H, Kambala A, Kwatra SG. A review on the safety of using JAK inhibitors in dermatology: clinical and laboratory monitoring. Dermatol Ther (Heidelb). 2023;13(3):729-749. doi: 10.1007/s13555-023-00892-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yates M, Mootoo A, Adas M, et al. Venous thromboembolism risk with JAK inhibitors: a meta-analysis. Arthritis Rheumatol. 2021;73(5):779-788. doi: 10.1002/art.41580 [DOI] [PubMed] [Google Scholar]
- 44.Bilal J, Riaz IB, Naqvi SAA, et al. Janus kinase inhibitors and risk of venous thromboembolism: a systematic review and meta-analysis. Mayo Clin Proc. 2021;96(7):1861-1873. doi: 10.1016/j.mayocp.2020.12.035 [DOI] [PubMed] [Google Scholar]
- 45.Chen TL, Lee LL, Huang HK, Chen LY, Loh CH, Chi CC. Association of risk of incident venous thromboembolism with atopic dermatitis and treatment with Janus kinase inhibitors: a systematic review and meta-analysis. JAMA Dermatol. 2022;158(11):1254-1261. doi: 10.1001/jamadermatol.2022.3516 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eNote. A brief introduction to Taiwan's National Health Insurance Research Database (NHIRD)
eTable 1. Diagnostic and procedure codes for covariates
eTable 2. Demographic data of the study population before propensity score matching
eTable 3. Follow-up time (in years) of the study population
eTable 4. Risks of venous thromboembolism in patients with severe atopic dermatitis and those with mild atopic dermatitis
eTable 5. Risks of venous thromboembolism in stratified analyses by age and sex
eTable 6. Risks of deep vein thrombosis in stratified analyses by age and sex
eTable 7. Risks of pulmonary embolism in stratified analyses by age and sex
eTable 8. Risks of venous thromboembolism in the sensitivity analyses excluding systemic steroid users
Data sharing statement