Dear Editor,
Introduction
Pain is among the most frequent reasons for seeking medical care worldwide and across all socioeconomic levels.1 Although many pain conditions are not immediately life-threatening, pain can significantly impact an individual’s daily life and wellbeing long-term.2–4 Despite significant advances in the understanding of pain mechanisms and ways to improve pain diagnosis, the currently available options for effective pain treatment and management inadequately address the scope of the pain burden.
Methods
The INTEGRATE-Pain Consortium (Innovative Medicine Initiative [IMI] – National Institutes of Health [NIH] Transatlantic Emphasis Group on Research and Translation-to-care Efforts for Pain) was created in 2020 as a US-EU (United States–European Union) consortium to advance the pain field, to enhance development of treatments, and to facilitate transfer of existing and new treatments into clinical practice, ultimately improving the lives of people with lived pain experience in the United States and European Union. The similarities in aims and scopes of the work being conducted by IMI-PainCare and NIH staff inspired the establishment of the INTEGRATE-Pain Consortium. While the consortium does not develop or discuss funding opportunities within pain research, members discuss current projects, potential advancements, and published work.
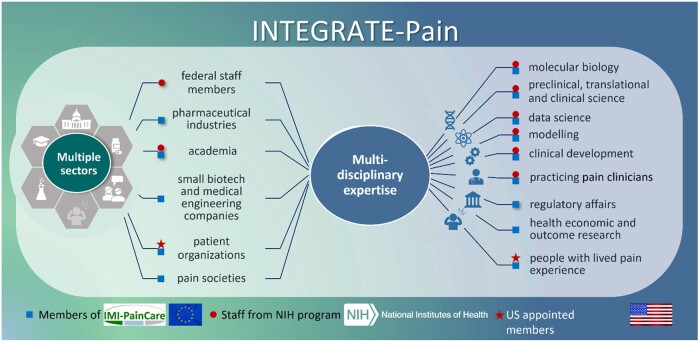
Figure 1 shows the INTEGRATE-Pain Consortium’s composition, with staff at the US NIH and the European IMI-PainCare Consortium. The NIH is the largest public funder of medical research and houses the largest medical research institute in the world. NIH staff involvement in pain research spans preclinical pain research to workforce enhancement and human clinical trials.5 The IMI-PainCare Consortium, composed of 41 partner organizations,6 is established within the IMI’s framework.7 IMI is a public-private partnership between the EU and the European Federation of Pharmaceutical Industries and Associations (EFPIA). It is engaged in the development of patient-reported outcome measures (PROM), development of functional biomarkers, and on improving the understanding of chronic pelvic pain.
Figure 1.
The INTEGRATE-pain consortium.
Formed by IMI-PainCare members and NIH program staff, INTEGRATE-Pain united multiple stakeholders from several sectors (pictured left) and with a broad spectrum of multidiscilinary expertise (pictured right) way to create a transatlantic US-EU ecosystem for advancing pain science and clinical management. IMI-PainCare members (indicated by blue squares) are involved in academia, industry (i.e., pharmaceutical and biotech/engineering), advocacy. IMI-PainCare also has multiple European societies related to pain involved in their initative. NIH staff members (incdicated by red circles) are involved in intramural research, federal policy, federal health science program administration; some staff members also have academic appointmentships, and/or are practicing clinicians. Further, NIH staff have appointed people with lived experience of pain as members of the consortium (indicated by red stars). On an ad-hoc basis, other stakeholders who are not INTEGRATE-Pain members have been or will be invited to participate in events to expand stakeholder representation in intiativites, such as US-based health economics, regulatory affairs, industry represenativites, and practicing clinicians.
NIH and EU leadership met with the INTEGRATE-Pain Consortium in September 2020 to discuss potential collaborative opportunities to advance the pain field. Based on existing core outcome set (COS) projects within the NIH and IMI-PainCare,8,9 leadership encouraged the consortium to establish overarching pain COS to enhance clinical pain management (ie, patient reported outcomes (PRO) and PROM for use in research and clinical practice) among the United States, European Union, and—eventually—globally. Leadership also expressed support of collaborations in: (1) studies on pain and co-morbidities across populations, (2) preclinical cooperation, back-translation, and multisite evaluation, (3) biomarker identification and validation, and (4) preclinical cooperation and clinical infrastructure for testing new non-addictive pain therapeutics.
Results
Achievements: The establishment of the INTEGRATE-Pain Consortium has led to cross-cultural exchange of information and ideas. Such partnerships are increasingly important, as they can help leverage resources and develop pain treatments with broad global impact. Next, based on the feedback from leadership, the consortium has prioritized establishing four overarching pain COS (acute—eg, post-surgical pain, transition, recurrent/episodic—eg, sickle cell disease, and chronic pain—eg, fibromyalgia) that indicate the minimal set of domains that should be used within clinical research and practice. A systematic literature review (SLR) helped inform a three-round Delphi voting process, which included 616 relevant stakeholders (eg, people with lived experience, researchers, and clinicians) throughout each step (Figure 2A). Most stakeholders were not INTEGRATE-Pain members, and although all continents were represented, most stakeholders resided in North America or Europe at the time of voting. Delphi voting ended in early 2023, and the aims, methods, and results from this process will be reported as a separate article.
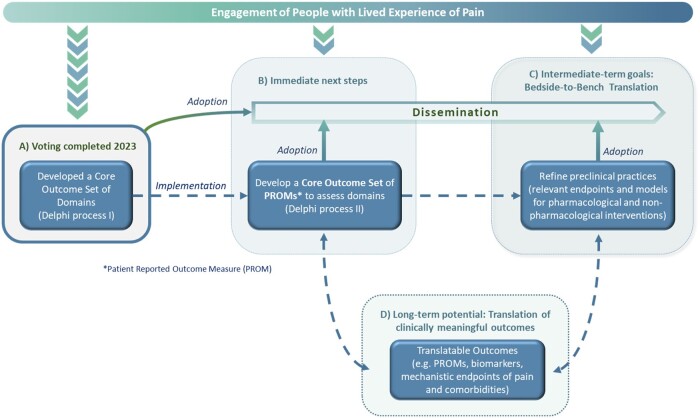
Figure 2.
Potential for the field to build off of INTEGRATE-Pain accomplishments.
INTEGRATE-Pain has completed one major effort and has additional short- and long-term goals. Because partnering with people who have lived experience of pain improves the relevance and impact of initiatives related to pain research and care, these stakeholders have been included early on and throughout the entire process of INTEGRATE-Pain s initiatives, deliverables, and events. A: In early 2023, INTEGRATE-Pain completed the final round of a Delphi process to achieve consensus on Core Outcome Sets (COS) of domains for pain research. B: The immediate next steps for consortium members will be to disseminate the results of the Delphi process to interested stakeholders. This includes two journal articles in preparation, presentations at scientific meetings, webinars for research, clinical, and advocacy organizations, and educational materials for people with lived experience of pain that will be co-produced with lived experience expert members of our Advisory Committee. To ultimately implement the COS, consortium members or other stakeholders, will determine an appropriate set of patient-reported outcomes (PROM). C: These COS can inform ongoing efforts for bedside-to-bench back-translation to refine condition-specific preclinical models and validate clinically relevant endpoints to better model the experience of pain in humans. D: To advance the field in the long-term, collaborative pre-clinical and clinical approaches of INTEGRATE-Pain and the international pain community might further improve clinically meaningful outcomes for pain conditions and comorbidities. The model undertaken by the INTEGRATE-Pain Consortium integrates deliverables from all levels of pain research and pain care to ultimately produce and deliver clinically meaningful outcomes for people living with pain.
Next Steps: Once the four COS have been disseminated to the research community, clinical organizations, and advocacy organizations, the consortium’s immediate next step is to determine an appropriate set of PROMs corresponding to each COS. This process will likely include a SLR, assessment of the psychometric properties of existing PROMs, and the conduct of a stakeholder-engaged Delphi process.10 Discussions among consortium members across the translational research spectrum has also highlighted the importance of back-translating the clinical COS into preclinical approaches for testing pharmacological and non-pharmacological interventions. Endpoints useable for such purposes (eg, facial pain expression or home-cage-analysis in animal models) may be verified in ongoing or future projects. Finally, an anticipated long-term next step for this consortium, as well as the pain community, might include continuing to refine and validate preclinical endpoints and disease models in multiple centers internationally. Refinements to preclinical studies based on back-translation can facilitate forward-translation into revised clinical outcomes. Having clinical and preclinical COS that reciprocally inform each other is a novel approach that bridges existing gaps. Figure 2B–D depicts these next steps and potential long-term impacts.
Discussion
The INTEGRATE-Pain Consortium is a group of scientists spanning the translational pain research spectrum and people with lived experience that aims to improve pain care globally. A unique asset of this group is the diversity of perspectives stemming from members’ disparate training backgrounds, cultural differences, and roles within pain research and engagement. Although not all sectors are currently represented among INTEGRATE-Pain members across the European Union and United States (eg, health economics, implementation science), future efforts will expand representation as relevant to the initiative. The consortium’s diversity has contributed to several strengths and considerations for future consortiums to heed. Strengths include information sharing across countries that has helped align pain research efforts broadly and creativity in generating novel pain research ideas. Pooled COS across countries, for example, can identify treatment targets with the broadest potential impact. Our experience confirms the importance of continuously refining expectations and goals, especially for large consortia. Similarly, identifying potential differences in cross-national regulatory processes early in the consortium facilitates collaborations. Consortia might also consider continuous collaborative approaches to integrate disease-specific preclinical models, clinically relevant models and endpoints, and people with lived pain experience in pain research and care. Finally, this and other consortia should strive to incorporate voices from countries and cultures across all continents to facilitate global equity in pain care. In sum, cross-continent, multiple stakeholder partnerships, such as this consortium, are becoming increasingly feasible and imperative to spark innovative solutions that will address longstanding, global pain care needs.
Acknowledgments
The authors want to thank the other speakers who presented at the INTEGRATE-Pain Scientific Meeting in August 2020: Francesca Bosetti, Ian Gilron, Andre Mouraux, Keith Phillips, Linda Porter, Andrew SC Rice, Cheryl Stucky, Tor Wager, and Mark Zylka. In addition, we express our gratitude to the participants of meeting in September 2020 of the NIH and EU pain leadership, composed of leaders from the National Institute of Neurological Disorders and Stroke (NINDS) and the Helping End Addiction Long-Term (HEAL) Initiative, the European Commission—Directorate General Research & Innovation, the Innovation Medicines Initiative (IMI), and the European Pain Federation (EFIC): Walter Koroshetz, Rebecca Baker, Linda Porter, Karim Berkouk, Pierre Meulien, Elisabetta Vaudano, Gisèle Pickering, Sam Kynman and Sara Badreh. Giulia Bova, Esther Pogatzki-Zahn, and Laura Wandner are preparing a separate pain domain Delphi manuscript that will be submitted for publication in 2023. The authors would like to thank Ralf Baron, Didier Bouhassira, Ulrike Kaiser, and Daniela Rosenberger for their assistance writing the Delphi manuscript. The INTEGRATE-Pain Pain Domain Delphi team would like to thank the INTEGRATE-Pain pain domain advisory committee for their assistance throughout the Delphi process: Adam Anicich, Judy Birch, George Casey, Katie Golden, Kate Nicholson, Peter Tugwell, Dennis Turk, and Paula Williamson. The INTEGRATE-Pain Consortium would like to thank all of the pain researchers, clinicians, people with lived experience, and patient organizations who attended the June 2022 Pain Domain meeting and have voted in the four Delphi surveys. We also want to thank Ursula Wirtz for revising the figures in the research letter.
Authors’ contributions: Laura D Wandner and Petra Bloms-Funke drafted the paper. All authors reviewed and revised the final version of the research letter.
Disclaimer: This letter does not represent the official view of the National Institute of Neurological Disorders and Stroke (NINDS), the National Institutes of Health (NIH), or any part of the US Federal Government. No official support or endorsement of this article by the NINDS or NIH is intended or should be inferred. The statements and opinions presented here reflect the authors’ view and neither IMI nor the European Union, EFPIA, or any Associated Partners are responsible for any use that may be made of the information contained therein.
Contributor Information
Laura D Wandner, Office of Pain Policy and Planning, National Institute of Neurological Disorders and Stroke (NINDS), National Institutes of Health (NIH), Bethesda, MD 20892, United States.
Petra Bloms-Funke, BlomsFunkeResearch GmbH, Würselen, Germany.
Giulia Bova, Office of Pain Policy and Planning, National Institute of Neurological Disorders and Stroke (NINDS), National Institutes of Health (NIH), Bethesda, MD 20892, United States.
Anthony Domenichiello, Office of Pain Policy and Planning, National Institute of Neurological Disorders and Stroke (NINDS), National Institutes of Health (NIH), Bethesda, MD 20892, United States.
Anja Hoffmann, Research & Development, Pharmaceuticals, Bayer AG, Berlin, Germany.
Smriti Iyengar, Division of Translational Research, National Institute of Neurological Disorders and Stroke (NINDS), National Institutes of Health (NIH), Bethesda, MD 20892, United States.
Barbara I Karp, Division of Clinical Research, National Institute of Neurological Disorders and Stroke (NINDS), National Institutes of Health (NIH), Bethesda, MD 20892, United States.
Janelle Letzen, Office of Pain Policy and Planning, National Institute of Neurological Disorders and Stroke (NINDS), National Institutes of Health (NIH), Bethesda, MD 20892, United States.
Hiltrud Liedgens, Global Market Access, Grunenthal GmbH, Aachen, Germany.
Durga P Mohapatra, Division of Neuroscience, National Institute of Neurological Disorders and Stroke (NINDS), National Institutes of Health (NIH), Bethesda, MD 20892, United States.
Jens Nagel, Research & Development, Pharmaceuticals, Bayer AG, Berlin, Germany.
Mary Ann Pelleymounter, Division of Translational Research, National Institute of Neurological Disorders and Stroke (NINDS), National Institutes of Health (NIH), Bethesda, MD 20892, United States.
Esther Pogatzki-Zahn, Department of Anesthesiology, Intensive Care and Pain Medicine, University Hospital Muenster, Muenster, Germany.
Leah Pogorzala, Office of Pain Policy and Planning, National Institute of Neurological Disorders and Stroke (NINDS), National Institutes of Health (NIH), Bethesda, MD 20892, United States.
Jan Vollert, Department of Anesthesiology, Intensive Care and Pain Medicine, University Hospital Muenster, Muenster, Germany; Department of Neurophysiology, Mannheim Center for Translational Neuroscience (MCTN), Heidelberg University, Mannheim, Germany; Department of Surgery & Cancer, Imperial College London, London, United Kingdom; Division of Neurological Pain Research and Therapy, Department of Neurology, University Hospital of Schleswig-Holstein, Campus Kiel, Kiel, Germany.
Sarah A Woller, Division of Translational Research, National Institute of Neurological Disorders and Stroke (NINDS), National Institutes of Health (NIH), Bethesda, MD 20892, United States.
Rolf-Detlef Treede, Department of Neurophysiology, Mannheim Center for Translational Neuroscience (MCTN), Heidelberg University, Mannheim, Germany.
Funding
The IMI-PainCare project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 777500. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA. The NIH and NIH staff did not receive any funding for the INTEGRATE-Pain Consortium.
Conflicts of interest: A.H. is employee of Bayer AG, Germany; E.M.P.-Z reports grants from Innovative Medicines Initiative 2 Joint Undertaking, during the conduct of the study; grants and personal fees from Grünenthal, personal fees from Novartis, personal fees from Medtronic, grants from DFG, grants from BMBF, grants from G-BA, outside the submitted work.; H.L. reports personal fees from Grünenthal GmbH, Aachen Germany, during the conduct of the study; personal fees from Grünenthal GmbH, Aachen Germany, outside the submitted work; J.N. is employee and stockholder of Bayer AG, Germany; J.V. reports personal fees from Casquar, personal fees from Embody Orthopeadics, personal fees from Vertex Pharmaceuticals, outside the submitted work.; P.B.-F. was an employee of the Grünenthal GmbH until 2020 and receives consultancy income from the ConsulTech GmbH since 2020.; R.D.T. reports grants from Innovative Medicines Initiative EU and EFPIA, during the conduct of the study; grants from Deutsche Forschungsgemeinschaft, grants from Esteve, TEVA, personal fees from Bayer, Grünenthal, GSK, Merz, Saluda Medical, Sanofi, outside the submitted work; SI—is an (1) Adjunct Senior Research Professor, Departments of Anesthesia and Clinical Pharmacology, Indiana University School of Medicine, Indianapolis, Indiana, USAÐ2) Retiree and stockholder, Eli Lilly and Company, Indianapolis, Indiana, USA.
References
- 1. Mäntyselkä P, Kumpusalo E, Ahonen R, et al. Pain as a reason to visit the doctor: a study in Finnish primary health care. Pain. 2001;89(2-3):175–180. 10.1016/s0304-3959(00)00361-4 [DOI] [PubMed] [Google Scholar]
- 2. Dureja GP, Jain PN, Shetty N, et al. Prevalence of chronic pain, impact on daily life, and treatment practices in India. Pain Pract. 2014;14(2):E51–E62. 10.1111/papr.12132 [DOI] [PubMed] [Google Scholar]
- 3. Rice ASC, Smith BH, Blyth FM.. Pain and the global burden of disease. Pain. 2016;157(4):791-796. 10.1097/j.pain.0000000000000454 [DOI] [PubMed] [Google Scholar]
- 4. Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D.. Survey of chronic pain in Europe: Prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10(4):287–333. 10.1016/j.ejpain.2005.06.009 [DOI] [PubMed] [Google Scholar]
- 5. NIH Pain Consortium. August 22, 2015. Accessed January 4, 2023. https://www.painconsortium.nih.gov/
- 6. IMI-Paincare. June 4, 2018. Accessed January 4, 2023. https://www.imi-paincare.eu/
- 7. IMI. Innovative Medicines Initiative. May 30, 2008. Accessed January 18, 2023. https://www.imi.europa.eu/
- 8. Pogatzki-Zahn EM, Liedgens H, Hummelshoj L, et al. Developing consensus on core outcome domains for assessing effectiveness in perioperative pain management: results of the PROMPT/IMI-PainCare Delphi meeting. Pain. 2021;162(11):2717–2736. 10.1097/j.pain.0000000000002254 [DOI] [PubMed] [Google Scholar]
- 9. Wandner LD, Domenichiello AF, Beierlein J, et al. NIH’s helping to end addiction long-termSM initiative (NIH HEAL initiative) clinical pain management common data element program. J Pain. 2022;23(3):370–378. 10.1016/j.jpain.2021.08.005 [DOI] [PubMed] [Google Scholar]
- 10. Williamson PR, Altman DG, Bagley H, et al. The COMET handbook: version 1.0. Trials. 2017;18(Suppl 3):280. 10.1186/s13063-017-1978-4 [DOI] [PMC free article] [PubMed] [Google Scholar]