Abstract
Zika virus (ZIKV) is a mosquito-borne flavivirus that causes congenital defects. Sexual transmission of ZIKV was confirmed in a recent epidemic; however, mechanisms behind ZIKV infection and persistence in the male reproductive tract (MRT) are unknown. Previously, we found that approximately 33% of men with symptomatic ZIKV infections shed ZIKV RNA in semen, and some men shed ZIKV RNA for >3 months. Here, we evaluated the semen of 49 ZIKV-infected men to identify immune factors correlating with long-term ZIKV shedding in semen and ZIKV-infected cell types in semen. We found that prolonged ZIKV RNA shedding in semen was associated with MRT inflammation, indicated by higher leukocyte counts and inflammatory cytokine concentrations in semen of long-term versus short-term shedders. In addition, we found ZIKV RNA in seminal leukocytes and epithelial cells. This study of human semen from ZIKV-infected men provides critical insights into the effects of ZIKV on MRT health.
Keywords: Zika virus, flow cytometry, inflammation, leukocytospermia, male reproductive tract, sexual transmission
In a large cohort of Zika virus (ZIKV)–infected men, higher leukocyte counts and inflammatory cytokine levels were present in semen from individuals with prolonged seminal shedding of ZIKV than in those with shorter-term ZIKV shedding.
(See the Editorial Commentary by Haddow, on pages 1125–6.)
Zika virus (ZIKV; Flaviviridae) is a public health concern because it can cause severe congenital abnormalities (eg, microcephaly) in up to 15% of maternal infections [1–4]. ZIKV is primarily transmitted by Aedes spp. mosquitoes; however, sexual transmission of ZIKV has been reported [5–9]. Mathematical models estimate that sexual transmission accounts for up to 23% of ZIKV infections in endemic areas [10–12]. In addition, mouse models of ZIKV infection demonstrate higher viral titers in fetuses when the female is infected via sexual transmission versus subcutaneous inoculation [13]. Thus, understanding the mechanisms behind sexual transmission of ZIKV is critical for decreasing negative fetal outcomes.
During the ZIKV epidemic in the Americas, we conducted a prospective study including 184 men with symptomatic and laboratory-confirmed ZIKV infection to determine how long ZIKV is detectable in semen after symptom onset [14]. Infectious ZIKV and ZIKV RNA were detected in the semen of 1.6% and 33% of participants, respectively. Infectious virus in semen was detected within 30 days of illness onset, whereas ZIKV RNA could be detected in semen for much longer (1 to 6+ months after illness onset). The mechanisms behind long-term shedding of ZIKV RNA in semen is unknown. In addition, the location of ZIKV RNA in human semen (seminal plasma vs cellular fraction) is largely unknown, though ZIKV has been sporadically detected in leukocytes, epithelial cells, germ cell precursors, and sperm cells in human semen [15, 16].
Sexually transmitted infections, whether bacterial or viral, can cause inflammation of the male reproductive tract (MRT), indicated by leukocytospermia (leukocyte count >1 × 106/mL semen) and higher concentrations of inflammatory cytokines (eg, interleukin 6 [IL-6], interleukin [IL-8], and tumor necrosis factor [TNF] α; reviewed in [17–19]). Inflammation may promote clearance of the infection or increase replication by recruiting target cells, as seen with human immunodeficiency virus (HIV) (reviewed in [20]). Chronic MRT inflammation is associated with male infertility (reviewed in [18, 19]). Animal models of ZIKV infection indicate that ZIKV causes inflammation in testes, epididymides, and prostate [21–23]. Leukocytospermia has been reported in men after ZIKV infection [24]. Case reports of 3 men revealed elevated concentrations of inflammatory cytokines in semen after ZIKV infection [25, 26].
In the current study, we sought to characterize semen samples from a large cohort of ZIKV-infected men to investigate why some individuals shed ZIKV RNA in their semen much longer than others and to determine the location of ZIKV RNA within semen. We show that prolonged seminal ZIKV RNA shedding was associated with higher inflammatory markers in the semen, including leukocyte counts and inflammatory cytokine concentrations. In addition, inflammatory markers were positively correlated with ZIKV RNA concentrations in the semen. Finally, we detected ZIKV RNA in seminal plasma as well as leukocytes, epithelial cells, and sperm cells in the semen. These results suggest an association between long-term seminal shedding of ZIKV and MRT inflammation.
METHODS
Estimation of Individual Shedding Times and Classification of Samples
Semen samples were collected from men (n = 184) who had symptomatic, laboratory confirmed ZIKV infections and were tested for ZIKV RNA using reverse-transcription quantitative polymerase chain reaction (RT-qPCR) [14]. We assumed 100% positivity in semen samples at day 0 after symptom onset and assumed that, once cleared of ZIKV, semen remained cleared. In men for whom we had only negative results, we assumed that the change from positive to negative semen occurred between day 0 and the day of first sample collection. In those for whom we had only positive results, we assumed that the change from positive to negative had not occurred and censored these data.
Data were analyzed by a fitting a Weibull distribution to the number of days after onset to clearance of ZIKV [14]. Using parameter estimates from this distribution, we estimated the duration of ZIKV RNA shedding for each individual. Individuals whose estimated duration of shedding was within the highest quartile were termed long-term shedders (shedding >77.5 days) and those with an estimated duration of shedding in the lowest quartile were termed short-term shedders (shedding <22.0 days). ZIKV RNA was not detected in semen samples from the short-term shedders. The study included 26 long-term and 23 short-term shedders. All samples were initial semen collections from different individuals. More details can be found in the Supplementary Materials.
Quantification of Inflammatory Markers in Semen
Semen samples were fractionated into cell pellets and seminal plasma via centrifugation. Cell pellets were prepared for flow cytometry by staining with Ghost Dye Violet 450 (Tonbo Biosciences), anti-human CD45 antibody (clone H130; PE; eBiosciences), and anti-human EpCam antibody (clone 9C4; BV421; Biolegend) to quantify cell populations. Semen cytokine levels in seminal plasma were quantified via human magnetic Luminex assay (R&D Systems). Cytokines analyzed included IL-6, IL-8, monocyte chemoattractant (or chemotactic) protein (MCP) 1, interleukin 1β (IL-1β), interferon (IFN) ɣ, RANTES (regulated on activation of normal T cells expressed and secreted), MCP-3, TNF-α, and interleukins 2 (IL-2), 4(IL-4), 13 (IL-13), and 17A (IL-17A). More details can be found in the Supplementary Materials.
Quantification of Viral RNA
For comparing ZIKV RNA levels in seminal plasma and seminal cell pellets of long-term shedders, RNA was extracted from plasma and cells using the QiaAmp viral RNA kit (Qiagen), following the manufacturer’s protocol. For quantifying RNA in sorted samples, the RNeasy FFPE kit (Qiagen) was used as described elsewhere [27]. RT-qPCR was performed as described elsewhere [28]. The limit of detection was 10 RNA copies per reaction. More details can be found in the Supplementary Materials.
Statistical Analysis
Statistical analyses and data visualization were performed using GraphPad Prism (version 9.3.1) or R (version 4.2.0) software. More details can be found in the Supplementary Materials.
Ethics Statement
The original study for which the semen samples were collected was approved by the Centers for Disease Control and Prevention Institutional Review Board and by the Office of Management and Budget. Additional information is provided in the Supplementary Materials.
RESULTS
Study Groups
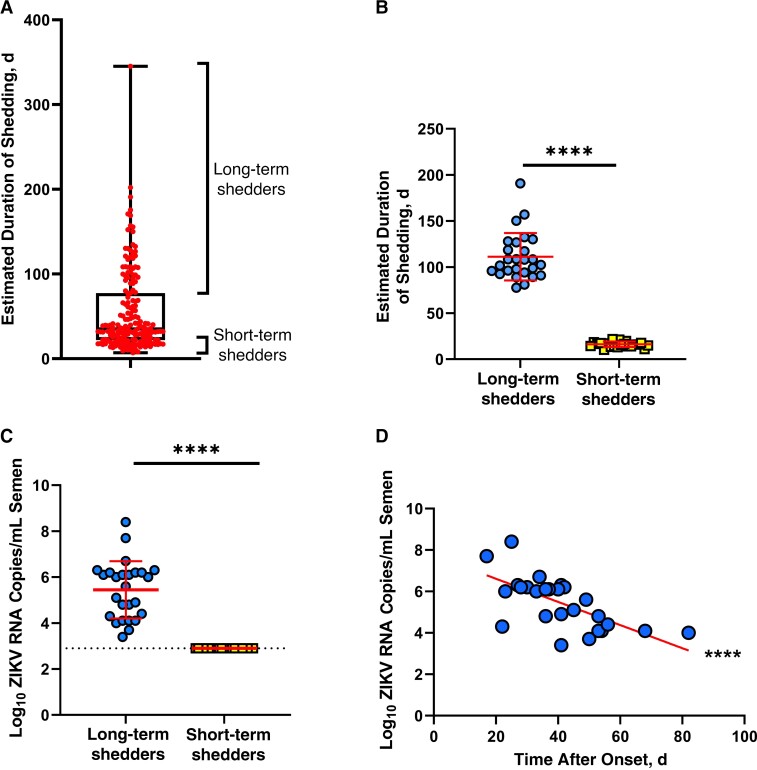
Semen samples used in this study were originally collected as part of a previous study investigating the duration of ZIKV shedding in men [14]. We estimated the duration of shedding of each individual and classified individuals as long-term shedders (highest quartile; n = 26) or short-term shedders (lowest quartile; n = 23) (Figure 1A). All samples were the initial semen sample donated by a given individual. Long-term and short-term shedders differed significantly in estimated duration of shedding (P < .001; Figure 1B), ZIKV RNA copies per milliliter of semen (P < .001; performed in [14]; Figure 1C), and number of ejaculations in the week before sample collection (P = .02; Supplementary Figure 1A).
Figure 1.
Characteristics of long-term and short-term Zika virus (ZIKV) shedders. A, Box-and-whiskers plot of estimated duration of shedding from all individuals whose semen samples were assessed in the original study (n = 184). Box represents interquartile range; whiskers, entire range; line bisecting box, median; and circles, data from individual samples. B, Estimated duration of shedding of long-term (circles; n = 26) and short-term (squares; n =23) shedders whose semen samples were included in this study. Horizontal lines represent medians. C, ZIKV RNA copies in semen samples of long-term and short-term shedders. Horizontal lines represent medians. D, Relationship between ZIKV RNA copies and days after symptom onset in semen from long-term shedders. Line represents result of simple linear regression analysis. ****P < .001.
The 2 groups did not differ in age (Supplementary Figure 1B) or time of sample collection after symptom onset (Supplementary Figure 1C). Initial ZIKV symptoms did not differ between the 2 groups except for a trend toward a higher incidence of joint pain in the short-term shedders (Supplementary Table 1), which is consistent with the absence of joint pain being associated with prolonged shedding, as reported elsewhere [14]. As was reported with the complete data set [14], ZIKV RNA copies in the semen of long-term shedders were significantly negatively correlated with days after symptom onset (P < .001; Figure 1D), indicating that ZIKV RNA copies in semen decreased as time after symptom onset increased.
Semen Leukocyte Counts in Long-term and Short-term ZIKV Shedders
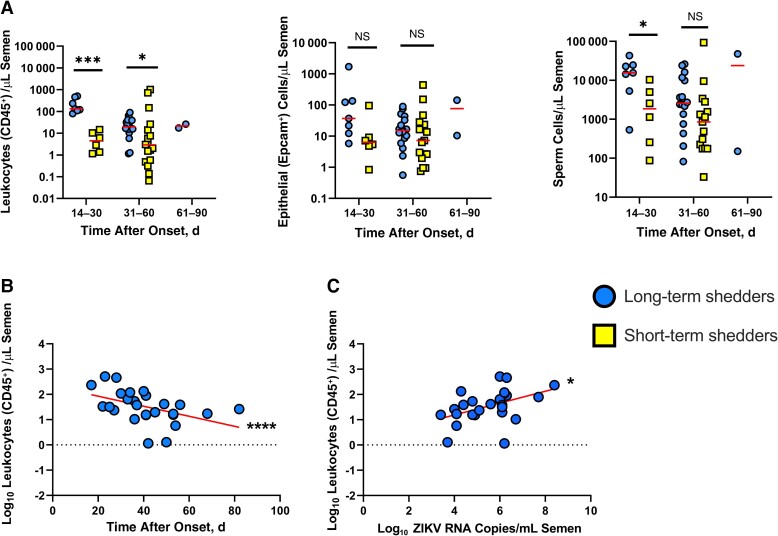
To determine whether long-term shedders had different seminal cell population frequencies than short-term shedders, we used flow cytometry, interrogating for leukocyte (CD45+), epithelial (EpCam+), and sperm (viability dye positive with low SSC intensity) cells. For cell population analysis, long-term and short-term shedders were further divided by time of initial sample collection after symptom onset. Long-term shedders had significantly higher leukocyte counts at 1 month (P = .001) and 2 months (P = .03) after symptom onset than short-term shedders. Leukocyte counts were significantly negatively correlated with days after onset in the long-term shedders (P < .001) but not in the short-term shedders (Figure 2B and Supplementary Figure 2A). There were no significant differences in epithelial cells counts between the 2 groups. Long-term shedders also had significantly higher sperm counts in the first month after symptom onset (P = .02; Figure 2A) Neither epithelial cell counts nor sperm cell counts were correlated with days after onset in either sample group (Supplementary Figure 2B, 2C, 2D, and 2E).
Figure 2.
Leukocyte (CD45+) cell counts are significantly higher in semen from long-term shedders compared to that of short-term shedders. A, Leukocyte (CD45+), epithelial (EpCam+), and sperm cell counts in semen samples from long-term (circles) and short-term (squares) Zika virus (ZIKV) shedders. Data were subgrouped by days after onset: 14–30 days after onset (long-term, n = 7; short-term, n = 6), 31–60 days after onset (long-term, n = 17; short-term, n = 17), and 61–90 days after onset (long-term, n = 2; short-term, n = 0). Horizontal lines represent medians. B, Relationship between leukocyte cell count (log-transformed) and days after onset in long-term shedders. Line represents result of linear regression analysis. C, Relationship between leukocyte cell count (log-transformed) and ZIKV RNA copies in semen (log-transformed). Line represents result of linear regression analysis. *P < .05; ***P < .005; ****P < .001; NS, not significant.
Because ZIKV RNA levels and leukocyte counts in semen of long-term shedders were both significantly negatively correlated with days after symptom onset, we investigated whether there was a relationship between ZIKV RNA levels in semen and cell counts. Leukocyte counts were significantly positively correlated with ZIKV RNA copies in long-term shedders (P = .02; Figure 2C). There were no correlations between ZIKV RNA copies and either epithelial or sperm cell counts (Supplementary Figure 2F and 2G). Taken together, these results indicate that long-term shedders have higher seminal leukocyte counts than short-term shedders and that higher leukocyte counts are associated with higher levels of ZIKV RNA.
Semen Inflammatory Concentrations in Long-term and Short-term ZIKV Shedders
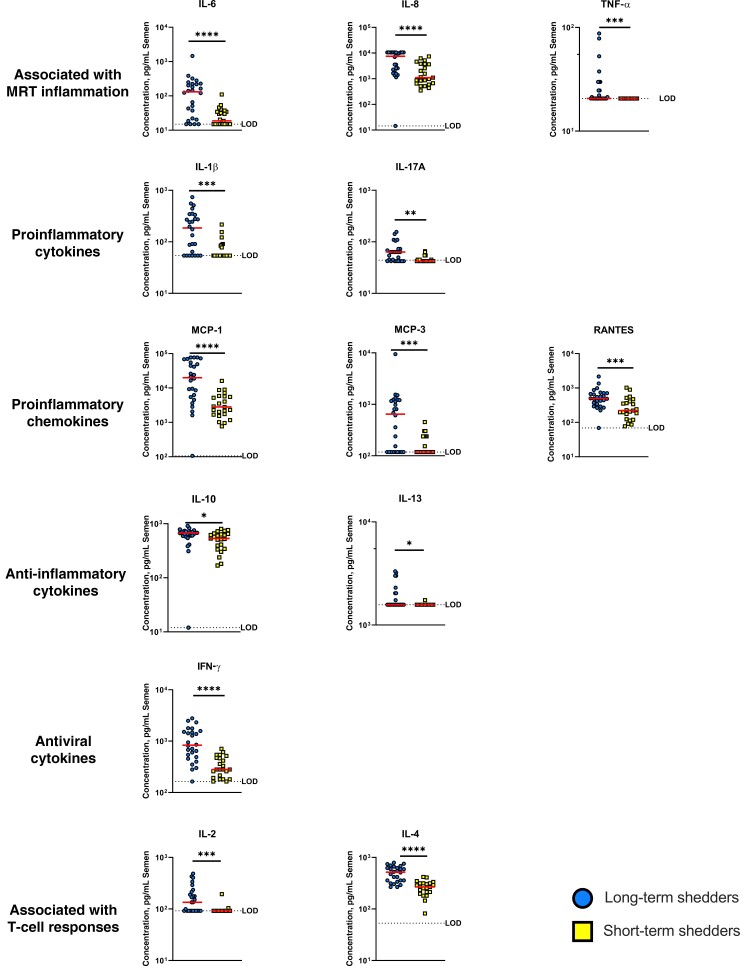
To determine whether long-term shedders have different seminal cytokine profiles than short-term shedders, we performed Luminex assay on seminal plasma samples. The cytokines assessed were chosen based on presence in semen, differential function, association with MRT inflammation, and association with other sexually transmitted infections [25, 26, 29–32] (reviewed in [17, 18, 20]). Compared with semen from short-term shedders, semen from long-term shedders had significantly higher concentrations of all cytokines assayed (P < .02), regardless of function (Figure 3). While still significant, the differences between concentrations of the anti-inflammatory cytokines IL-10 and IL-13 (both P = .01) in semen of long-term versus short-term shedders were smaller than those of all other cytokine groups (Figure 3).
Figure 3.
Concentrations of cytokines in semen of long-term (circles) or short-term (squares) Zika virus shedders. Long-term shedders have higher concentrations of proinflammatory cytokines in semen than short-term shedders. Horizontal lines represent medians. *P < .05; **P < .01; ***P < .005; ****P < .001. Abbreviations: IFN, interferon; IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-13, IL-17A, interleukin 1β, 2, 4, 6, 8, 10, 13, and 17A; LOD, limit of detection; MCP, monocyte chemoattractant (or chemotactic) protein; MRT, male reproductive tract; RANTES, regulated on activation of normal T cells expressed and secreted; TNF, tumor necrosis factor.
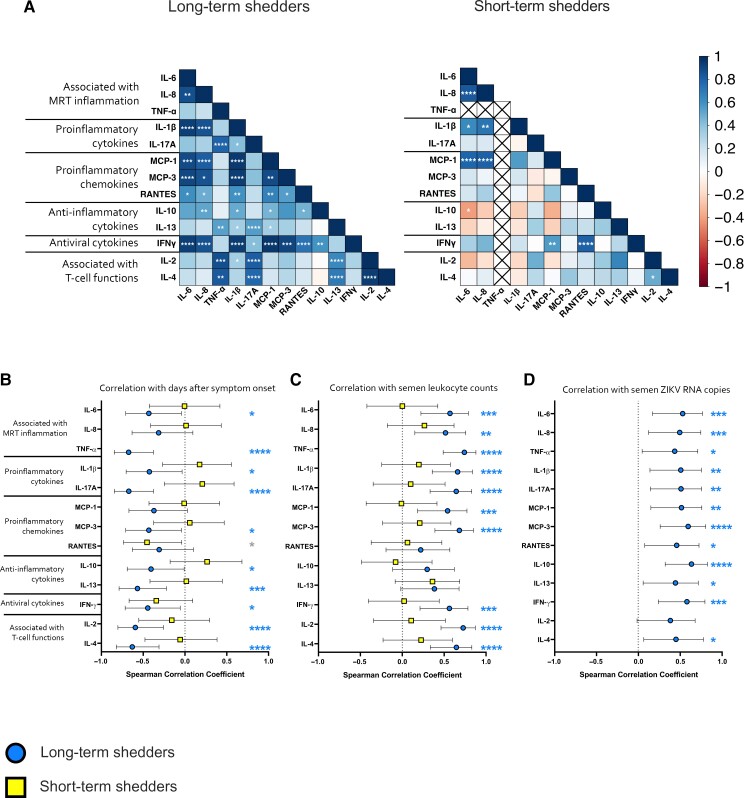
Next, we investigated whether any correlations existed between cytokine concentrations in the long-term shedders and short-term shedders (Figure 4A). In general, there were more significant correlations among semen cytokines in long-term shedders (53.8% of potential correlations) than in short-term shedders (18.2% of potential correlations). In long-term shedders, there were relationships between many of the proinflammatory cytokines and chemokines, including IL-6, IL-8, IL-1β, and MCP-1. The antiviral cytokine IFN-γ was significantly correlated with the anti-inflammatory cytokine IL-10 and the proinflammatory cytokines and chemokines IL-6, IL-8, IL-1β, interleukin 17A, MCP-3, and RANTES. All significant cytokine correlations observed in the short-term shedders were also observed in the long-term shedders, including that observed among IL-6, IL-8, IL-1β, and MCP-1.
Figure 4.
Cytokine concentrations are correlated with other cytokines, days after symptom onset, leukocyte cell counts, and Zika virus (ZIKV) RNA copies in long-term shedders. A. Cytokine networks in long-term and short-term shedders. Data are displayed in a heat map where blue represents a strong positive correlation (r = 1.0); red, a strong negative correlation (r = –1.0); and white, no correlation (r = 0). B–D, Correlations between semen cytokine concentrations and days after symptom onset (B), leukocyte counts in semen (C), or ZIKV RNA copies in semen (D) for long-term (circles) or short-term (squares) shedders. Spearman correlation coefficients and 95% confidence intervals are plotted for each cytokine assessed in each group. Asterisks (blue [long-term shedders] or gray [short-term shedders]) indicate a correlation coefficient significantly different from 0. *P < .05; **P < .01; ***P < .005; ****P < .001. Abbreviations: IFN, interferon; IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-13, IL-17A, interleukin 1β, 2, 4, 6, 8, 10, 13, and 17A; MCP, monocyte chemoattractant (or chemotactic) protein; MRT, male reproductive tract; RANTES, regulated on activation of normal T cells expressed and secreted; TNF, tumor necrosis factor.
Finally, we investigated whether there were any correlations between semen cytokine concentrations and days after symptom onset (Figure 4B), semen leukocyte counts (Figure 4C), or ZIKV RNA copies in semen (Figure 4D). For long-term shedders, there were significant correlations between days after symptom onset and all semen cytokine concentrations except for IL-8, MCP-1, and RANTES. All cytokines except for RANTES, IL-10, and IL-13 were significantly correlated with semen leukocyte counts in long-term shedders. Finally, all cytokines except IL-2 were significantly correlated with semen ZIKV RNA copies in long-term shedders. For short-term shedders, only the concentration of RANTES was significantly correlated with days after symptom onset. No significant correlations were found between any of the cytokines and semen leukocyte counts in short-term shedders. We did not perform correlation analyses for ZIKV RNA copies in short-term shedders because ZIKV RNA was undetectable in these samples. Finally, we found no significant correlations between initial ZIKV symptoms and cell counts or cytokine concentrations in either study group (data not shown).
Taken together, these results show long-term shedders have higher concentrations of cytokines in semen than short-term shedders. In addition, the overall cytokine network in semen of long-term shedders was altered compared to that of short-term shedders. For long-term shedders, cytokine concentrations were negatively correlated with days after symptom onset and positively correlated with semen leukocyte counts and ZIKV RNA copies.
Presence of ZIKV RNA in Seminal Plasma, Leukocytes, Epithelial Cells, and Sperm
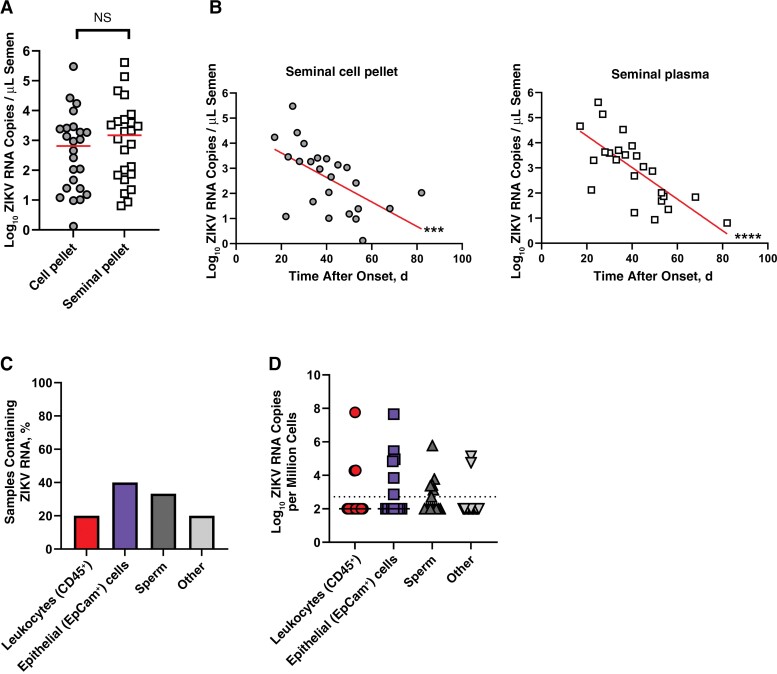
To determine which component of semen contained ZIKV RNA, we performed RT-qPCR on cell pellet and seminal plasma fractions from long-term shedders. We detected similar amounts of ZIKV RNA in both the cell pellet and seminal fractions (approximately 3 log10 RNA copies/mL semen; Figure 5A). In addition, ZIKV RNA copies in both the cell pellet and seminal plasma fractions were significantly negatively correlated with days after onset (Figure 5B).
Figure 5.
Zika virus (ZIKV) RNA is found in leukocytes and epithelial cells in semen of long-term shedders. A, Quantification of ZIKV RNA in semen cell pellets (circles) and seminal plasma (squares) of long-term shedders. Horizontal lines represent medians. B, Relationship between ZIKV RNA and days after onset in semen cell pellets and seminal plasma. Line represents the result of linear regression analysis. C, Frequency of fluorescence-activated cell sorted samples containing ZIKV RNA (n = 15). D, Quantification of ZIKV RNA in fluorescence-activated cell sorted samples. Dotted line represents limit of detection of the RT-qPCR assay.
Next, we investigated what cell types in semen contained ZIKV RNA. Leukocytes (CD45+), epithelial cells (CD45−, EpCam+), unidentified nonsperm cells (other; CD45−, EpCam−), and sperm cells were isolated from seminal cell pellets using fluorescence-activated cell sorting. We used RT-qPCR to quantify the amount of ZIKV RNA within each cell type. We detected ZIKV RNA in the leukocyte populations of 20% of samples, in epithelial cell populations of 40% of samples, in other cells of 20% of samples, and in sperm cells of 33% of samples (Figure 5C). The median concentrations of ZIKV RNA detected were 4.3 log10 RNA copies per million leukocytes, 4.9 log10 RNA copies per million epithelial cells, 4.8 log10 RNA copies per million other cells, and 3.4 log10 RNA copies per million sperm cells. While there were no significant differences in ZIKV RNA copies in these cell types, epithelial cells trended toward having more ZIKV RNA copies per million cells than the remaining cell types (P = .1). Only 2 individuals had detectable ZIKV RNA in all cell compartments tested, and infectious ZIKV was detected in the semen of both of these individuals in the initial study [14]. Taken together, these results indicate that ZIKV RNA is present in the cellular and seminal plasma fractions of semen. Within the cell pellet, leukocytes, epithelial cells, sperm, and other cells may harbor ZIKV RNA.
DISCUSSION
ZIKV RNA can be found in semen of infected men for >6 months after symptom onset [14]. The primary goal of the current study was to characterize immunological differences between semen samples from long-term and short-term shedders. Here, we have shown that semen from long-term shedders contain higher levels of inflammatory markers, including leukocytes and the inflammatory cytokines IL-6 and IL-8, than semen from short-term shedders. The short-term shedders did have significantly more ejaculation events than long-term shedders. However, ejaculation frequency is likely not responsible for lower leukocyte counts and cytokine concentrations in short-term shedders because epithelial cell counts and sperm cell counts (31–60 days after onset group) did not differ significantly between long-term and short-term shedders. These results indicate that long-term shedding of ZIKV in semen is associated with inflammation of the MRT, which can be identified via semen analysis.
A secondary goal of this study was to identify semen cells infected by ZIKV. We detected ZIKV RNA at similar levels within both the seminal plasma and cellular fractions of semen. Some of the ZIKV RNA in the seminal plasma may be due to cell lysis during freeze-thaw of samples. Within the cellular fraction, ZIKV RNA was detected in leukocytes, epithelial cells, sperm cells, and other cells. The other cells may be progenitor germ cells or Sertoli cells, both of which are present in human semen [17] but we were unable to be assess this in our assay due to technical limitations. Although all semen samples had detectable levels of ZIKV RNA in the cell pellet, in some samples ZIKV RNA was not detected in any of the cell compartments investigated. We were able to detect ZIKV RNA only in non-sperm cell populations of ≥10 000 cells. We did not reach this threshold among all samples, likely due to a relatively low recovery rate of cells during sorting (40%–80%). Overall, our results are consistent with those of similar studies investigating ZIKV-infected cells in semen, except that our study included a larger sample size than the aforementioned case reports [15, 16].
Whether ZIKV infection induces inflammation in semen or whether pre-existing inflammatory conditions allowed for the long-term shedding of ZIKV in semen remains unknown. Inflammation of the MRT has been observed after ZIKV infection in animal models. Orchitis (inflammation of the testes) has been reported in mouse models of ZIKV infection, and prostatitis (inflammation of the prostate) has been observed in both mouse and nonhuman primate models of ZIKV infection [21–23]. Prostatitis, but not orchitis, has been observed in humans infected with ZIKV [5, 6, 33]. While we detected inflammatory markers in the semen of ZIKV-infected men, we were unable to determine the source of these seminal leukocytes and cytokines. The inflammatory cytokine IL-8 has previously been associated with inflammation of the prostate during sexually transmitted infections [19, 34]. Leydig and Sertoli cells in the testes are known to produce various cytokines during normal function and in response to infection [18, 19, 35]. ZIKV infection of human Sertoli cells and testicular explants results in up-regulation of proinflammatory genes, including the cytokines IL-6, and IFNs [31, 32]; however, it is unknown whether these cytokines from the testes transit to the semen during ZIKV infection. Studies using animal models are necessary to determine the source of the inflammatory markers within the MRT.
Inflammatory cytokines have been previously detected in the semen of ZIKV-infected men; however, these previous studies included samples from a total of 3 individuals [25, 26]. Our study assessed semen from 49 ZIKV-infected men. Of the cytokines that were investigated by both us and others (IL-6, IL-8, IFN-ɣ, RANTES, and MCP-1), trends were similar between our study and theirs; notably, higher levels of these specific cytokines were associated with higher levels of ZIKV RNA in semen. The levels of cytokines in ZIKV RNA–positive individuals in these previous studies are similar, within 1 log of the median values we reported. The median cytokine concentrations for all cytokines in long-term ZIKV shedders and IL-1β, IL-10, and IFN-γ in short-term shedders were higher than those reported in the semen of otherwise healthy individuals [29, 30]. Intriguingly, the cytokine levels we reported in the semen of ZIKV-infected men also differ from those reported in the serum of ZIKV-infected individuals [25, 36], indicating that ZIKV may induce different immune responses in different compartments.
MRT inflammation negatively affects fertility by decreasing sperm counts and sperm motility (reviewed in [17–19]). Whether ZIKV infection affects fertility is largely unknown. In a prospective-observational study of 15 ZIKV-infected men, total and motile sperm counts were significantly lower at days 11–120 after symptom onset than on day 7 [37]. In our study, we did not observe a decreasing trend in sperm cells over time in either long-term or short-term shedders; however, most of our samples were collected after acute infection, and we did not have pre-infection samples from the study participants to determine their baseline sperm counts. In addition, a majority of the semen samples that we assessed had lower sperm cell counts than normal (15 000–200 000/μL ejaculate); however, it is possible that sperm cells were destroyed during sample processing or storage. It should be noted that some men with lower than normal sperm counts can still conceive without reproductive assistance [38].
Leukocytospermia has been associated with subfertile semen (reviewed in [17]). While we did observe higher leukocyte counts in long-term shedders versus short-term shedders, only 2 samples tested had high enough leukocyte counts to be classified as leukocytospermic (leukocyte count >1000/μL sperm). In addition, high cytokine concentrations of TNF-α (>100 pg/mL) and IFN-γ (>1000 pg/mL) affect sperm motility and fertilization potential in vitro [39, 40]. In some of the long-term shedders, seminal TNF-α and IFN-γ reached or exceeded the concentration necessary to negatively affect sperm functions. Unfortunately, we were unable to address sperm motility or function because all cells in our samples were nonviable, as indicated by uptake of viability dye during flow cytometry and lack of motile cells via microscopy. The impact of ZIKV-associated seminal cytokines on sperm function could be addressed in future studies.
In addition to affecting MRT health, ZIKV-associated inflammation in the MRT may affect transmissibility to sexual partners. While several studies have shown that human semen inhibits ZIKV infection in various anogenital cell lines and vaginal explants, these studies used semen from healthy men and infected cell lines and explants with extracellular ZIKV [41, 42]. Our results suggest that the cellular and cytokine composition of semen from ZIKV-infected men differs from that in uninfected men and that ZIKV may be contained within specific cell types in the semen in addition to the extracellular virus found in the seminal plasma. Intracellular virus may be protected from the inhibitory effects of semen described in the aforementioned studies. Seminal cytokines and intracellular virus can increase transmissibility of HIV to female sexual partners by recruiting susceptible cells to the vaginal mucosa and temporarily inhibiting vaginal immune responses (reviewed in [20]).
Indeed, our results showed increased levels of the chemokines MCP-1 and MCP-3, whose primary functions are recruitment of monocytes and macrophages, cell types known to be susceptible to ZIKV [43]. In addition, HIV-infected seminal macrophages may directly contribute to infection of the vaginal mucosal epithelium by secreting virus directly onto the epithelial cells or invading and migrating through the vaginal epithelium to infect susceptible cells on the basal side of the epithelium (reviewed in [44]). While these processes have not been studied yet in ZIKV infection, they are feasible, considering that ZIKV-infected leukocytes are present in the semen of ZIKV-infected men. Future studies will investigate the roles of seminal cytokines and infected leukocytes in transmission of ZIKV to sexual partners.
The mechanisms behind ZIKV infection and persistence in the MRT are still largely unknown. The study presented here provides insights into the relationship between MRT inflammation and prolonged ZIKV RNA shedding in semen. In addition, the current study illuminated target cells for ZIKV in the semen. Understanding how MRT inflammation is associated with ZIKV infection will allow for a better understanding of ZIKV sexual transmission, including prediction of shedding length, transmission dynamics, and risk assessments. This knowledge is crucial to reduce ZIKV spread and incidence of congenital ZIKV syndrome.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Megan B Vogt, Department of Biomedical Sciences and Pathobiology, Virginia-Maryland College of Veterinary Medicine, Virginia Polytechnic Institute and State University, Blacksburg, Virginia, USA.
Erin M McDonald, Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases, Fort Collins, Colorado, USA.
Mark Delorey, Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases, Fort Collins, Colorado, USA.
Paul S Mead, Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases, Fort Collins, Colorado, USA.
Sarah A Hook, Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases, Fort Collins, Colorado, USA.
Alison F Hinckley, Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases, Fort Collins, Colorado, USA.
Stephen R Werre, Department of Population Health Sciences, Virginia-Maryland College of Veterinary Medicine, Virginia Polytechnic Institute and State University, Blacksburg, Virginia, USA.
Aaron C Brault, Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases, Fort Collins, Colorado, USA.
Nisha K Duggal, Department of Biomedical Sciences and Pathobiology, Virginia-Maryland College of Veterinary Medicine, Virginia Polytechnic Institute and State University, Blacksburg, Virginia, USA.
Notes
Acknowledgments. The authors thank Melissa Makris for her help with flow cytometry and cell sorting. They also thank Xin Luo, PhD, and Xavier Cabana Puig for their help with Luminex assays.
Disclaimer. The conclusions are those of the authors and do not represent the opinions of the Centers for Disease Control and Prevention.
Financial support. This work was supported by the National Institute of Allergy and Infectious Disease, National Institutes of Health (grant R21AI142504).
References
- 1. Honein MA, Dawson AL, Petersen EE, et al. Birth defects among fetuses and infants of US women with evidence of possible Zika virus infection during pregnancy. JAMA 2017; 317:59–68. [DOI] [PubMed] [Google Scholar]
- 2. Reynolds MR, Jones AM, Petersen EE, et al. Vital signs: update on Zika virus-associated birth defects and evaluation of all U.S. infants with congenital Zika virus exposure—U.S. Zika pregnancy registry, 2016. MMWR Morb Mortal Wkly Rep 2017; 66:366–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shapiro-Mendoza CK, Rice ME, Galang RR, et al. Pregnancy outcomes after maternal Zika virus infection during pregnancy—U.S. territories, January 1, 2016-April 25, 2017. MMWR Morb Mortal Wkly Rep 2017; 66:615–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smoots AN, Olson SM, Cragan J, et al. Population-based surveillance for birth defects potentially related to Zika virus infection—22 states and territories, January 2016–June 2017. MMWR Morb Mortal Wkly Rep 2020; 69:67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Foy BD, Kobylinski KC, Chilson Foy JL, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis 2011; 17:880–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Musso D, Roche C, Robin E, Nhan T, Teissier A, Cao-Lormeau VM. Potential sexual transmission of Zika virus. Emerg Infect Dis 2015; 21:359–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hills SL, Russell K, Hennessey M, et al. Transmission of Zika virus through sexual contact with travelers to areas of ongoing transmission—continental United States, 2016. MMWR Morb Mortal Wkly Rep 2016; 65:215–6. [DOI] [PubMed] [Google Scholar]
- 8. D'Ortenzio E, Matheron S, Yazdanpanah Y, et al. Evidence of sexual transmission of Zika virus. N Engl J Med 2016; 374:2195–8. [DOI] [PubMed] [Google Scholar]
- 9. Freour T, Mirallie S, Hubert B, et al. Sexual transmission of Zika virus in an entirely asymptomatic couple returning from a Zika epidemic area, France, April 2016. Euro Surveill 2016; 21(23). doi: 10.2807/1560-7917.ES.2016.21.23.30254. [DOI] [PubMed] [Google Scholar]
- 10. Towers S, Brauer F, Castillo-Chavez C, Falconar AKI, Mubayi A, Romero-Vivas CME. Estimate of the reproduction number of the 2015 Zika virus outbreak in Barranquilla, Colombia, and estimation of the relative role of sexual transmission. Epidemics 2016; 17:50–5. [DOI] [PubMed] [Google Scholar]
- 11. Gao D, Lou Y, He D, et al. Prevention and control of Zika as a mosquito-borne and sexually transmitted disease: a mathematical modeling analysis. Sci Rep 2016; 6:28070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Counotte MJ, Kim CR, Wang J, et al. Sexual transmission of Zika virus and other flaviviruses: a living systematic review. PLoS Med 2018; 15:e1002611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Duggal NK, McDonald EM, Ritter JM, Brault AC. Sexual transmission of Zika virus enhances in utero transmission in a mouse model. Sci Rep 2018; 8:4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mead PS, Duggal NK, Hook SA, et al. Zika virus shedding in semen of symptomatic infected men. N Engl J Med 2018; 378:1377–85. [DOI] [PubMed] [Google Scholar]
- 15. Mahe D, Bourgeau S, Frouard J, et al. Long-term Zika virus infection of non-sperm cells in semen. Lancet Infect Dis 2020; 20:1371. [DOI] [PubMed] [Google Scholar]
- 16. Vanegas H, Gonzalez F, Reyes Y, et al. Zika RNA and flavivirus-like antigens in the sperm cells of symptomatic and asymptomatic subjects. Viruses 2021; 13:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fedder J. Nonsperm cells in human semen: with special reference to seminal leukocytes and their possible influence on fertility. Arch Androl 1996; 36:41–65. [DOI] [PubMed] [Google Scholar]
- 18. Loveland KL, Klein B, Pueschl D, et al. Cytokines in male fertility and reproductive pathologies: immunoregulation and beyond. Front Endocrinol (Lausanne) 2017; 8:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fraczek M, Kurpisz M. Cytokines in the male reproductive tract and their role in infertility disorders. J Reprod Immunol 2015; 108:98–104. [DOI] [PubMed] [Google Scholar]
- 20. Nguyen PV, Kafka JK, Ferreira VH, Roth K, Kaushic C. Innate and adaptive immune responses in male and female reproductive tracts in homeostasis and following HIV infection. Cell Mol Immunol 2014; 11:410–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ma W, Li S, Ma S, et al. Zika virus causes testis damage and leads to male infertility in mice. Cell 2016; 167:1511–24 e10. [DOI] [PubMed] [Google Scholar]
- 22. McDonald EM, Duggal NK, Delorey MJ, Oksanish J, Ritter JM, Brault AC. Duration of seminal Zika viral RNA shedding in immunocompetent mice inoculated with Asian and African genotype viruses. Virology 2019; 535:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Halabi J, Jagger BW, Salazar V, et al. Zika virus causes acute and chronic prostatitis in mice and macaques. J Infect Dis 2020; 221:1506–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huits R, De Smet B, Arien KK, Van Esbroeck M, Bottieau E, Cnops L. Zika virus in semen: a prospective cohort study of symptomatic travellers returning to Belgium. Bull World Health Organ 2017; 95:802–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mansuy JM, El Costa H, Gouilly J, et al. Peripheral plasma and semen cytokine response to Zika virus in humans. Emerg Infect Dis 2019; 25:823–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Oliveira DBL, Durigon GS, Mendes É, et al. Persistence and intra-host genetic evolution of Zika virus infection in symptomatic adults: a special view in the male reproductive system. Viruses 2018; 10:615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Russell JN, Clements JE, Gama L. Quantitation of gene expression in formaldehyde-fixed and fluorescence-activated sorted cells. PLoS One 2013; 8:e73849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lanciotti RS, Kosoy OL, Laven JJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, yap state, Micronesia, 2007. Emerg Infect Dis 2008; 14:1232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vanpouille C, Frick A, Rawlings SA, et al. Cytokine network and sexual human immunodeficiency virus transmission in men who have sex with men. Clin Infect Dis 2020; 71:2655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Olivier AJ, Masson L, Ronacher K, et al. Distinct cytokine patterns in semen influence local HIV shedding and HIV target cell activation. J Infect Dis 2014; 209:1174–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Matusali G, Houzet L, Satie AP, et al. Zika virus infects human testicular tissue and germ cells. J Clin Invest 2018; 128:4697–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Strange DP, Green R, Siemann DN, Gale MJR, Verma S. Immunoprofiles of human Sertoli cells infected with Zika virus reveals unique insights into host-pathogen crosstalk. Sci Rep 2018; 8:8702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kurscheidt FA, Mesquita CSS, Damke G, et al. Persistence and clinical relevance of Zika virus in the male genital tract. Nat Rev Urol 2019; 16:211–30. [DOI] [PubMed] [Google Scholar]
- 34. Mazzoli S, Cai T, Rupealta V, et al. Interleukin 8 and anti-Chlamydia trachomatis mucosal IgA as urogenital immunologic markers in patients with C. trachomatis prostatic infection. Eur Urol 2007; 51:1385–93. [DOI] [PubMed] [Google Scholar]
- 35. Chen Q, Deng T, Han D. Testicular immunoregulation and spermatogenesis. Semin Cell Dev Biol 2016; 59:157–65. [DOI] [PubMed] [Google Scholar]
- 36. Zuniga J, Choreno-Parra JA, Jimenez-Alvarez L, et al. A unique immune signature of serum cytokine and chemokine dynamics in patients with Zika virus infection from a tropical region in southern Mexico. Int J Infect Dis 2020; 94:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Joguet G, Mansuy JM, Matusali G, et al. Effect of acute Zika virus infection on sperm and virus clearance in body fluids: a prospective observational study. Lancet Infect Dis 2017; 17:1200–8. [DOI] [PubMed] [Google Scholar]
- 38. Guzick DS, Overstreet JW, Factor-Litvak P, et al. Sperm morphology, motility, and concentration in fertile and infertile men. N Engl J Med 2001; 345:1388–93. [DOI] [PubMed] [Google Scholar]
- 39. Perdichizzi A, Nicoletti F, La Vignera S, et al. Effects of tumour necrosis factor-alpha on human sperm motility and apoptosis. J Clin Immunol 2007; 27:152–62. [DOI] [PubMed] [Google Scholar]
- 40. Carrasquel G, Camejo MI, Michelangeli F, Ruiz MC. IFN-gamma alters the human sperm membrane permeability to Ca2+. Syst Biol Reprod Med 2014; 60:21–7. [DOI] [PubMed] [Google Scholar]
- 41. Muller JA, Harms M, Kruger F, et al. Semen inhibits Zika virus infection of cells and tissues from the anogenital region. Nat Commun 2018; 9:2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang R, Gornalusse GG, Kim Y, Pandey U, Hladik F, Vojtech L. Potent restriction of sexual Zika virus infection by the lipid fraction of extracellular vesicles in semen. Front Microbiol 2020; 11:574054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Michlmayr D, Andrade P, Gonzalez K, Balmaseda A, Harris E. CD14+CD16+ monocytes are the main target of Zika virus infection in peripheral blood mononuclear cells in a paediatric study in Nicaragua. Nat Microbiol 2017; 2:1462–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Anderson DJ, Politch JA, Nadolski AM, Blaskewicz CD, Pudney J, Mayer KH. Targeting Trojan horse leukocytes for HIV prevention. AIDS 2010; 24:163–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.