Abstract
Objectives
Pneumococcal vaccine recommendations have become increasingly complex. This study aims to understand how national immunization technical advisory groups (NITAGs) and health technology assessment (HTA) agencies of 5 European countries and the United States formed their pneumococcal vaccine recommendations, by providing reviewed evidence and key drivers for new recommendations.
Methods
Centers for Disease Control and Prevention, European Centre for Disease Prevention and Control, and National Health Authorities Web sites were screened to capture the evolution of pneumococcal recommendations. A narrative review was conducted on NITAGs and HTA bodies’ Web sites. Assessments of pneumococcal vaccines published from 2009 to 2022 were included.
Results
Thirty-four records were identified including 21 assessments for risk groups, 17 for elderly, and 12 for children. Burden of disease and vaccine characteristics were almost systematically reviewed during assessments. All 6 countries recommended the use of higher-valent pneumococcal vaccine (PCV; i.e., PCV10 and PCV13) in childhood vaccination programs, given their broader serotype coverage and their comparable profile to PCV7. PCV13 was progressively added to the vaccine schedule (in addition to polysaccharide vaccine) in at least the high-risk group, given the high burden in this population and expected additional benefits of PCV13. For the elderly, unlike the United States, European countries issued negative recommendation for PCV13 routine use because of substantial herd effects from childhood vaccination program making PCV13 likely not cost-effective.
Conclusions
This research provides an overview of decision-making processes for higher-valent PCVs recommendations and could be of interest to anticipate the place of next generation of PCVs in the vaccination landscape.
Highlights
By describing evidence-based criteria for decision making, this study emphasizes the framework analysis of NITAGs and HTA bodies when assessing pneumococcal vaccines and demonstrates that variation exists between countries and also according to population evaluated.
While the burden of disease and immunogenicity/efficacy data were almost systematically reviewed by national stakeholders, economic assessments were reported to a lesser extent but played a major role in the limited use of PCV13 in the adult population.
Keywords: pneumococcal vaccine, recommendations, HTA, NITAG, Europe, US
Pneumococcal diseases caused by Streptococcus pneumoniae are a major public health problem worldwide. 1 In high-income countries, young children, older adults, an individuals with underlying conditions are the most affected population.1,2S. pneumoniae is responsible for serious infections including pneumonia, meningitis, and febrile bacteriemia so-called invasive pneumococcal diseases (IPD) and more common but less serious manifestations such as acute otitis media (AOM), sinusitis, and bronchitis.3,4
Two types of pneumococcal vaccines are currently available: pneumococcal polysaccharide vaccine (PPV) represented by PPV23 (PNEUMOVAX 23, Merck & Co.), approved and marketed since 1983, and pneumococcal conjugate vaccine (PCV). In 2000 and 2001, PCV7 (PREVENAR, Wyeth Pharmaceuticals) was approved for use in young children in the United States and Europe, respectively. Thereafter, PCV7 was introduced in childhood national immunization programs (NIPs) of many countries worldwide. In 2009 and 2010, 2 additional PCVs, PCV10 (SYNFLORIX, GSK plc.) and PCV13 (PREVENAR 13/PREVNAR 13, Pfizer Inc.), were licensed5,6 and progressively replaced PCV7 in childhood NIPs, which was withdrawn. A few years later, PCV13 was approved for use in adults. In 2021, PCV15 (VAXNEUVANCE, Merck & Co.) and PCV20 (PREVENAR 20/APEXXNAR, Pfizer Inc.) were licensed in the United States and Europe7–10 and recommended for use in US adults at the end of 2021. 11 In June 2022, PCV15 was also recommended for children in the United States. 12
The use of PCVs for more than 2 decades reduced the pneumococcal disease burden considerably, with a simultaneous increase in the incidence of nonvaccine serotypes. 13 The serotype replacement has been particularly observed in Europe, whereas the United States reported stable rates of non-PCV13 serotype IPD after PCV13 introduction.13–15 Emerging new clinical isolates encouraged the development of higher-valent PCVs to maintain a low disease burden.
Vaccine recommendations are generally issued at the national level by health technology assessment (HTA) agencies and/or national immunization technical advisory groups (NITAGs). HTA agencies issue specific assessments during the pricing and reimbursement process, and NITAGs provide independent evidence-based recommendations on vaccines to guide national stakeholders in their decision. However, roles and responsibilities vary across countries, making the vaccine market access pathways heterogenous and complex, as described in European countries. 16
Different pneumococcal vaccine recommendations exist across Europe and other countries, and the regulatory approval of PCVs for adults has increased their complexity.17,18 The recommendations vary according to countries and populations, with different vaccines and vaccination schedules.
With the recent market authorization of PCV15 and PCV20 and the future arrival of other vaccine candidates, health authorities will be required to evaluate the potential introduction of upcoming vaccines in NIPs. The disparities in processes evaluation and diverse pneumococcal recommendations make it difficult to predict the place of new vaccines in the pneumococcal vaccination landscape. Therefore, it is useful to understand the decision-making criteria of past recommendations to anticipate future strategies.
This study aimed to describe the process by which HTA agencies and NITAGs have formulated their pneumococcal vaccine recommendations by 1) providing an overview of recommendation evolutions in 5 Western European countries and the United States and 2) identifying the main criteria assessed and the key drivers or barriers for new recommendations since licensure of PCV10 and PCV13.
Methods
Search Strategy and Data Sources
A narrative literature review of HTA agencies and NITAGs’ published assessments on pneumococcal recommendations was conducted in 6 countries: France, Germany, Spain, the Netherlands, the United Kingdom, and the United States. We included all national recommendation reports for pneumococcal vaccination in the 3 main eligible populations, defined as young children younger than 2 y of age, older adults aged over 60 or 65 y (so-called elderly), and children or adults with a risk for pneumococcal disease (so-called risk groups). Risk groups are defined at country level, and subrisk groups could benefit from different vaccine recommendations. To simplify the analysis, risk groups were divided into low-risk groups (generally defined as individuals with respiratory, heart, or kidney chronical diseases, alcoholism, etc.), and high-risk groups (generally defined as individuals with immunocompromising conditions, cochlear implants, asplenia, etc.).
Two search strategies were performed to answer the study objectives. The first search was conducted on the Center for Disease Control and Prevention (CDC), 19 the European Centre for Disease Prevention and Control, 20 and National Health Authorities (i.e., health ministries, government sites, etc.) Web sites, to capture the evolution of pneumococcal recommendations for children, elderly, and risk groups in each country without time restriction. The second search was conducted on national HTA bodies and NITAGs’ Web sites to capture assessment reports including criteria and evidence reviewed when formulating pneumococcal vaccination recommendations. The search, conducted by 2 reviewers, included pneumococcal vaccines related terms, and was adapted to each country and Web site explored. Search details are available in Supplementary Table 1. All documents reporting justification for new recommendations and published by NITAGs or HTA bodies between January 2009 and March 2022 were included. The 2009 threshold date was chosen to match PCV10 and PCV13 market authorizations5,6 and subsequently the update of NIPs. Documents that did not evaluate pneumococcal vaccines, did not report justification for change, or that covered an assessment already captured were excluded. For Spain, the research was focused on recommendations and assessments at national level. For the United Kingdom and the United States, according to the way of working of the Joint Committee on Vaccination and Immunisation (JCVI) and the Advisory Committee on Immunization Practices (ACIP), respectively, meeting minutes were consulted for potential additional information, but they were not extracted in the main research. Records published in the local language were translated into English.
Data extraction and analysis
For the first search, country-specific data pertaining to each targeted population were collected. For the second search, relevant data were extracted into a prespecified data extraction grid (Table 1). Extracted criteria were based on the most common decision-making criteria included in the national frameworks for vaccine assessment identified by Donadel et al. 21 and defined as burden of disease, vaccine efficacy/effectiveness, vaccine safety, economic evaluation, vaccine impact on health and nonhealth outcomes, and cost-effectiveness of alternatives. Adjustments were made to fit specific assessment of pneumococcal vaccine, and criteria were subdetailed by disease type (IPD, pneumonia, and AOM).
Table 1.
Data Extraction Framework
| General information |
| 1. Source |
| 2. Country |
| 3. Date |
| 4. Vaccine |
| 5. Population |
| 6. Purpose |
| 7. Final decision |
| Criteria for decision making |
| 8. Burden of disease • IPD, pneumonia, AOM |
| 9. Impact of current vaccination program • Direct impact on targeted population, indirect impact of children vaccination on other population (herd effects) |
| 10. Vaccine safety |
| 11. Vaccine efficacy/effectiveness and
immunogenicity • Against IPD, pneumonia, AOM |
| 12. Public health impact of alternative
strategy • Direct vaccination impact on targeted population, indirect impact on other age groups (herd effects) |
| 13. Economic evaluation • With IPD, pneumonia, AOM as clinical inputs of models |
AOM, acute otitis media; IPD, invasive pneumococcal diseases.
Results
Search Results
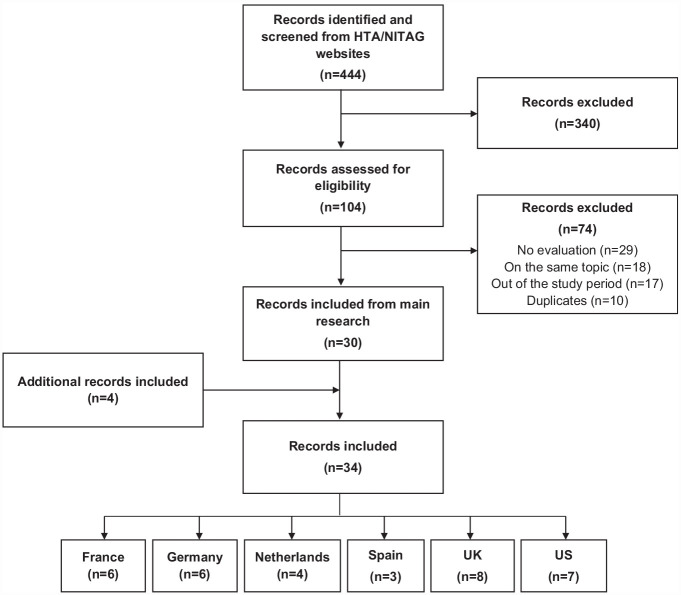
Overall, 444 records were identified, among which 414 were excluded. Details of included and excluded documents are available in Supplementary Table 2. A total of 30 records were included from the main search. Four additional records from JCVI meeting minutes and archives were included to highlight major changes not captured through the main search. For the ACIP, recommendation evolutions were covered by the Morbidity and Mortality Weekly Reports. Overall, 34 records were included for analysis (Figure 1). A summary of population and vaccines assessed by country is provided in Supplementary Table 3.
Figure 1.
Inclusion/exclusion flowchart.
HTA, Health Technology Assessment; NITAG, National Immunization Technical Advisory Group.
Chronological Evolution of Vaccine Recommendations
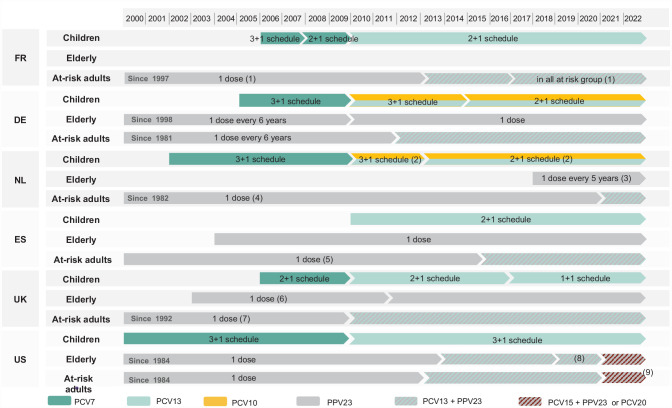
The evolution of recommendations in children, elderly, and adult risk groups are detailed in Figure 2.
Figure 2.
Overview of pneumococcal vaccine recommendations in children, elderly, and adult risk groups.
PCV, Pneumococcal conjugate vaccine; PPV, Pneumococcal polysaccharide vaccine.
(1) Additional dose of PPV23 could be administered after 5 years. (2) PCV10 won national tender and is the only vaccine used in the national childhood immunization program. (3) Revaccination recommended every 5 years in adults from 60 to 75 years of age. (4) Revaccination after 5 years is desirable in people with asplenia, sickle cell disease, liquor leakage; should be consider for Hodgkin disease; may be considered for non-Hodgkin lymphoma. (5) Additional dose of PPV23 could be administered after 5 years for some immunocompromised persons. (6) In March 2011, the JCVI issued a statement for the discontinuation of the PPV23 elderly vaccination program. In July 2011, the JCVI finally recommended to maintain this vaccination strategy. (7) Additional dose of PPV23 should be administered after 5 years in people with long-term immunosuppression, chronical kidney disease and asplenia. (8) In 2019, the elderly recommendation was modified and PCV13 administration was recommended based on shared clinical decision-making. (9) Repeat PPV23 after 5 years for those with immunocompromising conditions.
Recommendations for Children
PCV7 was introduced in the United States as a childhood NIP in 2000 22 and between 2002 and 2006 in European countries23–26 (except in Spain, which did not advise the routine use of PCV7 nationally 27 ). All countries recommended replacing PCV7 with PCV10 or PCV13 soon after their market authorization. France, Spain, the United Kingdom, and the United States recommended PCV13,26,28–30 whereas the Netherlands and Germany did not issue a preferential recommendation.31,32
In all countries, PCV7 was first recommended with a 4-dose regimen (3 doses and a booster [i.e., 3+1 schedule]), except in the United Kingdom, which directly recommended a reduced 2+1 schedule 26 (and later a 1+1 schedule with PCV13). Gradually, other European countries reduced the 3+1 schedule to a 2+1 schedule,28,31,33 and Spain directly recommended PCV13 with a 2 + 1 schedule. 29 The United States maintained the 3+1 schedule. 30
Recommendations for the Elderly
Except in France,34,35 an elderly vaccination program with PPV23 was introduced in all countries36–39 between 1984 (the United States) and 2018 (the Netherlands). In 2011, the United Kingdom advised the discontinuation of the vaccination program and eventually decided to maintain it.40,41
In 2014, the United States recommended the use of PCV13 in series with PPV23 for adults aged older than 65 y. 42 In 2019, the recommendation was limited to shared clinical decision making. 43 Recently, PCV20 alone or PCV15 in series with PPV23 was recommended in the elderly. 11
Recommendations for Risk Groups
In all 6 countries, PPV23 was recommended in risk groups before 2000.22,23,37–39,44 Revaccination with additional PPV23 doses was commonly recommended in some high-risk groups (generally defined as individuals with immunocompromising conditions). The PPV23 vaccination program was gradually extended to additional children and adult risk groups.22,23,37,38,45–49
After its licensure, PCV13 was progressively introduced in immunization programs but generally limited to high-risk groups.38,39,50 54–55 In France, all risk groups were offered PCV13 in addition to PPV23 since 2017. 35 In 2021, PCV20 alone or PCV15 in series with PPV23 was proposed to all adult risk groups in the United States. 11 Details on risk group vaccine recommendations by country are provided in Supplementary Table 4.
Criteria Assessed and Key Drivers/Barriers for Recommendations for Children
Recommendations for children were assessed in 12 records. The most frequent topics covered in NITAG and HTA bodies assessments were the introduction of higher-valent PCVs (n = 6) and the vaccination schedule reduction (n = 5). All records reported the disease burden and the direct and indirect impact of current childhood NIPs, and 11 reported immunogenicity or efficacy data. Safety data (n = 6), economic evaluations (n = 6), and the public health impact of alternative strategies (n = 5) were less frequently reviewed (Table 2).
Table 2.
Overview of Criteria Assessed for Recommendations for Children
| Country | Date | Vaccine | Purpose | Final Recommendation | Burden of Disease | Impact of Current Program | Safety | Immunogenicity/Efficacy | Public Health Impact of Alternatives | Cost-effectiveness a | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|
| France | 2009 | PCV13 | PCV13 introduction | PCV13 at 2, 4, and 12 mo | ✓IPD | ✓direct, indirect | ✓ | ✓ | ✓direct, indirect | 28 | |
| France | 2012 | PCV13 | PCV13 reassessment | PCV13 at 2, 4, and 12 mo | ✓IPD | ✓direct, indirect | 56 | ||||
| France | 2018 | PCV10 | PCV10 introduction | PCV13 at 2, 4, and 12 mo | ✓IPD | ✓direct, indirect | ✓ | ✓IPD, pneumonia, AOM | 57 | ||
| Germany | 2015 | PCV10 PCV13 |
Reduced schedule | PCV10 or PCV13 at 2, 4, and 11–14 mo | ✓IPD | ✓direct, indirect | ✓IPD, pneumonia | 31 | |||
| Netherlands | 2010 | PCV10 PCV13 |
PCV10/13 introduction Reduced schedule |
PCV10 or PCV13 at 2, 3, 4, and 11 mo | ✓IPD | ✓direct, indirect | ✓ | ✓IPD, pneumonia, AOM |
✓direct, indirect |
✓IPD, pneumonia, ± AOM | 32 |
| Netherlands | 2013 | PCV10 PCV13 |
PCV10/13 reassessment Reduced schedule |
PCV10 or PCV13 at 2, 4, and 11 mo | ✓IPD | ✓direct, indirect | ✓ | ✓IPD, pneumonia, AOM |
✓direct, indirect |
✓IPD, pneumonia, ±AOM | 33 |
| Spain | 2009 | PCV10 PCV13 |
PCV10/13 introduction Reduced schedule |
PCV13 at 2,4, and 12–15 mo | ✓IPD, pneumonia | ✓direct, indirect | ✓ | ✓IPD, pneumonia, AOM | ✓ a | 29 | |
| United Kingdom | 2009 | PCV10 PCV13 |
PCV10/13 introduction | In process b | ✓IPD | ✓direct, indirect | ✓ | ✓ | 26 | ||
| United Kingdom | 2010 | PCV10 PCV13 |
PCV10/13 introduction | PCV13 at 2, 4, and 12 mo c | ✓IPD | ✓direct, indirect | ✓ | ✓ | 58 | ||
| United Kingdom | 2014 | PCV10 | PCV10 introduction | PCV13 at 2, 4, and 12 mo | ✓IPD | ✓direct, indirect | ✓AOM | 59 | |||
| United Kingdom | 2018 | PCV13 | Reduced schedule | PCV13 at 3 and 12 mo d | ✓IPD | ✓direct, indirect | ✓IPD, pneumonia | ✓direct, indirect | 60 | ||
| United States | 2010 | PCV13 | PCV13 introduction | PCV13 at 2, 4, 6, and 12–15 mo | ✓IPD | ✓direct, indirect | ✓ | ✓IPD, pneumonia, AOM |
✓direct |
✓IPD, pneumonia, AOM e | 30 |
AOM, acute otitis media; IPD, invasive pneumococcal diseases; JCVI, Joint Committee on Vaccination and Immunisation.
Cost-effectiveness criterion was considered through a literature review of cost-effectiveness models.
Advice from subcommittee was reviewed and validated by the JCVI in February 2010, 61 and a final decision was stated in December 2010. 58
The final decision was stated by the JCVI in February 2011. 40
Introduction of Higher-Valent PCVs (Children)
Evaluation of PCV10 and PCV13 were identified for France, the Netherlands, Spain, and the United Kingdom. In the United States, only PCV13 was evaluated, as PCV10 was never registered. The 5 NITAGs and HTA bodies issued positive recommendations for the use of PCV13, and the Netherlands also recommended the use of PCV10.28–30,32,58
The switch to higher-valent PCVs was mainly driven by the epidemiological context showing a substantial impact of PCV7 vaccination programs but also an increase of IPD due to non-PCV7 serotypes, which could be partially covered by PCV10 and PCV13. Both vaccines also demonstrated immunogenicity and safety profiles comparable with PCV7. Cost-effectiveness evaluations were in favor of using higher-valent PCVs instead of PCV7 in the Netherlands, 32 the United Kingdom,26,58,59 and the United States 30 (no incremental cost-effectiveness ration [ICER] reported in the Netherlands and the United Kingdom—strategy being either dominant or with an ICER less than $35,000/quality-adjusted life-year [QALY] according to scenarios in the United States).
France, Spain, and the United Kingdom advised against the use of PCV1029,57–59 and issued a preferential recommendation for PCV13, given the large proportion of IPD, serotype replacement, and antimicrobial resistance due to the 3 additional serotypes included in PCV13. Potential additional benefits of PCV10 (prevention of AOM due to Haemophilus influenzae; cross-protection between the serotype 19F, included in PCV10, and the serotype 19A) were evaluated, but the evidence was considered limited. Conversely, the Netherlands advised for the use of both vaccines (based on the comparable immunogenicity and safety profiles and expected health benefits) with slight preference for PCV13, mainly due to its broader serotype coverage.32,33
Reduced Vaccination Schedule (Children)
After regulatory approval of the alternative 2-dose primary series, this vaccination schedule was assessed in the 6 countries to reduce the needle burden in children and ensure pneumococcal NIPs at lower cost. From 2009 onward, Spain, the Netherlands, and Germany implemented a reduced 2+1 schedule.29,31–33 This decision was driven by favorable immunogenicity and/or efficacy data. Germany also acknowledged other benefits such as improved vaccine acceptance, less painful vaccination, and cost savings. 31 The Netherlands initially issued a negative recommendation for a reduced schedule given the uncertainty around the indirect protection provided by PCVs at time of NIP implementation, 32 although they finally concluded that solid herd effects have been built up after several years of PCVs use. 33 In the United States, immunogenicity/efficacy data regarding reduced schedules (2+1 or 3+0) were assessed through the GRADE approach and discussed by the working group. At that time, the committee concluded that some evidence was lacking (e.g., head-to-head comparisons between the reduced and full-dose schedule), and the decision was delayed. However, this topic was not covered in other ACIP meetings, and the 3+1 schedule is still currently in use. 66
A 1+1 schedule instead of the 2+1 schedule was implemented in the United Kingdom. 60 This decision was driven by significant reductions in vaccine-type carriage and disease in all age groups, immunogenicity data of the 1+1 schedule indicating that the postbooster responses were preserved for most serotypes, and public health impact estimates indicating a limited increase in number of IPDs. The strategy would also rationalize health service resources, simplify the vaccine schedule, reduce the needle burden in infants, and allow space for new vaccines to be introduced.
Criteria Assessed and Key Drivers/Barriers for Recommendations for the Elderly
Recommendations for elderly individuals were assessed in 17 records. Topics covered were the introduction of PCVs or PPV23 (n = 12) and the reassessment of the current vaccination program (n = 7). The disease burden and the immunogenicity/efficacy data (n = 17) were reported in all records, with specific attention to pneumococcal pneumonia disease. The indirect impact of childhood NIPs (n = 14), direct impact of the current elderly program (n = 11), economic evaluations (n = 12), safety data (n = 11), and the public heath impact of alternative strategies (n = 8) were also largely reported (Table 3).
Table 3.
Overview of Criteria Assessed for Recommendations for the Elderly
| Country | Date | Vaccines | Purpose | Final Recommendation | Burden of Disease | Impact of Current Program | Safety | Immunogenicity/ Efficacy | Public Health Impact of Alternatives | Cost-effectiveness | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|
| France | 2017 | PCV13, PPV23 | PCV13 introduction PPV23 introduction |
No vaccination | ✓ IPD, pneumonia | ✓ indirect | ✓ | ✓IPD, pneumonia | ✓ direct | ✓IPD, pneumonia | 35 |
| Germany | 2009 | PPV23 | PPV23 reassessment | One dose of PPV23 in ≥60 y | ✓ IPD | ✓ | ✓IPD, pneumonia | 45 | |||
| Germany | 2012 | PCV13 | PCV13 introduction | One dose of PPV23 in ≥60 y | ✓ IPD | ✓IPD, pneumonia | 67 | ||||
| Germany | 2016 | PCV13, PPV23 | PCV13 introduction | One dose of PPV23 in ≥60 y | ✓IPD, pneumonia | ✓direct, indirect | ✓ | ✓IPD, pneumonia | ✓ direct | ✓IPD, pneumonia | 68 |
| Netherlands | 2018 | PCV13, PPV23 | PCV13 introduction PPV23 introduction |
PPV23 every 5 y in 60–75 y | ✓ IPD, pneumonia | ✓ indirect | ✓ | ✓IPD, pneumonia | ✓ direct | ✓IPD, pneumonia | 69 |
| Spain | 2015 | PCV13, PPV23 | PCV13 introduction | One dose of PPV23 in ≥65 y | ✓IPD, pneumonia | ✓direct, indirect e | ✓ | ✓IPD, pneumonia | ✓ IPD, pneumonia f | 53 | |
| Spain | 2017 | PCV13, PPV23 | PCV13 introduction | One dose of PPV23 in ≥65 y | ✓IPD, pneumonia | ✓ | ✓IPD, pneumonia | ✓ direct | ✓ IPD, pneumonia | 70 | |
| United Kingdom | 2009 | PPV23 | PPV23 reassessment | One dose of PPV23 in ≥65 y | ✓ IPD | ✓ direct, direct | ✓ IPD | ✓ | 26 | ||
| United Kingdom | 2010 | PPV23 | PPV23 reassessment | In process | ✓ IPD | ✓direct, indirect | ✓ IPD, pneumonia | 58 | |||
| United Kingdom | 2011 | PPV23 | PPV23 reassessment | No vaccination a | ✓ IPD, pneumonia | ✓direct, indirect | ✓ | 71 | |||
| United Kingdom | 2011 | PPV23 | PPV23 reassessment | One dose of PPV23 in ≥65 y b | ✓ | ✓direct, indirect | ✓ IPD, pneumonia | ✓ | 72 | ||
| United Kingdom | 2013 | PCV13 | PCV13 introduction | P One dose of PPV23 in ≥65 y c | ✓ IPD, pneumonia | ✓direct, indirect | ✓ IPD, pneumonia | ✓ | ✓IPD, pneumonia | 54 | |
| United Kingdom | 2015 | PCV13, PPV23 | PCV13 introduction | One dose of PPV23 in ≥65 y d | ✓IPD, pneumonia | ✓direct, indirect | ✓ | ✓ IPD, pneumonia | ✓direct | ✓IPD, pneumonia | 39 |
| United States | 2010 | PPV23 | PPV23 reassessment | One dose of PPV23 in ≥65 y | ✓IPD | ✓indirect | ✓ | ✓IPD, pneumonia | 48 | ||
| United States | 2014 | PCV13 | PCV13 introduction | PCV13+PPV23 (after 1 year) in ≥65 y | ✓IPD, pneumonia | ✓ direct, indirect | ✓ | ✓ IPD, pneumonia | ✓direct | ✓IPD, pneumonia g | 42 |
| United States | 2019 | PCV13 | PCV13 reassessment | PCV13 (after SCDM) + PPV23 (after 1 y) in ≥65 y | ✓IPD, pneumonia | ✓direct, indirect | ✓ | ✓ IPD, pneumonia | ✓IPD, pneumonia h | 43 | |
| United States | 2021 | PCV15, PCV20 | PCV15/20 introduction | PCV20 or PCV15+PPV23 (after 1 y) in ≥65 y | ✓IPD, pneumonia | ✓direct, indirect | ✓ | ✓ IPD, pneumonia |
✓direct |
✓IPD, pneumonia | 11 |
AOM, acute otitis media; IPD, invasive pneumococcal diseases; JCVI, Joint Committee on Vaccination and Immunisation; SCDM, shared clinical decision making.
The final decision was the result of deep evidence reviews by JCVI in February 2011 40 and the Pneumococcal Sub-committee in May 2011. 73
The final decision was the result of deep evidence reviews by JCVI in June 2011. 41
The final decision was the result of deep evidence reviews by JCVI in the Pneumococcal Sub-committee in May 2012 74 and June 2012. 75
The final decision was the result of deep evidence reviews by JCVI in February 2015, 76 October 2015, 77 and the Pneumococcal Sub-committee in January 2015, 78 May 2015, 79 and June 2015. 80
Cost-effectiveness analysis considered through literature review instead of modeling.
Even though the PCV13 childhood vaccination program was just implemented in Spain at the time of the assessment, it was assumed that the vaccination strategy would reduce the pneumococcal burden in all age groups.
Clinical inputs (IPD, pneumonia) of the cost-effectiveness analysis were detailed in the October 2018 meeting. 83
PCVs Introduction (Elderly)
The potential introduction of PCV13 in the routine elderly vaccination program was assessed by the NITAGs and HTA bodies of the 6 countries. In the United States, PCV15 and PCV20 were also considered.
France, Germany, the Netherlands, Spain, and the United Kingdom issued negative recommendations for PCV13 use (alone or in series with PPV23) in the elderly.35,38,39,53,54,67–69 The key barriers were unfavorable cost-effectiveness results compared with PPV23 alone, the decreasing trend of IPD cases attributable to PCV13 serotypes in the elderly (because of herd effect from childhood NIPs), and its inferior serotype coverage. The lack of efficacy data against clinically relevant endpoints (IPD, pneumococcal pneumonia, and deaths) in adults was also mentioned in evaluations54,67 conducted before the CAPiTA study, which was a randomized, double-blind, controlled trial that demonstrated the efficacy of PCV13 against vaccine serotype pneumococcal pneumonia and IPD in adults aged 65 y or older. 84 The use of PCV13 was reassessed several times in Germany,67,68 Spain,38,53 and the United Kingdom39,54 given its potential clinical benefits compared with PPV23 (efficacy against pneumococcal pneumonia, stronger and longer-lasting immunity). However, these benefits were insufficient to counterbalance the high price of PCV13 vaccination strategies.
Conversely, the United States advised the use of PCV13 in the elderly given the remaining burden caused by PCV13 serotypes in this population, its efficacy against IPD and pneumonia, and cost-effectiveness evaluations demonstrating that PCV13 in series with PPV23 was likely the optimal strategy. 42 However, the ACIP noted the potential time-limited utility of routine PCV13 use in the elderly given the growing herd effect. After 5 y of program implementation, given minimal changes in the incidence of pneumococcal disease among adults and less favorable economic evaluation, PCV13 administration was limited to shared clinical decision making between clinicians and patients based on clinical evidence that balances risks and expected outcomes with patient preferences and values. 43
Recently, the United States introduced PCV15 and PCV20 in the elderly vaccination program, driven by expected public health benefits, positive economic evaluations (the CDC model found cost savings in all scenarios for both strategies in adults aged ≥ 65 y), and comparable safety and immunogenicity profiles between PCV20, PCV15, and PCV13. No preferential recommendation was issued for PCV20 due to the lack of a head-to-head trial with PCV15 and the uncertainty of the impact of PCV20 alone. 11
PPV23 Introduction (Elderly)
Although the PPV23 elderly vaccination program was mainly implemented prior to 2009, its potential introduction has been assessed in France and the Netherlands only after 2009.35,69 In both countries, economic analysis was a major criterion of the evaluations but, given different methodological approaches, resulted in opposite conclusions. France advised against the use of PPV23 in the elderly, whereas the Netherlands recommended PPV23 every 5 y in those aged 65 to 75 y. In France, the elderly vaccination strategies were compared with vaccination strategies for adult risk groups and were found to be less cost-effective (according to the acceptable cost-effectiveness threshold set at €100,000/QALY saved). 35 In the Netherlands, the use of PPV23 in the elderly was compared with strategies with either PCV13 in the elderly or PCV13 in children (instead of PCV10). The analysis concluded that PPV23 was the most cost-effective strategy assuming a limit at €20,000/QALY (ICER equal to €9,000/QALY compared with €45,000/QALY for PCV13 in the elderly and >€80,000/QALY for PCV13 in children). 69
PPV23 Reassessment (Elderly)
Reassessment of the current PPV23 routine vaccination program in the elderly was conducted in the United Kingdom and the United States, and the need for a booster dose was evaluated in Germany. Although the United States maintained its current recommendation (1 dose of PPV23 at age 65 y), 48 the United Kingdom questioned at length the benefits of the PPV23 vaccination program in the elderly.26,58,71,72 Given the poor and declining effectiveness of PPV23, its relatively short duration of protection, and the absence of a discernable impact on the IPD incidence in this population, the program was initially discontinued.58,71 After additional analyses showing some short-term protection against IPD with possibly longer-lasting protection in the youngest age cohorts (65–74 y), the likely positive cost-effectiveness results, and the fact that an alternative risk group–based program may be more difficult to implement, the program was eventually maintained. 72 Germany removed the booster doses given evidence demonstrating high reactogenicity and hyporesponsiveness of additional PPV23 doses. 45
Criteria Assessed and Key Drivers/Barriers for Risk Group Recommendations
Risk group recommendations were assessed in 21 records. Topics covered were the introduction or extension of PCV13 or PPV23 (n = 16 and 13, respectively), the introduction of PCV10 in children risk groups (n = 1), and the introduction of PCV15 and PCV20 in adults risk groups (n = 1). Almost all records reported the disease burden and immunogenicity/efficacy data (n = 20). Safety data were reported more than for other populations (n = 16). The indirect impact of childhood NIPs (n = 15), direct impact of the current vaccination program (n = 11), economic evaluations (n = 9), and public health impact of alternative strategies (n = 6) were less reported (Table 4).
Table 4.
Overview of Criteria Assessed for Risk Group Recommendations
| Country | Date | Vaccine | Population | Main Purpose | Final Recommendation | Burden of Disease | Impact of Current Program | Safety | Immunogenicity/ Efficacy | Cost -effectiveness | Public health Impact of Alternatives | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| France | 2009 | PPV23 | All | PPV23 extension | PPV23 extended to additional risk groups | ✓ IPD, pneumonia | ✓ | ✓ IPD | 85 | |||
| France | 2009 | PCV13 | Children | PCV13 introduction | PCV13+PPV23 in some risk groups | ✓ IPD | ✓ direct, indirect | ✓ | ✓ | ✓ direct, indirect | 28 | |
| France | 2012 | PCV13 | Children | Program reassessment | PCV13+PPV23 in some risk groups | ✓ IPD | ✓ direct, indirect | 56 | ||||
| France | 2013 | PCV13, PPV23 | All | PCV13 extension | PCV13+PPV23 extended to additional groups | ✓IPD, pneumonia | ✓direct, indirect | ✓ | ✓ | 86 | ||
| France | 2017 | PCV13, PPV23 | Adults | PCV13 extension | PCV13+PPV23 extended to all risk groups | ✓ IPD, pneumonia | ✓ indirect | ✓ | ✓IPD, pneumonia | ✓ IPD, pneumonia | ✓ direct, indirect | 35 |
| France | 2018 | PCV10 | Children | PCV10 introduction | PCV13+PPV23 in all children risk groups | ✓IPD | ✓ direct, indirect | ✓ | ✓IPD, pneumonia, AOM | 57 | ||
| Germany | 2009 | PPV23 | All | PPV23 reassessment | PPV23 maintained in risk groups | ✓ IPD | ✓ | ✓IPD, pneumonia | 45 | |||
| Germany | 2014 | PCV13 | All | PCV13 extension | PCV13+PPV23 extended to additional risk groups | ✓ IPD |
✓ |
✓ | 50 | |||
| Germany | 2016 | PCV13, PPV23 | All | PCV13 extension | PCV13+PPV23 extended to additional risk groups | ✓IPD, pneumonia | ✓ | ✓IPD, pneumonia | 51 | |||
| Netherlands | 2021 | PCV13 | All | PCV13 introduction | PCV13+PPV23 in some risk groups | a | ✓ BIA | 52 | ||||
| Spain | 2015 | PCV13, PPV23 | Adults | PCV13 introduction | PCV13+PPV23 in some risk groups | ✓IPD, pneumonia | ✓direct, indirect b | ✓ | ✓IPD, pneumonia | ✓ IPD, pneumonia c | 53 | |
| Spain | 2017 | PCV13, PPV23 | Adults | PCV13 extension | PCV13+PPV23 not extended to additional risk groups | ✓IPD, pneumonia | ✓ | ✓IPD, pneumonia | ✓ IPD, pneumonia | ✓ direct | 70 | |
| United Kingdom | 2009 | PCV13, PPV23 | All | PCV13 introduction | PCV13+PPV23 in some risk groups | ✓ IPD | ✓ direct, indirect | ✓ IPD | 26 | |||
| United Kingdom | 2010 | PVC13, PPV23 | All | PCV13 extension | PCV13+PPV23 extended to additional high risk groups | ✓ IPD | ✓direct, indirect | ✓ IPD, pneumonia | 58 | |||
| United Kingdom | 2013 | PCV13 | Adults | PCV13 extension | PCV13+PPV23 not extended to additional risk groups d | ✓ IPD, pneumonia | ✓direct, indirect | ✓ IPD, pneumonia | ✓IPD, pneumonia | 54 | ||
| United Kingdom | 2015 | PCV13, PPV23 | Adults | PCV13 extension | PCV13+PPV23 not extended to additional risk groups e | ✓ IPD, pneumonia | ✓direct, indirect | ✓ | ✓ IPD, pneumonia | ✓IPD, pneumonia | ✓direct | 39 |
| United States | 2010 | PPV23 | Adults | Reassessment of PPV23 | PPV23 in US adults risk groups | ✓IPD | ✓indirect | ✓ | ✓IPD, pneumonia | 48 | ||
| United States | 2010 | PCV13 | Children | PCV13 introduction | PCV13+PPV23 in some children risk groups | ✓IPD | ✓direct, indirect | ✓ | ✓ IPD, pneumonia, AOM | ✓ IPD, AOM, pneumonia f | ✓direct | 30 |
| United States | 2012 | PCV13, PPV23 | Adults | PCV13 introduction | PCV13+PPV23 in some adult risk groups | ✓IPD | ✓indirect | ✓ | ✓ IPD | ✓ IPD, pneumonia | 49 | |
| United States | 2013 | PCV13, PPV23 | Children | PCV13 extension | PCV13+PPV23 extended to additional children risk groups | ✓IPD | ✓indirect | ✓ | ✓IPD, pneumonia | 55 | ||
| United States | 2021 | PCV15, PCV20 | Adults | PCV15/20 introduction | PCV20 or PCV15+PPV23 in all risk groups | ✓IPD, pneumonia | ✓direct, indirect | ✓ | ✓ IPD, pneumonia | ✓IPD, pneumonia | ✓direct | 11 |
AOM, acute otitis media; IPD, invasive pneumococcal diseases; JCVI, Joint Committee on Vaccination and Immunisation.
Full assessment of the vaccination program was detailed in another report.
Cost-effectiveness analysis considered through literature review instead of modeling.
Even though the PCV13 childhood vaccination program was just implemented in Spain at the time of the assessment, it was assumed that the vaccination strategy would reduce the pneumococcal burden in all age groups.
The final decision was the result of deep evidence reviews by the Pneumococcal Sub-committee in May 2012 74 and the JCVI in June 2012. 75
PCV Introduction (Risk Groups)
The 6 countries assessed and advised for the introduction of PCV13 in series with PPV23 in some high-risk groups given the high IPD incidence among those populations and the stronger and longer immune response of PCV13 compared with PPV23. All noted the lack of immunogenicity and efficacy data in many subgroups. However, the high probability of the occurrence of disease-related complications outweighed uncertain efficacy data, leading to PCV13 introduction in at least the highest risk groups. When conducted, economic evaluations were favorable to PCV13 use in some high-risk groups.30,35,38,49,53 The United Kingdom advised against the extension of PCV13 to additional risk groups, given the uncertain effectiveness and cost-effectiveness (ICER > £20,000/QALY saved threshold) of such a program.39,54
Unlike the other countries, France extended the PCV13 program in all risk groups. This decision was justified by the important pneumonia and IPD burden observed in adults with comorbidities and a cost-effectiveness analysis that demonstrated that the vaccination of all risk groups was the most efficient strategy (ICER < €100,000/QALY saved). 35
France also assessed whether PCV10 could be an option in addition to PCV13 in risk groups of children younger than 5 y and advised against its introduction due to the epidemiological context not in favor of PCV10 long-term use. 57
The United States was the first country to assess and advise the use of PCV15 in series with PPV23 and PCV20 alone in adults aged 19 to 64 y with certain underlying conditions. The recommendation was conducted simultaneously to the elderly one and was driven by the same criteria and with the willingness to simplify the risk group recommendations to improve vaccine coverage among adults. 11
PPV23 Reassessment (Risk Groups)
All 6 countries, except the Netherlands, reassessed the PPV23 vaccination program in risk groups. In 2009–2010, France, Germany, and the United States advised for the extension of PPV23 to additional moderate-risk groups.45,48,85 Since then, PPV23 was generally compared with other strategies including PCV13.26,30,35,38,39,49,53,55,58,68 Although the PCV13 in series with PPV23 was generally reserved to the high-risk groups, PPV23 alone was largely recommended in moderate-risk groups due to its lower price and its wider serotype coverage. Unlike in the elderly, the high incidence of pneumococcal disease in risk groups generally outweighed the reactogenicity and hyporesponsiveness issues, and repeat doses of PPV23 were generally recommended.
Discussion
By capturing the historical evolution of national vaccination programs, the evidence reviewed by NITAGs and HTA bodies and drivers or barriers for changes, this study details criteria used for the decision-making process for pneumococcal vaccination recommendations in the 6 countries considered.
After the introduction of PCV7, 2 major developments were observed in child vaccination policies: first, switching to higher-valent PCVs and then reduction of the dose schedule. The licensure of PCV10 and PCV13 enabled health authorities to update their recommendations by replacing PCV7 by a vaccine offering a broader serotype coverage. Following the control of pneumococcal vaccine-type disease and nasopharyngeal carriage through vaccination, a reduced schedule appeared sufficient, for European countries, to sustain that control at reduced costs. By contrast, the United States maintained the 3+1 schedule. The IPD burden, the impact of the PCV vaccination program, as well as vaccine characteristics were key determinants supporting the new vaccination strategies. When conducted, the economic evaluation was also a major criterion for decision making. These results are consistent with the findings from a recent HTA landscape evaluation for pediatric PCV in Europe. 87 The authors demonstrated that epidemiological IPD data were included in all assessments for children and that health economic analyses were performed in 13 of the 31 European countries studied. Of the 6 countries analyzed in our study, most advised for the use of PCV13 instead of PCV10 given its wider serotype coverage and the important burden associated with these additional serotypes. This preferential use of PCV13 reflects the global pneumococcal vaccination landscape in children, 88 but in some European countries, both vaccines can be used. Avoiding a monopoly situation could become more frequent in the future, since it could allow for competitive pricing and overcome supply chain issues. This trend was recently observed in the United States, where the ACIP recommended the use of either PCV13 or PCV15 in the childhood vaccination program. 12
Recommendations for people with underlying conditions evolved similarly in the 6 countries, with a broad use of PPV23 often coupled with sequential administration of PCV13 for some high-risk groups. Nevertheless, eligible risk groups vary between countries. Recommendation updates were generally driven by the need to protect people at increased risk of IPD despite a lack of immunogenicity or efficacy data in each specific population.
The recommendations for the elderly were more heterogenous between the 6 countries. Although the United States introduced PCVs as part of the immunization options, France did not recommend pneumococcal vaccination in the elderly, and the other European countries studied recommended PPV23 alone. These 3 vaccination strategies reflect the diversity of recommendations that exist in Europe for the elderly, with about one-third of countries recommending PPV23 alone, one-third recommending PCV13 in series with PPV23, and one-third without a pneumococcal vaccine recommendation for the elderly. 20 Given the expected benefits of PCV13 in comparison with PPV23 (long-lasting immunogenicity, efficacy data against pneumonia) and the remaining pneumococcal burden in the elderly, the potential introduction of PCV13 was largely assessed by the national stakeholders. The economic criterion was the major barrier for PCV13 introduction in vaccination programs for the elderly. In the European countries, strategies involving PCV13 appeared to be not cost-effective compared with the use of PPV23 alone, primarily due to the importance of the herd effect from childhood NIPs, its higher cost, and its lower serotype coverage. Conversely, when PCV13 was registered in the United States for adults, PCV13 in series with PPV23 had an acceptable ICER even if additional benefits of PCV13 use among adults was predicted to decline over time with continued use of PCV13 among children. 82 This difference may be explained by various methodological approaches and assumptions (e.g., vaccine efficacy, the indirect effects of childhood vaccination assumptions), which greatly affected cost-effectiveness estimates. 89
Differences between NITAGs processes in terms of role, transparency, and the decision analysis framework have been largely described in the published literature.90,91 Our study results demonstrated that for the pneumococcal vaccines, the assessment frameworks vary according to the target population (children, elderly, risk groups) and the type of recommendation (recommendation extension, introduction of a new vaccine). Focusing on the criteria for decision making defined by Donadel et al., 21 we observed that the burden of disease and the immunogenicity/efficacy data were almost systematically reported whatever the topic and population assessed. Although the effectiveness or the efficacy against specific clinical outcomes (IPD, pneumonia, AOM, etc.) are the most robust evidence, it was acceptable to evaluate the vaccine performances through immunogenicity data. The other criteria for decision making were less reported. Economic evaluations were not considered by all countries when assessing major recommendation changes. Safety data were not reviewed in depth. The public heath impact of alternative vaccine strategies, corresponding to the number of cases avoided, was the least reported criterion.
There are few limitations worth noting in this study. First, the restricted number of countries selected did not provide a comprehensive overview of the global landscape of pneumococcal vaccination strategies. Second, the data extraction was based on a prespecified list of common criteria used for decision making and therefore did not cover all criteria that played a role in the final decisions. During the analysis, it was observed that other criteria such as antimicrobial resistance, a review of the World Health Organization’s recommendations, and a review of vaccination programs of other countries also influenced some decisions. To a lesser extent, ethical, acceptability, and feasibility aspects could also be considered during assessments. 21 Third, data collection was purely based on publicly available online resources. Therefore, the collected data may not capture all information regarding policy changes or discussions. In addition, PCV7 was already included in the NIP for children when PCV13 was launched; therefore, criteria that could have been evaluated at the time of PCV7 evaluation and that were still considered as valid for PCV13 were not captured in this study.
New higher-valent pneumococcal vaccines (PCV15 and PCV20) were recently evaluated by the regulatory bodies in Europe and the United States and recommended by the ACIP in the United States. 12 These next-generation pneumococcal vaccines may be evaluated by European HTA bodies or NITAGs in the near future to determine their place in countries’ NIPs. Considering the history of recommendations, the introduction of these new PCVs in European childhood NIPs is largely probable, as they are expected to prevent more IPD cases and deaths. 92 Their introduction in vaccination programs for the elderly could be more challenging, and new PCVs might face the same issues as those encountered by PCV13. Despite important herd immunity and more than 30 y of vaccination against pneumococcal disease, morbidity and mortality remain high in adults. 18 The use of PPV23 still appears to offer suboptimal protection, and new PCVs seem to be the most promising field for improvement in the management of pneumococcal infections in adults. 4 However, given the price differential between polysaccharide and conjugate vaccines, cost-effectiveness analyses will be needed to examine the monetary value of replacing the PPV23 by new PCVs. Furthermore, additional aspects such as the benefit of vaccination in reducing antibiotic resistance could be considered to accurately reflect the full value of PCVs in elderly. 93
Conclusion
Pneumococcal vaccination policy is a dynamic field in constant evolution. To ensure the optimal level of disease prevention while streamlining budget allocations, national stakeholders periodically assess their strategies and adapt their recommendations to cater to the needs of each population, resulting in various vaccination strategies. This study highlights that framework analyses and assessment methodologies vary between countries, which contributes to the diversity of pneumococcal vaccine recommendations.
Supplemental Material
Supplemental material, sj-docx-1-mpp-10.1177_23814683231174432 for Evolution of Pneumococcal Vaccine Recommendations and Criteria for Decision Making in 5 Western European Countries and the United States by Roxane Noharet-Koenig, Katarzyna Lasota, Pascaline Faivre and Edith Langevin in MDM Policy & Practice
Acknowledgments
The authors would like to thank Alok Vyas, PhD (Sanofi), for providing editorial assistance; Anirban Sanyal, PhD (Sanofi), for manuscript coordination and editorial support; and Fabian Alvarez (Sanofi) for revision and intellectual contribution. The authors indicate that data, analytic methods, and/or study materials could be provided to other researchers upon request to a specific named author.
Footnotes
Author Contributions: All authors contributed to the concept and design of the manuscript. RNK and KL were in charge of the data acquisition. RNK and EL wrote the manuscript. All authors revised the article for intellectual content and approved the final draft prior to submission.
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: RN-K was an intern at Sanofi during this work. KL and PF are employees of Certara. EL is an employee of Sanofi and may hold shares/stocks in Sanofi. No other disclosures were reported.
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was done in Sanofi by the Health Economics & Value assessment team in partnership with Certara team.
ORCID iD: Roxane Noharet-Koenig  https://orcid.org/0000-0003-4426-0464
https://orcid.org/0000-0003-4426-0464
Supplemental Material: Supplementary material for this article is available on the MDM Policy & Practice Web site at https://journals.sagepub.com/home/mpp.
Contributor Information
Roxane Noharet-Koenig, Faculté de Pharmacie, Université Bourgogne Franche-Comté, Besançon, France; Sanofi, Lyon, France.
Katarzyna Lasota, Certara Poland, Cracow, Poland.
Pascaline Faivre, Certara France, SARL, Paris, France.
Edith Langevin, Sanofi, Lyon, France.
References
- 1.World Health Organization (WHO). Pneumococcal disease. Available from: https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/vaccine-standardization/pneumococcal-disease [Accessed 6 May, 2022].
- 2.Muhammad RD, Oza-Frank R, Zell E, et al. Epidemiology of invasive pneumococcal disease among high-risk adults since the introduction of pneumococcal conjugate vaccine for children. Clin Infect Dis. 2013;56:e59–67. [DOI] [PubMed] [Google Scholar]
- 3.Drijkoningen JJC, Rohde G.Pneumococcal infection in adults: burden of disease. Clin Microbioloy Infect. 2014;20:45–51. [DOI] [PubMed] [Google Scholar]
- 4.Blasi F, Mantero M, Santus P, Tarsia P.Understanding the burden of pneumococcal disease in adults. Clin Microbiol Infect. 2012;18:7–14. [DOI] [PubMed] [Google Scholar]
- 5.European Medicines Agency (EMA). PREVENAR 13 - summary of product characteristics. Available from: https://www.ema.europa.eu/en/documents/product-information/prevenar-13-epar-product-information_en.pdf [Accessed 4 May, 2022].
- 6.European Medicines Agency (EMA). SYNFLORIX - summary of product characteristics. Available from: https://www.ema.europa.eu/en/documents/product-information/synflorix-epar-product-information_en.pdf [Accessed 4 May, 2022].
- 7.Food and Drug Administration (FDA). VAXNEUVANCE - approval letter. Available from: https://www.fda.gov/media/150820/download [Accessed 4 May, 2022].
- 8.Food and Drug Administration (FDA). PEVENAR 20 - approval letter. Available from: https://www.fda.gov/media/150021/download [Accessed 4 May, 2022].
- 9.Merck. European Commission approves Merck’s VAXNEUVANCE™ (pneumococcal 15-valent conjugate vaccine) for individuals 18 years of age and older. Available from: https://www.merck.com/news/european-commission-approves-mercks-vaxneuvance-pneumococcal-15-valent-conjugate-vaccine-for-individuals-18-years-of-age-and-older/ [Accessed 10 May, 2022].
- 10.Pfizer. European Medicines Agency approves Pfizer’s 20-valent pneumococcal conjugate vaccine against invasive pneumococcal disease and pneumonia in adults Available from: https://www.pfizer.com/news/press-release/press-release-detail/european-medicines-agency-approves-pfizers-20-valent [Accessed 12 April, 2022].
- 11.Kobayashi M, Farrar JL, Gierke R, et al. Use of 15-valent pneumococcal conjugate vaccine and 20-valent pneumococcal conjugate vaccine among U.S. adults: updated recommendations of the Advisory Committee on Immunization Practices - United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merck. CDC’s ACIP unanimously votes to provisionally recommend use of Merck’s VAXNEUVANCE™ (pneumococcal 15-valent conjugate vaccine) as an option for pneumococcal vaccination in infants and children. Available from: https://www.merck.com/news/cdcs-acip-unanimously-votes-to-provisionally-recommend-use-of-mercks-vaxneuvance-pneumococcal-15-valent-conjugate-vaccine-as-an-option-for-pneumococcal-vaccination-in-infa/ [Accessed 10 May, 2022].
- 13.Lewnard JA, Hanage WP.Making sense of differences in pneumococcal serotype replacement. Lancet Infect Dis. 2022;19:e213–20. [DOI] [PubMed] [Google Scholar]
- 14.Hanquet G, Krizova P, Dalby T, et al. Serotype replacement after introduction of 10-valent and 13-valent pneumococcal conjugate vaccines in 10 countries, Europe. Emerg Infect Dis J. 2022;28:137–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tin Tin Htar M, Christopoulou D, Schmitt H-J. Pneumococcal serotype evolution in Western Europe. BMC Infect Dis. 2015;15:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laigle V, Postma MJ, Pavlovic M, et al. Vaccine market access pathways in the EU27 and the United Kingdom−analysis and recommendations for improvements. Vaccine. 2021;39:5706–18. [DOI] [PubMed] [Google Scholar]
- 17.Chapman TJ, Pichichero ME, Kaur R.Comparison of pneumococcal conjugate vaccine (PCV-13) cellular immune responses after primary and booster doses of vaccine. Hum Vaccines Immunother. 2020;16:3201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah AA.Simplifying pneumococcal immunizations for adults. Am Fam Physician. 2022;105:580–1. [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC). Immunization schedule. Available from: https://www.cdc.gov/vaccines/schedules/index.html [Accessed 3 July, 2022].
- 20.European Centre for Disease Prevention and Control (ECDC). Vaccine scheduler. Available from: https://vaccine-schedule.ecdc.europa.eu/Scheduler/ByDisease?SelectedDiseaseId=25&SelectedCountryIdByDisease=-1 [Accessed 3 July, 2022].
- 21.Donadel M, Panero MS, Ametewee L, et al. National decision-making for the introduction of new vaccines: a systematic review, 2010–2020. Vaccine. 2021;39:1897–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Advisory Committee on Immunization Practices (ACIP). Preventing pneumococcal disease among infants and young children. MMWR Morb Mortal Wkly Rep. 2000;49:1–35. [PubMed] [Google Scholar]
- 23.Haut Conseil de la Santé Publique (HCSP). Avis relatif à la vaccination par le vaccin anti-pneumococcique conjugué chez les enfants de moins de deux ans et les enfants de deux à cinq ans. Available from: https://www.hcsp.fr/Explore.cgi/Telecharger?NomFichier=a_mt_190506_pneumo_enf.pdf [Accessed 25 April, 2022].
- 24.Ständigen Impfkommission (STIKO). Epidemiologisches bulletin 31/2006. Available from: https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2006/Ausgabenlinks/31_06.pdf?__blob=publicationFile [Accessed 25 April, 2022].
- 25.Health Council of the Netherlands (HCN). Algemene vaccinatie tegen meningokokken C en pneumokokken - Advies - Gezondheidsraad. Available from: https://www.gezondheidsraad.nl/documenten/adviezen/2002/01/09/algemene-vaccinatie-tegen-meningokokken-c-en-pneumokokken [Accessed 25 April, 2022].
- 26.The Joint Committee on Vaccination and Immunisation (JCVI). Minute of the pneumococcal subgroup on Tuesday 15 January 2009. Available from: https://webarchive.nationalarchives.gov.uk/ukgwa/20120907151247mp_/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@ab/documents/digitalasset/dh_097593.pdf [Accessed 29 April, 2022]. [Google Scholar]
- 27.Grupo de trabajo de la Ponencia de Registro (PdV). Enfermedad invasora por Streptococcus Pneumoniae - Implicación de la Vacunación con la Vacuna Conjugada Heptavalente. Available from: https://www.sanidad.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/comoTrabajamos/docs/neumo.pdf [Accessed 25 April, 2022].
- 28.Haut Conseil de la Santé Publique (HCSP). Avis relatif à la vaccination par le vaccin pneumococcique conjugué 13-valent. Available from: https://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=172 [Accessed 22 March, 2022].
- 29.Grupo de trabajo de la Ponencia de Registro (PdV). Nuevas vacunas antineumocócicas conjugadas. MSCBS. Available from: https://www.sanidad.gob.es/en//profesionales/saludPublica/prevPromocion/vacunaciones/comoTrabajamos/docs/Vacunas_Antineumococicas_conjugadas2010.pdf [Accessed 10 March, 2022].
- 30.Nuorti JP, Whitney CG; Centers for Disease Control and Prevention (CDC). Prevention of pneumococcal disease among infants and children - use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine- recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2010;59:1–18. [PubMed] [Google Scholar]
- 31.Ständigen Impfkommission (STIKO). Epidemiologisches bulletin 36/2015. Available from: https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2015/Ausgaben/36_15.pdf?__blob=publicationFile [Accessed 22 March, 2022].
- 32.Health Council of the Netherlands (HCN). Vaccinatie van zuigelingen tegen pneumokokkeninfecties (2) - Advies - Gezondheidsraad. Available from: https://www.gezondheidsraad.nl/documenten/adviezen/2010/03/10/vaccinatie-van-zuigelingen-tegen-pneumokokkeninfecties-2 [Accessed 24 March, 2022].
- 33.Health Council of the Netherlands (HCN). Vaccinatie van zuigelingen tegen pneumokokkeninfecties (3) - Advies - Gezondheidsraad. Available from: https://www.gezondheidsraad.nl/documenten/adviezen/2013/11/27/vaccinatie-van-zuigelingen-tegen-pneumokokkeninfecties-3 [Accessed 24 March, 2022].
- 34.Comité Technique de Vaccination (CTV). Efficacité du vaccin polysaccharidique pneumococcique chez les sujets ages. Available from: https://www.hcsp.fr/Explore.cgi/avisrapports3?clef=14&clefr=135 [Accessed 5 May, 2022].
- 35.Haut Conseil de la Santé Publique (HCSP). Avis relatif aux recommendations vaccinales contre les infections à pneumocoque pour les adultes. Available from: https://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=614 [Accessed 22 March, 2022].
- 36.Centers for Disease Control. Recommendations of the immunization practices advisory committee (ACIP) update: pneumococcal polysaccharide vaccine usage—United States. MMWR Morb Mortal Wkly Rep. 1984;33:273–76. [PubMed] [Google Scholar]
- 37.Health Council of the Netherlands (HCN). Vaccinatie tegen pneumokokken bij ouderen en risicogroepen - Advies - Gezondheidsraad. Available from: https://www.gezondheidsraad.nl/documenten/adviezen/2003/08/18/vaccinatie-tegen-pneumokokken-bij-ouderen-en-risicogroepen [Accessed 29 April, 2022].
- 38.Grupo de trabajo de la Ponencia de Registro (PdV). Vacunación en Adultos Recommendaciones. Available from: https://www.sanidad.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/programasDeVacunacion/docs/recoVacunasAdultos.pdf [Accessed 10 March, 2022].
- 39.The Joint Committee on Vaccination and Immunisation (JCVI). Interim JCVI statement on adult pneumococcal vaccination in the UK. November 2015. Available from: https://www.gov.uk/government/publications/jcvi-interim-statement-on-adult-pneumococcal-vaccination [Accessed 29 April, 2022].
- 40.The Joint Committee on Vaccination and Immunisation (JCVI). Minute of the meeting held on Wednesday 2 February 2011. Available from: https://webarchive.nationalarchives.gov.uk/ukgwa/20120907095358/http://www.dh.gov.uk/ab/JCVI/DH_123529 [Accessed 29 April, 2022]. [Google Scholar]
- 41.The Joint Committee on Vaccination and Immunisation (JCVI). Minutes of the meeting held on Wednesday 8 June 2011. Available from: https://webarchive.nationalarchives.gov.uk/ukgwa/20120907095358/http://www.dh.gov.uk/ab/JCVI/DH_123529#_3 [Accessed 29 April, 2022]. [Google Scholar]
- 42.Tomczyk S, Bennett NM, Stoecker C, et al. ; Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014;63:822–5. [PMC free article] [PubMed] [Google Scholar]
- 43.Matanock A, Lee G, Gierke R, Kobayashi M, Leidner A, Pilishvili T.Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:1069–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ständigen Impfkommission (STIKO). Epidemiologisches bulletin 28/2001. Available from: https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2001/Ausgabenlinks/28_01.pdf?__blob=publicationFile [Accessed 2 May, 2022].
- 45.Ständigen Impfkommission (STIKO). Epidemiologisches bulletin 32/2009. Available from: https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2006/Ausgabenlinks/31_06.pdf?__blob=publicationFile [Accessed 22 April, 2022].
- 46.Health Council of the Netherlands (HCN). COVID-19 en vaccinatie tegen pneumokokken - Advies - Gezondheidsraad. Available from: https://www.gezondheidsraad.nl/documenten/adviezen/2020/04/20/covid-19-en-vaccinatie-tegen-pneumokokken [Accessed 24 March, 2022].
- 47.Advisory Committee on Immunization Practices (ACIP). Notice to readers: pneumococcal vaccination for cochlear implant recipients. Available from: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5141a5.htm [Accessed 15 March, 2022].
- 48.Centers for Disease Control and Prevention (CDC), Advisory Committee on Immunization Practices (ACIP). Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23). MMWR Morb Mortal Wkly Rep. 2010;59:1102–6. [PubMed] [Google Scholar]
- 49.Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2012;61:816–9. [PubMed] [Google Scholar]
- 50.Ständigen Impfkommission (STIKO). Epidemiologisches bulletin 36/2014. Available from: https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2014/Ausgaben/36_14.pdf?__blob=publicationFile [Accessed 22 March, 2022].
- 51.Ständigen Impfkommission (STIKO). Epidemiologisches bulletin 37/2016. Available from: https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2016/Ausgaben/37_16.pdf?__blob=publicationFile [Accessed 22 March, 2022].
- 52.Zorginstituut Nederland (ZIN). GVS assessment of 13-valent pneumococcal conjugate vaccine (Prevenar13®) for medical high-risk groups - report - National Health Care Institute. Available from: https://english.zorginstituutnederland.nl/publications/reports/2021/03/03/gvs-assessment-of-13-valent-pneumococcal-conjugate-vaccine-prevenar13 [Accessed 24 March, 2022].
- 53.Grupo de trabajo de la Ponencia de Registro (PdV). Vacunación frente a neumococo en grupos de riesgo. Available from: https://www.sanidad.gob.es/ca/profesionales/saludPublica/prevPromocion/vacunaciones/programasDeVacunacion/docs/Neumococo_Gruposriesgo.pdf [Accessed 22 March, 2022].
- 54.The Joint Committee on Vaccination and Immunisation (JCVI). Statement on the wider use of pneumococcal conjugate vaccines in the UK. July 2013. https://www.gov.uk/government/publications/jcvi-statement-on-the-wider-use-of-pneumococcal-conjugate-vaccines [Accessed 29 April, 2022]. [Google Scholar]
- 55.Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among children aged 6–18 years with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2013;62:521–24. [PMC free article] [PubMed] [Google Scholar]
- 56.Haut Conseil de la Santé Publique (HCSP). Avis relatif à la vaccination des nourrissons contre les infections invasives à pneumocoque par le vaccin pneumococcique conjugué 13 valent. Available from: https://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=255 [Accessed 22 March, 2022].
- 57.Haute Autorité de Santé (HAS). Place de SYNFLORIXTM dans la stratégie vaccinale contre les infections à pneumocoques chez l’enfant âgé de moins de 5 ans. Available from: https://www.has-sante.fr/jcms/c_2838905/fr/place-du-vaccin-synflorix-dans-la-strategie-vaccinale-contre-les-infections-a-pneumocoques-chez-l-enfant-age-de-moins-de-5-ans#:∼:text=La%20HAS%20consid%C3%A8re%20donc%20qu,des%20vaccinations%20et%20recommandations%20vaccinales [Accessed 24 April, 2022].
- 58.The Joint Committee on Vaccination and Immunisation (JCVI). Pneumococcal sub-committee. minute of the meeting held on 15 December 2010. Available from: https://webarchive.nationalarchives.gov.uk/ukgwa/20120907095400/http://www.dh.gov.uk/ab/JCVI/DH_107556 [Accessed 29 April, 2022].
- 59.The Joint Committee on Vaccination and Immunisation (JCVI). Minute of the meeting held on 4 June 2014. Available from: https://app.box.com/s/iddfb4ppwkmtjusir2tc/file/229171860655 [Accessed 29 April, 2022].
- 60.The Joint Committee on Vaccination and Immunisation (JCVI). Minute of the meeting held on 06 June 2018. Available from: https://app.box.com/s/iddfb4ppwkmtjusir2tc/file/305779572165 [Accessed 29 April, 2022].
- 61.The Joint Committee on Vaccination and Immunization (JCVI). Minutes of the meeting held on 3 February 2010. Available from: https://webarchive.nationalarchives.gov.uk/ukgwa/20120907151219mp_/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@ab/documents/digitalasset/dh_118114.pdf [Accessed 29 April, 2022]. [Google Scholar]
- 62.The Joint Committee on Vaccination and Immunisation (JCVI). Minute of the meeting on 04 October 2017. Available from: https://app.box.com/s/iddfb4ppwkmtjusir2tc/file/247634612957 [Accessed 29 April, 2022].
- 63.The Joint Committee on Vaccination and Immunisation (JCVI). Pneumococcal subcommittee. Minute of the meeting on Thursday 10 May 2018. Available from: https://app.box.com/s/1mrhw4tnughfvbbvujt5thww5f0mjhpv/file/305778551993 [Accessed 29 April, 2022].
- 64.Advisory Committee on Immunization Practices (ACIP). ACIP meeting minutes June 2009. Available from: https://www.cdc.gov/vaccines/acip/meetings/downloads/min-archive/min-jun09-508.pdf [Accessed 24 July, 2022].
- 65.Advisory Committee on Immunization Practices (ACIP). Summary report October 21-22, 2009. Available from: https://www.cdc.gov/vaccines/acip/meetings/downloads/min-archive/min-2009-10-508.pdf [Accessed 10 March, 2022].
- 66.Advisory Committee on Immunization Practices (ACIP). Summary report August 13, 2014. Available from: https://www.cdc.gov/vaccines/acip/meetings/downloads/min-archive/min-2014-08.pdf [Accessed 15 March, 2022].
- 67.Ständigen Impfkommission (STIKO). Epidemiologisches bulletin 7/2012. Available from: https://gpk.de/downloadp/STIKO_2012_Bulletin07_120220_Stellungnahme_zur_Impfung_Erwachsener_gegen_Pneumokokken.pdf [Accessed 22 March, 2022].
- 68.Ständigen Impfkommission (STIKO). Epidemiologisches bulletin 36/2016. Available from: https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2016/Ausgaben/36_16.pdf?__blob=publicationFile [Accessed 22 March, 2022].
- 69.Health Council of the Netherlands (HCN). Vaccinatie van ouderen tegen pneumokokken - Advies - Gezondheidsraad. Available from: https://www.gezondheidsraad.nl/documenten/adviezen/2018/02/28/vaccinatie-van-ouderen-tegen-pneumokokken [Accessed 24 March 2022].
- 70.Ministerio de Sanidad, Consumo y bienestar Social(MSCBS). Coste-efectividad de la vacunación antineumocócica en adultos > 60 años. Available from: https://www3.gobiernodecanarias.org/sanidad/scs/content/db30c019-c554-11e8-8288-2bf81093cafe/SESCS_2017_VacunaNeumococo_NIPO.pdf [Accessed 24 March, 2022].
- 71.The Joint Committee on Vaccination and Immunisation (JCVI). Advice on pneumococcal polysaccharide vaccination programme. March 2011. Available from: https://webarchive.nationalarchives.gov.uk/ukgwa/20120907095410/http://www.dh.gov.uk/ab/JCVI/DH_094744 [Accessed 29 April, 2022]. [Google Scholar]
- 72.The Joint Committee on Vaccination and Immunisation (JCVI). Advice on the pneumococcal vaccination programme for people aged 65 years and older. July 2011. Available from: https://webarchive.nationalarchives.gov.uk/ukgwa/20120907095410/http://www.dh.gov.uk/ab/JCVI/DH_094744 [Accessed 29 April, 2022]. [Google Scholar]
- 73.The Joint Committee on Vaccination and Immunisation (JCVI). Pneumococcal subcommittee. Note of teleconference on 25 May 2011 and subsequent discussions by correspondence. Available from: https://webarchive.nationalarchives.gov.uk/ukgwa/20120907095358/http:/www.dh.Gov.uk/ab/JCVI/DH_123529 [Accessed 29 April, 2022]. [Google Scholar]
- 74.The Joint Committee on Vaccination and Immunisation (JCVI). Meeting minutes held on Wednesday 30 May 2012. Available from: https://webarchive.nationalarchives.gov.uk/ukgwa/20120907105454mp_/https://www.wp.dh.gov.uk/transparency/files/2012/07/JCVI-minutes-Pneumococcal-sub-committee-meeting-held-on-30-May-2012.pdf [Accessed 29 April, 2022].
- 75.The Joint Committee on Vaccination and Immunisation (JCVI). Minute of the meeting on Wednesday 13 June 2012. Available from: https://webarchive.nationalarchives.gov.uk/ukgwa/20120907093619/http://transparency.dh.gov.uk/category/minutes-2/jcvi-minutes/ [Accessed 29 April, 2022].
- 76.The Joint Committee on Vaccination and Immunisation (JCVI). Minute of the meeting on 4 February 2015. Available from: https://app.box.com/s/iddfb4ppwkmtjusir2tc/file/229171832189 [Accessed 24 July, 2022].
- 77.The Joint Committee on Vaccination and Immunisation (JCVI). Minute of the meeting on 7 October 2015. Available from: https://app.box.com/s/iddfb4ppwkmtjusir2tc/file/229171824302 [Accessed 24 July, 2022].
- 78.The Joint Committee on Vaccination and Immunisation (JCVI). Pneumococcal subcommittee. Minute of the meeting on Wednesday 28 January 2015. Available from: https://app.box.com/s/1mrhw4tnughfvbbvujt5thww5f0mjhpv/file/44126256797 [Accessed 29 April, 2022].
- 79.The Joint Committee on Vaccination and Immunisation (JCVI). Pneumococcal sub-committee. minute of the meeting on Friday 22 May 2015. Available from: https://app.box.com/s/1mrhw4tnughfvbbvujt5thww5f0mjhpv/file/44129299893
- 80.The Joint Committee on Vaccination and Immunisation (JCVI). Pneumococcal sub-committee. Minute of the meeting on 22 June 2015. Available from: https://app.box.com/s/1mrhw4tnughfvbbvujt5thww5f0mjhpv/file/44129297485 [Accessed 29 April, 2022].
- 81.Advisory Committee on Immunization Practices (ACIP). ACIP meeting minutes June 2014. Available from: https://www.cdc.gov/vaccines/acip/meetings/downloads/min-archive/min-2014-06.pdf [Accessed 24 July, 2022].
- 82.Advisory Committee on Immunization Practices (ACIP). ACIP meeting minutes August 2014. Available from: https://www.cdc.gov/vaccines/acip/meetings/downloads/min-archive/min-2014-08.pdf [Accessed 5 July, 2022].
- 83.Advisory Committee on Immunization Practices (ACIP). ACIP meeting minutes October 2018. Available from: https://www.cdc.gov/vaccines/acip/meetings/downloads/min-archive/min-2018-10-508.pdf [Accessed 24 July, 2022].
- 84.Bonten MJM, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372:1114–25. [DOI] [PubMed] [Google Scholar]
- 85.Haut Conseil de la Santé Publique (HCSP). Avis relatif à la vaccination contre le pneumocoque chez l’adulte et l’enfant âgé de 5 ans et plus infectés par le VIH. Available from: https://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=85 [Accessed 22 March, 2022].
- 86.Haut Conseil de la Santé Publique (HCSP). Avis relatif aux recommandations vaccinales contre les infections à pneumocoque pour les personnes à risqué. Available from: https://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=355 [Accessed 22 March, 2022].
- 87.Bencina G, Wahl HF, Tsoumani E, Salomonsson S.Recommendations and health technology assessment (HTA) landscape evaluation for pediatric pneumococcal conjugate vaccines (PCV) in Europe: a systematic literature review. Hum Vaccines Immunother. 2022;18:2060017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.International Vaccine Access Center (IVAC), Johns Hopkins Bloomberg School of Public Health. ViewHub. Available from: https://view-hub.org [Accessed 31 July, 2022].
- 89.Treskova M, Scholz SM, Kuhlmann A.Cost effectiveness of elderly pneumococcal vaccination in presence of higher-valent pneumococcal conjugate childhood vaccination: systematic literature review with focus on methods and assumptions. PharmacoEconomics. 2019;37:1093–127. [DOI] [PubMed] [Google Scholar]
- 90.Piso B, Zechmeister I, Geiger-Gritsch S.Criteria for vaccine introduction: results of a DELPHI discussion among international immunisation experts on a stepwise decision-making procedure. J Public Health. 2010;19:73–80. [Google Scholar]
- 91.Ricciardi GW, Toumi M, Weil-Olivier C, et al. Comparison of NITAG policies and working processes in selected developed countries. Vaccine. 2015;33:3–11. [DOI] [PubMed] [Google Scholar]
- 92.Rodgers GL, Whitney CG, Klugman KP.Triumph of Pneumococcal conjugate vaccines: overcoming a common foe. J Infect Dis. 2021;224:S352–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vyse A, Campling J, Czudek C, et al. A review of current data to support decision making for introduction of next generation higher valency pneumococcal conjugate vaccination of immunocompetent older adults in the UK. Expert Rev Vaccines. 2021;20:1311–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-mpp-10.1177_23814683231174432 for Evolution of Pneumococcal Vaccine Recommendations and Criteria for Decision Making in 5 Western European Countries and the United States by Roxane Noharet-Koenig, Katarzyna Lasota, Pascaline Faivre and Edith Langevin in MDM Policy & Practice