Abstract
Background
Macrophages, upon encounter with micro-organisms or stimulated by cytokines, produce various effector molecules aimed at destroying the foreign agents and protecting the organism. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are front line molecules exerting strong cytotoxic activities against micro-organisms and many cells, including macrophages themselves. Using cells of the murine macrophage cell line (RAW 264.7) stimulated in vitro with lipopolysaccharide (LPS) and/or interferon (IFN-γ), which induce strong endogenous NO production, we examined by which mechanisms a fraction of activated macrophages protect themselves from nitrosative stress and manage to escape destruction?
Results
We observed that survivors (10–50% depending on the experiments) had acquired a resistant phenotype being capable to survive when further exposed in vitro to an apoptosis inducing dose of the NO donor compound DETA-NO. These cells expressed an increased steady-state levels of Mn SOD, CuZn SOD and catalase mRNA (130–200%), together with an increased activity of the corresponding enzymes. Intracellular concentration of glutathione was also increased (× 3.5 fold at 6 hours, still maintained × 5.2 fold at 48 hours). Neither mRNA for glutathione peroxydase, γ-glutamylcysteine synthase and glutathione reductase, nor thioredoxine and thioredoxine reductase, were significantly modified. Additional experiments in which RAW 264.7 cells were stimulated with LPS and/or IFN-γ in the presence of relatively specific inhibitors of both Mn and Cu/Zn SOD, aminotriazol (ATZ) catalase inhibitor and buthionine sulfoximine (BSO) glutathione inhibitor, showed that inhibiting LPS-induced up-regulation of intracellular redox buffering systems also prevented acquisition of the resistant phenotype.
Conclusions
Our data suggest a direct causal relationship between survival of a fraction of macrophages and a up-regulation of key sets of auto-protective intracellular redox buffering systems, occurring simultaneously with modulation of expression of apoptotic molecules of the Bcl2-Bcl-XL/Bax-Bad family.
Background
Macrophages, and their circulating form monocytes, are potent defenders of the integrity of our body by mediating crucial physiological and protective functions. Just to mention some : they are central actors in innate immunity and inflammatory reactions; they process and present foreign antigens either by themselves, or through their lineage descendents; they are also dead cells scavengers. Strikingly, their role as front line defense against myriad of potentially pathogenic infectious agents in the outside environment, is essential in many species from insects to humans. Macrophages use various sets of receptor proteins to react to these agents, a central role being plaid by the Toll family molecules [1,2]. Such receptors recognize microbial lipids [3], lipoproteins [4,5], microbial carbohydrates [6] and bacterial DNA specific patterns [7]. Whatever the specific type of Toll like receptors involved, their engagements induce macrophages activation. This results in the production and release of a broad variety of specialized molecules aimed at limiting the multiplication and propagation of the infectious agents (innate immunity). The front line effector molecules produced by activated macrophages are reactive oxygen species (ROS) and reactive nitrogen species (RNS), highly diffusible molecules which have strong cytotoxic activities, including against macrophages themselves [8-10]. Such toxic effects are potentially dangerous since extensive macrophage destructions in the body can lead to the development of the fatal complication known as septic shock. Most of the time however, auto-protective redox buffering mechanisms prevent extensive destruction of activated macrophages. The aim of the present work was to elucidate by which mechanisms macrophages stimulated by bacterial products, manage to avoid massive auto-destruction caused by RNS? Experiments were performed with cells of the murine macrophage cell line (RAW 264.7) stimulated in vitro with lipopolysaccharide (LPS) and/or interferon (IFN-γ), two stimuli which induce strong endogenous NO production [11]. Our results directly establish that macrophage resistance is tightly regulated by the expression of definite sets of auto-protective redox buffering molecules.
Results
LPS and/or IFN-γ in vitro, stimulate cells of the murine RAW264.7 line along the differentiation pathway of secreting macrophages : induction of TNFα and NO production
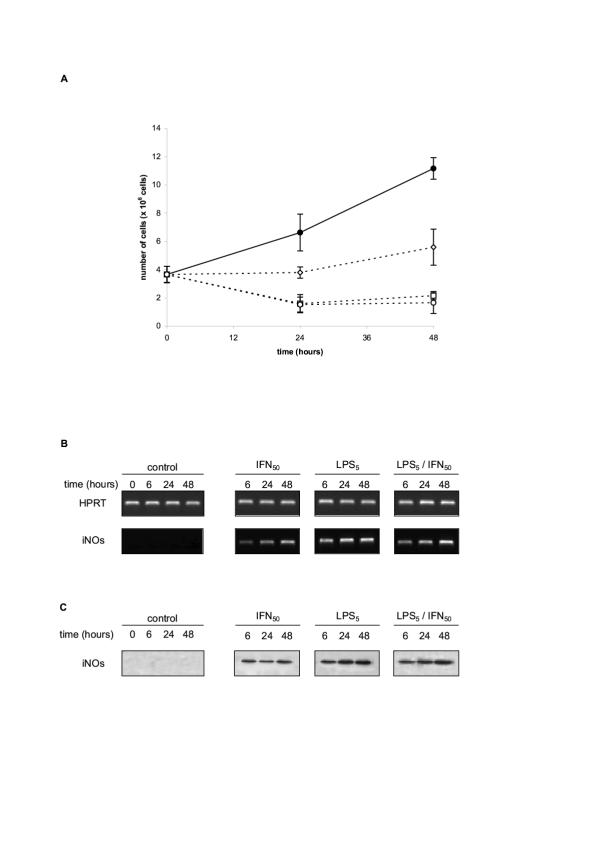
We first made a detailed kinetics analysis of the response of RAW 264.4 cells to in vitro stimulation by LPS, or IFN, or both. We confirmed that these products stimulated the differentiation of the cells toward the phenotype of activated macrophages releasing NO and secreting TNFα. In our experiments, RAW 264.7 cells were exposed in vitro to either 50 units/ml of IFN-γ (IFN50), or 5 μg/ml LPS (LPS5), or a mixture of both stimulating products. Cell free culture supernatants were harvested at 0, 6, 24 and 48 H and assessed for the production of TNFα, nitrites/nitrates and citrullin. Titrating TNFα production by ELISA, the strongest stimulation was observed with either LPS or LPS + IFN, leading to abundant secretions of TNFα as early as 6 H after the beginning of the culture, with a subsequent plateau at 24 and 48 H. The quantities of TNFα released by 0.25 × 106 cells initially seeded were in the range of 1400–1700 pg/ml at 24 and 48 H. Stimulation by IFN alone was less efficient inducing roughly only half of the above TNFα production (table 2). In the same supernatants, the production of NO was evaluated by quantifying citrulline and the total nitrite/nitrate with the Griess reagent. Both products were titrated in quantity (15–30 μM range and 30–70 μM range respectively) in 24 and 48 H culture supernatants of RAW 264.7 cells stimulated by either LPS or LPS+IFN. Smaller quantities were titrated in supernatants of cells stimulated by IFN alone (Table 2). Parallel experiments were performed to monitor NOs2 (iNOs) mRNA induction and NOs2 (iNOs) protein synthesis in RAW 264.7 cells exposed in vitro to either 50 units/ml of IFN-γ (IFN50), or 5 μg/ml LPS (LPS5), or a mixture of both stimulating products. Cells were pelleted at 0, 6, 24 H and 48 H. NOs2 (iNOs) mRNA was evaluated by semi-quantitative RT-PCR (Figure 1B), and NOs2 (iNOs) protein intracellular level by immunoblot (Figure 1C). NOs2 (iNOs) was undetectable both at the mRNA and protein levels in unstimulated RAW 264.7 cells. It was strongly induced at times 6, 24 and 48 H with the three stimulation protocols (Figure 1A &1B). Only slight variations were noted depending of the stimulations used; IFN-γ alone being as previously, the weakest inducing stimulus compared to LPS and LPS + IFN-γ.
Table 2.
Differentiation of RAW 264 7 cells toward secreting-activated macrophages following culture with LPS, IFN-γ or LPS+IFN-γ
| nitric oxide metabolism | ||||||||||||
| TNF production (pg/ml) | nitrites/nitrates (μM) | citruline (μM) | ||||||||||
| Sample type | 0 h | 6 h | 24 h | 48 h | 0 h | 6 h | 24 h | 48 h | 0 h | 6 h | 24 h | 48 h |
| medium | 156+/ 12 | 215+/ 18 | 332+/ 168 | 462 +/ 250 | ND | ND | ND | ND | 20+/ 4 | 22+/ 5 | 25+/ 7 | 33+/ 8 |
| LPS | 156+/ 12 | 1480+/ 187 | 1646+/ 266 | 1733+/ 284 | ND | ND | 26+/ 5 | 38+/ 18 | 20+/ 4 | 35+/ 12 | 47+/ 11 | 86+/ 31 |
| IFN | 156+/ 12 | 402+/ 28 | 681 +/ 42 | 819+/ 126 | ND | ND | 4+/ 2 | 12+/ 3 | 20+/ 4 | 25+/ 5 | 34+/ 9 | 42+/ 8 |
| LPS/IFN | 156+/ 12 | 1392+/ 224 | 1502+/ 335 | 1754+/ 201 | ND | ND | 13+/ 9 | 33+/ 15 | 20+/ 4 | 37+/ 12 | 43+/ 7 | 72+/ 22 |
ND: not detectable RAW 264.7 cells (250 000 cells/well) were cultured with medium or medium supplemented with either IFN-γ (50 U/ml), or LPS (5 μg/ml) or a mixture of IFN-γ (50 U/ml) + LPS (5 μg/ml). Cell-free culture supernatants were harvested at 0, 6, 24, and 48 hours. The concentrations of TNF-α (measured using an ELISA kit), nitrites/nitrates (measured using the Griess reagent) and citrulline (measured using colorimetric reaction), were evaluated in each culture supernatant as described in Methods. Data represent means +/- SEM of five independent experiments.
Figure 1.
A/ Growth rate and survival of RAW 264.7 cells cultured in medium, or medium supplemented with LPS or IFN-γ or LPS+IFN-γ. RAW 264.7 cells were cultured (4 × 106 cells in 20 ml) in medium (• œ) or medium supplemented with either IFN-γ (50 U/ml -.), or LPS (5 μg/ml -) or a mixture of IFN-γ (50 U/ml) + LPS (5 μg/ml -). The number of live cells recovered at the indicated times was estimated using trypan blue exclusion. Similar results were obtained estimating the cell survival either by 3H-thymidine incorporation, or by the MTT reduction test. B/ NOS2 (iNOs) mRNA induction in RAW 264.7 cultured with LPS or IFN-γ or LPS+IFN-γ. RAW 264.7 cells seeded into six-well plates (2.5 × 106 cells/well) were treated with the different stimuli as indicated above for the indicated times and analyzed by RT-PCR with specific primers for murine NOS2 (iNOs) (Table 1). HPRT was used as internal control for semi-quantitative estimation. C/ NOS2 (iNOs) protein in RAW 264.7 cultured with LPS or IFN-ã or LPS+IFN-γ. RAW 264.7 cells were treated with the different stimuli as indicated above for various times and cell pellets (1 × 106 cells) were lysed with lysis buffer. Protein concentrations in samples were adjusted and electrophoresed on 15% SDS-polyacrylamide gel, then transferred to nitro-cellulose membrane and Western bloted using polyclonal rabbit anti-murine NOS2 (iNOs), as described in Materials and Methods.
Death induction of RAW 264.7 cells stimulated by IFN-γ and/or LPS in vitro, with selection of a fraction of surviving activated RAW 264.7
In vitro exposure of cells of the murine macrophage cell line RAW 264.7 to either 5 μg/ml LPS (LPS5), or 50 units/ml of IFN-γ (IFN50), or a mixture of both stimulating products, led to a substantial reduction of the growth rate and number of surviving cells after 24 H or 48 H in culture (Figure 1A). The strongest deleterious effects were observed culturing the cells with either LPS alone or both IFN and LPS, resulting in the recovery of only approximatively 20% viable cells as estimated by MTT assay (or trypan blue test) at 24 H, and 13% at 48 H. IFN alone induced less cytoxicity with 54% and 50% viable cells recovered at 24 H and 48 H respectively (Figure 1A). The surviving cells subsequently recovered an in vitro growth rate comparable to untreated parental RAW 264.7 cells in culture (not shown). Additional experiments indicated however that those surviving activated macrophages had acquired peculiar resistance capacity to exogenous oxydative and nitrosative stress. More experiments were performed to characterize this resistance phenotype and the differentiation induced cellular metabolic events responsible for this resistance.
Nitrosative stress resistance of RAW 264.7 macrophages surviving LPS, or IFN, or LPS+ IFN, stimulation
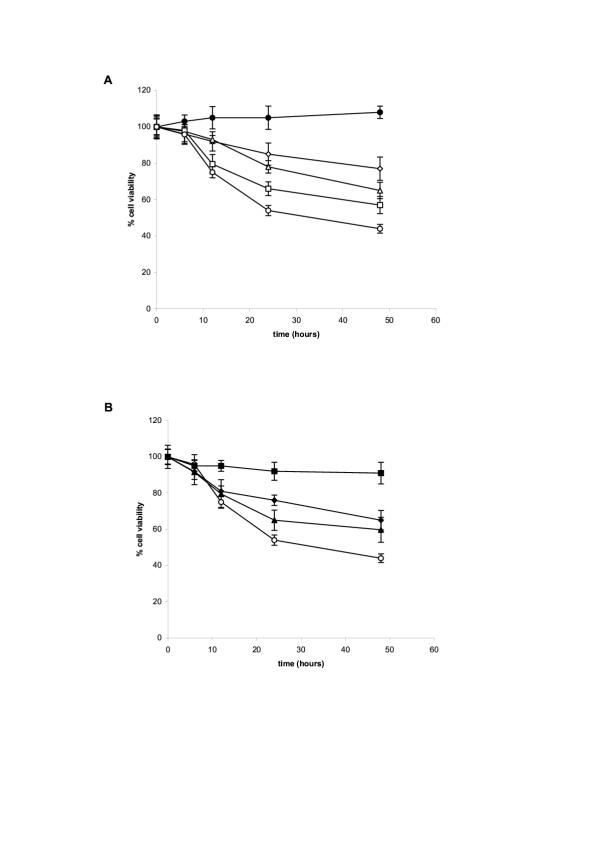
We next established that the selected population of activated macrophages surviving in vitro stimulation by either LPS, or IFN, or LPS+ IFN, had acquired a nitrosative stress resistance phenotype. RAW 264.7 cells, recovered from 24 H stimulated cultures with LPS, or IFN, or LPS+ IFN, or from unstimulated cultures (control parental cells), were washed and seeded in fresh culture medium in the presence of 1 mM of the NO releasing compound diethylenetriamine nitric oxide (DETA-NO). This concentration had been established previously to reproducibly induce the apoptotic death of about 50% unstimulated parental RAW 264.7 cells after 48 H in vitro (Figure 2A). The susceptibility of LPS, or IFN, or LPS+ IFN activated macrophages to the NO releasing compound DETA-NO, was evaluated in vitro by measuring the number of metabolically active cells (reducing Formazan = MTT assay) or trypan blue negative cells, recovered at the indicated time. We established previously that the MTT test provides a reproducible quantitative estimate of cell viability giving comparable results with the trypan blue dye exclusion method. As can be seen in Figure 2B, unselected parental RAW 264.7 cells were susceptible to DETA-NO death induction in vitro, with only 55% cells surviving after 24 H, and 45% after 48 hours. By contrast, LPS, or IFN, or LPS+ IFN activated RAW 264.7 macrophages were less susceptible to DETA-NO death induction in vitro, with variations depending upon the stimulation protocol.(Fig 2B). Activated-macrophages recovered from cultures stimulated with LPS alone were almost completely resistant to the nitrosative stress. Activated-macrophages recovered from IFN, or IFN + LPS stimulated cultures, exhibited an intermediate NO resistance, with about 70% and 60% metabolically active cells, or trypan blue negative cells, recovered after 24 or 48 H in culture with DETA-NO.
Figure 2.
A / Susceptibility of RAW 264.7 undifferentiated cells to various doses of the NO donor compound DETA-NO in culture. RAW 264.7 cells seeded at 5.104 per well in 96 wells plates were cultured in medium alone (●) or medium supplemented with 125 μM (◇), 250 μM (△), 500 μM (□), 1000 μM (O) DETA-NO. The number of live cells recovered at the indicated times was estimated using the MTT reduction test. Similar results were obtained estimating the cell survival either by 3H-thymidine incorporation, or by trypan blue exclusion. B/ Susceptibility of RAW 264.7 either undifferentiated, or differentiated cells, to 1 mM dose of the NO donor compound DETA-NO in culture. RAW 264.7 cells were cultured (10 × 106 cells in 50 ml) in medium alone (○) or medium supplemented with either IFN-γ (50 U/ml – ◆), or LPS (5 μg/ml – ■) or a mixture of IFN-γ (50 U/ml) + LPS (5 μg/ml – ▲). After 24 h, the cells were recovered, washed, seeded at 5.104 cells per well in 96 wells plate, and exposed to 1 mM dose of the NO donor compound DETA-NO for the indicated times. The percentage of living cells was assessed after 48 hours exposure to the NO donor compound using the MTT assay. The experiments were performed in triplicate and the results presented are representative of four separate experiments.
Upregulation of redox protection/detoxification systems in LPS-differentiated NO resistant RAW 264.7 cells
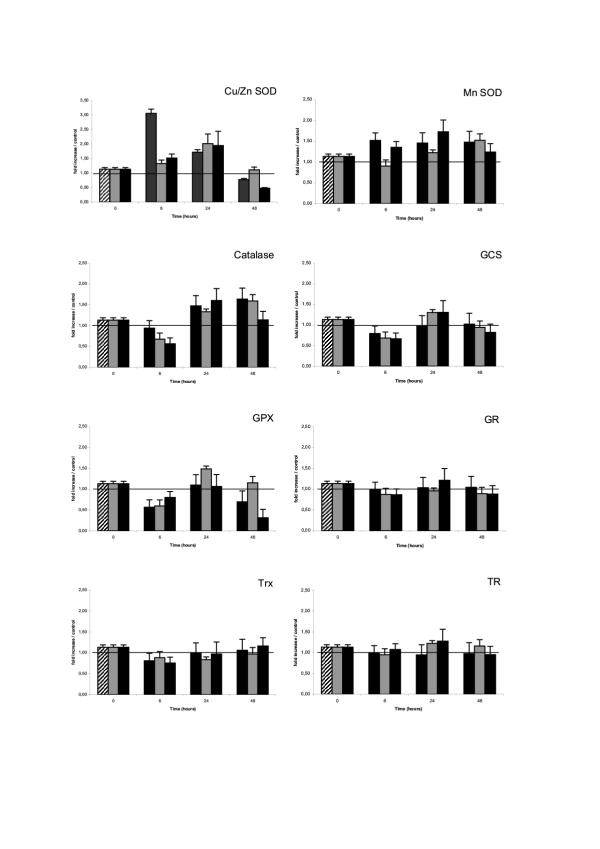
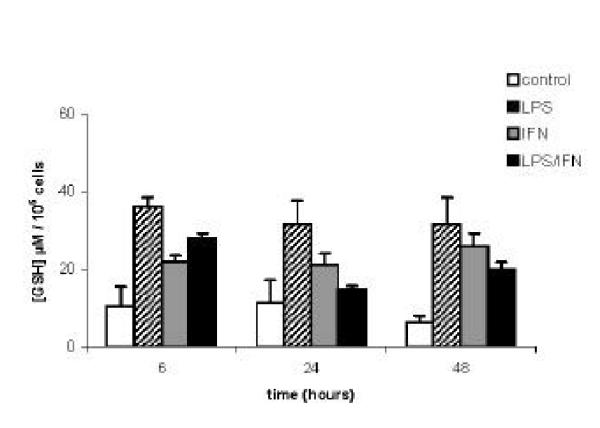
LPS (and/or IFN) induced NO resistance of differentiated RAW 264.7 macrophages could result from an up-regulation of their cellular redox protection/detoxification systems. We therefore evaluated the LPS (and/or IFN) induced modulation of a number of redox proteins, critical in the three major systems protecting cells against oxydative and nitrosative stress : the superoxide dismutase/catalase system (Cu/Zn SOD, Mn SOD and catalase); the glutathione system (glutathione (GSH), γ-glutamyl cystein synthase (γ-GCS), glutathione peroxidase (Gpx), gluthatione reductase (GR); and the thioredoxin system (thioredoxin (Trx), thioredoxin reductase (TR). The transcriptional modulation of several of these redox proteins was evaluated by semi-quantitative RT-PCR on 24 hours cell extracts, using the specific primers described in table 1. As shown in Figure 3, Cu/Zn SOD, Mn SOD, and catalase, were significantly upregulated (130 to 200 % increased) in NO resistant activated RAW 264.7 macrophages obtained following 24 hours stimulation with LPS (and/or IFN). By contrast, γ-glutamyl cystein synthase (γ-GCS) and glutathion peroxidase (Gpx) were only marginally increased with IFN, while glutathione reductase (GR), thioredoxin (Trx) and thioredoxin reductase (TR) mRNA were not significantly affected. This gene transcription study was further documented by measuring cellular concentrations of glutathione (GSH) in lysates of activated RAW 264.7 macrophages, recovered 6, 24 or 48 hours after LPS (and/or IFN) stimulations. As can be seen in Figure 4, glutathione level was approximatively 3.5 folds increased at 6 hours in LPS stimulated RAW 264.7 cells, compared to basal level in unstimulated control RAW 264.7 cells. This LPS induced high glutathione level was still detected at 24 and 48 hours (2.6 and 5.2 fold increased respectively). IFN50 and IFN50/LPSs stimulations also increased intra cellular glutathione levels in RAW 264.7 cells, with the same kinetics than LPS alone, although with variable efficiency (Figure 4). Additional experiments were performed to quantify the cellular specific activity of several other redox protection proteins in lysates of RAW 264.7 macrophages, recovered 6, 24 or 48 hours after LPS (and/or IFN) stimulations. As can be seen in table 3, catalase activity was approximately two folds increased at 6 and 24 hours after LPS stimulation of RAW 264.7 cells, compared to basal level in unstimulated cells, (but not after IFN50 or LPS5/IFN50 treatments), returning to basal level at 48 H. A sharp seven fold increased SOD activity was observed at 6 H in RAW 264.7 cells stimulated with IFN50 and IFN50/LPS5, which was not observed at any other time point, nor with the other stimulation protocols. No significant changes in Gpx activity was observed in stimulated cells.
Table 1.
Pairs of synthetic primers used in RT-PCR to amplify the different molecular systems studied.
| Gene studied | Sens sequence | Anti-sens sequence | size (pb) |
| HPRT | 5'TGG AAT CCT GTG GCA TCC ATG AAA C 3' | 5' TAA AAC GCA GCT CAG TAA CAG TCC G 3' | 348 |
| catalase | 5' GCA GAT ACC TGT GAA CTG TC 3' | 5' GTA GAA TGT CCG CAC CTG AG 3' | 229 |
| GCS | 5' CCT TCT GGC ACA GCA CGT TG 3' | 5' TAA GAC GGC ATC TCG CTC CT 3' | 346 |
| Gpx | 5' CCT CAA GTA CGT CCG ACC TG 3' | 5' CAA TGT CGT TGC GGC ACA CC 3' | 197 |
| GR | 5' AGC CGC CTG AAC ACC ATC TA 3' | 5' CCG TCT GAA TGC CCA CTT TA 3' | 601 |
| NOS(2) | 5' ACG CTT CAC TTC CAA TGC AAC 3' | 5' TGA GGG CTG ACA CAA GGC CTC 3' | 511 |
| SOD(Cu/Zn) | 5' AAG GCC GTG TGC GTG CTG AA 3' | 5' CAG GTC TCC AAC ATG CCT CT 3' | 246 |
| SOD(Mn) | 5' GCA CAT TAA CGC GCA GAT CA 3' | 5' AGC CTC CAG CAA CTC TCC TT 3' | 241 |
| Trx | 5' CCC TTC TTC CAT TCC CTC TG 3' | 5' AAC TCC CCC ACC TTT TGA CC 3' | 149 |
| TR | 5' TCC TCT TTT TCT ACC CAC TG 3' | 5' GTA TTC CTT GCT GTC ATC CA 3' | 464 |
Figure 3.
Transcriptional modulation of important redox proteins in RAW 264.7 either undifferentiated, or differentiated cells. RAW 264.7 cells were cultured (2.5 × 106 cells/well) in medium, or medium supplemented with either LPS (5 μg/ml – hached boxes), or IFN-γ (50 U/ml – green boxes) or a mixture of IFN-γ (50 U/ml) + LPS (5 μg/ml) (black boxes). After 24 h, the cells were recovered and washed. Total cellular RNA were extracted and used for semi-quantitative RT-PCR evaluation of the redox proteins indicated. Vertical axis indicate the ratio of the redox protein mRNA in cells cultured in medium with the three stimulating conditions, to the same redox protein mRNA in cells cultured in medium alone. HPRT mRNA was used as internal standard.
Figure 4.
Glutathione concentrations in RAW 264.7 either undifferentiated, or differentiated cells. Cells were cultured for the indicated times in medium, or medium supplemented with 5 μg/ml LPS, 50 U/ml IFN-γ or a mixture of IFN-γ (50 U/ml) + LPS (5 μg/ml). Total glutathione was quantified using "Glutathione Cellular Assay" kit (specific evaluation of glutathione-SH groups). Results are expressed as means +/- SEM of five independent experiments.
Table 3.
Activities of the redox enzymes catalase, Gpx and SOD, in differentiated NO resistant RAW 264 7 cells
| catalase (U/mg) | Gpx (U/mg) | SOD (U/mg) | |||||||
| time (hours) | 6 | 24 | 48 | 6 | 24 | 48 | 6 | 24 | 48 |
| control | 873+/-100 | 761+/-128 | 767+/-96 | 257+/-116 | 379+/-95 | 300+/-32 | 249+/-111 | 263+/-47 | 326+/-76 |
| LPS5 | 1563+/-408 | 1036+/-670 | 738+/-90 | 335+/-206 | 249+/-55 | 323+/-52 | 454+/-174 | 205+/-136 | 231+/-169 |
| IFN50 | 598+/81 | 555+/ 92 | 612+/76 | 420+/ 30 | 218+/46 | 248+/17 | 1637+/512 | 288+/ 85 | 66+/37 |
| LPS5/IFN50 | 778+/67 | 698+/167 | 849+/ 33 | 280+/13 | 213+/61 | 268+/ 18 | 1833+/1601 | 284+/ 190 | 236+/115 |
RAW 264.7 cells were cultured (10 × 106 cells in 50 ml) with medium or medium supplemented with either IFN-γ (50 U/ml), or LPS (5 μg/ml) or a mixture of IFN-γ (50 U/ml) + LPS (5 μg/ml). Cells were harvested at 6, 24, and 48 hours. Activities of the enzymes : catalase (decomposition of H2O2), Gpx (oxidation of NADPH) and SOD (reaction with chromophore) were determined on cell lysates as described in Methods. Results are expressed as means +/- SEM of five independent experiments.
LPS induced NO resistance of differentiated RAW 264.7 macrophages is abrogated by chemical inhibitors affecting the intra-cellular redox protection/detoxification systems
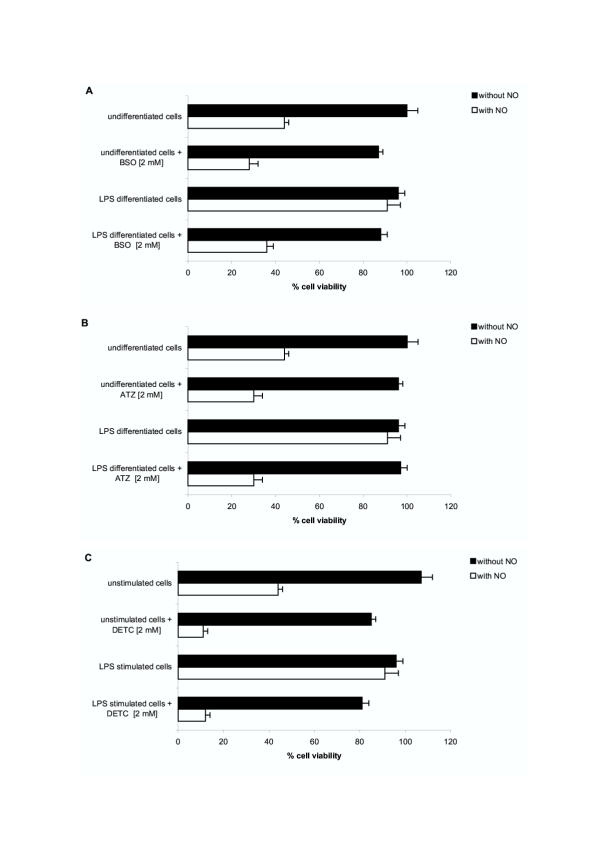
The causal relationship between the acquisition of NO resistance and the upregulation of a selective set of redox protection/detoxification systems in LPS differentiated RAW 264.7 macrophages was established using chemical inhibitors targeting those redox systems. The compounds used were relatively specific inhibitors affecting the SOD/catalase system and the glutathione system, the two redox protection/detoxification biochemical sets significantly up-regulated in LPS-differentiated macrophages. DETC is an inhibitor of both Mn SOD and Cu/Zn SOD, ATZ is a catalase inhibitor, and BSO reduces the intra-cellular level of glutathione by inhibiting the γ-glutamyl cysteine synthase. In our experiments, RAW 264.7 cells were first differentiated in vitro, as previously, by stimulation with an optimal dose of LPS (5 μg/ml) for 48 h. Selected-surviving-LPS-differentiated RAW 264.7 macrophages (and control unstimulated RAW 264.7 cells) were washed and incubated for an additional 2 hours period in the presence of the redox inhibitors (or not). Subsequently, nitrosative stress resistance of the treated cells was evaluated by culturing them in the presence of 1 mM DETA-NO and assessing cell viability after 24 hours (number of metabolically active cells reducing formazan = MTT test). The results are presented in Figure 5. LPS differentiated macrophages were 90–100% resistant to the toxic effects of NO released by 1 mM DETA-NO in vitro, while unstimulated cells were susceptible to NO, with 45% cells only surviving to the same in vitro treatment. Noteworthy, incubation of LPS differentiated cells in the presence of the redox inhibitors abrogated their NO resistance and restored their susceptibility to the toxic effects of DETA-NO. The SOD inhibitor DETC was the most effective compound. It completely reversed the NO resistance status of LPS-differentiated macrophages : only 10% of DETC-treated LPS-differentiated macrophages were recovered at 48 hours following exposure to DETA-NO; compared to 90% recovery of LPS-differentiated macrophages cultured without DETC (Figure 5C). The two others redox inhibitors BSO (γ-GCS inhibitor) and ATZ (catalase inhibitor), also very significantly abrogated NO resistance in LPS-differentiated macrophages (with only 35% and 30% viable cells recovery following DETA-NO exposure of LPS-differentiated macrophages treated with these two compounds, respectively) (Figure 5A &5B). In addition we noted that the SOD inhibitor DETC substantially sensitized unstimulated RAW 264.7 cells to the toxic effects of DETA-NO : only 10% DETC treated unstimulated RAW 264.7 cells survived NO exposure, compared to 40% control unstimulated RAW 264.7 not incubated with DETC (Figure 5C).
Figure 5.
LPS induced NO resistance in differentiated RAW 264.7 macrophages is abrogated by chemical inhibitors affecting the intra-cellular redox protection/detoxification systems. RAW 264.7 cells were first treated in vitro, as previously, culturing them in medium (undifferentiated cells), or medium supplemented with 5 μg/ml LPS. After for 24 hours, surviving-LPS-differentiated RAW 264.7 macrophages, and control undifferentiated RAW 264.7 cells, were washed and incubated for an additional 2 hours period in the presence of the redox inhibitors BSO (an inhibitor of γ-glutamyl cysteine synthase), or ATZ (a catalase inhibitor), or DETC (an inhibitor of both Mn SOD and Cu/Zn SOD). Subsequently, the resistance of the treated cells to exogenous NO was evaluated by culturing them (or not) in the presence of 1 mM DETA-NO (a dose previously established to be toxic for undifferentiated RAW 264.7 cells, Figure 2A). The % viability of cell exposed to exogenous NO was assessed after 48 hours as the percent of metabolically active cells reducing formazan (MTT test) compared to control (number of RAW 264.7 cells undifferentiated, not exposed to exogeneous NO, surviving at the end of the culture period = 100% cell viability).
Discussion
Macrophages mediate crucial functions protecting the organism against pathogenic infectious agents. Upon encounter with micro-organisms or stimulation by cytokines, they produce and release various sets of effector molecules aimed at destroying the foreign agents. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are their front line effector molecules [13,14]. These highly diffusible products exert strong cytotoxic activities against micro-organisms and many cells, including against macrophages themselves. However, a fraction of activated macrophages exposed to redox stress manage to escape destruction. Our results establish that nitrosative resistance results from the adapatative tuning of the redox buffers in these cells.
Reactive oxygen species and nitrogen monoxide are the earliest cytotoxic defense molecules produced by activated murine macrophages after contact with the infectious agents. Elevated oxygen production in macrophages (and polymorpho nuclear cells) relies upon the enzyme NaDPH oxydase [15]. Elevated and sustained production of nitric oxide (NO), a short-lived radical molecule generated from the guanido nitrogen group of L-arginine, is controled by an inducible NO synthase isoform (iNOS) [15]. This iNOS enzyme is tightly regulated in macrophages at variance with the two additional constitutive isoforms of NO synthase (cNOS) which are expressed in different tissues and function in basal physiological conditions. These later enzymes produce low levels of NO involved respectively in neurotransmission, and vascular relaxation [16-19]. Elevated production of NO in activated macrophages by the inducible NOS isoform following cytokines and/or bacterial lipopolysaccharide (LPS) challenge has been recognized as an essential anti-bacterial defense mechanism in rodent [14]. NO is not a strong oxidant by itself. However, NO can react with transition metals and reactive oxygen species (ROS) leading to the secondary generation of very toxic and reactive products like peroxynitrite (ONOO-) [20]. All these products, NO and its related nitrosocompounds, mediate toxicity in bacterial assailants. They also mediate growth arrest and apoptosis in normal mammalian cells [13] (including autocrine and paracrine toxicity for activated macrophages) and tumor cells [21-23].
Mammalian cells are equiped with intracellular protection systems, « redox buffer », which protect them from endogen oxygen radicals produced by the respiration in mitochondria [24,25]. Three biochemical sytems are the most important redox buffers. A predominant protection is by the Cu/Zn-dependent or Mn-dependent superoxide dismutase enzymes (SOD) which convert superoxide ions to hydrogen peroxide, then detoxified by catalase [26,27]. A second protection is by gluthatione (GSH), an intracellular peptidic thiol molecule with both oxidant scavenger and redox regulating capacities [28-30]. Thiols on gluthatione combine with NO and form the less reactive product S-nitrosoglutathione (GSNO). A third protection is performed by thioredoxine (Trx) which can buffer ROS and RNS through oxidation of its intra chain disulfide bridge, reduced thioredoxine being then regenerated by the enzyme thioredoxine reductase (TR) [31-33]. The respective roles of these intracellular antioxidant autoprotective systems against ROS and/or RNS-mediated auto-injury in activated macrophages have not been defined in details. Using a murine macrophage cell line (RAW 264.7), abundantly producing NO upon stimulation with lipopolysaccharide (LPS) and/or interferon (IFN-γ), we examined changes in those intracellular redox homeostatic systems and, using relatively specific inhibitors, identified the most important redox protection systems.
Our experiments indicate that macrophage stimulation induce an up-regulation of the two major intracellular redox buffering systems : the enzymatic SOD-catalase system and the RNS acceptor-neutralizer molecule glutathione. Such differentiated macrophages are resistant to exogenous NO This activation-induced new redox status is stable since the resistant phenotype of RAW 264.7 cells persisted after induction for at least one week in culture (data not shown). The regulation is both at the transcriptional and protein levels for SOD and catalase. The increased glutathione content in cells following stimulation is not explained by an increased transcription of the enzyme γ-glutamylcysteine synthase controling its synthesis; nor by an increased transcription of the regenerating enzyme glutathione reductase. It might result from the post translational stress-induced allosteric activation of the glutathione generating enzyme γ-glutamylcysteine synthase, or (and) from intra-cellular mobilization of an undefined inactive pool [34]. We did not observed any modulation of the third redox buffering system thioredoxine-thioredoxine reductase. This is at variance with our own previous results with THP1 monocytic human cells which heavily relied upon the thioredoxine system to maintain their redox homeostasis [33]. Such variation might represent inter species difference. The present data with RAW 264.7 cells are in good agreement with previous work from Brockaus & Brune group who reported that stably transfected RAW 264.7 cells overexpressing CuZn SOD were more resistant to endogenous or exogenous NO [35].
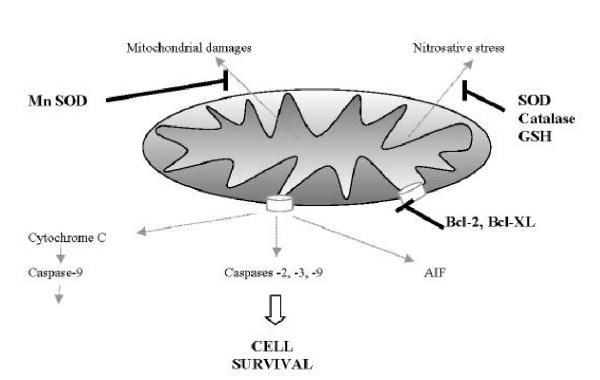
Using relatively specific inhibitors, we were able to establish a causal relationship between the upregulation of the intra cellular redox buffering systems mentioned above and the acquisition of their nitrosative stress resistant phenotype. The SOD inhibitor DETC almost completely reversed the NO resistance status of LPS-differentiated macrophages. It also substantially sensitized unstimulated RAW 264.7 cells to the toxic effects of DETA-NO. The two others inhibitors BSO (γ-GCS inhibitor) and ATZ (catalase inhibitor), also very significantly abrogated NO resistance in LPS-differentiated macrophages. Indeed the activation-induced resistance of macrophages has been studied by many groups (also designated as endotoxin-tolerized macrophages). Their results linked the resistance of macrophages to several, non exclusive, mechanisms such as : complex impaired expression and/or function of common signaling pathways [36] increased expression of Bcl-xL anti-apototic proteins [37], increased expression of Bfl-1 anti-apototic gene and simultaneous down regulation of caspase-8 mRNA [38], alteration of the Fas-Fas ligand transduction pathway [39], over-expression of CD14 receptor [40], autocrine cytokine regulatory network between IL-12 and IL-10 [41,42]. The multiplicity and complexity of the macrophage responses was recently documented using the gene array technology which detected a broad spectrum of genes overexpressed in response to S. typhimurium and S. typhimurium LPS in RAW 264.7 cells [43]. We also observed in our experiments an increased expression of Bcl2 and Bcl-XL and decreased expression of Bax and Bad in NO-resistant activated macrophages (not shown). Nevertheless, our results clearly demonstrated the crucial auto-protective importance of the up-regulation of the two major redox buffering systems SOD-catalase and glutathione, for nitrosative stress resistance of activated macrophage. A schematic representation of the many factors regulating survival of activated macrophages is presented in Figure 6.
Figure 6.
Schematic representation of the many factors regulating nitrosative stress resistance and survival of activated macrophage.
Conclusion
Activated macrophages exposed to endogenous NO are submited to a redox nitrosative stress which acts as a strong selection process allowing the survival of the fraction of cells up-regulating key sets of autoprotective redox buffering molecules. What are the cascade of events and the mechanisms leading to the transcriptional activation of SOD genes and increased glutathione availability in activated macrophages, remain to be determined. Such findings might help define new strategies aimed at improving the induction of nitrosative stress resistant macrophages which could be the best efficient defenders of the organisms against infection and possibly cancer.
Materials and Methods
Materials
The monoclonal antibody (MoAb) against NOs2 (iNOs) was purchased from Pharmingen (San Diego, CA). The horseradish peroxydase-conjugated goat-anti-rabbit antibody was purchased from BIO-RAD (Bio-Rad laboratories, California). The NO donor compound 2,2'(hydroxynitrosohydrazino)bis-ethanamine (DETA/NO) was purchased from Calbiochem. Purified LPS from Escherichia Coli, Leupeptin, pepstatin, phenylmethylsulfonyl fluoride (PMSF), EDTA, Hepes, CHAPS, 3-(4,5-dimethylthiazol-2-yl)-2-5-diphenyltetrazolium bromide (MTT), ATZ (aminotriazol), BSO (Buthionine sulfoximine), DETC (Diethylthiocarbamate) and DTT, were from Sigma. ECL chemoluminescence enhancer reagents were from Amersham.
Cell cultures
Cells of the mouse macrophage-RAW 264.7 line were obtained from the American Type Culture Collection [11] and cultured in RPMI 1640 medium supplemented with glutamax 1 (GIBCO/BRL/Life Technologies), 7% (v/v) heat-inactivated foetal calf serum (HyClone), 100 units/ml penicillin and 100 μg/ml streptomycin, at 37°C in a humidified air/CO2 (5%) atmosphere. Standart bulk cultures were with 10 × 106 cells in 150 cm2/50 ml vials (Corning, polystyrene) in culture medium, or culture medium supplemented with either 5 μg/ml Escherichia Coli LPS (serotype O127:B8, from Sigma), or 50 units/ml recombinant mouse IFN-γ (Genzyme), or the combinaison of both (LPS 5 μg/ml plus IFN-γ 50 units/ml).
Titration of macrophage-derived pro-inflammatory products
TNF-α were measured in undiluted supernatants using ELISA kit purchased from Medgenix Diagnostics (Rungis, France) according to manufacter's instructions, in triplicates for each sample. The total amount of nitrite/nitrate, the stable products of NO, was determined in supernatants using the Griess reagent. Briefly, 50 μl of culture supernatants collected at 6, 24 and 48 hours were mixed with 150 μl of Griess reagent (1% sulfanilamide/0,1% naphtylethylenediamine-dihydrochloride) at RT for 30 s. The absorbance at 543 nm was immediately determined on Dynex Revelation F 3.21 microplate reader. Nitrite concentrations in each sample were determined by extrapolation from a sodium nitrite standart curve.
L-citrulline levels were determined by the colorimetric reaction of carbamido groups with diacetyl monoxime in acid solution [10]. Briefly, 30 μl of urease (25 U/ml) was added to 300 μl of supernatant, and incubated for 1 hour at 37°C. After addition of 37.5 μl trichloroacetic acid (TCA, 59% vol/vol), the precipitated proteins were pelleted by 5 minutes centrifugation at 12 000 g. 250 μl of supernatant was harvested and mixed with 300 μl of a 1:1 (vol/vol) mixture of 240 mmol diacetylmonoxime and a solution of phenazone (3 g in 104 ml H2O reacted with 12 mg FeSO4 and 21 ml H2SO4 36N, and incubated 15 minutes at 90°C in dark. 200 μl of the reaction mixture was transferred to a microtitration plate for measurement of the optical density at 492 nm using Dynex Revelation F 3.21 microplate reader. Citrulline concentrations were determined by extrapolation from a citrulline standart curve.
RNA isolation and RT-PCR analysis
Following in vitro stimulation for the indicated times with the indicated stimulus, total cellular RNAs were extracted from 2.5 × 106 RAW 264.7 cells using TRI reagent (Euromedex, Souffelweyersheim, France) according to manufacturer's instructions. cDNAs were synthesised in 40 μl reaction mixtures containing 2 μg of RNA, 8 μl of 5X buffer (250 mM TRIS-HCL, 250 mM KCL, 50 mM MgCl2, 50 mM DTT and 2.5 mM spermidine), 2 μl of each dNTP 25 mM, 0.4 μl of 50 mM oligo-dT primers, 0.4 μl of RNAsin at 22 000 U/ml and 0.6 μl of Avian Myeloblastosis Virus-reverse transcriptase (Appligene, Strasbourg, France) at 42°C for 2 hours. PCR was performed using 1/20 of the cDNA reaction mixture in a 100 μl reaction volume containing 10 μl of 10X buffer (10 mM TRIS-HCL, 50 mM KCL, 1.5 mM MgCl2, 0.1% Triton X 100 and 0.2 mg/ml gelatine), 0.5 μl of each dNTP 25 mM, 0.4 μl of Taq Polymerase 5 000 U/ml (Thermus aquaticus, Appligene) and 1 μl of each primer 20 μM. The RT-PCR products were subjected to electrophoresis on agarose gel, stained with ethidium bromide, scanned and quantified by densitometry. The sequences of sense and anti-sense PCR-primers used for amplification and the predicted size of products are described on Table 1. HPRT mRNA was used as internal standard. Modulation of mRNA expression in RAW 264.7 cells either undifferentiated, or differentiated, was standardized as follow. Bands were scanned and quantified by densitometry. The ratios : redox protein mRNA band intensity / HPRT mRNA band intensity were calculated for each stimulation conditions. The ratio values were then standardized taking the relevant mRNA band intensity ratio in undifferentiated RAW 264.7 cells as reference value 1.
Immunoblot analysis
Pellets of 1 × 106 cells were lysed in lysis buffer (25 mM HEPES, 0.5% Nonidet P40, 0.1% SDS, 0.5 M NaCl, 5 mM EDTA, 0.1 mM sodium deoxycholate, 1 mM PMSF, and 0.1 mg/ml leupeptin/pepstatin, pH 7.8) at 4°C for 30 min. Nuclei and membranes were removed by centrifugation at 12 000 g for 15 min. The amount of protein in each lysate was measured using the BSA microbiuret assay from Pierce. Loading buffer (42 mM tris-HCl, pH 6.8, 10% glycerol, 2.3% SDS, 5% 2-mercaptoethanol, and 0.002% bromophenol blue) was added to each lysate, which was subsequently boiled for 3 min and electrophoresed on an 15% SDS-polyacrylamide gel. Proteins were transferred to nitro-cellulose membrane. The membrane was saturated with TBS (150 mM NaCl, 10 mM tris HCl, pH 7.5) containing 10% skim milk overnight, washed twice 15 min with TTBS 0.1% (150 mM NaCl, 10 mM tris HCl, 0.1% Tween 20, pH 7.5), and incubated with a polyclonal rabbit-anti-murine NOS2 (iNOs) (1/1000 dilution), in TTBS 0.1% containing 3% skimmed milk for 1 h. The membrane was subsequently washed twice 15 min with TTBS 0.1%, and incubated for 45 min with the second antibody, peroxidase conjugated goat-anti-rabbit (1/5000). Bound antibodies were visualised by chemiluminescence using an ECL Western Immunoblotting Kit (Amersham).
Susceptibility-resistance of RAW 264.7 cells to NO-mediated cell injury
The susceptibility-resistance of RAW 264.7 cells exposed in vitro to exogenous NO was evaluated on cells distributed in 96 wells plates (4.104 per well). Diethylene triamine-nitric oxide (DETA-NO) 1 mM was added to each well at t0 and incubation processed for the indicated times. This 1 mM dose induced substantial cytotoxicity of undifferentiated RAW 264.7 cells after 24 h (Figure 2A). After various incubation times, cell viability was assessed using 3 methods: trypan blue dye exclusion test, 3H thymidine incorporation and MTT assay (mitochondria-dependant reduction of 3-(4,5-dimethylthiazol-2-yl) 2,5-diphenyltetrazolium bromide (MTT) to formazan. The 3 tests give highly concordant results in every experiments, with or without NO-donor molecules. Results with the MTT assay only are presented. It was performed as follow : 20 μl of MTT (5 mg/ml in PBS 1X) was added in each wells and incubated at 37°C for 4 hours. Then, 150 μl of medium was removed from each well, and 100 μl of 0,5% HCl/propanol-1 was added to dissolve cristals. The net absorbance ratio (ratio A550-A630) in each well was immediately recorded using an ELISA microplate reader. The net absorbance in wells containing cells cultured in control medium was taken as the 100% viability value. The percent of viable cells exposed to DETA-NO was calculated by comparison.
Determination of cellular content of glutathion
The cellular concentration of glutathion was evaluated using the reagent kit Bioxytech GSH-400 (Bioproducts, Gagny, France) according to manufacturer instructions', a glutathione colorimetric quantification test based upon specific evaluation of glutathione-SH groups.
Activities of redox enzymes
In vitro cultures of RAW 264.7 cells, in medium or medium supplemented with the differentiating agents, were interruped at the indicated times. Cells were pelleted, washed three times and lysed by three successive freezing-thawing cycles. The enzymatic activities were evaluated on cell lysates recovered after centrifugation at 12 000 g for 15 mn. The catalase activity was assayed by monitoring the decreased absorbance at 240 nm resulting from the decomposition of H2O2 for 1 min at 37°C. The assay mixture consisted of 2.98 ml of H2O2 solution from a stock solution of 0,1 ml of 30% (w/v) H2O2 diluted in 50 ml of sodium phosphate buffer, pH 7, and 20 μl of the incubation solution (0.025 mg of enzyme/ml of buffer). All assays were performed in triplicate. Activity is expressed in comparision with control activitis determined for each incubation time set as 100% value [12]. SOD and Gpx activities were determined using the reagent kits SOD-525 and Gpx-340 kits (from Alexi and Calbiochem respectively) following manufacturer's instructions.
Statistical analysis
Results are reported as mean ± SD except where indicated, and differences between groups are calculated by means of the nonpaired Student's t-test. Experiments were performed in triplicate.
Abbreviations
NO : nitric oxide
GSH : reduced glutathione
GSSG : oxidized glutathione
LPS : lipopolysaccharide
IFN-γ : interferon γ
INOs : inducible isoform of nitric-oxide synthase
GCS : γ-glutamylcysteine synthase
GR : glutathione reductase
GPx : glutathione peroxydase
Trx : thioredoxin
TR : thioredoxin reductase
Cu/Zn SOD : Cu/Zn-dependent superoxide dismutase
Mn SOD: Mn-dependent superoxide dismutase
DETC: Diethylthiocarbamate
ATZ: Aminotriazol
BSO: Buthionine sulfoximine
Acknowledgments
Acknowledgements
This work was supported by the Institut National de la Sante et de la Recherche Medicale, the Association pour la Recherche contre le Cancer (Grants n° 9974 and n° 5483), the Ministère de l'Education Nationale de la Recherche et de la Technologie. PJF has received a research grant from the Association Française pour la Recherche Thérapeutique.
Contributor Information
Pierre-Jacques Ferret, Email: pjferret@gmx.net.
Emmanuelle Soum, Email: fradeli@cochin.inserm.fr.
Olivier Negre, Email: fradeli@cochin.inserm.fr.
Didier Fradelizi, Email: fradeli@cochin.inserm.fr.
References
- Beutler B, Poltorak A. Positional cloning of Lps, and the general role of toll-like receptors in the innate immune response. Eur Cytokine Netw. 2000;11:143. [PubMed] [Google Scholar]
- Beutler B. Endotoxin, toll-like receptor 4, and the afferent limb of innate immunity. Curr Opin Microbiol. 2000;3:23. doi: 10.1016/S1369-5274(99)00046-6. [DOI] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, Huffel CV, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Brightbill HD, Libraty DH, Krutzik SR, Yang RB, Belisle JT, Bleharski JR, Maitland M, Norgard MV, Plevy SE, Smale ST, Brennan PJ, Bloom BR, Godowski PJ, Modlin RL. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285:732. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- Aliprantis AO, Yang RB, Mark MR, Suggett S, Devaux B, Radolf JD, Klimpel GR, Godowski P, Zychlinsky A. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285:736. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- Ozinsky AD, Underhill M, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, Schroeder L, Aderem A. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci U S A. 2000;97:13766. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- Zhuang JC, Wogan GN. Growth and viability of macrophages continuously stimulated to produce nitric oxide. Proc Natl Acad Sci U S A. 1997;94:11875. doi: 10.1073/pnas.94.22.11875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMicking JXQ, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–50. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- Sarih M, Souvannavong V, Adam A. Nitric oxide synthase induces macrophage death by apoptosis. Biochem Biophys Res Commun. 1993;191:503. doi: 10.1006/bbrc.1993.1246. [DOI] [PubMed] [Google Scholar]
- Stuehr DJ, Marletta MA. Synthesis of nitrite and nitrate in murine macrophage cell lines. Cancer Res. 1987;47:5590. [PubMed] [Google Scholar]
- Yan H, Harding JJ. Glycation-induced inactivation and loss of antigenicity of catalase and superoxide dismutase. Biochem J. 1997;328:599. doi: 10.1042/bj3280599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuehr DJ, Nathan CF. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989;169:1543. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan CF, Hibbs JB., Jr Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991;3:65. doi: 10.1016/0952-7915(91)90079-G. [DOI] [PubMed] [Google Scholar]
- Bastian NR, Hibbs JB., Jr Assembly and regulation of NADPH oxidase and nitric oxide synthase. Curr Opin Immunol. 1994;6:131. doi: 10.1016/0952-7915(94)90044-2. [DOI] [PubMed] [Google Scholar]
- Schmidt HH, Walter U. NO at work. Cell. 1994;78:919. doi: 10.1016/0092-8674(94)90267-4. [DOI] [PubMed] [Google Scholar]
- Nathan C. Natural resistance and nitric oxide. Cell. 1995;82:873. doi: 10.1016/0092-8674(95)90019-5. [DOI] [PubMed] [Google Scholar]
- Nathan C, Xie QW. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994;78:915. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109. [PubMed] [Google Scholar]
- Ischiropoulos H, al-Mehdi AB. Peroxynitrite-mediated oxidative protein modifications. FEBS Lett. 1995;364:279. doi: 10.1016/0014-5793(95)00307-U. [DOI] [PubMed] [Google Scholar]
- Drapier JC, Hibbs JB., Jr Murine cytotoxic activated macrophages inhibit aconitase in tumor cells. Inhibition involves the iron-sulfur prosthetic group and is reversible. J Clin Invest. 1986;78:790. doi: 10.1172/JCI112642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui S, Reichner JS, Mateo RB, Albina JE. Activated murine macrophages induce apoptosis in tumor cells through nitric oxide-dependent or -independent mechanisms. Cancer Res. 1994;54:2462. [PubMed] [Google Scholar]
- Gal A, Tamir S, Kennedy LJ, Tannenbaum SR, Wogan GN. Nitrotyrosine formation, apoptosis, and oxidative damage: relationships to nitric oxide production in SJL mice bearing the RcsX tumor. Cancer Res. 1997;57:1823. [PubMed] [Google Scholar]
- Bast A, Haenen GR, Doelman CJ. Oxidants and antioxidants: state of the art. Am J Med. 1991;91:2S. doi: 10.1016/0002-9343(91)90278-6. [DOI] [PubMed] [Google Scholar]
- Lewin G, Popov I. The antioxidant system of the organism. Theoretical basis and practical consequences. Med Hypotheses. 1994;42:269. doi: 10.1016/0306-9877(94)90130-9. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Canada AT. Catalase: its role in xenobiotic detoxification. Pharmacol Ther. 1989;44:297. doi: 10.1016/0163-7258(89)90069-7. [DOI] [PubMed] [Google Scholar]
- Canada AT, Calabrese EJ. Superoxide dismutase: its role in xenobiotic detoxification. Pharmacol Ther. 1989;44:285. doi: 10.1016/0163-7258(89)90068-5. [DOI] [PubMed] [Google Scholar]
- Duval DL, Sieg DJ, Billings RE. Regulation of hepatic nitric oxide synthase by reactive oxygen intermediates and glutathione. Arch Biochem Biophys. 1995;316:699. doi: 10.1006/abbi.1995.1093. [DOI] [PubMed] [Google Scholar]
- Meister A. On the antioxidant effects of ascorbic acid and glutathione. Biochem Pharmacol. 1992;44:1905. doi: 10.1016/0006-2952(92)90091-V. [DOI] [PubMed] [Google Scholar]
- Meister A. Biosynthesis and functions of glutathione, an essential biofactor. J Nutr Sci Vitaminol (Tokyo) Spec. 1992. p. 1. [DOI] [PubMed]
- Holmgren A. Thioredoxin and glutaredoxin systems. J Biol Chem. 1989;264:13963. [PubMed] [Google Scholar]
- Fernando MR, Nanri H, Yoshitake S, Nagata-Kuno K, Minakami S. Thioredoxin regenerates proteins inactivated by oxidative stress in endothelial cells. Eur J Biochem. 1992;209:917. doi: 10.1111/j.1432-1033.1992.tb17363.x. [DOI] [PubMed] [Google Scholar]
- Ferret PJ, Soum E, Negre O, Wollman EE, Fradelizi D. Protective effect of thioredoxin upon NO-mediated cell injury in THP1 monocytic human cells. Biochem J. 2000;346:759. doi: 10.1042/0264-6021:3460759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith OW. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic Biol Med. 1999;27:922. doi: 10.1016/S0891-5849(99)00176-8. [DOI] [PubMed] [Google Scholar]
- Brockhaus F, Brune B. Overexpression of CuZn superoxide dismutase protects RAW 264.7 macrophages against nitric oxide cytotoxicity. Biochem J. 1999;338:295. doi: 10.1042/0264-6021:3380295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvedev AE, Kopydlowski KM, Vogel SN. Inhibition of lipopolysaccharide-induced signal transduction in endotoxin-tolerized mouse macrophages: dysregulation of cytokine, chemokine, and toll-like receptor 2 and 4 gene expression. J Immunol. 2000;164:5564. doi: 10.4049/jimmunol.164.11.5564. [DOI] [PubMed] [Google Scholar]
- Okada S, Zhang H, Hatano M, Tokuhisa T. A physiologic role of Bcl-xL induced in activated macrophages. J Immunol. 1998;160:2590. [PubMed] [Google Scholar]
- Perera LP, Waldmann TA. Activation of human monocytes induces differential resistance to apoptosis with rapid down regulation of caspase-8/FLICE. Proc Natl Acad Sci U S A. 1998;95:14308. doi: 10.1073/pnas.95.24.14308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiener PA, Davis PM, Starling GC, Mehlin C, Klebanoff SJ, Ledbetter JA, Liles WC. Differential induction of apoptosis by Fas-Fas ligand interactions in human monocytes and macrophages. J Exp Med. 1997;185:1511. doi: 10.1084/jem.185.8.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidenreich S, Schmidt M, August C, Cullen P, Rademaekers A, Pauels HG. Regulation of human monocyte apoptosis by the CD14 molecule. J Immunol. 1997;159:3178. [PubMed] [Google Scholar]
- Karp CL, Wysocka M, Ma X, Marovich M, Factor RE, Nutman T, Armant M, Wahl L, Cuomo P, Trinchieri G. Potent suppression of IL-12 production from monocytes and dendritic cells during endotoxin tolerance. Eur J Immunol. 1998;28:3128. doi: 10.1002/1521-4141(199810)28:10<3128::AID-IMMU3128>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Shnyra A, Brewington R, Alipio A, Amura C, Morrison DC. Reprogramming of lipopolysaccharide-primed macrophages is controlled by a counterbalanced production of IL-10 and IL-12. J Immunol. 1998;160:3729. [PubMed] [Google Scholar]
- Rosenberger CM, Scott MG, Gold MR, Hancock RE, Finlay BB. Salmonella typhimurium infection and lipopolysaccharide stimulation induce similar changes in macrophage gene expression. J Immunol. 2000;164:5894. doi: 10.4049/jimmunol.164.11.5894. [DOI] [PubMed] [Google Scholar]