Abstract
Bacterial translocation is a pathological process involving migration of pathogenic bacteria across the intestinal barrier to enter the systemic circulation and gain access to distant organs. This phenomenon has been linked to a diverse range of diseases including inflammatory bowel disease, pancreatitis, and cancer. The intestinal barrier is an innate structure that maintains intestinal homeostasis. Pathogenic infections and dysbiosis can disrupt the integrity of the intestinal barrier, increasing its permeability, and thereby facilitating pathogen translocation. As translocation represents an essential step in pathogenesis, a clear understanding of how barrier integrity is disrupted and how this disruption facilitates bacterial translocation could identify new routes to effective prophylaxis and therapy. In this comprehensive review, we provide an in-depth analysis of bacterial translocation and intestinal barrier function. We discuss currently understood mechanisms of bacterial–enterocyte interactions, with a focus on tight junctions and endocytosis. We also discuss the emerging concept of bidirectional communication between the intestinal microbiota and other body systems. The intestinal tract has established ‘axes’ with various organs. Among our regulatory systems, the nervous, immune, and endocrine systems have been shown to play pivotal roles in barrier regulation. A mechanistic understanding of intestinal barrier regulation is crucial for the development of personalized management strategies for patients with bacterial translocation-related disorders. Advancing our knowledge of barrier regulation will pave the way for future research in this field and novel clinical intervention strategies.
Keywords: bacterial translocation, intestinal barrier, microbiota-immune-endocrine-nervous-axis
Introduction
The human intestine contains an enormous and diverse microbiota, predominantly comprising bacteria. The symbiotic relationship between humans and their intestinal bacteria profoundly affects multiple aspects of quality of life. The intestinal microbiota contributes to food digestion, nutrient absorption, and protection against pathogenic infections.1–3 Pathogenic infection and infection-induced microbial dysbiosis can permeabilize the intestinal barrier, allowing bacterial translocation exiting the intestine. 4 Such translocation contributes to inflammatory and immunity-related diseases including colitis, liver cirrhosis, pancreatitis, and rheumatoid arthritis. 5 The intestinal barrier is a complex structure that separates the lumen from the host’s interior, protecting against pathogenic microbial invasion while facilitating the absorption of nutrients, electrolytes, and solutes, thereby maintaining the host’s health status.2,6,7 When multiple defences fail, translocated bacteria can spread through adjacent lymph nodes or circulating blood to distant organs, leading to fatal bacteremia, sepsis, and tumorigenesis. 8 Pathogenic bacteria can also evade immune responses upon exposure to immune cells.8,9 This barrier is also regulated by the enteric nervous system, inflammatory responses, external antigen stimulation, metabolic disorder, and autoimmunity.4,10 Understanding mechanisms of bacterial translocation mainly concerns elucidating how bacteria damage and become translocated across the intestinal barrier. Owing to the complexity of involved regulatory factors and the diversity of pathogenic bacteria, our understanding of bacterial translocation is still very limited. People with different dietary habits, ages, and health conditions display extremely varied microbiota composition, making statistical data analysis challenging. A better understanding of bacterial translocation will clarify its role in pathogenesis and facilitate the prevention and treatment of intestinal-derived infections. This review focuses on how pathogenic bacteria increase intestinal barrier permeability and mediate bacterial translocation, thereby providing possible targets for the treatment of bacterial translocation.
The intestinal barrier and bacterial translocation
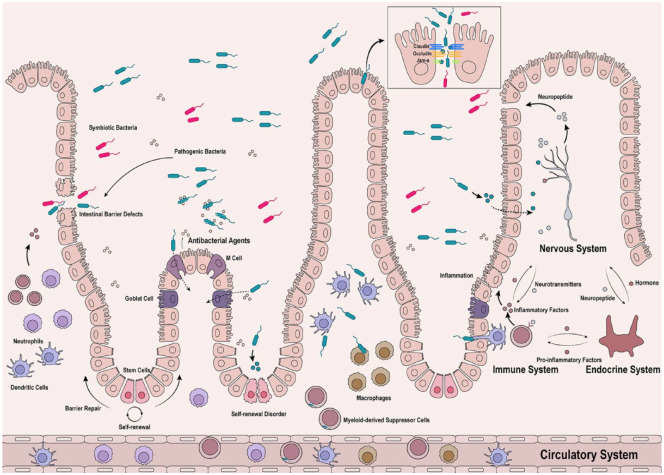
The intestinal barrier is a fundamental innate defense system that regulates the intestinal environment and protects against invasion by pathogenic bacteria. It incorporates multiple barriers, including mechanical, chemical, microbial, and immune. The mechanical barrier, also known as the physical barrier, depends on the structures of the intestinal epithelium, lamina propria, and muscular mucosae. 11 The intestinal epithelium is composed of several cell types, including Enterocytes, Goblet Cells, Paneth Cells, Cupped Cells, Enteroendocrine Cells, Microfold (M) cells, and tuft cells, 12 collectively referred to as intestinal epithelial cells (IECs). 13 An important role of IECs is to maintain intestinal barrier integrity, which allows solvent, electrolytes, and nutrients to pass through and impedes access to pathogens. 14 IECs contribute to the physical and chemical barriers against microbial infection by secreting mucus, digestive juices, mucopolysaccharides, glycoproteins, glycolipids, and other antibacterial agents. 14 Mucus-secreting goblet cells are interspersed among the intestinal mucosal epithelial cells, and a translucent layer of mucus is continuously distributed on the surface of the intestinal mucosa, which competes for IEC binding sites, preventing bacteria from adhering to the intestinal epithelium. Intestinal antimicrobial substances, including bile, mucopolysaccharides, lysozymes, and glycoproteins, also play roles in maintaining intestinal barrier function. 15 IECs are continuously generated and replaced every 4–5 days from intestinal stem cells (ISCs) through renewal and migration. 16 Aged IECs undergo apoptosis and slough off into the intestinal lumen. ISCs residing in the crypts and expressing leucine-rich repeat-containing G-protein-coupled receptor 5 (Lgr5) are responsible for the steady replenishment of surface-resident cells. This replenishment is vital for intestinal homeostasis and protection against pathogenic bacterial invasion. 17 A structurally and functionally undamaged barrier is foundational to intestinal health. Impairment of the intestinal barrier increases host susceptibility to various infectious and inflammatory diseases. Bacterial translocation indicates increased permeability of the monolayer cell barrier of the intestinal epithelium.
The concept of translocation of intestinal pathogenic bacteria was first proposed by Wolochow et al. 18 Bacterial translocation is now generally acknowledged that the microbiota initially colonizes the intestine, where endotoxins, peptidoglycans, and/or metabolites penetrate the intestinal mucosal barrier and reach distant organs and tissues. 19 Bacterial translocation may occur during microbial dysbiosis, intestinal barrier damage, and systemic immune suppression. A wide range of other diseases have also been linked to bacterial translocation, including depression, anxiety, malignancies, heart failure, and cardiopulmonary bypass.20–24 Bifidobacteria induce necrotic enterocolitis and increase intestinal permeability before the onset of disease, indicating that intestinal permeability can be enhanced before symptomatic disease is observed. 25 Intestinal pathogen diseases are not always triggered solely by altered tight junction integrity. Abnormal immune responses within the host may also increase disease predisposition by increasing tissue inflammation. Pathogenic bacterial translocation occurs mainly through tight junctions and endocytosis but can also induce a neuroimmune response through necrosis or disorders of IEC renewal. Interactions between immune cells and IECs play a significant role in maintaining intestinal barrier integrity. Defects in the intestinal barrier are often accompanied by inflammation, which recruits immune cells including immature dendritic cells, neutrophils, macrophages, and myeloid-derived suppressor cells.8,26–28 Myeloid-derived cells possess the ability to harbor pathogenic bacteria, which may be a way by which translocated bacteria evade immune clearance.29,30 Altered immune cell frequencies can increase intestinal barrier permeability. Pathogenic bacteria cross through the mucosal epithelium, causing various intestine-associated diseases, including inflammatory bowel disease (IBD) and pancreatitis, and cancer. 31 For example, in patients with IBD, the concentration of total bacterial DNA in intestinal tissues and blood samples is increased, suggesting the occurrence of bacterial translocation and microbial dysbiosis 32 It has also been shown that the ruin of the intestinal barrier leads to bacteria translocation where they are thought not to be present. Through the Apcmin mouse infection with Helicobacter hepaticus, it was found that H. hepaticus DNA was present in the mammary gland, but not in the uninfected Apcmin mouse, and the incidence of breast cancer was higher than the uninfected mouse. MDSC were found to migrate from the gut to the mammary gland, and the reinfusion of MDSC from the infected group to the uninfected group increased mammary tumor number, suggesting that MDSC may play a synergistic role in H. hepaticus metastasis and promoting tumorigenesis. 8 Colitis and gut dysbiosis reduce the barrier permeability, allowing the presence of gut-derived bacteria and lipopolysaccharides (LPS) in liver,33,34 which recruited the CXCR2+ PMN-MDSCs formatting an immunosuppressive microenvironment in liver, thereby promoting the development of hepatocellular carcinoma. 35 Bacteria in the tumor microenvironment may be associated with gene expression and pathway activity. 36 Removed intratumoral bacteria could significantly reduce lung metastasis of breast cancer, but without affecting primary tumor growth. Further confirmed that circulating tumor cells harboring intratumoral bacteria enhanced their resistance to fluid shear stress, thereby promoting survival. 37 Nowadays, more and more diseases are found to be related to bacterial translocation, and more evidence is still needed to prove it.
Mechanisms of bacterial translocation
Intestinal barrier defects
Paracellular routes
Intestinal barrier integrity is based on the presence of a series of intercellular junctions composed of apical junction complexes and desmosomes. 38 The former includes Tight Junctions (TJs) and Adherens Junctions (AJs). TJs play crucial roles in preventing paracellular transit. Solutes and small molecules can be transported paracellularly through TJs; however, proteins, lipids, and peptides cannot.39,40 TJ proteins include transmembrane proteins, such as occludin, claudin, JAM-A (JAM-A), and intracellular scaffold proteins, such as zonula occludins (ZO) and tricellsin. 41 These proteins interact with the cytoskeleton, resulting in complex structures. 42 To maintain barrier integrity, TJ proteins act in concert with intracellular signaling and membrane-spanning proteins.43,44 Occludin was the first discovered tight junction protein. 45 TJ barrier function depends heavily on occludin for structural integrity. A close relationship has been observed between occludin expression and barrier properties. 46 Claudins have diverse functions. Barrier function is strengthened by claudins -1, -3, -4, -5, and -8, and weakened by claudins -2, -7, -10, and -23. 47 To date, the roles of the other TJ proteins remain unclear. AJs are formed by the transmembrane proteins E-cadherin and nectin, and intracellular components including p120-catenin, α-catenin and β-catenin. 38 Nectin is an adhesion molecule, and afadin is an actin filament-binding protein. Afadin links nectin with the actin cytoskeleton, while catenin links E-cadherin to the actin cytoskeleton. 48
Under pathological conditions, TJs and AJS can be disrupted. Bacteria, toxins, LPS, and enzymes are likely to breach the intestinal barrier through paracellular pathways to facilitate bacterial translocation. Pathogenic bacteria frequently manipulate paracellular barriers using LPS, virulence factors, and enzymes, to directly downregulate the expression of TJ and AJ proteins and activate pro-inflammatory cytokines that disrupt tight junctions or recruit immune cells to disrupt the barrier. Bacteria utilize multiple mechanisms to alter permeability, leading to progressive changes concurrent with infection progression.
Campylobacter jejuni secretes a serine protease, high-temperature requirement A (htrA), that downregulates claudin-8 to breach the intestinal barrier.49–51C. jejuni, co-infected with non-invasive bacterial strains facilitates barrier crossing by the non-invasive bacteria, 51 which can be attributed to opening of IEC junctions local to C. jejuni infection. Fusobacterium nucleatum upregulates myosin light chain phosphorylation and triggers actomyosin contraction, which distributes ZO-1, occludin, and E-cadherin to increase barrier permeability. 52 Colonic epithelial barrier dysfunction is exacerbated by C. concisus activating M1-macrophage by reducing the expression of occludin and tricellsulin in the tricellular proteins of three-cell TJs. 53 Helicobacter hepaticus downregulates ZO-1 and Claudin-1, decreases numbers of lysozyme-positive immune cells in the colonic submucosa, and damages the intestinal barrier in Il-17a-/- mice. 54 Salmonella serovar virulence relies on Salmonella plasmid virulence (Spv) genes. Through F-actin rearrangements and suppression of protein kinase C signaling, SpvB rearranges claudin-1, occludin, and E-cadherin, which play key roles in impairing the intestinal epithelial barrier and facilitating bacterial translocation. 55 Listeria monocytogenes secretes the Listeria adhesion protein which interacts with the host cell receptor heat shock protein 60. This process activates the NF-κB pathway, expediting the opening of the epithelial barrier mediated by myosin light-chain kinase (MLCK) via cellular redistribution of junctional proteins, including claudin-1, occludin, and E-cadherin, thereby promoting bacterial translocation.56,57 In addition, LPS decreases occludin and ZO-1 expression and activates Ca2+-activated Cl- channels and epithelial Na+ channels, thereby depolarizing the apical membrane and initiating ZO-1 tyrosine phosphorylation.58,59 LPS effects depend on mast cell degranulation. 60 Both can disrupt barrier integrity, significantly increasing paracellular permeability. Infection triggers release of the proinflammatory cytokine tumor necrosis factor α (TNF-α).8,61,62 TNF-α stimulates barrier permeability by activating MLCK II and inducing occludin endocytosis.63,64
In addition, pathogenic bacteria and their virulence factors can directly damage TJ and AJ proteins. Group A Streptococcus (GAS) can translocate through the intestinal barrier. Streptococcal pyrogenic exotoxins B and S cleave E-cadherin to promote GAS translocation.65,66Clostridium perfringens secretes pore-forming delta-toxin, which stimulates a-disintegrin and metalloprotease 10, causing E-cadherin cleavage 67 and an enterotoxin that targets claudin -9 and -4 also causes barrier damage.68,69Vibrio cholerae hemagglutinin/protease and Aeromonas hydrophila pore-forming aerolysin can directly cleave occludin, damaging the barrirer. 70 Aeromonas sobria serine protease (ASP), secreted by A. sobria, degrades tight junction components including ZO-1, 2, 3, and claudin-7, and facilitates bacterial translocation through the intestinal epithelial cell line, T84. 11 In addition, ASP destroys components including nectin-2 and afadin. 71 Haderer et al. 72 reported that a novel bacterial protease produced by Escherichia coli and Proteus mirabilis in patients with spontaneous bacterial peritonitis was responsible for the cleavage of E-cadherin structures.
Transcellular route
Microbiota-Mediated Translocation can occur via Epithelial Endocytosis. Antibiotic-resistant commensal bacteria spread via epithelial transcytosis as a result of microbial dysbiosis. 73 M cells are important entrances for bacterial translocation. M cells are specialized epithelial cells in the mucosal immune system that are scattered among the epithelial cells of the intestinal mucosa. 74 M cells sample pathogens in contact with their apical membranes, enclose them in vesicles, and deliver them to the immune system through the basolateral membrane. 75 Pathogenic bacteria use M cells as a portal of entry to invade the host and cause infection. 76 Transcytosis subversion via M cells may be pivotal for evasion of adaptive immunity by intracellular pathogens. 77
In addition to opening the TJ barrier, L. monocytogenes crosses the intestinal barrier by undergoing transcytosis. The bacterial invasion protein internalin A interacts with M cells in the Peyer’s patch and the E-cadherin receptor, prompting goblet cell exocytosis and epithelial extrusion. 56 Yersinia pseudotuberculosis (Yptb) exhibiting low type three secretion system (T3SS) expression shows higher transcytosis than Yptb exhibiting high expression. 78 Yersinia outer proteins as effectors of T3SS hinder the uptake function of M cells in early-stage infections. 79 Therefore, Yptb alters the interplay between the immune system and IECs to subvert intestinal barrier function. Moreover, Salmonella typhimurium invades the intestinal barrier via M cells.80,81 Invasion by S. typhimurium involves epithelium-sampling lamina propria phagocytes as well as type III secretion system-2-dependent epithelium traversal and basolateral exit. S. typhimurium type III effector protein SopB induces epithelial-mesenchymal transition (EMT) of follicle-associated epithelial enterocytes into M cells by inducing the EMT-regulating transcription factor Slug. Oral Brucella abortus infects the host via uptake through the prion protein PrP (C) on the apical surface of M cells. 82 All of these factors enable barrier penetration.
Cell apoptosis
Pathogenic bacteria cause epithelial cell death in numerous ways, through activation of receptors leading to membrane lysis or mitochondrial dysfunction-mediated cell death. Clostridium difficile TcdA-induced enteritis depends on prostaglandin E2, which upregulates the Fas/FasL ligand to induce apoptosis in colonocytes. 83 Kim and Kim proved that prostaglandin E2, via the EP1 receptor, upregulates BCL-2 homologous antagonist/killer (Bak), which could result in apoptosis in mitochondrial outer membrane pores. 84 Additionally, toxin B (tcdB) could induce cell apoptosis and increase levels of IL-6 and TNF-α in fetal human colon epithelial cells through the AKT/FOXO3/PPM1B pathway. 85 In colonocytes, TcdA, and TcdB disassemble actin microfilaments via glucosylation of Rho family proteins, causing epithelial cells to die and causing TJs to open. 86 Cronobacter sakazakii increases intestinal cAMP and protein kinase A phosphorylation following IEC apoptosis after infection. 87 EPEC induces intestinal epithelial cell line T84 apoptosis through oligonucleosome formation, PARP cleavage, and activation of caspases-3, -6, -8, and -9. 88 Serapio-Palacios and Navarro-Garcia reported that the serine protease motif of EPEC-secreted protein C, an autotransporter protein, can cleave procaspase-3, stimulating the mitochondria-mediated apoptotic pathway. 89 Moreover, MAP, the effector of EPEC T3SS, triggers dissolution of the host mitochondrial membrane potential, followed by Ca2+ efflux into the cytoplasm and activation of metalloproteinase domain-containing protein 10, which stimulates a mitogen-activated protein kinase cascade, ultimately causing cell apoptosis. 90 Vibrio vulnificus secretes many virulence factors, including VvhA and VvpM. Recombinant (r) VvhA protein activates PKCα and ERK/JNK leading to an increase in production of reactive oxygen species, eventually stimulating NF-κB-dependent mitochondrial cell death. 91 Furthermore, VvpM activates extracellular signal-regulated kinase (ERK signaling, caspases-9, -3, and cytochrome release to induce IEC apoptosis. 92
Klebsiella oxytoca colonization of the intestine plays a role in antibiotic-associated hemorrhagic colitis.93–95K. oxytoca produces enterotoxins including tilivalline (TV) and tilimycin (TM) during colitis. Targets that induce cell apoptosis differ between TV and TM. TV causes mitotic arrest by binding tubulin and stabilizing microtubules, whereas TM induces strand breaks in DNA by activating damage repair mechanisms. 96 SpvB mediates the accumulation of receptor-interacting protein kinase 3 (RIPK3) by reducing its degradation in an autophagy-dependent manner. RIPK3 increases MLKL phosphorylation and promotes necroptosis in IECs. 97
Repair and renewal disorder of the intestinal barrier
Maintenance of the intestinal barrier relies on rigorous regulation of IEC death and renewal, and the intestinal epithelium can be almost entirely renewed every 4–5 days.16,98 This tremendous capacity for renewal relies on ISCs residing at the bottom of the crypts. 17 ISCs include crypt base columnar cells (CBCs) and cells at position +4. 99 The CBC-specific gene Lgr5 has been identified as a vital marker of active ISCs. 100 Lgr5+ ISCs can generate all types of intestinal cells. 17 Several signaling pathways contribute to regulating ISC activity. Wnt and Notch signaling are considered the major driving pathways that contribute to intestinal maintenance, proliferation of ISCs, and differentiation of post-mitotic cells. Wnt/β-catenin signaling is required for physiological functions such as stem cell maintenance. 101 Interference with this signaling pathway leads to ISC functional disorder. Notch signaling targets CBC stem cells, and Notch inhibition causes a reduction in CBC number. 102
Bacteria impair intestinal epithelial repair, increasing intestinal barrier permeability and bacterial translocation. They can induce barrier defects that cause lesions in the intestinal barrier, and can also affect intestinal epithelium repair and renewal by impacting ISCs. 103 ISCs coexist with bacteria, placing bacteria in close contact with ISCs and affecting ISC proliferation and differentiation.100,104Lm and C. difficile directly damage ISCs by reducing the number of Lgr5+ stem cells, which greatly influences intestinal integrity.105,106 Some bacteria also impair the intestinal barrier by disturbing signaling pathways. For instance, Enterotoxigenic Escherichia coli produces heat-stable enterotoxins that suppress Fzd7 expression and downregulate the Wnt/β-catenin signaling pathway to inhibit ISC exposure. 107 Conversely, S. typhimurium protein, AvrA, activates the Wnt/β-catenin pathway and S. typhimurium infection upregulates the level of p53 in vivo, which promotes the progression of intestinal adenomas. 108 L. monocytogenes infection affects the differentiation of ISCs into paneth cells. 105 In summary, pathogenic bacteria can not only directly decrease the number of ISCs to inhibit their function but can also interfere with the signaling pathway to disturb ISC activity.
The microbiota-immune-endocrine-nervous-axis
As the intestine has the abundant lymphatic tissue and nervous tissue interacting with the microbiota, intestinal barrier permeability is regulated by various internal and external factors. 109 Nerve fibers permeate the intestinal tissue, forming the enteric nervous system. The enteric nervous system (ENS) is composed of intestinal peripheral neuronal cells, including sympathetic, parasympathetic, and sensory neurons, which convey signals from the central nervous system (CNS) to the gut and induce mutual communication.110,111 The ENS, which coordinates symbiotic bacterial colonization and pathogenic microbe infections, plays an important role in regulating mucosal immunity, contributing to inflammation through the regulation of immunomodulatory neuropeptides, neurotransmitters, and inflammatory cytokines.6,10,112,113 The intestine and CNS are constantly involved in bidirectional communication, and the intestinal microbiota play a critical role in this communication.1,114,115 The intestinal microbiota has the ability to interact with the ENS. Interfacing with the vagus nerve can relay signals of peripheral inflammation to the CNS, and can secrete signal peptides or other molecules to the gut, modulating the composition of the microbiota.116,117 The microbiota can also generate neuroactive metabolites which can travel in the circulation to the CNS. 118 However, neural pathways are not the only bidirectional pathways involved. The gut is rich in lymphoid tissue, and its mucosal immune system constantly communicates with the gut microbiota to modulate inflammation and immune functions. The metabolic capacity of the intestinal flora also significantly affects the endocrine environment of the body. The hypothalamic-pituitary-adrenal (HPA) axis which regulates the endocrine system, is in constant communication with the flora.117,119–121 In this setting, microbiota, immune system, endocrine system, and CNS function as a mutually coordinating inter-organ communication network to foster healthy metabolism, mental health, and neuroimmune regulation. This network could be called ‘The Microbiota-Immune-Endocrine-Brain-Axis’. Increased intestinal permeability caused by pathogenic infection, which activates immune-inflammatory responses resulting from reciprocal interactions between the intestinal tract and microbiota, has important implications for brain function, metabolism, and mental health.4,122
In the presence of pathogenic bacteria, both humans and animals are susceptible to dysbiosis, LPS-induced depression, anxiety, and cognitive decline.4,123 The microbiota involved in microglial homeostasis and metabolism appear to be key to the nervous system and several behavioral phenotypes.2,124–126 The two-way regulation of nerves and flora, psychological stress, including depression, anxiety, and possible feedback, causes the intestinal barrier to become more permeable, mediating flora translocation. A likely mechanism is sympathetic activation, which leads to increased intercellular permeability in the gut.127,128 Translocated flora can negatively impact mucosal immunity and immune-inflammatory responses.2,4,117,118,129,130 TLR4 recognizes LPS, which is widely expressed in intestinal tissues. 131 LPS has been reported to promote immune-inflammatory responses by activating Toll-like transmembrane receptor (TLR4).4,132 LPS also activates RAGE, leading to endothelial hyperpermeability. 133 Pathogens may directly interact with the intestinal epithelium, causing bacterial translocation, and subsequent intestinal inflammation. The local immune-inflammatory microenvironment reduces barrier integrity and leads to bacterial translocation. Microbial dysbiosis caused by pathogenic infections may mediate stress feedback, leading to increased intestinal barrier permeability. Additionally, toxins secreted by pathogenic bacteria can simultaneously regulate neuroimmunity. C. difficile produces two cytotoxic and enterotoxic proteins, toxin A (TcdA) and toxin B (TcdB), which are its two most potent virulence toxins. 134 MIP-2 and IL-8 are released from IECs in response to toxin A exposure.135,136 Cytokines activate macrophages and enteric nerves to release substance P, calcitonin gene-related peptide, and neurotensin, resulting in interaction between IECs and immune cells, and amplification of the inflammatory response. 86 The intestinal microbiota is essential for development and function of the HPA axis. Imbalances in the HPA axis caused by the intestinal microbiota can affect the endocrine system and behavior, including anxiety or depression.120,121,137 Stress-induced increased intestinal permeability allows bacteria and bacterial antigens to cross the epithelial barrier and activate mucosal immune responses, which in turn alters microbiome composition and enhances HPA drive. Psychological factors can also increase the efficacy of the intestinal barrier by inducing mast cells and corticotropin-releasing hormone eosinophils.138,139 Corticotropin-releasing hormone can also return to stimulate mast cell degranulation to release TNF-α and protease. 140 Moreover, increased levels of specific proinflammatory cytokines such as IFN-γ, TNF-α, IL-1β, and IL-6, via activation of HPA axis can disrupt TJ protein function, thereby resulting in bacterial translocation (Figure 1). 4
Figure 1.
Pathogens cause defects in the intestinal barrier to translocate or disrupt the self-renewal of ISCs located at crypts, resulting in barrier renewal disorders. Some bacteria exploit M cells and goblet cells as their portal of entry through endocytosis. Pathogenic bacteria and their productions regulate nervous, endocrine and immune systems to regulate inflammatory responses, leading to barrier hyperpermeability. The entire regulatory process is bidirectional, and the regulatory network can be called ‘the nervous-endocrine-immune systems axis’. In addition, the immune cells phagocytose pathogenic bacteria which produce inflammatory factors and then enter the circulatory system.
Clinical treatment
Reshaping the gut microbial microenvironment is an important strategy for preventing and treating bacterial translocation. In this review, the mechanisms of barrier integrity regulation reveal promising ways to alleviate disease symptoms associated with bacterial translocation. Reinforcement of the intestinal barrier has become increasingly attractive with the identification and development of novel therapeutic targets.
Numerous studies have demonstrated the efficacy of probiotics and prebiotics in ameliorating microbial dysbiosis and enhancing intestinal barrier integrity to abrogate bacterial translocation. Using one type of probiotic alone or different probiotics simultaneously can strengthen the intestinal barrier. For instance, Lactobacillus species exert well-documented beneficial effects on the intestinal barrier.141–149 The concurrent use of different probiotics can alleviate dextran Sulfate sodium salt-induced colitis by rectifying microbial dysbiosis and compromised barrier function.150,151 Fecal bacterial transplantation to treat intestinal barrier defects has received considerable attention. A randomized controlled study involving 70 individuals that investigated whether probiotics can reduce bacterial translocation and cause changes in the intestinal flora found that probiotics reduced the count of live intestinal bacteria in the blood. Reducing translocation with probiotic intervention occurred late in the intervention at 16 weeks. 152 Other reports indicate that the intestinal barrier and translocation levels do not change significantly after probiotic intervention.153,154 Owing to the different observation times and interventions, it is challenging to ascertain the promise of probiotics or fecal microbiota transplantation therapy. An increased number of larger cohort studies are required to fill this gap. We continue to face difficulties in sample collection and analysis, as well as a lack of high-efficiency targeted strains in this field. The virulence of the transplanted bacteria and possible infection require more attention and clinical studies to support it. 155 Natural derivatives of many plants, including emodin, matrine, galangin, Kaempferol, and Pinocembrin are displaying promising bioactivities.156–160 Traditional Chinese medicines, including aqueous extracts of Paeoniae Radix Alba (Paeonia lactiflora Pall), herbal medicines including Scutellaria-coptis, Astragalus membranaceus, and Scutellaria baicalensis Georgi, ameliorate DSS-induced colitis in mice by tuning the intestinal physical barrier, immune responses, and microbiota composition.161–164 Gas-based therapeutics have seen rapid recent development, and some have displayed high therapeutic efficiency and biosafety. H2 alleviates colitis by reprograming colonocyte metabolism and reinforcing the intestinal barrier. 165 Additionally, the intake of small molecules such as Butyrate,166–168 Brain orexin, 169 vitamins A and D,170,171 and Galactooligosaccharides172–174 has improved intestinal barrier function. A high-fat diet, hypertension, and hyperglycemia drive intestinal barrier dysfunction and increase risk of enteric infection.175–177 Antihyperglycemic Agents (e.g. metformin) can improve intestinal barrier function.178–181 Therapies targeting ISCs have also been developed. The environmental sensor, aryl hydrocarbon receptor, protects from barrier damage by guarding the stem cell niche to maintain intestinal barrier integrity. 182 An important research direction for cancer treatment is to engineer bacterial vaccine carriers by utilizing bacterial characteristics that foster translocation. 183 Further research is required to better understand the role of intestinal barrier integrity in various diseases and therapeutic interventions. By elucidating the signaling pathways that regulate barrier integrity, novel barrier-restoring agents that are critical for deciphering novel treatment strategies and potent approaches for disease treatment can be identified. Early diagnosis, intervention, or prevention of bacterial translocation could provide a new avenue for the treatment of intestinal-derived infections.
Future perspectives
Clinical strain profiling, typically from stool samples, is commonly used to analyse intestinal microbiota. However, discrepancies in the analysis have been documented compared to samples taken directly from the intestinal contents. To facilitate the reproducibility and comparability of microbiota between different sites of the digestive tract, it is imperative to further develop sample collection and analysis methods. The ability to identify and annotate bacterial strains is critical for detecting bacterial translocation events and determining translocation mechanisms. Identifying high-risk strains, such as Enterotoxigenic Bacteroides fragilis, Helicobacter hepaticus, and Listeria before translocation may prevent disease exacerbation. Most steps in strain identification and annotation require standardized and accurate reference genomes. However, many strains lack high-quality references, leading to identification failures. In addition, bacterial strains entering the intestinal microenvironment in vivo undergo genomic changes, which is a significant obstacle to identifying translocated bacteria. It is thus important to improve current methods of strain annotation, identification, and tracking. Combinatorial use of multiomics may offer breakthroughs in the detection of translocated bacteria and identification of their translocation mechanisms. By combining omics technologies, such as 16S, metagenomics, and metabolomics, more microenvironment-specific characteristics of bacteria can be obtained, which can help us learn from different angular analyses to compare the characteristics of the bacteria, identify and annotate bacteria, and greatly improve accuracy rates.
To date, most studies have focused on the effects of a single or a few species of bacteria local to specific body tissues. Understanding host-microbial interactions is imperative to understand the underlying mechanisms of intestine-derived diseases. To understand how bacteria translocate and how these new environments affect strain behavior, we must understand what factors enable these microbes to translocate. The general mechanisms by which pathogenic bacteria foster flora translocation, including direct action on the intestinal barrier or passing through the microbiota-immune-endocrine-nervous axis, have been elucidated above. The complexity of these regulatory mechanisms makes it difficult to generalize them through a single theory or target. The bidirectional regulatory mechanism of the microbiota-immune-endocrine-nervous axis may underlie a positive feedback loop that further amplifies the translocation of the flora. Currently, an increasing number of researchers are realizing the impacts of psychological status on the intestinal tract. External stress may make patients more prone to eating disorders, unclean food intake, and pathogenic bacterial infections. Pathogen infection may cooperate with stress psychology to further damage the intestinal barrier and translocate bacteria.
Current treatment plans incorporating human experiments and probiotic (fecal bacteria) transplantation still exhibit disadvantages of poor stability, limited curative outcomes, and large individual differences. Symptoms including diarrhea and nausea may also occur. If the patient has severe intestinal leakage and flora translocation events, rash introduction of foreign flora may aggravate the condition or even cause bacteremia. Probiotic transplantation therapy may take a long time to enter the clinic, and it is also necessary to investigate the safety of probiotic transplantation replacement therapy for some patients with severe barrier impairment. Much work is still needed to prove reliable safety and efficacy.
Translocated bacteria are often found circulating in blood, and in distant organs, indicating that translocated bacteria not only exert local impacts but also trigger distal system diseases. An increasing number of immune cells is associated with the spread of translocated bacteria into the circulatory system. Little is known about the immune cells that carry translocated bacteria or help them to evade immune responses.
Acknowledgments
None.
Footnotes
ORCID iD: Lin-Zhen Shu  https://orcid.org/0000-0002-1137-6754
https://orcid.org/0000-0002-1137-6754
Contributor Information
Lin-Zhen Shu, Medical College, Nanchang University, Nanchang, Jiangxi Province, China.
Yi-Dan Ding, Medical College, Nanchang University, Nanchang, Jiangxi Province, China.
Qing-Ming Xue, Medical College, Nanchang University, Nanchang, Jiangxi Province, China.
Wei Cai, Medical College, Nanchang University, Nanchang, Jiangxi Province, China; Department of Pathology, the Fourth Affiliated Hospital of Nanchang University, Nanchang, Jiangxi Province, China.
Huan Deng, Department of Pathology, The Fourth Affiliated Hospital of Nanchang University, No. 133 South Guangchang Road, Nanchang 330003, Jiangxi Province, China; Tumor Immunology Institute, Nanchang University, Nanchang, China.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Lin-Zhen Shu: Conceptualization; Writing – original draft.
Yi-Dan Ding: Writing – original draft; Writing – review & editing.
Qing-Ming Xue: Writing – review & editing.
Wei Cai: Writing – review & editing.
Huan Deng: Conceptualization; Funding acquisition; Project administration; Supervision; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Science Foundation of China, Nos.82160546; and the Science Foundation of Jiangxi Province, No. 20202BBG73027.
The authors declare that there is no conflict of interest.
Availability of data and materials: Not applicable.
References
- 1.Choi J, Kim BR, Akuzum B, et al. TREGking from Gut to brain: the control of regulatory T cells along the Gut-Brain axis. Front Immunol 2022; 13: 916066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foster JA, Baker GB, Dursun SM. The relationship between the gut microbiome-immune system-Brain axis and major depressive disorder. Front Neurol 2021; 12: 721126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi N, Li N, Duan X, et al. Interaction between the gut microbiome and mucosal immune system. Mil Med Res 2017; 4: 14–20170427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rudzki L, Maes M. From “Leaky Gut” to impaired glia-neuron communication in depression. Adv Exp Med Biol 2021; 1305: 129–155. [DOI] [PubMed] [Google Scholar]
- 5.Camilleri M. Leaky gut: mechanisms, measurement and clinical implications in humans. Gut 2019; 68: 1516–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jarret A, Jackson R, Duizer C, et al. Enteric nervous system-derived IL-18 orchestrates mucosal barrier immunity. Cell 2020; 180: 50–63.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosi A, Banfi D, Bistoletti M, et al. Hyaluronan: a neuroimmune modulator in the microbiota-Gut axis. Cells 2021; 11: 20211231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng H, Muthupalani S, Erdman S, et al. Translocation of Helicobacter hepaticus synergizes with myeloid-derived suppressor cells and contributes to breast carcinogenesis. OncoImmunology 2022; 11: 2057399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maudet C, Kheloufi M, Levallois S, et al. Bacterial inhibition of Fas-mediated killing promotes neuroinvasion and persistence. Nature 2022; 603: 900–906. [DOI] [PubMed] [Google Scholar]
- 10.Lei C, Sun R, Xu G, et al. Enteric VIP-producing neurons maintain gut microbiota homeostasis through regulating epithelium fucosylation. Cell Host Microbe 2022; 30: 1417–1434.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ueda M, Kobayashi H, Seike S, et al. Aeromonas sobria serine protease degrades several protein components of tight junctions and assists bacterial translocation across the T84 monolayer. Front Cell Infect Microbiol 2022; 12: 824547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Callaghan AA, Corr SC. Establishing boundaries: the relationship that exists between intestinal epithelial cells and Gut-Dwelling bacteria. Microorganisms 2019; 7: 11. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akiyama T, Oishi K, Wullaert A. Bifidobacteria prevent tunicamycin-induced endoplasmic reticulum stress and subsequent barrier disruption in human intestinal epithelial caco-2 monolayers. PLoS One 2016; 11: e0162448–20160909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gieryńska M, Szulc-Dąbrowska L, Struzik J, et al. Integrity of the intestinal barrier: the involvement of epithelial cells and microbiota-A mutual relationship. Animals 2022; 12: 20220108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deplancke B, Gaskins HR. Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. Am J Clin Nutr 2001; 73: 1131S–1141S. [DOI] [PubMed] [Google Scholar]
- 16.van der Flier LG, van Gijn ME, Hatzis P, et al. Transcription factor achaete scute-like 2 controls intestinal stem cell fate. Cell 2009; 136: 903–912. [DOI] [PubMed] [Google Scholar]
- 17.Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007; 449: 1003–1007. [DOI] [PubMed] [Google Scholar]
- 18.Wolochow H, Hildebrand GJ, Lamanna C. Translocation of microorganisms across the intestinal wall of the rat: effect of microbial size and concentration. J Infect Dis 1966; 116: 523–528. [DOI] [PubMed] [Google Scholar]
- 19.Mielcarek C, Romond PC, Romond MB, et al. Modulation of bacterial translocation in mice mediated through lactose and human milk oligosaccharides. Anaerobe 2011; 17: 361–366. [DOI] [PubMed] [Google Scholar]
- 20.Loman BR, Russart KLG, Grant CV, et al. Mammary tumors alter the fecal bacteriome and permit enteric bacterial translocation. BMC Cancer 2022; 22: 245–20220305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simeonova D, Stoyanov D, Leunis JC, et al. Increased serum immunoglobulin responses to gut commensal Gram-negative bacteria in unipolar major depression and bipolar disorder Type 1, especially when melancholia is present. Neurotox Res 2020; 37: 338–348. [DOI] [PubMed] [Google Scholar]
- 22.Zhou X, Li J, Guo J, et al. Gut-dependent microbial translocation induces inflammation and cardiovascular events after ST-elevation myocardial infarction. Microbiome 2018; 6: 66–20180403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oyama N, Winek K, Bäcker-Koduah P, et al. Exploratory investigation of intestinal function and bacterial translocation after focal cerebral ischemia in the mouse. Front Neurol 2018; 9: 937–20181119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woodhouse CA, Patel VC, Singanayagam A, et al. Review article: the gut microbiome as a therapeutic target in the pathogenesis and treatment of chronic liver disease. Aliment Pharmacol Ther 2018; 47: 192–202. [DOI] [PubMed] [Google Scholar]
- 25.Bergmann KR, Liu SX, Tian R, et al. Bifidobacteria stabilize claudins at tight junctions and prevent intestinal barrier dysfunction in mouse necrotizing enterocolitis. Am J Pathol 2013; 182: 1595–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lakritz JR, Poutahidis T, Mirabal S, et al. Gut bacteria require neutrophils to promote mammary tumorigenesis. Oncotarget 2015; 6: 9387–9396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tse BCY, Welham Z, Engel AF, et al. Genomic, microbial and immunological microenvironment of colorectal polyps. Cancers 2021; 13: 3382. DOI: 10.3390/cancers13143382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dorhoi A, Du Plessis N. Monocytic myeloid-derived suppressor cells in chronic infections. Front Immunol 2017; 8: 1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barbosa Bomfim CC, Pinheiro Amaral E, Santiago-Carvalho I, et al. Harmful effects of granulocytic myeloid-derived suppressor cells on tuberculosis caused by hypervirulent mycobacteria. J Infect Dis 2021; 223: 494–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knaul JK, Jörg S, Oberbeck-Mueller D, et al. Lung-residing myeloid-derived suppressors display dual functionality in murine pulmonary tuberculosis. Am J Respir Crit Care Med 2014; 190: 1053–1066. [DOI] [PubMed] [Google Scholar]
- 31.Jin S, Wetzel D, Schirmer M. Deciphering mechanisms and implications of bacterial translocation in human health and disease. Curr Opin Microbiol 2022; 67: 102147–20220420. [DOI] [PubMed] [Google Scholar]
- 32.Vrakas S, Mountzouris KC, Michalopoulos G, et al. Intestinal bacteria composition and translocation of bacteria in inflammatory bowel disease. PLoS One 2017; 12: e0170034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iida N, Mizukoshi E, Yamashita T, et al. Chronic liver disease enables gut Enterococcus faecalis colonization to promote liver carcinogenesis. Nat Cancer 2021; 2: 1039–1054. [DOI] [PubMed] [Google Scholar]
- 34.Komiyama S, Yamada T, Takemura N, et al. Profiling of tumour-associated microbiota in human hepatocellular carcinoma. Sci Rep 2021; 11: 10589–20210519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Q, Ma C, Duan Y, et al. Gut microbiome directs hepatocytes to recruit MDSCs and promote cholangiocarcinoma. Cancer Discov 2021; 11: 1248–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghaddar B, Biswas A, Harris C, et al. Tumor microbiome links cellular programs and immunity in pancreatic cancer. Cancer Cell 2022; 40: 1240–1253.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fu A, Yao B, Dong T, et al. Tumor-resident intracellular microbiota promotes metastatic colonization in breast cancer. Cell 2022; 185: 1356–1372.e26. [DOI] [PubMed] [Google Scholar]
- 38.Mehta S, Nijhuis A, Kumagai T, et al. Defects in the adherens junction complex (E-cadherin/ β-catenin) in inflammatory bowel disease. Cell Tissue Res 2015; 360: 749–760. [DOI] [PubMed] [Google Scholar]
- 39.Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol 2009; 1: a002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chelakkot C, Ghim J, Ryu SH. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp Mol Med 2018; 50: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paradis T, Bègue H, Basmaciyan L, et al. Tight junctions as a key for pathogens invasion in intestinal epithelial cells. Int J Mol Sci 2021; 22: 20210302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Günzel D, Yu AS. Claudins and the modulation of tight junction permeability. Physiol Rev 2013; 93: 525–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hossain Z, Hirata T. Molecular mechanism of intestinal permeability: interaction at tight junctions. Mol Biosyst 2008; 4: 1181–1185. [DOI] [PubMed] [Google Scholar]
- 44.Miyoshi J, Takai Y. Molecular perspective on tight-junction assembly and epithelial polarity. Adv Drug Deliv Rev 2005; 57: 815–855. [DOI] [PubMed] [Google Scholar]
- 45.Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol 1963; 17: 375–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cummins PM. Occludin: one protein, many forms. Mol Cell Biol 2012; 32: 242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Serek P, Oleksy-Wawrzyniak M. The effect of bacterial infections, probiotics and zonulin on intestinal barrier integrity. Int J Mol Sci 2021; 22: 20211021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takai Y, Ikeda W, Ogita H, et al. The immunoglobulin-like cell adhesion molecule nectin and its associated protein afadin. Annu Rev Cell Dev Biol 2008; 24: 309–342. [DOI] [PubMed] [Google Scholar]
- 49.Sharafutdinov I, Esmaeili DS, Harrer A, et al. Campylobacter jejuni serine protease HtrA cleaves the tight junction component Claudin-8. Front Cell Infect Microbiol 2020; 10: 590186–20201208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harrer A, Bücker R, Boehm M, et al. Campylobacter jejuni enters gut epithelial cells and impairs intestinal barrier function through cleavage of occludin by serine protease HtrA. Gut Pathog 2019; 11: 4–20190213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharafutdinov I, Tegtmeyer N, Müsken M, et al. Campylobacter jejuni serine protease HtrA induces paracellular transmigration of microbiota across polarized intestinal epithelial cells. Biomolecules 2022; 12: 20220330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu H, Hong XL, Sun TT, et al. Fusobacterium nucleatum exacerbates colitis by damaging epithelial barriers and inducing aberrant inflammation. J Dig Dis 2020; 21: 385–398. [DOI] [PubMed] [Google Scholar]
- 53.Nattramilarasu PK, Lobo de Sá FD, Schulzke JD, et al. Immune-mediated aggravation of the Campylobacter concisus-Induced epithelial barrier dysfunction. Int J Mol Sci 2021; 22: 20210219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu L, Wu Z, Zhu C, et al. The deletion of IL-17A enhances Helicobacter hepaticus colonization and triggers colitis. J Inflamm Res 2022; 15: 2761–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun L, Yang S, Deng Q, et al. Salmonella effector SpvB disrupts intestinal epithelial barrier integrity for bacterial translocation. Front Cell Infect Microbiol 2020; 10: 606541–20201217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Drolia R, Bhunia AK. Crossing the intestinal barrier via listeria adhesion protein and Internalin A. Trends Microbiol 2019; 27: 408–425. [DOI] [PubMed] [Google Scholar]
- 57.Drolia R, Tenguria S, Durkes AC, et al. Listeria adhesion protein induces intestinal epithelial barrier dysfunction for bacterial translocation. Cell Host Microbe 2018; 23: 470–484.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bein A, Zilbershtein A, Golosovsky M, et al. LPS induces hyper-permeability of intestinal epithelial cells. J Cell Physiol 2017; 232: 381–390. [DOI] [PubMed] [Google Scholar]
- 59.Kim M, Lee SW, Kim J, et al. LPS-induced epithelial barrier disruption via hyperactivation of CACC and ENaC. Am J Physiol Cell Physiol 2021; 320: C448–c461. [DOI] [PubMed] [Google Scholar]
- 60.Sun T, Wang Y, Hu S, et al. Lipopolysaccharide induces the early enhancement of mice colonic mucosal paracellular permeability mainly mediated by mast cells. Histol Histopathol 2019; 34: 191–200. [DOI] [PubMed] [Google Scholar]
- 61.Zhou C, Zhang Y, Bassey A, et al. Expansion of intestinal secretory cell population induced by Listeria monocytogenes infection: accompanied with the inhibition of NOTCH Pathway. Front Cell Infect Microbiol 2022; 12: 793335–20220325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Si X, Jia H, Liu N, et al. Alpha-ketoglutarate attenuates colitis in mice by increasing Lactobacillus abundance and regulating stem cell proliferation via Wnt-Hippo Signaling. Mol Nutr Food Res 2022; 66: e2100955. [DOI] [PubMed] [Google Scholar]
- 63.Turner JR, Buschmann MM, Romero-Calvo I, et al. The role of molecular remodeling in differential regulation of tight junction permeability. Semin Cell Dev Biol 2014; 36: 204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schoultz I, Keita ÅV. The intestinal barrier and current techniques for the assessment of gut permeability. Cells 2020; 9: 1909. DOI: 10.3390/cells9081909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sumitomo T, Nakata M, Higashino M, et al. Group A streptococcal cysteine protease cleaves epithelial junctions and contributes to bacterial translocation. J Biol Chem 2013; 288: 13317–13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sumitomo T, Nakata M, Higashino M, et al. Streptolysin S contributes to group A streptococcal translocation across an epithelial barrier. J Biol Chem 2011; 286: 2750–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seike S, Takehara M, Kobayashi K, et al. Clostridium perfringens delta-toxin damages the mouse small intestine. Toxins 2019; 11: 20190422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vecchio AJ, Stroud RM. Claudin-9 structures reveal mechanism for toxin-induced gut barrier breakdown. Proc Natl Acad Sci USA 2019; 116: 17817–17824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eichner M, Augustin C, Fromm A, et al. In colon epithelia, Clostridium perfringens enterotoxin causes focal leaks by targeting Claudins which are apically accessible due to tight junction derangement. J Infect Dis 2017; 217: 147–157. [DOI] [PubMed] [Google Scholar]
- 70.Roxas JL, Viswanathan VK. Modulation of intestinal paracellular transport by bacterial pathogens. Compr Physiol 2018; 8: 823–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kobayashi H, Seike S, Yamaguchi M, et al. Aeromonas sobria serine protease decreases epithelial barrier function in T84 cells and accelerates bacterial translocation across the T84 monolayer in vitro. PLoS One 2019; 14: e0221344–20190816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Haderer M, Neubert P, Rinner E, et al. Novel pathomechanism for spontaneous bacterial peritonitis: disruption of cell junctions by cellular and bacterial proteases. Gut 2022; 71: 580–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yu LC, Shih YA, Wu LL, et al. Enteric dysbiosis promotes antibiotic-resistant bacterial infection: systemic dissemination of resistant and commensal bacteria through epithelial transcytosis. Am J Physiol Gastrointest Liver Physiol 2014; 307: G824–G835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miller H, Zhang J, Kuolee R, et al. Intestinal M cells: the fallible sentinels? World J Gastroenterol 2007; 13: 1477–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kanaya T, Williams IR, Ohno H. Intestinal M cells: tireless samplers of enteric microbiota. Traffic 2020; 21: 617–644. [DOI] [PubMed] [Google Scholar]
- 76.Clark MA, Jepson MA. Intestinal M cells and their role in bacterial infection. Int J Med Microbiol 2003; 293: 17–39. [DOI] [PubMed] [Google Scholar]
- 77.Rey C, Chang YY, Latour-Lambert P, et al. Transcytosis subversion by M cell-to-enterocyte spread promotes Shigella flexneri and Listeria monocytogenes intracellular bacterial dissemination. PLoS Pathog 2020; 16: e1008446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fasciano AC, Dasanayake GS, Estes MK, et al. Yersinia pseudotuberculosis YopE prevents uptake by M cells and instigates M cell extrusion in human ileal enteroid-derived monolayers. Gut Microbes 2021; 13: 1988390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jung C, Meinzer U, Montcuquet N, et al. Yersinia pseudotuberculosis disrupts intestinal barrier integrity through hematopoietic TLR-2 signaling. J Clin Investig 2012; 122: 2239–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Richards AF, Torres-Velez FJ, Mantis NJ. Salmonella uptake into gut-associated lymphoid tissues: implications for targeted mucosal vaccine design and delivery. Methods Mol Biol (Clifton, NJ) 2022; 2410: 305–324. DOI: 10.1007/978-1-0716-1884-4_15. [DOI] [PubMed] [Google Scholar]
- 81.Müller AJ, Kaiser P, Dittmar KE, et al. Salmonella gut invasion involves TTSS-2-dependent epithelial traversal, basolateral exit, and uptake by epithelium-sampling lamina propria phagocytes. Cell Host Microbe 2012; 11: 19–32. [DOI] [PubMed] [Google Scholar]
- 82.Nakato G, Hase K, Suzuki M, et al. Cutting Edge: Brucella abortus exploits a cellular prion protein on intestinal M cells as an invasive receptor. J Immunol 2012; 189: 1540–1544. [DOI] [PubMed] [Google Scholar]
- 83.Kim H, Rhee SH, Pothoulakis C, et al. Inflammation and apoptosis in Clostridium difficile enteritis is mediated by PGE2 up-regulation of Fas ligand. Gastroenterology 2007; 133: 875–886. [DOI] [PubMed] [Google Scholar]
- 84.Kim YH, Kim H. Clostridium difficile toxin A upregulates Bak expression through PGE2 pathway in human colonocytes. J Microbiol Biotechnol 2019; 29: 1675–1681. [DOI] [PubMed] [Google Scholar]
- 85.Xu Q, Li Y, Zheng Y, et al. Clostridium difficile toxin B-induced colonic inflammation is mediated by the FOXO3/PPM1B pathway in fetal human colon epithelial cells. Am J Transl Res 2020; 12: 6204–6219. [PMC free article] [PubMed] [Google Scholar]
- 86.Pothoulakis C, Richards AF, Torres-Velez FJ, et al. Salmonella uptake into Gut-associated lymphoid tissues: implications for targeted mucosal vaccine design and delivery. Methods Mol Biol (Clifton, NJ) 2022; 2410: 305–324. [DOI] [PubMed] [Google Scholar]
- 87.Blackwood BP, Wood DR, Yuan C, et al. A role for camp and protein kinase A in experimental necrotizing enterocolitis. Am J Pathol 2017; 187: 401–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Flynn AN, Buret AG. Caspases-3, -8, and -9 are required for induction of epithelial cell apoptosis by enteropathogenic E. coli but are dispensable for increased paracellular permeability. Microb Pathog 2008; 44: 311–319. [DOI] [PubMed] [Google Scholar]
- 89.Serapio-Palacios A, Navarro-Garcia F. EspC, an autotransporter protein secreted by enteropathogenic Escherichia coli, causes apoptosis and necrosis through caspase and calpain activation, including direct procaspase-3 Cleavage. mBio 2016; 7: e00479–16. DOI: 10.1128/mBio.00479-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ramachandran RP, Spiegel C, Keren Y, et al. Mitochondrial targeting of the enteropathogenic Escherichia coli map triggers calcium mobilization, ADAM10-MAP kinase signaling, and host cell apoptosis. mBio 2020; 11: e01397–20. DOI: 10.1128/mBio.01397-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee SJ, Jung YH, Oh SY, et al. Vibrio vulnificus VvhA induces NF-κB-dependent mitochondrial cell death via lipid raft-mediated ROS production in intestinal epithelial cells. Cell Death Dis 2015; 6: 1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee MA, Kim JA, Yang YJ, et al. VvpM, an extracellular metalloprotease of Vibrio vulnificus, induces apoptotic death of human cells. J Microbiol 2014; 52: 1036–1043. [DOI] [PubMed] [Google Scholar]
- 93.Beaugerie L, Metz M, Barbut F, et al. Klebsiella oxytoca as an agent of antibiotic-associated hemorrhagic colitis. Clin Gastroenterol Hepatol 2003; 1: 370–376. [DOI] [PubMed] [Google Scholar]
- 94.Högenauer C, Langner C, Beubler E, et al. Klebsiella oxytoca as a causative organism of antibiotic-associated hemorrhagic colitis. New Engl J Med 2006; 355: 2418–2426. [DOI] [PubMed] [Google Scholar]
- 95.Hering NA, Fromm A, Bücker R, et al. Tilivalline- and tilimycin-independent effects of Klebsiella oxytoca on tight junction-mediated intestinal barrier impairment. Int J Mol Sci 2019; 20: 20191108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Unterhauser K, Pöltl L, Schneditz G, et al. Klebsiella oxytoca enterotoxins tilimycin and tilivalline have distinct host DNA-damaging and microtubule-stabilizing activities. Proc Natl Acad Sci USA 2019; 116: 3774–3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dong K, Zhu Y, Deng Q, et al. Salmonella pSLT-encoded effector SpvB promotes RIPK3-dependent necroptosis in intestinal epithelial cells. Cell Death Discov 2022; 8: 44–20220202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Piscaglia AC, Novi M, Campanale M, et al. Stem cell-based therapy in gastroenterology and hepatology. Minim Invasive Ther Allied Technol 2008; 17: 100–118. [DOI] [PubMed] [Google Scholar]
- 99.Barker N, van Oudenaarden A, Clevers H. Identifying the stem cell of the intestinal crypt: strategies and pitfalls. Cell Stem Cell 2012; 11: 452–460. [DOI] [PubMed] [Google Scholar]
- 100.Hou Q, Ye L, Huang L, et al. The research progress on intestinal stem cells and its relationship with intestinal Microbiota. Front Immunol 2017; 8: 599–20170523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Albrecht LV, Tejeda-Muñoz N, De Robertis EM. Cell Biology of canonical Wnt Signaling. Annu Rev Cell Dev Biol 2021; 37: 369–389. [DOI] [PubMed] [Google Scholar]
- 102.VanDussen KL, Carulli AJ, Keeley TM, et al. Notch signaling modulates proliferation and differentiation of intestinal crypt base columnar stem cells. Development 2012; 139: 488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.National survey of tuberculosis notifications in England and Wales in 1983: characteristics of disease. Report from the Medical Research Council Tuberculosis and Chest Diseases Unit. Tubercle 1987; 68: 19–32. [DOI] [PubMed] [Google Scholar]
- 104.Markandey M, Bajaj A, Ilott NE, et al. Gut microbiota: sculptors of the intestinal stem cell niche in health and inflammatory bowel disease. Gut Microbes 2021; 13: 1990827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Huang J, Zhou C, Zhou G, et al. Effect of Listeria monocytogenes on intestinal stem cells in the co-culture model of small intestinal organoids. Microb Pathog 2021; 153: 104776. [DOI] [PubMed] [Google Scholar]
- 106.Mileto SJ, Jardé T, Childress KO, et al. Clostridioides difficile infection damages colonic stem cells via TcdB, impairing epithelial repair and recovery from disease. Proc Natl Acad Sci USA 2020; 117: 8064–8073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhou JY, Huang DG, Gao CQ, et al. Heat-stable enterotoxin inhibits intestinal stem cell expansion to disrupt the intestinal integrity by downregulating the Wnt/β-catenin pathway. Stem Cells 2021; 39: 482–496. [DOI] [PubMed] [Google Scholar]
- 108.Liu X, Lu R, Wu S, et al. Salmonella regulation of intestinal stem cells through the Wnt/beta-catenin pathway. FEBS Lett 2010; 584: 911–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Spencer J, Finn T, Isaacson PG. Gut associated lymphoid tissue: a morphological and immunocytochemical study of the human appendix. Gut 1985; 26: 672–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Walsh KT, Zemper AE. The enteric nervous system for epithelial researchers: basic anatomy, techniques, and interactions with the epithelium. Cell Mol Gastroenterol Hepatol 2019; 8: 369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zeng W, Yang F, Shen WL, et al. Interactions between central nervous system and peripheral metabolic organs. Sci China Life Sci 2022; 65: 1929–1958. [DOI] [PubMed] [Google Scholar]
- 112.Barajon I, Serrao G, Arnaboldi F, et al. Toll-like receptors 3, 4, and 7 are expressed in the enteric nervous system and dorsal root ganglia. J Histochem Cytochem Off J Histochem Soc 2009; 57: 1013–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Coquenlorge S, Duchalais E, Chevalier J, et al. Modulation of lipopolysaccharide-induced neuronal response by activation of the enteric nervous system. J Neuroinflammation 2014; 11: 202–20141212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mishima Y, Ishihara S. Molecular mechanisms of microbiota-mediated pathology in irritable bowel syndrome. Int J Mol Sci 2020; 21: 8664. DOI: 10.3390/ijms21228664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cryan JF, O’Riordan KJ, Cowan CSM. The microbiota-Gut-brain axis. Physiol Rev 2019; 99: 1877–2013. [DOI] [PubMed] [Google Scholar]
- 116.Konsman JP, Luheshi GN, Bluthé RM, et al. The vagus nerve mediates behavioural depression, but not fever, in response to peripheral immune signals; a functional anatomical analysis. Eur J Neurosci 2000; 12: 4434–4446. [DOI] [PubMed] [Google Scholar]
- 117.Flux MC, Lowry CA. Finding intestinal fortitude: integrating the microbiome into a holistic view of depression mechanisms, treatment, and resilience. Neurobiol Dis 2020; 135: 104578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Martin CR, Osadchiy V, Kalani A, et al. The Brain-Gut-microbiome axis. Cell Mol Gastroenterol Hepatol 2018; 6: 133–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Powell N, Walker MM, Talley NJ. The mucosal immune system: master regulator of bidirectional gut-brain communications. Nat Rev Gastroenterol Hepatol 2017; 14: 143–159. [DOI] [PubMed] [Google Scholar]
- 120.Huo R, Zeng B, Zeng L, et al. Microbiota modulate anxiety-like behavior and endocrine abnormalities in hypothalamic-pituitary-adrenal axis. Front Cell Infect Microbiol 2017; 7: 489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Farzi A, Fröhlich EE, Holzer P. Gut Microbiota and the neuroendocrine system. Neurother 2018; 15: 5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Laissue JA, Gebbers JO. The intestinal barrier and the gut-associated lymphoid tissue. Curr Stud Hematol Blood Transfus 1992; 59: 19–43. DOI: 10.1159/000429607 [DOI] [PubMed] [Google Scholar]
- 123.Reichenberg A, Yirmiya R, Schuld A, et al. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry 2001; 58: 445–452. [DOI] [PubMed] [Google Scholar]
- 124.Kronsten VT, Tranah TH, Pariante C, et al. Gut-derived systemic inflammation as a driver of depression in chronic liver disease. J Hepatol 2022; 76: 665–680. [DOI] [PubMed] [Google Scholar]
- 125.Erny D, Hrabeˇ de Angelis AL, Jaitin D, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 2015; 18: 965–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ramirez V, Swain S, Murray K, et al. Neural immune communication in the control of host-bacterial pathogen interactions in the gastrointestinal tract. Infect Immun 2020; 88: e00928–19. DOI: 10.1128/IAI.00928-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Turner JR.Show me the pathway! Regulation of paracellular permeability by Na(+)-glucose cotransport. Adv Drug Deliv Rev 2000; 41: 265–281. [DOI] [PubMed] [Google Scholar]
- 128.de Punder K, Pruimboom L. Stress induces endotoxemia and low-grade inflammation by increasing barrier permeability. Front Immunol 2015; 6: 223–20150515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol 2016; 16: 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pellegrini C, Antonioli L, Colucci R, et al. Interplay among gut microbiota, intestinal mucosal barrier and enteric neuro-immune system: a common path to neurodegenerative diseases? Acta Neuropathol 2018; 136: 345–361. [DOI] [PubMed] [Google Scholar]
- 131.Vaure C, Liu Y. A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front Immunol 2014; 5: 316–20140710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang YS, White TD. The bacterial endotoxin lipopolysaccharide causes rapid inappropriate excitation in rat cortex. J Neurochem 1999; 72: 652–660. [DOI] [PubMed] [Google Scholar]
- 133.Wang L, Wu J, Guo X, et al. RAGE plays a role in LPS-Induced NF-κB activation and endothelial hyperpermeability. Sensors 2017; 17: 722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Voth DE, Ballard JD. Clostridium difficile toxins: mechanism of action and role in disease. Clin Microbiol Rev 2005; 18: 247–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Castagliuolo I, Keates AC, Wang CC, et al. Clostridium difficile toxin A stimulates macrophage-inflammatory protein-2 production in rat intestinal epithelial cells. J Immunol 1998; 160: 6039–6045. [PubMed] [Google Scholar]
- 136.Mahida YR, Makh S, Hyde S, et al. Effect of Clostridium difficile toxin A on human intestinal epithelial cells: induction of interleukin 8 production and apoptosis after cell detachment. Gut 1996; 38: 337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liu Y, Wu Z, Cheng L, et al. The role of the intestinal microbiota in the pathogenesis of host depression and mechanism of TPs relieving depression. Food Funct 2021; 12: 7651–7663. [DOI] [PubMed] [Google Scholar]
- 138.Vanuytsel T, van Wanrooy S, Vanheel H, et al. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut 2014; 63: 1293–1299. [DOI] [PubMed] [Google Scholar]
- 139.Zheng PY, Feng BS, Oluwole C, et al. Psychological stress induces eosinophils to produce corticotrophin releasing hormone in the intestine. Gut 2009; 58: 1473–1479. [DOI] [PubMed] [Google Scholar]
- 140.Overman EL, Rivier JE, Moeser AJ. CRF induces intestinal epithelial barrier injury via the release of mast cell proteases and TNF-α. PLoS One 2012; 7: e39935–20120629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li A, Zhang C, Chi H, et al. 2’-fucosyllactose promotes Lactobacillus rhamnosus KLDS 8001 to repair LPS-induced damage in Caco-2 cells. J Food Biochem 2022; 46: e14059–20220203. [DOI] [PubMed] [Google Scholar]
- 142.Singh TP, Tehri N, Kaur G, et al. Cell surface and extracellular proteins of potentially probiotic Lactobacillus reuteri as an effective mediator to regulate intestinal epithelial barrier function. Arch Microbiol 2021; 203: 3219–3228. [DOI] [PubMed] [Google Scholar]
- 143.Niu H, Zhou X, Gong P, et al. Effect of Lactobacillus rhamnosus MN-431 producing indole derivatives on complementary feeding-induced diarrhea rat pups through the enhancement of the intestinal barrier function. Mol Nutr Food Res 2022; 66: e2100619. [DOI] [PubMed] [Google Scholar]
- 144.Xu C, Yan S, Guo Y, et al. Lactobacillus casei ATCC 393 alleviates enterotoxigenic Escherichia coli K88-induced intestinal barrier dysfunction via TLRs/mast cells pathway. Life Sci 2020; 244: 117281–20200108. [DOI] [PubMed] [Google Scholar]
- 145.Liu Z, Zhao J, Sun R, et al. Lactobacillus plantarum 23-1 improves intestinal inflammation and barrier function through the TLR4/NF-κB signaling pathway in obese mice. Food Funct 2022; 13: 5971–5986. [DOI] [PubMed] [Google Scholar]
- 146.Wei CX, Wu JH, Huang YH, et al. Lactobacillus plantarum improves LPS-induced Caco2 cell line intestinal barrier damage via cyclic AMP-PKA signaling. PLoS One 2022; 17: e0267831–20220531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gao Y, Liu Y, Ma F, et al. Lactobacillus plantarum Y44 alleviates oxidative stress by regulating gut microbiota and colonic barrier function in Balb/C mice with subcutaneous d-galactose injection. Food Funct 2021; 12: 373–386. [DOI] [PubMed] [Google Scholar]
- 148.Kaur H, Gupta T, Kapila S, et al. Protective effects of potential probiotic Lactobacillus rhamnosus (MTCC-5897) fermented whey on reinforcement of intestinal epithelial barrier function in a colitis-induced murine model. Food Funct 2021; 12: 6102–6116. [DOI] [PubMed] [Google Scholar]
- 149.Zhai Z, Torres-Fuentes C, Heeney DD, et al. Synergy between probiotic Lactobacillus casei and milk to maintain barrier integrity of intestinal epithelial cells. J Agric Food Chem 2019; 67: 1955–1962. [DOI] [PubMed] [Google Scholar]
- 150.Wang Y, Xie Q, Zhang Y, et al. Combination of probiotics with different functions alleviate DSS-induced colitis by regulating intestinal microbiota, IL-10, and barrier function. Appl Microbiol Biotechnol 2020; 104: 335–349. [DOI] [PubMed] [Google Scholar]
- 151.Liu Q, Yu Z, Tian F, et al. Surface components and metabolites of probiotics for regulation of intestinal epithelial barrier. Microb Cell Fact 2020; 19: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sato J, Kanazawa A, Azuma K, et al. Probiotic reduces bacterial translocation in type 2 diabetes mellitus: a randomised controlled study. Sci Rep 2017; 7: 12115–20170921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Stadlbauer V, Horvath A, Komarova I, et al. Dysbiosis in early sepsis can be modulated by a multispecies probiotic: a randomised controlled pilot trial. Benef Microbes 2019; 10: 265–278. [DOI] [PubMed] [Google Scholar]
- 154.Horvath A, Leber B, Schmerboeck B, et al. Randomised clinical trial: the effects of a multispecies probiotic vs. Placebo on innate immune function, bacterial translocation and gut permeability in patients with cirrhosis. Aliment Pharmacol Ther 2016; 44: 926–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Li X, Li X, Shang Q, et al. Fecal microbiota transplantation (FMT) could reverse the severity of experimental necrotizing enterocolitis (NEC) via oxidative stress modulation. Free Radic Biol Med 2017; 108: 32–43. [DOI] [PubMed] [Google Scholar]
- 156.Zhang M, Lian B, Zhang R, et al. Emodin ameliorates intestinal dysfunction by maintaining intestinal barrier integrity and modulating the Microbiota in Septic Mice. Mediators Inflamm 2022; 2022: 5026103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yao H, Shi Y, Yuan J, et al. Matrine protects against DSS-induced murine colitis by improving gut barrier integrity, inhibiting the PPAR-α signaling pathway, and modulating gut microbiota. Int Immunopharmacol 2021; 100: 108091. [DOI] [PubMed] [Google Scholar]
- 158.Fan J, Zhao XH, Li TJ. Heat treatment of galangin and kaempferol inhibits their benefits to improve barrier function in rat intestinal epithelial cells. J Nutr Biochem 2021; 87: 108517–20201002. [DOI] [PubMed] [Google Scholar]
- 159.Cao C, Zhu B, Liu Z, et al. An arabinogalactan from Lycium barbarum attenuates DSS-induced chronic colitis in C57BL/6J mice associated with the modulation of intestinal barrier function and gut microbiota. Food Funct 2021; 12: 9829–9843. [DOI] [PubMed] [Google Scholar]
- 160.Yue B, Ren J, Yu Z, et al. Pinocembrin alleviates ulcerative colitis in mice via regulating gut microbiota, suppressing TLR4/MD2/NF-κB pathway and promoting intestinal barrier. Biosci Rep 2020; 40: BSR20200986. DOI: 10.1042/bsr20200986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yan BF, Chen X, Chen YF, et al. Aqueous extract of Paeoniae Radix Alba (Paeonia lactiflora Pall.) ameliorates DSS-induced colitis in mice by tunning the intestinal physical barrier, immune responses, and microbiota. J Ethnopharmacol 2022; 294: 115365–20220518. [DOI] [PubMed] [Google Scholar]
- 162.Zhang B, Yue R, Chen Y, et al. The Herbal Medicine Scutellaria-Coptis alleviates intestinal mucosal barrier damage in diabetic rats by inhibiting inflammation and modulating the gut Microbiota. Evid Based Complement Alternat Med 2020; 2020: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Xie S, Yang T, Wang Z, et al. Astragaloside IV attenuates sepsis-induced intestinal barrier dysfunction via suppressing RhoA/NLRP3 inflammasome signaling. Int Immunopharmacol 2020; 78: 106066–20191210. [DOI] [PubMed] [Google Scholar]
- 164.Wu D, Ding L, Tang X, et al. Baicalin protects against hypertension-associated intestinal barrier impairment in part through enhanced microbial production of short-chain fatty acids. Front Pharmacol 2019; 10: 1271–20191028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ge L, Qi J, Shao B, et al. Microbial hydrogen economy alleviates colitis by reprogramming colonocyte metabolism and reinforcing intestinal barrier. Gut Microbes 2022; 14: 2013764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fachi JL, Felipe JS, Pral LP, et al. Butyrate protects mice from Clostridium difficile-Induced colitis through an HIF-1-Dependent mechanism. Cell Rep 2019; 27: 750–761.e7. [DOI] [PubMed] [Google Scholar]
- 167.Okumura T, Nozu T, Ishioh M, et al. Centrally administered butyrate improves gut barrier function, visceral sensation and septic lethality in rats. J Pharmacol Sci 2021; 146: 183–191. [DOI] [PubMed] [Google Scholar]
- 168.Beisner J, Filipe Rosa L, Kaden-Volynets V, et al. Prebiotic inulin and sodium butyrate attenuate obesity-induced intestinal barrier dysfunction by induction of antimicrobial peptides. Front Immunol 2021; 12: 678360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Okumura T, Nozu T, Ishioh M, et al. Brain orexin improves intestinal barrier function via the vagal cholinergic pathway. Neurosci Lett 2020; 714: 134592. [DOI] [PubMed] [Google Scholar]
- 170.Xiao S, Li Q, Hu K, et al. Vitamin A and retinoic acid exhibit protective effects on necrotizing enterocolitis by regulating intestinal flora and enhancing the intestinal epithelial barrier. Arch Med Res 2018; 49: 1–9. [DOI] [PubMed] [Google Scholar]
- 171.Wang PF, Yao DH, Hu YY, et al. Vitamin D improves intestinal barrier function in cirrhosis rats by upregulating heme oxygenase-1 expression. Biomol Ther 2019; 27: 222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wang G, Wang H, Jin Y, et al. Galactooligosaccharides as a protective agent for intestinal barrier and its regulatory functions for intestinal microbiota. Food Res Intern 2022; 155: 111003. [DOI] [PubMed] [Google Scholar]
- 173.Wang G, Sun W, Pei X, et al. Galactooligosaccharide pretreatment alleviates damage of the intestinal barrier and inflammatory responses in LPS-challenged mice. Food Funct 2021; 12: 1569–1579. [DOI] [PubMed] [Google Scholar]
- 174.Engstler AJ, Sellmann C, Jin CJ, et al. Treatment with alpha-galactosylceramide protects mice from early onset of nonalcoholic steatohepatitis: role of intestinal barrier function. Mol Nutr Food Res 2017; 61: 20170222. [DOI] [PubMed] [Google Scholar]
- 175.Thaiss CA, Levy M, Grosheva I, et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science 2018; 359: 1376–1383. [DOI] [PubMed] [Google Scholar]
- 176.Kim S, Goel R, Kumar A, et al. Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clin Sci 2018; 132: 701–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Rahman K, Desai C, Iyer SS, et al. Loss of junctional adhesion molecule A promotes severe steatohepatitis in mice on a diet high in saturated fat, fructose, and cholesterol. Gastroenterology 2016; 151: 733–746.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang YF, Li JW, Wang DP, et al. Anti-hyperglycemic agents in the adjuvant treatment of sepsis: improving intestinal barrier function. Drug Des Devel Ther 2022; 16: 1697–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jang H, Kim S, Kim H, et al. Metformin protects the intestinal barrier by activating goblet cell maturation and epithelial proliferation in radiation-induced enteropathy. Int J Mol Sci 2022; 23: 5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wu W, Wang S, Liu Q, et al. Metformin protects against LPS-Induced intestinal barrier dysfunction by activating AMPK pathway. Mol Pharm 2018; 15: 3272–3284. [DOI] [PubMed] [Google Scholar]
- 181.Deng J, Zeng L, Lai X, et al. Metformin protects against intestinal barrier dysfunction via AMPKα1-dependent inhibition of JNK signalling activation. J Cell Mol Med 2018; 22: 546–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Metidji A, Omenetti S, Crotta S, et al. The environmental sensor AHR protects from inflammatory damage by maintaining intestinal stem cell homeostasis and barrier integrity. Immunity 2018; 49: 353–362.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lou X, Chen Z, He Z, et al. Bacteria-mediated synergistic cancer therapy: small microbiome has a Big Hope. Nano-Micro Lett 2021; 13: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]