Abstract
Background:
Revision procedures for recurrent Dupuytren disease (DD) can be difficult and carry a high risk of complications. Our goal was to describe surgical strategies used for cases of recurrence and report on their outcomes.
Methods:
We reviewed 1 surgeon’s operative cases for recurrent DD performed at 1 institution. Prior procedures included collagenase injection, percutaneous needle fasciotomy, or open surgical fasciectomy in the same digit or area of the hand.
Results:
From January 1981 to December 2020, 54 procedures were performed on 33 patients for recurrent DD. Most patients were men (82%), had bilateral involvement (64%) and family history (52%), and some had ectopic disease in their feet (24%). The small finger was involved in 76% of the cases, and the proximal interphalangeal (PIP) joint was involved in 83% of these digits. The procedures included 38 partial fasciectomies (72%), 12 dermofasciectomies (23%), 3 radical fasciectomies (6%), 1 of each needle fasciotomy, ray amputation, and PIP joint arthrodesis (2%). Twenty-three patients (43%) required full thickness skin grafts with an average area of 7.1 cm2 (range: 1-20 cm2).
Conclusions:
This study highlights the complexity of recurrent DD case management and found the treatment required for 95% of patients in this series was open partial fasciectomy with or without demofasciectomy. Full thickness skin grafting was necessary in nearly half of the cases.
Keywords: Dupuytren, recurrent, fasciotomy, revision, hand, anatomy, surgery, specialty
Introduction
Dupuytren disease (DD) was first described by the Swiss physician Felix Plater in 1614 and eventually received its current name after the French physician Baron Guillaume Dupuytren’s detailed lecture on the subject in 1831. 1 Dupuytren disease is a benign progressive disorder of the hand involving the palmar fascial complex which develops nodules, cords, and subsequent digital joint contractures.2,3
Dupuytren disease origin has been attributed to the Nordic, Saxon, Celtic, and Viking ethnic groups who resided in the northern European continent.4,5 However, this hypothesis was refuted in a genome-wide association study on the ethnic origin of DD from the British Isles where the authors found no evidence for an excess of Norse ancestry in DD and concluded that there is no genetic evidence for a Viking origin of DD. 6
A study in Norway found a 46% prevalence of the disease in a 65- to 74-year-old patients. 7 There was a prevalence of 33% in a similar age group in Iceland. 8 Lennox studied a Scottish population and found a 39% prevalence in patients over 60 years old. 9 Hueston found a 28% prevalence in Australia, presuming to be related to the large number of northern Europeans who immigrated there over the last few centuries. 4 The disease prevalence has been shown to be lower in areas further from Northern Europe, such as a 14% and 19% prevalence in England and Spain, respectively.10,11 Even in Japan, a prevalence of 12% in patients 60 to 69 years old has been reported. Dupuytren disease can be found throughout the world, 12 but it is rare among African and Middle Eastern populations. In addition, a “Non-Dupuytren’s palmar fascial disease” has been described in epidemiologic studies to have no genetic predisposition and more likely to be associated with previous trauma, surgery, or systemic conditions. 13 Inclusion of non-DD within the classic disease further confounds the precise incidence of the disease which may explain the disparity in reported prevalence studies.
Surgical treatments of DD can range from limited needle aponeurectomy, open or percutaneous fasciotomy, to different methods of open fasciectomy.1,14 Newly introduced collagenase injections have become recently a popular treatment option. 15 Following open procedures and especially after needle and enzymatic treatments, the disease can recur and may require revision surgery. 16 These secondary procedures can be more difficult and carry a higher risk of complications than primary surgeries. Patients with Dupuytren diathesis are a population with greater genetical predisposition than the classic DD patients and have more extensive and severe disease with higher rate of recurrence after surgery. 17 A recent meta-analysis described that a consensus on the efficacy of treatment for recurrent DD is not yet available. 18
The purpose of our study was to include demographic information and describe surgical strategies used for cases of recurrent DD and report on their treatment outcomes.
Materials and Methods
After obtaining approval from the institutional review board at our institution, we reviewed the total registry of operative cases for DD performed at 1 institution by 1 surgeon spanning 40 years from beginning of 1981 to end of 2020. This included review of recent electronic medical records and operative records from paper charts, scheduling surgery books, and a registry of operative reports. All procedures performed for recurrent DD were selected. Out of this list, surgeries for recurrent cases were separated and studied. Recurrent surgeries were performed for any patients who had prior collagenase injection, percutaneous needle fasciotomy, or any open surgical fasciectomy procedure in the same area of the palm or digit. The primary surgeries for the recurrent cases were included whether performed by the senior author or at other institutions. Demographic information was collected, including the patient’s sex, age at the time of each operation, family history, and presence of ectopic disease, side affected, digits involved, cords identified, joints involved, and type of procedure performed. Preoperative and postoperative range of motion and flexion contracture examination data were collected from charts retrospectively. Only procedures that were performed in the same area or finger ray were included as recurrences.
Results
Over the period of the study, there were 54 procedures performed on 33 patients for recurrent DD (Table 1). Of these patients, 27 (82%) were men and 6 (18%) were women. The average age was 60.5 years old (27-84). There were 17 patients (52%) with a family history of DD and 8 (24%) with ectopic disease (affecting feet and 1 genitalia). Twenty-one patients (64%) had bilateral and 12 (36%) had unilateral involvement. Two (6%) of the initial procedures were performed by the study surgeon, while the other 31 patients (94%) had surgery at an outside facility.
Table 1.
Demographics: Characteristics of Patients Undergoing Revision Surgery for Recurrent Dupuytren Disease.
| Demographic / Comorbidity | Number/Mean | Percentage (%)/Range |
|---|---|---|
| Patients | 33 | |
| Procedures | 54 | |
| Age | 60.5 | 27-84 |
| Male | 27 | 81.8 |
| Female | 6 | 18.2 |
| Bilateral involvement | 21 | 63.6 |
| Unilateral involvement | 12 | 36.4 |
| Family history | 17 | 51.5 |
| Ectopic disease | 8 | 24.2 |
| Foot nodules | 8 | 24.2 |
| Penile contracture | 1 | 3.0 |
| Tobacco use | 9 | 27.3 |
| Alcohol use | 13 | 39.4 |
| Diabetes mellitus type 2 | 7 | 21.2 |
| Hypertension | 30 | 90.9 |
| Hyperlipidemia | 12 | 36.4 |
Demographics included 30 patients with hypertension (91%), 12 with hyperlipidemia (36%), 13 with a history of alcohol consumption (39%), 9 with a history of smoking (27%), and 7 with type 2 diabetes mellitus (21%).
The treatments before the recurrent procedures (Figure 1) were 26 partial fasciectomies (79%), 10 collagenase injections (30%), and 1 needle aponeurotomy (3%). The procedures performed for recurrence (Figure 2) were 38 open partial fasciectomies (72%), 12 dermofasciectomies (23%), 3 radical fasciectomies for diffuse involvement (6%), 1 needle fasciotomy (2%) for well-defined recurrent cord, 1 ray amputation (2%), and proximal interphalangeal (PIP) joint arthrodesis (2%). Twenty-three patients (43%) required adjunct full thickness skin graft (including dermofasciectomy) with an average area of 7.1 cm2 (range: 1-20 cm2).
Figure 1.
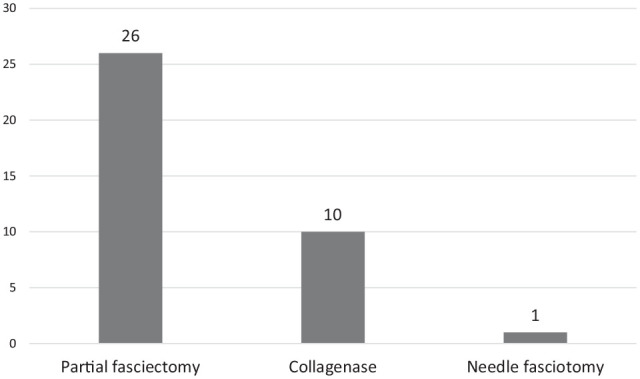
Initial procedures: the number of initial treatments that patients underwent before their recurrent Dupuytren disease treatments.
Figure 2.
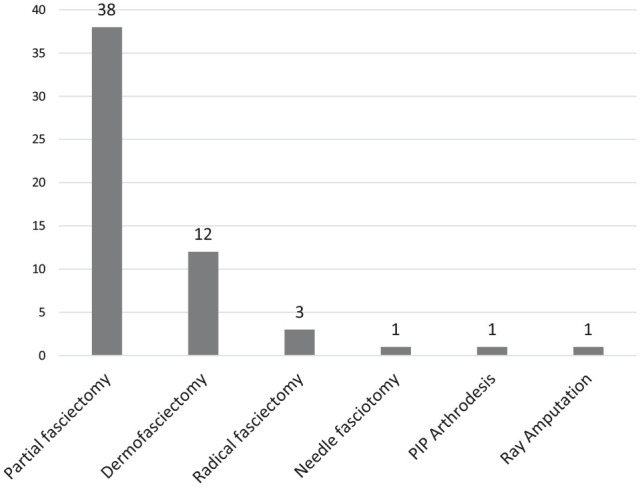
Revision procedures: the number of surgical procedures performed for recurrent Dupuytren disease.
Note. PIP = proximal interphalangeal.
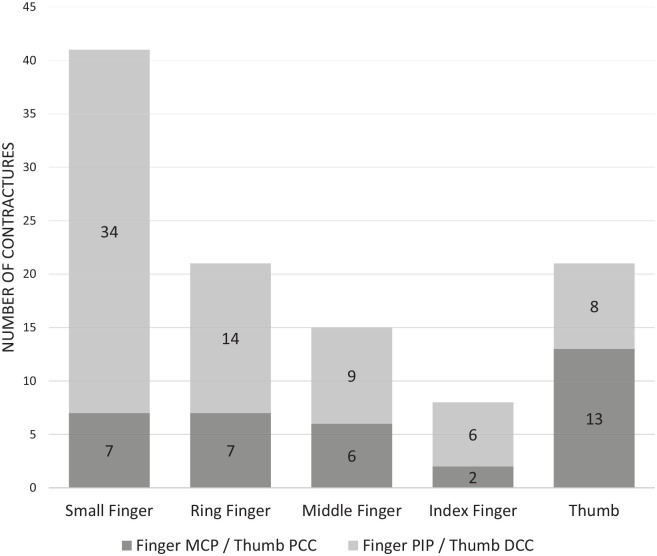
Before the revision surgery, the small finger was involved in 41 cases (76%) and, of those, 7 (17%) affected the metacarpophalangeal (MCP) joints and 34 (83%) affected the PIP joints (Figure 3). The ring finger was involved in 21 cases (39%) and, of those, 7 affected the MCP (37%) and 14 affected the PIP (74%) joints. The middle finger was involved in 15 cases (28%) and, of those, 6 affected the MCP (43%) and 9 affected the PIP (64%) joints. The index finger was involved in 8 cases (15%) and, of those, 2 affected the MCP (25%) and 6 affected the PIP (75%) joints. Finally, the thumb-index Web space was involved in 21 cases (39%) and, of those, 13 (68%) affected the proximal commissural cords and 8 (42%) affected the distal commissural cords.
Figure 3.
Finger/thumb contractures: the finger joints with flexion contractures and thumb commissural cords that were addressed during revision procedures.
Note. PIP = proximal interphalangeal; MCP = metacarpophalangeal; DCC = distal commissural cord; PCC = proximal commissural cord.
Postoperative follow-up after recurrent surgery averaged 30 months (range: 0.2-210 months). Small finger MCP joint contractures improved from average 43° (0-90°) preoperatively to 2° (0°-10°) postoperatively. Small finger PIP joint contractures improved from average 58° (0°-120°) preoperatively to 20° (0°-90°) postoperatively. Ring finger MCP joint contractures improved from 31° (0°-85°) preoperatively to 1° (0°-10°) postoperatively. Ring finger PIP joint contractures improved from 52° (0°-90°) preoperatively to 13° (0°-60°) postoperatively. Middle finger MCP joint contractures improved from 23° (0°-45°) preoperatively to 1° (0°-10°) postoperatively. Middle finger PIP contractures improved from 53° (0°-90°) preoperatively to 4° (0°-20° postoperatively. Index finger MCP contractures improved from 12° (5°-15°) preoperatively to 2° (0°-10°) postoperatively. Index finger PIP contractures improved from 45° (25°-80°) preoperatively to 7° (0°-30°) postoperatively.
Discussion
Although a recent Delphi Group defined recurrence as “more than 20 degrees of contracture recurrence in any treated joint at one year post-treatment compared to six weeks post-treatment,” DD does not have a widely agreed upon definition for recurrance. 19 The recurrence can be diagnosed by the appearance of any nodule or cord after a procedure whether it is in the operative field17,19,20 or outside of the operative field.21,22 However, appearance of disease outside the area of surgery generally referred to as disease extension. The recurrence can also be based on the degree of joint contracture. 23 Finally, recurrence can be self-reported by the patients 24 or can be based on whether a repeat procedure was performed. 25 It is important to differentiate between true recurrence caused by diseased cord and residual PIP flexion deformity, which is not uncommon, especially in the small finger. In our study, we reported on patients who underwent DD release in the same area that had been operated on previously with recurrence of diseased tissue causing joint flexion deformity.
A balance between minimally invasive procedures and risk of recurrence of DD has always been a driving force behind its treatment. Collagenase clostridium histolyticum uses enzymatic cleavage of the pathologic cords and delayed manual manipulation to achieve contracture correction. 26 Despite promising early results, Zhang found that 80% of patients subjectively perceived recurrence within 5 years after collagenase and 53% of patients required additional treatments within that time period. 27 Peimer reported 47% recurrence and 18% requiring additional treatments within 5 years after collagenase. 28 Werlenrud found that 21% of patients required additional treatment for MCP joint contractures and 51% of PIP joint contractures. 29
Percutaneous needle fasciotomy is a technique in which the pathologic cord is divided mechanically through repeated perforations by a needle. 30 Unfortunately, this procedure has a high risk of recurrence as well, albeit it is less intrusive than collagenase. Stromberg found 12% recurrence at the MCP joint and 8% at the PIP joint within 2 years after needle aponeurotomy. 30 Van Rijssen found an 85% recurrence within 5 years after such treatment. 31 The senior author performs needle fasciotomy often, yet rarely if any of those cases had residual tissue severe enough to require recurrent open procedure.
Partial fasciectomy involves excision of diseased tissue and remains the most common surgical technique performed for DD. Van Rissjen reports 21% recurrence within 5 years after fasciectomy and Mavrogenis reported 20% recurrence within 7 years and 7.3% of patients requiring additional treatments in that time period.23,31 Even the most invasive surgical technique, radical open fasciectomy, has been found to have residual contracture in 20% of cases and can have 17.5% chance of recurrence within 7 years. 32 Dupuytren disease recurrence has more to do with patient’s diathesis or genetic predisposition than with the original surgical procedure performed. Also, in the senior author’s experience, there seems to be less tendency for disease recurrence beneath skin graft when it is used during the primary surgery.
There have been 12 studies that describe outcomes of treatment for recurrent DD.16,21,24,33-41 Only 2 of those studies included more patients than our study.16,33 One of those studies included 31 patients but was limited to repeat collagenase injections, 33 and the other study included 131 patients, but only had 3-month follow-up. 16 Therefore, we believe our study will add significantly to the literature on treatment for recurrent DD.
Our study has described the experience of 1 surgeon performing surgery for patients with recurrent DD over a 40-year period at 1 institution. Demographic characteristics from this study showed that most patients who had DD diathesis manifested with the presence of family history and bilateral disease in more than half the cases. In addition, one-quarter of those patients had ectopic disease. Two-thirds of the recurrent cases involved the small finger and PIP joint flexion deformity was present in 83%.
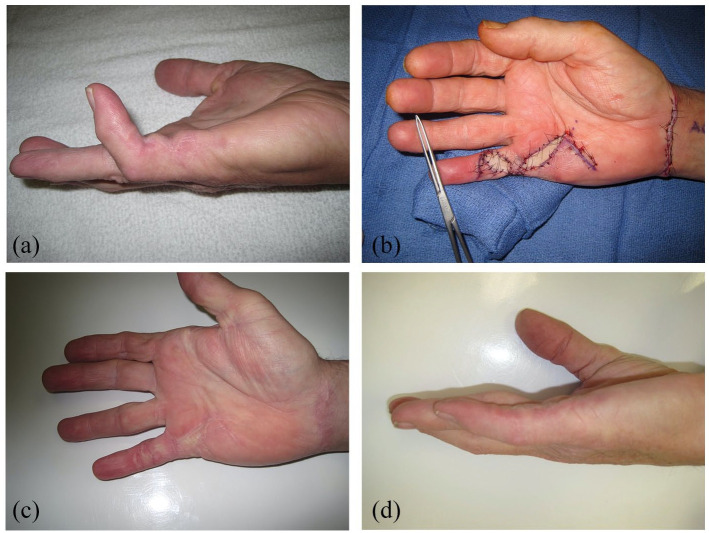
We found that the majority of these patients were men (84%), with an average age of 60 years, had bilateral involvement (65%) and family history (55%). Ectopic disease in the feet was present in 26% of these patients. Likewise, the majority of our reported patients who underwent revision surgeries had involvement of the small finger (75%) and the PIP joint (82%) of small finger. In all fingers, the PIP joints were affected in greater flexion deformity than the MCP joints. In the first Web space, the proximal commissural cord was involved more than the distal commissural cord. The surgical trend that we found necessary for these patients was revision open fasciectomy and nearly half required full thickness skin grafting (Figure 4a-d). About half of those requiring skin grafts were in the form of dermofasciectomy. Several patients required more involved resection such as radical fasciectomy with full thickness skin grafting (Figure 5). With this approach, satisfactory outcomes with substantial correction of digital flexion deformities were achieved.
Figure 4.
(a) Partial fasciectomy with skin grafting: preoperative image of a patient who had a recurrent disease with proximal interphalangeal (PIP) joint flexion contracture. (b) Intraoperative photograph during partial fasciectomy and full thickness skin grafting from the volar wrist. (c) Postoperative photograph of well-healed incisions and well-incorporated skin grafts. (d) Postoperative photograph of restoration of full extension of the PIP joint. The patient also had full PIP joint flexion.
Figure 5.
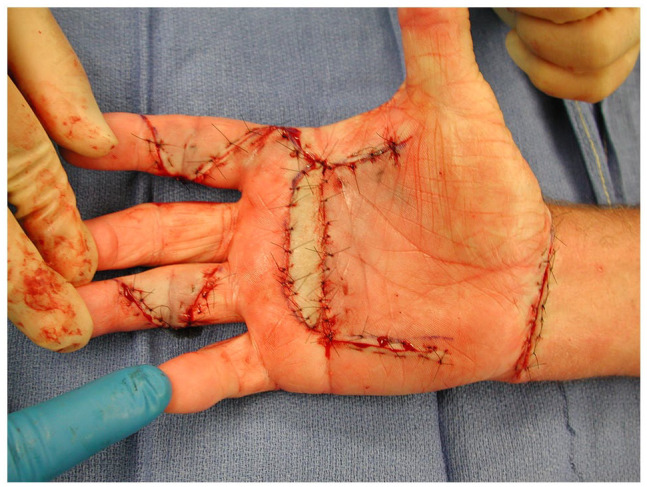
Extensive fasciectomy: intraoperative photograph of an extensive fasciectomy with full thickness skin grafting from the volar wrist.
Dermofasciectomy is typically indicated for recurrent cases when the skin is adherent to the diseased tissue and should be excised with the disease cords. This will leave skin shortage that requires full thickness skin grafting. Skin grafting is also indicated in primary cases with severe PIP joint contracture. The correction of which will necessitate skin coverage with a graft. These indications have not changed over the last 4 generations.
In this study, we observed that needle fasciotomy, with or without enzymatic material injection, has no role in management of recurrent DD. The findings from the Dupuytren’s Interventions Surgery versus Collagenase trial which is underway in England is expected to shed some light on whether the injection for primary cases is no worse than surgery and whether the effects are sustained in the long term.
Small finger disease with spiral digital cord and severe contracture of the PIP joint may not allow full correction of the flexion deformity due to associated involvement of the neurovascular structures. These cases may recur after primary partial fasciectomy. However, patients with Dupuytren diathesis and strong genetic predisposition for the disease are more likely to have recurrence after primary surgery, regardless of the involved digit.
This is a retrospective chart review, and it is therefore limited by the documentation that is currently available. The patients show a wide range of clinical challenges prohibiting this population from an in-depth statistical analysis. However, this study offers epidemiologic information about patients with recurrent DD and the risk factors associated with recurrence. It also offers treatment strategy and highlights the complexity of this condition.
Footnotes
Ethical Approval: This study was approved by our institutional review board.
Statement of Human and Animal Rights: Procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation.
Statement of Informed Consent: No informed consent was able to be obtained because the research was done retrospectively.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Bryan A. Hozack  https://orcid.org/0000-0002-5780-5796
https://orcid.org/0000-0002-5780-5796
Ghazi M. Rayan  https://orcid.org/0000-0003-0400-6269
https://orcid.org/0000-0003-0400-6269
References
- 1.Elliot D.Pre-1900 literature on Dupuytren’s disease. Hand Clin. 1999;15(1):175-181. [PubMed] [Google Scholar]
- 2.Riolo J, Young VL, Ueda K, et al. Dupuytren’s contracture. South Med J. 1991;84(8):983-986. [DOI] [PubMed] [Google Scholar]
- 3.Ross DC.Epidemiology of Dupuytren’s disease. Hand Clin. 1999;15(1):53vi-62. [PubMed] [Google Scholar]
- 4.Hueston JT.Further studies on the incidence of Dupuytren’s contracture. Med J Aust. 1962;49(1):586-588. [PubMed] [Google Scholar]
- 5.McFarlane RM.On the origin and spread of Dupuytren’s disease. J Hand Surg Am. 2002;27(3):385-390. [DOI] [PubMed] [Google Scholar]
- 6.Ng M, Lawson DJ, Winney B, et al. Is Dupuytren’s disease really a “disease of the Vikings.” J Hand Surg Eur Vol. 2020;45(3):273-279. [DOI] [PubMed] [Google Scholar]
- 7.Mikkelsen OA.The prevalence of Dupuytren’s disease in Norway. A study in a representative population sample of the municipality of Haugesund. Acta Chir Scand. 1972;138(7):695-700. [PubMed] [Google Scholar]
- 8.Gudmundsson KG, Arngrímsson R, Sigfússon N, et al. Epidemiology of Dupuytren’s disease: clinical, serological, and social assessment. The Reykjavik Study. J Clin Epidemiol. 2000;53(3):291-296. [DOI] [PubMed] [Google Scholar]
- 9.Lennox IA, Murali SR, Porter R.A study of the repeatability of the diagnosis of Dupuytren’s contracture and its prevalence in the Grampian region. J Hand Surg Br. 1993;18(2):258-261. [DOI] [PubMed] [Google Scholar]
- 10.Early PF.Population studies in Dupuytren’s contracture. J Bone Joint Surg. 1962;44B:602-613. [Google Scholar]
- 11.Guitian AQ.Quelques Aspects Epidemiologiques de la Maladie de Dupuytren. Ann Chir Main. 1988;7:256-262. [DOI] [PubMed] [Google Scholar]
- 12.Egawa T, Horiki H, Senrui H. Dupuytren’s contracture in Japan. In: Hueston JT, Tubiana R, eds. Dupuytren’s disease. Edinburgh, England: Churchill Livingstone; 1985:100-103. [Google Scholar]
- 13.Rayan GM, Moore J.Non-Dupuytren’s disease of the palmar fascia. J Hand Surg Br. 2005;30(6):551-556. [DOI] [PubMed] [Google Scholar]
- 14.Shaw RB, Jr, Chong AKS, Zhang A, et al. Dupuytren’s disease: history, diagnosis, and treatment. Plast Reconstr Surg. 2007;120(3):44e-54e. [DOI] [PubMed] [Google Scholar]
- 15.Hurst LC, Badalamente MA.Nonoperative treatment of Dupuytren’s disease. Hand Clin. 1999;15(1):97-107, vii. [PubMed] [Google Scholar]
- 16.Mendelaar NHA, Poelstra R, van Nieuwenhoven CA, et al. Outcome of recurrent surgery in Dupuytren’s disease: comparison with initial treatment. Plast Reconstr Surg. 2019;144(5):828e-835e. [DOI] [PubMed] [Google Scholar]
- 17.Hindocha S, Stanley JK, Watson S, et al. Dupuytren’s diathesis revisited: evaluation of prognostic indicators for risk of disease recurrence. J Hand Surg Am. 2006;31(10):1626-1634. [DOI] [PubMed] [Google Scholar]
- 18.Wong CR, Huynh MNQ, Fageeh R, et al. Outcomes of management of recurrent Dupuytren contracture: a systematic review and meta-analysis. Hand. 2021;1558944721994220. doi:10.1177/1558944721994220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kan HJ, Verrijp FW, Hovius SER, et al. Recurrence of Dupuytren’s contracture: a consensus-based definition. PLoS ONE. 2017;12(5):e0164849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abe Y, Rokkaku T, Ofuchi S, et al. An objective method to evaluate the risk of recurrence and extension of Dupuytren’s disease. J Hand Surg Br. 2004;29(5):427-430. [DOI] [PubMed] [Google Scholar]
- 21.Abe Y, Rokkaku T, Kuniyoshi K, et al. Clinical results of dermofasciectomy for Dupuytren’s disease in Japanese patients. J Hand Surg Eur Vol. 2007;32(4):407-410. [DOI] [PubMed] [Google Scholar]
- 22.Moermans JP.Recurrences after surgery for Dupuytren’s disease. Eur J Plast Surg. 1997;20(5):240e5. [Google Scholar]
- 23.Mavrogenis AF, Spyridonos SG, Ignatiadis IA, et al. Partial fasciectomy for Dupuytren’s contractures. J Surg Orthop Adv. 2009;18(2):106-110. [PubMed] [Google Scholar]
- 24.Degreef I, De Smet L.Dupuytren’s disease: a predominant reason for elective finger amputation in adults. Acta Chir Belg. 2009;109(4):494-497. [DOI] [PubMed] [Google Scholar]
- 25.Wilbrand S, Ekbom A, Gerdin B.The sex ratio and rate of reoperation for Dupuytren’s contracture in men and women. J Hand Surg Br. 1999;24(4):456-459. [DOI] [PubMed] [Google Scholar]
- 26.Hurst LC, Badalamente MA, Hentz VR, et al. Injectable collagenase clostridium histolyticum for Dupuytren’s contracture. N Engl J Med. 2009;361(10):968-979. [DOI] [PubMed] [Google Scholar]
- 27.Zhang D, Earp BE, Benavent KA, et al. Collagenase treatment of Dupuytren’s Disease with minimum 5-year follow-up: recurrence, reintervention, and satisfaction. Plast Reconstr Surg. 2020;146(5):1071-1079. [DOI] [PubMed] [Google Scholar]
- 28.Peimer CA, Blazar P, Coleman S, et al. Dupuytren contracture recurrence following treatment with collagenase clostridium histolyticum (CORDLESS [Collagenase Option for Reduction of Dupuytren Long-Term Evaluation of Safety Study]): 5-year data. J Hand Surg Am. 2015;40(8):1597-1605. [DOI] [PubMed] [Google Scholar]
- 29.Werlinrud JC, Hansen KL, Larsen S, et al. Five-year results after collagenase treatment of Dupuytren disease. J Hand Surg Eur Vol. 2018;43(8):841-847. [DOI] [PubMed] [Google Scholar]
- 30.Strömberg J, Ibsen Sörensen A, Fridén J.Percutaneous needle fasciotomy versus collagenase treatment for Dupuytren contracture: a randomized controlled trial with a two-year follow-up. J Bone Joint Surg Am. 2018;100(13):1079-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Rijssen AL, Ter Linden H, Werker PMN.Five-year results of a randomized clinical trial on treatment in Dupuytren’s disease: percutaneous needle fasciotomy versus limited fasciectomy. Plast Reconstr Surg. 2012;129(2):469-477. [DOI] [PubMed] [Google Scholar]
- 32.Roulet S, Bacle G, Guéry J, et al. Outcomes at 7 and 21 years after surgical treatment of Dupuytren’s disease by fasciectomy and open-palm technique. Hand Surg Rehabil. 2018;37(5):305-310. [DOI] [PubMed] [Google Scholar]
- 33.Bear BJ, Peimer CA, Kaplan FTD, et al. Treatment of recurrent Dupuytren contracture in joints previously effectively treated with collagenase clostridium histolyticum. J Hand Surg Am. 2017;42(5):391e1-391. [DOI] [PubMed] [Google Scholar]
- 34.Eberlin KR, Kobraei EM, Nyame TT, et al. Salvage palmar fasciectomy after initial treatment with collagenase clostridium histolyticum. Plast Reconstr Surg. 2015;135(6):1000e-1006e. [DOI] [PubMed] [Google Scholar]
- 35.Hohendorff B, Spies CK, Müller LP, et al. Supplementary arthrolysis of the proximal interphalangeal finger joint in Dupuytren’s contracture: primary operation versus revision. Arch Orthop Trauma Surg. 2016;136(3):435-439. [DOI] [PubMed] [Google Scholar]
- 36.Könneker S, Broelsch GF, Krezdorn N, et al. multiple recurrences in aggressive forms of Dupuytren’s disease-can patients benefit from repeated selective fasciectomy. Plast Reconstr Surg Glob Open. 2017;5(2):e1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Molenkamp S, Schouten TAM, Broekstra DC, et al. Early postoperative results of percutaneous needle fasciotomy in 451 patients with Dupuytren disease. Plast Reconstr Surg. 2017;139(6):1415-1421. [DOI] [PubMed] [Google Scholar]
- 38.Novoa-Parra CD, Montaner-Alonso D, Pérez-Correa JI, et al. Arthrodesis of the proximal interphalangeal joint of the 4th and 5th finger using an interlocking screw device to treat severe recurrence of Dupuytren’s disease. Rev Esp Cir Ortop Traumatol. 2018;62(3):216-221. [DOI] [PubMed] [Google Scholar]
- 39.Roush TF, Stern PJ.Results following surgery for recurrent Dupuytren’s disease. J Hand Surg Am. 2000;25(2):291-296. [DOI] [PubMed] [Google Scholar]
- 40.Spies CK, Hahn P, Müller LP, et al. The efficacy of open partial aponeurectomy for recurrent Dupuytren’s contracture. Arch Orthop Trauma Surg. 2016;136(6):881-889. [DOI] [PubMed] [Google Scholar]
- 41.van Rijssen AL, Werker PMN.Percutaneous needle fasciotomy for recurrent Dupuytren disease. J Hand Surg Am. 2012;37(9):1820-1823. [DOI] [PubMed] [Google Scholar]