Abstract

3,4- and 3,5-Dinitropyrazoles (DNPs) were substituted with acryl and allyl groups on the N1 nitrogen atom, resulting in three novel energetic materials. These compounds are all liquids at room temperature with melting points ranging from −60.2 to −38.6 °C and were fully characterized by high-resolution mass spectrometry, elemental analysis, proton and carbon nuclear magnetic resonance spectroscopy, and Fourier transform infrared spectroscopy. These materials were also tested for electrostatic discharge, friction, and impact sensitivities and then compared to DNP starting materials and to the explosive nitroglycerin (NG). These results indicate that the synthesized compounds are less sensitive to impact compared to NG and have higher thermal stabilities to decomposition.
1. Introduction
Current efforts in the synthesis of new energetic materials focus on compounds that have high performance with low sensitivities, compared to conventional explosives. Low-melting or liquid explosives are an important class of materials that can be cast or poured, which is useful in munitions as they can be easily shaped or used as plasticizers.1 The liquid explosive nitroglycerin (NG), most notable for its use in dynamite, has been one of the most thoroughly studied explosives since its discovery in 1847.2 However, its high sensitivity has driven the need for new liquid explosives. Additionally, nitrate ester-containing compounds, like NG, usually have poor thermal stability and high impact sensitivity, which make these materials dangerous to handle and prone to accidental detonation.3 For example, when NG is heated above 200 °C, it will explode, and when stored long term, it becomes unstable at temperatures exceeding 70–80 °C.4
Chemists routinely target high nitrogen-containing heterocycles because they have high thermal stabilities and low sensitivities as a result of their aromatic character. Also, the N–N and C–N bonds in these materials result in high heats of formation and favor N2 formation upon decomposition.5−10 Dinitropyrazoles (DNPs) are high nitrogen materials with good oxygen balances (−30.4%) and high densities (1.7 and 1.8 g cm–3 at 293 K), with positive heats of formation (20–24 kcal mol–1). DNPs are also easily functionalizable, and replacing the acidic N–H with alkyl groups has been shown to increase thermal stability while reducing the melting point. The improvements to thermal stability and reducing the melting point are sought after for applications in niche demand environments, where ideally the liquid would have difficulties solidifying and could potentially change the energetic properties of the material. For example, N-methyl-3,4-DNP (3,4-MDNP) is thermal stabile up to 300 °C, 24 °C higher than 3,4-DNP (3,4-DNP), and has a melting point of 20–23 °C, 67 °C lower than 3,4-DNP due to the substitution of the H with a methyl group.7,11N-Methyl-3,5-DNP (3,5-MDNP) has similar energetic properties to its 3,4-MDNP regioisomer, but it has a much higher melting point (∼60 °C), highlighting the influence of molecular structure on physical properties.8 Despite its higher molecular weight, the N-allyl substituted 3,4-DNP has an even lower melting point and a very low glass transition temperature (−72.1 °C), making it an excellent candidate as an energetic plasticizer.13 However, no other alkene-based substitutions have been explored. Herein, we report the derivatization of N-substituted 3,4-DNP and 3,5-DNP via allylation and acrylation to produce novel liquid explosives. The properties of these three new novel materials are compared against 3,4-DNP, 3,5-DNP, and NG.12
2. Results and Discussion
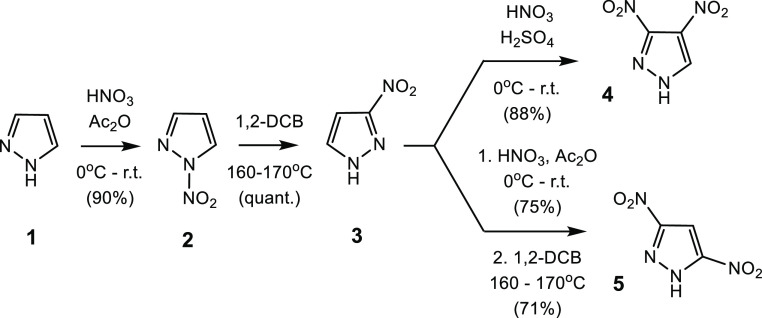
The syntheses of both DNP isomers followed the literature procedure and began with the nitration of pyrazole (1) using a mixture of 90% HNO3 and acetic anhydride (Ac2O) affording N-nitropyrazole (2) in 90% isolated yield. Compound 2 was thermally isomerized in refluxing 1,2-dichlorobenzene to give 3-nitropyrazole (3) in quantitative yield.14 Subsequent steps involved the direct nitration of 3 with mixed acid, affording 3,4-DNP (4),15 or an additional N-nitration followed by a second thermal rearrangement providing 3,5-DNP16 (5) in 88 and 71% yields, respectively (Scheme 1).
Scheme 1. Synthesis of 3,4-DNP 4 and 3,5-DNP 5.
With isomers 4 and 5 in hand, we subjected these materials to acylation and alkylation conditions. Compounds 4 and 5 were both acylated and allylated under basic conditions using a 1:1 solvent mixture of acetonitrile (MeCN) and triethylamine (TEA) chilled to 0 °C via an ice bath, with the dropwise addition of either acryloyl chloride or allyl bromide (Scheme 2). After allowing the reactions to warm to room temperature over the course of 12 h, compounds 4a–4b and 5a–5b were isolated in yields ranging from 48% to near-quantitative amounts. Initial characterization of allyl-substituted compounds 4a and 5a using proton NMR showed new distinctive alkene splitting patterns between 6.0 and 4.8 ppm. Additionally, the appearance of new C–H stretches in the Fourier transform infrared (FT-IR) spectra of 3140 and 3150 cm–1 further indicate that the allyl substitution had occurred. Acrylated materials 4b and 5b also showed similar acryloyl substitutions through proton NMR characterization of alkene patterns between 7.4 and 5.0 ppm and carbon NMR of strong carbonyl signals between 169 and 174 ppm. FT-IR spectra also illustrated the presence of carbonyls by strong C=O stretches between 1700 and 1710 cm–1 for compounds 4b and 5b. All compounds 4a–4b and 5a–5b had strong N–O stretching from the nitro functional groups indicated by stretches between 1550 and 1500 cm–1 in the FT-IR data (see the Supporting Information).
Scheme 2. Synthetic Route to Energetic Plasticizers 4a–4b and 5a–5b.
To determine thermal and energetic viability of the newly synthesized compounds 4a–4b and 5a–5b, physiochemical parameters of compounds 4a–4b and 5a–5b were measured and are outlined in Figure 1 and Table 1. For clarity of data comparisons in Figure 1, the compounds have consistent coloring for each plot with 4a as green, 4b as red, 5a as gray, and 5b as blue. Thermal analyses for 4a–4b and 5a–5b were conducted using differential scanning calorimetry (DSC), with a heating rate of 10 °C min–1 and data point acquisition every 0.18 s, at ranges between −85 to 20 °C and 50 to 400 °C (Figure 1, top and center plots). The lower-temperature-range DSC was collected to measure the melting temperature, indicated by the endotherm, for each material, and the higher range was collected to determine decomposition temperature, indicated by the exotherm. Similarities in the melting temperatures of allyl-substituted materials 4a (green) and 5a (gray) were measured at −56.7 and −56.5 °C, respectively. The melting temperature differences between materials 4a (green) and 5a (gray) indicate that despite the difference in one nitro position on each ring, the allyl functional group had a larger effect on the melting temperature for these materials. However, the 3,4-DNP acryl-substituted material, 4b (red), had a higher melt temperature at −39.8 °C than the 3,5-DNP acyl compound, 5b (blue), at −59.8 °C. These melting temperature differences between acryl materials 4b (red) and 5b (blue) show that the acryl groups establish a higher melting temperature than the allyl materials but are also effected by the nitro group position on the ring. This is likely a result of additional stability from electrostatic interactions from additional carbonyl oxygens coupled with steric hinderance considerations comparing nitro group substitution on both systems. However, these significantly lower melt temperatures for 4a–4b and 5a–5b ranging from −39.8 to −56.7 °C confirm that the materials would remain as a liquid in extremely cold environments. The DSC measurements collected from 50 to 400 °C for compounds 4a–4b and 5a–5b (Figure 1, center plot) show a variety of endotherms for each material prior to the major exotherm or decomposition peak. Decomposition temperature (Td) comparisons show that the 3,4-DNP compounds 4a (green) and 4b (red) have lower decomposition temperatures at 201.2 and 194.8 °C than the 3,5-DNP compounds 5a (gray) and 5b (blue) at 217.4 and 255.1 °C, respectively. From our data, 4 decomposes at a lower temperature (275 °C) than 5 (296 °C). These results match the trend for substituted materials 5a–5b and 4a–4b, with 5a–5b having higher decomposition temperatures than 4a–4b. Last, additional thermogravimetric analyses (TGAs) were collected at a heating rate of 5 °C min–1 between the range of 35–400 °C on 4a–4b and 5a–5b (Figure 1, bottom plot). Any residual weight percent left after the TGA measurement past the decomposition temperatures is due to pyrolysis with char residue left in the pan. The differences of heating rates between DSC (10 °C min–1) and TGA (5 °C min–1) measurements are significant enough to impact the decomposition temperatures, so these sets of data cannot be directly compared.
Figure 1.
DSC curves from −85 to 20 °C (top) and 50 to 400 °C (middle), along with TGA curves (bottom) for compounds 4a (green), 4b (red), 5a (gray), and 5b (blue).
Table 1. Calculated and Measured Energetic Properties of 4, 5, 4a–4b, and 5a–5b.
| 4a | 4b | 5a | 5b | 4 3,4-DNP | 5 3,5-DNP | NG | |
|---|---|---|---|---|---|---|---|
| ρ (g·cm–3)a | 1.57 | 1.65 | 1.55 | 1.64 | 1.81 | 1.84 | 1.59 |
| Dp (GPa)a | 18.93 | 20.70 | 18.39 | 20.54 | 29.75 | 32.70 | 20.96 |
| Dv (km·s–1)a | 7.23 | 7.34 | 7.15 | 7.31 | 8.35 | 8.39 | 7.6 |
| OBCO %a | –40.4 | –22.6 | –40.4 | –22.6 | 0 | 0 | 24.7 |
| Td (°C) | 201.2 | 194.8 | 217.4 | 255.1 | 275 | 296 | 165 |
| Tm (°C) | –56.7 | –39.8 | –56.5 | –54.8 | 71 | 169 | |
| IS (cm)b | >320 | 131.6 | 140.3 | >320 | 47.7 | 19.2 | 11.1 |
| FS (N)c | >360 | >360 | >360 | >360 | >360 | >360 | >360 |
| ESD (J)d | 0.025 | 0.062 | 0.025 | 0.062 | 0.025 | 0.125 | <0.025 |
Calculated values.
LANL type 12, 50% drop height, and 2.5 kg weight.
50% load Bruceton up/down method.
ABL spark, 3.4% threshold initiation level.
All materials 4a–4b and 5a–5b were found to be significantly more thermally stable compared to NG (decomposition temperature of 165 °C), with decomposition temperatures ranging from 185 to 255 °C (Table 1). Decomposition temperatures for 4a–4b are between 74 and 81 °C lower than that for parent compound 4. These lower decomposition temperatures are also measured for 5a–5b and are anywhere between 41 and 79 °C lower than 5. The lower decomposition temperatures are likely a result of the weak bonding between the pyrazolyl nitrogen atom to the allyl and carbonyl carbon atoms. Despite all substituted molecules having a lower decomposition temperature, the melt temperatures (Tm) for 4a–4b and 5a–5b are lower than those for both parent compounds 4 and 5. The sensitivities of these materials were probed through impact, friction, and electrostatic discharge (ESD) measurements. Generally, the substituted DNPs were found to be less sensitive to impact, ESD, and friction compared to 3,4-DNP, 3,5-DNP, and NG. The densities and heats of formation of compounds 4a–4b and 5a–5b were calculated using the method developed by Byrd and Rice (see the Supporting Information) and entered into the CHEETAH thermochemical code to determine the detonation properties.17,18 The CHEETAH code uses the traditional Chapman–Jouguet thermodynamic detonation theory to calculate detonation pressure, volume, and shock velocity. These calculations revealed that 4b and 5b have the highest densities (1.6 g cm–3), detonation pressures (20 GPa), detonation velocities (7.3 km s–1), and oxygen balances (−22%) compared to the other allyl-substituted DNPs. The addition of allyl and acryl groups in 4a–4b and 5a–5b results in a decrease in all calculated energetic properties compared to the parent compounds 4 and 5; however, this is accompanied by a decrease in sensitivity. The experimental impact sensitivity comparisons show that compound 4 is less sensitive with a “go” measurement at 47.7 cm than compound 5 with its “go” measurement at 19.2 cm. The impact sensitivity for compounds 4a–4b and 5a–5b do not follow an obvious trend. While there is a large error associated with sensitivity testing, the difference in impact sensitivities of 4b and 5a to 4a and 5b is well outside the standard deviation. However, the allyl and acryl groups on compounds 4a–4b and 5a–5b have a more profound effect on spark sensitivity than nitro group position on the ring, with the allyl compounds 4a and 5a having higher sensitivities to ESD than 4b and 5b. All synthesized compounds 4a–4b and 5a–5b are less sensitive than NG. The increase in spark sensitivities coupled with low melting temperatures for these new materials has the potential for further applications in niche demand environments. While compound 4a has been reported, no energetic properties were given.13
3. Conclusions
3,4- and 3,5-DNP-based compounds were substituted and explored as novel energetic materials. Each pyrazole isomer was substituted with acryl and allyl groups to the N1 nitrogen atom forming liquid explosives with low melting points, making them candidates for applications in extremely cold environments. Each compound was characterized by high-resolution mass spectrometry (HRMS), FT-IR, 1H, and 13C{1H} NMR spectroscopies. The sensitivities of the new compounds to ESD, friction, and impact indicate that the new compounds are less sensitive to impact and friction than 3,4-DNP, 3,5-DNP, and NG and have higher thermal stabilities than NG. Additionally, compounds 5a–5b have higher thermal stabilities than compounds 4a–4b, which is surprising due to the higher thermal stability of 4 compared to that of 5.
4. Experimental Methods
NMR data was collected using a Bruker Avance III 400 MHz NMR spectrometer at room temperature. Spectra were referenced to residual dimethyl sulfoxide (DMSO) (proton 2.50 ppm and carbon 39.52 ppm) or chloroform, CDCl3 (proton 7.26 ppm and carbon 77.16 ppm) with chemical shifts reported in δ values (ppm) and J values in hertz (Hz). The following abbreviations are used to describe peaks: d (doublet), brs (broad singlet), and m (multiplet). FT-IR spectra were measured with a Thermo-Nicolet iS5 FTIR spectrophotometer using OMNIC software, and data collected are reported in reciprocal centimeters (cm–1). IR spectra measurements were made using an ATR cell. DSC measurements were conducted on DSC Q-2000 from TA Instruments with temperature sweeps ranging from −80 to 400 °C. Melting temperatures were acquired using the DSC Q-2000 from TA Instruments by heating the materials to 120 °C, followed by cooling to −80 °C and warming at 10 °C·min–1 ramp to 100 °C. TGA measurements were collected from the TGA Q-5000 (TA Instruments) from 25 to 400 °C at a heating rate of 5 °C·min–1.
4.1. 3,4-DNP (4) and 3,5-DNP (5) Were Synthesized According to Literature Procedures15,16
Caution! The compounds presented are highly energetic with sensitivity to various stimuli. While we encountered no issues while working with this material, proper protective measures (Kevlar gloves, face shield, and grounded equipment) should still be used at all times.
4.1.1. Allyl-3,4-DNP (4a)
3,4-DNP (1.639 g, 0.010 mol) is added to a round-bottom flask, followed by the addition of acetonitrile (10 mL) and TEA (10 mL). The solution is stirred for 5 min at room temperature, followed by the dropwise addition of allyl bromide (1.60 mL, 0.018 mol). The reaction mixture is stirred at room temperature for another 5 min and then heated to 60 °C for 12 h. After cooling to room temperature, the reaction mixture is diluted with dichloromethane (20 mL) and DI water (20 mL) and poured into a separatory funnel. The organic layer is extracted and washed with water three times (100 mL), dried with magnesium sulfate, filtered, and rotary evaporated to give 4a as a brown oil (79% yield, 1.584 g, 0.008 mol). 1H NMR (CDCl3, 400 MHz): δ 8.69 (s, 1H), 6.06 (m, J = 6 Hz 1H), 5.45 (dd, J = 10 Hz 2H), 4.85 (d, J = 6 Hz 2H). 13C NMR (400 MHz, DMSO-d6): δ 137.3, 133.8, 127.3, 126.1, 119.2, 55.7. FTIR cm–1 3152, 1569, 1500, and 1332. Anal. Calcd: C, 36.37; H, 3.05; N, 28.28. Found: C, 34.55; H, 2.70; N, 24.77. Quadrupole time-of-flight (QTOF)-HRMS [M + CH3COO]− calcd: 257.0527 m/z; found, 257.0528 m/z.
4.1.2. Acryloyl-3,4-DNP (4b)
3,4-DNP (2.023 g, 0.013 mol) is added to a round-bottom flask, followed by the addition of acetonitrile (25 mL) and TEA (10 mL). The solution is stirred for 5 min at room temperature, followed by the dropwise addition of acryloyl chloride (1.39 mL, 0.017 mol). The reaction mixture is stirred at room temperature for another 5 min and then heated to 60 °C for 12 h. After cooling to room temperature, the reaction mixture is diluted with dichloromethane (50 mL) and DI water (30 mL) and poured into a separatory funnel. The organic layer is extracted and washed with water three times (100 mL), dried with magnesium sulfate, filtered, and rotary evaporated to give 4b as a yellow-orange oil (80% yield, 2.145 g, 0.010 mol). 1H NMR (CDCl3, 400 MHz): δ 9.03 (s, 1H), 7.45 (dd, J = 10 Hz 1H), 7.02 (d, J = 16 Hz 1H), 6.43 (d, J = 10 Hz 1H). 13C NMR (400 MHz, DMSO-d6): δ 172.05, 134.65, 133.13, 127.79, 126.31, 119.68. FTIR cm–1 3141, 1713, 1570, and 1319. Anal. Calcd: C, 33.97; H, 1.90; N, 26.41. Found: C, 30.09; H, 2.12; N, 22.68. QTOF-HRMS [M + H]− calcd: 211.0108 m/z; found, 211.0107 m/z.
4.1.3. Allyl-3,5-DNP (5a)
3,5-DNP (0.564 g, 0.004 mol) is added to a round-bottom flask, followed by the addition of acetonitrile (10 mL) and TEA (10 mL). The solution is stirred for 5 min at room temperature, followed by the dropwise addition of allyl bromide (0.50 mL, 0.006 mol). The reaction mixture is stirred at room temperature for another 5 min and then heated to 60 °C for 12 h. After cooling to room temperature, the reaction mixture is diluted with dichloromethane (50 mL) and DI water (50 mL) and poured into a separatory funnel. The organic layer is extracted and washed with water three times (50 mL), dried with magnesium sulfate, filtered, and rotary evaporated to give 5a as a yellow oil (48% yield, 0.339 g, 0.002 mol). 1H NMR (CDCl3, 400 MHz): δ 7.68 (s, 1H), 6.04 (m, J = 6 Hz 1H), 5.42 (dd, J = 5 Hz, 2H), 5.35 (d, J = 5 Hz, 2H). 13C NMR (400 MHz, CDCl3): δ 137.3, 133.7, 129.5, 121.4, 102.5, 57.1. FTIR cm–1 3156, 1572, 1507, and 1333. Anal. Calcd: C, 36.37; H, 3.05; N, 28.28. Found: C, 34.95; H, 3.13; N, 27.40. QTOF-HRMS [M + H]− calcd: 197.0316 m/z; found, 197.0316 m/z.
4.1.4. Acryloyl-3,5-DNP (5b)
3,5-DNP (0.152 g, 0.001 mol) is added to a round-bottom flask, followed by the addition of acetonitrile (10 mL) and TEA (10 mL). The solution is stirred for 5 min at room temperature, followed by the dropwise addition of acryloyl chloride (0.1 mL, 0.001 mol). The reaction mixture is stirred at room temperature for another 5 min and then heated to 60 °C for 12 h. After cooling to room temperature, the reaction mixture is diluted with dichloromethane (20 mL) and DI water (30 mL) and poured into a separatory funnel. The organic layer is extracted and washed with water three times (100 mL), dried with magnesium sulfate, filtered, and rotary evaporated to give 5b as a yellow oil [quant. yield, 0.233 g (slightly wet with dichloromethane), 0.001 mol]. Further purification can be conducted with polar solvent silica plug. 1H NMR (DMSO, 400 MHz): δ 7.95 (s, 1H), 6.23 (bm, J = 16 Hz, 1H), 6.05 (bm, 1H), 5.84 (bm, 1H). 13C NMR (400 MHz, DMSO-d6): δ 172.04, 152.29, 130.99, 129.81, 102.98, 100.19. FTIR cm–1 3154, 1703, 1562, and 1508. Anal. Calcd: C, 33.97; H, 1.90; N, 26.41. Found: C, 31.69; H, 3.10; N, 25.27. QTOF-HRMS [M + CH3COO]− calcd: 271.0320 m/z; found, 271.0397 m/z.
Acknowledgments
Funding for this work was graciously provided by Harold Agnew National Security Postdoctoral Fellowship for V.A.K. and A.H.C. We would like to thank Geoff Brown, Hongzhao Tian, Lisa Klamborowski, Chris Freye, and Taylor Dehner of the Los Alamos National Laboratory for providing thermal stability, sensitivity, HRMS, and molecular weight analyses. We would also like to thank Edward (Ed) Byrd for contributing the density and heat of formation calculations for the compounds reported. Document was released for publication under LA-UR-22-28267.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c07390.
1H and 13C NMR spectra, FT-IR data, DSC, and TGA for all compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Liu Z.; Zhao X. Application of Melt-Cast Explosive with High Solid Contents in HEAT Projectile. IOP Conf. Ser.: Mater. Sci. Eng. 2019, 493, 012026. 10.1088/1757-899X/493/1/012026. [DOI] [Google Scholar]
- Marsh N.; Marsh A. A Short History of Nitroglycerine and Nitric Oxide in Pharmacology and Physiology. Clin. Exp. Pharmacol. Physiol. 2000, 27, 313–319. 10.1046/j.1440-1681.2000.03240.x. [DOI] [PubMed] [Google Scholar]
- Kumari D.; Balakshe R.; Banerjee S.; Singh H. Energetic plasticizers for gun & rocket propellants. Rev. J. Chem. 2012, 2, 240–262. 10.1134/s207997801203003x. [DOI] [Google Scholar]
- Fordham S.High Explosives and Propellants; Pergamon Press: Oxford, 1996; Vol. I. [Google Scholar]
- a Stepanov A. I.; Sannikov V. S.; Dashko D. V.; Roslyakov A. G.; Astrat’ev A. A.; Stepanova E. V. A rational method of synthesis and chemical properties of 5-(4-aminofurazan-3-yl)-1-hydroxytetrazole. Chem. Heterocycl. Compd. 2017, 53, 746–759. 10.1007/s10593-017-2121-x. [DOI] [Google Scholar]; b Schulze M. C.; Scott B. L.; Chavez D. E. A high density pyrazolo-triazine explosive (PTX). J. Mater. Chem. A 2015, 3, 17963–17965. 10.1039/c5ta05291b. [DOI] [Google Scholar]
- Göbel M.; Klapötke T. M. Development and Testing of Energetic Materials: The Concept of High Densities Based on the Trinitroethyl Functionality. Adv. Funct. Mater. 2009, 19, 347–365. 10.1002/adfm.200801389. [DOI] [Google Scholar]
- Zhang S.; Gao Z.; Lan D.; Jia Q.; Liu N.; Zhang J.; Kou K. Recent Advances in Synthesis and Properties of Nitrated-Pyrazoles Based Energetic Compounds. Molecules 2020, 25, 3475. 10.3390/molecules25153475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J.; Tang Y.; Cheng G.; Imler G. H.; Parrish D. A.; Shreeve J. M. Energetic Derivatives of 8-Nitropyrazolo[1,5- a ] [1,3,5]Triazine-2,4,7-Triamine: Achieving Balanced Explosives by Fusing Pyrazole with Triazine. Org. Lett. 2020, 22, 1321–1325. 10.1021/acs.orglett.9b04642. [DOI] [PubMed] [Google Scholar]
- Xue H.; Gao Y.; Twamley B.; Shreeve J. M. New Energetic Salts Based on Nitrogen-Containing Heterocycles. Chem. Mater. 2005, 17, 191–198. 10.1021/cm048864x. [DOI] [Google Scholar]
- Yin P.; Zhang J.; He C.; Parrish D. A.; Shreeve J. M. Polynitro-Substituted Pyrazoles and Triazoles as Potential Energetic Materials and Oxidizers. J. Mater. Chem. A 2014, 2, 3200. 10.1039/c3ta15057g. [DOI] [Google Scholar]
- Tingxing Z.; Lei L.; Zhan D.; Yong Z.; Guangquan Z.; Ming H.; Hongbo L. Research Progress on the Synthesis of Energetic Nitroazoles. Chin. J. Org. Chem. 2014, 34, 304–315. 10.6023/cjoc201307050. [DOI] [Google Scholar]
- Zhang Y.; Parrish D. A.; Shreeve J. M. 4-nitramino-3,5-dinitropyrazole-based Energetic Salts. Chem.—Eur. J. 2012, 18, 987–994. 10.1002/chem.201102773. [DOI] [PubMed] [Google Scholar]
- Ek S.; Wahlström Y. L.; Latypov N. J. Chem. Chem. Eng. 2011, 5, 929–935. [Google Scholar]
- Janssen J. W. A. M.; Koeners H. J.; Kruse C. G.; Habrakern C. L. Pyrazoles. XII. Preparation of 3(5)-Nitropyrazoles by Thermal Rearrangement of N-Nitropyrazoles. J. Org. Chem. 1973, 38, 1777–1782. 10.1021/jo00950a001. [DOI] [Google Scholar]
- Biffin M. E. C.; Brown D. J.; Porter Q. N. A Novel Route from 4-methoxy-5-nitropyrimidine to 3-amino-4-nitropyrazole and Pyrazolo[3,4-b]pyrazine. Tetrahedron Lett. 1967, 8, 2029–2031. 10.1016/s0040-4039(00)90779-2. [DOI] [Google Scholar]
- Cho J. R.; Kim K. J.; Cho S. G.; Kim J. K. Synthesis and Characterization of 1-Methyl-2,4,5-Trinitroimidazole (MTNI). J. Heterocycl. Chem. 2002, 39, 141–147. 10.1002/jhet.5570390121. [DOI] [Google Scholar]
- Byrd E. F. C.; Rice B. M. Improved Prediction of Heats of Formation of Energetic Materials Using Quantum Mechanical Calculations. J. Phys. Chem. A 2006, 110, 1005–1013. 10.1021/jp0536192. [DOI] [PubMed] [Google Scholar]
- a Byrd E. F. C.; Rice B. M. Improved Prediction of Heats of Formation of Energetic Materials Using Quantum Mechanical Calculations. J. Phys. Chem. A 2009, 113, 5813. 10.1021/jp806520b. [DOI] [PubMed] [Google Scholar]; J. Phys. Chem. A 2022, 126, 1787; Fried L. A.; Howard W. M.; Bastea S.; Glaesmann K.; Souers P. C.; Vitello P. A.; Kuo L. F.. CHEETAH Thermochemical Code; Lawrence Livermore National Laboratory: Livermore, CA.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.