Abstract
Nanotechnology has yielded nanostructure-based drug delivery approaches, among which nanofibers have been explored and researched for the potential topical delivery of therapeutics. Nanofibers are filaments or thread-like structures in the nanometer size range that are fabricated using various polymers, such as natural or synthetic polymers or their combination. The size or diameter of the nanofibers depends upon the polymers, the techniques of preparation, and the design specification. The four major processing techniques, phase separation, self-assembly, template synthesis, and electrospinning, are most commonly used for the fabrication of nanofibers. Nanofibers have a unique structure that needs a multimethod approach to study their morphology and characterization parameters. They are gaining attention as drug delivery carriers, and the substantially vast surface area of the skin makes it a potentially promising strategy for topical drug products for various skin disorders such as psoriasis, skin cancers, skin wounds, bacterial and fungal infections, etc. However, the large-scale production of nanofibers with desired properties remains challenging, as the widely used electrospinning processes have certain limitations, such as poor yield, use of high voltage, and difficulty in achieving in situ nanofiber deposition on various substrates. This review highlights the insights into fabrication strategies, applications, recent clinical trials, and patents of nanofibers for different skin disorders in detail. Additionally, it discusses case studies of its effective utilization in the treatment of various skin disorders for a better understanding for readers.
1. Introduction
Skin as the outermost sheath of the human body serves as a protective barrier from the outer environment, microbial infections, and other hazards like UV radiation. The skin is composed of three different layers: the epidermis, the dermis, and the hypodermis which are responsible for different functions and ultimately maintain homeostasis.1 It provides physiological and mechanical support to the underlying cells and organs and prevents the entry of foreign substances. Any kind of damage and infections can destroy this protective layer, causing redness, itchiness, and inflammation resulting in different skin conditions and autoimmune skin disorders like atopic dermatitis, ichthyosis, acne, scleroderma, epidermolysis bullosa, psoriasis, vitiligo, pemphigus, etc.2 Mostly, in these disorders, topical delivery of drugs becomes the first line of therapy to achieve localized or targeted therapeutic action. Decrease in systemic side effects, avoidance of first-pass metabolism, ability to provide site-specific delivery, and minimization of dosing frequency by boosting the drug levels at the point of action are some of the noted advantages of topical drug delivery approaches.3 Also, the large surface area of the skin makes it a potential route for the delivery of pharmaceutical products like local anesthetics, antiseptics, antibacterial, antifungal, and anti-inflammatory agents for localized therapeutic action.4
The skin itself acts as a major challenge for topical drug delivery as it restricts the entry of the therapeutic substances to reach the target site, hence scientists have been looking for ways to make topical drug delivery more effective. Creams and ointments are the most commonly used conventional topical formulations with limited permeation and retention. Hence, permeation enhancers have been used in the conventional formulations, which cause side effects like irritation due to their high concentration and disrupt the skin barriers, temporarily inviting chances of further infections.5 In searching for other alternatives, researchers have found that small molecules can easily permeate through the skin, and hence efforts have been put into exploring the use of nanotechnology, particularly nanofibers and nanoparticulate systems for transdermal and topical delivery of the therapeutics.6
Nanofibers are nanostructures that are fibrous in nature, forming threads of size ranging from micrometers to a few nanometers and exhibiting a high ratio of surface area to volume, allowing both hydrophilic and hydrophobic drugs to be delivered efficiently. It shows tunable porosity, and biological and therapeutic molecules can be easily functionalized on them.7 Polymers, ceramics, and their mixtures are among the numerous materials that are utilized to produce nanofibers. They are commonly fabricated from natural or synthetic polymers or their blend as they offer a wide range of therapeutic applications. For greater permeability through different channels of the skin, size reduction of the drug carriers acts as a rule of thumb. The distribution of the active therapeutic moiety using nanofibers works on the principle of increased drug dissolution rate because of the increased surface area and exposure of the drug-loaded carriers as a consequence of small particle size.8
Nanofibers have shown the ability to improve physicochemical properties and provide good penetration of drugs due to their nanosize. In an experiment, hydroxypropyl-β-cyclodextrin (HPBCD) and polyvinylpyrrolidone (PVP) nanofibers loaded with a drug (resveratrol) exhibited improved aqueous solubility as compared to pure resveratrol by more than 20,000-fold due to the conversion of a crystalline form of the drug to an amorphous form (intermolecular bond formation) following nanofiber fabrication. Additionally, the skin permeation study was performed using Franz diffusion cells, which showed that nanofiber formulation resulted in higher penetration into the epidermis and dermis layers than pure resveratrol and thus displayed good anti-inflammatory activity.9
Generally, in nanofibers, the drug molecules are entrapped between the mesh frameworks formed by the polymeric nanofibers and therefore can provide controlled delivery of drugs. Jalvandi et al. through a study revealed that designing nanofibers using poly(vinyl alcohol) (PVA) resulted in a controlled release approach of the drug (levofloxacin (LVF)). The drug, being covalently attached to chitosan via a cleavable amide bond, was electrospun with PVA to form nanofibers. They found 27% release in the case of the PVA-conjugated chitosan-loaded LVF nanofiber-mats as compared to 90% release with PVA-LVF (control) after 8 h which exhibited a reduction of in vitro burst release. The possible mechanism behind this was inferred to be the amide bond between LVF and chitosan which hydrolyzed at neutral pH, resulting in a controlled release over 72 h.10 The findings suggested that drug conjugation to the polymer backbone is an effective method to achieve prolonged delivery with reduced burst release of the drug.
Using the nanofiber sandwich technology, prolonged release of drugs can be achieved. In this technology, a drug-loaded layer of nanofibers is put in between the drug-free nanofiber layers. The external layers which are devoid of the drug act as a barrier and are thus responsible for prolonged drug release.11 Recently, a group of researchers prepared a multilayered electrospun nanofibers mesh in which the gelatin nanofiber with drug was sandwiched with another layer of nanofibers without drug with cross-linking. Results showed a zero-order drug release up to 48 h due to the formation of a diffusional barrier.12 This shows that nanofibers may be modulated to design the drug release profile to suit the desired individual clinical application. Another advantage of nanofibers is the possibility to modify the nanofibers’ functionality through surface modification.13 Gautam et al. prepared a tripolymer composite nanofibrous scaffold by modifying the surface of nanofibers with collagen type I grafting which improved the fibroblast and keratinocyte cells adhesion and ensured good cell proliferation as found in the study for wound healing and skin tissue regeneration.14 The modulation in drug release can be achieved with a change in nanofibers diameter and the effective contact surface area15
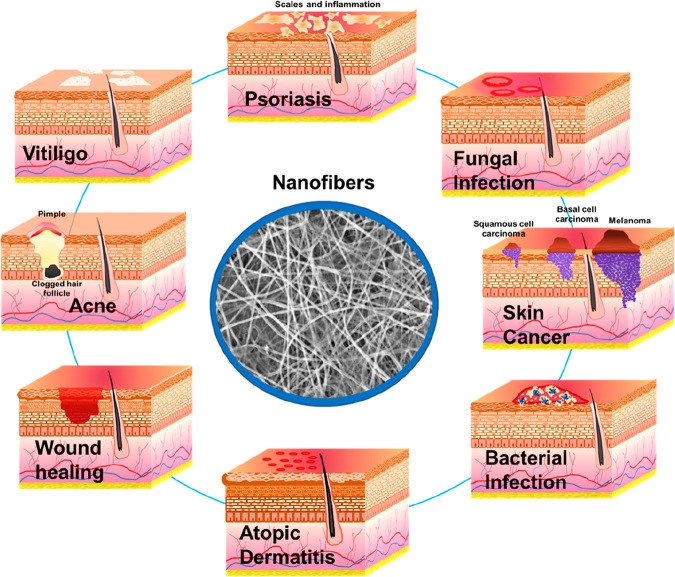
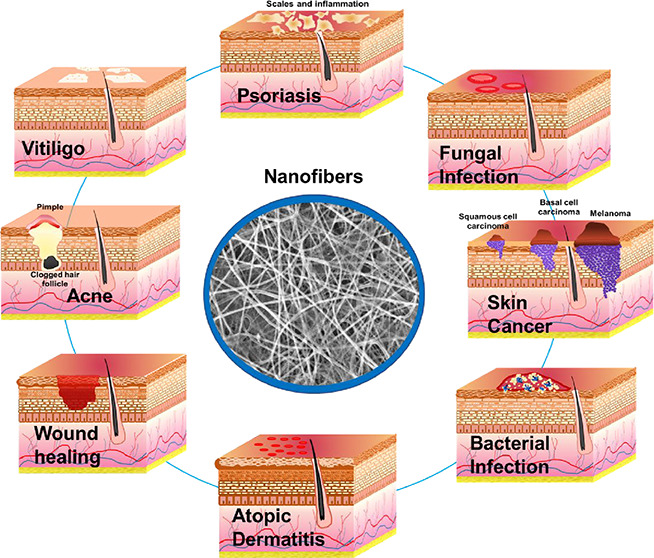
Smart nanofibers, also known as on-demand drug release systems, are the most recent advanced drug delivery systems designed to release drugs due to stimuli such as temperature, enzyme, pH, etc.16 In 2021, researchers fabricated a ketoconazole-loaded temperature-responsive nanofibrous film made of poly(N-isopropylacrylamide) (PNIPAM) and polycaprolactone which showed a shift from a hydrophilic to a hydrophobic condition when the temperature increased from 25 to 37 °C (room temperature to body temperature). This resulted in a sustained drug release behavior at body temperature and with a lower temperature resulting in faster drug delivery.17 Similarly blends of PVA and PNIPAM nanofibrous membranes were fabricated for delivering Levothyroxine topically with temperature change.18 Hence, with the help of temperature-responsive properties, the fabricated nanofibers are useful for both the on-demand and prolonged drug release approach. Therefore, nanofibers with their unique properties of high porosity, good interconnectivity, and the flexibility of molding the nanofiber meshes are suitable for topical delivery of therapeutics in the management of skin cancer, bacterial infections, inflammatory conditions, and wounds.19 Several published reports are available on materials and techniques utilized in the fabrication of nanofibers and the individual application of nanofibers in the management of specific skin disorders. However, there is a need to bring intense attention to the utilization of nanofibers in the management of various skin disorders in one place. Therefore, in this work, the authors provide insights into the compilation of the application of nanofibers in therapeutic delivery in skin disorders such as wound healing, bacterial infection, fungal infection, psoriasis, acne, atopic dermatitis, vitiligo, and skin cancer (Figure 1). Additionally, various case studies, clinical trials, and patents are compiled for extensive knowledge to readers.
Figure 1.
Application of nanofibers for topical delivery of therapeutics in skin disorders.
2. Fabrication of Nanofibers
Nanofibers are mainly fabricated using different types of polymers. The size or diameter of the nanofibers depends upon the polymers, the techniques of preparation, and the design specification.
2.1. Materials Utilized in the Fabrication of Nanofibers
Numerous polymers have been employed to develop nanofibers, which can be broadly divided into three categories: natural, synthetic, and mixed or blended polymers.
2.1.1. Natural Polymers
Polymers of natural origin are mainly utilized in the preparation of nanofibers due to the advantages they offer such as being biodegradable, biocompatible, and nontoxic.
Natural origin polymers belonging to proteins such as gelatin, collagen, and polysaccharides like chitosan, cellulose, dextran, hyaluronic acid, and alginate are most commonly used in nanofibers so far with a wide range of applications.20 Gelatin electrospun nanofibers scaffold containing tellurium nanoparticles were developed for wound dressing to avoid immunogenic responses due to the natural origin of gelatin.21 Also in another experiment, gelatin was combined with bacterial cellulose to produce nanofibers and was tested for diabetic wound healing achieving a system made up of an all-natural polymer construct.22
2.1.2. Synthetic Polymers
Polymers derived synthetically have also been employed in making nanofibers as they can be easily modified according to their requirements during the synthesis process to offer mechanical strength, stability, durability, and other properties.23 Although their biocompatibility is low, they are easy to produce, and their reproducibility gives them an advantage over natural polymers. Some examples of synthetic polymers that have found their application in nanofibers are polylactic acid, PVA, poly(ethylene oxide), polycaprolactone, poly(lactic-co-glycolic acid), PVP, etc. Zinc oxide and silver bimetallic nanoparticles encapsulated in PVP, and polycaprolactone-based nanofibers have shown enhanced antibacterial activity.24 PVA nanofibers loaded with cinnamaldehyde phytoconstituent were fabricated to tackle the irritant effect associated with cinnamaldehyde and also to boost its therapeutic efficacy for eradicating Candida biofilm by solving the problem of high volatility and low aqueous solubility.25 In a recent development, antimicrobial peptides (AMPs) were also fabricated as nanofibers using poly(ethylene oxide) for the purpose of wound healing.26
2.1.3. Mixed or Blended Polymers
Mixed polymers are combinations of both natural-origin and synthetically made polymers used together to fabricate nanofibers. They have been blended and used to overcome the limitations of each other by modifying their properties, such as the biocompatibility of natural polymers with the high mechanical strength of synthetic polymers. For example, polycaprolactone, chitosan, and PVA were used together in nanofibers for treating burns and cuts, whereas Ferulic acid-loaded collagen hydrolysate and polycaprolactone nanofibers were developed.27 Even two different synthetic or natural polymers can be blended together for the preparation of nanofibers. Amphotericin B-loaded coaxial nanofibers were fabricated using PVA, chitosan, poly(ethylene oxide), and gelatin to make a new dressing for cutaneous leishmaniasis and superficial mycoses.28 As a result, polymers are crucial in the development of nanofibers that serve the intended applications.
2.2. Preparation Methods
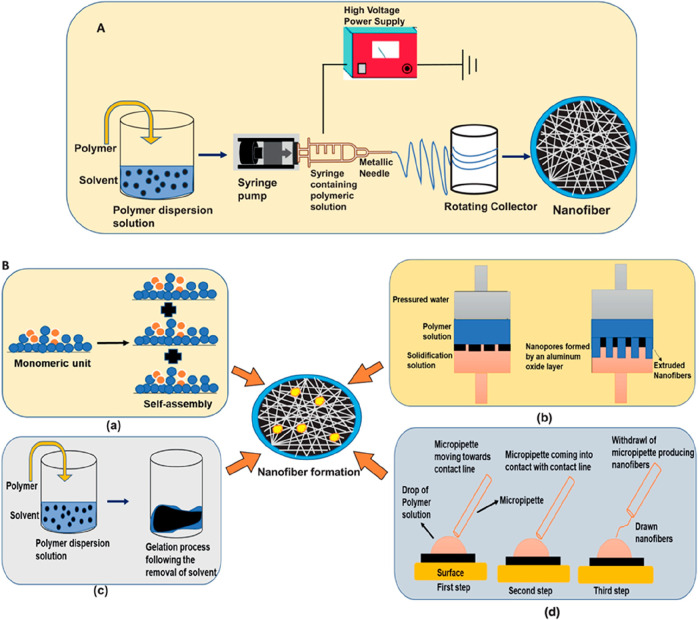
Nanofibers are prepared using different processing approaches.29 However, the four major fabrication techniques, i.e., self-assembly method, phase separation technique, template synthesis, and electrospinning approach are widely utilized for biomedical applications (Figure 2).8 Electrospinning is one of the convenient techniques which has been used industrially for commercial-scale production, and it has provided numerous ways to control the properties of the produced nanofibers as a result of its instrumental setup and its influencing parameters.30 There is one other technique, i.e., the drawing technique, that mainly utilizes viscoelastic materials for the fabrication of nanofibers and is also limited to only a laboratory scale.8 A comparison between various fabrication techniques is included in Table 1.
Figure 2.
(A) Schematic representation of the fabrication of nanofibers by the electrospinning technique. (B) Different fabrication techniques of nanofibers: (a) self-assembly, (b) template synthesis, (c) phase separation, and (d) drawing. Adapted with permission from ref (8). Copyright 2022 Elsevier.
Table 1. Comparison between Different Methods Used for Fabricating Nanofibers.
| Method | Characteristics | Advantages | Limitations |
|---|---|---|---|
| Self-Assembly Method31 | • Bottom-up process where individual molecules are arranged in specific ways to generate macromolecular nanofibers. | Molecules can gather into organized structures like monolayers, tubes, or porous structures such as honeycomb microfilms. | Complex process and takes a longer time for preparation. |
| • Yields nanofibers in the 100 nm range. | |||
| • Intermolecular interactions connecting these molecules determine the shape of the nanofiber. | |||
| Phase Separation Method32 | • Dissolution of polymer, gel formation, extraction of solvent, and freeze-drying are the fundamental phases involved in this technique. | This approach produces nanofibers with a 50–500 nm diameter and a shorter length. | Time-consuming and tiresome procedure. |
| • The instability or physical incompatibility of the polymer within the solvent causes them to split into two distinct phases. | |||
| • Changing polymer composition can change the properties of nanofibers. | |||
| Template Method33 | • Targeting material is placed in contact with a nanostructured ceramic or polymeric membrane used as a template to form nanofibers. | Uses commercially available nanoporous membranes as templates to fabricate nanoscale fibers 100s nm in size. | Not scalable. |
| • Anodized aluminum oxide (AAO) and silica are commonly used ceramic templates which are easily available. Fiber characteristics can be changed using various templates. | |||
| Electrospinning34 | • Top-down methodwhich has the ability to fabricate fibers with diameters from a few nanometers to several microns. | • Cost-effective as compared to others, scalable. | Jet instability, toxic solvents used, and handling not easy. |
| • Various factors which affect the size and shape of nanofibers produced are: polymer type, applied operating voltage, the distance between the collector and the tip of the needle, polymeric solution, concentration, its viscosity and flow rate, solution conductivity, temperature conditions, etc. | • Possible to control the thickness, composition, and porosity of nanofiber meshes. |
2.3. Loading of Drugs in Nanofibers and Drug Release Mechanism
For developing drug delivery systems, drugs can be loaded onto nanofibers using physical adsorption, blend-electrospinning, coelectrospinning, and covalent immobilization methods.35 The drug loading process is determined through the drug’s characteristics and the application of the systems. Different drug release patterns are expected depending on the drug loading mechanism within or onto the nanofibers. The most crucial method for drug loading into nanofibers is blending the medicament with a polymeric solution using an electrospinning (single-phase) process.36 The compatibility of the drug and polymer, as well as their physicochemical characteristics, must be taken into account in order to ensure effective drug encapsulation, drug distribution, and optimal release kinetics. Hydrophilic drugs are loaded into hydrophilic polymer solutions, whereas lipophilic drugs are placed into lipophilic polymer solutions. An alternative technique includes chemically or physically immobilizing the molecule. Dipping nanofibers into the drug solution causes physical adsorption, which includes electrostatic interaction.37 However, the drug release profile cannot be controlled with this approach. Chemical processes involve infusing nanofibers with acid or base for a predetermined amount of time. Through the chemical alteration of the functional groups on the surface, the drug molecule is covalently bonded to the nanofibers’ surface.38
Emulsion and coaxial electrospinning techniques have recently acquired popularity, owing to a lower risk of first burst release and superior shielding of loaded drugs.36 These techniques yield nanofibers with a core–shell structure. A spinneret with separate chambers for each of the components is used to load the polymer and drug solution. The active moiety is delimited in the central core of the coaxial nanofibers, which is surrounded by a polymer shell.39 The drug solution and the polymer solution form the aqueous and oil phase, respectively, in the emulsion electrospinning. When employing low molecular weight drugs, the drug disperses inside the fibers after electrospinning. However, with macromolecules, the drug forms a core–shell kind of nanofibrous structure. The emulsification process may cause delicate medicinal substances like plasmid DNA or proteins to degrade, which is a drawback of this approach.40 Coaxial electrospinning has been referred to as a new technique to prevent initial burst release, and instability as the drug or bioactive agents are incorporated into a double-layered nanofiber structure. It is a two-fluid electrospinning technique where the structures have shown a long-lasting release profile due to the absence of any free drug molecules on or near the exposed surfaces of the nanofibers. This encapsulation not only enables sustained drug release but also shields the agent from outside influences and supports the maintenance of its biological activity.41
Degradation, diffusion, or swelling are the mechanisms involved in releasing the drug from the polymeric network. Physical and chemical degradation are the two ways by which polymer degradation or erosion of polymeric carrier occurs. In some cases, enzymatic cleavage, hydrolytic cleavage, or oxidation may happen to convert the polymeric fibers into small monomers or oligomers.42 These parameters also recommend the duration of time the nanofibers can be applied topically. However, it also depends upon the specific product and the type of drug delivery. Some nanofiber-based drug delivery systems with a sustained release over an extended period of time, such as for several hours or even days, can be left on the skin for a longer duration to ensure a steady release of the drug. The recommended duration of application also depends on the dose and concentration of the drug being delivered, as well as the condition being treated. For example, a wound dressing containing nanofibers with an antibacterial drug may need to be applied for a longer period of time to ensure complete eradication of the infection.21 For a controlled release profile, a diffusion-based mechanism is generally exhibited, where an encapsulated drug is released by diffusion along with matrix erosion.43 Smart nanofibers diffuse the drug in response to stimuli or any change in pH, temperature, enzyme, light, and other conditions. The factors which influence the release mechanism or behavior are the polymer mixture composition, polymeric blend interaction, and the methods of preparation.44
3. Therapeutic Applications of Nanofibers
Encapsulating active molecules in nanocarrier drug delivery systems is a viable strategy for reducing side effects, improving solubility, achieving controlled release, improving biocompatibility, and also preventing drug degradation.45 Additionally, nanofibers are designed to have biological activity on their own or to be integrated with and utilized to administer active pharmaceutical ingredients in a controlled, regulated way. Incorporating biological products such as cells, microbes, proteins and peptides, genes, and nucleic acids as well as drugs needs the employment of diverse loading techniques to achieve the required release profile.46 Several studies have been summarized in Table 2 exploring nanofibers as a potential therapeutic delivery system for skin disorders.
Table 2. Summarized Topical Applications of Nanofibers in Skin Disorders.
| Disease | Active Moiety Incorporated | Polymers Used and Fabricating Technique | Outcomes |
|---|---|---|---|
| Antifungal | Eucalyptol | Ellan/polyvinyl alcohol (Electrospinning) | • Smooth, bead-free, uniform nanofibers with 219.23 ± 30.93 nm as average diameter and 40 ± 6.2% of encapsulation efficiency were prepared. |
| • 67–74% developing biofilms inhibition and 45–47% matured biofilms eradication of Candida strains. | |||
| • Enhanced antifungal activity due to nanofibers hydrophobic encapsulation of oils decreasing its degradation and increasing its therapeutic efficacy.84 | |||
| Ketoconazole | Polycaprolactone (Electrospinning) | • Ketoconazole-functionalized nanofibers (with a mean diameter of 526 ± 148 nm) consistently inhibited more fungal growth as compared to the pure drug. | |
| • The drug load was found to be 45.3 ± 1.7 μg of ketoconazole per mg of nanofiber. | |||
| • Enhanced the activity of a poorly water-soluble drug.85 | |||
| Wound dressing/healing | Ciprofloxacin hydrochloride | Polyurethane and soy protein (Electrospinning) | • Biocompatible, flexible nanofibers with an average diameter of 312 nm, tensile strength of 4.5 MPa, and tensile strain of 105.5%. |
| • Antibactericidal activity against Gram-positive (inhibition rate about 70%) and Gram-negative bacteria (inhibition rate about 90%) and efficiency in wound healing.86 | |||
| Chlorhexidine acetate | Polyurethane/clay nanocomposite (Electrospinning) | • Nanofiber diameter ranging between 325 and 375 nm with a mean pore diameter of the nanofibrous web as 0.357 μm. | |
| • With 5% of drug loading, nanofibers exhibited a maximum of 91% drug release at pH 7.4. | |||
| • Maintained wound moisture, prevented dehydration during wound healing, and sustained release with long-term activity.87 | |||
| Doxycycline (DCH) | Polylactide (PLA) (Electrospinning) | • The bead-free, continuous, and smooth nanofibers were obtained with 5–30% of loading efficiency. Also, it was observed that with an increase in loading to 30%, the DCH-encapsulated nanofibers’ mean diameter also increased to 424 ± 62nm. | |
| • Exhibited faster healing due to half of the wound beds being filled with tissue, and it regenerated epidermis.88 | |||
| Psoriasis | Methotrexate magnetic nanoparticles | Polyvinyl alcohol polymer (Electrospinning) | • Smooth surface and uniform fibers with 330 to 480 nm diameter range and 88.4% drug entrapment efficiency. |
| • Smart and sensitive stimuli-responsive release of drugs (5 min of alternate magnetic field released ∼10% of the drug as temperature increased by ∼5 °C) in a controlled manner (once a day, h, etc.).89 | |||
| Acne | Clindamycin | Polyvinyl alcohol and tamarind seed gum (Electrohydrodynamic atomization, i.e., electrospraying and electrospinning) | • Fabricated nanofibers with smooth surfaces had an average diameter between 202.65 ± 26.45 nm and 277.80 ± 27.05 nm depending upon the voltage applied, PVA concentration, and amount of clindamycin loaded (range between 1 to 2.5% w/w) into it. |
| • Drug-loaded fibers showed slightly greater antibacterial activity, with a bacterial inhibition zone approximately greater than 3 cm2 as compared to commercial 1% clindamycin gel (inhibition zone around 2.7 cm2).90 | |||
| Zinc oxide nanoparticles | Polyvinyl alcohol cross-linked by citric acid (Electrospinning) | • Nanofibers with 325 ± 48 nm diameter for 7 wt % of ZnO concentration incorporated and swelling ratio 780 ± 58:77%, suggesting its potential use as a facial mask. | |
| • PVA/ZnO (7%) reported antibacterial activity against Staphylococcus aureus and Candida acne bacterial strains with 1.5 mm and 2.25 mm inhibition zones, respectively.91 | |||
| Resveratrol nanocrystals | Polycaprolactone (Single nozzle electrospinning) | • Nanofibers with mean diameters of 1457 ± 648 nm and 1506 ± 527 nm for 0.2 mg/cm2 and 1 mg/cm2 nanocrystals, respectively, with 89.32 ± 1.0% and 71.73 ± 9.0% adsorption efficiency, respectively. | |
| • Effective antimicrobial activity against acne with 1.3 ± 0.02 cm and 1.6 ± 0.1 cm inhibition zone, respectively.92 | |||
| Atopic dermatitis | Pioglitazone | Polyvinylpyrrolidone (Electrospinning) | • Nanofiber mats with 0.230 ± 0.0196 mm as the mean thickness, and 82 to 88% drug loading efficiency. |
| • 5-fold enhanced drug permeation flux and highly retained (677.7 mg/cm2) in the epidermis skin layer as compared to casted film.93 | |||
| Natural oils (borage, black cumin seed, and evening primrose oil) | Poly(vinyl butyral-co-vinyl alcohol-co-vinyl acetate) (PVB) | • 335 ± 86 nm was the average diameter of the prepared nanofibers with a porosity value of 92.6%, which affected the sorption, helped in the adhesion of oils with the skin, and provided better permeability. | |
| • The average maximum tensile stress and the average maximum elongation of the nanofibers were observed to be 0.66 ± 0.11 MPa and 59 ± 9% respectively. | |||
| • In direct contact with fibroblasts, nanofibers exhibited high biocompatibility, with the rapid spread of oils aiding oil delivery for the desired period of time providing moisturization to the skin.94 | |||
| Skin Cancer | Doxorubicin (Dox) hydrochloride | Polycaprolactone (Electrospinning) | • Fe3O4 magnetic nanoparticles encapsulated polycaprolactone nanofibrous mat-based bandages with diameters in the range of 100–1000 nm. |
| • 20 μg of Dox was incorporated in 10 mg of a fibrous mat, and a 1.8-fold higher release of Dox was observed in the presence of a magnetic field as compared to normal. | |||
| • Irreversible necrotic tumor cell death was observed through 5 heating doses and specific parameters, and the treated mice exhibited complete recovery within 2 weeks of the treatment. | |||
| • Designed bandages dissipated heat energy locally and enhanced the activity of the drug through elevated temperature, which may potentially kill Dox-resistant cells as well.95 | |||
| Gold nanoparticles (AuNPs) and curcumin | Polyvinyl alcohol (PVA) and polycaprolactone (PCL) (Electrospinning) | • Smooth and continuous PVA loaded with AuNPs and PCL loaded with curcumin nanofibers were produced in the diameter range of 300 nm and 600–800 nm, respectively, with tensile strengths of 4.5 MPa and 2–3 MPa, respectively. | |
| • High drug entrapment efficiency of 95.60% was observed in the case of PCL–curcumin nanofibers. | |||
| • Reported cancer cell selective toxicity by the mechanism of apoptosis and lesser toxicity on normal cells better than as compared to free formulations due to sustained release with high antioxidant efficacy.96 | |||
| Bioactive compounds of Terminalia catappa (TC) (extract) | Sodium alginate (SA) (Electrospinning) | • Nanofibers formed with interconnecting pores. | |
| • In SA+TC, nanofibrous scaffold cell death was found to be at a maximum with an approximately 2-fold increase in the expression of Bax, Cyt C, p53, p21, Cas9, and Cas3 genes (apoptosis markers).97 | |||
| Doxorubicin hydrochloride | Chitosan/cobalt ferrite/titanium oxide (Electrospinning) | • Smooth, uniform nanofibers 110 nm in diameter on average and with 96.5 ± 1% drug loading efficiency. | |
| • At acidic pH, the release of the drug was faster (more than 40% in 10 h) in the presence of magnetic field from the magnetic nanofibers as compared to physiological pH, showing efficacy toward tumor cells with an acidic environment. | |||
| • Highest cytotoxicity and improved antitumor efficacy due to synergism in the case of the designed nanofibers with 58% and 78% cell deaths after 2 and 3 days, respectively.98 |
3.1. Nanofibers in Wound Healing and Surgical Dressings
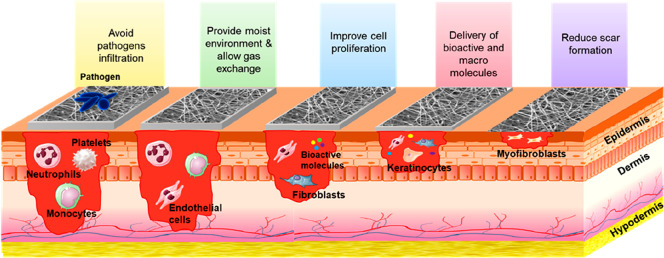
Wound dressings are used for treating injuries and wounds. However, standard dressings are inefficient since they restrict the restoration of the wounded skin’s structure and functions, which can lead to wound infection and dehydration and also interfere with the healing process as a whole.47 As a result, there is a need for novel wound dressings that could promote skin regeneration as well. Electrospun polymeric nanofibers have been viewed as promising materials for enhancing skin regeneration because of their structural resemblance to the extracellular matrix of healthy skin, ability to stimulate cell growth and proliferation, bacteriostatic activity, and suitability to deliver bioactive molecules to the wound site as shown in Figure 3. In a study conducted by Doostmohammadi et al., in vivo wound healing capabilities of tellurium nanoparticles embedded in polycaprolactone and gelatin nanofibers (electrospun) were investigated on wounds of Wistar rats using histopathology examination.21 Scaffolds containing nanoparticles showed the maximum healing with a score of 15 out of 19. In addition, the stated scaffold had dose-dependent beneficial effects on collagen production and collagen horizontalization and decreased the edema and inflammation at the injury site. The results obtained strongly confirmed their healing activity, which can be useful for wound dressing.21
Figure 3.
Representation of the properties that electrospun membranes must display to be used as wound dressings.
In addition to promoting cellular motility and morphological changes at wound sites, the topographic surface conditions of scaffolds also impact cellular behavior by promoting tissue regeneration.48 Controlling the shape and morphology of fibers and selecting combinations that do not disrupt regular physiological function are critical for the production of scaffolds in tissue engineering applications. Collagen, a well-known biopolymer, is commonly used in electrospinning nanofiber compositions. It is the most prevalent protein in the human body with a high degree of in vivo stability and can also maintain an excellent biomechanical strength over time; thus, it is mostly used to prepare scaffolds.49 Some researchers formed ferulic acid (FA)-loaded polycaprolactone and collagen hydrolysate (CH)-based composite electrospun nanofibers to treat chronic wounds. The average tensile strength of the nanofibers was 0.46 ± 0.3 MPa, and CH addition boosted their hydrophilicity (with a reduced contact angle to 58.3°), which improved cell adhesion and increased fibroblast proliferation, which led to cell growth and regeneration of injured tissue.27 Thus, in addition to the objective of the study, to find the wound healing efficacy, it also resulted that the prepared system could be used as a protective cover for wounds.
Biomolecules and natural substances have also been incorporated into the nanofibers to increase their effectiveness. In 2015, a group of scientists synthesized N,O-carboxymethyl chitosan (CMCS) and poly(ethylene oxide) blend nanofibrous samples and mixed them with phenytoin sodium and vitamin C to make wound dressings. As compared to the phenytoin sodium and vitamin C 1% ointment, the nanofiber formulation showed better efficiency in wound healing as it was observed that the wound area was decreased to 12.5% (in the case of ointment) and 3.8% (in the case of nanofibers) after 14 days. Also, the wounds treated with optimized nanofiber formulation resulted in epidermal layer regeneration because of the generation of granulating tissues without necrosis before collagen fiber accumulation.50 Chinatangkul et al.51 performed an experiment in which Shellac and polyvinylpyrrolidone (SHL-PVP) blended fibers were used to fabricate a desirable wound dressing material with a natural antimicrobial lipid, monolaurin (ML) to avoid microbial infection-induced delayed wound healing. It was found that the presence of PVP improved drug loading capacity (the maxiumum concentration of ML loaded onto SHL fibers without polymers was 7% w/w, but with PVP addition 5%–20% w/w could be completely encapsulated according to Powder X-ray diffractometry results). The manufactured fibers had excellent antimicrobial action against Staphylococcus aureus and Candida albicans, as well as improved cell adherence was observed. The nanofibers’ size-dependent antimicrobial efficacy was also reported as improved activity with finer SHL-PVP blended fibers at 27 kV than those prepared at 9 kV attributing to the fact that high specific surface area with the smaller diameter fibers leads to enhanced release of the drug.51
In a recent study by Zhang et al.,52 dendritic mesoporous bioglass nanoparticles loaded with 5-fluorouracil were prepared and fabricated in poly(ethylene oxide) and poly(ether-ester-urethane) urea-based coaxially electrospun nanofibers. The nanoparticles with 23% loading efficiency were found to have a smooth surface and good adsorption rate to protein and also showed similar mechanical properties and degradation rates when compared to autologous skin. The nanofibers exhibited a prolonged drug release profile for 20 days closely related to its degradation profile. The therapeutic effectiveness of nanofibers was evaluated in a rat wound model and with collagen I labeling. All of the findings demonstrated that 5- fluorouracil-loaded nanofibers showed a superior wound therapy influence for the reconstruction of scar skin tissue, implying the significant potential for clinical wound healing.52
3.2. Nanofibers in Treatment of Bacterial Infections
Bacterial infections may occur in different skin conditions as the amount of bacteria on the surfaces of the human body is said to be equivalent to the number of human cells. Microbes are effortlessly capable of triggering an infection by permeating the skin or mucosa, and nanofibers can be applied as a film that acts as a barrier to further infections and prevents their growth by releasing the therapeutic moiety over a period of time.53 In an experimental study, vancomycin-loaded nanofibers were applied on the wound of an animal model topically and checked for topical skin infections treatment against methicillin-resistant Staphylococcus aureus (MRSA) and compared with free drug activity. A stronger antibacterial action was seen in the animals treated with nanofibers than free vancomycin.54 This provides evidence that nanofibers could be an efficient approach to treat MRSA-induced skin infections by playing the role of a potential topical delivery vehicle for drugs.
In another study, Hajiali and co-workers55 prepared alginate–lavender nanofibers having an anti-inflammatory and antibacterial action as a dressing to be applied on burn wounds to diminish the risk of microbial infection. They controlled and reduced the inflammatory response as well as repressed the Staphylococcus aureus growth in the induced human foreskin fibroblasts, and in the rodents. As compared to the control animals (not treated with the dressing), 4, 10, and 7 times lower levels of interleukins (IL)-6, IL-1β, and tumor necrosis factor-alpha (TNF-α), respectively, were reported with nanofibers treatment after 24 h, and the levels further decreased to 7, 24, and 19 times lower after 48 h, respectively.55 The decreased level of cytokines production, reduced erythema, and inhibited growth of bacterial infection on the treated animal skin support the use of nanofibrous dressings for burn management. Recent work in 2022 emphasized the preparation of nanofibrous materials with dual properties, like antibacterial and tissue repair, which would treat wounds with bacterial infection followed by scar formation. Therefore, chitosan nanofibers composed of nanoparticles containing silver (Ag) and curcumin were prepared and showed antibacterial activity against Gram-negative and positive bacteria (Figure 4).56 Moreover, studies revealed that it showed higher rates of healing and wound-closing properties (wound contraction up to 95% within 12 days with a small scar) in comparison with a commercial formulation (Figure 5).56 Thus, it reported synergism with the dual action of antibacterial activity and wound healing.56
Figure 4.
Antibacterial activity of chitosan/Cur@β-CD/AgNPs dressing. (a) Bacterial viability of P. aeruginosa, S. aureus and E. coli treated with four nanoparticles. (b) Bacterial viability of P. aeruginosa, S. aureus, and E. coli treated with different concentrations of Cur@β-CD/AgNPs chitosan nanofibers. (c) The inhibition zone of nanofibers on P. aeruginosa, S. aureus, and E. coli. Adapted with permission from ref (56). Copyright 2022 Taylor & Francis.
Figure 5.
Photographic images of the extent of wound healing and graphical illustration of the changes in wound size. Adapted with permission from ref (56). Copyright 2022 Taylor & Francis.
Recently, Wu et al.57 fabricated biosafe nanofibers to achieve long-term antibacterial therapeutic effects using chitosan and lysozyme. The lysozyme was incorporated into nanofibers, and chitosan and polydopamine were deposited on the surface of layers. Lysozyme and chitosan showed synergistic effects against Escherichia coli, and Staphylococcus aureus showed a long-term release for 14 days with strong tensile stress of 6.7 MPa, demonstrating the biomaterial’s enormous promise for wound dressings.57
3.3. Nanofibers in Treatment of Fungal Infections
Skin in a diseased condition and with reduced immunity is prone to microbial infections. Fungal skin infections have emerged as one of the leading causes of morbidity, mainly in immune-impaired individuals, and have become a serious health concern on a worldwide scale. Nanotechnology-derived carriers have gained huge attention for therapeutic delivery at the disease site,58 and nanofibers can be incredibly helpful in curing such infections. Misra et al.42 formed an antifungal tolnaftate-loaded nanofiber scaffold to be applied topically at the site of infection for the elimination of dermatophytes, a topical fungal infection. Eudragit was used for better adhesion and, along with the nanofibrous structure, ensured the controlled release of the drug. Nanofibers with an average diameter of 402.3 ± 65 nm showed 71.52% extended drug release over 8 h (in vitro) due to high eudragit concentration, and when its efficacy was compared in vivo with pure tolnaftate, it was found that mice treated with pure tolnaftate had dermatophytosis symptoms on the dorsal side, which was not, however, the case with nanofibers-treated mice as shown in Figure 6.42 Thus, in vivo studies demonstrated the fungal pathogen inhibition and dermatophytosis management effectively.42
Figure 6.
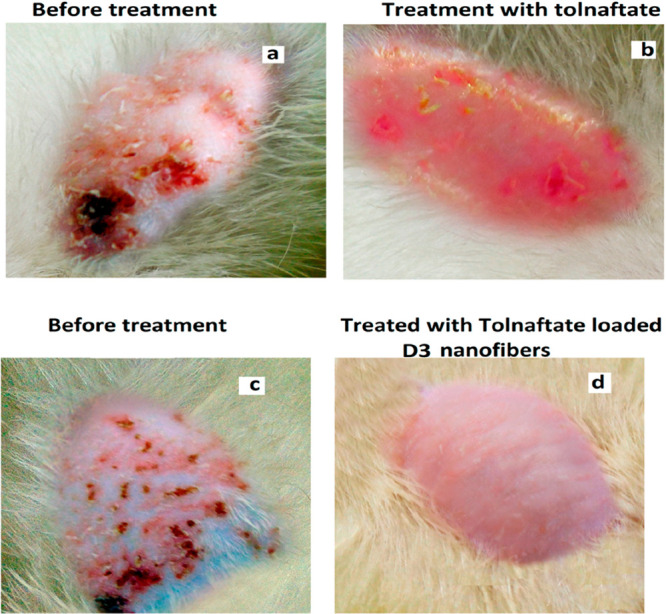
In vivo study on Swiss albino mice infected by T. rubrum dermatophyte (a,c) and their treatment with the tolnaftate drug (b) and tolnaftate-loaded nanofibers (d). Adapted with permission from ref (42). Copyright 2017 MDPI.
Candida has been identified as one of the utmost lethal fungal pathogens, causing health concerns from skin infections to bloodstream infections. Its antifungal treatment shows some side effects, and multidrug resistance is more common. Nanofibers ensure targeted and effective drug delivery in such infections. In a recent approach to target vulvovaginal candidiasis pathogen (Candida albicans), poly(lactic-co-glycolic acid) nanofibers with amphotericin B were fabricated. According to the obtained results of in vitro and in vivo fungicidal activity, controlled delivery of the drug provided effective antifungal activity and decreased the fungal burden from 3.43 ± 0.83 CFU mL–1 (100%) at 0 h to 0 ± 0 CFU mL–1 (0%) at 72 h with a single local application within 3 days of treatment.59 Hence, nanofibers offered an antifungal-resistance-free strategy for its treatment. One study reported that when cross-linked PVA/sodium alginate nanofibers loaded with voriconazole were prepared, a slower release rate (38% release in the first 30 min) was observed as compared to non-cross-linked nanofibers (50% release in the first 30 min). Also, the penetration of the formulation was found to be deeper into the skin layers as compared to the control formulation (voriconazole solution in 1% w/v propylene glycol). Further, it also exhibited good antifungal activity with minimum inhibitory concentration (MIC) values of 0.4 μg/mL against Candida albicans on topical treatment of mouse fibroblast cells.60
In a study by Ilhan et al.,61 layered nanofibers using gelatin and PVA were developed incorporating cinnamaldehyde (CA) and fluconazole (FLU) by the electrospinning technique to treat fungal keratitis. The nanofibers were further evaluated for tensile strength, swelling and disintegration behavior, and drug release rates with an encapsulation efficiency of 73.84% and 68.58% of CA and FLU, respectively. The release profile demonstrated the controlled release of CA for up to 96 h after a burst release of 87% within 8 h. In the case of FLU, 61% of the drug was released in 12 h, followed by extended release up to 168 h. The MTT assay demonstrated the noncytotoxic effect of the fabricated nanofibers, implying it has potential as an effective long-term treatment strategy for fungal keratitis.61
3.4. Nanofibers in Treatment of Psoriasis
Psoriasis is a prevalent inflammatory skin condition marked by keratinocyte hyperproliferation mediated by T cells. It is a chronic autoimmune disorder with scaly lesions on the skin exhibiting characteristics of a typical inflammatory reaction: stinging sensation, pain, and bleeding. Chronic plaque psoriasis, the most prevalent form, is caused by a combined play of both genetic predisposition and environmental triggers including stress, damage, infections, etc.62,63 In research by Mart et al.,64 three compounds (capsaicin, salicylic acid, and methyl salicylate) were utilized in the fabrication of nanofibers of poly(methyl vinyl ether-alt-maleic ethyl monoester) (PMVEMA-ES) for the treatment of psoriasis dermal symptoms. With respect to the amount of polymer, 3.5% w/w concentration of the active constituents was loaded with 100% encapsulation efficiency. Analysis of its activity was performed by applying these nanofibers as adhesive dressings for topical application on the arm of volunteers which reported release of the contents and reddening of the skin due to capsaicin action followed by degradation of nanofibers 8 h after the application.64
In another interesting experiment, antipsoriatic medicine tazarotene was delivered topically through magnetic nanoparticles embedded within a polycaprolactone nanofibers patch. A smart nanofiber system was designed that stimulated the drug release based on alternating magnetic-field-induced hyperthermia. Results demonstrated that the permeation of tazarotene was enhanced up to 70% with the magnetic field, possibly through the Arrhenius mechanism.65 These findings open up future approaches for remote-controlled drug delivery devices for more effective psoriasis topical treatment. Corticosteroids are also applied in the treatment of psoriasis to decrease inflammation, but they have reduced drug bioavailability when given topically at the desired site of action. Hence, to overcome this problem, in a recent study,66 pH-responsive nanofibers of poly-l-glutamic acid and fluocinolone acetonide conjugate were prepared and applied with hyaluronic acid-poly-l-glutamate cross polymer for enhancement of skin permeability. This system reduced the symptoms of psoriasis with a decrease in the pro-inflammatory cytokines levels (reduction of 51% in interferon-gamma (INF-γ) levels and 34% in IL-23 levels) in the tissue of the in vivo mouse model displaying the anti-inflammatory capacity effectively.66 In another study, cellulose nanofibrous film was fabricated for topical delivery of curcumin (cur)-loaded nanostructured lipid carriers. In comparison to the films without lipids, the curcumin-loaded nanofiber films displayed a greater than 2-fold surge in the amount of curcumin deposited to the skin’s epidermis in a psoriatic mouse model. The decrease in pro-inflammatory cytokine levels was found to be almost as effective as corticosteroid topical creams. These findings suggest cellulose nanofiber films as a viable drug delivery strategy for topical psoriasis treatment due to the improved drug retention and skin hydration benefit of the films (Figure 7).67
Figure 7.
Schematic illustration of the curcumin (cur)-loaded lipid-hybridized CNF film for the treatment of IMQ-induced psoriasiform dermatitis. Adapted with permission from ref (67). Copyright 2018 Elsevier.
A synergistic treatment approach for psoriasis was evaluated by a group of researchers through developing and evaluating tazarotene and calcipotriol (CPT) co-loaded PVA/PVP-nanofibers which were incorporated in a hydrogel film. The electrospinning technique was used to fabricate the nanofibers with an average diameter of 244.67 ± 58.11 and 252.31 ± 35.50 nm. The developed nanofibers were found to have a good tensile strength of 14.02 ± 0.54 to 22.50 ± 0.03 MPa and biodegrade within 2 weeks, indicating its biocompatible nature, with a sustained release of the drug up to 72 h. In contrast to tazarotene-loaded nanofibers, the hydrogel film made of tazarotene and CPT-loaded nanofibers showed a strong capacity for antipsoriatic activity in Wistar rats exposed to imiquimod.68
3.5. Nanofibers in Treatment of Acne
Acne is one of the common conditions of the skin caused by inflammation of sebaceous glands due to bacterial infection, mostly seen in young people and generally treated by antibacterial and anti-inflammatory drugs. Recently, nanofibers have been explored as an alternative treatment strategy with enhanced contact time and drug permeation. Currently, in 2022 for the treatment of acne vulgaris, chitosan, and melittin nanofibers were fabricated. These nanofibers helped in decreasing redness and inflammation when they were applied topically as a dressing and showed 98.02 ± 3.53% inhibition in bacterial growth in both animal studies and in vitro assessment.69 Khoshbakht and co-workers70 designed tretinoin-loaded polycaprolactone nanofibers for topical skin delivery to treat acne. It showed a biphasic release, i.e., burst release followed by slow sustained release over 90 h, and the formulation provided stability to the vulnerable unstable tretinoin. Also, slightly higher inhibition diameters of the bacterial growth were observed for tretinoin and erythromycin-loaded nanofibers (32 mm) when compared with standard erythromycin discs (30 mm) because of the higher surface-to-volume ratio of the nanofibers.70 This puts the nanofibers in the list of suitable candidates as potential antiacne patches.
In an approach to designing a depot formulation for the development of an easily permeable skin delivery system, PVA/quercetin/essential oils-based nanofibers were fabricated for activity against acne. After obtaining positive results from the laboratory experiments, a clinical trial was also performed on patients with acne. Nanofiber formulation exhibited antibacterial activity more efficiently as compared to quercetin alone. The percentage decrease in total acne lesions, comedonal, and inflammation were found to be 52.9 ± 9.9%, 14.7 ± 16.5%, and 61.2 ± 10.2%, respectively, with nanofibers as compared to 15.3 ± 10.7%, 22.5 ± 19.9%, and 12.5 ± 15.2%, respectively, for commercial cream on clinical assessment of the subjects.71 This shows the development of a potential antiacne topical delivery system using nanofibers.
3.6. Nanofibers in Treatment of Atopic Dermatitis
Atopic Dermatitis (AD), also known as eczema is an inflammatory chronic skin disorder characterized by dry skin and pruritus, and therefore its treatment includes antibacterial protection, increasing skin moisture, and reducing irritation.72 With the aim of designing a patient-friendly topical formulation providing biocompatibility, flexibility, breathability, and efficacy using nanofibers that could be used as a patch for its treatment, tacrolimus loaded into a polymeric nanofibrous membrane was developed. Nanofibers helped to achieve a sustained drug release profile and provided a desirable tensile strength, and its efficacy was analyzed using AD animal model. The results reported that the same therapeutic effect was seen in the case of nanofibrous membranes administered after every 2 days as compared to the commercially available tacrolimus 0.03% ointment given daily.73 This suggests that a tacrolimus-loaded nanofibrous system with the advantage of less frequent application could be an alternative for tacrolimus ointment. In 2021, researchers developed patches of blackcurrant seed oil (rich in gamma linoleic acid (GLA)) loaded polyimide membranes (electrospun) for the hydration of the atopic skin which reported more than 350% stretchability and high (95.6%) membrane porosity, making it desirable for skin patch development. In vivo skin hydration tests revealed that more delivery of GLA was seen, with longer patch application durations exhibiting enhanced skin hydration in the case of subjects with very dry skin (deficient of GLA).74 These systems can be tailored-made according to individual AD patients based on their levels of skin dryness.
3.7. Nanofibers in Treatment of Vitiligo
Vitiligo is a depigmentation skin disorder caused due to malfunctioning of melanocytes, and its treatment lies in restoring the normal functioning of the melanocytes and decreasing the immunological response toward them.75 This requires the delivery of bioactive molecules, cytokines, and growth factors in the skin, and nanofibers have been explored for such applications.76 Rahnama et al.77 designed a delivery system using polycaprolactone nanofibrous scaffolds to achieve prolonged activity of bioactive molecules released from platelet-rich plasma, paving a way for a low-cost and safe regenerative therapy. The surface functionalization of scaffolds was done with platelets of different concentrations, a prolonged 14-day release of the bioactive molecules was seen, and the amount released was almost 2-fold greater in the case of electrospun scaffolds as compared to centrifugally spun ones. The metabolic activity was found to be improved, with bioactive molecules’ higher concentrations leading to the better proliferation of melanocytes because of the functionalized nanofibers.77 This shows evidence of onsite activity of the nanofibers. Another similar study reported platelets functionalized polycaprolactone electrospun scaffolds for the delivery of different growth factors like epidermal growth factor (EGF), platelet-derived growth factor (PDGF), etc. The results depicted cell proliferation, cell spreading, and metabolic activity of the skin cells (melanocytes, keratinocytes, fibroblasts).78 This can be used as a base scaffold to design treatment options for vitiligo with desired cells and growth factors.
3.8. Nanofibers in Treatment of Skin Cancer
Skin cancer or tumor cells of the skin have become one of the major skin problems as a result of UV ray penetration due to environmental degradation of the protective ozone layer. The most prevalent kinds of skin cancer are squamous cell carcinomas, basal cell carcinomas (nonmelanocytic skin cancer), and cutaneous malignant melanomas (skin melanoma).79 Nanotechnology can provide better treatment options for melanoma skin cancer through targeted delivery of nanofibers to the desired site, diminishing the side effects as compared to other treatment options like surgical removal, chemotherapy, and radiation. A group of researchers prepared molybdenum nanoparticles loaded on a polycaprolactone nanofibrous scaffold as a targeted approach for skin cancer with the objective to achieve selective apoptosis of the tumor cells. They observed more than 50% cancer cell viability reduction by the mechanism of mitochondria-dependent apoptosis, probably by the molybdenum oxide. The mechanism was explored via Real-Time Polymerase Chain Reaction (RT-PCR) through the analysis of three major genes. Expression of P53 genes, pro-apoptotic marker BAX, and Caspase 3 executioner protein increased in the Mol–polycaprolactone nanofibers treated A431 cells. In vivo experiment results reported cancer progression reduction by more than 30% within 14 days in the zebrafish.80
Rengifo and co-workers81 prepared poly(ethylene oxide)-chitosan nanofiber mats encapsulated with pyrazoline-loaded carboxymethyl-hexanoyl chitosan and dodecyl sulfate nanoparticles for treating skin cancer. It showed controlled release over a period of 120 h with an enhanced rate of transportation through the epidermis, probably by increasing solubilization of the drug and drug partitioning in the skin. An approximately 2-fold increase in the amount of drug retained was seen in the epidermis (3.08 ± 0.67 μg/cm2) as compared to the dermis layer (1.60 ± 0.32 μg/cm2), attributed to the fact that the epidermis, being the most lipophilic skin layer, has a higher affinity for the lipophilic drug. A high intensity of fluorescence was observed for skin treated with the nanofiber formulation as compared to the free drug, confirming that nanofiber mats can potentially be used for local chemotherapy in targeting melanoma cells as shown in Figure 8.81 These results exhibited the fact that nanofibers can be helpful in treating skin cancers with fewer side effects because of the possibility of targeted action of anticancer drugs topically.
Figure 8.

(a) Retention (μg/cm2) of H3TM04-loaded nanoparticles in human skin after 7 h of permeation assay. * Indicates statistical difference (p < 0.05). (b) Fluorescence microscopy images of the penetration of H3TM04 in human skin sections (20 μm) after 7 h of treatment: (i) control, (ii) H3TM04, (iii) Nps CHC-SDS-H3, (iv) PEOChH3, and (v) PEOChNps. Adapted with permission from ref (81). Copyright 2019 Elsevier.
Recently, Jiang et al.82 have explored the potential of electrospun hybrid nanofibers for the postsurgical treatment of glioblastomas. The curcumin-loaded zeolite Y nanoparticles were synthesized and incorporated into a polycaprolactone–gelatin-based nanofibrous mat. The fabricated nanofibers were further characterized and evaluated for in vitro release and for anticancer activity through an MTT assay, 4′,6-diamidino-2-phenylindole (DAPI) staining, and a cell migration test using a U87-MG cell line. The zeolite-nanoparticle-loaded nanofibers showed more antimigratory activity as compared to the free-drug-loaded nanofibers, with cell migration of 29.5% and 68%, respectively. Similarly, it also showed superior activity, as indicated by the decreased intensity of DAPI and high nuclear fragmentation with a reduction in the number of glioblastoma cells. The characterized bead-free nanofibers showed a curcurmin release of up to 33.5% in 72 h, and 47% within 14 days of analysis. Additionally, the cells exposed to the nanofibers demonstrated better cytotoxicity and pro-apoptotic impact against the glioblastoma, depicting the conceivable fabrication of curcumin-loaded zeolite Y nanoparticles incorporated into nanofibers for glioblastoma treatment.82
For the treatment of melanoma skin cancer, a synergistic approach was devised with coaxial nanofibers prepared by electrospinning, where chitosan-loaded poly(ε-caprolactone) and 5-fluorouracil (FU)-loaded poly(N-vinyl-2-pyrrolidone) represented core and shell, respectively. It initially reported 5-FU burst release, ensuring early apoptosis in B16F10 skin cancer cells by increasing apoptosis cell numbers from 0.8% to 62.2%, and later sustained chitosan release displayed “remedying effects” on L929 normal skin cells by showing an increase in the number of vital cells from 68.9% to 77.0%.83 This demonstrates the capability of nanofibers in generating a synergism for developing a potential strategy for chemotherapeutic therapy of skin cancer.
4. Clinical Trials and Patents
A human clinical trial (NCT04325789) was organized in March 2020, titled “Rotator Cuff Healing Using a Nanofiber Scaffold in Patients Greater Than 55 Years” in the United States. It is still in the recruiting phase, planning to assess 240 patients in 6 different sites in the US, and it would use an FDA-approved, Rotium nanofiber scaffold (FDA 510 (K) #K183236) made up of poly(l-lactide-co-caprolactone) and polyglycolide microfiber matrix which has reportedly demonstrated improvement in the healing of tendons in the preclinical studies. It is effective because of its possibility to resemble the extracellular matrix, which provides concentrates and holds together the cells at the site of repair, representing an efficient healing tissue organizational structure. With increasing age, vascularity decreases, leading to poor tendon healing following rotator cuff surgery. Hence, the objective of the trial aims to find if the scaffold application can bring any improvement in the healing process to minimize the perioperative failure of the repair risks.99
Another human clinical trial (NCT02680106) was designed in February 2016 and completed in December 2017, with the title “Evaluation of the SPINNER Device for the Application of Wound Dressing: Treatment of Split Skin Graft Donor Sites (SPINNER01)”. It was aimed at the safety and performance assessment of the device, as well as the analysis of the potential treatment of the wound dressing generated from it, and any side effects of them. The hand-held electrospinning device, Spinner, produces in situ nanofiber dressings that are personalized according to the user for the treatment of external wounds and burns. High absorbance, nonadherence, and easy peel-off without any pain are some of its advantages. Also, it provides protection from bacterial infection and shows outstanding conformability while covering the wound properly.100 Several patents have been filed for nanofibers in the area of skin disorders as summarized in Table 3.
Table 3. Summarized Table for Patents of Nanofibers in Skin Disorders.
| Patent Title | Application No. and Grant Date | Description of the Patent | Application |
|---|---|---|---|
| Composite wound cover containing nanofiber active layer | JP3239763U | It is a composite, multilayered structure which carries hyaluronic acid with or without pharmaceutical salts and derivatives thereof. | Local wound healing.101 |
| 2022–11–08 | |||
| Fiber–hydrogel composite surgical meshes for tissue repair | US11338062B2 | Nanofiber–hydrogel composite offers a surgical scaffold or mesh which would decrease foreign body response and manage the interface of tissue. | Damaged tissue repair.102 |
| 2022–05–24 | |||
| Biomaterial devices and topical compositions for guided tissue regeneration | US20190388586A1 | Devices patented for tissue regeneration made up of chitosan bonded with collagen (mutable collagen tissue - MCT). This composite can be fabricated as a biofilm, nanofibers, or in a hydrogel system. | Wound healing, tissue engineering, and burns.103 |
| 2021–02–09 | |||
| Antimicrobial fibers and compositions | EP3253424A1 | The nanofibers consist of an enzyme responsible for converting substrates to release hydrogen peroxide, which shows activity against microbes. | Antimicrobial activity.104 |
| 2020–12–09 | |||
| Self-assembled ultrashort peptide hydrogels for wound healing, skin care and cosmetic applications | CN105189532B | Hydrogels which contain amphiphilic linear peptides/peptoids, self-assemble into three-dimensional macromolecular nanofiber networks, and provide support to damaged tissue. | Cell culture, tissue regeneration, wound healing, and the release of bioactive moieties.105 |
| 2020–11–03 | |||
| Treatment of disease with poly-N- acetylglucosamine nanofibers | US10765698B2 | Compositions comprising shortened fibers of poly-N-acetylglucosamine and/or a derivative thereof (“sNAG nanofibers”). | Treatment of atopic dermatitis in a human subject.106 |
| 2020–09–08 | |||
| Wound dressing | CN107106720B | Comprising a lyophilized Hyaluronic Acid (HA) hydrogel and a plurality of devices embedded within the lyophilized hyaluronic acid hydrogel, each of which comprises chitosan and hypromellose and may be formed as a biofilm and/or electrospun fiber mat. | Wound dressing, and more particularly, a stimulus-responsive wound dressing.107 |
| 2020–06–16 | |||
| Antibacterial applications of poly(N-acetylglucosamine) nanofibers | KR101883797B1 | A nanofibrous composition, consisting of shortened fibers of poly-N acetylglucosamine (nNAF) for treating bacterial infections or its symptoms. | Topical antibacterial therapy.108 |
| 2018–08–30 | |||
| Topical skin preparation | JP6308531B2 | Cellulose nanofibers and one or more polyvalents selected from polyethylene glycol, 1,3-butylene glycol, 1,2-pentanediol, isoprene glycol, propylene glycol, dipropylene glycol, glycerin, and diglycerin. | A skin external preparation suitable for retaining moisture in the stratum corneum.109 |
| 2018–04–11 | |||
| Tamper-resistant opioid composition for treating skin lesions | CA2723307C | A tamper-resistant topical pharmaceutical composition, comprising a matrix made of polymeric nanofibers, at least one opioid agonist, and/or at least one pharmaceutically acceptable salt and a gel former. | Treat skin lesions.110 |
| 2017–08–22 |
5. Challenges and Future Perspectives
Different unique technologies and approaches for the scale-up of nanofibers are under investigation, even though some of these implementations are restricted to lab-scale studies. Nanofiber-based formulations have yet to make a successful transfer into the market, despite the potential benefits and advances made in this field, highlighting the need for more intensive study to address numerous restrictions and further in vivo studies to better understand the effectiveness of nanofiber compositions.111 In addition, the in vivo stability of nanofibers, durability, and biocompatibility are some important factors for nanofibers to consider. The large-scale production of nanofibers with desired properties remains a difficult issue, as the widely used electrospinning processes have certain limitations, such as poor yield, high voltage requirement, and difficulties achieving in situ nanofiber deposition.112 Electrospinning, as one of the most used methods, is known to be influenced by many parameters which in turn influence the fiber diameter and surface area. Although theoretical approaches have been utilized to predict the electrospinning process, it is extremely difficult to control the nature of the fibers generated through it. Systematic studies on the multiple parameters that act during the process are difficult to conduct and often fall short of predicting how certain parameters affect the fiber’s nature due to the delicate interaction between them.113 Different experimental parameters are also known to pair in subtle ways, making identification difficult for simple correlations between these parameters and the resulting fiber characteristics.
The significance of optimizing the release of active constituents from nanofiber architectures cannot be disputed, and for a suitable continuous release target, burst release must be carefully regulated. Layer-by-layer technologies, the incorporation of a hydrophobic and hydrophilic polymer into the framework of nanofibers, and the notion of composite nanofibers have all been investigated to optimize the release characteristic of nanofibers.111 Thus, some of the challenges associated with these systems include scaling up the technology, producing affordable formulations, tailoring the quantity of drug loading, and regulating the shape and porosity of nanofibers for product uniformity.114 Formulation concerns such as controlled porosity, homogeneous drug loading in the fiber architecture, and regulated nanofiber thickness must be monitored to generate cost-effective and reliable end-products. Recently, the usage of nanofibers for various therapeutic and biomedical applications has garnered a lot of interest. Many poly blended nanofibers, made by electrospinning synthetic and natural polymer combinations, have been used to offer strong biochemical, structural, and mechanical capabilities that no single polymer can match alone.115 Therefore, researchers are working on modifying these nanofibers in ways that they could more closely resemble local tissue systems. Poly blend nanofibers may play an important role in regenerating functional tissues like muscle tissue, bone tissue, heart tissue, nerves, etc. Additionally, new developments are being made in the simultaneous stimulation of cellular functions during tissue regeneration, induction of differentiation of stem cells, and preventive medicine by the integration of numerous biomolecules into poly blend solutions to fabricate nanofibers.
6. Conclusion
Nanofibers consist of a network of fibers in the nanometric size range which owe them their intertwined ultrafine structure, high surface area, and permeability, and so they have been widely used in a variety of scientific domains for decades. To customize the drug release profile for every therapeutic application, a variety of properties can be modified, including the drug-to-polymer ratio, diameter range of nanofibers, shape, and/or porosity. Small molecules are found to permeate easily through the skin layers, and thus researchers have explored this nanotechnology as a therapeutic delivery system for topical and other applications. Electrospun nanofibers have high porosity, interconnectivity, and flexibility, making them ideal for topical drug delivery; hence, they are gaining popularity as drug delivery carriers that can also be targeted to deliver drugs to a specific location for localized treatment. Therefore, they are being explored for tissue regeneration, scaffold preparation, wound healing applications, and treatment of different types of skin disorders. Thus, the use of nanofibers has become a rapidly developing domain that has made a huge contribution toward revolutionizing medicine technologies as well.
Acknowledgments
The authors gratefully acknowledge the support received from Science & Engineering Research Board (SERB), Department of Science and Technology, Government of India CRG/2021/003631.
Author Contributions
§ Authors contributed equally
The authors declare no competing financial interest.
References
- Waghule T.; Gorantla S.; Rapalli V. K.; Shah P.; Dubey S. K.; Saha R. N.; Singhvi G. Emerging trends in topical delivery of curcumin through lipid nanocarriers: effectiveness in skin disorders. AAPS PharmSciTechnol. 2020, 21, 1–2. 10.1208/s12249-020-01831-9. [DOI] [PubMed] [Google Scholar]
- Skin Diseases, Conditions & Disorders|. NIAMS. Accessed December 30, 2022. https://www.niams.nih.gov/health-topics/skin-diseases.
- Priya S.; Tomar Y.; Desai V. M.; Singhvi G. Enhanced skin drug delivery using dissolving microneedles: A potential approach for the management of skin disorders. Expert Opin Drug Delivery 2023, 1. 10.1080/17425247.2023.2190095. [DOI] [PubMed] [Google Scholar]
- Goyal R.; Macri L. K.; Kaplan H. M.; Kohn J. Nanoparticles and nanofibers for topical drug delivery. J. Controlled Release 2016, 240, 77–92. 10.1016/j.jconrel.2015.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waghule T.; Rapalli V. K.; Gorantla S.; Saha R. N.; Dubey S. K.; Puri A.; Singhvi G. Nanostructured lipid carriers as potential drug delivery systems for skin disorders. Current Pharmaceutical Design. 2020, 26 (36), 4569–79. 10.2174/1381612826666200614175236. [DOI] [PubMed] [Google Scholar]
- Priya S.; Desai V. M.; Singhvi G. Surface Modification of Lipid-Based Nanocarriers: A Potential Approach to Enhance Targeted Drug Delivery. ACS omega. 2023, 8, 74–86. 10.1021/acsomega.2c05976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahriar S.; Mondal J.; Hasan M.; Revuri V.; Lee D.; Lee Y.-K. Electrospinning Nanofibers for Therapeutics Delivery. Nanomaterials 2019, 9, 532. 10.3390/nano9040532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priya S.; Batra U.; Samshritha R. N.; Sharma S.; Chaurasiya A.; Singhvi G. Polysaccharide-based nanofibers for pharmaceutical and biomedical applications: A review. Int. J. Biol. Macromol. 2022, 218, 209–224. 10.1016/j.ijbiomac.2022.07.118. [DOI] [PubMed] [Google Scholar]
- Lin Y. C.; Hu S. C. S.; Huang P. H.; Lin T. C.; Yen F. L. Electrospun resveratrol-loaded polyvinylpyrrolidone/cyclodextrin nanofibers and their biomedical applications. Pharmaceutics 2020, 12 (6), 552. 10.3390/pharmaceutics12060552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalvandi J.; White M.; Gao Y.; Truong Y. B.; Padhye R.; Kyratzis I. L. Polyvinyl alcohol composite nanofibres containing conjugated levofloxacin-chitosan for controlled drug release. Materials Science and Engineering C 2017, 73, 440–446. 10.1016/j.msec.2016.12.112. [DOI] [PubMed] [Google Scholar]
- Kajdic S.; Planinsek O.; Gasperlin M.; Kocbek P. Electrospun nano fi bers for customized drug-delivery systems. Journal of Drug Delivery Science and Technology 2019, 51, 672–681. 10.1016/j.jddst.2019.03.038. [DOI] [Google Scholar]
- Laha A.; Sharma C. S.; Majumdar S. Sustained drug release from multi-layered sequentially crosslinked electrospun gelatin nanofiber mesh. Materials Science and Engineering C 2017, 76, 782–786. 10.1016/j.msec.2017.03.110. [DOI] [PubMed] [Google Scholar]
- Miguel S. P.; Figueira D. R.; Simões D.; et al. Electrospun polymeric nanofibres as wound dressings: A review. Colloids Surf. B Biointerfaces. 2018, 169, 60–71. 10.1016/j.colsurfb.2018.05.011. [DOI] [PubMed] [Google Scholar]
- Gautam S.; Chou C. F.; Dinda A. K.; Potdar P. D.; Mishra N. C. Surface modification of nanofibrous polycaprolactone/gelatin composite scaffold by collagen type i grafting for skin tissue engineering. Materials Science and Engineering C 2014, 34 (1), 402–409. 10.1016/j.msec.2013.09.043. [DOI] [PubMed] [Google Scholar]
- Rezaei S.; Valipouri A.; Hosseini Ravandi S. A.; Kouhi M.; Ghasemi Mobarakeh L. Fabrication, characterization, and drug release study of vitamin C-loaded alginate/polyethylene oxide nanofibers for the treatment of a skin disorder. Polym. Adv. Technol. 2019, 30 (9), 2447–2457. 10.1002/pat.4692. [DOI] [Google Scholar]
- Calamak S.; Shahbazi R.; Eroglu I.; Gultekinoglu M.; Ulubayram K. An overview of nanofiber-based antibacterial drug design. Expert Opin Drug Discovery 2017, 12 (4), 391–406. 10.1080/17460441.2017.1290603. [DOI] [PubMed] [Google Scholar]
- Li C.; Qiu Y.; Li R.; Li M.; Qin Z.; Yin X. Preparation of poly (N-isopropylacrylamide)/ polycaprolactone electrospun nanofibres as thermoresponsive drug delivery systems in wound dressing. International Journal of Polymeric Materials and Polymeric Biomaterials. 2023, 72, 243–251. 10.1080/00914037.2021.2006654. [DOI] [Google Scholar]
- Fathi Azarbayjani A.; Venugopal J. R.; Ramakrishna S.; Lim F. C.; Chan Y. W.; Chan S. Y. Smart polymeric nanofibers for topical delivery of levothyroxine. Journal of Pharmacy and Pharmaceutical Sciences. 2010, 13 (3), 400–410. 10.18433/J3TS3G. [DOI] [PubMed] [Google Scholar]
- Stojanov S.; Berlec A. Electrospun Nanofibers as Carriers of Microorganisms, Stem Cells, Proteins, and Nucleic Acids in Therapeutic and Other Applications. Front Bioeng Biotechnol. 2020, 8, 1–16. 10.3389/fbioe.2020.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra N. S.; Gorantla S.; Priya S.; Singhvi G. Insight on updates in polysaccharides for ocular drug delivery. Carbohydr. Polym. 2022, 297, 120014. 10.1016/j.carbpol.2022.120014. [DOI] [PubMed] [Google Scholar]
- Doostmohammadi M.; Forootanfar H.; Shakibaie M.; et al. Polycaprolactone/gelatin electrospun nanofibres containing biologically produced tellurium nanoparticles as a potential wound dressing scaffold: Physicochemical, mechanical, and biological characterisation. IET Nanobiotechnol. 2021, 15 (3), 277–290. 10.1049/nbt2.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cam M. E.; Crabbe-Mann M.; Alenezi H.; et al. The comparision of glybenclamide and metformin-loaded bacterial cellulose/gelatin nanofibres produced by a portable electrohydrodynamic gun for diabetic wound healing. Eur. Polym. J. 2020, 134 (June), 109844. 10.1016/j.eurpolymj.2020.109844. [DOI] [Google Scholar]
- Juncos Bombin A. D.; Dunne N. J.; McCarthy H. O. Electrospinning of natural polymers for the production of nanofibres for wound healing applications. Materials Science and Engineering C 2020, 114, 110994. 10.1016/j.msec.2020.110994. [DOI] [PubMed] [Google Scholar]
- Hu M.; Li C.; Li X.; et al. Zinc oxide/silver bimetallic nanoencapsulated in PVP/PCL nanofibres for improved antibacterial activity. Artif Cells Nanomed Biotechnol. 2018, 46 (6), 1248–1257. 10.1080/21691401.2017.1366339. [DOI] [PubMed] [Google Scholar]
- Mishra P.; Gupta P.; Pruthi V. Materials Science & Engineering C Cinnamaldehyde incorporated gellan/PVA electrospun nanofibers for eradicating Candida biofilm. Materials Science & Engineering C 2021, 119, 111450. 10.1016/j.msec.2020.111450. [DOI] [PubMed] [Google Scholar]
- Afshar A.; Yuca E.; Wisdom C.; et al. Next-generation Antimicrobial Peptides (AMPs) incorporated nanofibre wound dressings. Med. Devices Sens. 2021, 4 (1), 1–11. 10.1002/mds3.10144. [DOI] [Google Scholar]
- Kumar C. S.; Soloman A. M.; Thangam R.; Perumal R. K.; Gopinath A.; Madhan B. Ferulic acid-loaded collagen hydrolysate and polycaprolactone nanofibres for tissue engineering applications. IET Nanobiotechnol. 2020, 14 (3), 202–209. 10.1049/iet-nbt.2019.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asgari Q.; Alishahi M.; Davani F.; et al. Fabrication of amphotericin B-loaded electrospun core-shell nanofibers as a novel dressing for superficial mycoses and cutaneous leishmaniasis. Int. J. Pharm. 2021, 606 (April), 120911. 10.1016/j.ijpharm.2021.120911. [DOI] [PubMed] [Google Scholar]
- Wang X.; Ding B.; Li B. Biomimetic electrospun nanofibrous structures for tissue engineering. Materials Today. 2013, 16 (6), 229–241. 10.1016/j.mattod.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra R. K.; Mishra P.; Verma K.; Mondal A.; Chaudhary R. G.; Abolhasani M. M.; Loganathan S. Electrospinning Production of Nanofibrous Membranes. Environmental Chemistry Letters 2019, 17, 767–800. 10.1007/s10311-018-00838-w. [DOI] [Google Scholar]
- Yan G.; Yu J.; Qiu Y.; et al. Self-assembly of electrospun polymer nanofibers: A general phenomenon generating honeycomb-patterned nanofibrous structures. Langmuir. 2011, 27 (8), 4285–4289. 10.1021/la1047936. [DOI] [PubMed] [Google Scholar]
- Wu D.; Xu F.; Sun B.; Fu R.; He H.; Matyjaszewski K. Design and preparation of porous polymers. Chem. Rev. 2012, 112 (7), 3959–4015. 10.1021/cr200440z. [DOI] [PubMed] [Google Scholar]
- Martin C. R.; Parthasarathy R.; Menon V. Template synthesis of electronically conductive polymers-preparation of thin films. Electrochim. Acta 1994, 39 (8–9), 1309–1313. 10.1016/0013-4686(94)E0052-2. [DOI] [Google Scholar]
- Valizadeh A.; Mussa Farkhani S. Electrospinning and electrospun nanofibres. IET Nanobiotechnol. 2014, 8 (2), 83–92. 10.1049/iet-nbt.2012.0040. [DOI] [PubMed] [Google Scholar]
- Son Y. J.; Kim W. J.; Yoo H. S. Therapeutic applications of electrospun nanofibers for drug delivery systems. Published online 2014, 37, 69–78. 10.1007/s12272-013-0284-2. [DOI] [PubMed] [Google Scholar]
- Kamble P.; Sadarani B.; Majumdar A.; Bhullar S. Nanofiber based drug delivery systems for skin: A promising therapeutic approach. J. Drug Deliv Sci. Technol. 2017, 41, 124–133. 10.1016/j.jddst.2017.07.003. [DOI] [Google Scholar]
- Ji W.; Sun Y.; Yang F.; et al. Bioactive electrospun scaffolds delivering growth factors and genes for tissue engineering applications. Pharm. Res. 2011, 28 (6), 1259–1272. 10.1007/s11095-010-0320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma J.; Lizu M.; Stewart M.; et al. Multifunctional Nanofibers towards Active Biomedical Therapeutics 2015, 7, 186. 10.3390/polym7020186. [DOI] [Google Scholar]
- Balaji A.; Vellayappan M. V.; John A. A.; et al. An insight on electrospun-nanofibers-inspired modern drug delivery system in the treatment of deadly cancers. RSC Adv. 2015, 5 (71), 57984–58004. 10.1039/C5RA07595E. [DOI] [Google Scholar]
- Sebe I.; Szabó P.; Kállai-Szabó B.; Zelkó R. Incorporating small molecules or biologics into nanofibers for optimized drug release: A review. Int. J. Pharm. 2015, 494 (1), 516–530. 10.1016/j.ijpharm.2015.08.054. [DOI] [PubMed] [Google Scholar]
- Salimbeigi G; McGuinness G.. Antibacterial and Nanostructured Sutures for Enhanced Healing and Tissue Regeneration. In Reference Module in Materials Science and Materials Engineering; Elsevier Ltd., 2022; Vol 1 10.1016/b978-0-12-815732-9.00051-6. [DOI] [Google Scholar]
- Misra S. K.; Pandey H.; Patil S.; Ramteke P. W.; Pandey A. C. Tolnaftate-loaded polyacrylateelectrospun nanofibers for an impressive regimen on dermatophytosis. Fibers. 2017, 5 (4), 41. 10.3390/fib5040041. [DOI] [Google Scholar]
- Priyanto A.; Hapidin D. A.; Suciati T.; Khairurrijal K. Current Developments on Rotary Forcespun Nanofibers and Prospects for Edible Applications. Food Engineering Reviews. 2022, 14 (3), 435–461. 10.1007/s12393-021-09304-w. [DOI] [Google Scholar]
- Abdul Hameed M. M.; Mohamed Khan S. A. P.; Thamer B. M.; Rajkumar N.; El-Hamshary H.; El-Newehy M. Electrospun nanofibers for drug delivery applications: Methods and mechanism. Polym. Adv. Technol. 2023, 34 (1), 6–23. 10.1002/pat.5884. [DOI] [Google Scholar]
- Feng X.; Li J.; Zhang X.; Liu T.; Ding J.; Chen X. Electrospun polymer micro/nano fi bers as pharmaceutical repositories for healthcare. J. Controlled Release 2019, 302 (March), 19–41. 10.1016/j.jconrel.2019.03.020. [DOI] [PubMed] [Google Scholar]
- Sabra S.; Ragab D. M.; Agwa M. M.; Rohani S. Recent advances in electrospun nanofibers for some biomedical applications. European Journal of Pharmaceutical Sciences. 2020, 144, 105224. 10.1016/j.ejps.2020.105224. [DOI] [PubMed] [Google Scholar]
- Ambekar R. S.; Kandasubramanian B. Advancements in nanofibers for wound dressing: A review. Eur. Polym. J. 2019, 117 (May), 304–336. 10.1016/j.eurpolymj.2019.05.020. [DOI] [Google Scholar]
- Mohd Razali N. A.; Lin W.-C.; Norzain N. A.; Yu Z.-W. Controlling cell elongation and orientation by using microstructural nanofibre scaffolds for accelerating tissue regeneration. Materials Science and Engineering C 2021, 128, 112321. 10.1016/j.msec.2021.112321. [DOI] [PubMed] [Google Scholar]
- Hall I. J.; Paladino E.; Szabó P.; et al. Electrospun collagen-based nano fi bres : A sustainable material for improved antibiotic utilisation in tissue engineering applications. Int. J. Pharm. 2017, 531 (1), 67–79. 10.1016/j.ijpharm.2017.08.071. [DOI] [PubMed] [Google Scholar]
- Zarandi M. A.; Zahedi P.; Rezaeian I.; Salehpour A.; Gholami M.; Motealleh B. Drug release, cell adhesion and wound healing evaluations of electrospun carboxymethyl chitosan/polyethylene oxide nanofibres containing phenytoin sodium and Vitamin C. IET Nanobiotechnol. 2015, 9 (4), 191–200. 10.1049/iet-nbt.2014.0030. [DOI] [PubMed] [Google Scholar]
- Chinatangkul N.; Tubtimsri S.; Panchapornpon D.; Akkaramongkolporn P.; Limmatvapirat C.; Limmatvapirat S. Design and characterisation of electrospun shellac-polyvinylpyrrolidone blended micro/nano fi bres loaded with monolaurin for application in wound healing. Int. J. Pharm. 2019, 562, 258–270. 10.1016/j.ijpharm.2019.03.048. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Guo M.; Zhu T.; Xiong H.; Zhu L. M. A careob-like nanofibers with a sustained drug release profile for promoting skin wound repair and inhibiting hypertrophic scar. Compos B Eng. 2022, 236, 109790. 10.1016/j.compositesb.2022.109790. [DOI] [Google Scholar]
- Vyas T.; Rapalli V. K.; Chellappan D. K.; Dua K.; Dubey S. K.; Singhvi G. Bacterial biofilms associated skin disorders: Pathogenesis, advanced pharmacotherapy and nanotechnology-based drug delivery systems as a treatment approach. Life Sci. 2021, 287, 120148. 10.1016/j.lfs.2021.120148. [DOI] [PubMed] [Google Scholar]
- Fathi H. A.; Abdelkader A.; AbdelKarim M. S.; et al. Electrospun vancomycin-loaded nanofibers for management of methicillin-resistant Staphylococcus aureus-induced skin infections. Int. J. Pharm. 2020, 586 (April), 119620. 10.1016/j.ijpharm.2020.119620. [DOI] [PubMed] [Google Scholar]
- Hajiali H.; Summa M.; Russo D.; et al. Alginate-lavender nanofibers with antibacterial and anti-inflammatory activity to effectively promote burn healing. J. Mater. Chem. B 2016, 4 (9), 1686–1695. 10.1039/C5TB02174J. [DOI] [PubMed] [Google Scholar]
- Liu C.; Zhu Y.; Lun X.; Sheng H.; Yan A. Effects of wound dressing based on the combination of silver@curcumin nanoparticles and electrospun chitosan nanofibers on wound healing. Bioengineered. 2022, 13 (2), 4328–4339. 10.1080/21655979.2022.2031415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J.; Liu F.; Chen C. Long-term antibacterial activity by synergistic release of biosafe lysozyme and chitosan from LBL-structured nanofibers. Carbohydr. Polym. 2023, 312, 120791. 10.1016/j.carbpol.2023.120791. [DOI] [PubMed] [Google Scholar]
- Waghule T.; Sankar S.; Rapalli V. K. Emerging role of nanocarriers based topical delivery of anti-fungal agents in combating growing fungal infections. Dermatol Ther. 2020, 33 (6), e13905 10.1111/dth.13905. [DOI] [PubMed] [Google Scholar]
- Souza R. O.; Henrique de Lima T.; Oréfice R. L.; et al. Amphotericin B-Loaded Poly(lactic-co-glycolic acid) Nanofibers: An Alternative Therapy Scheme for Local Treatment of Vulvovaginal Candidiasis. J. Pharm. Sci. 2018, 107 (10), 2674–2685. 10.1016/j.xphs.2018.06.017. [DOI] [PubMed] [Google Scholar]
- Esentürk I; Balkan T.; Özhan G.; et al. Voriconazole incorporated nanofiber formulations for topical application: preparation, characterization and antifungal activity studies against Candida species. Pharm. Dev Technol. 2020, 25 (4), 440–453. 10.1080/10837450.2019.1706563. [DOI] [PubMed] [Google Scholar]
- Ilhan E.; Cesur S.; Sulutas R. B.; et al. The Role of Multilayer Electrospun Poly(Vinyl Alcohol)/Gelatin nanofibers loaded with Fluconazole and Cinnamaldehyde in the Potential Treatment of Fungal Keratitis. Eur. Polym. J. 2022, 176, 111390. 10.1016/j.eurpolymj.2022.111390. [DOI] [Google Scholar]
- Tomar Y.; Gorantla S.; Singhvi G. Insight into the pivotal role of signaling pathways in psoriasis pathogenesis, potential therapeutic molecules and drug delivery approaches. Drug Discov Today. 2023, 28 (2), 103465. 10.1016/j.drudis.2022.103465. [DOI] [PubMed] [Google Scholar]
- Rapalli V. K.; Sharma S.; Roy A.; Singhvi G. Design and dermatokinetic evaluation of Apremilast loaded nanostructured lipid carriers embedded gel for topical delivery: A potential approach for improved permeation and prolong skin deposition. Colloids Surf. B Biointerfaces. 2021, 206, 111945. 10.1016/j.colsurfb.2021.111945. [DOI] [PubMed] [Google Scholar]
- Martinez-Ortega L.; Mira A.; Fernandez-Carvajal A.; Mateo C. R.; Mallavia R.; Falco A. Development of A New Delivery System Based on Drug-Loadable Electrospun Nanofibers for Psoriasis Treatment. Pharmaceutics 2019, 11 (1), 1–14. 10.3390/pharmaceutics11010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andryskova N.; Sourivong P.; Babincova M.; Simaljakova M. Controlled Release of Tazarotene from Magnetically Responsive Nanofiber Patch : Towards More Efficient Topical Therapy of Psoriasis. Applied Sciences 2021, 11 (22), 11022. 10.3390/app112211022. [DOI] [Google Scholar]
- Dolz-Pérez I.; Sallam M. A.; Masiá E.; et al. Polypeptide-corticosteroid conjugates as a topical treatment approach to psoriasis. J. Controlled Release 2020, 318, 210–222. 10.1016/j.jconrel.2019.12.016. [DOI] [PubMed] [Google Scholar]
- Kang N. W.; Kim M. H.; Sohn S. Y.; et al. Curcumin-loaded lipid-hybridized cellulose nanofiber film ameliorates imiquimod-induced psoriasis-like dermatitis in mice. Biomaterials. 2018, 182, 245–258. 10.1016/j.biomaterials.2018.08.030. [DOI] [PubMed] [Google Scholar]
- Thakur S.; Anjum M. M.; Jaiswal S.; Kumar A.; Deepak P.; Anand S.; Singh S.; Rajinikanth P. S. Novel Synergistic Approach: Tazarotene-Calcipotriol-Loaded-PVA/PVP-Nanofibers Incorporated in Hydrogel Film for Management and Treatment of Psoriasis. Mol. Pharm. 2023, 20 (2), 997–1014. 10.1021/acs.molpharmaceut.2c00713. [DOI] [PubMed] [Google Scholar]
- Rahnama S.; Movaffagh J.; Shahroodi A.; et al. Development and characterization of the electrospun melittin-loaded chitosan nanofibers for treatment of acne vulgaris in animal model. Journal of Industrial Textiles. 2022, 52, 152808372211124. 10.1177/15280837221112410. [DOI] [Google Scholar]
- Khoshbakht S.; Asghari-Sana F.; Fathi-Azarbayjani A.; Sharifi Y. Fabrication and characterization of tretinoin-loaded nanofiber for topical skin delivery. Biomater Res. 2020, 24 (1), 1–7. 10.1186/s40824-020-00186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amer S. S.; Mamdouh W.; Nasr M.; et al. Quercetin loaded cosm-nutraceutical electrospun composite nanofibers for acne alleviation: Preparation, characterization and experimental clinical appraisal. Int. J. Pharm. 2022, 612, 121309. 10.1016/j.ijpharm.2021.121309. [DOI] [PubMed] [Google Scholar]
- Krysiak Z. J.; Stachewicz U. Electrospun fibers as carriers for topical drug delivery and release in skin bandages and patches for atopic dermatitis treatment. Wiley Interdiscip Rev. Nanomed Nanobiotechnol. 2023, 15 (1), e1829 10.1002/wnan.1829. [DOI] [PubMed] [Google Scholar]
- Shams G.; Rad A. N.; Safdarian M.; Rezaie A.; Bavarsad N.; Abbaspour M. Self-microemulsification-assisted incorporation of tacrolimus into hydrophilic nanofibers for facilitated treatment of 2,4-dinitrochlorobenzene induced atopic dermatitis like lesions. J. Drug Deliv Sci. Technol. 2021, 62, 102326. 10.1016/j.jddst.2021.102326. [DOI] [Google Scholar]
- Sroczyk E. A.; Berniak K.; Jaszczur M.; Stachewicz U. Topical electrospun patches loaded with oil for effective gamma linoleic acid transport and skin hydration towards atopic dermatitis skincare. Chemical Engineering Journal. 2022, 429, 132256. 10.1016/j.cej.2021.132256. [DOI] [Google Scholar]
- Lin S. J.; Jee S. H.; Hsaio W. C.; Lee S. J.; Young T. H. Formation of melanocyte spheroids on the chitosan-coated surface. Biomaterials. 2005, 26 (12), 1413–1422. 10.1016/j.biomaterials.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Dicks L. M. T.; Heunis T. D. J. Nanofibers offer alternative ways to the treatment of skin infections. J. Biomed Biotechnol. 2010, 2010, 1. 10.1155/2010/510682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vocetkova K.; Sovkova V.; Buzgo M.; et al. A simple drug delivery system for platelet-derived bioactive molecules, to improve melanocyte stimulation in vitiligo treatment. Nanomaterials 2020, 10 (9), 1801. 10.3390/nano10091801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vocetkova K.; Buzgo M.; Sovkova V.; Bezdekova D.; Kneppo P.; Amler E. Nanofibrous polycaprolactone scaffolds with adhered platelets stimulate proliferation of skin cells. Cell Prolif. 2016, 49 (5), 568–578. 10.1111/cpr.12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R.; Dubey S. K.; Singhvi G. The Hedgehog pathway and its inhibitors: Emerging therapeutic approaches for basal cell carcinoma. Drug Discov Today. 2022, 27 (4), 1176–1183. 10.1016/j.drudis.2021.12.005. [DOI] [PubMed] [Google Scholar]
- Janani I.; Lakra R.; Kiran M. S.; Korrapati P. S. Selectivity and sensitivity of molybdenum oxide-polycaprolactone nanofiber composites on skin cancer: Preliminary in-vitro and in-vivo implications. Journal of Trace Elements in Medicine and Biology. 2018, 49 (April), 60–71. 10.1016/j.jtemb.2018.04.033. [DOI] [PubMed] [Google Scholar]
- Rengifo A. F. C.; Stefanes N. M.; Toigo J.; et al. PEO-chitosan nanofibers containing carboxymethyl-hexanoyl chitosan/dodecyl sulfate nanoparticles loaded with pyrazoline for skin cancer treatment. Eur. Polym. J. 2019, 119, 335–343. 10.1016/j.eurpolymj.2019.08.001. [DOI] [Google Scholar]
- Jiang B.; Yang Z.; Shi H.; Turki Jalil A.; Mahmood Saleh M.; Mi W. Potentiation of Curcumin-loaded zeolite Y nanoparticles/PCL-gelatin electrospun nanofibers for postsurgical glioblastoma treatment. J. Drug Deliv Sci. Technol. 2023, 80, 104105. 10.1016/j.jddst.2022.104105. [DOI] [Google Scholar]
- Zhu L. F.; Zheng Y.; Fan J.; Yao Y.; Ahmad Z.; Chang M. W. A novel core-shell nanofiber drug delivery system intended for the synergistic treatment of melanoma. European Journal of Pharmaceutical Sciences. 2019, 137 (July), 105002. 10.1016/j.ejps.2019.105002. [DOI] [PubMed] [Google Scholar]
- Mishra P.; Gupta P.; Srivastava A. K.; Poluri K. M.; Prasad R. Eucalyptol/β -cyclodextrin inclusion complex loaded gellan/PVA nanofibers as antifungal drug delivery system. Int. J. Pharm. 2021, 609, 121163. 10.1016/j.ijpharm.2021.121163. [DOI] [PubMed] [Google Scholar]
- Veras F. F.; Roggia I.; Pranke P.; Pereira C. N.; Brandelli A. Inhibition of filamentous fungi by ketoconazole-functionalized electrospun nanofibers. European Journal of Pharmaceutical Sciences 2016, 84, 70–76. 10.1016/j.ejps.2016.01.014. [DOI] [PubMed] [Google Scholar]
- Sampath Kumar N.; Santhosh C.; Vathaluru Sudakaran S.; et al. Electrospun polyurethane and soy protein nanofibres for wound dressing applications. IET Nanobiotechnol. 2018, 12, 94–98. 10.1049/iet-nbt.2017.0022. [DOI] [Google Scholar]
- Saha K.; Butola B. S.; Joshi M. Drug-loaded polyurethane/clay nanocomposite nanofibers for topical drug-delivery application. J. Appl. Polym. Sci. 2014, 131 (10), 1–9. 10.1002/app.40230. [DOI] [Google Scholar]
- Cui S.; Sun X.; Li K. Polylactide nanofibers delivering doxycycline for chronic wound treatment. Materials Science and Engineering C 2019, 104 (July), 109745. 10.1016/j.msec.2019.109745. [DOI] [PubMed] [Google Scholar]
- Babincová N.; Jirsák O.; Babincová M.; Babinec P.; Šimaljaková M. Remote magnetically controlled drug release from electrospun composite nanofibers: design of a smart platform for therapy of psoriasis. Zeitschrift fur Naturforschung - Section A Journal of Physical Sciences. 2020, 75 (7), 587–591. 10.1515/zna-2020-0087. [DOI] [Google Scholar]
- Sangnim T.; Limmatvapirat S.; Nunthanid J.; et al. Design and characterization of clindamycin-loaded nanofiber patches composed of polyvinyl alcohol and tamarind seed gum and fabricated by electrohydrodynamic atomization. Asian J. Pharm. Sci. 2018, 13 (5), 450–458. 10.1016/j.ajps.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong S.; Oh S. G. Antiacne Effects of PVA/ZnO Composite Nanofibers Crosslinked by Citric Acid for Facial Sheet Masks. Int. J. Polym. Sci. 2022, 2022, 4694921. 10.1155/2022/4694921. [DOI] [Google Scholar]
- Karakucuk A.; Tort S. Preparation, characterization and antimicrobial activity evaluation of electrospun PCL nanofiber composites of resveratrol nanocrystals. Pharm. Dev Technol. 2020, 25 (10), 1216–1225. 10.1080/10837450.2020.1805761. [DOI] [PubMed] [Google Scholar]
- Obaidat R.; Shameh A. A.; Aljarrah M.; Hamed R. Preparation and Evaluation of Polyvinylpyrrolidone Electrospun Nanofiber Patches of Pioglitazone for the Treatment of Atopic Dermatitis. AAPS PharmSciTechnol. 2022, 23 (1), 1–19. 10.1208/s12249-021-02204-6. [DOI] [PubMed] [Google Scholar]
- Krysiak Z. J.; Kaniuk Łu.; Metwally S.; Szewczyk P. K.; Sroczyk E. A.; Peer P.; Lisiecka-Graca P.; Bailey R. J.; Bilotti E.; Stachewicz U. Nano-and Microfiber PVB Patches as Natural Oil Carriers for Atopic Skin Treatment. ACS Appl. Bio Mater. 2020, 3 (11), 7666–7676. 10.1021/acsabm.0c00854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suneet K.; De T.; Rangarajan A.; Jain S. Magnetic nanofibers based bandage for skin cancer treatment: a non-invasive hyperthermia therapy. Cancer Rep. 2020, 3 (6), 1–9. 10.1002/cnr2.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balashanmugam P.; Sucharithra G.; Agnes Mary S.; Tamil Selvi A. Efficacy of biopolymeric PVA-AuNPs and PCL-Curcumin loaded electrospun nanofibers and their anticancer activity against A431 skin cancer cell line. Mater. Today Commun. 2020, 25, 101276. 10.1016/j.mtcomm.2020.101276. [DOI] [Google Scholar]
- Muthulakshmi L.; Prabakaran S.; Ramalingam V.; et al. Sodium alginate nanofibers loaded Terminalia catappa scaffold regulates intrinsic apoptosis signaling in skin melanoma cancer. Process Biochemistry. 2022, 118 (April), 92–102. 10.1016/j.procbio.2022.04.004. [DOI] [Google Scholar]
- Radmansouri M.; Bahmani E.; Sarikhani E.; Rahmani K.; Sharifianjazi F.; Irani M. Doxorubicin hydrochloride - Loaded electrospun chitosan/cobalt ferrite/titanium oxide nanofibers for hyperthermic tumor cell treatment and controlled drug release. Int. J. Biol. Macromol. 2018, 116, 378–384. 10.1016/j.ijbiomac.2018.04.161. [DOI] [PubMed] [Google Scholar]
- Hoyer R.; Harris D.; Kruse K.; Badman B.. A Prospective Randomized Evaluation of Rotator Cuff Healing Using a Nanofiber Scaffold in Patients Greater than 55 Years. 2020; ( (317), ), 1–15.
- Evaluation of the SPINNER Device for the Application of Wound Dressing: Treatment of Split Skin Graft Donor Sites - Tabular View - ClinicalTrials.gov. U.S. National Library of Medicine. 2016. Accessed January 3, 2023. https://clinicaltrials.gov/ct2/show/record/NCT02680106?term=nanofibers&draw=2&rank=9.
- Composite Wound Cover Containing Nanofiber Active Layer. JP3239763U, 2022. Accessed January 3, 2023.
- Martin R.; Reddy S.; Sacks J.; Li X.; Cho B. H.; Mao H.-q.. Fiber-hydrogel composite surgical meshes for tissue repair. US11338062B2, 2022. Accessed January 3, 2023.
- Qiu D.; Carballo S. M.. Biomaterial Devices and Topical Compositions for Guided Tissue Regeneration. US20190388586A1, 2021. Accessed January 3, 2023.
- Patton T.; Brennan J.; Staples I.; Elder I.; Callaghan A.; Dryden M.; Barrett J. R.; Kershaw D.; Salib R.. Antimicrobial fibers and compositions. EP3253424B1, 2020. Accessed April 27, 2022. https://patents.google.com/patent/EP3253424B1/en?q=nanofibres+used+topically&oq=nanofibres+used+topically.
- Self-assembled ultrashort peptide hydrogels for wound healing, skin care and cosmetic applications. CN105189532B, 2020. Accessed April 27, 2022.
- Vournakis J. N.; Finkielsztein S.. Treatment of disease with poly-N- acetylglucosamine nanofibers. US10765698B2, 2020. Accessed January 3, 2023.
- Wound dressing. CN107106720B, 2020. Accessed January 3, 2023.
- Anti-bacterial applications of poly-n-acetylglucosamine nanofibers. KR101883797B1, 2018. Accessed April 27, 2022.
- Topical skin preparation. JP6308531B2, 2018. Accessed January 3, 2023.
- Oksche A.; Smith K. J.; Prater D.; Walden M.; Heath W.; Kennedy B.; Addison V.; Mohammad H.. Tamper resistant opioid composition for treating skin lesions. CA2723307C, 2017. Accessed January 3, 2023.
- Topcu B.; Gultekinoglu M.; Timur S. S.; Eroglu I.; Ulubayram K.; Eroglu H. Current approaches and future prospects of nanofibers: a special focus on antimicrobial drug delivery. J. Drug Target. 2021, 29 (6), 563–575. 10.1080/1061186X.2020.1867991. [DOI] [PubMed] [Google Scholar]
- Bhagwan J; Kumar N; Sharma Y. Fabrication, Characterization, and Optimization of MnxOy Nanofibers for Improved Supercapacitive Properties. Nanomaterials Synthesis 2019, 451–481. 10.1016/B978-0-12-815751-0.00013-4. [DOI] [Google Scholar]
- Williams G. R; Chatterton N. P; Nazir T.; Yu D.-G.; Zhu L.-M.; Branford-White C. J Electrospun nanofibers in drug delivery: recent developments and perspectives. Therapeutic Delivery 2012, 3 (4), 515–533. 10.4155/tde.12.17. [DOI] [PubMed] [Google Scholar]
- Sofi H. S.; Abdal-hay A.; Ivanovski S.; Zhang Y. S.; Sheikh F. A. Electrospun nanofibers for the delivery of active drugs through nasal, oral and vaginal mucosa: Current status and future perspectives. Materials Science and Engineering C 2020, 111, 110756. 10.1016/j.msec.2020.110756. [DOI] [PubMed] [Google Scholar]
- Gunn J.; Zhang M. Polyblend nanofibers for biomedical applications: Perspectives and challenges. Trends Biotechnol. 2010, 28 (4), 189–197. 10.1016/j.tibtech.2009.12.006. [DOI] [PubMed] [Google Scholar]