Abstract
Introduction
Metformin-treated patients may experience severe hyperlactatemia or lactic acidosis (LA). LA often requires intensive-care-unit (ICU) treatment, and mortality rates are high. Here, we investigate the impact of renal dysfunction and renal replacement therapy (RRT) on the outcomes of critically ill patients with metformin-associated LA (MALA). Furthermore, we assessed associations between mortality and metformin dose, metformin plasma/serum concentrations, lactate level, and arterial pH. Finally, we investigated whether the recommended classification in MALA, metformin-unrelated LA, metformin-induced LA, and LA in metformin therapy appears useful in this regard.
Methods
We performed a retrospective analysis based on a systematic PubMed search for publications on hyperlactatemia/LA in metformin-treated ICU patients from January 1995 to February 2020. Case-level data including demographics and clinical conditions were extracted, and logistic regression analyses were performed.
Results
A total of 92 ICU patients were reported. Two of these patients had no comorbidities interfering with lactate metabolism. In the overall group, arterial pH, lactate levels, and metformin plasma/serum concentrations were similar in survivors versus non-survivors. Ingested daily metformin doses and plasma/serum creatinine levels were significantly higher in survivors versus non-survivors (p = 0.007 vs. p = 0.024, respectively). Higher plasma/serum creatinine levels, higher lactate levels, and lower arterial pH were all associated with patients receiving RRT (all p < 0.05). Overall mortality was 22% (20 out of 92 patients) and did not differ between the RRT and non-RRT groups.
Conclusion
Mortality is high in ICU patients with metformin-associated hyperlactatemia/LA. Unexpectedly, higher ingested metformin dose and plasma/serum creatinine were associated with a better outcome. Survival was similar in patients with or without need for RRT.
Keywords: Hyperlactatemia, Lactic acidosis, Metformin, Critically ill, Intensive care unit
Introduction
Metformin is an oral anti-hyperglycemic drug of the biguanide class used in Europe since 1957 [1]. It is recommended as first-line therapy for the treatment of diabetes mellitus type 2 [2, 3]. A prominent side effect in metformin-treated patients is lactic acidosis (LA), with an estimated incidence of 1–10 per 100,000 patient-years [4, 5]. While rare, metformin-associated LA (MALA) is potentially life-threatening and often requires intensive care support [6] and reported mortality is high [7, 8, 9]. Although LA is often observed in the intensive care unit (ICU), the evaluation of a potential impact of metformin and the clinical management represent a challenge for intensivists.
Metformin's standard therapeutic dose ranges from 500 to 2,000 mg daily with a maximal dose of 3,000 mg daily [10]. Once absorbed, it has a large volume of distribution of 1–5 L/kg with main accumulation intracellularly, especially in intestinal cells and erythrocytes [11, 12, 13]. Steady-state plasma concentrations with routine clinical doses are generally <1.5 µg/mL but can be as high as 5 µg/mL [14].
Importantly, metformin is excreted unmetabolized via the kidney both via glomerular filtration and tubular secretion (normal clearance is 500 mL/min, i.e., x5 normal GFR) [15]. Thus, in patients with impaired kidney function, standard metformin doses may lead to increased plasma/serum metformin concentrations [15, 16, 17] and is contraindicated in patients with severely reduced renal function (estimated glomerular filtration rate <30 mL/min/1.73 m2, respectively [10]). Metformin interferes with lactate metabolism and augments lactate production by inhibiting the electron transport chain and thus shifts aerobic to anaerobic metabolism. The resulting increase in NADH prevents gluconeogenesis, leading to reduced lactate clearance [18]. Under physiologic conditions, lactate produced by therapeutic metformin doses is cleared without significant lactate accumulation [19].
Several additional medical conditions may lead to hyperlactatemia, classified as type A or type B according to Cohen and Woods [20]. This includes reduced tissue perfusion/oxygenation, resulting in hyperlactatemia (type A) or hypoxic conditions (type B). Additional medical drugs or medical conditions include, e.g., adrenaline, metformin, and/or renal/hepatic impairment that are responsible for lactate clearance [21, 22] as 70% of lactate clearance is performed by the liver and 30% by the kidneys [23, 24]. The terms hyperlactatemia and LA are often used as synonyms in the literature. However, it seems important that hyperlactatemia is not always associated with LA (defined as a serum lactate concentration >5 mmol/L and arterial pH < 7.35) [25].
Development of hyperlactatemia/LA in metformin-treated patient is influenced by multiple conditions, whereby metformin can play a causal, co-responsible, and/or coincidental role. A new diagnostic approach was postulated by Lalau et al. [19] to categorize the role of metformin in hyperlactatemia/LA in metformin-treated patients. Depending on plasma metformin concentrations and comorbidities, it was proposed to distinguish between MALA, metformin-unrelated LA (MULA), metformin-induced LA (MILA), and LA in metformin therapy (LAMT). In MALA, metformin accumulation plays a co-responsible role with one or several concomitant diseases, e.g., kidney disease, hepatic impairment, and/or acute/chronic heart failure. In MULA, blood metformin levels are not augmented, i.e., either normal to low or even undetectable [19]. Here, metformin has a coincidental role, and additional systemic disease is responsible for development of LA. MILA is a condition exclusively caused by metformin. In respective cases, blood metformin levels are increased due to metformin intoxication and/or acute kidney injury (AKI). Here, metformin plays a causal role. LAMT describes a condition where plasma metformin concentration was not assessed [19]. Renal dysfunction seems of particular interest in metformin intoxication with renal replacement therapy (RRT) successfully applied in some respective cases [4, 22].
Here, we investigated the influence of renal dysfunction and RRT on the outcomes of critically ill patients. We searched previous reports on hyperlactatemia/LA in metformin-treated ICU patients to analyze whether metformin dose, metformin plasma/serum concentrations, lactate levels, and/or arterial pH are associated with adverse patient outcomes. Finally, we assess the published cases regarding the terminology MALA, MULA, MILA, and LAMT and investigate whether this classification reflects prognosis and/or outcome in the critically ill.
Methods
Search Strategy and Study Selection
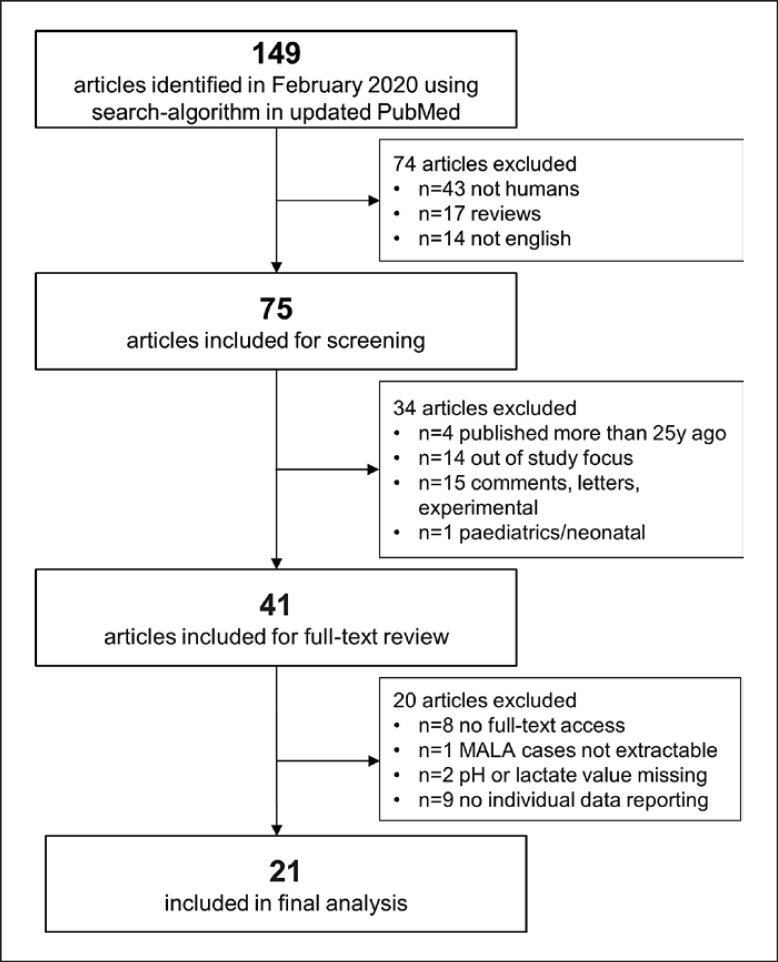
A search was performed according to PRISMA [21]. Literature search was performed in PubMed (censor date February 4, 2020) using the following search terms: (metformin OR biguanid) AND (LA OR lactic acidemia OR lactic acidaemia OR hyperlactatemia OR hyperlactatemia OR toxicity OR intoxication) AND (critical care OR intensive care OR critically ill). Articles older than 25 years (before January 1995), articles not involving humans, articles not reporting data on adults (e.g., pediatrics/neonatal reports), reviews/comments/letters, and/or language other than English were excluded. Further, articles with no full-text access, articles not reporting data of ICU patients, and/or articles not reporting about cases fulfilling our LA definition and/or different study focus (e.g., no patient-level data) were excluded (Fig. 1). Abstracts of identified articles were screened by two independent reviewers. The study was performed in adherence to local guidelines. Formal ethics approval was not required.
Fig. 1.
Study flowchart. PRISMA flowchart. Three steps were conducted: search strategy/identification, screening, and eligibility. Respective exclusion criteria are listed separately.
Data Extraction
Individual data from each case report/series were collected. Age, median plasma/serum creatinine levels, arterial pH, and lactate levels are important parameters concerning the choice of renal treatment (i.e., RRT vs. conservative treatment) and were included in the extraction process. For each report, the following information was extracted (i.e., if provided by the articles): age, gender, metformin daily doses, metformin plasma/serum concentration, plasma/serum creatinine level at admission, arterial pH, lactate and bicarbonate concentration, the presence or absence of following clinical conditions: diarrhea and vomiting before admission, acute or chronic renal impairment, liver impairment/alcoholism, infection, other reasons for hypoxia. Further, classifications including MALA, MULA, MILA, LAMT, RRT use, and clinical outcome were noted as shown in online supplementary Table S1 (for all online suppl. material, see www.karger.com/doi/10.1159/000528252) (raw data). For analytical purposes, we categorized the metformin levels based on our literature search into three groups: low (≤5 µg/mL) [19], moderate (>5 µg/mL and ≤50 µg/mL) [24], and high (>50 µg/mL). In online supplementary Figure S2, we used a creatinine level above 4 mg/dL (i.e., its converted value in µmol/L) to separate patients with/without kidney failure for our analysis.
Statistical Methods
Analyses were performed with R version 4.1.0 (2021-05-18, R core Team). Continuous variables were presented as median and interquartile ranges and categorical variables as numbers and percentages. Between-group differences were tested with the Kruskal-Wallis test and Fisher's exact test. Pairwise comparisons of means were computed using the Wilcoxon rank sum test and Fisher's exact test for continuous and categorical data, respectively. Correlations between variables and metformin dose and metformin serum/plasma concentrations were computed using Spearman rank correlation (ρ). Directional changes of lactate levels and arterial pH between groups were tested with one-sided Wilcoxon rank sum tests. Multiple logistic regression was used to test for independent predictors with the clinical outcome as the dependent variable. p < 0.05 was considered as statistically significant.
Results
Seventy five full-text articles were identified. After assessment of pre-defined in- and exclusion criteria, 21 articles remained in the final analysis set as shown in Figure 1 and Table 1 ([17, 23, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44]). The included 21 articles consisted of 11 case reports, 6 case series, 4 retrospective clinical studies. A total of 92 patients met the inclusion criteria for the final analysis.
Table 1.
Description of included studies
| Author (year) | Study type | Patients, n |
|---|---|---|
| Carlon (2010) [31] | Case series | 3 |
| Chiew (2018) [44] | Case report (intentional overdose) | 1 |
| Dichtwald (2012) [36] | Retrospective clinical study | 6 |
| Galea (2007) [28] | Case report (intentional overdose) | 1 |
| Giuliani (2010) [32] | Case report | 1 |
| Keller (2011) [35] | Retrospective clinical study | 6 |
| Krzymien (2013) [37] | Retrospective clinical study | 8 |
| Lalau (1995) [23] | Case series | 14 |
| Lemyze (2010) [33] | Case report | 1 |
| McNamara (2015) [39] | (Retrospective) case series (intentional overdose) | 4 (2 excluded; missing pH/lactate level) |
| Nakamura (2017) [42] | Case report | 1 |
| Ncomanzi (2014) [38] | Case report | 1 |
| Pan (2009) [29] | Case report | 1 |
| Protti (2010) [34] | Case series | 23 (1 excluded; phenformin) |
| Runge (2008) [17] | Case series | 4 |
| Schure (2003) [26] | Case report | 1 |
| Schwetz (2017) [43] | Case series | 3 |
| Sehra (2016) [40] | Case report (intentional overdose) | 1 |
| von Mach (2004) [27] | Case report | 1 |
| Wen (2009) [30] | Retrospective clinical study | 10 |
| White (2016) [41] | Case report | 1 |
|
| ||
| Total | 92 | |
Patient Demographics and Clinical Conditions
Demographics of included patients are given in Table 2. In brief, median age was 67 years (IQR 58, 74). 41 patients (59%) were female, 28 patients (41%) were male, and 23 patients were unknown. In 7 cases (15%), the ingested metformin doses were intentional (14,000–132,000 mg/days); in 39 cases (83%), in the recommended therapeutic range (500–3,000 mg/days); and in one case (2%), a prescribed overdose (3,400 mg/days) was reported (n = 45 unknown). Cases were classified as LA (defined as pH < 7.35 and lactate >5 mmol/L) in 89 patients (96.7%) and as hyperlactatemia (defined as lactate >2.4 mmol/L) in 3 patients (3.3%). The overall median plasma/serum creatinine level was 528 µmol/L (IQR 277, 799), median arterial pH was 6.93 (IQR 6.80, 7.12), and median lactate concentration, 16 mmol/L (IQR 12, 21). The median plasma bicarbonate concentration was 5.0 mmol/L. The median metformin plasma/serum concentration was 53 µg/mL (IQR 14, 69; n = 39 patients), with 6 patients (15.4%) having concentrations of ≤5 µg/mL, 12 patients (30.8%) between >5 µg/mL and ≤50 µg/mL, and 21 patients (53.8%) > 50 µg/mL.
Table 2.
Comparison of ICU patients along all-cause mortality
| Overall, (N = 92) | Survivors, (N = 72) | Non-survivors, (N = 20) | p value | |
|---|---|---|---|---|
| Age, years | 67 (58, 74) | 66 (57, 72) | 74 (60, 76) | 0.2 |
| Unknown | 23 | 18 | 5 | |
| Gender, n (%) | ||||
| Female | 41 (59) | 33 (61) | 8 (53) | 0.6 |
| Male | 28 (41) | 21 (39) | 7 (47) | |
| Unknown | 23 | 18 | 5 | |
| Metformin doses, mg/days | 2,500 (1,700, 3,000) | 2,550 (1,700, 3,000) | 1,700 (1,700, 1,700) | 0.007 |
| Unknown | 45 | 36 | 9 | |
| Metformin concentration, µg/mL (plasma/serum) | 53 (14, 69) | 55 (36, 70) | 29 (2, 62) | 0.2 |
| Unknown | 53 | 43 | 10 | |
| Creatinine (median), µmol/L (plasma/serum) | 528 (277, 799) | 589 (371, 824) | 313 (191, 624) | 0.024 |
| Arterial pH | 6.93 (6.80, 7.12) | 6.95 (6.80, 7.12) | 6.89 (6.80, 7.13) | 0.6 |
| Lactate (median), mmol/L | 16 (12, 21) | 16 (12, 21) | 17 (13, 24) | 0.2 |
| Bicarbonate, mmol/L | 5.0 (3.0, 9.3) | 5.0 (3.0, 8.7) | 6.0 (4.9, 8.4) | 0.5 |
| Unknown | 50 | 37 | 13 | |
| Acute renal impairment, n (%) | ||||
| No | 20 (34) | 10 (23) | 10 (71) | 0.002 |
| Yes | 38 (66) | 34 (77) | 4 (29) | |
| Unknown | 34 | 28 | 6 | |
| Diarrhea/vomiting days before admission, n (%) | ||||
| No | 23 (50) | 13 (37) | 10 (91) | 0.002 |
| Yes | 23 (50) | 22 (63) | 1 (9.1) | |
| Unknown | 46 | 37 | 9 | |
| Chronic renal impairment, n (%) | ||||
| No | 39 (75) | 30 (79) | 9 (64) | 0.3 |
| Yes | 13 (25) | 8 (21) | 5 (36) | |
| Unknown | 40 | 34 | 6 | |
| Liver impairment/alcoholism, n (%) | ||||
| No | 32 (76) | 23 (77) | 9 (75) | >0.9 |
| Yes | 10 (24) | 7 (23) | 3 (25) | |
| Unknown | 50 | 42 | 8 | |
| Infection, n (%) | ||||
| No | 24 (59) | 16 (57) | 8 (62) | >0.9 |
| Yes | 17 (41) | 12 (43) | 5 (38) | |
| Unknown | 51 | 44 | 7 | |
| Other reasons for hypoxia, n (%) | ||||
| No | 28 (56) | 22 (59) | 6 (46) | 0.5 |
| Yes | 22 (44) | 15 (41) | 7 (54) | |
| Unknown | 42 | 35 | 7 |
Of the 92 patients, all but 2 patients had at least one acute and/or chronic organ dysfunction before or at ICU admission. In summary, 38 patients (66%) had AKI (n = 34 unknown), 23 patients (46%) experienced gastroenteritis days before admission (n = 46 unknown), 17 patients (40%) suffered from an infection or sepsis (n = 51 unknown), 13 patients (25%) had chronic renal impairment (n = 40 unknown), 10 patients (23.8%) had hepatic impairment or alcohol abuse (n = 50 unknown). Based on plasma metformin concentrations and comorbidities, we classified cases into MALA, MULA, MILA, and LAMT according to Lalau et al. [19] shown in online supplementary Table S1.
Renal (Dys)function and Metformin Plasma/Serum Concentrations, Metformin Doses, Lactate Levels, Arterial pH
Ingested daily metformin doses correlated with metformin plasma/serum concentrations (rho (ρ) = 0.51, p = 0.011, online suppl. Fig. S1a). Metformin plasma/serum concentrations showed no significant correlation with lactate concentrations (ρ = 0.22, p = 0.18, online suppl. Fig. S1b), arterial pH (ρ = −0.33, p = 0.84, online suppl. Fig. S1c), and plasma/serum creatinine levels (ρ = 0.16, p = 0.32, online suppl. Fig. S1d).
Ingested daily metformin doses showed no correlation with lactate concentrations (ρ = −0.085, p = 0.57) and arterial pH (ρ = 0.054, p = 0.72) (online suppl. Fig. S2a, S2c). Patients with a plasma/serum creatinine ≤354.2 µmol/L displayed a negative association between the ingested metformin dose and lactate concentrations (ρ = −0.46, p = 0.073) and a positive correlation between the metformin dose and arterial pH (ρ = 0.43, p = 0.093). In patients with a plasma/serum creatinine >354.2 µmol/L, there was no significant association with lactate levels (ρ = 0.22, p = 0.24) and arterial pH (ρ = −0.13, p = 0.5), as shown in online supplementary Figure S2b, S2d.
Renal Dysfunction and Outcome Parameters
All-cause ICU mortality in the 92 ICU patients was 22% (n = 20 non-survivors, Table 2). Non-survivors did not differ from survivors regarding age, gender, arterial pH, lactate concentration levels, and metformin plasma/serum concentrations. In particular, median arterial pH was comparable in survivors versus non-survivors (pH 6.95 vs. pH 6.89). Significant differences were observed regarding the ingested metformin dose and plasma/serum creatinine levels between survivors and non-survivors (p = 0.007 and p = 0.024, respectively). Survivors more often had AKI with previous episodes of diarrhea and/or vomiting when compared to non-survivors (p = 0.002). The available data revealed no differences between survivors and non-survivors regarding further predisposing risk factors for developing hyperlactatemia/LA (please note missing data).
RRT and Clinical Outcome
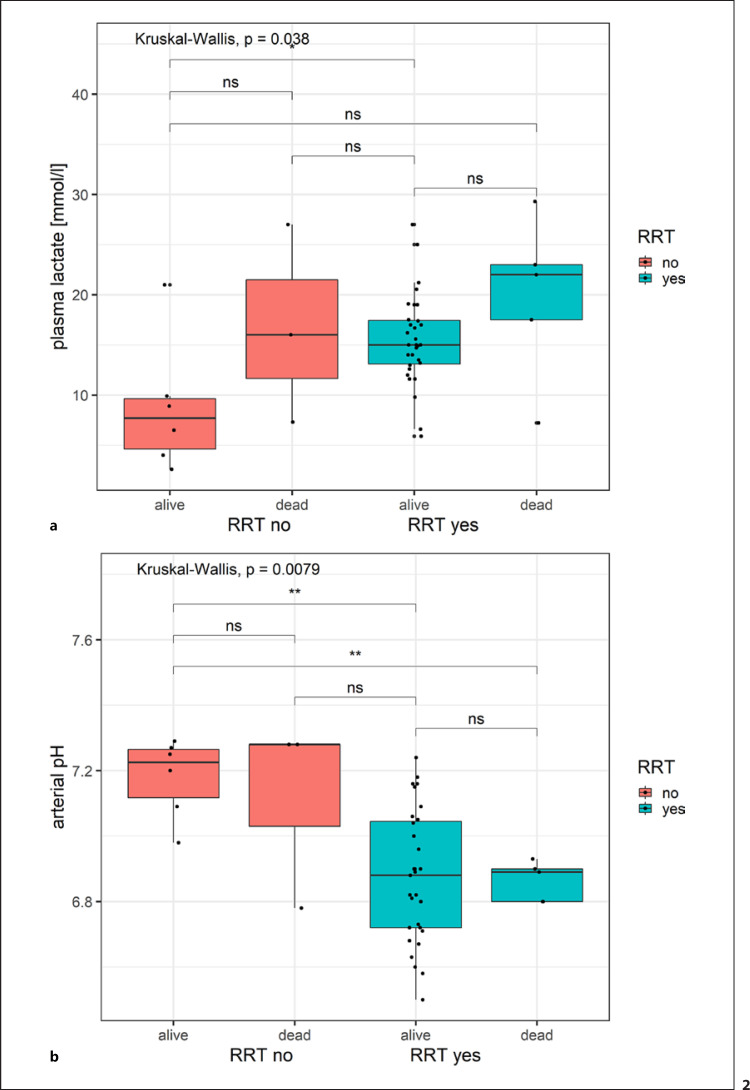
Data on mortality and RRT were only available in a subset of 45 patients. Table 3 shows a comparison between ICU patients treated with RRT versus without RRT. Patients in the RRT group had advanced age (Z = −2.4564, p = 0.007017), higher plasma/serum creatinine levels (Z = −3.9587, p < 0.0001), higher lactate levels (Z = −1.916, p = 0.02769), and lower arterial pH (Z = 3.3937, p = 0.0003447) when compared to the non-RRT group. Significant differences in lactate (p = 0.038) and arterial pH (p = 0.0079) were found in patients grouped by RRT and mortality (Fig. 2). Pairwise group comparisons of lactate values by RRT and mortality showed significant mean differences to be restricted to alive patients with and without RRT (Fig. 2a). Significant differences in means of arterial plasma values stratified by RRT and mortality are found between the presence and absence of RRT and not mortality (Fig. 2b).
Table 3.
Demographics of ICU patients treated with RRT versus without RRT
| Overall, (N = 45) | RRT(−), (N = 9) | RRT(+), (N = 36) | p value | |
|---|---|---|---|---|
| Outcome, n (%) | ||||
| Survivor | 37 (82) | 6 (67) | 31 (86) | 0.3 |
| Non-survivor | 8 (18) | 3 (33) | 5 (14) | |
| Age, years | 67 (55, 74) | 55 (32, 60) | 70 (58, 75) | 0.015 |
| Gender, n (%) | 0.7 | |||
| Female | 29 (64) | 5 (56) | 24 (67) | |
| Male | 16 (36) | 4 (44) | 12 (33) | |
| Metformin doses, mg/days | 2,550 (1,775, 3,000) | 15,000 (14,500, 15,000) | 2,550 (1,700, 3,000) | 0.037 |
| Unknown | 11 | 6 | 5 | |
| Metformin concentration, µg/mL (plasma/serum) | 60 (42, 80) | NA (NA, NA) | 60 (42, 80) | NA |
| Unknown | 32 | 9 | 23 | |
| Creatinine (median), µmol/L (plasma/serum) | 511 (246, 779) | 126 (93, 250) | 647 (371, 799) | <0.001 |
| Arterial pH | 6.90 (6.80, 7.09) | 7.25 (7.09, 7.28) | 6.88 (6.73, 7.01) | <0.001 |
| Lactate (median), mmol/L | 15 (12, 19) | 9 (6, 16) | 15 (13, 19) | 0.057 |
| Bicarbonate, mmol/L | 5.2 (2.5, 11.0) | 14.0 (11.5, 14.8) | 4.3 (2.4, 7.0) | 0.006 |
| Unknown | 13 | 3 | 10 | |
| Acute renal impairment, n (%) | ||||
| No | 10 (29) | 7 (78) | 3 (12) | <0.001 |
| Yes | 25 (71) | 2 (22) | 23 (88) | |
| Unknown | 10 | 0 | 10 | |
| Diarrhea/vomiting days before admission, n (%) | ||||
| No | 9 (41) | 0 (NA) | 9 (41) | >0.9 |
| Yes | 13 (59) | 0 (NA) | 13 (59) | |
| Unknown | 23 | 9 | 14 | |
| Chronic renal impairment, n (%) | ||||
| No | 18 (64) | 6 (100) | 12 (55) | 0.062 |
| Yes | 10 (36) | 0 (0) | 10 (45) | |
| Unknown | 17 | 3 | 14 | |
| Liver impairment/alcoholism, n (%) | ||||
| No | 14 (78) | 4 (67) | 10 (83) | 0.6 |
| Yes | 4 (22) | 2 (33) | 2 (17) | |
| Unknown | 27 | 3 | 24 | |
| Infection, n (%) | ||||
| No | 10 (59) | 2 (33) | 8 (73) | 0.2 |
| Yes | 7 (41) | 4 (67) | 3 (27) | |
| Unknown | 28 | 3 | 25 | |
| Other reasons for hypoxia, n (%) | ||||
| No | 12 (46) | 2 (33) | 10 (50) | 0.7 |
| Yes | 14 (54) | 4 (67) | 10 (50) | |
| Unknown | 19 | 3 | 16 |
Fig. 2.
Boxplot of lactate and arterial pH stratified by RRT and mortality. Boxplot of lactate levels (a) and arterial pH (b), stratified by patients with and without RRT and clinical outcomes (survivors/non-survivors). Overall differences in group means is indicated by Kruskal-Wallis test statistics. Significance levels of difference for pairwise group comparisons are indicated with stars (**: 0.001 < p < 0.01; *: 0.01 < p < 0.05, ns: p < 0.05).
AKI was, as expected, more often observed in the RRT group compared to non-RRT-treated ICU patients (p = 0.001). Overall mortality (i.e., all-cause) was somewhat lower in the RRT group when compared to the non-RRT group, but this did not reach statistical significance (14% vs. 33%, p = 0.3). To assess overall mortality in the subgroup of 45 patients with RRT information, multivariable logistic regression analysis (included variables were age, arterial pH, RRT [yes/no], plasma/serum creatinine, and acute renal impairment [yes/no]) did not find any significant factors (data not shown).
Discussion
In this retrospective analysis, we investigate data from 92 metformin-treated ICU patients with hyperlactatemia/LA. In the absence of comorbidities interfering with lactate metabolism, metformin seems rarely to cause hyperlactatemia/LA. In particular, we observe that renal dysfunction seems to be associated with higher lactate levels, lower arterial pH, and, somewhat surprisingly, lower mortality. In the current analysis, we observed that reduced plasma/serum creatinine levels on admission are associated with a rather “indolent” disease course with moderate arterial pH and lactate derangement, even after ingestion of rather high metformin doses. Importantly, however, improved renal function was not associated with lower all-cause mortality with plasma/serum creatinine levels higher in the survivor versus the non-survivor group.
Clearly, the proposed terminology MALA, MULA, MILA, and LAMT by Lalau et al. [19] cannot be challenged in the light of this retrospective analysis given all its limitations. Measurement of metformin concentrations may be logistically challenging (e.g., often not rapidly available), its interpretation may be considered complex, nevertheless are drug levels worth requesting in order to better understand the patients' mechanism of disease and estimate the patients' prognosis.
MILA cases may allow to study associations between metformin, lactate, and pH more precisely and may elucidate the influence of metformin on development of hyperlactatemia/LA in metformin-treated ICU patients. However, in light of the fact that only 2 out of 92 patients (1 MILA and 1 LAMT) included in this study had no other comorbidities, this question cannot be answered in the context of the current analysis.
The present study included ICU patients with an all-cause mortality rate of 22%, which seems rather in line with previously published data (17.2% mortality rate [45]) but lower when compared to other publications (about 50% mortality rates [46, 47]). Since arterial pH, lactate level, metformin plasma/serum concentration were similar in survivors versus non-survivors in the current analysis, a direct link between respective laboratory parameters and mortality appears unlikely, although uncertainties remain with regard to the role of RRT given the sample size in respective cases. Somewhat counterintuitively, the present study identified higher plasma/serum creatinine levels in survivors versus non-survivors, which is also a finding in a previous investigation [45]. Another key finding of our study is the observation that the survivor group had a significantly higher ingested metformin dose than in the non-survivor group. Furthermore, acute renal dysfunction and diarrhea prior to ICU admission were observed more often in survivors versus non-survivors. Hence, a potential reversible cause (dehydration, i.e., prerenal AKI) seems to be associated with a favorable clinical outcome. Again, it should be kept in mind that patients with high doses of ingested metformin, low arterial pH, increased lactate level, high plasma/serum creatinine level, and/or AKI at ICU admission are likely to be treated with RRT on the ICU, and RRT may impact significantly on the clinical outcome, in particular, in severe cases. This is consistent with a proposed decision-making algorithm for RRT in metformin-treated patients with hyperlactatemia/LA [22]. RRT indications may include, among others, severe acidosis [22]. Further, in contrast to a previous study [45], our data do not show gender-related differences in survivors versus non-survivors.
Additionally, we observed high mortality in patients who did not receive RRT, had a high metformin intake, good renal function, and an only minimal acid-base disorder. This would raise the question if some of these patients could be undertreated and if clinical practice should address this aspect in future studies more thoroughly and consider RRT treatment in such a setting. One potential explanation could also be reduced resources at the respective centers. Given the limited information provided in the articles, this question remains unanswered.
A number of important limitations of our analysis require discussion. First, by nature of severe intoxications leading to critical illness, we present data from a retrospective analysis relying on observational, non-randomized investigations (often case series), mostly with a limited sample size, resulting in uneven distribution of, e.g., the cohorts of patients treated with RRT versus without RRT. Second, potential confounders to our observational study include a large heterogeneity of reports with at least partly inconsistent definitions regarding hyperlactatemia; LA; and classification in MALA, MULA, MILA, and LAMT. Third, the data set available may be biased by heterogenous data reporting with (partly) important clinical (e.g., preexisting organ dysfunctions, comorbidities) and/or laboratory data missing. Fourth, baseline plasma/serum creatinine levels, which would be of great importance for exact assessment of progression of renal (dys)function, were often unavailable. Hence, classification of a stated “acute kidney injury” by the articles according to the KDIGO guidelines was not possible. Although this may be considered typical in the context of critical illness, the course of renal dysfunction may be difficult to determine in respective cases. Nevertheless, even in cases of reported plasma/serum creatinine levels, it may be important to keep in mind that renal function often is not in a steady state, and creatinine assessment may therefore only reflect the estimated glomerular filtration rate at a given point in time. In addition, best timing, optimal dose, and/or (RRT) mode in hyperlactatemia/LA under metformin therapy is currently unknown. Fifth, data extraction from the articles shows that in the majority of patients (n = 60), it was not specified in what type of medium (serum or plasma) laboratory analyses were performed, which limits comparative research. Sixth, variation in standard medical practice may impose bias on our data. Seventh, and importantly, by nature of the retrospective observational study design, we demonstrate associations but not causality.
The strength of the present study is that among the cases analyzed, misidentification of hyperlactatemia/LA in metformin-treated patients can likely be excluded since all cases meet preset definitions and were classified according to the classification by Lalau et al. [19]. In addition, we screened all articles thoroughly and meticulously tried to extract certain preconditions for organ dysfunction acting as cofactors impacting metformin metabolism (online suppl. Table S1).
Conclusions
Our data indicate that metformin rarely causes hyperlactatemia/LA in non-frail patients, when no comorbidities interfere with lactate metabolism. Established renal dysfunction, as suggested previously, appears of key importance for developing hyperlactatemia/LA. In contrast, our study reveals an increased all-cause mortality in ICU patients presenting with lower plasma/serum creatinine levels at admission, which may be confounded by interference of renal replacement strategies.
Statement of Ethics
An ethics statement is not applicable because this study is based exclusively on the published literature.
Conflict of Interest Statement
The authors report departmental grants (full disclosure): from Orion Pharma, Abbott Nutrition International, B. Braun Medical A.G., CSEM A.G., Edwards Lifesciences Services GmbH, Kenta Biotech Ltd, Maquet Critical Care A.B., Omnicare Clinical Research A.G., Nestle, Pierre Fabre Pharma A.G., Pfizer, Bard Medica S.A., Abbott A.G., Anandic Medical Systems, Pan Gas A.G. Healthcare, Bracco, Hamilton Medical A.G., Fresenius Kabi, Getinge Group Maquet A.G., Dräger A.G., Teleflex Medical GmbH, Glaxo Smith Kline, Merck Sharp and Dohme A.G., Eli Lilly and Company, Baxter, Astellas, AstraZeneca, C.S.L. Behring, Novartis, Covidien, Nycomed, Phagenesis, and Cytel outside the submitted work. The money was paid into departmental funds. No personal financial gain applied.
Funding Sources
No study-specific funding applies for this analysis.
Author Contributions
Patrick Zuercher is the guarantor of the paper and takes responsibility for the integrity of the work as a whole, from inception to publication. Livia Mueller, Patrick Zuercher, Daniel G. Fuster, and Joerg C. Schefold developed the research strategy and drafted the manuscript with Josef Prazak, Daniel G. Fuster, Joerg C. Schefold, and Michel Moser. Michel Moser performed all statistical analyses. All the authors helped to finalize the manuscript, revised it for important intellectual content, and approved the final version for publication. All the authors meet the criteria for authorship as recommended by the International Committee of Medical Journal Editors.
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author.
Supplementary Material
Supplementary data
Supplementary data
Supplementary data
Funding Statement
No study-specific funding applies for this analysis.
References
- 1.Bailey CJ. Metformin historical overview. Diabetologia. 2017 Sep;60((9)):1566–1576. doi: 10.1007/s00125-017-4318-z. [DOI] [PubMed] [Google Scholar]
- 2.Goodarzi MO, Bryer-Ash M. Metformin revisited re-evaluation of its properties and role in the pharmacopoeia of modern antidiabetic agents. Diabetes Obes Metab. 2005 Nov;7((6)):654–665. doi: 10.1111/j.1463-1326.2004.00448.x. [DOI] [PubMed] [Google Scholar]
- 3.Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, et al. Medical management of hyperglycemia in type 2 diabetes a consensus algorithm for the initiation and adjustment of therapy − a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes care. 2009 Jan;32((1)):193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lalau JD. Lactic acidosis induced by metformin incidence, management and prevention. Drug Saf. 2010 Sep 1;33((9)):727–740. doi: 10.2165/11536790-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Richy FF, Sabido-Espin M, Guedes S, Corvino FA, Gottwald-Hostalek U. Incidence of lactic acidosis in patients with type 2 diabetes with and without renal impairment treated with metformin a retrospective cohort study. Diabetes care. 2014 Aug;37((8)):2291–2295. doi: 10.2337/dc14-0464. [DOI] [PubMed] [Google Scholar]
- 6.Lalau JD, Race JM. Lactic acidosis in metformin therapy. Drugs. 1999;58((Suppl 1)):55–60. doi: 10.2165/00003495-199958001-00013. discussion 75-82. [DOI] [PubMed] [Google Scholar]
- 7.Peters N, Jay N, Barraud D, Cravoisy A, Nace L, Bollaert PE, et al. Metformin-associated lactic acidosis in an intensive care unit. Crit Care. 2008;12((6)):R149. doi: 10.1186/cc7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kajbaf F, Lalau JD. The prognostic value of blood pH and lactate and metformin concentrations in severe metformin-associated lactic acidosis. BMC Pharmacol Toxicol. 2013 Apr 12;14:22. doi: 10.1186/2050-6511-14-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kajbaf F, Lalau JD. Mortality rate in so-called “metformin-associated lactic acidosis” a review of the data since the 1960s. Pharmacoepidemiol Drug Saf. 2014 Nov;23((11)):1123–1127. doi: 10.1002/pds.3689. [DOI] [PubMed] [Google Scholar]
- 10. www.compendium.ch. Metformin Helvepharm Filmtabl, Glucophage Filmtabl, 15.02.2022.
- 11.Lalau JD, Lacroix C. Measurement of metformin concentration in erythrocytes clinical implications. Diabetes Obes Metab. 2003 Mar;5((2)):93–98. doi: 10.1046/j.1463-1326.2003.00241.x. [DOI] [PubMed] [Google Scholar]
- 12.Bailey CJ, Wilcock C, Scarpello JHB. Metformin and the intestine. Diabetologia. 2008 Aug;51((8)):1552–1553. doi: 10.1007/s00125-008-1053-5. [DOI] [PubMed] [Google Scholar]
- 13.Graham GG, Punt J, Arora M, Day RO, Doogue MP, Duong JK, et al. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 2011 Feb;50((2)):81–98. doi: 10.2165/11534750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 14.Sutkowska E, Fortuna P, Wisniewski J, Sutkowska K, Hodurek P, Gamian A, et al. Low metformin dose and its therapeutic serum concentration in prediabetes. Sci Rep. 2021 Jun 3;11((1)):11684. doi: 10.1038/s41598-021-91174-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scheen AJ. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 1996 May;30((5)):359–371. doi: 10.2165/00003088-199630050-00003. [DOI] [PubMed] [Google Scholar]
- 16.Assan R, Heuclin C, Ganeval D, Bismuth C, George J, Girard JR. Metformin-induced lactic acidosis in the presence of acute renal failure. Diabetologia. 1977 May;13((3)):211–217. doi: 10.1007/BF01219702. [DOI] [PubMed] [Google Scholar]
- 17.Runge S, Mayerle J, Warnke C, Robinson D, Roser M, Felix SB, et al. Metformin-associated lactic acidosis in patients with renal impairment solely due to drug accumulation? Diabetes. Diabetes Obes Metab. 2008 Jan;10((1)):91–93. doi: 10.1111/j.1463-1326.2006.00657.x. [DOI] [PubMed] [Google Scholar]
- 18.Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin an overview. Clin Sci. 2012 Mar;122((6)):253–270. doi: 10.1042/CS20110386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lalau JD, Kajbaf F, Protti A, Christensen MM, De Broe ME, Wiernsperger N. Metformin-associated lactic acidosis (MALA) moving towards a new paradigm. Diabetes Obes Metab. 2017 Nov;19((11)):1502–1512. doi: 10.1111/dom.12974. [DOI] [PubMed] [Google Scholar]
- 20.Cohen RD, Woods HF. Clinical and biochemical aspects of lactic acidosis. Oxford Blackwell Scientific. 1976 [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses the PRISMA statement. Ann Intern Med. 2009 Aug 18;151((4)):64–69. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 22.Calello DP, Liu KD, Wiegand TJ, Roberts DM, Lavergne V, Gosselin S, et al. Extracorporeal treatment for metformin poisoning systematic review and recommendations from the extracorporeal treatments in poisoning workgroup. Crit Care Med. 2015 Aug;43((8)):1716–1730. doi: 10.1097/CCM.0000000000001002. [DOI] [PubMed] [Google Scholar]
- 23.Lalau JD, Lacroix C, Compagnon P, de Cagny B, Rigaud JP, Bleichner G, et al. Role of metformin accumulation in metformin-associated lactic acidosis. Diabetes care. 1995 Jun;18((6)):779–784. doi: 10.2337/diacare.18.6.779. [DOI] [PubMed] [Google Scholar]
- 24.Dell'Aglio DM, Perino LJ, Kazzi Z, Abramson J, Schwartz MD, Morgan BW. Acute metformin overdose examining serum pH, lactate level, and metformin concentrations in survivors versus nonsurvivors: a systematic review of the literature. Ann Emerg Med. 2009 Dec;54((6)):818–823. doi: 10.1016/j.annemergmed.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 25.Fall PJ, Szerlip HM. Lactic acidosis from sour milk to septic shock. J Intensive Care Med. 2005 Sep-Oct;20((5)):255–271. doi: 10.1177/0885066605278644. [DOI] [PubMed] [Google Scholar]
- 26.Schure PJCM, de Gooijer A, van Zanten ARH. Unexpected survival from severe metformin-associated lactic acidosis. Neth J Med. 2003 Oct;61((10)):331–333. [PubMed] [Google Scholar]
- 27.von Mach MA, Sauer O, Sacha Weilemann L. Experiences of a poison center with metformin-associated lactic acidosis. Exp Clin Endocrinol Diabetes. 2004 Apr;112((4)):187–190. doi: 10.1055/s-2004-817931. [DOI] [PubMed] [Google Scholar]
- 28.Galea M, Jelacin N, Bramham K, White I. Severe lactic acidosis and rhabdomyolysis following metformin and ramipril overdose. Br J Anaesth. 2007 Feb;98((2)):213–215. doi: 10.1093/bja/ael347. [DOI] [PubMed] [Google Scholar]
- 29.Pan LTT, MacLaren G. Continuous venovenous haemodiafiltration for metformin-induced lactic acidosis. Anaesth Intensive Care. 2009 Sep;37((5)):830–832. doi: 10.1177/0310057X0903700520. [DOI] [PubMed] [Google Scholar]
- 30.Wen YK. Impact of acute kidney injury on metformin-associated lactic acidosis. Int Urol Nephrol. 2009 Dec;41((4)):967–972. doi: 10.1007/s11255-009-9549-6. [DOI] [PubMed] [Google Scholar]
- 31.Carlon R, Dal Cero P, Corbanese U, Possamai C. Three cases of severe metformin-related lactic acidosis. Eur J Anaesthesiol. 2010 Jul;27((7)):666–667. doi: 10.1097/EJA.0b013e3283350c68. [DOI] [PubMed] [Google Scholar]
- 32.Giuliani E, Albertini G, Vaccari C, Barbieri A. pH 6.68--surviving severe metformin intoxication. QJM. 2010 Nov;103((11)):887–890. doi: 10.1093/qjmed/hcq049. [DOI] [PubMed] [Google Scholar]
- 33.Lemyze M, Baudry JF, Collet F, Guinard N. Life threatening lactic acidosis. BMJ. 2010 Mar 24;340((mar24 2)):c857. doi: 10.1136/bmj.c857. [DOI] [PubMed] [Google Scholar]
- 34.Protti A, Russo R, Tagliabue P, Vecchio S, Singer M, Rudiger A, et al. Oxygen consumption is depressed in patients with lactic acidosis due to biguanide intoxication. Crit Care. 2010;14((1)):R22. doi: 10.1186/cc8885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keller G, Cour M, Hernu R, Illinger J, Robert D, Argaud L. Management of metformin-associated lactic acidosis by continuous renal replacement therapy. PLoS One. 2011;6((8)):e23200. doi: 10.1371/journal.pone.0023200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dichtwald S, Weinbroum AA, Sorkine P, Ekstein MP, Dahan E. Metformin-associated lactic acidosis following acute kidney injury. Efficacious treatment with continuous renal replacement therapy. Diabet Med. 2012 Feb;29((2)):245–250. doi: 10.1111/j.1464-5491.2011.03474.x. [DOI] [PubMed] [Google Scholar]
- 37.Krzymień J, Karnafel W. Lactic acidosis in patients with diabetes. Pol Arch Med Wewn. 2013;123((3)):91–97. doi: 10.20452/pamw.1619. [DOI] [PubMed] [Google Scholar]
- 38.Ncomanzi D, Sicat RMR, Sundararajan K. Metformin-associated lactic acidosis presenting as an ischemic gut in a patient who then survived a cardiac arrest a case report. J Med Case Rep. 2014 May 21;8((1)):159. doi: 10.1186/1752-1947-8-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McNamara K, Isbister GK. Hyperlactataemia and clinical severity of acute metformin overdose. Intern Med J. 2015 Apr;45((4)):402–408. doi: 10.1111/imj.12713. [DOI] [PubMed] [Google Scholar]
- 40.Sehra S, Jaggi S, Sehra D, Aggarwal R, Saraswat V, Juneja D. Management of sitagliptin and metformin combination toxic overdose. J Assoc Physicians India. 2016 Nov;64((11)):80–81. [PubMed] [Google Scholar]
- 41.White S, Driver BE, Cole JB. Metformin-associated lactic acidosis presenting as acute ST-elevation myocardial infarction. J Emerg Med. 2016 Jan;50((1)):32–36. doi: 10.1016/j.jemermed.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 42.Nakamura A, Suzuki K, Imai H, Katayama N. Metformin-associated lactic acidosis treated with continuous renal replacement therapy. BMJ Case Rep. 2017 Feb 10;:bcr2016218318. doi: 10.1136/bcr-2016-218318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwetz V, Eisner F, Schilcher G, Eller K, Plank J, Lind A, et al. Combined metformin-associated lactic acidosis and euglycemic ketoacidosis. Wien Klin Wochenschr. 2017 Sep;129((17–18)):646–649. doi: 10.1007/s00508-017-1251-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chiew AL, Wright DFB, Dobos NM, McArdle K, Mostafa AA, Newth A, et al. “Massive” metformin overdose. Br J Clin Pharmacol. 2018 Dec;84((12)):2923–2927. doi: 10.1111/bcp.13582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yeh HC, Ting IW, Tsai CW, Wu JY, Kuo CC. Serum lactate level and mortality in metformin-associated lactic acidosis requiring renal replacement therapy a systematic review of case reports and case series. BMC Nephrol. 2017 Jul 10;18((1)):229. doi: 10.1186/s12882-017-0640-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seidowsky A, Nseir S, Houdret N, Fourrier F. Metformin-associated lactic acidosis a prognostic and therapeutic study. Crit Care Med. 2009 Jul;37((7)):2191–2196. doi: 10.1097/CCM.0b013e3181a02490. [DOI] [PubMed] [Google Scholar]
- 47.Friesecke S, Abel P, Roser M, Felix SB, Runge S. Outcome of severe lactic acidosis associated with metformin accumulation. Crit Care. 2010;14((6)):R226. doi: 10.1186/cc9376. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author.