Abstract
Background
Intestinal Immunoglobulin A (IgA) is crucial in maintaining host-microbiota mutualism and gut homeostasis. It has been shown that many species of gut bacteria produce cyclic dinucleotides, along with an abundance of microbiota-derived DNA present within the intestinal lumen, which triggers the tonic activation of the cytosolic cGAS-STING pathway. However, the role of STING in intestinal IgA remains poorly understood. We further investigated whether and how STING affects intestinal IgA response.
Methods
Intestinal IgA was determined between wild-type (WT) mice and Sting-/- mice in steady conditions and upon enteric Citrobacter rodentium infection. STING agonists were used to stimulating B cells or dendritic cells in vitro. Gut microbiota composition was examined by 16S ribosomal RNA gene sequencing. Bacteria metabolomics functional analyses was performed by PICRUSt2. Fecal short-chain fatty acid (SCFA) was determined by Mass spectrometry and Cedex Bio Analyzer. Gut bacteria from WT mice and Sting-/- mice were transferred into germ-free mice and antibiotic-pretreated mice.
Results
Intestinal IgA response was impaired in Sting-/- mice. However, STING agonists did not directly stimulate B cells or dendritic cells to induce IgA. Interestingly, Sting-/- mice displayed altered gut microbiota composition with decreased SCFA-producing bacteria and downregulated SCFA fermentation pathways. Transfer of fecal bacteria from Sting-/- mice induced less IgA than that from WT mice in germ-free mice and antibiotic-pretreated mice, which is mediated by GPR43. Acetate, the dominant SCFA, was decreased in Sting-/- mice, and supplementation of acetate restored intestinal IgA production in Sting-/- mice.
Conclusions
STING promotes intestinal IgA by regulating acetate-producing gut bacteria.
Keywords: gut IgA, fermentation, microbiota
Graphical Abstract
This graphical abstract is also available at Tidbit: https://tidbitapp.io/tidbits/sting-promotes-intestinal-iga-production-by-regulating-acetate-producing-bacteria-to-maintain-host-microbiota-mutualism
Key Messages.
What is already known?
Intestinal IgA has been crucial in maintaining host-microbiota mutualism and intestinal homeostasis.
What is new here?
STING promotes intestinal IgA response by promoting short-chain fatty acid–producing gut bacteria.
How can this study help patient care?
Enhancing STING signaling could protect patients with IBD.
Introduction
Gut microbiota manipulates the development and function of the host immune system.1,2 Intestinal homeostasis depends on immune regulation and tolerance to gut microbiota. It has been well-established that intestinal Immunoglobulin A (IgA) response is a crucial mechanism that ensures the host-microbiota mutualism and maintains intestinal homeostasis. IgA suppresses the overgrowth of intestinal pathogens and gut microbiota to maintain the mucosa barrier function and reduces the invasion of extracellular pathogens by neutralizing and preventing adherence to underlying epithelial surfaces.3 Meanwhile, the maintenance of IgA relies on commensal microbiota to protect the host from infection.4,5 It is clear that proper IgA levels contribute to the maintenance of intestinal homeostasis; however, how intestinal IgA response is regulated is still not completely understood.
Microbiota-derived pathogen-associated molecular patterns (PAMPs) are recognized by immune cells through pattern recognition receptors (PRRs), thereby inducing immune responses against pathogens and microbiota.6,7 Among PRRs, the stimulator of interferon genes (STING), which acts as an intracellular DNA sensor, plays a critical role in both innate and adaptive immune responses to pathogen infection.8–10 STING signaling can be activated by viral and bacterial dsDNA and cytosolic cyclic-dinucleotides (CDNs) produced by bacteria.8,11 STING signals to TANK binding kinase 1 (TBK1) to activate IRF3 and NF-κB to promote type 1 interferon (IFN) and other pro-inflammatory cytokines (ie, IL-6), which is essential for thwarting infectious pathogens and disease progression.9,12–14 The abundance of microbiota-derived DNA is present within the intestinal lumen, which is produced by many species of gut bacteria and triggers the tonic activation of the cytosolic cGAS-STING pathway.15,16 Accumulating evidence indicates the role of STING in regulating intestinal inflammation and tumorigenesis in that STING-deficient mice are more susceptible to intestinal inflammation and tumorigenesis.12,17 However, whether and how STING regulates intestinal IgA production to contribute to the host-microbiota mutualism and the maintenance of intestinal homeostasis is still unclear.
In this study, we found that STING deficiency leads to decreased intestinal IgA response in steady conditions and upon enteric Citrobacter rodentium infection. Sting-/- mice demonstrated an altered gut microbiota composition characterized by reduced acetate-producing gut bacteria, which mediated STING induction of the intestinal IgA response. Our findings demonstrate that STING regulates intestinal IgA by promoting acetate-producing bacteria, providing insight into how the host-microbiota interaction contributes to the maintenance of intestinal homeostasis.
Materials and Methods
Mice
All specific pathogen-free (SPF) mice were maintained at the University of Texas Medical Branch (UTMB) SPF animal facility on a 12-hour light-dark cycle with a temperature of 20°C to 26°C and 30% to 70% humidity. The C57BL/6J wild-type (WT) mice and B6(Cg)-Sting1tm1.2Camb/J (Sting-/-) mice were purchased from Jackson Laboratory. The GPR43-/- (Ffar2tmLex) mice were a gift from Bristol-Myers Squibb. Wild type and Sting-/- littermates were separately caged or cohoused for 4 weeks after weaning and used as indicated in the text.
Germ-free C57BL/6 mice were bred and housed in flexible film gnotobiotic isolators (Class Biologically Clean, Ltd.) in the germ-free (GF) mouse facility at UTMB. Mice were housed in flexible film vinyl isolators, which were maintained with filtered air and a sterilization port combined with sterilization cylinders to import sterilized supplies for all experiments. All GF mice were housed with a 12-hour light-dark cycle and allowed ad libitum access to a sterile diet and water.
Mice 8 to 12 weeks old of age and sex-matched were used in this study according to protocols approved by the Institutional Animal Care and Use Committee of UTMB.
Citrobacter rodentium Infection Model
Wild type and Sting-/- mice were orally infected with Citrobacter rodentium (ATCC, DBS100) by gavage (5 × 108 colony-forming unit [CFU]/mouse). Fecal samples were collected on day 7 post-infection for fecal IgA and Citrobacter rodentium CFU measurement. Mice were killed on day 10 post-infection, and colons were extracted and stained with H&E for histopathologic analysis.
Anti-type 1 Interferon Antibody Treatment
Wild type mice were treated with anti-IFNα (1 mg per mouse) and anti-IFNβ (0.5 mg per mouse) antibodies (Leinco Technologies) by intraperitoneal injection twice a week. Mice were killed after 2 weeks of treatment. Feces and serum samples were collected before and after treatment.
Mice Antibiotic Treatment
Wild type and Gpr43-/- mice were administered an antibiotic cocktail of 1 g/L Ampicillin (Fisher Bioreagents), 1 g/L Kanamycin (Fisher Bioreagents), 0.5 g/L Vancomycin (ACROS), and 1 g/L Metronidazole (ACROS) in drinking water for 2 weeks. The drinking water containing antibiotic was prepared and refreshed twice a week.
Fecal Microbiota Transplantation Experiment
Fresh fecal pellets were collected from WT and Sting-/- mice. Fecal samples (100mg) were resuspended with 1mL cold-ice reducing phosphate-buffered saline (PBS) (containing 0.05% L-Cysteine hydrochloride, Sigma-Aldrich) and then homologized and centrifuged 50 g at 4°C for 15 minutes to remove larger particles. The bacteria solution was used for gavage after homogenate supernatants were filtered by a 70-μM cell strainer. All the procedures were performed on ice.
All GF mice were pooled together and randomly assigned to different cages. These GF mice were then colonized with 200 μL aliquot of bacteria suspension via oral gavage once a week for 2 weeks. Bacteria-colonized mice stay alone in the separated flexible film vinyl isolators for an additional 2 weeks. Mice were killed 4 weeks after gavage. Fecal samples were collected at 0 and 4 weeks, and serum samples were collected at 4 weeks.
Wild type and Gpr43-/- recipient mice that received pretreatment with antibiotics were switched to regular drinking water 12 hours before microbiota transplantation. The mice were colonized with a 200-μL aliquot of bacteria suspension via oral gavage once a week for 4 weeks. The colonized mice stayed alone for an additional 2 weeks and were killed 6 weeks after gavage. Fecal samples were collected at 0 and 6 weeks, and serum samples were collected at 6 weeks.
Feces Supernatant Preparation
Mice fecal pellets were collected and weighed. Fecal samples (20 mg) were dissolved in 1 mL sterile collection medium (25 mM ethylenediaminetetraacetic acid [EDTA] and 0.05 mg/mL Soybean trypsin inhibitor in PBS), homologized, and centrifuged at 4°C to remove particles. The supernatants were collected and resuspended in 20% glycerol (Fisher BioReagents) with 2 mM PMSF (ACROS). The feces supernatants were stored at −80°C for later use of ELISA. The supernatants used for acetate determination were stored without glycerol.
Quantification of Immunoglobulins by ELISA
Microplates with 96 wells were coated with 1 µg/mL of goat anti-mouse IgA capture antibody or IgG capture antibody (KPL) at 4°C overnight. Plates were washed and blocked with 1% bovine serum albumin in PBS for 1 hour at room temperature. Diluted samples and standards (SouthernBiotech) were incubated in the wells for 2 hours and washed. The wells were next incubated with 2 µg/mL of goat anti-mouse IgA detection antibody or IgG detection antibody (KPL) and the addition of horseradish peroxidase (HRP)-labeled Streptavidin for 1 hour. After adding 3,3',5,5'-Tetramethylbenzidine (TMB) substrate (KPL) and quenched with 1 M H2SO4, IgA or IgG concentrations were measured at 450 nm using the Gene5 instrument (BioTek) according to the manufacturer’s instructions.
Intestinal Epithelial Cells and Lamina Propria Cells Isolation
Intestinal epithelial cells (IECs) and lamina propria cells were isolated as described previously.18 Briefly, small intestines and colons were excised, sliced into 1-cm pieces, and washed with ice-cold PBS. After washing, tissues were incubated with 0.5 mM of EDTA in PBS with agitation for 40 minutes at 37°C. The supernatants were filtered with a 100-µm cell strainer, and IECs were isolated from the interface between 20% and 40% Percoll layer. The remaining tissues were washed and then digested in dissociation buffer (0.5 mg/mL collagenase 4 and 2 µg/mL DNase in RPMI 1640 medium) using the gentleMACSTM Dissociator system (Miltenyi Biotec). Cell suspensions were filtered with a 100-µm cell strainer, and lamina propria cells were separated from the interface between 40% and 75% Percoll layer.
B Cells Isolation and Culture
Splenic cells were obtained by grinding the spleen with the rough surface of 2 glass slides followed by filtration with a 100-µm cell strainer. The red blood cells were removed using Tris-NH4CL buffer. Resting B cells were isolated using Mouse B Lymphocyte Enrichment Set-DM (BD Biosciences) according to the manufacturer’s instructions. The B cells were cultured with or without STING agonists in the presence of 5 µg/mL anti-μ and 5 µg/mL anti-CD40 antibodies. Cells were cultured at 37°C with 5% CO2. Culture supernatants were collected on day 5 to measure IgA and IgG.
Bone Marrow-derived DC Generation
As previously described (Feng et al, 2010), bone marrow cells were isolated from mice and then cultured in 6-well plates with 10% FBS RPMI 1640 medium containing 20 ng/mL GM-CSF (R&D system). The nonadherent cells were collected as BMDCs on day 8, and purity was > 90%. BMDCs were cocultured with B cells with a ratio of 1:5 (BMDCs: B cells) in the presence of 5 µg/mL anti-μ for 5 days. For DMXAA treatment, BMDCs were stimulated with 10 µg/mL DMXAA for 6 hours and then cocultured with B cells.
Flow Cytometry
Isolated spleen and lamia propria cells were washed with PBS and incubated with LIVE/DEAD Fixable Near-IR Dead Cell Stain dye (Invitrogen) to distinguish dead cells. Anti-mouse CD16/32 antibody (Biolegend) was used to block the nonspecific binding to Fc receptors. Cells were stained with a mix of surface antibodies (anti-mouse CD45.2 and anti-mouse B220 antibodies [Biolegend]) in the staining buffer at 4°C for 30 minutes. After washing, cells were fixed and permeabilized using Intracellular Fixation and Permeabilization Buffer Set (ThermoFisher) according to the manufacturer’s instructions. Finally, cells were stained with anti-mouse IgA antibody (SouthernBiotech) in permeabilization buffer at room temperature for 30 minutes.
Bone marrow-derived DCs (BMDCs) were incubated with live/dead dye and anti-mouse CD16/32 antibody. Cells were then stained in the staining buffer at 4°C for 30 minutes with a mix of surface antibodies (anti-mouse CD11b, anti-mouse CD11c, anti-mouse CD45.2, anti-mouse CD40, anti-mouse CD80, anti-mouse CD86 antibodies (Biolegend)).
Cells were collected by BD LSR II/Fortessa and FACS Diva software (BD Biosciences) and analyzed by FlowJo software (Tree Star, Inc).
Hematoxylin/Eosin Staining
Colon and cecum samples were collected from mice, rolled into Swiss rolls, and put into tissue cassettes. The tissues were then fixed in 10% neutral-buffered formalin (Azer scientific) for 24 hours, embedded with paraffin, and sliced into 5-µM sections. After dewaxing and hydration, the slides were stained with modified Mayers Hematoxylin (Epredia) and Eosin Y-solution (Sigma-Aldrich). The Leica ICC50W microscope acquired the image of tissue sections (Leica Microsystems). Pathology scores of Citrobacter rodentium infection were calculated with the following criteria: epithelium change (normal, 0; mild, 1; moderate, 2; severe, 3), lamina propria inflammation (normal, 0; mild, 1; moderate, 2; severe, 3), the extent of the affected area (none, 0; 0-25%, 1; 25-50%, 2; >50%, 3), and severe markers (none, 0; mild submucosal inflammation or <5 crypt abscesses, 1; mild submucosal inflammation and <5 crypt abscesses, 2; severe submucosal inflammation or >5 crypt abscesses or crypt branching, 2; severe submucosal inflammation and >5 crypt abscesses or crypt branching, 3; ulceration or extensive fibrosis, 3).
Quantitative Real-time Polymerase Chain Reaction
Total RNA was extracted from IECs using Trizol (ThermoFisher), followed by cDNA synthesis using the qScript cDNA Synthesis Kit (Quanta Bio) according to the manufacturer’s instructions. Mouse Gapdh primer included forward (GGTTGTCTCCTGCGACTTCA) and reverse (TGGTCCAGGGTTTCTTACTCC). Mouse Pigr primer included forward (ATGAGGCTCTACTTGTTCACGC) and reverse (CGCCTTCTATACTACTCACCTCC). Quantitative real-time polymerase chain reaction was performed for analysis of gene expression by using iTaq universal SYBR Green supermix (Bio-Rad) in the CFX Real-Time 96 PCR detection system (Bio-Rad).
16S rRNA Gene PCR and Electrophoresis
Fecal pellets were collected and stored at −80°C. DNA was isolated from fecal pellets using a MoBio PowerFecal kit (MoBio) according to the manufacturer’s guidelines. The bacteria 16S ribosomal RNA gene was amplified through a universal 16S ribosomal RNA target primer with the product length 447bp (forward, TCCTACGGGAGGCAGCAG; reverse, GGACTACCAGGGTATCTAATCCTGTT). The reaction conditions for the amplification of DNA were 95°C for 5 minutes and 40 cycles of 95°C for 10 seconds and 60°C for 20 seconds. Polymerase chain reaction products were visualized by electrophoresis on 1.5% agarose gels.
Western Blot
The B cells and BMDCs were prepared as described previously. The total protein of cells was extracted by sonication with RIPA lysis buffer (ThermoFisher). After centrifugation, cell lysates were collected to measure the concentration using the BCA kit (ThermoFisher). The cell lysates (20 µg per sample) were then loaded into the lanes of gels (Bio-Rad), and the separated proteins in the gel were electronically transferred to PVDF membranes (ThermoFisher). After blocking with 1% casein buffer (Bio-Rad), membranes were incubated with primary antibodies (STING rabbit mAb 1:1000, conjugated secondary antibody [CST]; β-actin rabbit mAb, 1:2000, CST) overnight at 4°C, followed by incubation with anti-rabbit horseradish peroxidase-conjugated secondary antibody for 1 hour at room temperature. Bands were detected by western ECL substrate (Bio-Rad) using ChemiDoc Imaging System (Bio-Rad). STING/β-actin relative expression was calculated using ImageJ 10.2 software.19
Immunofluorescence Staining
After dewaxing and hydration, the paraffin sections underwent heat antigen retrieval with 0.1 M citrate buffer (pH, 6). The slides were permeabilized and blocked with 10% goat serum and 0.2% Triton X-100 in PBS for 30 minutes at room temperature and incubated with rat anti-mouse IgA primary antibody (1:200, Southern Biotech) at 4°C overnight. Slides were washed and incubated with Texas Red goat anti-rat secondary antibody (1:400, ThermoFisher) for 2 hours at room temperature and finally mounted with ProLong gold antifade reagent with DAPI (ThermoFisher). Fluorescence images of sections were acquired with a Cytation 5 Multi-Mode Reader (BioTek).
Citrobacter rodentium Colony-forming Units Counting
Mice feces were collected on day 7 after Citrobacter rodentium infection. Feces were weighed and homogenized in sterile PBS. Fecal supernatants were inoculated on MacConkey agar-plates (BD Biosciences) at a series of concentrations at 37°C overnight. Colony-forming units were counted and normalized to the weight of feces.
Quantification of Fecal Organic Acid by LC–MS
Mice fecal samples were extracted with 80% isopropanol solution (50 mg per 3 mL) using Beadbeater (BioSpec) and 1-mm zirconia/silica beads (Biospec). Each sample was pulsed for 20 seconds followed by 1 minute on ice. This was done twice for a total of 60 seconds of pulse. After the derivatization of the samples with 3-NPH, LC-MS analysis was performed. Standard analyte solutions (acetate, butyric acid, citric acid, fumaric acid, lactate, malic acid, propionic acid, and succinic acid) were run at 10 different concentrations. Then, D7-butyric acid was used as an internal standard and added to each sample and standard before derivatization. The data were analyzed using Multiquant (SCIEX). The concentration of each sample was normalized to the weight of feces.
Acetate Measurement by Cedex Bio Analyzer
Fecal supernatants (100 µL) without glycerol were used to measure fecal acetate levels using the Acetate V2 Bio kit (Roche Diagnostics) by Cedex Bio Analyzer (Roche Diagnostics) according to the manufacturer’s instructions.
Microbiota 16S rRNA Gene Sequencing and Analysis
Mice fecal bacterial DNA was extracted using a MoBio PowerFecal kit (MoBio). The isolated DNA was amplified using universal 16S rRNA V3-V4 region primers.20 Sequencing was performed with an Illumina MiSeq instrument resulting in 20 000 to 140 000 base pair paired-end reads according to the manufacturer’s guidelines. The raw sequencing data were analyzed by the QIIME2 pipeline.21 The subsequences were analyzed to determine taxonomy assignment using a pretrained Silva 138 V3-V4 hypervariable region naïve Bayes classifier with a sequence identity preclustered at 99%.22 The alpha and beta diversity analyses were generated by the q2-diversity plugin. The alpha diversity was measured with the Shannon diversity index. The beta diversity was calculated from weighted Bray-Curtis dissimilarity matrices and visualized using PCoA plots. The taxonomic biomarkers of WT mice and Sting-/- mice were determined by linear discriminant analysis effect size (LEfSe).23 Linear discriminant analysis effect size analysis was performed with an α value of 0.05 for the factorial Kruskal-Wallis test, and an linear discriminant analysis (LDA) score effect size threshold of 2.5 for the taxa from WT and Sting-/- mice. Bar charts of the relative abundance were created by Atima2, developed by Center for Metagenomics and Microbiome Research of Baylor College of Medicine.
Microbial Gene Functional Pathway Analysis
The prediction of bacterial functional pathways was performed from 16S operational taxonomic unit (OTU) data by PICRUSt2.24 Briefly, 16S OTUs were aligned to amplicon sequence variant (ASV) and an inferred gene family copy ASV. Gene family copy numbers of ASV prediction were mapped to the MetaCyc pathway, which contains pathways involved in both primary and secondary metabolism, as well as associated metabolites, reactions, enzymes, and genes including microorganisms and hosts.25 Differential pathways between groups were examined with FDR adjusted P value <.05 and fold change >1.5 through 2 ways of differential expression analysis, edgeR26 and limma-voom.27
Statistical Analysis
Statistical significance between 2 groups was assessed by an unpaired 2-tailed Student t test or Mann-Whitney U test. Comparisons among 3 or more groups were performed using 1-way ANOVA followed by Dunnett’s test. All data are presented as mean ± SD, and the P value <.05 was considered statistically significant. Data plotting, interpolation, and statistical analysis were performed using GraphPad Prism 9.0 (GraphPad Software, Inc).
Results
STING Pathway Promotes Intestinal IgA Response
To determine whether STING regulates the intestinal IgA response, we compared IgA production between WT mice and Sting-/- mice in steady conditions. We found that Sting-/- mice displayed a lower level of fecal IgA and IgG than WT mice (Figure 1A, B). Serum IgA was also decreased in Sting-/- mice, whereas there was no difference in serum IgG (Figure 1C, D). The IgA + B cells were significantly decreased in the small intestine and colon of Sting-/- mice (Figure 1E, F). Consistently, immunofluorescence staining confirmed that IgA+ Bcells were decreased in the small intestine and colon of Sting-/- mice (Figure 1G). However, there was no difference in the number of splenic IgA+ B cells between WT mice and Sting-/- mice (Figure S1A, B). These results indicate that STING pathway promotes the intestinal IgA response to microbiota.
Figure 1.
Sting-/- mice produce less intestinal IgA in steady conditions and upon enteric infection. A-D, Fecal IgA (A), IgG (B), serum IgA (C), and IgG (D) of WT and Sting-/- mice in steady conditions (n = 8/group). Representative flow cytometry (E) and quantification (F) of IgA + B cells gated on CD45 + population in the small intestine and colon lamina propria of WT and Sting-/- mice (n = 8/group). G, Representative immunofluorescence staining of IgA + cells in the small intestine and colon of WT and Sting-/- mice. The scale bar represents 400 µM. H-K, WT and Sting-/- mice (n = 8/group) were infected with Citrobacter rodentium (CR; 5 × 108 CFU/mouse). Colonic histopathology (H) and pathological scores (I) of WT and Sting-/- mice 10 days after CR infection. The scale bar represents 200 µM. CR CFU counts (J) and CR-specific fecal IgA (K) of WT and Sting-/- mice at day 7 postinfection. Data are presented as mean ± SD and are pooled from 2 independent experiments. Unpaired Student t test (A-D, F, J, and K); Mann-Whitney U test (I). *P < .05, **P < .01, ***P < .001.
To determine whether STING regulates the intestinal IgA response to enteric pathogens and the development of colitis, we infected WT mice and Sting-/- mice with Citrobacter rodentium by gavage, which mimics the colitis induced by human enteropathogenic and enterohaemorrhagic Escherichia coli.28 Mice were killed 10 days after infection. Infected Sting-/- mice developed more severe colitis than WT mice, as evidenced by exacerbated colonic histopathology (Figure 1H, I). Feces were collected to determine bacteria clearance and Citrobacter rodentium–specific IgA. We found that Sting-/- mice showed a higher CFU number than WT mice (Figure 1J), indicating that STING deficiency impairs bacteria clearance. Notably, intestinal Citrobacter rodentium–specific IgA production was significantly decreased in Sting-/- mice (Figure 1K). These results suggest that STING promotes a Citrobacter rodentium–specific IgA response and inhibits colitis development.
STING Does Not Directly Act on B Cells or DCs to Induce IgA Production
To investigate how STING promotes IgA production, we first determined whether STING directly acts on B cells to promote IgA production. STING expression in B cells and other immune cells including CD4 + T cells and bone marrow-derived DCs was determined by western blot. The B cells expressed STING at higher levels than DCs (Figure 2A). To determine whether intrinsic STING signaling promotes B cell IgA production, we activated purified WT and Sting-/- splenic B cells with anti-μ and anti-CD40 antibodies, and IgA production in the supernatant was determined 5 days later. There were no differences in IgA and IgG production between WT and Sting-/- B cells (Figure 2B, C). To determine whether STING directly acts on B cells to promote IgA production, we activated purified WT B cells with anti-μ and anti-CD40 antibodies in the presence or absence of a series of STING agonists, 5,6-Di-methylxanthenone-4-acetic acid (DMXAA) and 10-carboxymethyl-9-acridanone (CMA). STING agonists did not induce IgA and IgG production by B cells (Figure 2D, E). As DCs have been shown as crucial in driving B cell IgA production,29,30 we next investigated whether STING acts on DCs to promote B cell IgA production. We cocultured WT B cells with WT and Sting-/- BMDCs (Figure S2A) in the presence or absence of STING agonist DMXAA. STING agonists did not promote B cell production of IgA and IgG in the presence of DCs (Figure 2F, G). We then pretreated WT BMDCs with DMXAA for 6 hours and cocultured them with B cells for 5 days. The DMXAA pretreatment increased BMDCs expression of CD40, CD80, and CD86 (Figure S2B-D), confirming that DMXAA activates BMDCs.31 However, DMXAA-pretreated BMDCs did not promote B cell production of IgA and IgG (Figure 2H, I). These data indicate that STING does not directly act on B cells or DCs to induce IgA production.
Figure 2.
STING agonists do not act directly on B cells, DCs, or IECs to promote IgA production. A, STING expression was determined by western blot (left pane), and relative STING expression against β-actin was determined (right pane). B-C, Splenic B cells (n = 5/group) from WT and Sting-/- mice were cultured with anti-µ (5 µg/mL) and anti-CD40 (5 µg/mL) for 5 days. IgA (B) and IgG (C) in culture supernatants were determined by ELISA. D-E, Splenic B cells (n = 4/group) were treated with different doses of STING agonists DMXAA and CMA in the presence of anti-µ (5 µg/mL) and anti-CD40 (5 µg/mL) for 5 days. IgA (D) and IgG (E) in culture supernatants were determined by ELISA. F-G) WT B cells were cocultured with WT and Sting-/- BMDCs in the presence of anti-µ (5 µg/mL) for 5 days, and IgA (F) and IgG (G) in culture supernatants were determined by ELISA. H-I, Splenic B cells (n = 4/group) were cocultured with BMDCs pretreated with or without DMXAA in the presence of anti-µ (5 µg/mL) for 5 days. IgA (H) and IgG (I) in culture supernatants were determined by ELISA. Pigr expression in IECs of small intestine (J) and colon (K) of WT and Sting-/- mice (n = 5/group). Data are presented as mean ± SD and are one representative of 3 independent experiments. Unpaired Student t test (B, C, and F-J); 1-way ANOVA test (D-E). *P < .05.
Intestinal luminal IgA is secreted from plasma cells in intestinal lamia propria and transported across intestinal epithelial cells via polymeric immunoglobulin receptor (pIgR).32,33 To investigate whether STING regulates IEC expression of Pigr, leading to more IgA in the intestinal lumen, we measured Pigr expression in IECs of WT and Sting-/- mice. Wild type and Sting-/- IECs expressed Pigr at similar levels in small intestine and colon (Figure 2J, K), indicating that the IgA transporter pIgR in IECs is not involved in the STING promotion of intestinal IgA.
Microbiota Composition Is Altered in Sting-/- Mice
Given that STING promotion of the IgA response does not happen directly by acting on B cells, DCs, or IECs, we investigated whether STING regulates gut microbiota, leading to promoting intestinal IgA response. We analyzed the gut microbiota composition between WT mice and Sting-/- mice using 16S ribosomal RNA gene sequencing. Alpha diversity, which represents microbiota richness and evenness, was decreased in Sting-/- mice (Figure 3A). An assessment of beta diversity showed that the intestinal bacteria communities were distinct in WT mice and Sting-/- mice (Figure 3B). We used linear discriminant analysis effect size to search biomarkers in various levels of taxonomy hierarchy between WT mice and Sting-/- mice microbiota.23 Consistent with the alpha diversity results, many bacteria taxa were decreased in Sting-/- mice (Figure 3C, S3). Furthermore, Sting-/- mice displayed a lower abundance of Firmicutes and Proteobacteria and a higher abundance of Desulfobacterota in the phylum level (Figure 3C). The relative abundance of Prevotellaceae, Rikenellaceae, Marinifilaceae, Peptostreptococcaceae, and Clostridiaceae at the family level was significantly decreased in Sting-/- mice (Figure 3D). Some genera of bacteria were absent in Sting-/- mice, including Dubosiella, Romboutsian, Alloprevotella, Prevotellaceae_UCG_001_group, and Clostridium_sensu_stricto_1 (Figure 3E). These results indicate that STING contributes to maintaining the typical bacteria richness in the intestines.
Figure 3.
Intestinal microbiota composition is altered in Sting-/- mice. A, Gut microbiota alpha diversity of WT and Sting-/- mice (n = 5). B, Principal coordinates analysis (PCoA) plots showed the distribution of bacterial communities between WT and Sting-/- mice gut microbiota (n = 5). C, Biomarkers from phylum to genus of WT and Sting-/- mice displayed as cladogram by LEfSe. The red taxonomical units indicated the increased bacteria, and the green ones indicated the decreased bacteria in Sting-/- mice (n = 5). D-E, Bar charts showed the relative abundance of the 14 most abundant families (D) and the 16 most abundant genera (E) of bacteria in WT and Sting-/- mice (n = 5/group). Data are presented as mean ± SD. Unpaired Student t test (A); weighted Bray-Curtis analysis (B). *P < .05.
STING Promotes the Intestinal IgA Response Through Microbiota
To determine whether the altered microbiota plays a role in STING promotion of IgA, we first cohoused WT mice and Sting-/- mice after weaning in order to normalize the microbial communities in those mice. We measured fecal IgA and IgG in these mice 4 weeks after cohousing. We observed that intestinal IgA and IgG production was restored in Sting-/- mice when cohoused with WT mice (Figure S4A, B). Further, serum IgA and IgG levels were similar in cohoused WT mice and Sting-/- mice (Figure S4C, D). In line with these results, we also found that levels of intestinal IgA+ B cells were similar between cohoused WT mice and Sting-/- mice (Figure S4E, F). To determine whether cohousing also affects enteric infection, we infected cohoused WT and Sting-/- with Citrobacter rodentium. Cohoused WT and Sting-/- mice developed colitis at similar levels 10 days after infection (Figure S4G, H). There were also no differences in Citrobacter rodentium CFU between the 2 groups of mice or in Citrobacter rodentium–specific IgA levels (Figure S4I, J).
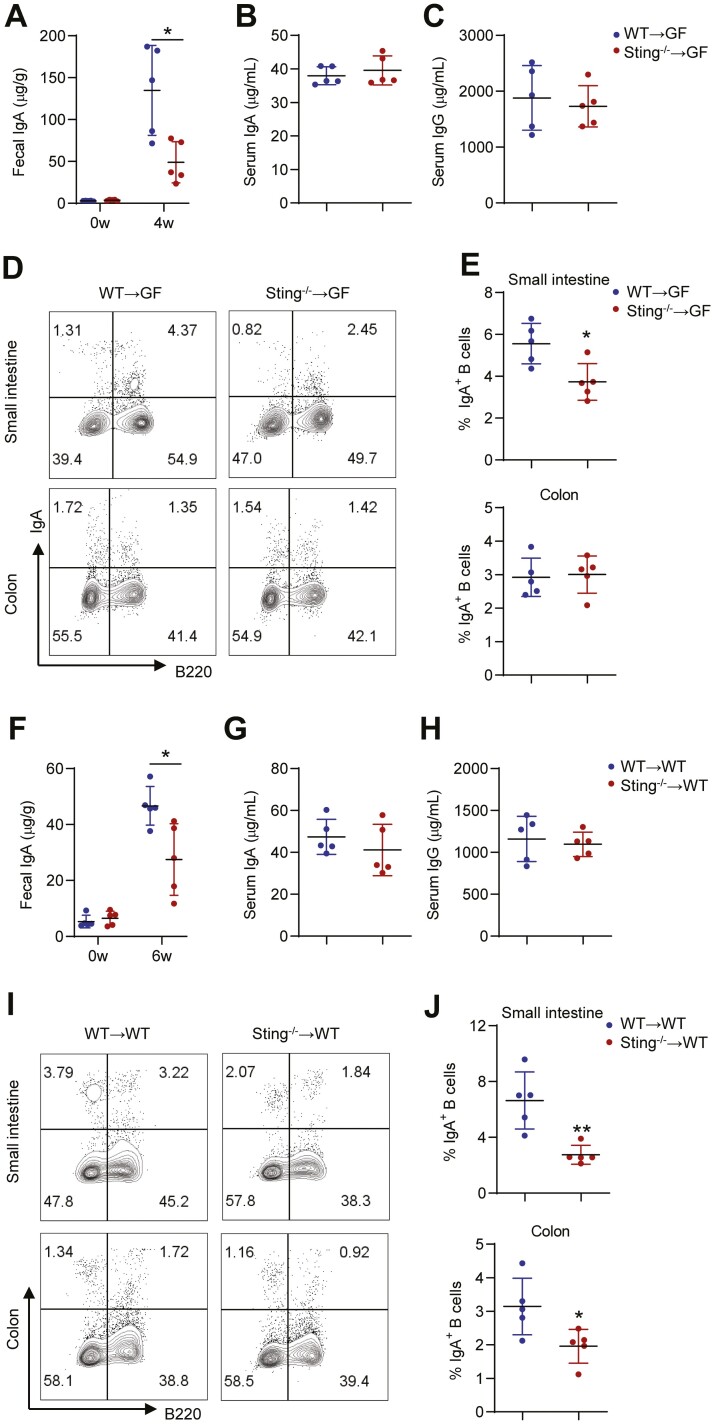
To further determine the role of microbiota in STING promotion of intestinal IgA production, we performed fecal bacteria transplantation. We first transplanted fecal bacteria from WT and Sting-/- mice into GF mice by gavage. These GF mice colonized with fecal bacteria of Sting-/- mice showed lower intestinal IgA than those who received fecal bacteria of WT mice (Figure 4A). There were no differences in serum IgA and IgG between GF recipient mice colonized with fecal bacteria from WT mice or Sting-/- mice (Figure 4B, C). Consistently, intestinal IgA-producing B cells were decreased in GF mice colonized with fecal bacteria of Sting-/- mice (Figure 4D, E). We also transferred fecal bacteria into antibiotic-treated mice. Wild type mice were first treated with a cocktail of antibiotics for 2 weeks. Antibiotic treatment effectively eliminated almost all gut bacteria, which was confirmed by 16S rRNA PCR (Figure S5). We then transferred fecal bacteria from WT mice and Sting-/- mice into the antibiotic-treated mice by gavage. We observed that fecal IgA of the mice receiving fecal bacteria of Sting-/- mice was lower than that of WT mice (Figure 4F). There were no differences in serum IgA and IgG between the 2 groups of recipient mice (Figure 4G, H). Intestinal IgA-producing B cells were decreased in recipient mice with fecal bacteria of Sting-/- mice (Figure 4I, J). These results demonstrate the crucial role of gut microbiota in mediating STING promotion of the intestinal IgA response.
Figure 4.
Fecal bacteria of Sting-/- mice induce less intestinal IgA in germ-free and antibiotic-treated mice. A-E, Fecal bacteria from WT and Sting-/- mice were transplanted to GF mice (n = 5/group). A, Fecal IgA of GF mice before and 4 weeks after colonization with fecal bacteria from WT and Sting-/- mice. Serum IgA (B) and IgG (C) of GF mice 4 weeks after colonization with fecal bacteria from WT and Sting-/- mice (n = 5/group). Representative flow cytometry (D) and quantification (E) of IgA + B cells gated on CD45 + population in the small intestine and colon lamina propria of GF mice colonized with fecal bacteria from WT and Sting-/- mice. F-J, Fecal bacteria from WT and Sting-/- mice were transplanted to antibiotic-treated WT mice (n = 5/group). F, Fecal IgA of antibiotic-treated WT mice before and 6 weeks after colonization with fecal bacteria from WT and Sting-/- mice. Serum IgA (G) and IgG (H) of antibiotic-treated WT mice 6 weeks after colonization with fecal bacteria from WT and Sting-/- mice. Representative flow cytometry (I) and quantification (J) of IgA + B cells gated on CD45 + population in the small intestine and colon lamina propria of antibiotic-treated WT mice colonization with fecal bacteria from WT and Sting-/- mice. Data are presented as mean ± SD and are one representative of 2 independent experiments. Unpaired Student t test (A-C, E-H, and J). *I < .05, **P < .01.
STING Promotion of Intestinal IgA Through Acetate Produced by Gut Bacteria
To investigate how STING-regulated microbiota promote intestinal IgA production, we performed bacteria metabolomics functional analyses from 16S sequencing data by PICRUSt224 and determined the differential microbiota metabolic pathways by edgeR and limma-voom analyses. There were 48 significantly differential pathways between microbiota from WT mice versus Sting-/- mice (Figure S6A). These 48 pathways belong to the major categories’ biosynthesis, degradation/utilization/assimilation, and generation of precursor metabolite and energy (Figure S6B). Among these pathways, pathways related to fermentation in the Generation of Precursor Metabolite and Energy were downregulated in Sting-/- mice, including bacterial metabolic pathway 4-aminobutanoate degradation V, glycerol degradation to butanol, L-lysine fermentation to acetate and butanoate, pyruvate fermentation to acetone, pyruvate fermentation to acetone, and succinate fermentation to butanoate (Figure 5A), indicating that bacterial the process of SCFA fermentation was reduced in Sting-/- mice.
Figure 5.
STING promotes intestinal IgA through acetate produced by gut bacteria. A, Differential pathways form the generation of precursor metabolite and energy of WT and Sting-/- mice bacteria. The x axis shows the log value of fold change expression of WT and Sting-/- mice bacterial pathways. B, Fecal acetate was determined in WT and Sting-/- mice by Cedex Bio Analyzer (n = 16/group). C, A correlation analysis was performed between the relative fecal IgA and fecal acetate in WT mice (n = 16). D, Fecal acetate of cohoused WT and Sting-/- mice was determined by Cedex Bio Analyzer (n = 9/group). E, Fecal acetate of GF mice before and 4 weeks after colonization with fecal bacteria from WT and Sting-/- mice (n = 5/group). F, Fecal acetate of antibiotic-treated WT mice before and 6 weeks after colonization with fecal bacteria from WT and Sting-/- mice (n = 5/group). G, Fecal IgA of Sting-/- mice before and after 3 weeks of feeding 300mM acetate (n = 5/group). Representative flow cytometry (H) and quantification (I) of IgA + B cells gated on CD45 + population in the small intestine and colon lamina propria of Sting-/- mice fed with or without 300 mM acetate. Data are presented as mean ± SD and are pooled from four independent experiments (B-C) or 2 independent experiments (D) or representative of 2 independent experiments (E-I). Unpaired Student t test (B, D-G, and I); Spearman’s rank correlation coefficient (C). *P < .05, **P < .01, ***P < .001.
SCFAs, especially acetate, have been shown to promote intestinal IgA production.34–36 To investigate whether STING regulates SCFA production by gut bacteria, we measured organic acids by mass spectrometry in WT and Sting-/- mice fecal contents. Fecal acetate and butyrate were significantly decreased in Sting-/- mice (Figure S7A, B), while there were no differences in other organic acids (Figure S7C-H). It has been shown that acetate, butyrate, and propionate are the majority of SCFAs in the colon and feces.37–39 Indeed, acetate was the most abundant SCFA in the intestines (Figure S7A-C). We also measured fecal acetate using a Cedex Bio Analyzer. We found that fecal acetate was decreased in Sting-/- mice (Figure 5B). Notably, fecal acetate was positively correlated with fecal IgA in WT mice (Figure 5C). Furthermore, cohousing Sting-/- mice with WT mice led to similar levels of acetate in both groups of mice (Figure 5D, S7I). These GF mice and antibiotic-treated mice colonized with gut microbiota from Sting-/- mice produced less fecal acetate than that with gut microbiota from WT mice (Figure 5E, F). This is consistent with decreased SCFA-producing bacteria in Sting-/- mice (Figure 3C-E), including different genera of Ruminococcaceae,40,41 Lachnospiraceae,40,42 and Clostridiaceae43,44 families from Firmicutes phylum, and Prevotellaceae45 family from Bacteroidota phylum.
To determine whether decreased acetate leads to lower intestinal IgA in Sting-/- mice, we supplemented Sting-/- mice with acetate in drinking water. Supplementation of acetate increased intestinal IgA and IgA-producing B cells in Sting-/- mice (Figure 5G-I). Overall, these data demonstrated that STING promotes growth of acetate-producing bacteria, which induce intestinal IgA.
STING Regulates Acetate-producing Bacteria Through Its Downstream Type 1 IFN
Given that type 1 IFN is the practical product of the cGAS-STING signaling pathway, we investigated whether STING regulated gut IgA through its downstream type 1 IFN. Interferonα and IFNβ are the major subtypes of type 1 IFN.46 Wild type mice were blockaded of type 1 IFN by intraperitoneal injection with anti-IFNα and anti-IFNβ antibodies twice a week. We found fecal IgA was decreased when mice received 2 weeks of anti-type 1 IFN treatment (Figure 6A). Interestingly, serum IgA was significantly downregulated, whereas serum IgG was upregulated considerably after anti-type 1 IFN treatment (Figure 6B, C). Furthermore, acetate fermented by gut bacteria was significantly reduced when WT mice received anti-IFN treatment (Figure 6D).These results indicated that STING may regulate gut IgA and acetate-producing bacteria through its downstream product type 1 IFN.
Figure 6.
STING promotes intestinal IgA and acetate through type 1 IFN. Wild type mice treated with anti-IFNα and anti-IFNβ antibodies treatment for 2 weeks. Fecal IgA (A), serum IgA (B), serum IgG (C), and fecal acetate (D) were determined in WT mice before and after treatment (n = 9/per group). Data are presented as mean ± SD and are pooled from 2 independent experiments. Unpaired Student t test (A-D). *P < .05, **P < .01, ***P < .001.
GPR43 Mediates STING Promotion of Intestinal IgA Response
We have previously shown that acetate induces IgA production in a GPR43-dependent manner.30,34 To investigate whether GPR43 mediates STING promotion of intestinal IgA, we transferred fecal bacteria from WT and Sting-/- mice into antibiotic-treated Gpr43-/- mice. Fecal IgA, serum IgA, and acetate levels were measured 6 weeks later. The levels of acetate were lower in Gpr43-/- recipient mice with fecal bacteria from Sting-/- mice than that from WT mice (Figure 7A). However, there were no differences in intestinal IgA, serum IgA, and serum IgG between these 2 groups of Gpr43-/- mice (Figure 7B-D). Additionally, there were no differences in intestinal IgA+ B cells detected (Figure 7E, F). These results suggest that STING promotes intestinal IgA through gut bacteria-produced acetate in a GPR43-dependent manner.
Figure 7.
STING promotes intestinal IgA in a GPR43-dependent manner. A, Fecal acetate of antibiotic-treated Gpr43-/- mice before and 6 weeks after colonization with fecal bacteria from WT and Sting-/- mice (n = 5/group). B, Fecal IgA of antibiotic-treated Gpr43-/- mice before and 6 weeks after colonization with fecal bacteria from WT and Sting-/- mice (n = 5/group). Serum IgA (C) and IgG (D) of antibiotic-treated Gpr43-/- mice 6 weeks after colonization with fecal bacteria from WT and Sting-/- mice (n = 5/group). Representative flow cytometry (E) and quantification (F) of IgA + B cells gated on CD45 + population in the small intestine and colon LP of antibiotic-treated Gpr43-/- mice colonized with fecal bacteria from WT and Sting-/- mice (n = 5/group). Data are presented as mean ± SD and are one representative of 2 independent experiments. Unpaired Student t test. *P < .05.
Discussion
As the major immunoglobulin isotype in the intestines, IgA is one of the most important factors for maintaining intestinal homeostasis. It has been well-established that gut microbiota plays a crucial role in the induction of the intestinal IgA response against microbiota. Several gut bacteria, including Akkermansia,47 segmented filamentous bacteria (SFB),48–50 and Bacteroides ovatus,51 have been shown to stimulate intestinal IgA production. However, how the microbiota is regulated to induce intestinal IgA is still not fully understood. In this report, we demonstrated that STING promotes SCFA-producing gut bacteria, leading to the induction of intestinal IgA through acetate in a GPR43-dependent manner.
As a cytoplasmic sensor for CDNs, STING plays a crucial role as an adaptor molecule for a number of intracellular DNA receptors. It is vital for host defense against viral, bacterial, and eukaryotic pathogens and also a major player in the development of autoimmune disease.52,53 Recent studies have shown that Sting-/- mice are more susceptible to dextran sulfate sodium-induced acute colitis and STING deficiency alters the gut microbiota,12 demonstrating the role of STING in regulating intestinal homeostasis and inflammation. However, it is still unclear whether such altered gut microbiota plays a role in the STING regulation of intestinal homeostasis. It is also unknown how STING regulates the intestinal IgA response to microbiota.
Our current study demonstrated that intestinal IgA in Sting-/- mice was lower than in WT mice under steady conditions and also upon enteric infection, indicating a role of STING in intestinal IgA production. However, STING agonists did not act directly on B cells, DCs, or IECs to induce IgA production by B cells. Instead, fecal bacteria transplantation in GF mice and antibiotic-treated mice demonstrated that gut microbiota mediates STING promotion of intestinal IgA production, as transfer of fecal bacteria from Sting-/- mice into GF mice and antibiotic-pretreated mice induced less intestinal IgA than fecal bacteria from WT mice. Cohoused WT and Sting-/- mice produced similar levels of intestinal IgA. Interestingly, Sting-/- mice developed more severe colitis than WT mice upon Citrobacter rodentium infection when the mice were housed separately, but WT and Sting-/- mice developed similar levels of intestinal inflammation when they were cohoused. Our study, therefore, provides an example of host-microbiota cross talk in regulating intestinal homeostasis.
Gut microbiota was altered in Sting-/- mice. Among the bacteria decreased in Sting-/- mice, many were SCFA-producing bacteria, including different genera of the Ruminococcaceae, Lachnospiraceae, Clostridiaceae families, and Prevotellaceae families. Although there were no differences in levels of other organic acids, the levels of acetate and butyrate—the most enriched SCFAs in the intestines—were decreased in Sting-/- mice. Mice with SCFAs fermented from gut microbiota have been shown to affect B cell activation and antibody production.30,34,35,54,55 We have shown previously that acetate but not butyrate promotes intestinal IgA production in vivo.34 Interestingly, fecal acetate was positively correlated with fecal IgA in WT mice, and supplementation of acetate could restore the intestinal IgA of Sting-/- mice to the level of WT mice, indicating that STING promotion of intestinal IgA production is mainly mediated by acetate-producing bacteria. Furthermore, gut IgA and fecal acetate were also decreased when type 1 IFN was blocked in WT mice. In a study of gut microbiome of multiple sclerosis patients found that IFNβ treatment could increase the level of SCFAs in multiple sclerosis patients.56 Considering that, cGAS-STING-type 1 IFN pathway contributes to the maintenance of SCFA-producing bacteria.
It has been shown that GPR43 mediates acetate-induction of intestinal IgA.34 Although fecal bacteria of WT mice induced more intestinal IgA than fecal bacteria of Sting-/- mice when transferred into antibiotic-treated WT mice, it induced similar levels of intestinal IgA in GPR43-/- mice. This data confirmed the role of acetate in mediating STING promotion of intestinal IgA production.
In summary, our study demonstrated a critical pathway by which STING regulates intestinal IgA response via maintaining the normal composition of gut microbiota, especially a group of SCFA-producing bacteria. This provides an example of host-microbiota interaction in the regulation of intestinal homeostasis and the pathogenesis of intestinal inflammation, in which STING acts as an adaptor protein to coordinate such host-microbiota mutualism.
Supplementary Material
Acknowledgments
We thank Dr. Qihong Zhao from Bristol-Myers Squibb for kindly providing us GPR43-/- (Ffar2tmLex) mice. We do appreciate Dr. Linsey Yeager of University of Texas Medical Branch for proof reading the manuscript.
Glossary
Abbreviations
- IgA
Immunoglobulin A
- STING
stimulator of interferon genes
- SCFA
short-chain fatty acid
- PAMPs
pathogen-associated molecular patterns
- PRRs
pattern recognition receptors
- FMT
fecal microbiota transplantation; IFN, type 1 interferon
- WT
wild-type
- CDNs
cyclic-dinucleotides; TBK1, TANK binding kinase 1
- CFU
colony-forming unit
- DMXAA
5,6-Di-methylxanthenone-4-acetic acid
- CMA
10-carboxymethyl-9-acridanone
- BMDCs
bone marrow-derived DCs
- IECs
intestinal epithelial cells
- pIgR
polymeric immunoglobulin receptor
- LEfSe
linear discriminant analysis effect size
- CR
Citrobacter rodentium
- SFB
segmented filamentous bacteria
- SPF
specific pathogen-free
- GF
germ-free
- PBS
phosphate-buffered saline
- EDTA
ethylenediaminetetraacetic acid
- LDA
Linear Discriminant Analysis
- OTU
Operational Taxonomic Unit
Contributor Information
Tianming Yu, Department of Microbiology and Immunology, University of Texas Medical Branch, Galveston, TX 77555, USA; Sealy Center for Microbiome Research, University of Texas Medical Branch, Galveston, TX, 77555, USA.
Wenjing Yang, Department of Microbiology and Immunology, University of Texas Medical Branch, Galveston, TX 77555, USA; Sealy Center for Microbiome Research, University of Texas Medical Branch, Galveston, TX, 77555, USA.
Suxia Yao, Department of Microbiology and Immunology, University of Texas Medical Branch, Galveston, TX 77555, USA; Sealy Center for Microbiome Research, University of Texas Medical Branch, Galveston, TX, 77555, USA.
Yanbo Yu, Department of Microbiology and Immunology, University of Texas Medical Branch, Galveston, TX 77555, USA.
Maki Wakamiya, Germ-free Mouse Facility, University of Texas Medical Branch, Galveston, TX 77555, USA.
George Golovko, Department of Pharmacology and Toxicology, University of Texas Medical Branch, Galveston, TX 77555, USA.
Yingzi Cong, Department of Microbiology and Immunology, University of Texas Medical Branch, Galveston, TX 77555, USA; Sealy Center for Microbiome Research, University of Texas Medical Branch, Galveston, TX, 77555, USA.
Author Contribution
Conceptualization, T.Y., W.Y., and Y.C.; formal analysis, T.Y., W.Y., and G.G.; investigation, T.Y., W.Y., S.Y., Y.Y., M.W., G.G., and Y.C.; methodology, T.Y., W.Y., and Y.C.; writing of original draft, T.Y., W.Y., and Y.C; review and editing of manuscript, T.Y., W.Y., and Y.C., with input from all other authors; funding acquisition, Y.C.; resources, Y.C..; supervision, Y.C.
Funding
This work was supported by National Institute of Diabetes and Digestive and Kidney Disease Grants DK105585, DK112436, and DK125011, and the University of Texas System STARs award.
Conflicts of Interest
No financial or conflicts of interest to disclose.
References
- 1. Honda K, Littman DR.. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535(7610):75-84. [DOI] [PubMed] [Google Scholar]
- 2. Wu HJ, Wu E.. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 2012;3(1):4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lycke NY, Bemark M.. The regulation of gut mucosal IgA B-cell responses: recent developments. Mucosal Immunol 2017;10(6):1361-1374. [DOI] [PubMed] [Google Scholar]
- 4. Hapfelmeier S, Lawson MA, Slack E, et al. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science. 2010;328(5986):1705-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Macpherson AJ, Uhr T.. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303(5664):1662-1665. [DOI] [PubMed] [Google Scholar]
- 6. Cao X. Self-regulation and cross-regulation of pattern-recognition receptor signalling in health and disease. Nat Rev Immunol. 2016;16(1):35-50. [DOI] [PubMed] [Google Scholar]
- 7. Takeuchi O, Akira S.. Pattern Recognition Receptors and Inflammation. Cell. 2010;140(6):805-820. [DOI] [PubMed] [Google Scholar]
- 8. Chen Q, Sun L, Chen ZJ.. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat Immunol. 2016;17(10):1142-1149. [DOI] [PubMed] [Google Scholar]
- 9. Vatner RE, Janssen EM.. STING, DCs and the link between innate and adaptive tumor immunity. Mol Immunol. 2019;110:13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang Z-D, Zhong B.. Regulation and function of the cGAS-MITA/STING axis in health and disease. Cell Insight 2022;1(1):100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li Y, Wilson HL, Kiss-Toth E.. Regulating STING in health and disease. J Inflamm (Lond) 2017;14:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Canesso MCC, Lemos L, Neves TC, et al. The cytosolic sensor STING is required for intestinal homeostasis and control of inflammation. Mucosal Immunol 2018;11(3):820-834. [DOI] [PubMed] [Google Scholar]
- 13. Huang L, Li L, Lemos H, et al. Cutting edge: DNA sensing via the STING adaptor in myeloid dendritic cells induces potent tolerogenic responses. J Immunol. 2013;191(7):3509-3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Larkin B, Ilyukha V, Sorokin M, Buzdin A, Vannier E, Poltorak A.. Cutting edge: activation of STING in T cells induces type 1 IFN responses and cell death. J Immunol. 2017;199(2):397-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rudlaff RM, Waters CM.. What is the role of cyclic di-GMP signaling within the human gut microbiome? Microbiome Sci. Med 2014;1:39-44. [Google Scholar]
- 16. Erttmann SF, Swacha P, Aung KM, et al. The gut microbiota prime systemic antiviral immunity via the cGAS-STING-IFN-I axis. Immunity. 2022;55(5):847-861.e10. [DOI] [PubMed] [Google Scholar]
- 17. Zhu Q, Man SM, Gurung P, et al. Cutting edge: STING mediates protection against colorectal tumorigenesis by governing the magnitude of intestinal inflammation. The Journal of Immunology 2014;193(10):4779-4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang W, Liu H, Xu L, et al. GPR120 inhibits colitis through regulation of CD4+ T cell interleukin 10 production. Gastroenterology. 2022;162(1):150-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abràmoff MD, Magalhães PJ, Ram SJ.. Image processing with ImageJ. Biophotonics international 2004;11(7):36-42. [Google Scholar]
- 20. Klindworth A, Pruesse E, Schweer T, et al. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2012;41(1):e1-e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bolyen E, Rideout JR, Dillon MR, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019;37(8):1091852-1091091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bokulich NA, Kaehler BD, Rideout JR, et al. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018;6(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Segata N, Izard J, Waldron L, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12(6):R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Douglas GM, Maffei VJ, Zaneveld JR, et al. PICRUSt2 for prediction of metagenome functions. Nat Biotechnol. 2020;38(6):685-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Caspi R, Billington R, Fulcher CA, et al. The MetaCyc database of metabolic pathways and enzymes. Nucleic Acids Res. 2017;46(D1):D633-D639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Robinson MD, McCarthy DJ, Smyth GK.. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ritchie ME, Phipson B, Wu D, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47-e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Collins JW, Keeney KM, Crepin VF, et al. Citrobacter rodentium: infection, inflammation and the microbiota. Nat Rev Microbiol. 2014;12(9):612-623. [DOI] [PubMed] [Google Scholar]
- 29. Tezuka H, Ohteki T.. Regulation of IgA production by intestinal dendritic cells and related cells. Front Immunol. 2019;10:1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang W, Xiao Y, Huang X, et al. Microbiota metabolite short-chain fatty acids facilitate mucosal adjuvant activity of cholera toxin through GPR43. J Immunol. 2019;203(1):282-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Corrales L, Glickman Laura H, McWhirter Sarah M, et al. Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Reports 2015;11(7):1018-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Phalipon A, Corthésy B.. Novel functions of the polymeric Ig receptor: well beyond transport of immunoglobulins. Trends Immunol. 2003;24(2):55-58. [DOI] [PubMed] [Google Scholar]
- 33. Johansen FE, Kaetzel CS.. Regulation of the polymeric immunoglobulin receptor and IgA transport: new advances in environmental factors that stimulate pIgR expression and its role in mucosal immunity. Mucosal Immunology 2011;4(6):598-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu W, Sun M, Chen F, et al. Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43. Mucosal Immunol 2017;10(4):946-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim M, Qie Y, Park J, Kim C H.. Gut microbial metabolites fuel host antibody responses. Cell Host Microbe. 2016;20(2):202-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Takeuchi T, Miyauchi E, Kanaya T, et al. Acetate differentially regulates IgA reactivity to commensal bacteria. Nature. 2021;595(7868):560-564. [DOI] [PubMed] [Google Scholar]
- 37. Hijova E, Chmelarova A.. Short chain fatty acids and colonic health. Bratisl Lek Listy. 2007;108(8):354-358. [PubMed] [Google Scholar]
- 38. Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT.. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28(10):1221-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Scott KP, Duncan SH, Flint HJ.. Dietary fibre and the gut microbiota. Nutrition Bulletin 2008;33(3):201-211. [Google Scholar]
- 40. Vital M, Karch A, Pieper DH.. Colonic butyrate-producing communities in humans: an overview using omics data. mSystems 2017;2(6):e00130-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang L, Liao Y, Yang R, et al. An engineered probiotic secreting Sj16 ameliorates colitis via Ruminococcaceae/butyrate/retinoic acid axis. Bioeng Transl Med. 2021;6(3):e10219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang J, Song L, Wang Y, et al. Beneficial effect of butyrate-producing Lachnospiraceae on stress-induced visceral hypersensitivity in rats. J Gastroenterol Hepatol. 2019;34(8):1368-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nogal A, Valdes AM, Menni C.. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes 2021;13(1):1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wüst PK, Horn MA, Drake HL.. Clostridiaceae and Enterobacteriaceae as active fermenters in earthworm gut content. ISME J. 2011;5(1):92-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Esquivel-Elizondo S, Ilhan ZE, Garcia-Peña EI, et al. Insights into butyrate production in a controlled fermentation system via gene predictions. mSystems 2017;2(4):e00051-e00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Boxx Gayle M, Cheng G.. The roles of type 1 Interferon in bacterial infection. Cell Host & Microbe 2016;19(6):760-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Planer JD, Peng Y, Kau AL, et al. Development of the gut microbiota and mucosal IgA responses in twins and gnotobiotic mice. Nature. 2016;534(7606):263-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Talham GL, Jiang H-Q, Bos NA, Cebra JJ.. Segmented filamentous bacteria are potent stimuli of a physiologically normal state of the murine gut mucosal immune system. Infect Immun. 1999;67(4):1992-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Umesaki Y, Setoyama H, Matsumoto S, Imaoka A, Itoh K.. Differential roles of segmented filamentous bacteria and clostridia in development of the intestinal immune system. Infect Immun. 1999;67(7):3504-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Palm Noah W, de Zoete Marcel R, Cullen Thomas W, et al. Immunoglobulin a coating identifies colitogenic bacteria in inflammatory bowel disease. Cell. 2014;158(5):1000-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yang C, Mogno I, Contijoch EJ, et al. Fecal IgA levels are determined by strain-level differences in bacteroides ovatus and are modifiable by gut microbiota manipulation. Cell Host & Microbe 2020;27(3):467-475.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Burdette DL, Monroe KM, Sotelo-Troha K, et al. STING is a direct innate immune sensor of cyclic di-GMP. Nature. 2011;478(7370):515-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ishikawa H, Barber GN.. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455(7213):674-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sanchez HN, Moroney JB, Gan H, et al. B cell-intrinsic epigenetic modulation of antibody responses by dietary fiber-derived short-chain fatty acids. Nat Commun. 2020;11(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tan J, McKenzie C, Vuillermin PJ, et al. Dietary fiber and bacterial SCFA enhance oral tolerance and protect against food allergy through diverse cellular pathways. Cell Rep 2016;15(12):2809-2824. [DOI] [PubMed] [Google Scholar]
- 56. Zhou X, Baumann R, Gao X, et al. Gut microbiome of multiple sclerosis patients and paired household healthy controls reveal associations with disease risk and course. Cell. 2022;185(19):3467-3486.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.