Abstract
Drinking water contamination by per- and polyfluoroalkyl substances (PFAS) is widespread near more than 300 United States (U.S.) military bases that used aqueous film-forming foams (AFFF) for fire training and firefighting activities. Much of the PFAS at these sites consist of precursors that can transform into terminal compounds of known health concern but are omitted from standard analytical methods. Here, we estimate the expected duration and contribution of precursor biotransformation to groundwater PFAS contamination at an AFFF-contaminated military base on Cape Cod, Massachusetts, United States, by optimizing a geochemical box model using measured PFAS concentrations from a multidecadal time series of groundwater and a soil survey in the source zone. A toolbox of analytical techniques used to reconstruct the mass budget of PFAS showed that precursors accounted for 46 ± 8% of the extractable organofluorine (a proxy for total PFAS) across years. Terminal PFAS still exceed regulatory limits by 2000-fold decades after AFFF use ceased. Measurements and numerical modeling show that sulfonamido precursors are retained in the vadose zone and their slow biotransformation into perfluoroalkyl sulfonates (half-life > 66 yr) sustains groundwater concentrations of perfluorobutane sulfonate (PFBS) and perfluorohexane sulfonate (PFHxS). The estimated PFAS reservoir in the vadose zone and modeled flux into groundwater suggest PFAS contamination above regulatory guidelines will persist for centuries without remediation.
Keywords: per- and polyfluoroalkyl substances, aquatic contamination, biogeochemistry, vadose zone, groundwater, numerical modeling
Short abstract
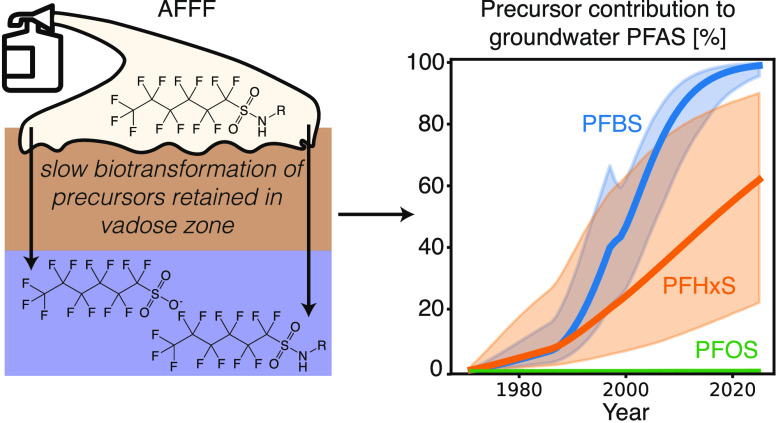
Slow transport and biotransformation of unregulated polyfluoroalkyl precursors are likely to sustain centurial timescales of PFAS contamination at AFFF-contaminated sites
Introduction
Severe contamination of freshwater resources at diverse locations globally has been caused by fire training and firefighting using aqueous film-forming foams (AFFF) that contain 1–5% by weight per- and polyfluoroalkyl substances (PFAS).1 Drinking water hotspots for one PFAS, perfluorooctane sulfonate (PFOS), have been detected near hundreds of United States (U.S.) military bases that repeatedly used AFFF.2 Suppressed immune function and other adverse health effects have been associated with exposures to PFOS at concentrations less than 1 ng L–1, which is more than 10 billion times lower than concentrations found in AFFF.3,4 Polyfluoroalkyl precursors that transform into PFOS and other terminal PFAS of regulatory concern make up the majority of PFAS in AFFF.1,5,6 For example, AFFF manufactured by 3M prior to 2001 (3M AFFF) contained hundreds of precursors that accounted for >50% of the total PFAS.5−7 However, most precursors are not routinely monitored, and all are currently unregulated. Thus, additional information on the physicochemical properties, environmental behavior, and persistence of precursors is urgently needed.
Commercial standards are available for <10 out of the hundreds of precursors previously detected in AFFF.7−10 This means a toolbox of analytical methods (Table S1) is needed to fully account for all of the PFAS in AFFF and present at AFFF-contaminated sites. In prior work, we reconstructed the mass budget for PFAS in AFFF and impacted surface waters, and developed a method for calculating the relative proportions of PFAS produced by 3M and other manufacturing processes in environmental samples.6,11 This method relies on a combined analytical and statistical method that applies the total oxidizable precursor assay (TOP) followed by Bayesian inference (BI). TOP+BI quantifies precursors grouped by their perfluorinated chain length (Cn) and manufacturing origin (electrochemical fluorination/3M versus fluorotelomerization/FT) from the change in perfluoroalkyl carboxylates (PFCA) before and after TOP based on the oxidation patterns of these precursor groups. The method propagates uncertainties associated with analytical measurements and chemical reactions into quantifiable confidence limits (see SI Methods for a detailed description of the technique).6,11 TOP+BI can reproduce extractable organofluorine measurements (EOF) in AFFF, thereby confirming that it quantitatively captures all PFAS derived from AFFF use.6 High-resolution mass spectrometry (HRMS) can confirm the identity and presence of individual precursors but is not a quantitative measurement. Here, we use this toolbox of methods to reconstruct the mass budget for PFAS in groundwater at an AFFF-contaminated site.
Prior field studies and theoretical models have explored PFAS fate and transport at AFFF-contaminated sites.5,12−14 These studies suggest that retention of PFAS in the vadose zone due to partitioning at the air–water interface and biotransformation of precursors may sustain long-term downstream contamination of the more mobile perfluoroalkyl sulfonates (PFSA) and PFCA. However, prior results are either theoretical or only include site measurements from a single snapshot in time. Moreover, they have not quantitatively assessed the impact of hundreds of precursors in AFFF on ongoing contamination because physicochemical properties needed for quantitative model assessment are unknown.
The main objectives of this study were to quantify: (1) the flux of PFAS from the vadose zone into groundwater and (2) the contributions of precursor transformation to groundwater contamination at an AFFF-contaminated military base on Cape Cod, Massachusetts (Joint Base Cape Cod: JBCC). We used multidecadal groundwater time series data in combination with a vadose zone survey conducted by the U.S. military to constrain a numerical model for precursor transport and transformation at the site. Results provide the first estimates of precursor physicochemical properties (sediment–water and air–water interface partitioning) and biotransformation rates grouped by chain length. We project modeling results forward in time to estimate the expected timescales of contamination in the absence of remediation efforts.
Materials and Methods
Field Site Description
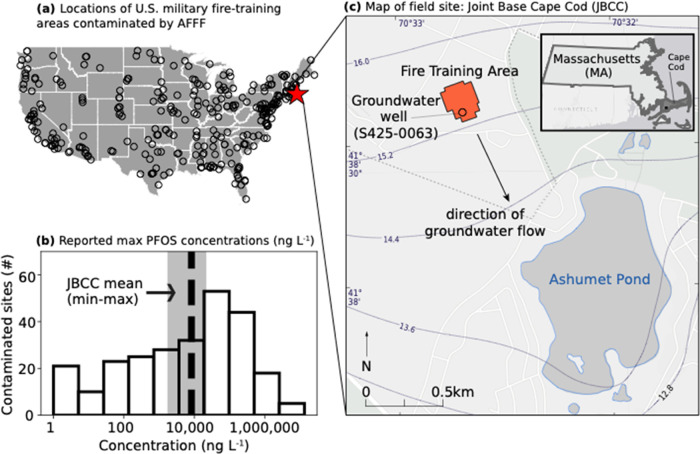
This study was conducted at one of the former fire training areas on JBCC (Figure 1). The hydrogeology of the fire training area has been well characterized through decades of research and data from thousands of groundwater wells near the site.15−18 Soil and sediment consist of quartz and feldspar sand with <0.1% organic carbon and a porosity of 0.39 (Figures S1–S3). The thickness of the vadose zone is approximately 17 m. The annual average recharge from precipitation is 0.73 m. The linear groundwater velocity is 0.4 m day–1. 3M AFFF was used routinely at this site for fire training between 1970 and 1985 and for firefighting once in 1997.12 Because the site’s hydrogeology does not support PFAS retention,19 chemical transport times are expected to be fast relative to other sites. This means the site provides a rapid transport endmember for evaluating the natural attenuation of PFAS contamination compared to most other AFFF-impacted systems.
Figure 1.
Prevalence and magnitude of 3M AFFF contamination at military bases across the continental U.S. (a) Location of 327 military fire training areas with known AFFF use, including the field site for this study (JBCC, red star).20 (b) Maximum concentrations of PFOS measured in groundwater at AFFF-contaminated military sites across the country.21 Gray shading in (b) shows the minimum, mean, and maximum PFOS concentrations at JBCC measured in well S425-0063 indicated in (c). (c) Site map of the fire training area at JBCC. The elevation of the groundwater table in meters is indicated by the contour lines. The land surface elevation of the fire training area is 32 meters above sea level, and the depth to groundwater is ∼17 meters.
Groundwater Time Series Data for 2007–2021
Groundwater samples from the fire training area were collected following established USGS protocols from well MA-SDW 425–0063 (30MW0417C) (short name: S25–0063, Figure 1c) between September 2007 and July 2021 (six time points; Figure 2a).22 All groundwater measurements in this work are from samples collected from the same well at the center of the plume below the fire training area to control for hydrological variability. The variability in the groundwater plume was the focus of previous research.12,23 The well is screened 16.2–19.3 m below ground surface, and the water table fluctuated between 16.0–17.5 m below ground surface over the sampling period. Therefore, samples represent concentrations in the upper 2–3 m of groundwater. All samples, except for the one from September 2007, were collected in high-density polyethylene (HDPE) or polypropylene bottles, stored on ice in the field, and transferred into a 4 °C refrigerator prior to analysis. The sample collected in September 2007 was filtered using a 0.45 μm polyvinylidene fluoride filter into an HDPE bottle and frozen at −20 °C prior to analysis. All archived USGS samples were analyzed for PFAS at Harvard University in July 2021. A subset of samples from the same well that were collected simultaneously with the archived subset in 2016 and analyzed without filtering and immediately after collection were compared to the results. We found minimal differences in PFAS (including precursors) between the two sampling and storage methods. We therefore directly compared concentrations of PFAS from the samples collected in 2007 and from all subsequent years without any corrections throughout this study (additional details of the sample intercomparison are contained in the SI and Figure S4).
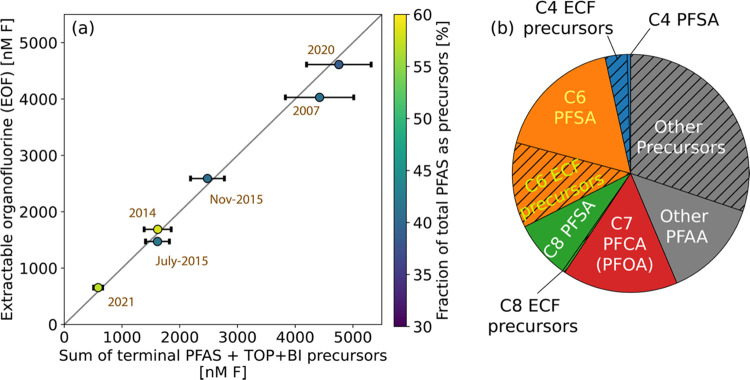
Figure 2.
Mass budget for PFAS in groundwater below the fire training area at Joint Base Cape Cod. (a) Comparison of measured concentrations of EOF to PFAS concentrations measured in JBCC groundwater. The x-axis represents the sum of terminal PFAS and oxidizable precursors inferred from the total oxidizable precursor assay using Bayesian Inference (TOP+BI). The sum of differences from duplicate extracts for targeted PFAS (9%; n = 1) and the standard deviation of inferred precursor concentrations are shown by x-axis error bars. The percent difference in EOF (y-axis) from duplicate extracts was 1% (n = 1). Circles are shaded in purple by the fraction of EOF explained by oxidizable precursors. The one-to-one line (gray) is used to compare the sum of terminal PFAS and precursors measured using targeted analysis and the TOP inference with EOF. (b) Average composition of JBCC groundwater below the fire training area across samples from multiple years (n = 6, 2007–2021). Raw data are presented in Table S7.
PFAS Analyses
Groundwater samples were analyzed for PFAS using four techniques described in detail in the SI Methods. The four analytical techniques included: (1) targeted analysis for 29 PFAS including 11 precursors with available standards using liquid chromatography-tandem mass spectrometry (LC-MS/MS) (Tables S2–S5); (2) the total oxidizable precursor (TOP) assay measured using LC-MS/MS and interpreted using Bayesian inference (TOP+BI, Tables S5–S6, Figure S5); (3) extractable organofluorine (EOF), a proxy for total PFAS, using combustion ion chromatography (Table S7); and (4) suspect screening for 252 PFAS in SCIEX’s database using high-resolution mass spectrometry (HRMS) (Tables S8–S11; comparison of measured and library MS/MS spectra are provided in the SI). The first three techniques provide quantitative information and were performed at Harvard University following previously published methods.6,11,12 The fourth technique, HRMS, provides qualitative information on precursor structures from suspect screening and was performed at SCIEX (Framingham, Massachusetts) on an X500R quadrupole time of flight mass spectrometry system.
We included in our analysis groundwater concentrations of targeted PFAS from the same well at the fire training area from the U.S. National Institute of Standards and Technology and the Air Force (n = 6; Table S5). We also used data from a vadose zone soil survey conducted by the Air Force in our analysis and modeling (Table S12). Details of the analytical methods used by other laboratories are reported in the SI.
Biogeochemical Box Model Development
Model Overview
We developed four first-order geochemical box models for each of the main terminal PFAS in 3M AFFF and their precursors (i.e., PFOA, C4 PFSA precursors + PFBS, C6 PFSA precursors + PFHxS, C8 PFSA precursors + PFOS). Each model simulates biotransformation from precursors into terminal PFSA represented by “boxes” for both the vadose zone and groundwater and quantifies discharges from the vadose zone into groundwater at the JBCC fire training area at steady state. Following optimization using site measurements, each model was applied to examine the temporal evolution of PFAS reservoirs in the vadose zone and groundwater. We did not consider a broader spatial domain for the model or use traditional groundwater models for flow through porous media that are more spatially resolved and highly parameterized because many parameters are unknown or too uncertain (including precursor physicochemical properties and soil moisture content time series) to conduct a meaningful simulation. In addition, these models are computationally expensive for simulations conducted over the timescales relevant for PFAS contamination and uncertainty analysis is infeasible as a result. Instead, we chose a simpler approach that enables us to explicitly quantify and propagate uncertainty in the major physical and chemical processes at the site. Quantified uncertainty bounds for model results show whether conclusions are robust to the large uncertainty in many of the modeled parameters.
Each model was set up as a system of first-order differential equations (eqs 1–4) forced by time-dependent PFAS inputs from 3M AFFF use at the fire training area (eq 5). PFAS inputs were estimated from measured PFAS concentrations in 3M AFFF (c) and yearly volumetric application during active fire training activities between 1970 and 1985 (Vtraining), as well as an additional application for firefighting in 1997 (Vfire) (see the SI for further information).12 Reservoirs M1 and M3 represent precursor masses in the vadose zone and groundwater, respectively. M2 and M4 represent the terminal PFAS masses in the vadose zone and groundwater, respectively. Details on how groundwater and vadose zone concentrations were converted to mass reservoirs are provided in the SI (eq S1, Figure S6, Table S13).
First-order rates in each model include terms for advection of water in the vadose zone (ksoil) and in groundwater (kGW), and PFSA precursor biotransformation rates into terminal PFSA (kbio). Eqs 1 and 3 and the term for kbio were not included in the model for PFOA (2-box model) because 3M AFFF does not contain substantial precursors for the C7 PFCA7,8 and the abundance of 8:2 FT precursors, which originate from other AFFF products, was <8% of PFOA (Table S6). Biotransformation of 8:2 FT precursors produces PFOA and other PFCA.24 Given the low ratio of 8:2 FT precursors to PFOA and multiple potential biotransformation products, it is unlikely that 8:2 FT precursors produced substantial amounts of PFOA at the field site. Production of other PFCA from 8:2 FT precursor transformation could be more important but is outside of the scope of this work. Site-averaged advective transport retardation coefficients with respect to water in the vadose zone (R1,2) and groundwater (R3,4) for terminal PFAS and precursors were used to calculate advection rates in water after accounting for the influence of PFAS sorption to solids and retention at the air–water interface.
Several of the model parameters described above are uncertain due to limited site record-keeping, limited laboratory data, and/or spatiotemporal variability. Therefore, we set up each of the four models with known and uncertain terms described in detail in the SI (Tables S14–S17). Known terms included PFAS concentrations (c) and water advection rates (ksoil and kGW). Uncertain terms that were initially specified as ranges include volumetric AFFF application (V(t)), precursor biotransformation (kbio), and retardation coefficients (R). The model was run to optimize values of the uncertain terms to minimize errors in time series observational constraints of the mass reservoirs in groundwater and the soil survey (M).
| 1 |
| 2 |
| 3 |
| 4 |
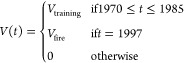 |
5 |
Model Optimization to Derive Uncertain Parameters
We implemented a statistical procedure (Bayesian optimization using Markov Chain Monte Carlo/MCMC simulations) to optimize the values of each uncertain term included in the models. Uniform prior probability distributions were used to parametrize the range of possible values for the uncertain parameters based on available field and laboratory data (Tables S14–S17; Figure S7; see the SI for further discussion). Large potential ranges for kbio, R1, and R2 allowed their values to be primarily constrained by the field measurements. The model was run with repeated random draws to construct scenarios that minimized the sum of squared errors between modeled PFSA/PFCA reservoirs and observational constraints based on vadose zone and groundwater measurements, while maintaining measured PFSA-to-precursor concentration ratios (Table S13). The Vtraining for PFOA in the optimized model was somewhat higher than for the three PFSA, which were in good agreement (overlapping IQR). This may reflect an additional (non-3M AFFF) source at the site or fewer constraints in the PFOA model relative to the PFSA because it does not include precursor biotransformation. Throughout this work, we report the expected value of model parameters (the most probable point estimate) and use the interquartile range to characterize modeled uncertainty. Additional details of the optimization and model evaluation are provided in the SI.
Future Model Projections
We ran the models forward in time for 5000 years to project timescales of natural attenuation. We forced the models using one unit of PFAS distributed between precursors and terminal PFAS by their relative composition in 3M AFFF (Tables S14–S17). We estimated the year that groundwater concentrations will fall below the Massachusetts maximum contaminant level (sum of C6 PFSA, C8 PFSA, and C6-C9 PFCA < 20 ng L–1) as when modeled vadose zone to groundwater fluxes decreased by 2000-times. This is the average amount PFAS measurements in this study of JBCC groundwater exceeded regulatory thresholds.
Site-Averaged Solid–Water (Kd) and Air–Water Interface (Kai) Partition Coefficients
Solid–water (Kd; L kg–1) and air–water interface (Kai; cm) partition coefficients (Table S18) were determined from the biogeochemical box models using eqs S2–S3.25 Optimized advective transport retardation coefficients in groundwater (RGW: R3 for precursors or R4 for terminal PFAS) and the vadose zone (Rsoil: R1 for precursors or R2 for terminal PFAS) were converted to Kd and Kai values using the one-dimensional advection-dispersion equation described in the SI (Tables S14–S17, eqs S2 and S3). The derived partition coefficients represent average site-wide values at steady state.
Results and Discussion
Groundwater PFAS Concentrations Greatly Exceed Drinking Water Guidelines
Measured PFOS concentrations in groundwater samples from the fire training area in this study (max = 22,000 ng L–1) were in the mid-range of those reported at military bases across the country (Figure 1b, Table S5).21 The sum of the six state-regulated PFAS (PFOS/C8 perfluoroalkyl sulfonate: PFSA, PFOA/C7 perfluoroalkyl carboxylate: PFCA, PFHxS/C6 PFSA, and the C6, C8, and C9 PFCA) in samples from well S425-0063 directly below the fire training area ranged between 4300–74,000 ng L–1 (Figure 1c, Table S5). These results show concentrations exceeded the state-level MCL by hundreds to thousands of times decades after active AFFF use at the site stopped.26 Even lower national primary drinking water standards for PFOS and PFOA (4 ng L–1) were proposed by the U.S. Environmental Protection Agency (U.S. EPA) in 2023, further emphasizing the discrepancy between contemporary health guidelines for PFAS in drinking water and concentrations in groundwater at AFFF-contaminated sites.27
Unregulated Precursors Account for Half of the Total PFAS in Groundwater
We measured extractable organofluorine (EOF) concentrations in groundwater that ranged from 700 to 5000 nanomolar fluorine equivalents (nM F) (Figure 2a, Table S7). Past work showed that EOF concentrations provide a good proxy for total PFAS in AFFF and range between (850–900) ×106 nM F in the 3M product.6 JBCC groundwater concentrations are therefore diluted by more than 5 orders of magnitude compared to 3M AFFF.
All measured EOF in groundwater below the fire training area (101 ± 8%) was accounted for by the sum of targeted terminal PFAS and precursor concentrations derived from the TOP+BI results (Figure 2a). This result shows that EOF serves as a good proxy for total PFAS in groundwater primarily contaminated by AFFF. The sum of six regulated PFAS on average accounted for less than half (46 ± 13%) of the EOF, and precursors with analytical grade standards only accounted for an additional 5 ± 1%, indicating that the TOP+BI analysis for detecting additional precursors was needed (Table S7).
TOP+BI results suggest that 3M products accounted for 81 ± 5% of the AFFF used at the fire training area (Table S19, eqs S4–S5). Perfluoroalkyl sulfonamido compounds (C4, C6, and C8) are the predominant precursors in 3M AFFF (46 ± 1% of total PFAS) and are biotransformed in the subsurface into PFSA of the same number of perfluorinated carbons.11,24 Together with their terminal PFSA, these three precursor groups make up 88 ± 1% of total PFAS in 3M AFFF and 40 ± 8% of total PFAS in groundwater beneath the JBCC fire training area.11 The other major terminal PFAS in groundwater was PFOA (C7 PFCA; 17 ± 8% of total PFAS), which is present in 3M AFFF but is not a major biotransformation endpoint for sulfonamido precursors.5,6,24 Another precursor group detected in JBCC groundwater was 6:2 fluorotelomer compounds (22 ± 7% of total PFAS). This class of precursors comprises >90% of PFAS in contemporary non-3M AFFF.6
Using HRMS, we identified 15 precursors in groundwater at a confidence level of 1–2a according to Schymanski et al.28 from a suspect list of 252 PFAS (Table S11). Eleven precursors identified using HRMS were not included in our targeted analyte panel. Almost all (14/15) were perfluoroalkyl sulfonamido compounds previously identified in 3M AFFF or suspected metabolites.7,8 None of the detected perfluoroalkyl sulfonamido precursors are included in current U.S. EPA methods and only one (perfluorooctane sulfonamide) is included in a new draft method.29−31 It has taken decades for other PFAS used in commerce and detected in the environment to be incorporated into standard analytical methods.6,32 TOP+BI is thus a useful intermediate tool for quantifying concentrations of unknown precursors in assessments of contaminated sites. The only non-perfluoroalkyl sulfonamido precursor identified through suspect screening was 6:2 fluorotelomer sulfonate. Using a larger suspect list would likely reveal even more precursors.
No Clear Temporal Trend in Groundwater PFAS Concentrations
In JBCC groundwater, temporal fluctuations in PFAS concentrations of up to an order of magnitude occurred between 2007–2021, but no consistent trends were observed for EOF, terminal PFAS, or precursors (Figure 2a, Figure S8, Table S5). Vertical profiles of PFAS at the site (Figure S9) showed that variability in concentrations reported in this work was not driven by the vertical interval of the fixed well screen relative to the naturally fluctuating vertical position of the groundwater table. Highest PFAS concentrations occurred in both the earliest (2007) and more recent (2020) samples, while lowest concentrations were detected in 2021 (see SI Results and Discussion). Together, these data suggest that PFAS discharges from the vadose zone into groundwater at the fire training area have not yet shown a sustained decline despite cessation of the source more than two decades ago.
Dynamic Soil Moisture Conditions Drive Variable Groundwater PFAS Concentrations
Theoretical simulations have identified variability in the air–water interface and associated partitioning of PFAS as the strongest controls on PFAS transport in the subsurface, but time series data to confirm this at field sites are lacking.33,34 Given the effect size in theoretical simulations, we hypothesized that periods with higher vadose zone moisture content would exhibit elevated groundwater PFAS concentrations over the temporal record in this study. Soil moisture was not recorded at the field site between 2007–2021, so we used several proxies to test this hypothesis. First, we examined county-level temporal data on the Drought Severity and Coverage Index (DSCI).35 Increasing DSCI reflects greater drought conditions, leading to drier soils and lower moisture content, which we hypothesized should result in lower groundwater PFAS concentrations. Increasing DSCI indicative of the onset of drought-like conditions (DSCI > 0) began in May 2021 and continued to rise until they reflected moderate drought conditions at the sampling date in July 2021 (DSCI = 123),35 the date when the lowest PFAS concentrations in groundwater were observed (Figures 2 and S8). DSCI increased from 13 to 64 between June and July 2014 and indicated abnormally dry conditions when we observed 16–44% declines in terminal PFAS between months (Table S20). These data are qualitatively consistent with the hypothesized influence of dynamic vadose zone moisture conditions on groundwater PFAS concentrations from theoretical simulations.34
Next, we examined the relationship between the water altitude of the downgradient groundwater-fed kettle lake (Ashumet Pond, MA, Figure 1c) and PFAS concentrations over time (Figures 3 and S10). We found statistically significant correlations between groundwater PFAS concentrations and the water altitude of the kettle lake for PFOA (C7 PFCA) and PFBS (C4 PFSA) but not for PFHxS (C6 PFSA), PFOS (C8 PFSA), or precursors. Past work indicates the water altitude of the kettle lake is controlled by the position of the water table, which itself reflects recent precipitation and high soil moisture conditions on timescales of weeks to months.15,18 Increasing the altitude of lake water level is therefore a reasonable proxy for higher soil moisture content. It is plausible that the strong positive relationship between the water altitude of the kettle lake and PFOA results in its relative enrichment in the groundwater well near the saturated/unsaturated interface at the groundwater table (Figure 2b).
Figure 3.
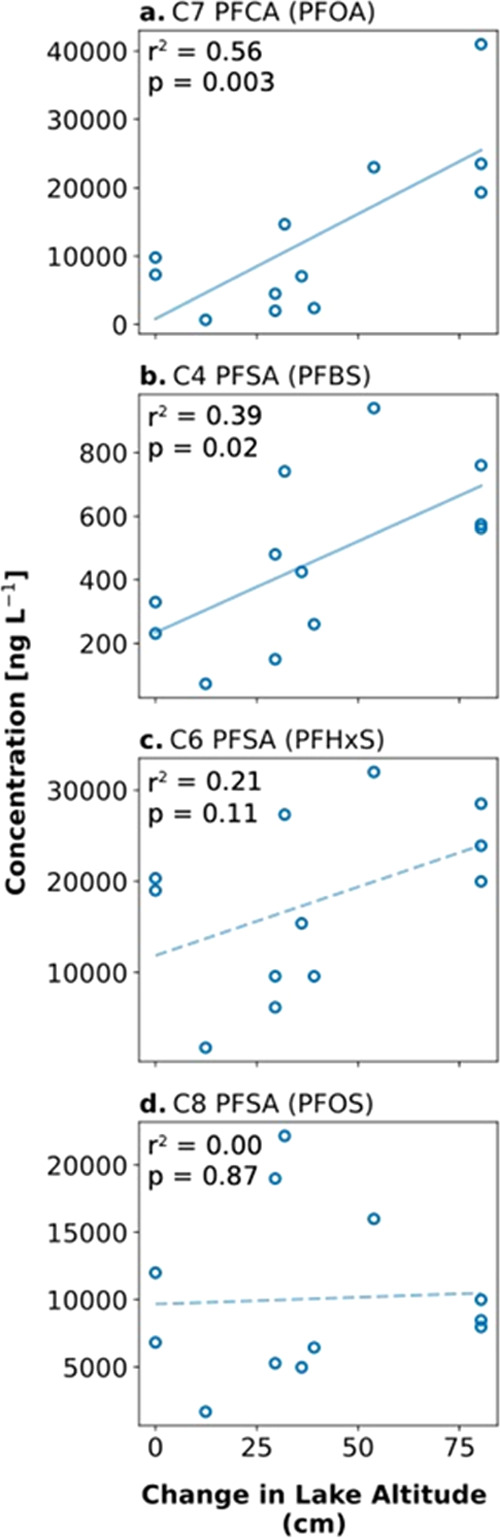
Relationship between PFAS concentrations and proxy for degree of soil saturation. The proxy for soil saturation is the water-level altitude of a proximate exclusively groundwater-fed kettle lake (Ashumet Pond, Figure 1c). The altitude of the water level of the kettle lake is from USGS Water Data for the Nation station number 413758070320501.37 The difference in average daily altitude of the lake level from measurements collected every 30 minutes on the same day as the groundwater sample is shown in the figure (0 = lowest recorded altitude). (a–d) Relationship between PFAS concentrations and the soil saturation proxy on the same day (n = 12). Lines represent the best fit ordinary least squares linear relationship (solid = p-value < 0.05; dashed = p-value > 0.05). Data for precursors are shown in Figure S10.
PFAS transport in the vadose zone and groundwater reflects the balance between sorption to the surfaces of soil/sediment solids, retention at the air–water interface in unsaturated soils, and advection in the aqueous phase.25 Precipitation leads to higher soil moisture content, which results in collapse of the air–water interface at saturation, promotes migration of surface active PFAS into the aqueous phase, and increases aquifer recharge.25,34 Results of this work suggest that variability in groundwater PFAS concentrations reflects site-specific hydrologic conditions and that increasing vadose zone saturation results in greater concentrations of PFAS in groundwater. The influence of soil moisture conditions on PFAS transport that is suggested by both proxies and previous theoretical simulations could be confirmed with site-specific lysimetric measurements in future work.34,36
Physicochemical Properties of PFAS Explain Relationships with Soil Moisture
Solid–water (Kd) and air–water interface (Kai) partition coefficients are physicochemical properties of PFAS and precursors that are essential for understanding field behavior. Precursors in AFFF and at 3M AFFF-contaminated sites occur in mixtures of tens/hundreds of unique compounds that share the same perfluorinated structure.5 The lack of analytical standards for precursors and low concentrations of diverse structures at AFFF-impacted sites has meant basic data on the physicochemical properties of precursors in 3M AFFF are lacking. Using the TOP+BI measurements and optimized box model simulations at the field site, we derived the first field estimates of Kd and Kai for precursors in AFFF grouped by perfluorinated chain length (Figure 4; eqs S2 and S3). Concentration dependency of these coefficients has been observed in prior experimental work but has been shown to have minimal impact on long-term environmental behavior.33
Figure 4.
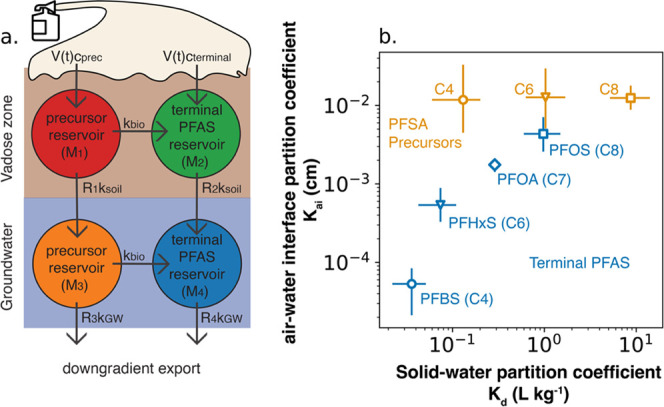
Box model and optimized solid–water (Kd) and air–water interface (Kai) partition coefficients. (a) Reservoirs and mass transfer processes represented by the biogeochemical box model of the fire training area (eqs 1–5). (b) Optimized site-averaged Kd and Kai values for terminal PFAS (blue) and precursors (orange). Symbols represent the mean modeled values, and error bars denote the interquartile ranges from model simulations and are calculated using eqs S2 and S3. Raw data are provided in Table S18.
Variability in Kd and Kai across precursor groups and terminal PFAS (Figure 4) helps to explain the effects of the vadose zone saturation proxy on groundwater PFAS concentrations (Figure 3). Among PFAS in this study, PFOA has relatively low affinity for sorption to solids (Kd < 1 L kg–1) and relatively high activity at the air–water interface (Kai > 10–3 cm) (Figure 4). Our estimates of Kai for PFOA (10–2.9–10–2.7 cm) and PFOS (10–2.6–10–2.1 cm) agree with values reported from laboratory studies conducted at field-relevant concentrations (Table S18).38,39 Groundwater concentrations of PFOA exhibited the strongest relationship with the proxy for soil moisture, suggesting it migrates quickly into groundwater when the air–water interface collapses and soil moisture content approaches saturation (Figure 3). PFBS and PFHxS have lower Kai compared to PFOA (Figure 3). This means they are less surface active and therefore less sensitive to the collapse of the air–water interface. By contrast, PFOS and the C6/C8 PFSA precursors have higher Kai (are more surface active) and higher Kd (are more sorptive to solids) compared to PFOA (Figure 4). This means they can outcompete PFOA for limited sites at the air–water interface under higher soil moisture content conditions and will be preferentially retained on solid surfaces instead of migrating into groundwater, explaining the lack of clear trends with the soil moisture proxy in this work. The combined role of Kd and Kai in producing the observed field trends is consistent with previous theoretical models that find competition at the air–water interface and greater sorption of PFOS leads to its extended retardation in the vadose zone compared to PFOA.40,41
Site-averaged Kd and Kai values from this work vary as a function of the nonfluorinated headgroup (Figure 4). Precursors have significantly greater Kd (ANOVA Type III; F-values > 200; p-values < 0.001) and Kai (ANOVA Type III; F-values > 70; p-values < 0.001) than the PFSA of the same perfluorinated chain length. Since solids at the field site have low organic matter content (<0.1%), sorption is strongly influenced by electrostatic interactions on the net negatively charged solid surfaces.12,17Kd is larger for precursors with neutral and positive charges compared to the negatively charged PFSA (Tables S11 and S18). Kai is larger for precursors than the PFSA because their nonfluorinated substituents are bulkier and less hydrated than sulfonates which enables a closer approach to the air–water interface.42
Similar to prior work, our results show site-averaged Kd increases significantly with the number of perfluorinated carbons for PFSA and precursors (Figure 4; ANOVA Type III; F-values > 5000; p-values < 0.001).19,43 Significant increases in site-averaged Kai were also observed for PFSA (ANOVA Type III; F-values > 2400; p-values < 0.001) but not precursors. Greater than 90% of the smallest chain length precursor group (C4) resides at the air–water interface at the mean site-averaged Kai. The lack of a relationship between precursor Kai and number of perfluorinated carbons may therefore reflect chemical saturation.42
To determine the contribution of each perfluorinated carbon to Kd and Kai, we calculated the change in Gibb’s free energy (ΔG; kJ mol–1) using eq 6, where R is the ideal gas constant (8.3145 × 10–3 kJ mol–1 K–1) and T is the mean annual air temperature at the site (283 K). Specifically, ΔG was calculated as the slope of the partition coefficients (K = Kd or Kai) using weighted least squares regression (weights = 1/interquartile range [IQR] from biogeochemical modeling results).44
| 6 |
For PFSA, we estimate an increase in ΔG of −2.0 to −2.5 kJ mol–1 per perfluorinated carbon. For precursors, we similarly estimate a change in ΔG of −2.5 kJ mol–1 per perfluorinated carbon. The ΔG associated with each additional perfluorinated carbon is much larger than for hydrocarbons (−0.75 kJ mol–1).44 Since ΔG is primarily driven by hydrophobicity, the lower polarizability of perfluorinated carbons compared to hydrocarbons limits their interactions with water and results in large differences in environmental behavior among homologous PFAS (such as the differing relationship among PFSA with the soil moisture proxy in Figure 3).44
In summary, the larger Kd and Kai of precursors result in lower mobility of precursors compared to their terminal PFSA and greater retention in the source zone. This means that the biotransformation of precursors retained near the source zone exerts an essential control on PFAS releases to downgradient groundwater. These first estimates of bulk precursor physicochemical properties enable a quantitative assessment of the impact of precursor biotransformation on groundwater PFAS concentrations, which previous site assessments were only able to do qualitatively.5,12,13
Precursor Biotransformation Sustains Groundwater PFAS Concentrations
We measured significantly lower precursor-to-terminal PFSA concentration ratios in JBCC groundwater compared to 3M AFFF for the C4 and C6 compounds (ANOVA type III; f > 10, p-values < 0.001). For the C4 and C6 compounds, the ratios of precursors to their terminal PFSA were 4.9 ± 1.6 in groundwater compared to 11 ± 4.7 in 3M AFFF (C4 compounds), and 0.7 ± 0.4 in groundwater compared to 6.7 ± 1.4 in 3M AFFF (C6 compounds).5,11 For the C8 compounds, we found no statistically significant difference in precursor-to-terminal PFSA ratios between groundwater (0.041 ± 0.011) and 3M AFFF (0.037 ± 0.001). These results are consistent with significantly greater partition coefficients for precursors compared to PFSA, leading to greater retention of precursors in the vadose zone, and/or production of PFSA through precursor biotransformation (Figure 4).
Using the box models, we estimated the overall residence times in the vadose zone and groundwater beneath the fire training area as 6 years for PFBS (C4) and 19 years for PFHxS (C6). This result suggests PFBS and PFHxS should have: (a) significantly declined in the decades after active AFFF use, and (b) exhibited minimal abundance in groundwater during the sampling period for this study between 2007–2021. Instead, PFHxS was the most abundant terminal PFAS in every sample collected as part of this study and PFBS was detectable in all samples (Table S5). We therefore hypothesized that the persistence of PFBS and PFHxS in groundwater is controlled by slow biotransformation of their precursors.
To determine the contribution of precursor biotransformation to PFSA, we ran the empirically optimized biogeochemical box models with and without precursor inputs and calculated the difference in PFSA groundwater reservoirs over time between the two simulations. Results suggest that the terminal C4 PFSA and C6 PFSA measured during sampling between 2007–2021 primarily reflect precursor biotransformation (Figure 5). In 2021, 98% (interquartile range IQR: 92–99%) of the C4 PFSA and 56% (IQR: 18–88%) of the C6 PFSA in groundwater were formed through precursor biotransformation, whereas C8 precursors only contributed 0.06% (IQR: 0.01–0.2%) to the terminal C8 PFSA. The substantial precursor contributions to the terminal C4 and C6 PFSA in groundwater, but not to the C8 PFSA, is consistent with the concentration ratios of precursors to PFSA in 3M AFFF and the relative mobility of these compounds in the vadose zone and groundwater. These results are additionally supported by the HRMS measurements, which identified C4 and C6 perfluoroalkyl sulfonamido precursors that are expected transformation intermediates of precursors that originated from 3M AFFF (Table S11).24
Figure 5.
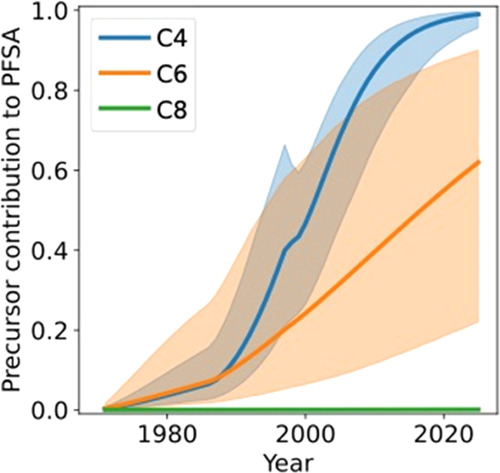
Contribution of precursor biotransformation to PFSA groundwater concentrations. The line represents the expected mean and the bounds represent the interquartile range (IQR) simulated using biogeochemical box models for the former fire training area at JBCC.
Our biogeochemical box modeling resulted in a data-optimized central estimate for first-order precursor biotransformation rates of 1 × 10–3 yr–1 (C4 PFSA precursors) to 2 × 10–3 yr–1 (C6 PFSA precursors) (IQR: 4 × 10–4–1 × 10–2 yr–1) (Table S21). Slow precursor biotransformation (expected half-lives = 340-670 yr; lower bound = 66 yr), indicates that some precursors persist and are transported to downstream environments where potential human and wildlife exposures occur. Slow precursor biotransformation rates help explain why previous microcosm studies (conducted over weeks/months timescales) have observed minimal production of terminal PFAS and have not been able to quantify rates.24,45,46 Instead, most of these studies observe rapid metabolism into other stable precursor intermediates such as the perfluoroalkyl sulfonamides, consistent with high concentrations of these compounds in groundwater at JBCC (Table S5).
Hazardous Concentrations Will Be Observed for Centuries without Vadose Zone Remediation
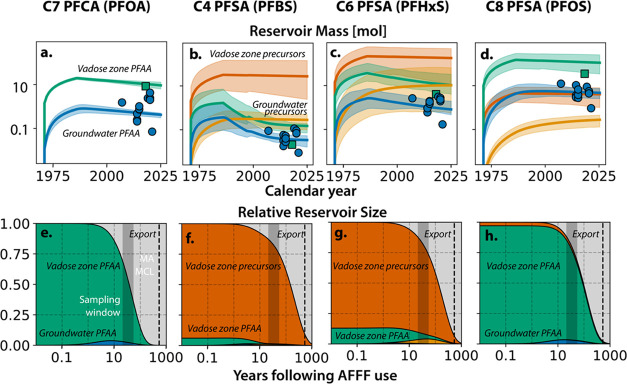
Monitoring data and modeling results both reinforce that the vadose zone at the fire training area is the primary reservoir for all PFAS (Figure 6, Table S13). Our modeling results suggest that 620 mol of PFOA, PFBS, PFHxS, PFOS, and their precursors were released in 3M AFFF at the fire training area (IQR = 330–830 mol). The optimized ranges of PFAS releases are 4 times smaller than the uncertainty bounds specified during model initialization (Tables S14–S17). Constraints from time series data suggest greater than 99% of the cumulative releases occurred between 1970–1985 when the site was actively used as a fire training area. Between 2007 and 2021, the expected mean vadose zone PFAS reservoirs exceeded those in groundwater by a factor of four (PFBS/C4 PFSA) to 97 (C4 PFSA precursors) (Figure 6a–d). These results agree with mathematical and experimental evidence showing much larger solids retention of PFAS under unsaturated compared to saturated conditions.14,33 Meta-analyses of contaminated sites globally, including those where AFFF was used, also show higher PFAS concentrations in the vadose zone compared to underlying groundwater.47,48
Figure 6.
Contemporary and projected future PFAS reservoirs beneath the fire training area. (a–d) Direct measurements as blue circles for groundwater (GW) and green squares for the vadose zone, and modeled reservoirs as lines (expected mean) and shaded regions (IQR) from the first use of the site to the present. (e–h) Projected distribution of PFAS in the vadose zone and groundwater following the use of 3M AFFF. The gray-shaded regions indicate the temporal range of sampling window in this study. The black dashed line indicates the year when concentrations in groundwater are expected to fall below the Massachusetts state-level MCL.
Results from theoretical models and laboratory experiments in prior work suggest that PFAS retention at the air–water interface in the vadose zone is likely to be a rate-limiting step mediating attenuation at AFFF-contaminated sites.14,49 Modeled transfer from the vadose zone to groundwater between 2007 and 2021 (2.6 ± 0.2 mol) represented only ∼0.4% yr–1 of all-time cumulative releases. This means proportionally small PFAS fluxes from the vadose zone have resulted in groundwater concentrations that exceed proposed health guidelines for PFAS in drinking water (Figure 2a). Further, relatively small changes in mass fluxes caused by temporal variability in hydrology (Figure 3) and/or precursor chemistry (Figure 5) have a large impact on groundwater reservoirs.
We ran the optimized biogeochemical box models forward in time to further explore the expected duration of elevated PFAS concentrations after AFFF use (Figure 6e–h). Simulated temporal changes in the PFAS reservoir indicated that in 2021, 57% (IQR = 57–70%) of PFAS releases remained in the vadose zone, 3% (IQR = 2–5%) were in groundwater beneath the fire training area, and 40% (IQR = 25–41%) had migrated to downgradient groundwater and surface waters. The modeled residence times of the C8 PFSA (expected mean = 130 yr) and all PFSA precursors (expected mean = 300-470 yr) occur on timescales of hundreds of years. Without remediation, groundwater concentrations are not expected to fall below the current Massachusetts state-level drinking water MCL before the year 2500. Error bounds for our analysis indicate these findings are robust to the large uncertainties in physicochemical and biotransformation parameters for PFAS considered in this work.
Implications
The U.S. military is the largest global user of AFFF.50 Military sites contaminated by AFFF manufactured by 3M are distinguishable by high PFOS concentrations in groundwater and downgradient drinking water sources (Figure 1a,b).2,21 PFOS is absent from contemporary foams and its production was phased out by 3M (the main global manufacturer) around 2002, indicating residual contamination is a legacy problem.5,6,8 Based on reported groundwater PFOS concentrations at military bases across the U.S., we estimate that 3M AFFF was used by at least two-thirds of the 327 presently identified PFAS-contaminated military sites.21
This study highlights the potential for elevated PFAS exposures downstream from AFFF source zones to persist for centuries. Since chemical transport is comparatively rapid on Cape Cod, other AFFF sites may experience even longer periods of elevated PFAS exposures. The combined effect of surface activity and sorption of PFAS to solids means most releases at AFFF-contaminated sites are retained in the vadose zone. This implies that remediation of the vadose zone is needed to prevent long-term contamination. Otherwise, groundwater contamination would need to be captured and treated for centuries to limit deleterious downstream exposures.
This work highlights the importance of unregulated and often overlooked precursors for total PFAS exposures near AFFF releases and their role in sustaining high concentrations of regulated terminal PFSA, especially PFBS and PFHxS. The U.S. EPA proposed a national primary drinking water standard for a mixture of PFAS which include PFBS and PFHxS but none of their precursors. Some of their perfluoroalkyl sulfonamido precursors persist over long transport distances and times (14 of the 15 precursors identified using HRMS: Table S11). Sulfonamido precursors are present in remote environments, strongly bioaccumulate, and are increasingly accumulating in wildlife.51−53 Additionally, sulfonamido precursors elicit some of the same toxicological responses at lower doses than their terminal PFSA biotransformation products.54 Results presented here provide the first estimates of bulk precursor physicochemical properties that can be used in the future to parameterize fate and transport models to better predict risks of harmful PFAS exposures.
Little is known about population-wide exposures to precursors. The U.S. EPA monitors drinking water for emerging contaminants under the Unregulated Contaminants Monitoring Rule (UCMR) but did not include any precursors in their first PFAS survey (UCMR 3 2012) and plans to include only six precursors (only 1 of the 15 identified in this work) in their second upcoming survey (UCMR 5, slated for 2023-2025).55 Using the toolbox of analytical methods combined in this study at other AFFF-contaminated sites could help address the critical gap in understanding PFAS precursor exposures.
Acknowledgments
Financial support for this work was provided by the Strategic Environmental Research and Development Program (SERDP ER18-1280) and the U.S. National Institute for Environmental Health Sciences Superfund Research Program (P4ES027706). Additional support was provided by the U.S. Geological Survey Toxic Substances Hydrology Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the U.S. National Institute for Environmental Health Sciences but does represent the views of the U.S. Geological Survey. The authors thank Alix E Robel and Katherine T Peter (NIST) for targeted PFAS measurements used for intercomparisons in this work; Philip Gschwend (MIT) for helpful feedback on earlier versions of this work and insights into the field site; and Richard H Anderson and Rose Forbes (Air Force Civil Engineer Center, AFCEC) for access to AFCEC data and reports and feedback on this work. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. This report contains CAS Registry Numbers, which is a registered trademark of the American Chemical Society. CAS recommends the verification of the CASRNs through CAS Client ServicesSM.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.3c00675.
Methods and Results and Discussion; lithology at the fire training area between 0 and 10.7 meters below land surface; lithology at the fire training area 10.7–21.3 meters below land surface; comparison of PFAS between two sample collection and storage procedures; interpolated cross section of PFAS concentrations in groundwater beneath the fire training area; and measured PFAS concentrations in groundwater (well S425-0063) (PDF)
Toolbox of analytical methods for PFAS at AFFF-contaminated sites; targeted analytes for LC-MS/MS determination; PFCA concentrations before and after TOP assay in groundwater (well S425-0063); measured PFAS concentration in vadose zone soil; two-box biogeochemical model parameters for PFOA simulation; precursor biotransformation rates; and half-lives from biogeochemical box modeling (XLSX)
Accession Codes
All code associated with this manuscript is written in Python. Code for TOP+BI is available at https://github.com/SunderlandLab/oxidizable-pfas-precursor-inference. Code for the biogeochemical box model code is available at https://github.com/SunderlandLab/pfas_afff_jbcc/.
Author Contributions
Conceptualization of study: B.J.R. and E.M.S. Methodology, software, validation: B.J.R. and C.P.T. Data curation and visualization: B.J.R. and C.M.B. Investigation and resources: B.J.R., C.M.B., D.R.L., A.K.T., E.M.S. Writing—original draft: B.J.R. and E.M.S. Review and editing: All coauthors. Supervision, project administration: E.M.S. Funding acquisition: A.K.T., E.M.S.
The authors declare no competing financial interest.
Supplementary Material
References
- Wang Z.; DeWitt J. C.; Higgins C. P.; Cousins I. T. A Never-Ending Story of Per- and Polyfluoroalkyl Substances (PFASs)?. Environ. Sci. Technol. 2017, 51, 2508–2518. 10.1021/acs.est.6b04806. [DOI] [PubMed] [Google Scholar]
- Hu X. C.; Andrews D. Q.; Lindstrom A. B.; Bruton T. A.; Schaider L. A.; Grandjean P.; Lohmann R.; Carignan C. C.; Blum A.; Balan S. A.; Higgins C. P.; Sunderland E. M. Detection of Poly- and Perfluoroalkyl Substances (PFASs) in U.S. Drinking Water Linked to Industrial Sites, Military Fire Training Areas, and Wastewater Treatment Plants. Environ. Sci. Technol. Lett. 2016, 3, 344–350. 10.1021/acs.estlett.6b00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P.; Andersen E. W.; Budtz-Jørgensen E.; Nielsen F.; Mølbak K.; Weihe P.; Heilmann C. Serum Vaccine Antibody Concentrations in Children Exposed to Perfluorinated Compounds. JAMA 2012, 307, 391–397. 10.1001/jama.2011.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton S. E.; Ducatman A.; Boobis A.; DeWitt J. C.; Lau C.; Ng C.; Smith J. S.; Roberts S. M. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ. Toxicol. Chem. 2021, 40, 606–630. 10.1002/etc.4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtz E. F.; Higgins C. P.; Field J. A.; Sedlak D. L. Persistence of Perfluoroalkyl Acid Precursors in AFFF-Impacted Groundwater and Soil. Environ. Sci. Technol. 2013, 47, 8187–8195. 10.1021/es4018877. [DOI] [PubMed] [Google Scholar]
- Ruyle B. J.; Thackray C. P.; McCord J. P.; Strynar M. J.; Mauge-Lewis K. A.; Fenton S. E.; Sunderland E. M. Reconstructing the Composition of Per- and Polyfluoroalkyl Substances in Contemporary Aqueous Film-Forming Foams. Environ. Sci. Technol. Lett. 2021, 8, 59–65. 10.1021/acs.estlett.0c00798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzen-Hanson K. A.; Roberts S. C.; Choyke S.; Oetjen K.; McAlees A.; Riddell N.; McCrindle R.; Ferguson P. L.; Higgins C. P.; Field J. A. Discovery of 40 Classes of Per- and Polyfluoroalkyl Substances in Historical Aqueous Film-Forming Foams (AFFFs) and AFFF-Impacted Groundwater. Environ. Sci. Technol. 2017, 51, 2047–2057. 10.1021/acs.est.6b05843. [DOI] [PubMed] [Google Scholar]
- Place B. J.; Field J. A. Identification of Novel Fluorochemicals in Aqueous Film-Forming Foams Used by the US Military. Environ. Sci. Technol. 2012, 46, 7120–7127. 10.1021/es301465n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backe W. J.; Day T. C.; Field J. A. Zwitterionic, Cationic, and Anionic Fluorinated Chemicals in Aqueous Film Forming Foam Formulations and Groundwater from U.S. Military Bases by Nonaqueous Large-Volume Injection HPLC-MS/MS. Environ. Sci. Technol. 2013, 47, 5226–5234. 10.1021/es3034999. [DOI] [PubMed] [Google Scholar]
- D’Agostino L. A.; Mabury S. A. Identification of Novel Fluorinated Surfactants in Aqueous Film Forming Foams and Commercial Surfactant Concentrates. Environ. Sci. Technol. 2014, 48, 121–129. 10.1021/es403729e. [DOI] [PubMed] [Google Scholar]
- Ruyle B. J.; Pickard H. M.; LeBlanc D. R.; Tokranov A. K.; Thackray C. P.; Hu X. C.; Vecitis C. D.; Sunderland E. M. Isolating the AFFF Signature in Coastal Watersheds Using Oxidizable PFAS Precursors and Unexplained Organofluorine. Environ. Sci. Technol. 2021, 55, 3686–3695. 10.1021/acs.est.0c07296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A. K.; Barber L. B.; LeBlanc D. R.; Sunderland E. M.; Vecitis C. D. Geochemical and Hydrologic Factors Controlling Subsurface Transport of Poly- and Perfluoroalkyl Substances, Cape Cod, Massachusetts. Environ. Sci. Technol. 2017, 51, 4269–4279. 10.1021/acs.est.6b05573. [DOI] [PubMed] [Google Scholar]
- Adamson D. T.; Nickerson A.; Kulkarni P. R.; Higgins C. P.; Popovic J.; Field J.; Rodowa A.; Newell C.; DeBlanc P.; Kornuc J. J. Mass-Based, Field-Scale Demonstration of PFAS Retention within AFFF-Associated Source Areas. Environ. Sci. Technol. 2020, 54, 15768–15777. 10.1021/acs.est.0c04472. [DOI] [PubMed] [Google Scholar]
- Guo B.; Zeng J.; Brusseau M. L. A Mathematical Model for the Release, Transport, and Retention of Per- and Polyfluoroalkyl Substances (PFAS) in the Vadose Zone. Water Resour. Res. 2020, 56, e2019WR026667 10.1029/2019WR026667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc D. R.; Guswa J. H.; Frimpter M. H.; Londquist C. J.. Ground-Water Resources of Cape Cod, Massachusetts; Hydrologic Atlas; USGS Numbered Series 692; United States Geological Survey, 1986.
- Garabedian S. P.; LeBlanc D. R.; Gelhar L. W.; Celia M. A. Large-Scale Natural Gradient Tracer Test in Sand and Gravel, Cape Cod, Massachusetts: 2. Analysis of Spatial Moments for a Nonreactive Tracer. Water Resour. Res. 1991, 27, 911–924. 10.1029/91WR00242. [DOI] [Google Scholar]
- Barber L. B.; Thurman E. M.; Runnells D. D. Geochemical Heterogeneity in a Sand and Gravel Aquifer: Effect of Sediment Mineralogy and Particle Size on the Sorption of Chlorobenzenes. J. Contam. Hydrol. 1992, 9, 35–54. 10.1016/0169-7722(92)90049-K. [DOI] [Google Scholar]
- Massey A. J.; Carlson C. S.; LeBlanc D. R.. Ground-Water Levels near the Top of the Water-Table Mound, Western Cape Cod, Massachusetts, 2002-04; Scientific Investigations Report; Scientific Investigations Report 2006–5054; U.S. Geological Survey: Reston, Virginia, 2006; p 14. https://pubs.er.usgs.gov/publication/sir20065054 (accessed April 19, 2022).
- Nguyen T. M. H.; Bräunig J.; Thompson K.; Thompson J.; Kabiri S.; Navarro D. A.; Kookana R. S.; Grimison C.; Barnes C. M.; Higgins C. P.; McLaughlin M. J.; Mueller J. F. Influences of Chemical Properties, Soil Properties, and Solution PH on Soil–Water Partitioning Coefficients of Per- and Polyfluoroalkyl Substances (PFASs). Environ. Sci. Technol. 2020, 54, 15883–15892. 10.1021/acs.est.0c05705. [DOI] [PubMed] [Google Scholar]
- Andrews D. Q.; Naidenko O. V. Population-Wide Exposure to Per- and Polyfluoroalkyl Substances from Drinking Water in the United States. Environ. Sci. Technol. Lett. 2020, 7, 931–936. 10.1021/acs.estlett.0c00713. [DOI] [Google Scholar]
- Northeastern University Social Science Environmental Health Research Institute (SSEHRI). PFAS Contamination Site Tracker. PFAS Contamintion Site Tracker. https://pfasproject.com/pfas-sites-and-community-resources/ (accessed April 19, 2022).
- Savoie J. G.; LeBlanc D. R.; Fairchild G. M.; Smith R. L.; Kent D. B.; Barber L. B.; Repert D. A.; Hart C. P.; Keefe S. H.; Parsons L. A.. Groundwater-Quality Data for a Treated-Wastewater Plume near the Massachusetts Military Reservation, Ashumet Valley, Cape Cod, Massachusetts, 2006–08; Data Series; Data Series 648; U.S. Geological Survey: Reston, Virginia, 2012; p 11. http://pubs.usgs.gov/ds/648 (accessed April 19, 2022).
- Tokranov A. K.; LeBlanc D. R.; Pickard H. M.; Ruyle B. J.; Barber L. B.; Hull R. B.; Sunderland E. M.; Vecitis C. D. Surface-Water/Groundwater Boundaries Affect Seasonal PFAS Concentrations and PFAA Precursor Transformations. Environ. Sci.: Processes Impacts 2021, 23, 1893–1905. 10.1039/D1EM00329A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. J.; Helbling D. E.; Liu J.; Olivares C. I.; Higgins C. P. Microbial Biotransformation of Aqueous Film-Forming Foam Derived Polyfluoroalkyl Substances. Sci. Total Environ. 2022, 824, 153711 10.1016/j.scitotenv.2022.153711. [DOI] [PubMed] [Google Scholar]
- Brusseau M. L. Assessing the Potential Contributions of Additional Retention Processes to PFAS Retardation in the Subsurface. Sci. Total Environ. 2018, 613–614, 176–185. 10.1016/j.scitotenv.2017.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (accessed 2022-04-19)
- (accessed 2022-04-19)
- Schymanski E. L.; Jeon J.; Gulde R.; Fenner K.; Ruff M.; Singer H. P.; Hollender J. Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. 10.1021/es5002105. [DOI] [PubMed] [Google Scholar]
- Shoemaker J. A.; Tettenhorst D. R.. Method 537.1 Determination of Selected Per- and Polyfluorinated Alkyl Substances in Drinking Water by Solid Phase Extraction and Liquid Chromatography/Tandem Mass Spectrometry (LC/MS/MS), 2018. https://cfpub.epa.gov/si/si_public_record_Report.cfm?dirEntryId=343042&Lab=NERL(accessed April 19, 2022).
- Rosenblum L.; Wendelken S. C.. Method 533: Determination of Per- and Polyfluoroalkyl Substances in Drinking Water by Isotope Dilution Anion Exchange Solid Phase Extraction and Liquid Chromatography/Tandem Mass Spectrometry, 2019. https://www.epa.gov/sites/default/files/2019-12/documents/method-533-815b19020.pdf(accessed April 19, 2022).
- Hanley A.Draft Method 1633 Analysis of Per- and Polyfluoroalkyl Substances (PFAS) in Aqueous, Solid, Biosolids, and Tissue Samples by LC-MS/MS, 2021. https://www.epa.gov/system/files/documents/2021-09/method_1633_draft_aug-2021.pdf(accessed May 04, 2022).
- Grandjean P. Delayed Discovery, Dissemination, and Decisions on Intervention in Environmental Health: A Case Study on Immunotoxicity of Perfluorinated Alkylate Substances. Environ. Health 2018, 17, 62 10.1186/s12940-018-0405-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J.; Brusseau M. L.; Guo B. Model Validation and Analyses of Parameter Sensitivity and Uncertainty for Modeling Long-Term Retention and Leaching of PFAS in the Vadose Zone. J. Hydrol. 2021, 603, 127172 10.1016/j.jhydrol.2021.127172. [DOI] [Google Scholar]
- Wallis I.; Hutson J.; Davis G.; Kookana R.; Rayner J.; Prommer H. Model-Based Identification of Vadose Zone Controls on PFAS Mobility under Semi-Arid Climate Conditions. Water Res. 2022, 225, 119096 10.1016/j.watres.2022.119096. [DOI] [PubMed] [Google Scholar]
- (accessed Feb 11, 2022)
- Anderson R. H. The Case for Direct Measures of Soil-to-Groundwater Contaminant Mass Discharge at AFFF-Impacted Sites. Environ. Sci. Technol. 2021, 55, 6580–6583. 10.1021/acs.est.1c01543. [DOI] [PubMed] [Google Scholar]
- (accessed Feb 25, 2022)
- Schaefer C. E.; Culina V.; Nguyen D.; Field J. Uptake of Poly- and Perfluoroalkyl Substances at the Air–Water Interface. Environ. Sci. Technol. 2019, 53, 12442–12448. 10.1021/acs.est.9b04008. [DOI] [PubMed] [Google Scholar]
- Stults J. F.; Choi Y. J.; Schaefer C. E.; Illangasekare T. H.; Higgins C. P. Estimation of Transport Parameters of Perfluoroalkyl Acids (PFAAs) in Unsaturated Porous Media: Critical Experimental and Modeling Improvements. Environ. Sci. Technol. 2022, 56, 7963–7975. 10.1021/acs.est.2c00819. [DOI] [PubMed] [Google Scholar]
- Silva J. A. K.; Šimůnek J.; McCray J. E. A Modified HYDRUS Model for Simulating PFAS Transport in the Vadose Zone. Water 2020, 12, 2758. 10.3390/w12102758. [DOI] [Google Scholar]
- Abraham J. E. F.; Mumford K. G.; Patch D. J.; Weber K. P. Retention of PFOS and PFOA Mixtures by Trapped Gas Bubbles in Porous Media. Environ. Sci. Technol. 2022, 56, 15489–15498. 10.1021/acs.est.2c00882. [DOI] [PubMed] [Google Scholar]
- Psillakis E.; Cheng J.; Hoffmann M. R.; Colussi A. J. Enrichment Factors of Perfluoroalkyl Oxoanions at the Air/Water Interface. J. Phys. Chem. A 2009, 113, 8826–8829. 10.1021/jp902795m. [DOI] [PubMed] [Google Scholar]
- Higgins C. P.; Luthy R. G. Sorption of Perfluorinated Surfactants on Sediments. Environ. Sci. Technol. 2006, 40, 7251–7256. 10.1021/es061000n. [DOI] [PubMed] [Google Scholar]
- Schwarzenbach R. P.; Gschwend P. M.; Imboden D. M.. Environmental Organic Chemistry, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, New Jersey,2017. [Google Scholar]
- Mejia Avendaño S.; Liu J. Production of PFOS from Aerobic Soil Biotransformation of Two Perfluoroalkyl Sulfonamide Derivatives. Chemosphere 2015, 119, 1084–1090. 10.1016/j.chemosphere.2014.09.059. [DOI] [PubMed] [Google Scholar]
- Ruyle B. J.; Schultes L.; Akob D. M.; Harris C. R.; Lorah M. M.; Vojta S.; Becanova J.; McCann S.; Pickard H. M.; Pearson A.; Lohmann R.; Vecitis C. D.; Sunderland E. M. Nitrifying Microorganisms Linked to Biotransformation of Perfluoroalkyl Sulfonamido Precursors from Legacy Aqueous Film-Forming Foams. Environ. Sci. Technol. 2023, 57, 5592–5602. 10.1021/acs.est.2c07178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter Anderson R.; Adamson D. T.; Stroo H. F. Partitioning of Poly- and Perfluoroalkyl Substances from Soil to Groundwater within Aqueous Film-Forming Foam Source Zones. J. Contam. Hydrol. 2019, 220, 59–65. 10.1016/j.jconhyd.2018.11.011. [DOI] [PubMed] [Google Scholar]
- Brusseau M. L.; Anderson R. H.; Guo B. PFAS Concentrations in Soils: Background Levels versus Contaminated Sites. Sci. Total Environ. 2020, 740, 140017 10.1016/j.scitotenv.2020.140017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusseau M. L.; Guo B. PFAS Concentrations in Soil versus Soil Porewater: Mass Distributions and the Impact of Adsorption at Air-Water Interfaces. Chemosphere 2022, 302, 134938 10.1016/j.chemosphere.2022.134938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin R.Estimated Quantities of Aqueous Film Forming Foam (AFFF) in the United States; Stockholm Convention: Baltimore, MD, 2004. https://www.informea.org/en/estimated-quantities-aqueous-film-forming-foam-afff-united-states-2004(accessed May 08, 2019). [Google Scholar]
- Chu S.; Letcher R. J.; McGoldrick D. J.; Backus S. M. A New Fluorinated Surfactant Contaminant in Biota: Perfluorobutane Sulfonamide in Several Fish Species. Environ. Sci. Technol. 2016, 50, 669–675. 10.1021/acs.est.5b05058. [DOI] [PubMed] [Google Scholar]
- Dassuncao C.; Hu X. C.; Zhang X.; Bossi R.; Dam M.; Mikkelsen B.; Sunderland E. M. Temporal Shifts in Poly- and Perfluoroalkyl Substances (PFASs) in North Atlantic Pilot Whales Indicate Large Contribution of Atmospheric Precursors. Environ. Sci. Technol. 2017, 51, 4512–4521. 10.1021/acs.est.7b00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett H.; Du X.; Houde M.; Lair S.; Verreault J.; Peng H. Suspect and Nontarget Screening Revealed Class-Specific Temporal Trends (2000–2017) of Poly- and Perfluoroalkyl Substances in St. Lawrence Beluga Whales. Environ. Sci. Technol. 2021, 55, 1659–1671. 10.1021/acs.est.0c05957. [DOI] [PubMed] [Google Scholar]
- Rericha Y.; Cao D.; Truong L.; Simonich M. T.; Field J. A.; Tanguay R. L. Sulfonamide Functional Head on Short-Chain Perfluorinated Substance Drives Developmental Toxicity. iScience 2022, 25, 103789 10.1016/j.isci.2022.103789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Environmental Protection Agency. 86 FR 13846: Revisions to the Unregulated Contaminant Monitoring Rule (UCMR 5) for Public Water Systems and Announcement of Public Meeting; 2021; Vol. 13846, pp 13846–13872.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.