Abstract
Drinking water contaminated by per- and polyfluoroalkyl substances (PFAS) is a widespread public health concern, and exposure–response relationships are known to vary across sociodemographic groups. However, research on disparities in drinking water PFAS exposures and the siting of PFAS sources in marginalized communities is limited. Here, we use monitoring data from 7873 U.S. community water systems (CWS) in 18 states to show that PFAS detection is positively associated with the number of PFAS sources and proportions of people of color who are served by these water systems. Each additional industrial facility, military fire training area, and airport in a CWS watershed was associated with a 10–108% increase in perfluorooctanoic acid and a 20–34% increase in perfluorooctane sulfonic acid in drinking water. Waste sector sources were also significantly associated with drinking water PFAS concentrations. CWS watersheds with PFAS sources served higher proportions of Hispanic/Latino and non-Hispanic Black residents compared to those without PFAS sources. CWS serving higher proportions of Hispanic/Latino and non-Hispanic Black residents had significantly increased odds of detecting several PFAS. This likely reflects disparities in the siting of PFAS contamination sources. Results of this work suggest that addressing environmental justice concerns should be a component of risk mitigation planning for areas affected by drinking water PFAS contamination.
Keywords: water quality, environmental justice, drinking water, disparities, pollution
Short abstract
Community water systems contaminated with per- and polyfluoroalkyl substances (PFAS) serve greater proportions of Hispanic/Latino and non-Hispanic Black populations and contain greater numbers of PFAS sources within their watersheds.
1. Introduction
Impoverished communities and communities of color are often disproportionately exposed to environmental pollution and more vulnerable to adverse health outcomes compared to other populations.1−5 These disparities reflect discrimination and segregation, which have shaped the spatial patterning of industrial sources of pollution around the U.S., as well as the technical, financial, and managerial capacity of communities to alleviate pollution.2,6,7 Per- and polyfluoroalkyl substances (PFAS) are a diverse class of highly fluorinated anthropogenic chemicals that are widely used in consumer products and industrial processes.8,9 PFAS exposures have been associated with numerous adverse health outcomes, including altered liver function, immunotoxicity, and increased cholesterol.10−15 Hundreds of millions of U.S. residents are exposed to drinking water with perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) concentrations above 1 ng/L, a benchmark based on immunotoxicity.16,17 However, empirical evidence of disparities in PFAS contamination and drinking water exposures across different populations is limited.
Preliminary work on environmental disparities and PFAS exposures suggests that PFAS point sources may be disproportionately sited next to low-income communities and communities of color.18 Between 2013 and 2015, nationwide monitoring data for PFAS in drinking water were collected as part of the Unregulated Contaminant Monitoring Rule (UCMR 3) program.19 PFAS detection in public drinking water systems was positively associated with the number of civilian airports, military fire training areas (MFTA), industrial sites, and wastewater treatment plants (WWTP) within a watershed.20 Limitations of this analysis included the relatively high minimum reporting limits for PFAS (10–90 ng/L) and sampling data that were primarily restricted to large public water systems (PWS) serving >10,000 individuals.20 No additional nationwide monitoring data were collected between 2015 and 2023 due the lack of a federal maximum contaminant limit (MCL) for PFAS in drinking water. Instead, many states have intensively sampled community water systems (CWS, i.e., PWS that supply water to the same population year-round), and data on PFAS sources have been updated.21 Findings based on the 2013–2015 UCMR 3 data have not yet been confirmed with newer, higher resolution state-level data sets, and the relationships among PFAS sources, detections in PWS, and sociodemographic factors have not been explored.
The main objective of this study was to evaluate potential sociodemographic disparities in exposures to PFAS from drinking water and the locations of PFAS sources. To do this, we integrated a data set of >40,000 PFAS measurements compiled from 18 statewide CWS monitoring programs. Our analysis consisted of three main components. First, we examined the associations between PFAS sources and drinking water PFAS concentrations. We compared results from this study (which included many small CWS serving <10,000 people) to associations observed at the national level from the earlier UCMR 3 analysis of mainly larger PWS serving >10,000 individuals.20 Second, we examined associations between PFAS sources and county-level sociodemographic factors related to socioeconomic status and racial/ethnic composition. Finally, we examined associations between sociodemographic factors and PFAS detections in drinking water, as well as concentrations above the lowest state-level MCL for individual PFAS. Our analysis provides insights into potential disparities in siting of PFAS sources and drinking water exposures of low-income individuals and communities of color.
2. Methods
2.1. PFAS Drinking Water Data
As part of this study, we developed an interactive map of sociodemographic factors, PFAS source locations, and PFAS concentrations that is available at: https://sunderlandlab.github.io/pfas_interactive_maps/PFAS_EJ_interactive_map.html.
To develop this map and answer our research questions, we synthesized U.S. data on PFAS concentrations reported by PWS in samples collected between January 2016 and August 2022 from publicly available sources or provided by state agencies upon request. Because our research questions examined the link between local communities and drinking water PFAS concentrations, only data from CWS were retained for our analysis. The final synthesized data set included 44,111 samples from 7873 CWS in 18 states that conducted either statewide monitoring campaigns (Colorado: CO, Illinois: IL, Indiana: IN, Kentucky: KY, Massachusetts: MA, Maine: ME, Michigan: MI, New Hampshire: NH, New Jersey: NJ, Ohio: OH, Vermont: VT, and Wisconsin: WI) or targeted monitoring next to contamination sources (California: CA, Maryland: MD, New York: NY, Pennsylvania: PA, South Carolina: SC, and Utah: UT).22 Additional details on data sources, synthesis, and processing are provided in the Supporting Information (Table S1 and Figures S1 and S2).
Concentrations of 6–40 targeted PFAS were analyzed by multiple laboratories contracted by the states using comparable methods (United States Environmental Protection Agency: U.S. EPA Method 537, 537.1, or 533). This study focuses on five PFAS that were quantified in all 18 states: PFOA, PFOS, perfluorononanoic acid (PFNA), perfluorohexanesulfonic acid (PFHxS), and perfluorobutanesulfonic acid (PFBS). Variable detection limits across analytes and laboratories pose a challenge for merging data from multiple sources. For all statistical analyses, we assigned a conservative uniform detection limit at the 98th percentile of all reported detection limits (5 ng/L, Table S2), following prior work. Samples with reported detection limits greater than five times the median detection limit (n = 179, 0.4% of samples) were omitted from subsequent statistical analyses.23
2.2. CWS Characteristics and Sociodemographic Data
Data on precise CWS locations are often restricted. We used zip codes from the U.S. EPA’s Safe Drinking Water Information System (SDWIS)24 to geocode CWS within 8-digit hydrologic unit codes (hereon referred to as watersheds) when exact coordinates of water source regions were not available in the state databases.20 We used these 8-digit watershed boundaries because they were the smallest spatial units that matched the PFAS source data, which are described in further detail below. Zip codes for some CWS were inaccurate, so we cross-checked geocoded locations by searching reported addresses and system names with the Google Maps Geocoding application program interface. For each watershed, we calculated the maximum concentration of each individual PFAS among all raw and finished water samples from all CWS located within a watershed. For each CWS, we obtained data on treatment technologies (ca. 2017) known to reduce PFAS concentrations.25 These treatment technologies included granular or powder activated carbon, membrane separation (nanofiltration or reverse osmosis), and ion exchange processes.26 For facilities with these treatment technologies, we used PFAS concentrations in finished water samples in our statistical models to represent drinking water exposures.
We included SDWIS data on water source type, county served, and total population served for each CWS.24 We excluded data from water sources that were inactive or on standby, as indicated in the state databases. We incorporated county-level measures of racial/ethnic composition and socioeconomic status, including the proportions of Hispanic or Latino residents (hereafter referred to as Hispanic/Latino), non-Hispanic/Latino Black, or African-American residents (hereafter referred to as non-Hispanic Black), and residents under the federal poverty line, from the 5 year (2014–2018) American Community Survey (ACS).27,28 We assigned these sociodemographic factors to each CWS using their county-served information.24 For systems serving multiple counties, we assigned population-weighted averages of each proportion to the system.
2.3. Sources of PFAS Contamination within CWS Watersheds
We obtained the geographic locations of PFAS sources from multiple databases. Within the 18 states included in our analysis, PFAS sources included (a) 193 airports certified for use of aqueous film forming foams (AFFF) that contain PFAS available from the U.S. Federal Aviation Administration;29 (b) 152 MFTA known or suspected to be sources of PFAS contamination available from the U.S. Department of Defense;30 (c) seven industrial facilities operated by companies that participated in U.S. EPA’s 2010/2015 PFOA Stewardship Program (“major industrial facilities”);31 (d) 5640 WWTP from the U.S. EPA’s Clean Watersheds Needs Survey;32 and (e) 939 municipal solid waste landfills (active and inactive) from the U.S. EPA’s Landfill Methane Outreach Program.33 Prior work has shown that the magnitude of PFAS releases from WWTP is proportional to the population within watershed basins but variable across years depending on shifts in PFAS production.34 To better represent magnitudes of potential PFAS releases from different WWTP, we used the total effluent discharged by each plant32 as a proxy for PFAS releases. This type of proxy variable for releases (number of PFAS sources in a watershed or volume of wastewater effluent released) is necessary because data on magnitudes of PFAS releases from different source types are not yet widely available.35,36 All data sources are summarized in Table S3.
2.4. Statistical Analysis
Our statistical analysis included three main components (Tables 1 and S4). The first analyzed the relationships between PFAS concentrations in drinking water and the number of PFAS sources/volumes of effluent discharged by WWTP in CWS watersheds. We chose a multivariable log-linear spatial error regression approach to enable direct comparison to past (2013–2015) analyses using the UCMR 3 data.20 The second component of our analysis examined associations between PFAS sources in CWS watersheds and sociodemographic characteristics of communities served, which are resolved at the county level based on data provided in SDWIS. For this analysis, we used multivariable logistic regression models that are described in further detail below. For the third component, we analyzed relationships between PFAS detection or detection above the lowest state-level MCL and sociodemographic factors at the county level, also using multivariable logistic regression models. We did not use a spatial modeling approach for the second and third components of our analysis because more specific service area boundaries for CWS in all 18 states needed to analyze sociodemographic factors are presently unavailable.
Table 1. Three Primary Components, Units of Analyses, and Modeling Approaches in This Study.
| analysis | unit of analysis | outcome variables | primary independent variables | primary modeling approach | sample size |
|---|---|---|---|---|---|
| (1) association between PFAS sources (number or total existing effluent from wastewater treatment plants) and PFAS concentrations in 8-digit hydrologic unit codes (watersheds) | watershed of CWS included in recent statewide sampling | PFAS concentrations (natural log transformed) | PFAS sources | log-linear spatial error regression models | 476 |
| (2) association between sociodemographic factors and the presence of PFAS sources in watersheds of CWS | CWS included in recent statewide sampling | PFAS contamination sources (binary indicators) | county-level sociodemographic factors | logistic regression models with clustered standard errors (clustered at the county level) | 7873 |
| (3) association between sociodemographic factors and PFAS detections (>5 ng/L) or concentrations above lowest state-level MCL in CWS | CWS included in recent statewide sampling | PFAS concentrations above a threshold (>5 ng/L or the lowest state-level MCL; binary indicators) | county-level sociodemographic factors | partially and fully adjusted logistic regression models with clustered standard errors (clustered at the county level) | 7873 |
The CWS watershed was the spatial unit of analysis for the first part of our analysis that examined relationships between PFAS sources and detections or concentrations of each PFAS. Multivariable log-linear spatial error regression models were used to adjust for spatial autocorrelation in the residuals.37 A natural log transformation was used to normalize the distributions of PFAS concentrations. PFNA was excluded from the spatial regressions due a low detection frequency (38 out of 464 watersheds). For the four other PFAS, watersheds where all CWS had concentrations below detection were set to the uniform detection limit divided by √2. Four watersheds did not have neighbors based on queen contiguity.38 These were assigned spatial weights of zero in the main model because excluding them did not impact the coefficient estimates.
For the second and third components of our analysis, we developed logistic regression models using CWS as the unit of analysis. For these analyses, we assigned watershed-level PFAS source information and sociodemographic attributes at the county level to each CWS. We analyzed associations with PFAS detection and detection above the lowest state-level MCL. The lowest state-level MCL were based on regulatory values published as of August 2022 and were as follows for the five PFAS included in our analysis: PFOS (established by NY: 10 ng/L), PFOA (MI: 8 ng/L), PFNA (MI: 6 ng/L), PFHxS (NH: 18 ng/L), and PFBS (MI: 420 ng/L).39−41 For the logistic regression, it was necessary to develop a binary value (1/0) for several variables. These included (a) the detection/non-detection of PFAS (using a uniform detection limit of 5 ng/L for each PFAS and ≥1 PFAS of five total); (b) concentrations of PFAS above or below the lowest state-level MCL (for the PFOA MCL, PFOS MCL, or ≥1 MCL of five total); (c) presence or absence of major sources (manufacturing locations, MFTA, and airports certified for AFFF use) in each CWS watershed; (d) above or below the median number of landfills in each CWS watershed; and (e) above or below the median quantity of the total wastewater effluent in each CWS watershed.
We examined statistical differences in the sociodemographic characteristics of CWS that did and did not contain watersheds with several PFAS sources. We used multivariable logistic regression with mutual adjustment for all sociodemographic factors. We adjusted for state-level differences in PFAS sources and sociodemographics by including state-level fixed effects.42 In all logistic regressions, we accounted for correlations between CWS within the same county (which would impact standard errors) by clustering standard errors at the county level.
For the third component of our analysis, we examined statistical differences in the sociodemographic characteristics of CWS with and without concentrations above 5 ng/L (for each PFAS and ≥1 PFAS of 5 total) and above and below the lowest state-level MCL (for each PFAS MCL or ≥1 MCL). Multivariable logistic regressions were adjusted for state-level fixed effects and were mutually adjusted for all sociodemographic factors. Standard errors were clustered at the county-served level. These regression models included several compositional sociodemographic factors, where the sum of all the proportions is 100%. Therefore, associations related to greater proportions of one of these factors, holding all else constant, would imply lower proportions of the compositional factors excluded from the models.
We compared results from the initial models to alternate models that additionally adjusted for PFAS sources (airports, MFTA, major industrial facilities, WWTP effluent, and landfill counts) and other CWS characteristics that may affect drinking water quality.20,43−45 These CWS characteristics included water source type (surface or groundwater), water system size (based on the EPA definitions for population served: small or very small: ≤3300; medium: 3301–10,000; and large or very large: >10,000),46 and a binary indicator for treatment for PFAS.
Pollution sources and financial capacity often differ between urban and rural communities and may affect drinking water quality.43,47,48 We therefore examined whether statistical associations varied between urban versus rural place of residence by stratifying logistic regressions (≥50% residents living in urban areas) based on the US 2010–2020 decennial census.49 All statistical analyses were conducted using R version 4.2.2.50
3. Results and Discussion
3.1. PFAS Detection in CWS
CWS included in this study (n = 7873) provide drinking water to an estimated 70.0 million U.S. residents, representing approximately 21% of the U.S. population (Table S5). Large and very large CWS were oversampled relative to their proportions across the 18 states and nationally, but this study includes greater proportions of small and very small systems compared to the 2013–2015 UCMR 3 data (Table S6).19 Among the five PFAS considered, PFOA was most frequently reported above the uniform detection limit derived for the 18 states in this work of 5 ng/L (10.9% of CWS), and PFNA was detected least frequently (1.0% of CWS). An estimated 26% of individuals (18.0 million) received drinking water with PFOS or PFOA concentrations above the 5 ng/L detection level in this work, which is closest to the proposed federal standards of 4 ng/L for PFOA and PFOS proposed in 2023 (Table S7).51
Approximately one in four people across the 18 states were served by CWS that detected at least one of the five PFAS (PFOS, PFOA, PFBS, PFHxS, and PFNA) above 5 ng/L. It is notable that the uniform detection limit in this work that reflects the detection limits across analytical laboratories contracted by the states is 250–1250 times greater than the 2022 interim lifetime health advisories (LHA) for PFOS (0.02 ng/L) and PFOA (0.004 ng/L) that were issued by the U.S. EPA in 2022 (Table S2).52 Furthermore, the uniform detection limit across laboratories for PFOA and PFOS in our synthesized state database exceeds the federal MCL for PFOS and PFOS of 4 ng/L proposed by the U.S. EPA in 2023.51 This discrepancy highlights the analytical challenges associated with consistent low-level PFAS detection in environmental samples and the present disconnect between public health advisories and environmental measurements.
3.2. Diverse PFAS Sources Are Statistically Significant Predictors of Detection in CWS
Watersheds containing a CWS with detectable PFAS concentrations had significantly greater numbers of PFAS sources (industrial sites, MFTA, AFFF-certified airports, above the median landfill count, and above the median WWTP effluent volume) compared to watersheds with concentrations below detection (Table S8). Similarly, results from spatial regression models generally showed positive and significant associations between the presence of PFAS sources within watersheds and CWS PFAS concentrations (Table 2).
Table 2. Spatial Regression Models for Drinking Water PFAS Concentrations and Point Sources.
| outcome variable | MFTAa | AFFF-certified airportsb | major industriesc | WWTPd | MSW landfillse | λf | R2 | sample size |
|---|---|---|---|---|---|---|---|---|
| PFOA | 10.4g [−1.0, 23.1] | 21.0 [7.1, 36.7] | 108.4 [30.2, 233.5] | 7.4 [2.8, 12.2] | 0.3 [−2.9, 3.7] | 65.2 [50.8, 80.9] | 0.38 | 476 |
| p-value | 0.074 | 0.002 | 0.002 | 0.001 | 0.849 | <0.001 | ||
| PFOA (Hu et al., 2016) | 10 | –6 | 81 | 2 | 52 | 0.38 | 128 | |
| p-value | 0.111 | 0.353 | <0.001 | 0.006 | <0.001 | |||
| PFOS | 33.9 [17.6, 52.3] | 32.3 [14.3, 53.2] | 20.5 [−31.4, 111.8] | 6.3 [1.2, 11.6] | 3.8 [−0.2, 7.8] | 36.0 [21.9, 51.7] | 0.31 | 476 |
| p-value | <0.001 | <0.001 | 0.516 | 0.014 | 0.061 | <0.001 | ||
| PFOS (Hu et al., 2016) | 35 | –6 | 46 | 2 | 79 | 0.46 | 114 | |
| p-value | <0.001 | 0.512 | 0.124 | 0.007 | <0.001 | |||
| PFBSg | 4.9 [−3.6, 14.2] | 10.3 [0.1, 21.4] | 2.5 [−29.1, 48.4] | 7.4 [4.0, 10.9] | 4.8 [2.2, 7.5] | 34.5 [20.5, 50.2] | 0.29 | 473 |
| p-value | 0.290 | 0.047 | 0.894 | <0.001 | <0.001 | <0.001 | ||
| PFHxS | 22.4 [9.2, 37.1] | 26.7 [11.2, 44.5] | –0.7 [−39.7, 63.5] | 7.0 [2.7, 11.5] | 3.8 [0.4, 7.3] | 15.5 [2.5, 30.3] | 0.24 | 474 |
| p-value | 0.001 | <0.001 | 0.977 | 0.001 | 0.029 | 0.019 | ||
| PFHxS (Hu et al., 2016) | 20 | –13 | 24 | 1 | 94 | 0.62 | 94 | |
| p-value | 0.002 | 0.073 | 0.249 | 0.045 | <0.001 |
MFTA: military fire training area.
AFFF: aqueous film-forming foam.
Major industries refer to facilities of companies that participated in the US EPA’s 2010/2015 PFOA Stewardship Program.
WWTP: wastewater treatment plants. For this study, this variable refers to a one log unit increase (equivalent to a doubling) in the total existing effluent from WWTP within the 8-digit hydrologic unit code (watershed). In Hu et al. 2016,20 this refers to an additional WWTP within the watershed.
MSW: municipal solid waste.
Denotes the spatial autoregression coefficient.
Coefficients (with 95% confidence intervals) represent the percent change in PFAS concentrations for one-unit increases in each independent variable (either number of sources or doubling of the total existing effluent from wastewater treatment plants in the 8-digit hydrologic unit code) from the log-linear spatial error regressions. Results presented here substitute samples below the uniform detection limits with the uniform detection limit (5 ng/L) divided by √2.
The increase in PFOA concentrations (108%, p = 0.002) associated with each additional major industrial facility within a watershed was the largest estimated effect across source categories considered (Table 2). Major industrial facilities were not significantly associated with drinking water concentrations of other PFAS (Table 2). All facilities were operated by companies that participated in the 2010/2015 PFOA Stewardship Program, indicating that they were major PFOA sources. Using the UCMR 3 data for large PWS, Hu et al.(20) noted an 81% increase in PFOA concentrations for each major industrial site and similarly did not find significant associations for other PFAS. Both results are consistent with elevated concentrations of PFOA reported near fluoropolymer facilities.53
Two of the source categories investigated in this work (MFTA and airports certified for AFFF use) reflect PFAS contamination from firefighting and fire-training activities.54 Spatial regression modeling for the 18 states showed that each additional MFTA (total n = 152) within a CWS watershed was significantly associated with increases in the maximum concentrations of PFOS (34%), PFHxS (22%), and PFOA (10%). The coefficients observed in this study for PFOS and PFHxS for MFTA are remarkably similar in magnitude to the national scale results for large U.S. PWS.20 In both studies, the largest and most significant associations (p < 0.01) are observed for PFOS and PFHxS, which are widely understood to be the main PFAS in legacy AFFF manufactured by 3M using electrochemical fluorination.55−57
Each additional airport certified for AFFF use (total n = 193) within a CWS watershed was associated with increases in PFOS (32%), PFHxS (27%), PFOA (21%), and PFBS (10%). Results for AFFF-certified airports in this study contrast the spatial regression results for primarily large U.S. PWS sampled between 2013 and 2015.20 Positive and significant associations between the presence of AFFF-certified airports and four PFAS considered in this study, including PFBS, may reflect greater contamination of smaller CWS that rely on groundwater by AFFF-certified airports compared to the primarily large PWS included in the UCMR 3 data.58 PFBS is a minor impurity in legacy 3M AFFF, but large quantities of precursors with four and six perfluorinated carbons that can degrade in the environment into PFBS and PFHxS have been reported in 3M AFFF and in impacted watersheds.55,59 These results reinforce the problem of drinking water PFAS contamination from historic use of AFFF across the U.S.
Spatial regression models for the 18 states revealed novel significant associations between waste sector sources within a watershed and CWS PFAS concentrations. Each additional landfill (n = 939 total locations) within a watershed was associated with increases in PFBS (4.8%), PFHxS (3.8%), and PFOS (3.8%) concentrations. A doubling of the WWTP effluent (n = 5640 plants considered) released within a watershed was associated with significant increases in PFBS (7.4%), PFOA (7.4%), PFHxS (7.0%), and PFOS (6.3%) concentrations. Landfills and WWTP are well-known sources of PFAS in watersheds.60,61 Results from this study highlight the smaller but significant contributions of waste sector sources to PFAS contamination in drinking water systems across the 18 states.
We found similar coefficient estimates and 95% confidence intervals using the standard models that included watersheds with PFAS concentrations below detection with simple imputation and those that omitted values below detection as a sensitivity analysis (Table S9, Figure S3). These results suggest that results were robust to our treatment of values below detection. Coefficient estimates for landfills decreased when excluding watersheds with non-detectable concentrations, which may imply that these sources are contributors to concentrations near the 5 ng/L threshold. Future analyses using detection limits below 5 ng/L may thus need to consider not only likely sources (such as landfills) but also the large number of potential PFAS sources identified in recent work.21
In summary, associations between PFAS sources at the watershed scale and detection in drinking water across the 18 states were similar in directionality to prior work that included primarily large U.S. PWS.20 Model coefficients in this work were generally larger and more consistently positive. In addition, new and more consistent associations were identified for landfills and AFFF-certified airports. These differences likely reflect the lower uniform detection limit for PFAS in this work (5 ng/L for CWS in 18 states compared to 10–90 ng/L for U.S. PWS in the UCMR 3 data) and inclusion of a greater proportion of small, groundwater-sourced systems. Smaller water systems and groundwater systems are thought to be particularly susceptible to localized contamination.62,63
Spatial models for PFAS concentrations based on watershed PFAS sources explained a higher proportion of the variance (higher R2 values) in the national scale UCMR 3 analysis20 compared to the present study (Table 2). This likely reflects the predominance of a few large point sources contributing to the relatively high PFAS concentrations above the reporting limits for that study (10–90 ng/L). By contrast, this study showed significant associations with a broader array of smaller sources at a lower detection level. These results indicate that improved models of drinking water PFAS contamination will require data on potential PFAS sources that have not yet been confirmed on a national scale.21,64 PFAS release data are now being collected by the U.S. EPA’s Toxics Release Inventory (TRI),35 and additional reporting and recordkeeping requirements are proposed under the Toxics Substances Control Act (TSCA).36 These data are urgently needed to improved understanding of the drivers of drinking water PFAS contamination and guide remediation efforts.
3.3. CWS Watersheds with PFAS Sources Serve Greater Proportions of People of Color
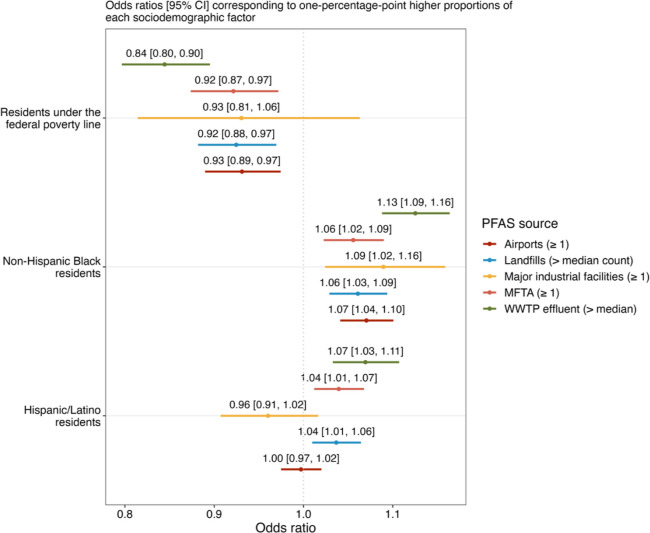
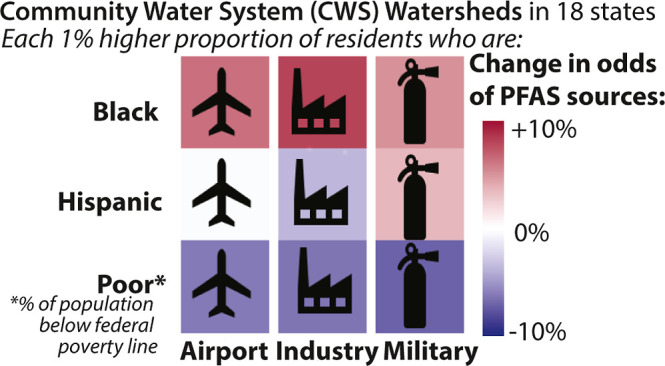
CWS watersheds serving higher proportions of Hispanic/Latino and non-Hispanic Black populations had significantly greater odds of containing PFAS sources (Figure 1). Each one percentage-point greater proportion of non-Hispanic Black residents was associated with a 6–9% increase in the odds of sharing a watershed with an industrial facility, MFTA, and civilian airport. The relationship was even more pronounced for waste sector sources. Each percentage-point greater proportion of non-Hispanic Black residents served by a CWS was associated with 7–13% higher odds of having greater than the median WWTP effluent discharge and greater than the median number of landfills across the 18 states.
Figure 1.
Association between sociodemographic factors and PFAS sources within watersheds of CWS from 18 states. Coefficients (with 95% confidence intervals) are percent changes in the odds of having PFAS sources within 8-digit hydrologic unit codes (watersheds) of CWS associated with one percentage-point higher proportions of each sociodemographic factor. Sources indicate a CWS sharing a watershed with ≥1 AFFF-certified airport, ≥1 major industrial facility, ≥1 MFTA, greater than median count of landfills, and greater than median WWTP effluent. Models are additionally adjusted for state-level fixed effects, and standard errors are clustered at the county level.
Explanations of these observed siting-related disparities include selective migration of people of color into urban and industrial regions with more potential PFAS sources and demographic changes that preferentially increase the proportion of people of color after a source is developed in a community. Past studies have reported that environmental hazards are disproportionately sited near marginalized communities.65,66 These communities may represent the “path of least resistance” due to limited political power, reduced access to information and resources, and restricted engagement in decision-making regarding siting.65,67−69 Historical segregation has also shaped the spatial patterning of pollution sources around the U.S.,70−73 and many areas remain highly segregated. Our analysis is consistent with preliminary reports suggesting that higher proportions of people of color live in proximity to sites potentially contaminated with PFAS.18
3.4. CWS with Detectable PFAS Serve Greater Proportions of People of Color
CWS with PFAS concentrations above 5 ng/L or above the lowest state-level MCL served communities with significantly greater proportions of Hispanic/Latino and non-Hispanic Black populations compared to CWS that did not have concentrations above these limits (Table S11). Proportions of Hispanic/Latino residents were 1.5–2 times greater among CWS with detectable PFAS (>5 ng/L) and detections above the lowest state-level MCL compared to those without detections above these thresholds. County-level proportions of non-Hispanic Black residents were significantly greater among systems with detectable levels of any PFAS and detections above the lowest state-level MCL (Table S11).
Logistic regression models showed 3–6% higher odds of detecting PFOA, PFOS, PFBS, and any of the five PFAS for each percentage-point greater proportion of Hispanic/Latino residents served by a CWS (Tables 3 and S12). Similar positive changes (4–6%) in the odds of a detection above the lowest state-level MCL were associated with each percentage greater proportion of Hispanic/Latino residents (Table S13). A one percentage-point greater proportion of non-Hispanic Black residents was associated with 3–6% greater odds of detecting PFOA, PFOS, and any of the five PFAS, and 4–6% greater odds of concentrations above the state-level MCL (Tables 2 and S13). After adjusting for PFAS sources and CWS characteristics (source water type, water system size, and treatment technologies relevant for PFAS), associations between the proportion of Hispanic/Latino residents and PFAS detections remained significant (Table 3).
Table 3. Associations between Sociodemographic Factors and Detection of PFAS in Community Water Systemsa,b.
| dependent variable |
||||||
|---|---|---|---|---|---|---|
| PFOAc | PFOAd | PFOSc | PFOSd | ≥1 PFAS (of 5 total)c,e | ≥1 PFAS (of 5 total)d,e | |
| (1) | (2) | (3) | (4) | (5) | (6) | |
| All Systems | ||||||
| % Hispanic/Latino | 6.1*** | 4.4*** | 3.8*** | 2.3** | 2.7** | 1.7* |
| (3.2, 9.0) | (2.1, 6.8) | (1.3, 6.4) | (0.3, 4.4) | (0.6, 4.8) | (−0.1, 3.5) | |
| % non-Hispanic Black | 3.9* | 1.6 | 5.8*** | 3.7** | 3.4** | 1.0 |
| (0.0, 8.0) | (−2.0, 5.2) | (2.5, 9.2) | (0.7, 6.8) | (0.5, 6.3) | (−1.7, 3.7) | |
| % under federal poverty line | –17.6*** | –13.1*** | –15.9*** | –11.5*** | –9.4*** | –5.7*** |
| (−21.8, −13.2) | (−17.4, −8.5) | (−19.7, −11.8) | (−15.9, −7.0) | (−13.2, −5.5) | (−9.8, −1.4) | |
| N | 7873 | 7873 | 7873 | 7873 | 6199 | 6199 |
| Stratification by Urban Versus Rural Status | ||||||
| Urban Systems (≥50% Residents in Urban Areas) | ||||||
| % Hispanic/Latino | 5.2*** | 3.6*** | 2.7* | 1.4 | 1.7 | 0.8 |
| (2.1, 8.3) | (1.1, 6.1) | (−0.1, 5.5) | (−0.7, 3.7) | (−0.6, 4.2) | (−1.1, 2.9) | |
| % non-Hispanic Black | 2.8 | 0.6 | 4.6** | 2.5 | 2.4 | 0.0 |
| (−1.8, 7.6) | (−3.3, 4.7) | (0.9, 8.4) | (−0.6, 5.8) | (−1.1, 6.0) | (−3.1, 3.2) | |
| % under federal poverty line | –16.8*** | –12.7*** | –14.5*** | –10.8*** | –10.6*** | –7.2*** |
| (−21.5, −12) | (−17.5, −7.7) | (−18.6, −10.2) | (−15.0, −6.3) | (−14.8, −6.2) | (−11.6, −2.5) | |
| N | 4735 | 4735 | 4735 | 4735 | 3933 | 3933 |
| Rural Systems (<50% Residents in Urban Areas) | ||||||
| % Hispanic/Latino | 2.0 | 1.1 | 5.3* | 3.9 | 0.6 | 0.3 |
| (−5.8, 10.4) | (−6.4, 9.2) | (−0.1, 10.9) | (−1.4, 9.5) | (−3.8, 5.1) | (−4.1, 4.8) | |
| % non-Hispanic Black | 4.3* | 4.9** | 2.5 | 2.3 | 2.6 | 3.1 |
| (−0.4, 9.3) | (0.5, 9.5) | (−1.8, 7.0) | (−2.0, 6.7) | (−1.6, 6.9) | (−1.0, 7.3) | |
| % under federal poverty line | 9.9** | 11.2*** | 0.0 | 4.3 | 10.3*** | 10.6*** |
| (2.2, 18.3) | (3.5, 19.4) | (−9.2, 10.1) | (−4.8, 14.2) | (4.0, 17.1) | (4.5, 17.1) | |
| N | 3138 | 3138 | 3138 | 3138 | 2266 | 2266 |
Results are from logistic regressions that are adjusted for state fixed effects and include clustered standard errors at the county level. Coefficients (with 95% confidence intervals) refer to percent changes in the odds of detecting PFAS (>5 ng/L) associated with one percentage-point higher proportions of each sociodemographic factor.
CWS are the unit of analysis. These CWS were included in statewide sampling from 18 states. *p < 0.1; **p < 0.05; and ***p < 0.01.
No additional adjustment for CWS characteristics and PFAS sources.
Includes adjustment for CWS characteristics (water source type, water system size, and a binary indicator for treatment for PFAS) and PFAS sources within the 8-digit hydrologic unit code of the CWS (airports, MFTA, major industrial facilities, WWTP total existing effluent, and landfills).
This outcome refers to detection of at least one of the five total PFAS (PFOA, PFOS, PFNA, PFHxS, and/or PFBS) over 5 ng/L. Systems that did not report measurements of all the five PFAS during their sampling periods are excluded from this model but are further analyzed in Figure S4 and Table S18.
Results of this study are consistent with prior findings that communities with greater proportions of Hispanic/Latino47,74 and non-Hispanic Black residents have elevated concentrations of other drinking water contaminants (arsenic;44,75,76 nitrate;43,77 and uranium, chromium, barium, and selenium78) and more frequent violations of the National Primary Drinking Water Regulations.74,79 Similar results were reported in analyses of arsenic MCL exceedances in Arizona (1–2% higher odds for each percentage-point greater proportion in Hispanic/Latino residents),75 health-based violations in Virginia (3% higher odds for each percentage-point greater proportion in Black residents),79 and an analysis of nitrate levels above 5 mg/L (2% higher odds for each one percentage-point greater proportion in Hispanic/Latino residents).43 However, no environmental justice studies on PFAS in drinking water are available for a more direct comparison.
The National Health and Nutritional Examination Survey (NHANES) is a probabilistic, nationally representative survey of U.S. individuals that routinely measures serum concentrations of several PFAS. Past work80 showed significantly elevated serum PFOS concentrations among certain racial/ethnic groups, including non-Hispanic Black and Asian individuals. However, these data reflect exposures from all PFAS sources rather than just drinking water. For example, fish consumption frequency has been identified as an important predictor of serum PFOS concentrations in NHANES,80 and freshwater fish contamination by PFAS may also be an environmental justice concern.81,82
3.5. Relationships with Proportion of Residents under the Federal Poverty Line
Significantly lower proportions of residents under the federal poverty line were served by CWS that shared watersheds with PFAS contamination sources (Table S10). Significantly lower proportions of residents under the poverty line were also served by CWS with PFAS levels >5 ng/L or above the lowest state-level MCL (Table S11). Model results indicated that a 6–18% decrease in the odds of detecting PFOS, PFOA, and at least one PFAS above 5 ng/L was observed for each percentage-point higher proportion of residents under the federal poverty line served by a CWS (Tables 3 and S12). Each percentage-point higher proportion of residents under the federal poverty line was associated with 13–18% lower odds of detection above the lowest state-level PFAS MCL (Table S13). Results with similar conclusions were observed when substituting the proportion of residents under the federal poverty line with median household income, the proportion of residents without a high school diploma, and other co-varying county-level variables (Tables S14–S16). Inverse associations with the proportion of residents under the federal poverty line may reflect the urban signature of PFAS since urban areas typically have lower average poverty rates at the county scale83 and may have more abundant pollution sources.
The directionality of the relationship between county-level residents under the federal poverty line and PFAS detection changed when stratified by areas with more or less than 50% of the population living in urban areas. Among rural water systems, each percentage-point greater proportion of residents under the federal poverty line was associated with 10% greater odds of detecting PFOA and any of the five PFAS (Table 2). However, in urban areas (and the overall data set) consistently negative associations were observed for PFAS detections above 5 ng/L and detections above the lowest state-level MCL (Table 3). These results suggest that the relationship between county-level residents under the federal poverty line and drinking water PFAS contamination is complex and varies across urban and rural areas.
Differences between urban and rural areas are consistent with the mixed (and sometimes inverse) relationships between socioeconomic status and drinking water quality that have been reported in prior studies.43,74,84,85 They may highlight a combination of factors that are not normally considered when evaluating drinking water quality such as economic isolation86 and poverty in rural areas. Results of this study indicate that PFAS sources may disproportionately impact CWS serving greater proportions of residents under the federal poverty line in rural areas but not urban areas. Among rural areas, associations between the proportion of residents under the federal poverty line after adjustment for PFAS sources and CWS characteristics were also significant (Table 3).
Inverse associations between county-level residents under the federal poverty line and PFAS sources may reflect the siting of PFAS sources within counties with high economic activity, where overall poverty levels are generally lower.86,87 However, each CWS may serve individuals with a wide range of incomes, and findings from this study reflect the county scale. At the sub-county level, prior work indicates that highly localized environmental conditions affect residential choices across income groups88 and that the presence of industrial facilities may decrease housing values.89 A negative association between income and Superfund sites was reported at the sub-county geographic scale, whereas results for race/ethnicity in the same study were more consistent across geographic scales.42 Different results across varying spatial units of analysis may reflect the modifiable areal unit problem.90 Results at the county level in this study therefore do not necessarily contradict preliminary analyses, suggesting that greater proportions of low-income individuals live within close proximity to PFAS sources.18 Future work should consider the relationships between PFAS sources and individuals under the federal poverty line at a higher spatial resolution. Such an analysis across the 18 states considered in this work is presently limited by the spatial resolution of available data.
3.6. Study Limitations
Our analyses of associations between PFAS sources, drinking water PFAS detections, detections above the lowest state-level MCL, and sociodemographic factors were limited by the spatial resolution of available data at the county level. CWS often serve communities at smaller geographic scales than the county, but presently nationwide data are only available at the county resolution. When geocoding each CWS into a watershed, we used administrative zip code information and cross-checked these locations using system names and addresses. However, in certain cases, this may have resulted in error if the geocoded location differed greatly from the location of the source water. The spatial coverage of PFAS concentrations in drinking water in this study was limited to 18 states, which restricts the nationwide generalizability of this study if unsampled regions differ greatly in terms of key characteristics. We observed suggestive evidence of disparities in PFAS drinking water contamination for additional racial/ethnic groups (Table S17). However, these analyses were limited by low variation in these sociodemographic factors among the counties included in this study, which did not include certain regions with large proportions of these groups. Future analyses with an expanded geographic scope should further consider these sociodemographic groups.
3.7. Study Implications
Results of this study highlight the current analytical challenge associated with detecting low-level concentrations of PFAS in drinking water that have been identified by the U.S. EPA in 2022 as a health concern (interim LHA for PFOS: 0.02 ng/L, PFOA: 0.004 ng/L).52 The proposed federal MCL for PFOS and PFOA of 4 ng/L is lower than the uniform detection limit for state data in this work of 5 ng/L.51 Our spatial analysis of PFAS sources showed that drinking water concentrations were most strongly associated with major industrial facilities and that a diverse group of additional sources were associated with smaller increases in PFAS concentrations. Strong and significant associations with MFTA and civilian airports certified for AFFF use highlight the broad scope of PFAS contamination from historic use of AFFF across the U.S. Waste sector sources (WWTP, landfills) were associated with smaller but significant contributions to PFAS contamination. Further improving large-scale models of drinking water PFAS contamination at lower detection levels will require additional spatial data on potential PFAS sources that have not yet been confirmed on a national scale. Updates to the U.S. EPA’s TRI35 and additional reporting and recordkeeping requirements under TSCA36 offer a solution to the urgent need to fill this data gap and should be prioritized.
Data considered in this study include a large proportion of small CWS that rely on groundwater. This contrasts prior national-scale analysis of UCMR 3 data that included primarily large PWS and a relative greater proportion of systems supplied by surface water. Significant associations between waste sector sources and civilian airports in CWS watersheds and PFAS detection were noted in this study for the first time, likely indicating the potential for groundwater contamination by a diverse array of PFAS pollution sources.
Both the locations of PFAS sources and PFAS concentrations in drinking water were positively and significantly associated with the proportion of non-Hispanic Black and Hispanic/Latino residents served. Although there are many additional PFAS exposure routes, disproportionate exposures may occur for these populations and should be considered when developing risk mitigation strategies for drinking water contamination. PFAS sources and detection were inversely related to the proportion of residents under the federal poverty line in urban areas but positively associated with the proportion of residents under the federal poverty line in rural areas. The relationship between drinking water quality and socioeconomic status appears to be complex, and the structural factors linking drinking water quality and area-level poverty in urban and rural areas warrant further consideration.
Acknowledgments
Research reported in this publication was supported by the National Institute of Environmental Health Sciences (NIEHS) under award number P42ES027706. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIEHS. L.A.S. received support from R01ES028311, and J.M.L. was supported by an NIEHS training grant (T32 E007069). We thank Gary Adamkiewicz, Brent Coull, John Evans, Francine Laden, and Tamarra James-Todd (HSPH) for feedback on earlier drafts of this work. We thank Richard Gunoskey (NJ Department of Environmental Protection) and Rosemarie Hewig (NY Department of Health) for access to drinking water monitoring data.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.2c07255.
Statewide sampling drinking water data; detection limit details; data source descriptions; sample comparison with CWS nationwide and UCMR 3; estimated populations served by CWS with detectable PFAS concentrations; results from all t-tests; additional regression tables (including additional PFAS outcome variables, other measures of socioeconomic status, and other racial/ethnic variables); study geographical coverage; and comparison of results excluding and including 8-digit hydrologic unit codes with non-detectable concentrations (PDF)
The authors declare no competing financial interest.
Notes
All code and data used for this analysis are available at the following site: https://github.com/SunderlandLab/pfas_sources_disparities.
Supplementary Material
References
- Evans G. W.; Kantrowitz E. Socioeconomic Status and Health: The Potential Role of Environmental Risk Exposure. Annu. Rev. Public Health 2002, 23, 303–331. 10.1146/annurev.publhealth.23.112001.112349. [DOI] [PubMed] [Google Scholar]
- Gee G. C.; Payne-Sturges D. C. Environmental Health Disparities: A Framework Integrating Psychosocial and Environmental Concepts. Environ. Health Perspect. 2004, 112, 1645–1653. 10.1289/ehp.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. Race, Class, and Environmental Health: A Review and Systematization of the Literature. Environ. Res. 1995, 69, 15–30. 10.1006/enrs.1995.1021. [DOI] [PubMed] [Google Scholar]
- Hajat A.; Hsia C.; O’Neill M. S. Socioeconomic Disparities and Air Pollution Exposure: A Global Review. Curr. Environ. Health Rep. 2015, 2, 440–450. 10.1007/s40572-015-0069-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicken M. T.; Gee G. C.; Morenoff J.; Connell C. M.; Snow R. C.; Hu H. A Novel Look at Racial Health Disparities: The Interaction Between Social Disadvantage and Environmental Health. Am. J. Public Health 2012, 102, 2344–2351. 10.2105/ajph.2012.300774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullard R. D.; Mohai P.; Saha R.; Wright B. Toxic Wastes and Race at Twenty: Why Race Still Matters after All of These Years Environmental Justice: Making It a Reality. Environ. Law 2008, 38, 371–412. [Google Scholar]
- Balazs C. L.; Ray I. The Drinking Water Disparities Framework: On the Origins and Persistence of Inequities in Exposure. Am. J. Public Health 2014, 104, 603–611. 10.2105/ajph.2013.301664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US EPA . EPA’s Per- and Polyfluoroalkyl Substances (PFAS) Action Plan. 2019, https://www.epa.gov/sites/production/files/2019-02/documents/pfas_action_plan_021319_508compliant_1.pdf (accessed March 27, 2020).
- US EPA . CompTox Chemicals Dashboard|PFASMASTER Chemicals. https://comptox.epa.gov/dashboard/chemical_lists/pfasmaster (accessed Feb 22, 2021).
- US EPA . Health Effects Support Document for Perfluorooctane Sulfonate (PFOS), 2016; p 245.
- US EPA . Health Effects Support Document for Perfluorooctanoic Acid (PFOA), 2016; p 322.
- Lopez-Espinosa M.-J.; Mondal D.; Armstrong B.; Bloom M. S.; Fletcher T. Thyroid Function and Perfluoroalkyl Acids in Children Living Near a Chemical Plant. Environ. Health Perspect. 2012, 120, 1036–1041. 10.1289/ehp.1104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P.; Andersen E. W.; Budtz-Jørgensen E.; Nielsen F.; Mølbak K.; Weihe P.; Heilmann C. Serum Vaccine Antibody Concentrations in Children Exposed to Perfluorinated Compounds. J. Am. Med. Assoc. 2012, 307, 391–397. 10.1001/jama.2011.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson J. W.; Hatch E. E.; Webster T. F. Exposure to Polyfluoroalkyl Chemicals and Cholesterol, Body Weight, and Insulin Resistance in the General U.S. Population. Environ. Health Perspect. 2010, 118, 197–202. 10.1289/ehp.0901165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello E.; Rock S.; Stratakis N.; Eckel S. P.; Walker D. I.; Valvi D.; Cserbik D.; Jenkins T.; Xanthakos S. A.; Kohli R.; Sisley S.; Vasiliou V.; La Merrill M. A.; Rosen H.; Conti D. V.; McConnell R.; Chatzi L. Exposure to Per- and Polyfluoroalkyl Substances and Markers of Liver Injury: A Systematic Review and Meta-Analysis. Environ. Health Perspect. 2022, 130, 046001. 10.1289/ehp10092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews D. Q.; Naidenko O. V. Population-Wide Exposure to Per- and Polyfluoroalkyl Substances from Drinking Water in the United States. Environ. Sci. Technol. Lett. 2020, 7, 931–936. 10.1021/acs.estlett.0c00713. [DOI] [Google Scholar]
- Grandjean P.; Budtz-Jørgensen E. Immunotoxicity of Perfluorinated Alkylates: Calculation of Benchmark Doses Based on Serum Concentrations in Children. Environ. Health 2013, 12, 35. 10.1186/1476-069x-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan A.; Carter J.; Kinser S.; Goldman G.. Abandoned Science, Broken Promises; Union of Concerned Scientists, 2019. https://www.ucsusa.org/sites/default/files/2019-10/abandoned-science-broken-promises-web-final.pdf (accessed July 12, 2021).
- US EPA . Third Unregulated Contaminant Monitoring Rule. https://www.epa.gov/dwucmr/third-unregulated-contaminant-monitoring-rule (accessed Dec 28, 2021).
- Hu X. C.; Andrews D. Q.; Lindstrom A. B.; Bruton T. A.; Schaider L. A.; Grandjean P.; Lohmann R.; Carignan C. C.; Blum A.; Balan S. A.; Higgins C. P.; Sunderland E. M. Detection of Poly- and Perfluoroalkyl Substances (PFASs) in U.S. Drinking Water Linked to Industrial Sites, Military Fire Training Areas, and Wastewater Treatment Plants. Environ. Sci. Technol. Lett. 2016, 3, 344–350. 10.1021/acs.estlett.6b00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews D. Q.; Hayes J.; Stoiber T.; Brewer B.; Campbell C.; Naidenko O. V. Identification of Point Source Dischargers of Per- and Polyfluoroalkyl Substances in the United States. AWWA Water Sci. 2021, 3, e1252 10.1002/aws2.1252. [DOI] [Google Scholar]
- Liddie J.PFAS Statewide Sampling Dataset; Harvard Dataverse, 2022. [Google Scholar]
- Hu X. C.; Ge B.; Ruyle B. J.; Sun J.; Sunderland E. M. A Statistical Approach for Identifying Private Wells Susceptible to Perfluoroalkyl Substances (PFAS) Contamination. Environ. Sci. Technol. Lett. 2021, 8, 596–602. 10.1021/acs.estlett.1c00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Environmental Protection Agency . Safe Drinking Water Information System (SDWIS) Federal Reporting Services; US EPA. https://www.epa.gov/ground-water-and-drinking-water/safe-drinking-water-information-system-sdwis-federal-reporting (accessed Feb 21, 2021).
- US EPA . Drinking Water Treatment Process Information, 2020.
- US EPA . Drinking Water Treatability Database: Per- and Polyfluoroalkyl Substances. https://tdb.epa.gov/tdb/contaminant?id=11020 (accessed Feb 22, 2021).
- US Census Bureau . 2014-2018 ACS 5-year Estimates; The United States Census Bureau. https://www.census.gov/programs-surveys/acs/technical-documentation/table-and-geography-changes/2018/5-year.html (accessed Nov 14, 2020).
- Krieger N.; Chen J.; Waterman P.. COVID-19 Resources. The Public Health Disparities Geocoding Project Monograph. https://www.hsph.harvard.edu/thegeocodingproject/covid-19-resources/ (accessed Nov 14, 2020).
- US FAA . Part 139 Airport Certification—Airports. https://www.faa.gov/airports/airport_safety/part139_cert/ (accessed Sept 18, 2020).
- US DoD . Aqueous Film Forming Foam (AFFF) Report to Congress, Washington, D.C., 2017; pp 16–28. https://www.denix.osd.mil/derp/denix-files/sites/26/2017/11/Aqueous-Film-Forming-Foam-AFFF-Report-to-Congress_DENIX.pdf (accessed Sept 18, 2020).
- Fact Sheet: 2010/2015 PFOA Stewardship Program|Assessing and Managing Chemicals under TSCA|US EPA. https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/fact-sheet-20102015-pfoa-stewardship-program (accessed Nov 14, 2020).
- US EPA . Clean Watersheds Needs Survey (CWNS)—2012 Report and Data; US EPA. https://www.epa.gov/cwns/clean-watersheds-needs-survey-cwns-2012-report-and-data (accessed Sept 18, 2020).
- US EPA . Landfill Methane Outreach Program (LMOP). https://www.epa.gov/lmop (accessed Nov 14, 2021).
- Zhang X.; Zhang Y.; Dassuncao C.; Lohmann R.; Sunderland E. M. North Atlantic Deep Water Formation Inhibits High Arctic Contamination by Continental Perfluorooctane Sulfonate Discharges. Global Biogeochem. Cycles 2017, 31, 1332–1343. 10.1002/2017gb005624. [DOI] [Google Scholar]
- US EPA . Toxics Release Inventory (TRI) Program. https://www.epa.gov/toxics-release-inventory-tri-program (accessed Sept 12, 2022).
- US EPA Office of Chemical Safety and Pollution Prevention . TSCA Section 8(a)(7) Reporting and Recordkeeping Requirements for Perfluoroalkyl and Polyfluoroalkyl Substances. https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/tsca-section-8a7-reporting-and-recordkeeping (accessed March 23, 2023).
- Anselin L. Spatial Externalities, Spatial Multipliers and Spatial Econometrics. Int. Reg. Sci. Rev. 2003, 26, 153–166. 10.1177/0160017602250972. [DOI] [Google Scholar]
- Getis A.; Aldstadt J. Constructing the Spatial Weights Matrix Using a Local Statistic. Geogr. Anal. 2004, 36, 90–104. 10.1111/j.1538-4632.2004.tb01127.x. [DOI] [Google Scholar]
- Michigan PFAS Action Response Team . PFAS Maximum Contaminant Levels (MCLs) and Drinking Water. https://www.michigan.gov/pfasresponse/drinking-water/mcl (accessed Jan 24, 2023).
- New York State Department of Environmental Conservation . DEC Releases New Guidance to Regulate PFOA, PFOS, and 1,4-Dioxane in State Waters; NYS Department of Environmental Conservation. https://www.dec.ny.gov/press/123915.html (accessed June 28, 2022).
- New Hampshire Department of Environmental Services . New Hampshire PFAS Response—Drinking Water. DES—PFAS Blog. https://www.pfas.des.nh.gov/drinking-water (accessed Jan 24, 2023).
- Baden B. M.; Noonan D. S.; Turaga R. M. R. Scales of Justice: Is There a Geographic Bias in Environmental Equity Analysis?. J. Environ. Plan. Manag. 2007, 50, 163–185. 10.1080/09640560601156433. [DOI] [Google Scholar]
- Schaider L. A.; Swetschinski L.; Campbell C.; Rudel R. A. Environmental Justice and Drinking Water Quality: Are There Socioeconomic Disparities in Nitrate Levels in U.S. Drinking Water?. Environ. Health 2019, 18, 3. 10.1186/s12940-018-0442-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balazs C. L.; Morello-Frosch R.; Hubbard A. E.; Ray I. Environmental Justice Implications of Arsenic Contamination in California’s San Joaquin Valley: A Cross-Sectional, Cluster-Design Examining Exposure and Compliance in Community Drinking Water Systems. Environ. Health 2012, 11, 84. 10.1186/1476-069x-11-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guelfo J. L.; Marlow T.; Klein D. M.; Savitz D. A.; Frickel S.; Crimi M.; Suuberg E. M. Evaluation and Management Strategies for Per- and Polyfluoroalkyl Substances (PFASs) in Drinking Water Aquifers: Perspectives from Impacted U.S. Northeast Communities. Environ. Health Perspect. 2018, 126, 065001. 10.1289/ehp2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US EPA . Drinking Water Dashboard|ECHO|US EPA. Enforcement and Compliance History Online. https://echo.epa.gov/help/drinking-water-qlik-dashboard-help (accessed Feb 20, 2021).
- Allaire M.; Wu H.; Lall U. National Trends in Drinking Water Quality Violations. Proc. Natl. Acad. Sci. U.S.A. 2018, 115, 2078–2083. 10.1073/pnas.1719805115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcillo C. E.; Krometis L.-A. H. Small Towns, Big Challenges: Does Rurality Influence Safe Drinking Water Act Compliance?. AWWA Water Sci. 2019, 1, e1120 10.1002/aws2.1120. [DOI] [Google Scholar]
- US Census Bureau . 2010–2020 Census, 2020.
- R Core Team . R: A Language and Environment for Statistical Computing. 2021, https://www.R-project.org/ (accessed Feb 22, 2021).
- US EPA Office of Water . Per- and Polyfluoroalkyl Substances (PFAS): Proposed PFAS National Primary Drinking Water Regulation. https://www.epa.gov/sdwa/and-polyfluoroalkyl-substances-pfas (accessed March 31, 2023).
- US EPA Office of Water . Drinking Water Health Advisories for PFOA and PFOS. https://www.epa.gov/sdwa/drinking-water-health-advisories-pfoa-and-pfos (accessed July 30, 2022).
- Hoffman K.; Webster T. F.; Bartell S. M.; Weisskopf M. G.; Fletcher T.; Vieira V. M. Private Drinking Water Wells as a Source of Exposure to Perfluorooctanoic Acid (PFOA) in Communities Surrounding a Fluoropolymer Production Facility. Environ. Health Perspect. 2011, 119, 92–97. 10.1289/ehp.1002503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Information Portal on Multilateral Environmental Agreements . Estimated Quantities of Aqueous Film Forming Foam (AFFF) in the United States, 2004. 2004, https://www.informea.org/en/estimated-quantities-aqueous-film-forming-foam-afff-united-states-2004 (accessed Aug 24, 2022).
- Ruyle B. J.; Pickard H. M.; LeBlanc D. R.; Tokranov A. K.; Thackray C. P.; Hu X. C.; Vecitis C. D.; Sunderland E. M. Isolating the AFFF Signature in Coastal Watersheds Using Oxidizable PFAS Precursors and Unexplained Organofluorine. Environ. Sci. Technol. 2021, 55, 3686–3695. 10.1021/acs.est.0c07296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzen-Hanson K. A.; Roberts S. C.; Choyke S.; Oetjen K.; McAlees A.; Riddell N.; McCrindle R.; Ferguson P. L.; Higgins C. P.; Field J. A. Discovery of 40 Classes of Per- and Polyfluoroalkyl Substances in Historical Aqueous Film-Forming Foams (AFFFs) and AFFF-Impacted Groundwater. Environ. Sci. Technol. 2017, 51, 2047–2057. 10.1021/acs.est.6b05843. [DOI] [PubMed] [Google Scholar]
- Houtz E. F.; Higgins C. P.; Field J. A.; Sedlak D. L. Persistence of Perfluoroalkyl Acid Precursors in AFFF-Impacted Groundwater and Soil. Environ. Sci. Technol. 2013, 47, 8187–8195. 10.1021/es4018877. [DOI] [PubMed] [Google Scholar]
- US EPA Office of Water . Data Summary of The Third Unregulated Contaminant Monitoring Rule. https://www.epa.gov/dwucmr/data-summary-third-unregulated-contaminant-monitoring-rule (accessed Dec 18, 2021).
- Ruyle B. J.; Thackray C. P.; McCord J. P.; Strynar M. J.; Mauge-Lewis K. A.; Fenton S. E.; Sunderland E. M. Reconstructing the Composition of Per- and Polyfluoroalkyl Substances in Contemporary Aqueous Film-Forming Foams. Environ. Sci. Technol. Lett. 2021, 8, 59–65. 10.1021/acs.estlett.0c00798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepburn E.; Madden C.; Szabo D.; Coggan T. L.; Clarke B.; Currell M. Contamination of Groundwater with Per- and Polyfluoroalkyl Substances (PFAS) from Legacy Landfills in an Urban Re-Development Precinct. Environ. Pollut. 2019, 248, 101–113. 10.1016/j.envpol.2019.02.018. [DOI] [PubMed] [Google Scholar]
- Helmer R. W.; Reeves D. M.; Cassidy D. P. Per- and Polyfluorinated Alkyl Substances (PFAS) Cycling within Michigan: Contaminated Sites, Landfills and Wastewater Treatment Plants. Water Res. 2022, 210, 117983. 10.1016/j.watres.2021.117983. [DOI] [PubMed] [Google Scholar]
- Guelfo J. L.; Adamson D. T. Evaluation of a National Data Set for Insights into Sources, Composition, and Concentrations of per- and Polyfluoroalkyl Substances (PFASs) in U.S. Drinking Water. Environ. Pollut. 2018, 236, 505–513. 10.1016/j.envpol.2018.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaider L. A.; Rudel R. A.; Ackerman J. M.; Dunagan S. C.; Brody J. G. Pharmaceuticals, Perfluorosurfactants, and Other Organic Wastewater Compounds in Public Drinking Water Wells in a Shallow Sand and Gravel Aquifer. Sci. Total Environ. 2014, 468–469, 384–393. 10.1016/j.scitotenv.2013.08.067. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Lohmann R.; Dassuncao C.; Hu X. C.; Weber A. K.; Vecitis C. D.; Sunderland E. M. Source Attribution of Poly- and Perfluoroalkyl Substances (PFASs) in Surface Waters from Rhode Island and the New York Metropolitan Area. Environ. Sci. Technol. Lett. 2016, 3, 316–321. 10.1021/acs.estlett.6b00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohai P.; Saha R. Which Came First, People or Pollution? A Review of Theory and Evidence from Longitudinal Environmental Justice Studies. Environ. Res. Lett. 2015, 10, 125011. 10.1088/1748-9326/10/12/125011. [DOI] [Google Scholar]
- Taylor D. E.Toxic Communities: Environmental Racism, Industrial Pollution, and Residential Mobility; NYU Press, 2014. [Google Scholar]
- Stretesky P.; Hogan M. J. Environmental Justice: An Analysis of Superfund Sites in Florida. Soc. Probl. 1998, 45, 268–287. 10.1525/sp.1998.45.2.03x0169m. [DOI] [Google Scholar]
- Cole L. W.; Foster S. R.. From the Ground Up: Environmental Racism and the Rise of the Environmental Justice Movement; New York University Press: New York, 2001. [Google Scholar]
- Bullard R.; Wright B.. The Wrong Complexion for Protection: How the Government Response to Disaster Endangers African American Communities; New York University Press: New York, 2012. [Google Scholar]
- Mohai P.; Lantz P. M.; Morenoff J.; House J. S.; Mero R. P. Racial and Socioeconomic Disparities in Residential Proximity to Polluting Industrial Facilities: Evidence From the Americans’ Changing Lives Study. Am. J. Public Health 2009, 99, S649–S656. 10.2105/ajph.2007.131383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane H. M.; Morello-Frosch R.; Marshall J. D.; Apte J. S. Historical Redlining Is Associated with Present-Day Air Pollution Disparities in U.S. Cities. Environ. Sci. Technol. Lett. 2022, 9, 345–350. 10.1021/acs.estlett.1c01012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morello-Frosch R.; Lopez R. The Riskscape and the Color Line: Examining the Role of Segregation in Environmental Health Disparities. Environ. Res. 2006, 102, 181–196. 10.1016/j.envres.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Wells E. C.; Vidmar A. M.; Webb W. A.; Ferguson A. C.; Verbyla M. E.; de los Reyes F. L.; Zhang Q.; Mihelcic J. R. Meeting the Water and Sanitation Challenges of Underbounded Communities in the U.S. Environ. Sci. Technol. 2022, 56, 11180–11188. 10.1021/acs.est.2c03076. [DOI] [PubMed] [Google Scholar]
- McDonald Y. J.; Jones N. E. Drinking Water Violations and Environmental Justice in the United States, 2011–2015. Am. J. Public Health 2018, 108, 1401–1407. 10.2105/ajph.2018.304621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory D. C.; Rahman T. Environmental Justice and Enforcement of the Safe Drinking Water Act: The Arizona Arsenic Experience. Ecol. Econ. 2009, 68, 1825–1837. 10.1016/j.ecolecon.2008.12.010. [DOI] [Google Scholar]
- Stone D.; Sherman J.; Hofeld E. Arsenic in Oregon Community Water Systems: Demography Matters. Sci. Total Environ. 2007, 382, 52–58. 10.1016/j.scitotenv.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Balazs C.; Morello-Frosch R.; Hubbard A.; Ray I. Social Disparities in Nitrate-Contaminated Drinking Water in California’s San Joaquin Valley. Environ. Health Perspect. 2011, 119, 1272–1278. 10.1289/ehp.1002878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravalli F.; Yu Y.; Bostick B. C.; Chillrud S. N.; Schilling K.; Basu A.; Navas-Acien A.; Nigra A. E. Sociodemographic Inequalities in Uranium and Other Metals in Community Water Systems across the USA, 2006–11: A Cross-Sectional Study. Lancet Planet. Health 2022, 6, e320–e330. 10.1016/s2542-5196(22)00043-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcillo C.; Krometis L.-A.; Krometis J. Approximating Community Water System Service Areas to Explore the Demographics of SDWA Compliance in Virginia. Int. J. Environ. Res. Public Health 2021, 18, 13254. 10.3390/ijerph182413254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H.; Calafat A. M.; Karvonen-Gutierrez C. A.; Park S. K. Isomer-Specific Serum Concentrations of Perfluorooctane Sulfonic Acid among U.S. Adults: Results from the National Health and Nutrition Examination Survey (NHANES) and the Study of Women’s Health Across the Nation Multi-Pollutant Study (SWAN-MPS). Environ. Sci. Technol. 2023, 57, 385–394. 10.1021/acs.est.2c04501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbo N.; Stoiber T.; Naidenko O. V.; Andrews D. Q. Locally Caught Freshwater Fish across the United States Are Likely a Significant Source of Exposure to PFOS and Other Perfluorinated Compounds. Environ. Res. 2023, 220, 115165. 10.1016/j.envres.2022.115165. [DOI] [PubMed] [Google Scholar]
- Liu M.; Nordstrom M.; Forand S.; Lewis-Michl E.; Wattigney W. A.; Kannan K.; Wang W.; Irvin-Barnwell E.; Hwang S.-A. Assessing Exposures to Per- and Polyfluoroalkyl Substances in Two Populations of Great Lakes Basin Fish Consumers in Western New York State. Int. J. Hyg. Environ. Health 2022, 240, 113902. 10.1016/j.ijheh.2021.113902. [DOI] [PubMed] [Google Scholar]
- Weber B.; Jensen L.; Miller K.; Mosley J.; Fisher M. A Critical Review of Rural Poverty Literature: Is There Truly a Rural Effect?. Int. Reg. Sci. Rev. 2005, 28, 381–414. 10.1177/0160017605278996. [DOI] [Google Scholar]
- Wallsten S.; Kosec K. The Effects of Ownership and Benchmark Competition: An Empirical Analysis of U.S. Water Systems. Int. J. Ind. Organ. 2008, 26, 186–205. 10.1016/j.ijindorg.2006.11.001. [DOI] [Google Scholar]
- Statman-Weil Z.; Nanus L.; Wilkinson N. Disparities in Community Water System Compliance with the Safe Drinking Water Act. Appl. Geogr. 2020, 121, 102264. 10.1016/j.apgeog.2020.102264. [DOI] [Google Scholar]
- Tickamyer A. R.; Duncan C. M. Poverty and Opportunity Structure in Rural America. Annu. Rev. Sociol. 1990, 16, 67–86. 10.1146/annurev.so.16.080190.000435. [DOI] [Google Scholar]
- Davide D. F.; Alessandra F.; Roberto P. Distributive Justice in Environmental Health Hazards from Industrial Contamination: A Systematic Review of National and near-National Assessments of Social Inequalities. Soc. Sci. Med. 2022, 297, 114834. 10.1016/j.socscimed.2022.114834. [DOI] [PubMed] [Google Scholar]
- Hanna B. G. House Values, Incomes, and Industrial Pollution. J. Environ. Econ. Manage. 2007, 54, 100–112. 10.1016/j.jeem.2006.11.003. [DOI] [Google Scholar]
- de Vor F.; de Groot H. L. F. The Impact of Industrial Sites on Residential Property Values: A Hedonic Pricing Analysis from the Netherlands. Reg. Stud. 2011, 45, 609–623. 10.1080/00343401003601925. [DOI] [Google Scholar]
- Buzzelli M.Modifiable Areal Unit Problem. In International Encyclopedia of Human Geography; Elsevier, 2020; pp 169–173. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.